Abstract
Background
Malignant airway obstruction (MAO) occurs in 30% of patients with advanced-stage lung cancer, leading to debilitating dyspnea, cough, and hemoptysis. Other than recanalization of the airways, these patients lack long-lasting palliative therapy. The goal of this study was to determine the safety and feasibility of local injection of paclitaxel into the airway wall with a novel microinjection catheter.
Methods
In this multicentered prospective trial, 23 patients with non-small cell lung cancer and MAO were enrolled from July 2014 through June 2016 to undergo rigid bronchoscopy with recanalization, followed by injection of 1.5 mg of paclitaxel with a novel injection catheter. Primary end points consisted of safety (adverse events, severe adverse events, and unanticipated adverse device effects) as well as feasibility (number of injections, injection success). Secondary end points consisted of airway patency improvement, quality of life metrics, and need for further interventions and/or stenting.
Results
Nineteen patients underwent rigid bronchoscopy with successful recanalization and paclitaxel injection. There were no adverse events, severe adverse events, or unanticipated adverse device effects. There was an average of 3.4 injections given for a total dose of 1.5 mg of paclitaxel in all patients. There was significantly less stenosis postprocedure vs preprocedure (25%-50% vs 75%-90%; P < .001), which was unchanged at 6 weeks (25%-50%). None of the participants required further interventions or airway stenting.
Conclusions
The injection of paclitaxel after recanalization of MAO in patients with non-small cell lung cancer is safe and feasible, using a novel airway injection device.
Trial Registry
ClinicalTrials.gov; No.: NCT02066103; URL: www.clinicaltrials.gov
Key Words: airway obstruction, chemotherapy, interventional bronchoscopy, lung cancer
Abbreviations: EITC, endobronchial intratumoral chemotherapy; FACT-L TOI, Functional Assessment of Cancer Therapy-Lung Trial Outcome Index; FACT-L TOT, Functional Assessment of Cancer Therapy-Lung total score; HRQOL, health-related quality of life; KPS, Karnofsky Performance Status Scale; MAO, malignant airway obstruction; MCAO, malignant central airway obstruction; TBNA, transbronchial needle aspiration
Lung cancer is the leading cause of cancer death among both men and women, with an estimated 234,030 new cases and 154,050 deaths from the disease and its associated complications in the United States in 2018.1 Over the course of their illness, an estimated 30% of patients with lung cancer will develop malignant airway obstruction (MAO) of their central airways including the trachea and mainstem bronchi.2 These patients often experience debilitating dyspnea with subsequent respiratory distress, bleeding, and infection contributing to a low 18.1% 5-year survival rate.1
For patients with MAO, the daily struggle to breathe can be extremely difficult even with supplemental oxygen.3 Bronchoscopic treatment options for MAO include airway mechanical debridement, stenting,4 photodynamic therapy,5, 6 electrocautery,7 argon plasma coagulation,8, 9 and laser resection.10 Radiation therapies include both external beam radiation therapy11 and brachytherapy.12 These therapeutic options carry inherent risk and are often only temporizing, leading to recurrent bouts of symptomatic MAO. A study of 95 patients who underwent treatment with external beam radiation therapy for MAO reported a 79% (75 of 95) response rate in relief of airway obstruction; however, 33 of 75 responders (44%) developed recurrent MAO, for an overall success rate of only 44%.11 Airway stenting of MAO, while often immediately effective, has been reported to have a complication rate as high as 42%.13 The complications most frequently reported include stent migration, formation of granulation tissue, mucus plugging, infection, stent fractures, and airway perforation.14
Over the past two decades, these palliative approaches have been the mainstay treatment of dyspnea due to MAO and have shown demonstrable effects on patient symptoms and quality of life. Unfortunately, current treatment paradigms often lead to a need for multiple procedures due to high early restenosis rates and/or airway stent complications.14, 15 A multicentered study of 947 patients found that only 48% of patients with MAO had an improvement in quality of life postprocedure.16
Other, novel approaches to improving and/or maintaining airway patency have investigated the administration of chemotherapeutic medications topically or via transbronchial needle injection. These methods remain poorly studied and limited in their ability to precisely deliver drug into the airway wall, potentially leading to pulmonary parenchymal toxicity.17 Photodynamic therapy is another modality that is currently being studied for the treatment of these patients; however, photodynamic therapy has delayed tumor-destructive effects requiring repeat bronchoscopy. The use of needles for transbronchial needle aspiration (TBNA) to perform endobronchial intratumoral chemotherapy has shown promise, but data remain limited by the lack of prospective trials and safety concerns.17, 18, 19, 20
The novel transbronchial microneedle injection catheter (Blowfish catheter; Mercator MedSystems, Inc.) used in this study is an endobronchial balloon drug delivery catheter that extrudes a 34-gauge microneedle perpendicularly into the bronchial wall, allowing direct therapeutic access to the submucosa and bronchial adventitia but not beyond the cartilage layer.21 This needle was used in porcine models and showed an advantage over the TBNA needle by its ability to inject circumferentially 60% of the airway wall per injection.21 Despite these advantages, in comparison with the conventional TBNA needle, this transbronchial microneedle injection catheter has not been studied in human trials.
