Abstract
Aims/Introduction
Our previous survey of two Japanese populations, with different lifestyles but identical genetic dispositions, showed that Japanese Americans had different dietary intakes and higher prevalences of obesity and diabetes mellitus, compared with the native Japanese population. The present study examined whether Westernized dietary habits could affect the development of obesity or diabetes.
Materials and methods
This study included 765 individuals with normal glucose tolerance at baseline medical examinations (1986 or 1989 in Los Angeles and in 1988 or 1992 in Hawaii) who subsequently completed follow‐up medical examinations several years later. The participants were categorized at baseline as “lean” (576 individuals, body mass index of <25 kg/m2) or “obese” (189 individuals, body mass index of ≥25 kg/m2). Nutrient intakes were analyzed for associations with the development of obesity or diabetes using Cox's proportional hazard model.
Results
A total of 41 lean participants developed diabetes, which was not associated with any nutrient intakes (mean follow up 10.8 ± 6.6 years). A total of 36 obese participants developed diabetes, which was positively associated with intakes of animal protein, animal fat and saturated fatty acid (mean follow up 10.7 ± 6.3 years). A total of 85 lean participants became obese, which was positively associated with intakes of simple carbohydrates, sugar and fructose, as well as inversely associated with intakes of vegetable protein and complex carbohydrates (mean follow up 10.4 ± 6.5 years).
Conclusions
In the Japanese Americans, different nutrient intakes affected the development of obesity and diabetes. Furthermore, the associations of nutrient intakes with diabetes development varied according to the presence or absence of obesity.
Keywords: Diabetes mellitus, Nutrient intake, Obesity
Introduction
The number of diabetes patients in the world is reported to be >425 million, and the explosive increase has become a global issue1. Similarly, the prevalence of obesity is increasing in the world, and it is an important risk factor for cardiovascular and kidney diseases, diabetes mellitus, some cancers and musculoskeletal disorders2. Recently, in Japan, the percentage of overweight individuals in the population is slightly increasing in men, but not in women3. Diabetes mellitus and obesity result from the interaction between a genetic predisposition and behavioral and environmental risk factors4, 5. Dietary factors might influence the risk of diabetes and obesity6, 7. A Westernized diet is characterized by a high consumption of red meat, processed meat, eggs, refined grains, sweets and dessert, French fries, and high‐fat dairy products8. A Westernized diet is associated with increased diabetes and obesity incidence9, 10, 11.
We have been carrying out an epidemiological study of Japanese Americans since 1970, which is titled the Hawaii‐Los Angeles‐Hiroshima Study, and have carried out medical surveys of Japanese individuals who are living in Hawaii, Los Angeles and Hiroshima (Japan) every few years12. For our study, Japanese Americans are defined as Japanese individuals who immigrated to the USA and their descendants. These individuals have a Japanese genetic disposition, but live in an American environment with corresponding eating and exercise habits. Thus, we have been able to investigate the effects of lifestyle Westernization (i.e., a sudden change from Japanese to American lifestyle) on the prevalences of metabolic diseases and atherosclerosis by comparing the Japanese American and native Japanese populations.
Between 1978 and 1988, the surveys showed that Japanese Americans had relatively high intakes of animal protein, animal fat, saturated fatty acid (SFA), cholesterol and simple carbohydrates (e.g., sugar and fructose), with relatively low complex carbohydrate intake, compared with the native Japanese population13. Between 1992 and 1995, we divided Japanese Americans into two subgroups (first‐generation and second‐ or later‐generation) for comparison of the percentage of nutrient intake14. As a result, the percentage of animal protein, animal fat and simple carbohydrate intake was highest in the second‐ or later‐generation Japanese Americans, followed by the first‐generation Japanese Americans and the native Japanese population; the percentage of complex carbohydrate intake was highest in the native Japanese population, followed by the first‐generation Japanese Americans and the second‐ or later‐generation Japanese Americans. Furthermore, we reported that the Westernized eating habits among Japanese Americans are associated with a trend towards insulin resistance as an atherosclerotic risk factor15, as well as higher prevalences of obesity, diabetes mellitus and metabolic syndrome16, 17, 18, compared with the native Japanese population. However, we did not consider whether differences in nutrient intakes were causally associated with the development of these lifestyle diseases among Japanese Americans. Therefore, the present study was carried out to examine longitudinal medical survey data from Japanese Americans who lived in Hawaii or Los Angeles during 1986–2010, in order to examine the associations between nutrient intakes and the development of obesity or diabetes mellitus.
