Abstract
Cardiac microvascular ischemia-reperfusion (IR) injury has been a neglected topic in recent decades. In the current study, we investigated the mechanism underlying microvascular IR injury, with a focus on mitochondrial homeostasis. We also explored the protective role of tanshinone IIA (Tan IIA) in microvascular protection in the context of IR injury. Through animal studies and cell experiments, we demonstrated that IR injury mediated microvascular wall destruction, lumen stenosis, perfusion defects, and cardiac microvascular endothelial cell (CMEC) apoptosis via inducing mitochondrial damage. In contrast, Tan IIA administration had the ability to sustain CMEC viability and microvascular homeostasis, finally attenuating microvascular IR injury. Function studies have confirmed that the SIRT1/PGC1α pathway is responsible for the microvascular protection from the Tan IIA treatment. SIRT1 activation by Tan IIA sustained the mitochondrial potential, alleviated the mitochondrial pro-apoptotic factor leakage, reduced the mPTP opening, and blocked mitochondrial apoptosis, providing a survival advantage for CMECs and preserving microvascular structure and function. By comparison, inhibiting SIRT1 abrogated the beneficial effects of Tan IIA on mitochondrial function, CMEC survival, and microvascular homeostasis. Collectively, this study indicated that Tan IIA should be considered a microvascular-protective drug that alleviates acute cardiac microcirculation IR injury via activating the SIRT1/PGC1α pathway and thereby blocking mitochondrial damage.
Keywords: Cardiac microvascular IR injury, CMEC, Tan IIA, SIRT1/PGC1α pathways, Mitochondrial apoptosis
Introduction
Although the incidence of acute myocardial infarction (AMI) has declined in recent years, AMI remains the leading cause of non-cancer-related deaths worldwide (Abukar et al. 2018; Ter Horst et al. 2018). Timely reperfusion therapy via opening blocked coronary arteries is the standard treatment for patients with AMI. Nevertheless, reperfusion treatment also causes additional damage to the heart, termed ischemia-reperfusion (IR) injury, which reduces the clinical benefits of reperfusion treatment for patients (Zhang et al. 2019). Over the past few decades, most studies have focused on the pathogenesis of cardiomyocyte IR injury and, thus, little attention has been paid to microvascular IR injury (Zhou et al. 2017d). In fact, based on several clinical and basic studies, microvascular IR injury occurs in 15~50% of patients during or after reperfusion treatment. More importantly, cardiac IR injury actually results from platelet microthrombosis and microcirculation perfusion defects despite successful revascularization of the occluded epicardial vessels (Bocci et al. 2019). In addition, the microvascular damage caused by endothelial cell apoptosis and capillary obstruction significantly reduces the effectiveness of reperfusion therapy and may compromise the clinical benefits in patients with AMI (Chrifi et al. 2019). Therefore, microvascular damage is upstream of cardiomyocyte damage (Aalto et al. 2019). At last, given the direct contact between the microvasculature and blood flow (Anderton et al. 2019), the cardiac microvasculature is actually more vulnerable than cardiomyocytes to IR injury. Accordingly, reducing microvascular IR injury is the key to increasing reperfusion treatment efficiency.
Tanshinone IIA (Tan IIA) is primarily isolated from the Chinese medicine Danshen. Several animal studies have found that Tan IIA increases the resistance of the myocardium to chronic hypoxia stress, alleviates cardiac hypertrophy, and attenuates acute myocardial ischemia-reperfusion injury (Xuan et al. 2017; Zhu et al. 2017). These studies illustrate that Tan IIA is important for protecting the myocardium against acute and chronic stress. However, the influences of Tan IIA on cardiac microvascular homeostasis and the underlying mechanisms of these effects remain unclear.
An increasing number of studies have suggested that mitochondrial damage is the primary pathogenesis for cardiac microvascular IR injury (Zhou et al. 2017a; Zhu et al. 2018). Mitochondrial apoptosis due to mitochondrial fission (Zhou et al. 2018a), mitophagy (Shi et al. 2018), or mitochondrial calcium overload (Zhu et al. 2018) induces cardiac microvascular endothelial cell (CMEC) apoptosis, leading to the collapse of microvascular structure and function (Zhou et al. 2017c). Therefore, a strategy for enhancing mitochondrial homeostasis is required. Based on the protective role of Tan IIA in the cardiovascular system, it is very important to determine whether Tan IIA has the ability to alleviate microvascular IR injury via sustaining mitochondrial homeostasis, and if so, what is molecular mechanism linking Tan IIA and mitochondrial protection in the context of cardiac IR injury.
Recently, silent information regulator 1 (SIRT1) was found to be associated with mitochondrial homeostasis and cardiac IR injury (Nopparat et al. 2017). SIRT1 can influence mitophagy activity, alleviate the myocardial inflammatory response, and promote cardiomyocyte survival. However, the role of SIRT1 in cardiac microvascular homeostasis remains unclear. In addition, whether Tan IIA controls cardiac microvascular IR injury via SIRT1 is incompletely understood. Therefore, the aim of this study is to explore the effect of Tan IIA on microvascular IR injury and the underlying mechanism involved in this effect, with a focus on mitochondrial homeostasis. Our data indicated that Tan IIA improved mitochondrial function, CMEC survival, and microvascular homeostasis under IR injury. Functional studies have demonstrated that SIRT1 and PGC1α were responsible for the protective role of Tan IIA in microvascular IR injury. SIRT1/PGC1α pathways activated by Tan IIA blocked mitochondrial apoptosis and sustained mitochondrial energy metabolism, favoring the pro-survival state that ultimately resulted in the inhibition of CMEC apoptosis and microvascular IR injury in cardiac IR injury. Therefore, our data identify the beneficial effects of Tan IIA on reperfused cardiac microvasculature via SIRT1-PGC1α signaling pathway.
Materials and methods
Animal treatment
The C57BL/6 background mice were purchased from Laboratory Animal Research Centre At Southern Medical University (Guangzhou, China). The above mice (12-week-old) were housed under standard laboratory conditions (27 °C, 40–60% humidity, a 12-h light and dark cycle) with fresh drinking water and a commercial pellet diet (Battistelli et al. 2019). Mice were intraperitoneally anesthetized with sodium pentobarbital (30 mg/kg) (Abeysuriya et al. 2018). The animals were subsequently incubated and ventilated with a volume-regulated respirator during surgery (Boga et al. 2018). The cardiac ischemia-reperfusion (IR) model was conducted via passing a 7-0 silk suture underneath the left anterior descending coronary artery with a knot for about 45 min according to a previous study (Zhou et al. 2018b). Subsequently, reperfusion was performed for about 4 h. The mice were randomly divided into the four groups, with n = 6 in each (no animals die before completion of testing): (i) Sham group, (ii) IR injury+PBS group, (iii) IR injury+low dose of Tan IIA (5 mg/kg body weight; standard analytical pure, cat. no. 568-72-9), and (iv) IR injury high dose of Tan IIA (25 mg/kg body weight) (Armartmuntree et al. 2018). To inhibit SIRT1 activation, selisistat (5 mg/kg body weight; Sigma-Aldrich; Merck KGaA) was administrated 4 h before IR injury. To activate SIRT1, SRT1720 (SRT; 1 mg/kg body weight; Sigma-Aldrich; Merck KGaA) was applied into mice 2 h before IR injury (Ba and Boldogh 2018). After cardiac IR injury, blood samples were obtained and the levels of lactate dehydrogenase (LDH) (Beyotime Institute of Biotechnology, Haimen, China; cat. no. A0133), troponin T (CUSABIO, Wuhan, China), and creatine kinase-MB (CK-MB) (CUSABIO, Wuhan, China) were examined via ELISA assay (Darden et al. 2019).
