Abstract
Background:
To evaluate local control for patients with intermediate-risk rhabdomyosarcoma (RMS) treated on Children’s Oncology Group (COG) protocol ARST0531.
Methods:
We analyzed 424 patients with intermediate-risk RMS. Patients were randomized to chemotherapy with either vincristine, dactinomycin, and cyclophosphamide (VAC) or VAC alternating with vincristine and irinotecan. With the goal of improving local control, radiation therapy (RT) was delivered early at week 4 and was concurrent with irinotecan on the experimental arm. Individualized local control plans for children ≤24 months were allowed. Local failure (LF) on ARST0531 was compared to LF on the preceding COG intermediate-risk study, D9803.
Results:
For patients with group I/II alveolar RMS (n=55), the 5-year cumulative incidence of LF was 13.4%; for group III alveolar RMS (n=141), 20.2%; and for group III embryonal RMS (n=228), 27.9% (p=0.03). Among patients with group III disease, LF did not differ by histology, site, nodal status, RT modality, or treatment arm. LF was worse for tumor size >5cm (32.3% vs 16.7%, p=0.001). Among patients with group III embryonal RMS, LF was higher on ARST0531 compared to D9803 (27.9% vs 19.4%, p=0.03). After excluding patients ≤24 months or patients who did not receive radiation, LF remained significantly increased on ARST0531 (p=0.02). After adjusting for clinical prognostic factors, event-free and overall survival were worse on ARST0531 (p=0.004 and p=0.05).
Conclusions:
Despite interventions designed to enhance local control, local control was inferior on ARST0531 compared to D9803. The reason for this is unclear but could be due to the reduced cyclophosphamide dose on ARST0531.
Keywords: rhabdomyosarcoma, local control, radiation therapy, cyclophosphamide, clinical trial
Precis:
One of the goals of the Children’s Oncology Group (COG) protocol for intermediate-risk rhabdomyosarcoma patients, ARST0531, was to improve local control via the early introduction of radiation and the concurrent delivery of radiation with irinotecan, a known radiosensitizer. Despite these interventions, local control, event-free survival, and overall survival were inferior on ARST0531 compared to the preceding COG intermediate-risk rhabdomyosarcoma study, D9803.
INTRODUCTION
Although two-thirds of children with intermediate-risk rhabdomyosarcoma (RMS) will become long-term survivors, local failure remains the dominant form of initial relapse and is a major obstacle to cure. Prior strategies explored by the Intergroup Rhabdomyosarcoma Study Group (IRSG) and Children’s Oncology Group (COG), including radiation therapy (RT) dose escalation via a hyperfractionated approach and technical advances in imaging and RT, have not resulted in a difference in local control, although early data suggests that proton therapy may improve toxicity rates.1–3 Given the young patient age and extensive morbidity associated with salvage options after local relapse,4–7 it is imperative to explore new strategies to improve local control. One goal of the most recent COG clinical trial for intermediate-risk RMS, ARST0531, was to maximize local control via early introduction of RT at week 4 and concurrent delivery of RT with irinotecan, a potential radiosensitizer.8 The incorporation of vincristine and irinotecan into the systemic therapy backbone also allowed for reduced systemic doses of cyclophosphamide, with the intent of minimizing both acute and late toxicity, such as hemorrhagic cystitis and infertility. With such interventions designed to improve local control, we hypothesized that local control on ARST0531 would be improved compared to historical cohorts. Thus, the objective of the current analysis was to determine the local failure rate on COG ARST0531 and compare to the immediately prior COG study for intermediate-risk RMS, D9803.
