Abstract
Alzheimer’s disease (AD) is a large and increasing unmet medical need with no disease-modifying treatment currently available. Genetic evidence from genome-wide association studies (GWASs) and gene network analysis has clearly revealed a key role of the innate immune system in the brain, of which microglia are the most important element. Single-nucleotide polymorphisms (SNPs) in genes predominantly expressed in microglia have been associated with altered risk of developing AD. Furthermore, microglia-specific pathways are affected on the messenger RNA (mRNA) expression level in post-mortem AD tissue and in mouse models of AD. Together these findings have increased the interest in microglia biology, and numerous scientific reports have proposed microglial molecules and pathways as drug targets for AD. Target identification and validation are generally the first steps in drug discovery. Both target validation and drug lead identification for central nervous system (CNS) targets and diseases entail additional significant obstacles compared to peripheral targets and diseases. This makes CNS drug discovery, even with well-validated targets, challenging. In this article, we will illustrate the special challenges of AD drug discovery by discussing the viability/practicality of possible microglia drug targets including cluster of differentiation 33 (CD33), KCa3.1, kynurenines, ionotropic P2 receptor 7 (P2X7), programmed death-1 (PD-1), Toll-like receptors (TLRs), and triggering receptor expressed in myeloid cells 2 (TREM2).
Keywords: drug target, microglia, target identification, target validation, screening cascade
Highlights:
Glial cell–based targets receive increased attention in drug development.
What makes a good drug target?
Steps and pitfalls for preclinical drug development.
Specific consideration for putative microglial drug targets in Alzheimer’s disease.
Introduction
Microglial reactivity has long been recognized as a pathological hallmark of a wide variety of neurological diseases. While morphological changes of microglia were initially interpreted as a reactive response to neuronal damage, growing evidence suggests that these changes are not merely “reactive” but indicate that glial pathology contributes to disease progression (Raj et al., 2014a; Verkhratsky et al., 2014; Ben Haim et al., 2015; Bergles and Richardson, 2015; Biber et al., 2015; Heneka et al., 2015; Heppner et al., 2015; Jain et al., 2015; Ransohoff and El Khoury, 2015; Robel and Sontheimer, 2015; Zuchero and Barres, 2015; Osborn et al., 2016). Especially with regard to Alzheimer’s disease (AD), numerous reviews cover various aspects of microglial biology in the context of disease (see for example: (Guedes et al., 2018; Hansen et al., 2018; Kinney et al., 2018; Shi and Holtzman, 2018; Thei et al., 2018; Ulland and Colonna, 2018; Henstridge et al., 2019). Using the search term “microglia Alzheimer,” PubMed lists close to 100 reviews that were published in the last 2 years (Jan 2017–Jan 2019). It is not the purpose of the current review to discuss the potential role of microglia in AD, but to focus on the challenges and practical aspects of microglial proteins or pathways as potential drug targets.
A considerable set of genetic risk factors for AD are predominantly expressed in microglia (Wes et al., 2016; Henstridge et al., 2019). Thus, it is very clear from human genetics that regardless of being “reactive,” “active,” “dystrophic,” “senescent,” “dysfunctional,” or “disease associated,” microglial involvement in AD offers a hitherto unexplored intervention point. Many publications concerning microglia in AD include conclusions like: “The presented data here suggest that protein ‘XYZ’ can be considered as a drug target for AD.” As there are some common misunderstandings between colleagues focused on biology vs. drug discovery regarding the definition of a target, we first aim to clarify what it takes to consider a microglial molecule suitable for a drug discovery program. We will then review some commonly suggested microglial targets and pathways, i.e., cluster of differentiation 33 (CD33), KCa3.1, kynurenines (KYN), ionotropic P2 receptor 7 (P2X7), programmed death-1 (PD-1), Toll-like receptors (TLRs), and triggering receptor expressed in myeloid cells 2 (TREM2), discussing their potential role in AD and their respective challenges in regard to drug discovery.
Target Identification
In general, preclinical drug discovery can be staged as follows: target identification, in which the validity of a target for modulating a disease process and the initial chemical matter along with the means of characterizing them are established; lead optimization, in which target validity is further strengthened while identifying candidate molecules with the full range of necessary drug-like properties; and finally, preclinical testing for safety and efficacy. Apart from target identification, we will not discuss the other stages of preclinical drug development ( Figure 1 ). The interested reader is referred to (Möller and Boddeke, 2016) for a detailed description of the whole process.
Figure 1.
Schematic representation of the preclinical drug discovery process. The preclinical drug discovery process can generally be divided into six unique steps from target identification to preclinical testing.
For potential targets, it is of utmost importance to demonstrate relevancy to the disease and that modulating the target would result in therapeutic benefit with an acceptable safety margin. Evidence for a role of a candidate target in disease might include i) altered expression in disease, ii) integral relationship to disease pathophysiology, iii) genetic association with disease, iv) having a mechanistic link to disease etiology, and v) demonstration of a beneficial effect when modulated in a disease-relevant in vitro or (preferably) in vivo model.
Unfortunately, generating expression data for the human central nervous system (CNS) is extremely difficult. While for peripheral diseases biopsies, or blood draws are routine, for the CNS, most parameters can only be derived from post-mortem brain tissue that at best represents end-stage disease, or by limited imaging methods, making it difficult to observe changes in earlier and therapeutically more relevant disease states. Non-human models can also provide insight, but with significant translational uncertainties.
A strong genetic association of a microglial molecule with AD, for example, CD33, complement receptor 1 (CR1), phospholipase C gamma 2 (PLCγ2), and TREM2 (Wes et al., 2016; Henstridge et al., 2019), also suggests promise as a drug target. If such evidence can further be strengthened by a mechanistic link to a disease model by in vitro or in vivo genetic or pharmacologic intervention studies [e.g., knocking out CD33 (Bradshaw et al., 2013)], that would serve to increase confidence in the target.
What Makes a Good (Microglial) Target?
Drugability
Besides linking a target to disease, there are other more pragmatic considerations for selecting a target. Admittedly, “drugable” is not a well-defined term; it usually includes chemical tractability with a ligand (small molecule or antibody) but can also refer more broadly to the properties of such ligands, e.g., pharmacokinetics, distribution, metabolism (and the pharmacologic properties of significant metabolites), pharmaceutical properties including chemical stability, and manufacturability.
Traditionally, a target would be considered chemically tractable if a small molecule or antibody can bind with high affinity and specificity and induce the desired biological effect. For example, G-protein coupled receptors (GPCRs) are generally considered chemically tractable; more than 30% of currently marketed drugs target GPCRs (Ma and Zemmel, 2002; Hauser et al., 2017). Ion channels or enzymes are also considered tractable as those targets often include structural features that permit potent and specific ligand binding, with consequent direct inhibition of conductance or catalytic activity. Blocking protein–protein interactions is generally more difficult; however, recent advances have challenged this view, and modulation of protein–protein interactions by small molecules is now an area of active research and will increase the number of targets considered drugable (Higueruelo et al., 2013). Furthermore, blocking protein–protein interactions can often be accomplished with therapeutic antibodies, and several innovative approaches have been devised to improve antibody penetration into the brain (Watts and Dennis, 2013; Salameh and Banks, 2014; Pardridge, 2015). Despite these recent advancements, developing therapeutics for the CNS remains challenging, and while there are many biologically interesting molecules related to microglia, only a small subset of them might be considered drugable.
Specificity
It is not merely enough to generate a potent pharmacological agent against a drug target; it is also of utmost importance that the molecule is selectively modulating only this one target to avoid off-target effects that may hamper the interpretation of experimental data. Unfortunately, many so-called “reference compounds,” such as commercially available inhibitors, are not as specific as advertised by the suppliers (Frye, 2010). A group of 50 scientists from different life science disciplines have covered this topic in detail in Commentary in Nature Chemical Biology (Arrowsmith et al., 2015). This lack of specificity makes interpretation of results gained with many compounds virtually impossible. A prominent example in microglia biology (although not directly related to a single target) is minocycline, which often is referred to as a “microglia inhibitor” (Möller et al., 2016).
Safety
Arguably the most important criterion for target selection, however, is safety. During clinical development, many drugs fail, not due to the lack of efficacy but due to adverse safety events inadequately predicted by preclinical studies. Toxicity might not only arise from modulating the target itself, meaning that the therapeutic window is small, but that there could be off-target effects of a drug, or that toxicity may also arise from a metabolite of the drug that itself is toxic. It is therefore important to select targets with the highest likelihood of being safe as early as possible. A common question is how broadly a target is expressed. Localized expression preferentially in the target cell and/or tissue is considered advantageous as there is less likelihood for adverse effects unrelated to the desired mechanism of action. Unfortunately, there are very few potential targets restricted to microglia as most potential targets are also expressed in peripheral myeloid cells, so effects on the peripheral immune system are a persistent concern. Safety concerns, whether based on side effects of a development compound, a target knockout phenotype, or suspected essential physiological roles of the target, need to be addressed as early as possible by experiments.
Taken together, there is a whole range of drugability considerations that must be met to make a target practical for drug discovery. There are few targets in microglia that are often discussed in the literature to be of value in AD. Here we will discuss the potential and challenges around these targets when it comes down to drugability aspects.
Currently Proposed Microglial Drug Targets
Proposed Role of CD33 in AD
In 2011, large-scale genome-wide association studies (GWASs) identified CD33, a member of the sialic acid–binding immunoglobulin-like lectins (siglecs) family, as a genetic locus associated with the risk to develop AD (Hollingworth et al., 2011; Naj et al., 2011). This association with AD risk has been confirmed in a variety of subsequent genetic studies in different ethnic groups, with odds ratios between 0.7 and 0.9; thus, the CD33 rs3865444A allele protects from the development of AD [for recent meta-analysis, see (Jiang et al., 2018)]. It was furthermore described that the protective single-nucleotide polymorphism (SNP) rs3865444A is in linkage disequilibrium with rs12459419T, which causes increased splicing of exon2, and therefore deletes the sialic acid–binding site of CD33. The protective SNP therefore leads to increased expression of a truncated, non-functional version of the receptor, called d2-CD33 or CD33m (Bradshaw et al., 2013; Malik et al., 2013; Raj et al., 2014b). A reduction in full-length CD33 expression was observed in myeloid cells of CD33 rs3865444A allele carriers, resulting in an overall reduced CD33 function (Bradshaw et al., 2013; Raj et al., 2014b).
CD33 signaling in humans depends on two intracellular inhibitory domains, one immunoreceptor tyrosine-based inhibitory motif (ITIM), and one ITIM-like domain. CD33 is therefore considered to be an inhibitory immune receptor. Indeed, when activated CD33 becomes tyrosine-phosphorylated by Src kinases, which subsequently recruit Src homology-2 domain (SH2)–containing tyrosine phosphatases (SHP1/2), this results in the dephosphorylation and inhibition of signaling cascades (see for review Macauley et al., 2014). CD33 is expressed in myeloid cells, and its signaling negatively regulates the function of these cells. Accordingly, knockdown of CD33 in human monocytes leads to spontaneous release of interleukin 1 beta (IL-1β), Tumor necrosis factor alpha (TNFα), and IL-8 (Lajaunias et al., 2005). In monocytes from carriers of the CD33 rs3865444A allele (decreased CD33 function), an increased uptake capacity of amyloid beta(1–42) (Aβ1–42) was described (Bradshaw et al., 2013).
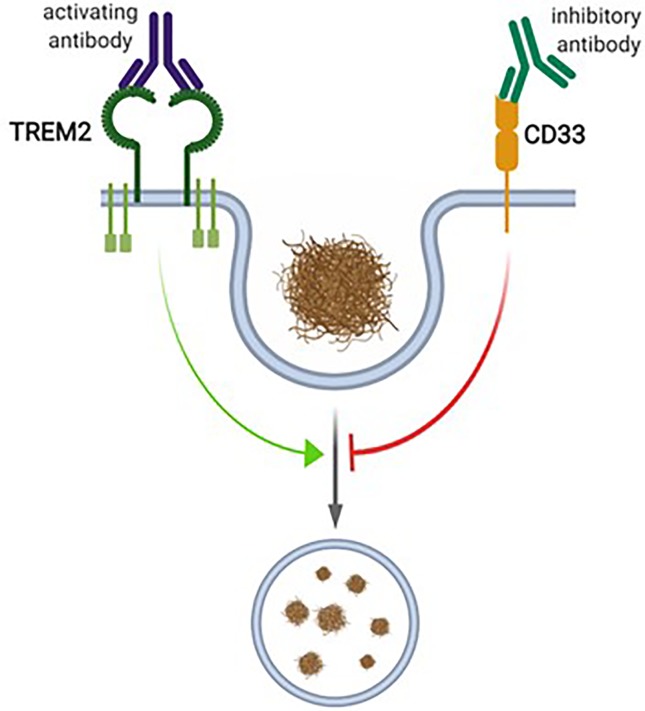
In the brain, CD33 is specifically expressed in microglia (Bradshaw et al., 2013; Griciuc et al., 2013; Malik et al., 2013; Walker et al., 2015), where an inverse relationship between CD33 expression and Aβ uptake capacity was observed (Griciuc et al., 2013). Accordingly, in the cortex of AD patients, a positive correlation of microglial CD33 expression and amyloid pathology was described, indicating that increased CD33 expression in microglia promotes plaque pathology (Griciuc et al., 2013). CD33-deficient Amyloid precursor protein (APP)/Presenilin 1 (PS1) mice showed reduced amyloid pathology compared to age-matched wildtype (WT) controls, supporting the idea that reduced microglial CD33 function is beneficial with respect to amyloid pathology (Griciuc et al., 2013). Given this variety of data concerning the role of CD33 as regulator of microglial Aβ uptake capacity, this siglec receptor is currently considered as drug target in AD. It is considered that inhibition of CD33 function in microglia would increase their amyloid uptake capacity, which in turn would be beneficial in AD Figure 2 (Heneka et al., 2015).
Figure 2.
