Abstract
Climate change is one of the biggest and most urgent challenges for the 21st century. Rising average temperatures and ocean levels, altered precipitation patterns and increased occurrence of extreme weather events affect not only the global landscape and ecosystem, but also human health. Multiple environmental factors influence the onset and severity of human diseases and changing climate may have a great impact on these factors. Climate shifts disrupt the quantity and quality of water, increase environmental pollution, change the distribution of pathogens and severely impacts food production – all of which are important regarding public health. This paper focuses on brain health and provides an overview of climate change impacts on risk factors specific to brain diseases and disorders. We also discuss emerging hazards in brain health due to mitigation and adaptation strategies in response to climate changes.
Keywords: climate change, environment, health, brain disease
1. Climate change as a brain health concern.
There is a consensus in the science community that climate change is a major scientific and medical challenge for the 21st century (WHO, 2018). Jointly with other global environmental changes: ozone layer depletion, soil degradation, pollution, and urbanization, changing climate creates an undeniable threat to our planet and human health (Paris Agreement, 2015). Three major components define climate change – global warming, changes in precipitation patterns and increased occurrence in extreme weather events. Global warming is a result of the increasing concentration of greenhouse gases (CO2, CH4, N2O). Current average concentrations of atmospheric CO2 levels – above 400 parts per million (ppm) (IPCC, 2018) – have climbed from 280 ppm in the pre-industrial times, and are predicted to reach 1000 ppm by the end of this century (Kiehl, 2011). The global mean surface temperature for 2018 amounts approximately 1°C above the pre-industrial levels and is predicted to rise 2–4°C more by 2100 (IPCC, 2018). Changes in precipitation include increased rainfall at higher latitudes and decreased at lower latitudes (IPCC, 2018). Increased frequency and greater intensity of extreme weather events, heat waves, droughts, hurricanes, tropical storms or floods, occur worldwide (IPCC, 2018). In consequence, sea levels continue to rise (3–4 mm/year with significant local variation) and the oceans are becoming more acidic. Wildfires and land degradation are more frequent and promote the release of environmental contaminants as well as alterations of the farming systems. The changing weather throughout the globe may severely affect biological systems, causing the extinction of some animal species, or promoting the expansion of others (IPCC, 2018). Climate change-related economic collapses, forced migrations, armed conflicts, and other social disruptions impose additional threat (Burrows and Kinney, 2016; Mach et al., 2019). Some groups are particularly vulnerable to the changing climate – primarily populations from low- and middle-income countries, with poor health and safety regulations, lack of infrastructure and environmental protection (Daoud et al., 2016; Hallegatte and Rozenberg, 2017). Geographically, coastal and marine regions are more susceptible to damaging impacts of changing climate and natural disasters (Lu et al., 2018). Urban areas (Misslin et al., 2016; Zhang et al., 2019), and areas close to industrial plants (Azuma et al., 2014) are also more likely to be affected.
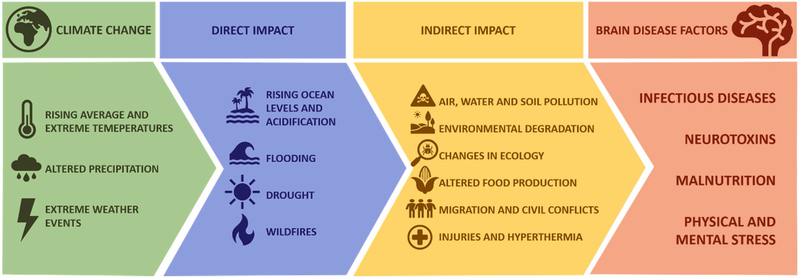
In light of the overwhelming evidence and broad scientific consensus of a changing climate, associated altered exposures to risk factors may affect human health, and thus also make it a public health concern (Kinney, 2018; Veenema et al., 2017). As numerous environmental factors are playing a role in the onset and severity of human diseases, understanding the modulatory effect of climate change is a priority. In this paper, we focus particularly on brain health. We provide an overview of the climate change impact on risk factors with implications for brain diseases, principally exposure to pathogens and hazardous pollutants, malnutrition, physical and psychological stress (Figure 1). We also discuss risks due to mitigation and adaptation activities in response to the changing climate.
Figure 1.
Direct and indirect consequences of global climate change contribute to increased occurrence of risk factors in brain disease.
2. Climate change-affected risk factors in brain disease.
2.1. Infectious diseases.
2.1.1. Vector-borne and zoonotic diseases.
Good examples of risks mediated by ecosystem changes are shifts in infectious diseases, particularly vector-borne and zoonotic diseases (VBZDs). The VBZDs ecology is complex and dependent upon multiple factors, including location, altitude, ecosystem, host, vector, weather and the climate. The VBZDs outbreaks are rising worldwide (e.g. avian influenza H5N1) and there is a strong evidence that the changing climate contributes to it (Canavan, 2019). Climate influences the occurrence, incubation period, survival, distribution, and transmission of pathogens and vectors. Changes in temperature, humidity or precipitation affect the VBZDs through host-vector interactions and through ecosystem changes. Climate shifts can also affect the epidemiological dynamics of the disease transmission indirectly, by changing social and cultural behaviors, as well as the economy (Caminade et al., 2019). Climate change is believed to promote the expansion of many tropical disease vectors in warming Europe (Semenza and Suk, 2018) and Northern America (Caminade et al., 2014). On the other hand, certain VBZDs may decrease in particular (warmer) regions, as habitats become less suitable for a host or vector survival and disease transmission (Cizauskas et al., 2017; Lafferty and Mordecai, 2016). The impact of climate change on various VBZDs transmission and epidemiology has been extensively studied and reviewed (Asad and Carpenter, 2018; Caminade et al., 2019; Campbell-Lendrum et al., 2015; Ebi and Nealon, 2016; Lafferty and Mordecai, 2016; Purse et al., 2017; Semenza and Suk, 2018). Herein, we focus on scenarios which contribute to climate-induced changes in geographic distribution and epidemiology of the VBZDs affecting the central nervous system (CNS). The pathogens may target the brain specifically, e.g. Japanese encephalitis and neuroborreliosis, or neurological outcomes may be secondary to the general infection, such as in malaria or yellow fever.
Aedes spp. mosquito is an example of a widespread tropical disease vector currently on a rise (Caminade et al., 2014). This is the major host for infectious arboviruses causing Dengue, Zika, Chikungunya, West Nile, and Yellow Fever (Kleinschmidt-DeMasters and Beckham, 2015). Mosquitoes reproduce and feed more frequently in higher temperatures (Carrington et al., 2013; Yang and Sarfaty, 2016), and with increasing global temperatures, the distribution of the Aedes spp. has drastically increased over the past few decades. Future predictions indicate further growth in Europe and North America, but local reductions in Southeast Asia and West Africa (Ebi and Nealon, 2016; Ryan et al., 2019). Dengue is currently the fastest spreading tropical infection in the world (Messina et al., 2019; Stanaway et al., 2016), exhibiting certain neurological outcomes in up to 20% of cases, mostly encephalitis and encephalopathy (Li et al., 2017). Both dengue virus and its vector (Aedes spp.) are sensitive to changing climate condition, as reviewed in (Ebi and Nealon, 2016; Li et al., 2018). Chikungunya occasionally affects the brain (Mehta et al., 2018) and its epidemiology is closely tied with weather patterns and climate change, as reviewed in (Meason and Paterson, 2014; Tjaden et al., 2017). Yellow fever can also lead to fatal encephalitis associated with acute inflammation and widespread neuronal damage (Almeida Bentes et al., 2019). West Nile virus is an important emerging neurotropic virus responsible for severe encephalitis outbreaks in humans and horses worldwide (Suen et al., 2014) – this neuroinvasive infection is a serious threat particularly to infants, elderly and immunocompromised populations (Kleinschmidt-DeMasters and Beckham, 2015). Zika virus has attracted considerable attention recently for its potential to cause microcephaly, cortical thinning and blindness during early development, while meningoencephalitis and Guillain-Barre syndrome in adults (Araujo et al., 2016; Russo and Beltrao-Braga, 2017). The effect of climate changes on the dynamically changing Zika epidemiology has been recently recognized and discussed (Asad and Carpenter, 2018; Depoux et al., 2018). Japanese encephalitis virus belongs to arboviruses transmitted by Aedes spp., but its primary host is another mosquito type, Culex spp. inhabiting Southeast Asia and the Western Pacific. The virus causes a severe infection of the brain, with about 68,000 symptomatic cases and 17,000 deaths per year (WHO). Weather conditions, particularly floods, were associated with an increased number of disease cases in China (Zhang et al., 2016).
Anopheles mosquitos transmit a protozoan parasite Plasmodium falciparum – the major cause of malaria in humans. This tropical infection kills approximately (approx.) one million people per year, mostly due to coma – cerebral malaria (CM). Survivors of the CM exhibit severe neurological deficits like epilepsy, cognitive impairment, and behavioral disorders, such as attencion deficit, hyperactivity and aggressive behavior, particularly common in young individuals (Hora et al., 2016; Postels and Birbeck, 2013). The impact of changing climate has been addressed in numerous recent studies and findings suggest a significant effect on malaria distribution and epidemiology (Boyce et al., 2016; Caminade et al., 2014; Dasgupta, 2018; Eikenberry and Gumel, 2018; Ivanescu et al., 2016; Leedale et al., 2016; Onyango et al., 2016).