PATIENTS AND METHODS:
Eligibility and systemic therapy
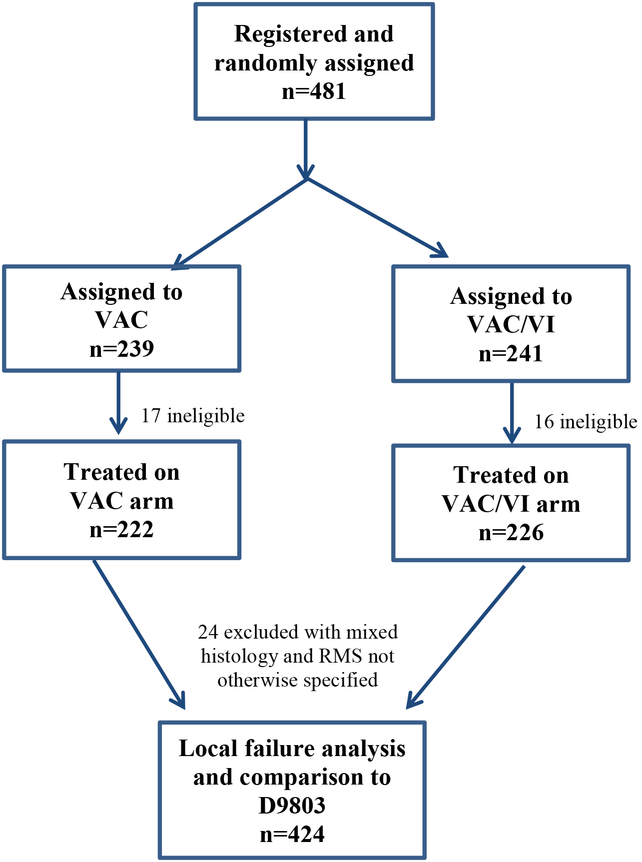
Between December 26, 2006 and December 7, 2012, COG ARST0531 enrolled 481 patients with intermediate-risk RMS (defined as non-metastatic alveolar RMS at any primary site [Group I-III, Stage 1–3] or incompletely excised embryonal RMS arising from an unfavorable site [Group III, Stage 2–3]).9 ARST0531 was a phase III study designed to evaluate the outcomes of patients treated with vincristine, dactinomycin and cyclophosphamide (VAC, cumulative dose of cyclophosphamide, 16.8 g/m2) chemotherapy vs VAC alternating with vincristine and irinotecan (VAC/VI, cumulative dose of cyclophosphamide, 8.4 g/m2). Because outcome was similar for the VAC and VAC/VI arms, the two treatment groups were combined in our analyses (other than the comparison of local failure by treatment arm). Thirty-three patients were ineligible and an additional 24 patients were excluded due to having a histology other than embryonal or alveolar RMS. Four hundred and twenty-four remaining patients were included in our analysis (Figure 1). Patients who met the same clinical group and stage, pathologic, and eligibility criteria and were enrolled on D9803,10 the most recent prior COG study for intermediate-risk RMS, were identified to provide a historic comparison. All D9803 treatment arms were combined for comparison to ARST0531.
Figure 1.
CONSORT
Abbreviations: VAC, vincristine, dactinomycin, and cyclophosphamide; VI, vincristine and irinotecan.
Local therapy
For all patients on ARST0531, RT began at week 4, with exceptions allowed for patients ≤24 months for whom individualized local control approaches were permitted at the discretion of the treating physician (n=75). On the experimental arm, irinotecan was given concurrently with RT during week 4 and again during week 7. Since the local control question on ARST0531 involved RT timing and irinotecan sensitization, delayed primary excision (DPE) after RT was discouraged but permitted.
