Abstract
Despite the advent of tyrosine kinase inhibitors, a proportion of chronic myeloid leukemia patients in chronic phase fail to respond to imatinib or to second-generation inhibitors and progress to blast crisis. Until now, improvements in the understanding of the molecular mechanisms responsible for chronic myeloid leukemia transformation from chronic phase to the aggressive blast crisis remain limited. Here we present a large parallel sequencing analysis of 10 blast crisis samples and of the corresponding autologous chronic phase controls that reveals, for the first time, recurrent mutations affecting the ubiquitin-conjugating enzyme E2A gene (UBE2A, formerly RAD6A). Additional analyses on a cohort of 24 blast crisis, 41 chronic phase as well as 40 acute myeloid leukemia and 38 atypical chronic myeloid leukemia patients at onset confirmed that UBE2A mutations are specifically acquired during chronic myeloid leukemia progression, with a frequency of 16.7% in advanced phases. In vitro studies show that the mutations here described cause a decrease in UBE2A activity, leading to an impairment of myeloid differentiation in chronic myeloid leukemia cells.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder with an incidence of 1-2 cases per 100,000/year. It is characterized by the presence of the BCR-ABL1 fusion gene, the product of the reciprocal translocation between chromosomes 9 and 22.1 After the translocation, the coding regions of BCR and ABL1 genes are juxtaposed, leading to an enhanced ABL1 tyrosine kinase activity.2 CML is a multi-step disease, evolving from a mild form that is easy to control, called chronic phase (CP), to a very aggressive and incurable acute phase called blast crisis (BC). The majority of CML-CP patients are successfully treated with drugs able to impair BCR-ABL1 kinase activity (tyrosine kinase inhibitors, TKI), thus confirming the central role of the oncogenic fusion protein in CML pathogenesis.3,4 However, a fraction of these patients fail to respond to the treatment (primary resistance) or become resistant after an initial response.4–6 The persistence of BCR-ABL1 activity typically drives the progression to the advanced phase of the disease within 3-5 years. One of the open issues in CML concerns the dissection of the molecular mechanisms driving the transformation to BC, commonly considered as a heterogeneous disease at the molecular level.7–9 BC is mainly characterized by the rapid expansion of the differentiation-arrested BCR-ABL1-positive blast cells,10 therefore resembling an acute leukemia. In most cases of BC cases (approx. 70%), blasts maintain myeloid features, while in 20-30% blast lineage is lymphoid. BCR-ABL1 expression, which increases during CML progression in conjunction with BCR transcription, seems to have a prominent role in this process, hyperactivating proliferative and anti-apoptotic signals and inducing genetic instability.5,11,12 Previous reports showed the existence of a heterogeneous molecular signature among distinct BC patients.5,7,8 However, these data were limited by the scarcity of matched CP/BC samples, due to the infrequent progression to BC after the advent of TKI. Here we analyzed ten paired CP/BC samples through a whole-exome sequencing (WES) approach, identifying somatic variants specific for BC progression since these were not present in the autologous CP controls. Along with several mutations previously identified as BC driver events,5,7,13 we detected, for the first time, recurrent BC-specific mutations occurring on the UBE2A gene. These data suggest that the appearance of UBE2A variants in CML cells could contribute to BC progression through the impairment of myeloid differentiation.
Methods
Cell lines
The BA/F3-BCR-ABL1 and 32Dcl3-BCR/ABL1 cell lines were generated and maintained as described by Puttini et al.14 and Piazza et al.15 K562 and 293FT were purchased from DSMZ (Braunschweig, Germany) and Thermo-Fisher-Scientific (Waltham, MA, USA) respectively, and were maintained according to the manufacturers’ instructions.
Patients
Diagnosis and staging were performed according to the World Health Organization WHO-2008 classification.16 Peripheral blood (PB) or bone marrow (BM) of ten matched CML chronic phase/blast crisis samples, 31 CP-CML, 14 AP/BC-CML, 38 atypical-CML (aCML), and 40 AML were collected at diagnosis and after obtaining written informed consent approved by the institutional ethics committee. The study was conducted in accordance with the Declaration of Helsinki. Samples were prepared as described by Piazza et al.17
Whole exome sequencing
Genomic DNA (gDNA) was extracted from purified cells with PureLink Genomic DNA kit (Thermo-Fisher-Scientific). 1 μg of gDNA from each sample was fragmented (500bp) with a Diagenode-Bioruptor sonicator system (Diagenode, Belgium) and processed according to the standard Illumina protocol. The Illumina TruSeq Exome Enrichment kit (Illumina Inc., San Diego, CA, USA) was used to enrich the genomic libraries for the exonic regions and samples were sequenced as described in the Online Supplementary Appendix.
Plasmids, transfections and lentiviral infections
BA/F3_BCR-ABL1-positive cells were transfected with pMIGR1_UBE2A vectors (Online Supplementary Appendix) as by Puttini et al.14 and were analyzed for GFP positivity with a FACSAria flow cytometer (BD Bioscience, San Jose, USA) and FACS-sorted when transfection efficiency was lower than 85%.
