Abstract
Genetic transformation of host plants by Agrobacterium tumefaciens and related species represents a unique model for natural horizontal gene transfer. Almost five decades of studying the molecular interactions between Agrobacterium and its host cells have yielded countless fundamental insights into bacterial and plant biology, even though several steps of the DNA transfer process remain poorly understood. Agrobacterium spp. may utilize different pathways for transferring DNA, which likely reflects the very wide host range of Agrobacterium. Furthermore, closely related bacterial species, such as rhizobia, are able to transfer DNA to host plant cells when they are provided with Agrobacterium DNA transfer machinery and T-DNA. Homologs of Agrobacterium virulence genes are found in many bacterial genomes, but only one non-Agrobacterium bacterial strain, Rhizobium etli CFN42, harbors a complete set of virulence genes and can mediate plant genetic transformation when carrying a T-DNA-containing plasmid.
Keywords: Agrobacterium, bacterium–plant interactions, horizontal gene transfer, macromolecular transport
INTRODUCTION
Agrobacterium tumefaciens is often described as a natural genetic engineer, equipped to horizontally transfer bacterial genes and genetically transform plant cells (45). Indeed, transfer of genetic material from A. tumefaciens and related species to their host plants represents the first known case of active horizontal gene transfer from prokarya to eukarya. The main factors conferring this ability to A. tumefaciens are located on the large Ti (tumor-inducing) plasmid, which contains a region with the vir (virulence) genes encoding most of the proteins required to mediate the DNA transfer and the T-DNA (transferred DNA) itself. The T-DNA sequences naturally transferred by several Agrobacterium spp. contain two types of genes under the control of promoters compatible with expression in eukaryotic cells. The first set of genes (oncogenes) encodes proteins that affect the biosynthesis of or plant cell response to growth regulators (auxins and cytokinins) and induce uncontrolled cellular division, resulting in tissue proliferation and formation of neoplastic growths (crown galls). The second set of genes encodes enzymes involved in the synthesis of small molecules (opines) composed of an amino acid and an organic acid or a carbohydrate, which can be used by Agrobacterium cells as a source of carbon and nitrogen (36). The ability of Agrobacterium to transfer DNA, either for transient expression or stable genetic transformation, is widely used as a tool in research and biotechnology (11). Although the mechanism of T-DNA transfer and integration has been studied extensively from the early 1970s (91), there are still many aspects of the process that are not completely understood. Recent discoveries indicate that there is a potentially important variability in the pathways used by Agrobacterium strains to deliver the T-DNA to the plant genome, which may also reflect adaptation to different hosts. Furthermore, the presence of homologs of the Agrobacterium genes required for virulence in related bacterial species suggests that DNA transfer to eukaryotes might be more widespread among bacteria outside the Agrobacterium genus.
MECHANISM OF AGROBACTERIUM-MEDIATED DNA TRANSFER AND INTEGRATION
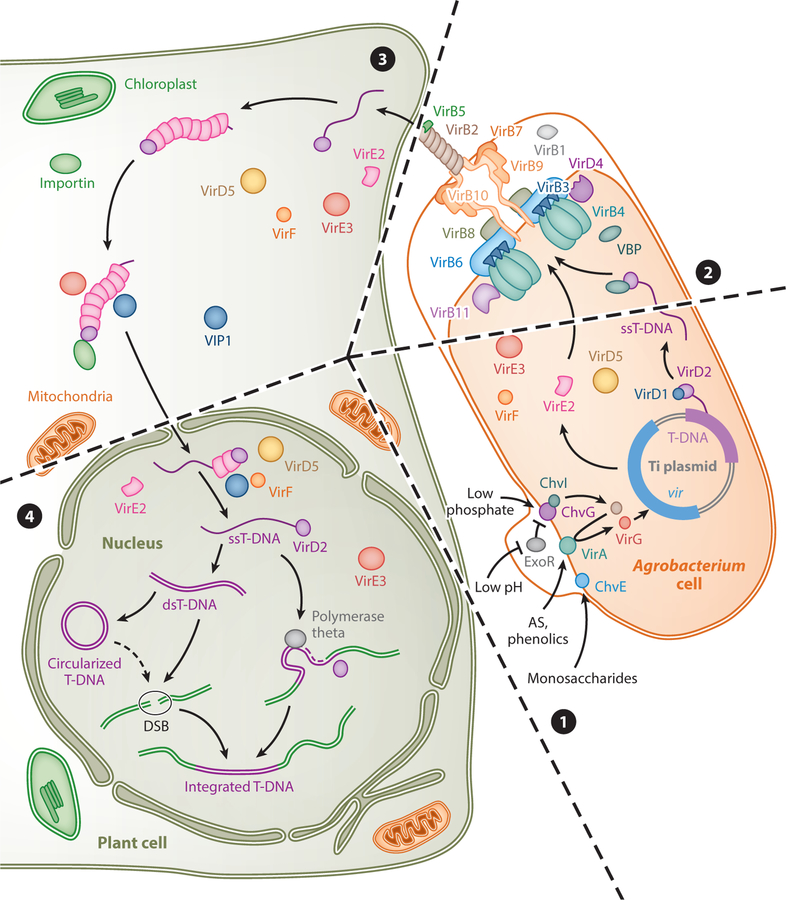
A. tumefaciens interactions with host plant cells represent a reference model for the transfer of DNA from bacteria to eukaryotic cell. The molecular mechanism of T-DNA transfer from Agrobacterium to its host plant cell genome has been reviewed in detail in several articles (45, 65); here, we provide a brief account of the current state of knowledge of this system. Several Agrobacterium species are known to genetically transform plants, resulting in distinct plant diseases (36); however, most of the research on the mechanism of DNA transfer has focused on a few strains of A. tumefaciens (i.e., the nopaline C58 and octopine A6 strains), which are presented in this section. For the purpose of this review, we divided the process of transfer of DNA from A. tumefaciens to its host cell genome into four steps (see Figure 1 for an overview).
Figure 1.
Schematic representation of the main steps of T-DNA transfer from Agrobacterium to the plant cell genome. Circled numbers represent the major steps in the pathway. ❶ Plant-derived and environmental signals activate the bacterial virulence system, resulting in the induction of vir (virulence) gene expression and the generation of the single-stranded T-DNA. ❷ The T-DNA covalently attaches to VirD2, and several vir-encoded effector proteins (VirD5, VirE2, VirE3, and VirF) are exported out of the bacterial cell via the VirB/VirD4 T4SS. ❸ T-DNA and effector proteins enter in the plant cell and are targeted into the nucleus. ❹ The T-DNA is processed in the nucleus and integrated into the plant cell chromosomal DNA. Abbreviations: AS, acetosyringone; Chv, chromosomal virulence protein; DSB, double-strand break; dsT-DNA, double-stranded transferred DNA; Exo, exocellular; ssT-DNA, single-stranded transferred DNA; T-DNA, transferred DNA; T4SS, type IV secretion system; Ti plasmid, tumor-inducing plasmid; vir, virulence gene region; Vir, virulence protein; VBP, VirD2 binding protein; VIP1, VirE2 interacting protein 1; VIP2, VirE2 interacting protein 2.
Step 1: Virulence Induction and Generation of Single-Stranded T-DNA
Upon induction by plant-emitted and environmental signals, the expression of Agrobacterium vir genes is activated, resulting in the synthesis of proteins required for DNA transfer and the generation of the single-stranded T-DNA.
Signal recognition and integration.
Agrobacterium cells can detect several plant-emitted signals and respond by modifying their lifestyle and adjusting the transcription level of their vir genes. The key regulator of vir gene expression is the two-component receptor system encoded by the virA and virG genes (120). The first-identified and major plant-produced molecule involved in vir activation is acetosyringone (AS; 3,5-dimethoxyacetophenone), a phenolic compound often found in plant exudates; AS activates the VirA/VirG two-component system, resulting in the induction of the vir gene expression (17, 118). Although virA and virG are expressed at low levels without induction, they are themselves inducible by AS (141), and VirA/VirG activation results in rapid and strong induction of all the vir genes. Reducing sugar monomers, such as d-glucose and d-galactose, can both increase the sensitivity of the VirA/VirG system to phenolics and elevate the saturating concentration of phenolics for virulence activation (21, 116). These monosaccharides bind to the periplasmic chromosome-encoded protein ChvE, which then interacts with the VirA periplasmic domain to enhance its vir gene–inducing activity (21, 115). VirA is also activated by low pH (between pH 5 and 6) either directly (88) or via its interaction with ChvE (41). In addition, low pH combined with low phosphate concentration activates a different two-component regulatory system (ChvG/ChvI), which results in increased expression of virG (24). The response to low pH relies on the periplasmic ChvG inhibitor ExoR, which is degraded under acidic conditions (52).
Activating vir gene expression.
Upon activation of the VirA/VirG system, VirG is phosphorylated and induces vir gene expression by direct binding to a 10- to 12-bp sequence (vir box) (121). One or several vir boxes are found in promoter regions of each of the vir operons, usually located between 50 and 200 bp upstream of the translation initiation of the first gene of each vir operon. Expression of vir promoters is observed a few hours after the initial induction by phenolics, and it generally reaches a plateau after 12 to 24 hours (141). In addition to several signaling pathways that converge to generate the activated (phosphorylated) VirG, there are other factors affecting vir gene expression. For example, the virC and virD operons are repressed by Ros, a chromosome-encoded transcriptional regulator (30). More recently, small RNAs regulating some of the vir genes were identified (33).