Paclitaxel is a US Food and Drug Administration-approved chemotherapeutic agent indicated for the treatment of non-small cell lung cancer. Paclitaxel binds microtubules and inhibits the late G2/M phase of mitosis, leading to cell death.22 The side effect profile is extensive for IV formulation, but until recently few data were available regarding submucosal use. A recent study in a porcine model showed that endobronchial injection of paclitaxel, at doses up to 1.5 mg/mL, caused minimal local injury in pig bronchi, and acceptable drug concentrations were found in bronchial tissues 28 days postinjection.23
The primary end point of this study was to assess the feasibility and safety of endobronchial intratumoral chemotherapy (EITC) via a new delivery device in patients undergoing bronchoscopy for malignant central airway obstruction (MCAO). Because of the extremely small gauge of the microneedle injection catheter, and its safety profile in animal studies,23 we hypothesized that there would be minimal risk posed to the participant through its use. As an exploratory analysis, we assessed airway patency and quality of life 6 weeks postprocedure.
Materials and Methods
Participants
This was a multicenter, prospective study conducted at Johns Hopkins University, the University of North Carolina, and Duke University, on the use of a novel EITC device to locally inject paclitaxel for the treatment of MCAO, from July 7, 2014 through June 20, 2016. Institutional review board approval was obtained from all three institutions (NA_00080371) prior to initiation of procedural and data collection. Participants with MCAO were identified by interventional pulmonary, medical oncology, radiation oncology, or thoracic surgery services, and were scheduled to undergo rigid bronchoscopy for recanalization of the airway after a multidisciplinary assessment. Inclusion and exclusion criteria can be found in e-Table 1. This trial was registered on ClinicalTrials.gov (NCT02066103).
Participants who met the eligibility criteria were approached at their visit to obtain informed consent. The potential risks and benefits to receiving intratumoral chemotherapy via a novel delivery device were explained, and their competency was assessed prior to signing the informed consent. On arrival for the procedure, the participants’ understanding of the research being conducted, as well as the risks and benefits, was once again assessed and the performing physician enrolled them into the trial if deemed appropriate. Participants were informed that they would be able to drop out of the clinical trial at any point thereafter.
Bronchoscopy and Imaging Procedures
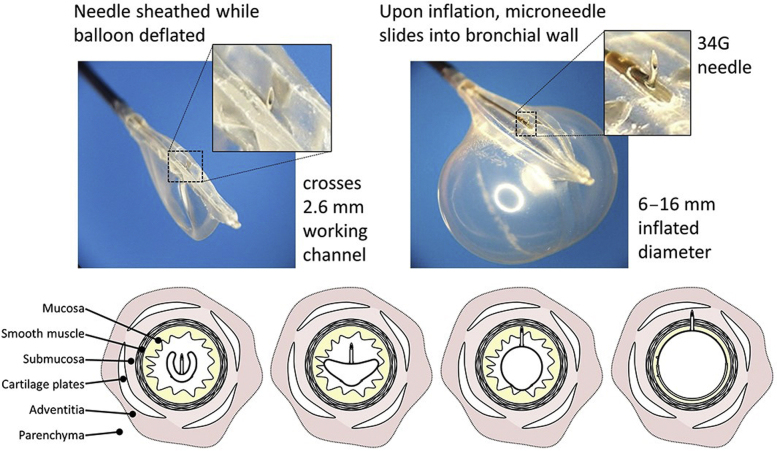
All participants were assessed bronchoscopically and underwent recanalization of the airway with rigid bronchoscopy, tumor debulking, argon plasma cautery, and/or electrocautery. After recanalization, the endobronchial balloon drug delivery device was placed through the working channel of a therapeutic flexible bronchoscope inserted through the lumen of the rigid bronchoscope. The device consists of a double-lumen catheter. One lumen is connected to an endobronchial balloon that, when inflated, extrudes a single 34-gauge microneedle into the bronchial wall, allowing direct therapeutic access to the submucosa and adventitia but not beyond the cartilage layer. Therapeutic agents can then be delivered via the second lumen attached to the microneedle, allowing precise drug delivery directly through the bronchial wall at the site of tumor (Fig 1). This technique allows an injected drug to be administered circumferentially over 60% of the airway wall per injection, offering controlled and complete delivery of medication.21
Figure 1.