32Dcl3-BCR/ABL1 cells were electroporated using a Gene Pulser® II Electroporation System (BIORAD) with pMIGR1_UBE2A WT and I33M vectors as described by Puttini et al.14 To obtain stable UBE2A WT or I33M cell lines, GFP positive population was FACS-sorted with a MoFlo Astrios cell sorter equipped with Summit 6.3 software (both from Beckman Coulter, Miami, FL, USA).
For UBE2A silencing, K562 cells were infected with lentivirus obtained from MISSION-shRNA pLKO.1-based vectors (TRCN0000320625) (Sigma-Aldrich, Missouri, USA) and packaged using 293FT cell line. As a control, a pLKO.1MISSION non-target control vector (SHC002) (Sigma-Aldrich) was used. After infection K562 cells were maintained in 2 μg/mL puromycin for selection of silenced (K562_shUBE2A) and control cells (K562_shNC).
Quantitative real-time polymerase chain reaction
Total RNA was extracted using Trizol (Thermo-Fisher-Scientific) following the manufacturer’s instructions. 1 μg of total RNA was used to synthesize cDNA using reverse transcription reagents (Thermo-Fisher-Scientific) after pre-treatment with DNAseI (Thermo-Fisher-Scientific) to avoid contamination from genomic DNA. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using TaqMan® Brilliant II QPCR Master Mix (Agilent Technologies, CA, USA) on a Stratagene-MX3005P (Agilent-Technologies) under standard conditions. The housekeeping gene glucoronidase β gene (GUSB) was used as an internal reference.12 TaqMan® Gene Expression Assays (Thermo-Fisher-Scientific) were used (Online Supplementary Table S1).
In vitro translation and ubiquitination assay
In vitro translation of UBE2A proteins was performed with 1-Step Human Coupled IVT Kit-DNA (Thermo-Fischer-Scientific) following the manufacturer’s instructions. For ubiquitination assay we incubated 15 μg of UBE2A proteins with 1 μg of GST-Ubiquitin (Enzo-Life-Sciences, NY, USA), 0.2 ng of ubiquitin activating enzyme (E1) (Enzo-Life-Sciences), 2 mM ATP, energy regeneration solution (BostonBiochem, MA, USA), 2 mM MgCl2, 2 mM KCl, 16 μg of BA/F3_BCR-ABL1 whole cell lysate in 50 mM TrisHCl (ph7.5). The reactions were incubated for 20 minutes at 37°C. The products were analyzed by western blotting.
For the enzymatic activity of WT and mutated UBE2A, 15 μg of UBE2A in vitro synthesized protein were used. The AMP-Glo Assay (Promega catalog v5011) was used in order to quantify the amount of AMP generated by the ubiquitin conjugation machinery, composed of 170 ng/μL ubiquitin protein, 15 ng/μL UBA1 and 50 μM ATP (SignalChem).
The production of AMP from ATP is directly proportional to the enzymatic activity of the ubiquitination machinery and therefore it was used to measure the ubiquitination in the presence of WT and mutated UBE2A. The AMP signal was detected using the AMP detection solution (Promega) and a TECAN reading plate (Infinite F200Pro TECAN).
Neutrophilic differentiation
For induction of neutrophilic differentiation, 32Dcl3-BCR/ABL1 UBE2A WT and I33M cells were treated as previously described.18
32Dcl3-BCR/ABL1 cells expressing UBE2A WT or I33M were seeded at a density of 2×105 cells per milliliter and cultured in the presence of imatinib mesylate (1 μM final concentration) in combination with human recombinant GCSF (10 ng/mL) or IL3 (0.5 ng/mL).
At days 3 and 6, cells were analyzed using FACS for CD11b surface expression and imaged with confocal microscopy (Online Supplementary Methods).
Results
Single nucleotide variants acquired during chronic myeloid leukemia progression
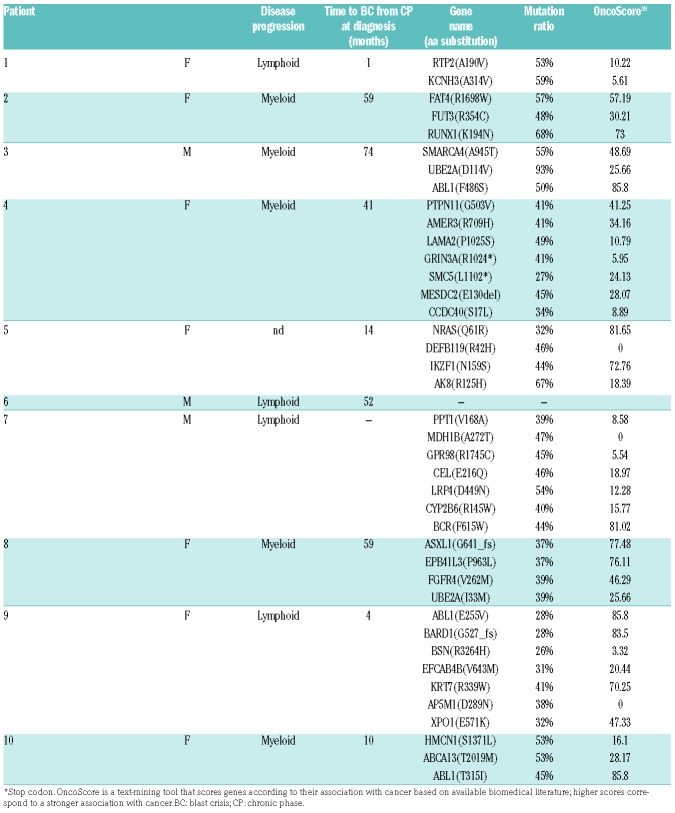
Genomic DNA (gDNA) from matched CP/BC samples was obtained for each patient at diagnosis (CP) and after progression to BC. Initially whole exome sequencing (WES) data from ten patients were analyzed. CP samples were used as baseline controls for each patient to identify somatic variants selectively occurring in BC (Table 1), thus allowing the recognition of molecular events occurring exclusively upon CML progression. By using this approach we identified mutations on genes already associated with BC, such as RUNX1, IKZF1, NRAS, ASXL1 and ABL1.7,13 A total of 41 non-synonymous single nucleotide variants (SNV) and small indels were identified, with a mean of 4.1 mutations/patient acquired upon BC progression. Of these events, 31 were transitions, seven transversions and three indels, with the C>T substitution being the most frequent (63.4%) (Online Supplementary Figure S1). In one patient (patient #7) no acquired exonic SNV could be detected during CML progression.