In addition to mosquitos, other vectors transmitting neurological diseases are affected by climate and weather. Geographic distribution of Ixodes Ricinus, a species of hard tick that transmits several important brain diseases in Europe and North Africa is influenced by climate change (Alkishe et al., 2017; Jore et al., 2014; Ostfeld and Brunner, 2015). Transmission by the tick lyme neuroborreliosis is caused by bacteria Borrelia burgdorferi, and manifests as lymphocytic meningoradiculitis (Bannwarth syndrome) (Garkowski et al., 2017). Tick-borne encephalitis (TBE) is a serious neuroinfection caused by a flavivirus. The disease is seasonal, dependent on the host-seeking activity of nymphs, and increased risk of the TBE has been linked to increasing temperatures (Daniel et al., 2018). Changing climate has been suggested to affect transmission and epidemiology of many other VBZDs exhibiting neurological outcomes: cerebrospinal bacterial meningitis (Codjoe and Nabie, 2014), tuberculosis (Sergi et al., 2019), syphilis (Marinho de Souza et al., 2019), cerebral schistosomiasis (McCreesh et al., 2015; Yang and Bergquist, 2018; Zhu et al., 2017), leishmaniasis (Azimi et al., 2017; Mendes et al., 2016; Purse et al., 2017; Ready, 2008), Chagas disease (Carmona-Castro et al., 2018; Garza et al., 2014), strongyloidiasis (Beknazarova et al., 2016; McMahon et al., 2012), toxoplasmosis (Yan et al., 2016), neurocysticercosis caused by soil-transmitted helminthiases (Weaver et al., 2010), and neurological diseases associated with rabies (Hayes and Piaggio, 2018), or human immunodeficiency virus (HIV) infections (Low et al., 2019).
2.1.2. Water-borne diseases.
Water-borne diseases (WBDs) are infectious diseases caused by a wide variety of pathogens transmitted through water and exhibiting strong dependence on climate and meteorological conditions, such as heavy rainfall, flooding, and other extreme events promoting the pathogen transmission (Levy et al., 2018; Levy et al., 2016; Walker, 2018). The WBDs are often related to food consumption due to a tight association of food and water. Infections are mostly gastrointestinal (diarrheal), although the pathogens may affect other systems, including the brain. Several previously mentioned VBZDs are also WBDs (e.g. malaria, dengue schistosomiasis, toxoplasmosis), but other infectious diseases associated with water exposure may lead to neurological damage.
Primary amebic meningoencephalitis (PAM) is a rare, but extremely fatal infection of the brain caused by Naegleria fowleri, known as the “brain-eating amoeba”, commonly found in warm freshwater. Infection usually occurs during recreational water activities, but exposure from drinking water has also been recorded (Cope et al., 2015). The clinical presentation of PAM is often indistinguishable from bacterial meningitis (headache, fever, nausea, and vomiting), thus the diagnosis is difficult and rarely on time – only 27% of cases are diagnosed before death due to the cerebral edema, and mortality rate is above 97% (Capewell et al., 2015). In the last decade, notable changes have been documented regarding PAM epidemiology. Secondary to increased temperatures, first PAM cases have been reported in the northern U.S. (Cope and Ali, 2016; Kemble et al., 2012). Increased incidence of hepatitis A virus (HAV) infection depends greatly on water-related extreme weather events (Gao et al., 2016; Gullon et al., 2017; Hu et al., 2004; Morand et al., 2013). The infection usually manifests as fatigue or jaundice, but sporadically the CNS impairment occurs (Alehan et al., 2004; Hegazi et al., 2011; Lee et al., 2011). Moreover, several bacterial infections producing neurological symptoms and transmitted by water have been affected by the changing global climate, e.g.: leptospirosis (Lau et al., 2010), shigellosis (Cheng et al., 2017; Liu et al., 2017; Song et al., 2018; Zhang et al., 2017), campylobacteriosis (Allard et al., 2011; Rosenberg et al., 2018; Soneja et al., 2016), salmonellosis (Lake, 2017; Wang et al., 2018; Welch et al., 2019), infections of Escherichia coli (Hellberg and Chu, 2016; Iqbal et al., 2019; Philipsborn et al., 2016), or Staphylococcus aureus (Hellberg and Chu, 2016).
2.2. Environmental neurotoxins.
Multiple environmental contaminants have a neurotoxic effect on the brain. Heavy metals such as mercury (Hg) (Farina and Aschner, 2017; Pletz et al., 2016), arsenic (As) (Escudero-Lourdes, 2016; Tolins et al., 2014), manganese (Mn) (Peres et al., 2016) and lead (Pb) (Andrade et al., 2017; Caito and Aschner, 2015; Singh et al., 2018), pesticides (Burke et al., 2017; Cassereau et al., 2017), persistent organic pollutants (POPs) (Costa et al., 2014; Winneke, 2011), endocrine disruptive chemicals (EDCs) (Ghassabian and Trasande, 2018; Pomara et al., 2015; Weiss, 2012), or biotoxins (Grant et al., 2010) – all have been associated with the development of neurological outcomes in humans. Their occurrence in the environment is due to natural or anthropogenic sources, and accumulation and recycling are subjected to climate and weather changes. How present and future climate shifts alter the transport, transfer, deposition, and fate of various environmental pollutants has been extensively reviewed elsewhere (Kallenborn et al., 2012; Macdonald et al., 2005; Noyes et al., 2009; Schiedek et al., 2007; Van Oostdam et al., 2005). Herein, we discuss the major concepts regarding climate impact on the circulation of environmental neurotoxins in the air and water.
The weather has a strong influence on the distribution and concentrations of air contaminants, and the changing climate likely accelerates air pollution, especially in urbanized areas. Extreme weather conditions, as well as increased temperature and humidity, promote the formation of particulate matter (PM) and changes in ozone (O3) levels (Doherty et al., 2017; Kinney, 2018). On the other hand, increased temperatures and locally decreased precipitation are projected to increase the frequency and expansion of wildfires during which the PM and other pollutants (Hg, As, Pb) are released into the environment (Cascio, 2018; Kinney, 2018; Liu et al., 2016). A growing body of epidemiological and modeling evidence suggests that global warming coupled with O3 and PM exposures will exacerbate the prevalence and severity of human disease and mortality (Noyes et al., 2009). While positive association between air pollution exposure and prevalence of neurological diseases is well established (Block and Calderon-Garciduenas, 2009; Costa et al., 2017; Lee et al., 2017; Myhre et al., 2018; Sram et al., 2017; Sunyer and Dadvand, 2019), the potentiating effect of climate change is still poorly studied. Lee et al. (2018) showed that increased air levels of PM, NO2, O3, and CO, enhanced the risk of migraine in Korean population (Seoul) and particles’ effects were significantly stronger on high-temperature days (Lee et al., 2018).
Intensified precipitation and extreme weather events may cause an overflow of contaminated land, which can lead to remobilization of contaminants from sediments and pollution of freshwater. Additionally, the increased temperature may enhance the volatility of contaminants from soils and water. Rising sea and ocean levels have been shown to intensify As release from contaminated coastal soils (LeMonte et al., 2017). Snow and ice melt release and remobilize sequestered pollutants – in Antarctic soil and permafrost are considered a sink for environmental contaminants, especially heavy metals and POPs, which once released, may disturb their ecological balance (Potapowicz et al., 2019). Climate change-driven oxygen limitation (hypoxia) may also alter neurotoxin deposition – hypoxic episodes reduced solid-phase manganese dioxide (MnO2) accumulated in the marine sediments causing a substantial increase of bioavailable Mn2+ concentrations in the water (Schiedek et al., 2007). Climate change may also influence how environmental contaminants accumulate in the aquatic organisms, enhancing toxicity in them and in humans who depend on seafood in diet. As discussed in (Kennedy and Walsh, 1997; Noyes et al., 2009; Schiedek et al., 2007; Van Oostdam et al., 2005), higher temperatures facilitate the bioavailability, uptake, biomagnifications, transport, degradation, volatilization, remobilization and metabolism of toxic chemicals. For instance, ocean warming intensifies methylation of mercury and subsequent uptake of methylmercury (MeHg) in fish and marine mammals by 3–5% for each 10°C rise in water temperature (Booth and Zeller, 2005). In turn, the amplification of food web bioaccumulation of MeHg and other emerging pollutants under climate change has been proposed (Alava et al., 2018; Taylor et al., 2019). Higher temperatures also facilitate the metabolism of aquatic species. In the light of reduced O2 concentration, the higher rate of water inflow into the body is needed to extract enough O2 – this may also increase the exposure to the dissolved pollutants. Bioavailability of contaminants is affected by salinity (McLusky et al., 1986) and acidification (low pH) (Riba et al., 2004), and climate-dependent changes in acidification may enhance bioaccumulation of some toxic metals, as found with clams (Lopez et al., 2010). Moreover, higher temperatures and lower salinity alter the aquatic species’ ability to cope with toxic stress (Heugens et al., 2001; Velasco et al., 2018).
The rise of precipitation, surface water temperature and nutrient loading accelerate growth of harmful algal blooms (e.g. Pseudonitzschia spp., blue-green cyanobacteria and dinoflagellates), which are increasing in frequency, intensity, and duration globally (Chapra et al., 2017; Goldstein et al., 2008; Huisman et al., 2018; Paerl, 2018). They produce neurotoxins – microcystin, saxitoxin, brevetoxin or domoic acid (Chernoff et al., 2017; Grant et al., 2010; Porojan et al., 2016), which accumulate in fish and other seafood increasing the risk of adverse shellfish poisonings, affecting the brain and other organs in humans (Grant et al., 2010; Watkins et al., 2008). The neurological effects include amnesia, epilepsy, parkinsonian- and dementia-like symptoms which may be severe, chronic, and even lethal (Ramsdell and Gulland, 2014; Wang, 2008); some biotoxins may cross the mammalian placenta and accumulate in the amniotic fluid disturbing neurodevelopment (Costa et al., 2010; Ramsdell and Zabka, 2008). Although the most frequent human exposure is via consumption of contaminated seafood, the poisoning also occurs through drinking water, consumption of plants irrigated with biotoxin-contaminated water, or swimming in polluted recreational waters.
