Abstract
Background
Metabolic syndrome (MetSyn) predicted future development of World Trade Center lung injury (WTC-LI) in a subgroup of firefighters who never smoked and were male. An intracohort validation of MetSyn as a predictor of WTC-LI is examined in the cohort exposed to the World Trade Center (WTC) that has been followed longitudinally for 16 years.
Methods
Results of pulmonary function tests (n = 98,221) in workers exposed to the WTC (n = 9,566) were evaluated. A baseline cohort of firefighters who had normal FEV1 before 9/11 and who had had serum drawn before site closure on July 24, 2002 (n = 7,487) was investigated. Case subjects with WTC-LI (n = 1,208) were identified if they had at least two measured instances of FEV1 less than the lower limit of normal (LLN). Cox proportional hazards modeled early MetSyn biomarker ability to predict development of FEV1 less than the LLN.
Results
Case subjects were more likely to smoke, be highly exposed, and have MetSyn. There was a significant exposure dose response; the individuals most highly exposed had a 30.1% increased risk of developing WTC-LI, having MetSyn increased risk of developing WTC-LI by 55.7%, and smoking increased risk by 15.2%. There was significant interaction between smoking and exposure.
Conclusions
We validated the usefulness of MetSyn to predict future WTC-LI in a larger population of individuals who were exposed. MetSyn defined by dyslipidemia, insulin resistance, and cardiovascular disease suggests that systemic inflammation can contribute to future lung function loss.
Key Words: lung injury, metabolic syndrome, validation, World Trade Center
Abbreviations: DBP, diastolic BP; FDNY, Fire Department of New York; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; LLN, lower limit of normal; MetSyn, metabolic syndrome; PFT, pulmonary function test; PM, particulate matter; SBP, systolic BP; WTC, World Trade Center; WTC-HP, WTC Health Program; WTC-LI, WTC Lung Injury
Metabolic syndrome (MetSyn) and particulate matter (PM) exposure are known independent risk factors for respiratory dysfunction, obstructive lung disease, and cardiovascular disease. MetSyn is a constellation of risk factors associated with end-organ disease affecting one-third of US adults.1, 2 Diagnosis is made by having three or more of following: abdominal obesity, insulin resistance, hypertriglyceridemia, low high-density lipoprotein (HDL) levels, and hypertension.1
The link between obesity and lung disease has been attributed partially to mechanical stress and mass loading. However, some studies have focused on the systemic effects of obesity and MetSyn through hormonal and immunoinflammatory biomarkers in the context of pollution exposure and respiratory decline.3, 4, 5, 6, 7, 8, 9 In the Cardiovascular Health Study, subjects with a systolic BP (SBP) > 160 mm Hg had significantly lower FEV1.10 Smoking also can induce insulin resistance and contribute to airways disease in the context of MetSyn.11 Mechanistic studies linking MetSyn risk and airway disease suggest biological plausibility.12, 13, 14, 15, 16, 17
We have focused on the well-phenotyped cohort from the Fire Department of New York (FDNY) exposed to the World Trade Center (WTC) who were enrolled in the WTC Health Program (WTC-HP).18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Our group has defined WTC lung injury (WTC-LI) as the development of FEV1 less than the lower limit of normal (LLN) (< 5th percentile on the basis of the Hankinson equations).17, 19, 20, 22, 30, 31, 32, 33, 34, 35, 36 In a pilot cohort of firefighters who were nonsmoking, had symptoms, and were exposed to the WTC, MetSyn biomarkers predicted WTC-LI.20, 30, 33, 37 We now assess the predictive abilities of MetSyn biomarkers in the development of WTC-LI in an expanded cohort that is more representative of FDNY rescue and recovery workers exposed to the WTC. We assess the effects of smoking and validate these biomarkers to identify prognostic indicators of disease. This assessment is a vital step in the investigation of MetSyn as a potentially modifiable risk factor for PM-associated lung disease.
Materials and Methods
Study Design and End Points
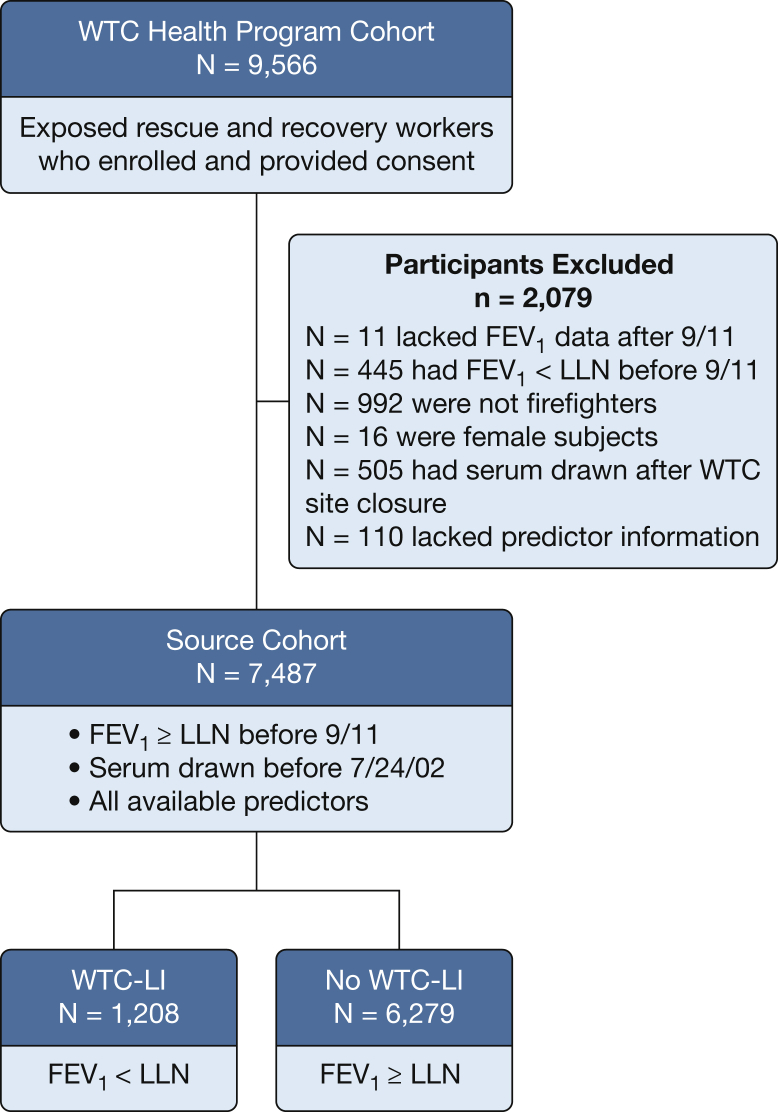
Serial pulmonary function tests (PFTs) were performed in rescue and recovery workers enrolled in the WTC-HP. Demographic characteristics, clinical information, and spirometric values were obtained from the electronic medical record or FDNY WTC database.38 Of the previously studied (n = 12,781) subjects, 9,566 had consented to physical health research. If pre-9/11 FEV1 was missing (n = 350), we reviewed subsequent FEV1 values, and we included them in our source cohort if the first FEV1 % predicted after 9/11 was ≥ 80%. We included only firefighters exposed to the WTC who had normal pre-9/11 FEV1 values; available post-9/11 PFT results; fasting blood drawn or banked before WTC site closure on July 24, 2002; and available clinical end points, which yielded a source cohort (n = 7,487) (Fig 1).33 Of this source cohort, 1,180 (15.8%) were from the previously published subspecialty cohort.19, 20, 35 All subjects were followed up longitudinally until August 1, 2017. As a primary end point, case subjects with WTC-LI (n = 1,208) were identified if their FEV1 was less than the LLN at least twice during this period; the remaining subjects (n = 6,279) were those with no WTC-LI. An alternative case definition was explored as a secondary end point and was defined as subjects having an FEV1 less than the LLN at any time point (n = 1,999), and these were compared with subjects with no WTC-LI (n = 5,488). The secondary end point was less restrictive and was used to mirror individuals from our prior work that met our original case definition of WTC-LI, which similarly included subjects having an FEV1 less than the LLN at one time point.20
