Abstract
With most hospitalized patients requiring peripheral intravenous catheters (PIVCs), PIVC-related process improvement may substantially affect the health, safety, and satisfaction of patients and health care workers, in addition to reducing costs. This study examined PIVC practice-related metrics before and after a comprehensive process improvement program, which included a change to closed catheter technology. Data were obtained from observations, clinician interviews, and patient records. Metrics included assessment of risk, especially blood exposure and contamination; measurement of insertion efficiency; and quantification of PIVC failure. A significant improvement in most metrics was achieved after the process improvement program.
Keywords: catheter safety, catheter stabilization, dwell time, first attempt, health care work safety, IV, needlestick injuries, outcomes, peripheral catheter, peripheral vascular access education
The placement of a peripheral intravenous catheter (PIVC) is one of the most frequently performed invasive patient interventions. Studies have estimated that 60% to 90% of hospitalized patients receive PIVCs during their stay,1 with as many as 70% of patients in acute care hospitals needing a PIVC.2 A total of 150 million PIVCs were used in the United States in 20093; this number ballooned to 330 million in 2013.2 Despite the fact that PIVCs are used frequently in patient care, there has been a lack of research investigating PIVCs and their associated risks.3,4
The duration for which an intravenous (IV) catheter remains in place and is functional, or dwell time, is believed to be associated with catheter-related complications. However, aside from routine replacement, dwell times are affected by factors including vein condition, types of drugs administered, and length of hospital stay.3 Therefore, while monitoring and evaluating catheters and vascular access sites daily for malfunction, irritation, or infection—and removing them, if necessary—are essential, replacement as clinically indicated could help prevent unnecessary catheter insertion and reduce equipment use, staff workload, and cost.
The average IV catheter dwell time is 44 hours,5 which falls short of current practice guidelines that recommend PIVC replacement every 72 to 96 hours.2,6 The Infusion Therapy Standards of Practice states that PIVCs should be removed when clinically indicated.7 A nurse-led, nationally funded multicenter study involving more than 3000 patients reported that PIVCs can be removed as clinically indicated instead of routinely replaced, as recommended in current practice guidelines.8 Other studies with smaller sample sizes have yielded similar results: overall complication rates did not appear to vary when catheters were changed as clinically indicated.9
Multiple risks and complications associated with the use of PIVCs exist for both patients and health care workers (HCWs). In patients, some of these complications include phlebitis, infiltration, and catheter-related infection. A large multicenter cohort study that compared conventional PIVC securement methods, such as tape, with a PIVC stabilization device showed that complications occurred in nearly half (47.6%) of the patients who had PIVCs with conventional securement.10 Phlebitis is a common PIVC-related complication that leads to catheter failure.11 This inflammation can arise from chemical or bacterial causes, as well as mechanical causes, such as improper catheter securement.11 Additionally, poor PIVC stabilization can damage the vein wall, which may lead to blood clot formation and occlusion. It may result in leakage of IV fluids or their infiltration into surrounding tissues as well, which could cause undesirable outcomes. Resiting PIVCs because of these complications also may increase the risk of infection. In 1 review including several studies, the incidence of catheter-related bloodstream infection was 0.0% to 2.2%, while insertion site infection rates ranged from 0.1% to 5.1%.1 Because of such complications, PIVCs often require unscheduled restarts and fail to achieve their recommended dwell time of 72 to 96 hours. Estimates of the PIVC failure rate range from 33% to as high as 69%.1,11
In addition, HCWs are at risk for needlestick injuries, blood splashes, and exposure to bloodborne pathogens during PIVC insertion. A systematic literature review by Hadaway12 identified risks to HCWs that were related to key challenges, such as knowledge deficits and lack of access to safety-engineered catheters. A survey conducted by Jagger et al4 to explore blood exposure risk during PIVC placement found that 46% of nurses reported at least 1 exposure per month during PIVC insertion.
