Abstract
Advanced respiratory disease imposes a greater symptom burden than many cancers but not does have comparable recognition of the need for supportive and palliative care or the infrastructure for its systematic delivery. Consequently, many people with advanced respiratory disease (and those closest to them) have a poor quality of life, disabled by chronic breathlessness, fatigue and other symptoms. They are socially isolated by the consequences of long-term illness and are often financially impoverished. The past decade has seen an increasing realisation that care for this group must improve and that symptom management must be prioritised. Clinical guidelines recommend person-centred care, including access to supportive and palliative care as needed, as part of standard medical practice. Advanced lung disease clinics and specialist breathlessness services (pioneered within palliative care) are developing within respiratory medicine services but are provided inconsistently.
This review covers the comprehensive assessment of the patient with advanced respiratory disease, the importance of supporting carers and the current best practice in the management of breathlessness, fatigue and cough. It also suggests ways to incorporate person-centred care into the general respiratory clinic, assisted by better liaison with specialist palliative and primary care. Emerging evidence shows that excellent symptom management leads to better clinical outcomes and reduces inappropriate use of emergency medical services.
Key points
People living with advanced respiratory disease and severe chronic breathlessness (and those closest to them) have a poor quality of life.
Chronic breathlessness is a disabling symptom, and acute-on-chronic/episodic breathlessness is frightening to experience and observe.
Chronic breathlessness imposes profound physical limitations and psychosocial burdens on those suffering from it or living with someone experiencing it.
Fatigue and cough are two other cardinal symptoms of advanced respiratory disease, with very detrimental effects on quality of life.
The impact of all these symptoms can be alleviated to a variable extent by a predominantly non-drug complex intervention.
Many of the interventions are delivered primarily by allied health or nursing professionals.
Doctors, nurses and other health professionals also need to play an active part in promoting quality of life as part of excellent medical care.
A person-centred, psychologically informed approach is needed by all clinicians treating patients with advanced respiratory disease.
Educational aims
To give specialist respiratory clinicians practical clinical tools to help improve the quality of life of their patients with advanced respiratory disease and chronic breathlessness.
To outline the evidence base for these interventions with reference to definitive sources.
To highlight the importance of person-centred care in people with respiratory disease at all stages of illness.
Short abstract
Improving quality of life in people with chronic breathlessness, with a combination of (mostly) non-drug and drug interventions, improves clinical outcomes and reduces patient/carer suffering and futile use of medical services http://bit.ly/30s9ckh
Introduction
People living with advanced respiratory diseases do not receive the supportive and palliative care that they need to have the best possible quality of life [1–4]. Globally, many millions of people live with advanced respiratory diseases [5], including chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD). Most affect middle-aged to older people but some (e.g. lymphangioleiomyomatosis (LAM) and cystic fibrosis) affect teenagers and young adults. The diseases may be rapidly progressive or have a more chronic course and younger patients may be awaiting hazardous treatments such as lung transplantation. However, in all, as the disease progresses, the patient becomes highly symptomatic and improvement is unlikely despite maximum treatment of the underlying condition.
While there is no comparable infrastructure to cancer medicine for providing supportive and generalist palliative care in respiratory medicine, it is recognised that the deficit in symptom control and psychosocial support leads to a poorer quality of life for patients not only with advanced respiratory disease [1, 4] but also across the general population [6, 7], and this is likely to have a negative effect on medical outcomes. It also leads to appreciable, futile use of expensive out-of-hours medical services, primarily associated with a failure to manage the severe breathlessness experienced by these patients [7]. It is therefore the responsibility of every respiratory clinician to provide the best “person-centred” care they can, by ensuring they use all the resources and expertise available to them. Typically, the “acute” of the acute-on-chronic breathlessness only is managed and the “chronic” is left untreated [4, 7–9]. The physical and mental demands of caring for someone with a chronic illness have long been established in the research literature, particularly if that person has breathlessness [10, 11]. These burdens lead to long-term health impacts in carers and increasing societal financial costs, as well as individual suffering; yet, there is very little formal recognition of the need to support carers.
Supportive care overlaps with palliative care, although patients receiving palliative care are generally expected to have advanced and progressive disease, often categorised as “death considered to be unsurprising within the next year” [12]. However, as most non-cancer conditions, and indeed now many cancer diagnoses, have a chronic, fluctuating trajectory, punctuated by relapses and remissions, with cumulating morbidity from the effects of treatment and disease, a needs-based approach rather than one based on prognosis is more appropriate in people with advanced respiratory disease. The principles of both supportive and palliative care are the same: provision of person-centred, integrated care for patient and family. Therefore, the term “person-centred care”, becoming recognised as a hallmark of excellent care, is used throughout this review and removes any discussion about when someone is “ready” for palliative care. Person-centred care also encompasses the choice of disease-directed treatments for the individual, and thus provides a basis on which to ensure patients receive individualised disease treatment and also have their symptoms and other concerns identified and addressed. If person-centred care is in place, appropriate supportive and palliative care should follow. The details of these definitions are summarised in table 1.
Table 1.
Terminology related to person-centred care
| Term | Definition | Comment |
| Person-centred care [13] | Person-centred care is a way of thinking and doing things that sees the people using health and social services as equal partners in planning, developing and monitoring care to make sure it meets their needs. | Least ambiguous term, unrelated to prognosis. Most important in chronic conditions where there is no disease-altering therapy and patient engagement in self-management is essential for best clinical outcomes. |
| Supportive care [14] | An approach “to prevent or treat as early as possible the symptoms of a disease, side-effects caused by treatment of a disease, and psychological, social and spiritual problems related to a disease or its treatment”. | Has become central to cancer medicine (and an expectation), where supportive services are advanced, although symptom burden in a number of cancers may be much less and disease may be completely curable. Accepted that “lifestyle” interventions will lead to greater long-term health. Many people with cancer live with “treatable but not curable” disease, like those with advanced respiratory disease, and the same problems are being recognised. |
| Palliative care [15] | Palliative care is an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual. Will enhance quality of life and may also positively influence the course of illness. | Favoured by the European Respiratory Society for people with advanced disease and where death would not be unexpected. The World Health Organization also states: “Is applicable early in the course of illness, in conjunction with other therapies that are intended to prolong life, […] and includes those investigations needed to better understand and manage distressing clinical complications”. |
This review is written for the specialist multiprofessional respiratory team, to help each member contribute to the delivery of excellent person-centred care for patients and families living with advanced respiratory disease. It outlines the current evidence for a person-centred approach and its beneficial impact on clinical outcomes, highlighting that a focus on quality of life needs to become an integral part of clinical practice. It briefly discusses how to give specific interventions as well the rationale for them, recognising the time pressures of routine clinical practice. Ultimately, good person-centred care is likely to reduce costs of money and time and improve clinical outcomes [16, 17]. This review does not consider care of the dying, nor improving quality of life in patients with cancer, both of which are well reviewed in standard texts [18, 19] and recent papers [20].
The cardinal symptoms of advanced respiratory disease are troublesome breathlessness, fatigue and cough [4, 21], sometimes called “the respiratory triad” [21]. All these symptoms can be invisible at rest, needing active elicitation to detect them. The science of symptom management has improved in recent years and failure to address these in specialist respiratory services should now be seen as inexcusable [19, 22]. Many patients have multimorbidities from the complications of their respiratory or unrelated illness(es), or their long-term treatment (e.g. steroids). A comprehensive assessment will encompass appraisal of all symptoms, even if only to ensure all are being actively managed by someone, e.g. in primary care.
Other factors that strongly affect quality of life and clinical outcomes, but which are mainly outside the clinical sphere of influence (e.g. social impacts on clinical outcomes), are shown in table 2 together with suggestions of how clinicians may at least direct patients and families to sources of help. Box 1 outlines suggestions for how well-being and general health may be improved.
Table 2.