Figure 1.
Study design. Fire Department of New York rescue and recovery workers exposed to World Trade Center particulates. LLN = lower limit of normal; WTC = World Trade Center; WTC-LI = WTC lung injury.
MetSyn Variables
MetSyn diagnosis was based on National Cholesterol Education Program Adult Treatment Panel III guidelines and optimized for this study cohort by having at least three criteria: SBP ≥ 130 mm Hg or diastolic BP (DBP) ≥ 85 mm Hg, HDL < 40 mg/dL, triglycerides ≥ 150 mg/dL, insulin resistance as glucose ≥ 100 mg/dL, or BMI > 30 kg/m2 at WTC-HP entry.39 Glucose also was examined at levels of ≥ 126 mg/dL (American Diabetes Association and World Health Organization guidelines).1, 40 Our current analysis uses SBP and DBP because they are clinically robust biomarkers and more closely align with MetSyn criteria. BMI > 30 kg/m2 was used as a surrogate for central adiposity (World Health Organization guidelines).41 Participants provided written informed consent, and the study was approved by the Institutional Review Boards of Montefiore Medical Center, Albert Einstein (07-09-320) and New York University (11-00439).
Statistical Analysis
We used software (SPSS 23; IBM) for primary data storage and statistics. Density plots also were developed using software (R version 3.4.3; R Project). Continuous variables were expressed as mean (SD) and were compared by using a two-sample t test. Categorical data were summarized as counts or proportions and were compared using a Pearson χ2 test. Smoking and exposure intensity were self-reported and collected from annual questionnaires. Furthermore, because distant history of smoking can predispose a person to nonresolving inflammation, our primary distinction was between ever and never smokers. Smoking data were categorized into a dichotomous variable of ever or never smokers.19, 20, 33, 34, 35, 36, 42, 43, 44, 45, 46 Self-reported arrival time was used as a proxy for WTC PM exposure; highest in the morning of 9/11, intermediate during the afternoon, and of lower intensity if workers arrived on or after 9/12.47 For the primary end point, the survival interval was determined as time from 9/11 to the time that the second FEV1 was less than the LLN; for the secondary end-point, the survival interval was calculated as the time between 9/11 and when the subjects first had an FEV1 less than the LLN.
Association of the end points and MetSyn, smoking, age, and exposure level were analyzed using Cox proportional hazard ratios (HRs [95% CI]). All models were adjusted for age at 9/11 and were significant if P < .05. Omnibus testing assessed the quality of the comparisons. Time-to-event curves (Kaplan-Meier curves) were assessed by means of log-rank tests. Time to divergence in Kaplan-Meier curves was assessed by means of a t test at each cumulative year interval after 9/11 for each MetSyn criterion. There was no loss to follow-up. Individual hazards were plotted against MetSyn criteria (3-D density plot) for a visual representation of the distribution of the odds of developing WTC-LI.
Results
FDNY Medical Monitoring and Treatment Cohort Characteristics
A total of 9,566 rescue and recovery workers who were exposed were enrolled in the WTC-HP and provided consent (Fig 1). We evaluated 98,221 PFTs performed in these subjects. A source cohort of 7,487 had available post-9/11 PFT and blood measurements at their first post-9/11 medical monitoring examination. We included FDNY firefighters with FEV1 greater than or equal to the LLN before 9/11 with available clinical end points (BP, lipid levels, and glucose measurements), demographic information (exposure group, smoking status), and serum drawn before closure of the WTC site on July 24, 2002.
WTC-LI Phenotype
Primary End Point
Demographic and clinical measures of WTC-LI case subjects (n = 1,208) and control subjects (n = 6,279) were compared (Table 1). Case subjects and control subjects were not significantly different in age, race, total duration at the site, SBP, glucose level, low-density lipoprotein (LDL) level, or cholesterol level. Case subjects were more likely to have higher BMI at WTC-HP entry, have MetSyn, be ever smokers, and have a high-intensity exposure than were subjects with no WTC-LI. They also had higher DBP and triglyceride levels and lower HDL levels. Subjects with WTC-LI who had at least two recorded FEV1 levels less than the LLN measurements (primary end point) had a mean (SD) survival interval of 91.2 (53.2) months after 9/11, whereas subjects with WTC-LI with at least one FEV1 level less than the LLN (secondary end point) had a mean (SD) survival interval of 59.1 (64.9) months.
Table 1.