The proposed interplay between CD33 and TREM2 in microglial phagocytosis. CD33 and TREM2 have been proposed to be inhibitors or activators of microglial phagocytosis, respectively. For TREM2, activating antibodies, and for CD33, inhibitory antibodies, have been proposed as therapeutic interventions. Figure created with Biorender.com
Target Challenges Around CD33
There are many challenges with respect to CD33 as a drug target in AD. In general, finding ligands for siglecs is difficult, as glycan binding often is of relatively low affinity and relies on multivalency to achieve adequate affinity (Varki, 2009). There is evidence that CD33 binds α2.3 sialic acid and α2.6 sialic acid; the corresponding glycosylated proteins that are decorated with these sialic acids, however, have not been identified (Linnartz-Gerlach et al., 2014; Macauley et al., 2014). As CD33 is the shortest of all known siglecs, it most likely is activated in a cis-manner (O’Reilly and Paulson, 2009; Linnartz-Gerlach et al., 2014; Macauley et al., 2014), meaning that the sialylated glycoproteins that activate CD33 would also be expressed in microglia. One report provides data that soluble sialyllactosamine in the mM range might activate CD33 in de-sialylated monocytes (Lajaunias et al., 2005). This finding awaits independent confirmation. Apart from our very limited understanding around natural ligands for CD33, functional tool compounds for CD33 have not yet been described. The only published molecule displaying a reasonable binding at CD33 is cpd22, which displaces bead-coupled α2.6 sialic acid with an half maximal effective concentration (EC50) of about 10 µM (Rillahan et al., 2014). It therefore is suggested that cpd22 binds CD33 at the sialic acid–binding domain; whether this binding affects CD33 signaling is not understood. Thus, it is not clear whether cpd22 might function as an agonist or antagonist for CD33. The lack of ligands or tool compounds seriously hampers the development of functional analysis of CD33 in cellular assays, making the development of functional inhibitors for CD33 difficult.
Therapeutic inhibitors of CD33 are hypothesized to increase phagocytic properties in microglia; from a drug development perspective, they might also be envisaged to inhibit the CD33 signaling cascade. It is not yet understood how recruitment of SHP1 leads to inhibition of cellular phagocytosis in CD33-expressing myeloid cells (Linnartz-Gerlach et al., 2014).
Another challenge concerns the large species difference of CD33. Mouse and human CD33 differ not only in the extracellular domain but also in the transmembrane domain as well as intracellularly. In contrast to human CD33, mouse CD33 only has one ITIM-like domain but lacks the ITIM domain (Cao and Crocker, 2011). Moreover, in the transmembrane region of mouse CD33, a positively charged residue is present, which might enable mouse CD33 to recruit immunoreceptor tyrosine-based activation motif (ITAM)-motif–containing adapter proteins like DNAX activating protein of 12 kDa (DAP12) (Linnartz-Gerlach et al., 2014). These data suggest that mouse CD33 can act as an activating receptor instead of being inhibitory, a hypothesis that awaits experimental evidence. In cultured microglia from CD33KO mice, it was shown that CD33 negatively couples to phagocytosis (Griciuc et al., 2013). Whether this is due to ITIM-like signaling or recruitment of ITAM-motif–containing adapter proteins is currently an open question. Potential drugs that inhibit CD33 function, however, need to be tested in animal models. Whether or not the mouse would be an adequate model system to do so is questionable given the species differences and the limited knowledge on CD33 function in mouse cells.
Taken together, despite the genetic association between CD33 and AD development and the straightforward hypothesis of how reduced CD33 function might protect from AD, CD33 remains a target with its unique set of challenges.
Proposed Role of KCa3.1 in AD
KCa3.1 is an intermediate-conductance potassium channel that is activated when intracellular calcium is increased. The Ca2+ binds to calmodulin, which is constitutively bound to the calmodulin binding domain of the channel, resulting in the opening of the K+ channel (Kd ∼ 300 nM) (Xia et al., 1998; Fanger et al., 1999; Bouhy et al., 2011). The KCa3.1-mediated K+ efflux augments the driving force for further Ca2+ influx via Ca2+ release-activated calcium (CRAC) channels and subsequent refilling of intracellular calcium stores. Thus, one of the primary functions of KCa3.1 is the augmentation of Ca2+ signaling (Feske et al., 2012). Expression of KCa3.1 in the CNS and its controversies have been reviewed (Dale et al., 2016). Here we will focus on microglia, as the expression of KCa3.1 was established using well-validated antibodies and demonstration of voltage-independent KCa3.1-like Ca2+-activated K+ currents.
Voltage-independent KCa3.1-like Ca2+-activated K+ currents in cultured murine microglia were first reported by Eder et al. (Eder et al., 1997). Khanna et al. subsequently demonstrated the presence of KCa3.1 mRNA in cultured rat and mouse microglia (Khanna et al., 2001). The conclusion that microglia express KCa3.1 was supported by pharmacological studies that demonstrated that charybdotoxin and clotrimazole could inhibit the voltage-independent KCa3.1-like Ca2+-activated K+ currents (Schilling et al., 2002; Schilling and Eder, 2004). There is further evidence that charybdotoxin and clotrimazole prevent the induced microglial oxidative burst (Khanna et al., 2001) and microglial migration (Schilling and Eder, 2004). The KCa3.1 inhibitor TRAM-34 reduced lipopolysaccharide (LPS)-induced microglial neurotoxicity (Kaushal et al., 2007), and inhibition of KCa3.1 using senicapoc (see below) blocked the LPS-induced IL-1β and Nitric oxide (NO) production by microglia (Staal et al., 2017a).
Early in vivo studies using antibodies validated in KCa3.1 WT and knock out (KO) mice did not provide any evidence of KCa3.1 expression on any cells in the mouse or human brain (including juvenile, adult, and aged mice) (Schilling and Eder, 2004; Schilling and Eder, 2007). However, studies using antibodies validated in this manner demonstrated KCa3.1 expression on CD68-positive cells (microglia or macrophages) upon injury to the CNS as induced by ischemic injury (Chen et al., 2011), suggesting that KCa3.1 is expressed by microglia only in response to injury. The study by Chen et al. also demonstrated the therapeutic efficacy of inhibiting KCa3.1 (using TRAM-34) in ischemic injury, a finding they validated using KCa3.1-null mice (Chen et al., 2011; Chen et al., 2015). Many other studies have since demonstrated the therapeutic efficacy of KCa3.1 inhibition in other models of neurological injury and disease in which microglia are believed to play a significant role, including experimental autoimmune encephalomyelitis, traumatic brain injury, spinal cord injury, optic nerve transection, glioblastoma multiforme, neuropathic pain, and AD (Dale et al., 2016; Staal et al., 2017b).
Taken together, there is convincing evidence for the expression of KCa3.1 in cultured rodent microglia. While the details of regulation of KCa3.1 await confirmation with more specific tool compounds, the available data suggest a role for KCa3.1 at the very least in microglia reactive oxygen species (ROS) production and signaling.
Challenges in KCa3.1 Inhibition as Therapeutic Intervention in AD
The presence of “activated” microglia identified by expression of markers of microglial activation [major histocompatibility complex II (MHCII), Iba1] or an activated morphology (enlarged cell bodies and shorter, thicker processes) in brains of AD is well established. The observation that microglial “activation” can occur prior to plaque deposition and neurofibrillary tangle formation, and correlates with cognitive deficits, is consistent with the theory that microglial activation occurs at the earliest stages of disease (perhaps even before disease onset) and is somehow tied to its progression (Okello et al., 2009a; Okello et al., 2009b; Sastre et al., 2011). Maezawa et al. demonstrated that TRAM-34 blocks Aβ-induced proliferation of microglia, p38 Mitogen-activated protein kinase (MAPK) phosphorylation, nuclear factor kappa b (NF-κb) activation, and NO generation in primary microglia cultures (Maezawa et al., 2012). Furthermore, TRAM-34 inhibited neurotoxic effects of Aβ oligomers in mixed microglia–neuron cultures and in organotypic hippocampal slices by decreasing microglial activation and partially preventing synaptic loss (Maezawa et al., 2011).
Senicapoc is a potent and selective inhibitor of KCa3.1 developed for the treatment of sickle cell anemia [half maximal inhibitory concentration (IC50) = 11 nM; screening of 57 targets yielded no hits below 1 µM other than KCa3.1] (Ataga et al., 2006; Ataga et al., 2008; Ataga and Stocker, 2009; Dale et al., 2016; Staal et al., 2017a). In a recent and comprehensive study, Maezawa and colleagues demonstrated that KCa3.1 is functionally upregulated in microglia in brains from 5xFAD mice as well as AD patients (Jin et al., In Press). The authors demonstrate that Aβ oligomers impair long-term potentiation (LTP) and that this effect is reversed by the KCa3.1 inhibitor TRAM-34 as well as senicapoc in hippocampal slices or in vivo. It was furthermore demonstrated in this paper that senicapoc can reduce the pro-inflammatory effects as well as rescue the hippocampal LTP affected by Aβ oligomer (Jin et al., In Press). Senicapoc was then tested in the 5xFAD mice, starting at 6 months of age and continuing for three months. Results show that treatment with senicapoc reduced the amyloid load as well as neuroinflammation while enhancing neuronal plasticity compared to vehicle-treated mice (Jin et al., In Press). Thus, the study provides a strong rationale for repurposing senicapoc for the treatment of AD.
In fact, the Alzheimer’s Drug Discovery Foundation has provided funding to manufacture clinical-grade senicapoc and conduct the required stability testing in support of an investigational new drug (IND) filing. Upon approval of the IND filing, senicapoc will be tested in a phase II clinical trial in prodromal and mild AD (https://www.alzdiscovery.org/news-room/announcements/1.8-million-in-new-funding-supports-clinical-stage-treatments).
Senicapoc has been through extensive preclinical safety testing as well as phase I, II, and III clinical trials with few significant side effects noted. Given our knowledge of the pharmacokinetics of senicapoc, including its CNS penetrance (Staal et al., 2017b), it is likely that a dose similar to that used in the previous clinical trials will be used. AD patients, however, are very different from patients with sickle cell disease. It is likely that the AD patients will be older and have more comorbidities than those with sickle cell anemia. The age and comorbidities could reveal underlying susceptibilities to KCa3.1 inhibition that may not have been problematic in younger patients with sickle cell disease (e.g., susceptibility to infections). On the other hand, many diseases or conditions afflicting the elderly have inflammatory components, and a mild immunosuppressant such as senicapoc may benefit those conditions as well (e.g., stroke, chronic and neuropathic pain, atherosclerosis).
Yet, the current AD study is still incredibly important to test the hypothesis that targeting neuroinflammation, specifically KCa3.1, can slow or halt the progression of AD. If the trial results demonstrated significant and convincing slowing of disease progression, pharmaceutical companies could initiate drug discovery projects to develop improved CNS penetrant KCa3.1 inhibitors.
Targeting the Kynurenine Pathway to Treat AD
Development of drugs that target products of tryptophan metabolism has transformed the treatment of psychiatric disorders, particularly with the advent of serotonin (5-HT) reuptake inhibitors (Wong et al., 1995). However, it is noteworthy that metabolism to 5-HT represents only a small fraction of the fate of free tryptophan, and in fact, the great majority is converted to KYN (Leklem, 1971) and its subsequent products by an array of enzymes located throughout the body and brain. Yet, surprisingly, there are no marketed medications that directly target metabolism of the KYN pathway.
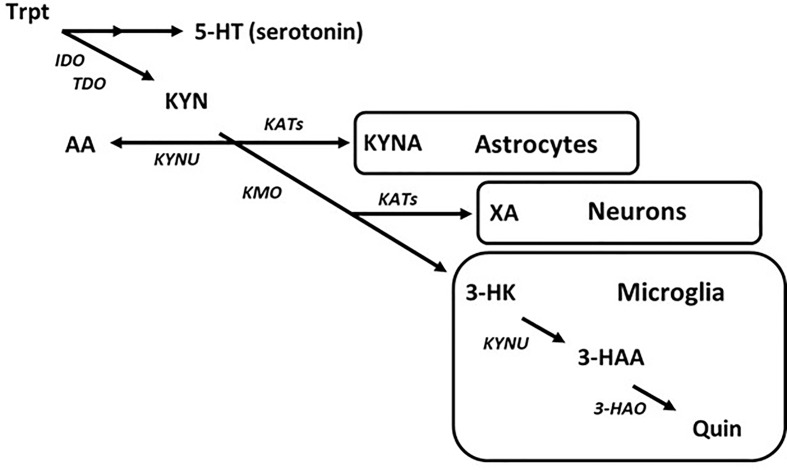
Entry into the KYN pathway begins with metabolism of tryptophan into N-formylkynurenine by indole-2,3-dioxygenase (IDO) or tryptophan-2,3-dioxygenase (TDO), which is then rapidly converted to L-kynurenine (Takikawa et al., 1986; Zhang et al., 2007). Metabolism of KYN produces a host of neuro-active products in the brain that may contribute to CNS diseases (Török et al., 2016; Lovelace et al., 2017; Schwarcz and Stone, 2017). The KYN pathway bifurcates into neurotoxic and neuroprotective metabolic routes. As an example, astrocytes largely express kynurenine aminotransferases (KATs) and so are the primary source of the neuroprotective metabolite, kynurenic acid (KYNA), in the brain (Guidetti et al., 2007). Alternatively, microglia express kynurenine 3-monooxygenase (KMO), which converts KYN to neurotoxic metabolites such as 3-hyroxykynurenine (3-HK) and subsequently quinolinic acid (Quin) (Heyes et al., 1996; Guillemin et al., 2003a) ( Figure 3 ). While the topic of this discussion is targeting the disruption in microglial KYN metabolism, one should understand that the various branches of KYN metabolism do not act in isolation, and so a change in one may cause a change in the others by altering the availability of substrate.
Figure 3.
The kynurenine pathway of tryptophan metabolism in the brain. Simplified diagram depicting the tryptophan–kynurenine pathway. Metabolism of tryptophan along the kynurenine pathway in the brain is regulated by a variety of enzymes that are largely segregated by cell type.
Inflammation triggers activation of microglia and dysregulation of KYN metabolism, leading to production of neurotoxic metabolites from the KMO branch, a process that is predicted to be involved in the development of AD pathology (Campbell et al., 2014; Zádori et al., 2018). However, direct evidence of microglial KYN metabolism from human AD subjects suggests that this effect may be limited to localized pathological events rather than global changes in CNS KYN metabolism. As an example, levels of KYN and 3-HK were both surprisingly reduced in CSF of AD patients with dementia (Tohgi et al., 1992). However, tryptophan, 5-HT, and melatonin were all also significantly reduced in the same study. In addition, the same group reported that KYN was reduced in a second cohort of patients with AD dementia but not in ones with vascular dementia (Tohgi et al., 1995). Furthermore, Quin (Mourdian et al., 1989; Heyes et al., 1992) and KYNA (Wennström et al., 2014) were reportedly unchanged in recent studies, though earlier evidence indicated that KYNA could also be decreased (Heyes et al., 1992; Hartai et al., 2007; Gulaj et al., 2010) in AD patients. Overall, there is limited evidence that CSF tryptophan/KYN metabolites are reproducibly altered in AD, though where it has been assessed, most studies point to either no consistent changes in metabolites or a reduction in KYN metabolism ( Table 1 ).