Social factors that negatively affect quality of life in patients with advanced respiratory disease, with possible solutions
| Contributory factor | Relevance in advanced respiratory disease | Possible alleviating factors | Sources of help that clinicians can recommend | Role of clinician |
| Poverty: anxiety about money, loss of accommodation, lack of food, difficulty in getting welfare/benefits, particularly if invisible. Concern regarding continuing benefit receipt may deter from health-promoting activities such as exercise, for fear of being reported as fraudulent. | Early loss of work, possibly low-skilled with lack of savings. Partner prevented from work by caring duties or morbidity of own. Increased costs (e.g. heating, transport) and decreased income. In some countries, medical costs are high. |
Benefits/welfare support, although complex bureaucratic system in most jurisdictions plus some rare illnesses unfamiliar to benefits system and common ones (e.g. COPD) may not be seen as serious. | Social workers where available. Citizens advice bureau (or equivalent charity) giving voluntary help. Charities such as Breathe Easy offer information from within network. Foodbanks: clinicians in UK can refer to foodbanks. If hospice/palliative care service involved, they may be able to support social referrals. |
Recommending to patient/carer or referral if within clinical services. Being aware of importance of poverty in reducing health outcomes. Endorsing need for health reasons for patient to use all support available. |
| Isolation: longstanding disabling illness, absence from work, high care needs and low income lead to isolation from social networks. Very important in young people, who find it more difficult to associate with people of similar age. | Isolation associated with poorer health outcomes in every illness as well as in normal health [23], through higher incidence of depression, lack of self-care, loss of social confidence, lack of mental/physical activity. | Company/friendship/support where costs can be helped where necessary and which have beneficial impact on health. | Pulmonary rehabilitation and other group activities. If palliative care involved, hospice day centre or breathlessness programme/voluntary sitters (also relieves carers). Singing for breathing, or other activities. Other clubs and societies/professional groups that may support, e.g. AGE UK, British Legion (for ex-service people). Other charities, e.g. Men in Sheds. |
Endorsing health benefit of activity to help symptoms and improve health, promoting access to information through the clinical services, e.g. at front desk. |
| Carer exhaustion and breakdown in family relationships. | Carers usually similar age to patient, often smokers, may have morbidity of own. Difficult to express resentment/ambiguity at caring role and carers' health needs often unsupported. Although may have feelings of “growth”, many sacrifices in caring role and patient partner may take out anger/frustration on carer. Where carers are parents of young adults, there are many complex feelings. |
Improvement in health/social connectedness of patient. Improved support for carer. |
Increased psychosocial support for carer, e.g. from member of clinical team (see text). Respite for carer, e.g. volunteer sitters. With young person, specialist family support. Hospice team may provide support for both patient and carer. Charities such as Breathe Easy may help. Citizens advice bureau for carer benefits. |
Getting permission to contact carers' GP/primary healthcare team to highlight difficulties for carer and refer to other agencies where possible. Impressing on carer the need to take care of own health. |
Box 1. The five ways to well-being
|
Be active Go for a walk or run. Step outside. Cycle. Play a game. Garden. Dance. Exercising makes you feel good. Most importantly, discover a physical activity you enjoy and that suits your level of mobility and fitness.
Exercise is good for reducing sensation of breathlessness. Connect Connect with the people around you. With family, friends, colleagues and neighbours. At home, work, school or in your local community. Think of these as the cornerstones of your life and invest time in developing them. Building these connections will support and enrich you every day.
Take notice Be curious. Catch sight of the beautiful. Remark on the unusual. Notice the changing seasons. Savour the moment, whether you are walking to work, eating lunch or talking to friends. Be aware of the world around you and what you are feeling. Reflecting on your experiences will help you appreciate what matters to you.
Give Do something nice for a friend or a stranger. Thank someone. Smile. Volunteer your time. Join a community group. Look out, as well as in. Seeing yourself, and your happiness, linked to the wider community, can be incredibly rewarding and creates connections with the people around you.
Keep learning Try something new. Rediscover an old interest. Sign up for that course. Take on a different responsibility at work. Fix a bike. Learn to play an instrument or how to cook your favourite food. Set a challenge you will enjoy achieving. Learning new things will make you more confident as well as being fun.
|
Compiled by the New Economics Foundation, within the UK Government’s Foresight project. Reproduced and modified from [24] with permission.
Some of the interventions recommended in this review are central to the practice of allied health professionals (e.g. breathing exercises), others to nurses (e.g. a supportive approach to patient and family with general health counselling), and others to physicians and other prescribers (e.g. drug therapy). All are discussed so that, even if not routinely delivered by an individual clinician, each clinician is equipped to endorse them and refer patients knowledgeably, which may help improve the intervention's impact and the patient's confidence in the multiprofessional team.
Breathlessness
The most commonly used definition of breathlessness is from an American Thoracic Society working group, which described it as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” [25]. Recently Johnson et al. [26] have defined chronic breathlessness syndrome as “breathlessness that persists despite optimal treatment of the underlying pathophysiology and that results in disability”, to make it easier for clinicians to identify and recognise it as a treatable condition, for patients to volunteer it in clinical encounters, and to drive service development. Treatable does not mean reversible: most breathlessness management aims to reduce the impact of the breathlessness on an individual's life or increase the threshold of activity at which breathlessness becomes limiting. It is important to clarify this when discussing treatment.
Chronic breathlessness in advanced respiratory disease
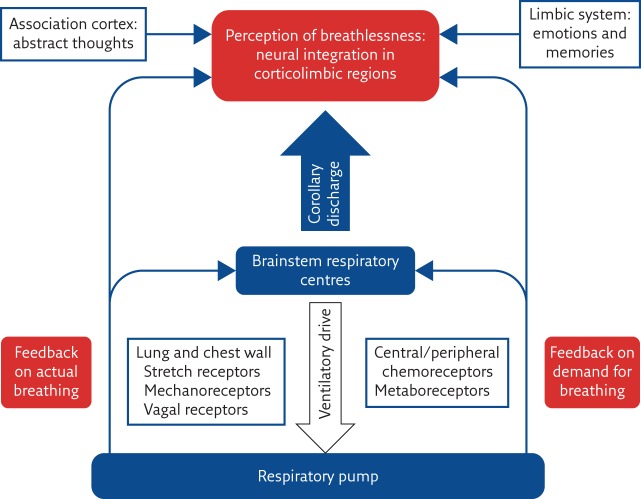
Although chronic breathlessness in advanced respiratory disease results from a number of pathophysiological processes, such as cachexia, deconditioning and airway narrowing [19], the sensation of breathlessness originates from complex neurophysiological interplays between the automaticity of the breathing centre in the brain stem, the generation of fear/anxiety in the amygdala and limbic system and the influence of the higher, cortical thinking and feeling areas (simplified in figure 1). The cortical areas that represent breathlessness are adjacent to other “alarm” centres for thirst and pain, for example. The areas involved in the generation of breathlessness do not “fire off” as independent units but interact as a complex network.
Figure 1.
Schematic diagram to outline the genesis of breathlessness. Reproduced and modified from [27] with permission.
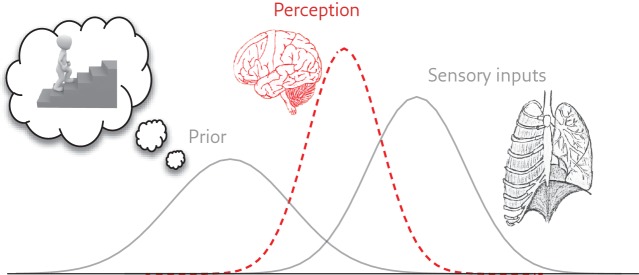
A recent paper by Ongaro and Kaptchuk [28] discussed ways in which symptoms may be generated within complex brain networks. They postulate that perception is “cognitively modulated (mostly unconsciously) and might best be viewed as a process of prediction based on sensory inputs, prior experience and contextual clues”. For example, according to the Bayesian brain hypothesis (figure 2) [29], someone who has always been very breathless on climbing stairs may well become anxious even at the sight of stairs and breathless early on in their climb. They may avoid stairs and become less fit. Practical help from a trusted clinician may help them find new ways of approaching this previously insuperable obstacle (e.g. by replacing old cognitions and perceptions and learning more efficient breathing techniques).
Figure 2.