Table 1.
Single nucleotide variants and indels identified by exome sequencing in blast crisis samples and absent in the paired chronic phase control.
Analysis of SNV data showed the presence of two recurrently mutated genes in this cohort: ABL1, with mutations F486S, E255V and T315I occurring on the BCR-ABL1 fusion gene and leading to TKI resistance (30%, 95%CI: 0.574, 0.026), and UBE2A (Xq24), an E2-ubiquitin conjugating enzyme required for post-replicative DNA damage repair15 (20%, 95%CI: 0.447, 0.000), which has never been previously reported as mutated in CML patients. UBE2A mutations occurred on two non-contiguous residues: D114V and I33M (Tables 1 and 2). Patient #3 (male) showed an UBE2A variant frequency of 93%, as expected given that the gene is localized on the X chromosome. Patient #8 (female) carried a heterozygous UBE2A mutation (mutation ratio: 39%). The high mutation ratio observed in both patients suggests that UBE2A is present in the dominant BC clone (Table 1).
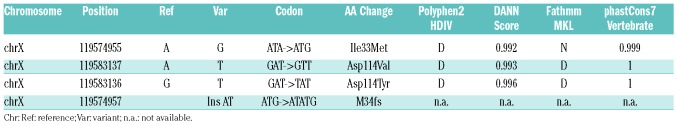
Table 2.
UBE2A single nucleotide variants and indels identified in blast crisis samples and absent in the paired chronic phase control.
UBE2A mutations are recurrent and acquired in late chronic myeloid leukemia
The evidence of recurrent, somatic UBE2A mutations has never been reported in BC cases; however, they had been previously found in other clonal disorders both of solid and hematopoietic origin, confirming their potential role in tumor progression.19 To further characterize the pattern and the frequency of UBE2A mutations in a larger cohort of patients, we sequenced 31 additional CML CP samples at onset, 14 AP/BC, 40 acute myeloid leukemia, and 38 aCML samples. No evidence of UBE2A mutations could be found in the CP, AML or aCML samples, while in two AP/BC samples, somatic UBE2A variants D114Y and M34fs were detected. Globally, acquired UBE2A mutations could be detected in a total of 16.7% (4 of 24) advanced (AP/BC) CML cases (95%CI: 1.78-31.62) (Table 2).
UBE2A mutations affect protein activity
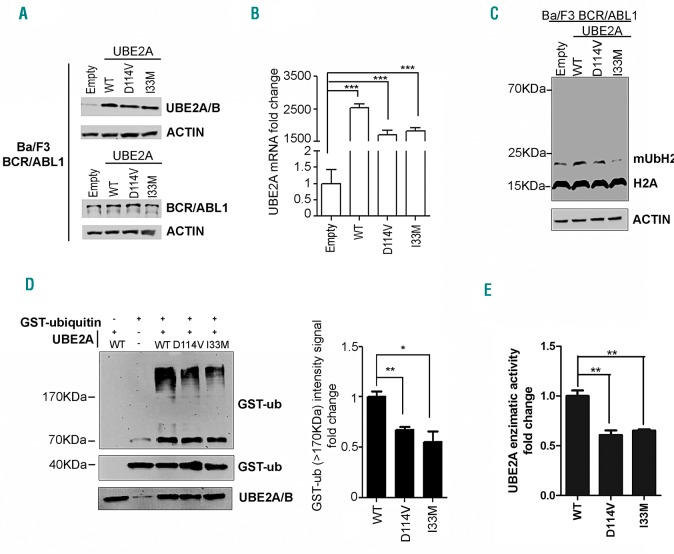
Polyphen-2 (http://genetics.bwh.harvard.edu/pph/),20 DANN11 and FATHMM-MKL21 analyses revealed that all the UBE2A variants identified were potentially damaging, as also suggested by the presence of a N-terminal frameshift variant (M34fs) in one of the patients (Table 2). To gain insight into the functional role of UBE2A mutations, we stably transfected the BA/F3_BCR-ABL1 cell line14 with the wild-type (WT) and the mutated UBE2A variants, I33M and D114V. The level of UBE2A expression in stable transfectants was verified both at protein (Figure 1A) and mRNA (Figure 1B) levels.