Researchers have reported varying first PIVC insertion attempt success rates, ranging from 18% to 79%.13 One study found that 27% of PIVC insertions required 3 or more attempts. Repeated venipuncture attempts can increase stress and frustration for staff, and decrease patient and family satisfaction and confidence in staff, staff professional satisfaction, and staff availability to attend to other patients' needs.14
Currently, there are several areas in infusion practice where gaps in clinical practice, product technology, or policy exist. These gaps may constitute risks to the patient, to the HCW, and to the economics of patient care. The field of PIVC technology has made strides in the advancement of such devices as safety PIVCs, which enhance patient and HCW safety by preventing needlestick injuries and providing closed catheter systems. These systems reduce microbial contamination and insertion discomfort compared with conventional catheters. The conventional (open) device is composed of catheter tubing that is one-half inch to 2 inches long connected to an open adapter, which serves as the connection point to an IV administration set. Currently available closed catheter systems, such as those used in this study, may incorporate multiple components—such as the catheter, a stabilization platform or feature, an extension set, and a needleless access site—in a preassembled manner. Typically, these parts would be assembled by the HCW during conventional PIVC insertion. While closed catheters are more costly than conventional catheters, reports suggest that they minimize the risk of accidental displacement, contamination, cross-contamination, and HCW exposure to hazardous bloodborne pathogens, such as the hepatitis C virus and human immunodeficiency virus. They also reduce the incidence of phlebitis and infection in patients with catheters, while increasing dwell times.15,16 Longer dwell times without increased incidence of adverse outcomes may facilitate replacement of catheters as clinically indicated (instead of routine replacement), which could result in cost reduction.
The purposes of this study were (1) to identify any areas of risk associated with PIVC insertion that could lead to needlestick injury, blood exposure, bloodstream infection, or other catheter-related complications; (2) to initiate process improvement projects and strategies to improve infusion practice and related laboratory specimen collection; and (3) to optimize workflow efficiencies to improve throughput, reduce cost, and improve the overall patient experience.
METHODS
The study was performed at University of Florida Health (UF Health) Jacksonville, a regional academic health center. UF Health Jacksonville is a 695-bed facility that employs almost 400 faculty physicians and more than 2200 nurses who practice across 75 areas of specialty care. UF Health Jacksonville sees more than 88 396 patients a year, with 23 376 hospital admissions. Studies were conducted across 15 patient care areas in June 2014 and across 22 patient care areas in June 2016.
After an assessment in June 2014 of the current PIVC practices of clinicians from 15 patient care areas, a comprehensive PIVC process improvement program began in June 2015. It included updating policies and procedures on the use of PIVCs, training nursing personnel on best practices regarding PIVCs, and adopting a closed PIVC product (Nexiva Closed IV Catheter System; Becton, Dickinson and Company [BD]; Franklin Lakes, New Jersey). Training included both instructor-led and learner-led continuing education courses on infusion therapy and hands-on support regarding proper techniques for the insertion and use of PIVC products.
Clinician Characteristics
Along with initial observations of PIVC insertions, interviews were conducted with 43 clinicians in June 2014. The composition of the team included frontline clinicians who actively participate in the insertion and maintenance of PIVCs. Physicians, nurses, and emergency department (ED) technicians were interviewed to develop a summary of the characteristics of current practice and compliance with use of insertion resources.
Data Collection and Analysis
Data were gathered from assessments of PIVC access sites, observations of PIVC insertions, clinician interviews, and reviews of patient health care records. Sample metrics included incidents of blood spillage and contamination, first insertion proficiency, PIVC dwell time, number of catheters per patient, and number of catheters failing within 48 hours of insertion.
To assess the significance of differences in the proportion of dichotomous events before and after the PIVC process improvement program, a 1-sided Fisher exact test was performed. Standard descriptive statistical calculations and a 2-sample t test were performed for continuous metrics.
RESULTS
Observation of Risks Associated With PIVC Use Before and After Process Improvement Program
PIVC-related practices at UF Health Jacksonville were assessed in June 2014, before a comprehensive PIVC process improvement program began; and in June 2016, after the program had been in place for approximately 1 year. PIVC access sites were observed in June 2014 and June 2016. A summary of risks is provided in Table 1. The incidence of PIVC risk decreased significantly between June 2014 and June 2016. The proportion of risk across all PIVCs observed also decreased significantly.
TABLE 1. Overall Risks Associated With Observed PIVC Sites.
| Overall Risk Assessmenta | June 2014 (n = 69) | June 2016 (n = 133) | P Value |
|---|---|---|---|
| PIVCs with any risk, % | 87 | 64 | <.001 |
| Risks per PIVC, n | 1.7 | 1.1 |
Abbreviation: PIVC, peripheral intravenous catheter.
aOverall risk was assessed relative to the number of PIVCs observed.