Schematic of the Bayesian brain hypothesis. Both prior expectations and incoming sensory information contribute to the resulting perception, where each is a distribution of possible values. Reproduced from [29] with permission.
It is also essential to remember that the central nervous system (CNS) and the endocrine and immune systems are intimately related and mutually influence each other; this mind–body integration is encapsulated by the concept of psychoneuroimmunology [30]. Usefully, this means that an improvement in one system (e.g. reduced impact of breathlessness) is likely to lead to an impact in another (e.g. reduced stress), but the opposite also holds, contributing to the impact of breathlessness on an individual's health.
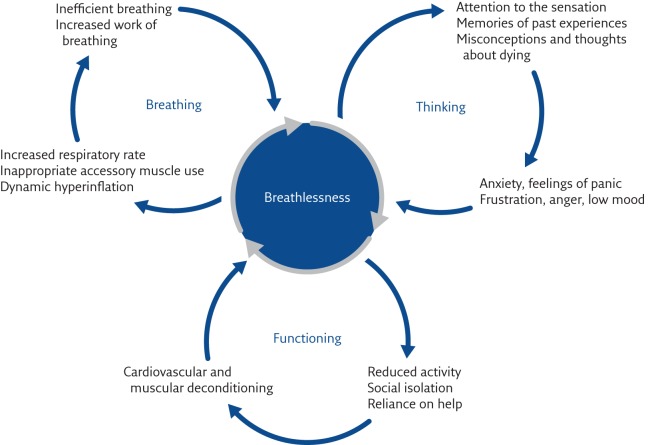
The genesis of breathlessness continues to be elucidated. The central concept is that the sensation comes from, and is a construct of, the CNS. When all possible treatment of pathophysiological processes has been accomplished, further management will involve the patient using a psychologically informed approach to their condition with the clinician helping them to improve peripheral generators of breathlessness, such as muscle deconditioning. Peripheral generators of breathlessness sometimes result from a secondary, ultimately unhelpful, behavioural consequence of breathlessness itself, such as inactivity or social isolation [25, 30]. These relationships are shown in figure 3 and are known as the spiral of disability. This concept was recognised many years ago by Comroe [31], who stated that breathlessness, “like pain, … involves both the perception of the sensation by the patient and his reaction to the sensation”. This has more recently been understood as the “sensory” and “affective” aspects of breathlessness [19, 32]. Understanding this offers pathways to symptom management through helping the individual to alter their learned response to breathlessness.
Figure 3.
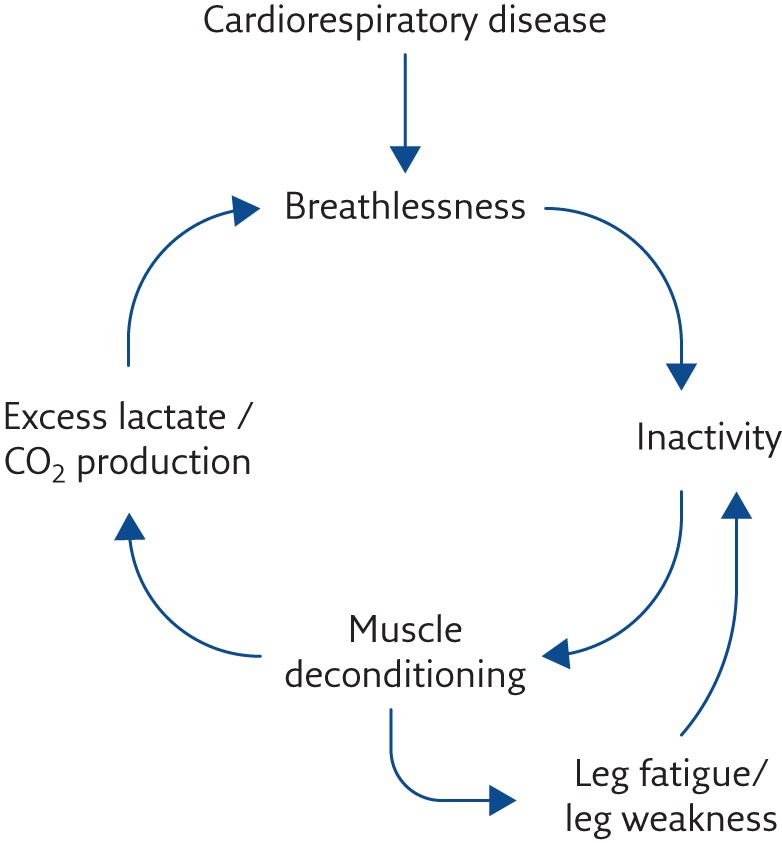
The spiral of disability.
Breathlessness severity is a better indicator of prognosis in advanced respiratory disease than lung function, as there is no consistent relationship between lung function and degree of breathlessness [33]. This is probably because breathlessness is related to many general health and social factors, such as degree of motivation, general fitness, burden of anxiety and depression, social isolation, etc., and again, understanding this opens ways to improve the symptom.
The idea of treating breathlessness “via the brain” [29, 34, 35], supporting and educating the breathless person in self-management [2], is gaining ground, and so is the understanding that “stimulation of the failing organ” alone [36] will be futile.
A conceptual model for the clinical management of breathlessness
Spathis et al. [37] have developed a “Breathing, Thinking, Functioning” (BTF) model to help clinicians, patients and carers understand the generation of breathlessness and the ways in which they can participate in and/or lead its management (figure 4). The model postulates that there are three broad “classes” of factors that contribute to the generation of breathlessness and can thus be understood and acted upon to increase or decrease the symptom. 1) The “Breathing” cycle: e.g. an inefficient, ineffective breathing pattern such as rapid shallow breaths, leading to a vicious cycle of increased breathlessness, often associated with perpetuating anxiety. 2) The “Thinking” cycle: problems with cognitions that the patient (and carer) hold about breathlessness, e.g. “one day I'm going to die gasping for breath”, which drives a vicious cycle of perpetuation through anxiety. The mistaken cognitions give rise to emotions that exacerbate breathlessness. 3) The “Functioning” cycle: the patient's dominant problem is in the area of fitness and reduced physical and mental activity, e.g. he/she has become deconditioned from inactivity, perhaps initiated by avoiding exercise to avoid breathlessness and/or anxiety, and a vicious cycle of deconditioning and increasing breathlessness ensues (figure 3).
Figure 4.
The BTF model of breathlessness used by the Cambridge Breathlessness Intervention Service (CBIS). ©2017 CBIS. Reproduced with permission from the CBIS and Cambridge University Hospitals NHS Foundation Trust. Modified from [37].
It is clear that these cycles are interconnected and not mutually exclusive, but one vicious cycle often predominates in an individual and drives others. Highlighting one cycle as a focus for treatment gives an early indication of which management strategies to prioritise, as well as making it easier to explain the rationale behind the treatment strategy to patient and family, facilitating engagement in self-management.
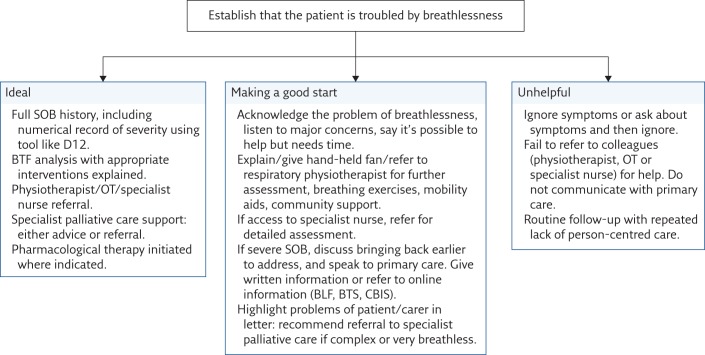
Patients who frequently attend the accident and emergency department, call their general practitioner (GP) out of hours, or come into hospital with nothing specifically disease-related to treat, should have a comprehensive assessment (see the ideal in figure 5). In these situations, lack of symptom control and/or psychosocial distress in the patient or carer are likely to be contributing to frequent futile use of clinical services, and change is unlikely unless these are addressed. Unless the “chronic” aspects of “acute-on-chronic” breathlessness are managed [7], the factors that led to an emergency presentation remain and may initiate a further emergency visit or frequent attendance in primary care.
