Abstract
Maternal stress during pregnancy adversely affects developmental fetal programming. Glucocorticoid excess is one of those conditions that underlie the prenatal stress and can lead to many pathological disorders later in life. Beyond the obvious use of mammalian model organisms to uncover the different mechanisms at the basis of prenatal stress effects, zebrafish represents a complementary fruitful model for this research field. Here we demonstrated that the application of an experimental paradigm, which simulates prenatal stress by exposing embryos to cortisol excess, produced an alteration of gene expression pattern. In particular, the transcript level of hsd11b2, a gene involved in the cortisol catabolism, was affected in prenatally stressed larvae, even after many hours from the removal of cortisol excess. Interestingly, the expression pattern of c-fos, a marker gene of neural activity, was affected in prenatally stressed larvae even in response to a swirling and osmotic stress challenge. Our data corroborate the idea of zebrafish as a useful model organism to study prenatal stress effects on vertebrate development.
Keywords: Prenatal stress, Zebrafish, c-fos, hsd11b2, Cortisol
Introduction
Physiological disorders could have a fetal origin when, in case of maternal stress during gestation, an adverse intrauterine environment is established, which can affect fetal brain development and potentially lead to various pathological conditions later in life. The developmental origins of diseases were stigmatized by the Barker theory, also known as “fetal origins” hypothesis (Barker and Osmond 1986; Barker and Osmond 1988; Barker et al. 1989; Barker 1998). During prenatal development, maternal stress, like a psychological status (i.e., stress, anxiety, and depression), has been shown to be associated with the development of a wide variety of behavioral and physiological disorders in the offspring (Monk et al. 2012; Schuurmans and Kurrasch 2013). The brain is one of the critical targets of stressors and is also the central organ responsible for stress responses; in this regard, the sustained high levels of glucocorticoids during maternal stress can lead to profound deleterious alteration of brain structure and functioning (Harris and Seckl 2011; McEwen 2008). These effects can depend on glucocorticoids regulation of expression patterns of several key molecules involved in neuronal development (Drake et al. 2007). A well-known target of prenatal stress is the hypothalamus-pituitary-adrenal axis (HPA axis) that is a fundamental mediator of stress response through the release of glucocorticoids. Prenatal stress, through relatively high level of glucocorticoids exposure, alters HPA axis development and function, leading to a heightened HPA axis sensitivity to stressful events in later life and, consequently, to permanently increased levels of glucocorticoids in offspring; this altered HPA axis activity enhances vulnerability to various neurobehavioral, neuropsychological, and neuropsychiatric disorders in postnatal life (Harris and Seckl 2011; Cottrell and Seckl 2009; Seckl and Meaney 2004; Bruin et al. 2010). Gene regulatory mechanisms are essential for proper development and function of the brain and are used to allow a fine adaptation of the nervous system to changes in the environment. Many examples of gene dysregulation because of prenatal stress have been produced and, in some cases, a clear role of epigenetic modifications has been shown (Gudsnuk and Champagne 2012). The long-term consequences of maternal stress during gestation for offspring neurobiological and physiological functioning have been previously studied in rodents. The need for an alternative and complementary experimental model to explore mechanisms underlying the prenatal stress effects was clearly discussed by Steenbergen et al. (2011). They highlighted some limits in the rodent model that may be overcome using the zebrafish model, which shares extensive similarities of the stress response with mammals in terms of anatomy, connectivity, and molecular constituents (Steenbergen et al. 2011), as exemplified by the homology and functional correspondence between mammalian HPA axis and zebrafish hypothalamic-pituitary-interrenal (HPI) axis (Alsop and Vijayan 2008; Alsop and Vijayan 2009a; Alsop and Vijayan 2009b). In support of the use of zebrafish as a model for prenatal stress studies, it was shown that cortisol microinjection into the yolk at one cell stage altered primary neurogenesis affecting early zebrafish brain development and behavioral phenotype at larval stages (Best et al. 2017). An experimental paradigm of prenatal stress in zebrafish was proposed by Steenbergen et al. (2011), in which a high level of maternal stress was mimicked by exposing newly fertilized eggs to high concentrations of synthetic corticosteroids during the first 2 days of embryogenesis; this prenatal stress condition produced a long-lasting impact on behavior in the offspring larvae (Steenbergen et al. 2011). Here we report that the application of this prenatal stress, induced by glucocorticoid excess, is able to affect gene expression pattern during embryogenesis and in larvae after swirling stress and osmotic stress, supporting the validity of that experimental paradigm in the zebrafish model organism. In particular, we assessed the effect of these stresses on the mRNA levels of two genes, hydroxysteroid 11-beta dehydrogenase 2 (hsd11b2) and c-fos, genes involved in the cortisol catabolism (Wilson et al. 2013) and a marker of neural activity (Stewart et al. 2014), respectively.