Table 2 lists the occurrence of specific risks as percentages of the total number of PIVCs observed. There was a statistically significant decrease in the percentage of incorrectly labeled dressings after the change to closed PIVCs. In addition, the percentage of PIVCs with nonocclusive dressings decreased significantly after the product change. After the transition to new PIVCs, there was little change in inappropriate site selection of PIVCs, including catheters placed in areas of flexion and lower extremities. The incidence of visible blood in the luer lock threads of the PIVC connections and blood leakage from the access site decreased after the change to the closed PIVC.
TABLE 2. Specific Risks Associated With Observed PIVC Sites.
| Risks, %a | June 2014 (n = 69) | June 2016 (n = 133) | P Value |
|---|---|---|---|
| Dressings not labeled correctly (in adherence with facility's policy) | 67 | 26 | <.001 |
| Inappropriate site selection | 38 | 39 | |
| Blood visible between connections and/or under dressing | 22 | 12 | |
| Blood or fluid leakage from access site | 8 | 3 | |
| No extension set used | 36 | 5 | <.001 |
| Nonocclusive dressings | 18 | 8 | <.05 |
| Swelling at or above access site | 5 | 0 | <.05 |
| Redness at or above access site | 3 | 1 |
Abbreviation: PIVC, peripheral intravenous catheter.
aSpecific risks are reported as a percentage of the number of PIVCs observed. Not all risks are reported.
Observation of PIVC Insertions Before and After PIVC Process Improvement Program Implementation
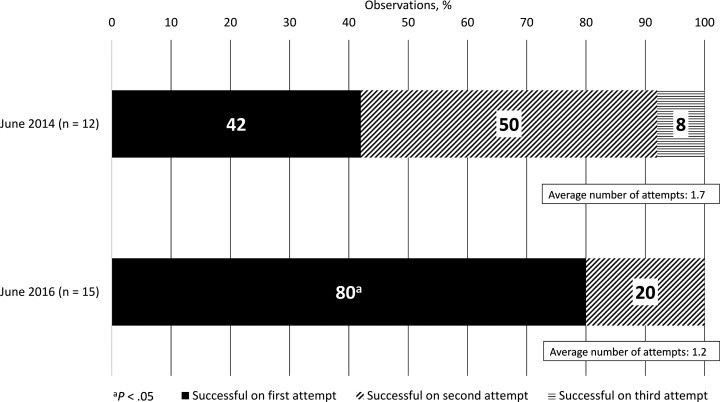
PIVC insertions were observed before and after implementation of the PIVC process improvement program. There was a 2-fold increase in the number of successful PIVC starts on the first attempt (Figure 1). Risk factors observed decreased after process improvement. For example, the incidence of inappropriate site selection decreased from June 2014 to June 2016, with corresponding decreases in the incidence of inadequate site preparation.
Figure 1.
Successful PIVC insertion by attempt before and after PIVC process improvement program implementation. Abbreviation: PIVC, peripheral intravenous catheter.
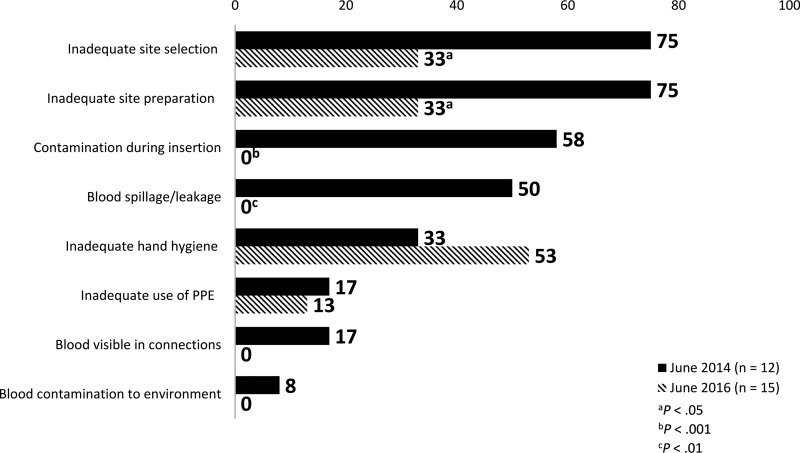
Furthermore, after the PIVC process improvement program, the incidence of PIVC site contamination, blood leakage from the access site, visible blood in the thread of the PIVC connections, and blood contamination to the environment were eliminated. After implementation of the comprehensive process improvement program, the incidence of inadequate hand hygiene increased, and the inadequate use of personal protective equipment decreased slightly. A summary of specific risks associated with observed PIVC insertions is shown in Figure 2.
