Significance
Neuronal nitric oxide synthase (nNOS) is believed to be activated primarily by Ca2+/calmodulin. Activation of nNOS by phosphorylation similar to endothelial NOS has been suggested but is technically difficult to confirm in vivo. We developed a knock-in mouse with nNOS serine1412 mutated to alanine and show using mouse ileum that nNOS phosphoserine1412 regulates gastrointestinal smooth muscle relaxation and functional motility. nNOS is phosphorylated by the protein kinase Akt during submaximal neuronal depolarization to augment NO-cGMP signaling in a manner tuned toward resting intracellular Ca2+ levels. Phosphorylation at nNOS serine1412 represents a previously unrecognized physiologic mechanism of nNOS regulation linking neuronal depolarization to gastrointestinal motility and clarifies characteristics of neuronal NO production.
Keywords: enteric nervous system, gasotransmitter, nitric oxide (NO), ileum
Abstract
Nitric oxide (NO) is a major inhibitory neurotransmitter that mediates nonadrenergic noncholinergic (NANC) signaling. Neuronal NO synthase (nNOS) is activated by Ca2+/calmodulin to produce NO, which causes smooth muscle relaxation to regulate physiologic tone. nNOS serine1412 (S1412) phosphorylation may reduce the activating Ca2+ requirement and sustain NO production. We developed and characterized a nonphosphorylatable nNOSS1412A knock-in mouse and evaluated its enteric neurotransmission and gastrointestinal (GI) motility to understand the physiologic significance of nNOS S1412 phosphorylation. Electrical field stimulation (EFS) of wild-type (WT) mouse ileum induced nNOS S1412 phosphorylation that was blocked by tetrodotoxin and by inhibitors of the protein kinase Akt but not by PKA inhibitors. Low-frequency depolarization increased nNOS S1412 phosphorylation and relaxed WT ileum but only partially relaxed nNOSS1412A ileum. At higher frequencies, nNOS S1412 had no effect. nNOSS1412A ileum expressed less phosphodiesterase-5 and was more sensitive to relaxation by exogenous NO. Under non-NANC conditions, peristalsis and segmentation were faster in the nNOSS1412A ileum. Together these findings show that neuronal depolarization stimulates enteric nNOS phosphorylation by Akt to promote normal GI motility. Thus, phosphorylation of nNOS S1412 is a significant regulatory mechanism for nitrergic neurotransmission in the gut.
Nitric oxide (NO) is a major nonadrenergic noncholinergic (NANC) inhibitory signal in the peripheral nervous system, including the gastrointestinal (GI) tract. The enteric nervous system (ENS) produces NO that relaxes GI smooth muscle to regulate physiologic peristalsis (1–3). Neuronal NO synthase (nNOS) is the primary source of NO in the gut (4, 5), and altered NO production can disrupt normal GI motility. In mice, NO-cyclic guanosine- 3′,5′-monophosphate (NO-cGMP) pathway antagonists inhibit GI smooth muscle relaxation (2), and genetic deletion of nNOS delays bowel transit (5, 6). In humans, nNOS inhibition increases GI contractility (7), while increased nitrergic neurons are found in idiopathic chronic constipation and other motility disorders (8). Diabetic patients exhibit defective nitrergic GI relaxation (9), but enteric nNOS expression appears unchanged (10, 11). This suggests that both nNOS protein expression and posttranslational regulation influence GI motility.
Depolarization of nitrergic enteric neurons leads to voltage-dependent calcium (Ca2+) entry, activation of calmodulin (CaM), and increased NO production by nNOS. NO stimulates soluble guanylate cyclase (sGC) to produce cGMP, which activates protein kinase G (PKG) to promote smooth muscle relaxation (12, 13). Elevated neuronal intracellular [Ca2+] is therefore a major stimulus for nitrergic relaxation (14). Sustained high [Ca2+] can damage cells (15), so generally only brief depolarization transiently activates Ca2+ entry (16). Because GI myocyte relaxation is prolonged (>5 s) (17), nNOS posttranslational modification may sustain NO production independently of Ca2+ entry. Ca2+-independent activation of endothelial NOS (eNOS) by Akt or PKA phosphorylation of eNOS serine1179 is well established in response to vascular shear stress and receptor activation (18–20). Some evidence suggests that phosphorylation of the equivalent Akt/PKA consensus site in nNOS, serine1412 (S1412), also stimulates neuronal NO synthesis (21). Because nNOS activity is more sensitive to [Ca2+] (21), and nNOS is more rapidly dephosphorylated than eNOS (22), it is technically difficult to evaluate nNOS S1412 phosphorylation in vivo. Nonetheless, studies implicate nNOS S1412 phosphorylation in hippocampal excitotoxicity (4), penile erection (22), and luteinizing hormone release (23).
Enteric neurons express high levels of nNOS, Akt, and PKA (24, 25), indicating that nNOS S1412 phosphorylation may regulate GI neurotransmission. To test this, we performed organ bath experiments using mouse ileum and electrical field stimulation (EFS) and created a knock-in mouse in which nNOS S1412 is replaced by nonphosphorylatable alanine (nNOSS1412A). We found that S1412 phosphorylation facilitates GI relaxation during minimal neuronal depolarization. Our results suggest depolarization activates Akt to promote Ca2+-independent nNOS activation. These findings offer targets to treat GI motility disorders and are consistent with depolarization-dependent Akt stimulation of nNOS as a general NO signaling mechanism in the autonomic nervous system and brain.
Results
ENS Depolarization Stimulates Akt Phosphorylation of nNOS S1412.
