Abstract
Dehydroepiandrosterone-sulfate (DHEAS) is an adrenal androgen that is, in part, aromatized to estradiol. It continues to be produced after menopause and provides estrogenicity after depletion of ovarian hormones. Estradiol depletion contributes to memory circuitry changes over menopause, including changes in hippocampal (HIPP) and dorsolateral- and ventrolateral-prefrontal cortex (DLPFC; VLPFC) function. Further, major depressive disorder (MDD) patients have, in general, lower levels of estradiol and lower DHEAS than healthy controls, thus potentially a higher risk of adverse menopausal outcomes. We investigated whether higher DHEAS levels after menopause is associated with better memory circuitry function, especially in women with MDD. 212 adults (ages 45-55, 50% women) underwent clinical and fMRI testing. Participants performed a working memory (WM) N-back task and an episodic memory verbal encoding (VE) task during fMRI scanning. DHEAS levels were significantly associated with memory circuitry function, specifically in MDD postmenopausal women. On the WM task, lower DHEAS levels were associated with increased HIPP activity. On the VE task, lower DHEAS levels were associated with decreased activity in the HIPP and VLPFC. In contrast, there was no association between DHEAS levels and memory circuitry function in MDD pre/perimenopausal women, men, and non-MDD participants regardless of sex and reproductive status. In fact, MDD postmenopausal women with higher levels of DHEAS were similar to MDD pre/perimenopausal women and men. Thus, memory circuitry deficits associated with MDD and a lower ability of the adrenal gland to produce DHEAS after menopause may contribute to a lower ability to maintain intact memory function with age.
Keywords: Memory, Adrenal hormones, Aging, Menopause, Depression, Sex difference
1. Introduction
Steroid hormones have pronounced effects on the brain including regulation of memory circuitry regions, such as the hippocampus (HIPP) and prefrontal cortex (PFC), which are highly sexually dimorphic (Goldstein et al., 2001). Dehydroepiandrosterone (DHEA) and its sulfate conjugate DHEAS (together referred to as DHEA/S) are the most abundant steroid hormones produced by the adrenal gland and bind with low affinity to androgen receptors. DHEA/S is an important precursor to estradiol and is the primary source of estrogen after menopause (Simpson and Davis, 2001). There is substantial preclinical and clinical literature demonstrating the impact of estradiol on memory circuitry and function (Barth et al., 2016; Daniel et al., 1997; Dumitriu et al., 2010; Hara et al., 2012; Hussain et al., 2016; Jacobs et al., 2015; Korol and Kolo, 2002; Korol, 2004; Lisofsky et al., 2015; Luine et al., 1998; McEwen et al., 1995; Packard and Teather, 1997; Protopopescu et al., 2008; Rosenberg and Park, 2002; Woolley et al., 1996a; Woolley et al., 1996b). With growing evidence for the neuroprotective role of estradiol and the detrimental effects of its depletion over menopause, it is important to consider the neuroprotective role of DHEA/S, which may contribute to maintaining estrogenicity in the brain after ovarian decline. Here, we show it further contributes to understanding sex differences in memory decline.
1.1. Estradiol and memory
There is a long history of preclinical evidence demonstrating the role of estradiol in HIPP structure and function. For example, in rodents, HIPP CA1 neurons show excitatory synaptic density changes that are modulated by estradiol levels (McEwen et al., 1995; Woolley et al., 1996b). Further, ovariectomization in rodents leads to degeneration in the HIPP, an effect that can be reversed with estrogen replacement (Dumitriu et al., 2010; Woolley et al., 1996a). Estrogen replacement also enhances spatial memory in rodents (Daniel et al., 1997; Korol and Kolo, 2002; Korol, 2004; Luine et al., 1998; Packard and Teather, 1997). In rhesus monkeys, reproductive age, rather than chronological age, is associated with lower recognition memory and synaptic density in the HIPP dentate gyrus (Hara et al., 2012). In population-level studies, fluctuating levels of estradiol across the menstrual cycle correlate with verbal memory performance (Hussain et al., 2016; Protopopescu et al., 2008; Rosenberg and Park, 2002), HIPP volume (Lisofsky et al., 2015; Protopopescu et al., 2008), and HIPP fractional anisotropy (Barth et al., 2016). Further, neuroimaging studies have demonstrated direct associations between ovarian steroid hormone levels and functional activity and connectivity in the memory circuitry (Jacobs et al., 2015; Lisofsky et al., 2015).
Modulatory effects of estradiol are also seen across the menopausal transition (Epperson et al., 2013; Jacobs et al., 2016a; Jacobs et al., 2016b; Morrison et al., 2006; Mosconi et al., 2017a; Mosconi et al., 2017b; Rentz et al., 2016; Ryan et al., 2012; Shanmugan and Epperson, 2014; Wroolie et al., 2015). In some women, the depletion of ovarian sex steroid hormones over menopause results in functional changes in these brain regions that may have long-term consequences on risk of cognitive impairment and Alzheimer’s disease (AD). In a series of analyses comparing men and women in early midlife, we replicated the finding that women outperform men on verbal and associative memory tasks (Rentz et al., 2016). Further, we demonstrated that this female advantage was attenuated postmenopause due, in part, to estradiol decline, underscoring the impact of ovarian decline in shaping memory function in women. Reproductive age also had a pronounced regional and network level impact on task-evoked activity in memory circuitry regions, independent of chronological age (Jacobs et al., 2016a; Jacobs et al., 2016b). Together, these findings demonstrated that sex steroid hormones alter memory circuity function and that menopause has a significant impact on aging of memory circuitry in women. In the current study, we build on these findings to examine the role of DHEAS in the face of reproductive aging.
1.2. DHEA/S and memory
There is strong preclinical evidence for the neuroprotective effects of DHEA/S on memory performance in aging studies (Chen et al., 2008; Flood and Roberts, 1988; Maurice et al., 2000; Maurice et al., 2001; Melchior and Ritzmann, 1996; Moriguchi et al., 2011; Reddy and Kulkarni, 1998; Shi et al., 2000). Oral DHEAS administration has been found to enhance longterm and working memory in aged mice (Markowski et al., 2001), and subcutaneous DHEA injection significantly improved spatial learning and memory in D-galactose-induced senescence rats (Chen et al., 2008). Similarly, other studies have found that DHEA/S treatment reduced impairments in spatial working memory induced by dizocilpine (NMDA receptor antagonist), scopolamine (anticholinergic agent), ethanol, and hypoxic insult from carbon monoxide exposure (Maurice et al., 2000; Maurice et al., 2001; Melchior and Ritzmann, 1996; Reddy and Kulkarni, 1998; Shi et al., 2000). While these preclinical studies have identified a significant impact of DHEA/S on memory function, they were primarily in male rodents who were not gonadectomized. Thus, it is unclear how DHEA/S and testosterone levels interact with or modulate one another. Several studies have also examined the impact of DHEA/S in ovariectomized female rodents, a model of ovarian decline. In response to DHEA/S treatment, ovariectomized females demonstrated improved HIPP-dependent memory performance and increased hippocampal spine density (Frye and Sturgis, 1995; Frye and Lacey, 1999; Hajszan et al., 2004). As such, preclinical studies have provided ample evidence for the beneficial impact of DHEA/S on HIPP-dependent memory in the presence of neuronal insult and ovarian steroid hormone depletion.
In contrast to rodent studies that have convincingly demonstrated the beneficial effects of DHEA/S treatment in enhancing spatial and working memory, studies in non-human primates and humans are less conclusive. Unlike rodents, humans and non-human primates have the capacity to produce DHEA/S in the adrenal gland (van Weerden et al., 1992), resulting in higher circulating levels of DHEA/S. In perimenopausal rhesus monkeys, DHEA administration does not correlate with improved memory performance (Sorwell et al., 2017). Further, a study examining endogenous DHEA/S levels in aged and young adult rhesus monkeys found that while DHEAS levels and memory performance predictably decreased with age, these factors together were not significantly correlated (Herndon et al., 1999). Several clinical studies in humans have found a positive association between memory performance and DHEA/S levels (Correa et al., 2016; Davis et al., 2008). Specifically, in postmenopausal women, higher DHEA/S levels were associated with better verbal and visual memory (Bojar et al., 2017) and better longterm memory recall (Barrett-Connor and Edelstein, 1994). However, the latter study did not find an association between midlife DHEA/S levels and subsequent diagnosis of dementia. Other studies have found no relationship between endogenous levels of DHEA/S and memory performance (Bo et al., 2006) or effects specific to men (Castanho et al., 2014; Hildreth et al., 2013). Overall, there is inconsistency in the literature regarding the role of DHEA/S and memory performance that requires further investigation. In part, this may be due to variation in the conversion of DHEAS into estradiol in the brain and other direct neuronal effects of DHEA/S (Bastianetto et al., 1999; Bergeron et al., 1996; Chen and Parker, 2004; Kalimi et al., 1994; Majewska, 1992; Naert et al., 2007; Park-Chung et al., 1999; Zhang et al., 2002), which were not measured in the human or non-human primate studies.
1.3. Steroid hormones and major depression
In addition to memory function, DHEA/S plays an important role in mood regulation. However, the exact relationship between DHEA/S and major depressive disorder (MDD) is unclear. Some studies report higher DHEA/S concentrations in clinically depressed patients compared to healthy controls (Heuser et al., 1998; Morita et al., 2014; Takebayashi et al., 1998), while others have shown that depressive symptomology is associated with lower (Barrett-Connor et al., 1999; Berr et al., 1996; Morsink et al., 2007) or unaltered (Erdincler et al., 2004; Glei et al., 2004; Morrison et al., 1998) circulating DHEA/S levels. We have previously demonstrated that estradiol levels are significantly lower in MDD patients (Holsen et al., 2011). Given that MDD is associated with overall lower levels of estradiol, we hypothesized that women with MDD may be at higher risk of adverse menopausal outcomes and therefore benefit most from their ability to maintain higher endogenous DHEAS levels. In the current study, we investigated the neural impact of DHEAS on memory circuitry function in MDD and healthy controls in the face of ovarian decline. We hypothesized that higher levels of DHEAS would have beneficial effects on memory circuitry activity and function, particularly among women with MDD.
2. Results
2.1. Demographics and clinical characteristics
The sample included 212 participants (106 women and 106 men). Eight women reported use of hormone replacement therapy and were removed from subsequent analyses. Serum measures were missing for an additional 10 participants (two women and eight men). Seven participants did not undergo sMRI/fMRI scanning, leaving a final sample of 187 participants (93 women and 94 men) for analysis.