Materials and methods
Ethics statement
The animal protocol for this study was approved by the Animal Care Review Board of the University of Naples Federico II (PROT. 2014/0020464).
Animals
Wild-type zebrafish were obtained from a local pet shop and housed in mixed-sex groups in static tanks (about 2 L/fish) with airlift-driven at 28.5 °C with a photoperiod of 14-h light (9:00 am–11:00 pm) and 10-h dark. The fish were fed two times daily with a mixture of tropical fish flakes and Artemia shrimps.
Prenatal stress paradigm
Fertilized eggs were obtained by natural spawning and incubated at 28.5 °C in 6-well plates containing embryo medium (25 eggs/well). To simulate prenatal stress, cortisol (H0888; Sigma-Aldrich®, Milan, Italy) dissolved in DMSO was added to embryo medium at a final concentration of 100 μM. Control zebrafish embryos were kept in embryo medium with an equal amount of DMSO (0.33%). Embryo medium + cortisol (or DMSO alone) was exchanged after 24 h with fresh embryo medium containing the respective treatment regimen. Embryos were exposed to cortisol (or DMSO alone) for a total time of 48 h after fertilization as reported in Steenbergen et al. (2011). After that, embryo medium + cortisol (or DMSO alone) was replaced with fresh embryo medium alone. Embryos/larvae at 48, 72, and 96 h post-fertilization (hpf) were anesthetized with 0.016% tricaine (A5040; Sigma-Aldrich®, Milan, Italy) and kept in ice for 1 min, then transferred in 2-mL tubes and snap frozen for RNA extraction. All the experiments were carried out in three biological replicates.
Embryos viability, hatching, and morphological analysis
Effects of the prenatal stress were assessed by evaluating different developmental features of prenatally stressed embryos/larvae versus control. Embryos viability was assessed by counting the number of live embryos at 24, 48, 72, and 96 hpf. Chorion self-hatch rate was assessed by counting the number of embryos that had hatched at 24, 48, 72, and 96 hpf. The morphological analysis included body length, swim bladder inflation, and yolk sac size. The analysis was performed by imaging 96 hpf larvae under a bright-field microscope and using ImageJ software.
Swirling and osmotic challenge
Larvae at 5 days post-fertilization (dpf, about 99 hpf) were subjected to a physical stressor (swirling stress) or osmotic stress for the analyses of prenatal stress effect on gene expression after stress challenge. For the swirling stress, 5 pools of cortisol-treated larvae and 5 pools of control larvae (25 larvae/pool) were transferred from the 6-well plates to a 50-mL beaker containing 10 mL of embryo medium and kept in a 28.5 °C incubator for 1.5 h before the challenge. After that, larvae were subjected to swirling stress at 250 rpm for 90 s by means of orbital shaker (ORBITAL SHAKER SO3, Stuart Scientific, UK). Subsequently, the larvae were collected at different time points (5 min, 10 min, 30 min, and 60 min) after the stress. Control samples were taken before applying the swirling stress (0 min). For the osmotic stress, 1 pool of cortisol-treated larvae and 1 pool of control larvae (25 larvae/pool) were transferred in embryo medium supplemented with NaCl to a final concentration of 0.56 M for 30 min. Corresponding control samples for the osmotic stress were obtained by transferring 1 pool of cortisol-treated larvae and 1 pool of control larvae (25 larvae/pool) in fresh embryo medium and collected in parallel with treated larvae. The larvae were anesthetized with 0.016% tricaine (Sigma-Aldrich®, Milan, Italy) and kept in ice for 1 min, then transferred in 2-mL tubes and snap-frozen for RNA extraction. All swirling and osmotic challenge experiments were carried out at the same time of day (12 pm). All the experiments were carried out in three biological replicates.