Figure 2.
Comparison of risks assessed during PIVC insertions before and after PIVC process improvement program implementation. Abbreviations: PIVC, peripheral intravenous catheter; PPE, personal protective equipment.
Analysis of Clinician Interviews
Clinicians were interviewed to determine the occurrence of key events related to PIVC practices before and after a comprehensive process improvement program, which included the adoption of a closed PIVC infusion product (Table 3). After the establishment of the institutional PIVC process improvement program, clinicians reported elimination of blood leakage from the hub during insertion. There was also a decrease in clinician-reported infiltration rates from 2014 to 2016, following the PIVC process improvement program, as well as decreases in IV catheter dislodgment and access site leakage. In addition, there was a decrease in clinicians reporting frequent replacement of PIVCs when patients were transferred from the ED or critical care or surgery departments. Furthermore, after the PIVC process improvement program, there was a decrease in clinicians reporting that they were inserting at least 10 PIVCs a week.
TABLE 3. Clinicians Reporting Key PIVC-Related Events.
| Key Event, %a | June 2014 (n = 42) | June 2016 (n = 23) | P Value |
|---|---|---|---|
| Blood leakage from catheter hub during insertion | 85 | 0 | <.001 |
| Blood leakage at hub caused patient concern | 59 | N/A | |
| Use of additional supplies to clean up spillage | 100 | 0 | <.001 |
| Use of syringe to draw blood from catheter | 80 | 53 | <.05 |
| Drawing back from catheter hub | 64 | N/A | |
| Use of blunt-fill or nonsafety needle to transfer blood to collection tube | 74 | 34 | |
| Reasons for restarts: | |||
| Infiltration or extravasation | 64 | N/A | |
| Dislodgment | 20 | N/A | |
| Leakage | 14 | N/A | |
| Frequent required IV catheter change at patient admission from ED or critical care or surgery department | 21 | 8 | |
| Initiation of different gauge, if patient suspected of needing to undergo computed tomography | 50 | 0 | |
| Frequent administration of IV push medications | 90 | N/A | |
| Placement of disinfectant caps on all catheters | 77 | 100 | <.01 |
| Use of same syringe to flush before and after medication administration | 8 | 0 | |
| Routine use of starter kit | 45 | 4 | <.001 |
| Routine use of extension set | 60 | 86 | |
| Use of dual or triple set | 62 | N/A | |
| Placement of ≥ 10 PIVCs/wk | 38 | N/A | |
Abbreviations: ED, emergency department; IV, intravenous; N/A, not applicable; PIVC, peripheral intravenous catheter; wk, week.
aSpecific perceptions are reported as a percentage of the number of clinicians interviewed.
Analysis of Health Care Record Reports
In addition to assessing existing PIVC access sites, observing PIVC insertion, and interviewing clinicians, patients' health care records were reviewed before and after the implementation of the comprehensive process improvement program. The resultant data are presented in Table 4. There was a significant reduction in PIVCs per patient after the process improvement program and a significant increase in mean dwell time. The mean length of patient stay was similar before and after the PIVC process improvement program. In addition, the percentage of PIVCs that failed within 24 hours and 48 hours decreased significantly.
TABLE 4. Results of Health Care Record Review.
| Variablesa | June 2014 (n = 69) | June 2016 (n = 60) | P Value |
|---|---|---|---|
| Mean dwell time, days (SD) | 2.4 (1.6) | 4.3 (1.9) | <.001 |
| Mean length of patient stay, days (SD) | 7.0 (15.3) | 6.4 (2.8) | |
| Mean PIVCs inserted per patient, n (SD) | 2.8 (1.9) | 1.6 (0.8) | <.001 |
| PIVCs failing within 24 hours, % | 32 | 10 | <.01 |
| PIVCs failing within 48 hours, % | 60 | 14 | <.001 |
| No record of number of IV access attempts, % | 32 | 24 | |
| No reason provided for catheter removal, % | 45 | 9 | <.001 |
Abbreviations: IV, intravenous; PIVC, peripheral intravenous catheter; SD, standard deviation.
aData are presented as mean (SD) or percentage of charts.