All participants were in early midlife (mean age = 49.97 ± 2.15; range: 45-55 years old), with an average verbal IQ of 116.72 ± 10.39, as assessed with the AMNART. Of these participants, 64.1% had a high school diploma/GED or higher. Among women, some were already in menopause with permanent amenorrhea, low estradiol levels, and elevated gonadotropins; some exhibited signs of follicular failure (elevated FSH and oligomenorrhea); and some showed normal cycling. Sixty-two (66.6%) were pre/perimenopausal and 31 (33.3%) were postmenopausal. In previous studies with this cohort (Jacobs et al., 2016a; Jacobs et al., 2016b; Rentz et al., 2016), premenopausal and perimenopausal women had similar performances on memory tests, and therefore, the two groups were combined to form a pre/perimenopausal group. Sixty-six (35.3%) participants had a history of MDD (single and recurrent). Table 1 reports demographic characteristics of the sample and group comparisons. Pre/peri and postmenopausal women were comparable for body mass index (BMI), ethnicity, education, and substance and alcohol abuse (allp’s n.s.). Pre/perimenopausal women were slightly younger than postmenopausal women (49.5 v. 50.5 years,p = 0.03) and MDD participants were more educated than non-MDD participants (Educational attainment above high school/GED: 76.9% v. 57,l%, p= 0.007). Men and women (pre/peri and post) were comparable for BMI, education, and ethnicity. Men tended to be older than pre/perimenopausal women (50.1 v. 49.5 years,p = 0.06) and a larger proportion reported current or past use of substance and alcohol abuse/dependence (62.8% v. 41.9%,p = 0.01).
Table 1.
Demographic and clinical characteristics of a sample of N = 187, men and women assessed at ages 45-55
| Men (n = 94) | Women (n = 93) | Pre/Peri (n = 62) | Post (n = 31) | MDD (n = 66) | Non-MDD (n = 121) | Comparison | |
|---|---|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | mean (SD) | mean (SD) | mean (SD) | ψp < 0.001; *p < 0.05; †p < 0.1 | |
| Age | 50.1 (2.4) | 49.8 (1.9) | 49.5 (1.7) | 50.5 (2.2) | 49.6 (2.1) | 50.2 (2.2) | Pre/Peri < Post*, Men† |
| BMIa | 28.8 (5.2) | 28.2 (5.9) | 28.4 (6.0) | 27.8 (5.8) | 29.2 (5.6) | 28.1 (5.5) | n.s. |
| Digit Span Forwarda | 9.3 (2.5) | 8.5 (2.3) | 8.4 (2.3) | 8.8 (2.3) | 9.0 (2.8) | 8.9 (2.3) | Men > Women*, Pre/Peri* |
| Digit Span Backwarda | 7.7 (2.8) | 7.1 (2.3) | 7.1 (2.3) | 7.1 (2.5) | 7.5 (2.9) | 7.3 (2.4) | n.s. |
| Verbal Fluency (FAS)a | 42.4 (11.4) | 41.2 (10.4) | 40.9 (8.5) | 41.9 (13.5) | 40.3 (11.5) | 42.7 (10.5) | Non-MDD > MDD* |
| Verbal Fluency (Categories)a | 44.8 (10.1) | 47.9 (8.4) | 48.8 (8.7) | 46.1 (7.8) | 45.6 (9.8) | 46.7 (9.2) | Men < Women*, Pre/Peri* |
| STAI STATEa | 28.6 (7.7) | 28.9 (8.4) | 28.8 (9.0) | 29.2 (7.2) | 30.9 (6.7) | 27.6 (8.5) | MDD > Non-MDDψ |
| STAI TRAITd | 34.1 (9.4) | 33.7 (8.1) | 33.9 (8.6) | 33.3 (6.9) | 38.6 (9.1) | 31.3 (7.3) | MDD > Non-MDDψ |
| POMSe | 0.4 (3.3) | −0.3 (24) | −04 (2.3) | 0.00 (2.8) | 1.1 (28) | −0.5 (2.8) | MDD > Non-MDDψ |
| d’ 2back (Performance on WM task)b | 2.0 (0.7) | 1.9 (0.7) | 1.9 (0.7) | 2.0 (0.7) | 1.9 (0.7) | 2.1 (0.7) | n.s. |
| d’ VR (Performance on VE task)c | 1.0 (0.5) | 1.0 (0.5) | 1.0 (0.6) | 0.9 (0.4) | 0.9 (0.5) | 1.0 (0.9) | n.s. |
| Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | ||
| DHEAS (ug/dL) | 139.2 (97.8, 199.4) | 100.0 ( 64.2, 138.0) | 101.8 (69.3, 141.8) | 100.0 ( 60.0, 135.4) | 121.3 (89.2, 173.1) | 118.9 ( 75.7, 173.9) | Men > Women*, Pre/Peri*, Post* |
| 17β-Estradiol (pg/ml): Only womena | - | 36.5 (11.6, 84.8) | 60.1 (21.8, 109.0) | 9.0 (3.9, 9.0) | 41.3 (8.9, 84.8) | 24.8 (11.8, 90.4) | Pre/Peri > Postψ |
| Progesterone (ng/ml): Only womend | - | 0.14 (0.06, 0.67) | 0.20 (0.09, 4.01) | 0.07 (0.05, 0.13) | 0.18 (0.06, 1.42) | 0.13 (0.06, 0.33) | Pre/Peri > Postψ |
| FSH (mlU/ml): Only women | - | 23.7 (6.9, 62.8) | 10.1 (5.5, 28.3) | 64.2 (45.5, 85.5) | 12.7 (6.8, 56.6) | 30.5 (6.6, 68.3) | Pre/Peri < Postψ |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Education (%>High school/GED)d | 63 (67.0%) | 55 (59.1%) | 37 (59.7%) | 18 (58.1%) | 50 (76.9%) | 68 (57.1%) | MDD > Non-MDD* |
| Ethnicity (%Caucasian) | 85 (90.4%) | 81 (87.1%) | 56 (90.3%) | 25 (80.6%) | 56 (84.8%) | 110 (90.9%) | n.s. |
| History of or Current substance or alcohol abuse/dependence (Yes) | 59 (62.8%) | 43 (46.2%) | 26 (41.9%) | 17 (54.8%) | 38 (57.6%) | 64 (52.9%) | Men > Women*, Pre/Peri* |
Missing n=1;
Missing n=5;
Missing n=11;
Missing n=3;
Missing n=2
Comparisons by group status were evaluated using nonparametric Wilcoxon rank-sum or chi-square test, for continuous and categorical data, respectively (n.s. = Not Significant).
p25, p75: 25th and 75th quartiles
BMI:Body Mass index; WM: Working memory; VE: Verbal encoding; STAI: Spielberger State-Trait Anxiety Inventory; POMS: Profile of Moods Questionnaire; DHEAS: Dehydroepiandrosterone-sulfate; FSH=Follcle-stimulating hormone
Men performed better than women on the Digit span forward task (Mean (SD): 9.3 (2.5) v. 8.5 (2.3); p = 0.02) but this effect was specific to the comparison with pre/perimenopausal women (9.3 (2.5) v. 8.4 (2.3); p = 0.02; Table 1). There was no difference in performance on the Digit span between men and postmenopausal women (9.3 (2.5) v. 8.8 (2.3); n.s.). In contrast, women performed better than men on the Verbal fluency categories test (47.9 (8.4) v. 44.8 (10.1); p = 0.01), again, an effect specific to pre/perimenopausal women (48.8 (8.7) v. 44.8 (10.1); p = 0.006). There was no difference between men and postmenopausal women on the Verbal fluency categories task (46.1 (7.8) v. 44.8 (10.1); n.s.).
MDD subjects performed worse on the Verbal fluency FAS task compared with non-MDD subjects (40.3 (11.5) v. 42.7(10.5); p = 0.05). Not surprisingly, MDD subjects scored higher on the STAI (State: 30.9 (6.7) v. 27.6 (8.5); p < 0.001; Trait: 38.6 (9.1) v. 31.3 (7.3);p < 0.001) and POMS questionnaires (1.1 (2.8) v. −0.5 (2.8); p < 0.001), demonstrating a higher degree of dysphoric mood.
2.2. Hormonal evaluations
As expected, postmenopausal women had significantly lower levels of serum estradiol (Median [p25, p75]: 9.0 [3.9, 9.0] v. 60.1 [21.8, 109.0];p < 0.001; Table 1) and progesterone (0.07 [0.05, 0.13] v. 0.20 [0.09, 4.01];p < 0.001) and significantly higher levels of FSH (64.2 [45.5, 85.5] v. 10.1 [5.5, 28.3]; p < 0.001) compared to pre/perimenopausal women.
Overall, MDD and non-MDD women did not differ in estradiol levels (41.3 [8.9, 84.8] v. 24.8 [11.8, 90.4]; n.s.; Fig. 1a; Table 1). However, in postmenopause, MDD women had significantly lower levels of estradiol compared to non-MDD women (4.3 [3.4, 10.1] v. 13.7 [5.9, 29.9p = 0.03). In pre/perimenopause, MDD women also had lower levels of estradiol (53.0 [29.2, 100.9) v. 60.6 [16.9, 122.5]) compared to non-MDD women, however, this was not significant and effect size was smaller than in postmenopausal women (post: d= −0.51; pre/peri: d=−0.34).
Figure 1.
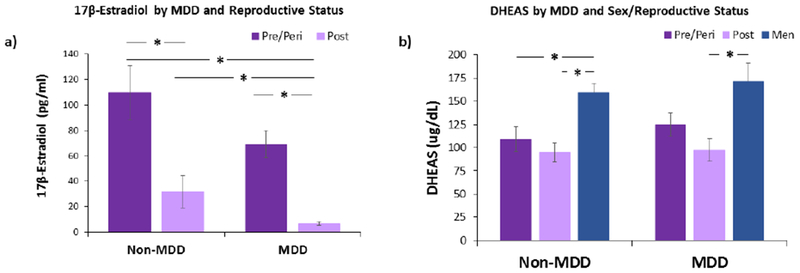
Mean 17β-estradiol and DHEAS concentrations by MDD and menopausal stage, a) Serum estradiol levels in MDD postmenopausal women were significantly lower than non-MDD postmenopausal women and pre/perimenopausal women, regardless of MDD status. As expected, non-MDD postmenopausal women had significantly lower levels of estradiol compared to non-MDD pre/perimenopausal women, b) In non-MDD subjects, men had significantly higher of DHEAS compared to pre/perimenopausal and postmenopausal women. In MDD, men had significantly higher levels of DHEAS compared to postmenopausal women. There were no differences in DHEAS levels between menopausal staging groups in both MDD status. Non-MDD: pre/perimenopause (n = 33), postmenopause (n = 21), men (n = 67); MDD: pre/perimenopause (n = 29), postmenopause (n = 10), men (n = 27). Error bars represent SEM. *p< 0.05.
Further, MDD pre/peri- and postmenopausal women did not differ in DHEAS levels (Fig. 1b; Table 2). Similarly, non-MDD pre/peri- and postmenopausal women did not differ in their DHEAS levels. Interestingly, MDD and non-MDD men also did not differ in DHEAS levels.
Table 2.
