The water channel aquaporin-4 (AQP4) is heavily enriched at glial barriers in the CNS, including at the glia limitans, the basolateral membrane of ependymocytes, and the perivascular face of astrocyte endfeet. Despite the impressive density of AQP4 at these sites, where it can occupy as much as 50% of the total membrane surface, the neurological phenotypes of Aqp4 deficient mice are surprisingly mild (Papadopoulos and Verkman, 2013). Some evidence points to a role for AQP4 in regulating brain extracellular space (ECS) structure, including increased ECS volume fraction in Aqp4 deficient mice (Yao et al., 2008), and altered diffusion of large molecules in the parenchyma (Binder et al., 2004). Additionally, a role for AQP4 in clearing edema fluid from the brain interstitium was suggested by experiments that showed more rapid intracranial pressure increase in Aqp4 deficient mice during parenchymal fluid infusion (Papadopoulos et al., 2004).
Building on these observations, and a longstanding body of work indicating that perivascular spaces constitute a critical route for solute transport through the interstitium (Rennels et al., 1985; Rennels et al., 1990; Abbott, 2004), Iliff and colleagues (Iliff et al., 2012) introduced the concept of an AQP4-dependent ‘glymphatic’ system of trans-astrocytic, convectional fluid flow through the brain interstitium from peri-arterial to peri-venous spaces (Figure 1A). It was proposed that this system plays an important role in clearing extracellullar Aβ and tau aggregates particularly during sleep (Xie et al., 2013), is enhanced by running (von Holstein-Rathlou et al., 2018) or moderate alcohol use (Lundgaard et al., 2018), and impaired following brain injury due to AQP4 mislocalization away from endfeet (Iliff et al., 2014). The glymphatic concept has been greeted with considerable enthusiasm in the popular press (Sample, 2013, Konnikova, 2014, Kohn, 2017); however, amongst researchers in the field of brain extracellular transport and AQP4 biology it has proved highly controversial (Hladky and Barrand, 2014, Spector et al., 2015, Smith and Verkman, 2017, Abbott et al., 2018).
Figure 1:
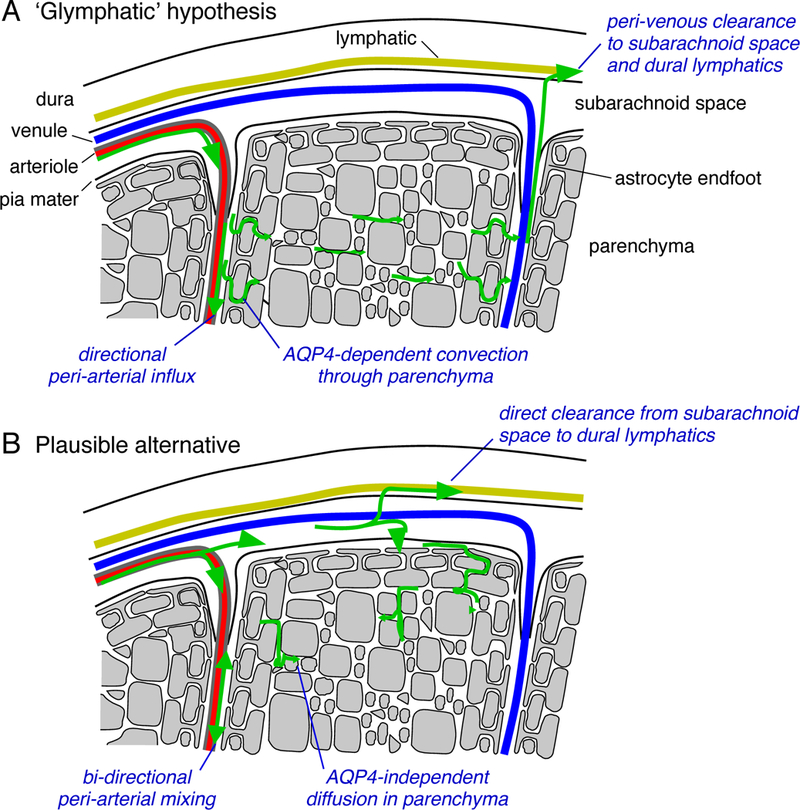
Mechanisms of tracer accumulation in brain parenchyma following bolus injection into the CSF. A. According to the ‘glymphatic’ hypothesis, CSF flows into the brain via the peri-arterial spaces of descending arterioles and is cleared via the peri-venous spaces toward the dural lymphatics. Tracer accumulation in the parenchyma is determined by the AQP4 content of the glial barrier at the parenchymal boundary, which promotes directional solute flow into, through and out of the parenchyma. B. A conventional and more plausible model of tracer accumulation proposes that some fraction of tracer enters the periarterial space and moves across the pial layer, basement membranes and astrocyte endfeet before reaching the parenchyma. Transport in the perivascular spaces is by facilitated diffusion (dispersion), and transport in the parenchyma is diffusional and non-directional. The extent of tracer accumulation in the parenchyma is determined by how much crosses the pial membrane before it is cleared from the sub-arachnoid space and is insensitive to AQP4 removal.