DISCUSSION
The purpose of the study was to examine the impact of a comprehensive performance improvement program on safety, patient satisfaction, and cost pertaining to hospital-based PIVC therapy at UF Health Jacksonville. To identify risks associated with PIVCs, UF Health Jacksonville collaborated with a team of 7 BD clinical consultants to review practices, processes, and products, including areas of risk that might lead to blood exposure, bloodstream infection, needlestick injury, or other catheter-related complications. An assessment was completed in June 2014 that consisted of observations of PIVC access sites and live catheter insertions, interviews with clinicians, and reviews of patient health care records. The assessment identified opportunities to improve PIVC-related practices, including minimizing blood exposure, increasing catheter dwell times, improving first-attempt insertion success rates, reducing complications, and decreasing catheter failure rates.
A process improvement program was developed to address these opportunities in collaboration with the BD clinical team. BD's integrated solution for vascular access management17 provided specific and practical recommendations, including policy updates, practice changes, extensive training on best practices for PIVC insertion, and product recommendations.
In June 2015, the BD clinical team offered a robust education and training program, which included instructor- and learner-led continuing education on infusion-related topics. Hands-on support and 1-on-1 training were provided to help clinicians gain proficiency with the new catheters and help ensure that clinicians were equipped to comply with institutional policies and best practices.
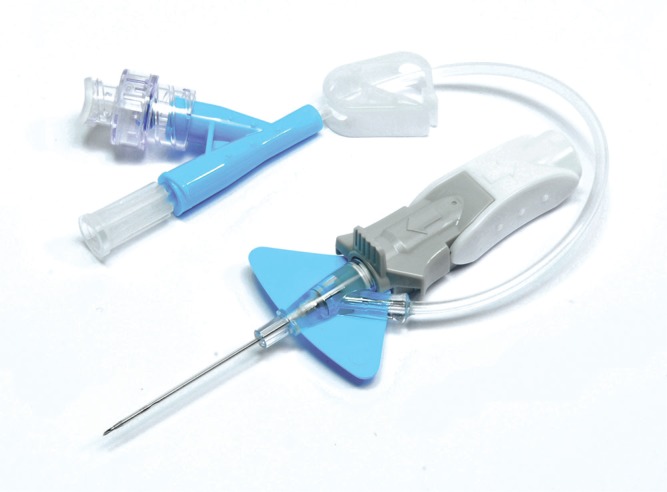
UF Health Jacksonville began using closed PIVC products in July 2015. The catheters (Figure 3) include a preattached extension set to contain blood, a safety mechanism to prevent needlesticks, and a stabilization platform, eliminating the need for a securement device.
Figure 3.
Example of Nexiva Closed PIVC System. Courtesy and © Becton, Dickinson and Company; Franklin, NJ. Reprinted with permission. Abbreviation: PIVC, peripheral intravenous catheter.
Outcomes of the Performance Improvement Program
Reassessment in June 2016 revealed improvements in safety and clinical efficiency. The process improvement program enhanced safety in several ways. The comprehensive policy revision and education program helped ensure that clinicians were aware of and implemented best practices. These changes, coupled with the use of a closed PIVC product, decreased risk across a broad range of areas, including use of proper dressings, use of extension sets, selection and preparation of access sites, and aseptic practice. While adherence to some safe practices decreased, notably hand hygiene, overall risk was reduced, and safe practice was increased. Especially impactful was the fact that exposure to blood and needles was reduced (Tables 3 and 4).
After the process improvement program was implemented, a significantly smaller proportion of PIVCs with risks was observed (Table 3), as well as fewer total risks versus observations from the preliminary assessment. It is worth noting that the incidence of blood visibility between connections and/or under the dressing was reduced by almost 50%, and the incidence of unused extension sets was reduced by 86%. In addition, with 85% of clinicians reporting blood leakage during insertion before the process improvement, none reported blood leakage after the process improvement. Leakage elimination was confirmed with PIVC insertion observations: at the preassessment observation, 50% of insertions yielded blood spillage or leakage, while 0% yielded blood spillage or leakage after the process improvement program and the adoption of the closed PIVC product.