2.3. Food contamination and malnutrition.
The quantity and nutritional quality of agricultural production depend on soil quality, sunlight, CO2, temperature, and water availability. Thus, due to increasing temperatures and water-dependent extreme weather events, changing climate will likely affect seasonal food availability, food contamination, or increased consumption of toxin in the diet (Myers et al., 2017). Increasing temperatures enhance soil erosion which facilitates pesticide run-off and pollution, and endorses the need for artificial fertilizers. Changing climate will likely promote weeds growth, the survival of some pests and diseases affecting plant and livestock, thus more herbicides, pesticides, insecticides, and other chemicals will be required, contributing to the even greater contamination of the environment, and subsequently the food (Boxall et al., 2009; Myers et al., 2017). Food is also an important vector of some infectious diseases affecting the brain, like previously mentioned toxoplasmosis (see 2.1.1.), shigellosis, campylobacteriosis, infections caused by Escherichia coli, Staphylococcus aureus or HAV (see 2.1.2.). The hot and humid climate is favorable for the growth of mycotoxin-producing fungal molds, thus weather and climate shifts may enhance contamination of food and environment (Paterson and Lima, 2011). Some mycotoxins exhibit severe neurotoxic effects, e.g. fumonisin B1 (Domijan, 2012), lolitrems, paspalitrems (Kozak et al., 2019; Plumlee and Galey, 1994), and several brain diseases have been linked to mycotoxins exposure (Bonnet et al., 2012; French et al., 2019; Ratnaseelan et al., 2018; Terciolo et al., 2018).
Future prognosis indicates that crop production will change and shift geographically, leaving some regions unsuitable for conventional farming. Climate change is likely to increase the area, frequency, and duration of extreme droughts. This will lead to changes in crop yield, higher food prices and consequently lower affordability, reduced calorie availability, and growing malnutrition in vulnerable populations from developing countries (Myers et al., 2017; Squire and Ryan, 2017). Malnutrition, particularly in early life, profoundly influences neurodevelopment, alters neurocognitive performances and cause severe neurological disorders, as reviewed in (Mattei and Pietrobelli, 2019). Moreover, it has been shown that the burden of conventional neurodevelopmental toxins (e.g. Pb) is exacerbated by malnutrition (Guerrant et al., 2008).
2.4. Brain (patho)physiology.
The brain is at the forefront of animals’ interactions with the environment, thus changing climate may have a direct effect on the CNS development and performance, thereby affecting behavior. From animal studies emerges that temperature modulates brain development – changing temperature can alter gene expression (Pallotta et al., 2017), neuronal structure, brain organization (Amiel et al., 2017; Groh et al., 2004), and learning ability (Dayananda and Webb, 2017; Wang et al., 2007). The thermal environment can influence neurogenesis in adult brain (Ramirez et al., 1997). Other abiotic conditions are shown to impair neural function in animals – lowering barometric pressure aggravates depression-like behaviors in rats (Kanekar et al., 2015; Mizoguchi et al., 2011) and induces neuronal activation in the superior vestibular nucleus in mice, linked to the generation of meteoropathy (Sato et al., 2019).
Climate change-related atmospheric conditions have been also associated with neurological issues in humans. Both increases and decreases in temperature lead to a significant (approx. 20% for 5°C change) increase in the number of migraine reports in German population in 2011–2012 (smartphone app and web form study) (Scheidt et al., 2013). Heat stroke is a life-threatening condition – severe increase in body temperature with central nervous system dysfunction that often includes combativeness, delirium, seizures, and coma. It primarily occurs in immunocompromised individuals during annual heat waves, but exertional heat stroke is observed in young fit individuals performing strenuous physical activity in hot environments (Leon and Bouchama, 2015). When the influence of weather on the incidence of primary spontaneous intracerebral hemorrhage was analyzed, changes in barometric pressure (Garg et al., 2019), the PM and O3 concentrations (Han et al., 2016), but not temperature, primarily affected the incidence of the condition. The impact of thermal conditions on intracerebral hemorrhage seems to be complex (Luo et al., 2018; Ma et al., 2018). Overall, the topic is little explored, moreover, it is still not clear how environmental conditions affect the core body and brain temperature (Cramer and Jay, 2016; Kiyatkin, 2018; Smith and Johnson, 2016; Szekely and Garai, 2018).
Although direct indications on the effect of climate change on brain (patho)physiology are limited, general understanding of the underlying mechanisms arise from in vivo and in vitro studies of the impact of heat stress and hyperthermia on brain metabolism. Given the exogenous cause of hyperthermia during current climate change, in this section we intentionally avoided discussion of the observations dealing with endogenous hyperthermia due to impaired brain thermoregulation (Kiyatkin, 2005).
Despite the observation of heat-induced increase in basal metabolic rate in certain brain regions, a significant decrease was observed in human caudate, putamen, insula, and posterior cingulum neuron metabolism (Nunneley et al., 2002). It is also notable that, even at a relatively stable global cerebral blood flow in environmental hyperthermia, regional (prefrontal cortex, somatosensory areas and limbic system) blood flow tended to decrease, being associated with mood state and cognitive changes (Qian et al., 2014). Heat-induced reduction in cerebral blood flow was also associated with reduced orthostatic tolerance (Crandall and Gonzalez-Alonso, 2010). Certain cerebrovascular effects of endogenous hyperthermia may be also mediated by heat-induced hyperventilation and hypocapnia (Ross et al., 2012) with subsequent respiratory-induced alkalosis (Bain et al., 2015). The latter were shown to reduce cerebral blood flow during passive hyperthermia (Bain et al., 2013). The patterns of cerebral blood flow under environmental hyperthermia also correspond to the observed functional heterogeneity of brain regions (Qian et al., 2013). Particularly, environmental hyperthermia (50 °C) was shown to impair functional connectivity of brain that may underlie alteration of cognitive performance and work behavior (Sun et al., 2013), as well as visual short-term memory (Jiang et al., 2013). Whole body hyperthermia induced long-term learning and memory deficits in rats with mild traumatic brain injury (Titus et al., 2015). Heat stress was also shown to impair blood-brain barrier and blood-cerebrospinal fluid barrier structure leading to an increase in their permeability and brain edema in rats (Sharma et al., 2010). These effects were found to be aggravated by diabetes (Muresanu et al., 2010a) and hypertension (Muresanu et al., 2010b).
Seizures are considered as the most common complications of hyperthermia and heat stroke (Leon and Bouchama, 2015), being at least partially associated with heat-induced activation of transient receptor potential cation channels (TRPV4) and N-methyl-D-aspartate receptors (NMDAR) signaling, as demonstrated in a zebrafish model (Hunt et al., 2012). High temperature exposure was shown to induce hyperthermic seizures along with inflammatory response in rats, that was aggravated by lipopolysaccharides treatment (Eun et al., 2015). Environmental hyperthermia was shown to aggravate adverse effects of brain trauma (Hermstad and Adams, 2010) even in the case of mild brain injury (Sakurai et al., 2012). Body hyperthermia (39°C) had a significant interactive effect with epileptic seizures in inducing neuronal injury in the amygdala and hippocampus (Suchomelova et al., 2015). Hyperthermia (39–40°C) was shown to induce epileptiform discharges in cortical neurons in vitro through interference with gamma-aminobutyric acid (GABA) receptor signaling (Wang et al., 2011). Structural changes due to heat-exposure (37–40°C) were observed in neurons and their axons, glia, as well as cerebral vascular endothelium (Sharma and Hoopes, 2003).
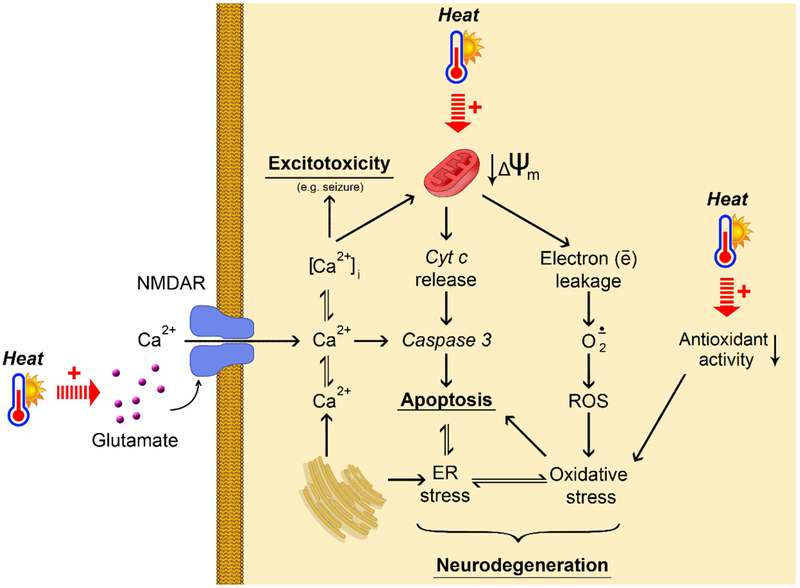
The intimate mechanisms of the observed effects of hyperthermia are still to be estimated, although some common pathways have been revealed (Figure 2). Heat stress is considered as an environmental prooxidant factor (Slimen et al., 2014). Heat exposure (44 °C) was shown to induce oxidative stress in brain and Tau pathology in laboratory rodents (Chauderlier et al., 2017), providing an additional link between hyperthermia and neurodegeneration. Correspondingly, high temperature exposure was shown to decrease antioxidant superoxide dismutase (SOD) expression and activity in neuronal HT-22 cells with subsequent cell death (El-Orabi et al., 2011). In addition to oxidative stress, exposure of primary cortical neurons to heat stress resulted in endoplasmic reticulum (ER) stress, inhibiting protective heat shock responses (Liu et al., 2012). These observations are generally in agreement with the indications of tight interplay between ER and oxidative stress in brain pathology (Thornton et al., 2017). Heat-induced mitochondrial dysfunction (irreversible mitochondrial membrane potential ΔѰm depolarization) with subsequent caspase-3 activation and apoptotic signaling was proposed as the potential mechanisms of hyperthermia-induced death of cultured rat neurons (White et al., 2012). Earlier studies revealed increased infarct volume and increased mortality in heat-exposed animals with ischemic stroke (Noor et al., 2003), that may be associated with heat-induced increase in matrix metalloproteinase (MMP-2) activity as well as basal membrane protein degradation and loss (Alam et al., 2011; Meng et al., 2012). Increased excitability of brain seems to play a significant role in heat-induced brain damage. For example, heat stress (38°C) significantly increased brain glutamate and aspartate levels in rats, whereas GABA and glycine concentrations were reduced, thus providing a shift to excitatory neurotransmitters (Sharma, 2006). These findings are in agreement with decreased hippocampal GABAergic synaptic transmission (Qu et al., 2007). Systemic glutamate levels were reduced in rats exposed to mild hyperthermia (37–39°C), whereas further heating (42°C) significantly elevated circulating glutamate concentrations (Zlotnik et al., 2010). Correspondingly, increased NMDAR signaling was also shown to contribute to heat-induced seizures (Morimoto et al., 1995), whereas NMDAR down-regulation had a protective effect in acclimation (Ely et al., 2015). Hyperthermia was shown to cause depolarization and reduced input resistance in parallel with increased synaptic activity of hippocampal pyramidal cells and inhibitory interneurons, being also indicative of higher excitability of the brain (Kim and Connors, 2012). It is expected that impaired calcium Ca2+ homeostasis may also contribute to neuronal damage under heat exposure, although the existing data are limited (White et al., 2012). Hyperthemic-dependent Ca2+ dysregulation has been shown in pathomechanisms of other systems, like smooth muscles (Burke and Hanani, 2012) and endothelial cells (Li et al., 2015). The latter may be involved in impaired cerebrovascular reactivity at heat stress exposure..