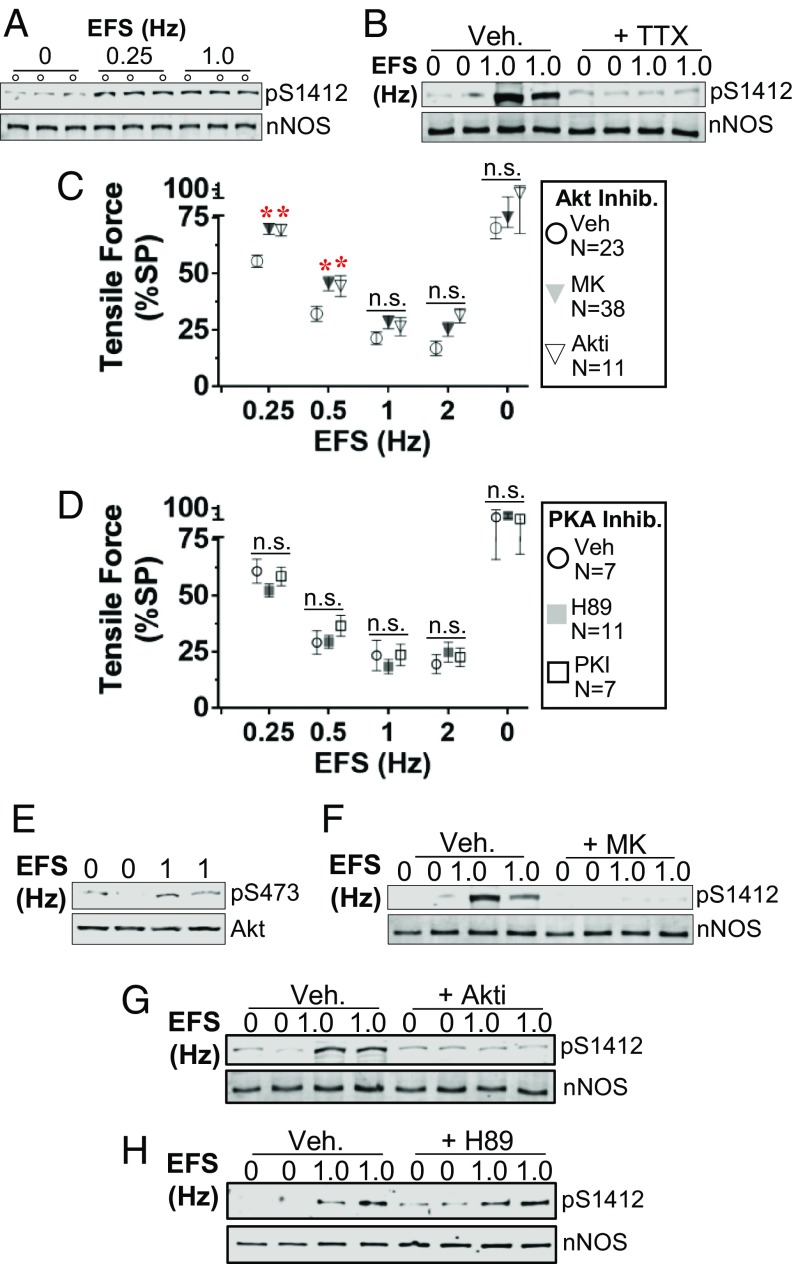
Glutamate stimulation of rat cortical neurons induces phosphorylation of nNOS S1412 by Akt (4), and direct electrical depolarization of rat pelvic ganglia increases nNOS S1412 phosphorylation by PKA (22). In vitro, the phosphomimetic mutant nNOSS1412D is more active than WT nNOS at low [Ca2+] typical of resting neurons (21). To test if depolarization increases nNOS phosphorylation in the ENS, we applied EFS to rings of wild-type (WT) mouse ileum suspended in physiologic organ bath under NANC conditions. Both low (0.25 Hz) and medium (1 Hz) frequency EFS increased S1412 phosphorylation by at least 2-fold compared to sham-treated unstimulated rings (Fig. 1A and SI Appendix, Fig. S1) (22). The pan-NOS inhibitor l-NAME blocked EFS-induced ileal relaxation (SI Appendix, Fig. S2), confirming that NOS was the predominant inhibitory mediator (4, 26). The voltage-gated Na+ channel antagonist tetrodotoxin (TTX), which blocks neuronal depolarization, also prevented EFS-stimulated nNOS S1412 phosphorylation (Fig. 1B) and ileal relaxation (SI Appendix, Fig. S2). These data suggest that neuronal depolarization can modulate nitrergic signaling via S1412 phosphorylation in the gut.
Fig. 1.
Neuronal depolarization stimulates nNOS S1412 phosphorylation by Akt in the ileum. (A and B) EFS promotes nNOS S1412 phosphorylation (pS1412), which is inhibited by TTX (10 μM). Veh: vehicle, 0.1% (vol/vol) DMSO. (C and D). Akt inhibitors (MK, 10 μM; Akti, 10 μM) suppress ileal relaxation at low EFS frequencies, but PKA inhibitors (H-89, 10 μM; PKI, 30 μM) do not. Error bars: SEM. N: ileal rings. *: P < 0.05 vs. Veh at each frequency by Dunnett test. n.s.: not significant. (E) EFS enhances Akt S473 phosphorylation (pS473). (F–H) Akt inhibition blocks EFS-induced S1412 phosphorylation, but PKA inhibition does not. A and B and F–H are blots of partially purified nNOS from pooled lysates of 4 to 8 ileal rings.
We next determined the role of Akt and PKA in mediating nNOS S1412 phosphorylation in the ileum during EFS stimulation by adding selective kinase inhibitors. Two Akt inhibitors (Akti and MK-2206 [MK]) significantly attenuated EFS relaxation at 0.25 and 0.5 Hz (Fig. 1C), suggesting that low-frequency depolarization promotes relaxation mediated by Akt. Activation of Akt by depolarization has been observed in cortical neurons, neuroblastoma cells, and cardiac myocytes (4, 27–29). In contrast to Akt inhibitors, the PKA inhibitors H89 and myristoylated PKA inhibitor peptide (PKI) had no effect on EFS responses (Fig. 1D). EFS stimulated Akt S473 phosphorylation by about 20 to 30% in total ileal lysates, an indication of increased Akt kinase activity (Fig. 1E and SI Appendix, Fig. S1). While Akt inhibitors blocked EFS-induced S1412 phosphorylation (Fig. 1 F and G), PKA inhibition by H89 did not (Fig. 1H). Thus, low-frequency neuronal depolarization stimulates Akt to phosphorylate S1412 and enhance nitrergic GI relaxation.
Mutating nNOS S1412 to Alanine Attenuates Akt-Dependent Nitrergic Relaxation.