As the importance of the peri-arterial route for pulsation-driven fluid transport in the brain is well-established (Abbott, 2004), the controversy has focused on the two novel and unconventional aspects of the glymphatic hypothesis – that convective flow clears interstitial fluid to the peri-venous spaces, and that this flow requires perivascular localization of AQP4. Independent experimental studies of brain clearance routes have shown that interstitially injected solutes are cleared to the ventricles (Bedussi et al., 2015) or peri-arterial spaces (Albargothy et al., 2018), but have not found evidence for clearance via the peri-venous route. Analysis of the size-dependence of solute transport into the parenchyma has demonstrated that solutes move at rates consistent with their diffusional mobility (Smith et al., 2017, Pizzo et al., 2018). Experiments to directly measure convective flow by multiphoton spot photobleaching of parenchymal fluorescent dextrans, with sensitivity down to 1 μm/min, could not find evidence for directional movement; additionally, parenchymal solute transport was not altered over a few minutes after sudden cessation of cardiac and respiratory pulsation (Smith et al., 2017).
These experimental studies are supported by structure-based fluid transport models that attempt to simulate the proposed glymphatic convection in brain ECS. We initially demonstrated that significant convection was implausible, even under very favorable assumptions, in a 2D model taking into account endfoot and parenchymal geometry (Jin et al., 2016). This conclusion was supported by subsequent modeling performed on a 3D reconstruction of the ECS from serial section electron micrographs (Holter et al., 2017), which found that the hydraulic resistance was much too large to permit significant convective flow. One study suggested that intracellular fluid flow through the astrocytic syncytium might provide a theoretically possible route for fluid transport (Asgari et al., 2015); however, this seems unlikely due to the high hydraulic resistance of the gel-like cytosol (Charras et al., 2005). While questions may persist regarding the extent to which low velocity (<1 μm/min) convective flow might occur in the parenchymal extracellular space, pericapillary space, or even astrocytic intracellular space, it is important to note that none of these possibilities are supported by the existing experimental data. A more plausible and conventional model supported by the experimental data and modeling is parenchymal diffusion coupled to dispersive mixing in the peri-arterial space (Figure 1B).
