Abstract
The Middle East respiratory syndrome coronavirus (MERS-CoV) presents an ideal example for developing One Health concepts. Dromedary camels are the principal reservoir for the virus. Infected camels shed the virus in body secretions, particularly nasal discharges. MERS-CoV has the potential to remain active in the environment for some time under optimum conditions of temperature and humidity. This shedding sustains the virus in endemic communities and thus contact with camels is considered a major risk factor for human infection. Reducing virus shedding from camels will have a great positive impact on reducing the human risk of infection. Our main objective is to highlight the potential aspects of reducing virus shedding from camels to the environment, thereby reducing the possibility of human infection. We will focus on the potential roles of camel markets, camel shows, importation, transportation and grazing in the amplification and shedding of the virus, providing some novel concepts for the control approaches for the MERS-CoV.
Keywords: MERS-CoV, One Health, Dromedary camel, Human, Transmission, Shedding
1. Introduction
MERS-CoV is an emerging viral pathogen of humans discovered in Saudi Arabia in late 2012 [1]. There are 2449 laboratory-confirmed cases from 27 countries reported to the World Health Organization (WHO) as of Aug 2nd, 2019. Of these, a total of 845 people passed away, with a case fatality rate of almost 34.5% [2]. Dromedary camels are the main reservoir of the virus. The infected camels shed the virus in large quantities into through their body secretions, particularly the nasal secretions [3]. Humans that come into close contact with dromedary camels or consume their products are at potential risk of infection [4].
The severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in southern China at Guangdong in 2012 [5]. MERS-CoV and SARS-CoV are members of the family Coronaviridae and the order Nidovirales. Asian palm civet Paradoxurus hermaphroditus was the main reservoir of SARS-CoV [6]. Eradication and culling of the palm civet in China contributed substantially to the eradication- of SARS-CoV [7]. Another example of the use of One Health approach to control zoonotic viral diseases was practiced in case of the Hendra virus in Australia. This virus causes serious problems for in close contact with Hendra virus infected or dead horses. The main reservoir of Hendra virus are the flying foxes (fruit bats). Adoption of some One health control strategies helped in the minimizing human exposure of this virus in Australia [[8], [9], [10]]. These strategies included the culling trials of bats as well as vaccination of horses. All these measures reduced the potential risk of human infection with Hendra virus [9,11]. Adoption of similar strategy for MERS-CoV in the light of the One Health approach could be a promising control trend.
2. Example for the design of typical animal markets in the Arabian Peninsula
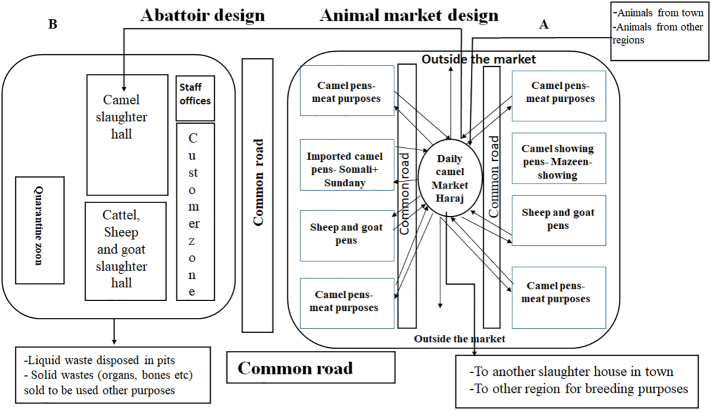
Most animal markets are usually designed to display, maintain and trade various species of animals especially sheep, goats, and camel. In many cases, the markets are located in close proximity to some regional slaughterhouses. The markets mainly consist of several pens. Each pen is assigned to hold and maintain one species of animals. The majority of the pens are assigned to dromedary camels, which are grouped according to the purpose of their admission to the market. Some are used for meat and are then slaughtered a short time after their admission. Some animals are displayed for the purpose of camel shows (Fig. 1). Some animals are sold for breeding purposes. This is in addition to the pens assigned for the imported camel breeds from Somalia and Sudan. All these pens are separated by metal fences and connected to each other (Fig. 1). Pens assigned for sheep and goats are co-located with the dromedary camel pens. Animals can be moved from one place to another across the market freely. Camel herders may visit several pens of different animals each day freely. Every afternoon, markets hold large-scale animal sales (Haraj) in the central square of the market. Animals are brought from different animal pens and even from outside the market from the neighboring regions as well as remote cities in the square. Thus, a large number of animals are mixed for at least 4 hours per day. They are sold for different purposes including slaughtering, breeding, and camel show. These animals are mixed together with animals from the market as well as from outside in one large square. At the end of the Haraj, some animals are returned to their pens in the market, some are sold and transferred to the regional or neighboring abattoirs to be slaughtered, and some are transferred to other regions for a variety purposes.