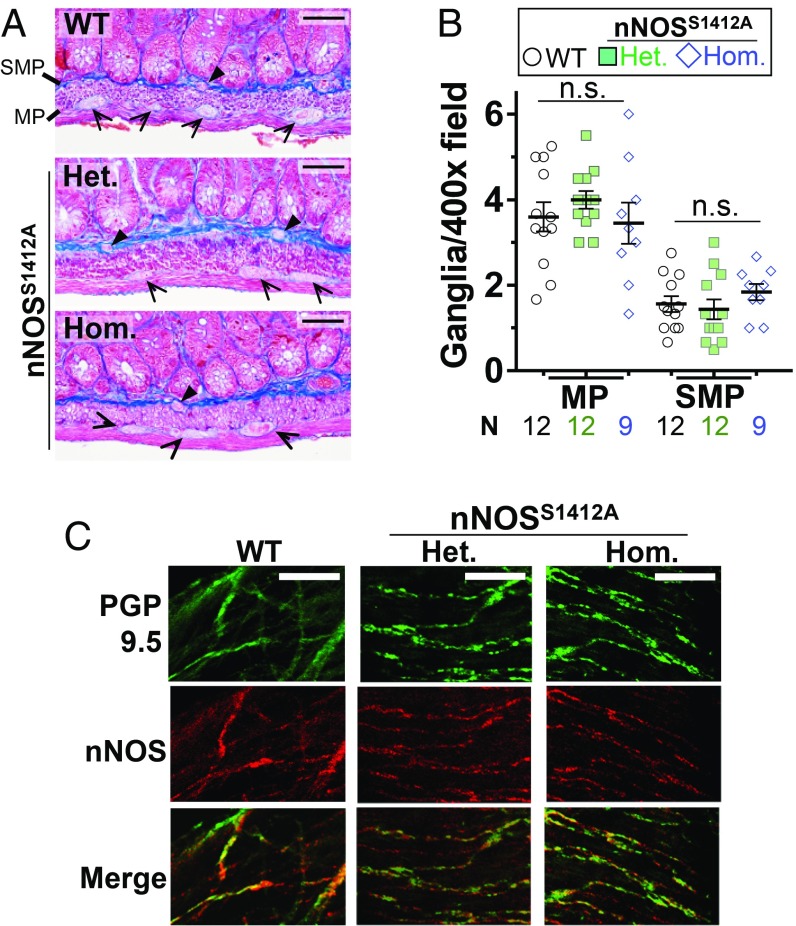
Because Akt inhibition blocks both nNOS S1412 phosphorylation and ileal relaxation during EFS, we hypothesized that S1412 phosphorylation is a physiologic mechanism by which depolarization sustains GI relaxation. To test this in detail, we created a knock-in mouse in which nNOS S1412 is replaced with alanine (nNOSS1412A), an amino acid that cannot be phosphorylated. We confirmed the mutation with mass spectrometry and in vitro kinase assays of purified nNOS from WT and nNOSS1412A heterozygous and homozygous mice (SI Appendix, Fig. S3). Mutant mice produced viable offspring, and male nNOSS1412A mice exhibited normal gross anatomy and organ histology (SI Appendix, Fig. S4 and Table S1). Because NO can influence ENS development (30), we quantified ganglia in ileal and colonic myenteric and submucosal plexi and found no difference between nNOSS1412A mutant and WT mice (Fig. 2 A and B and SI Appendix, Fig. S5). Similarly, we found no difference in the colocalization of nNOS and a neuronal marker, PGP9.5, in whole mounts of myenteric neuronal processes for WT and nNOSS1412A heterozygote or homozygote siblings (Fig. 2C and SI Appendix, Fig. S6).
Fig. 2.
nNOSS1412A mice exhibit normal ileal enteric nervous system organization. (A) The nNOSS1412A mutation does not change the number of ganglia in the ileal myenteric plexus (MP) or submucosal plexus (SMP). Representative trichrome-stained longitudinal sections are shown. Arrowheads: ganglia. (B) Quantification of A. (C) The nNOSS1412A mutation does not change expression of nNOS (red) with respect to the neuronal marker PGP9.5 (green). Representative whole mount neuronal processes are shown. Het: nNOSS1412A heterozygote. Hom: nNOSS1412A homozygote. n.s.: not significant by 1-way ANOVA (Scale bar, 50 µm.) N: number of 400× micrographs examined.
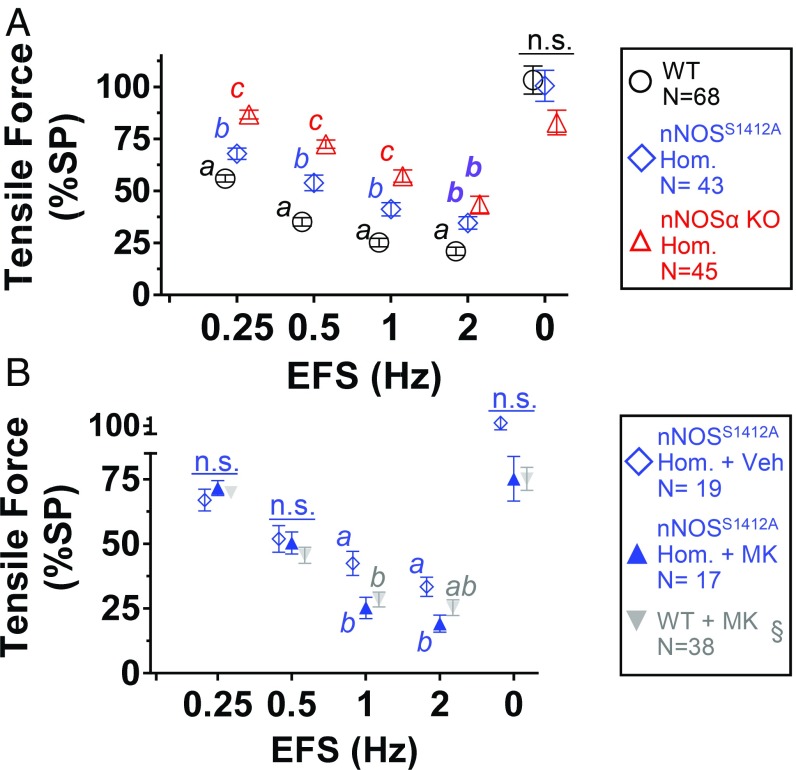
To determine the contribution of nNOS S1412 phosphorylation to inhibitory NANC neurotransmission, we tested EFS relaxation of ileal rings from WT, nNOSS1412A homozygote, and nNOS exon 2-null (nNOSα knockout [KO]) mice. nNOSα KO mice retain 5 to 10% of WT nNOS activity due to expression of N terminus truncated nNOS isoform β (5, 31). At frequencies below 2 Hz, EFS relaxed nNOSS1412A less than WT ileum, but more than nNOSα KO (Fig. 3A). Both l-NAME and TTX blocked EFS relaxation of nNOSS1412A ileum, and all 3 genotypes were indistinguishable at EFS above 2 Hz (SI Appendix, Fig. S7 and Fig. S8). These data demonstrate that low-frequency EFS-stimulated nitrergic relaxation requires nNOS S1412 phosphorylation. With stronger neuronal depolarization during high-frequency EFS, S1412 is not critical (including in nNOSα KO). To confirm that Akt phosphorylation of nNOS S1412 is the mechanism mediating low-frequency nitrergic relaxation, we applied EFS to ileal rings from nNOSS1412A mice in the presence of the Akt inhibitor MK. At 0.25 and 0.5 Hz, EFS relaxed MK- and vehicle-treated nNOSS1412A rings similarly (Fig. 3B). At 1 and 2 Hz, EFS relaxed MK-treated nNOSS1412A rings slightly more than vehicle-treated controls, but similarly to MK-treated WT rings (Fig. 3B). Consistent with our findings comparing WT ileal relaxation with and without Akt inhibitors (Fig. 1C), Akt appears to augment nitrergic relaxation only during low-frequency EFS.