Clinical Measures for First and Second End Points
| Measure | WTC-LI |
None (n = 6,279) |
P Valuea |
||
|---|---|---|---|---|---|
| First End Point (n = 1,208) | Second End Point (n = 1,999) | First End Point | Second End Point | ||
| Age on 9/11, y | 39.3 (7.3) | 39.3 (7.3) | 39.6 (7.5) | .152 | .029 |
| BMI kg/m2 (at WTC-HP entry) | 29.0 (3.6) | 28.9 (3.4) | 28.5 (3.2) | < .001 | < .001 |
| Ever smokers, No. (%) | 476 (39) | 758 (38) | 2,246 (36) | .016 | .090 |
| Race, No. (%) | |||||
| White | 1,148 (95) | 1,882 (94) | 5,894 (94) | .184 | .195 |
| African American | 20 (2) | 41 (2) | 172 (3) | ||
| Hispanic | 37 (3) | 71 (4) | 195 (3) | ||
| Asian or Other | 3 (0.2) | 5 (0.3) | 18 (0.3) | ||
| Exposure group, No. (%) | |||||
| Morning of 9/11 | 225 (19) | 378 (19) | 1,022 (16) | .006 | .001 |
| Afternoon of 9/11 | 675 (56) | 1,090 (55) | 3,392 (54) | ||
| After 9/12 | 308 (26) | 531 (27) | 1,865 (30) | ||
| Total duration, mo | 3.7 (2.7) | 3.8 (2.7) | 3.7 (2.7) | .376 | .051 |
| BP, mm Hg | |||||
| Systolic | 117.7 (13.0) | 117.7 (13.0) | 117.0 (12.4) | .072 | .013 |
| Diastolic | 74.0 (8.7) | 73.9 (8.6) | 73.3 (8.4) | .016 | .013 |
| Glucose, mg/dL | 91.6 (14.9) | 91.9 (14.9) | 91.7 (14.0) | .924 | .433 |
| Lipids, mg/dL | |||||
| Triglycerides | 198.0 (136.4) | 198.7 (143.2) | 183.8 (136.7) | .001 | < .001 |
| HDL | 47.2 (11.8) | 47.1 (11.6) | 48.2 (11.8) | .007 | < .001 |
| LDL | 128.2 (33.7) | 128.0 (33.7) | 128.6 (33.5) | .749 | .384 |
| Cholesterol, mg/dL | 212.3 (38.7) | 211.6 (38.6) | 210.9 (38.7) | .250 | .505 |
| MetSyn definition, No. (%) | 280 (23) | 460 (23) | 1,173 (19) | < .001 | < .001 |
| Pre-9/11 FEV1 % predicted | 93.7 (8.8) | 96.5 (10.2) | 108.4 (12.4) | < .001 | < .001 |
| FVC % predicted | 90.6 (9.6) | 92.4 (10.1) | 101.2 (11.7) | < .001 | < .001 |
| FEV1/FVC | 82.9 (5.7) | 83.6 (5.5) | 85.6 (4.7) | < .001 | < .001 |
| WTC-HP entry FEV1 % Predicted | 84.3 (10.0) | 86.3 (10.8) | 100.2 (12.3) | < .001 | < .001 |
| FVC % Predicted | 82.5 (9.7) | 83.9 (10.5) | 94.1 (11.4) | < .001 | < .001 |
| FEV1/FVC | 82.0 (6.0) | 82.4 (6.0) | 84.9 (4.8) | < .001 | < .001 |
Data are presented as mean (SD) unless otherwise indicated. Percentages do not necessarily sum to 100% because of rounding. HDL = high-density lipoprotein; LDL = low-density lipoprotein; MetSyn = metabolic syndrome; WTC-HP = World Trade Center Health Program; WTC-LI = WTC lung injury.
P values were calculated by means of a t test or χ2 test, as appropriate, between the end point indicated and the population with no WTC-LI, respectively.
Secondary End Point
Similarly, demographic and clinical measures were compared for the case subjects with WTC-LI as defined by the secondary end point and those with no WTC-LI (Table 1). Similar to the case subjects for the primary end point, the case subjects for the secondary end point were not significantly different in race, total duration at the site, glucose level, LDL level, or total cholesterol level and had significant differences in their BMI at WTC-HP entry, exposure, DBP, triglyceride levels, HDL levels, and spirometric values at both time points when compared with case subjects with no WTC-LI. However, these case subjects differed from the case subjects for the primary end point in that their age on 9/11 and SBP were significantly different compared with those with no WTC-LI (Table 1).
Comparison of Primary and Secondary End Point Case Subjects
The entire population of those with an FEV1 less than the LLN at least twice (primary end point; n = 1,208) is included in the secondary end-point population whose FEV1 was less than the LLN at least once (n = 1,999). There was no significant difference between the populations as defined by these two end points in age, BMI, smoking, race, exposure, duration at site, SBP, DBP, glucose levels, triglyceride levels, HDL levels, LDL levels, cholesterol levels, or percentage who met MetSyn criteria (Table 1). There was a statistically significant difference in spirometric values before 9/11 and at WTC-HP entry (P < .001 for all values except for FEV1/FVC at WTC-HP entry, which was P = .04). Those with a value less than the LLN at least once had slightly higher FEV1 % predicted, FVC % predicted, and FEV1/FVC than did those with a value less than the LLN at least twice.
Model Development
Cox proportional hazard models were used to estimate the HRs of individual components of MetSyn on the development of WTC-LI (Table 2). Most components of MetSyn were significant individual risk factors in the development of WTC-LI. The highest risks of having FEV1 less than the LLN at least twice were associated with BMI ≥ 30 (33% increased risk), DBP ≥ 85 mm Hg (26%), and triglycerides ≥ 150 (23%). Both cut points of glucose (American Diabetes Association guidelines) were not significant.1, 39, 40 These associations maintained significance for both primary and secondary end points.
Table 2.