Fig. 1.
Design showing the construction of an animal market and a slaughterhouse in the Arabian Peninsula.
(A) The animal market consists of several pens to accommodate several species of animals especially dromedary camels, sheep, and goat. Each pen is assigned for one group of animals on the two sides of the market. Some pens assigned to accommodate the foreign imported breed of camels from Sudan and Somalia. The daily Haraj market is designed at the center of the market. Some animals from all pens in the market and from outside from the neighboring regions participate in this daily sale held in every afternoon. Some of the sold animals are transferred directly to the adjacent abattoir. Some other animals transported to the other regions for various purposes (breeding, slaughtering, showing, etc). The non-sold animals return to their pens in the market. (B) Design of a slaughterhouse in the Arabian Peninsula. It consists of several slaughtering halls assigned for each species. Some halls are connected to each other. There is an animal quarantine zone in front of the market. There is a customer hall, which is connecting the slaughtering halls and separated with glass partitions. Some animals transferred from the local adjacent market to the abattoir for further slaughtering and processing.
3. Design of abattoirs
Most of the abattoirs are designed to have several slaughtering halls (Fig. 1). Each hall is used for slaughtering and processing of one species of animals (Fig. 1). In many cases however, the slaughtering halls are physically connected. Animals are usually admitted to the entrance region where they subjected to physical examination. Sick animals, or those for which they have concerns, are kept in a quarantine area for further testing and action. The customer hall, is connected with the main slaughtering and processing halls but separated by glass partitions. The veterinarians, staff, and employees in the abattoirs usually move freely between slaughtering halls and come in close contact with live animals and their body fluids and tissues before and after slaughtering.
4. Camel transportation nationally and internationally
Live dromedary camels are usually transported from one place to another for different purposes including sale, treatment in regional veterinary clinics, etc. The imported animals are transferred from the ports to their destinations in various regions. Animals may be moved from one place to another. Animals are usually packed together and transported in open vehicles. Sometimes large trucks are used to transfer a large number of animals in one shipment. Camel transportation by truck is not common in most of the cases especially during grazing time. Usually, camels are driven by camel herders parallel to national highways over large distances and passing, sometime, in close proximity to many cities on their way.
5. Camel show (Mazaeen)
Camel shows are one of the traditions in the Arabian Peninsula. Mazaeen involves a mass gathering of dromedary camels from across the Arabian Peninsula and is held annually at different places. In this, gathered animals are used in several multistage competitions. Animals are kept in only large place for almost two months. Mazaeen attracts a large number of people to attend for entertainment in addition to the camel owners, staff and employees.
6. Camel grazing
Camels are outdoor animals. They graze in deserts for most of their time. In the Arabian Peninsula, animals are moved for grazing after the rainy seasons in search of pasture. Animals usually move across the borders of different countries in the Arabian Gulf.
7. Camel reproduction and breeding
Breeding in dromedary camels is mainly practiced through natural insemination. Usually a common high pedigree male camel is used to inseminate several female animals within the herd and from other herds. In most of the cases, some camel owners may transfer their female camels for long distance in the sake of high pedigree male camel. On the other hands, a high pedigree male camel may be transferred over a long distance to another in a far distance to inseminate large number of female animals. This procedure usually occur during the ratting season from November to April each year. During the process of mating both male and female animals, come in close contact. Each mating time between the male and female camels is about 20 minutes. It is usually accompanied by gurgling and frothing which may exaggerate virus shedding [12]. This may pose a risk of transmission of MERS-CoV from infected to the non-infected animal. This may contribute to the amplification of MERS-CoV in certain region.
8. One Health based control strategies for MERS-CoV
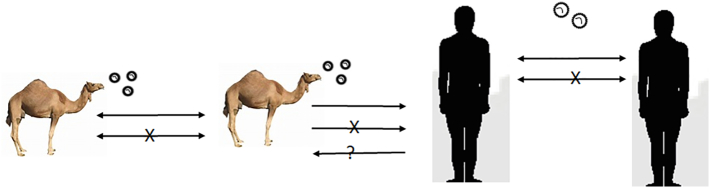
The main theme of these One Health control strategies is to reduce the MERS-CoV transmission from camel to camel and from camel to human thus, reducing the shedding from camel to camel and from camel to human thus the potential risk to human can be minimized (Fig. 2).