Fig. 3.
Akt-dependent nitrergic relaxation requires nNOS S1412. (A) Lack of nNOSα (nNOSα KO) reduces EFS relaxation more than nNOSS1412A mutation. (B) Akt inhibition does not reduce EFS relaxation of nNOSS1412A. Different letters: P < 0.05 via Tukey test. Veh: 0.1% (vol/vol) DMSO. n.s.: not significant. N: ileal rings. §: Akt inhibitor data repeated from Fig. 1 for comparison.
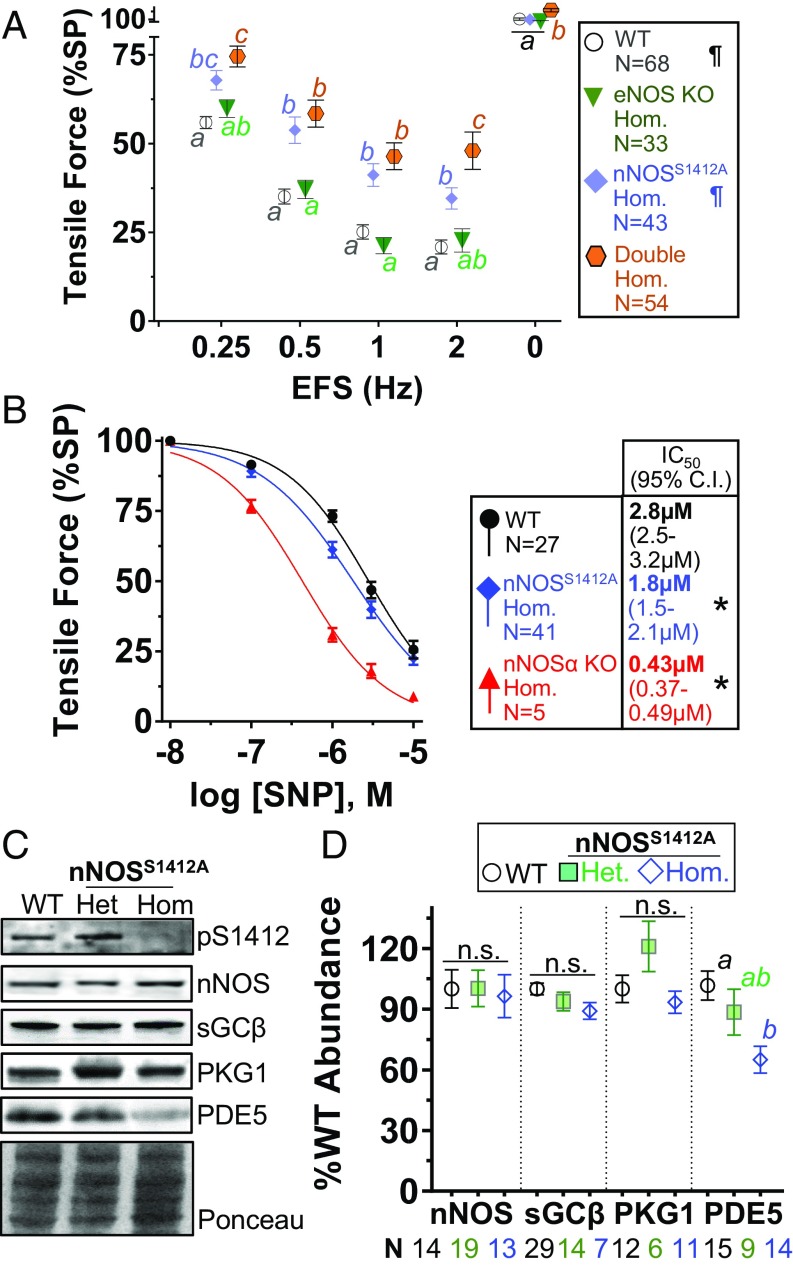
Because eNOS is abundant in enteric blood vessels and might contribute to NO production (32), we examined EFS relaxation in eNOS-null (eNOS KO) ileal rings and found the responses were identical to WT up to 2 Hz (Fig. 4A). Presumably, this is because nNOS expression is normal in the eNOS KO mouse, and EFS stimulation only activates neuronal NOS. We confirmed this using ileal rings from double mutant mice homozygous for eNOS KO and nNOSS1412A. At low-frequency EFS (0.25 to 1 Hz), the double mutant response was indistinguishable from homozygous nNOSS1412A ileum (Fig. 4A). Double mutant relaxation was less than nNOSS1412A at 2 Hz and higher frequency (SI Appendix, Fig. S8), suggesting that deletion of eNOS may increase the effect of nNOS S1412 phosphorylation on ileal relaxation. These data confirm that physiologic low-frequency stimulation of Akt-dependent nitrergic relaxation occurs only in enteric neurons and does not involve eNOS.
Fig. 4.