Materials and Methods
Study participants
The Japanese American participants underwent medical examinations in Los Angeles (during 1986 and 1989) and/or in Hawaii (during 1988 and 1992). If an individual completed examinations in both locations, the earlier examination was defined as the baseline examination. The same participants subsequently underwent medical examinations that were carried out in Los Angeles (during 1993, 1996, 2000, 2004 and 2010) or in Hawaii (during 1995, 1998, 2002 and 2007) one or more times. The initial examination involved a nutrient intake survey and a 75‐g oral glucose tolerance test (OGTT), and follow up was subsequently initiated. The present study examined data from 765 individuals (325 men and 440 women) who had normal glucose tolerance at the baseline examination. The study was carried out with the approval of the ethics committee of Hiroshima University.
Research methods
After an overnight fast, each participant was interviewed, provided written informed consent, and underwent a physical examination and venous blood collection. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). The development of obesity was defined as an increase in BMI from <25 kg/m2 to ≥25 kg/m2. The presence or development of diabetes mellitus was based on self‐reported diabetes mellitus, a fasting serum glucose (FSG) level of ≥126 mg/dL or a 2‐h serum glucose after OGTT level of ≥200 mg/dL.
In order to examine the associations between nutrient intakes and the development of obesity, the participants were classified into two groups using their baseline BMI: the “lean” group (576 individuals, including 222 men and 354 women, with a BMI of <25 kg/m2), which was assessed for the development of obesity and diabetes mellitus, and the “obese” group (189 individuals, including 103 men and 86 women, with a BMI of ≥25 kg/m2), which was assessed for the development of diabetes mellitus.
Dietary information at baseline was collected with the food frequency method. During the nutrient intake survey, the frequency of intake, amount of intake per meal and the cooking method for each food group were ascertained using food models and personal interviews that were carried out by a dietitian. Food models were used for portion sizes. All food items were itemized into 80 groups, which were derived from the nutrient survey. The mean daily intake of each food group was calculated as (mean amount of intake per meal) × (frequency of intake per day), and the nutrition intake from each food group was calculated as (nutritional value per g of each food) × (the mean daily intake of each food group). The nutritional value of each food group was determined according to the Nutritive Value of American Foods in Common Units19. Values for daily total energy and intake amount were calculated for individual nutritional elements (i.e., total protein, animal protein, vegetable protein, total fat, animal fat, vegetable fat, total carbohydrates, simple carbohydrates, complex carbohydrates, sugar, fructose, polyunsaturated fatty acid and SFA). Simple carbohydrate intake was defined as the sum of the sugar and fructose intakes.
Statistical analysis
Data were expressed as number, mean ± standard deviation or median (interquartile range). Skewed data distributions were detected for triglycerides (TG), fasting immunoreactive insulin (FIRI), homeostasis model assessment of insulin resistance and homeostasis model assessment of β‐cell function. Thus, those parameters were logarithmically transformed before being analyzed. Continuous variables were compared using Student's unpaired t‐test. We analyzed each nutrient as a percentage of total energy (energy from each nutrient / total energy × 100). The corresponding risks of developing obesity or diabetes mellitus for each nutrient were evaluated using its intake level relative to total energy, based on Cox's proportional hazard model, which was adjusted for age, sex, BMI, systolic blood pressure, total cholesterol (T‐cho), TG, FSG and FIRI. P‐values of <0.05 were considered statistically significant. All analyses were carried out using IBM SPSS Statistics for Windows (IBM Corp., Armonk, New York, USA).
Results
During the follow‐up period, 41 lean participants developed diabetes mellitus (mean follow up 10.8 ± 6.6 years) and 36 obese participants developed diabetes mellitus (mean follow up 10.7 ± 6.3 years; Figure 1).
Figure 1.
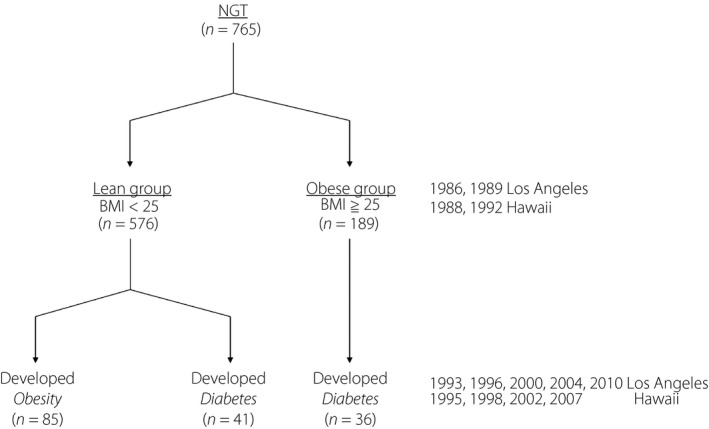
Study participants. BMI, body mass index; NGT, normal glucose tolerance.
Table 1 shows the comparisons of the lean and obese participants according to whether they did or did not develop diabetes mellitus. In the lean group, participants who developed diabetes mellitus had significantly higher FSG and 2‐h serum glucose after OGTT levels, compared with participants who did not develop diabetes mellitus. In the obese group, participants who developed diabetes mellitus had significantly higher T‐cho and TG levels, compared with participants who did not develop diabetes mellitus. Compared with lean participants who developed diabetes mellitus, obese participants who developed diabetes mellitus had significantly higher values for BMI, TG, FIRI and homeostasis model assessment of insulin resistance.