Cardiac microvascular endothelial cells isolation and hypoxia/reoxygenation (HR) model in vitro
In our study, 45-min hypoxia and 4-h reoxygenation model was applied to cardiac microvascular endothelial cells (CMECs) to mimic IR injury in vitro. CMECs were isolated from mice with trypsin and collagenase according to a previous report (Zhang et al. 2016). The CMECs (3.5 × 106 cells/well) were placed into a hypoxic incubator (95% N2 and 5% CO2) at 37 °C with fresh Hank’s solution (Beyotime Institute of Biotechnology) for 45 min (Zhou et al. 2018d). Then, CMECs were under normal culture condition for about 4 h to induce the reoxygenation injury (Zhou et al. 2018e). To activate the SIRT1 pathway, SRT1720 (SRT; 10 μM; Sigma-Aldrich; Merck KGaA) was treated for 4 h before HR injury. In contrast, to block SIRT1 pathway, selisistat (10 μM; Sigma-Aldrich; Merck KGaA) was used for 6 h before HR injury.
Sample preparation and histological analysis
The hearts were excised and rapidly frozen in optimal cutting temperature medium at room temperature (Agar Scientific Ltd., Stansted, UK) for the preparation of frozen sections (5-μm thickness). Hematoxylin-eosin (HE) staining was performed according to a previous study (Zhou et al. 2018f).
Immunofluorescence staining
The samples were first washed with cold PBS and then were permeabilized in 0.1% Triton X-100 for 10 min at 4 °C. Then, 10% goat serum albumin (Invitrogen, USA) was used to block the samples for 1 h at room temperature (Farber et al. 2019). Subsequently, samples were incubated with primary antibodies overnight at 4 °C. After three rinses in PBS, secondary antibodies were added to the samples for 1 h at room temperature (DeLeon-Pennell et al. 2018). The following primary antibodies were used in this study: Gr1 (1:1000, Abcam, #ab ab25377), troponin T (1:1000, Abcam, #ab ab8295), and Cyt-c (1:1000, Abcam, #ab ab133504). Images were observed with an inverted microscope (magnification, × 40; BX51; Olympus Corp., Tokyo, Japan) (Bittremieux et al. 2019).
BrdU assay
To evaluate cellular proliferation, the BrdU assay (RiboBio Co., Guangzhou, China) was used according to a previous study. First, cells were fixed via 4% paraformaldehyde at 4 °C, followed by permeabilization with 0.5% Triton X-100 for approximately 20 min at room temperature (Erland et al. 2018a). Subsequently, samples were incubated in 2N HCl solution for 30 min at 37 °C to unmask the antigen, followed by a neutralization step with 0.1 M sodium tetraborate. Then, the BrdU antibody (1:200; Abcam, #ab8152) was used to incubate with samples overnight (Erland et al. 2018b). After three rinses in PBS, secondary antibodies were added to the samples for 1 h at room temperature (Chandra et al. 2018). At last, the cells were stained with DAPI (Sigma-Aldrich) for 5 min to distinguish the nuclei (Cheignon et al. 2018). Subsequently, the samples were viewed under a fluorescence microscope. Photos were captured, and the number of BrdU positive cells was measured via counting at least three random separate fields (Chen et al. 2018).
Western blotting
Total protein (40–60 μg) was loaded onto a 12–15% SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane (Roche Applied Science, Penzberg, Germany) (Fukumoto et al. 2018). Bands were detected using an enhanced chemiluminescence substrate (Applygen Technologies, Inc., Beijing, China) (Edwards et al. 2018). Band intensities were normalized to the respective internal standard signal intensity (β-actin, 1:2000; Abcam; cat. no. ab8224). The experiment was repeated three times. The primaries antibodies used in our study were as follows: Complex III subunit core (CIII-core2, 1:1000, Invitrogen, #459220), complex II (CII-30, 1:1000, Abcam, #ab110410), complex IV subunit II (CIV-II, 1:1000, Abcam, #ab110268), SIRT1 (1:1000; Abcam; #ab121903) PGC1α (1:1000; Abcam; #aab176328), Bax (1:1000; Abcam; #ab32503), Bcl2 (1:1000, Cell Signaling Technology, #3498), Bad (1:1000; Abcam; #ab32455), caspase9 (1:1000, Cell Signaling Technology, #9504), x-IAP (1:1000; Abcam; #ab28151), survivin (1:1000, Cell Signaling Technology, #2808), p-eNOS (Ser1117) (1:1000, Abcam, #ab184154), and ET-1 (1:1000, Abcam, #ab2786) (Denton et al. 2019).
Mitochondrial ROS
To observe the mitochondrial ROS levels, the ROS probe (5 mg/ml, MitoSOX red mitochondrial superoxide indicator, Molecular Probes, USA) was incubated with the cells for approximately 30 min at 37 °C in the dark (Frank and Vince 2019). Subsequently, the cells were washed with PBS to remove the ROS probe (Fan et al. 2018). Then, the cells were immediately analyzed under a fluorescence microscope (Li et al. 2018c).
mPTP opening assay, JC-1 staining, and ATP production
mPTP opening is an early event in mitochondrial apoptosis. In our study, mPTP opening was measured via tetramethylrhodamine ethyl ester fluorescence (Zhou et al. 2018b). Samples were washed with PBS approximately three times and then were loaded with tetramethylrhodamine ethyl ester (Sajib et al. 2018). The baseline fluorescence of tetramethylrhodamine ethyl ester was recorded using the microplate reader set to a wavelength of 450 nm (Epoch 2; BioTek Instruments, Inc.) (Meyer and Leuschner 2018). After 30 min, the tetramethylrhodamine ethyl ester fluorescence was recorded again using the microplate reader set to a wavelength of 450 nm (Epoch 2; BioTek Instruments, Inc.) (Kim et al. 2019). According to a previous study, the mPTP opening rate was expressed as the ratio of 30-min fluorescence intensity to the baseline fluorescence intensity (Proietti et al. 2018). Mitochondrial potential was assessed using a JC-1 probe, a sensitive fluorescent dye used to detect alterations in mitochondrial potential. Following HR injury, cells were incubated with 10 mg/ml JC-1 for 10 min at 37 °C in the dark and monitored with a fluorescence microscope (magnification, × 100; BX51; Olympus Corp., Tokyo, Japan) (Li et al. 2018a). Red-orange fluorescence was attributable to potential-dependent dye aggregation in the mitochondria. Green fluorescence, reflecting the monomeric form of JC-1, appeared in the cytosol following mitochondrial membrane depolarization (Man et al. 2019). ATP production was detected to reflect mitochondrial function. First, the samples were washed with cold PBS three times (Shen et al. 2018). Then, the samples were lysed, and the luciferase-based ATP assay kit (Beyotime, China) was used. ATP production was measured via a microplate reader (Zhu et al. 2018).