RT doses varied by histology and clinical group at time of study entry. For patients with Group I or II alveolar RMS, 36 Gy was used for node negative patients, and 41.4 Gy was used for node positive patients. For patients with group III disease, 50.4 Gy was used for both embryonal and alveolar RMS, with the exception of those with orbital primary sites, who received 45 Gy. All RT was delivered in 1.8 Gy daily fractions. There was no difference in RT dosing guidelines between ARST0531 and D9803, although ARST0531 allowed a cone-down for tumors with a rapid response after 36 Gy. See Supplemental Table 1 for more details regarding RT dosing, volume guidelines, and timing. Megavoltage photons (with either 3-D conformal or intensity modulated radiation) electrons, protons, and brachytherapy were all permitted for use. All radiation plans were reviewed by the Imaging and Radiation Oncology Core Group (IROC) at the start of RT to minimize deviations. For further quality assurance and final determination of compliance, radiation oncology members of the COG Soft Tissue Sarcoma Committee also reviewed the radiation plans.
Endpoints and statistical methods
Local failure was defined as progression or relapse at the primary tumor site occurring as a first event (with or without concurrent regional and/or distant failure). Time to local failure was calculated from time of study enrollment. A competing-risks analysis11 was used to assess the cumulative incidence of local failure, treating regional and/or distant failures as competing events. Local failure on ARST0531 was compared to historical rates on D9803.12 Local failure was also evaluated by treatment arm (VAC vs VAC/VI), histology, tumor size, tumor site, nodal status, and radiation modality (protons vs photons). Patient characteristics were compared using the Chi-Square test.
Event-free survival (EFS) was defined as the time from study enrollment to disease progression, disease recurrence, second malignant neoplasm, or death from any cause. Overall survival (OS) was defined as the time from study enrollment to death from any cause. EFS and OS were censored at the patient’s last contact date, and evaluated using a Cox proportional hazards regression model to account for prognostic factors including age, group, histology, primary site, and tumor size. The median time of follow-up was calculated based on the Kaplan-Meier estimates.13 Patient follow-up was current through June 30, 2016.
RESULTS
Patient population
In total, 424 patients were analyzed, including 228 patients with group III embryonal RMS (54%), 141 patients with group III alveolar RMS (33%), and 55 patients with group I/II alveolar RMS (13%) (Table 1). The most common primary sites included parameningeal (n=191, 45%), bladder/prostate (n= 55, 13%), extremity (n=56, 13%) and retroperitoneal/perineal (n=49, 12%). Median age was 5.3 years (range: 0.01–40.2 years) and median follow up was 4.9 years (range: 0.1–8.8 years). There were no significant differences in baseline characteristics in terms of histology, group, tumor size, or nodal status between the ARST0531 cohort and D9803 cohort, although there were slightly more patients with parameningeal primary site on ARST0531 (p=0.08). Forty-seven patients (12%) on ARST0531 received proton RT compared to 9 patients (2%) on D9803 (p<0.001). Additionally, 8% of patients on ARST0531 did not receive RT as planned per protocol, compared to 12% on D9803 (p=0.02). The rate of major deviations in RT on ARST0531 was 4.9% compared to 9.1% on D9803.
Table 1.
Patient characteristics on ARST0531 and D9803
| ARST0531 | D9803 | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
|
Primary site Favorable Unfavorable |
44 (10) 380 (90) |
56 (12) 417 (88) |
0.49 |
|
Group Group I/II ARMS Group III ERMS Group III ARMS |
55 (13) 228 (54) 141 (33) |
74 (16) 252 (53) 147 (31) |
0.48 |
|
Histology
Embryonal Alveolar |
228 (54) 196 (46) |
221 (47) 252 (53) |
0.88 |
|
Tumor size
≤ 5cm > 5cm |
202 (48) 219 (52) |
215 (46) 249 (54) |
0.62 |
|
Parameningeal
Yes No |
191 (45) 233 (55) |
186 (39) 287 (61) |
0.08 |
|
Nodal Status
N0 N1 |
327 (78) 92 (22) |
383 (81) 90 (19) |
0.28 |
|
Modality of RT
Protons Photons |
47 (12) 345 (88) |
9 (2) 407 (98) |
<0.001 |
|
RT omission
Yes No |
32 (8) 392 (92) |
57 (12) 416 (88) |
0.02 |
Abbreviations: ARMS, alveolar rhabdomyosarcoma; ERMS, embryonal rhabdomyosarcoma; RT, radiation therapy
Clinical group I/II alveolar RMS
Fifty-five patients had gross total resection prior to chemotherapy initiation and were considered group I/II. The 5-year cumulative incidence of local failure among group I/II patients on ARST0531 was 13.4%, compared to 8.6% on D9803 (p=0.39).