Table 1.
Baseline clinical characteristics
| Lean (BMI of <25 kg/m2) | Obese (BMI of ≥25 kg/m2) | |||
|---|---|---|---|---|
| Did not develop DM | Developed DM | Did not develop DM | Developed DM | |
| n | 535 | 41 | 153 | 36 |
| Men/women | 202/333 | 20/21 | 84/69 | 19/17 |
| Age (years) | 58.2 ± 14.0 | 61.2 ± 11.0 | 58.4 ± 13.4 | 57.0 ± 11.1 |
| BMI (kg/m2) | 21.6 ± 2.1 | 22.1 ± 2.0 | 27.3 ± 2.2 | 27.6 ± 2.0§ |
| SBP (mmHg) | 128.0 ± 18.3 | 129.0 ± 16.6 | 136.0 ± 18.9 | 133.0 ± 14.4 |
| DBP (mmHg) | 75.9 ± 10.0 | 76.9 ± 9.5 | 79.3 ± 10.7 | 78.1 ± 10.6 |
| T‐cho (mg/dL) | 220.0 ± 39.6 | 222.0 ± 31.5 | 223.0 ± 36.6 | 237.0 ± 44.4‡ |
| TG (mg/dL) | 104.0 (75.0–148.0) | 106.0 (81.0–185.0) | 123.0 (92.5–161.0) | 169.0 (110.0–242.0)‡ § |
| FSG (mg/dL) | 86.6 ± 8.3 | 92.9 ± 9.3† | 89.6 ± 8.6 | 92.8 ± 9.1 |
| 2‐h SG (mg/dL) | 97.5 ± 21.6 | 113.0 ± 22.3† | 105.0 ± 19.8 | 112.0 ± 17.0 |
| FIRI (μU/mL) | 12.0 (9.0–14.0) | 13.0 (9.5–14.5) | 15.0 (11.0–19.0) | 16.0 (11.3–19.8)§ |
| HOMA‐IR | 2.46 (1.80–3.18) | 3.02 (2.07–3.42) | 3.16 (2.42–3.99) | 3.67 (2.41–4.57)§ |
| HOMA‐β | 180.0 (128.0–246.0) | 144.0 (110.0–180.0) | 203.0 (137.0–272.0) | 175.0 (129.0–284.0) |
Data are expressed as number, mean ± standard deviation or median (interquartile range). P‐values were calculated using unpaired t‐test. Parameters were transformed logarithmically before the analysis. † P < 0.05 between lean individuals who did and did not develop diabetes mellitus (DM). ‡ P < 0.05 between obese individuals who did and did not develop DM. § P < 0.05 between obese and lean individuals who developed DM. 2‐h SG, 2‐h serum glucose after the oral glucose tolerance test; BMI, body mass index; DBP, diastolic blood pressure; FIRI, fasting immunoreactive insulin; FSG, fasting serum glucose; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment‐insulin resistance; SBP, systolic blood pressure; T‐cho, total cholesterol; TG, triglycerides.
The baseline intakes of each nutrient were similar for participants who did and did not develop diabetes mellitus in the lean and obese groups (Table 2).
Table 2.
Baseline nutrient intakes
| Lean (BMI of <25 kg/m2) | Obese (BMI of ≥25 kg/m2) | |||
|---|---|---|---|---|
| Did not develop DM | Developed DM | Did not develop DM | Developed DM | |
| Total energy (kcal) | 1,960 ± 603 | 2,050 ± 593 | 2,190 ± 750 | 2,120 ± 685 |
| Total protein (g) | 69.4 ± 21.1 | 73.6 ± 20.8 | 79.4 ± 27.8 | 78.7 ± 22.6 |
| Animal protein (g) | 39.4 ± 15.7 | 41.5 ± 15.0 | 45.9 ± 21.2 | 47.1 ± 16.2 |
| Vegetable protein (g) | 30.1 ± 9.1 | 32.2 ± 9.0 | 33.5 ± 11.1 | 31.6 ± 12.6 |
| Total fat (g) | 65.1 ± 25.7 | 69.3 ± 28.1 | 77.8 ± 34.2 | 76.8 ± 25.9 |
| Animal fat (g) | 31.2 ± 15.5 | 32.9 ± 15.2 | 35.8 ± 20.2 | 38.0 ± 16.7 |
| Vegetable fat (g) | 33.9 ± 14.3 | 36.4 ± 16.9 | 42.0 ± 18.9 | 38.8 ± 15.6 |
| Total carbohydrates (g) | 259.0 ± 76.8 | 271.0 ± 72.8 | 274.0 ± 82.9 | 267.0 ± 101.0 |
| Simple carbohydrates (g) | 82.7 ± 40.5 | 83.1 ± 40.2 | 87.5 ± 43.4 | 87.6 ± 50.0 |
| Sugar (g) | 37.3 ± 25.7 | 39.7 ± 25.4 | 38.4 ± 25.8 | 44.0 ± 35.0 |
| Fructose (g) | 45.4 ± 29.7 | 43.4 ± 28.9 | 49.1 ± 31.2 | 43.6 ± 33.6 |
| Complex carbohydrates (g) | 176.0 ± 59.8 | 188.0 ± 54.7 | 186.0 ± 65.1 | 180.0 ± 68.8 |
| PUFA (g) | 19.1 ± 6.9 | 20.7 ± 8.2 | 23.3 ± 9.2 | 22.1 ± 8.3 |
| SFA (g) | 20.9 ± 9.2 | 22.1 ± 9.3 | 24.6 ± 12.2 | 25.1 ± 10.1 |