Hormone assessment of sample by MDD and sex/reproductive status
| Men | Women | Pre/Perimenopause | Postmenopause | Comparisons | |
|---|---|---|---|---|---|
| Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | Median (p25, p75) | ψp < 0,001; *p < 0.05; †p < 0.1 | |
| DHEAS (ug/dL) | |||||
| MDD | 132.0 (99.3, 202.8) | 114.9 (S5.0, 149.0) | 121.0 (71.5, 156.0) | 103.5 (58.8, 126.9) | Men > Women†, Post* |
| Non-MDD | 150.0 (97.1, 198.3) | 87.0 ( 63.1, 129.5) | 85.9 (68.5, 124.7) | 93.6 (56.8, 139.8) | Men > Womenψ, Pre/Periψ, Postψ |
| n.s. | n.s. | n.s. | n.s. | ||
| 17β-Estradiol (pg/ml)a | |||||
| MDD | - | 41.3 (8.9, 84.8) | 53.0 (29.2, 100.9) | 4.3 (3.4, 10.1) | Pre/Peri >Postψ |
| Non-MDD | - | 24.8 (11.8, 90.4) | 60.6 (16.9, 122.5) | 13.7 (5.9, 29.9) | Pre/Peri>Postψ |
| n.s. | n.s. | non-MDD>MDD* | |||
| Progesterone (ng/ml)b | |||||
| MDD | - | 0.18 (0.06, 1.42) | 0.19 (0.08, 3.41) | 0.07 (0.04, 0.38) | Pre/Peri > Post* |
| Non-MDD | - | 0.13 (0.06, 0.33) | 0.20 (0.10, 4.80) | 0.07 (0.05, 0.09) | Pre/Peri >Postψ |
| n.s. | n.s. | n.s. | |||
| FSH (mIU/ml) | |||||
| MDD | - | 12.7 (6.8, 56.6) | 10.1 (6.5, 16.2) | 65.5 (48.2, 73.6) | Post> Pre/Periψ |
| Non-MDD | - | 30.5 (6.6, 68.3) | 9.1 (4.8, 35.0) | 64.2 (43.7, 92.3) | Post> Pre/Periψ |
| n.s. | n.s. | n.s. | |||
Missing n=1;
Missing n=3
Comparisons by group status were evaluated using nonparametric Wilcoxon rank-sum (n.s. = Not Significant).
p25, p75: 25th and 75th quartiles
2.3. Behavioral Performance
Behavioral performances on the WM and VE tasks were comparable across sex/menopausal staging and MDD groups (Table 1). There was also no association between DHEAS levels and task performance on the WM and VR tasks in the total sample (WM: β = 7.21, n.s.; VR: β = 15.79, n.s.) as well as in each sex/menopausal staging and MDD group (all p’s n.s.).
2.4. Regional BOLD response in Working Memory (N-back) circuitry
In our previous work with the same cohort presented here (Jacobs et al., 2016a), we demonstrated that the N-back paradigm evoked robust responses throughout working memory circuitry in this cohort. The N-back paradigm assesses working memory and the ability to maintain and manipulate information. It requires subjects to continuously update their short-term memory with new stimuli to reference. We conducted blood-oxygen-level-dependent (BOLD) contrast imaging to assess task-evoked activity. The BOLD response is an indirect measure of neuronal activity and is based on the relative concentration of oxygenated and deoxygenated blood. When neurons become active in response to a task, blood flow increases through that region to replenish the oxygen supply that is used by the cells. This leads to a relative surplus of local blood oxygen levels and is the basis for the BOLD signal. Regions of interest included task-evoked activity within bilateral DLPFC and systematic deactivation of the HIPP. We demonstrated that task-evoked activity in DLPFC increased over the menopausal transition, with postmenopausal women exhibiting greater activity relative to premenopausal women. Similarly, pronounced deactivation in the HIPP was observed in premenopausal women, with increasingly attenuated deactivation (failure to disengage) over the menopausal transition in women. Changes in memory circuitry function were strongly related to a decline in endogenous estradiol levels (Jacobs et al., 2016a) In the current study, we extended these findings to examine working memory function in the context of MDD and DHEAS.
2.4.1. Total sample
In the total sample (N=187), we found a significant association between DHEAS levels and activity in the left HIPP (β (SE) = −0.0007 (0,0004), pchsq = 0.04), whereby, higher levels of DHEAS were associated with lower activity in the HIPP. In all other regions of the working memory circuitry, irrespective of sex/reproductive status, we found no significant associations between DHEAS levels and activity in the working memory circuitry in healthy middle-aged adults (all p’s n.s.). We then examined the interaction between sex/reproductive status and DHEAS levels on HIPP and DLPFC fMRI BOLD activity and found no significant interactions (all p’s n.s.).
2.4.2. MDD
Group differences emerged when we examined the association of MDD and reproductive status on memory circuitry activity and function. First, in MDD, significant interactions in the left and right HIPP were observed as a function of women’s reproductive status (pre/peri v. post), despite minimal difference in chronological age, and DHEAS levels (left: χ2 = 5.97, pchsq = 0.029; right: χ2 = 8.04, pchsq = 0.005; Table 3). Interaction in the left HIPP did not pass correction for multiple comparison. Specifically, in MDD postmenopausal women, lower DHEAS levels were significantly associated with a failure to disengage the right HIPP (β (SE) = −0.007 pchsq = 0.05; Fig. 2a). In contrast, higher levels of DHEAS were associated with disengagement of the HIPP, similar to patterns of activity previously observed in premenopausal women (Jacobs et al., 2016a). Trends were observed in the right DLPFC, whereby lower DHEAS levels were associated with increased task-evoked activity in the left DLPFC (β (SE) = −0.009 (0.005), p = 0.08). However, these effects did not persist after controlling for age, substance dependence, and assay type (Table 3). In MDD pre/perimenopausal women, there were no associations between DHEAS levels and activity in the DLPFC (left: β (SE) = 0.002 (0.002), n.s.; right: β (SE) = −0.00005 (0.002), n.s.) or HIPP (left: β (SE) = −0.0004 (0.001), n.s.; right: β (SE) = 0.001 (0.001), n.s., Fig. 2b).
Table 3.
MDD and Non-MDD: Association between DHEAS and adult regional fMRI BOLD activity in the HIPP and PFC by sex and reproductive status.
| Comparisons |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Pre-/Peri-Menopause | Post-Menopause | Men V. Women | Men v. Pre/Peri- | Men v. Post | Pre-/Peri- v. Post- | ||||||||||||||
| B | S.E. | X2 | p | B | S.E. | X2 | p | B | S.E. | X2 | p | B | S.E. | X2 | p | p | p | p | p | ||
| MDD | Working memory fMRI BOLD activity | ||||||||||||||||||||
| Hippocampus | |||||||||||||||||||||
| Left | −0.0001 | 0.001 | 0.02 | 0.88 | −0.001 | 0.001 | 1.2 | 0.27 | −0.0004 | 0.001 | 0.2 | 0.65 | −0.007 | 0.005 | 1.58 | 0.21 | 0.24 | 0.62 | c1.11 | 0.029* | |
| Right | −0.00001 | 0.001 | 0.00 | 0.99 | b0.0007 | 0.001 | 0.24 | 0.62 | 0.001 | 0.001 | 1.28 | 0.26 | −0.007 | 0.003 | 3.83 | 0.05* | 0.8 | 0.64 | 0.07† | 0.005Ψ | |
| DLPFC | |||||||||||||||||||||
| Left | a0.001 | 0.002 | 0.37 | 0.55 | 0.0005 | 0.002 | 0.03 | 0.78 | 0002 | 0.002 | 0.47 | 0.49 | −0.009 | 0.009 | 1.07 | 0.3 | b0.77 | 0.68 | c0.37 | 0.14 | |
| Right | a−0.001 | 0.002 | 0.59 | 0.44 | −0.001 | 0.002 | 0.5 | 0.48 | −0.00005 | 0.002 | 0.00 | 0.98 | −0.01 | 0.006 | 2.26 | 0.13 | 0.42 | 0.84 | 0.19 | 0.11 | |
| Verbal encoding fMRI BOLD activity | |||||||||||||||||||||
| Hippocampus | |||||||||||||||||||||
| Left | −0.0018 | 0.001 | 5.94 | 0.01* | −0.0005 | 0.001 | 0.26 | 0.61 | −0.0008 | 0.001 | 0.35 | 0.56 | 0.007 | 0.002 | 12.47 | 0.0004* | 0.28 | c0.66 | 0.06† | 0.073† | |
| VLPFC | |||||||||||||||||||||
| Left Dorsal | 0.002 | 0.001 | 1.19 | 0.27 | 0.0001 | 0.002 | 0 | 0.95 | −0.001 | 0.002 | 0.3 | 0.58 | 0.013 | 0.009 | 2.49 | 0.11 | 0.98 | 0.54 | 0.06† | 0.02* | |
| Left Mid | −0.002 | 0.001 | 1.35 | 0.24 | −0 0002 | 0.002 | 0.01 | 0.91 | −0.0003 | 0.002 | 0.02 | 0.89 | 0.013 | 0.005 | 5.49 | 0.02* | 0.44 | 0.84 | 0.01Ψ | 0.01Ψ | |
| Left Ventral | 0.0003 | 0.001 | 0.05 | 0.83 | −0.0009 | 0.002 | 0.23 | 0.63 | 0.0002 | 0.002 | 0.01 | 0.91 | 0.005 | 0.008 | 0.33 | 0.57 | 0.91 | 0.65 | 0.26 | 0.2 | |
| Non-MDD | Working memory fMRI BOLD activity | ||||||||||||||||||||
| Hippocampus | |||||||||||||||||||||
| Left | −0.0001 | 0.001 | 0.02 | 0.9 | −0.0008 | 0.001 | 1.01 | 0.31 | −0.001 | 0.001 | 1.01 | 0.32 | 0.0007 | 0.002 | 0.15 | 0.7 | 0.26 | 0.15 | 0.76 | 0.53 | |
| Right | −0.0006 | 0.001 | 0.54 | 0.46 | −0.0002 | 0 001 | 0.07 | 0.79 | −0.00004 | 0.001 | 000 | 0.97 | −0.001 | 0.002 | 0.24 | 0.62 | 0.89 | 0.91 | 0.98 | 0.97 | |
| DLPFC | |||||||||||||||||||||
| Left | 0.0006 | 0.002 | 0.14 | 0.71 | 0.001 | 0.002 | 0.69 | 0.4 | 0.0009 | 0.002 | 0.21 | 0.65 | 0.006 | 0.004 | 1.99 | 0.16 | 0.76 | 1.00 | 0.54 | 0.35 | |
| Right | 0.0008 | 0.001 | 0.67 | 0.41 | 0.0008 | 0 001 | 0.52 | 0.47 | 0.0009 | 0.001 | 0.56 | 0.46 | 0.0005 | 0.003 | 0.03 | 0.87 | 0.75 | 0.87 | 0.65 | 0.89 | |
| Verbal encoding fMRI BOLD activity | |||||||||||||||||||||
| Hippocampus | |||||||||||||||||||||
| Left | 0.0001 | 0.001 | 0.04 | 0.84 | 0.001 | 0.001 | 3.15 | 0.08† | 0.002 | 0.001 | 6.56 | 0.01* | −0.001 | 0.002 | 0.22 | 0.64 | 0.71 | 0.57 | 0.67 | 0.25 | |
| VLPFC | |||||||||||||||||||||
| Left Dorsal | 0.001 | 0.002 | 0.38 | 0.54 | 0.002 | 0.001 | 6.54 | 0.01* | 0.002 | 0.002 | 1.9 | 0.17 | −0.003 | 0.005 | 0.26 | 0.61 | 0.87 | 0.94 | 0.37 | 0.44 | |
| Left Mid | −0.0001 | 0.001 | 0.01 | 0.91 | 0.0008 | 0.001 | 0.27 | 0.6 | 0.002 | 0.001 | 1.5 | 0.22 | −0.002 | 0.005 | 0.14 | 0.7 | 0.42 | 0.33 | 0.68 | 0.89 | |
| Left Ventral | 0.002 | 0.002 | 2.12 | 0.15 | −0.0009 | 0.001 | 0.63 | 0.43 | 0.000 | 0.001 | 0.00 | 0.99 | −0.002 | 0.004 | 0.16 | 0.69 | 0.31 | 0.28 | 0.49 | 0.99 | |
Comparisons in linear mixed models adjusted for intrafamilial correlation among siblings, along with potential confounding by age, substance or alcohol abuse or dependence, and CLIA assay.
p<0.05;
p< 0.1;
Comparisons that meet statistical significance after correcting for multiple comparisons.