Clinical group III RMS
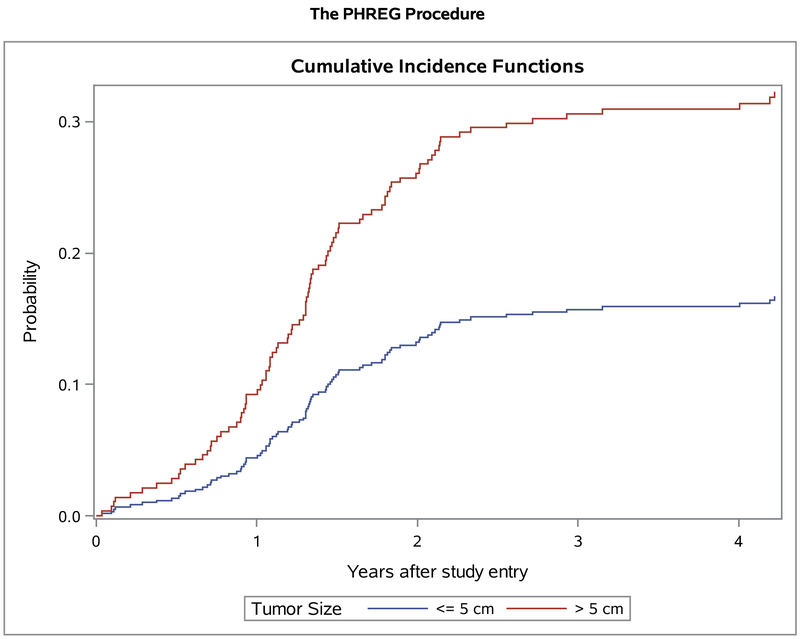
Of the 369 patients with group III disease, the 5-year cumulative incidence of local failure on ARST0531 was 25.4%. Similar to the analysis on D9803, local failure was not affected by histology, primary site, or nodal status, although there was a trend towards increased local failure with embryonal vs alveolar RMS (28.4 vs. 20.2%, respectively, p=0.08). Consistent with findings on D9803, there was a significant difference in local failure by tumor size. The 5-year cumulative incidence was 16.7% for tumor size ≤ 5cm vs 32.3% for >5cm, (p<0.001, Figure 2). The cumulative incidence of local failure was similar on the VAC and VAC/VI arms (21.3% vs 26.1%, p=0.26). The modality of radiation (protons vs photons) also had no effect on local failure (24.1% vs 22.0%, p=0.75). Thirty-two patients (8%) who did not receive RT had a higher local failure cumulative incidence compared to patients who did receive RT (44.2% vs. 22.1%, p=0.007). For patients with tumors at traditionally resectable sites (bladder dome, extremity and trunk), primary tumor resection after induction chemotherapy for Group III tumors was lower for patients on ARST0531 (27/163 or 16% of patients with tumors at resectable sites) compared to D9803 (73/161 or 45%).
Figure 2.