Fig. 2.
Proposed model for the Middle East respiratory syndrome coronavirus/dromedary camels/human interaction.
The MERS-CoV is transmitted among dromedary camels through the respiratory routes. Strong evidence of the transmission of the virus from dromedary camels to human. Human to human transmission is well documented. Blockage or minimizing the virus shedding from camels will reduce the potential risk of infection with MERS-CoV, pathways marked by the symbol (X). The non-confirmed pathway is marked by a question mark (?).
8.1. Strategies related to the animal markets and slaughterhouses
Several studies showed a high prevalence of the MERS-CoV in dromedary camels in camel markets and animals admitted to the abattoirs in the Arabian Peninsula, North and central Africa and Pakistan [[13], [14], [15], [16]]. Camel market thus enable amplification and recombination of the MERS-CoV [[17], [18], [19]]. There are several potential ways to reduce the animal-to-animal transmission in the markets, thus reducing the potential human risk. The animal markets should be relocated to a far distance from major cities and villages. The ideal location of the market should be downstream of the prevailing wind. The market should be re-organized into separate isolated pens per each group of animals. Special consideration should be paid to ensure enough space for each animal and between adjacent animal pens. Overcrowdings of animals may facilitate the virus transmission between animals particular among the active viral shedders. Thus, reducing the number of animals per each pen will minimize the risk of transmission of the virus between animals in the same pen and to neighboring pens. It is recommended to separate the pens of dromedary camels from other species as sheep and goat. Restriction of animal movement between markets should be encouraged. Imported breed of camels should be placed in separate pens apart from the local breeds. One study showed an absence of specific antibodies in sera of some camel herders, veterinarians, and abattoir staff [20]. However, several studies showed the detection of MERS-CoV antibodies in sera of people, camel particular camel workers, veterinarians and abattoir employees [15,16,[21], [22], [23], [24]]. One recent study reported a high prevalence of MERS-CoV antibodies among camel workers in Saudi Arabia [25]. Regardless of the discrepancy between the research group and the sensitivity of the techniques to detect the virus-specific antibodies as well as antigen in the at-risk people, special precautions should be taken by these groups of people who get in close contact with dromedary camels.
People in the abattoirs should wear special PPE, particularly N95 mask, gowns, and goggles during their active contact with camels during the whole process of slaughtering including inspection, slaughtering evisceration, etc. A separate hall should be assigned for slaughtering each species of animal. Customers should not be allowed in close contact with slaughtered animals, until they receive the final meat. The carcasses should be transported from the abattoir in well-equipped leak-proof vehicles, which do not allow any leakage of the body fluids of the slaughtered animals. The liquid wastes from the abattoirs should not be allowed to be merged with the common municipal sewage system. Special decontamination processes should be practiced to get rid of the animal waste after slaughtering. Animal skins should be chemically treated before leaving the abattoir to avoid harboring any contamination from the body fluids of the animals.
8.2. Strategies related to camel racing and show (Mazaeen)
More recently, most animals in shows are microchipped, with such information as birth date, sex, breed, and owner information recorded. This is a very useful tool in careful monitoring all animals through the duration of the camel show, which may extend to more than two months. Special attention should be paid to test the animals prior to the introduction to the camel shows. Active MERS-CoV shedding animals should be placed in a remote quarantine area. They should be sampled regularly at weekly interval until they show no virus shedding in their body secretions. Random sampling of animals participating in the shows and exhibition during the period of the show should be conducted to ensure the absence of active shedders in a certain group of animals. People who come in close contact with animals should wear PPE. People attending the show are advised not to come in close contact with camels.