The nNOSS1412A mutation enhances classical NO-cGMP signaling. (A) Lack of eNOS (eNOS KO) does not alter low-frequency EFS relaxation of nNOSS1412A. (B) nNOSS1412A and nNOSα KO ilea are more sensitive than WT to SNP-induced relaxation. IC50 (μM SNP) and 95% confidence intervals are in symbol legend. (C) PDE5 is decreased in nNOSS1412A compared with WT ilea. Total nNOS, sGCβ, and PKG1 expression are unchanged. (D) Quantification of C. Different letters: P < 0.05 via Tukey test. ¶: Data repeated from Fig. 3 for comparison. Double Hom: eNOS KO nNOSS1412A double mutant homozygote. *: IC50 significantly different from WT. n.s.: not significant. N: ileal rings.
nNOSS1412A Mice Exhibit Enhanced NO-cGMP Signaling.
Because null mutation of nNOS or eNOS may induce compensatory changes in cGMP signaling (33), we explored whether the decreased EFS response in nNOSS1412A is due to lower NO sensitivity. When we added the exogenous NO donor sodium nitroprusside (SNP), nNOSS1412A ileal rings relaxed slightly more than WT rings. The IC50 for SNP with nNOSS1412A was 1.6-fold lower than WT but 4.2-fold higher than nNOSα KO (Fig. 4B). Thus, despite decreased relaxation with low-frequency EFS, both nNOSS1412A and nNOSα KO ilea respond more robustly to exogenous NO. Enhanced downstream NO-cGMP signal transduction or down-regulation of signal terminators in nNOSS1412A gut could explain this. When we quantified ileal expression of NO-cGMP pathway transducers in sibling-matched nNOSS1412A homozygotes, heterozygotes, and WT mice, we found similar nNOS, sGCβ, and PKG1 expression. However, nNOSS1412A homozygotes expressed significantly less of the cGMP catabolizing enzyme phosphodiesterase-5 (PDE5; Fig. 4 E and F). Reduced PDE5 activity prolongs cGMP signals, which augments the NO-cGMP pathway (33, 34).
nNOSS1412A Ilea Display Faster Spontaneous GI Motility.
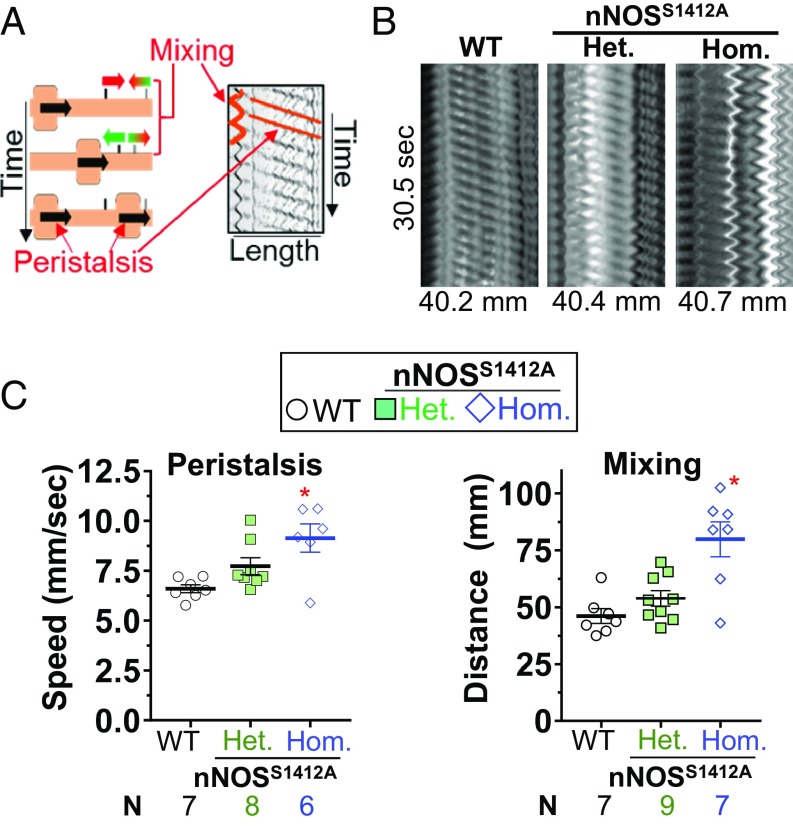
EFS experiments are performed under NANC conditions because depolarization releases other neurotransmitters such as acetylcholine and norepinephrine that can obscure nitrergic effects (35). Therefore, to study the influence of nNOS S1412 phosphorylation on physiologic GI motility, we determined ileal peristalsis and segmentation under non-NANC conditions using the Gastrointestinal Motility Monitor (GIMM) (36, 37). From spatiotemporal maps of spontaneously contracting ileum, we quantified propagating peristalsis and mixing segmentation for sibling-matched WT and nNOSS1412A homozygous or heterozygous mice (Fig. 5A). nNOSS1412A homozygotes had faster anterograde propagating peristalsis than WT (1.4-fold) and nNOSS1412A heterozygotes, and a smaller proportion of the nNOSS1412A ileum exhibited peristalsis (SI Appendix, Table S2). nNOSS1412A homozygotes also exhibited faster mixing (1.7-fold) than WT and nNOSS1412A heterozygotes (Fig. 5 B and C). We found no association between mouse age, weight, and propagation speed or mixing distance to explain these differences (SI Appendix, Fig. S9). These data show that nNOS S1412 is physiologically relevant for ENS control of GI motility.
Fig. 5.
nNOSS1412A ilea display faster gastrointestinal motility. (A) Ileal propagating-peristaltic and mixing-segmental contractions (Left), respectively, produce parallel lines and sinusoids in spatiotemporal maps (Right). (B) nNOSS1412A ilea exhibit higher propagation speeds and mixing distances. Representative spatiotemporal maps are shown. (C) Quantification of B. *: P < 0.05 vs. WT via Dunnett test. N: mice.