Caspase-3/-9 activity detection
To analyze changes in caspase-3 and caspase-9, caspase-3/-9 activity kits (Beyotime Institute of Biotechnology, China) were used according to the manufacturer’s protocols (Mehra et al. 2018). To analyze caspase-3 activity, 5 μL of DEVD-p-NA substrate (4 mM, 200 μM final concentration) was added to the samples for 2 h at 37 °C (Tabish et al. 2018). To measure caspase-9 activity, 5 μl of LEHD-p-NA substrate (4 mM, 200 μM final concentration) was added to the samples for 1 h at 37 °C. Then, the wavelength at 400 nm was recorded via a microplate reader to reflect the caspase-3 and caspase-9 activities.
MTT and TUNEL assays
MTT experiments were performed in 96-well plates. Samples were washed 3 times with PBS, and 50 μl of MTT reagent was added to each well (Wei et al. 2018). The samples were subsequently incubated for 4 h at 37 °C in a humid atmosphere containing 5% CO2. The MTT solution was removed, 200 μl of dimethyl sulfoxide was added to each sample (Staudacher et al. 2018), and the samples were incubated for 10 min. Following the addition of Sorensen’s buffer (Ren et al. 2018), the absorbance at the wavelength of 570 nm was determined (Jeelani et al. 2018). To detect DNA fragmentation in the cell nuclei (a marker of apoptosis in testicular tissue) (Kazakov et al. 2018), a TUNEL assay was performed using an In Situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. DAPI was used to label the nuclei (at room temperature for approximately 30 min) (Lee et al. 2018).
RNA isolation and qPCR
Trizol reagent (Invitrogen, Carlsbad, CA) was used to isolate total RNA in CMEC (Montoya-Zegarra et al. 2019). Subsequently, the Reverse Transcription Kit (Eurogentec, Fremont, CA) was applied to transcribe RNA (1 μg in each group) into cDNA (Li et al. 2019). The qPCR was performed with primers and matched probes from the Universal Fluorescence-labeled Probe Library (Roche, Basel, Switzerland) (Nwadozi et al. 2019). The primers used in the present study were as follows: TNFα (forward, 5′-AGATGGAGCAACCTAAGGTC-3′; reverse, 5′-GCAGACCTCGCTGTTCTAGC-3′), IL6 (forward, 5′-CAGACTCGCGCCTCTAAGGAGT-3′; reverse, 5′-GATAGCCGATCCGTCGAA-3′), MCP1 (forward, 5′-GGATGGATTGCACAGCCATT-3′; reverse, 5′-GCGCCGACTCAGAGGTGT-3′), and MMP9 (forward, 5′-GAGAGACGTCTGGTAGATCG-3′; reverse, 5′-GTGCCAGCATGTGTCGTAGT-3′)(Li et al. 2018b).
Statistical analysis
All data are expressed as the means ± standard error of mean (SEM) at least 3 independent experiments. Statistical analyses were performed with SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). The results from more than two groups were evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Tan IIA sustains cardiac microvascular structure in the context of IR injury
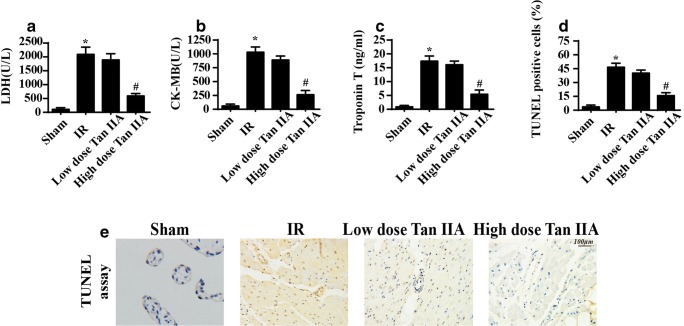
First, ischemia for 45 min and reperfusion for 4 h were used to establish the cardiac IR injury according to a previous study. Subsequently, the low and high dose of Tan IIA were administered 12 h before the IR injury. Then, cardiac damage markers were observed. The lactate dehydrogenase (LDH), troponin T, and creatine kinase-MB (CK-MB) levels were significantly increased in the reperfused hearts compared with the sham hearts (Fig. 1a–c), confirming the success of cardiac IR injury model. However, the Tan IIA treatment reduced the LDH, troponin T, and CK-MB content (Fig. 1a–c), confirming that Tan IIA alleviates myocardial damage under IR stress. Then, TUNEL assays were used to observe endothelial apoptosis. A greater number of TUNEL-positive endothelial cells were observed after IR injury (Fig. 1d, e), and this effect was reversed by Tan IIA treatment. Therefore, the above information indicated that Tan IIA sustained microvascular patency and reduced microvascular damage in the setting of cardiac IR injury.
Fig. 1.
Tan IIA sustained cardiac microvascular structure in the context of IR injury. a–c The cardiac damage markers were detected via ELISA assay. Tan IIA treatment attenuated the increased LDH, CK-MB, and troponin T levels induced by IR injury. d, e TUNEL assay was used to quantify the cellular apoptosis under cardiac IR injury. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group. The data represent the mean ± SEM. n = 6 mice per group
Tan IIA preserves microvascular function
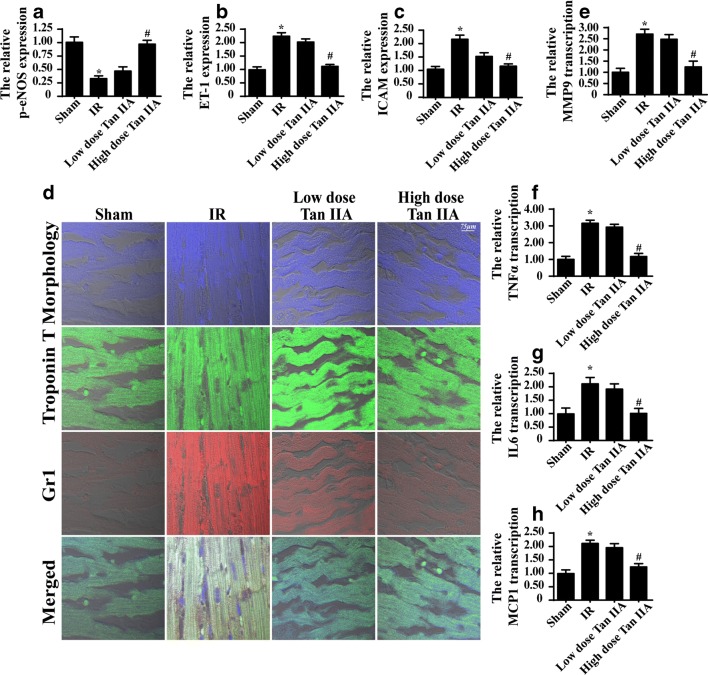
Alterations in microvascular structure disorder result in functional damage. The primary role of the microvasculature is to provide blood and energy to cardiomyocytes via eNOS-mediated vasodilation. Through western blot analysis, we found that IR injury significantly reduced p-eNOS expression and increased ET-1 expression (Fig. 2a–c), indicative of vasoconstriction. In contrast, Tan IIA treatment reversed the effect on p-eNOS expression and reduced the ET-1 level (Fig. 2a–c). In addition, IR injury also increased the expression of adhesion molecules, such as ICAM1, as determined via western blots (Fig. 2a–c). The higher expression of ICAM1 indicates an increased susceptibility for thrombus formation. In contrast, Tan IIA reduced ICAM1 expression.