Turning off vir gene expression.
After successful infection of a host plant by Agrobacterium cells, i.e., when the virulence system is no longer needed, the virulence system should be shut off. Indeed, the energy cost of virulence induction is high, and the activation of virulence results in a decrease in the population growth rate (103). Among potential factors negatively affecting vir gene expression, IAA (indole acetic acid) interferes with vir gene induction, probably as a competitive inhibitor of AS binding to VirA (79). The role of IAA, synthesized at high levels during the development of Agrobacterium-induced crown gall tumors, could be to turn off virulence induction as well as to inhibit additional transformation by competing bacterial strains or by the initially infecting strain. In addition, the change of lifestyle of the bacteria between free-living, nonpathogenic bacteria and pathogenic cells attached to the plant cell surface and embedded in the biofilm matrix may also affect the vir gene expression.
Generation of single-stranded T-DNA.
The T-DNA is a segment of the Ti plasmid, delimited by two short (24–25 bp) direct repeat sequences, the left border (LB) and right border (RB) (102, 144). T-DNA is mobilized from the Ti plasmid and transferred into the host cell as a single-stranded DNA (ssDNA) intermediate, termed the T-strand (119). Two essential proteins for T-DNA processing are VirD1 and VirD2. VirD2 is an endonuclease (2, 147), which, in association with the VirD1 DNA topoisomerase (47), mediates the mobilization of the transferable T-DNA from the Ti plasmid via a strand replacement mechanism. Importantly, the T-DNA borders are the only sequences required for recognition by VirD2/VirD1, and, thus, the sequences between these borders may be modified at will. Two other Vir proteins, VirC1 and VirC2, have been shown to increase the number of T-strand molecules, most likely by binding to sequences, termed overdrive, close to the T-DNA borders (34). At the end of the process, VirD2 remains covalently linked to the 5′ end of the T-strand (148), forming an immature T-complex.
Step 2: Export of the T-DNA and Effector Proteins and Cell-to-Cell Interactions
Macromolecules are translocated across the bacterial membranes via a T4SS (type IV secretion system) composed of the 11 proteins encoded by the virB operon and VirD4 by a mechanism closely resembling plasmid transfer during bacterial conjugation. T4SSs are molecular complexes that mediate the transport of proteins and nucleoprotein complexes, usually comprising an ssDNA with a protein molecule at its 5′ end, through the membranes and cell walls of gram-negative bacteria (27).
Targeting of exported macromolecules to type IV secretion system.
Interactions with bacterial factors are likely required to mediate targeting of the exported substrates—i.e., the VirD2–T-strand complex and the effector proteins VirD5, VirE2, VirE3, and VirF—to the VirB/D4 T4SS. Protein export from Agrobacterium may occur independently of DNA export, and it depends on the presence of an arginine-rich export signal found in all exported Vir proteins (133, 134). Several factors have been suggested to mediate targeting of the VirD2–T-strand complex and individual translocated proteins to the T4SS machinery. For example, VirC1 and VirC2 may assist targeting of VirD2 and the T-strand to the bacterial membrane at the cell poles where T4SS is thought to be assembled (7). VirE2 might be recruited to the cell poles via its association with the coupling protein VirD4 (8). Other bacterial factors, VBPs (VirD2 binding proteins), appear important for VirD2 recruitment to T4SS as well as for recruitment of diverse relaxase proteins in other T4SS systems (51). Indeed, VBPs conserved in Agrobacterium spp. can target VirD2 and the associated T-strand to the energizing components of the T4SS, i.e., VirD4, VirB4, and VirB11, and, thus, to the T-DNA export machinery (49, 51). Furthermore, VBPs, which do not interact with the other exported effector proteins, appear to be important for the recruitment of conjugative DNA transfer intermediates to T4SS during conjugation (51).
For the most part, the transport pathway of the VirD2–T-strand complex through bacterial membranes has been deciphered, and it comprises sequential interactions with different protein components of T4SS (23). The lumen size of the VirB2 pilus of T4SS appears sufficient to accommodate passage of ssDNA and partially unfolded proteins (62); indeed, in other bacterial species, relaxases transported through T4SS channels have been reported to unfold during transport (124).
Attachment and close-range cell-to-cell interactions.
Close-range interaction and attachment between bacterial and host cells are thought to be required for the transfer of T-DNA and effector proteins (84). Although under laboratory conditions, bacterial virulence can be induced without interaction with the host cell surface, in nature these two events are likely linked, and the induction of virulence is coincidental with a change in the bacterial cell lifestyle from free bacteria in the rhizosphere to bacteria attached at the surface of the host cell and embedded in a biofilm. Indeed, the same signals that trigger vir gene expression also induce chemotaxis. Specifically, Agrobacterium cells respond to phenolics and sugars secreted by plants by moving toward their source via chemotaxis (50); at low concentration of these molecules, chemotaxis is activated, whereas high concentrations result in virulence activation. Furthermore, in addition to its positive effect on virulence, low phosphate concentration enhances biofilm formation and cellular adhesion (143).
Analogous to other host–plant interactions, such as Rhizobium–legume symbiosis (106), the cellular interaction is believed to occur in two steps. The initial contact between bacteria and eukaryotic cells usually relies on host cell-surface receptors and represents a reversible interaction. The bacterial attachment is then stabilized via the synthesis of cellulose fibrils, and bacterial cells are embedded in a biofilm at the surface of the plant tissue. In the case of Agrobacterium, the precise role of the different factors affecting attachment during the infection process is not completely understood. The Agrobacterium T4SS components, i.e., VirB2 (pilin) and VirB5, exposed to the bacterial cell surface represent good candidates for interaction with potential host cell receptors (9). Four Arabidopsis proteins were identified to interact with VirB2 (59) and shown to affect the efficiency of the T-DNA transfer. However, it is not clear whether these VirB2-interacting proteins are involved in cellular attachment or in other steps of the DNA transfer process, such as signal transmission or passage of the T-DNA through the host-cell membrane. Interestingly, pilin homologs encoded by Agrobacterium, CtpA and PilA, appear to be involved in the early stages of Agrobacterium cell-surface attachment, although their role in virulence remains unknown (138). VirB5 localizes at the tip of the VirB2 pilus (4) and may have a dual function: (a) during T4SS biogenesis, which requires VirB5 expression in the bacterial cell, and (b) outside the bacterial cell (64), although it plays no obvious role in cellular attachment. Exocellular polysaccharides produced by Agrobacterium are important for attachment and biofilm formation. Synthesis and export of cyclic 1,2-β-d-glucan, which relies on proteins encoded by the chvA, chvB, and exoC genes, are involved in attachment and virulence (22, 32); UPPs (unipolar polysaccharides) and cellulose also may play a role in bacterial adhesion and biofilm formation (83, 143). However, plant receptors that, similarly to the plant lectins facilitating Rhizobium–plant cell recognition (53), bind these exopolysaccharides and are involved in Agrobacterium-mediated transformation have not been identified.
Step 3: Entry and Subcellular Sorting of T-DNA and Effector Proteins in the Host Cell
The entry of T-DNA and effector proteins in the host cell cytoplasm, across plant cell plasma membrane, is not completely understood; different hypotheses are presented below. Multiple interactions with host factors mediate the nuclear import of these macromolecules.
Entry of T-DNA and associated proteins into the host cell cytoplasm.
The mechanism by which the VirD2–T-strand complex and effector proteins pass through the host cell wall and plasma membrane is unknown. Although wounding of the plant tissue enhances the Agrobacterium-mediated transformation efficiency, T-DNA transfer from Agrobacterium without wounding the host plant cell has been reported (18). Several mechanisms are possible for entry through the host plasma membrane. First, similar to a mechanism proposed for bacterial conjugation, depolymerization of the VirB2 pilus may bring the bacterial outer membrane and the host cell plasma membrane together, resulting in temporary membrane fusion and allowing the transfer of cargo (20). Second, the VirB2 pilus may act as a needle via a mechanism similar to type III secretion system (T3SS)-mediated effector protein transport (96); in this scenario, macromolecular substrates pass through the pilus, and the pilus interacts with the host membrane or integral membrane proteins to allow the entry of the cargo. So far, however, no interactions with host membrane proteins or bacterial factors able to form a pore in the host membrane have been identified in the Agrobacterium–host plant cell system. Furthermore, Agrobacterium mutants unable to form pili still retain a low-level virulence, demonstrating that T-DNA transfer can occur in the absence of the VirB2 pilus (108). Third, macromolecules could be first exported into the intercellular space and then internalized by the host cell, for example, via an endocytosis-like mechanism, which might involve recognition between the exported macromolecules and a potential host receptor. Indeed, a recent study suggested that VirE2 associates with early endosomes in the host plant cell and that endocytosis inhibitors affected both VirE2 transport and transformation efficiency (76). Thus, endocytosis might be involved in the internalization of VirE2 and potentially other translocated molecules. In addition, VirE2 has been shown to form channels through artificial membranes (35); although formation of VirE2 pores has not been demonstrated in infected plant cells, such pores might mediate transport of other macromolecules through the host cell membranes.
Nuclear import.