Cox Adjusted Univariate Hazard Ratios (95% CI)
| MetSyn Variable | FEV1 Less Than Lower Limit of Normal |
|
|---|---|---|
| At Least Twice First End Point (n = 1,208) | At Least Once Second End Point (n = 1,999) | |
| BMI 30 kg/m2 | 1.33a (1.18-1.49) | 1.24a (1.13-1.36) |
| Lipids, mg/dL | ||
| HDL < 40 | 1.17a (1.03-1.33) | 1.20a (1.09-1.32) |
| Triglycerides150 | 1.23a (1.09-1.37) | 1.26a (1.15-1.38) |
| BP, mm Hg | ||
| Systolic130 | 1.17a (1.02-1.35) | 1.14a (1.02-1.27) |
| Diastolic85 | 1.26a (1.05-1.52) | 1.18a (1.02-1.37) |
| Glucose, mg/dL | ||
| 100 | 1.00 (0.87-1.16) | 1.05 (0.94-1.18) |
| 126 | 1.31 (0.88-1.97) | 1.34 (0.98-1.85) |
See Table 1 legend for expansion of abbreviations.
Significant hazard.
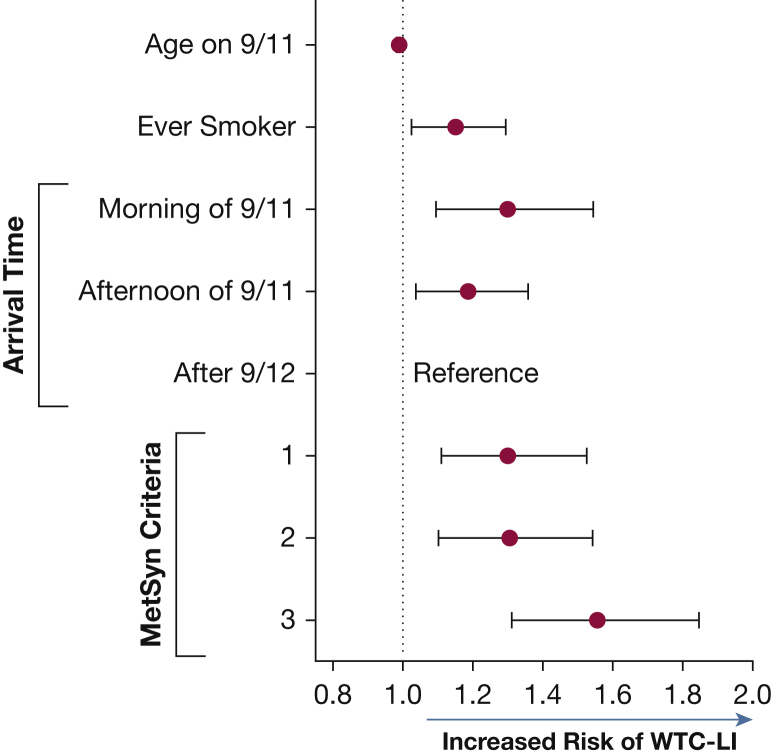
The final model used the total number of MetSyn risk factors to avoid effects from multicollinearity because many of the definitional components of MetSyn (such as SBP and DBP) are related intrinsically to each other (Fig 2).48 This analysis examined the contribution of BMI to the development of WTC-LI as a component of the MetSyn criteria. Increasing age was associated with a small but significant reduction of risk by 1% for every additional year. Smoking increased risk of developing WTC-LI by 15.2%. The final model had a significant exposure dose response; the individuals who were most highly exposed who were present on the morning of 9/11 had a 30.1% increased risk of developing WTC-LI and an 18.7% increased risk if the individual arrived in the afternoon of 9/11 compared with values in the least exposed group. There was also a significant increased risk associated with the number of MetSyn criteria in a dose-response relationship. Having one or two of five MetSyn criteria would increase risk by 30.2% and 30.5%, respectively; having three or more criteria increased the risk of developing WTC-LI by 55.7%. There was a significant interaction between most highly exposed and smoking (P = .03). These relationships were maintained at the secondary end point.
Figure 2.
MetSyn final model hazard ratios. The final model was adjusted for age at 9/11, smoking, and exposure. The final model had a significant exposure dose response (P = .007). There was also a significant increased risk associated with the number of MetSyn criteria met in a dose-response fashion (P < .001). MetSyn = metabolic syndrome. See Figure 1 legend for expansion of other abbreviation.
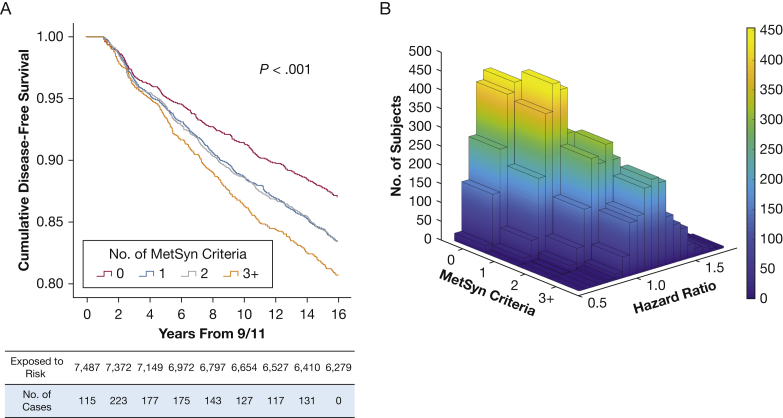
Case subjects with MetSyn began diverging from the cohort at approximately year 3 after exposure (Fig 3A). Having one or two MetSyn risk factors overlapped but had significant separation from zero risk factors; having three or more MetSyn risk factors showed separation from all other groups (log-rank P < .001). A 3-D density plot was used to visualize the distribution of the cohort (Fig 3B). Many of the population with zero MetSyn points had an HR < 1, indicating potential protective effects. The population with three or more MetSyn risk factors had the highest hazards of developing of WTC-LI of the cohort.
Figure 3.
A, Kaplan-Meier curve shows MetSyn criteria stratified time to development of WTC-LI. Cumulative disease-free survival is expressed on the y-axis and time in years from their WTC exposure is on the x-axis. B, A 3-D density plot of hazard ratio and number of MetSyn criteria met demonstrates a large proportion of patients with zero MetSyn criteria with hazards < 1, in contrast to those with three or more MetSyn criteria who had more significant risk of developing WTC-LI. See Figure 1 and 2 legends for expansion of abbreviations.