Fig. 2.
Tan IIA maintained the microvascular function under IR injury. a–c The expression of p-eNOS and ET-1,ICAM1 was detected via western blots under IR injury with Tan IIA treatment or not. d Immunofluorescence assay of Gr1. IR injury induced more Gr1-positive inflammatory cells migration into the myocardial tissue, and this effect was inhibited by Tan IIA treatment. e–h The transcriptional alterations of inflammation factors. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group. The data represent the mean ± SEM. n = 6 mice per group
The microvasculature is the first barrier for limiting inflammatory cell permeation into myocardial tissue. Through immunofluorescence assays of Gr1 (a marker of inflammatory cells), we demonstrated that IR injury increased the immunosignals of Gr1 (Fig. 2d). However, this effect was inhibited by Tan IIA treatment. Furthermore, as a consequence of excessive inflammatory cell accumulation, IR injury increased the transcriptional expression of inflammatory factors, including MMP9, TNFα, IL6, and MCP1, as determined through qPCR assays (Fig. 2e–h). In contrast, Tan IIA treatment alleviated the inflammatory factor transcription levels (Fig. 2e–h). Therefore, our data revealed that Tan IIA administration sustained microvascular vasodilation and barrier function under IR injury.
Tan IIA alleviates CMEC apoptosis under IR injury
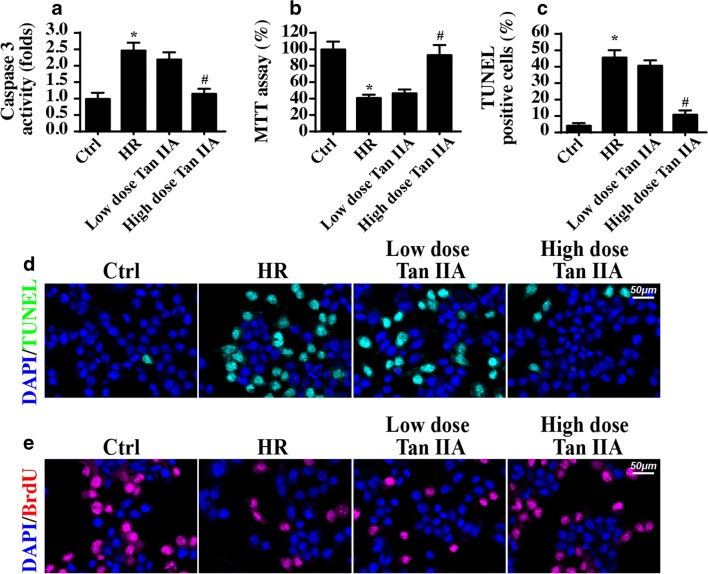
To explore the mechanism by which Tan IIA suppresses microvascular IR injury, CMECs were isolated from mice and exposed to 45 min of hypoxia and 4 h of reoxygenation (HR) with or without Tan IIA treatment in vitro. First, cell apoptosis and viability were detected via caspase-3 activity and MTT assay, respectively. As shown in Fig. 3 a and b, compared with the control group, HR injury reduced cellular viability and increased CMEC’s apoptosis rate, whereas the Tan IIA treatment promoted CMEC survival (Fig. 3a, b). Furthermore, the TUNEL assay was used to evaluate cell death in response to HR injury. Compared with the control group, the HR injury group presented with a greatly increased number of TUNEL-positive cells (Fig. 3c, d), and this effect was abolished by the Tan IIA treatment. Apart from cell death, we also evaluated CMEC proliferation, which is vital for post-infarction angiogenesis. Through the BrdU assay, we demonstrated that IR injury strongly attenuated the proliferative capacity of CMECs (Fig. 3e), and this effect was reversed by the Tan IIA treatment. Therefore, this information indicated that Tan IIA reduced the IR-mediated CMEC apoptosis and promoted CMEC proliferation.
Fig. 3.
Tan IIA protected the CMEC against the HR attack in vitro. a The cellular apoptosis was measured via caspase-3 activity. b The cellular viability was detected via MTT assay. c–d The TUNEL assay was used to observe the cellular apoptosis. e BrdU staining for the proliferated cells under HR treatment. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group. Experiments were repeated three times, and the data represent the mean ± SEM
Tan IIA attenuates IR injury–induced mitochondrial damage
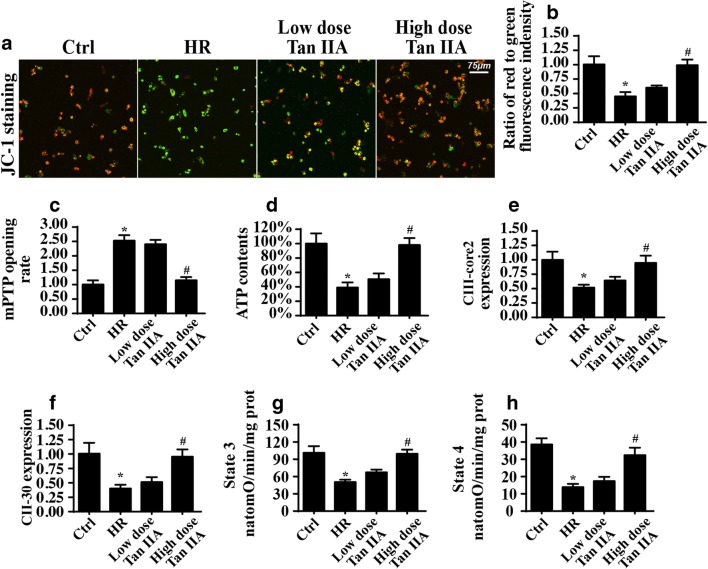
A previous study indicated that mitochondrial damage is the pathogenesis of microvascular IR injury (Zhou et al. 2017d). Thus, we observed mitochondrial function under IR injury. First, the HR treatment reduced the mitochondrial potential, as evidenced by the JC-1 assay (Fig. 4a, b). Normal mitochondria exhibited more red fluorescence and less green fluorescence, whereas HR-treated cells displayed more green fluorescence. In contrast, Tan IIA treatment reversed the mitochondrial potential (Fig. 4a, b). In addition, HR promoted mPTP opening (Fig. 4c), which is considered to be the initiating signal for mitochondrial apoptosis. However, this conformational alteration was reversed by the Tan IIA treatment (Fig. 4c). This information established that Tan IIA protects mitochondrial structure under IR injury.
Fig. 4.