Before integration can occur, the T-DNA, as well as translocated effector proteins with a nuclear function, must be imported into the nucleus. Efficient nuclear import via simple diffusion is unlikely for large molecules such as T-strands. Genetic transformation of plant cells using protocols that do not involve Agrobacterium implies that nuclear import of foreign DNA can occur without exogenous effector proteins, most likely using cellular DNA-binding proteins that facilitate import; such transformation techniques are considered less efficient than the Agrobacterium-mediated transformation, although it is difficult to compare efficiency between such different methods. The nuclear import step can be circumvented altogether if the transformation process occurs during cell division, when the nuclear envelope is disrupted (135). However, transient expression of T-DNA, which obviously requires its nuclear import, occurs efficiently in nondividing cells following agroinfiltration (146). Thus, active nuclear import most likely is involved in most cases of Agrobacterium-mediated genetic transformation. Generally, bacterial proteins interacting with the T-DNA are presumed to mediate its nuclear uptake via the importin alpha-mediated import pathway. First, VirD2, attached to the 5′ end of the T-DNA, interacts directly with importin alpha via its NLS sequences and is targeted to the host cell nucleus (10). VirE2, an ssDNA-binding protein, is also thought to interact with the T-DNA after its entry in the host cell cytoplasm, forming the mature T-complex (28, 44). Although the VirE2–T-strand complex has not been directly shown to form in living cells, a significant amount of data suggests that such formation occurs. First, the T-DNA integrated in absence of VirE2 displays increased truncations, suggesting that VirE2 associates with and protects the T-strand against degradation (107). VirE2 then has a strong affinity for ssDNA in vitro (26, 28), producing helical ssDNA–VirE2 filaments with well-defined structure (1). Initially, several studies demonstrated that VirE2 tagged with different markers was, at least partially, targeted to the nucleus in plant cells (29, 78, 151). Other studies showed that fusion of VirE2 with fluorescent proteins remained largely cytoplasmic (73, 113). Because of its strong homopolymerization, VirE2 tends to form aggregates when expressed ectopically in plant cells, which hinders assessment of its localization; it is also possible that only a fraction of VirE2 is directed to the nucleus but that this fraction is sufficient for VirE2 functionality in the T-strand import process. VirE2 was shown to interact with several plant proteins likely to affect its intracellular distribution and/or function: VIP1 (VirE2 interacting protein 1) (126), VIP2 (VirE2 interacting protein 2) (5), importins alpha (14), and core histones (69, 81). Furthermore, VirE2 also interacts with VirE3 (71, 77), and this interaction likely assists accumulation of VirE2 at the sites of entry into the host cell (77) and/or subsequent nuclear import of VirE2 (71). Both VirD2 and VirE2 have been shown to mediate nuclear import of short segments of ssDNA (152). Potentially, these two proteins participate in T-DNA nuclear import; VirD2, alone or with the help of VirE2, targets the T-strand to the nuclear pores, whereas VirE2 packages the T-strand and mediates its movement through host cell cytoplasm (152) and through the nuclear pore. It has also been suggested that the T-strand and its associated proteins could interact with the host cell cytoskeleton and endoplasmic reticulum during its transport to the nucleus (145).
Step 4: T-DNA Integration in the Host Chromosomal DNA
The mechanism of T-DNA integration into the host genome remains largely obscure (46). We first present the main known facts about the integration process and then incorporate them into potential integration pathways. Two main approaches have been used to characterize T-DNA integration: (a) analysis of the locations of the integrated T-DNA and its patterns of integration and (b) studies of plant and bacterial factors that may affect integration. In the first approach, early studies showed that integrated T-DNAs were preferentially located in transcriptionally active chromatin (3); however, these studies relied on analyses of transgenic plants regenerated under antibiotic selection. This made it virtually impossible to detect integration into heterochromatin, which does not support the expression of the antibiotic-resistant reporter. Indeed, a completely different result was obtained in studies performed without selection, which showed that T-DNA integrated randomly in all regions of chromatin (63), although a local bias might occur toward specific epigenetic markers (114). Nucleosomal histones have been suggested to be involved in the targeting of the T-DNA complex to the host chromatin by allowing interaction between the T-complex and the host chromatin before integration. First, histone H2A was found to be important for T-DNA integration, as an Arabidopsis mutant in this gene displayed lower transformation efficiency (90) and, later, other histones were shown to increase T-DNA integration (122). Interaction between VIP1 and different histones was also demonstrated (81), and VirD2 was found to interact with histones (142).
Unlike many integrating viruses, Agrobacterium does not encode a dedicated integrase among its effector proteins. Although early studies suggested that VirD2 might act as an integrase (98, 123); integration was later shown to be mediated by host factors (153). Yet it cannot be excluded that VirD2 or another Agrobacterium translocated effector protein facilitates T-DNA integration by interacting with the host factors that directly mediate integration. Furthermore, the analysis of integration in various host species, particularly with different yeast mutants, has shown that the integration of T-DNA relies mostly on the host cell pathways. Several studies have suggested a role for the host cell DNA repair pathways in T-DNA integration, and double-strand breaks (DSBs) were shown to represent preferred sites for T-DNA integration (25, 110, 125). Measuring T-DNA integration rates in Arabidopsis mutants in different genes encoding DNA repair proteins yielded inconclusive results (see below). Using a combination of these two approaches, it was recently reported that an Arabidopsis mutant in the DNA polymerase theta was deficient in T-DNA integration, suggesting an integration mechanism based on microhomologies (132). DNA polymerase theta was first identified as a suppressor of genome instability, and it is known for its role in genomic DNA ligation in the microhomology-mediated end-joining [MMEJ; or alternative end-joining (alt-EJ)] DSB repair pathway (16). However, this mechanism does not explain the integration of double-stranded T-DNA and recombination between several T-DNAs in different orientation, suggesting that several concurrent integration pathways may underlie transformation events (46).
Potential pathways for T-DNA integration.
T-DNA enters the nucleus as a segment of ssDNA; it may either be converted to a double-stranded DNA (dsDNA) before integration, most likely into a DSB in the genomic DNA, or anneal partially to the host genomic DNA via microhomologies before synthesis of its second strand and ligation. There is direct proof that T-DNA can integrate into DSBs as a dsDNA; by introducing a rare cutting dsDNA endonuclease site in both the T-DNA and the host genome and transiently expressing this enzyme, precise reconstruction of the original restriction site at junctions between T-DNA and host DNA was observed (25, 125). Interestingly, it has been shown that the formation of circularized T-DNA (T-circles) occurs after T-DNA transfer into the plant cell (117), although there is no indication that these T-circles act as a substrate for integration. The observation of microhomologies at the junction of some integration sites suggests that the second pathway is also possible, and recent implication of DNA polymerase theta in T-DNA integration (132) shows involvement of this pathway in integration.
Experiments using T-DNA transfer into yeast (Saccharomyces cerevisiae)—this model host allows the use of numerous viable mutants in different DSB repair pathways—demonstrated that the integration pathway depends mostly on the host mechanisms. Taking advantage of the ability of yeast cells to support DNA integration via either homologous recombination (HR) or nonhomologous end-joining (NHEJ), depending on the presence of homologous sequences in the target genome and the T-DNA, T-DNA integration was assessed in mutants impaired in these pathways. Disruption of Rad52 or Rad51 resulted in integration only via NHEJ, whereas in the absence of Ku70 or Mre11 expression, only integration via HR was observed (130, 131). In plants, HR occurs only at extremely low rates (48, 85), and NHEJ is believed to be the main pathway for foreign DNA integration. However, studies using Arabidopsis mutants in the NHEJ pathways yielded conflicting results. AtLig4 and AtKu80 were found to be dispensable for T-DNA integration in one study (40) but were required in two other studies (39, 75). More recently, a systematic survey of Arabidopsis mutants impaired in different genes involved in the known pathways of NHEJ reported that T-DNA integration efficiency was not reduced in any of these lines, and it was even increased in some of the mutants (100). In rice, however, reduced rates of overall integration were observed in plant lines with downregulated Ku70, Ku80, and Lig4 (93). Because of high levels of redundancy between DNA repair pathways, it is difficult to prove their specific involvement in T-DNA integration. Yet when several NHEJ pathways were mutated in Arabidopsis, the resulting viable plants supported only very low levels of T-DNA integration (89). The involvement of DSB repair pathways in T-DNA integration also appears to be complex and may vary at different time points during the infection process. For example, targeting of the incoming T-DNA to open DSBs may be achieved in a less efficient repair pathway, but subsequent ligation of the T-DNA into the DSB may require efficient DSB repair. Furthermore, the host NHEJ machinery may be manipulated by Agrobacterium effector proteins; for example, VirE2 interacted with XRCC4, a component of the NHEJ pathway, and potentially prevented DSB repair, allowing the T-DNA to be targeted to an available DSB site (129). Other host nuclear proteins, such as the transcriptional regulator VIP2 (VirE2 interacting protein 2), might play a role in T-DNA integration (5). Finally, histone post-translational modification (specifically, methylation) was shown to affect T-DNA integration (60).
VARIABILITY OF THE MOLECULAR PATHWAY FOR AGROBACTERIUM INFECTION
Different strains and species of Agrobacterium use different pathways for the transfer of DNA to different eukaryotic organisms. Besides the wide range of plant species that serve as hosts to Agrobacterium spp. in nature (31), under artificial conditions this range extends further to species from all the clades of the plant kingdom (92) as well as to non-plant cells, such as yeast, other fungi, and animal cultured cells (70 and references therein). This possibility to transfer DNA to virtually all eukaryotic cell types (70) reflects Agrobacterium adaptability beyond plant-specific factors.