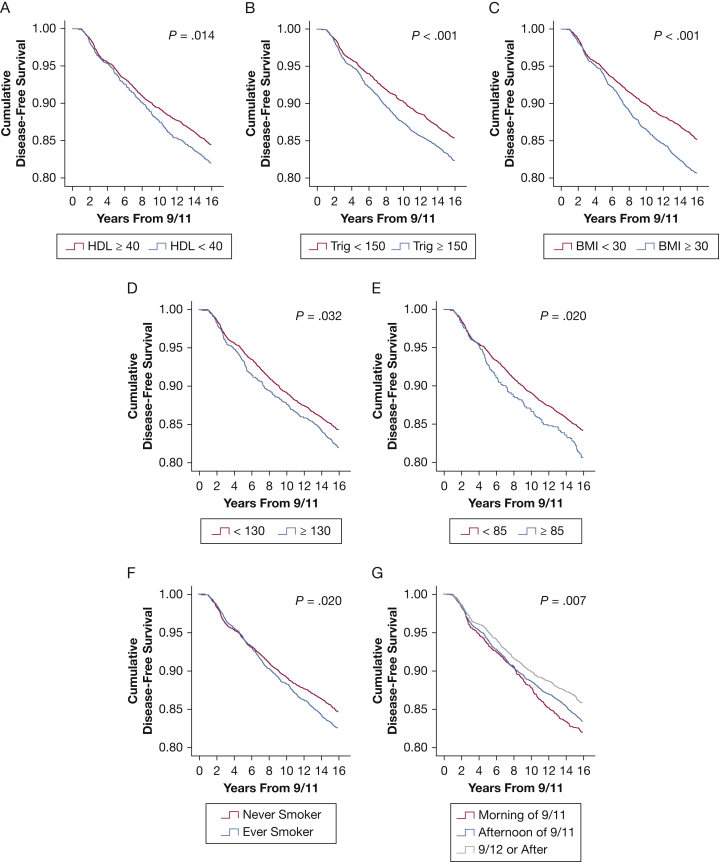
Additionally, we assessed Kaplan-Meier curves for each of MetSyn biomarker (Fig 4). Divergence analyses at each year interval were calculated by using a t test and showed significant difference in disease-free survival curves in HDL < 40 and triglycerides ≥ 150 at 10 and 6 years, P = .014 and < .0001, respectively (Figs 4A, 4B). BMI > 30 showed significant separation at 6 years (P < .001) (Fig 4C). Hypertension (either SBP ≥ 130 or DBP ≥ 85) showed significant separation at 6 years (P = .032 and .020) (Figs 4D, 4E). Smoking did not show significant separation until 13 years after 9/11 (P = .020) (Fig 4F). There was no significant change point between morning and afternoon exposure on 9/11, but morning of 9/11 and after 9/12 had a separation at 5 years and between afternoon of 9/11 and after 9/12 had a separation at 6 years (P = .007) (Fig 4G). Insulin resistance as measured by means of fasting serum glucose levels was not a significant risk factor.
Figure 4.
Cumulative disease-free survival curves by individual metabolic syndrome biomarkers. Cumulative disease-free survival is expressed on the y-axis, and time in years from World Trade Center exposure is on the x-axis. A, High-density lipoprotein (HDL) (mg/dL). B, Triglycerides (Trig) (mg/dL). C, BMI (kg/m2). D, Systolic BP (mm Hg). E, Diastolic BP (mm Hg). F, Smoking status. G, Arrival time.
Discussion
The FDNY cohort exposed at the WTC continues to have their health adversely impacted even after 16 years.49, 50, 51 Our previous pilot study focused on the contribution of MetSyn to the development of WTC-LI in a select group of nonsmokers requiring subspecialty pulmonary evaluation.22 To our knowledge, our current work is the first study to validate MetSyn biomarkers as a predictor of WTC-LI in a more representative cohort. Our study indicates that MetSyn predicts WTC-LI, in contrast to other studies that purport possible lung injury predicting incident MetSyn.52 We show this temporality based on blood samples drawn early in the disease process, up to site closure on July 24, 2002, whereas the diagnosis of WTC-LI is after at least two PFT results showing a consistent FEV1 less than the LLN (mean of 92 months after 9/11). Dyslipidemia maintains its ability to predict WTC-LI in the larger cohort exposed to WTC PM. To our knowledge, ours is also the longest longitudinal study to show that MetSyn risk factors impart a higher risk of developing loss of FEV1 than does either PM or smoking exposure.
Our earlier model showed that dyslipidemia and heart rate independently increased the odds of developing WTC-LI. Our current investigation shows the continued associations of MetSyn biomarkers and WTC-LI. Moreover, we show that MetSyn biomarkers are more predictive than are smoking and WTC exposure intensity in predicting the development of WTC-LI. MetSyn risk factors are classically predictors of cardiovascular disease, but their implications in affecting future lung disease are novel and indicate reversible risks that may be therapeutic targets.
Similar to the results in our pilot, glucose level was not a significant predictor of WTC-LI.20 Specifically, there was no increased risk of lung injury with cut points of either 100 or 126 mg/dL. We had hypothesized in our earlier work that insulin resistance would be a significant predictor if sufficiently powered in the larger group. This validation model suggests that the impact of glucose on lung function is either indirect or weaker than that of dyslipidemia. This finding is in contrast to those of other studies that similarly have investigated insulin resistance and lung function.53 This result is unlikely to be from a healthy worker effect, given that the prevalence of insulin resistance was approximately 18% in both case subjects and control subjects. Rather, this result may indicate that dyslipidemia is present earlier in the disease process than is insulin resistance.
The study design allowed us to explore multiple aspects of WTC-LI. In our pilot, we described WTC-LI as FEV1 less than the LLN at the time of subspecialty evaluation.20 We now confirm in this more representative cohort that MetSyn biomarkers remain valid predictors of developing FEV1 less than the LLN at a single time point (secondary end point). By using the stricter definition for WTC-LI of being less than the LLN at least twice (primary end point), we potentially have identified a population with greater long-term pulmonary health consequences of WTC exposure. Despite having the same baseline demographic characteristics, those in the primary end-point group had significantly lower PFT values at baseline and at WTC-HP entry. Although having slightly lower baseline PFT values conceivably can increase the likelihood of developing an FEV1 less than the LLN, the clinical difference is minute and likely would not have altered medical treatment.