Figure 1.
Activity of UBE2A mutants. (A) Western blot analysis of total cell lysates from BA/F3_BCR-ABL cell lines stably transfected with pMIGR-UBE2A vectors encoding for wild-type (WT) or mutated (D114V or I33M) UBE2A. Empty vector has been used as negative control. (B) Real-time quantitative polymerase chain reaction (RT-qPCR) of total RNA extracted from BA/F3_BCR-ABL_pMIGR/UBE2A cell lines. The values are normalized on the EMPTY cells (***P<0.001). (C) Western blot of total cell lysates from BA/F3_BCR-ABL_pMIGR/UBE2A cell lines. The signal at 14KDa corresponds to histone H2A. The signal at ~23KDa corresponds to monoubiquitinated histone H2A (mUbH2A) according to Wu et al.37 (D) Western blot analysis of the in vitro ubiquitination reaction performed with in vitro translated UBE2A (WT and mutated forms) and GST-ubiquitin on total BA/F3_BCR-ABL lysate. (Right) The densitometric analysis of GST-ub signal (>170KDa) from three independent experiments obtained with ImageJ software.38 The fold change is obtained normalizing the signal on the WT sample (WT vs. D114V *P=0.022; WT vs. I33M **P=0.0069). (E) Histogram showing the enzymatic activity of in vitro expressed UBE2A using the AMP Glow assay (WT vs. D114V *P=0.0056; WT vs. I33M **P=0.0036).
The analysis of the levels of ubiquitin-conjugated H2A, a known UBE2A substrate,22 in total cell lysate revealed a decreased H2A ubiquitination for both UBE2A variants compared to WT (Figure 1C), with the effect of I33M being more prominent. In line with these findings, suggesting a decreased UBE2A activity for both variants, ubiquitination assay performed with in vitro translated WT and mutated UBE2A proteins confirmed a decrease in ubiquitin-conjugating activity for mutants compared to the WT form (Figure 1D). To further support this indication, we developed a new in vitro assay based on the measurement of the AMP concentration as a proxy to assess the overall level of ubiquitination. This test was performed in the presence of GST-ubiquitin and of the E1 ubiquitin activating enzyme UBA1 together with WT or mutated UBE2A; this revealed a significant decrease in ATP consumption, and therefore in ubiquitin-conjugating activity, for UBE2A mutants compared to the WT form [see Figure 1E: enzyme specific activity assay, 1.55-fold (P<0.01) and 1.53-fold (P<0.01) decrease in UBE2A D114V and I33M AMP concentration compared to UBE2A WT].
Transcriptome analysis of UBE2A cellular models shows significant perturbation of downstream pathways related to myeloid development
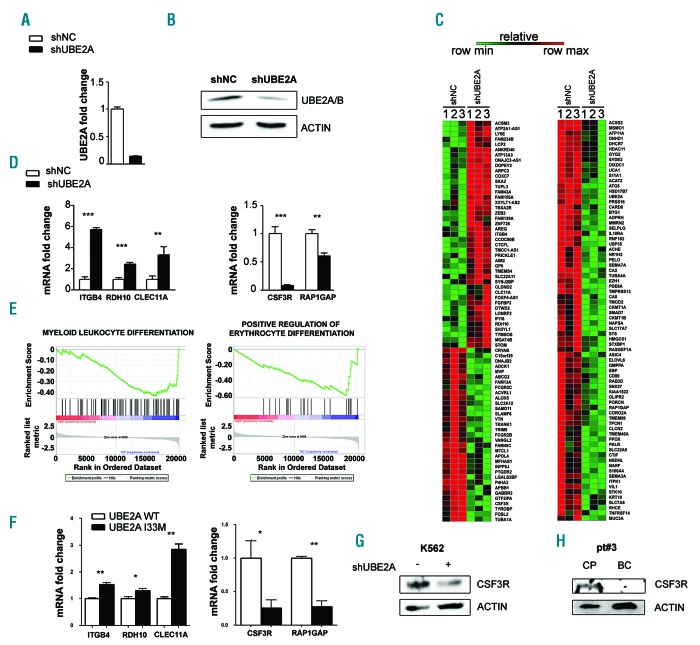
To identify the gene networks perturbed by the UBE2A knock-out, stable lentiviral UBE2A silencing models were generated (Figure 2A and B) in the human myeloid K562 cell line (K562-shUBE2A and K562-shNC cells for UBE2A silencing and scrambled control, respectively) (Online Supplementary Figure S2). Whole-transcriptome analysis (RNA-Seq) highlighted the presence of 168 differentially expressed genes, with 117 of them being down-regulated and 51 up-regulated (Figure 2C). Gene set enrichment analysis (GSEA) showed significant enrichment for ontologies related to myeloid differentiation (Figure 2D) and neural development (Online Supplementary Figure S3). RT-qPCR on K562shNC/shUBE2A cell lines on a set of five differentially expressed genes (ITGB4, RDH10, CLEC11A, CSF3R, RAP1GAP) confirmed RNA-Seq data (Figure 2E). Interestingly, the colony stimulating factor 3 receptor (CSF3R) was potently down-regulated in shUBE2A both at mRNA (12.5-fold downregulation; Figure 2E) and protein (Figure 2F) levels, hence suggesting that its downmodulation may play a role in the differentiation block that is ultimately responsible for the onset of the BC. Immunoblot analysis on CP/BC mononuclear cells from patient #3, which acquired the D114V-UBE2A mutation in BC phase, confirmed CSF3R downmodulation (Figure 2G). To confirm the expression signature identified in the UBE2A silencing models, we stably over-expressed UBE2A WT and I33M in the 32Dcl3-BCR/ABL1 murine