RNA extraction and qPCR analysis
Total RNA from embryos and larvae was isolated using the Trizol® reagent (Invitrogen™, Monza, Italy) as reported by the manufacturer. The RNA was quantified by NanoDrop 1000 (Thermo Scientific, Wilmington, DE) and quality checked by 260/280 and 260/230 ratios, whereas integrity was evaluated by agarose gel electrophoresis. First strand cDNA was synthesized from 1 μg of total RNA in 20 μL of total volume reaction with High-Capacity cDNA Reverse Transcription Kit (Life Technologies™, Monza, Italy); afterward, it was diluted to 50 μL. Two microliters of diluted cDNA was used for each reaction of the qPCR analysis. The primers used for the expression pattern analysis were as follows: for c-fos gene (ENSDARG00000031683), forward (5′-GTGCAGCACGGCTTCACCGA-3′) and reverse (5′-TTGAGCTGCGCCGTTGGAGG-3′); for hsd11b2 gene (ENSDARG00000001975), forward (5′-GGGGGTCAAAGTTTCCACTA-3′) and reverse (5′-TGGAAGAGCTCCTTGGTCTC-3′); for actb2 gene (reference gene, ENSDARG00000037870), forward (5′-AAGGCCAACAGGGAAAAGAT-3′) and reverse (5′-GTGGTACGACCGGAGGCATAC-3′). All the primers were used at a final concentration of 400 nM in the qPCR reaction volume of 15 μL. The gene expression analysis was performed by the use of SYBR Green PCR master mix (Life Technologies™, Monza, Italy) in the Applied Biosystem 7500 real-time PCR System (Life Technologies™, Monza, Italy). The following amplification conditions were used: holding the stage at 95 °C for 10′, 40 cycles of 95 °C for 30″, 58 °C for 35″, 72 °C for 40″ (signal detection at this step), followed by melt curve analysis to check amplification specificity. qPCR reactions were performed in two technical replicates for each sample. The mean of technical replicates was used for the fold change calculation and for the statistical analysis. In particular, the fold change was calculated by the efficiency-corrected ΔΔCt method. The qPCR efficiency (E) was evaluated by means of standard curve method and calculated from the slope: E = (10−1/slope−1) × 100 (Ec-fos = 99.0%, Ehsd11b2 = 90.0%, Eactb2 = 99.5).
Statistical significance was tested by using the REST2009 software with 10,000 iterations and the cutoff for significance was P = 0.05 (Pfaffl et al. 2002).
Results
Embryo viability, spontaneous hatching, and morphological analysis
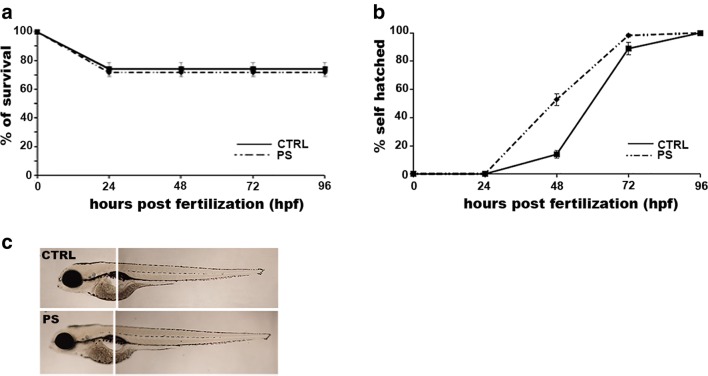
As a starting point, we assessed the effect of cortisol treatment on different developmental landmarks. First of all, we determined the percentage of survival of prenatally stressed embryos compared to vehicle-treated controls. No significant differences were observed at any analyzed developmental stage (Fig. 1a). Another considered landmark was the spontaneous chorion hatching. In this case, we observed an increase of hatching rate for prenatally stressed embryos (52.8 ± 4.1%) over the control (13.9 ± 2.7%) at 48 hpf (Fig. 1b). Finally, no significant differences were revealed between prenatally stressed 96 hpf larvae and control larvae under various morphological features such as body length, swim bladder inflation, and yolk sac size (Fig. 1c). Overall, these results demonstrated that cortisol treatment did not evidently affect embryonic development.
Fig. 1.