Figure 2.
Hypothetical mechanisms of the impact of heat exposure on brain (patho)physiology. Heat exposure results in mitochondrial dysfunction decreased mitochondrial membrane potential (↓Δѱm) causing increased electron leakage. The latter is associated with increased superoxide (O2·−) generation and further increase in reactive oxygen species (ROS) production resulting in oxidative stress together with decreased antioxidant enzymes activity. Both oxidative stress and heat exposure impair endoplasmic reticulum (ER) functioning ultimately leading to ER stress. Increased cytochrome c (Cyt c) release due to mitochondrial dysfunction induces caspase-3 activation and apoptotic signaling. The latter is aggravated by oxidative and ER stress. Hypothetically, a tight interplay between mitochondrial dysfunction, apoptosis, oxidative stress and ER stress may underlie heat-induced neurodegeneration. The overall effect of heat exposure is also associated with increased brain glutamate levels, although the particular mechanisms are unclear. Elevated glutamate levels induce NMDA receptor signaling causing intracellular Ca2+ flux. Taken together with Ca2+ release from ER, glutamate-induced Ca2+ uptake results in increasing intracellural calcium levels ([Ca2+]i) levels, leading to excitotoxicity and seizures. Moreover, increased [Ca2+]i levels aggravate apoptotic signaling through caspase-3 activation.
Prolonged heat exposure in mice resulted in a proinflammatory milieu being characterized by increased nuclear factor NF-κβ signaling and increased expression of interleukin IL-1β, tumor necrosis factor (TNF-α), cyclooxygenase-2, and inducible nitric oxide synthase (iNOS) in hippocampus with subsequent decrease in neuronal and synaptic density, and gliosis (Lee et al., 2015). Neuroinflammation was also associated with altered hypothalamic monoamine content and glutamate levels in heat-stressed (42°C) animals (Chauhan et al., 2017). Systemic inflammatory response syndrome is considered as an important pathway in heat stroke (Leon and Helwig, 2010).
Taken together, heat exposure induces complex metabolic changes in brain, resulting in formation of pathogenetic cascades including heat-induced oxidative stress, ER stress, mitochondrial dysfunction, apoptosis, excitotoxicity, neuroinflammation, and impaired brain microcirculation, being all implicated in neurodegeneration and other brain diseases.
2.5. Mental health.
Climate-related environmental changes may profoundly impact psychological well-being and mental health, particularly among those with pre-existing vulnerabilities or living in ecologically sensitive areas. Climate change may affect physical health (heat stress, injury, disease, disruption to food supply), or endorse mental health issues directly, by exposing people to the psychological trauma. Such trauma can be induced by multiple factors, particularly those related to extreme weather events and natural disasters: personal loss; destroyed environment, landscapes, infrastructure, and communities; decreased food access; depressed economy and impaired financial security; forced migration or social conflicts. Moreover, psychological distress may result from acknowledging climate change as a global environmental threat (Fritze et al., 2008). Depression and anxiety (Mamun et al., 2019), post-traumatic stress disorder (PTSD) (Hanigan et al., 2018; LaJoie et al., 2010; Pietrzak et al., 2012; Schwartz et al., 2017), increased substance use (Rohrbach et al., 2009), and suicide rates (Carleton, 2017; Fountoulakis et al., 2016a; Fountoulakis et al., 2016b; Hanigan et al., 2012) are increasing with changes in climate conditions. For instance, depression symptoms were eight times higher among people in flooded homes (Azuma et al., 2014). Short-term exposure to extreme weather, climate warming, or tropical cyclone was associated with worsened mental health, as concluded from data drawn from nearly 2 million U.S. residents between 2002 and 2012 (Obradovich et al., 2018): shifting from monthly temperatures between 25 °C and 30 °C to >30 °C increased the probability of mental health issues by 0.5% points, 1°C of 5-year warming was associated with a 2% increase in the prevalence of mental health issues, and exposure to Hurricane Katrina was associated with a 4% increase in this metric (Obradovich et al., 2018). PTSD and psychological distress have been observed years after a hurricane, particularly among vulnerable populations (LaJoie et al., 2010; Paxson et al., 2012). For a more comprehensive reading on the climate change impact on human mental health, we recommend recent reviews (Berry et al., 2018; Bourque and Willox, 2014; Burke et al., 2018; Dyregrov et al., 2018; Hayes et al., 2018; Torres and Casey, 2017; Trombley et al., 2017).
3. Mitigation and adaptation to climate change produce additional risk factors for brain diseases.
Emerging health risks related to changing climate can be minimized and avoided via effective mitigation and adaptation pathways. One of the major targets of the Paris Agreement is to limit global warming to no more than 2°C above the pre-industrial levels by 2100 (Paris Agreement, 2015). There are many potential paths to reach this goal, although no current strategy can prevent the change in climate that has already occurred (Portier et al., 2010). The major strategy of climate change mitigation aims to reduce global greenhouse emissions, through reduction of energy consumption and the use of fossil fuels, development and expansion of alternative energy sources and energy conservation technologies. Moreover, carbon capture and storage, changes in land use (reforestation) and actions aiming to preserve ecosystems and conserve biodiversity are introduced, as reviewed in (Tong and Ebi, 2019; Woodward, 2019). Climate change is very complex and difficult to predict – it occurs fast and manifests differently in different places. Analogously, the indirect impact and health risks associated with human responses and undertaken actions, due to the scale and speed, are also uncertain and challenging (Carney, 2016). Mitigation and adaptation responses to changing climate will likely disturb the environment and consequently human health – numerous strategies may have both positive and negative effects, most of which are poorly recognized or understood.
For instance, reduction in the use of fossil fuels will likely reduce the release of neurotoxicants such as As, Hg, and other contaminants into the environment (Gustin and Ladwig, 2004; Hu and Cheng, 2016; Ito et al., 2006). But it may also open new routes of human exposure, e.g. due to improper disposal of energy-saving fluorescent light bulbs containing Hg, or heavy metals release associated with manufacturing and disposal of batteries used in electric vehicles (Bronstein et al., 2009; Noyes et al., 2009). Nuclear power plants are potential sources of contamination and they have strong environmental impacts on water availability and quality. Increased reliance on hydroelectric power, which typically requires the construction of dams, may change local VBZDs ecologies and alter diseases transmission (Zhou et al., 2016). Mitigation focused on the preservation of forests and wetlands are also likely to impact VBZDs ecology and transmission.
Adaptation efforts may increase environmental contamination and human exposure to neurotoxic compounds, due to e.g. the increased use of insecticides to cope with transmission of VBZDs vectors or application of (new) pesticides and herbicides in response to changed requirements for food production (Noyes et al., 2009). Interestingly, the indirect consequences of climate change, e.g. shifts in agriculture and resource exploitation may have a more pronounced impact on contaminants presence in the environment, than direct climate change, as shown on the example of POPs (Kallenborn et al., 2012). Capture and storage of water runoff to adapt to drought may provide more suitable breeding habitat for mosquitoes, thereby increasing incidence of VBZDs. Moreover, increased application of biofuels as alternative energy sources or genetically modified organisms, may have a questionable effect on food-borne diseases and human nutrition. Climate change-related, drought-triggered famines may lead to increased consumption of resilient plants such as grass pea, cassava or cycad seeds, containing neurotoxicants which are associated with a high-burden of neurological diseases. Unbalanced grass pea (Lathyrus sativus) consumption due to substantial amount of neurotoxic β-N-oxalyl-L-α,β-diaminopropionic acid (ODAP) has been associated with neurolathyrism, a neurodegenerative disease that causes paralysis of the lower body. In normal socio-economic and environmental situations, in which grass pea is part of a balanced diet, neurolathyrism is virtually non-existent (Lambein et al., 2019). Cassava (Manihot esculenta, also known as mandioca, yuca) is a root vegetable resistant to poor soil and drought, which is an important staple, particularly among people who live in poverty and remote tropical areas (Gleadow et al., 2016). Unprocessed cassava contains high amounts of neurotoxic cyanogenic glucosides (linamarin and lotaustralin), associated with development of myeloneuropathy and konzo (Kashala-Abotnes et al., 2019). Edible cycads seeds contain multiple neurotoxins (methylazoxymethanol, β-methylamino-l-alanine, β-alanine-l-oxalylamino and cycasin) and their consumption has been associated with the development of neurodegenerative diseases with motor impairment, such as amyotrophic lateral sclerosis or Parkinson’s disease (Rivadeneyra-Dominguez and Rodriguez-Landa, 2014). Thus, in the light of such complex climate-environment-human interactions, scrutinized examination of the neurological health risks associated with mitigation and adaptation strategies is needed.