Tan IIA alleviated the HR-mediated mitochondrial damage. a, b The mitochondrial potential was observed via JC-1 staining. The red fluorescence is indicative of the normal and healthy mitochondrial potential, whereas the green fluorescence suggests the damaged or collapsed mitochondrial potential. c The opening of mPTP. d The ATP production was detected and Tan IIA reversed the ATP levels in HR-treated cells. e, f The expression of mitochondrial respiratory complex via western blots assay. g, h Mitochondrial respiratory function was measured via state 3 and state 4 respiratory rate. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group. Experiments were repeated three times, and the data represent the mean ± SEM
Structural alterations are closely associated with functional changes. The primary function of mitochondria is ATP production. However, ATP production was substantially decreased in response to HR injury (Fig. 4d), whereas the Tan IIA treatment maintained the ATP level in CMECs under HR exposure. Furthermore, cellular ATP is mostly synthesized via the mitochondrial respiratory complex. Interestingly, HR stress largely abated the expression of the mitochondrial respiratory complex (Fig. 4e, f). This effect was strongly reversed by the Tan IIA treatment (Fig. 4e, f). Furthermore, due to the downregulation of mitochondrial respiratory complex expression, the mitochondrial state 3 and state 4 respiratory rates were severely perturbed by HR but were returned to normal levels following the Tan IIA incubation (Fig. 4g, h). These data indicated that the Tan IIA treatment robustly sustained the mitochondrial structure and function under HR stress.
Tan IIA inhibits mitochondria-mediated cellular apoptosis
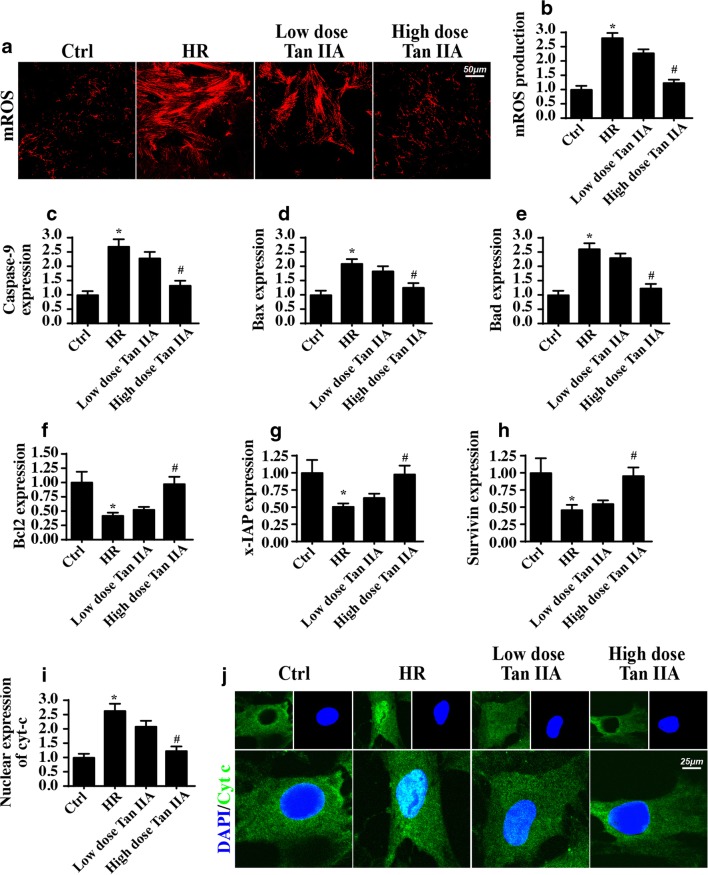
Mitochondrial damage is tightly linked to mitochondrial apoptosis that occurs, at least in part, through mitochondrial ROS release, mitochondrial pro-apoptotic factor leakage, and caspase family activation (Hu et al. 2017). Through mROS staining, we confirmed that HR stress increased mitochondrial ROS production (Fig. 5a, b). The mROS release was correlated with apoptotic signaling amplification, as evidenced by the increased expression of pro-apoptotic proteins such as caspase-9, Bax, and Bad (Fig. 5c–h). In contrast, the anti-apoptotic factors including Bcl2, survivin, and x-IAP were unfortunately repressed by HR stress (Fig. 5c–h), as determined via western blotting. In fact, caspase family activation is primarily derived from mitochondrial pro-apoptotic factor leakage (Zhou et al. 2018c). Considering this finding, immunofluorescence assays of cyt-c expression were performed to analyze mitochondrial cyt-c diffusion. Compared with the control group, the HR-treated group presented with more cyt-c release into the nucleus (Fig. 5i, j). Based on the above data, we confirmed that HR stress initiates mitochondrial apoptosis pathways in CMECs. Interestingly, the Tan IIA treatment reduced mROS production (Fig. 5a, b), inhibited caspase family activation (Fig. 5c–h), limited mitochondrial cyt-c leakage (Fig. 5i, j), favoring a pro-survival state that blocked mitochondrial apoptosis pathways in CMECs. This evidence explained the beneficial role of Tan IIA in mitochondrial homeostasis and suggested the ROS-clearing and anti-apoptotic effects of Tan IIA under cardiac microvascular IR injury.
Fig. 5.
Tan IIA blocked the mitochondrial apoptosis induced by HR stress. a, b The mROS production was detected via MitoSOX red mitochondrial superoxide indicator. c–h The mitochondrial apoptosis activation was confirmed via western blots analysis. i, j The cyt-c liberation from mitochondrion into the nuclear was identified via the co-staining of cyt-c and nuclear. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group. Experiments were repeated three times, and the data represent the mean ± SEM
Tan IIA protects CMEC viability and mitochondrial homeostasis via the SIRT1/PGC1α signaling pathway
To identify the signaling pathways responsible for the microvascular protection effect of Tan IIA, we focused on the role of SIRT1-PGC1α pathway since SIRT1-PGC1α pathway activation by several pharmacological approaches inhibits cellular apoptosis and preserves mitochondrial function in cardiovascular disorders. Through western blot analysis, we first demonstrated that SIRT1 and PGC1α expression levels were decreased in response to the HR injury, suggestive of SIRT1/PGC1α inactivation upon exposure to HR stress (Fig. 6a, b). In contrast, Tan IIA administration rescued the SIRT1 and PGC1α levels in CMECs exposed to HR (Fig. 6a, b).
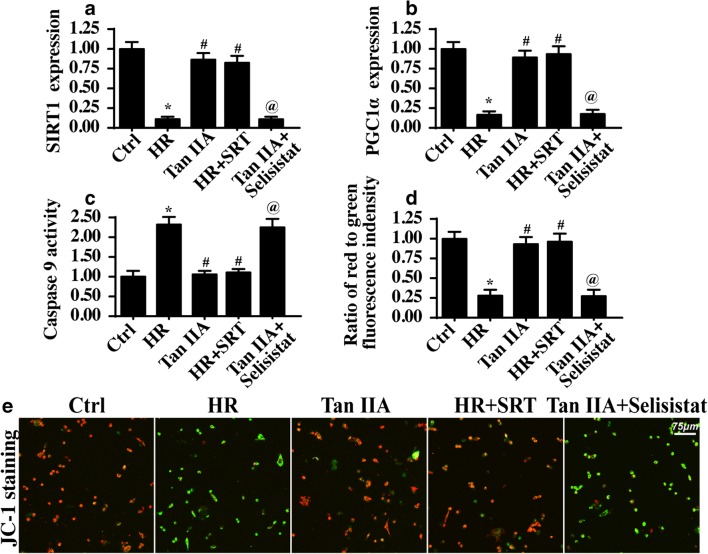
Fig. 6.