The second area of major controversy relates to the proposed role of AQP4 in the glymphatic system. Vascular pulsation-driven, AQP4-dependent, trans-astrocytic flow appears unlikely, given that AQP4 transports only water; however, a broader question remains about whether AQP4 has any role in CSF/ISF exchange. Iliff et al (2012) reported that following bolus injection interstitial uptake of CSF-delivered fluorescent albumin was decreased at 30 min but not at 60 min in Aqp4 deficient mice and that Aqp4 deletion did not alter solute transport in the peri-arterial spaces. We performed similar experiments, but found that the extent of interstitial tracer uptake in the cortex was substantially less than that observed by Iliff et al, and insensitive to Aqp4 deletion (Smith et al., 2017). In response to this, a consortium of authors (Mestre et al., 2018) variously found: (i) a much more limited parenchymal albumin uptake with some sensitivity to Aqp4 deletion (URMC group); (ii) that tracer uptake that was sensitive to deletion of α-syntrophin (OHSU); (iii) alterations in paravascular uptake of Texas Red 3 kDa dextran (NMU); (iv) failure of intrathecally injected dyes to reach the cortical surface in AQP4 deficient animals (UNC); and (v) a small effect of Aqp4 deletion on tracer penetration in the cortex (Riken). Notably, none of the accompanying images reproduce the dramatically reduced tracer accumulation originally reported (Iliff et al., 2012) and instead document much more subtle, limited tracer uptake in the parenchyma in both genotypes. Additionally, while results in α-syntrophin deficient mice have been attributed to loss of perivascular AQP4, it should be noted that α-syntrophin is widely expressed and has been implicated in a number of physiological processes including regulation of cardiac rhythm (Ueda et al., 2008).
Differences in results between labs are potentially attributable to a number of factors. Mestre et al. (2018) have suggested that the use of ketamine/xylazine (rather than avertin as used by Smith et al. (2017) as an anesthetic is required for CSF tracers to enter the interstitium. However, the importance of ketamine/xylazine was not supported by an independent study that found inhibition of tracer uptake into parenchyma by ketamine/xylazine (Gakuba et al., 2018). Intrathecally injected solutes must cross significant serial barriers before reaching the endfoot/parenchyma, including entering the peri-arterial spaces, then exiting across the pial cells and their basement membrane that surround descending arterioles, dispersal in the subpial space, and crossing the astrocyte basement membrane. It remains to be determined if AQP4-mediated endfoot structural plasticity, or local osmotic pumping, facilitate interstitial uptake of perivascular solutes on the faster time scales associated with neuronal excitation. The astrocyte endfeet may act as a reversible diffusion barrier under some circumstances (Nuriya et al., 2013, Kutuzov et al., 2018); however, AQP4 does not appear to play a significant role in endfoot structural plasticity (Rosic et al., 2019).
A reasonable conclusion from these studies is that only a small fraction of CSF solutes enter the parenchyma, and that measurement of fluorescent solute transfer to the interstitium is extremely sensitive to experimental conditions such as choice of anesthetic, injection method, fixation rate, and analysis details. Tissue autofluorescence may also be a significant confounding factor when experiments are done using visible wavelength dyes. The pitfalls associated with interpreting tracer accumulation in fixed tissue were well-illustrated by a recent study showing that the degree to which bolus-injected CSF solutes are delivered to the parenchyma is highly sensitive to changes in the rate of clearance from the sub-arachnoid space to the dural lymphatics, and occurs mostly during fixation (Ma et al., 2019). This view is supported by quantitative MRI measurements of small paramagnetic tracers infused into the CSF of live rats demonstrating slow parenchymal uptake that is mostly confined to the ventral surface of the brain (Lee et al., 2017). With regards to clearance from the interstitium, the advocates of the glymphatic hypothesis recently proposed that intraparenchymal injection disrupts the putative glymphatic flow (Mestre et al., 2018); how this new finding can be reconciled with the AQP4-dependent clearance of intra-parenchymally injected Aβ, reported as a key function of the glymphatic system in their original paper (Iliff et al., 2012), remains to be determined.
The glymphatic hypothesis originally proposed that AQP4 mediates a brain-wide directional clearance pathway that removes toxic protein aggregates from the interstitium. The evidence reviewed here demonstrate that long-range, convective transport through the parenchymal grey matter, as proposed in the glymphatic hypothesis, is unlikely to occur. Parenchymal uptake of CSF delivered solutes is determined by the rates of pial penetration and clearance from the subarachnoid space and is largely insensitive to AQP4 removal. Further work is needed to investigate possible roles of AQP4 in glial barrier function and to optimize routes for therapeutic macromolecule delivery to the CNS.
Acknowledgements
Supported by NIH grants EY13574, EY029881, DK72517, DK101373 and EB00415, and grants from the Guthy-Jackson Charitable Foundation, Bright Focus Foundation, Focused Ultrasound Foundation and Cystic Fibrosis Foundation.