4. Conclusions.
Climate change rapidly and extensively disrupts global ecosystems with yet unknown consequences for human. Rising average temperatures and sea levels, and intensification of extreme weather events impact environmental factors which directly or indirectly affect human health. Moreover, the impact of human responsive actions associated with mitigation and adaptation strategies poses additional hazard through intensification of some health risk factors. Many of them, separate or jointly contribute to increased occurrence of brain diseases. Changing climate conditions promote transmitting of pathogens infecting the brain; intensify environmental pollution increasing risk of exposure to the harmful neurotoxicants; create food contamination and shortage potentially leading to brain-affecting malnutrition and poisoning. Climate-driven natural disasters and their socio-economic consequences have a strong and persistent impact on the mental health of affected populations. Moreover, drastically changing weather conditions may directly disturb brain physiology. Yet scientific evidence is sparse, and more research is needed to recognize and effectively address all these emerging and complex challenges associated with climate change-driven environmental risk factors of neurological diseases.
Acknowledgments
This work has been supported by the National Institute of Environmental Health Sciences (R01ES07331, R01ES10563 and R01ES020852).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam M, et al. , 2011. Hyperthermia up-regulates matrix metalloproteinases and accelerates basement membrane degradation in experimental stroke. Neurosci Lett. 495, 135–9. [DOI] [PubMed] [Google Scholar]
- Alava JJ, et al. , 2018. Projected amplification of food web bioaccumulation of MeHg and PCBs under climate change in the Northeastern Pacific. Sci Rep. 8, 13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alehan FK, et al. , 2004. Acute disseminated encephalomyelitis associated with hepatitis A virus infection. Ann Trop Paediatr. 24, 141–4. [DOI] [PubMed] [Google Scholar]
- Alkishe AA, et al. , 2017. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One. 12, e0189092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard R, et al. , 2011. The reported incidence of campylobacteriosis modelled as a function of earlier temperatures and numbers of cases, Montreal, Canada, 1990–2006. Int J Biometeorol. 55, 353–60. [DOI] [PubMed] [Google Scholar]
- Almeida Bentes A, et al. , 2019. Neurological manifestations of pediatric arboviral infections in the Americas. J Clin Virol. 116, 49–57. [DOI] [PubMed] [Google Scholar]
- Amiel JJ, et al. , 2017. The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim Cogn. 20, 117–125. [DOI] [PubMed] [Google Scholar]
- Andrade VM, et al. , 2017. Neurotoxicity of Metal Mixtures. Adv Neurobiol. 18, 227–265. [DOI] [PubMed] [Google Scholar]
- Araujo AQ, et al. , 2016. Zika virus-associated neurological disorders: a review. Brain. 139, 2122–30. [DOI] [PubMed] [Google Scholar]
- Asad H, Carpenter DO, 2018. Effects of climate change on the spread of zika virus: a public health threat. Rev Environ Health. 33, 31–42. [DOI] [PubMed] [Google Scholar]
- Azimi F, et al. , 2017. Impact of climate variability on the occurrence of cutaneous leishmaniasis in Khuzestan Province, southwestern Iran. Geospat Health. 12, 478. [DOI] [PubMed] [Google Scholar]
- Azuma K, et al. , 2014. Effects of water-damaged homes after flooding: health status of the residents and the environmental risk factors. Int J Environ Health Res. 24, 158–75. [DOI] [PubMed] [Google Scholar]
- Bain AR, et al. , 2015. Cerebral Vascular Control and Metabolism in Heat Stress. Compr Physiol. 5, 1345–80. [DOI] [PubMed] [Google Scholar]
- Bain AR, et al. , 2013. Regional changes in brain blood flow during severe passive hyperthermia: effects of PaCO2 and extracranial blood flow. J Appl Physiol (1985). 115, 653–9. [DOI] [PubMed] [Google Scholar]
- Beknazarova M, et al. , 2016. Strongyloidiasis: A Disease of Socioeconomic Disadvantage. Int J Environ Res Public Health. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry HL, et al. , 2018. The case for systems thinking about climate change and mental health. Nature Climate Change. 8, 282–290. [Google Scholar]
- Block ML, Calderon-Garciduenas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MS, et al. , 2012. Advances in deoxynivalenol toxicity mechanisms: the brain as a target. Toxins (Basel). 4, 1120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth S, Zeller D, 2005. Mercury, food webs, and marine mammals: implications of diet and climate change for human health. Environ Health Perspect. 113, 521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque F, Willox AC, 2014. Climate change: the next challenge for public mental health? Int Rev Psychiatry. 26, 415–22. [DOI] [PubMed] [Google Scholar]
- Boxall AB, et al. , 2009. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ Health Perspect. 117, 508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R, et al. , 2016. Severe Flooding and Malaria Transmission in the Western Ugandan Highlands: Implications for Disease Control in an Era of Global Climate Change. J Infect Dis. 214, 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein J, et al. , 2009. Meeting report: consensus statement-Parkinson’s disease and the environment: collaborative on health and the environment and Parkinson’s Action Network (CHE PAN) conference 26–28 June 2007. Environ Health Perspect. 117, 117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, et al. , 2017. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J Neurochem. 142 Suppl 2, 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S, Hanani M, 2012. The actions of hyperthermia on the autonomic nervous system: central and peripheral mechanisms and clinical implications. Auton Neurosci. 168, 4–13. [DOI] [PubMed] [Google Scholar]
- Burke SEL, et al. , 2018. The Psychological Effects of Climate Change on Children. Curr Psychiatry Rep. 20, 35. [DOI] [PubMed] [Google Scholar]
- Burrows K, Kinney PL, 2016. Exploring the Climate Change, Migration and Conflict Nexus. Int J Environ Res Public Health. 13, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S, Aschner M, 2015. Neurotoxicity of metals. Handb Clin Neurol. 131, 169–89. [DOI] [PubMed] [Google Scholar]
- Caminade C, et al. , 2014. Impact of climate change on global malaria distribution. Proc Natl Acad Sci U S A. 111, 3286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade C, et al. , 2019. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 1436, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Lendrum D, et al. , 2015. Climate change and vector-borne diseases: what are the implications for public health research and policy? Philos Trans R Soc Lond B Biol Sci. 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan BC, 2019. Opening Pandora’s Box at the roof of the world: Landscape, climate and avian influenza (H5N1). Acta Trop. 196, 93–101. [DOI] [PubMed] [Google Scholar]
- Capewell LG, et al. , 2015. Diagnosis, Clinical Course, and Treatment of Primary Amoebic Meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc. 4, e68–75. [DOI] [PubMed] [Google Scholar]
- Carleton TA, 2017. Crop-damaging temperatures increase suicide rates in India. Proc Natl Acad Sci U S A.114, 8746–8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Castro O, et al. , 2018. Impact of climate change on vector transmission of Trypanosoma cruzi (Chagas, 1909) in North America. Med Vet Entomol. 32, 84–101. [DOI] [PubMed] [Google Scholar]
- Carney M, Resolving the Climate Paradox. Berlin, 2016, pp. 1–13. [Google Scholar]
- Carrington LB, et al. , 2013. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One. 8, e58824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio WE, 2018. Wildland fire smoke and human health. Sci Total Environ. 624, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassereau J, et al. , 2017. Neurotoxicity of Insecticides. Curr Med Chem. 24, 2988–3001. [DOI] [PubMed] [Google Scholar]
- Chapra SC, et al. , 2017. Climate Change Impacts on Harmful Algal Blooms in U.S. Freshwaters: A Screening-Level Assessment. Environ Sci Technol. 51, 8933–8943. [DOI] [PubMed] [Google Scholar]
- Chauderlier A, et al. , 2017. In Vivo Hyperthermic Stress Model: An Easy Tool to Study the Effects of Oxidative Stress on Neuronal Tau Functionality in Mouse Brain. Methods Mol Biol. 1523, 369–373. [DOI] [PubMed] [Google Scholar]
- Chauhan NR, et al. , 2017. Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience. 358, 79–92. [DOI] [PubMed] [Google Scholar]
- Cheng J, et al. , 2017. Impacts of ambient temperature on the burden of bacillary dysentery in urban and rural Hefei, China. Epidemiol Infect. 145, 1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff N, et al. , 2017. A critical review of the postulated role of the non-essential amino acid, beta-N-methylamino-L-alanine, in neurodegenerative disease in humans. J Toxicol Environ Health B Crit Rev. 20, 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas CA, et al. , 2017. Parasite vulnerability to climate change: an evidence-based functional trait approach. R Soc Open Sci. 4, 160535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codjoe SN, Nabie VA, 2014. Climate change and cerebrospinal meningitis in the Ghanaian meningitis belt. Int J Environ Res Public Health. 11, 6923–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope JR, Ali IK, 2016. Primary Amebic Meningoencephalitis: What Have We Learned in the Last 5 Years? Curr Infect Dis Rep. 18, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope JR, et al. , 2015. The first association of a primary amebic meningoencephalitis death with culturable Naegleria fowleri in tap water from a US treated public drinking water system. Clin Infect Dis. 60, e36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, et al. , 2017. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Curr Environ Health Rep. 4, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, et al. , 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett. 230, 282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, et al. , 2010. Domoic acid as a developmental neurotoxin. Neurotoxicology. 31, 409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Gonzalez-Alonso J, 2010. Cardiovascular function in the heat-stressed human. Acta Physiol (Oxf). 199, 407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, et al. , 2018. Increased Relative Risk of Tick-Borne Encephalitis in Warmer Weather. Front Cell Infect Microbiol. 8, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud A, et al. , 2016. What Is the Association between Absolute Child Poverty, Poor Governance, and Natural Disasters? A Global Comparison of Some of the Realities of Climate Change. PLoS One. 11, e0153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, 2018. Burden of climate change on malaria mortality. Int J Hyg Environ Health. 221, 782–791. [DOI] [PubMed] [Google Scholar]