Table 1.
Kynurenine metabolite profile from patients with Alzheimer’s Disease.
In post-mortem AD brain tissue, early studies reported a lack of change in KYN metabolism. Indeed, Quin levels were shown to be unchanged (Moroni et al., 1986; Mourdian et al., 1989; Sofic et al., 1989; Pearson and Reynolds, 1992), as were KYNA concentrations (Flint Beal et al., 1992). However, more recent analysis revealed regional changes that indicated greater production of KYN as well as shunting of KYN metabolism toward production of neurotoxic microglial metabolites. Immunohistochemical analysis showed that both TDO and IDO were elevated in the hippocampus of AD patients (Guillemin et al., 2005; Wu et al., 2013). Importantly, the increase in IDO expression was found in microglia, astrocytes, and neurons, with the primary product found in proximity to senile plaques and neurofibrillary tangles in post-mortem AD brain being Quin, the microglial product (Guillemin et al., 2005; Bonda et al., 2010). These data are in line with reports that treatment of microglia with Aβ1–42 induces IDO expression (Guillemin et al., 2003a; Guillemin et al., 2003b) and that Quin may seed α-synuclein aggregation (Tavassoly et al., 2018) and induce tau hyperphosphorylation (Rahman et al., 2009). Given the well-documented evidence that tryptophan and KYN metabolism is sensitive to inflammatory stimuli (Campbell et al., 2014; Strasser et al., 2017), it is plausible to predict that neuroinflammation centered around plaques and tangles in the brains of AD patients produces localized pro-inflammatory activation of microglia, resulting in dysregulation of KYN metabolism, which favors production of neurotoxic metabolites such as 3-HK and Quin. A deleterious impact resulting in local brain circuit disruption and longer-term neurodegeneration is hypothesized, but further analysis in longitudinal studies is needed to confirm or disprove this.
Challenges of Developing Kynurenine Pathway Drugs for AD
The main challenge for developing KYN metabolite inhibitors for AD is a clear understanding of the role of KYN metabolic products in the development and/or progression of AD, as well as the availability of agents to test the efficacy of CNS inhibition of neurotoxic KYN enzymes, specifically KMO. Peripheral measures of KYN metabolism in serum and plasma support the hypothesis that the KYN pathway is induced in AD, leading to increased production of KYN and products associated with the microglial metabolic branch. However, it is unclear whether this peripheral pattern of metabolism reflects CNS KYN metabolism or contributes to neural pathology. Indeed, distribution of metabolites in the CNS (brain, CSF) suggest a more nuanced pattern of regulation that also supports a shunting of tryptophan metabolism toward neurotoxic KYN metabolites, but only in proximity to plaques and tangles, indicating a need for brain-penetrable KMO inhibitors to act locally at the site of pathology. As discussed above, there is also a lack of longitudinal data on KYN metabolism in AD patients and an analysis of how these data correlate with disease progression or severity state. The limited correlation between blood and CSF metabolite profiles, as well as with post-mortem brain patterns, highlights the need for methodologies that correlate disease progression with direct measurements of brain KYN metabolites and/or enzymes in living AD patients. A concerted effort to develop positron-emission tomography (PET) ligands and/or other imaging agents targeting the KYN pathway would be a great benefit toward understanding the role of this pathway in AD and improving the rationale for the substantial investment required to develop brain-penetrant drugs.
While KMO has been targeted by the pharmaceutical industry for at least 3 decades, there are no published agents with good CNS drug exposure. Importantly, targeting KMO in the periphery creates a substantial increase in substrate, KYN, which is actively transported into the brain, where it is converted to substances such as KYNA, which could be neuroprotective, but also into 3-HK and Quin (Beaumont et al., 2016), which may increase oxidative stress and neuronal damage. This could be especially problematic in neurodegenerative disorders such as AD where neuroinflammation associated with plaques and tangles is predicted to increase KMO expression, thus more readily converting KYN to 3-HK and Quin (Guillemin et al., 2003a; Guillemin et al., 2005; Bonda et al., 2010). Therefore, it will be necessary to focus drug discovery efforts on identifying KMO inhibitor chemotypes with a high degree of CNS penetrance to test their therapeutic benefit in AD. Indeed, it may even be advantageous to attempt developing non-competitive KMO inhibitors along the lines of recent advances in KAT II inhibitors (Dounay et al., 2012) since they would not compete with increased KYN pumped into the brain from the periphery that could otherwise limit their efficacy. Finally, while the paucity of brain-penetrant drugs targeting the KMO branch of the KYN pathway is discouraging, advances have been made in developing agents that stimulate the neuroprotective KAT branch, such as 4-Chlorokynurenine (4CL-KYN; AV-101). Peripheral administration of 4Cl-KYN is transported into the brain, where it is converted to 7-chloro-KYNA, thereby acting as a locally synthesized KYNA mimetic (Hokari et al., 1996), where it appears to have promising neuroprotective effects (Wu et al., 2000; Wu et al., 2002). These data are encouraging and support the hypothesis that development of centrally acting KMO inhibitors could be neuroprotective in AD since blocking microglial KYN metabolism would not only prevent production of neurotoxic metabolites such as 3-HK and Quin but also indirectly increase endogenously produced KYNA. Thus, a greater understanding of the impact of rebalancing the neurotoxic and neuroprotective branches of the KYN pathway in AD may provide additional mechanistic evidence to support development of a new generation of neuroprotective brain-penetrant KMO inhibitors.
The P2X7–NLRP3 Axis in Alzheimer’s Disease
The P2X7–NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain–containing inflammasome complex 3) pathway has received extensive attention, with several publications pointing to its potential role in neuroinflammatory disorders, from psychiatry to neurodegenerative diseases. Microglia express several cell-surface and cytoplasmic sensors of danger signals that react to invading “dangers” and cause neuroinflammation. The ATP-gated ion channel P2X7 is expressed abundantly in microglia and responds to extracellular ATP, which often occurs during tissue distress or damage and can lead to activation of the NLRP3 inflammasome complex, producing pro-inflammatory cytokines, IL-1β and IL-18 (Bhattacharya and Biber, 2016). As such, the P2X7–NLRP3 pathway has been the focus of intense drug discovery efforts: while brain-penetrant P2X7 antagonists have made progress and are in the clinic ( Table 2 ), brain-penetrant, drug-like NLRP3 inhibitors are the focus of medicinal chemistry discovery programs.
Table 2.
Clinical strategies used to dampen IL-1β signaling.
| P2X7 Antagonists | Indication(s) | Comments |
|---|---|---|
| CE-224,535 (Pfizer) |
Rheumatoid arthritis Not in development |
Failed efficacy study (Ph-II) Not brain penetrant Blocked ex-vivo human IL-1β |
| AZD-9056 (AstraZeneca) |
Rheumatoid arthritis Crohn’s Not in development |
+ve signal in Chron’s +ve & -ve signals in RA Blocked ex vivo human IL-1β Not brain penetrant |
| GSK-1482160 (GlaxoSmithKline) |
Pain (intended) Not in development |
Phase-I safety study Blocked ex-vivo human IL-1β Brain penetrant |
| SGM-1019 (Second Genome) |
NASH (intended) In active development |
Phase-I safety study No information on compound |
| JNJ-54175446 (Janssen) |
CNS In active development |
Phase-I safety study Blocked ex-vivo human IL-1β Brain penetrant |
| JNJ-55308942 (Janssen) |
CNS In active development |
Phase-I safety study Brain penetrant |
| NLRP3 Inhibitors | Indication(s) | Comments |
| OLT-1177 (Olatec) |
Inflammatory disorders In active development |
Phase-II (CAPS) No ex-vivo human IL-1β data In-vitro blockade of IL-1β No brain penetration data |
| Caspase-1 inhibitors | Indications | Comments |
| VX-765 (Vertex) |
Epilepsy Psoriasis Not in development |
Brain penetrant |
| Vx-740 (Vertex) |
Rheumatoid arthritis Psoriasis Not in development |
Pro-drug |
| IL-1β biologics | Indications | Comments |
| Anakinra | Rheumatoid arthritis | IL-1r antibody Marketed product (injection) |
| Rilonacept | Inflammatory disorders CAPS, FCAS MWS |
IL-1β and IL-1α antibody Marketed product (injection) |
| Canakinumab (Novartis) |
Atherosclerosis Lung cancer |
IL-1β antibody Phase-III |
P2X7 belongs to the P2X family of trimeric ligand–gated cation channels. Its activation by ATP allows for the influx of several cations, including Ca2+, Na+, and K+ (Bartlett et al., 2014). Even though P2X7 expression is abundant in microglia (and in peripheral immune cells), it is “silent” under normal physiology, where ATP concentrations do not reach the high micromolar levels required to activate its ion channel (Bhattacharya and Biber, 2016). As such, P2X7 is an attractive drug target as antagonism of a silent channel by true neutral antagonists would not cause any serious target mediated (adverse) effects: antagonism will only be evident when the channel is activated by high ATP concentrations, which is believed to occur during neuroinflammatory disorders of the CNS. NLRP3, on the other hand, is a convergent point of inflammasome activation, both dependent and independent of P2X7 activation (Bhattacharya and Jones, 2018), and as such is an attractive drug target. The advantages and disadvantages of targeting P2X7 vs. NLRP3 in neuroinflammatory disorders remain to be determined.
The P2X7–NLRP3 pathway is a case in point where microglia-driven neuroinflammation may play a role in AD causality (Heneka et al., 2013), and there has been growing interest in probing the role of pathogenic Aβ in neuroinflammation, although the exact mechanism of its involvement is still unknown (Illes et al., 2019; Martin et al., 2019; Thawkar and Kaur, 2019). P2X7 activation and consequent release of IL-1β is one such mechanism of interest in the AD field in the quest to understand the role of neuroinflammation in AD (Sanz et al., 2009). P2X7 upregulation has been reported in post-mortem AD brains and in animal models of tauopathy and amyloid deposition, mostly around the areas of pathology (Parvathenani et al., 2003; McLarnon et al., 2006; Lopez-Gonzalez et al., 2015). For example, P2X7 upregulation was seen in cortical samples from AD patients compared to healthy volunteers (Martin et al., 2019). With the recent discoveries of clinically available P2X7 PET ligands (Bhattacharya, 2018), the detection of P2X7 upregulation in humans becomes possible. In a mouse model of AD/frontotemporal lobar degeneration (FTLD) (P301S), enhanced uptake of P2X7 PET signal was detected in brain regions of tauopathy (Jin et al., 2018). P2X7 activation has also been linked to neuronal damage and synaptotoxicity in transgenic AD models (Lee et al., 2011). There are signals of an active P2X7–NLRP3 axis in animal models of AD. For example, in the J20 mouse model of AD, which overexpresses the human APP gene with two familial AD-linked mutations, the authors noted that P2X7 activation prevents APP processing by α-secretase, facilitating the formation of toxic Aβ via P2X7-mediated activation of glycogen synthase kinase 3 (GSK-3) (Diaz-Hernandez et al., 2012). Supporting these data, inhibition of P2X7 with Brilliant Blue G (BBG) in the same mouse model increased α-secretase activity and reduced the formation of Aβ plaques, supporting a beneficial role of P2X7 antagonism in AD (Chen et al., 2014). Recently, it was reported that mice lacking P2X7 have reduced Aβ load and cognitive impairment in another AD model overexpressing both human mutated APP and mutated presenilin-1 (APP–PSEN1) (Martin et al., 2018). In addition to P2X7, the downstream intracellular NLRP3 inflammasome has been receiving special attention through several publications, and there is a growing interest to bring forward NLRP3 inhibitors for clinical testing in AD (Heneka, 2017; Heneka et al., 2018). For example, MCC950 (NLRP3 inhibitor) has been reported to prevent cognitive decline in APP/PS1 mice (Dempsey et al., 2017). A comprehensive study by Venegas et al. demonstrated mechanistically that ASC [adapter protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD)] specks (involved in NLRP3 activation) contributed to the spreading of Aβ plaques in APP/PSEN1 double-mutant mice (Venegas et al., 2017). The study by Martin et al. points to the unique role of P2X7, outside the NLRP3–IL-1β signaling cascade. While support for the NLRP3 pathway being involved in disease is gaining momentum, there are subtleties of targeting P2X7 vs. NLRP3 inflammasome with therapeutics. P2X7 antagonism may bring additional benefits as highlighted in the study by Martin et al. (2018). While NLRP3 is downstream of P2X7 and may be a critical mediator of IL-1β release through the inflammasome, P2X7 antagonism may provide additional benefits such as modulation of synaptic plasticity, modulation of chemokine release (e.g., CCL3), and consequent effects on CD8+ T cell recruitment to focal regions of the diseased brain.
Challenges Around P2X7/NLRP3
While the scientific community continues to generate data supporting the role of P2X7–NLRP3 in models of AD, there must be a healthy balance of caution and opportunistic investments in clinical trials to test these new mechanisms. The biggest challenge of NLRP3 for AD is a lack thus far of a suitable clinical compound with acceptable brain penetration. For P2X7, several brain-penetrant antagonists have been disclosed, some of which are in clinical development ( Table 2 ). With the availability of brain-penetrant P2X7 antagonists and P2X7 PET ligands, these clinical tools could be tested in a patient population, perhaps with the help of PET and associated biomarkers (CSF, blood, genomics) that would enrich AD patients with an active P2X7–NLRP3 axis. In addition to using P2X7 PET ligands as imaging tools to enrich patient clusters for proof-of-concept testing, one must also demonstrate target engagement at clinically achievable doses. Target engagement in patients receiving a clinical candidate for proof-of-concept testing is critical to the evaluation of primary end points. Emerging data supporting the role of microglia-driven processes (neuroinflammation, phagocytosis), and description of different phenotypes of microglia in normal and disease states has prompted renewed thinking regarding their role in AD. To that end, it is of some comfort to witness that the role of microglia and microglia-driven neuroinflammation in AD has been the subject of some high-profile publications in the last few years, including significant progress with clinical compounds. While brain-penetrant P2X7 compounds are clinically available ( Table 2 ), NLRP3 inhibitors that adequately demonstrate central target engagement (with NLRP3 PET ligands) are currently lacking. Demonstration of central target engagement is fundamental to drug development; once a dose-occupancy is established, moving to AD patients is the next step, with the challenge of selecting patients who can be intervened in early on in the disease process.