Essential and Optional Virulence-Associated Genes
Agrobacterium’s virulence factors fall into three main categories: (a) the core factors absolutely essential for T-DNA transfer, i.e., the two-component regulatory system (VirA, VirG), the T-strand processing machinery (VirD1, VirD2), and T4SS (VirB1-VirB11, VirD4); (b) the important but not absolutely essential factors, such that in their absence the T-DNA transfer occurs only at very low efficiency, i.e., VirE2 effector, VirC1, and VirC2; and (c) the nonessential factors that likely play a role in determination of host range and/or in further facilitating infection, for example, in competition with other microorganisms, i.e., the effector proteins VirD3, VirD5, VirE3, and VirF as well as some bacterial strains containing additional Vir proteins that fall into this nonessential category, such as VirH, VirJ, VirK, VirL, and VirM. Several proteins, usually termed Chv, encoded by the bacterial chromosome also play an important role in Agrobacterium interactions with plant cells; they are involved in different steps of infection, such as virulence activation (e.g., ChvE, ChvG, ChvI, ChvH) or cellular adhesion and biofilm formation (e.g., ChvB, ChvA, ExoC). Finally, in addition to the Ti plasmid, some Agrobacterium strains carry a large At plasmid; its function appears not essential for DNA transfer, although it encodes factors with activities related to survival in the competitive rhizosphere environment, such as quorum-sensing mechanisms that regulate plasmid exchange in bacterial populations (104). Although all species and strains of Agrobacterium share a common general mechanism for T-DNA transfer, there is a certain degree of variability between them, which translates into differences in the bacterial virulence factors and affects the outcome of infection. As described above, the transfer of T-DNA by Agrobacterium relies on a core of essential factors. Presumably, the function of these proteins is conserved between different virulent Agrobacterium strains, although they may interact with different host factors. Nonessential genes were defined based on virulence of the corresponding mutants in highly susceptible hosts, such as tobacco or kalanchoe (57). Thus, although they are not absolutely required for transformation of these plants, they may be necessary to infect other plant species or they may provide a competitive advantage to achieve successful infection in nature.
Variability in Inducers and Repressors of the vir Genes
The outcome of the interactions between a specific Agrobacterium strain and its specific host plant is also affected by the signal molecules emitted by the host and by the response of the bacteria to these signals. Perception of inducing signals varies between different Agrobacterium strains, which may reflect adaptation to specific hosts. A wide variety of phenolic compounds, related to AS, can activate vir gene expression (87), including glycoside derivatives (61). Genetic studies identified the protein able to recognize these phenolic compounds as VirA via swapping virA genes between different strains of Agrobacterium, thereby modifying the range of recognized phenolic molecules (74). That different Agrobacterium strains show variable responses to different phenolic compounds may confer onto each strain a specific inducibility corresponding to the phenolics emitted by the strain’s specific host species. This sensing of phenolics by VirA in different Agrobacterium strains may also be affected by specific monosaccharides that are sensed by the chromosomal virulence protein ChvE (101).
Other plant-produced molecules also affect Agrobacterium’s virulence, likely contributing to the variability of T-DNA transfer efficiencies in different plant species or tissues. Among the signal molecules emitted by plants in response to biotic or abiotic stresses, salicylic acid (SA) inhibits vir gene expression, probably by attenuating the VirA protein kinase activity (149). Tobacco or Arabidopsis plants overproducing SA or treated with exogenous SA displayed increased resistance to Agrobacterium, whereas plants deficient in SA accumulation were more sensitive (6, 149). Furthermore, ethylene might also inhibit Agrobacterium’s virulence, although its direct effect on vir gene expression has not been demonstrated (95). Some plant species emit chemicals that inhibit Agrobacterium virulence, most likely contributing to the variability of susceptibility of different species to Agrobacterium. For example, DIMBOA [2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazine-3(4H)-one] and MDIBOA (2-hydroxy-4,7-dimethoxybenzoxazin-3-one), two chemical compounds found in maize homogenates, are strong inhibitors of Agrobacterium AS-induced virulence and growth (109).
VirE2 versus GALLS
VirE2 is not absolutely essential for Agrobacterium-mediated transformation, although transformation efficiency of virE2 mutants is very low and results in a greater proportion of truncated T-DNA (107). Interestingly, some strains of Agrobacterium rhizogenes do not contain the virE2 gene but carry the GALLS gene instead, which complements virE2 in a virE2-deficient strain of Agrobacterium, although its mode of action appears to be different from that of VirE2 (54). GALLS encodes two proteins, corresponding to the full-length coding sequence or to its C-terminal part, that interact with VirD2 and are targeted to the host cell nucleus (55). Their exact function in the transformation process remains unknown.
Interaction of VirE2 with VIP1 and its Homologs
The interaction between VirE2 and VIP1 was first discovered via yeast-two-hybrid screening (126). Using transgenic tobacco overexpressing VIP1 from Arabidopsis (AtVIP1), it was shown that VIP1 overexpression increased the transformation rate and that VIP1 likely facilitated the nuclear import of VirE2 and thus of the T-complex (126, 127). However, other studies using Arabidopsis mutants reached a different conclusion, i.e., that VIP1 was not required for Agrobacterium-mediated T-DNA transfer (113). Recently, VirE2 proteins from four different Agrobacterium strains were all shown to interact with AtVIP1 and one or more of its close Arabidopsis homologs; interestingly, binding efficiency for the different AtVIP1 homologs was different among the different VirE2 proteins (137). This interaction between VirE2 and several AtVIP1 homologs was confirmed in a more recent paper (72). This study also showed that plants expressing a dominant negative mutant of AtVIP1, e.g., AtVIP1 fused to an SRDX transcriptional repression domain, did not affect transformation; however, SRDX inhibits only the function of AtVIP1 as transcriptional activator rather than as a VirE2 binding partner. An Arabidopsis mutant with three disrupted AtVIP1 homologs showed a modest reduction in transformation efficiency (72), suggesting that functional redundancy might mask the role of these proteins in transformation.
Involvement of the Ubiquitin–Proteasome System: Roles of VirF
The VirF effector from the A. tumefaciens A6 octopine-type strain (A6-VirF) was shown to contain an F-box domain and bind several Arabidopsis ASK proteins, the Skp1 homologs that function in the SCF pathway for proteasomal degradation (111). The F-box protein activity of A6-VirF was demonstrated in yeast and in plant cells, and one of its potential substrates and interacting partners was identified; specifically, VirF was shown to bind VIP1 and destabilize via the ubiquitin–proteasome system (UPS) both VIP1 and its associated VirE2 (128). More recently, several other Arabidopsis interactors of VirF were identified (42, 43), one of which, VFP4, a transcriptional regulator of genes involved in stress or defense response, was targeted by VirF for proteasomal degradation (42). In addition, VirF itself is destabilized via UPS, and this destabilization is prevented by VirF interaction with another effector, VirD5 (82). Historically, the C58 nopaline-type strain of A. tumefaciens has not been considered to encode an active VirF (86), but more recent data suggested that the C58–VirF can bind Arabidopsis ASK proteins via its predicted F-box domain, which suggests that it is a bona fide F-box protein (66). Unlike A6-VirF, C58-VirF did not interact with VIP1, suggesting that it has a different set of targets. Most bacterial species able to transfer DNA (e.g., A. rhizogenes, Agrobacterium vitis, and R. etli) encode a VirF homolog with potentially different target specificities, likely contributing to the host range specificity of these bacteria. Also, an Arabidopsis F-box protein VBF was shown to substitute to A6-VirF in targeting VIP1 for degradation, potentially explaining the dispensability of VirF for infection of this plant species (150). Interestingly, VBF was among the plant genes whose expression is activated by VirE3, which has transcriptional regulator activity in plants (94).
T-DNA TRANSFER BY NON-AGROBACTERIUM BACTERIAL SPECIES
The most common pathogenic Agrobacterium species include A. tumefaciens, A. rhizogenes, and A. vitis. The taxonomy of Rhizobiaceae is still subject to revision, and we follow the commonly used classification in which Agrobacterium species are named according to their pathogenic interactions with plants (37). Their pathogenicity relies on Ti plasmids or, in the case of A. rhizogenes, on Ri plasmids, which are highly diverse and present a mosaic structure (97). Furthermore, because these plasmids can be transmitted by conjugation within or even between these bacterial species, other non-Agrobacterium species could gain the Ti-plasmid features that allow transfer DNA to eukaryotic hosts.