8.3. Strategies related to animal transportation and movement
Camel movement could be from one region to another within the same country or between different countries. There is an active international trade marketing between countries from the Arabian peninsula and countries from north and central Africa particularly Sudan, Somalia, Kenya, Mali, Ethiopia, Burkina Faso, Nigeria, Morocco, etc. Saudi Arabia is the largest camel importer from these countries especially during the pilgrimage season every year. Dromedary camels from these countries show high MERS-CoV seroprevalence and virus shedding [3,13,15,[26], [27], [28], [29], [30], [31]]. A recent study showed the circulating strains of MERS-CoV in Africa are somewhat different in the genomic level from the currently circulating strains in the Arabian Peninsula [32]. The African strains lack the ORF-4 of the MERS-CoV. Thus, this may explain the possibility of recombination and mutations between different strains of MERS-CoV. Special attention should be paid to the importation and movement of dromedary camels between different countries. Imported camels should be sampled and tested for the presence of MERS-CoV antibodies and antigens at the country of origin. They must be quarantined upon arrival, sampled and tested by serology and for the presence of virus in nasal secretions in their nasal secretions. Sero-positive or virus-shedding camels should be quarantined before being allowed into the country. Special attention should be given to camels imported for other purposes such as breeding and shows. These animals will eventually be mixed with local breeds and therefore poses greater risk of MERS-CoV to the local camel breeds. Internal transport of animals should be done in well-equipped vehicles, which should be ventilated and closed. Overcrowding of animals in one vehicle should be prohibited. Shipping act as one of the stress factors posed on the animals and the very close contact between animals favor the transmission not only of MERS-CoV but also other pathogens they may harbor.
Dromedary camels usually spend most of their times on pasture for grazing. They are moved from one place to another in a search of pasture. Several herds of animals may get into close proximity and share the same land for grazing at the same time. Some countries have banned grazing in their lands or in certain regions. Camels move across the border of the countries and are mixed with the animals from the neighboring countries. Theoretically, there should be rules governing the grazing and crossing of borders. Animals should be monitored not only for MERS-CoV but also for other pathogens before being allowed contact with other animals. Regular monitoring of animals on the open grazing regions is highly recommended to follow up on the health status of animals. This will help in the identification of active shedders of the virus, which may contaminate the pasture and act as a source of infection of any subsequent grazing by other herds.
8.4. Strategies related to the consumption of various camel meat and dairy products
One study showed the stability of MERS-CoV experimentally introduced into camel milk [33]. Another study showed the detection of some MERS-CoV neutralizing antibodies and RNAs in camel milk collected by the traditional methods used in Qatar [34]. An expert opinion study was recently conducted to assess the hazard posed by the consumption of raw camel milk and raw or undercooked camel meat [35]. Thorough boiling or pasteurization of camel milk before consumption should be practiced to avoid any potential risk of transmission of MERS-CoV through the ingestion of contaminated milk. Similarly, thorough cooking of camel meat and meat products before consumption is highly recommended.
8.5. Continuous surveillance and monitoring of MERS-CoV among dromedary camel
Several studies reported the molecular and serological surveillance of MERS-CoV among dromedary camels in many countries in Asia and Africa [13,15,16,26,27,36,37]. However, these studies have been conducted on a random basis. Some of them included only a partial population of a camel at a certain region in a country. Moreover, there is a lack of information from some African countries that have large camel population such as Sudan, Mauritania, and Somalia. There is an urgent need for a more systematic and well-designed surveillance system where intensive dromedary camels are raised and exist. These surveillance systems will help in mapping the camel population that shed or seroconvert to MERS-CoV in various regions. Careful monitoring of the genetic changes of MERS-CoV in both camel and human population should be done at a regular intervals.
8.6. Strategies for dealing with active MERS-CoV dromedary camels herd shedders
WHO established a standard operating protocol (SOP) for the handling of the active human MERS-CoV cases [38,39]. However, there are no established strategies for dealing with active shedder animals as is the in case for other viral pathogens such as the highly pathogenic avian influenza viruses. Although, some countries have started to develop their own protocols, they are not applicable on a large scale across the world. One major burden is that MERS-CoV does not produce obvious clinical signs in camels despite mild nasal discharge and lacrimation [3,40]. This makes it very difficult to identify MERS-CoV infected camels or to assess their health conditions based on the clinical signs. The gold standard for the identification of the active infected animal is laboratory testing. WHO established another SOP for laboratory examination, processing and testing of suspected human cases with MERS-CoV [35]. Animal to animal transmission is reported during the course of MERS-CoV infection in a certain animal population V [41]. Animal to animal transmission is reported during the course of MERS-CoV infection in certain animal populations [3,27,42]. Ideally, regular monitoring of the dromedary camel population should be scheduled. Special precautions should be adopted for dealing with the positive active shedder animals either within a herd or in camel markets. When positive animals are detected in camel markets, application of quarantine measures should be applied in that market. None of the animals should get in or out of the market during the course of the viral infection. Periodic testing of a statistically significant number of animals from all corners as well as the center of the market should be also conducted. Special precautions should be paid from handlers and camel attendants as well as veterinarians. The workers must wear the appropriate PPE all the time when dealing with animals. Testing and data analysis of animals on a weekly basis should be performed. Thorough cleaning and disinfection of the market should be practiced regularly. The market should not be opened again to the public until all animals are tested free from shedding the virus for at least two consecutive time interval with two weeks apart. A similar approach could be adopted in case of detection of positive MERS-CoV animal in a particular herd. Additionally, the positive camel herd should not be moved during the active course of virus shedding. They should not allowed to graze in an open pasture to avoid spreading of the virus through their secretions.