Tan IIA activated the SIRT1-PGC1α pathways to sustain the mitochondrial homeostasis and alleviate the damage to cardiac microvasculature under IR injury. a, b Western blot was used to detect the alterations about SIRT1 and PGC1α. c The caspase-9 activity was measured to reflect the mitochondrial apoptosis with SIRT1 activation or inhibition. d, e Mitochondrial potential was observed via JC-1 staining with SIRT1 activation or inhibition. *P < 0.05 vs. Ctrl group; #P < 0.05 vs. IR group; @P < 0.05 vs. Tan IIA group. Experiments were repeated three times, and the data represent the mean ± SEM
To determine the role of the SIRT1-PGC1α pathway in Tan IIA–mediated endothelial protection, SIRT1 loss- and gain-of-function assays were carried out. SRT (10 μM for 4 h), an activator of SIRT1, was applied to HR-treated cells to activate SIRT1 expression. In contrast, selisistat (10 μM for 6 h), a SIRT1 inhibitor, was used in Tan IIA–treated cells to inhibit SIRT1 expression. The activation and inhibition efficiencies were confirmed via western blotting, as shown in Fig. 6a–c. After SIRT1 reactivation in HR-treated cells, the activity of the mitochondrial apoptosis marker caspase-9 was significantly reduced compared with that of the HR group (Fig. 6c), suggesting that SIRT1 activation is vital for mitochondrial homeostasis. In contrast, in Tan IIA–treated cells with SIRT1 activity being inhibited, caspase-9 activity was re-elevated (Fig. 6c), suggesting that SIRT1 inhibition negates the protective effect of Tan IIA in mitochondrial apoptosis. Subsequently, mitochondrial potential assays via JC-1 staining also reconfirmed that SIRT1, activated by Tan IIA, is an endothelial-protective agent via maintaining mitochondrial function and reducing mitochondrial apoptosis (Fig. 6d, e).
Discussion
Although the reperfusion strategy is the standard treatment for AMI patients, reperfusion injury occurs in 15–50% of patients during and after revascularization. More importantly, IR injury accounts for the infarction area expansion and additional myocardial damage and is associated with increased mortality and readmission rate in patients with AMI (Zhang et al. 2016). The pathogenesis of cardiac IR injury involves microvascular perfusion defects due to the microthrombus formation and endothelial swelling/apoptosis (Jin et al. 2018; Zhou et al. 2017b), which eventually lead to a second ischemic attack for cardiomyocytes albeit successful epicardial vessel reperfusion. Therefore, regulating microvascular IR injury is vital for alleviating the incidence of IR injury (Zhou et al. 2017a). In the current study, we explored the role and mechanism of Tan IIA treatment in a murine model of microvascular IR injury. The results indicated that Tan IIA had the ability to protect cardiac microcirculation against IR injury. Mechanistically, Tan IIA activated the SIRT1-PGC1α signaling pathway to sustain mitochondrial homeostasis. Through blocking mitochondrial apoptosis and maintaining mitochondrial function in vitro, Tan IIA activated pro-survival signaling in CMECs and preserved the microvascular structure and function in the context of cardiac IR injury. As far as we are aware, this is the first study to show the beneficial role of Tan IIA in microvascular injury.
Tan IIA is primarily isolated from the root of the Chinese medicine Danshen. Compelling evidence has emerged to support the protective role of Tan IIA in cardiac IR injury. Tan IIA can activate the PI3K/Akt/mTOR signaling pathway to reduce cardiomyocyte death under IR injury. Moreover, Tan IIA has been reported to alleviate oxidative stress, inhibit excessive inflammatory responses (Feng et al. 2016), regulate calcium balance (Fan et al. 2011), and promote stem cell recruitment into infarcted myocardium (Tong et al. 2011). These findings indicate that Tan IIA protects reperfused hearts. However, no evidence is available to explain the role of Tan IIA in cardiac microvascular protection. The current study provides this answer. We confirmed that Tan IIA sustained microvascular perfusion, maintained the endothelial barrier, and reduced the inflammatory response in vivo. Cell experiments demonstrated that Tan IIA treatment was associated with improved mitochondrial function and increased CMEC survival. Therefore, this study suggests that the functions of Tan IIA extend beyond the capacity to reduce cardiomyocyte death and include the regulation of microvascular IR damage. Therefore, our data add more information to support the use of Tan IIA for treating cardiac IR injury, especially microvascular IR injury.
We confirmed that Tan IIA enhanced mitochondrial function and blocked mitochondrial apoptosis in CMECs under IR injury. These results were similar to a previous study that suggested mitochondria are the potential target of CMEC damage under IR stress (Zhu et al. 2018). Thoughtful studies from several researchers have confirmed that excessive mitochondrial fission (Zhou et al. 2017a), mitochondrial calcium (Zhang et al. 2016), mitochondrial oxidative stress, and fatal mitophagy (Zhou et al. 2017e) initiate caspase-9-mediated mitochondrial apoptosis pathways in the setting of cardiac IR injury. However, in the current study, we provided evidence to support the inhibitory role of Tan IIA in mitochondrial apoptosis. In addition, this beneficial effect of Tan IIA was dependent on SIRT1 activation. SIRT1, a kind of deacetylase, has been reported to modify the activity of pro-apoptotic factor p53 and abate that of the pro-inflammatory mediator NF-κB, inhibiting cellular apoptosis and tissue inflammation. In this study, we found that SIRT1 activation was related to CMEC survival and microvessel protection, identifying a novel role for SIRT1 in cardiac microvascular IR injury. Furthermore, we also found that SIRT1 activation was associated with PGC1α upregulation. PGC1α, the regulator of mitochondrial biogenesis, is vital for mitochondrial renewal. The increase in PGC1α expression may explain the possible protective role of SIRT1 in mitochondrial homeostasis. However, more studies are needed in the future to explain the mechanism by which PGC1α protects the mitochondria.
Altogether, our study mainly explored the role of Tan IIA in microvascular IR injury, a neglected topic in cardiac IR injury, for the first time. SIRT1-PGC1α signaling pathway activation by Tan IIA recused mitochondrial function and blocked CMEC apoptosis, preserving microvascular structure and function.
Funding information
This study was supported by the Natural Science Foundation of Guangdong Province of China (NO: 2018A030313067) , Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006;FS0AA-KJ218-1301-0010), Key Specialist Department Training Project of Foshan City, Guangdong Province of China ( NO: FSPY3-2015034).
Data availability
All data generated or analyzed during this study are included in this published article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiankai Zhong and Haichun Ouyang contributed equally to this work.
Contributor Information
Ying Tan, Email: tanying1115@163.com.
Yunzhao Hu, Email: sdhuyz@163.com.