Effect of prenatal stress on different developmental landmarks. Percentage of survival (a) and hatching (b) determined at different developmental stages indicated as hours post-fertilization (hpf). c Lateral view of larvae at 96 hpf. CTRL, control embryos/larvae; PS, prenatally stressed embryos/larvae. Data are reported as mean ± SEM
Effect of prenatal stress on the transcript level of a stress-related gene
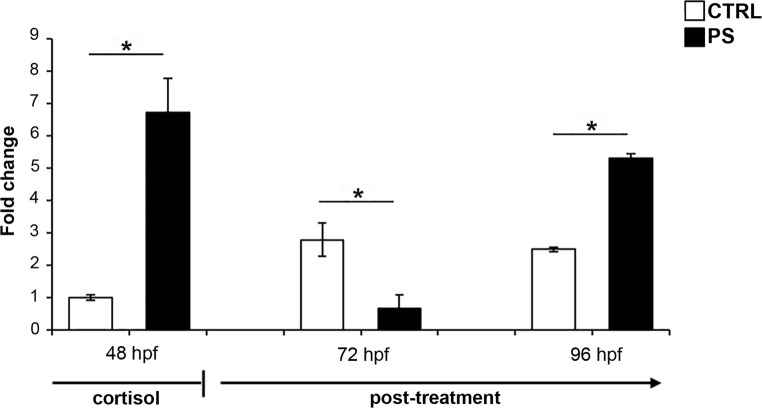
In order to evaluate the efficacy of cortisol treatment, we analyzed the transcript level of hsd11b2 gene. In fact, this gene encodes for a protein involved in the cortisol catabolism and it is known to be induced after cortisol challenge (Tokarz et al. 2013). We evaluated the transcript level by means of RT-qPCR at the end of cortisol treatment (48 hpf, hatching stage). As shown in Fig. 2, the mRNA level of prenatally stressed embryos resulted in 5.8-folds higher than control embryos at the end of cortisol treatment. Different transcript levels were also revealed after cortisol removal in the post-treatment period. In particular, we analyzed the hsd11b2 gene expression at two post-treatment time points. One of that represents the time point used by Steenbergen et al. (2011) to perform a behavioral test (96 hpf or 5 dpf). At this time point, we revealed a higher level of hsd11b2 mRNA in prenatally stressed larvae compared to control (Fig. 2). We also revealed a change in the expression pattern at 72 hpf, which represents a middle time point in the cortisol-free period, spanning between the end of cortisol treatment (48 hpf) and the 96 hpf stage. In particular, we observed a decrease of hsd11b2 mRNA in prenatally stressed larvae compared to control (Fig. 2).
Fig. 2.
mRNA expression pattern by means of RT-qPCR for hsd11b2 gene in prenatal stress condition. The analysis was carried out at different developmental stages indicated as hours post-fertilization (hpf). The mRNA level was reported as fold change by using actb2 as a reference gene and control 48 hpf embryos as a calibrator. CTRL, control embryos/larvae; PS, prenatally stressed embryo/larvae. Data are reported as mean ± SEM. *Significant differences as determined by REST2009 (P < 0.05)
Effect of prenatal stress on the transcript level of a neuronal activity marker gene
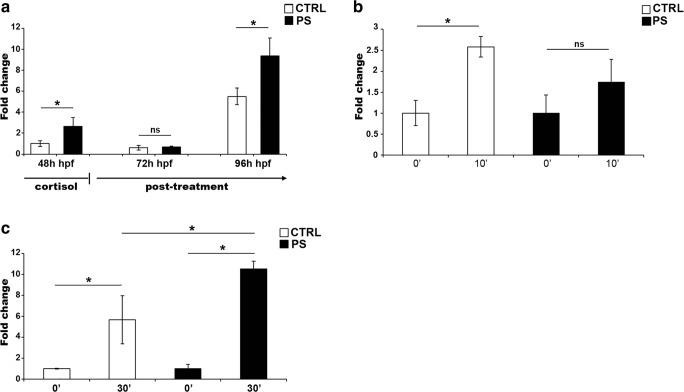
c-fos is considered an immediate early gene whose transcript level increases after neuronal activation (Stewart et al. 2014) and is often used as a mirror of brain activity. In fact, the analysis of c-fos mRNA level in zebrafish larvae under swirling stress condition revealed a significant increase of the transcript amount after 10 min following the end of the stress treatment (CTRL in the Fig. 3b). In order to assess if cortisol treatment affected c-fos expression pattern during zebrafish development, we performed RT-qPCR analysis at the same time points used for hsd11b2 gene. In prenatally stressed embryos, we found an altered c-fos mRNA level at the end of cortisol treatment, when higher transcript level was revealed compared to control embryos (Fig. 3a). In the post-treatment period, no difference was present at 72 hpf, whereas higher level in prenatally stressed larvae, as compared to controls, was detected at 96 hpf (Fig. 3a). The latter is particularly interesting since it highlighted a long-lasting effect of cortisol treatment on the expression of c-fos gene that might reflect a fine perturbation in the zebrafish brain development. In fact, when prenatally stressed larvae were subjected to swirling stress, we detected no significant changes in c-fos levels after swirling stress in larvae grown in cortisol excess (Fig. 3b). In order to confirm the different response observed between prenatally stressed and control larvae, we carried out an additional challenge paradigm, i.e., an osmotic stress experiment. As shown in Fig. 3c, the expression level of c-fos increased in control larvae as a consequence of the osmotic stress. In parallel, the prenatally stressed larvae showed an increased c-fos level significantly higher than the level detected in the control larvae, corroborating the idea of an affected ability of prenatally stressed larvae to properly respond the different challenges.