Transbronchoscopic microneedle balloon drug delivery catheter (Blowfish catheter; Mercator MedSystems, Inc.). When the balloon is not inflated, the 34G needle is sheathed. On inflation of the balloon, the 34G needle inserts itself into the adjacent airway wall, allowing injection of paclitaxel.
All participants received 1.5 mg of paclitaxel at the site of prior MCAO, using the microneedle injection catheter. Paclitaxel was chosen because of its common use among malignancies, as well as its efficacy in reducing or preventing tumor growth in animals and humans.20, 23 Injections were given to each participant at a single level in the airway, with the goal of circumferential paclitaxel distribution. This dose was chosen because of its proven safety profile, and tissue levels, as shown in a porcine model.23 By delivering paclitaxel directly in the tumor, the dosage necessary to induce cytotoxic effects can be reduced, and the specificity of treatment is greater. Delivery was performed with multiple injections around the circumference of the bronchus at the location of recanalization. CT scans were scheduled to be obtained prior to the intervention, immediately postintervention, 6 weeks postintervention, and 12 weeks postintervention. Radiation therapy was not given in the area of the airway obstruction.
Data Collection
Data collection included the number and location of injections necessary for the delivery of paclitaxel at the time of bronchoscopy. The procedure was broken into the following duration time segments: bronchoscope insertion, recanalization of the airway, paclitaxel injection, bronchoscope removal, total anesthesia time, and postanesthesia care unit recovery. Postprocedure clinic visits were schedule at 1, 4, 6, and 12 weeks. Adverse event data were gathered via interview, and a physical examination and laboratory work (CBC, complete metabolic panel) were performed. Systemic levels of paclitaxel were not obtained at visits because of the acceptable tissue and plasma concentrations found in previous animal models.23 Any participant deaths that occurred during the trial were reviewed by an independent panel of medical oncologists and pulmonologists to determine whether the deaths were study procedure related.
The lesion was assessed bronchoscopically on the day of treatment (before and after), and at the 6-week visit. Karnofsky Performance Status Scale scores24, 25 and Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaires26, 27 were used to assess the participant’s quality of life before the intervention, 4 weeks after the intervention, 6 weeks after the intervention, and 12 weeks after the intervention in person or by phone if the patient was unable to present for a clinic visit. Tumor volume and invasion were evaluated by CT scan and assessed using built-in tools of the Picture Archiving and Communications System (PACS v12.1; Carestream Health, Inc.), which calculates volumetric data in a multiplanar fashion on the basis of the border of the tumor.
The primary outcome for this study was to assess safety after local injection of paclitaxel into the bronchial wall and airway recanalization as well as feasibility (number of injections, injection success), in patients with MCAO at 6 weeks. Six weeks was chosen given the known complication rates, rates of reobstruction (approximately 27% by 4-6 weeks), and limited life span of patients with airway obstruction.28 Adequacy of paclitaxel delivery was assessed by demonstration of the entire solution being injected into the submucosa without any spillage, and confirmation that all 1.5 mg was injected. Secondary outcomes were to determine the longitudinal effects of this procedure by bronchoscopic measurements of the tumor at 6 weeks, and to assess longitudinal changes in quality of life and need for further interventions and/or stenting for up to 12 weeks.
Data Analysis
All baseline demographic information was summarized using means and standard deviations if continuous variables or as a percentage if dichotomous. The Wilcoxon signed-ranks test was used to determine whether there was a significant change in airway patency before and after the procedure, as well as after the procedure and at 6 weeks. Airway patency was determined by the bronchoscopist performing the rigid bronchoscopy, and was rated on a scale of 0%-25%, 25%-50%, 50%-75%, 75%-90%, or completely occluded. The Wilcoxon signed-ranks test was also used to compare CT scan volume, Karnofsky Performance Status Scale scores, and FACT-L scores over two discrete time points. Friedman’s analysis of variance was used to look at the change in tumor volume by CT scan, Karnofsky Performance Status Scale score, and FACT-L score over a minimum of two longitudinal time points. The number of injections was averaged with a standard deviation, and the location of injection was calculated as a percentage, with the locations corresponding to time locations on a clock. Adverse event data were recorded as to whether each event was likely or unlikely due to the procedure, and are presented as count data. A P value of .05 was considered significant. All statistics were done with STATA version 14.2 (StataCorp).