Covariate ageentered ascategorkal ratherthan as continuous
Not enough variation in the response to attribute any variation to the random effect. Analyses not adj for sibs, but otherwise fully adjusted. Results are still non-sgnificant if we remove siblings altogether (4 sets [2 sets 2 men, 1 set 2 pre-, 1 set pre- & man]) and fully adjusted (all p’s >0.05).
Model overspecified. Too many likeihood variations Analyses not adj for sibs, but otherwise fully adjusted. Results are still non-significant if we remove siblings altogether (4 sets [2 sets 2 men, 1 set 2 pre-1 set pre-6 man]) and fully adjusted (all p’s >0.05).
Figure 2.
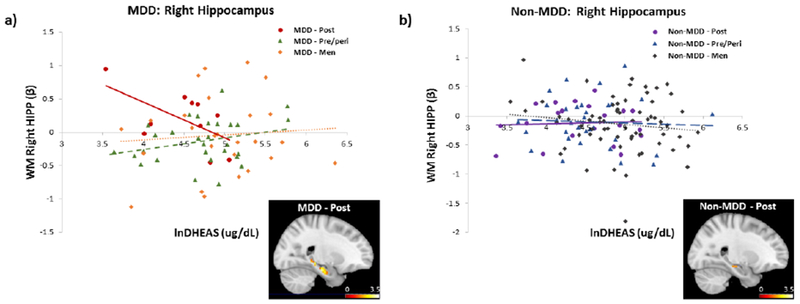
Relationship between working memory-related activity in the right HIPP and endogenous levels of DHEAS by sex/reproductive status, a) In MDD, task-related HIPP activity decreased with increasing levels of DHEAS in postmenopausal women, reaching levels similar to pre/perimenopausal women and men. Brain image displays negative correlation between DHEAS and fMRI BOLD activity in the right hippocampus in postmenopausal women with MDD (to illustrate full spatial extent, findings are displayed at p< 0.05, uncorrected), b) In non-MDD, there was no relationship between task-related HIPP activity and DHEAS levels in any group. Brain image displays negative correlation between DHEAS and fMRI BOLD activity in the right hippocampus in postmenopausal women with no MDD (to illustrate full spatial extent, findings are displayed at p< 0.05, uncorrected). Functional ROI was generated from an anatomically-defined mask of the HIPP (2-back > 0-back). DHEAS levels were natural log transformed.
We tested the impact of DHEAS on memory circuitry in MDD postmenopausal women compared to men. We found a trend level interaction between DHEAS levels and sex (men vs. postmenopausal women) on functional activity in the right HIPP (χ2 = 3.40, pchsq = 0.065). MDD postmenopausal women with higher levels of DHEAS had similar levels of fMRI activity in the right HIPP as MDD men (Fig. 2a). DHEAS levels were not significantly associated with memory circuitry function in MDD men, although this was not surprising given lack of difference in DHEAS levels in MDD and non-MDD men and the presence of testosterone in both. Overall, importantly, MDD postmenopausal women with higher levels of DHEAS looked similar to MDD pre/perimenopausal women and men.
2.4.3. Non-MDD
In non-MDD, there were no significant interactions between menopausal staging and DHEAS levels on memory circuitry function (all p ’s n.s.; Table 3), demonstrating the specificity of the above results to MDD. Similarly, there were no significant interactions between DHEAS levels and sex (men vs. women; men vs pre/peri; men vs. post) on regional fMRI BOLD activity in the HIPP and DLPFC (all p’s n.s.; Fig. 2b; Table 3).
2.5. Regional BOLD response in Episodic memory (VE) circuitry
In previous work on memory circuitry activity in our cohort (Jacobs et al., 2016b), we have demonstrated that the verbal encoding paradigm evoked robust responses within episodic memory circuitry regions. The verbal encoding paradigm assesses long-term memory and the ability to encode and recall verbal stimuli. Region of interest included task-evoked activity within VLPFC subdivisions (along a dorsal/posterior to ventral/anterior gradient) and HIPP. We demonstrated significant changes in HIPP activity as a function of women’s reproductive stage, with premenopausal and perimenopausal women exhibiting greater activity relative to postmenopausal women. In women, better verbal retrieval was also related to increased activity in dorsal-VLPFC (Jacobs et al., 2016b). In the current study, we extended these findings to examine the impact of DHEAS and MDD on episodic memory function.
2.5.1. Total sample
In the total sample (N = 187), irrespective of sex/reproductive status, we found no significant associations between DHEAS levels and episodic memory circuitry function in healthy middle-aged adults (allp’s n.s.). Only in pre/perimenopausal women, we found that higher levels of DHEAS was significantly associated with higher activity in the left HIPP (β (SE) = 0.001 (0.0006), pchsq = 0.05). When we examined whether there was an interaction between sex/reproductive status and DHEAS levels on task-evoked activity, we found no other significant interactions in the HIPP or VLPFC (all p’s n.s.).
2.5.2. MDD
Again, a significant impact of DHEAS on episodic memory circuitry activity emerged when examining reproductive staging in the face of MDD status. In MDD, significant interactions in the left VLPFC (Dorsal: χ2 = 5.29, pchsq = 0.02; Mid: χ2 = 5.98,pchsq = 0.01) and a trend in the left HIPP (χ2 = 3.21, pchsq = 0.07) were observed as a function of women’s reproductive stage and DHEAS levels (Table 3). Interaction in the dorsal-VLPFC did not pass correction for multiple comparisons. Specifically, in postmenopausal women with MDD, lower DHEAS levels were significantly associated with lower task-evoked activity in the mid-VLPFC (β (SE) = 0.013 (0.005), pchsq = 0.02; Fig. 3a) and left HIPP (β (SE) = 0.007 (0.002), pchsq = 0.0004; Fig. 4a). In contrast, higher levels of DHEAS were associated with increased task-evoked activity in the VLPFC and HIPP, similar to patterns of activity previously observed in premenopausal women (Jacobs et al., 2016b).
Figure 3.
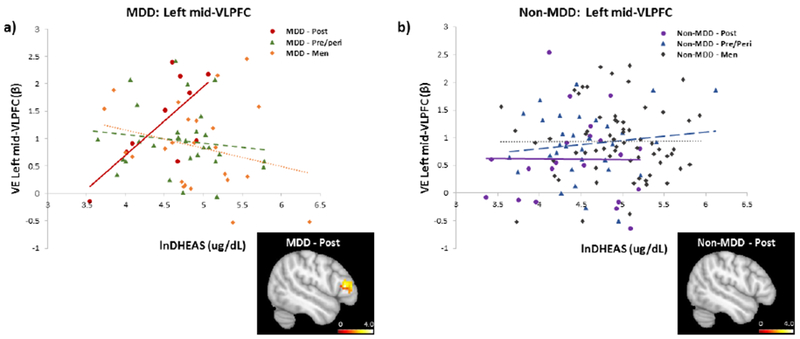
Relationship between verbal encoding-related activity in the left mid-VLPFC and endogenous levels of DHEAS by sex/reproductive status, a) In MDD, task-related activity in the mid-VLPFC increased with higher levels of DHEAS in postmenopausal women. Brain image displays positive correlation between DHEAS and fMRI BOLD activity in the left mid-VLPFC in postmenopausal women with MDD (to illustrate full spatial extent, findings are displayed at p< 0.05, uncorrected), b) In non-MDD, there was no relationship between task-related activity in the mid-VLPFC and DHEAS levels in any group. Brain image displays positive correlation between DHEAS and fMRI BOLD activity in the left mid-VLPFC in postmenopausal women with no MDD (to illustrate full spatial extent, findings are displayed at p< 0.05, uncorrected). From a supergroup activity map in the larger sample, a 10mm spheres around peak loci in the mid-VLPFC (BA45; MNI coordinates −51, 30, 10) was used to generate a functional ROI (Novel > Repeat). DHEAS levels were natural log transformed.
Figure 4.
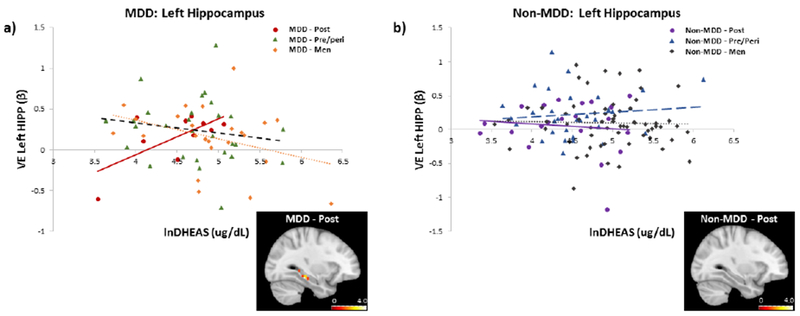
Relationship between verbal encoding-related activity in the left HIPP and endogenous levels of DHEAS by sex/reproductive status, a) In MDD, task-related HIPP activity increased with higher levels of DHEAS in postmenopausal women, reaching levels similar to pre/perimenopausal women and men. Brain image displays positive correlation between DHEAS and fMRI BOLD activity in the left hippocampus in postmenopausal women with MDD (to illustrate full spatial extent, findings are displayed at p< 0.05, uncorrected), b) In non-MDD, there was no relationship between task-related HIPP activity and DHEAS levels in any group. Brain image displays positive correlation between DHEAS and fMRI BOLD activity in the left hippocampus in postmenopausal women with no MDD (to illustrate full spatial extent, findings are displayed at p < 0.05, uncorrected). Functional ROI was generated from an anatomically-defined mask of the HIPP (Novel > Repeat). DHEAS levels were natural log transformed.
As with working memory activity (Table 3), in MDD, a significant interaction between DHEAS levels and sex (men vs. postmenopausal women) was found in the left mid-VLPFC (χ2 = 6.60, pchsq = 0.01) and trends were found in the left dorsal-VLPFC (p = 0.06) and left HIPP (χ2 = 3.54, pchsq = 0.06). MDD postmenopausal women with higher levels of DHEAS had similar levels of fMRI activity in the left HIPP as MDD men (Fig. 4a). In MDD men, we observed a negative association between DHEAS levels and activity in the left HIPP (β (SE) = −0.002 (0.001), pchsq = 0.01). Again, overall, MDD postmenopausal women with higher levels of DHEAS looked similar to MDD pre/perimenopausal women and men.