Discussion
The major finding of this study is that neuronal depolarization stimulates phosphorylation of nNOS S1412 to enhance nitrergic neurotransmission in the gut and regulate physiologic GI motility. Neurons are the relevant source of NO because TTX blocks nNOS S1412 phosphorylation and ileal relaxation, whereas eNOS ablation has no effect. EFS activates Akt, not PKA, to phosphorylate nNOS S1412. Cell type and stimulus likely determine which kinases phosphorylate nNOS S1412, as PKA (22) and AMPK (38) phosphorylate S1412 in pelvic ganglia and cardiomyocytes, respectively. The degree of depolarization and cytosolic [Ca2+] may determine the effect of nNOS S1412 phosphorylation in vivo because the difference in relaxation between WT and nNOSS1412A was prominent only with low-frequency EFS. While phosphorylation of S1179 increases eNOS activity over a broad [Ca2+] range (19), phosphorylation of nNOS S1412 increases activity only at lower [Ca2+] (21). Thus, S1412 phosphorylation may be a signal integrator for neuronal depolarization, Ca2+/CaM stimulation, and Akt activation that increases NO output only within a discrete lower range of intracellular [Ca2+] (Fig. 6). nNOS S1412 phosphorylation may also be a critical mechanism to sustain NO-cGMP signals longer than a brief neuronal depolarization.
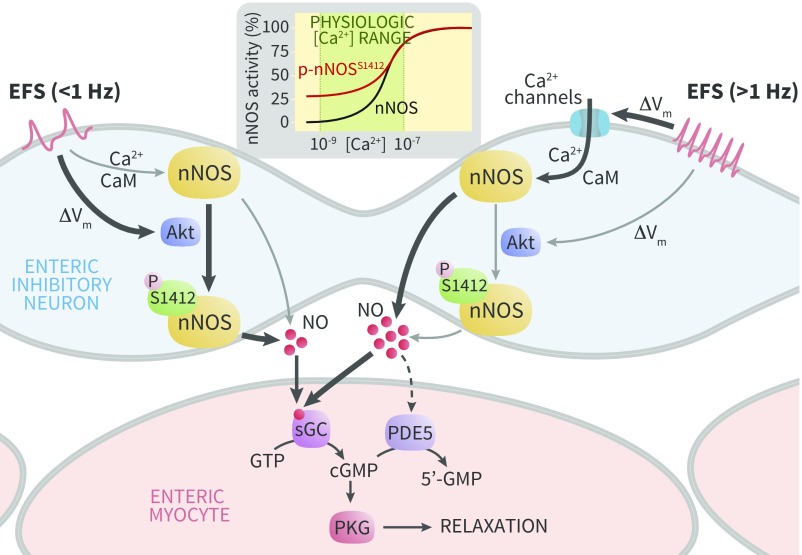
Fig. 6.
nNOS S1412 phosphorylation promotes enteric neuron NO signaling during low frequency depolarization. High frequency EFS (Right) promotes Ca2+ channel opening, which increases NO production via Ca2+/CaM dependent nNOS activation (thick arrows). Low frequency EFS (Left) slightly depolarizes nitrergic neurons, which activates Akt to phosphorylate nNOS S1412 and increase NO production (thick arrows). This pathway is independent of classical Ca2+/CaM activation. Regardless of nNOS stimulation, NO relaxes smooth muscle via a common final pathway in enteric myocytes by increasing cGMP synthesis. Through unknown mechanisms, NO bioavailability regulates PDE5 expression to modulate NO responses in smooth muscle cells. Thick or thin black pathway lines denote major and minor mechanisms of NO production. Dotted line represents unknown mechanisms.
The physiologic relevance of nNOS S1412 phosphorylation has been difficult to assess previously. Studies with saturating [Ca2+] (200 µM to 1 mM) concluded that S1412 phosphorylation does not alter nNOS activity (39). However, a phosphomimetic nNOSS1412D mutant is more active than WT nNOS at low total [Ca2+] (<10 μM) in vitro (21), and resting intracellular [Ca2+] is typically much lower (<100 nM). Using a knock-in mouse (nNOSS1412A) allowed us to determine if depolarization modulates nNOS activity via S1412 phosphorylation or Ca2+/CaM (14), exploring mechanisms reported for NMDA receptor (NMDAR) regulation of nNOS S1412 and S847 phosphorylation (4, 40). EFS frequency correlates with the extent of depolarization, with higher frequencies raising intracellular [Ca2+] (41). We show that Akt inhibition and nNOSS1412A mutation reduce NO signaling at low-frequency EFS when Ca2+ entry is low, but not at high-frequency EFS when Ca2+ entry is high. Therefore, nNOS S1412 phosphorylation may be relevant when neurons depolarize minimally or during metabotropic receptor-mediated Akt activation. The effect of Akt inhibition or nNOSS1412A mutation in our EFS experiments is modest, but there is clear physiologic influence on ileal motility in GIMM experiments. Low-frequency stimulation (0.25 and 0.5 Hz) induces nitrergic relaxation of gastric smooth muscle (2), and depolarization with glutamate or low-frequency EFS (0.5 Hz) activates Akt in cortical neurons and cardiomyocytes (4, 28). We found no direct studies of Akt activation by depolarization in enteric neurons, however, and the relevance of depolarization frequency for GI motility has not been reported. Recent work examining myenteric plexus Ca2+ waves suggests a wide depolarization frequency range (0.2 to 1.3 Hz) for nitrergic neurons that overlaps the 0.25 to 0.5 Hz EFS at which we saw Akt inhibitor effects (16). Importantly, our results show that Akt-dependent nNOS S1412 augmentation of nitrergic relaxation is detected only when intracellular [Ca2+] is expected to be low. At high-frequency depolarization and increased [Ca2+], our model supposes that the classical Ca2+/CaM mechanism activating nNOS is predominant (Fig. 6).