Results
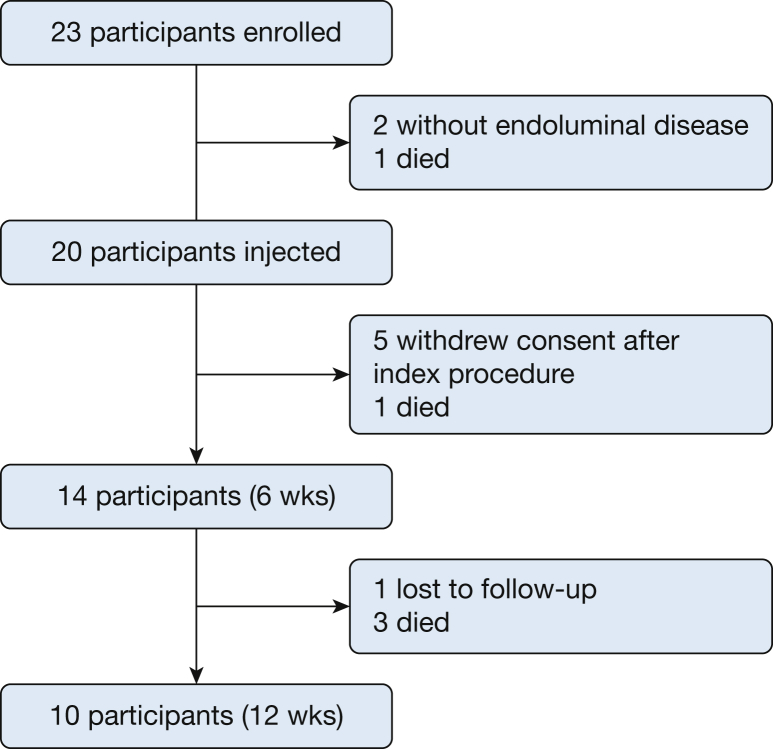
There were 23 participants initially enrolled in this study. Two participants did not have endoluminal disease on airway inspection and no longer met inclusion criteria, and one participant withdrew after an attempted procedure where the operator overinflated the balloon on the microneedle injection catheter and was unable to perform EITC. Twenty participants underwent balloon EITC, 14 completed 6 weeks of follow-up, and 10 patients completed 12 weeks of follow-up (Fig 2). Table 1 shows the baseline characteristics of all participants. Characteristics of each participant who completed the study, including stage of cancer, location of obstruction, whether they received concomitant chemotherapy, and debulking technique used, can be found in Table 2.
Figure 2.
Flowchart of participant enrollment.
Table 1.
Baseline Characteristics
| Characteristic | Participants Enrolled |
Participants Completed 6 Weeks |
Participants Completed 12 Weeks |
|---|---|---|---|
| (N = 23) | (n = 14) | (n = 10) | |
| Age, y, mean | 59.9 | 59.6 | 60.3 |
| Male sex, % | 52.1 | 50.0 | 36.4 |
| Race, % | |||
| White | 73.9 | 71.4 | 81.8 |
| Black | 17.4 | 21.4 | 9.1 |
| COPD, % | 39.1 | 50.0 | 45.5 |
| Asthma, % | 8.7 | 14.3 | 18.2 |
| Diabetes, % | 17.4 | 7.1 | 9.1 |
| Smoking history | |||
| Current, % | 4.4 | 7.1 | 9.1 |
| Former, % | 91.3 | 92.9 | 90.9 |
| Pack-years | 34.3 | 36.8 | 36.4 |
| Type of NSCLC, % | |||
| Adenocarcinoma | 43.5 | 50.0 | 54.5 |
| Squamous cell carcinoma | 52.2 | 42.9 | 45.5 |
| Adenosquamous | 4.4 | 7.1 | 0 |
| Receiving chemotherapy, % | 87.0 | 85.7 | 82.8 |
| Lesion size, mm | 20.6 | 16.8 | 17.6 |
NSCLC = non-small cell lung cancer.
Table 2.