References
- Aalto AL, Mohan AK, Schwintzer L, Kupka S, Kietz C, Walczak H, Broemer M, Meinander A. M1-linked ubiquitination by LUBEL is required for inflammatory responses to oral infection in Drosophila. Cell Death Differ. 2019;26:860–876. doi: 10.1038/s41418-018-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeysuriya RG, Lockley SW, Robinson PA, Postnova S. A unified model of melatonin, 6-sulfatoxymelatonin, and sleep dynamics. J Pineal Res. 2018;64:e12474. doi: 10.1111/jpi.12474. [DOI] [PubMed] [Google Scholar]
- Abukar Y, Ramchandra R, Hood SG, McKinley MJ, Booth LC, Yao ST, May CN. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol. 2018;113:35. doi: 10.1007/s00395-018-0695-9. [DOI] [PubMed] [Google Scholar]
- Anderton H, Bandala-Sanchez E, Simpson DS, Rickard JA, Ng AP, di Rago L, Hall C, Vince JE, Silke J, Liccardi G, Feltham R. RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development. Cell Death Differ. 2019;26:877–889. doi: 10.1038/s41418-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armartmuntree N, Murata M, Techasen A, Yongvanit P, Loilome W, Namwat N, Pairojkul C, Sakonsinsiri C, Pinlaor S, Thanan R. Prolonged oxidative stress down-regulates early B cell factor 1 with inhibition of its tumor suppressive function against cholangiocarcinoma genesis. Redox Biol. 2018;14:637–644. doi: 10.1016/j.redox.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase 1: beyond repair of the oxidatively modified base lesions. Redox Biol. 2018;14:669–678. doi: 10.1016/j.redox.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistelli C, Sabarese G, Santangelo L, Montaldo C, Gonzalez FJ, Tripodi M, Cicchini C. The lncRNA HOTAIR transcription is controlled by HNF4alpha-induced chromatin topology modulation. Cell Death Differ. 2019;26:890–901. doi: 10.1038/s41418-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittremieux M, la Rovere RM, Akl H, Martines C, Welkenhuyzen K, Dubron K, Baes M, Janssens A, Vandenberghe P, Laurenti L, Rietdorf K, Morciano G, Pinton P, Mikoshiba K, Bootman MD, Efremov DG, de Smedt H, Parys JB, Bultynck G. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ. 2019;26:531–547. doi: 10.1038/s41418-018-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci M, Sjölund J, Kurzejamska E, Lindgren D, Marzouka NAD, Bartoschek M, Höglund M, Pietras K. Activin receptor-like kinase 1 is associated with immune cell infiltration and regulates CLEC14A transcription in cancer. Angiogenesis. 2019;22:117–131. doi: 10.1007/s10456-018-9642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boga JA, Caballero B, Potes Y, Perez-Martinez Z, Reiter RJ, Vega-Naredo I, Coto-Montes A. Therapeutic potential of melatonin related to its role as an autophagy regulator: a review. J Pineal Res. 2018;66:e12534. doi: 10.1111/jpi.12534. [DOI] [PubMed] [Google Scholar]
- Chandra M, Escalante-Alcalde D, Bhuiyan MS, Orr AW, Kevil C, Morris AJ, Nam H, Dominic P, McCarthy KJ, Miriyala S, Panchatcharam M. Cardiac-specific inactivation of LPP3 in mice leads to myocardial dysfunction and heart failure. Redox Biol. 2018;14:261–271. doi: 10.1016/j.redox.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Dai SH, Li X, Luo P, Zhu J, Wang YH, Fei Z, Jiang XF. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018;14:229–236. doi: 10.1016/j.redox.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrifi I, Louzao-Martinez L, Brandt MM, van Dijk CGM, Bürgisser PE, Zhu C, Kros JM, Verhaar MC, Duncker DJ, Cheng C. CMTM4 regulates angiogenesis by promoting cell surface recycling of VE-cadherin to endothelial adherens junctions. Angiogenesis. 2019;22:75–93. doi: 10.1007/s10456-018-9638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden J, Payne LB, Zhao H, Chappell JC. Excess vascular endothelial growth factor-a disrupts pericyte recruitment during blood vessel formation. Angiogenesis. 2019;22:167–183. doi: 10.1007/s10456-018-9648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeon-Pennell KY, et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. 2018;113:40. doi: 10.1007/s00395-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Xu T, Dayan S, Nicolson S, Kumar S. Dpp regulates autophagy-dependent midgut removal and signals to block ecdysone production. Cell Death Differ. 2019;26:763–778. doi: 10.1038/s41418-018-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KS, Ashraf S, Lomax TM, Wiseman JM, Hall ME, Gava FN, Hall JE, Hosler JP, Harmancey R. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res Cardiol. 2018;113:47. doi: 10.1007/s00395-018-0707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erland LAE, Shukla MR, Singh AS, Murch SJ, Saxena PK (2018a) Melatonin and serotonin: mediators in the symphony of plant morphogenesis. J Pineal Res 64. 10.1111/jpi.12452 [DOI] [PubMed]
- Erland LAE, Yasunaga A, Li ITS, Murch SJ, Saxena PK. Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J Pineal Res. 2018;66:e12527. doi: 10.1111/jpi.12527. [DOI] [PubMed] [Google Scholar]
- Fan G, Zhu Y, Guo H, Wang X, Wang H, Gao X. Direct vasorelaxation by a novel phytoestrogen tanshinone IIA is mediated by nongenomic action of estrogen receptor through endothelial nitric oxide synthase activation and calcium mobilization. J Cardiovasc Pharmacol. 2011;57:340–347. doi: 10.1097/FJC.0b013e31820a0da1. [DOI] [PubMed] [Google Scholar]
- Fan T, Pi H, Li M, Ren Z, He Z, Zhu F, Tian L, Tu M, Xie J, Liu M, Li Y, Tan M, Li G, Qing W, Reiter RJ, Yu Z, Wu H, Zhou Z (2018) Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis in tongue squamous cell carcinoma. J Pineal Res 64. 10.1111/jpi.12457 [DOI] [PubMed]
- Farber G, Parks MM, Lustgarten Guahmich N, Zhang Y, Monette S, Blanchard SC, di Lorenzo A, Blobel CP. ADAM10 controls the differentiation of the coronary arterial endothelium. Angiogenesis. 2019;22:237–250. doi: 10.1007/s10456-018-9653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Li S, Chen H. Tanshinone IIA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: possible role of silent information regulator 1. Eur J Pharmacol. 2016;791:632–639. doi: 10.1016/j.ejphar.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto M, Kondo K, Uni K, Ishiguro T, Hayashi M, Ueda S, Mori I, Niimi K, Tashiro F, Miyazaki S, Miyazaki JI, Inagaki S, Furuyama T. Tip-cell behavior is regulated by transcription factor FoxO1 under hypoxic conditions in developing mouse retinas. Angiogenesis. 2018;21:203–214. doi: 10.1007/s10456-017-9588-z. [DOI] [PubMed] [Google Scholar]
- Hu S, Gao Y, Zhou H, Kong F, Xiao F, Zhou P, Chen Y. New insight into mitochondrial changes in vascular endothelial cells irradiated by gamma ray. Int J Radiat Biol. 2017;93:470–476. doi: 10.1080/09553002.2017.1286048. [DOI] [PubMed] [Google Scholar]
- Jeelani R, Maitra D, Chatzicharalampous C, Najeemuddin S, Morris RT, Abu-Soud HM. Melatonin prevents hypochlorous acid-mediated cyanocobalamin destruction and cyanogen chloride generation. J Pineal Res. 2018;64:e12463. doi: 10.1111/jpi.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakov A, Hall RA, Werner C, Meier T, Trouvain A, Rodionycheva S, Nickel A, Lammert F, Maack C, Böhm M, Laufs U. Raf kinase inhibitor protein mediates myocardial fibrosis under conditions of enhanced myocardial oxidative stress. Basic Res Cardiol. 2018;113:42. doi: 10.1007/s00395-018-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, Kurita T. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ. 2019;26:502–515. doi: 10.1038/s41418-018-0151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Hwang OJ, Reiter RJ, Back K. Flavonoids inhibit both rice and sheep serotonin N-acetyltransferases and reduce melatonin levels in plants. J Pineal Res. 2018;65:e12512. doi: 10.1111/jpi.12512. [DOI] [PubMed] [Google Scholar]