Figure 5.
First-step algorithm for a busy general respiratory clinic without specialist breathlessness/supportive care service. SOB: shortness of breath; D12: Dyspnoea-12; OT: occupational therapist; BLF: British Lung Foundation; BTS: British Thoracic Society.
The consultation: assessing patients with severe respiratory disease
Assessment is the step that defines the quality of person-centred care given to the individual with advanced respiratory disease. If it is inadequate then major symptoms (and other concerns) will not be elicited and potentially, if carried out insensitively, the clinician–patient relationship will be impaired, with a negative effect on clinical outcomes. It is essential to find out the patients' main concerns. There is extensive work to show that there are marked differences between patients' and physicians' concerns in a consultation [38]. In an incurable, highly symptomatic illness where improving quality of life is the main goal, the patient's goals of care are paramount.
Eliciting symptoms
Even people with severe breathlessness may not look breathless when sitting quietly in the consultation room; this is part of the phenomenon which Gysels and Higginson [9] called “the invisibility of breathlessness”. They highlighted that this may contribute to the reasons that patients with COPD do not get appropriate care. Only those with the most severe disease and worst prognosis are breathless at rest. They are unlikely to be well enough to visit the outpatient department. Patients may not volunteer difficult symptoms if they are completely exhausted from the strain of reaching the hospital outpatient clinic and may not feel emotionally or even cognitively powerful enough to make their needs felt or explain their symptoms. They may think that the clinician is not interested in how they feel, as accepted treatments for chronic breathlessness, fatigue and cough have only recently been established. Patients may have discerned from previous consultations that mentioning symptoms was futile [39]. Some patients may have episodic breathlessness [8] and, although this is overwhelming at the time, it is not made evident in the outpatient clinic unless information is explicitly sought.
Therefore, it is essential that the clinician asks about symptoms actively [22]. Evidence suggests that consultation time is saved when simple open questions are asked to enable the patient to make clear their needs and the impact of breathlessness on their everyday life and that of their family. After a general opening, e.g. addressing recent acute events, direct enquiry about symptoms is crucial (see box 2 for suggestions). This should include the activities that precipitate the symptom and the activities they have stopped or reduced in order to remain comfortable. A simple “Are you breathless?” may elicit a “No”. The clinician then misses not only the presence of the symptom, but also the severe and widespread limitations that follow from chronic breathlessness.
Box 2. Assessment of chronic breathlessness: areas to be covered
| Introduction |
| Read the notes and greet the patient/family by name. It is very distressing for patients if the clinician appears to have no knowledge of the patient or even makes basic mistakes, e.g. in diagnosis. This does not preclude you from making your own assessment of key issues. |
| Questions to establish history of breathlessness |
| Are you troubled by breathlessness? |
| What makes you breathless? |
| What helps your breathlessness? |
| What have you stopped/reduced doing to prevent you getting breathless? |
| Are you breathless when you are sitting completely still? |
| What happens when you feel breathless? (e.g. How does it come on? How do you try to improve it? How long before you feel better? What makes it better/worse? Are you taking any medications to help it?) |
| (Ask carer) What do you notice when patient X becomes breathless? |
| How do you feel when you become breathless? Some people say being breathless makes them feel very anxious, some people even use the word panicky. Does that sound familiar? Have you always been troubled by anxiety? |
| What do you think is causing your breathlessness? (Ask the carer the same question) |
| Do you have times when a worsening of breathlessness does not settle as usual, or when it seems to come out of the blue/from nowhere? (Start asking about feelings/emotions/thoughts about breathlessness, which may be triggering breathlessness without the patient being aware of what is preceding it) [40] |
| Was there a particular episode after which your breathlessness seemed to get much worse? (It is not uncommon for there to be an episode of breathlessness that is particularly frightening or associated with panic, after which breathlessness seems to get much worse generally, i.e. a “trigger” episode. Unpicking this, e.g. with the BTF model, may help bring about improvement.) |
| What do you when this kind of crisis happens? And your wife/husband/carer/partner or other carer? (Gives a basis for education and “ritual for crises”) [18, 41] |
| Have you attended pulmonary rehabilitation? If yes: how you did/felt about it/changes made afterwards. If not: why was this? e.g. lack of confidence, not referred, not available, etc. and rectify or consider specialist palliative care, advanced lung clinic, etc. |
| What is the worst thing for you at the moment? (Ask carer too. Note: this may not be breathlessness, may be cough, fatigue or other concern. Ask about cough and fatigue using similar questions. See text and other boxes.) |
| Summarise and plan |
| Summarise what you have learned, for the patient to check. |
| Lead onto management plans, for example: |
| This is a very difficult symptom and sounds as if it is making life very hard for you. There has been a lot of research in this area in recent years and now we do have ways we can help. |
| It does take a bit of time and work and different skills from different members of the team working together. |
| I would like you to meet… |
It is important to avoid a quick-fire litany of questions, without waiting for the patient to give answers. If the patient does have troublesome chronic breathlessness, but there is no time for a comprehensive symptom assessment and management, start by acknowledging the importance of what the patient is telling you, explain that it is possible to help reduce the impact of symptoms and that this will need help and some extra time to accomplish, possibly on another day.
This does not need to take long but provides a powerful signal that someone authoritative is listening. It is also important to gain an understanding of how the patient manages their chronic breathlessness and seeks help. Those who actively engage in their care using a problem-solving approach and pro-active self-management [42] are most likely to maximise their quality of life. This has recently been discussed in a framework concept called “breathing space” [43]. Specialist psychological help may be needed to help people achieve this. Effective symptom control improves clinical outcomes, for example moving an individual from category D in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification to category C [1]. No drug therapy currently achieves this.
Managing chronic breathlessness
An understanding of the generation of breathlessness underlines the need for the clinician to:
Listen to the patient's experience of chronic breathlessness (box 2), which will give clues to “priors” (e.g. previous experiences) that trigger breathlessness and the possible predominant vicious cycle, which can be tackled first.
Understand how the patient (and carer) view the genesis of breathlessness to help overcome misconceptions that lead to cognitions and emotions that may drive or exacerbate the sensation.
Help the patient and carer to understand the genesis of breathlessness so that when psychological or behavioural interventions are suggested, rather than a drug treatment, the patient and carer can understand the possible benefits.
Support the patient and carer to self-manage breathlessness where they can, which often requires difficult changes in their own approach and behaviour. Motivational interviewing techniques can be very helpful. The importance of the clinician's attention to the symptom in addition to the medical condition has been highlighted [4, 7, 18].
Develop a “ritual for crises” with the patient and carer [18, 41]. This will enable patient and carer to be ready to tackle an exacerbation of breathlessness, which helps shifts the power balance towards patient and carer and away from seemingly random breathlessness [40]. Mularski et al. [41] discussed an approach to managing acute-on-chronic breathlessness episodes and “rituals for crises” have been a mainstay of the CBIS management approach since its inception [18]. Involving the carer is critical (as discussed later), as a feeling of powerlessness in the face of terrifying suffering in a loved one is extremely stressful and more likely to lead to an out-of-hours admission. As the work of Linde et al. [40] has demonstrated, there is usually a precipitator of apparently inexplicable episodes of breathlessness.
Refer to specialist palliative care. This is advised when 1) breathlessness is having a very significant impact on the quality of life of the patient or family, 2) there have been multiple hospital admissions or consultations irrelevant to disease management [4, 44–46], 3) there are extra barriers to giving the patient and family excellent care (box 3 and table 2), and 4) where there is insufficient expertise within the respiratory service to provide adequate symptom control [4, 44].
Box 3. Patients with advanced respiratory disease particularly at risk of not receiving appropriate person-centred care
Those with rare diseases (e.g. LAM) in areas where multiprofessional respiratory support is organised around common diseases; therefore, these patients are ineligible for respiratory nurses whose remit is only for COPD/ILD.