Fig. 3.
mRNA expression by means of RT-qPCR for the c-fos gene in response to prenatal stress and after swirling and osmotic stress challenges. a Prenatal stress condition. The analysis was carried out at different developmental stages indicated as hours post-fertilization (hpf). The mRNA level was reported as fold change by using actb2 as a reference gene and control 48 hpf embryos as a calibrator. b Swirling stress condition. The analysis was carried out on larvae at 5 dpf (99 hpf). Time after stressor was indicated as minutes (0′ and 10′). The mRNA level was reported as fold change by using actb2 as a reference gene and non-stressed larvae as a calibrator (indicated as 0′). c Osmotic stress condition. The analysis was carried out on larvae at 5 dpf (99 hpf). Time after stressor was indicated as minutes (30′), while 0′ represent control larvae maintained in only embryo medium and not submitted to osmotic stress. The mRNA level was reported as fold change by using actb2 as a reference gene and non-stressed larvae as a calibrator (indicated as 0′). Data are reported as mean ± SEM. *Significant differences as determined by REST2009 (P < 0.05)
Discussion
During the lifetime of an organism, cells forming tissues and organs can be affected by several types of intrinsic and extrinsic stresses (Alessio et al. 2018). Among these, prenatal stress refers to an adverse intrauterine environment that can exert profound rewriting of the developmental program of the fetus and that leads to different pathological conditions later in life. Maternal glucocorticoids excess can alter developmental trajectories, affecting normal development and function of various organs, the brain among them (Harris and Seckl 2011). The vast majority of information on prenatal stress effects has been acquired on mammalian organisms; in particular, rodent models, thanks to their strong similarity to humans at cellular and molecular levels, allowed to explore molecular mechanisms involved in gene expression regulation that may mediate the long-lasting effects of prenatal stress. In agreement with Steenbergen et al. (2011), we underline the need of a complementary and less complex model organism for those fields, such as stress studies, where the control of experimental conditions for rodent organisms could be very difficult. Complementary and less complex model organisms are useful tools to uncover different aspects of the molecular mechanisms underlying compromised (stress-exposed) brain development. Currently, zebrafish represents one of the most popular model organisms, which is employed in many research fields based on different characteristics. Our own experience showed a high degree of evolutionary conservation of gene expression pattern for relaxin ligand/receptor system between zebrafish and mammals, highlighting its suitability for the study of mechanisms underlying gene expression regulation during embryonic development (Donizetti et al. 2008; Donizetti et al. 2009; Donizetti et al. 2010; Fiengo et al. 2012; Fiengo et al. 2013; Donizetti et al. 2015a; Donizetti et al. 2015b; Venditti et al. 2018). In the case of the stress research field, a key feature is a large similarity between zebrafish and mammals in those neural anatomo-functional elements involved in stress response, among which a well-known is represented by HPA/HPI axis (Alsop and Vijayan 2008; Alsop and Vijayan 2009a; Alsop and Vijayan 2009b). In addition, in our laboratory, we characterized a new brain structure involved in stress response, the nucleus incertus, which has been hypothesized in zebrafish (Donizetti et al. 2008), corroborating the idea that it could be a powerful model in the stress research field. In the present paper, we investigated the effect of prenatal stress in zebrafish larvae by reproducing the experimental design reported in Steenbergen et al. (2011). In that article, the authors reported the simulation of prenatal stress by means of glucocorticoids excess exposure of fertilized eggs for the first 2 days of development, a period during which embryos are exclusively exposed to maternally derived stress hormones; interestingly, they showed that although prenatally stressed larvae had similar growth index and basal locomotor activity compared to control larvae, their responsiveness to a behavioral test was different with a