- Dayananda B, Webb JK, 2017. Incubation under climate warming affects learning ability and survival in hatchling lizards. Biol Lett. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoux A, et al. , 2018. A multi-faceted pandemic: a review of the state of knowledge on the Zika virus. Public Health Rev. 39, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty RM, et al. , 2017. Climate change impacts on human health over Europe through its effect on air quality. Environ Health. 16, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domijan AM, 2012. Fumonisin B(1): a neurotoxic mycotoxin. Arh Hig Rada Toksikol. 63, 531–44. [DOI] [PubMed] [Google Scholar]
- Dyregrov A, et al. , 2018. Children and natural disasters. Eur J Psychotraumatol. 9, 1500823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL, Nealon J, 2016. Dengue in a changing climate. Environ Res. 151, 115–123. [DOI] [PubMed] [Google Scholar]
- Eikenberry SE, Gumel AB, 2018. Mathematical modeling of climate change and malaria transmission dynamics: a historical review. J Math Biol. 77, 857–933. [DOI] [PubMed] [Google Scholar]
- El-Orabi NF, et al. , 2011. Heat-induced inhibition of superoxide dismutase and accumulation of reactive oxygen species leads to HT-22 neuronal cell death. . J Therm Biol. 36, 49–56. [Google Scholar]
- Ely BR, et al. , 2015. Can targeting glutamate receptors with long-term heat acclimation improve outcomes following hypoxic injury? Temperature (Austin). 2, 51–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero-Lourdes C, 2016. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology. 53, 223–235. [DOI] [PubMed] [Google Scholar]
- Eun BL, et al. , 2015. Lipopolysaccharide potentiates hyperthermia-induced seizures. Brain Behav.5, e00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Aschner M, 2017. Methylmercury-Induced Neurotoxicity: Focus on Pro-oxidative Events and Related Consequences. Adv Neurobiol. 18, 267–286. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, et al. , 2016a. Relationship of suicide rates with climate and economic variables in Europe during 2000–2012. Ann Gen Psychiatry. 15, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, et al. , 2016b. Climate change but not unemployment explains the changing suicidality in Thessaloniki Greece (2000–2012). J Affect Disord. 193, 331–8. [DOI] [PubMed] [Google Scholar]
- French PW, et al. , 2019. Fungal Neurotoxins and Sporadic Amyotrophic Lateral Sclerosis.Neurotox Res. 35, 969–980. [DOI] [PubMed] [Google Scholar]
- Fritze JG, et al. , 2008. Hope, despair and transformation: Climate change and the promotion of mental health and wellbeing. Int J Ment Health Syst. 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, et al. , 2016. Projections of hepatitis A virus infection associated with flood events by 2020 and 2030 in Anhui Province, China. Int J Biometeorol. 60, 1873–1884. [DOI] [PubMed] [Google Scholar]
- Garg RK, et al. , 2019. The Influence of Weather on the Incidence of Primary Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 28, 405–411. [DOI] [PubMed] [Google Scholar]
- Garkowski A, et al. , 2017. Cerebrovascular Manifestations of Lyme Neuroborreliosis-A Systematic Review of Published Cases. Front Neurol. 8, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza M, et al. , 2014. Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. PLoS Negl Trop Dis. 8, e2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Trasande L, 2018. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne). 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadow R, et al. , 2016. Resilience of cassava (Manihot esculenta Crantz) to salinity: implications for food security in low-lying regions. J Exp Bot. 67, 5403–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein T, et al. , 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci. 275, 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KS, et al. , 2010. Domoic acid: neurobehavioral consequences of exposure to a prevalent marine biotoxin. Neurotoxicol Teratol. 32, 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh C, et al. , 2004. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc Natl Acad Sci U S A. 101, 4268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant RL, et al. , 2008. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 66, 487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullon P, et al. , 2017. Association between meteorological factors and hepatitis A in Spain 2010–2014. Environ Int. 102, 230–235. [DOI] [PubMed] [Google Scholar]
- Gustin MS, Ladwig K, 2004. An assessment of the significance of mercury release from coal fly ash. J Air Waste Manag Assoc. 54, 320–30. [DOI] [PubMed] [Google Scholar]
- Hallegatte S, Rozenberg J, 2017. Climate change through a poverty lens. Nature Climate Change. 7, 250. [Google Scholar]
- Han MH, et al. , 2016. Association between hemorrhagic stroke occurrence and meteorological factors and pollutants. BMC Neurol. 16, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan IC, et al. , 2012. Suicide and drought in New South Wales, Australia, 1970–2007. Proc Natl Acad Sci U S A. 109, 13950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan IC, et al. , 2018. Drought and Distress in Southeastern Australia. Ecohealth. 15, 642–655. [DOI] [PubMed] [Google Scholar]
- Hayes K, et al. , 2018. Climate change and mental health: risks, impacts and priority actions. Int J Ment Health Syst. 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MA-O, Piaggio AJ, 2018. Assessing the potential impacts of a changing climate on the distribution of a rabies virus vector. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazi MA, et al. , 2011. Hepatitis A virus presenting as fatal encephalitis in a child. Pediatr Infect Dis J. 30, 1012. [DOI] [PubMed] [Google Scholar]
- Hellberg RS, Chu E, 2016. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit Rev Microbiol. 42, 548–72. [DOI] [PubMed] [Google Scholar]
- Hermstad E, Adams B, 2010. Traumatic brain injury complicated by environmental hyperthermia. J Emerg Trauma Shock. 3, 66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heugens EH, et al. , 2001. A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Crit Rev Toxicol. 31, 247–84. [DOI] [PubMed] [Google Scholar]
- Hora R, et al. , 2016. Cerebral malaria--clinical manifestations and pathogenesis. Metab Brain Dis. 31, 225–37. [DOI] [PubMed] [Google Scholar]
- Hu W, et al. , 2004. El Nino Southern Oscillation and the transmission of hepatitis A virus in Australia. Med J Aust. 180, 487–8. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cheng H, 2016. Control of mercury emissions from stationary coal combustion sources in China: Current status and recommendations. Environ Pollut. 218, 1209–1221. [DOI] [PubMed] [Google Scholar]
- Huisman J, et al. , 2018. Cyanobacterial blooms. Nat Rev Microbiol. 16, 471–483. [DOI] [PubMed] [Google Scholar]
- Hunt RF, et al. , 2012. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol. 237, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC, Global Warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty 2018. [Google Scholar]
- Iqbal MS, et al. , 2019. The impact of socio-economic development and climate change on E. coli loads and concentrations in Kabul River, Pakistan. Sci Total Environ. 650, 1935–1943. [DOI] [PubMed] [Google Scholar]
- Ito S, et al. , 2006. Emissions of mercury and other trace elements from coal-fired power plants in Japan. Sci Total Environ. 368, 397–402. [DOI] [PubMed] [Google Scholar]
- Ivanescu L, et al. , 2016. Climate Change Is Increasing the Risk of the Reemergence of Malaria in Romania. Biomed Res Int. 2016, 8560519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, et al. , 2013. Hyperthermia impaired human visual short-term memory: an fMRI study. Int J Hyperthermia. 29, 219–24. [DOI] [PubMed] [Google Scholar]
- Jore S, et al. , 2014. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit Vectors. 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenborn R, et al. , 2012. The influence of climate change on the global distribution and fate processes of anthropogenic persistent organic pollutants. J Environ Monit. 14, 2854–69. [DOI] [PubMed] [Google Scholar]
- Kanekar S, et al. , 2015. Hypobaric hypoxia induces depression-like behavior in female Sprague Dawley rats, but not in males. High Alt Med Biol. 16, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashala-Abotnes E, et al. , 2019. Konzo: a distinct neurological disease associated with food (cassava) cyanogenic poisoning. Brain Res Bull. 145, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble SK, et al. , 2012. Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 54, 805–9. [DOI] [PubMed] [Google Scholar]
- Kennedy CJ, Walsh PJ, 1997. Effects of temperature on xenobiotic metabolism. 303–324. [Google Scholar]
- Kiehl J, 2011. Lessons from Earth’s Past. Science. 331, 158–159. [DOI] [PubMed] [Google Scholar]