Data are expressed as mean ± standard deviation. Unpaired t‐tests were used to compare the groups that did and did not develop diabetes mellitus (DM). PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
In the lean group, the nutrient intakes were not associated with the development of diabetes mellitus (Table 3). In the obese group, the development of diabetes mellitus was significantly positively associated with the intakes of total protein (hazard ratio [HR] 1.22, 95% confidence interval [CI] 1.03–1.45, P = 0.025), animal protein (HR 1.20, 95% CI 1.04–1.38, P = 0.011), animal fat (HR 1.09, 95% CI 1.02–1.17, P = 0.010) and SFA (HR 1.14, 95% CI 1.00–1.30, P = 0.047). These associations were independent of the baseline values for age, sex, BMI, systolic blood pressure, T‐cho, TG, FSG and FIRI (Table 4).
Table 3.
Risks of developing diabetes mellitus according to nutrient intakes/total energy (body mass index of <25 kg/m2)
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Total energy/IBW (kcal/kg) | 1.00 (0.97–1.03) | 0.974 |
| Total protein/TE (%) | 1.08 (0.93–1.24) | 0.308 |
| Animal protein/TE (%) | 1.04 (0.90–1.21) | 0.590 |
| Vegetable protein/TE (%) | 1.11 (0.87–1.43) | 0.396 |
| Total fat/TE (%) | 1.02 (0.96–1.07) | 0.574 |
| Animal fat/TE (%) | 1.01 (0.94–1.09) | 0.736 |
| Vegetable fat/TE (%) | 1.02 (0.95–1.09) | 0.671 |
| Total carbohydrates/TE (%) | 1.03 (0.98–1.07) | 0.276 |
| Simple carbohydrates/TE (%) | 0.99 (0.94–1.04) | 0.694 |
| Sugar/TE (%) | 1.02 (0.94–1.10) | 0.725 |
| Fructose/TE (%) | 0.98 (0.93–1.04) | 0.509 |
| Complex carbohydrates/TE (%) | 1.04 (0.99–1.08) | 0.136 |
| PUFA/TE (%) | 1.06 (0.91–1.23) | 0.469 |
| SFA/TE (%) | 1.04 (0.91–1.19) | 0.587 |
The hazard ratios were calculated after adjusting for age, sex, body mass index, systolic blood pressure, total cholesterol, triglycerides, fasting serum glucose and fasting immunoreactive insulin. The mean duration of the follow‐up period was 10.8 ± 6.6 years (mean ± standard deviation). CI, confidence interval; IBW, ideal bodyweight; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TE, total energy.
Table 4.
Risks of developing diabetes mellitus according to nutrient intakes/total energy (body mass index of ≥25 kg/m2)
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Total energy/IBW (kcal/kg) | 0.96 (0.93–1.00) | 0.059 |
| Total protein/TE (%) | 1.22 (1.03–1.45) | 0.025 |
| Animal protein/TE (%) | 1.20 (1.04–1.38) | 0.011 |
| Vegetable protein/TE (%) | 0.82 (0.62–1.09) | 0.173 |
| Total fat/TE (%) | 1.04 (0.99–1.10) | 0.138 |
| Animal fat/TE (%) | 1.09 (1.02–1.17) | 0.010 |
| Vegetable fat/TE (%) | 0.98 (0.91–1.05) | 0.527 |
| Total carbohydrates/TE (%) | 0.98 (0.94–1.03) | 0.523 |
| Simple carbohydrates/TE (%) | 0.99 (0.93–1.05) | 0.696 |
| Sugar/TE (%) | 1.06 (0.98–1.14) | 0.133 |
| Fructose/TE (%) | 0.94 (0.88–1.01) | 0.089 |
| Complex carbohydrates/TE (%) | 0.99 (0.94–1.05) | 0.726 |
| PUFA/TE (%) | 0.99 (0.84–1.15) | 0.848 |
| SFA/TE (%) | 1.14 (1.00–1.30) | 0.047 |
The hazard ratios were calculated after adjusting for age, sex, body mass index, systolic blood pressure, total cholesterol, triglycerides, fasting serum glucose and fasting immunoreactive insulin. The mean duration of the follow‐up period was 10.7 ± 6.3 years (mean ± standard deviation). CI, confidence interval; IBW, ideal body weight; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TE, total energy.