Those with respiratory symptoms secondary to a non-respiratory disease, e.g. breathlessness associated with neurological or renal disease.
Young people with chronic disease and breathlessness, who often have a rare disease.
Elderly people with multimorbidities, who may be considered too old to improve or too complicated to manage.
Those with sensory disabilities such as blindness and deafness, because of inadequate training of clinical staff or inadequate support for clinical staff in caring for people with these needs.
Those who have learning disabilities, who need specialist partnerships between those in respiratory medicine/palliative care and learning disability care.
Socially disadvantaged patients, e.g. with low income, unemployed, on benefits, travellers, the homeless.
Chronic breathlessness interventions
The most common interventions (and those with greatest evidence base individually) are shown in table 3. Three Cochrane reviews of non-pharmacological interventions (to map against the three BTF cycles) are under way [46, 52, 53] and will report soon.
Table 3.
Key non-pharmacological interventions used in managing breathlessness, with selected references
| Intervention | Predominant cycle of the BTF model | Evidence strength | Evidence origin | Practical comment |
| Pulmonary rehabilitation | Functioning | ++++ | Cochrane [47] | Patient may lack confidence and need one-to-one support or breathlessness service first. |
| Hand-held fan | Breathing | +++ | [48, 49] | Evidence suggests this reduces breathlessness recovery time, supports exercise, increases self-efficacy. No important adverse effects, use in all patients, giving advice on how/why used. |
| Cognitive behavioural therapy | Thinking | ++ | [9, 50] | May require specialist psychological support. |
| Breathing techniques | Breathing | ++ | [9] | Need to be personalised, specialist respiratory physiotherapy advice required. |
| Inspiratory muscle training | Breathing | ++ | [9] | Needs to be personalised, specialist respiratory physiotherapy advice required. |
| Pedometer | Functioning | ++ | [9] | Pedometer training, e.g. as used by CBIS, increasing activity by 5% weekly from baseline. |
| Mindfulness-based stress reduction | Thinking | ++ | [9] | Requires 8-week course in standard evaluated form. Needs formal teaching even in abbreviated form. |
| Relaxation | Breathing | ++ | [9] | Various techniques, needs to be personalised. |
| Walking aids | Functioning | ++ | [51] | Should be standard assessment for every breathless individual, also possibly affects thinking via confidence. |
| Positioning | Breathing | + | [9] | Best position for individual may not fit standard ideas. |
| Acupuncture | Breathing? | + | [9] | Needs specialist training. |
It is likely that active listening and promotion of the therapeutic alliance [18] involved in effective assessment is an intervention in itself. Specific breathlessness interventions are not all provided at once [37], and not everyone needs every intervention. Even so, many respiratory physicians working in outpatient clinics, or clinicians with a priority such as managing inhaler technique, will be concerned about how they will find time to integrate them into practice. Remember, when very time-constrained, specific interventions are initially less important to a patient than the attention, support and affirmation of their difficulties from an interested, knowledgeable, professional, kindly clinician [4, 16, 17]. Specific interventions should be part of the care, not a list of actions or techniques given as a substitute for excellent personalised assessment and support.
Best practice is a fully integrated respiratory service with a multiprofessional team who have an interest in advanced respiratory disease and access to specialist palliative care when needed [2–4, 54, 55], or access to a specialist breathlessness service [56, 57] or to a palliative care service working closely with respiratory medicine [16, 17, 44, 45].
Where these do not exist, the patient may still be given good care for breathlessness and other symptoms by ensuring the resources within respiratory medicine, palliative care and primary care work closely together.
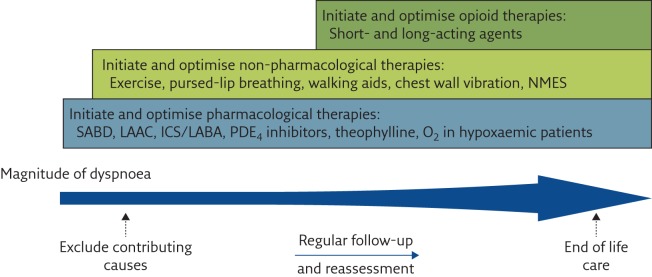
A suggested algorithm for care where no specialist advanced respiratory disease clinic or breathlessness service operates is shown in figure 5. More information on each step has been given in the text and boxes 2, 4 and 5, and figure 6 [61] highlights the balance between drug and non-drug interventions.
Box 4. Role of specialist breathlessness services
There is increasing evidence for the effectiveness of a complex mostly non-pharmacological intervention to help breathlessness of all severities, delivered by a specialist breathlessness or advanced respiratory disease specialist clinic.
A complex intervention is one that combines several components that may be used together or separately, by an individual or different individuals.
The most effective treatments for breathlessness are delivered by a multiprofessional team. This a process, rather than an event, which may need to be revisited from time to time after the initial treatment period is completed.
There is now good evidence that specialist breathlessness services and/or early integration of palliative care can improve care quality [4, 56], and emerging evidence that advanced respiratory disease clinics can improve quality of care and patient satisfaction while maintaining or reducing healthcare costs [54, 58].
Box 5. Practical tips for busy clinics: referring to palliative care
If you do not practise in an advanced lung disease clinic [54, 59, 60], assessing and managing breathlessness will need to be a staged, shared procedure with someone taking a coordinating role. Clearly this cannot be a rushed discussion brought up as an afterthought with severe time pressures. It does not have to be completed in one session. If your patient is very symptomatic and basic breathlessness interventions have not helped, or if the patient has had several recent hospital admissions unrelated to exacerbation or severe infection, is very cachectic or rarely leaves the house, or if you feel that another episode of ventilation in an intensive therapy unit will not be of long-term benefit, they may benefit from specialist palliative care [4, 44, 45]. Family or carer distress is another possible reason for referral.
Figure 6.
The breathlessness ladder. Comprehensive approach to the management of dyspnoea in patients with advanced COPD. NMES: neuromuscular electrical stimulation; SABD: short-acting bronchodilators; LAAC: long-acting anticholinergics; ICS: inhaled corticosteroids; LABA: long-acting β2-agonists; PDE4: phosphodiesterase-4. Reproduced and modified from [61] with permission.
Drug therapy for chronic breathlessness
There is a limited range of drugs used in the management of chronic breathlessness. The principal one in routine clinical practice is morphine (box 6). Mirtazapine is beginning to be more widely used on the basis of its neurochemistry, therapeutic profile, and some clinical experience. Data collection in a phase 3 trial is starting soon (BETTER-B; ISRCTN registry, identifier ISRCTN32236160). A recent phase 3 placebo-controlled randomised controlled trial of sertraline failed to demonstrate benefit over placebo [65]. Benzodiazepines are used appropriately for dying patients [66] but their dependency potential and lack of evidence for effectiveness makes them unsuitable for long-term use for symptom control. Furosemide and cannabinoids show promise [67] but will not be further discussed as they are not used outside trials. Drug therapy aims to improve quality of life by reducing breathlessness, and patient assessment is key. It is crucial that patients are given drug therapy for chronic breathlessness when needed, but not as a first option, except when patients are extremely breathless and have no prospect of improvement (figure 6) [61].
Box 6. Morphine: drug portrait
|
|
|
|
|
|
|
|
|
|
|
|
| Licensed preparation (Therapeutic Goods Administration, Australia) |
|
|
|
|
#: although many countries do not have access to Kapanol, most have access to modified/sustained-release preparations that allow similar steady-state blood levels; total initial daily starting dose can be 10 mg in 24 h on Kapanol.
The recent scandals around inappropriate use of opioids in chronic non-malignant pain (see box 7) are likely to increase patients' and some physicians' fear of opioids, so it is important that the reason for prescribing is clear.