Proposed Role of PD-1 Immune Checkpoint Blockade in AD
PD-1 (or CD279) is a member of the CD28 family of T cell regulators (Riley and June, 2005). It is weakly expressed on naive T cells but can be induced upon activation in several types of immune cells, including B cells, T cells, natural killer cells, dendritic cells, and monocytes. PD-1 plays an important role in immune responses related to B cell activity and/or T cell activity, such as antibody production, immune tolerance, and autoimmunity (Okazaki et al., 2013). PD-1 function is important to preserve immune tolerance. Taken together, PD-1 signaling ameliorates immune overreaction.
Recent studies have indicated that boosting the immune response in the brain might have therapeutic potential. It was shown that inhibition of forkhead box protein P3 (FOXP3)-positive regulatory T cells increased interferon-γ (IFNγ)–dependent leukocyte trafficking to the brain, promoting clearance of Aβ and improving cognitive function in a mouse model of amyloidosis (Baruch et al., 2015). As PD-1 checkpoint blockade was previously reported to stimulate IFNγ-dependent immune responses in cancer immunotherapy, the therapeutic potential of PD-1 blockade in AD models was explored (Baruch et al., 2016).
In their studies, the authors treated the 5XFAD mouse model of amyloidosis at 10 months of age (an age with advanced pathology) with two intraperitoneal injections of a PD-1–specific antibody with 3-day intervals. One week after the first treatment, the mice were reported to exhibit a systemic IFNγ immune response. Further analysis of the myeloid cell populations in the brain upon treatment documented IFNγ-dependent recruitment of monocyte-derived macrophages as characterized by high expression of lymphocyte antigen 6c and expression of the chemokine receptor CCR2. Intriguingly, treatment also resulted in reduced cognitive deficits. In detail, spatial learning and memory in the radial-arm water-maze task was improved 1 month after treatment. Furthermore, 2 months after initial treatment, 5XFAD mice that had received 2 rounds of the anti–PD-1 treatment at a 1-month interval performed indistinguishably from non-transgenic mice. In comparison, mice with a single round of PD-1 blockade that were examined 2 months later were not different from placebo control and hardly showed improved memory. These results indicated that repeated treatment was needed to maintain improved cognition. Treatment with the PD-1–specific antibodies also caused a reduction in plaque load in the hippocampus and in the cortex. This treatment effect was more pronounced after two rounds of anti–PD-1 treatment. In addition, astrogliosis was reduced in the hippocampus of 5XFAD mice with either one or two rounds of anti–PD-1 treatment. The beneficial effects described in 5xFAD mice after PD-1 blockade were largely confirmed in a second mouse model of amyloidosis (APP/PS1APPswe, PSEN1dE9), in which treatment reduced plaque deposition in hippocampus.
Given the fact that various antibodies or small molecules targeting PD-1/PD-1L are already marketed or under development (Kobold et al., 2018; Shaabani et al., 2018), the findings by Baruch et al. triggered considerable interest in immune checkpoint blockade as a novel therapeutic strategy for AD. Accordingly, this study stimulated evaluation of the PD-1 immune checkpoint blockade for AD by three independent pharmaceutical companies.
Challenges of PD-1 Immune Checkpoint Blockade as Therapeutic Intervention in AD
In subsequent studies jointly published in Glia (Latta-Mahieu et al., 2018) by Sanofi, Janssen, and Eli Lilly, the consequences of PD-1 immunotherapy in a range of different APP transgenic models (ThyAPP/PS1M146L, ThyAPP/PS1A246E, PD-APPAPPV717F) with the same anti–PD-1 antibody were documented. In addition to the antibody used by Baruch et al., two mouse chimeric variants and corresponding Immunglobulin G (IgG) controls were tested. In accordance with Baruch et al. (2016), PD-1 immunotherapy caused a systemic IFNγ-dependent immune response (Latta-Mahieu et al., 2018). However, in contrast to Baruch et al. (2016) there was no effect of anti–PD-1 treatment either on amyloid pathology or on monocyte-derived macrophage infiltration into the brain. This result point is consistent with data from another laboratory that explored the consequences of PD-1 deficiency in a murine prion disease model of neurodegeneration (ME7). In this study, there was no detectable infiltration of peripheral myeloid cells into the brain (Obst et al., 2018).
Latta-Mahieu and coworkers used, in addition to the rat mouse antibody (mAb) used by Baruch et al., two mouse chimeric variants with mouse IgG1 and IgG2a Fc domains as additional controls to evaluate whether observed effects were directly related to PD-1 target engagement. The in vivo studies were designed to ensure high statistical power (n = 9/group at Sanofi, n = 20/group at Janssen, and n = 25/group at Eli Lilly) and carried out in a blinded fashion. Furthermore, additional cohorts of animals for baseline measurement of pathology at the start of the anti–PD-1 treatment have been included. This way, age-dependent increases in amyloid pathology in each model of amyloidosis could be compared to anti–PD-1 treatment effects at the end of the study (Latta-Mahieu et al., 2018).
The reason for the different findings between Baruch et al. and Latta-Mathieu et al. is unclear, and more research is needed to understand the potential of PD-1 as a target for AD. It should be noted that in the study by Baruch et al., a xenogeneic rat antibody was used that potentially made the interpretation of the results difficult. Thus, it remained uncertain whether PD-1 target engagement or stimulation of the immune system, independent of PD-1, was causing the reduction in pathology and the functional improvements.
The Proposed Role of Toll-Like Receptors in AD
The innate immune system is the first line of defense against pathogens and tissue damage and elicits a rapid and robust response. Within the CNS, the innate immune system performs a similar role that is mediated by microglial and astroglial cells (Ransohoff and Brown, 2012). In the case of CNS infection or injury, microglial cells detect pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) via specialized cell-surface pattern recognition receptors (PRRs). PRRs transmit information about the CNS microenvironment to the glial cells, which then orchestrate an appropriate immune response (Kigerl et al., 2014). Glia direct the neuroimmune response by migrating toward lesions or pathology, producing pro- and anti-inflammatory cytokines and chemokines, releasing growth factors and up regulating phagocytic mechanisms (Hansen et al., 2018). After a period, there is a resolution signal, which returns glia to their homeostatic state (Chavan et al., 2017). However, in AD, where Aβ, hyperphosphorylated tau, and neuronal damage persist for decades, there is no resolution, resulting in chronic activation of the innate immune system. This persistent alteration of glial cell phenotype is believed to contribute to AD progression and severity, and therefore, the identification of drugable molecular targets within the innate immune system of the CNS is of paramount interest.
TLRs are a major class of PRRs characterized by an extracellular leucine-rich repeat domain and an intracellular TIR domain (Kielian, 2006). Ten mammalian TLRs have been identified, each with a particular ligand specificity. Complicating the picture, TLRs can hetero-dimerize with additional co-receptors, resulting in an expansion of their ligand repertoire (Kielian, 2006). TLRs are expressed in microglia (Laflamme and Rivest, 2001; Laflamme et al., 2001; Bsibsi et al., 2002; Dalpke et al., 2002; Olson and Miller, 2004; Zhang et al., 2012), and several have been shown to have increased expression both in the brains of AD transgenic animals and in human AD patients (Liu et al., 2005; Walter et al., 2007; Frank et al., 2009; Letiembre et al., 2009; Wirths et al., 2010; Rangasamy et al., 2018). The primary consensus among researchers is that persistent TLR signaling in AD models is detrimental; however, conflicting evidence also suggests beneficial roles of TLR signaling. The most extensively studied TLRs, in the context of AD, are TLR2 and TLR4 along with their co-receptor, CD14.
TLR2 and its co-receptor CD14 are upregulated in AD transgenic mouse models and in human AD brain sections (Letiembre et al., 2009). Functional characterization of TLR2 in primary mouse microglia and the mouse BV-2 microglia cell line demonstrated that fibrillar preparations of Aβ peptide can increase expression of NO synthase, pro-inflammatory cytokines, and integrin markers in a TLR2-dependent manner (Jana et al., 2008). Consistent with this, primary microglia and bone marrow–derived macrophages from TLR2 knockout mice show a reduced pro-inflammatory reaction and an enhanced phagocytic response when stimulated with aggregated Aβ1–42 peptide (Liu et al., 2012). Anti-TLR2 antibodies, administered over seven months, improved spatial learning, reduced gliosis [as assessed by immunoreactivity of CD68 and glial fibrillary acidic protein (GFAP)], and decreased Aβ plaque burden (McDonald et al., 2016). Anti-TLR2 antibodies can prevent a metabolic switch in microglia toward glycolysis, which results in an increase in phagocytosis (Rubio-Araiz et al., 2018). A peptide corresponding to the TLR2-interacting domain of myeloid differentiation primary response 88 (MyD88) (TIDM) disrupted the interaction of TLR2 with MyD88, resulting in a blockade of TLR2 signaling (Rangasamy et al., 2018). When administered intranasally to 5xFAD transgenic mice, the peptide could be detected in the hippocampus, where it reduced glial activation and Aβ plaque burden (Rangasamy et al., 2018). In contrast, several studies have postulated that TLR2 can promote beneficial responses in AD models. TLR2 deficiency accelerated spatial and contextual memory deficits while increasing levels of Aβ1–42 in the brains of PS1/APP transgenic mice (Richard et al., 2008). In addition, the TLR2 agonist peptidoglycan (PGN) enhanced uptake of Aβ1–42 in primary mouse microglia (Chen et al., 2006).
Like TLR2, TLR4 is expressed in microglial cells and can mediate binding and internalization of Aβ1–42 in vitro (Tahara et al., 2006). TLR4 was originally identified as an endotoxin receptor, mediating immune response to stimuli such as LPSs (Janssens and Beyaert, 2003). Studies using LPS as a tool to activate TLR4 signaling in AD transgenic mice have produced conflicting results. Intra-hippocampal administration of LPS to Tg2576 or PS1/APP mice activated microglia (as assessed by CD45 and CR3 or MHCII immunoreactivity) and reduced diffuse Aβ staining but not compact plaque (DiCarlo et al., 2001; Herber et al., 2007). Multiple doses of peripheral LPS improved cognitive function and reduced tau pathology in the P301S model (Qin et al., 2016). In contrast, repeated peripheral administration of LPS to WT mice was shown to increase Aβ levels, activate microglia, and induce cognitive impairment (Lee et al., 2008; Lee et al., 2012). Studies with in APPswe PS1dE9 and PS1/APP TLR4 knockout mice confirm an increase in plaque pathology (Tahara et al., 2006; Song et al., 2011), resulting in reduced glial activation and improved cognitive function (Song et al., 2011). Therapeutic blockade of TLR4 signaling using the cyanobacterial product CyP (Macagno et al., 2006) injected intracerebroventricularly (ICV) was able to reduce memory impairment and glial cell activation induced by ICV administration of Aβ oligomers (Balducci et al., 2017).
Challenges Targeting TLRs
Over the last two decades, considerable attention has been paid to understanding the role of TLRs in innate immunity, but their role in AD initiation and progression remains elusive. As highlighted above, the transgenic models used, the timing of the intervention, and the end points measured can impact the conclusions made. AD is a complex disease, and the effects of modulating TLRs may differ depending on the stage of the disease during intervention. For instance, memory impairment was reduced after ICV injection of Aβ oligomers by pre-treatment with TLR2 and TLR4 agonists (Pourbadie et al., 2018). In contrast co-administration of TLR4 antagonist produced similar results (Balducci et al., 2017). Additional complexities in targeting TLRs arise from their ability to downregulate the immune response following repeated exposures to TLR agonists like LPS. This “endotoxin tolerance” has been described in animals and a variety of human diseases (Lopez-Collazo and del Fresno, 2013): this might confound interpretation of experimental data using TLR agonists. This is highlighted by recent work that demonstrated that the microglia response to LPS increases in aged WT animals compared to young WT mice. However, in the PS1/APP model, the microglia response to LPS is decreased in aged animals compared to WT (Go et al., 2016). This altered TLR signaling (i.e., tolerance) could affect the efficacy of TLR agonists or antagonists especially where chronic administration of the drug will be needed, such as in AD.
TLRs are expressed on a wide variety of cells, including peripheral immune cells and non-immune cells within the CNS such a neurons (Kielian, 2009). With traditional small molecule approaches, selectively modulating CNS and not peripheral TLRs is not feasible. Therefore, a suitable therapeutic window will need to be established for any TLR therapeutic developed. This will require a delicate balance between achieving the brain exposures needed to drive a therapeutically beneficial effect and minimizing peripheral exposures to reduce the risk for potential toxicities. The typically lower brain exposure compared to the periphery that is observed for many drugs adds to this challenge.
Several small molecule TLR agonists and antagonists have been described (Zhu et al., 2018). Discovery of TLR8 inhibitors was greatly facilitated by structure-based design (Hu et al., 2018). Similar approaches may be useful in designing small molecules for additional TLRs. Rather than drugging TLRs directly, others have sought to interfere with key protein–protein interactions required for TLR signaling. The TIDM peptide serves as a MyD88 decoy, essentially capping TLR2, preventing assembly of the myddosome and subsequent TLR2 signaling (Rangasamy et al., 2018). TAK-242 is a small molecule that interacts with cysteine-747 in TLR4, preventing interaction with TIR domain–containing adaptor protein (TIRAP) or TRAM resulting in blockade of TLR4 signaling (Matsunaga et al., 2011). Degrader approaches may also be useful to selectively shut down TLR signaling, but the use of such technology has not been demonstrated on TLRs. The therapeutic potential of TLRs in AD remains compelling, and the recent development of selective TLR ligands will support investigations of the role of TLRs in AD while helping to frame a robust therapeutic hypothesis.
Proposed Role of TREM2 in AD
TREM2 is an immunoreceptor of partially understood function, expressed in myeloid cells in the periphery including dendritic cells, tissue macrophages and osteoclasts, and brain microglia (Colonna and Wang, 2016). TREM2 is a prominent AD risk gene (Pimenova et al., 2018) and is remarkable in the context of AD for at least two reasons: 1) its selective brain expression in microglia clearly links microglia specifically to neurodegenerative disease, and 2) there is the possibility of pharmacologic modulation of TREM2 to treat neurodegenerative disease.