Agrobacterium T-DNA Transfer Machinery in Related Bacterial Species
Introducing the Agrobacterium vir region and T-DNA into several species of plant-associated bacteria—pathogenic, symbiotic, and nitrogen-fixing—related to Agrobacterium spp. has conferred onto these bacteria the ability to genetically transform plants. So far, all such bacterial species have belonged to two families of the Rhizobiales order, Rhizobiaceae and Phyllobacteriaceae. For example, conjugative transfer of the Ti plasmid from a virulent Agrobacterium strain to Rhizobium trifolii resulted in virulent bacterial cells able to induce crown gall formation in several plant species (56). Several other bacterial species became capable of transforming plants after they have received two plasmids: a helper plasmid, carrying the Agrobacterium vir region, and a binary plasmid with an engineered T-DNA. Using this strategy, Arabidopsis, tobacco, and rice were transformed by three different bacterial species, Rhizobium leguminosarum, R. trifolii, and Phyllobacterium myrsinacearum (19), whereas Sinorhizobium meliloti, Rhizobium sp. NGR234, and Mesorhizobium loti were used to transform potato (140). Similarly, Ensifer adherens (syn. Sinorhizobium adherens), harboring a cointegrated plasmid and carrying the vir region from Agrobacterium and an engineered T-DNA, was able to transform potato and rice plants (139, 154). Thus, all these bacterial species likely possess the chromosomally encoded function required for transformation but not the vir gene functionalities. Attempts to transform plants or other eukaryotes by introducing plasmids carrying a vir region and a T-DNA into bacteria outside of the Rhizobiales order, such as Escherichia coli, have been unsuccessful (80, 99). Agrobacterium spp. are facultative pathogens (12) with a possible transition between pathogenic and nonpathogenic lifestyles. Horizontal gene transfer between bacterial species via the exchange of plasmids by conjugation is well documented (13); thus, vir regioncarrying plasmids may be shared among a pool of related bacteria in the rhizosphere, thereby conferring pathogenicity to the recipient cells.
Rhizobium etli CFN42 Contains Functional vir Genes
Although DNA transfer to plants can be achieved using different bacterial species provided with Agrobacterium’s vir region, these bacterial species do not encode an endogenous complete and functional DNA transfer machinery, making Agrobacterium the only species with natural genetic transformation capability. This notion has been altered by the observations that the Rhizobium etli CFN42 strain, a symbiotic nitrogen-fixing bacterium associated with host plants such as beans, contains in its p42a plasmid a complete and functional vir region and is able to mediate DNA transfer and stable integration into the plant genome, albeit with a low efficiency, when a vector carrying a T-DNA with a reporter gene is provided (67). The R. etli CFN42 strain with mutated virG or virE2 and R. leguminosarum, a very similar bacterial strain that does not contain close homologs of the Agrobacterium vir genes, were incapable of T-DNA transfer. The vir regions of R. etli and Agrobacterium share extensive similarity yet exhibit two significant differences: The R. etli virB2 gene is not a part of the virB operon but constitutes a separated operon with its own promoter, and there are two virF operons in R. etli. Analysis of vir gene expression in R. etli showed a pattern of expression close to that observed in Agrobacterium, notably induction by AS, except for the virB2 gene that was expressed constitutively at low levels and was not induced by AS (136). Interestingly, the R. etli p42a plasmid was shown to be exchanged between R. etli and related species, including Agrobacterium spp. (15). Although R. etli has evolved to encode and preserve the functional vir machinery, it remains unknown whether this species also contains endogenous T-DNA-like sequences that could be transferred to the plant hosts.
CONCLUSIONS
There is a wide diversity in the pathways underlying each step of DNA transfer from pathogenic Agrobacterium and related species. This unique capability relies on a core of essential bacterial factors and on their interactions with different host cell factors. In addition, many other bacterium-encoded proteins represent facultative virulence factors that are not essential for DNA transfer to model plants highly susceptible to Agrobacterium. Rather, these nonessential factors may be required for infection of specific hosts as well as for achieving maximally successful infection in the competitive rhizosphere environment. The virulence genes are mostly located on a large plasmid, transmissible between bacterial cells by conjugation, but functions encoded by the bacterial genome are also important for efficient T-DNA transfer under natural conditions. The diversity of pathways, as well as the large array of bacterial factors presumed to facilitate and optimize infection, likely confers to Agrobacterium spp. their seemingly unlimited range of host cells under natural or experimental conditions.
Our present knowledge of the Agrobacterium-mediated T-DNA transfer raises an interesting question: What constitutes the minimal T-DNA transfer machinery? Among the essential vir-encoded proteins, most represent pathways common to many bacterial species. For example, the virB and virD operons encode a DNA transfer machinery similar to those involved in plasmid transfer by conjugation, and the VirA/VirG sensors regulating expression of vir genes are representative of the widespread bacterial two-component regulatory systems. These common pathways may allow easy addition of the genetic transformation capability by other bacterial species via acquisition of the functional vir region. Such gene transfer possibly occurs within natural bacterial populations, and it would render pathogenic those bacterial species that are not normally considered as such, e.g., rhizobia, as they are usually engaged in symbiotic relationships with their host plants.
Interestingly, by introducing an artificial transferable DNA into several human pathogens, DNA transfer to cultured human cells was achieved under laboratory conditions (38, 112) in a T4SS-dependent manner. Analyses of complete eukaryotic genome sequences, which are becoming increasingly available, have shown that they contain a significant number of sequences originating from prokaryotes and resulting from horizontal gene transfer (58, 68). It makes biological sense that at least some of these sequences have been acquired from bacteria via a mechanism similar to the Agrobacterium-mediated T-DNA transfer. In some cases, bacterial sequences present in genomes from several plant species of the Nicotiana and Linaria genera, as well as sweet potato, can be traced back to their Agrobacterium-like donor bacteria that share homologies with today’s Agrobacterium pathogenic species (reviewed in 105).
UNANSWERED QUESTIONS
Some of the fundamental questions about the mechanism of the Agrobacterium-mediated T-DNA transfer remain unanswered. How do the T-DNA and its associated proteins pass through the host cell plasma membrane? What is the exact role of VirE2, and its interacting plant proteins, in packaging the T-DNA and facilitating T-DNA subcellular transport and fate within the host cell? How are the multiple pathways for T-DNA integration into the host cell genome regulated? Besides plant genetic transformation by Agrobacterium spp., the question is whether other natural cases of DNA transfer, via a similar mechanism, from different bacterial species to their eukaryotic hosts exist or have existed in past evolutionary times and whether such events may have contributed to the gene flux from bacteria to eukaryotes. From a biotechnological viewpoint, a better understanding of DNA transfer mechanisms will help expand our toolbox for Agrobacterium-mediated transformation, for example, for improvement of the genetic transformation of recalcitrant plant species or non-plant eukaryotic cells or for better control of the integration sites and integration patterns within the target genome. In this respect, the ultimate feat would be using synthetic biology to refactor the entire Ti plasmid (and, potentially, even the bacterial chromosome) to eliminate all pathogenic and transformation-unrelated (e.g., bacterial conjugation) abilities and to include nonbacterial (e.g., plant) genes known to facilitate transformation and/or transgene expression by refactoring them for optimal prokaryotic expression and export.
Ti plasmid: tumor-inducing plasmid
vir: virulence
T-DNA: transferred DNA
AS: acetosyringone
IAA: indole acetic acid
LB: left border
RB: right border
ssDNA: single-stranded DNA
T4SS: type IV secretion system
VBPs: VirD2 binding proteins
UPPs: unipolar polysaccharides
T3SS: type III secretion system
VIP1: VirE2 interacting protein 1
VIP2: VirE2 interacting protein 2
DSBs: double-strand breaks
MMEJ: microhomology-mediated end-joining
dsDNA: double-stranded DNA
HR: homologous recombination
NHEJ: nonhomologous end-joining
UPS: ubiquitin–proteasome system
ACKNOWLEDGMENTS
We apologize to our colleagues whose works could not be cited because of format restrictions. The work in the V.C. laboratory is supported by grants from USDA/NIFA, NIH, NSF, and BARD to V.C.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, membership, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abu-Arish A, Frenkiel-Krispin D, Fricke T, Tzfira T, Citovsky V, et al. 2004. Three-dimensional reconstruction of Agrobacterium VirE2 protein with single-stranded DNA. J. Biol. Chem 279:25359–63 [DOI] [PubMed] [Google Scholar]
- 2.Albright LM, Yanofsky MF, Leroux B, Ma DQ, Nester EW. 1987. Processing of the T-DNA of Agrobacterium tumefaciens generates border nicks and linear, single-stranded T-DNA. J. Bacteriol 169:1046–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–57 [DOI] [PubMed] [Google Scholar]