2.5.3. Non-MDD
In non-MDD, there were no significant interactions between menopausal staging and DHEAS levels (all p’s n.s.; Table 3), again demonstrating the specificity of the above results to MDD. Similarly, there were no significant interactions between DHEAS levels and sex (men vs. women; men vs pre/peri; men vs. post) on regional BOLD activity in the HIPP or VLPFC (all p’s n.s.; Fig. 3b & 4b; Table 3). However, in non-MDD women, we found that higher levels of DHEAS were significantly associated with higher activity in the left VLPFC (Dorsal: β (SE) = 0.002 (0.001), pchsq = 0.01) and a trend in the left HIPP (β (SE) = 0.001 (0.001), pchsq = 0.08). Effects in the left HIPP appeared to be driven by pre/perimenopausal women (β (SE) = 0.002 (0.001), pchsq = 0.01).
3. Discussion
In this population-based study, we identified the impact of DHEAS on memory circuitry in MDD, as a function of reproductive status in women, specifically in postmenopausal women with MDD compared with pre- and peri-menopausal women with MDD and non-MDD women. On the WM N-back task, higher levels of DHEAS were associated with decreased task-dependent activity in the HIPP, a finding that we previously demonstrated was associated with better working memory performance (Jacobs et al., 2016a). On the episodic memory VE task, higher levels of DHEAS were associated with increased activity in the HIPP and VLPFC, again in our previous work showing associations with better episodic memory performance (Jacobs et al., 2016b). In contrast, there was no association between DHEAS levels and memory circuitry function in MDD pre/perimenopausal women and non-MDD women, regardless of reproductive status. In fact, memory circuitry function in MDD postmenopausal women with higher levels of DHEAS was similar to men. These results suggest that in the face of deficits in memory circuitry associated with MDD, the ability of the adrenal gland to produce DHEAS after ovarian decline may contribute to better ability to maintain intact memory function with age. We would argue, this is, in part, due to the conversion of DHEAS into estradiol in the brain.
Although DHEAS levels significantly decrease with age, they do not deplete over menopause in the same manner as ovarian steroid hormones such as estradiol. DHEAS is aromatizated to estradiol in the brain and is the primary source of estrogen after ovarian decline (Simpson and Davis, 2001). A major contributor to neuronal changes over menopause is the depletion of estradiol. As such, we hypothesized that higher levels of DHEAS would help women maintain “estrogenicity” after menopause and be neuroprotective against memory decline.
Aside from DHEA/S conversion to estradiol, DHEA/S has many other direct neurobiological actions that may underlie some of our results. DHEA/S has anti-glucocorticoid (Kalimi et al., 1994), antioxidant (Bastianetto et al., 1999), and anti-inflammatory (Chen and Parker, 2004) properties. It also inhibits apoptosis (Zhang et al., 2002) and GABAA receptor activity (Majewska, 1992; Park-Chung et al., 1999). In addition, it facilitates catecholamine synthesis and secretion (Perez-Neri et al., 2008) and activates NMDA receptors (Bergeron et al., 1996), which in turn influences neurite growth. DHEA/S promotes neurogenesis and neuronal survival, potentially by increasing Brain-Derived Neurotrophic Factor (BDNF) levels in the HIPP (Naert et al., 2007). These additional pathways in which DHEA/S work are particularly important in the face of MDD, given that they have been implicated in MDD pathophysiology. While it is possible that these other mechanisms also contribute to our current findings, we believe that given the specificity of our results to postmenopausal women, a substantial contributor is the conversion of DHEAS to estradiol. Group differences were primarily reproductive status and estradiol levels with very minimal differences in chronological age or demographic characteristics. As such, while DHEA/S has multiple effects, both in the brain and periphery, across the menopausal transition it may help women maintain estrogenicity and reduce the impact of reproductive aging.
Menopause presents a critical period for neurological changes in women that have longterm implications for risk of cognitive impairment and AD. Over the menopausal transition, some women experience a decline in memory performance (Bleecker et al., 1988; Kerschbaum et al., 2017; Rentz et al., 2016), functional changes in episodic and working memory networks (Jacobs et al., 2016a; Jacobs et al., 2016b), reduced cerebral glucose metabolism (Mosconi et al., 2017a), increased amyloid beta deposition (Mosconi et al., 2017b), and reduced volumes of grey and white matter in regions vulnerable in AD (Mosconi et al., 2017b). These studies suggest that pathological changes occur in some women over menopause, which chronologically aligns with the emergence of early AD-related pathology. Our group (Jacobs et al., 2016b) and others (Kerschbaum et al., 2017; Wolf et al., 1999) have demonstrated that there is a direct association between decline in estradiol and memory performance. Further, early surgical menopause, without hormone replacement, significantly increases the risk of memory decline and AD (Bove et al., 2014; Phung et al., 2010; Rocca et al., 2007; Rocca et al., 2011). Our current results extend these findings and suggest that the ability to maintain estrogenicity after menopause, with higher endogenous levels of DHEAS, may be important for reducing risk of cognitive impairment and potentially even AD in later life.
Results in the current study were unique to postmenopausal women with MDD, suggesting that certain populations may be more sensitive to the early impact of ovarian decline than others. We previously demonstrated that MDD women have lower overall levels of estradiol compared to healthy controls (Holsen et al., 2011). In the current study, we found these effects to be exacerbated by reproductive aging, whereby postmenopausal women with MDD had the lowest levels of estradiol compared to pre/perimenopausal women with MDD and non-MDD women of all reproductive stages. Thus, the added impact of reproductive aging in the face of MDD may make these women particularly sensitive to the estrogenic effects that DHEAS may provide after menopause. The women in the current study were in early postmenopause. Thus, it may also be that with time and decreasing levels of estradiol, non-MDD postmenopausal women may also benefit from higher endogenous levels of DHEAS. We are following these women as they age further and will test this hypothesis.
We further identified specific memory circuitry activity impacted by DHEAS in a task-dependent manner. On the WM task, lower DHEAS levels were associated with higher fMRI BOLD activity in the HIPP, while on the VE task, lower DHEAS levels were associated with lower fMRI BOLD activity in the VLPFC and HIPP. These results demonstrated that lower levels of DHEAS were not associated with an overall decrease in functional activity but rather modulated activation in response to task demands. Given that task performance did not differ between groups and was not associated with DHEAS levels, these results suggest that altered task-dependent activity associated with DHEAS contributes to maintain task performance. With increasing levels of endogenous DHEAS, patterns of activity in MDD postmenopausal women resembled those of pre/perimenopausal women and men. These results suggest that higher levels of DHEAS may reduce the impact of reproductive aging on memory circuitry function in early midlife and contribute to understanding sex differences in memory decline with age.
There is large discrepancy in the literature regarding the relationship between endogenous levels of DHEAS and memory function. While several studies have found that higher endogenous levels of DHEAS are associated with better memory performance (Barrett-Connor and Edelstein, 1994; Bojar et al., 2017; Correa et al., 2016; Davis et al., 2008), others have found no association (Bo et al., 2006; Koyama et al., 2016). Inconsistencies may result from demographic differences in study populations as well as measures used to assess memory and cognitive function. Some studies exclusively looked at postmenopausal women, controlling (Barrett-Connor and Edelstein, 1994; Castanho et al., 2014) and not controlling (Bojar et al., 2017; Davis et al., 2008; Koyama et al., 2016) for hormone use, while others looked at both men and women across various age ranges (Correa et al., 2016; Hildreth et al., 2013). As demonstrated in the current study, reproductive age is an important factor to consider when examining the role of DHEAS on brain function. Further, disease state is also important to consider, given that certain clinical populations, like major depression, may be more vulnerable than others to memory decline which may underscore the impact of DHEAS.
Memory measures are also a critical factor to consider. In the current study, we found an impact selectively on memory circuitry function and not memory performance. We examined brain function in key memory circuitry regions known to alter in function with reproductive aging and AD. In AD, functional changes are known to precede cognitive changes (Jack et al., 2010; Jagust, 2013; Putcha et al., 2011; Reagh and Yassa, 2017; Reitz et al., 2009) and therefore, early changes that are not detectable by standard neuropsychological tests such as the Mini Mental State Examination (MMSE) and Digit Span may be detectable using neuroimaging. Tests such as the MMSE, which as been frequently used to assess the impact of DHEA/S (Barrett-Connor and Edelstein, 1994; Hirshman et al., 2004; Leblhuber et al., 1993; Merritt et al., 2012; Stangl et al., 2011; Yaffe et al., 1998a; Yamada et al., 2010), are blunt instruments of cognitive function and may explain null findings in many studies.
Our study was unique in that we examined the impact of DHEAS on brain activity using neuroimaging memory challenges. Our results suggest that in early midlife, brain activity may be a more sensitive measure than cognitive function in identifying the neuronal impact of DHEAS, and this may be particularly true only for postmenopausal women. The relatively young age of our postmenopausal women may explain why we did not observe an impact of DHEAS on cognitive function. In early midlife, there is less variability in cognitive performance and a general absence of cognitive impairment. With increasing age, variability increases and there may be a stronger association between DHEAS and cognitive function. Further, DHEAS decreases with age and thus being able to maintain estrogenicity (i.e. higher DHEAS levels) in older age may have a larger impact. This is of interest to pursue in our future follow up studies with this cohort.
The preclinical literature, specifically rodent studies, has convincingly demonstrated the beneficial effects of exogenous DHEA/S treatment in enhancing HIPP-dependent memory. Interestingly, many of these studies were in male rodents who are not gonadectomized and thus the impact of testosterone was not dissociated from DHEAS. In contrast, clinical research outcomes have been inconsistent and complex with discrepancies regarding the impact of exogenous DHEA/S (DHEA/S supplementation) on memory function. While DHEA treatment is effective in elevating circulating levels of DHEA/S and other steroid metabolites (Barnhart et al., 1999; Hirshman et al., 2004; Merritt et al., 2012; Parsons et al., 2006; Schmidt et al., 2005; Stangl et al., 2011; Yamada et al., 2010), its impact on memory function has yielded inconsistent results. Several studies report exogenous DHEA supplementation improves memory (Hirshman et al., 2004; Stangl et al., 2011; Yamada et al., 2010), while others report no beneficial effects (Hirshman et al., 2004; Kritz-Silverstein et al., 2008; Merritt et al., 2012; Wolf et al., 1997; Wolkowitz et al., 2003) or negative effects (Parsons et al., 2006; Wolf et al., 1998). The discrepancy in potential benefits of DHEA/S treatment between preclinical and clinical work may, in part, be attributed to differing pathways through which DHEA/S is synthesized. Humans (Lacroix et al., 1987) and rodents (Corpechot et al., 1981) have higher concentrations of DHEA/S in the brain compared to plasma. However, unlike humans who produce DHEA/S in the adrenal gland, rodents lack the capacity to synthesize DHEA/S in their adrenal gland (van Weerden et al., 1992), resulting in lower peripheral circulating concentrations of DHEA/S (Cutler et al., 1978; van Weerden et al., 1992). As such, clinical studies of DHEA/S treatment are far more complex and require further investigation and discussion.