Malignancy Characteristics, by Participant
| ID | Type of NSCLC | Stage | Chemotherapy | Location of Obstruction | Device Used to Recannulate |
|---|---|---|---|---|---|
| 1 | Adenocarcinoma | 4 | Pemetrexed, carboplatin | RUL | Forceps |
| 2 | Squamous cell carcinoma | 3A | Paclitaxel | RMS | Forceps, argon plasma coagulation |
| 3 | Squamous cell carcinoma | 2B | Paclitaxel | RMS | Forceps, argon plasma coagulation |
| 4 | Squamous cell carcinoma | 4 | Cisplatin, etoposide | LUL | Forceps |
| 5 | Adenocarcinoma | 4 | Pemetrexed, carboplatin | RUL | Forceps, argon plasma coagulation |
| 6 | Adenocarcinoma | 3C | Pemetrexed, carboplatin, bevacizumab | RML | Forceps |
| 7 | Adenocarcinoma | 3B | Cisplatin | RUL | Forceps |
| 8 | Adenocarcinoma | 4 | Docetaxel | BI | Forceps |
| 9 | Adenocarcinoma | 1B | None | BI | Forceps |
| 10 | Adenocarcinoma | 4 | Erlotinib | RML | Forceps |
| 11 | Adenocarcinoma | 4 | Pemetrexed, carboplatin | RUL | Forceps |
| 12 | Squamous cell carcinoma | 4 | Paclitaxel, carboplatin | LUL | Forceps |
| 13 | Squamous cell carcinoma | 3A | Paclitaxel, carboplatin | BI | Forceps, argon plasma coagulation |
| 15 | Squamous cell carcinoma | 3B | None | RLL | Forceps |
| 17 | Squamous cell carcinoma | 4 | Carboplatin, paclitaxel | RML | Forceps |
| 19 | Adenocarcinoma | 3A | Cisplatin | RMS | Electrocautery, cryotherapy, forceps |
| 20 | Squamous cell carcinoma | 3A | Erlotinib | RMS | Forceps |
| 21 | Adenosquamous | 4 | Carboplatin, paclitaxel | LMB | Forceps |
| 22 | Squamous cell carcinoma | 3B | Nivolumab | LMB | Forceps |
| 23 | Squamous cell carcinoma | 2B | None | LMB | Forceps, argon plasma coagulation |
BI = bronchus intermedius; LMB = left mainstem bronchus; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RMS = right mainstem bronchus; RUL = right upper lobe. See Table 1 legend for expansion of other abbreviation.
There were no serious adverse events related to the procedure throughout the study period. There was one death within 4 weeks due to septic shock and one participant who suffered hair loss, which began prior to the index procedure. Two participants withdrew from the study after electing to pursue comfort care between the index procedure and the 6-week follow-up. There were no adverse events from 4 weeks through 6 weeks postprocedure. There were three deaths from 6 weeks through 12 weeks postprocedure, one of which was due to progression of the participant’s underlying malignancy with failure to thrive without respiratory symptoms, and the other two were due to septic shock. No deaths in this study were deemed secondary to the index procedure by the independent safety review panel.
Overall procedure times can be found in Table 3. There was an average of 3.9 injections per participant for a total dose of 1.5 mg of paclitaxel. There was a total of 77 injections in all participants. The majority of injections were located at the 12 o’clock position (23%), 6 o’clock position (16%), 9 o’clock position (15%), and 3 o’clock positions (15%).
Table 3.
Procedure Times
| Intervention | Time (min) |
|---|---|
| Total bronchoscopy, mean (SD) | 46.8 (16.6) |
| Recanalization, mean (SD) | 15.7 (12.1) |
| Paclitaxel injection, mean (SD) | 8.9 (6.4) |
| Anesthesia, mean (SD) | 69.1 (26.5) |
| Recovery, mean (SD) | 162.6 (104.9) |
| Entire procedure, mean (SD) | 243.4 (108.3) |
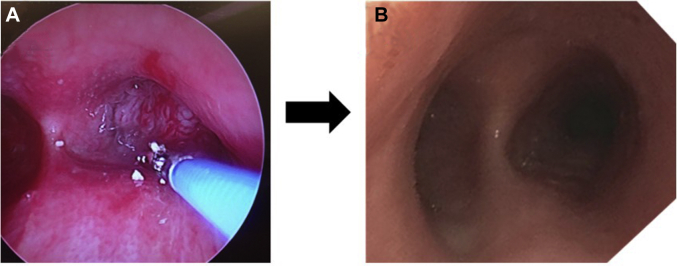
No patients had clinically significant restenosis after drug injection during the study period. There was no evidence of any local or airway mucosal disruption, ulceration, or inflammatory response at any of the follow-up bronchoscopies. There were no airway stents, dilation, or therapeutic interventions on subsequent follow-up procedures in any patient enrolled in the trial (Fig 3). The degree of stenosis, as determined by the bronchoscopist, was significantly less postprocedure in comparison with preprocedure (25%-50% vs 75%-90%, respectively; P < .005).