8.7. Reducing the shedding of MERS-CoV from dromedary camels through vaccination
There is a controversial discussion about the idea of using vaccines against MERS-CoV in camels and humans. Some studies showed a high seroprevalence of MERS-CoV among dromedary camels in Asia and Africa [13,15,16,26,36,37,[43], [44], [45]]. Other research showed the possibility of reinfection of dromedary camels with MERS-CoV in the presence of neutralizing antibodies in sera of the infected animals [42]. However, several experimental approaches were adopted to prepare and test the efficacy of some vaccine candidates for MERS-CoV in experimental animals, dromedary camels and alpaca [[46], [47], [48], [49], [50]]. A recent study showed that application of an adjuvant-MERS-CoV-S1 vaccine in dromedary camels and alpaca resulted in the production of robust neutralizing antibodies [46]. This study supported the notion that vaccinating young camels under two years will reduce the virus shedding from these naïve animals. Thus, the risk of human infection could be minimized by vaccination.
8.8. Reducing the infection among at risk group of people
WHO issued some standard operating protocols for dealing with positive MERS-CoV human and dromedary camels [51]. The elder and immunocompromised people suffering from some chronic diseases such as diabetes, high blood pressure, cancer should practice extreme precautions if they visit camel barns or market especially in the Middle East. People come in close contact with dromedary camels on daily basis such as camel owners, veterinarians; slaughterhouse employees should protect themselves by adopting standard personal hygiene. This include wearing the proper PPE, taking showers after finishing their work, leaving their clothes and gowns in special laundry where they can be thoroughly decontaminated [51]. They should not accompany these clothes to their homes or outside the camel market or slaughterhouse.
9. Conclusions
Although MERS-CoV had emerged almost seven years ago, there are many aspects of the virus/human/animal that interactions are not well identified. Furthermore, there is no effective medication or vaccine to treat or prevent viral infection in both human and animals. The One Health approach has many applications, which can be of great help in the control of the pathogens of animal origin. Adoption of the above-mentioned strategies will have a great impact on the reduction of the virus shedding from the dromedary camels to the environment and subsequently to the human. Thus, the risk of human infection with MERS-CoV could be minimized.
Authors' contribution
MGH and AAA conceived the idea, did data analysis and drafted the manuscript.
Declaration of Competing Interest
Authors declared there is no conflict of interest.
Acknowledgments
We wish to thank the King Abdul-Aziz City for Science and Technology (KACST) for their generous funding through the MERS-CoV research grant program (number 20-0004), which is a part of the Targeted Research Program (TRP).