- Kim JA, Connors BW, 2012. High temperatures alter physiological properties of pyramidal cells and inhibitory interneurons in hippocampus. Front Cell Neurosci. 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL, 2018. Interactions of Climate Change, Air Pollution, and Human Health. Curr Environ Health Rep. 5, 179–186. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, 2005. Brain hyperthermia as physiological and pathological phenomena. Brain Res Brain Res Rev. 50, 27–56. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Beckham JD, 2015. West Nile Virus Encephalitis 16 Years Later. Brain Pathol. 25, 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L, et al. , 2019. Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: biosynthetic diversity, threats and applications. Appl Microbiol Biotechnol. 103, 1599–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, Mordecai EA, 2016. The rise and fall of infectious disease in a warmer world. F1000Res. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJoie AS, et al. , 2010. Long-term effects of Hurricane Katrina on the psychological well-being of evacuees. Disasters. 34, 1031–44. [DOI] [PubMed] [Google Scholar]
- Lake IR, 2017. Food-borne disease and climate change in the United Kingdom. Environ Health. 16, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambein F, et al. , 2019. Grass pea (Lathyrus sativus L.): orphan crop, nutraceutical or just plain food? Planta. [DOI] [PubMed] [Google Scholar]
- Lau CL, et al. , 2010. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Trans R Soc Trop Med Hyg. 104, 631–8. [DOI] [PubMed] [Google Scholar]
- Lee H, et al. , 2017. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci Rep. 7, 44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, et al. , 2011. Encephalitis associated with acute hepatitis a. J Epilepsy Res. 1, 27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, et al. , 2015. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J Neuroinflammation. 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedale J, et al. , 2016. Projecting malaria hazard from climate change in eastern Africa using large ensembles to estimate uncertainty. Geospat Health. 11, 393. [DOI] [PubMed] [Google Scholar]
- LeMonte JJ, et al. , 2017. Sea Level Rise Induced Arsenic Release from Historically Contaminated Coastal Soils. Environ Sci Technol. 51, 5913–5922. [DOI] [PubMed] [Google Scholar]
- Leon LR, Bouchama A, 2015. Heat stroke. Compr Physiol. 5, 611–47. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG, 2010. Heat stroke: role of the systemic inflammatory response. J Appl Physiol (1985). 109, 1980–8. [DOI] [PubMed] [Google Scholar]
- Levy K, et al. , 2018. Climate Change Impacts on Waterborne Diseases: Moving Toward Designing Interventions. Curr Environ Health Rep. 5, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy K, et al. , 2016. Untangling the Impacts of Climate Change on Waterborne Diseases: a Systematic Review of Relationships between Diarrheal Diseases and Temperature, Rainfall, Flooding, and Drought. Environ Sci Technol. 50, 4905–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. , 2018. Climate change and dengue fever transmission in China: Evidences and challenges. Sci Total Environ. 622–623, 493–501. [DOI] [PubMed] [Google Scholar]
- Li GH, et al. , 2017. Neurological Manifestations of Dengue Infection. Front Cell Infect Microbiol.7, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. , 2015. Heat Stress Induces Apoptosis through a Ca2+-Mediated Mitochondrial Apoptotic Pathway in Human Umbilical Vein Endothelial Cells. PLOS ONE. 9, e111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, et al. , 2016. Particulate Air Pollution from Wildfires in the Western US under Climate Change. Clim Change. 138, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, et al. , 2017. Projected burden of disease for bacillary dysentery due to flood events in Guangxi, China. Sci Total Environ. 601–602, 1298–1305. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2012. Heat stress activates ER stress signals which suppress the heat shock response, an effect occurring preferentially in the cortex in rats. Mol Biol Rep. 39, 3987–93. [DOI] [PubMed] [Google Scholar]
- Lopez IR, et al. , 2010. Influence of sediment acidification on the bioaccumulation of metals in Ruditapes philippinarum. Environ Sci Pollut Res Int. 17, 1519–28. [DOI] [PubMed] [Google Scholar]
- Low AJ, et al. , 2019. Association between severe drought and HIV prevention and care behaviors in Lesotho: A population-based survey 2016–2017. PLoS Med. 16, e1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, et al. , 2018. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ Pollut. 239, 670–680. [DOI] [PubMed] [Google Scholar]
- Luo Y, et al. , 2018. The cold effect of ambient temperature on ischemic and hemorrhagic stroke hospital admissions: A large database study in Beijing, China between years 2013 and 2014-Utilizing a distributed lag non-linear analysis. Environ Pollut. 232, 90–96. [DOI] [PubMed] [Google Scholar]
- Ma P, et al. , 2018. Differences of hemorrhagic and ischemic strokes in age spectra and responses to climatic thermal conditions. Sci Total Environ. 644, 1573–1579. [DOI] [PubMed] [Google Scholar]
- Macdonald RW, et al. , 2005. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ. 342, 5–86. [DOI] [PubMed] [Google Scholar]
- Mach KJ, et al. , 2019. Climate as a risk factor for armed conflict. Nature. [DOI] [PubMed] [Google Scholar]
- Mamun MA, et al. , 2019. Prevalence of depression among Bangladeshi village women subsequent to a natural disaster: A pilot study. Psychiatry Res. 276, 124–128. [DOI] [PubMed] [Google Scholar]
- Marinho de Souza J, et al. , 2019. Mother-to-child transmission and gestational syphilis: Spatial-temporal epidemiology and demographics in a Brazilian region. PLoS Negl Trop Dis. 13, e0007122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D, Pietrobelli A, 2019. Micronutrients and Brain Development. Curr Nutr Rep. 8, 99–107. [DOI] [PubMed] [Google Scholar]
- McCreesh N et al. , 2015. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors. 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLusky DD, et al. , 1986. The effect of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanographic Marine Biology Annual Reviews. 24, 481–520. [Google Scholar]
- McMahon C, et al. , 2012. The effects of climate change on ovine parasitic gastroenteritis determined using veterinary surveillance and meteorological data for Northern Ireland over the period 1999–2009. Vet Parasitol. 190, 167–77. [DOI] [PubMed] [Google Scholar]
- Meason B, Paterson R, 2014. Chikungunya, climate change, and human rights. Health Hum Rights. 16, 105–12. [PubMed] [Google Scholar]
- Mehta R, et al. , 2018. The neurological complications of chikungunya virus: A systematic review. Rev Med Virol. 28, e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, et al. , 2016. [The impact of climate change on leishmaniasis in Brazil]. Cien Saude Colet. 21, 263–72. [DOI] [PubMed] [Google Scholar]
- Meng Q, et al. , 2012. Hyperthermia worsens ischaemic brain injury through destruction of microvessels in an embolic model in rats. Int J Hyperthermia. 28, 24–32. [DOI] [PubMed] [Google Scholar]
- Messina JP, et al. , 2019. The current and future global distribution and population at risk of dengue. Nat Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslin R, et al. , 2016. Urban climate versus global climate change-what makes the difference for dengue? Ann N Y Acad Sci. 1382, 56–72. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, et al. , 2011. Lowering barometric pressure aggravates depression-like behavior in rats. Behav Brain Res. 218, 190–3. [DOI] [PubMed] [Google Scholar]
- Morand S, et al. , 2013. Climate variability and outbreaks of infectious diseases in Europe. Sci Rep. 3, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, et al. , 1995. The pathogenic role of the NMDA receptor in hyperthermia-induced seizures in developing rats. Brain Res Dev Brain Res. 84, 204–7. [DOI] [PubMed] [Google Scholar]
- Muresanu DF, et al. , 2010a. Diabetes aggravates heat stress-induced blood-brain barrier breakdown, reduction in cerebral blood flow, edema formation, and brain pathology: possible neuroprotection with growth hormone. Ann N Y Acad Sci. 1199, 15–26. [DOI] [PubMed] [Google Scholar]
- Muresanu DF, et al. , 2010b. Chronic hypertension aggravates heat stress-induced brain damage: possible neuroprotection by cerebrolysin. Acta Neurochir Suppl. 106, 327–33. [DOI] [PubMed] [Google Scholar]
- Myers SS, et al. , 2017. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu Rev Public Health. 38, 259–277. [DOI] [PubMed] [Google Scholar]
- Myhre O, et al. , 2018. Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): Need for novel strategies for mechanisms and causalities. Toxicol Appl Pharmacol. 354, 196–214. [DOI] [PubMed] [Google Scholar]
- Noor R, et al. , 2003. Effects of hyperthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett. 349, 130–2. [DOI] [PubMed] [Google Scholar]
- Noyes PD, et al. , 2009. The toxicology of climate change: environmental contaminants in a warming world. Environ Int. 35, 971–86. [DOI] [PubMed] [Google Scholar]
- Nunneley SA, et al. , 2002. Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol (1985). 92, 846–51. [DOI] [PubMed] [Google Scholar]
- Obradovich N, et al. , 2018. Empirical evidence of mental health risks posed by climate change. Proc Natl Acad Sci U S A. 115, 10953–10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango EA, et al. , 2016. An integrated risk and vulnerability assessment framework for climate change and malaria transmission in East Africa. Malar J. 15, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Brunner JL, 2015. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, 2018. Mitigating Toxic Planktonic Cyanobacterial Blooms in Aquatic Ecosystems Facing Increasing Anthropogenic and Climatic Pressures. Toxins (Basel). 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta MM, et al. , 2017. Brain Gene Expression is Influenced by Incubation Temperature During Leopard Gecko (Eublepharis macularius) Development. J Exp Zool B Mol Dev Evol. 328, 360–370. [DOI] [PubMed] [Google Scholar]
- Paris Agreement. 2015.