Figure 3.
Progression of the procedure. A, a 100% MAO in the right main-stem bronchus. B, the same patient 6 weeks after debulking and injection with paclitaxel, with a fully patent right mainstem.
There were five participants with measurable CT scans pre- and postprocedure, with no statistically significant difference between tumor volumes (35.3 vs 27.38 cm3, respectively; P = .23). Four patients had CT scan measurements postprocedure at 6 weeks, with no significant difference in tumor volume (33.85 vs 52.88 cm3, respectively; P = .21). The tumor volume as a radiologic surrogate for disease progression also did not significantly change from immediately postprocedure through 12 weeks postprocedure (P = .45).
Health-related quality of life (HRQOL) metrics, including Karnofsky Performance Status Scale (KPS) scores, FACT-L total scores (FACT-L TOT), and FACT-L Trial Outcome Index (FACT-L TOI), are provided in Table 4. The average KPS scores were 71.3 vs 71.0 (P = .83) at enrollment and at 12 weeks postprocedure, respectively. In addition, there was no significant change in KPS scores before the procedure and throughout the entirety of the study (P = .85). There was no statistically significant change in FACT-L TOI scores throughout the study (P = .67). FACT-L TOI scores averaged 41.1 at enrollment and 50.2 at 12 weeks (P = .92). FACT-L TOT scores did not significantly change throughout the course of the study (P = .71). FACT-L TOT scores were 78.1 at enrollment and 89.3 at 12 weeks (P = .96).
Table 4.
Health-Related Quality of Life Scores
| HRQOL Metric | Enrollment | 4 weeks | 6 weeks | 12 weeks |
|---|---|---|---|---|
| KPS, mean ± SD (No.) | 71.3 ± 13.2 (23) | 73.3 ± 16.8 (15) | 70.8 ± 17.1 (13) | 71.0 ± 20.8 (10) |
| FACT-L TOI, mean ± SD (No.) | 41.1 ± 16.1 (23) | 47.3 ± 11.3 (13) | 53.5 ± 16.4 (12) | 50.2 ± 18.8 (10) |
| FACT-L TOT, mean ± SD (No.) | 78.1 ± 23.1 (23) | 84.7 ± 16.1 (13) | 92.4 ± 22.6 (12) | 89.3 ± 25.8 (10) |
FACT-L TOI = Functional Assessment of Cancer Therapy-Lung Trial Outcome Index; FACT-L TOT = Functional Assessment of Cancer Therapy-Lung total score; HRQOL = health-related quality of life; KPS = Karnofsky Performance Status Scale score.
Discussion
In this study, we demonstrated that EITC using a novel microneedle injection catheter designed to optimize drug delivery in the airway wall after airway recanalization is both feasible and safe. Nearly all participants with the intention to treat received 1.5 mg of paclitaxel from an average of 3.4 injections. None of the patients who received drug injection had evidence of restenosis on subsequent procedures. There were no deaths, unanticipated adverse device effects, or clinically significant adverse events that were directly due to the procedure. The length of the procedure was only 8.9 min longer than recanalization alone.
Although the study was not powered to assess a clinical response, it was noted that no patients in the study required additional airway interventions, no airway stents were needed, and there were no procedure-related complications. This is in sharp contrast to historical data with high complication rates in patients with MAO requiring stent placement, many of whom required additional therapeutic procedures.29 In addition, conventional TBNA or endobronchial aspiration needles have been of limited utility for EITC because of needle design. Conventional transbronchial needles have a limited needle insertion angle, which can only be controlled by the flexible bronchoscope angulation because of tangential needle deployment in relation to the long axis of the catheter. This design limitation may have been a significant factor in prior studies because of the lack of precision and distribution of drug. Use of the endobronchial balloon-based drug delivery catheter in this study offered a novel perpendicular injection capability that may have had a significant impact on the results presented. Future studies are needed to appropriately assess clinical response as well as needle approaches, but the results presented here are promising.