Local failure on ARST0531 for group III patients with tumors ≤ 5cm (n=161) >5cm (n=205)
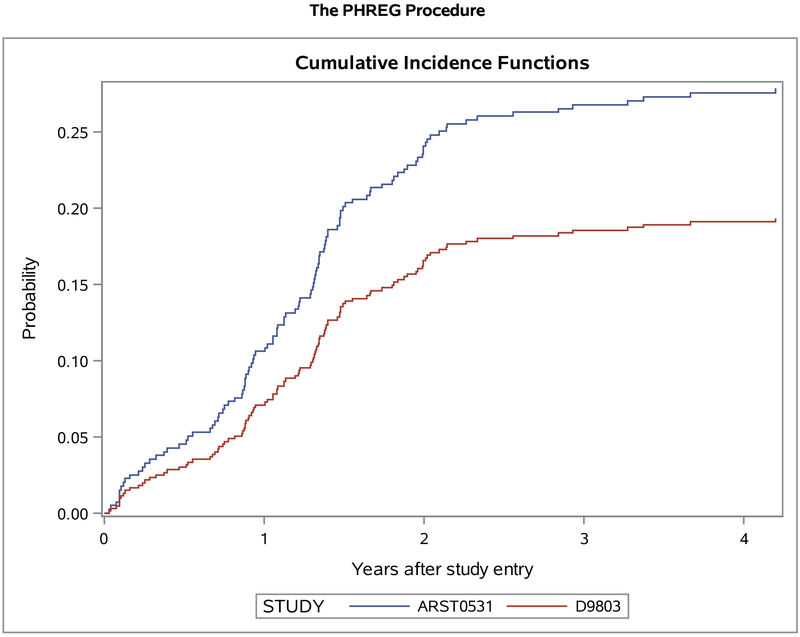
When compared to the 5-year local failure cumulative incidence of 19.4% for Group III embryonal patients on D9803, cumulative incidence on ARST0531 was 27.9% (p=0.03, Figure 3). The 5-year cumulative incidence of local failure for Group III alveolar patients was 20.2% on ARST0531 vs 17.7% on D9803 (p=0.58). For patients with parameningeal disease, the 5-year cumulative incidence of local failure was 27.7% on ARST0531 vs 19.5% on D9803 (p=0.06), and for patients with non-parameningeal disease, 25.2% vs 15.6% (p=0.19). Because ARST0531 allowed for individualized local control for patients ≤24 months, we excluded these patients; local failure still remained increased on ARST0531 compared to D9803 (23.4% vs 16.7%, p=0.02). Additionally, after excluding all patients who did not receive RT, local failure continued to be worse on ARST0531 (22.2% vs 15.5%, p=0.02).
Figure 3.
Local failure on ARST0531 (n=228) vs D9803 (n=252) for group III embryonal RMS
The 5-year cumulative incidence of regional and/or distant failure was 16.6%. After adjusting for covariates, EFS was inferior on ARST0531 compared to D9803 (hazard ratio [HR] 1.4, p=0.004, Table 2). Similar to EFS, OS was also inferior on ARST0531 on multivariable analysis (HR 1.3, p=0.05, Supplemental Table 2 and 3).
Table 2.
Multivariable analysis of event-free survival
| Covariate | HR | 95% CI | P value |
|---|---|---|---|
| Study (ARST0531 vs D9803) | 1.4 | 1.11–1.73 | 0.004 |
| Histology (alveolar vs embryonal) | 1.4 | 1.10–1.84 | 0.007 |
| Site (favorable vs unfavorable) | 0.8 | 0.52–1.20 | 0.26 |
| Size (≤ 5cm vs >5cm) | 0.6 | 0.50–0.80 | <0.001 |
| Group (I vs II) | 0.5 | 0.21–1.20 | 0.25 |
| (I vs III) | 0.5 | 0.22–1.13 | |
| Age (1–10 vs <1 and ≥10 years) | 0.6 | 0.49–0.78 | <0.001 |
DISCUSSION
Local relapse has remained the most common pattern of failure for children with intermediate-risk RMS on previous IRSG and COG trials.9,12 The objective of the current analysis was to determine local control among patients treated on ARST0531 compared to those treated on D9803. On ARST0531, interventions designed to influence local control were the timing of RT (delivered early at week 4) as well as the addition of concurrent irinotecan in the experimental arm. However, local control was lower for patients treated on ARST0531 compared to D9803. This was most pronounced for those with group III embryonal RMS.