- 4.Aly KA, Baron C. 2007. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 153:3766–75 [DOI] [PubMed] [Google Scholar]
- 5.Anand A, Krichevsky A, Schornack S, Lahaye T, Tzfira T, et al. 2007. Arabidopsis VIRE2 INTERACTING PROTEIN2 is required for Agrobacterium T-DNA integration in plants. Plant Cell 19:1695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand A, Uppalapati SR, Ryu CM, Allen SN, Kang L, et al. 2008. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol 146:703–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atmakuri K, Cascales E, Burton OT, Banta LM, Christie PJ. 2007. Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions. EMBO J 26:2540–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atmakuri K, Ding Z, Christie PJ. 2003. VirE2, a type IV secretion substrate, interacts with the VirD4 transfer protein at cell poles of Agrobacterium tumefaciens. Mol. Microbiol 49:1699–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backert S, Fronzes R, Waksman G. 2008. VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol 16:409–13 [DOI] [PubMed] [Google Scholar]
- 10.Ballas N, Citovsky V. 1997. Nuclear localization signal binding protein from Arabidopsis mediates nuclear import of Agrobacterium VirD2 protein. PNAS 94:10723–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banta L, Montenegro M. 2008. Agrobacterium and plant biotechnology. In Agrobacterium: From Biology to Biotechnology, ed. Tzfira T, Citovsky V, pp. 72–147. New York: Springer [Google Scholar]
- 12.Barton IS, Fuqua C, Platt TG. 2018. Ecological and evolutionary dynamics of a model facultative pathogen: Agrobacterium and crown gall disease of plants. Environ. Microbiol 20:16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beiko RG, Harlow TJ, Ragan MA. 2005. Highways of gene sharing in prokaryotes. PNAS 102:14332–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharjee S, Lee LY, Oltmanns H, Cao H, Veena, et al. 2008. IMPa-4, an Arabidopsis importin alpha isoform, is preferentially involved in Agrobacterium-mediated plant transformation. Plant Cell 20:2661–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittinger MA, Gross JA, Widom J, Clardy J, Handelsman J. 2000. Rhizobium etli CE3 carries vir gene homologs on a self-transmissible plasmid. Mol. Plant-Microbe Interact 13:1019–21 [DOI] [PubMed] [Google Scholar]
- 16.Black SJ, Kashkina E, Kent T, Pomerantz RT. 2016. DNA polymerase theta: a unique multifunctional end-joining machine. Genes 7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton GW, Nester EW, Gordon MP. 1986. Plant phenolic compounds induce expression of the Agrobacterium tumefaciens loci needed for virulence. Science 232:983–85 [DOI] [PubMed] [Google Scholar]
- 18.Brencic A, Angert ER, Winans SC. 2005. Unwounded plants elicit Agrobacterium vir gene induction and T-DNA transfer: transformed plant cells produce opines yet are tumor free. Mol. Microbiol 57:1522–31 [DOI] [PubMed] [Google Scholar]
- 19.Broothaerts W, Mitchell HJ, Weir B, Kaines S, Smith LM, et al. 2005. Gene transfer to plants by diverse species of bacteria. Nature 433:629–33 [DOI] [PubMed] [Google Scholar]
- 20.Cabezón E, Ripoll-Rozada J, Peña A, de la Cruz F, Arechaga I. 2015. Towards an integrated model of bacterial conjugation. FEMS Microbiol. Rev 39:81–95 [DOI] [PubMed] [Google Scholar]
- 21.Cangelosi GA, Ankenbauer RG, Nester EW. 1990. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. PNAS 87:6708–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cangelosi GA, Martinetti G, Leigh JA, Lee CC, Thienes C, Nester EW. 1989. Role for [corrected] Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J. Bacteriol 171:1609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascales E, Christie PJ. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charles TC, Nester EW. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol 175:6614–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilton MD, Que Q. 2003. Targeted integration of T-DNA into the tobacco genome at double-strand breaks: new insights on the mechanism of T-DNA integration. Plant Physiol 133:956–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie PJ, Ward JE, Winans SC, Nester EW. 1988. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J. Bacteriol 170:2659–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie PJ, Whitaker N, González-Rivera C. 2014. Mechanism and structure of the bacterial type IV secretion systems. Biochim. Biophys. Acta 1843:1578–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Citovsky V, Wong ML, Zambryski PC. 1989. Cooperative interaction of Agrobacterium VirE2 protein with single stranded DNA: implications for the T-DNA transfer process. PNAS 86:1193–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Citovsky V, Zupan J, Warnick D, Zambryski PC. 1992. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science 256:1802–5 [DOI] [PubMed] [Google Scholar]
- 30.Close TJ, Rogowsky PM, Kado CI, Winans SC, Yanofsky MF, Nester EW. 1987. Dual control of Agrobacterium tumefaciens Ti plasmid virulence genes. J. Bacteriol 169:5113–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Cleene M, De Ley J. 1976. The host range of crown gall. Bot. Rev 42:389–466 [Google Scholar]
- 32.de Iannino NI, Ugalde RA. 1989. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1–2)glucan. J. Bacteriol 171:2842–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dequivre M, Diel B, Villard C, Sismeiro O, Durot M, et al. 2015. Small RNA deep-sequencing analyses reveal a new regulator of virulence in Agrobacterium fabrum C58. Mol. Plant-Microbe Interact 28:580–89 [DOI] [PubMed] [Google Scholar]
- 34.De Vos G, Zambryski PC. 1989. Expression of Agrobacterium nopaline specific VirD1, VirD2, and VirC1 proteins and their requirement for T-strand production in E. coli. Mol. Plant-Microbe Interact 2:43–52 [DOI] [PubMed] [Google Scholar]
- 35.Dumas F, Duckely M, Pelczar P, Van Gelder P, Hohn B. 2001. An Agrobacterium VirE2 channel for transferred-DNA transport into plant cells. PNAS 98:485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escobar MA, Dandekar AM. 2003. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 8:380–86 [DOI] [PubMed] [Google Scholar]
- 37.Farrand SK, Van Berkum PB, Oger P. 2003. Agrobacterium is a definable genus of the family Rhizobiaceae. Int. J. Syst. Evol. Microbiol 53:1681–87 [DOI] [PubMed] [Google Scholar]
- 38.Fernández-González E, de Paz HD, Alperi A, Agúndez L, Faustmann M, et al. 2011. Transfer of R388 derivatives by a pathogenesis-associated type IV secretion system into both bacteria and human cells. J. Bacteriol 193:6257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friesner J, Britt AB. 2003. Ku80- and DNA ligase IV–deficient plants are sensitive to ionizing radiation and defective in T-DNA integration. Plant J 34:427–40 [DOI] [PubMed] [Google Scholar]
- 40.Gallego ME, Bleuyard JY, Daoudal-Cotterell S, Jallut N, White CI. 2003. Ku80 plays a role in non-homologous recombination but is not required for T-DNA integration in Arabidopsis. Plant J 35:557–65 [DOI] [PubMed] [Google Scholar]
- 41.Gao R, Lynn DG. 2005. Environmental pH sensing: resolving the VirA/VirG two-component system inputs for Agrobacterium pathogenesis. J. Bacteriol 187:2182–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Cano E, Hak H, Magori S, Lazarowitz SG, Citovsky V. 2018. The Agrobacterium F-box protein effector VirF destabilizes the Arabidopsis GLABROUS1 enhancer/binding protein-like transcription factor VFP4, a transcriptional activator of defense response genes. Mol. Plant-Microbe Interact 31:576–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Cano E, Magori S, Sun Q, Zhang S, Lazarowitz SG, Citovsky V. 2015. Interaction of Arabidopsis trihelix-domain transcription factors VFP3 and VFP5 with Agrobacterium virulence protein VirF. PLOS ONE 10:e014212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelvin SB. 1998. Agrobacterium VirE2 proteins can form a complex with T strands in the plant cytoplasm. J. Bacteriol 180:4300–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gelvin SB. 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev 67:16–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gelvin SB. 2017. Integration of Agrobacterium T-DNA into the plant genome. Annu. Rev. Genet 51:195–217 [DOI] [PubMed] [Google Scholar]
- 47.Ghai J, Das A. 1989. The virD operon of Agrobacterium tumefaciens Ti plasmid encodes a DNA-relaxing enzyme. PNAS 86:3109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gheysen G, Villarroel R, Van Montagu M. 1991. Illegitimate recombination in plants: a model for T-DNA integration. Genes Dev 5:287–97 [DOI] [PubMed] [Google Scholar]
- 49.Guo M, Hou Q, Hew CL, Pan SQ. 2007. Agrobacterium VirD2-binding protein is involved in tumorigenesis and redundantly encoded in conjugative transfer gene clusters. Mol. Plant-Microbe Interact 20:1201–12 [DOI] [PubMed] [Google Scholar]
- 50.Guo M, Huang Z, Yang J. 2017. Is there any crosstalk between the chemotaxis and virulence induction signaling in Agrobacterium tumefaciens? Biotechnol. Adv 35:505–11 [DOI] [PubMed] [Google Scholar]
- 51.Guo M, Jin S, Sun D, Hew CL, Pan SQ. 2007. Recruitment of conjugative DNA transfer substrate to Agrobacterium type IV secretion apparatus. PNAS 104:20019–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heckel BC, Tomlinson AD, Morton ER, Choi JH, Fuqua C. 2014. Agrobacterium tumefaciens exoR controls acid response genes and impacts exopolysaccharide synthesis, horizontal gene transfer, and virulence gene expression. J. Bacteriol 196:3221–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirsch AM. 1999. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol 2:320–26 [DOI] [PubMed] [Google Scholar]