Box 7. The opioid epidemic in the USA, and its possible global impact
In 2018, there were 6000 deaths from opioid overdose in the USA. These deaths were mainly in young people who had first become addicted to opioids from legally available drugs, some then switching to other more potent opioids from illegal sources. Many of those affected came from stable, middle-class backgrounds where a family member had a short, limited course of opioids, e.g. after a surgical intervention. The epidemic of addiction has had profound societal effects and is beginning to affect the status of opioids for medical use, e.g. shorter post-operative prescription of drugs, withdrawal of use of opioids for non-malignant pain. It may yet have further effects as legal action is taken against drug companies for use of misleading information in “selling” of opioids. It's important to be aware of the dependency potential of opioids and to ensure that family members know the dangers of using the patient's pain and breathlessness medication. On 28 April 2019, the Department of Health (UK) announced it was putting health warnings on drug packages containing opioids, both prescribed and those sold “over the counter”.
Prescribing drugs should not be a lazy reflex because non-drug interventions (appropriate for every patient) seem too complex or time-consuming. Drug interventions are not required for every breathless patient. Different non-drug interventions will be needed at different stages of the disease, along a continuum from principally rehabilitative to principally supportive. Slowing the rate of deterioration and optimising general health in a progressive disease is improvement. Morphine is the drug with the best evidence base [68] and is prescribed orally, with the largest effect sizes seen in steady state. After review of effectiveness and safety data, it now has an extended licensed indication in Australia (from the Therapeutic Goods Administration) that includes chronic breathlessness, to use at 10–30 mg·day−1 of sustained-release oral morphine (Kapanol) after individual assessment (box 6). Start on the lowest possible dose [63], increasing where necessary to 20 mg daily modified-release form and titrate up, if needed, to a maximum of 30 mg daily, treating adverse effects actively. Most patients in clinical trials have required no more than 30 mg·day−1, but it should be noted that two-thirds of patients had stopped morphine at 3 months because of adverse effects or lack of response [64], so it is clear that it does not help everyone. There are some non-placebo-controlled reports of benefit from lower doses [18, 59, 61].
Oral morphine (box 6) is indicated: 1) in the palliative care of patients with distressing breathlessness due to severe COPD, heart failure, cancer or other causes, 2) after treatments for the underlying cause(s) of the breathlessness have been optimised and non-pharmacological treatments are not effective, and 3) when initiated by a specialist knowledgeable in its use.
It is important to note that a recent study indicated that in patients with pulmonary arterial hypertension [58], morphine was unhelpful, even making the sensation worse in some individuals. We cannot assume that morphine will be beneficial in all diseases or in all individuals, so careful monitoring is needed. Most of the research in morphine has been conducted in people with COPD and there is still much to learn. Fortunately, there are a number of trials underway in patients with cancer, ILD and COPD (e.g. ClinicalTrials.gov NCT02429050, for COPD).
Ongoing review is important, and it may be possible to stop morphine when the patient has improved and has, for example, been able to engage with exercise or cognitive approaches to their breathlessness. Once prescribed, patients therefore need to be monitored to see if the dose needs increasing, decreasing or stopping, and to optimise management of adverse effects. Most people with breathlessness who are prescribed morphine will have progressive disease or end-stage disease, as in other patients, non-pharmacological approaches will be the safest and most appropriate choice. Respiratory depression has been a concern historically, but in selected patients with appropriate, individualised dose schedules and monitoring, these fears appear to be unfounded [63]. There is no indication to use other opioids outside clinical trials or specialist centres. Mirtazapine may be a better first alternative if depression, severe anxiety or sleeplessness predominate, although phase 3 data are lacking [69].
Fatigue
Fatigue is the most common symptom of every illness and equally commonly overlooked. It is highly prevalent in advanced respiratory disease [70, 71]. Fatigue is not simple tiredness but “incorporates total body feelings, ranging from tiredness to exhaustion, creating an overall body condition which is unrelenting and alters the person's ability to function” [71]. Characteristically, it is not improved by sleep or rest. In spite of this, fatigue is often seen by clinicians as normal, or to be expected, and untreatable, only remediable by recovery from illness (impossible in advanced respiratory disease). Fortunately, many of the treatments for breathlessness help fatigue as well, particularly exercise, cognitive behavioural therapy and pacing and prioritising [72]. It is also important to assess patients' sleep patterns and quality, as poor sleep contributes to fatigue.
Many patients who mention fatigue feel belittled by clinicians, relatives or the general public, who confuse fatigue with tiredness. It is particularly difficult in these circumstances to explain that appropriate exercise is likely to help (initially supported by a physiotherapist, then integrated into day-to-day living). It is counter-intuitive and may be interpreted as implying that patients have not tried hard enough to help themselves. It is useful that pulmonary rehabilitation, backed by a wealth of evidence, can improve breathlessness [47]. As an exercise-based intervention it is the ideal place to start for those fit enough. Participants also make social connections, another benefit to health. If the patient is too breathless for this (rarer now as there is often specialist provision for severe disease), then a hospice rehabilitation service may help to co-design a personalised exercise programme with the patient. It is important to remember that rest rather than exercise during periods of deterioration or infection is needed as the inflammatory response is likely to be active at these times.
Cough
This can be a very distressing symptom, and a precipitator and exacerbator of breathlessness, anxiety and poor sleep in both the sufferer and those closest to them [73]. The social embarrassment and discomfort can lead to social isolation; it may even be associated with impatience and irritation in those around the patient, which adds to the distress of the symptom.
A non-pharmacological intervention based on speech and language therapy has been used in chronic (idiopathic) cough [74] with some success. Yorke et al. [21] have also used a psychoeducational approach in cough, fatigue and breathlessness in cancer patients, which showed promise in a large pilot study and is currently being tested in a randomised controlled trial. While both of these approaches need training, some elements will be available within standard respiratory services (e.g. physiotherapy) or within the wider service (e.g. speech and language service).
Drug therapy is imperfect: gabapentin, a central modulator of cough generation, is used but there is no guidance for its use in advanced respiratory disease [75]. Taking 10 mg of oral modified-release morphine daily has been shown to cause a statistically significant 40% reduction in cough scores compared with placebo [76]. Considerations apply with regard to individualising dose, active monitoring of dosing regimen and adverse effects, similar to those for breathlessness management. Long-term use of opioids is not recommended. More promising agents are being evaluated, such as P2X3 and aprepitant (in cancer patients), but have not been tested in people with advanced respiratory disease.
Psychological aspects of care
Anxiety and breathlessness are inextricably linked, as humans are hard-wired to breathe, and avoidance of suffocation is an evolutionary response partly imprinted by feelings as an adaptive response [77]. For many patients (and carers), being listened to is an important first step [4, 7, 10, 16, 17], and education about breathlessness is the second step in reducing anxiety. Use of the BTF model can be helpful in understanding and explaining how anxiety feeds in to breathlessness and how it is fed by the sensation, as well as ways to break these cycles. Access to specialist psychological support, where the therapist understands breathlessness, can be invaluable where this is needed. A recent systematic review demonstrated that drug treatment can be very effective for “trait” (i.e. part of the individual's make-up) anxiety and may have the additional benefit of helping to contain breathlessness [78].
Depression
Depression is associated with poorer clinical outcomes in advanced respiratory disease [79]. Again, it needs to be actively sought, and many clinics now use the Hospital Anxiety and Depression Scale (HADS) as a screening tool. If it is detected, depression must be actively treated.
Nutrition
The role and importance of the gut and lung microbiomes for psychological and physical health, including containing the inflammatory response, are becoming increasingly understood [80]. While there are no clear guidelines for the health of the lung microbiome, it is known that, for optimum gut health, individuals should eat a wide variety of foods, with a high proportion of fibre, wholegrains, vegetables and fruit, and with a low proportion of processed foods and those high in refined sugars, particularly fructose [81]. A healthy diet will also prevent the onset of metabolic syndrome, facilitate activity and reduce the impact of cumulative morbidity of both disease and drugs such as steroids.
Adequate sleep
Good quality and adequate amounts of sleep are needed for optimum health, both psychological and physical, including symptom control (e.g. fatigue) and containing inflammatory risk [82]. There is an increased incidence of sleep problems in people with chronic illness and some may be particularly important in advanced respiratory disease (e.g. nocturnal hypoxia). It is important that a detailed sleep history is taken, as some problems may need specialist help. All clinic patients (and carers) need to be aware of the importance of good sleep habits.