In 2013, two landmark papers (Guerreiro et al., 2013; Jonsson et al., 2013) identified, by exome sequencing, rare mutants in TREM2 associated with an AD risk similar to that of a single allele of the strongest known risk factor, the E4 isoform of Apolipoprotein E (ApoE). Specifically, they showed that the mutation R47H, present in about 0.2% of the human population, elevated the AD odds ratio to at least 3.0. Several subsequent studies found slightly lower odds ratios but have confirmed the TREM2 R47H observations and identified additional TREM2 risk alleles and variants of unknown consequence (Bellenguez et al., 2017; Sims et al., 2017) that, in total, are present in about 1.5% of the human population (http://exac.broadinstitute.org/). Many other genes of modest AD risk have been identified before and since (Pimenova et al., 2018). But TREM2 is the strongest risk factor expressed in the brain only in microglia, unambiguously linking innate immune mechanisms in general, and microglial biology in particular, to AD. This observation has focused a great deal of attention on the role of microglia in AD and encouraged exploration of novel microglia-specific approaches to AD therapy (Hansen et al., 2018; Song and Colonna, 2018).
Functions of TREM2 in health and disease are summarized in several excellent reviews (for example, Hansen et al., 2018). AD-associated TREM2 mutants confer at least partially reduced ligand affinity, signaling, phagocytosis, and microgliosis (Ulrich et al., 2017). Significant evidence suggests that TREM2 is a direct phagocytic receptor (Takahashi et al., 2005; N’Diaye et al., 2009), which implies that failure to phagocytose aggregated proteins is a key driver of AD and related diseases. Note, however, that TREM2 is but one of many phagocytic receptors in microglia (Sierra et al., 2013), and it is unclear why the contribution of TREM2 in particular to phagocytosis would so strongly affect AD risk. Perhaps TREM2 has a prominent role in the phagocytosis of substrates specific to AD, e.g., Aβ or tau, or perhaps compromised TREM2 function has other pathological consequences ( Figure 4 ). TREM2 also seems necessary for microglial plaque association in mouse models and in human disease (Wang et al., 2016; Yuan et al., 2016). Functional studies have suggested that TREM2 is a lipid receptor, and that the binding of some but not all lipids is compromised by AD-associated mutations (Wang et al., 2015). Evidence for other ligands including ApoE (Atagi et al., 2015; Bailey et al., 2015; Yeh et al., 2016) and Aβ peptide (Lessard et al., 2018; Zhao et al., 2018; Zhong et al., 2018) has been presented. These results should be interpreted cautiously. Aβ peptide is markedly hydrophobic, making specific interactions difficult to interpret. Also, depending on the presence and nature of bound lipids, ApoE is likely to have different interaction properties. The key ligand(s) for TREM2 in AD may not yet be known, or TREM2 may simply be a promiscuous receptor (Kober and Brett, 2017). The phenotype of mouse ApoE knockouts mimics TREM2 knockouts with respect to microglial plaque association (Shi and Holtzman, 2018; Ulrich et al., 2018), supporting ApoE as a TREM2 ligand and suggesting a model in which TREM2-mediated effects on plaque may require or be enhanced by a ternary complex with ApoE. Structural studies provide some insight into the specific effects of AD risk of TREM2 mutations. R47, for example, is a surface residue that may constitute part of the TREM2 ligand binding surface (Kober et al., 2016). In mutant form (R47H), histidine substantially alters the conformation of a surface loop, which may change the energetics of ligand binding (Sudom et al., 2018). It should be noted that some TREM2 mutants, including ones that eliminate expression by chain termination, are associated with a different neurologic condition called Nasu–Hakola disease (NHD). AD-associated mutations change amino acids on the outer surface of the protein and are predicted to have subtle structural effects. In contrast, NHD sequence variants are buried in the core of the protein and are predicted to have profound effects on protein structure and expression (Kober et al., 2016). Thus, relatively conservative mutations increase the risk of AD, while protein elimination is associated with NHD.
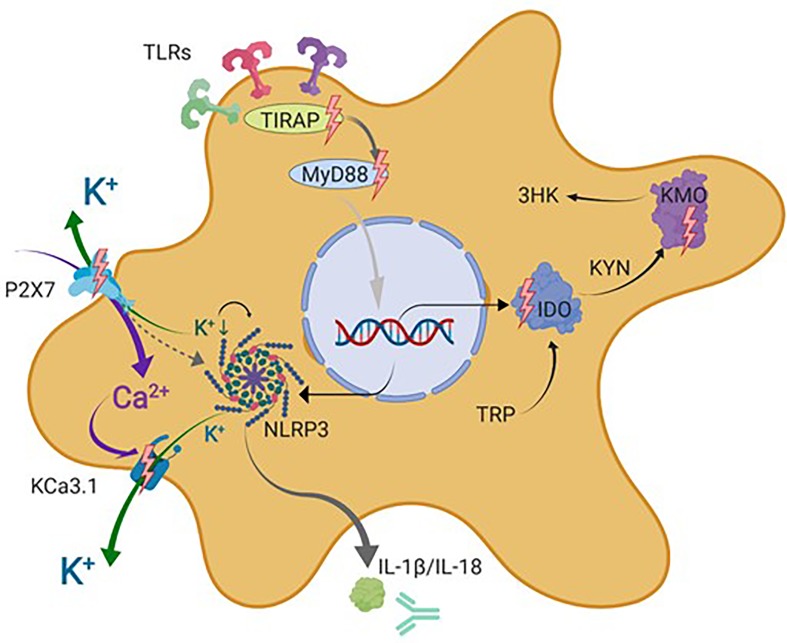
Figure 4.
Schematic representation of microglial drug targets discussed in this review. This simplified schematic does not contain all signal transduction molecules known to be involved in the described signaling cascades but focuses on the microglial drug targets discussed in this review indicated by lighting bolts and antibody symbol. IDO, indolamine-2,3-dioxygenase; IL-1β, interleukin 1 beta; IL-18, interleukin 18; KCa3.1, intermediate-conductance calcium-activated potassium channel 3.1; KYN, kynurenine; AA, anthranilic acid; 3-HK, 3-hydroxykynurenine; KATs, kynurenine aminotransferases; KMO, kynurenine 3-monooxygenase (kynurenine 3-hydroxylase); NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain–containing inflammasome complex 3; P2X7, ionotropic P2 receptor 7; TIRAP, TIR domain–containing adaptor protein; MyD88, myeloid differentiation primary response protein 88; TDO, tryptophan-2,3-dioxygenase; TRP, tryptophan. Figure created with Biorender.com
Some challenges in the TREM2 field have slowed progress in our understanding of its role in AD. Many studies are in cells other than microglia—often, peripheral macrophages. Many studies are done with TREM2 knockouts. As noted above, such knockouts are associated with NHD rather than AD, and the mechanistic relationships between the two are essentially not known. Some studies rely on overexpression of TREM2 (e.g., Jiang et al., 2016; Lee et al., 2018), which risks overwhelming TREM2 signaling partners in these cells, with uncertain consequences. Much of this is understandable. A major problem is that the phenotype of the AD risk sequence variants is quite subtle, making differential readouts and clear conclusions difficult. Some investigators use heterozygous as well as homozygous knockouts to reveal trends that are complex to interpret. Likewise, the relationship between a heterozygous knockout and, for example, the R47H sequence variant is not clear, and the latter is the preferable experimental target (for example, Cheng et al., 2018; Song et al., 2018). Compounding the TREM2 phenotype problem are difficulties common to all studies of microglia; these cells are extremely sensitive to their environment, rapidly changing their gene expression profile ex vivo (Bohlen et al., 2017). Human primary cells are available, of course, only from cadavers, and even then, only after several hours of hypoxia, with uncertain impact. Inducible pluripotent stem cell (iPSC)-derived “microglia-like” cells have, at best, an uncertain relationship with human microglia in their natural setting. Finally, due largely to the lack of reagents, intervention studies with pharmacologic modulators are rare: one such study using an antibody reported to activate TREM2 signaling caused significant increases in microglial survival and migration (Cheng et al., 2018). More studies with better-characterized reagents are needed.
TREM2 as Drug Target: Challenges
Besides directing attention to microglia as an approach to AD drug discovery, TREM2 itself may have promise as a target if the appropriate pharmacologic modulation can be identified. As mutants that increase AD risk seem to have a partial loss of TREM2 function, and considering that overexpression studies with TREM2 seem to enhance its function in processes associated with AD (Takahashi et al., 2005; Lee et al., 2018), one hypothesis is that augmenting the amount or function of TREM2 might oppose AD progression. However, increasing the function of a protein is challenging. Antibodies stabilizing TREM2 on the cell surface might be another possibility, including those that increase cell-surface TREM2 by blocking its proteolytic cleavage and release (Schlepckow et al., 2017; Thornton et al., 2017). Small molecule binders that stabilize TREM2 by blocking proteolytic cleavage or other mechanisms are conceivable but likely quite difficult to find. More promising, but further off, would be modulation of TREM2 regulatory factors. Such signaling or regulatory mechanisms specific enough to TREM2 to expect avoidance of undesired pleiotropic effects have not been reported. These may be revealed by ongoing research, including phenotypic screens for genes or compounds that boost TREM2 function. But as a caveat to this whole strategy, recall that TREM2 mutants that increase AD risk are quite rare. The vast majority of AD patients carry the common variant sequence of TREM2. There is no direct evidence in humans, and only limited evidence in model systems, that boosting the function of “normal” TREM2 would have beneficial effects for most AD patients. There is much to be learned about TREM2 and its promise as a drug target.
Conclusions
To date, no disease-modifying treatment for AD has achieved Food and Drug Administration (FDA) approval despite many studies showing their efficacy in mouse models. Aβ and tau are key features of AD, and either could be a “disease-modifying” target in principle. Multiple failures to stop AD by facilitating Aβ clearance or inhibition of production have considerably increased the interest in other modalities to target this devastating disease, such as neuroinflammation.
Microglia were first recognized as key players in neurodegenerative diseases more than two decades ago. More recently, unambiguous genetic evidence clearly links microglia function to AD pathogenesis. A large percentage of disease-associated SNPs are present in genes that are specifically expressed in microglia, a finding that has boosted the interest in understanding how microglia could be targeted to treat AD. Accordingly, numerous microglia proteins or pathways have been suggested to be drug targets. Here we reviewed several target candidates and their drugability challenges. Ideal drug targets should have a strong biological case, ideally combining genetic evidence with a well-understood mechanism of action that is supported by in vitro and in vivo data. However, even targets which fullfill this criteria need to be evaluated on whether they can be drugged safely. For the targets that have been briefly discussed in this review, these high hurdles have not yet been overcome. We remain optimistic that microglia-driven mechanisms will eventually bear clinical success and that one of the many discussed targets in this article will find a drug in the market helping AD patients.
Author Contributions
KB, AB, BC, JP, MR, RS, RT, TM wrote the manuscript. KB, RT, and TM edited the manuscript.
Conflict of Interest Statement
JP, RT, and TM are full-time employees of the Abbvie Foundational Neuroscience Center, Cambridge, MA, US. KB and MR are or have been full-time employees of AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany at the time of writing this article,. AB is a full-time employee of Janssen Research & Development LLC, San Diego, CA, US. BC is a full-time employee of Sage Therapeutics, Cambridge, MA, US. RS is a full-time employee of Paracelsus Neuroscience, Metuchen, NJ, US.