- 54.Hodges LD, Cuperus J, Ream W. 2004. Agrobacterium rhizogenes GALLS protein substitutes for Agrobacterium tumefaciens single-stranded DNA-binding protein VirE2. J. Bacteriol 186:3065–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodges LD, Lee LY, McNett H, Gelvin SB, Ream W. 2008. Agrobacterium rhizogenes GALLS gene encodes two secreted proteins required for genetic transformation of plants. J. Bacteriol 191:355–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rorsch A. 1977. Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent agrobacteria and to Rhizobium ex planta. J. Gen. Microbiol 98:477–84 [Google Scholar]
- 57.Horsch RB, Klee HJ, Stachel S, Winans SC, Nester EW, et al. 1986. Analysis of Agrobacterium tumefaciens virulence mutants in leaf discs. PNAS 83:2571–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husnik F, McCutcheon JP. 2018. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol 16:67–79 [DOI] [PubMed] [Google Scholar]
- 59.Hwang HH, Gelvin SB. 2004. Plant proteins that interact with VirB2, the Agrobacterium tumefaciens pilin protein, mediate plant transformation. Plant Cell 16:3148–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwakawa H, Carter BC, Bishop BC, Ogas J, Gelvin SB. 2017. Perturbation of H3K27me3-associated epigenetic processes increases Agrobacterium-mediated transformation. Mol. Plant-Microbe Interact 30:35–44 [DOI] [PubMed] [Google Scholar]
- 61.Joubert P, Beaupère D, Wadouachi A, Chateau S, Sangwan RS, Sangwan-Norreel BS. 2004. Effect of phenolic glycosides on Agrobacterium tumefaciens virH gene induction and plant transformation. J. Nat. Prod 67:348–51 [DOI] [PubMed] [Google Scholar]
- 62.Kado CI. 2000. The role of the T-pilus in horizontal gene transfer and tumorigenesis. Curr. Opin. Microbiol 3:643–48 [DOI] [PubMed] [Google Scholar]
- 63.Kim SI, Veena, Gelvin SB. 2007. Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–91 [DOI] [PubMed] [Google Scholar]
- 64.Lacroix B, Citovsky V. 2011. Extracellular VirB5 enhances T-DNA transfer from Agrobacterium to the host plant. PLOS ONE 6:e25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lacroix B, Citovsky V. 2013. The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. Int. J. Dev. Biol 57:467–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lacroix B, Citovsky V. 2015. Nopaline-type Ti plasmid of Agrobacterium encodes a VirF-like functional F-box protein. Sci. Rep 5:16610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lacroix B, Citovsky V. 2016. A functional bacterium-to-plant DNA transfer machinery of Rhizobium etli. PLOS Pathog 12:e1005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lacroix B, Citovsky V. 2016. Transfer of DNA from bacteria to eukaryotes. mBio 7:00863–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacroix B, Loyter A, Citovsky V. 2008. Association of the Agrobacterium T-DNA-protein complex with plant nucleosomes. PNAS 105:15429–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lacroix B, Tzfira T, Vainstein A, Citovsky V. 2006. A case of promiscuity: Agrobacterium’s endless hunt for new partners. Trends Genet 22:29–37 [DOI] [PubMed] [Google Scholar]
- 71.Lacroix B, Vaidya M, Tzfira T, Citovsky V. 2005. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J 24:428–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lapham R, Lee LY, Tsugama D, Lee S, Mengiste T, Gelvin SB. 2018. VIP1 and its homologs are not required for Agrobacterium-mediated transformation, but play a role in Botrytis and salt stress responses. Front. Plant Sci 9:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee LY, Fang MJ, Kuang LY, Gelvin SB. 2008. Vectors for multi-color bimolecular fluorescence complementation to investigate protein–protein interactions in living plant cells. Plant Methods 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YW, Jin S, Sim WS, Nester EW. 1995. Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. PNAS 92:12245–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Vaidya M, White C, Vainstein A, Citovsky V, Tzfira T. 2005. Involvement of KU80 in T-DNA integration in plant cells. PNAS 102:19231–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Pan SQ. 2017. Agrobacterium delivers VirE2 protein into host cells via clathrin-mediated endocytosis. Sci. Adv 3:e1601528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Tu H, Pan SQ. 2018. Agrobacterium delivers anchorage protein VirE3 for companion VirE2 to aggregate at host entry sites for T-DNA protection. Cell Rep 25:302–11.e6 [DOI] [PubMed] [Google Scholar]
- 78.Li X, Yang Q, Tu H, Lim Z, Pan SQ. 2014. Direct visualization of Agrobacterium-delivered VirE2 in recipient cells. Plant J 77:487–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu P, Nester EW. 2006. Indoleacetic acid, a product of transferred DNA, inhibits vir gene expression and growth of Agrobacterium tumefaciens C58. PNAS 103:4658–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lohrke SM, Yang H, Jin S. 2001. Reconstitution of acetosyringone-mediated Agrobacterium tumefaciens virulence gene expression in the heterologous host Escherichia coli. J. Bacteriol 183:3704–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loyter A, Rosenbluh J, Zakai N, Li J, Kozlovsky SV, et al. 2005. The plant VirE2 interacting protein 1. A molecular link between the Agrobacterium T-complex and the host cell chromatin? Plant Physiol 138:1318–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magori S, Citovsky V. 2011. Agrobacterium counteracts host-induced degradation of its F-box protein effector. Sci. Signal 4:ra69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matthysse AG. 1983. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J. Bacteriol 154:906–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matthysse AG. 1987. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J. Bacteriol 169:313–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayerhofer R, Koncz-Kalman Z, Nawrath C, Bakkeren G, Crameri A, et al. 1991. T-DNA integration: a mode of illegitimate recombination in plants. EMBO J 10:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melchers LS, Maroney MJ, den Dulk-Ras A, Thompson DV, van Vuuren HA, et al. 1990. Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol. Biol 14:249–59 [DOI] [PubMed] [Google Scholar]
- 87.Melchers LS, Regensburg-Tuink AJ, Schilperoort RA, Hooykaas PJJ. 1989. Specificity of signal molecules in the activation of Agrobacterium virulence gene expression. Mol. Microbiol 3:969–77 [DOI] [PubMed] [Google Scholar]
- 88.Melchers LS, Regensburg-Tuink TJ, Bourret RB, Sedee NJ, Schilperoort RA, Hooykaas PJ. 1989. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J 8:1919–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mestiri I, Norre F, Gallego ME, White CI. 2014. Multiple host-cell recombination pathways act in Agrobacterium-mediated transformation of plant cells. Plant J 77:511–20 [DOI] [PubMed] [Google Scholar]
- 90.Mysore KS, Nam J, Gelvin SB. 2000. An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. PNAS 97:948–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nester EW, Gordon MP, Kerr A, eds. 2005. Agrobacterium tumefaciens: From Plant Pathology to Biotechnology St. Paul, MN: APS Press [Google Scholar]
- 92.Newell CA. 2000. Plant transformation technology. Developments and applications. Mol. Biotechnol 16:53–65 [DOI] [PubMed] [Google Scholar]
- 93.Nishizawa-Yokoi A, Nonaka S, Saika H, Kwon YI, Osakabe K, Toki S. 2012. Suppression of Ku70/80 or Lig4 leads to decreased stable transformation and enhanced homologous recombination in rice. New Phytol 196:1048–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niu X, Zhou M, Henkel CV, van Heusden GP, Hooykaas PJJ. 2015. The Agrobacterium tumefaciens virulence protein VirE3 is a transcriptional activator of the F-box gene VBF. Plant J 84:914–24 [DOI] [PubMed] [Google Scholar]
- 95.Nonaka S, Yuhashi K, Takada K, Sugaware M, Minamisawa K, Ezura H. 2008. Ethylene production in plants during transformation suppresses vir gene expression in Agrobacterium tumefaciens. New Phytol 178:647–56 [DOI] [PubMed] [Google Scholar]
- 96.Notti RQ, Stebbins CE. 2016. The structure and function of type III secretion systems. Microbiol. Spectr 10.1128/microbiolspec.VMBF-0004-2015 [DOI] [PMC free article] [PubMed]
- 97.Otten L, De Ruffray P. 1994. Agrobacterium vitis nopaline Ti plasmid pTiAB4: relationship to other Ti plasmids and T-DNA structure. Mol. Gen. Genet 245:493–505 [DOI] [PubMed] [Google Scholar]
- 98.Pansegrau W, Schoumacher F, Hohn B, Lanka E. 1993. Site-specific cleavage and joining of single-stranded DNA by VirD2 protein of Agrobacterium tumefaciens Ti plasmids: analogy to bacterial conjugation. PNAS 90:11538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pappas KM, Winans SC. 2003. Plant transformation by coinoculation with a disarmed Agrobacterium tumefaciens strain and an Escherichia coli strain carrying mobilizable transgenes. Appl. Environ. Microbiol 69:6731–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park SY, Vaghchhipawala Z, Vasudevan B, Lee LY, Shen Y, et al. 2015. Agrobacterium T-DNA integration into the plant genome can occur without the activity of key non-homologous end-joining proteins. Plant J 81:934–46 [DOI] [PubMed] [Google Scholar]
- 101.Peng WT, Lee YW, Nester EW. 1998. The phenolic recognition profiles of the Agrobacterium tumefaciens VirA protein are broadened by a high level of the sugar binding protein ChvE. J. Bacteriol 180:5632–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peralta EG, Ream LW. 1985. T-DNA border sequences required for crown gall tumorigenesis. PNAS 82:5112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Platt TG, Bever JD, Fuqua C. 2012. A cooperative virulence plasmid imposes a high fitness cost under conditions that induce pathogenesis. Proc. R. Soc. B 279:1691–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Platt TG, Morton ER, Barton IS, Bever JD, Fuqua C. 2014. Ecological dynamics and complex interactions of Agrobacterium megaplasmids. Front. Plant Sci 5:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quispe-Huamanquispe DG, Gheysen G, Kreuze JF. 2017. Horizontal gene transfer contributes to plant evolution: the case of Agrobacterium T-DNAs. Front. Plant Sci 8:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriguez-Navarro DN, Dardanelli MS, Ruiz-Sainz JE. 2007. Attachment of bacteria to the roots of higher plants. FEMS Microbiol. Lett 272:127–36 [DOI] [PubMed] [Google Scholar]