We confirm the relevance of MetSyn as a risk for a persistent loss of FEV1 to less than the LLN. Long-term exposure to PM significantly increases LDL, cholesterol, and triglyceride levels and decreases HDL levels in pregnant rats and their offspring.54 Additionally, adult women exposed to tobacco smoke in utero had higher triglyceride and lower HDL levels than did unexposed women, and female adolescents exposed in utero had significantly higher BMIs and waist circumference percentiles than did those who were unexposed.55, 56 The constellation of respiratory diseases related to PM exposure through burn pits, improvised explosive devices, and sandstorms has been studied extensively and shows great potential as comparable cohorts.57, 58 Ambient pollution in China and its association with MetSyn also has been investigated.59 Our findings can be applied to these cohorts to examine the effect of MetSyn characteristics as a potential screening tool for the development of future FEV1 less than the LLN.
Using the clinical markers of MetSyn as predictors of lung disease is advantageous in several ways. These biomarkers are easily attainable, are cost-effective to obtain, and can be replicated in many cohorts. Dyslipidemia, insulin resistance, obesity, and hypertension are also all potentially reversible causes of end-organ disease. Extending statin therapy and increasing glycemic control can be the targets of future studies.
There are several limitations to this study. Smoking status was self-reported, and no pack-year history or secondhand smoke exposure data were available. Also, case subjects with WTC-LI and control subjects had significantly different pre-9/11 FEV1 measurements. However, their average PFT values were well within the clinically expected normal values. It is not within the scope of this study to examine whether there was some genetic or innate predisposition to MetSyn or lung disease. However, studying the relevant multiomics of this cohort is an active area of research.22 Serum samples were not available from before WTC exposure or from when subjects first joined the FDNY.
It remains unclear to what extent, if any, diet, alcohol consumption, and exercise contributed to the metabolic signature of subjects with WTC-LI or whether a dietary modification would be useful in preventing, managing, or treating WTC-LI and similar processes. Specifically, a diet that is low in saturated fat intake and has a low ratio of omega-6 to omega-3 fatty acids may help to correct high ceramide levels and the imbalance in phospholipid-derived long-chain polyunsaturated fatty acid metabolites, which could have downstream beneficial effects on inflammatory and insulin signaling pathways.60, 61, 62 In patients with advanced lung diseases such as COPD, branched chain amino acid supplements improve health outcomes.63, 64 Dietary interventions that have focused on weight loss in patients with obstruction show improvement of both FEV1 and FVC by as much as 22% in as little as 15 days.65, 66 With use of a very low-calorie diet, investigators have been able to achieve a 20-kg loss over a 6-month period; every 10% relative loss of weight led to a significant improvement of FVC by 92 mL and of FEV1 by 73 mL.67 As patients decreased their BMI from 37 to 32 kg/m2, the mean morning FEV1 and FVC increased.68 Additionally, a 2008 study showed the effectiveness of calorie restriction and Mediterranean diets in reducing lipid levels.69 Although moderating fats can be essential to maintaining a healthy diet, there is extensive literature that explores the potential health benefits of fats, such as n-3 and n-6 polyunsaturated fatty acids, that are high in a Mediterranean diet.70, 71 Additional research is needed to examine the impact of diet and other modifiable risks on WTC-LI progression.
This study lacks further assessment of biomarkers such as amylin and leptin.20 In 2004, the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints group investigated the repeatability of 34 biomarkers and found that only some were reproducible and correlated with measures of lung dysfunction.72 However, we are strongly encouraged by our result that pathways involved in metabolism have broad impacts on the immune and hormonal environment in the lung. Future investigations will seek to validate these biomarkers in this study’s cohort and identify mechanistic pathways that lead to WTC-LI.
Examination of the primary end point shows that, compared with the other MetSyn criteria, having a BMI ≥ 30 is associated with the highest risk of developing WTC-LI. BMI is a surrogate for abdominal waist circumference, and BMI has been shown to be flawed as a biomarker in numerous studies.73 However, we also see that this association is less powerful in the secondary end point and that dyslipidemia, particularly hypertriglyceridemia, is a pervasive predictor. We also showed that the cumulative effect of at least three MetSyn criteria is more powerful than any one factor alone. Therefore, we are cautious to not overinterpret the association of BMI > 30 as the strongest predictor of WTC-LI. The 3-D plot that shows stratification with other components of MetSyn supports this interpretation.
Conclusions
In summary, we have validated the usefulness of MetSyn biomarkers as predictors of WTC-LI in a larger more representative population of firefighters exposed at the WTC. These biomarkers are associated with dyslipidemia, insulin resistance, and cardiovascular disease and suggest MetSyn can contribute to future loss of lung function. Our data contribute to the growing body of literature investigating the complex associations between lung injury and reversible MetSyn risk factors.
Acknowledgments
Author contributions: S. K. and A. N. participated in study conception and design; A. N. was the primary investigator and guarantor of the paper. S. K., R. Z. O., T. M. S., D. J. P., and A. N. were responsible for data collection. S. K. and A. N. were responsible for data validation. S. K., G. C., S. H. H., A. V., and A. N. participated in data analysis. S. K., G. C., L. Y., M. L., and A. N. undertook the statistical analysis. E. J. C. and R. L. were responsible for data acquisition. All authors participated in data interpretation, writing and revision of the report, and approval of the final version.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Heart, Lung, and Blood Institute [Grant R01HL119326]; the Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health [Grant U01-OH11300]; the Clinical Center of Excellence [Grant 200-2017-93426]; and the Data Center [Grant 200-2017-93326].