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; 2019. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)http://www.who.int/emergencies/mers-cov/en/2019 Available: [Google Scholar]
- 3.Hemida M.G., Chu D.K., Poon L.L., Perera R.A., Alhammadi M.A., Ng H.Y. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg. Infect. Dis. 2014;20(7):1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 5.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10(12 Suppl):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 2005;11(12):1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng V.C., Chan J.F., To KK, Yuen K.Y. Clinical management and infection control of SARS: lessons learned. Antivir. Res. 2013;100(2):407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendez D., Buttner P., Speare R. Hendra virus in Queensland, Australia, during the winter of 2011: veterinarians on the path to better management strategies. Prev Vet Med. 2014;117(1):40–51. doi: 10.1016/j.prevetmed.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton D., Pallister J., Klein R., Feng Y.R., Haining J., Arkinstall R. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014;20(3):372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiethoelter A.K., Schembri N., Dhand N.K., Sawford K., Taylor M.R., Moloney B. Australian horse owners and their biosecurity practices in the context of Hendra virus. Prev Vet Med. 2017;148:28–36. doi: 10.1016/j.prevetmed.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Kung N.Y., Field H.E., McLaughlin A., Edson D., Taylor M. Flying-foxes in the Australian urban environment-community attitudes and opinions. One Health. 2015;1:24–30. doi: 10.1016/j.onehlt.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanvilkar A.V., Samant S.R., Ambore B.N. Reproduction in Camel. Veterinary World. 2009;2(2):72–73. [Google Scholar]
- 13.Ali M.A., Shehata M.M., Gomaa M.R., Kandeil A., El-Shesheny R., Kayed A.S. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg Microbes Infect. 2017;6(1):e1. doi: 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamau E., Ongus J., Gitau G., Galgalo T., Lowther S.A., Bitek A. Knowledge and practices regarding Middle East Respiratory Syndrome Coronavirus among camel handlers in a Slaughterhouse, Kenya, 2015. Zoonoses Public Health. 2019;66(1):169–173. doi: 10.1111/zph.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect. Dis. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zohaib A., Saqib M., Athar M.A., Chen J., Sial A.U., Khan S. Countrywide survey for MERS-coronavirus antibodies in dromedaries and humans in Pakistan. Virol. Sin. 2018;33(5):410–417. doi: 10.1007/s12250-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo P.C., Lau S.K., Fan R.Y., Lau C.C., Wong E.Y., Joseph S. Isolation and characterization of dromedary camel coronavirus UAE-HKU23 from dromedaries of the Middle East: minimal serological cross-reactivity between MERS coronavirus and dromedary camel coronavirus UAE-HKU23. Int. J. Mol. Sci. 2016;17(5) doi: 10.3390/ijms17050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusof M.F., Eltahir Y.M., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50(3):509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusof M.F., Queen K., Eltahir Y.M., Paden C.R., Al Hammadi Z., Tao Y. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg Microbes Infect. 2017;6(11):e101. doi: 10.1038/emi.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemida M.G., Al-Naeem A., Perera R.A., Chin A.W., Poon L.L., Peiris M. Lack of middle East respiratory syndrome coronavirus transmission from infected camels. Emerg. Infect. Dis. 2015;21(4):699–701. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Kahlout R.A., Nasrallah G.K., Farag E.A., Wang L., Lattwein E., Muller M.A. Comparative serological study for the prevalence of anti-MERS coronavirus antibodies in high- and low-risk groups in Qatar. J Immunol Res. 2019;2019 doi: 10.1155/2019/1386740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farag E.A., Reusken C.B., Haagmans B.L., Mohran K.A., Stalin Raj V., Pas S.D. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 2015;5 doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reusken C.B., Farag E.A., Haagmans B.L., Mohran K.A., GJt Godeke, Raj S. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013-2014. Emerg. Infect. Dis. 2015;21(8):1422–1425. doi: 10.3201/eid2108.150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikkema R.S., Farag E., Himatt S., Ibrahim A.K., Al-Romaihi H., Al-Marri S.A. Risk factors for primary middle east respiratory syndrome coronavirus infection in Camel workers in Qatar during 2013-2014: a case-control study. J. Infect. Dis. 2017;215(11):1702–1705. doi: 10.1093/infdis/jix174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshukairi A.N., Zheng J., Zhao J., Nehdi A., Baharoon S.A., Layqah L. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. MBio. 2018;9(5) doi: 10.1128/mBio.01985-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu D.K., Oladipo J.O., Perera R.A., Kuranga S.A., Chan S.M., Poon L.L. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Nigeria, 2015. Euro Surveill. 2015;20(49) doi: 10.2807/1560-7917.ES.2015.20.49.30086. [DOI] [PubMed] [Google Scholar]