The number of successful first attempts for PIVC insertion nearly doubled after the process improvement program, and the mean number of attempts at insertion was reduced by 30%. Because repeated venipunctures and difficulty obtaining access may be associated with failure and complications,1 improving first-attempt success rates for PIVCs may result in improved patient outcomes. In a similar fashion, the improved dwell times achieved by adopting more sophisticated PIVC technology, as noted in this and other studies, may reduce the frequency of venipunctures and potentially improve patient outcomes.15,16
In conjunction with improving patient outcomes with fewer venipunctures, needing fewer attempts to achieve insertion may translate to fewer venipuncture injuries and corresponding risks to HCWs. For any institution, reducing blood and needle exposure reduces safety risks, which include complications resulting from blood contamination. However, for an institution such as UF Health Jacksonville, which serves a relatively large patient population with HIV/AIDS, the hepatitis C virus, or both, reducing exposure to blood and needles reduces risk to patients who may be more acutely affected by complications, decreases the risk of infection, and reduces the risk of HCWs acquiring bloodborne disease.
Some of the benefits noted after the process improvement program may influence patient satisfaction positively, in addition to increasing safety. Fewer attempts needed to insert PIVCs and longer dwell times contribute to fewer venipunctures, which results in less pain and discomfort for the patient.18
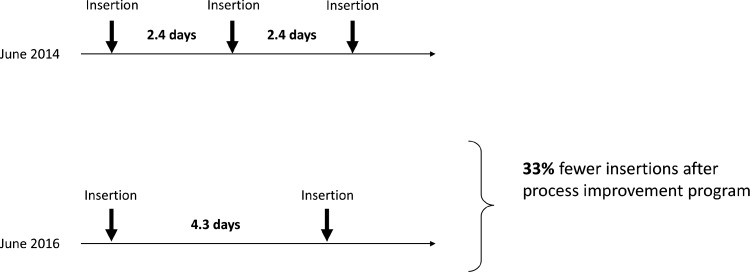
In addition to the benefits found in reducing the frequency of venipunctures, the process improvement program at UF Health Jacksonville resulted in several economic efficiencies. The institution saw an 80% increase in mean catheter dwell times (from 2.4 to 4.3 days). This improvement alone reduced the number of catheters needed during a patient's average hospital stay (approximately 7 days) by 33% (Figure 4). As mentioned earlier, UF Health Jacksonville experienced a reduction of 30% in the mean number of attempts needed to gain vascular access. Reducing the number of attempts needed to insert a PIVC and improving safety may result in fewer catheters being needed. The institution realized a total 42% reduction in PIVCs needed per patient during a hospital stay. Additional savings will be gained from reduced nursing time and cost of care and supplies, and potential litigations related to complications.15,16,19 Finally, because the Patient Protection and Affordable Care Act of 2010 includes Hospital Consumer Assessment of Healthcare Providers and Systems surveys of patient satisfaction, adopting catheter-related practices to improve patient satisfaction may have important implications for calculations of value-based incentive payments.20
Figure 4.
Over an average hospital stay of 7 days, longer dwell times translate to fewer needed insertions.
The potential safety and cost improvements identified in the current study are similar to those suggested in previous reports on PIVC process improvement. For example, Delp and Hadaway21 found that switching to a catheter with an integrated stabilization platform, a preattached extension set, and a passive safety mechanism produced benefits for clinicians and cost saving without adversely affecting patient outcomes. González López et al16 reported that use of closed-system PIVCs reduced phlebitis and infection risk while reducing cost relative to other PIVCs.
Because as many as 70% of patients in acute care hospitals require a PIVC,2,3 and 330 million PIVCs are used annually in the United States,2 implementation of a comprehensive process improvement program may result in substantial savings by avoiding complications, other health risks to patients and HCWs, and a reduction in nursing time and needed supplies.
LIMITATIONS
This study was limited to a single institution and a single study arm, and statistical analyses were post hoc. Performing a prospective statistically powered study with controls at multiple institutions might allow broader conclusions to be drawn. In addition, data input was largely binary (eg, yes or no), which has been shown to reduce statistical power and increase the likelihood of false-positive data, rather than allowing degrees of data.22
CONCLUSION
Because a majority of hospitalized patients require PIVCs, improving processes could substantially affect the US health care system. This study examined a comprehensive PIVC process improvement program's potential impact on safety, patient satisfaction, and cost at UF Health Jacksonville. Process improvement included updating policies, a broad education campaign, and a change to a closed PIVC product.