References
- 1.Grundy S.M., Cleeman J.I., Daniels S.R. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 3.Wallwork R.S., Colicino E., Zhong J. Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am J Epidemiol. 2017;185(1):30–39. doi: 10.1093/aje/kww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook R.D., Sun Z., Brook J.R. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: the Air Pollution and Cardiometabolic Disease Study. Hypertension. 2016;67(1):77–85. doi: 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leone N., Courbon D., Thomas F. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 6.Fiordelisi A., Piscitelli P., Trimarco B., Coscioni E., Iaccarino G., Sorriento D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev. 2017;22(3):337–347. doi: 10.1007/s10741-017-9606-7. [DOI] [PubMed] [Google Scholar]
- 7.Zammit C., Liddicoat H., Moonsie I., Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baffi C.W., Wood L., Winnica D. Metabolic syndrome and the lung. Chest. 2016;149(6):1525–1534. doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters U., Suratt B.T., Bates J.H.T., Dixon A.E. Beyond BMI: obesity and lung disease. Chest. 2018;153(3):702–709. doi: 10.1016/j.chest.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith K.A., Sherrill D.L., Siegel E.M., Manolio T.A., Bonekat H.W., Enright P.L. Predictors of loss of lung function in the elderly: the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163(1):61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 11.Piazzolla G., Castrovilli A., Liotino V. Metabolic syndrome and chronic obstructive pulmonary disease (COPD): the interplay among smoking, insulin resistance and vitamin D. PLoS One. 2017;12(10):e0186708. doi: 10.1371/journal.pone.0186708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H.J., Leem A.Y., Lee S.H. Comorbidities in obstructive lung disease in Korea: data from the fourth and fifth Korean National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2015;10:1571–1582. doi: 10.2147/COPD.S85767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham T.J., Ford E.S., Rolle I.V., Wheaton A.G., Croft J.B. Associations of self-reported cigarette smoking with chronic obstructive pulmonary disease and co-morbid chronic conditions in the United States. COPD. 2015;12(3):276–286. doi: 10.3109/15412555.2014.949001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowler R.P., Jacobson S., Cruickshank C. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med. 2015;191(3):275–284. doi: 10.1164/rccm.201410-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoonhorst S.J., Lo Tam Loi A.T., Hartman J.E. Advanced glycation end products in the skin are enhanced in COPD. Metabolism. 2014;63(9):1149–1156. doi: 10.1016/j.metabol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Yonchuk J.G., Silverman E.K., Bowler R.P. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192(7):785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 17.Caraher E.J., Kwon S., Haider S.H. Receptor for advanced glycation end-products and World Trade Center particulate induced lung function loss: a case-cohort study and murine model of acute particulate exposure. PLoS One. 2017;12(9):e0184331. doi: 10.1371/journal.pone.0184331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho S.J., Echevarria G.C., Kwon S. One airway: biomarkers of protection from upper and lower airway injury after World Trade Center exposure. Respir Med. 2014;108(1):162–170. doi: 10.1016/j.rmed.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan A., Naveed B., Comfort A.L. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest. 2012;142(2):412–418. doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naveed B., Weiden M.D., Kwon S. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med. 2012;185(4):392–399. doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prezant D.J., Weiden M., Banauch G.I. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 22.Crowley G., Kwon S., Haider S.H. Metabolomics of World Trade Center-Lung Injury: a machine learning approach. BMJ Open Respir Res. 2018;5(1):e000274. doi: 10.1136/bmjresp-2017-000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lioy P.J., Weisel C.P., Millette J.R. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110(7):703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin S., Herbert R., Skloot G. Health effects of World Trade Center site workers. Am J Ind Med. 2002;42(6):545–547. doi: 10.1002/ajim.10154. [DOI] [PubMed] [Google Scholar]
- 25.Banauch G.I., Dhala A., Alleyne D. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 suppl):S102–S106. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 26.Landrigan P.J., Lioy P.J., Thurston G. Health and environmental consequences of the World Trade Center disaster. Environ Health Perspect. 2004;112(6):731–739. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farfel M., DiGrande L., Brackbill R. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008;85(6):880–909. doi: 10.1007/s11524-008-9317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldrich T.K., Vossbrinck M., Zeig-Owens R. Lung function trajectories in World Trade Center-exposed New York City firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niles J.K., Webber M.P., Cohen H.W. The respiratory pyramid: from symptoms to disease in World Trade Center exposed firefighters. Am J Ind Med. 2013;56(8):870–880. doi: 10.1002/ajim.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukiji J., Cho S.J., Echevarria G.C. Lysophosphatidic acid and apolipoprotein A1 predict increased risk of developing World Trade Center-lung injury: a nested case-control study. Biomarkers. 2014;19(2):159–165. doi: 10.3109/1354750X.2014.891047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho S.J., Nolan A., Echevarria G.C. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013;33(6):1134–1142. doi: 10.1007/s10875-013-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 33.Weiden M.D., Naveed B., Kwon S. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust-exposed firefighters. Eur Respir J. 2013;41(5):1023–1030. doi: 10.1183/09031936.00077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiden M.D., Kwon S., Caraher E. Biomarkers of World Trade Center particulate matter exposure: physiology of distal airway and blood biomarkers that predict FEV(1) decline. Semin Respir Crit Care Med. 2015;36(3):323–333. doi: 10.1055/s-0035-1547349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiden M.D., Ferrier N., Nolan A. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon S., Weiden M.D., Echevarria G.C. Early elevation of serum MMP-3 and MMP-12 predicts protection from World Trade Center-lung injury in New York City firefighters: a nested case-control study. PLoS One. 2013;8(10):e76099. doi: 10.1371/journal.pone.0076099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho S.J., Echevarria G.C., Lee Y.I. YKL-40 is a protective biomarker for fatty liver in World Trade Center particulate matter-exposed firefighters. J Mol Biomark Diagn. 2014;5(174):10000174. doi: 10.4172/2155-9929.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelman P., Osterloh J., Pirkle J. Biomonitoring of chemical exposure among New York City firefighters responding to the World Trade Center fire and collapse. Environ Health Perspect. 2003;111(16):1906–1911. doi: 10.1289/ehp.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang P.L. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genuth S., Alberti K.G., Bennett P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva, Switzerland: World Health Organization; 1999. https://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf;jsessionid=9CC692F8D168C27C8070D5BC89F1A05C?sequence=1. Accessed April 2, 2019.