- 27.Chu D.K., Poon L.L., Gomaa M.M., Shehata M.M., Perera R.A., Abu Zeid D. MERS coronaviruses in dromedary camels, Egypt. Emerg. Infect. Dis. 2014;20(6):1049–1053. doi: 10.3201/eid2006.140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu D.K.W., Chan S.M.S., Perera R., Miguel E., Roger F., Chevalier V. A46 MERS-CoV in Arabian camels in Africa and Central Asia. Virus Evol. 2017;3 doi: 10.1093/ve/vew036.045. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiambi S., Corman V.M., Sitawa R., Githinji J., Ngoci J., Ozomata A.S. Detection of distinct MERS-Coronavirus strains in dromedary camels from Kenya, 2017. Emerg Microbes Infect. 2018;7(1):195. doi: 10.1038/s41426-018-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miguel E., Chevalier V., Ayelet G., Ben Bencheikh M.N., Boussini H., Chu D.K. Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015. Euro Surveill. 2017;22(13) doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Doremalen N., Hijazeen Z.S., Holloway P., Al Omari B., McDowell C., Adney D. High prevalence of Middle East respiratory coronavirus in young dromedary camels in Jordan. Vector Borne Zoonotic Dis. 2017;17(2):155–159. doi: 10.1089/vbz.2016.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu D.K.W., Hui K.P.Y., Perera R., Miguel E., Niemeyer D., Zhao J. MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity. Proc. Natl. Acad. Sci. U. S. A. 2018;115(12):3144–3149. doi: 10.1073/pnas.1718769115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Doremalen N., Bushmaker T., Karesh W.B., Munster V.J. Stability of Middle East respiratory syndrome coronavirus in milk. Emerg. Infect. Dis. 2014;20(7):1263–1264. doi: 10.3201/eid2007.140500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reusken C.B., Farag E.A., Jonges M., Godeke G.J., El-Sayed A.M., Pas S.D. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 2014;19:23. doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- 35.Funk A.L., Goutard F.L., Miguel E., Bourgarel M., Chevalier V., Faye B. MERS-CoV at the animal-human interface: inputs on exposure pathways from an expert-opinion elicitation. Front Vet Sci. 2016;3:88. doi: 10.3389/fvets.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falzarano D., Kamissoko B., de Wit E., Maiga O., Cronin J., Samake K. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health. 2017;3:41–43. doi: 10.1016/j.onehlt.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harcourt J.L., Rudoler N., Tamin A., Leshem E., Rasis M., Giladi M. The prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health. 2018 Sep;65(6):749–754. doi: 10.1111/zph.12482. Epub 2018 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Clinical management of severe acute respiratory infections when novel coronavirus is suspected: What to do and what not to do. 2013. http://wwwwhoint/entity/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13updf Available online.
- 39.WHO Infection prevention and control during health care for probable or confirmed cases of novel coronavirus (nCoV) infection Interim guidance. 2013. http://wwwwhoint/entity/csr/disease/coronavirus_infections/IPCnCoVguidance_06May13pdf Available online.
- 40.Khalafalla A.I., Lu X., Al-Mubarak A.I., Dalab A.H., Al-Busadah K.A., Erdman D.D. MERS-CoV in upper respiratory tract and lungs of dromedary camels, Saudi Arabia, 2013-2014. Emerg. Infect. Dis. 2015;21(7):1153–1158. doi: 10.3201/eid2107.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO 2012. http://www.who.int/entity/csr/disease/coronavirus_infections/LaboratoryTestingNovelCoronavirus_21Dec12.pdf Available online.
- 42.Hemida M.G., Alnaeem A., Chu D.K., Perera R.A., Chan S.M., Almathen F. Longitudinal study of Middle East Respiratory Syndrome coronavirus infection in dromedary camel herds in Saudi Arabia, 2014-2015. Emerg Microbes Infect. 2017;6(6) doi: 10.1038/emi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemida M.G., Perera R.A., Al Jassim R.A., Kayali G., Siu L.Y., Wang P. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19(23) doi: 10.2807/1560-7917.es2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemida M.G., Perera R.A., Wang P., Alhammadi M.A., Siu L.Y., Li M. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18(50) doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 45.Reusken C.B., Haagmans B.L., Muller M.A., Gutierrez C., Godeke G.J., Meyer B. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adney D.R., Wang L., van Doremalen N., Shi W., Zhang Y., Kong W.P. Efficacy of an adjuvanted Middle East Respiratory Syndrome coronavirus spike protein vaccine in dromedary camels and alpacas. Viruses. 2019;11(3) doi: 10.3390/v11030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35(30):3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munster V.J., Wells D., Lambe T., Wright D., Fischer R.J., Bushmaker T. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines. 2017;2:28. doi: 10.1038/s41541-017-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song F., Fux R., Provacia L.B., Volz A., Eickmann M., Becker S. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J. Virol. 2013;87(21):11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H. Protective efficacy of recombinant modified vaccinia virus ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2015;89(16):8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO . WHO/MERS/RA/August18 Geneva, Switzerland: World Health Organization; 2018 Licence: CC BY-NC-SA 30 IGO. 2018. MERS-CoV global summary and assessment of risk. [Google Scholar]