The current results pertain to endogenous levels of DHEAS and not exogenous DHEAS administration. DHEA/S is a steroid hormone that works on multiple tissues and caution should be taken when administering DHEA/S as a treatment. Many aging studies use women 65+ in age (Carlson and Sherwin, 1999; Yaffe et al., 1998a; Yamada et al., 2010). However, based on evidence from the Women’s Health Initiative-Memory Study (Shumaker et al., 2003; Shumaker et al., 2004), we now know that there are critical windows for hormone therapy that may also apply for DHEAS. Initiation of estradiol in late perimenopause or early menopause can have positive effects on brain activity and memory function in the healthy brain (Espeland et al., 2013; Gleason et al., 2015; Henderson et al., 2016; LeBlanc et al., 2001; Phung et al., 2010; Rocca et al., 2007; Yaffe et al., 1998b; Zandi et al., 2002). In contrast, initiation of estradiol in late menopause (65+) may have adverse effects on the brain and increase risk of AD (Shumaker et al., 2003; Shumaker et al., 2004). As such, if the neuroprotective effects of DHEAS are, in part, through its metabolism to estradiol, then DHEAS administration may not be beneficial after a certain age. Further research is required for understanding which populations may benefit most from treatment and in which populations it may be harmful. In the same manner as other hormone therapies, further investigation on the optimal dosage and timing as well as the most effective form, route, and duration of administration are needed. This is particularly important given that DHEA is sold over-the-counter and used extensively by the public.
The current results should be viewed in light of certain limitations. The first is that the sample sizes when broken down by menopausal and MDD status, are relatively small and thus future studies should aim to replicate these findings in larger cohorts. The study design is also cross-sectional, and as such, causality cannot be determined. Future longitudinal studies can prospectively assess DHEAS levels in premenopausal women and examine the neuroprotective effects of higher DHEAS levels across the menopausal transition. We intend to continue our investigation of this as our cohort ages. Also, steroid hormone levels were assessed from serum, presenting limitations for drawing conclusion about neuronal levels of the steroid hormones. Finally, when measuring circulating levels of DHEAS, we are also tracking circulating androstenediol which is both an androgen and a weak estrogen (Lasley et al., 2013). Androstenediol can transduce a signal through the androgen receptor (AR), the estrogen receptors alpha (ER-α), as well as the estrogen receptor beta (ER-β). Circulating concentrations move up and down with DHEAS because they are both produced and secreted from the adrenal gland together. Therefore, when measuring peripheral levels of DHEA, one cannot distinguish its effects from that of androstenediol, and therefore, androstenediol may be partially responsible for some of the observed effects in the current study.
At a human population-level, these results contribute to a broader understanding of the impact of adrenal hormones on aging of memory circuitry in women. Our results suggest that higher levels of DHEAS may maintain intact memory function across the menopausal transition, in particular for women with major depression who may have particularly low levels, shedding light on possible mechanisms for resilience in women, and understanding sex differences in memory decline with age and the impact of major depression.
4. Experimental Procedure
4.1. Participants
Adult participants were selected from 17,741 pregnancies in the New England Family Study (NEFS; subsidiary of the National Collaborative Perinatal Project), a representative sample of those receiving prenatal care in Boston-Providence from 1959 tol966. In a series of studies over 20 years and ongoing, we have followed the offspring of these pregnancies to investigate the fetal programming of adult psychiatric and general medical disorders and sex differences therein. We recently completed a study investigating prenatal immune antecedents to sex differences in memory circuitry in early midlife (NIMH R01 MH090291, Goldstein, PI). In that study, same-sex siblings were recruited from NEFS, discordant for preeclampsia or fetal growth restriction. When a discordant sibling was unavailable, an unrelated and unexposed offspring was individually matched based on maternal age, ethnicity, socioeconomic status, offspring sex, and gestational age.
212 offspring (equally divided by sex) were recruited between 45-55 years of age and underwent clinical, cognitive, and neuropsychological assessments. 201 completed functional and structural magnetic resonance imaging (fMRI/sMRI). The community-based sample was 88.6% White, 8.5% African American, 2.8% Other (primarily Hispanic). Exclusion criteria included any history of neurological disease, central nervous system (CNS) damage, head injury with loss of consciousness, endocrine disorders, heart disease, alcohol-related diseases, current or past history of psychosis, other medical illnesses that may significantly alter CNS function, or any MRI contraindication. Demographic information, including body mass index (BMI), marital status, race/ethnicity, psychiatric history, and alcohol and substance abuse dependence, were collected as part of the clinical interview via self-report. The Partners Human Research Committee and Brown University’s Institutional Review Board granted Human Studies participants’ approval. All volunteers gave written informed consent and were paid for their participation.
4.2. Study design and procedures
Participants were seen at the Brigham and Women’s Hospital (BWH) Outpatient Clinical Research Center. Women who were still menstruating were scheduled within the early follicular menstrual cycle phase (days 3-5). Participants fasted for ≥8 hours prior to their morning baseline blood draw. They were offered a light standardized breakfast (excluding caffeine) before MRI scanning. MRI scanning was followed by neuropsychological testing and structured clinical interviews administered by an experienced clinical interviewer/clinician. Clinical interviews assessed history of alcohol or substance disorders, family medical history, and reproductive history.
4.3. Menopausal staging
Timing of menopause between the first clinical appearance of decreased ovarian function (i.e., shorter inter-menstrual time periods) to menstrual irregularity and final amenorrhea is highly variable and can occur over several years. Women in this sample were between the ages of 45-55 years and were in various stages of ovarian decline, ranging from oligoamenorrhea to permanent amenorrhea as well as to normal cycling. Reproductive histories and hormonal evaluations (see Endocrine assessments below) were used to determine reproductive stage following the Stages of Reproductive Aging Workshop (STRAW)-IO guidelines (Harlow et al., 2012). Women were categorized into the following groups: late reproductive (“premenopause”), menopausal transition (“perimenopause”), and early postmenopausal (“postmenopause”). Eight women reported current use of hormone therapy and were excluded from analyses.
4.4. Sample collection
Trained nurses inserted a saline-lock IV line in the non-dominant forearm of participants to acquire serum and plasma samples. Approximately 18.5 ml of blood was collected at the BWH Center for Clinical Investigation. Fasting morning blood was drawn at approximately 0800h for optimal evaluation of hormones reflecting the hypothalamic-pituitary-gonadal axis, including sex steroids (estradiol, progesterone, and testosterone) and gonadotropins (luteinizing hormone and follicle-stimulating hormone (FSH)), and the hypothalamic-pituitary-adrenal axis, including dehydroepiandrosterone-sulfate (DHEAS) and cortisol. For endocrine assessments (see below), blood was collected into SST tubes. Serum aliquots were stored at −80°C for later evaluations and analyzed at the Brigham Research Assay Core (BRAC) laboratory for sex steroid determinations.
4.5. Endocrine assessments
17β-estradiol, progesterone, and testosterone concentrations were determined via liquid chromatography-mass spectrometry (LC-MS) at BRAC. Assay sensitivities, dynamic range and intra-assay coefficients of variation (CV) were as follows (respectively): Estradiol (1 pg/mL, 1-500 pg/mL, <5% RSD); Progesterone (0.05 ng/mL, 0.05-10ng/mL, 5.75% RSD); Testosterone (1.0 ng/dL, 1-2000 ng/dL, <2% RSD). FSH levels were determined via chemoluminescent immunoassay (CLIA, Beckman Coulter), with assay sensitivity of 0.2 mlU/mL, dynamic range 0.2-200 mlU/mL, and intra-assay CV 3.1-4.3%.
DHEAS concentrations were also determined via CLIA (Beckman Coulter & Abbott). Samples were analyzed using different assays and thus, CLIA assay type was controlled for in analyses. The Beckman Coulter Access CLIA has an assay sensitivity of 2 ug/dL, dynamic range 2-1000 ug/dL, and intra-assay CV 1.6-8.3%. The Abbott Architect CLIA has an assay sensitivity of 3 ug/dL and dynamic range of 3-1500 ug/dL.
4.6. Working Memory fMRI Paradigm
201 participants performed a verbal working memory N-back task during fMRI scanning (Jacobs et al., 2016a). The task consisted of two conditions, 0-back and 2-back. In each condition, participants were presented with a sequence of white upper-case letters on a black background presented centrally (200msec duration, 1800msec interstimulus interval) in a pseudo-random order. Participants performed two experimental runs of the task, with each run lasting 5min44sec. Each run contained six 32sec blocks. Each block was preceded by a 20sec fixation period and a 4sec instruction screen. During 0-back blocks, participants responded to every letter using one of two buttons to indicate whether or not the target letter (X) appeared. During 2-back blocks, participants responded to every letter using one of two buttons to indicate whether it matched or did not match the letter seen two previously. Response times and accuracy (d’ 2back) were recorded. Response time (RT) values < 100msec were considered null and not included in the computation of participants’ average RT. The sensitivity index d’ 2back was calculated (Wickens, 2001) as d’ 2back = z[probability(hits)] - z[probability(false alarms)]. In accordance with signal detection theory, a higher d’ represents a greater distinction between signal and noise (i.e., better signal detection).
4.7. Verbal encoding fMRI paradigm and subsequent memory retrieval task
The same 201 participants performed a verbal encoding task during fMRI scanning (Golby et al., 2001; Jacobs et al., 2016b; Stone et al., 2005). The task consisted of two conditions, “Novel” and “Repeat.” In each condition, subjects were presented with a pair of common nouns on a black background presented centrally (4000 msec duration) with a variable interstimulus interval (600-1500 msec). Subjects were asked to silently generate a sentence using both words and were instructed to remember the stimuli for a later test. In the Repeat condition, subjects viewed the same noun pair repeated throughout each block of a run and were instructed to generate the same sentence each time they saw the word pair. In the Novel condition, subjects viewed novel word pairs and generated a new sentence in response to each pair. Subjects were instructed to respond to every word pair with a single button press (pointer finger) to indicate that they had successfully formed a sentence in their mind. Subjects performed two experimental runs of the task. Each run contained three Repeat blocks and three Novel blocks, for a total of six blocks per condition.
A subsequent recognition memory task was administered immediately following the encoding task using the same response box and while the subject remained in the scanner. Subjects viewed single nouns, including 24 previously presented nouns and 24 foils. Each stimulus was presented for 4000 msec with a variable interstimulus interval. Subjects were instructed to indicate, using one of two buttons, whether they had seen the item on the screen in the previous task (yes) or not (no). Response times (RT) and accuracy (d’ VR) were recorded. RT values <100 msrc were considered null and not included in the computation of subjects’ average RT. Same as above, the sensitivity index d’ VR was calculated (Wickens, 2001) as d’ VR = z[probability(hits)] - z[probability(false alarms)].
4.8. fMRI Data Acquisition
MRI data were acquired with a Siemens 3T Tim Trio scanner (Siemens, Erlangen, Germany), equipped with a 12-channel head coil. Functional data were obtained using a T2*-weighted echo-planar imaging sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (repetition time, 2000msec; echo time, 30msec; field of view, 200mm; flip angle, 90°; voxel size, 3.1 × 3.1 × 3.0). Each functional volume consisted of 33 (3mm) oblique axial slices. A Tl-weighted image was collected using a high-resolution 3D Multi-Echo (ME) MPRAGE sagittal sequence with an isotropic resolution of 1mm3. Following acquisition, MRI data were converted to NifTI format and preprocessed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included realignment and geometric unwarping of echo-planar imaging images using magnetic field maps, correction for head motion, nonlinear volume-based spatial normalization (Montreal Neurological Institute template MNI-152), and spatial smoothing with a Gaussian filter (6mm [full width at half maximum]). Additional software (http://web.mit.edu/swg/software.htm) was used to identify and exclude outliers in the global mean image time series (threshold 3.0 standard deviation (SD) from the mean) and movement (threshold 1.0mm; measured as scan-to-scan movement, separately for translation and rotation) parameters. Statistical parametric maps of BOLD activation were calculated in SPM8 using the general linear model approach (Worsley and Friston, 1995).