significant blunt response for those specimens exposed to a high dose of glucocorticoids (Steenbergen et al. 2011). The analysis of vitality showed no significant differences between prenatally stressed and control embryos/larvae. On the contrary, prenatally stressed embryos showed a higher percentage of hatching at 48 hpf compared to control. Our results are in agreement with those obtained by Wilson et al. (2013), who showed an increased proportion of hatched embryos at 48 hpf when incubated in dexamethasone, likely because of different hatching gland maturation. This correspondence provided a preliminary evidence of the efficacy of the cortisol treatment. For what concerns morphological features, the 96 hpf prenatally stressed larvae appeared similar to the control larvae in terms of swim bladder inflation and body length, showing no difference in growth index as also reported by Steenbergen et al. (2011). We additionally assessed the size of the yolk sac and also in this case, no difference was revealed between 96 hpf prenatally stressed and control larvae. Despite those similarities between prenatally stressed and control embryos/larvae, a different scenario was revealed looking at gene expression pattern. We observed that significant differences are detected in the mRNA level of two genes, chosen based on their specific involvement in stress response. The first one is hsd11b2, a gene encoding for 11βHSD2, which catalyzes the oxidation of cortisol leading to its inactivation. This enzyme is likely involved in controlling the disposal of cortisol, whose level is regulated by a fine balance between biosynthesis and catabolism (Wilson et al. 2013; Alderman and Vijayan 2012; Tokarz et al. 2012, 2013). During zebrafish embryogenesis, hsd11b2 mRNA level increases significantly from 48 to 72 hpf (Wilson et al. 2013) and is upregulated by cortisol excess (Tokarz et al. 2013). Similarly, we found an increased level of hsd11b2 mRNA from 48 hpf in control embryos/larvae and an upregulation of gene expression in the prenatally stressed embryo at 48 hpf (Fig. 2). Tokartz et al. (2013) showed that after 24 h post-treatment in the 48 hpf embryos, the induction of hsd11b2 was even higher and decreased to normal level at 72 hpf, 48 h after the cortisol challenge. Interestingly, in our case, where the cortisol challenge was carried out for 48 h, the hsd11b2 mRNA level was lower in prenatally stressed larvae than controls at 72 hpf (24 h after cortisol removal) and resulted particularly dysregulated at 96 hpf (48 h after cortisol removal), when prenatally stressed larvae showed higher transcript level than the controls (Fig. 2). In zebrafish, the HPI axis can respond to stressor starting at 72/96 hpf, when osmotic stress (Alderman and Bernier 2009) or swirling stress (Alsop and Vijayan 2008) is able to induce cortisol increase. Until these time points, zebrafish is unable to increase cortisol level, suggesting for the existence of an analog of the stress-hyporesponsive period (SHRP) (Steenbergen et al. 2011). Our results showed a decreased expression level of hsd11b2 in prenatally stressed embryos/larvae at 72 hpf that might be explained by an alteration of the components of HPI axis affecting the time window of SHRP around the 72 hpf. In this regard, further experiments are needed to better investigate this hypothesis. For what concern the altered expression pattern at 96 hpf, we may consider it as a long-lasting effect on the hsd11b2 transcript level that further corroborated the efficacy of the cortisol treatment and showed that prenatal stress affected the development of zebrafish. To further investigate this perturbation, we extended our analysis to another gene, c-fos. It is an immediate early gene that is often used as a marker of neural activity; in fact, it is upregulated in different challenge conditions (Stewart et al. 2014). In this regard, the analysis of c-fos expression is particularly informative at 96 hpf, when zebrafish brain reaches a proper level of maturation and it can be used as a useful tool for various stress tests, so c-fos may represent a mirror of neuronal activity. In addition, we analyzed the expression of c-fos at the same time points used for hsd11b2 gene, in order to assess if cortisol treatment may affect its expression at early time points. The analysis of expression