References
- Arrowsmith C. H., Audia J. E., Austin C., Baell J., Bennett J., Blagg J., et al. (2015). The promise and peril of chemical probes. Nat. Chem. Biol. 11 (8), 536–541. 10.1038/nchembio.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga K. I., Orringer E. P., Styles L., Vichinsky E. P., Swerdlow P., Davis G. A., et al. (2006). Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy 26 (11), 1557–1564. 10.1592/phco.26.11.1557 [DOI] [PubMed] [Google Scholar]
- Ataga K. I., Smith W. R., De Castro L. M., Swerdlow P., Saunthararajah Y., Castro O., et al. (2008). Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood 111 (8), 3991–3997. 10.1182/blood-2007-08-110098 [DOI] [PubMed] [Google Scholar]
- Ataga K. I., Stocker J. (2009). Senicapoc (ICA-17043): a potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin. Investig. Drugs 18 (2), 231–239. 10.1517/13543780802708011 [DOI] [PubMed] [Google Scholar]
- Atagi Y., Liu C.-C., Painter M. M., Chen X.-F., Verbeeck C., Zheng H., et al. (2015). Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem. 290 (43), 26043–26050. 10.1074/jbc.M115.679043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atukeren P., Cengiz M., Yavuzer H., Gelisgen R., Altunoglu E., Oner S., et al. (2018). The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer's disease. Biomed. Pharmacother. 90, 786–795. 10.1016/j.biopha.2017.03.101 [DOI] [PubMed] [Google Scholar]
- Bailey C. C., DeVaux L. B., Farzan M. (2015). The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem. 290 (43), 26033–26042. 10.1074/jbc.M115.677286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C., Frasca A., Zotti M., La Vitola P., Mhillaj E., Grigoli E., et al. (2017). Toll-like receptor 4–dependent glial cell activation mediates the impairment in memory establishment induced by beta-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav. Immun. 60, 188–197. 10.1016/j.bbi.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Baran H., Jellinger K., Deecke L. (1999). Kynurenine metabolism in Alzheimer's disease. J. Neural. Transm. (Vienna). 106 (2), 165–181. 10.1007/s007020050149 [DOI] [PubMed] [Google Scholar]
- Bartlett R., Stokes L., Sluyter R. (2014). The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 66 (3), 638–675. 10.1124/pr.113.008003 [DOI] [PubMed] [Google Scholar]
- Baruch K., Deczkowska A., Rosenzweig N., Tsitsou-Kampeli A., Sharif A. M., Matcovitch-Natan O., et al. (2016). PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22 (2), 135–137. 10.1038/nm.4022 [DOI] [PubMed] [Google Scholar]
- Baruch K., Rosenzweig N., Kertser A., Deczkowska A., Sharif A. M., Spinrad A., et al. (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967. 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F., Matson W. R., Storey E., Milbury P., Ryan E. A., Ogawa T., et al. (1992). Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J. Neurol. Sci. 108 (1), 80-87. 10.1016/0022-510x(92)90191-m [DOI] [PubMed] [Google Scholar]
- Beaumont V., Mrzljak L., Dijkman U., Freije R., Heins M., Rassoulpour A., et al. (2016). The novel KMO inhibitor CHDI-340246 leads to a restoration of electrophysiological alterations in mouse models of Huntington’s disease. Exp. Neurol. 282, 99–118. 10.1016/j.expneurol.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Bellenguez C., Charbonnier C., Grenier-Boley B., Quenez O., Le Guennec K., Nicolas G., et al. (2017). Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol. Aging. 59, 220.e1-220.e9. 10.1016/j.neurobiolaging.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Ben Haim L., Carrillo-de Sauvage M. A., Ceyzeriat K., Escartin C. (2015). Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 9, 278. 10.3389/fncel.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles D. E., Richardson W. D. (2015). Oligodendrocyte development and plasticity. Cold Spring Harb. 8 (2), a020453. 10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A. (2018). Recent advances in CNS P2X7 physiology and pharmacology: focus on neuropsychiatric disorders. Front. Pharmacol. 9, 30. 10.3389/fphar.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Biber K. (2016). The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64 (10), 1772–1787. 10.1002/glia.23001 [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Jones D. N. C. (2018). Emerging role of the P2X7–NLRP3–IL1beta pathway in mood disorders. Psychoneuroendocrinology 98, 95–100. 10.1016/j.psyneuen.2018.08.015 [DOI] [PubMed] [Google Scholar]
- Biber K., Moller T., Boddeke E., Prinz M. (2015). Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat. Rev. Drug Discov. 15 (2), 110–124. 10.1038/nrd.2015.14 [DOI] [PubMed] [Google Scholar]
- Bohlen C. J., Bennett F. C., Tucker A. F., Collins H. Y., Mulinyawe S. B., Barres B. A. (2017). Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94 (4), 759–773 e758. 10.1016/j.neuron.2017.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda D. J., Mailankot M., Stone J. G., Garrett M. R., Staniszewska M., Castellani R. J., et al. (2010). Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 15 (4), 161–168. 10.1179/174329210X12650506623645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhy D., Ghasemlou N., Lively S., Redensek A., Rathore K. I., Schlichter L. C., et al. (2011). Inhibition of the Ca(2)(+)-dependent K(+) channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. J. Neurosci. 31 (45), 16298–16308. 10.1523/JNEUROSCI.0047-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw E. M., Chibnik L. B., Keenan B. T., Ottoboni L., Raj T., Tang A., et al. (2013). CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 16 (7), 848–850. 10.1038/nn.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M., Ravid R., Gveric D., van Noort J. M. (2002). Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 61 (11), 1013–1021. 10.1093/jnen/61.11.1013 [DOI] [PubMed] [Google Scholar]
- Campbell B. M., Charych E., Lee A. W., Möller T. (2014). Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 8, 12. 10.3389/fnins.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Crocker P. R. (2011). Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology 132 (1), 18–26. 10.1111/j.1365-2567.2010.03368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan S. S., Pavlov V. A., Tracey K. J. (2017). Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46 (6), 927–942. 10.1016/j.immuni.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Iribarren P., Hu J., Chen J., Gong W., Cho E. H., et al. (2006). Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease–associated amyloid beta peptide. J. Biol. Chem. 281 (6), 3651–3659. 10.1074/jbc.M508125200 [DOI] [PubMed] [Google Scholar]
- Chen X., Hu J., Jiang L., Xu S., Zheng B., Wang C., et al. (2014). Brilliant Blue G improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-beta–induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience 279, 94–101. 10.1016/j.neuroscience.2014.08.036 [DOI] [PubMed] [Google Scholar]
- Chen Y. J., Raman G., Bodendiek S., O’Donnell M. E., Wulff H. (2011). The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 31 (12), 2363–2374. 10.1038/jcbfm.2011.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Wallace B. K., Yuen N., Jenkins D. P., Wulff H., O’Donnell M. E. (2015). Blood–brain barrier KCa3.1 channels: evidence for a role in brain Na uptake and edema in ischemic stroke. Stroke 46 (1), 237–244. 10.1161/STROKEAHA.114.007445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Danao J., Talreja S., Wen P., Yin J., Sun N., et al. (2018). TREM2-activating antibodies abrogate the negative pleiotropic effects of the Alzheimer’s disease variant TREM2R47H on murine myeloid cell function. J. Biol. Chem. 293 (32), 12620–12633. 10.1074/jbc.RA118.001848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouraki V., Preis S. R., Yang Q., Beiser A., Li S., Larson M. G., et al. (2017). Association of amine biomarkers with incident dementia and Alzheimer's disease in the Framingham Study. Alzheimers Dement. 13 (12), 1327–1336. 10.1016/j.jalz.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Wang Y. (2016). TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 17 (4), 201–207. 10.1038/nrn.2016.7 [DOI] [PubMed] [Google Scholar]
- Dale E., Staal R. G., Eder C., Moller T. (2016). KCa 3.1—a microglial target ready for drug repurposing? Glia 64 (10), 1733–1741. 10.1002/glia.22992 [DOI] [PubMed] [Google Scholar]
- Dalpke A. H., Schafer M. K., Frey M., Zimmermann S., Tebbe J., Weihe E., et al. (2002). Immunostimulatory CpG-DNA activates murine microglia. J. Immunol. 168 (10), 4854–4863. 10.4049/jimmunol.168.10.4854 [DOI] [PubMed] [Google Scholar]
- Dempsey C., Rubio Araiz A., Bryson K. J., Finucane O., Larkin C., Mills E. L., et al. (2017). Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 61, 306–316. 10.1016/j.bbi.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez J. I., Gomez-Villafuertes R., Leon-Otegui M., Hontecillas-Prieto L., Del Puerto A., Trejo J. L., et al. (2012). In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3beta and secretases. Neurobiol. Aging 33 (8), 1816–1828. 10.1016/j.neurobiolaging.2011.09.040 [DOI] [PubMed] [Google Scholar]
- DiCarlo G., Wilcock D., Henderson D., Gordon M., Morgan D. (2001). Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging 22 (6), 1007–1012. 10.1016/S0197-4580(01)00292-5 [DOI] [PubMed] [Google Scholar]
- Dounay A. B., Anderson M., Bechle B. M., Campbell B. M., Claffey M. M., Evdokimov A., et al. (2012). Discovery of brain-penetrant, irreversible kynurenine aminotransferase II inhibitors for schizophrenia. ACS Med. Chem. Lett. 3 (3), 187–192. 10.1021/ml200204m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C., Klee R., Heinemann U. (1997). Pharmacological properties of Ca2+-activated K+ currents of ramified murine brain macrophages. Naunyn Schmiedebergs Arch. Pharmacol. 356 (2), 233–239. 10.1007/PL00005046 [DOI] [PubMed] [Google Scholar]
- Fanger C. M., Ghanshani S., Logsdon N. J., Rauer H., Kalman K., Zhou J., et al. (1999). Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 274 (9), 5746–5754. 10.1074/jbc.274.9.5746 [DOI] [PubMed] [Google Scholar]
- Feske S., Skolnik E. Y., Prakriya M. (2012). Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 12 (7), 532–547. 10.1038/nri3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint Beal M., Matson W. R., Storey E., Milbury P., Ryan E. A., Ogawa T., et al. (1992). Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J. Neurol. Sci. 108 (1), 80–87. 10.1016/0022-510X(92)90191-M [DOI] [PubMed] [Google Scholar]
- Frank S., Copanaki E., Burbach G. J., Muller U. C., Deller T. (2009). Differential regulation of toll-like receptor mRNAs in amyloid plaque–associated brain tissue of aged APP23 transgenic mice. Neurosci. Lett. 453 (1), 41–44. 10.1016/j.neulet.2009.01.075 [DOI] [PubMed] [Google Scholar]
- Frye S. V. (2010). The art of the chemical probe. Nat. Chem. Biol. 6 (3), 159–161. 10.1038/nchembio.296 [DOI] [PubMed] [Google Scholar]
- Giil L. M., Midttun Ø., Refsum H., Ulvik A., Advani R., Smith A. D., et al. (2017). Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimers Dis. 60 (2), 495–504. 10.3233/JAD-170485 [DOI] [PubMed] [Google Scholar]
- Go M., Kou J., Lim J. E., Yang J., Fukuchi K. I. (2016). Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer’s mouse model: implication of TLR4 signaling in disease progression. Biochem. Biophys. Res. Commun. 479 (2), 331–337. 10.1016/j.bbrc.2016.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilberger J., Fuchs D., Leblhuber F., Greilberger M., Wintersteiger R., Tafeit E. (2010). Carbonyl proteins as a clinical marker in Alzheimer’s disease and its relation to tryptophan degradation and immune activation. Clin. Lab. 56 (9–10), 441–448. [PubMed] [Google Scholar]
- Griciuc A., Serrano-Pozo A., Parrado A. R., Lesinski A. N., Asselin C. N., Mullin K., et al. (2013). Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78 (4), 631–643. 10.1016/j.neuron.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes J. R., Lao T., Cardoso A. L., El Khoury J. (2018). Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer’s disease–associated amyloid-beta and tau pathologies. Front. Neurol. 9, 549. 10.3389/fneur.2018.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., et al. (2013). TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368 (2), 117–127. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P., Hoffman G. E., Melendez-Ferro M., Albuquerque E. X., Schwarcz R. (2007). Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 55 (1), 78–92. 10.1002/glia.20432 [DOI] [PubMed] [Google Scholar]
- Guillemin G. J., Brew B. J., Noonan C. E., Takikawa O., Cullen K. M. (2005). Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol. Appl. Neurobiol. 31 (4), 395–404. 10.1111/j.1365-2990.2005.00655.x [DOI] [PubMed] [Google Scholar]
- Guillemin G. J., Smith D. G., Smythe G. A., Armati P. J., Brew B. J. (2003. a). Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 527, 105–112. 10.1007/978-1-4615-0135-0_12 [DOI] [PubMed] [Google Scholar]
- Guillemin G. J., Smythe G. A., Veas L. A., Takikawa O., Brew B. J. (2003. b). Aβ1–42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport 14 (18), 2311–2315. 10.1097/00001756-200312190-00005 [DOI] [PubMed] [Google Scholar]
- Gulaj E., Pawlak K., Bien B., Pawlak D. (2010). Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 55 (2), 204–211. 10.2478/v10039-010-0023-6 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Hanson J. E., Sheng M. (2018). Microglia in Alzheimer’s disease. J. Cell Biol. 217 (2), 459–472. 10.1083/jcb.201709069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartai Z., Juhász A., Rimanóczy A., Janáky T., Donkó T., Dux L., et al. (2007). Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem. Int. 50 (2), 308–313. 10.1016/j.neuint.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Hauser A. S., Attwood M. M., Rask-Andersen M., Schioth H. B., Gloriam D. E. (2017). Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16 (12), 829–842. 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T. (2017). Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. 27 (2), 220–222. 10.1111/bpa.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14 (4), 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493 (7434), 674–678. 10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., McManus R. M., Latz E. (2018). Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 19 (10), 610–621. 10.1038/s41583-018-0055-7 [DOI] [PubMed] [Google Scholar]
- Henstridge C. M., Hyman B. T., Spires-Jones T. L. (2019). Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 20 (2), 94–108. 10.1038/s41583-018-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F. L., Ransohoff R. M., Becher B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16 (6), 358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Herber D. L., Mercer M., Roth L. M., Symmonds K., Maloney J., Wilson N., et al. (2007). Microglial activation is required for Abeta clearance after intracranial injection of lipopolysaccharide in APP transgenic mice. J. Neuroimmune Pharmacol. 2 (2), 222–231. 10.1007/s11481-007-9069-z [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Achim C. L., Wiley C. A., Major E. O., Saito K., Markey S. P. (1996). Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 320 (2), 595–597. 10.1042/bj3200595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Crowley J. S., Davis L. E., Demitrack M. A., Der M., et al. (1992). Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115 (5), 1249–1273. 10.1093/brain/115.5.1249 [DOI] [PubMed] [Google Scholar]
- Higueruelo A. P., Jubb H., Blundell T. L. (2013). Protein–protein interactions as druggable targets: recent technological advances. Curr. Opin. Pharmacol. 13 (5), 791–796. 10.1016/j.coph.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Hokari M., Wu H.-Q., Schwarcz R., Smith Q. R. (1996). Facilitated brain uptake of 4-chlorokynurenine and conversion to 7-chlorokynurenic acid. Neuroreport 8 (1), 15–18. 10.1097/00001756-199612200-00004 [DOI] [PubMed] [Google Scholar]
- Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J. C., Carrasquillo M. M., et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43 (5), 429–435. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Tanji H., Jiang S., Zhang S., Koo K., Chan J., et al. (2018). Small-molecule TLR8 antagonists via structure-based rational design. Cell Chem. Biol. 25 (10), 1286–1291 e1283. 10.1016/j.chembiol.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Rubini P., Huang L., Tang Y. (2019). The P2X7 receptor: a new therapeutic target in Alzheimer’s disease. Expert Opin. Ther. Targets. 23 (3), 165–176. 10.1080/14728222.2019.1575811 [DOI] [PubMed] [Google Scholar]
- Jain P., Wadhwa P. K., Jadhav H. R. (2015). Reactive astrogliosis: role in Alzheimer’s disease. CNS Neurol. Dis. Drug Targets 14 (7), 872–879. 10.2174/1871527314666150713104738 [DOI] [PubMed] [Google Scholar]
- Jana M., Palencia C. A., Pahan K. (2008). Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J. Immunol. 181 (10), 7254–7262. 10.4049/jimmunol.181.10.7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Beyaert R. (2003). Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16 (4), 637–646. 10.1128/CMR.16.4.637-646.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Zhang Y. D., Chen Q., Gao Q., Zhu X. C., Zhou J. S., et al. (2016). TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 105, 196–206. 10.1016/j.neuropharm.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Jiang Y. T., Li H. Y., Cao X. P., Tan L. (2018). Meta-analysis of the association between CD33 and Alzheimer’s disease. Ann. Transl. Med. 6 (10), 169. 10.21037/atm.2018.04.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Han J., Resing D., Liu H., Yue X., Miller R. L., et al. (2018). Synthesis and in vitro characterization of a P2X7 radioligand [(123)I]TZ6019 and its response to neuroinflammation in a mouse model of Alzheimer disease. Eur. J. Pharmacol. 820, 8–17. 10.1016/j.ejphar.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. D. L., Nguyen H., Singh V., Sing L., Chavez M., et al. (In Press). Repurposing the KCa3.1 inhibitor senicapoc as a microglia-targeted therapeutic candidate for Alzheimer’s disease. Brain.
- Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P. V., Snaedal J., et al. (2013). variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368 (2), 107–116. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V., Koeberle P. D., Wang Y., Schlichter L. C. (2007). The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide–dependent neurodegeneration. J. Neurosci. 27 (1), 234–244. 10.1523/JNEUROSCI.3593-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Roy L., Zhu X., Schlichter L. C. (2001). K+ channels and the microglial respiratory burst. Am. J. Physiol. Cell Physiol. 280 (4), C796–C806. 10.1152/ajpcell.2001.280.4.C796 [DOI] [PubMed] [Google Scholar]
- Kielian T. (2006). Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 83 (5), 711–730. 10.1002/jnr.20767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. (2009). Overview of toll-like receptors in the CNS. Curr. Top. Microbiol. Immunol. 336, 1–14. 10.1007/978-3-642-00549-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K. A., de Rivero Vaccari J. P., Dietrich W. D., Popovich P. G., Keane R. W. (2014). Pattern recognition receptors and central nervous system repair. Exp. Neurol. 258, 5–16. 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J. W., Bemiller S. M., Murtishaw A. S., Leisgang A. M., Salazar A. M., Lamb B. T. (2018). Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 4, 575–590. 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober D. L., Brett T. J. (2017). TREM2–ligand interactions in health and disease. J. Mol. Biol. 429 (11), 1607–1629. 10.1016/j.jmb.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober D. L., Alexander-Brett J. M., Karch C. M., Cruchaga C., Colonna M., Holtzman M. J., et al. (2016). Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. Elife 5, e20391. 10.7554/eLife.20391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobold S., Pantelyushin S., Rataj F., Vom Berg J. (2018). Rationale for combining bispecific T cell activating antibodies with checkpoint blockade for cancer therapy. Front. Oncol. 8, 285. 10.3389/fonc.2018.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N., Rivest S. (2001). Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 15 (1), 155–163. 10.1096/fj.00-0339com [DOI] [PubMed] [Google Scholar]
- Laflamme N., Soucy G., Rivest S. (2001). Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J. Neurochem. 79 (3), 648–657. 10.1046/j.1471-4159.2001.00603.x [DOI] [PubMed] [Google Scholar]
- Lajaunias F., Dayer J. M., Chizzolini C. (2005). Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase–mediated intracellular signaling. Eur. J. Immunol. 35 (1), 243–251. 10.1002/eji.200425273 [DOI] [PubMed] [Google Scholar]
- Latta-Mahieu M., Elmer B., Bretteville A., Wang Y., Lopez-Grancha M., Goniot P., et al. (2018). Systemic immune-checkpoint blockade with anti-PD1 antibodies does not alter cerebral amyloid-β burden in several amyloid transgenic mouse models. Glia 66 (3), 492–504. 10.1002/glia.23260 [DOI] [PubMed] [Google Scholar]
- Lee C. Y. D., Daggett A., Gu X., Jiang L. L., Langfelder P., Li X., et al. (2018). Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron 97 (5), 1032–1048 e1035. 10.1016/j.neuron.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. G., Won S. M., Gwag B. J., Lee Y. B. (2011). Microglial P2X₇ receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Exp. Mol. Med. 43 (1), 7–14. 10.3858/emm.2011.43.1.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Lee Y. K., Yuk D. Y., Choi D. Y., Ban S. B., Oh K. W., et al. (2008). Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 5, 37. 10.1186/1742-2094-5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Choi D. Y., Choi I. S., Kim K. H., Kim Y. H., Kim H. M., et al. (2012). Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J. Neuroinflammation 9, 35. 10.1186/1742-2094-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leklem J. E. (1971). Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am. J. Clin. Nutr. 24 (6), 659–672. 10.1093/ajcn/24.6.659 [DOI] [PubMed] [Google Scholar]
- Lessard C. B., Malnik S. L., Zhou Y., Ladd T. B., Cruz P. E., Ran Y., et al. (2018). High affinity interactions and signal transduction between Aβ oligomers and TREM2. EMBO Mol. Med. 10 (11), e9027. 10.15252/emmm.201809027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M., Liu Y., Walter S., Hao W., Pfander T., Wrede A., et al. (2009). Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol. Aging 30 (5), 759–768. 10.1016/j.neurobiolaging.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Linnartz-Gerlach B., Kopatz J., Neumann H. (2014). Siglec functions of microglia. Glycobiology 24 (9), 794–799. 10.1093/glycob/cwu044 [DOI] [PubMed] [Google Scholar]
- Liu S., Liu Y., Hao W., Wolf L., Kiliaan A. J., Penke B., et al. (2012). TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J. Immunol. 188 (3), 1098–1107. 10.4049/jimmunol.1101121 [DOI] [PubMed] [Google Scholar]
- Liu Y., Walter S., Stagi M., Cherny D., Letiembre M., Schulz-Schaeffer W., et al. (2005). LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain 128 (Pt 8), 1778–1789. 10.1093/brain/awh531 [DOI] [PubMed] [Google Scholar]
- Lopez-Collazo E., del Fresno C. (2013). Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit. Care 17 (6), 242. 10.1186/cc13110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez I., Schluter A., Aso E., Garcia-Esparcia P., Ansoleaga B., Llorens F., et al. (2015). Neuroinflammatory signals in Alzheimer disease and APP/PS1 transgenic mice: correlations with plaques, tangles, and oligomeric species. J. Neuropathol. Exp. Neurol. 74 (4), 319–344. 10.1097/NEN.0000000000000176 [DOI] [PubMed] [Google Scholar]
- Lovelace M. D., Varney B., Sundaram G., Lennon M. J., Lim C. K., Jacobs K., et al. (2017). Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 112, 373–388. 10.1016/j.neuropharm.2016.03.024 [DOI] [PubMed] [Google Scholar]
- Ma P., Zemmel R. (2002). Value of novelty? Nat. Rev. Drug Discov. 1 (8), 571–572. 10.1038/nrd884 [DOI] [PubMed] [Google Scholar]
- Macagno A., Molteni M., Rinaldi A., Bertoni F., Lanzavecchia A., Rossetti C., et al. (2006). A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J. Exp. Med. 203 (6), 1481–1492. 10.1084/jem.20060136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M. S., Crocker P. R., Paulson J. C. (2014). Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14 (10), 653–666. 10.1038/nri3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I., Jenkins D. P., Jin B. E., Wulff H. (2012). Microglial KCa3.1 Channels as a potential therapeutic target for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 868972. 10.1155/2012/868972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I., Zimin P. I., Wulff H., Jin L. W. (2011). Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 286 (5), 3693–3706. 10.1074/jbc.M110.135244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M., Simpson J. F., Parikh I., Wilfred B. R., Fardo D. W., Nelson P. T., et al. (2013). CD33 Alzheimer’s risk–altering polymorphism, CD33 expression, and exon 2 splicing. J. Neurosci. 33 (33), 13320–13325. 10.1523/JNEUROSCI.1224-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Amar M., Dalle C., Youssef I., Boucher C., Le Duigou C., et al. (2018). New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry. 24 (1), 108–125. 10.1038/s41380-018-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Amar M., Dalle C., Youssef I., Boucher C., Le Duigou C., et al. (2019). New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol. Psychiatry 24 (1), 108–125. 10.1038/s41380-018-0108-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. (2011). TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 79 (1), 34–41. 10.1124/mol.110.068064 [DOI] [PubMed] [Google Scholar]
- McDonald C. L., Hennessy E., Rubio-Araiz A., Keogh B., McCormack W., McGuirk P., et al. (2016). Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 58, 191–200. 10.1016/j.bbi.2016.07.143 [DOI] [PubMed] [Google Scholar]
- McLarnon J. G., Ryu J. K., Walker D. G., Choi H. B. (2006). Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 65 (11), 1090–1097. 10.1097/01.jnen.0000240470.97295.d3 [DOI] [PubMed] [Google Scholar]
- Möller T., Boddeke H. W. (2016). Glial cells as drug targets: what does it take? Glia 64 (10), 1742–1754. 10.1002/glia.22993 [DOI] [PubMed] [Google Scholar]
- Möller T., Bard F., Bhattacharya A., Biber K., Campbell B., Dale E., et al. (2016). Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64 (10), 1788–1794. 10.1002/glia.23007 [DOI] [PubMed] [Google Scholar]
- Moroni F., Lombardi G., Robitaille Y., Etienne P. (1986). Senile dementia and Alzheimer’s disease: lack of changes of the cortical content of quinolinic acid. Neurobiol. Aging 7 (4), 249–253. 10.1016/0197-4580(86)90003-5 [DOI] [PubMed] [Google Scholar]
- Mourdian M. M., Heyes M. P., Pan J. B., Heuser I. J. E., Markey S. P., Chase T. N. (1989). No changes in central quinolinic acid levels in Alzheimer’s disease. Neurosci. Lett. 105 (1–2), 233–238. 10.1016/0304-3940(89)90043-8 [DOI] [PubMed] [Google Scholar]
- Muguruma Y., Tsutsui H., Noda T., Akatsu H., Inoue K. (2018). Widely targeted metabolomics of Alzheimer’s disease postmortem cerebrospinal fluid based on 9-fluorenylmethyl chloroformate derivatized ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1091, 53–66. 10.1016/j.jchromb.2018.05.031 [DOI] [PubMed] [Google Scholar]
- N’Diaye E.-N., Branda C. S., Branda S. S., Nevarez L., Colonna M., Lowell C., et al. (2009). TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J. Cell Biol. 184 (2), 215–223. 10.1083/jcb.200808080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A. C., Jun G., Beecham G. W., Wang L. S., Vardarajan B. N., Buros J., et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 43 (5), 436–441. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M. K., Paulson J. C. (2009). Siglecs as targets for therapy in immune-cell–mediated disease. Trends Pharmacol. Sci. 30 (5), 240–248. 10.1016/j.tips.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst J., Mancuso R., Simon E., Gomez-Nicola D. (2018). PD-1 deficiency is not sufficient to induce myeloid mobilization to the brain or alter the inflammatory profile during chronic neurodegeneration. Brain Behav. Immun. 73, 708–716. 10.1016/j.bbi.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Chikuma S., Iwai Y., Fagarasan S., Honjo T. (2013). A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14 (12), 1212–1218. 10.1038/ni.2762 [DOI] [PubMed] [Google Scholar]
- Okello A., Edison P., Archer H. A., Turkheimer F. E., Kennedy J., Bullock R., et al. (2009. a). Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 72 (1), 56–62. 10.1212/01.wnl.0000338622.27876.0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A., Koivunen J., Edison P., Archer H. A., Turkheimer F. E., Nagren K., et al. (2009. b). Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 73 (10), 754–760. 10.1212/WNL.0b013e3181b23564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. K., Miller S. D. (2004). Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173 (6), 3916–3924. 10.4049/jimmunol.173.6.3916 [DOI] [PubMed] [Google Scholar]
- Osborn L. M., Kamphuis W., Wadman W. J., Hol E. M. (2016). Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 144, 121–141. 10.1016/j.pneurobio.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Oxenkrug G., van der Hart M., Roeser J., Summergrad P. (2017). Peripheral Tryptophan - Kynurenine Metabolism Associated with Metabolic Syndrome is Different in Parkinson’s and Alzheimer’s Diseases. Endocrinol. Diabetes Metab. J. 1 (4). [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. (2015). Targeted delivery of protein and gene medicines through the blood–brain barrier. Clin. Pharmacol. Ther. 97 (4), 347–361. 10.1002/cpt.18 [DOI] [PubMed] [Google Scholar]
- Parvathenani L. K., Tertyshnikova S., Greco C. R., Roberts S. B., Robertson B., Posmantur R. (2003). P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 278 (15), 13309–13317. 10.1074/jbc.M209478200 [DOI] [PubMed] [Google Scholar]
- Pearson S. J., Reynolds G. P. (1992). Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington’s disease. Neurosci. Lett. 144 (1–2), 199–201. 10.1016/0304-3940(92)90749-W [DOI] [PubMed] [Google Scholar]
- Pimenova A. A., Raj T., Goate A. M. (2018). Untangling genetic risk for Alzheimer’s disease. Biol. Psychiatry 83 (4), 300–310. 10.1016/j.biopsych.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbadie H. G., Sayyah M., Khoshkholgh-Sima B., Choopani S., Nategh M., Motamedi F., et al. (2018). Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer’s disease–related cognitive deficit in rats: behavioral, molecular, and electrophysiological evidence. Neurobiol. Aging 70, 203–216. 10.1016/j.neurobiolaging.2018.06.020 [DOI] [PubMed] [Google Scholar]
- Qin Y., Liu Y., Hao W., Decker Y., Tomic I., Menger M. D., et al. (2016). Stimulation of TLR4 attenuates Alzheimer’s disease–related symptoms and pathology in tau-transgenic mice. J. Immunol. 197 (8), 3281–3292. 10.4049/jimmunol.1600873 [DOI] [PubMed] [Google Scholar]
- Rahman A., Ting K., Cullen K. M., Braidy N., Brew B. J., Guillemin G. J. (2009). The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One 4 (7), e6344. 10.1371/journal.pone.0006344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D. D., Jaarsma D., Holtman I. R., Olah M., Ferreira F. M., Schaafsma W., et al. (2014. a). Priming of microglia in a DNA-repair deficient model of accelerated aging. Neurobiol. Aging 35 (9), 2147–2160. 10.1016/j.neurobiolaging.2014.03.025 [DOI] [PubMed] [Google Scholar]
- Raj T., Ryan K. J., Replogle J. M., Chibnik L. B., Rosenkrantz L., Tang A., et al. (2014. b). CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer’s disease susceptibility. Hum. Mol. Genet. 23 (10), 2729–2736. 10.1093/hmg/ddt666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy S. B., Jana M., Roy A., Corbett G. T., Kundu M., Chandra S., et al. (2018). Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J. Clin. Invest. 128 (10), 4297–4312. 10.1172/JCI96209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R. M., Brown M. A. (2012). Innate immunity in the central nervous system. J. Clin. Invest. 122 (4), 1164–1171. 10.1172/JCI58644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R. M., El Khoury J. (2015). Microglia in health and disease. Cold Spring Harb. Perspect. Biol. 8 (1), a020560. 10.1101/cshperspect.a020560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard K. L., Filali M., Prefontaine P., Rivest S. (2008). Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 28 (22), 5784–5793. 10.1523/JNEUROSCI.1146-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J. L., June C. H. (2005). The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood 105 (1), 13–21. 10.1182/blood-2004-04-1596 [DOI] [PubMed] [Google Scholar]