4.9. fMRI Data Analyses
4.9.1. Working Memory fMRI Paradigm
Hemodynamic responses were modeled using a gamma function and convolved with onset times of 2-back and 0-back blocks to form the general linear model (GLM) at the single subject level. Outlier time points and the 6 rigid-body movement parameters were included in the GLM as covariates of no interest. To test a priori hypotheses targeting the HIPP and dorsolateral prefrontal cortex (DLPFC), anatomically-defined masks of the HIPP and bilateral DLPFC (BA9/46; MNI coordinates, left: −42,26,30; right: 44,32,28) (10mm spheres around peak loci) were used. Functional regions of interest (ROIs) in the DLPFC were defined from “supergroup” whole-brain analyses in the larger sample based on peak task-evoked activity generated at p<10−10. The left and right HIPP ROIs was anatomically defined using a manually segmented MNI-152 brain (based on methods previously published by the Center for Morphometric Analysis at Massachusetts General Hospital and Harvard Medical School; see Makris et al. (2013). ROIs were created with the Wake Forest University PickAtlas ROI toolbox for SPM (Maldjian et al., 2003). β weights from the right and left HIPP and DLPFC were extracted for each participant as a function of WM load (2-back > 0-back) using the REX toolbox (Whitfield-Gabrieli, 2009) and were used for subsequent analyses. For each participant and ROI, β estimates were entered into a mixed model analysis with sex or reproductive status and DHEAS levels as independent variables.
4.9.2. Verbal encoding fMRI paradigm and subsequent memory retrieval task
Hemodynamic responses were modeled using a gamma function and convolved with onset times of Novel and Repeat blocks to form the general linear model (GLM) at the single subject level. Outlier time points and the six rigid-body movement parameters were included in the GLM as covariates of no interest. To test a priori hypotheses targeting ROIs within memory encoding circuitry (Blumenfeld and Ranganath, 2007; Golby et al., 2001; Spaniol et al., 2009; Uncapher and Wagner, 2009), we conducted ROI analyses (10mm spheres around peak loci) on functionally defined masks of left dorsal/posterior ventrolateral prefrontal cortex (VLPFC; BA44/BA45/BA9), mid-VLPFC (BA45), ventral/anterior VLPFC (BA47; MNI coordinates, −51, 20, 25; −51, 30, 10; and −39, 26, −2, respectively), and anatomically defined masks of the HIPP. Functional ROIs were defined from whole-brain analyses in the larger sample based on peak task-evoked activity generated atp<10−16. The HIPP ROI was anatomically defined using a manually segmented MNI-152 brain (based on methods previously published by the Center for Morphometric Analysis at Massachusetts General Hospital and Harvard Medical School (Makris et al., 2013). ROIs were implemented as overlays on the SPM8 canonical brain using the Wake Forest University Pick Atlas ROI toolbox for SPM (Maldjian et al., 2003). β weights from the ROIs were extracted for each participant as a function of encoding load (Novel - Repeat) using the REX toolbox (Whitfield-Gabrieli, 2009). For each participant and ROI, β estimates were entered into a mixed model analysis with sex or reproductive status and DHEAS levels as independent variables.
4.10. Neuropsychological and Clinical assessments
Participants completed a basic mood and neuropsychological battery that included the following: a digit span (Wechsler, 1997), a Controlled Oral Word Association Test for verbal fluency to the letters F, A, and S (FAS) and categories (Benton, 1968), the American National Adult Reading Test (AMNART; Nelson, 1982), the Spielberger State-Trait Anxiety Inventory (STAI), and the Profile of Moods Questionnaire (POMS).
All subjects underwent a systematic psychiatric diagnostic interview using the SCID (First MB, 2015), and mood (Hamilton, 1967; McNair, 1992) and anxiety (Hamilton, 1959; Spielberger, 1983) traits. Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnoses were obtained by a trained senior clinical interviewer with three decades of experience assessing AXIS I disorders. SCIDs and state/trait instruments were administered along with demographic and medical histories. Clinical narratives were written, provisional diagnoses made, and then all materials were reviewed by a Clinical Psychologist and J.M.G. DSM diagnoses were obtained by group consensus, including a study psychiatrist who oversaw any disagreements. History of MDD included both single episode and recurrent MDD subjects.
4.11. Statistical Analyses
Descriptive statistics of study sample characteristics - age, BMI, education (above v. below a high school level of attainment), ethnicity (Caucasian v. not Caucasian), substance or alcohol abuse or dependence (history of or current, yes v. no) – were reported by group status (i.e., sex (men and women), reproductive status (pre/peri-menopausal and post-menopausal), and MDD status (MDD and non-MDD)). Similarly reported were, neuropsychological and clinical assessments – task performance (d’ 2back and d’ VR), Digit span (Forward and Backward), Verbal fluency (FAS and Category), STAI (State and Trait), and POMS. Comparisons by group status were evaluated using nonparametric Wilcoxon rank-sum or chi-square test, for continuous and categorical data, respectively.
The associations between DHEAS levels and fMRI BOLD activity were assessed overall and by individual group status using linear mixed models adjusted for intrafamilial correlation among siblings, along with potential confounding by age, substance or alcohol abuse or dependence, and CLIA assay type. Models were run for the overall sample, as well as stratified by group status. Group differences in the association between DHEAS and fMRI BOLD activity were assessed by including in the model an interaction term between group status and DHEAS. Tests of our hypotheses were two-sided with an α = 0.05. To address multiple comparisons across sex/reproductive status, we used a Bonferroni-corrected α = (0.05/4 comparisons) = 0.0125. All analyses were performed using SAS 9.4 software (SAS institute, Cary, NC).
Highlights:
Steroid hormones have pronounced effects on the brain including regulation of memory circuitry regions, which are highly sexually dimorphic.
DHEAS levels were significantly associated with memory circuitry function, specifically in postmenopausal women with major depression.
Memory circuitry deficits associated with major depression and a lower ability of the adrenal gland to produce DHEAS after menopause may contribute to a lower ability to maintain intact memory function with age.
Acknowledgment
This work was supported by NIMH RO1 MH090291 (J.M.G.). Additional support for K.K was provided by the Harvard Translational Research in Aging Training Program T32AG023480 and for E.G.J. was previously provided by ORWH-NICHD (BIRCWH) K12HD051959. The Harvard Clinical and Translational Science Center (NIH UL1 RR025758) provided support for serological acquisition and evaluations. We thank Dr. Anne Klibanski for her collaboration with our team on designing the neuroendocrine component of the original study. We greatly appreciate her contribution to the study. We are also grateful for the support of the BrightFocus Foundation, with whom we are partnering on our Women, Heart and Brain Global Initiative, specifically on the studies involving sex differences in the risk for Alzheimer’s disease in early midlife.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE, 1999. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab 84, 3896–902. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein SL, 1994. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: the Rancho Bernardo Study. J Am Geriatr Soc 42, 420–3. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A, 1999. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc 47, 685–91. [DOI] [PubMed] [Google Scholar]
- Barth C, Steele CJ, Mueller K, Rekkas VP, Arelin K, Pampel A, Burmann I ., Kratzsch J, Villringer A, Sacher J, 2016. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci Rep 6, 32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Poirier J, Quirion R, 1999. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Brain Res Mol Brain Res 66, 35–41. [DOI] [PubMed] [Google Scholar]
- Benton AL, 1968. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 6, 53–60. [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G, 1996. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci 16, 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE, 1996. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 93, 13410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA, 1988. Age-related sex differences in verbal memory. J Clin Psychol 44, 403–11. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C, 2007. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 13, 280–91. [DOI] [PubMed] [Google Scholar]
- Bo M, Massaia M, Zannella P, Cappa G, Ferrario E, Rainero I, Arvat E, Giordano R, Molaschi M, 2006. Dehydroepiandrosterone sulfate (DHEA-S) and Alzheimer’s dementia in older subjects. Int J Geriatr Psychiatry. 21, 1065–70. [DOI] [PubMed] [Google Scholar]
- Bojar I, Pinkas J, Gujski M, Owoc A, Raczkiewicz D, Gustaw-Rothenberg K, 2017. Postmenopausal cognitive changes and androgen levels in the context of apolipoprotein E polymorphism. Arch Med Sci 13, 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL, 2014. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 82, 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB, 1999. Relationships among cortisol (CRT), dehydroepiandrosterone-sulfate (DHEAS), and memory in a longitudinal study of healthy elderly men and women. Neurobiol Aging. 20, 315–24. [DOI] [PubMed] [Google Scholar]
- Castanho TC, Moreira PS, Portugal-Nunes C, Novais A, Costa PS, Palha JA, Sousa N, Santos NC, 2014. The role of sex and sex-related hormones in cognition, mood and well-being in older men and women. Biol Psychol 103, 158–66. [DOI] [PubMed] [Google Scholar]
- Chen C, Lang S, Zuo P, Yang N, Wang X, 2008. Treatment with dehydroepiandrosterone increases peripheral benzodiazepine receptors of mitochondria from cerebral cortex in D-galactose-induced aged rats. Basic Clin Pharmacol Toxicol 103, 493–501. [DOI] [PubMed] [Google Scholar]
- Chen CC, Parker CR Jr., 2004. Adrenal androgens and the immune system. Semin Reprod Med 22, 369–77. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE, 1981. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 78, 4704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa MS, Giacobbo BL, Vedovelli K, Lima DB, Ferrari P, Argimon II, Walz JC, Bromberg E, 2016. Age Effects on Cognitive and Physiological Parameters in Familial Caregivers of Alzheimer’s Disease Patients. PLoS One. 11, e0162619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler GB Jr., Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL, 1978. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 103, 2112–8. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP, 1997. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32, 217–25. [DOI] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ, 2008. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab 93, 801–8. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH, 2010. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci 1204, 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Sammel MD, Freeman EW, 2013. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab 98, 3829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdincler D, Bugay G, Ertan T, Eker E, 2004. Depression and sex hormones in elderly women. Arch Gerontol Geriatr 39, 239–44. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, Wactawski-Wende J, Stefanick ML, Li W, Resnick SM, Group WS, 2013. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med 173, 1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, W J, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5-Research Version (SCID-F for DSM-5, Research Version; SCID-5-RV). Arlington, VA, American Psychiatric Association. [Google Scholar]
- Flood JF, Roberts E, 1988. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res 448, 178–81. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD, 1995. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem 64, 83–96. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH, 1999. The neurosteroids DHEA and DHEAS may influence cognitive performance by altering affective state. Physiol Behav 66, 85–92. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S, 2015. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med 12, e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Weinstein M, Liu IW, 2004. Dehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex? Exp Gerontol 39, 321–31. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JD, 2001. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 124, 1841–54. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr., Faraone SV, Tsuang MT, 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 11, 490–7. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C, 2004. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 145, 1042–5. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. British Journal of Medical Psychology. 32, 50–5. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1967. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 6, 278–96. [DOI] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR, 2012. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 33, 421 e17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, 2012. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric: The Journal of the International Menopause Society. 15, 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ, 2016. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 87, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Lacreuse A, Ladinsky E, Killiany RJ, Rosene DL, Moss MB, 1999. Age-related decline in DHEAS is not related to cognitive impairment in aged monkeys. Neuroreport. 10, 3507–11. [DOI] [PubMed] [Google Scholar]
- Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B, 1998. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J Clin Endocrinol Metab 83, 3130–3. [DOI] [PubMed] [Google Scholar]