Our finding that nNOSα KO ileum relaxes during high-frequency EFS likely reflects activation of alternatively spliced nNOS isoforms (31). nNOSβ, expressed in nNOSα KO mice, lacks the PDZ domain and therefore is not localized to the plasma membrane where Akt is also activated. At high-frequency depolarization, [Ca2+] may rise distant from membrane channels and activate nNOSβ, causing attenuated but significant relaxation in nNOSα KO. Our data have implications for other physiologic systems in which nNOS S1412 phosphorylation could tune increased NO production selectively to low intracellular [Ca2+] and might explain inconsistent findings in other areas of neuronal NO biology such as long-term potentiation (LTP). NO mediates LTP in the rat hippocampus via both NMDARs and voltage-gated L-type Ca2+ channels (VGCCs). Low-frequency stimulation induces NMDAR LTP, while high frequency induces VGCC-dependent LTP (42, 43), and NMDAR and VGCC activation, respectively, promote and reduce nNOS S1412 phosphorylation (4). Our findings in the gut suggest that nNOS S1412 phosphorylation with low-frequency depolarization is physiologically relevant. Akt modulation of NO signaling has been proposed in other areas such as opioid receptor signaling in dorsal root ganglia (44), where depolarization-dependent nNOS activation may occur. Our observation that Akt inhibition enhances EFS relaxation at 1 to 2 Hz raises the possibility that other downstream signaling is altered in nNOSS1412A mice. Ca2+/CaM stimulates CaMKII, which can activate Akt (45) and also phosphorylate nNOS at S847 to decrease NO production (40). Akt activation or inhibition may also have different effects in different cell types. While deletion of the PI3K-Akt signal terminator PTEN in enteric neurons slows GI motility (46) consistent with our findings, manipulation of Akt within smooth muscle cells may produce different effects. Because we cannot apply pharmacologic inhibitors selectively during EFS, this is a technical limitation that might be addressed using cell-specific genetic manipulation. Interactions among nNOS posttranslational modifications require further investigation.
In addition to altered acute inhibitory responses during EFS, nNOS S1412 appears to regulate PDE5 expression (47). GI myocytes and interstitial cells of Cajal express PDE5 (47), and both cell types are critical for normal GI motility. PDE5 inhibitors alleviate esophageal achalasia symptoms (48), implicating NO mediators in GI dysmotility. Prior studies showed that conditions with NO deficiency cause compensatory PDE5 reduction. For example, penile corpus cavernosa of nNOSα KO and eNOS KO mice produce less NO and express less PDE5 than WT mice (33). A simple explanation is that less NO requires less PDE5, but no mechanism has been identified. Decreased PDE5 in the ileum of nNOSS1412A mice suggests that even slight changes in nitrergic output can alter the cGMP pathway. Lower PDE5 in nNOSS1412A mice also indicates that the reduced relaxation we observed during low-frequency EFS occurred despite decreased cGMP catabolism, and relaxation may have been more suppressed with WT levels of PDE5.
Our findings have implications for functional gastroenterology and the treatment of GI motility disorders. NO and other key NANC inhibitory transmitters (e.g., purines, vasoactive intestinal peptide) regulate peristalsis and segmentation (49). Functional constipation, small intestinal pseudoobstruction, and achalasia may exhibit abnormal nNOS signaling in humans (7, 8, 50), but the number of nitrergic neurons and expression of nNOS protein do not adequately explain GI motility dysfunction. For example, impaired nitrergic gastric relaxation in diabetes does not consistently correlate with nNOS expression (9–11). Animal models suggest that enteric nNOS activation depends on subcellular location and phosphorylation; mice lacking the nNOS plasma membrane-targeting PDZ domain (nNOSα KO) exhibit pyloric stenosis and slow colonic transit (5, 6), and phosphorylation of nNOS serine847 (S847) impairs enteric nNOS membrane association and reduces nNOS activation by Ca2+/CaM (39). We observed significantly impaired EFS relaxation of both the nNOSα KO and nNOSS1412A ileum, suggesting that nNOS expression and posttranslational activation are both important for GI neuromuscular signaling. Pharmacologic therapies that selectively alter nNOS S1412 phosphorylation in nitrergic neurons might alleviate some GI motility disorders.
nNOS S1412 phosphorylation may sustain NO synthesis beyond the brief depolarization of neurons (16, 17), but S1412 does not appear relevant at saturating [Ca2+] during high-frequency depolarization (51). nNOS S1412 tunes increased NO production to low resting [Ca2+], thereby integrating several cellular signals. These observations extend beyond GI physiology and indicate that nNOS S1412 phosphorylation may be an important nitrergic signaling mechanism.
Materials and Methods
See SI Appendix for detailed methods. The University of Colorado Institutional Animal Care and Use Committee approved all animal experiments (Protocol #90).
Organ Bath Studies.
We performed EFS (20 V, 2-ms pulse) with an S88 Grass stimulator on ileal rings mounted in an organ bath with oxygenated Krebs buffer under NANC conditions (1 μM atropine, 1 μM phentolamine, 1 μM propranolol, 10 μM indomethacin). We recorded and analyzed data with Powerlab and LabChart (AD Instruments). For Li-Cor Odyssey immunoblotting, we homogenized ileal rings in Tris-EDTA plus protease and phosphatase inhibitors and resolved lysates by SDS/PAGE as before (22). For nNOS and pS1412 blots, we partially purified crude lysates with 2′5′ ADP Sepharose (22).
Histology and Immunofluorescence.
We obtained whole mounts of ileal myenteric plexus and longitudinal muscle (52) and imaged whole mount and paraffin-embedded tissue with Olympus BH-2 and FV1000 microscopes.
Gastrointestinal Motility Monitor Experiments.
We quantified contraction speed and frequency of terminal ilea without NANC inhibitors in the GIMM (Catamount R&D) using ImageJ (36, 37).
Statistics.
We calculated statistics with GraphPad Prism and defined significance as P < 0.05. We evaluated EFS relaxation by 1-way ANOVA and then Dunnett or Tukey posttest. We calculated IC50 values by nonlinear regression to Hill equations. Experimental units (N) are given in figure legends.
Supplementary Material
Acknowledgments
We thank Crystal Woods and Jaime Belkind-Gerson for assistance with ileal whole mounts and GIMM experiments. We appreciate manuscript input from Thomas Jansson, Andrew Bradford, Nathen Bopp, and Diane Beckles. This work was supported by an NIH T32 training grant (5T32HD007186-37, D.D.G.) and a Society for Maternal Fetal Medicine/American Association of Obstetricians and Gynecologists Foundation Scholar Award (K.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905902116/-/DCSupplemental.