- Li D, Wang X, Huang Q, Li S, Zhou Y, Li Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-kappaB pathway in vivo and in vitro. Redox Biol. 2018;15:62–73. doi: 10.1016/j.redox.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cai SX, He Q, Zhang H, Friedberg D, Wang F, Redington AN. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res Cardiol. 2018;113:36. doi: 10.1007/s00395-018-0694-x. [DOI] [PubMed] [Google Scholar]
- Li R, Xin T, Li D, Wang C, Zhu H, Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, Li H, Zhang SR, Xu JZ, Qi ZH, Ni QX, Yu XJ, Liu L. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22:15–36. doi: 10.1007/s10456-018-9645-2. [DOI] [PubMed] [Google Scholar]
- Man S, Sanchez Duffhues G, Ten Dijke P, Baker D. The therapeutic potential of targeting the endothelial-to-mesenchymal transition. Angiogenesis. 2019;22:3–13. doi: 10.1007/s10456-018-9639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra P, Guo Y, Nong Y, Lorkiewicz P, Nasr M, Li Q, Muthusamy S, Bradley JA, Bhatnagar A, Wysoczynski M, Bolli R, Hill BG. Cardiac mesenchymal cells from diabetic mice are ineffective for cell therapy-mediated myocardial repair. Basic Res Cardiol. 2018;113:46. doi: 10.1007/s00395-018-0703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer IS, Leuschner F. The role of Wnt signaling in the healing myocardium: a focus on cell specificity. Basic Res Cardiol. 2018;113:44. doi: 10.1007/s00395-018-0705-y. [DOI] [PubMed] [Google Scholar]
- Montoya-Zegarra JA, Russo E, Runge P, Jadhav M, Willrodt AH, Stoma S, Nørrelykke SF, Detmar M, Halin C. AutoTube: a novel software for the automated morphometric analysis of vascular networks in tissues. Angiogenesis. 2019;22:223–236. doi: 10.1007/s10456-018-9652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopparat C, Sinjanakhom P, Govitrapong P (2017) Melatonin reverses H2 O2 -induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-kappaB. J Pineal Res 63. 10.1111/jpi.12407 [DOI] [PubMed]
- Nwadozi E, Ng A, Stromberg A, Liu HY, Olsson K, Gustafsson T, Haas TL. Leptin is a physiological regulator of skeletal muscle angiogenesis and is locally produced by PDGFRalpha and PDGFRbeta expressing perivascular cells. Angiogenesis. 2019;22:103–115. doi: 10.1007/s10456-018-9641-6. [DOI] [PubMed] [Google Scholar]
- Proietti S, Catizone A, Masiello MG, Dinicola S, Fabrizi G, Minini M, Ricci G, Verna R, Reiter RJ, Cucina A, Bizzarri M. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J Pineal Res. 2018;64:e12467. doi: 10.1111/jpi.12467. [DOI] [PubMed] [Google Scholar]
- Ren W, Wang P, Yan J, Liu G, Zeng B, Hussain T, Peng C, Yin J, Li T, Wei H, Zhu G, Reiter RJ, Tan B, Yin Y (2018) Melatonin alleviates weanling stress in mice: involvement of intestinal microbiota. J Pineal Res 64. 10.1111/jpi.12448 [DOI] [PubMed]
- Sajib S, Zahra FT, Lionakis MS, German NA, Mikelis CM. Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis. 2018;21:1–14. doi: 10.1007/s10456-017-9583-4. [DOI] [PubMed] [Google Scholar]
- Shen YQ, Guerra-Librero A, Fernandez-Gil BI, Florido J, García-López S, Martinez-Ruiz L, Mendivil-Perez M, Soto-Mercado V, Acuña-Castroviejo D, Ortega-Arellano H, Carriel V, Diaz-Casado ME, Reiter RJ, Rusanova I, Nieto A, López LC, Escames G (2018) Combination of melatonin and rapamycin for head and neck cancer therapy: suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J Pineal Res 64. 10.1111/jpi.12461 [DOI] [PubMed]
- Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018;14:59–71. doi: 10.1016/j.redox.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudacher V, Trujillo M, Diederichs T, Dick TP, Radi R, Morgan B, Deponte M. Redox-sensitive GFP fusions for monitoring the catalytic mechanism and inactivation of peroxiredoxins in living cells. Redox Biol. 2018;14:549–556. doi: 10.1016/j.redox.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabish TA, Zhang S, Winyard PG. Developing the next generation of graphene-based platforms for cancer therapeutics: the potential role of reactive oxygen species. Redox Biol. 2018;15:34–40. doi: 10.1016/j.redox.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst EN, et al. Elevated monocyte-specific type I interferon signalling correlates positively with cardiac healing in myocardial infarct patients but interferon alpha application deteriorates myocardial healing in rats. Basic Res Cardiol. 2018;114(1):1. doi: 10.1007/s00395-018-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Xu W, Han H, Chen Y, Yang J, Qiao H, Hong D, Wu Y, Zhou C. Tanshinone IIA increases recruitment of bone marrow mesenchymal stem cells to infarct region via up-regulating stromal cell-derived factor-1/CXC chemokine receptor 4 axis in a myocardial ischemia model. Phytomedicine. 2011;18:443–450. doi: 10.1016/j.phymed.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chang Y, Zeng H, Liu G, He C, Shi H (2018) RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J Pineal Res 64. 10.1111/jpi.12454 [DOI] [PubMed]
- Xuan Y, Gao Y, Huang H, Wang X, Cai Y, Luan QX. Tanshinone IIA attenuates atherosclerosis in apolipoprotein E knockout mice infected with Porphyromonas gingivalis. Inflammation. 2017;40:1631–1642. doi: 10.1007/s10753-017-0603-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jin B, Faber JE. Mouse models of Alzheimer’s disease cause rarefaction of pial collaterals and increased severity of ischemic stroke. Angiogenesis. 2019;22:263–279. doi: 10.1007/s10456-018-9655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F, Chen Y. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 2016;95:278–292. doi: 10.1016/j.freeradbiomed.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Zhou H, du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018a) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res 64. 10.1111/jpi.12450 [DOI] [PubMed]
- Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017a) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc 6. 10.1161/JAHA.116.005328 [DOI] [PMC free article] [PubMed]
- Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017b) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63. 10.1111/jpi.12438 [DOI] [PubMed]
- Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y. Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res. 2018;65:e12503. doi: 10.1111/jpi.12503. [DOI] [PubMed] [Google Scholar]
- Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018c) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res 64. 10.1111/jpi.12471 [DOI] [PubMed]
- Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21:599–615. doi: 10.1007/s10456-018-9611-z. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol. 2018;113:23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2017;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63:e12413. doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23:101–113. doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Xu Y, Ren G, Hu X, Wang C, Yang Z, Li Z, Mao W, Lu D. Tanshinone IIA sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol. 2017;815:427–436. doi: 10.1016/j.ejphar.2017.09.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.