- Hildreth KL, Gozansky WS, Jankowski CM, Grigsby J, Wolfe P, Kohrt WM, 2013. Association of serum dehydroepiandrosterone sulfate and cognition in older adults: sex steroid, inflammatory, and metabolic mechanisms. Neuropsychology. 27, 356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman E, Merritt P, Wang CC, Wierman M, Budescu DV, Kohrt W, Templin JL, Bhasin S, 2004. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Horm Behav 45, 144–55. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM, 2011. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord 131, 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain D, Hanafi S, Konishi K, Brake WG, Bohbot VD, 2016. Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology. 70, 108–17. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ, 2010. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM, 2015. 17beta-estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology. 40, 566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss B, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2016a. Reorganization of Functional Networks in Verbal Working Memory Circuitry in Early Midlife: The Impact of Sex and Menopausal Status. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2016b. Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife. J Neurosci 36, 10163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, 2013. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 77, 219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W, 1994. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem 131, 99–104. [DOI] [PubMed] [Google Scholar]
- Kerschbaum HH, Hofbauer I, Gfollner A, Ebner B, Bresgen N, Bauml KT, 2017. Sex, age, and sex hormones affect recall of words in a directed forgetting paradigm. J Neurosci Res 95, 251–259. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL, 2002. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116, 411–20. [DOI] [PubMed] [Google Scholar]
- Korol DL, 2004. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82, 309–23. [DOI] [PubMed] [Google Scholar]
- Koyama AK, Tworoger SS, Eliassen AH, Okereke OI, Weisskopf MG, Rosner B, Yaffe K, Grodstein F, 2016. Endogenous sex hormones and cognitive function in older women. Alzheimers Dement 12, 758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritz-Silverstein D, von Muhlen D, Laughlin GA, Bettencourt R, 2008. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc 56, 1292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix C, Fiet J, Benais JP, Gueux B, Bonete R, Villette JM, Gourmel B, Dreux C, 1987. Simultaneous radioimmunoassay of progesterone, androst-4-enedione, pregnenolone, dehydroepiandrosterone and 17-hydroxyprogesterone in specific regions of human brain. J Steroid Biochem 28, 317–25. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Crawford SL, McConnell DS, 2013. Ovarian adrenal interactions during the menopausal transition. Minerva Ginecol 65, 641–51. [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD, 2001. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 285, 1489–99. [DOI] [PubMed] [Google Scholar]
- Leblhuber F, Neubauer C, Peichl M, Reisecker F, Steinparz FX, Windhager E, Dienstl E, 1993. Age and sex differences of dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels in normal controls and Alzheimer’s disease (AD). Psychopharmacology (Berl). 111, 23–6. [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Martensson J, Eckert A, Lindenberger U, Gallinat J, Kuhn S, 2015. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 118, 154–62. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD, 1998. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34, 149–62. [DOI] [PubMed] [Google Scholar]
- Majewska MD, 1992. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38, 379–95. [DOI] [PubMed] [Google Scholar]
- Makris N, Swaab DF, van der Kouwe A, Abbs B, Boriel D, Handa RJ, Tobet S, Goldstein JM, 2013. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: normative data and sex differences. Neuroimage. 69, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Markowski M, Ungeheuer M, Bitran D, Locurto C, 2001. Memory-enhancing effects of DHEAS in aged mice on a win-shift water escape task. Physiol Behav 72, 521–5. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan V, Sandillon F, Urani A, 2000. Differential effect of dehydroepiandrosterone and its steroid precursor pregnenolone against the behavioural deficits in CO-exposed mice. Eur J Pharmacol 390, 145–55. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Guillemain I, 2001. Differential involvement of the sigma(1) (sigma(1)) receptor in the anti-amnesic effect of neuroactive steroids, as demonstrated using an in vivo antisense strategy in the mouse. Br J Pharmacol 134, 1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS, 1995. Oestrogens and the structural and functional plasticity of neurons: Implications for memory, ageing, and neurodegenerative processes. Ciba Foundation Symposium. 191, 52–66; discussion 66-73. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, & Droppleman L , 1992. Profile of Mood States. Vol., ed.^eds. EdITS/Educational and Industrial Testing Service, Inc, San Diego, pp. 1–40. [Google Scholar]
- Melchior CL, Ritzmann RF, 1996. Neurosteroids block the memory-impairing effects of ethanol in mice. Pharmacol Biochem Behav 53, 51–6. [DOI] [PubMed] [Google Scholar]
- Merritt P, Stangl B, Hirshman E, Verbalis J, 2012. Administration of dehydroepiandrosterone (DHEA) increases serum levels of androgens and estrogens but does not enhance short-term memory in post-menopausal women. Brain Res 1483, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S, Yamamoto Y, Ikuno T, Fukunaga K, 2011. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J Neurochem 117, 879–91. [DOI] [PubMed] [Google Scholar]
- Morita T, Senzaki K, Ishihara R, Umeda K, Iwata N, Nagai T, Hida H, Nabeshima T, Yukawa K, Ozaki N, Noda Y, 2014. Plasma dehydroepiandrosterone sulfate levels in patients with major depressive disorder correlate with remission during treatment with antidepressants. Hum Psychopharmacol 29, 280–6. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC, 2006. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 26, 10332–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T, 1998. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 6, 277–84. [PubMed] [Google Scholar]
- Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW, Health A.B.C.s., 2007. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology. 32, 874–83. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Swerdlow RH, Brinton RD, 2017a. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS One. 12, e0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD, 2017b. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 89, 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Maurice T, Tapia-Arancibia L, Givalois L, 2007. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 32, 1062–78. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA, 1997. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 8, 3009–13. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH, 1999. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res 830, 72–87. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Kratz KM, Thompson E, Stanczyk FZ, Buckwalter JG, 2006. Dhea supplementation and cognition in postmenopausal women. Int J Neurosci 116, 141–55. [DOI] [PubMed] [Google Scholar]
- Perez-Neri I, Montes S, Ojeda-Lopez C, Ramirez-Bermudez J, Rios C, 2008. Modulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: mechanism of action and relevance to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 32, 1118–30. [DOI] [PubMed] [Google Scholar]
- Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G, 2010. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord 30, 43–50. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E, 2008. Hippocampal structural changes across the menstrual cycle. Hippocampus. 18, 985–8. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R, 2011. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci 31, 17680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh Z, Yassa M, 2017. Selective vulnerabilities and biomarkers in neurocognitive aging. F1000Res 6, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK, 1998. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res 791, 108–16. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, Mayeux R, 2009. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol 66, 1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, Aizley H, Goldstein JM, 2016. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ 3rd, 2007. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 69, 1074–83. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT, 2011. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 1379, 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Park S, 2002. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 27, 835–41. [DOI] [PubMed] [Google Scholar]
- Ryan J, Stanczyk FZ, Dennerstein L, Mack WJ, Clark MS, Szoeke C, Kildea D, Henderson VW, 2012. Hormone levels and cognitive function in postmenopausal midlife women. Neurobiol Aging. 33, 1138–47. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR, 2005. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 62, 154–62. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN, 2014. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp 35, 847–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Schulze S, Lardy HA, 2000. The effect of 7-oxo-DHEA acetate on memory in young and old C57BL/6 mice. Steroids. 65, 124–9. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Investigators W, 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 289, 2651–62. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH, Women’s Health Initiative Memory, S., 2004. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 291, 2947–58. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR, 2001. Minireview: aromatase and the regulation of estrogen biosynthesis--some new perspectives. Endocrinology. 142, 4589–94. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Renner L, Weiss AR, Neuringer M, Kohama SG, Urbanski HF, 2017. Cognition in aged rhesus monkeys: effect of DHEA and correlation with steroidogenic gene expression. Genes Brain Behav 16, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL, 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 47, 1765–79. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA , 1983. Manual for the State-Trait Anxiety Inventory Vol., ed.^eds. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Stangl B, Hirshman E, Verbalis J, 2011. Administration of dehydroepiandrosterone (DHEA) enhances visual-spatial performance in postmenopausal women. Behav Neurosci 125, 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Thermenos HW, Tarbox SI, Poldrack RA, Seidman LJ, 2005. Medial temporal and prefrontal lobe activation during verbal encoding following glucose ingestion in schizophrenia: A pilot fMRI study. Neurobiol Learn Mem 83, 54–64. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S, 1998. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm (Vienna). 105, 537–42. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD, 2009. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem 91, 139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroder FH, 1992. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci 50, 857–61. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997. Manual for the Wechsler Memory Scale - III. Vol., Psychological Corporation, San Antonio, TX. [Google Scholar]
- Whitfield-Gabrieli S, 2009. Region of Interest Extraction (REX) Toolbox. Vol., ed.^eds., Boston, MA. [Google Scholar]
- Wickens TD, 2001. Elementary Signal Detection Theory. Oxford: Oxford University Press. [Google Scholar]
- Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke KM, Kirschbaum C, 1997. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab 82, 2363–7. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C, 1998. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 23, 617–29. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Torber S, McEwen BS, Kirschbaum C, 1999. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 24, 727–41. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, De Souza E, Sadowsky C, Roberts E, Research, D.H.-A.s.D.C., 2003. DHEA treatment of Alzheimer’s disease: a randomized, doubleblind, placebo-controlled study. Neurology. 60, 1071–6. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA, 1996a. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol 373, 108–17. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA, 1996b. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. Journal of Comparative Neurology. 373, 108–17. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ, 1995. Analysis of fMRI time-series revisited--again. Neuroimage. 2, 173–81. [DOI] [PubMed] [Google Scholar]
- Wroolie TE, Kenna HA, Williams KE, Rasgon NL, 2015. Cognitive Effects of Hormone Therapy Continuation or Discontinuation in a Sample of Women at Risk for Alzheimer Disease. Am J Geriatr Psychiatry. 23, 1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S, 1998a. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol Psychiatry. 43, 694–700. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D, 1998b. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 279, 688–95. [DOI] [PubMed] [Google Scholar]
- Yamada S, Akishita M, Fukai S, Ogawa S, Yamaguchi K, Matsuyama J, Kozaki K, Toba K, Ouchi Y, 2010. Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatr Gerontol Int. 10, 280–7. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC, Cache County Memory Study, I., 2002. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 288, 2123–9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li B, Ma W, Barker JL, Chang YH, Zhao W, Rubinow DR, 2002. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing ways. Brain Res Mol Brain Res 98, 58–66. [DOI] [PubMed] [Google Scholar]