- 42.Webber M.P., Glaser M.S., Weakley J. Physician-diagnosed respiratory conditions and mental health symptoms 7-9 years following the World Trade Center disaster. Am J Ind Med. 2011;54(9):661–671. doi: 10.1002/ajim.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiden M.D., Naveed B., Kwon S. Comparison of WTC dust size on macrophage inflammatory cytokine release in vivo and in vitro. PLoS One. 2012;7(7):e40016. doi: 10.1371/journal.pone.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldrich T.K., Ye F., Hall C.B. Longitudinal pulmonary function in newly hired, non-World Trade Center-exposed fire department City of New York firefighters: the first 5 years. Chest. 2013;143(3):791–797. doi: 10.1378/chest.12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolan A., Kwon S., Cho S.J. MMP-2 and TIMP-1 predict healing of WTC-lung injury in New York City firefighters. Respir Res. 2014;15:5. doi: 10.1186/1465-9921-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenck E.J., Echevarria G.C., Girvin F.G. Enlarged pulmonary artery is predicted by vascular injury biomarkers and is associated with WTC-Lung Injury in exposed fire fighters: a case-control study. BMJ Open. 2014;4(9):e005575. doi: 10.1136/bmjopen-2014-005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Yip J., Zeig-Owens R. The effect of World Trade Center exposure on the timing of diagnoses of obstructive airway disease, chronic rhinosinusitis, and gastroesophageal reflux disease. Front Public Health. 2017;5:2. doi: 10.3389/fpubh.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song M.K., Lin F.C., Ward S.E., Fine J.P. Composite variables: when and how. Nurs Res. 2013;62(1):45–49. doi: 10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landgren O., Zeig-Owens R., Giricz O. Multiple myeloma and its precursor disease among firefighters exposed to the World Trade Center disaster. JAMA Oncol. 2018;4(6):821–827. doi: 10.1001/jamaoncol.2018.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haider S.H., Kwon S., Lam R. Predictive biomarkers of gastroesophageal reflux disease and Barrett's esophagus in World Trade Center exposed firefighters: a 15 year longitudinal study. Sci Rep. 2018;8(1):3106. doi: 10.1038/s41598-018-21334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hena K.M., Yip J., Jaber N. Clinical course of sarcoidosis in World Trade Center-exposed firefighters. Chest. 2018;153(1):114–123. doi: 10.1016/j.chest.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moualla M., Qualls C., Arynchyn A. Rapid decline in lung function is temporally associated with greater metabolically active adiposity in a longitudinal study of healthy adults. Thorax. 2017;72(12):1113–1120. doi: 10.1136/thoraxjnl-2016-209125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagun G., Gedik C., Ekiz E., Karagoz E., Takir M., Oguz A. The relation between insulin resistance and lung function: a cross sectional study. BMC Pulm Med. 2015;15:139. doi: 10.1186/s12890-015-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y., Zhang J.J., Li Z. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 2016;30(6):2115–2122. doi: 10.1096/fj.201500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cupul-Uicab L.A., Skjaerven R., Haug K. Exposure to tobacco smoke in utero and subsequent plasma lipids, ApoB, and CRP among adult women in the MoBa cohort. Environ Health Perspect. 2012;120(11):1532–1537. doi: 10.1289/ehp.1104563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens D.R., Malek A.M., Laggis C., Hunt K.J. In utero exposure to tobacco smoke, subsequent cardiometabolic risks, and metabolic syndrome among U.S. adolescents. Ann Epidemiol. 2018;28(9) doi: 10.1016/j.annepidem.2018.06.010. 619.e611-624.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szema A., Mirsaidi N., Patel B. Proposed Iraq/Afghanistan War-Lung Injury (IAW-LI) Clinical Practice Recommendations: National Academy of Sciences' Institute of Medicine Burn Pits Workshop. Am J Mens Health. 2017;11(6):1653–1663. doi: 10.1177/1557988315619005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrington A.D., Schmidt M.P., Szema A.M. The role of Iraqi dust in inducing lung injury in United States Soldiers: an interdisciplinary study. Geohealth. 2017;1(5):237–246. doi: 10.1002/2017GH000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B.Y., Qian Z.M., Li S. Long-term exposure to ambient air pollution (including PM1) and metabolic syndrome: the 33 Communities Chinese Health Study (33CCHS) Environ Res. 2018;164:204–211. doi: 10.1016/j.envres.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 60.Holland W.L., Bikman B.T., Wang L.P. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lands W.E., Libelt B., Morris A. Maintenance of lower proportions of (n - 6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n - 3) fatty acids. Biochim Biophys Acta. 1992;1180(2):147–162. doi: 10.1016/0925-4439(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 62.Fekete K., Marosvolgyi T., Jakobik V., Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr. 2009;89(6):2070S–2084S. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 63.Chuang Y.C., Shaw H.M., Chen C.C., Pan H.J., Lai W.C., Huang H.L. Short-term glutamine supplementation decreases lung inflammation and the receptor for advanced glycation end-products expression in direct acute lung injury in mice. BMC Pulm Med. 2014;14:115. doi: 10.1186/1471-2466-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kutsuzawa T., Shioya S., Kurita D., Haida M. Plasma branched-chain amino acid levels and muscle energy metabolism in patients with chronic obstructive pulmonary disease. Clin Nutr. 2009;28(2):203–208. doi: 10.1016/j.clnu.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Angelillo V.A., Bedi S., Durfee D., Dahl J., Patterson A.J., O'Donohue W.J., Jr. Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann Intern Med. 1985;103(6 pt 1):883–885. doi: 10.7326/0003-4819-103-6-883. [DOI] [PubMed] [Google Scholar]
- 66.McDonald V.M., Gibson P.G., Scott H.A. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology. 2016;21(5):875–882. doi: 10.1111/resp.12746. [DOI] [PubMed] [Google Scholar]
- 67.Aaron S.D., Fergusson D., Dent R., Chen Y., Vandemheen K.L., Dales R.E. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125(6):2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- 68.Hakala K., Stenius-Aarniala B., Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118(5):1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- 69.Shai I., Schwarzfuchs D., Henkin Y. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz J. Role of polyunsaturated fatty acids in lung disease. Am J Clin Nutr. 2000;71(1 suppl):393S–396S. doi: 10.1093/ajcn/71.1.393s. [DOI] [PubMed] [Google Scholar]
- 71.Seegmiller A.C. Abnormal unsaturated fatty acid metabolism in cystic fibrosis: biochemical mechanisms and clinical implications. Int J Mol Sci. 2014;15(9):16083–16099. doi: 10.3390/ijms150916083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gan W.Q., Man S.F., Senthilselvan A., Sin D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pories W.J., Dohm L.G., Mansfield C.J. Beyond the BMI: the search for better guidelines for bariatric surgery. Obesity (Silver Spring) 2010;18(5):865–871. doi: 10.1038/oby.2010.8. [DOI] [PubMed] [Google Scholar]