- 107.Rossi L, Hohn B, Tinland B. 1996. Integration of complete transferred DNA units is dependent on the activity of virulence E2 protein of Agrobacterium tumefaciens. PNAS 93:126–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagulenko V, Sagulenko E, Jakubowski S, Spudich E, Christie PJ. 2001. VirB7 lipoprotein is exocellular and associates with the Agrobacterium tumefaciens T pilus. J. Bacteriol 183:3642–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sahi SV, Chilton MD, Chilton WS. 1990. Corn metabolites affect growth and virulence of Agrobacterium tumefaciens. PNAS 87:3879–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salomon S, Puchta H. 1998. Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J 17:6086–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schrammeijer B, Risseeuw E, Pansegrau W, Regensburg-Tuïnk TJG, Crosby WL, Hooykaas PJJ. 2001. Interaction of the virulence protein VirF of Agrobacterium tumefaciens with plant homologs of the yeast Skp1 protein. Curr. Biol 11:258–62 [DOI] [PubMed] [Google Scholar]
- 112.Schröder G, Schuelein R, Quebatte M, Dehio C. 2011. Conjugative DNA transfer into human cells by the VirB/VirD4 type IV secretion system of the bacterial pathogen Bartonella henselae. PNAS 108:14643–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi Y, Lee LY, Gelvin SB. 2014. Is VIP1 important for Agrobacterium-mediated transformation? Plant J 79:848–60 [DOI] [PubMed] [Google Scholar]
- 114.Shilo S, Tripathi P, Melamed-Bessudo C, Tzfadia O, Muth TR, Levy AA. 2017. T-DNA-genome junctions form early after infection and are influenced by the chromatin state of the host genome. PLOS Genet 13:e1006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimoda N, Toyoda-Yamamoto A, Aoki S, Machida Y. 1993. Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J. Biol. Chem 268:26552–58 [PubMed] [Google Scholar]
- 116.Shimoda N, Toyoda-Yamamoto A, Nagamine J, Usami S, Katayama M, et al. 1990. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. PNAS 87:6684–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singer K, Shiboleth YM, Li J, Tzfira T. 2012. Formation of complex extrachromosomal T-DNA structures in Agrobacterium tumefaciens–infected plants. Plant Physiol 160:511–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stachel SE, Messens E, Van Montagu M, Zambryski PC. 1985. Identification of the signal molecules produced by wounded plant cell that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–29 [Google Scholar]
- 119.Stachel SE, Timmerman B, Zambryski PC. 1986. Generation of single-stranded T-DNA molecules during the initial stages of T-DNA transfer for Agrobacterium tumefaciens to plant cells. Nature 322:706–12 [Google Scholar]
- 120.Stachel SE, Zambryski PC. 1986. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell 46:325–33 [DOI] [PubMed] [Google Scholar]
- 121.Steck TR, Morel P, Kado CI. 1988. Vir box sequences in Agrobacterium tumefaciens pTiC58 and A6. Nucleic Acids Res 16:8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tenea GN, Spantzel J, Lee LY, Zhu Y, Lin K, et al. 2009. Overexpression of several Arabidopsis histone genes increases Agrobacterium-mediated transformation and transgene expression in plants. Plant Cell 21:3350–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tinland B, Schoumacher F, Gloeckler V, Bravo-Angel AM, Hohn B. 1995. The Agrobacterium tumefaciens virulence D2 protein is responsible for precise integration of T-DNA into the plant genome. EMBO J 14:3585–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trokter M, Waksman G. 2018. Translocation through the conjugative type 4 secretion system requires unfolding of its protein substrate. J. Bacteriol 200:e00615–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tzfira T, Frankmen L, Vaidya M, Citovsky V. 2003. Site-specific integration of Agrobacterium T-DNA via double-stranded intermediates. Plant Physiol 133:1011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tzfira T, Vaidya M, Citovsky V. 2001. VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J 20:3596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tzfira T, Vaidya M, Citovsky V. 2002. Increasing plant susceptibility to Agrobacterium infection by over-expression of the Arabidopsis VIP1 gene. PNAS 99:10435–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tzfira T, Vaidya M, Citovsky V. 2004. Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature 431:87–92 [DOI] [PubMed] [Google Scholar]
- 129.Vaghchhipawala ZE, Vasudevan B, Lee S, Morsy MR, Mysore KS. 2012. Agrobacterium may delay plant nonhomologous end-joining DNA repair via XRCC4 to favor T-DNA integration. Plant Cell 24:4110–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Attikum H, Bundock P, Hooykaas PJJ. 2001. Non-homologous end-joining proteins are required for Agrobacterium T-DNA integration. EMBO J 20:6550–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Attikum H, Hooykaas PJJ. 2003. Genetic requirements for the targeted integration of Agrobacterium T-DNA in Saccharomyces cerevisiae. Nucleic Acids Res 31:826–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Kregten M, de Pater S, Romeijn R, van Schendel R, Hooykaas PJJ, Tijsterman M. 2016. T-DNA integration in plants results from polymerase-theta-mediated DNA repair. Nat. Plants 2:16164. [DOI] [PubMed] [Google Scholar]
- 133.Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CMT, Regensburg-Tuink TJ, Hooykaas PJJ. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290:979–82 [DOI] [PubMed] [Google Scholar]
- 134.Vergunst AC, van Lier MCM, den Dulk-Ras A, Stüve TA, Ouwehand A, Hooykaas PJJ. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. PNAS 102:832–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villemont E, Dubois F, Sangwan RS, Vasseur G, Bourgeois Y, Sangwan-Norreel BS. 1997. Role of the host cell cycle in the Agrobacterium-mediated genetic transformation of Petunia: evidence of an S-phase control mechanism for T-DNA transfer. Planta 201:160–72 [Google Scholar]
- 136.Wang L, Lacroix B, Guo J, Citovsky V. 2017. Transcriptional activation of virulence genes of Rhizobium etli. J. Bacteriol 199:e00841–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, Lacroix B, Guo J, Citovsky V. 2018. The Agrobacterium VirE2 effector interacts with multiple members of the Arabidopsis VIP1 protein family. Mol. Plant Pathol 19(5):1172–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Haitjema CH, Fuqua C. 2014. The Ctp type IVb pilus locus of Agrobacterium tumefaciens directs formation of the common pili and contributes to reversible surface attachment. J. Bacteriol 196:2979–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wendt T, Doohan F, Mullins E. 2012. Production of Phytophthora infestans–resistant potato (Solanum tuberosum) utilising Ensifer adhaerens OV14. Transgenic Res 21:567–78 [DOI] [PubMed] [Google Scholar]
- 140.Wendt T, Doohan F, Winckelmann D, Mullins E. 2011. Gene transfer into Solanum tuberosum via Rhizobium spp. Transgenic Res 20:377–86 [DOI] [PubMed] [Google Scholar]
- 141.Winans SC, Kerstetter RA, Nester EW. 1988. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J. Bacteriol 170:4047–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wolterink-van Loo S, Escamilla Ayala AA, Hooykaas PJJ, van Heusden GP. 2015. Interaction of the Agrobacterium tumefaciens virulence protein VirD2 with histones. Microbiology 161:401–10 [DOI] [PubMed] [Google Scholar]
- 143.Xu J, Kim J, Danhorn T, Merritt PM, Fuqua C. 2012. Phosphorus limitation increases attachment in Agrobacterium tumefaciens and reveals a conditional functional redundancy in adhesin biosynthesis. Res. Microbiol 163:674–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yadav NS, Vanderleyden J, Bennett DR, Barnes WM, Chilton MD. 1982. Short direct repeats flank the T-DNA on a nopaline Ti plasmid. PNAS 79:6322–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Q, Li X, Tu H, Pan SQ. 2017. Agrobacterium-delivered virulence protein VirE2 is trafficked inside host cells via a myosin XI-K-powered ER/actin network. PNAS 114:2982–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang Y, Li R, Qi M. 2000. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–51 [DOI] [PubMed] [Google Scholar]
- 147.Yanofsky MF, Porter SG, Young C, Albright LM, Gordon MP, Nester EW. 1986. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell 47:471–77 [DOI] [PubMed] [Google Scholar]
- 148.Young C, Nester EW. 1988. Association of the VirD2 protein with the 5’ end of T-strands in Agrobacterium tumefaciens. J. Bacteriol 170:3367–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, et al. 2007. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. PNAS 104:11790–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zaltsman A, Lacroix B, Gafni Y, Citovsky V. 2013. Disassembly of synthetic Agrobacterium T-DNA-protein complexes via the host SCFVBF ubiquitin-ligase complex pathway. PNAS 110:169–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ziemienowicz A, Görlich D, Lanka E, Hohn B, Rossi L. 1999. Import of DNA into mammalian nuclei by proteins originating from a plant pathogenic bacterium. PNAS 96:3729–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ziemienowicz A, Merkle T, Schoumacher F, Hohn B, Rossi L. 2001. Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins. Plant Cell 13:369–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ziemienowicz A, Tinland B, Bryant J, Gloeckler V, Hohn B. 2000. Plant enzymes but not Agrobacterium VirD2 mediate T-DNA ligation in vitro. Mol. Cell. Biol 20:6317–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuniga-Soto E, Mullins E, Dedicova B. 2015. Ensifer-mediated transformation: an efficient non-Agrobacterium protocol for the genetic modification of rice. SpringerPlus 4:600. [DOI] [PMC free article] [PubMed] [Google Scholar]