- Rillahan C. D., Macauley M. S., Schwartz E., He Y., McBride R., Arlian B. M., et al. (2014). Disubstituted sialic acid ligands targeting siglecs CD33 and CD22 associated with myeloid leukaemias and B cell lymphomas. Chem. Sci. 5 (6), 2398–2406. 10.1039/c4sc00451e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S., Sontheimer H. (2015). Glia as drivers of abnormal neuronal activity. Nat. Neurosci. 19 (1), 28–33. 10.1038/nn.4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Araiz A., Finucane O. M., Keogh S., Lynch M. A. (2018). Anti-TLR2 antibody triggers oxidative phosphorylation in microglia and increases phagocytosis of beta-amyloid. J. Neuroinflammation 15 (1), 247. 10.1186/s12974-018-1281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh T. S., Banks W. A. (2014). Delivery of therapeutic peptides and proteins to the CNS. Adv. Pharmacol. 71, 277–299. 10.1016/bs.apha.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J. M., Chiozzi P., Ferrari D., Colaianna M., Idzko M., Falzoni S., et al. (2009). Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J. Immunol. 182 (7), 4378–4385. 10.4049/jimmunol.0803612 [DOI] [PubMed] [Google Scholar]
- Sastre M., Richardson J. C., Gentleman S. M., Brooks D. J. (2011). Inflammatory risk factors and pathologies associated with Alzheimer’s disease. Curr. Alzheimer Res. 8 (2), 132–141. 10.2174/156720511795256062 [DOI] [PubMed] [Google Scholar]
- Schilling T., Eder C. (2004). A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J. Physiol. 559 (Pt 1), 35–40. 10.1113/jphysiol.2004.070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T., Eder C. (2007). TRAM-34 inhibits nonselective cation channels. Pflugers Arch. 454 (4), 559–563. 10.1007/s00424-007-0232-4 [DOI] [PubMed] [Google Scholar]
- Schilling T., Repp H., Richter H., Koschinski A., Heinemann U., Dreyer F., et al. (2002). Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca(2+)-dependent K(+) channels. Neuroscience 109 (4), 827–835. 10.1016/S0306-4522(01)00534-6 [DOI] [PubMed] [Google Scholar]
- Schlepckow K., Kleinberger G., Fukumori A., Feederle R., Lichtenthaler S. F., Steiner H., et al. (2017). An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol. Med. 9 (10), 1356–1365. 10.15252/emmm.201707672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M. J., Guillemin G. J., Teipel S. J., Buerger K., Hampel H. (2013). Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer's disease patients from controls. Eur. Arch. Psychiatry Clin. Neurosci. 263 (4), 345–352. 10.1007/s00406-012-0384-x [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Stone T. W. (2017). The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112, 237–247. 10.1016/j.neuropharm.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaabani S., Huizinga H. P. S., Butera R., Kouchi A., Guzik K., Magiera-Mularz K., et al. (2018). A patent review on PD-1/PD-L1 antagonists: small molecules, peptides, and macrocycles (2015–2018). Expert Opin. Ther. Pat. 28 (9), 665–678. 10.1080/13543776.2018.1512706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Holtzman D. M. (2018). Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18 (12), 759–772. 10.1038/s41577-018-0051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Abiega O., Shahraz A., Neumann H. (2013). Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 7, 6. 10.3389/fncel.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R., van der Lee S. J., Naj A. C., Bellenguez C., Badarinarayan N., Jakobsdottir J., et al. (2017). Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. J. Prev. Alzheimers Dis. 4 (4), 247–254. 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E., Halket J., Przyborowska A., Riederer P., Beckmann H., Sandler M., et al. (1989). Brain quinolinic acid in Alzheimer’s dementia. Eur. Arch. Psychiatry Clin. Neurosci. 239 (3), 177–179. 10.1007/BF01739651 [DOI] [PubMed] [Google Scholar]
- Song W. M., Colonna M. (2018). The microglial response to neurodegenerative disease. Adv. Immunol. 139, 1–50. 10.1016/bs.ai.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Song M., Jin J., Lim J. E., Kou J., Pattanayak A., Rehman J. A., et al. (2011). TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflammation 8, 92. 10.1186/1742-2094-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. M., Joshita S., Zhou Y., Ulland T. K., Gilfillan S., Colonna M. (2018). Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 215 (3), 745–760. 10.1084/jem.20171529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal R. G. W., Khayrullina T., Zhang H., Davis S., Fallon S. M., Cajina M., et al. (2017. a). Inhibition of the potassium channel KCa3.1 by senicapoc reverses tactile allodynia in rats with peripheral nerve injury. Eur. J. Pharmacol. 795, 1–7. 10.1016/j.ejphar.2016.11.031 [DOI] [PubMed] [Google Scholar]
- Staal R. G. W., Weinstein J. R., Nattini M., Cajina M., Chandresana G., Moller T. (2017. b). Senicapoc: repurposing a drug to target microglia KCa3.1 in stroke. Neurochem. Res. 42 (9), 2639–2645. 10.1007/s11064-017-2223-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B., Becker K., Fuchs D., Gostner J. M., (2017). Kynurenine pathway metabolism and immune activation: peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology 112, Pt B, 286–296. 10.1016/j.neuropharm.2016.02.030 [DOI] [PubMed] [Google Scholar]
- Sudom A., Talreja S., Danao J., Bragg E., Kegel R., Min X., et al. (2018). Molecular basis for the loss-of-function effects of the Alzheimer’s disease–associated R47H variant of the immune receptor TREM2. J. Biol. Chem. 293 (32), 12634–12646. 10.1074/jbc.RA118.002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara K., Kim H. D., Jin J. J., Maxwell J. A., Li L., Fukuchi K. (2006). Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 129 (Pt 11), 3006–3019. 10.1093/brain/awl249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Rochford C. D. P., Neumann H. (2005). Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 201 (4), 647–657. 10.1084/jem.20041611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa O., Yoshida R., Kido R., Hayaishi O. (1986). Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 261 (8), 3648–3653. [PubMed] [Google Scholar]
- Tavassoly O., Sade D., Bera S., Shaham-Niv S., Vocadlo D. J., Gazit E. (2018). Quinolinic acid amyloid-like fibrillar assemblies seed α-synuclein aggregation. J. Mol. Biol. 430 (20), 3847–3862. 10.1016/j.jmb.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Thawkar B. S., Kaur G. (2019). Inhibitors of NF-kappaB and P2X7/NLRP3/caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 326, 62–74. 10.1016/j.jneuroim.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Thei L., Imm J., Kaisis E., Dallas M. L., Kerrigan T. L. (2018). Microglia in Alzheimer’s disease: a role for ion channels. Front. Neurosci. 12, 676. 10.3389/fnins.2018.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P., Sevalle J., Deery M. J., Fraser G., Zhou Y., Stahl S., et al. (2017). TREM2 shedding by cleavage at the H157–S158 bond is accelerated for the Alzheimer’s disease–associated H157Y variant. EMBO Mol. Med. 9 (10), 1366–1378. 10.15252/emmm.201707673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H., Abe T., Takahashi S., Kimura M., Takahashi J., Kikuchi T. (1992). Concentrations of serotonin and its related substances in the cerebrospinal fluid in patients with Alzheimer type dementia. Neurosci. Lett. 141 (1), 9–12. 10.1016/0304-3940(92)90322-X [DOI] [PubMed] [Google Scholar]
- Tohgi H., Abe T., Takahashi S., Saheki M., Kimura M. (1995). Indoleamine concentrations in cerebrospinal fluid from patients with Alzheimer type and Binswanger type dementias before and after administration of citalopram, a synthetic serotonin uptake inhibitor. J. Neural Transm. Park. Dis. Dement. Sect. 9 (2-3), 121–131. 10.1007/BF02259654 [DOI] [PubMed] [Google Scholar]
- Török N., Majláth Z., Fülöp F., Toldi J., Vécsei L. (2016). Brain aging and disorders of the central nervous system: kynurenines and drug metabolism. Curr. Drug Metab. 17 (5), 412–429. 10.2174/1389200217666151222155043 [DOI] [PubMed] [Google Scholar]
- Ulland T. K., Colonna M. (2018). TREM2—a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14 (11), 667–675. 10.1038/s41582-018-0072-1 [DOI] [PubMed] [Google Scholar]
- Ulrich J. D., Ulland T. K., Colonna M., Holtzman D. M. (2017). Elucidating the role of TREM2 in Alzheimer’s disease. Neuron 94 (2), 237–248. 10.1016/j.neuron.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Ulrich J. D., Ulland T. K., Mahan T. E., Nystrom S., Nilsson K. P., Song W. M., et al. (2018). ApoE facilitates the microglial response to amyloid plaque pathology. J. Exp. Med. 215 (4), 1047–1058. 10.1084/jem.20171265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. (2009). Natural ligands for CD33-related siglecs? Glycobiology 19 (8), 810–812. 10.1093/glycob/cwp063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C., Kumar S., Franklin B. S., Dierkes T., Brinkschulte R., Tejera D., et al. (2017). Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552 (7685), 355–361. 10.1038/nature25158 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Parpura V., Pekna M., Pekny M., Sofroniew M. (2014). Glia in the pathogenesis of neurodegenerative diseases. Biochem. Soc. Trans. 42 (5), 1291–1301. 10.1042/BST20140107 [DOI] [PubMed] [Google Scholar]
- Walker D. G., Whetzel A. M., Serrano G., Sue L. I., Beach T. G., Lue L. F. (2015). Association of CD33 polymorphism rs3865444 with Alzheimer’s disease pathology and CD33 expression in human cerebral cortex. Neurobiol. Aging 36 (2), 571–582. 10.1016/j.neurobiolaging.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S., Letiembre M., Liu Y., Heine H., Penke B., Hao W., et al. (2007). Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell. Physiol. Biochem. 20 (6), 947–956. 10.1159/000110455 [DOI] [PubMed] [Google Scholar]
- Wang Y., Cella M., Mallinson K., Ulrich, Jason D., Young, et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160 (6), 1061–1071. 10.1016/j.cell.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ulland T. K., Ulrich J. D., Song W., Tzaferis J. A., Hole J. T., et al. (2016). TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213 (5), 667–675. 10.1084/jem.20151948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R. J., Dennis M. S. (2013). Bispecific antibodies for delivery into the brain. Curr. Opin. Chem. Biol. 17 (3), 393–399. 10.1016/j.cbpa.2013.03.023 [DOI] [PubMed] [Google Scholar]
- Wennström M., Nielsen H. M., Orhan F., Londos E., Minthon L., Erhardt S. (2014). Kynurenic acid levels in cerebrospinal fluid from patients with Alzheimer’s disease or dementia with Lewy bodies. Int. J. Tryptophan. Res. 7, 1–7. 10.4137/IJTR.S13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes P. D., Holtman I. R., Boddeke E. W., Möller T., Eggen B. J. (2016). Next generation transcriptomics and genomics elucidate biological complexity of microglia in health and disease. Glia 64 (2), 197–213. 10.1002/glia.22866 [DOI] [PubMed] [Google Scholar]
- Widner B., Leblhuber F., Walli J., Tilz G. P., Demel U., Fuchs D. (1999). Degradation of tryptophan in neurodegenerative disorders. Adv. Exp. Med. Biol. 467, 133–138. 10.1007/978-1-4615-4709-9_19 [DOI] [PubMed] [Google Scholar]
- Widner B., Leblhuber F., Walli J., Tilz G. P., Demel U., Fuchs D. (2000). Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural. Transm. (Vienna). 107 (3), 343–353. 10.1007/s007020050029 [DOI] [PubMed] [Google Scholar]
- Wirths O., Breyhan H., Marcello A., Cotel M. C., Bruck W., Bayer T. A. (2010). Inflammatory changes are tightly associated with neurodegeneration in the brain and spinal cord of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiol. Aging 31 (5), 747–757. 10.1016/j.neurobiolaging.2008.06.011 [DOI] [PubMed] [Google Scholar]
- Wong D. T., Bymaster F. P., Engleman E. A. (1995). Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 57 (5), 411–441. 10.1016/0024-3205(95)00209-O [DOI] [PubMed] [Google Scholar]
- Wu H.-Q., Lee S.-C., Scharfman H. E., Schwarcz R. (2002). L-4-chlorokynurenine attenuates kainate-induced seizures and lesions in the rat. Exp. Neurol. 177 (1), 222–232. 10.1006/exnr.2002.7971 [DOI] [PubMed] [Google Scholar]
- Wu H. Q., Lee S. C., Schwarcz R. (2000). Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 390 (3), 267–274. 10.1016/S0014-2999(00)00024-8 [DOI] [PubMed] [Google Scholar]
- Wu W., Nicolazzo J. A., Wen L., Chung R., Stankovic R., Bao S. S., et al. (2013). Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One 8 (4), e59749. 10.1371/journal.pone.0059749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J. E., et al. (1998). Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395 (6701), 503–507. 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Yeh F., Wang Y., Tom I., Gonzalez L., Sheng M. (2016). TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91 (2), 328–340. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Yuan P., Condello C., Keene C. D., Wang Y., Bird T. D., Paul S. M., et al. (2016). TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron 90 (4), 724–739. 10.1016/j.neuron.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádori D., Veres G., Szalárdy L., Klivényi P., Vécsei L. (2018). Alzheimer’s disease: recent concepts on the relation of mitochondrial disturbances, excitotoxicity, neuroinflammation, and kynurenines. J. Alzheimers Dis. 62 (2), 523–547. 10.3233/JAD-170929 [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang L. Z., Yu J. T., Chi Z. F., Tan L. (2012). Increased expressions of TLR2 and TLR4 on peripheral blood mononuclear cells from patients with Alzheimer’s disease. J. Neurol. Sci. 315 (1-2), 67–71. 10.1016/j.jns.2011.11.032 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kang S. A., Mukherjee T., Bale S., Crane B. R., Begley T. P., et al. (2007). Crystal structure and mechanism of tryptophan 2,3-dioxygenase, a heme enzyme involved in tryptophan catabolism and in quinolinate biosynthesis. Biochemistry 46 (1), 145–155. 10.1021/bi0620095 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wu X., Li X., Jiang L. L., Gui X., Liu Y., et al. (2018). TREM2 is a receptor for beta-amyloid that mediates microglial function. Neuron 97 (5), 1023–1031 e1027. 10.1016/j.neuron.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Wang Z., Wang D., Wang Z., Martens Y. A., Wu L., et al. (2018). Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol. Neurodegener. 13 (1), 15. 10.1186/s13024-018-0247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Xu Y., Cen X., Nandakumar K. S., Liu S., Cheng K. (2018). Targeting pattern-recognition receptors to discover new small molecule immune modulators. Eur. J. Med. Chem. 144, 82–92. 10.1016/j.ejmech.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Zuchero J. B., Barres B. A. (2015). Glia in mammalian development and disease. Development 142 (22), 3805–3809. 10.1242/dev.129304 [DOI] [PMC free article] [PubMed] [Google Scholar]