As a secondary exploratory aim, we measured health-related quality of life over the course of this study. Given the severity of disease, and the poor prognosis associated with it, we would have expected the quality of life of patients to decrease over the course of this study.26, 30, 31 Despite this, the use of endobronchial paclitaxel after recanalization kept HRQOL scores stable over the course of 12 weeks. Although not statistically significant, there was an increase in FACT-L scores over the course of the study. FACT-L scores have been shown to decrease over time in patients receiving systemic chemotherapy,26, 30, 31 which is not what we observed in this study. Our population also had much lower baseline FACT-L scores, likely due to the severity of the MAO. Unfortunately, with only 10 participants with preprocedure and 12-week postprocedure HRQOL scores, it is difficult to make a full conclusion as to whether there is a HRQOL benefit.
Airway patency, as measured by the bronchoscopist, was significantly higher from pre- to postprocedure, which continued to the 6-week bronchoscopy. Although the measurement was subjective, providers were given a choice of no obstruction; 1%-25%, 26%-50%, 51%-75%, 76%-90% obstruction; and complete obstruction. Given the range within the measurements, it was likely an accurate estimation was made as to the amount of obstruction present.
This study has several limitations. The high mortality rate makes this a difficult patient population to study. All patients injected with paclitaxel survived to the primary end point of 6 weeks, but three of the participants enrolled died prior to the end of the study. The study was conducted only at academic medical centers, and of the three, the majority (74%) were from a single center, which limits the generalizability. This was not a randomized controlled trial, so we do not have a control group for comparison; however, the study was designed as a feasibility pilot and based on the results of this pilot, randomized controls studies are being designed. The study was also not powered to detect a difference in HRQOL, as the main goal of this trial was to assess the safety and feasibility of the use of a novel endobronchial microneedle balloon catheter to inject a cytotoxic chemotherapeutic agent into recanalized MAO.
Conclusions
This study showed that localized endobronchial microneedle injection of paclitaxel into a recanalized malignant airway obstruction was both feasible and safe. Participants did not experience any adverse effects from the paclitaxel injection. Importantly, given the palliative intent of these procedures, participants who were able to complete the study appeared to have a sustained quality of life, a finding that warrants further evaluation in an appropriately designed and powered study. Given these findings a trial that randomizes patients with MAO to either rigid bronchoscopy and debulking alone vs rigid bronchoscopy followed by endobronchial injection of paclitaxel should be undertaken to establish whether this novel approach is a more effective method of palliating malignant central airway obstruction than current approaches.
Acknowledgments
Author contributions: L. Y., K. S., J. A., and M. M. W. contributed to the design, analysis, and writing of the manuscript. C. M., C. T. L., H. L., D. F.-K., D. E., R. H., K. R. V. and R. S. contributed to data analysis, manuscript drafting, and final approval.
Financial/nonfinancial disclosures: K. S. is an employee at Mercator MedSystems receiving compensation and stock options. D. E. is a consultant for BeyondSpring Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly & Co., Genentech, Golden Biotech, Corp., and Guardant Health, Inc. None declared (L. Y., C. M., J. A., C. T. L., R. H., K. R. V., H. L., D. F.-K., R. S., M. M. W.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The study was supported by the National Cancer Institute [Award No. R42CA141907]. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award No. T32HL007534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mercator MedSystems supplied the Blowfish catheters used in this study.
Supplementary Data
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017 [based on November 2016 SEER data submission, posted to the SEER website]. https://seer.cancer.gov/csr/1975_2014/. Accessed February 14, 2019.