One possible explanation for the poor local control on ARST0531 includes the significantly lower cyclophosphamide dose on ARST0531 compared to that used on D9803 (cumulative dose of 8.4–16.8 g/m2 vs 25.1–30.8 g/m2).10 A reduction in cyclophosphamide dose (from 26.4–28.6 g/m2 on IRS-IV and D9602 to 4.8 g/m2 on ARST0331) was associated with inferior local control in low-risk embryonal RMS,14,15 and similar findings have been observed in single-institution studies of patients with intermediate-risk disease.16,17 However, there was no difference in local control between the two treatment arms of ARST0531 (with cumulative cyclophosphamide doses of 8.4 and 16.8 g/m2) or the two randomized treatment arms of D9803 (with cumulative cyclophosphamide doses of 25.1 and 30.8 g/m2). Thus, the cyclophosphamide dose to achieve optimal local control in intermediate-risk patients remains elusive. Additionally, after adjusting for clinical prognostic factors such as age, group, histology, primary site, and tumor size, EFS and OS were inferior on ARST0531, suggesting that the worse outcomes may be secondary to inherent differences between the trials rather than a difference in patient characteristics. It is also possible that the higher doses of cyclophosphamide on IRS-IV may have negated any potential clinical improvement in local control with altered fractionated schemes. As such, hyperfractionation may provide a clinical benefit in the setting of lower cyclophosphamide doses such as those used on ARST0531, although this remains to be explored.
Regarding the addition of concurrent irinotecan, there was no improvement in local control with this intervention on ARST0531. Irinotecan is a camptothecin that targets the nuclear enzyme topoisomerase I, and in doing so, inhibits resealing of topoisomerase I single-stranded DNA breaks and prevents DNA re-ligation. In both in vitro and in vivo studies, camptothecins have been shown to have radiosensitization effects.8,18–20 Irinotecan has also shown activity in pre-clinical models of resistant RMS and in patients with untreated metastatic RMS on a recent COG phase II window study.21–23 Although the addition of concurrent irinotecan to RT did not improve local control on the experimental arm, the dose of cyclophosphamide used in this arm was lower than the control arm (8.4 g/m2 vs 16.8 g/m2), making evaluation of the specific impact of irinotecan difficult.
With the goal of improving local control, besides the addition of concurrent irinotecan, we also introduced local therapy with RT early at week 4. This is compared to the timing of RT on more recent trials including D9803 (week 13) and IRS-IV (week 10). The decision to move RT earlier was in part based on the improved local control seen in children with high-risk parameningeal RMS on IRS II-IV with early vs delayed RT (local failure rate of 18% vs 33%).24,25 In addition, early experiences with delayed RT in parameningeal patients utilized increased doses of systemic therapy.26 In spite of the move to early RT, local failure rates on ARST0531 were inferior to those seen after week 13 RT on D9803. However, 47% of patients with parameningeal RMS on D9803 also received early (week 1) RT. Given the insufficient power to appropriately compare local control on D9803 vs ARST0531 if one were to exclude patients with parameningeal primaries who received early RT on D9803, as well as the historical results from IRS II-IV,24 it is difficult to draw definitive conclusions regarding the effect of timing. For patients with parameningeal disease, though, the impact of cyclophosphamide dosing and timing of RT on leptomeningeal failure rates must be further evaluated.
Regarding the differences in volume reduction allowed on ARST0531 vs D9803, while a cone down after 36 Gy to the remaining disease after induction chemotherapy was allowed on ARST0531, most patients did not have a significant reduction in volume of their disease at week 4 (with radiation planning typically happening 1–2 weeks earlier). As such, volume reduction secondary to the use of cone downs is an unlikely sole explanation for the inferior outcomes.