References
- 1.Ward S. M., et al. , NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am. J. Physiol. 263, G277–G284 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre R. A., Smits G. J., Timmermans J. P., Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br. J. Pharmacol. 116, 2017–2026 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah S., et al. , Gastrointestinal motility during pregnancy: Role of nitrergic component of NANC nerves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R1478–R1485 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Rameau G. A., et al. , Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J. Neurosci. 27, 3445–3455 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang P. L., Dawson T. M., Bredt D. S., Snyder S. H., Fishman M. C., Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273–1286 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Dickson E. J., Heredia D. J., McCann C. J., Hennig G. W., Smith T. K., The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G222–G232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo A., Fraser R., Adachi K., Horowitz M., Boeckxstaens G., Evidence that nitric oxide mechanisms regulate small intestinal motility in humans. Gut 44, 72–76 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortesini C., Cianchi F., Infantino A., Lise M., Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig. Dis. Sci. 40, 2450–2455 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Min Y. W., Ko E.-J., Lee J.-Y., Rhee P.-L., Impaired neural pathway in gastric muscles of patients with diabetes. Sci. Rep. 8, 7101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover M., et al. , Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 140, 1575–1585.e8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhury A., De Miranda-Neto M. H., Pereira R. V. F., Zanoni J. N., Myosin Va but not nNOSα is significantly reduced in jejunal musculomotor nerve terminals in diabetes mellitus. Front. Med. 1, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignarro L. J., Nitric oxide is not just blowing in the wind. Br. J. Pharmacol. 176, 131–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra D. D., Hurt K. J., Gasotransmitters in pregnancy: From conception to uterine involution. Biol. Reprod. 101, 4–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franck H., Sweeney K. M., Sanders K. M., Shuttleworth C. W., Effects of a novel guanylate cyclase inhibitor on nitric oxide-dependent inhibitory neurotransmission in canine proximal colon. Br. J. Pharmacol. 122, 1223–1229 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orrenius S., Gogvadze V., Zhivotovsky B., Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 460, 72–81 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Spencer N. J., et al. , Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. J. Neurosci. 38, 5507–5522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama H., Thapaliya S., Takewaki T., Cyclic GMP-associated apamin-sensitive nitrergic slow inhibitory junction potential in the hamster ileum. Br. J. Pharmacol. 128, 830–836 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boo Y. C., et al. , Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. J. Biol. Chem. 277, 3388–3396 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Chen C. A., Druhan L. J., Varadharaj S., Chen Y. R., Zweier J. L., Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J. Biol. Chem. 283, 27038–27047 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurt K. J., et al. , Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. U.S.A. 99, 4061–4066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adak S., et al. , Neuronal nitric-oxide synthase mutant (Ser-1412 –> Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. J. Biol. Chem. 276, 1244–1252 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Hurt K. J., et al. , Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. U.S.A. 109, 16624–16629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellefontaine N., et al. , Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Invest. 124, 2550–2559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anitha M., et al. , GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 116, 344–356 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe D. G., et al. , Inhibition of protein kinase A in murine enteric neurons causes lethal intestinal pseudo-obstruction. J. Neurobiol. 66, 256–272 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Shah S., Nathan L., Singh R., Fu Y. S., Chaudhuri G., E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: Implications in pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1546–R1554 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Curran J., et al. , Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS One 9, e87495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dedkova E. N., et al. , Signalling mechanisms in contraction-mediated stimulation of intracellular NO production in cat ventricular myocytes. J. Physiol. 580, 327–345 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y. I., et al. , Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3beta, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 280, 22044–22052 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Arab H. A., Muhammadnejad S., Faghihi S. M., Hassanpour H., Muhammadnejad A., Effects of nitric oxide modulating activities on development of enteric nervous system mediated gut motility in chick embryo model. J. Biosci. 39, 835–848 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Hurt K. J., et al. , Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. U.S.A. 103, 3440–3443 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yazji I., et al. , Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 9451–9456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champion H. C., Bivalacqua T. J., Takimoto E., Kass D. A., Burnett A. L., Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc. Natl. Acad. Sci. U.S.A. 102, 1661–1666 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagoda G., et al. , Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J. 28, 76–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekelund K. M., Ekblad E., Structural, neuronal, and functional adaptive changes in atrophic rat ileum. Gut 45, 236–245 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swaminathan M., et al. , Video imaging and spatiotemporal maps to analyze gastrointestinal motility in mice. J. Vis. Exp. 108, 53828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGill M., Klinger-Lawrence M., Herrera G., Disruption of serotonin signaling results in loss of coordinated tone development and subsequent inhibition of peristalsis in Guinea pig distal colon. FASEB J. 30, 1254.6 (2016). [Google Scholar]
- 38.Kar R., Kellogg D. L. 3rd, Roman L. J., Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochem. Biophys. Res. Commun. 459, 393–397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao Y. M., Chaudhury A., Goyal R. K., Active and inactive pools of nNOS in the nerve terminals in mouse gut: Implications for nitrergic neurotransmission. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G627–G634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rameau G. A., Chiu L. Y., Ziff E. B., Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J. Biol. Chem. 279, 14307–14314 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Oliveria S. F., Dittmer P. J., Youn D. H., Dell’Acqua M. L., Sather W. A., Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J. Neurosci. 32, 15328–15337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pigott B. M., Garthwaite J., Nitric oxide is required for L-type Ca(2+) channel-dependent long-term potentiation in the Hippocampus. Front. Synaptic Neurosci. 8, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavuş I., Teyler T., Two forms of long-term potentiation in area CA1 activate different signal transduction cascades. J. Neurophysiol. 76, 3038–3047 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Cunha T. M., et al. , Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 4442–4447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gocher A. M., et al. , Akt activation by Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J. Biol. Chem. 292, 14188–14204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puig I., et al. , Deletion of Pten in the mouse enteric nervous system induces ganglioneuromatosis and mimics intestinal pseudoobstruction. J. Clin. Invest. 119, 3586–3596 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groneberg D., Aue A., Lies B., Friebe A., Cell-specific modulation of gastrointestinal NO-induced relaxation by phosphodiesterases. BMC Pharmacol. Toxicol. 16 (suppl. 1), A56 (2015). [Google Scholar]
- 48.Bortolotti M., Mari C., Lopilato C., Porrazzo G., Miglioli M., Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology 118, 253–257 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Sanders K. M., Enteric inhibitory neurotransmission, starting down under. Adv. Exp. Med. Biol. 891, 21–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shteyer E., et al. , Truncating mutation in the nitric oxide synthase 1 gene is associated with infantile achalasia. Gastroenterology 148, 533–536.e4 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Zheng H., et al. , Generation and characterization of functional phosphoserine-incorporated neuronal nitric oxide synthase holoenzyme. J. Biol. Inorg. Chem. 24, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belkind-Gerson J., et al. , Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 7, 2525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.