pattern in the prenatal stress paradigm revealed that embryos that were raised in cortisol excess for 48 h showed higher transcript level than the controls, whereas no difference was detected after 24 h of cortisol-free period (72 hpf), when we revealed a relatively low level of expression in both control and prenatally stressed larvae (Fig. 3a). After 48 h from cortisol challenge (96 hpf), an upregulated gene expression was evident in prenatally stressed larvae (96 hpf) (Fig. 3a). As aforementioned for hsd11b2 gene, the different expression patterns of c-fos observed at these different time points might be related to the development of the HPI axis and to the emergence time from SHRP. As expected, the c-fos level increased in response to swirling stress in normally grown control larvae after 10 min from the end of the challenge (Fig. 3b). On the contrary, this transcript increases resulted in blunted in prenatally stressed larvae (Fig. 3b). Interestingly, in the osmotic stress paradigm, we observed that control and prenatally stressed larvae responded to the challenge by increasing c-fos expression level (Fig. 3c). Worthy of note, the increased level of c-fos mRNA was significantly higher in prenatally stressed larvae than in control. This data represents a symptomatic effect that supports the idea of an altered ability of prenatally stress larvae to properly respond to stress challenges. Taken together, the analyses of hsd11b2 and c-fos gene expression pattern intriguingly showed that the prenatal stress condition altered their transcript levels during cortisol treatment, and that, in addition, this alteration is a long-lasting change still present in 5 dpf larvae. This developmental time is particularly interesting, since it is the time when the brain development reaches an enough grade of maturation to allow the zebrafish larvae to response to different environmental stimuli. In fact, at this developmental stage, when prenatally stressed larvae were subjected to swirling stress or osmotic stress, c-fos expression induction appeared different from that observed in control larvae. In conclusion, in this paper, we confirmed that hsd11b2 and c-fos gene expression is modulated in response to cortisol treatment, so we may hypothesize that they could be used as useful markers to study the effects of stress conditions. Moreover, we added further insights on the use of zebrafish as a model for prenatal stress studies.
Compliance with ethical standards
The animal protocol for this study was approved by the Animal Care Review Board of the University of Naples Federico II (PROT. 2014/0020464).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Serena D’Agostino, Email: serena.dagostino1@gmail.com.
Martino Testa, Email: martino.testa@yahoo.com.
Vincenza Aliperti, Email: vincenza.aliperti@unina.it.
Massimo Venditti, Email: massimo.venditti@unicampania.it.
Sergio Minucci, Email: sergio.minucci@unicampania.it.
Francesco Aniello, Email: faniello@unina.it.
Aldo Donizetti, Phone: +39081679082, Email: aldo.donizetti@unina.it.
References
- Alderman SL, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol. 2009;164:61–69. doi: 10.1016/j.ygcen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Alderman SL, Vijayan MM. 11β-Hydroxysteroid dehydrogenase type 2 in zebrafish brain: a functional role in hypothalamus-pituitary-interrenal axis regulation. J Endocrinol. 2012;215:393–402. doi: 10.1530/JOE-12-0379. [DOI] [PubMed] [Google Scholar]
- Alessio N, Squillaro T, Özcan S, Di Bernardo G, Venditti M, Melone M, Peluso G, Galderisi Stress and stem cells: adult Muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget. 2018;9(27):19328–19341. doi: 10.18632/oncotarget.25039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Phys Regul Integr Comp Phys. 2008;294:R711–R719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan M. The zebrafish stress axis: molecular fallout from the teleost-specific genome duplication event. Gen Comp Endocrinol. 2009;161:62–66. doi: 10.1016/j.ygcen.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Molecular programming of the corticosteroid stress axis during zebrafish development. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:49–54. doi: 10.1016/j.cbpa.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, babies and health in later life. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Best C, Kurrasch DM, Vijayan MM. Maternal cortisol stimulates neurogenesis and affects larval behaviour in zebrafish. Sci Rep. 2017;7:40905. doi: 10.1038/srep40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donizetti A, Grossi M, Pariante P, D’Aniello E, Izzo G, Minucci S, Aniello F. Two neuron clusters in the stem of postembryonic zebrafish brain specifically express relaxin-3 gene: first evidence of nucleus incertus in fish. Dev Dyn. 2008;237:3864–3869. doi: 10.1002/dvdy.21786. [DOI] [PubMed] [Google Scholar]