In the lean group, 85 participants developed obesity (mean follow up 10.4 ± 6.5 years; Figure 1). After adjusting for baseline age, sex, BMI, systolic blood pressure, T‐cho, TG, FSG and FIRI, the development of obesity was significantly positively associated with the intakes of total carbohydrates (HR 1.04, 95% CI 1.01–1.07, P = 0.018), simple carbohydrates (HR 1.07, 95% CI 1.04–1.10, P < 0.001), sugar (HR 1.09, 95% CI 1.04–1.14, P < 0.001) and fructose (HR 1.05, 95% CI 1.01–1.08, P = 0.011). The development of obesity was significantly inversely associated with the intakes of total protein (HR 0.89, 95% CI 0.81–0.98, P = 0.017), vegetable protein (HR 0.82, 95% CI 0.68–0.99, P = 0.049) and complex carbohydrates (HR 0.97, 95% CI 0.93–0.99, P = 0.047; Table 5).
Table 5.
Risks of developing obesity according to nutrient intakes/total energy
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Total energy/IBW (kcal/kg) | 0.99 (0.97–1.01) | 0.461 |
| Total protein/TE (%) | 0.89 (0.81–0.98) | 0.017 |
| Animal protein/TE (%) | 0.94 (0.85–1.03) | 0.182 |
| Vegetable protein/TE (%) | 0.82 (0.68–0.99) | 0.049 |
| Total fat/TE (%) | 0.98 (0.95–1.02) | 0.347 |
| Animal fat/TE (%) | 0.98 (0.93–1.03) | 0.375 |
| Vegetable fat/TE (%) | 0.99 (0.94–1.04) | 0.661 |
| Total carbohydrates/TE (%) | 1.04 (1.01–1.07) | 0.018 |
| Simple carbohydrates/TE (%) | 1.07 (1.04–1.10) | <0.001 |
| Sugar/TE (%) | 1.09 (1.04–1.14) | <0.001 |
| Fructose/TE (%) | 1.05 (1.01–1.08) | 0.011 |
| Complex carbohydrates/TE (%) | 0.97 (0.93–0.99) | 0.047 |
| PUFA/TE (%) | 0.96 (0.87–1.07) | 0.488 |
| SFA/TE (%) | 0.96 (0.88–1.06) | 0.436 |
The hazard ratios were calculated after adjusting for age, sex, body mass index, systolic blood pressure, total cholesterol, triglycerides, fasting serum glucose and fasting immunoreactive insulin. The mean duration of the follow‐up period was 10.4 ± 6.5 years (mean ± standard deviation). CI, confidence interval; IBW, ideal body weight; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TE, total energy.
Discussion
The present longitudinal analysis (1986–2010) showed that the development of obesity among Japanese Americans was significantly associated with a higher intake of simple carbohydrates, and a lower intake of vegetable protein and complex carbohydrates. In addition, the development of diabetes mellitus was not significantly associated with nutrition in the lean group (BMI of <25 kg/m2 at baseline), although it was associated with higher intakes of animal protein, animal fat and SFA in the obese group (BMI of ≥25 kg/m2 at baseline).
The results show that simple carbohydrate intake can lead to the development of obesity, whereas intakes of vegetable protein and complex carbohydrates can help prevent the development of obesity. Many reports have shown that simple carbohydrate intake leads to weight gain and obesity, and meta‐analyses have shown that the sugar in sweetened beverages and soft drinks is associated with weight gain20, 21, 22. Several reports have described a mechanism through which fructose causes obesity. First, unlike glucose, fructose acts as an unregulated de novo substrate for fatty acid synthesis in the liver23. Next, fructose does not stimulate insulin or leptin, and does not suppress ghrelin, which can impair satiety signaling24. Furthermore, compared with glucose, fructose differentially stimulates the hypothalamic centers that are associated with food intake and reward regulation25.
A study of overweight individuals with metabolic syndrome showed that a diet that was high in complex carbohydrates provided significant weight loss, compared with the normal diet or a diet that was high in simple carbohydrates26. In addition, the intake of dietary fiber, particularly cereal fiber, in complex carbohydrates is inversely correlated with bodyweight, waist circumference and BMI, which can protect against obesity27, 28.
Large amounts of vegetable proteins are found in soybeans, nuts and cereals. A study of individuals in the USA and Canada showed an inverse correlation between the intake of nuts and the prevalence of obesity29. In addition, a study of Chinese women showed that soy protein intake was associated with reduced bodyweight and body fat percentage30. Wistar rats that were fed with soy protein showed a reduction in adipose cell size, and elevated expression of adiponectin messenger ribonucleic acid and protein31, 32. Furthermore, β‐conglycinin in soybean has been reported to increase FGF21 levels and suppress weight gain33.