myeloid cell line (Online Supplementary Figure S4). In line with the expression profile shown in K562 UBE2A silenced cells, also in these cell lines we observed a comparable modulation in the previous analyzed set of five differentially expressed genes (ITGB4 1.52 P<0.01, RDH10 1.30 P<0.05, CLEC11A 2.84 P<0.01, CSF3R 0.25 P<0.05, RAP1GAP 0.27 P<0.01) (Figure 2H; data are reported as fold-change in UBE2A I33M compared to UBE2A WT), therefore supporting the hypotheses that: 1) UBE2A mutations modulate the activity of the target protein in a loss of function manner; and 2) UBE2A mutations probably act as dominant negative variants. Comparison of our signature with known BC data (GEO_GSE47927 -HSC data were used for BC vs. CP calculation) indicated the presence of a moderate positive linear correlation (R2 = 0.234) (Online Supplementary Figure S5). Notably, CSF3R expression level was seen to be markedly decreased also in the reference BC database, with a Log2 fold-change of -2.19. Globally, these data indicate that UBE2A mutations are directly responsible for the modulation of CSF3R, ITGB4, RDH10, CLEC11A and RAP1GAP expression. This hypothesis is also corroborated by the 32Dcl3 cell models.
Figure 2.
UBE2A silencing in K562 cells. (A) Real-time quantitative polymerase chain reaction (RT-qPCR) analysis of total RNA extracted from K562 cell lines infected with a lentiviral based system for UBE2A silencing (shNC: scrambled negative control; shUBE2A: UBE2A silenced cells). Values are normalized on shNC cells (***P<0.0001). (B) Western blot analysis of total cell lysates from K562_shNC and K562_shUBE2A cells. (C) Heat map of RNA-sequencing data showing color-coded expression levels of differentially expressed genes in three distinct populations of K562-shUBE2A compared to control (shNC). (D) RT-qPCR analysis in K562 cell lines of a subset of differentially expressed genes identified by RNA-sequencing. (E) Gene set enrichment analysis of the shUBE2A transcriptome. (F) RT-qPCR analysis in the 32Dcl3 cell line of a subset of differentially expressed genes identified by RNA-sequencing. (G and H) CSF3R protein levels in total cell lysate of K562 cells (G) and of BC/CP samples from patient #3, carrying UBE2A mutation in the BC phase (H).
UBE2A activity is involved in myeloid differentiation
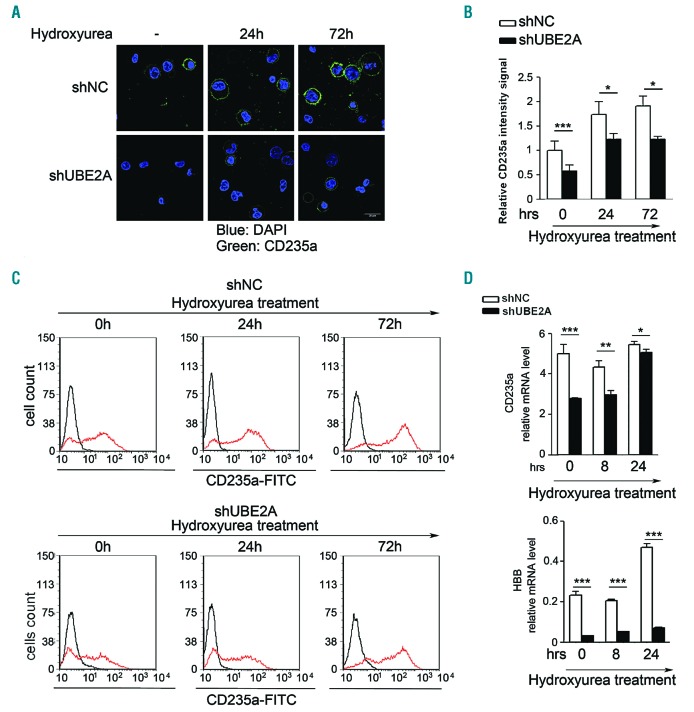
Erythrocytes and megakaryocyte differentiation can be induced in K562 cells by treating with hydroxyurea or phorbol 12-myristate 13-acetate (PMA), respectively.23,24 Treatment of UBE2A-silenced K562 cells with hydroxyurea showed a significant delay in the ability to differentiate into erythrocytes, as assessed by glycophorin A (GYPA-CD235a) expression levels when compared with the scrambled control (Figure 3A-C) (relative CD235a expression compared to shNC fold-change at day 0: 0.54±0.13, data are reported as fold-change in UBE2A I33M compared to UBE2A WT<0.001; day 1: 0.70 ± 0.12, data are reported as fold-change in UBE2A I33M compared to UBE2A WT <0.05; day 3: 0.61 ± 0.15, data are reported as fold-change in UBE2A I33M compared to UBE2A WT <0.05). Fluorescence-activated cell sorting analysis (FACS) showed a 45% decrease in GYPA surface expression in silenced cells compared to controls after 24 hours (h) of treatment (Figure 3B). In line with these findings, induction of hemoglobin-subunit-β (HBB) production was almost completely suppressed in shUBE2A cells [6.6-fold relative decrease of HBB mRNA level at 24 (h) of treatment: data are reported as fold-change in UBE2A I33M compared to UBE2A WT<0.001] further confirming the negative effect of UBE2A silencing on erythroid differentiation (Figure 3C). Similarly, treatment of K562 cells with the megakaryocytic-inducing agent PMA showed significant impairment of megakaryocyte differentiation in shUBE2A cells, as assessed by the expression levels of CD41 (33% downregulation of surface protein expression; P<0.001) and CD44 (38% decrease at mRNA level; P<0.01) after PMA treatment (Online Supplementary Figure S6).