- 2.Stohr S., Bolliger C.T. Stents in the management of malignant airway obstruction. Monaldi Arch Chest Dis. 1999;54:264–268. [PubMed] [Google Scholar]
- 3.Chhajed P.N., Baty F., Pless M., Somandin S., Tamm M., Brutsche M.H. Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest. 2006;130:1803–1807. doi: 10.1378/chest.130.6.1803. [DOI] [PubMed] [Google Scholar]
- 4.Dalar L., Ozdemir C., Abul Y. Therapeutic bronchoscopic interventions for malignant airway obstruction: a retrospective study from experience on 547 patients. Medicine (Baltimore) 2016;95:e3886. doi: 10.1097/MD.0000000000003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghissi K., Parsons R.J., Dixon K. Photodynamic therapy (PDT) for bronchial carcinoma with the use of rigid bronchoscope. Lasers Med Sci. 1992;7(1-4):381–385. [Google Scholar]
- 6.Ross P., Jr., Grecula J., Bekaii-Saab T., Villalona-Calero M., Otterson G., Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg Med. 2006;38:881–889. doi: 10.1002/lsm.20444. [DOI] [PubMed] [Google Scholar]
- 7.Wahidi M.M., Unroe M.A., Adlakha N., Beyea M., Shofer S.L. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol. 2011;6:1516–1520. doi: 10.1097/JTO.0b013e3182242142. [DOI] [PubMed] [Google Scholar]
- 8.Morice R.C., Ece T., Ece F., Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781–787. doi: 10.1378/chest.119.3.781. [DOI] [PubMed] [Google Scholar]
- 9.Okada S., Yamauchi H., Ishimori S., Satoh S., Sugawara H., Tanaba Y. Endoscopic surgery with a flexible bronchoscope and argon plasma coagulation for tracheobronchial tumors. J Thorac Cardiovasc Surg. 2001;121:180–182. doi: 10.1067/mtc.2001.109544. [DOI] [PubMed] [Google Scholar]
- 10.Li C.H., Huang S.F., Li H.Y. Bronchoscopic Nd-YAG laser surgery for tracheobronchial mucoepidermoid carcinoma: a report of two cases. Int J Clin Pract. 2004;58:979–982. doi: 10.1111/j.1742-1241.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.W., Lee J.H., Kim H.K., Shim B.Y., An H.J., Kim S.H. The efficacy of external beam radiotherapy for airway obstruction in lung cancer patients. Cancer Res Treat. 2015;47:189–196. doi: 10.4143/crt.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallick I., Sharma S.C., Behera D. Endobronchial brachytherapy for symptom palliation in non-small cell lung cancer: analysis of symptom response, endoscopic improvement and quality of life. Lung Cancer. 2007;55:313–318. doi: 10.1016/j.lungcan.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Wood D.E., Liu Y.H., Vallieres E., Karmy-Jones R., Mulligan M.S. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76:167–172. doi: 10.1016/s0003-4975(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 14.Chin C.S., Litle V., Yun J., Weiser T., Swanson S.J. Airway stents. Ann Thorac Surg. 2008;85:S792–S796. doi: 10.1016/j.athoracsur.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Kizilgöz D., Aktaş Z., Yilmaz A., Öztürk A., Seğmen F. Comparison of two new techniques for the management of malignant central airway obstruction: argon plasma coagulation with mechanical tumor resection versus cryorecanalization. Surg Endosc. 2018;32(4):1879–1884. doi: 10.1007/s00464-017-5877-2. [DOI] [PubMed] [Google Scholar]
- 16.Ost D.E., Ernst A., Grosu H.B. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest. 2015;147:1282–1298. doi: 10.1378/chest.14-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta H.J., Begnaud A., Penley A.M. Restoration of patency to central airways occluded by malignant endobronchial tumors using intratumoral injection of cisplatin. Ann Am Thorac Soc. 2015;12:1345–1350. doi: 10.1513/AnnalsATS.201503-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celikoglu F., Celikoglu S.I., Goldberg E.P. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008;61(1):1–12. doi: 10.1016/j.lungcan.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hohenforst-Schmidt W., Zarogoulidis P., Darwiche K. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther. 2013;7:571–583. doi: 10.2147/DDDT.S46393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B., Sun L., Yan X., Ai Z., Xu J. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: a new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med Oncol. 2015;32:345. doi: 10.1007/s12032-014-0345-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsukada H., Seward K.P., Rafeq S., Kocher O., Ernst A. Experimental pilot study of a novel endobronchial drug delivery catheter. J Bronchology Interv Pulmonol. 2015;22(4):312–318. doi: 10.1097/LBR.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma P., Mumper R.J. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol. 2013;4:1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukada H., Entcheva-Dimitrov P., Ernst A. Pharmacokinetics and safety of paclitaxel delivery into porcine airway walls by a new endobronchial drug delivery catheter. Respirology. 2018;23(4):399–405. doi: 10.1111/resp.13214. [DOI] [PubMed] [Google Scholar]
- 24.Karnofsky D.A., Abelmann W.H., Craver L.F., Burchenal J.H. The use of the nitrogen mustards in the palliative treatment of carcinoma: with particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 25.Karnofsky D.A., Burchenal J.H. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C., editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; New York: 1949. [Google Scholar]
- 26.Cella D.F., Bonomi A.E., Lloyd S.R., Tulsky D.S., Kaplan E., Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 27.Webster K., Cella D., Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H.J., Labaki W., Yu D.H. Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis. 2017;9:4651–4659. doi: 10.21037/jtd.2017.09.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung F.T., Chen H.C., Chou C.L. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg. 2011;6:46. doi: 10.1186/1749-8090-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonomi P., Kim K., Fairclough D. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 31.Cella D., Eton D.T., Fairclough D.L. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy–Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.