Meta‐analyses have shown that the risk of developing diabetes mellitus is associated with the consumption of red meat and processed meat, which is rich in animal protein, animal fat and SFA34, 35. The present study also confirmed that higher intakes of total protein, animal protein, animal fat and SFA were only significantly associated with the development of diabetes mellitus in the obese group. Similarly, total protein and animal protein intake are risk factors for the development of diabetes mellitus, based on the findings of European and American studies36, 37, 38, 39, as well as a meta‐analysis40. It is possible that amino acids reduce the endocrine function of the pancreas, act as gluconeogenic precursors in the liver and increase insulin resistance through the hexosamine biosynthetic pathway41, 42.
European and American studies have also confirmed that intakes of total fat, animal fat and SFA are risk factors for the development of diabetes mellitus 43, 44, 45. In addition, a high‐fat diet or high proportion of SFA in the diet can lead to accumulation of fat in the adipose tissue, muscle and liver, which can cause insulin resistance46, 47. Our study of mice that received a high‐fat diet also confirmed that it impaired the insulin–protein kinase B signaling pathway, and reduced the phosphorylation/activation of adenosine monophosphate‐activated protein kinase, which led to insulin resistance48.
Several previous Japanese cohort studies have shown the association of carbohydrate intake with the development of diabetes mellitus. One study showed that rice intake was associated with an increased risk of diabetes mellitus in women49, another study showed that higher carbohydrate intake was associated with a higher risk of diabetes mellitus in obese men50, the other study showed that a low‐carbohydrate diet was associated with a decreased risk of diabetes mellitus in women, and the association might be partly attributable to high intake of white rice51. In contrast, a study of approximately 36,000 American women showed that intakes of total dietary and cereal fiber helped prevent the development of diabetes mellitus, and that total carbohydrate intake was not correlated with the development of diabetes mellitus52. In the present study, the association between nutrient intakes and the development of diabetes mellitus in the obese group of Japanese Americans was similar to that in previous Western studies, but different from that in previous Japanese studies.
These results showed that, even among a Japanese population with a relatively low prevalence of obesity (vs Western populations), an obese group of Japanese Americans are likely to develop obesity‐related diabetes mellitus, which is caused by insulin resistance in response to an American diet, which is high in animal protein, animal fat and SFA. However, the lean group did not show an association between nutrient intakes and the development of diabetes mellitus. In the lean group, the subpopulation that developed diabetes mellitus had significantly higher baseline FSG and 2‐h serum glucose after OGTT levels, with insignificantly lower baseline homeostasis model assessment of β‐cell function values, compared with the subpopulation that did not develop diabetes mellitus (Table 1). In this context, Japanese Americans are considered genetically Japanese, and are included in an Asian population that has a relatively low ability to secrete insulin. Thus, among non‐obese Japanese Americans, the development of non‐obese diabetes seems to be more strongly related to the presence of mild glucose intolerance caused by impaired insulin secretion, rather than the intake of insulin resistance‐inducing nutrients, such as animal protein, fat and SFA.
The present study had several limitations. First, we did not evaluate various factors that are related to the development of diabetes mellitus, such as family history of diabetes mellitus, exercise, physical activity and socioeconomic status. In the participants who had information regarding family history of diabetes mellitus (317 individuals), we carried out the analysis after adjusting for family history of diabetes mellitus, but the results were almost same (data not shown). Second, we evaluated total energy, three major nutrients, fructose, polyunsaturated fatty acid and SFA, but not other nutrients (e.g., vitamins, magnesium etc.). Furthermore, diet information was collected through interviews during 1986–1992. However, the nutrient intake profile of Japanese Americans might have changed over time, and this possibility was not considered in the analysis. Third, there is a minor environmental difference between Hawaii and Los Angeles, despite these locations being in the same country15, 16, although these regions were not separated in the analysis.
In conclusion, the present study examined longitudinal observational data from 765 Japanese Americans over a >10‐year period, and showed that the development of obesity was associated with American dietary habits, such as a high intake of simple carbohydrates, and low intakes of vegetable protein and complex carbohydrates. Furthermore, among obese Japanese Americans, the development of diabetes mellitus was associated with higher intakes of animal protein, animal fat and SFA. However, nutrient intakes were not associated with the development of diabetes mellitus among non‐obese Japanese Americans, which indicates that obesity can modulate the association between nutrient intakes and the development of diabetes mellitus.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This survey was supported by the members of the Hiroshima Kenjin‐Kai Associations of Hilo and Kona, Hawaii, and of Los Angeles, California.