Figure 3.
Induction of erythroid differentiation in UBE2A-silenced K562 cell line. K562 cells were treated with 400 μM hydroxyurea. (A) CD235a immunofluorescence staining for UBE2A-silenced K562 (shUBE2A) and control (shNC) cells after hydroxyurea or mock (-) treatments for the indicated times. (scale bar: 25 μm). (B) Average intensity of CD235a signal obtained acquiring ten fields from two independent experiments for each sample (approx. 80 cells each). (C) Fluorescence-activated cell sorting analysis (FACS) analysis of CD235a levels in K562 cells in presence (red line) or absence (black line) of hydroxyurea. (D) Quantification of CD235a and hemoglobin mRNA relative levels (HBB: Hemoglobin-subunit-β) through real-time quantitative polymerase chain reaction (RT-qPCR) after hydroxyurea treatment.
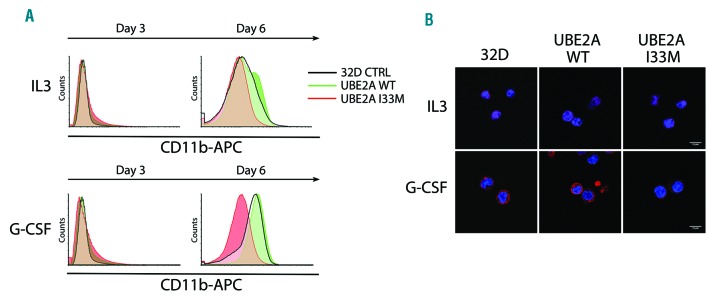
Neutrophilic differentiation was similarly tested in the 32Dcl3 BCR/ABL1 cell lines over-expressing UBE2A WT or I33M. Treatment of the UBE2A I33M cell line with GCSF + IL-3 showed a delay in neutrophilic differentiation, as assessed by CD11b expression levels when compared with both UBE2A WT or control (Figure 4A). Cells treated with IL-3 alone were used as an internal control. FACS showed no difference in CD11b surface expression in UBE2A I33M cell line compared to controls after three days of treatment but showed a 37% decrease at day 6 which was also confirmed by confocal microscopy analysis (Figure 4B).
Figure 4.
Induction of neutrophilic differentiation in UBE2A wild-type (WT) or I33M 32Dcl3 cell line. Cells were treated with IL-3 or granulocyte-colony stimulating factor (GCSF). (A) Fluorescence-activated cell sorting analysis (FACS) analysis of CD11b staining after induction of differentiation at days 3 and 6. (B) CD11b immunofluorescence staining for 32Dcl3 control (CTRL), UBE2A WT and I33M at day 6 showing a clear reduction in UBE2A I33M CD11b staining (scale bar: 20 μm).
Discussion
In line with previous results,5,7,9 our analysis performed on matched CP/BC CML samples showed considerable somatic heterogeneity in BC phase. In all the samples, we detected a very low number of acquired SNV, corresponding to an average of 4.1 non-synonymous mutations per patient, a frequency far below the average reported for other hematopoietic neoplasms, such as acute myeloid leukemia (AML: 7.8) and chronic lymphocytic leukemia (CLL: 11.9).25 This can in part be explained by the characteristics of our analysis, where somatic variants occurring in BC were filtered against those in CP, therefore filtering-out all the driver and passenger variants pre-existing the evolution to BC. All BC samples showed the prevalence of transition events and, in particular, of C:G>T:A substitutions, accounting for 66.7% of all the SNV (Online Supplementary Figure S1). Approximately 85% of the C:G>T:A transitions were part of a CpG dinucleotide. Cytosines in CpG sites are known to be affected by a high mutation rate, caused by a spontaneous deamination of methylated cytosines.26 This mutation pattern is also in accordance with a BCR-ABL1 dependent mutation signature, characterized by inhibition of the mismatch repair system (MMR) and by accumulation of reactive oxygen species (ROS), as previously reported.27 Mutations in RUNX1 and IKZF1, both involved in hematopoietic differentiation, have already been detected in the advanced stages of CML7 and are confirmed here as specific markers for BC progression. Along with this, the XPO1 gene (exportin-1) mutated here in a single patient with the E571K substitution, is also frequently mutated in clonal hematologic disorders, with the E571K mutation widely represented in chronic lymphocytic leukemia.28 SNV analysis showed the presence of a recurrent mutation affecting the UBE2A gene (Xq24) (pt#3 and pt#8). UBE2A is an E2-ubiquitin conjugating enzyme that has never been found mutated in CML. Interestingly, the two patients harboring UBE2A mutations lacked any recognizable copy number alteration (Table 2). WES and targeted resequencing of a broader cohort showed that somatic UBE2A mutations are found in a significant fraction (16.7%) of advanced CML phases, thus confirming the initial exome analysis and suggesting a driver role for UBE2A loss of function during disease progression.