Biography
Alex J Smith, Ph.D. studied mechanisms of stimulus-secretion coupling in mast cells during his graduate work and the role of cholesterol in synaptic function during his post-doc. An interest in the physiological consequences of altered membrane organization led him to work on AQP4 and the unique orthogonal arrays formed by this protein. Having previously demonstrated that orthogonal arrays are required for AQP4 polarization in astrocytes, he has been investigating the role of AQP4 in glial barrier function and how this is altered in neurodegenerative and neuroinflammatory disease.
Alan S Verkman, M.D., Ph.D. is Professor of Medicine and Physiology at UCSF. He has extensively studied the mechanisms and physiology of fluid and solute transport in and around cells. He was responsible for the original discovery of AQP4 and the generation of AQP4 knockout mice, and elucidation of the roles of AQP4 in brain water transport, neuroexcitation, glial scarring and neuroinflammation.
References
- Abbott NJ (2004). Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45, 545–552. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Pizzo ME, Preston JE, Janigro D & Thorne RG (2018). The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol, 135, 387–407 [DOI] [PubMed] [Google Scholar]
- Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA & Carare RO (2018). Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari M, de Zelicourt D & Kurtcuoglu V (2015). How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep 5, 15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E & Bakker EN (2015). Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 12, 23–015-0019–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM & Verkman AS (2004). In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci 24, 8049–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT, Yarrow JC, Horton MA, Mahadevan L & Mitchison TJ (2005). Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A, Martinez de Lizarrondo S, Vivien D & Gauberti M (2018). General anesthesia inhibits the activity of the “glymphatic system”. Theranostics. 8, 710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky SB & Barrand MA (2014). Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal KA & Pettersen KH (2017). Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A, 114 9894–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R & Nedergaard M (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA & Nedergaard M (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin BJ, Smith AJ & Verkman AS (2016). Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn D (2017). When scientists saw the mouse heads glowing they knew the discovery was big. Washington Post; May 21. [Google Scholar]
- Konnikova M (2014). Goodnight. Sleep clean. The New York Times January 11. [Google Scholar]
- Kutuzov N, Flyvbjerg H & Lauritzen M (2018). Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A 115, E9429–E9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R & Nedergaard M (2018). Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep 8, 2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ries M, Decker Y, Muller A, Riner C, Bucker A, Fassbender K, Detmar M & Proulx ST (2019). Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Pla V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, Thomas JH, Xiao M, Hirase H, Asokan A, Iliff JJ & Nedergaard M (2018). Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7, e40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Shinotsuka T & Yasui M (2013). Diffusion properties of molecules at the blood-brain interface: potential contributions of astrocyte endfeet to diffusion barrier functions. Cereb Cortex 23, 2118–2126. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S & Verkman AS (2004). Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. Faseb J 18, 1291–1293. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC & Verkman AS (2013). Aquaporin water channels in the nervous system. Nat Rev Neurosci 14, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, Abbott NJ, Meyerand ME, Sorokin L, Stanimirovic DB & Thorne RG (2018). Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol 596, 445–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K & Grady PA (1985). Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326, 47–63. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Blaumanis OR & Grady PA (1990). Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 52, 431–9. [PubMed] [Google Scholar]
- Rosic B, Dukefoss DB, Abjorsbraten KS, Tang W, Jensen V, Ottersen OP, Enger R & Nagelhus EA (2019). Aquaporin-4-independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia doi: 10.1002/glia.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample I (2013). Why do we sleep? The Guardian October18. [Google Scholar]
- Smith AJ & Verkman AS (2017). The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? Faseb J 32, 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Yao X, Dix JA, Jin BJ & Verkman AS (2017). Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6, e27679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R, Snodgrass SR & Johanson CE (2015). A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol 273, 57–68 [DOI] [PubMed] [Google Scholar]
- Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. (2008) Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA. 105, 9355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, Petersen NC & Nedergaard M (2018). Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett 662, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R & Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Hrabetova S, Nicholson C & Manley GT (2008). Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J Neurosci 28, 5460–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]