We explored other potential causes to explain the difference in local control from D9803 to ARST0531. ARST0531 allowed for individualized treatment plans in children ≤ 24 months, including the omission of RT. However, even after excluding patients ≤ 24 months or patients for whom RT was omitted, local failure remained increased on ARST0531. Even though more patients on ARST0531 received proton therapy, we did not see a difference in local failure by RT modality. Finally, DPE was less common on ARST0531 compared to D9803, in part due to the early timing of RT. An analysis of D9803 demonstrated that local control after DPE with reduced RT dosing was similar to historical rates with RT without DPE.27 Therefore, the impact of less frequent DPE on local control is difficult to determine.
The findings of our analysis should be considered within the context of their limitations. First, ARST0531 and D9803 were sequential clinical trials conducted in different decades; patients were not randomized to one study or the other. It is not possible to determine all differences in therapy delivery between the two clinical trials that could have accounted for the observed differences in outcome. The two study populations may also have had differences for which we are unable to adjust. While we did control for multiple factors through our multivariable model, no regression model can fully account for unknown or unrecognized confounding. However, it is notable that ARST0531 was explicitly designed to improve local control rates; even after correcting for confounding factors, the statistically significant increase in local failure rates and decrease in EFS on ARST0531 persisted.
In the design of future trials, such as the ongoing ARST1431 (NCT01222715), efforts to improve local control and other disease outcomes include boosting all tumors >5cm to a dose of 59.4 Gy given the significant impact of tumor size on local control seen on both ARST0531 and D9803 (despite the increased cyclophosphamide dose in the latter). In addition, ARST1431 encourages DPE at selected anatomic sites as a local control option similar to D9803. Changes in systemic chemotherapy to improve local control could include adding 24 weeks of low-intensity maintenance chemotherapy, recently reported to improve OS by the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG); although the additional cumulative dose of cyclophosphamide with maintenance as per EpSSG was modest, a survival benefit was nevertheless seen.28 Consideration could also be given to increasing dosage or intensity of other control modalities, such as cyclophosphamide dose-intensity or cumulative dose of cyclophosphamide. Importantly, it is unknown whether the cumulative cyclophosphamide dose, dose-intensity and/or dose per cycle is the most significant feature of cyclophosphamide therapy. Novel systemic therapies that may improve local control are also of great need for localized RMS. Clearly new approaches are needed to increase local control to improve outcome for children with RMS.
Supplementary Material
Acknowledgments
Conflicts of interest: Dr. Hawkins reports grants and non-financial support from Loxo Oncology, grants and non-financial support from Bayer, non-financial support from Celgene, grants and non-financial support from Bristol Myers Squibb, grants from Merck Sharpe Dohme, grants from Lilly, grants from Eisai, grants from Glaxo Smith Kline, grants from Novartis, grants from Sanofi, outside the submitted work. Dr. Yock reports grants from Protom, Elekta, IBA, and MIM, outside the submitted work. Dr. Wolden reports personal fees from YmAbs therapeutics, outside the submitted work. Dr. Arndt reports stock ownership in Merck and Pfizer, outside the submitted work.
Research support: Children’s Oncology Group Grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413; St. Baldrick’s Foundation; Seattle Children’s Foundation, from Kat’s Crew Guild through the Sarcoma Research Fund; Imaging and Radiation Oncology Core Group (IROC) and Quality Assurance Review Center (QARC); NIH grant P30 CA 008748.