- Paterson RRM, Lima N, 2011. Further mycotoxin effects from climate change. Food Res Int. 44, 2555–2566. [Google Scholar]
- Paxson C, et al. , 2012. Five years later: recovery from post traumatic stress and psychological distress among low-income mothers affected by Hurricane Katrina. Soc Sci Med. 74, 150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres TV, et al. , 2016. “Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies”. BMC Pharmacol Toxicol. 17, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsborn R, et al. , 2016. Climatic Drivers of Diarrheagenic Escherichia coli Incidence: A Systematic Review and Meta-analysis. J Infect Dis. 214, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, et al. , 2012. Resilience in the face of disaster: prevalence and longitudinal course of mental disorders following hurricane Ike. PLoS One. 7, e38964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletz J, et al. , 2016. Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury-Implications for toxic effects in the developing brain. Toxicology. 347–349, 1–5. [DOI] [PubMed] [Google Scholar]
- Plumlee KH, Galey FD, 1994. Neurotoxic mycotoxins: a review of fungal toxins that cause neurological disease in large animals. J Vet Intern Med. 8, 49–54. [DOI] [PubMed] [Google Scholar]
- Pomara C, et al. , 2015. Neurotoxicity by synthetic androgen steroids: oxidative stress, apoptosis, and neuropathology: A review. Curr Neuropharmacol. 13, 132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porojan C, et al. , 2016. Overview of the potent cyanobacterial neurotoxin beta-methylamino-L-alanine (BMAA) and its analytical determination. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 33, 1570–1586. [DOI] [PubMed] [Google Scholar]
- Portier CJ, et al. , 2010. A human health perspective on climate change: A report outlining the research needs on the human health effects of climate change. Research Triangle Park, NC: Environmental Health Perspectives/National Institute of Environmental Health Sciences; . https://doi.org/10.1289/ehp.1002272 , Retrieved from https://doi.org/10.1289/ehp.1002272www.niehs.nih.gov/climatereport, Retrieved from www.niehs.nih.gov/climatereport. [Google Scholar]
- Postels DG, Birbeck GL, 2013. Cerebral malaria. Handb Clin Neurol. 114, 91–102. [DOI] [PubMed] [Google Scholar]
- Potapowicz J, et al. , 2019. The influence of global climate change on the environmental fate of anthropogenic pollution released from the permafrost: Part I. Case study of Antarctica. Sci Total Environ. 651, 1534–1548. [DOI] [PubMed] [Google Scholar]
- Purse BV, et al. , 2017. How will climate change pathways and mitigation options alter incidence of vector-borne diseases? A framework for leishmaniasis in South and Meso-America. PLoS One. 12, e0183583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, et al. , 2014. Effects of short-term environmental hyperthermia on patterns of cerebral blood flow. Physiol Behav. 128, 99–107. [DOI] [PubMed] [Google Scholar]
- Qian S, et al. , 2013. Altered topological patterns of large-scale brain functional networks during passive hyperthermia. Brain Cogn. 83, 121–31. [DOI] [PubMed] [Google Scholar]
- Qu L, et al. , 2007. Hyperthermia decreases GABAergic synaptic transmission in hippocampal neurons of immature rats. Neurobiol Dis. 27, 320–7. [DOI] [PubMed] [Google Scholar]
- Ramirez C, et al. , 1997. Photoperiod-temperature and neuroblast proliferation-migration in the adult lizard cortex. Neuroreport. 8, 2337–42. [DOI] [PubMed] [Google Scholar]
- Ramsdell JS, Gulland FM, 2014. Domoic acid epileptic disease. Mar Drugs. 12, 1185–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell JS, Zabka TS, 2008. In utero domoic acid toxicity: a fetal basis to adult disease in the California sea lion (Zalophus californianus). Mar Drugs. 6, 262–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaseelan AM, et al. , 2018. Effects of Mycotoxins on Neuropsychiatric Symptoms and Immune Processes. Clin Ther. 40, 903–917. [DOI] [PubMed] [Google Scholar]
- Ready PD, 2008. Leishmaniasis emergence and climate change. Rev Sci Tech. 27, 399–412. [PubMed] [Google Scholar]
- Riba I, et al. , 2004. The influence of pH and salinity on the toxicity of heavy metals in sediment to the estuarine clam Ruditapes philippinarum. Environ Toxicol Chem. 23, 1100–7. [DOI] [PubMed] [Google Scholar]
- Rivadeneyra-Dominguez E, Rodriguez-Landa JF, 2014. Cycads and their association with certain neurodegenerative diseases. Neurologia. 29, 517–22. [DOI] [PubMed] [Google Scholar]
- Rohrbach LA, et al. , 2009. Impact of hurricane Rita on adolescent substance use. Psychiatry. 72, 222–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, et al. , 2018. Ambient temperature and age-related notified Campylobacter infection in Israel: A 12-year time series study. Environ Res. 164, 539–545. [DOI] [PubMed] [Google Scholar]
- Ross EZ, et al. , 2012. Cerebrovascular and corticomotor function during progressive passive hyperthermia in humans. J Appl Physiol (1985). 112, 748–58. [DOI] [PubMed] [Google Scholar]
- Russo FB, Beltrao-Braga PCB, 2017. The impact of Zika virus in the brain. Biochem Biophys Res Commun. 492, 603–607. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, et al. , 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 13, e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, et al. , 2012. Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J Neurotrauma. 29, 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, et al. , 2019. Lowering barometric pressure induces neuronal activation in the superior vestibular nucleus in mice. PLoS One. 14, e0211297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt J, et al. , 2013. Influence of temperature changes on migraine occurrence in Germany. Int J Biometeorol. 57, 649–54. [DOI] [PubMed] [Google Scholar]
- Schiedek D, et al. , 2007. Interactions between climate change and contaminants. Mar Pollut Bull. 54, 1845–56. [DOI] [PubMed] [Google Scholar]
- Schwartz RM, et al. , 2017. Longitudinal Impact of Hurricane Sandy Exposure on Mental Health Symptoms. Int J Environ Res Public Health. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza JC, Suk JE, 2018. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi C, et al. , 2019. Tuberculosis evolution and climate change: How much work is ahead? Acta Trop. 190, 157–158. [DOI] [PubMed] [Google Scholar]
- Sharma HS, 2006. Hyperthermia influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. An experimental study in the rat using behavioural, biochemical, pharmacological, and morphological approaches. J Neural Transm (Vienna). 113, 497–519. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Hoopes PJ, 2003. Hyperthermia induced pathophysiology of the central nervous system. Int J Hyperthermia. 19, 325–54. [DOI] [PubMed] [Google Scholar]
- Sharma HS, et al. , 2010. Cerebrolysin reduces blood-cerebrospinal fluid barrier permeability change, brain pathology, and functional deficits following traumatic brain injury in the rat. Ann N Y Acad Sci. 1199, 125–37. [DOI] [PubMed] [Google Scholar]
- Singh G, et al. , 2018. Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front Genet. 9, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimen IB, et al. , 2014. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 30, 513–23. [DOI] [PubMed] [Google Scholar]
- Soneja S, et al. , 2016. Extreme precipitation events and increased risk of campylobacteriosis in Maryland, U.S.A. Environ Res. 149, 216–221. [DOI] [PubMed] [Google Scholar]
- Song YJ, et al. , 2018. The Epidemiological Influence of Climatic Factors on Shigellosis Incidence Rates in Korea. Int J Environ Res Public Health. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire SA, Ryan U, 2017. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors. 10, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram RJ, et al. , 2017. The impact of air pollution to central nervous system in children and adults. Neuro Endocrinol Lett. 38, 389–396. [PubMed] [Google Scholar]
- Stanaway JD, et al. , 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. The Lancet Infectious Diseases. 16, 712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchomelova L, et al. , 2015. Hyperthermia aggravates status epilepticus-induced epileptogenesis and neuronal loss in immature rats. Neuroscience. 305, 209–24. [DOI] [PubMed] [Google Scholar]
- Suen WW, et al. , 2014. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses.6, 2796–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, et al. , 2013. Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One. 8, e61157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Dadvand P, 2019. Pre-natal brain development as a target for urban air pollution. Basic Clin Pharmacol Toxicol. [DOI] [PubMed] [Google Scholar]
- Taylor VF, et al. , 2019. Organic carbon content drives methylmercury levels in the water column and in estuarine food webs across latitudes in the Northeast United States. Environ Pollut. 246, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terciolo C, et al. , 2018. Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins. Food Chem Toxicol. 121, 701–714. [DOI] [PubMed] [Google Scholar]
- Thornton C, et al. , 2017. Oxidative stress and endoplasmic reticulum (ER) stress in the development of neonatal hypoxic-ischaemic brain injury. Biochem Soc Trans. 45, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus DJ, et al. , 2015. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp Neurol. 263, 254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden NB, et al. , 2017. Modelling the effects of global climate change on Chikungunya transmission in the 21(st) century. Sci Rep. 7, 3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolins M, et al. , 2014. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 80, 303–14. [DOI] [PubMed] [Google Scholar]
- Tong S, Ebi K, 2019. Preventing and mitigating health risks of climate change. Environ Res. 174, 9–13. [DOI] [PubMed] [Google Scholar]
- Torres JM, Casey JA, 2017. The centrality of social ties to climate migration and mental health. BMC Public Health. 17, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley J, et al. , 2017. Climate Change and Mental Health. Am J Nurs. 117, 44–52. [DOI] [PubMed] [Google Scholar]
- Van Oostdam J, et al. , 2005. Human health implications of environmental contaminants in Arctic Canada: A review. Sci Total Environ. 351–352, 165–246. [DOI] [PubMed] [Google Scholar]
- Veenema TG, et al. , 2017. Climate Change-Related Water Disasters’ Impact on Population Health. J Nurs Scholarsh. 49, 625–634. [DOI] [PubMed] [Google Scholar]
- Velasco J, et al. , 2018. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos Trans R Soc Lond B Biol Sci. 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JT, 2018. The influence of climate change on waterborne disease and Legionella: a review. Perspect Public Health. 138, 282–286. [DOI] [PubMed] [Google Scholar]
- Wang DZ, 2008. Neurotoxins from marine dinoflagellates: a brief review. Mar Drugs. 6, 349–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, et al. , 2018. Associations of Salmonella hospitalizations with ambient temperature, humidity and rainfall in Hong Kong. Environ Int. 120, 223–230. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. , 2007. Thermal disruption of mushroom body development and odor learning in Drosophila. PLoS One. 2, e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, et al. , 2011. Hyperthermia induces epileptiform discharges in cultured rat cortical neurons. Brain Res. 1417, 87–102. [DOI] [PubMed] [Google Scholar]
- Watkins SM, et al. , 2008. Neurotoxic shellfish poisoning. Mar Drugs. 6, 431–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver HJ, et al. , 2010. Soil-transmitted helminthiases: implications of climate change and human behavior. Trends Parasitol. 26, 574–81. [DOI] [PubMed] [Google Scholar]
- Weiss B, 2012. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology. 33, 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K, et al. , 2019. Salmonella and the changing environment: systematic review using New York State as a model. J Water Health. 17, 179–195. [DOI] [PubMed] [Google Scholar]
- White MG, et al. , 2012. Mitochondrial dysfunction induced by heat stress in cultured rat CNS neurons. J Neurophysiol. 108, 2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. COP24 Special report: Health & Climate Change. [Google Scholar]
- Winneke G, 2011. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci. 308, 9–15. [DOI] [PubMed] [Google Scholar]
- Woodward A, 2019. Climate change: Disruption, risk and opportunity. Global Transitions. 1, 44–49. [Google Scholar]
- Yan C, et al. , 2016. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasit Vectors. 9, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Bergquist R, 2018. Potential Impact of Climate Change on Schistosomiasis: A Global Assessment Attempt. Trop Med Infect Dis. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YT, Sarfaty M, 2016. Zika virus: A call to action for physicians in the era of climate change. Prev Med Rep. 4, 444–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, et al. , 2016. Short-term effects of floods on Japanese encephalitis in Nanchong, China, 2007–2012: A time-stratified case-crossover study. Sci Total Environ. 563–564, 1105–10. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. , 2017. Environmental Drivers and Predicted Risk of Bacillary Dysentery in Southwest China. Int J Environ Res Public Health. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, et al. , 2019. Measuring urban vulnerability to climate change using an integrated approach, assessing climate risks in Beijing. PeerJ. 7, e7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YB, et al. , 2016. The Three Gorges Dam: Does it accelerate or delay the progress towards eliminating transmission of schistosomiasis in China? Infect Dis Poverty. 5, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, et al. , 2017. Schistosoma japonicum transmission risk maps at present and under climate change in mainland China. PLoS Negl Trop Dis. 11, e0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, et al. , 2010. The effect of hyperthermia on blood glutamate levels. Anesth Analg. 111, 1497–504. [DOI] [PubMed] [Google Scholar]