- Donizetti A, Fiengo M, Minucci S, Aniello F. Duplicated zebrafish relaxin-3 gene shows a different expression pattern from that of the co-orthologue gene. Develop Growth Differ. 2009;51(8):715–722. doi: 10.1111/j.1440-169X.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Donizetti A, Fiengo M, del Gaudio R, Di Giaimo R, Minucci S, Aniello F. Characterization and developmental expression pattern of the relaxin receptor rxfp1 gene in zebrafish. Develop Growth Differ. 2010;52:799–806. doi: 10.1111/j.1440-169X.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- Donizetti A, Fiengo M, Iazzetti G, del Gaudio R, Di Giaimo R, Pariante P, Minucci S, Aniello F. Expression analysis of five zebrafish RXFP3 homologues reveals evolutionary conservation of gene expression pattern. J Exp Zool B Mol Dev Evol. 2015;324:22–29. doi: 10.1002/jez.b.22591. [DOI] [PubMed] [Google Scholar]
- Donizetti A, Fiengo M, Del Gaudio R, Iazzetti G, Pariante P, Minucci S, Aniello F. Expression pattern of zebrafish rxfp2 homologue genes during embryonic development. J Exp Zool B Mol Dev Evol. 2015;324(7):605–613. doi: 10.1002/jez.b.22637. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond) 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Fiengo M, Donizetti A, del Gaudio R, Minucci S, Aniello F. Characterization, cDNA cloning and expression pattern of relaxin gene during embryogenesis of Danio rerio. Develop Growth Differ. 2012;54:579–587. doi: 10.1111/j.1440-169X.2012.01361.x. [DOI] [PubMed] [Google Scholar]
- Fiengo M, Del Gaudio R, Iazzetti G, Di Giaimo R, Minucci S, Aniello F, Donizetti A. Developmental expression pattern of two zebrafish rxfp3 paralogue genes. Develop Growth Differ. 2013;55:766–775. doi: 10.1111/dgd.12093. [DOI] [PubMed] [Google Scholar]
- Gudsnuk K, Champagne FA. Epigenetic influence of stress and the social environment. ILAR J. 2012;53:279–288. doi: 10.1093/ilar.53.3-4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol. 2012;24:1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36–e336 [DOI] [PMC free article] [PubMed]
- Schuurmans C, Kurrasch DM. Neurodevelopmental consequences of maternal distress: what do we really know? Clin Genet. 2013;83:108–117. doi: 10.1111/cge.12049. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Steenbergen PJ, Richardson MK, Champagne DL. The use of the zebrafish model in stress research. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:1432–1451. doi: 10.1016/j.pnpbp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 2014;37:264–278. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz J, Mindnich R, Norton W, Möller G, Hrabé de Angelis M, Adamski J. Discovery of a novel enzyme mediating glucocorticoid catabolism in fish: 20 beta-hydroxysteroid dehydrogenase type 2. Mol Cell Endocrinol. 2012;349:202–213. doi: 10.1016/j.mce.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Tokarz J, Norton W, Möller G, Hrabé de Angelis M, Adamski J. Zebrafish 20β-hydroxysteroid dehydrogenase type 2 is important for glucocorticoid catabolism in stress response. PLoS One. 2013;8:e54851. doi: 10.1371/journal.pone.0054851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti M, Donizetti A, Fiengo M, Fasano C, Santillo A, Aniello F, Minucci S. Temporal and spatial expression of insulin-like peptide (insl5a and insl5b) paralog genes during the embryogenesis of Danio rerio. J Exp Zool B Mol Dev Evol. 2018;330:33–40. doi: 10.1002/jez.b.22787. [DOI] [PubMed] [Google Scholar]
- Wilson KS, Matrone G, Livingstone DE, Al-Dujaili EA, Mullins JJ, Tucker CS, Hadoke PW, Kenyon CJ, Denvir MA. Physiological roles of glucocorticoids during early embryonic development of the zebrafish (Danio rerio) J Physiol. 2013;591:6209–6220. doi: 10.1113/jphysiol.2013.256826. [DOI] [PMC free article] [PubMed] [Google Scholar]