Footnotes
Clinical trial registration number:
REFERENCES
- 1.Donaldson SS, Meza J, Breneman JC, et al. : Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys 51:718–28, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Lin C, Donaldson SS, Meza JL, et al. : Effect of radiotherapy techniques (IMRT vs. 3D-CRT) on outcome in patients with intermediate-risk rhabdomyosarcoma enrolled in COG D9803--a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 82:1764–70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladra MM, Szymonifka JD, Mahajan A, et al. : Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol 32:3762–70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakefield DV, Eaton BR, Dove APH, et al. : Is there a role for salvage re-irradiation in pediatric patients with locoregional recurrent rhabdomyosarcoma? Clinical outcomes from a multi-institutional cohort. Radiother Oncol 129:513–519, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappo AS, Anderson JR, Crist WM, et al. : Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol 17:3487–93, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Raney RB Jr., Crist WM, Maurer HM, et al. : Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response. A report from the Intergroup Rhabdomyosarcoma Study I. Cancer 52:44–50, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Hayes-Jordan A, Doherty DK, West SD, et al. : Outcome after surgical resection of recurrent rhabdomyosarcoma. J Pediatr Surg 41:633–8; discussion 633–8, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Rich TA, Kirichenko AV: Camptothecin dose, schedule, and timing of administration for clinical radiation sensitization. Ann N Y Acad Sci 922:334–9, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Hawkins DS, Chi YY, Anderson JR, et al. : Addition of Vincristine and Irinotecan to Vincristine, Dactinomycin, and Cyclophosphamide Does Not Improve Outcome for Intermediate-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol 36:2770–2777, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arndt CA, Stoner JA, Hawkins DS, et al. : Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J Clin Oncol 27:5182–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 94:496–509, 1999 [Google Scholar]
- 12.Wolden SL, Lyden ER, Arndt CA, et al. : Local Control for Intermediate-Risk Rhabdomyosarcoma: Results From D9803 According to Histology, Group, Site, and Size: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 93:1071–6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–6, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Ermoian RP, Breneman J, Walterhouse DO, et al. : 45 Gy is not sufficient radiotherapy dose for Group III orbital embryonal rhabdomyosarcoma after less than complete response to 12 weeks of ARST0331 chemotherapy: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer 64, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walterhouse DO, Pappo AS, Meza JL, et al. : Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Cancer 123:2368–2375, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas JT Jr., Pappo AS, Wu J, et al. : Excessive Treatment Failures in Patients With Parameningeal Rhabdomyosarcoma With Reduced-dose Cyclophosphamide and Delayed Radiotherapy. J Pediatr Hematol Oncol 40:387–390, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey DL, Wexler LH, Wolden SL: Worse Outcomes for Head and Neck Rhabdomyosarcoma Secondary to Reduced-Dose Cyclophosphamide. Int J Radiat Oncol Biol Phys, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boothman DA, Wang M, Schea RA, et al. : Posttreatment exposure to camptothecin enhances the lethal effects of x-rays on radioresistant human malignant melanoma cells. Int J Radiat Oncol Biol Phys 24:939–48, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Ilson DH, Bains M, Kelsen DP, et al. : Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol 21:2926–32, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Negoro S, Kudoh S, et al. : Phase I/II study of weekly irinotecan and concurrent radiation therapy for locally advanced non-small cell lung cancer. Br J Cancer 79:1462–7, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furman WL, Stewart CF, Poquette CA, et al. : Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol 17:1815–24, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Houghton PJ, Cheshire PJ, Hallman JD 2nd, et al. : Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 36:393–403, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Pappo AS, Lyden E, Breitfeld P, et al. : Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol 25:362–9, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Michalski JM, Meza J, Breneman JC, et al. : Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys 59:1027–38, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Minn AY, Lyden ER, Anderson JR, et al. : Early treatment failure in intermediate-risk rhabdomyosarcoma: results from IRS-IV and D9803--a report from the Children’s Oncology Group. J Clin Oncol 28:4228–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dharmarajan KV, Wexler LH, Wolden SL: Concurrent radiation with irinotecan and carboplatin in intermediate- and high-risk rhabdomyosarcoma: a report on toxicity and efficacy from a prospective pilot phase II study. Pediatr Blood Cancer 60:242–7, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Rodeberg DA, Wharam MD, Lyden ER, et al. : Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer 137:204–11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisogno G, Jenney M, Bergeron C, et al. : Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.