J Diabetes Investig 2019; 10: 1229–1236
References
- 1. IDF . IDF Diabetes Atlas, 8th edn IDF; Available from: http://www.diabetesatlas.org/ Accessed November 14, 2018. [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD‐RisC) . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishi N. Monitoring obesity trends in health Japan 21. J Nutr Sci Vitaminol 2015; 61(Suppl): S17–S19. [DOI] [PubMed] [Google Scholar]
- 4. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962; 14: 353–362. [PMC free article] [PubMed] [Google Scholar]
- 5. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med 2007; 357: 370–379. [DOI] [PubMed] [Google Scholar]
- 6. Tuomilehto J, Lindström J, Eriksson JG, et al Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 7. O'Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr 2011; 93: 314–321. [DOI] [PubMed] [Google Scholar]
- 8. Salas‐Salvadó J, Martinez‐González MÁ, Bulló M, et al The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis 2011; 21(Suppl 2): B32–B48. [DOI] [PubMed] [Google Scholar]
- 9. van Dam RM, Rimm EB, Willet WC, et al Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 2002; 136: 201–209. [DOI] [PubMed] [Google Scholar]
- 10. Fung TT, Schulze M, Manson JE, et al Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 2004; 164: 2235–2240. [DOI] [PubMed] [Google Scholar]
- 11. Schulze MB, Fung TT, Manson JE, et al Dietary patterns and changes in body weight in women. Obesity 2006; 14: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 12. Kawate R, Yamakido M, Nishimoto Y, et al Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care 1979; 2: 161–170. [DOI] [PubMed] [Google Scholar]
- 13. Egusa G, Murakami F, Ito C, et al Westernized food habits and concentrations of serum lipids in the Japanese. Atherosclerosis 1993; 100: 249–255. [DOI] [PubMed] [Google Scholar]
- 14. Egusa G, Watanabe H, Ohshita K, et al Influence of the extent of westernization of lifestyle on the progression of preclinical atherosclerosis in Japanese subjects. J Atheroscler Thromb 2002; 9: 299–304. [DOI] [PubMed] [Google Scholar]
- 15. Nakanishi S, Okubo M, Yoneda M, et al A comparison between Japanese‐Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed Pharmacother 2004; 58: 571–577. [DOI] [PubMed] [Google Scholar]
- 16. Hara H, Egusa G, Yamakido M, et al The high prevalence of diabetes mellitus and hyperinsulinemia among the Japanese‐Americans living in Hawaii and Los Angeles. Diabetes Res Clin Pract 1994; 24: S37–S42. [DOI] [PubMed] [Google Scholar]
- 17. Yoneda M, Yamane K, Jitsuiki K, et al Prevalence of metabolic syndrome compared between native Japanese and Japanese‐Americans. Diabetes Res Clin Pract 2008; 79: 518–522. [DOI] [PubMed] [Google Scholar]
- 18. Shiwa M, Yoneda M, Nakanishi S, et al Japanese lifestyle during childhood prevents the future development of obesity among Japanese‐Americans. PLoS One 2015; 10: e0120804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Department of Agriculture, The Agricultural Research Service . Nutritive Value of American Foods in Common Units, Agriculture Handbook No.456. Washington, DC: U.S. Dept of Agriculture, 1975. [Google Scholar]
- 20. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta‐analysis. Am J Public Health 2007; 97: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta‐analyses of randomised controlled trials and cohort studies. BMJ 2012; 346: e7492. [DOI] [PubMed] [Google Scholar]
- 22. Khan TA, Sievenpiper JL. Controversies about sugars: results from systematic reviews and meta‐analyses on obesity, cardiometabolic disease and diabetes. Eur J Nutr 2016; 55(suppl. 2): 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanhope KL, Schwarz JM, Keim NL, et al Consuming fructose‐sweetened, not glucose‐sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Investig 2009; 119: 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teff KL, Elliott SS, Tschöp M, et al Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004; 89: 2963–2972. [DOI] [PubMed] [Google Scholar]
- 25. Page KA, Chan O, Arora J, et al Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013; 309: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poppitt SD, Keogh GF, Prentice AM, et al Long‐term effects of ad libitum low‐fat, high‐carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr 2002; 75: 11–20. [DOI] [PubMed] [Google Scholar]
- 27. Feskens EJ, Sluik D, Du H. The association between diet and obesity in Specific European Cohorts: DiOGenes and EPIC‐PANACEA. Curr Obes Rep 2014; 3: 67–78. [DOI] [PubMed] [Google Scholar]
- 28. Murakami K, Sasaki S, Okubo H, et al Dietary fiber intake, dietary glycemic index and load, and body mass index: across‐sectional study of 3931 Japanese woman aged 18‐20 years. Eur J Clin Nutr 2007; 61: 986–995. [DOI] [PubMed] [Google Scholar]