The Saccaromyces Cerevisiae UBE2A homolog Rad6 participates in DNA repair, sporulation and cell cycle regulation;29 in mammals a role for UBE2A in the regulation of transcription and chromatin reorganization through post-translational histone modifications has recently been hypothesized.30 Germline mutations of the UBE2A gene in humans have been associated with the X-linked Nascimento-type intellectual disability syndrome.31–33 In order to understand the effect of UBE2A mutations in a BCR-ABL1-positive model, we tested the activity of exogenous UBE2A both in the WT or mutated forms (D114V and I33M) in BA/F3 BCR-ABL1-positive cell lines. We observed a reduced amount of mono-ubiquitinated histone H2A, a known UBE2A substrate, after over-expression of mutated UBE2A compared to the WT (Figure 1C), which indicates that the UBE2A mutations analyzed in this study decrease the activity of the enzyme. This result has been further confirmed by in vitro assays for ubiquitination and enzymatic activity on total cell lysates (Figure 1D and E), thus providing evidence of a damaging effect of the two mutations on UBE2A function. Accordingly, one of the four variants identified in our cohort is a N-terminal frameshift mutation, thus supporting this hypothesis. This evidence is further strengthened by the distribution of UBE2A mutations throughout the entire protein, a pattern that is more common for genes undergoing inactivation. Mutations in the UBE2A paralog UBE2B were not detected in this study, which suggests a specific role for UBE2A in chronic myeloid leukemia. Stable silencing of UBE2A in the BCR-ABL-positive K562 cell line or overexpression of the I33M mutated form in a BCR-ABL1-positive 32Dcl3cl3 myeloid cell line showed profound downmodulation of CSF3R, a critical regulator of myeloid lineage differentiation and development.34,35 CSF3R, also known as granulocyte colony-stimulating factor receptor (GCSFR), is a member of the hematopoietin receptor superfamily35 and plays a key role in promoting neutrophilic differentiation but may also support the development of different types of hematopoietic progenitors.34 This suggests a potential role for CSF3R modulation in the suppression of myeloid differentiation in BC. Although the precise mechanism by which UBE2A controls CSF3R expression is still unknown, our data suggest that UBE2A-mediated CSF3R regulation occurs at transcriptional level. Alteration of CSF3R transcription could occur either by a direct activity of UBE2A on CSF3R promoter through epigenetic mechanisms36 or indirectly by UBE2A-mediated ubiquitination of specific transcription factors. Further studies will be needed to clarify this process and to establish the relevance of CSF3R deregulation in the impairment of CML cell differentiation. In line with these findings, we showed that impairment of UBE2A function induces a delay in the differentiation of K562 and 32Dcl3-BCR/ABL1 cells after PMA, hydroxyurea or GCSF treatment, suggesting an important role for UBE2A as a modulator of myeloid differentiation.
In conclusion, in this work we identified recurrent, somatic UBE2A mutations occurring in a significant proportion of advanced CML cases. We propose that the acquisition of somatic UBE2A mutations affects myeloid developmental pathways, promoting a differentiation blockade. Further studies will be required to thoroughly dissect the molecular mechanisms responsible for these effects and to define possible therapeutic strategies for UBE2A-mutated BC-CML cases.
Acknowledgments
The authors would like to thank Manuela Carrera and Giuliana Laurenza for technical assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/9/1789
Funding
This work was supported by Associazione Italiana Ricerca sul Cancro (IG-14249 to CGP, IG-17727 to RP, IG-22082 to RP), by the European Union’s Horizon 2020 Marie Skłodowska-Curie Innovative Training Networks (ITN-ETN) with grant agreement No.: 675712CGP and by Giovani Ricercatori #GR-2011-02351167 to AM. CGP is a member of the European Research Initiative for ALK-Related Malignancies (www.erialcl.net). JB acknowledges support from Bloodwise-UK.