The program demonstrated that overall adherence to PIVC policies was improved, and it revealed opportunities to improve patient satisfaction while reducing cost. Less blood and needle exposure were the most notable safety improvements. In addition, the medical facility saw increases in dwell times and a decrease in the number of attempts needed to place PIVCs successfully. These improvements have the potential to reduce patient complications and delays in treatment, improve patient satisfaction, reduce risk to HCWs with regard to bloodborne pathogens, and reduce costs.
ACKNOWLEDGMENTS
The authors thank Alyssa Garrelts, PhD, of FORCE Communications, LLC, for providing support in the preparation of this manuscript.
Footnotes
Becton, Dickinson and Company provided financial assistance for writing and editorial services in the preparation of this manuscript.
REFERENCES
- 1.Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189–203. [DOI] [PubMed] [Google Scholar]
- 2.Keogh S. New research: change peripheral intravenous catheters as clinically indicated, not routinely. JAVA. 2013;18(3):153–154. [Google Scholar]
- 3.Zingg W, Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34(suppl 4):S38–S42. 10.1016/S0924-8579(09)70565-5. [DOI] [PubMed] [Google Scholar]
- 4.Jagger J, Perry J, Parker G, Phillips EK. Nursing2011 survey results: blood exposure risk during peripheral I.V. catheter insertion and removal. Nursing2011. 2011;41(12):45–49. [DOI] [PubMed] [Google Scholar]
- 5.Frey AM, Schears GJ. Why are we stuck on tape and suture? A review of catheter securement devices. J Infus Nurs. 2006;29(1):34–38. [DOI] [PubMed] [Google Scholar]
- 6.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–e193. 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infus Nurs. 2016;39(suppl 1):S1–S159. [Google Scholar]
- 8.Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066–1074. [DOI] [PubMed] [Google Scholar]
- 9.Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8(53):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schears GJ. Summary of product trials for 10,164 patients: comparing an intravenous stabilizing device to tape. J Infus Nurs. 2006;29(4):225–231. [DOI] [PubMed] [Google Scholar]
- 11.Marsh NM, Webster J, Mihala G, Rickard CM. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database Syst Rev. 2015;(6):CD011070. 10.1002/14651858.CD011070.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadaway L. Needlestick injuries, short peripheral catheters, and health care worker risks. J Infus Nurs. 2012;35(3):164–178. [DOI] [PubMed] [Google Scholar]
- 13.Carr PJ, Rippey JC, Budgeon CA, Cooke ML, Higgins N, Rickard CM. Insertion of peripheral intravenous cannulae in the emergency department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182–190. [DOI] [PubMed] [Google Scholar]
- 14.Barton AJ, Danek G, Johns P, Coons M. Improving patient outcomes through CQI: vascular access planning. J Nurs Care Qual. 1998;13(2):77–85. [DOI] [PubMed] [Google Scholar]
- 15.van Zundert A. IV catheter technology: benefits of closed IV catheter systems. Hosp Pharm Europe. 2012;62(3):1–3. [Google Scholar]
- 16.González López JL, Arribi Vilela A, Fernández del Palacio E, Olivares Corral J, Benedicto Martí C, Herrera Portal P. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117–126. [DOI] [PubMed] [Google Scholar]
- 17.Becton, Dickinson and Company (BD). Vascular access management for peripheral IV care. BD website. https://www.bd.com/en-us/offerings/integrated-solutions/vascular-access-management/vascular-access-management-for-peripheral-iv-care. Accessed February 1, 2017.
- 18.Anderson NR. Influencing patient satisfaction scores: prospective one-arm study of a novel intravenous catheter system with retractable coiled-tip guidewire compared with published literature for conventional peripheral intravenous catheters. J Infus Nurs. 2016;39(4):201–209. [DOI] [PubMed] [Google Scholar]
- 19.Kokotis K. Cost containment and infusion services. J Infus Nurs. 2005;28(3 suppl):S22–S32. [DOI] [PubMed] [Google Scholar]
- 20.Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Project Team. HCAHPS fact sheet. HCAHPS Hospital Survey website. http://www.hcahpsonline.org/globalassets/hcahps/facts/hcahps_fact_sheet_november_2017a.pdf. Accessed July 21, 2018.
- 21.Delp J, Hadaway L. New product decisions—the process and outcome for a community health system. JAVA. 2011;16(2):74–76, 78,–79, 82–84. [Google Scholar]
- 22.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]