Carers
Living with someone with advanced respiratory disease is emotionally stressful, physically demanding [10, 11, 16] and imposes serious restrictions on the individual aspirations of the carer. Health services do not generally fund carer support as an intrinsic part of service delivery. Education and training about optimising symptom control, improving self-management, initiating a ritual for crises that involves the carer, and facilitating discussion of difficult issues (like the strain the carer is under) may all help to ease these. Farquhar et al. [83] are developing some tools (Support Needs Assessment for Patients (SNAP)) and educational programmes (Living With Breathlessness (LWB)) that may help in this area in the future. It is always important to listen to carers' concerns, acknowledge their efforts and encourage them to look after their own health and take some time for themselves [4, 16, 17]. This may be an area where specialist palliative care can help, as carer support is intrinsic to the specialty.
Optimising general health
An additional advantage of the non-pharmacological approach to breathlessness and fatigue (including better sleep and nutrition) is that it builds an individual's general health. People with chronic illness live stressful lives (and so do their carers). The stress response, if prolonged, will contribute to “inflammaging” [84, 85] and symptom exacerbation (well-reviewed elsewhere [60]). This is another rationale to help patients see the value of committing to necessary behaviour change. It is also helpful to explain that mind, body and brain are interconnected [86] and that improvement in one helps to drive improvement in another. It also makes it easier for the carer to understand the need to look after their own health actively. The “five ways to well-being” outlined in box 1 provide a framework to help individuals optimise their general health.
Advanced care planning
Although this might seem like an odd topic for an article on improving quality of life, it is clear that, with good reason, 1) many patients with advanced respiratory disease and their carers worry about their future and how they will be if/when the disease gets worse, and 2) patients expect doctors to initiate relevant discussions about end-of-life care [87]. The respiratory clinician who knows the patient and their illness is well placed to help patients make informed choices. There are also some patients who do not realise that advanced COPD, for example, is a life-threatening illness. All the available evidence suggests that patients with advanced respiratory disease would like to discuss possible options for end-of-life care and the use of invasive potentially life-sustaining interventions if they are gravely ill [87]. Support from specialist palliative care will help improve the choices available to the patient and family for care and support. The respiratory clinician needs to initiate these discussions. There are many papers in the literature that discuss possible timings of these discussions [24, 44, 87] and it may be decided with primary care or specialist palliative care that they are best placed to talk about options.
Conclusions
Person-centred care of those suffering with advanced respiratory disease is the most effective way of improving quality of life and clinical outcomes, and of reducing wasteful ineffective care that further exhausts and demoralises patients and families. It is not a one-off event but a process, and involves the whole respiratory multidisciplinary team working closely with primary and specialist palliative care.
Advanced respiratory disease is associated with some of the most difficult symptom-control and quality-of-life problems in medicine, sometimes over many years. The respiratory clinician, with their specialist knowledge and personal relationship with the patient, has an obligation to ensure that advanced respiratory disease is managed effectively. Every clinical encounter is an opportunity to improve quality of life for that patient and family. Use of specialist skills of other teams, like palliative and primary care, as soon as possible [4], will ensure that person-centred care is seamless, reducing suffering and optimising health throughout the patient's life.
Footnotes
Conflict of interest: S. Booth has nothing to disclose.
Conflict of interest: M.J. Johnson reports institutional payment of honoraria from Novartis, and institutional payment for clinical consultancy from Mayne Pharma, outside the submitted work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. Available from: https://goldcopd.org
- 2.Wouters EFM, Augustin IML. COPD health-care delivery: a holistic and dynamic approach is needed. Lancet Respir Med 2016; 4: e30–e31. [DOI] [PubMed] [Google Scholar]
- 3.Complexities of care in COPD. Lancet 2017; 389: 574. [DOI] [PubMed] [Google Scholar]
- 4.Maddocks M, Lovell N, Booth S, et al. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017; 390: 988–1002. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Chronic Respiratory Diseases. www.who.int/respiratory/en/
- 6.Currow DC, Dal Grande E, Ferreira D, et al. Chronic breathlessness associated with poorer physical and mental health-related quality of life (SF-12) across all adult age groups. Thorax 2017; 72: 1151–1153. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson A, Johnson MJ, Currow D. Acute-on-chronic breathlessness: recognition and response. J Pain Symptom Manage 2019; 57: e4–e5. [DOI] [PubMed] [Google Scholar]
- 8.Simon ST, Bausewein C, Schildmann E, et al. Episodic breathlessness in patients with advanced disease: a systematic review. J Pain Symptom Manage 2013; 45: 561–578. [DOI] [PubMed] [Google Scholar]
- 9.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage 2008; 36: 451–460. [DOI] [PubMed] [Google Scholar]
- 10.Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliat Support Care 2003; 1: 337–344. [DOI] [PubMed] [Google Scholar]
- 11.Farquhar M. Supporting informal carers In: Bausewein C, Currow DC, Johnson MJ, eds. Palliative Care in Respiratory Disease (ERS Monograph). Sheffield, European Respiratory Society, 2016; pp. 51–69. [Google Scholar]
- 12.The Gold Standards Framework Proactive Identification Guidance. 6th Edn. 2016. www.goldstandardsframework.org.uk/cd-content/uploads/files/PIG/NEW%20PIG%20-%20%20%2020.1.17%20KT%20vs17.pdf
- 13.Health Education England. Person-Centred Care. www.hee.nhs.uk/our-work/person-centred-care
- 14.National Cancer Institute. NCI Dictionary of Cancer Terms. www.cancer.gov/publications/dictionaries/cancer-terms/def/supportive-care
- 15.Sepúlveda C, Marlin A, Yoshida T, et al. Palliative care: the World Health Organization's global perspective. J Pain Symptom Manage 2002; 24: 91–96. [DOI] [PubMed] [Google Scholar]
- 16.Farquhar MC, Prevost AT, McCrone P, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med 2014; 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2: 979–987. [DOI] [PubMed] [Google Scholar]
- 18.Booth S, Burkin J, Moffat C, et al. Managing Breathlessness in Clinical Practice. London, Springer, 2014. [Google Scholar]
- 19.Bausewein C, Currow DC, Johnson MJ, eds. Palliative Care in Respiratory Disease (ERS Monograph). Sheffield, European Respiratory Society, 2016. [Google Scholar]
- 20.Booth S, Chin C, Spathis A, et al. Non-pharmacological interventions for breathlessness in people with cancer. Expert Rev Qual Life Cancer Care 2018; 10.1080/23809000.2018.1524708. [DOI] [Google Scholar]
- 21.Yorke J, Lloyd-Williams M, Smith J, et al. Management of the respiratory distress symptom cluster in lung cancer: a randomised controlled feasibility trial. Support Care Cancer 2015; 23: 3373–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banzett RB, O'Donnell CR. Should we measure dyspnoea in everyone? Eur Respir J 2014; 43: 1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacioppo JT, Cacioppo S. The growing problem of loneliness. Lancet 2018; 391: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth S, Ryan R, Clow A, et al. Enhancing well-being and building resilience in people living with cancer part 2: a central role for nurses. Cancer Nursing Practice 2018; 10.7748/cnp.2018.e1596. [DOI] [Google Scholar]
- 25.American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med 1999; 159: 321–340. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J 2017; 49: 1602277. [DOI] [PubMed] [Google Scholar]
- 27.Moosavi S, Booth S. The Cambridge BIS Manual. Cambridge, Breathlessness Intervention Service, 2011. Available from www.cuh.nhs.uk/breathlessness-intervention-service-bis/resources/practical-tools [Google Scholar]
- 28.Ongaro G, Kaptchuk TJ. Symptom perception, placebo effects, and the Bayesian brain. Pain 2019; 160: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faull OK, Marlow L, Finnegan SL, et al. Chronic breathlessness: re-thinking the symptom. Eur Respir J 2018; 51: 1702238. [DOI] [PubMed] [Google Scholar]