References
- 1.Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph’ translocation. Nature. 1985;315(6022):758–761. [DOI] [PubMed] [Google Scholar]
- 2.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–830. [DOI] [PubMed] [Google Scholar]
- 3.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–561. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376(10):917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol. 2003;4(2):75–85. [DOI] [PubMed] [Google Scholar]
- 7.Grossmann V, Kohlmann A, Zenger M, et al. A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia. 2011;25(3):557–560. [DOI] [PubMed] [Google Scholar]
- 8.Boultwood J, Perry J, Zaman R, et al. High-density single nucleotide polymorphism array analysis and ASXL1 gene mutation screening in chronic myeloid leukemia during disease progression. Leukemia. 2010;24(6):1139–1145. [DOI] [PubMed] [Google Scholar]
- 9.Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107(2):76–94. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian HM, Keating MJ, Talpaz M, et al. Chronic myelogenous leukemia in blast crisis. Analysis of 242 patients. Am J Med. 1987;83(3):445–454. [DOI] [PubMed] [Google Scholar]
- 11.Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31(5):761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marega M, Piazza RG, Pirola A, et al. BCR and BCR-ABL regulation during myeloid differentiation in healthy donors and in chronic phase/blast crisis CML patients. Leukemia. 2010;24(8):1445–1449. [DOI] [PubMed] [Google Scholar]
- 13.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. [DOI] [PubMed] [Google Scholar]
- 14.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66(23):11314–11322. [DOI] [PubMed] [Google Scholar]
- 15.Piazza RG, Magistroni V, Gasser M, et al. Evidence for D276G and L364I Bcr-Abl mutations in Ph+ leukaemic cells obtained from patients resistant to Imatinib. Leukemia. 2005;19(1):132–134. [DOI] [PubMed] [Google Scholar]
- 16.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. [DOI] [PubMed] [Google Scholar]
- 17.Piazza R, Valletta S, Winkelmann N, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat Genet. 2013;45(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster C, Forster K, Dierks H, et al. The effects of Bcr-Abl on C/EBP transcription-factor regulation and neutrophilic differentiation are reversed by the Abl kinase inhibitor imatinib mesylate. Blood. 2003;101(2):655–663. [DOI] [PubMed] [Google Scholar]
- 19.de Miranda NF, Georgiou K, Chen L, et al. Exome sequencing reveals novel mutation targets in diffuse large B-cell lymphomas derived from Chinese patients. Blood. 2014;124(16):2544–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30(17):3894–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shihab HA, Rogers MF, Gough J, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015;31(10):1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung P, Prakash S, Prakash L. The RAD6 protein of Saccharomyces cerevisiae polyu-biquitinates histones, and its acidic domain mediates this activity. Genes Dev. 1988;2(11):1476–1485. [DOI] [PubMed] [Google Scholar]
- 23.Kim KW, Kim SH, Lee EY, et al. Extracellular signal-regulated kinase/90-KDA ribosomal S6 kinase/nuclear factor-kappa B pathway mediates phorbol 12-myristate 13-acetate-induced megakaryocytic differentiation of K562 cells. J Biol Chem. 2001;276(16):13186–13191. [DOI] [PubMed] [Google Scholar]
- 24.Park JI, Choi HS, Jeong JS, Han JY, Kim IH. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562 cells. Cell Growth Differ. 2001;12(9):481–486. [PubMed] [Google Scholar]
- 25.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013; 339(6127):1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan BK, Miller JH. Mutagenic deamination of cytosine residues in DNA. Nature. 1980;287(5782):560–561. [DOI] [PubMed] [Google Scholar]
- 27.Stoklosa T, Poplawski T, Koptyra M, et al. BCR/ABL inhibits mismatch repair to protect from apoptosis and induce point mutations. Cancer Res. 2008;68(8):2576–2580. [DOI] [PubMed] [Google Scholar]
- 28.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekhar MP, Lyakhovich A, Visscher DW, Heng H, Kondrat N. Rad6 overexpression induces multinucleation, centrosome amplification, abnormal mitosis, aneuploidy, and transformation. Cancer Res. 2002;62(7):2115–2124. [PubMed] [Google Scholar]
- 30.Roest HP, Baarends WM, de Wit J, et al. The ubiquitin-conjugating DNA repair enzyme HR6A is a maternal factor essential for early embryonic development in mice. Mol Cell Biol. 2004;24(12):5485–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad DM, Vilain S, Vos M, et al. Mutations in the intellectual disability gene Ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell. 2013;50(6):831–843. [DOI] [PubMed] [Google Scholar]
- 32.Budny B, Badura-Stronka M, Materna-Kiryluk A, et al. Novel missense mutations in the ubiquitination-related gene UBE2A cause a recognizable X-linked mental retardation syndrome. Clin Genet. 2010;77(6):541–551. [DOI] [PubMed] [Google Scholar]
- 33.Nascimento RM, Otto PA, de Brouwer AP, Vianna-Morgante AM. UBE2A, which encodes a ubiquitin-conjugating enzyme, is mutated in a novel X-linked mental retardation syndrome. Am J Hum Genet. 2006;79(3):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang FC, Tsuji K, Oda A, et al. Differential effects of human granulocyte colony-stimulating factor (hG-CSF) and thrombopoietin on megakaryopoiesis and platelet function in hG-CSF receptor-transgenic mice. Blood. 1999;94(3):950–958. [PubMed] [Google Scholar]
- 35.Cosman D. The hematopoietin receptor superfamily. Cytokine. 1993;5(2):95–106. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Guermah M, McGinty RK, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137(3):459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Huen MS, Lu LY, et al. Histone ubiquitination associates with BRCA1-dependent DNA damage response. Mol Cell Biol. 2009;29(3):849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piazza R, Ramazzotti D, Spinelli R, et al. OncoScore: a novel, Internet-based tool to assess the oncogenic potential of genes. Sci Rep. 2017;7(7):46290. [DOI] [PMC free article] [PubMed] [Google Scholar]