- 29. Jaceldo‐Siegl K, Haddad E, Oda K, et al Tree nuts are inversely associated with metabolic syndrome and obesity: the Adventist Health Study‐2. PLoS One 2014; 9: e85133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu ZM, Ho SC, Chen YM, et al A mild favorable effect of soy protein with isoflavones on body composition–a 6‐month double‐blind randomized placebo‐controlled trial among Chinese postmenopausal women. Int J Obes 2010; 34: 309–318. [DOI] [PubMed] [Google Scholar]
- 31. Oliva ME, Selenscig D, D'Alessandro ME, et al Soya protein ameliorates the metabolic abnormalities of dysfunctional adipose tissue of dyslipidaemic rats fed a sucrose‐rich diet. Br J Nutr 2011; 105: 1188–1198. [DOI] [PubMed] [Google Scholar]
- 32. Chen JH, Song J, Chen Y, et al The effect of Vegan protein‐based diets on metabolic parameters, expressions of adiponectin and its receptors in Wistar rats. Nutrients 2016; 8: E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hashidume T, Kato A, Tanaka T, et al Single ingestion of soy β‐conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Sci Rep 2016; 6: 28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes : a systematic review and meta‐analysis of cohort studies. Diabetologia 2009; 52: 2277–2287. [DOI] [PubMed] [Google Scholar]
- 35. Pan A, Sun Q, Bernstein AM, et al Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta‐analysis. Am J Clin Nutr 2011; 94: 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sluijs I, Beulens JW, van der A DL, et al Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European prospective investigation into cancer and nutrition (EPIC)‐NL study. Diabetes Care 2010; 33: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pounis GD, Tyrovolas S, Antonopoulou M, et al Long‐term animal‐protein consumption is associated with an increased prevalence of diabetes among elderly: the Mediterranean islands (MEDIS) study. Diabetes Metab 2010; 36: 484–490. [DOI] [PubMed] [Google Scholar]
- 38. van Nielen M, Feskens EJ, Mensink M, et al Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC‐InterAct case‐cohort study. Diabetes Care 2014; 37: 1854–1862. [DOI] [PubMed] [Google Scholar]
- 39. Malik VS, Li Y, Tobias DK, et al Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol 2016; 183: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shang X, Scott D, Hodge AM, et al Dietary protein intake and risk of type 2 diabetes: results from the Melbourne Collaborative Cohort Study and a meta‐analysis of prospective studies. Am J Clin Nutr 2016; 104: 1352–1365. [DOI] [PubMed] [Google Scholar]
- 41. Tremblay F, Lavigne C, Jacques H, et al Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007; 27: 293–310. [DOI] [PubMed] [Google Scholar]
- 42. Promintzer M, Krebs M. Effects of dietary protein on glucose homeostasis. Curr Opin Clin Nutr Metab Care 2006; 9: 463–468. [DOI] [PubMed] [Google Scholar]
- 43. Lovejoy LC, Windhauser MM, Rood JC, et al Effect of a controlled high‐fat versus low‐fat diet on insulin sensitivity and leptin levels in African‐American and Caucasian women. Metabolism 1998; 47: 1520–1524. [DOI] [PubMed] [Google Scholar]
- 44. Vessby B, Uusitupa M, Hermansen K, et al Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia 2001; 44: 312–319. [DOI] [PubMed] [Google Scholar]
- 45. van Dam RM, Willett WC, Rimm EB, et al Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002; 25: 417–424. [DOI] [PubMed] [Google Scholar]
- 46. Thanopoulou AC, Karamanos BG, Angelico FV, et al Dietary fat intake as risk factor for the development of diabetes: multinational, multicenter study of the Mediterranean Group for the Study of Diabetes (MGSD). Diabetes Care 2003; 26: 302–307. [DOI] [PubMed] [Google Scholar]
- 47. Guasch‐Ferré M, Becerra‐Tomás N, Ruiz‐Canela M, et al Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr 2017; 105: 723–735. [DOI] [PubMed] [Google Scholar]
- 48. Shiwa M, Yoneda M, Okubo H, et al Distinct time course of the decrease in hepatic AMP‐activated protein kinase and Akt phosphorylation in mice fed a high fat diet. PLoS One 2015; 10: e0135554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hirata A, Ohnaka K, Tashiro N, et al Effect modification of green tea on the association between rice intake and the risk of diabetes mellitus: a prospective study in Japanese men and women. Asia Pac J Clin Nutr 2017; 26: 545–555. [DOI] [PubMed] [Google Scholar]
- 50. Sakurai M, Nakamura K, Miura K, et al Dietary carbohydrate intake, presence of obesity and the incident risk of type 2 diabetes in Japanese men. J Diabetes Investig 2016; 7: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nanri A, Mizoue T, Kurotani K, et al Low‐carbohydrate diet and type 2 diabetes risk in Japanese men and women: the Japan Public Health Center‐Based Prospective Study. PLoS One 2015; 10: e0118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meyer KA, Kushi LH, Jacobs DR Jr, et al Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000; 71: 921–930. [DOI] [PubMed] [Google Scholar]