- 30.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comroe JH., Jr Some theories of the mechanisms of dyspnoea In: Howell JBL, Campbell EJM, eds. Breathlessness. Oxford, Blackwell Scientific, 1966; pp. 1–5. [Google Scholar]
- 32.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol 2009; 167: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 34.Herigstad M, Faull OK, Hayen A, et al. Treating breathlessness via the brain: changes in brain activity over a course of pulmonary rehabilitation. Eur Respir J 2017; 50: 1701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth S, Chin C, Spathis A. The brain and breathlessness: understanding and disseminating a palliative care approach. Palliat Med 2015; 29: 396–398. [DOI] [PubMed] [Google Scholar]
- 36.Coats AJ. Teaching heart-failure patients how to breathe. Lancet 1998; 351: 1299–1300. [DOI] [PubMed] [Google Scholar]
- 37.Spathis A, Booth S, Moffat C, et al. The Breathing, Thinking, Functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med 2017; 27: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark NM, Gong M. Management of chronic disease by practitioners and patients: are we teaching the wrong things? BMJ 2000; 320: 572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs 1993; 16: 310–320. [PubMed] [Google Scholar]
- 40.Linde P, Hanke G, Voltz R, et al. Unpredictable episodic breathlessness in patients with advanced chronic obstructive pulmonary disease and lung cancer: a qualitative study. Support Care Cancer 2018; 26: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 41.Mularski RA, Reinke LF, Carrieri-Kohlman V, et al. An official American Thoracic Society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc 2013; 10: S98–S106. [DOI] [PubMed] [Google Scholar]
- 42.de Silva D. Helping People Help Themselves. London, The Health Foundation, 2011. [Google Scholar]
- 43.Hutchinson A, Barclay-Klingle N, Galvin K, et al. Living with breathlessness: a systematic review and qualitative synthesis. Eur Respir J 2018; 51: 1701477. [DOI] [PubMed] [Google Scholar]
- 44.Landers A, Wiseman R, Pitama S, et al. Severe COPD and the transition to a palliative approach. Breathe 2017; 13: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cawley D, Horne C. A hospice breathlessness management intervention service – a blend of the old with the new! BMJ Support Palliat Care 2016; 6: Suppl. 1, A51–A52. [Google Scholar]
- 46.Bolzani A, Rolser SM, Kalies H, et al. Respiratory interventions for breathlessness in adults with advanced diseases. Cochrane Database Syst Rev 2017; 6: CD012683. [Google Scholar]
- 47.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luckett T, Phillips J, Johnson MJ, et al. Contributions of a hand-held fan to self-management of chronic breathlessness. Eur Respir J 2017; 50: 1700262. [DOI] [PubMed] [Google Scholar]
- 49.Swan F, Newey A, Bland M, et al. Airflow relieves chronic breathlessness in people with advanced disease: an exploratory systematic review and meta-analyses. Palliat Med 2019; 33: 618–633. [DOI] [PubMed] [Google Scholar]
- 50.Howard C, Dupont S. “The COPD breathlessness manual”: a randomised controlled trial to test a cognitive-behavioural manual versus information booklets on health service use, mood and health status, in patients with chronic obstructive pulmonary disease. NPJ Prim Care Respir Med 2014; 24: 14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bausewein C, Booth S, Gysels M, et al. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 2008; 2: CD005623. [DOI] [PubMed] [Google Scholar]
- 52.Bolzani A, Rolser SM, Kalies H, et al. Cognitive-emotional interventions for breathlessness in adults with advanced diseases. Cochrane Database Syst Rev 2017; 6: CD012682. [Google Scholar]
- 53.Bolzani A, Rolser SM, Kalies H, et al. Physical interventions for breathlessness in adults with advanced diseases. Cochrane Database Syst Rev 2017; 6: CD012684. [Google Scholar]
- 54.Smallwood N, Thompson M, Warrender-Sparkes M, et al. Integrated respiratory and palliative care may improve outcomes in advanced lung disease. ERJ Open Res 2018; 4: 00102-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocker GM, Cook D. “INSPIRED” approaches to better care for patients with advanced COPD. Clin Invest Med 2013; 36: E114–E120. [DOI] [PubMed] [Google Scholar]
- 56.Brighton LJ, Miller S, Farquhar M, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019; 74: 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bausewein C, Schunk M, Schumacher P, et al. Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis 2018; 15: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira DH, Ekström M, Sajkov D, et al. Extended-release morphine for chronic breathlessness in pulmonary arterial hypertension – a randomized, double-blind, placebo-controlled, crossover study. J Pain Symptom Manage 2018; 56: 483–492. [DOI] [PubMed] [Google Scholar]
- 59.Roberts MM, Leeder SR, Robinson TD. Nurse-led 24-h hotline for patients with chronic obstructive pulmonary disease reduces hospital use and is safe. Intern Med J 2008; 38: 334–340. [DOI] [PubMed] [Google Scholar]
- 60.Janssen DJA, Spruit MA, Schols JMGA, et al. A call for high-quality advance care planning in outpatients with severe COPD or chronic heart failure. Chest 2011; 139: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 61.Marciniuk DD, Goodridge D, Hernandez P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011; 18: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016; 3: CD011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Currow DC, McDonald C, Oaten S, et al. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage 2011; 42: 388–399. [DOI] [PubMed] [Google Scholar]
- 64.Rocker G, Horton R, Currow D, et al. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax 2009; 64: 910–915. [DOI] [PubMed] [Google Scholar]
- 65.Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2016; 10: CD007354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbetta C, Currow DC, Johnson MJ. Non-opioid medications for the relief of chronic breathlessness: current evidence. Expert Rev Respir Med 2017; 11: 333–341. [DOI] [PubMed] [Google Scholar]
- 67.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med 2019; 380: 2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 2014; 348: g445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Currow DC, Ekström M, Louw S, et al. Sertraline in symptomatic chronic breathlessness: a double blind, randomised trial. Eur Respir J 2019; 53: 1801270. [DOI] [PubMed] [Google Scholar]
- 70.Vanfleteren LEGW, Spruit MA, Wouters EFM, et al. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016; 4: 911–924. [DOI] [PubMed] [Google Scholar]
- 71.Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: a phenomenological enquiry. Int J Nurs Stud 1997; 34: 44–53. [DOI] [PubMed] [Google Scholar]
- 72.Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3: CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irwin RS, French CT, Lewis SZ, et al. Overview of the management of cough: CHEST Guideline and Expert Panel Report. Chest 2014; 146: 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017; 72: 129–136. [DOI] [PubMed] [Google Scholar]
- 75.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 76.Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med 2007; 175: 312–315. [DOI] [PubMed] [Google Scholar]
- 77.Damasio A, Carvalho DB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 2013; 14: 143–152. [DOI] [PubMed] [Google Scholar]
- 78.Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet 2019; 393: 768–777. [DOI] [PubMed] [Google Scholar]
- 79.Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med 2007; 167: 60–67. [DOI] [PubMed] [Google Scholar]
- 80.Han MK, Huang YJ, Lipuma JJ, et al. Significance of the microbiome in obstructive lung disease. Thorax 2012; 67: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015; 66: 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farquhar M, Penfold C, Benson J, et al. Six key topics informal carers of patients with breathlessness in advanced disease want to learn about and why: MRC phase I study to inform an educational intervention. PLoS One 2017; 12: e0177081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan R, Spathis A, Booth S. Correlates between basic science and therapeutic interventions: the theory and the practice. Curr Opin Support Palliat Care 2014; 8: 200–207. [DOI] [PubMed] [Google Scholar]
- 85.Booth S, Ryan R, Clow A, et al. Enhancing well-being and resilience in people living with cancer. Part 1. Cancer Nursing Practice 2018; 10.7748/cnp.2018.e1484. [DOI] [Google Scholar]
- 86.Daruna JH. Introduction to Psychoneuroimmunology. 2nd Edn Elsevier, 2012. [Google Scholar]
- 87.Cawley D, Billings J, Oliver D, et al. Potential triggers for the holistic assessment of people with severe chronic obstructive pulmonary disease: analysis of multiperspective, serial qualitative interviews. BMJ Support Palliat Care 2014; 4: 152–160. [DOI] [PubMed] [Google Scholar]