Abstract
Serine Protease Inhibitors (Serpins) control tightly regulated physiological processes and their dysfunction is associated to various diseases. Thus, increasing interest is given to these proteins as new therapeutic targets. Several studies provided functional and structural data about human serpins. By comparison, only little knowledge regarding bacterial serpins exists. Through the emergence of metagenomic studies, many bacterial serpins were identified from numerous ecological niches including the human gut microbiota. The origin, distribution and function of these proteins remain to be established. In this report, we shed light on the key role of human and bacterial serpins in health and disease. Moreover, we analyze their function, phylogeny and ecological distribution. This review highlights the potential use of bacterial serpins to set out new therapeutic approaches.
Keywords: Serine protease inhibitors, Disease, Homeostasis, Function, Human gut microbiota
Introduction
Serpins were first discovered in 1980 when Hunt and Dayhoff noticed similarities between ovalbumin, an egg white protein and two human proteins: antithrombin and α1-antitrypsin (α1-AT) (Hunt & Dayhoff, 1980). The acronym serpin was coined in 1985 to designate serine protease inhibitors (Carrell & Travis, 1985). Serpins constitute a superfamily displaying different functions and are divided into 16 clades (named A-P) (Heit et al., 2013). Although serpin acronym initially derived from their main function, which is the inhibition of serine proteases (Gettins, 2002; Huntington, 2011), cross-class inhibition was also demonstrated (Schick et al., 1998; Bao et al., 2018). However, several serpins do not exhibit any inhibitory activity but coordinate a wide range of other biological functions (Hammond et al., 1987; Clarke et al., 1991; Gettins, 2002; Carrell & Read, 2017). In human, serpins are well studied and their dysregulation is often associated to many pathologies including inflammation, cardiovascular diseases, cancer and neurological disorders (Ho et al., 1994; Wolf et al., 1999; Vecchi et al., 2008). Many reports stressed the key role of serpins in human health leading to their suggestion as potential therapeutic targets (Richardson, Viswanathan & Lucas, 2006; Zheng et al., 2013; Al-Horani, 2014).
Unlike eukaryotic serpins, the discovery of their prokaryotic counterparts is relatively recent. Indeed, until 2002, serpins were believed to be restricted to eukaryotes, but based on phylogenetic analysis, Irving et al. (2002) evidenced that such proteins are also encoded by prokaryotes (Irving et al., 2002). Despite these findings, bacterial serpins remain poorly studied and data about their origin and functions need to be established.
In this review, we report a concise overview of serpin functions in human and outline the current knowledge on bacterial serpins. Moreover, we provide the first analysis of serpins encoded by human gut microbiota and their impact on host wellbeing.
Survey methodology
In this review, we discussed the current literature related to serpins and their functions in health and disease, with a focus on the human gut microbiota. References mentioned in this review were retrieved from PubMed up to 2019. We used the research terms such as serpin, microbiota, health and diseases. Considered references will provide more information about serpins and their impact on the human health. We excluded the studies related to the serpin engineering and the improvement of their biochemical behaviors. Protein sequences encoding for serpins were isolated from the NCBI public database using the key word “serpin”. Phylogenetic tree was built with PhyloT (https://phylot.biobyte.de/) and ITOL.
Human serpins
Serpins were extensively studied in eukaryotes. Since 2012, the number of genes encoding eukaryotic serpins listed in NCBI has increased from 6,628 to 12,953 (Gaci et al., 2013). Human genome encodes 37 serpins, among them 30 are functional inhibitors (Rau et al., 2007; Lucas et al., 2018; Sanrattana, Maas & De Maat, 2019). They act at various cellular compartments and they are involved in many physiological functions. In fact, these inhibitors are encoded by 10 different chromosomes and belong to the A-I clades (Heit et al., 2013). Most serpins from clade A i.e., extracellular serpins, are encoded by a group of genes located on chromosome 14 and act through the regulation of protease activities involved mainly in: pathogen invasion, injury and inflammation (Olson & Gettins, 2011). While in clade B, serpins (known as ov-serpins) are intracellular and are encoded by genes from chromosomes 6 and 18 (Gettins, 2002; Olson & Gettins, 2011). Given their mechanism of inhibition, serpins were selected to control tightly regulated physiological processes (Huntington, 2011) such as the blood coagulation cascade (Anti-thrombin) (Pickering & Hewitt, 1922; Quinsey et al., 2004; Pike et al., 2005; Hepner & Karlaftis, 2013) and tissue remodeling (Plasminogen Activator Inhibitor-1 and 2) (Diebold et al., 2008). Serpins also play key roles in other processes including the control of the inflammatory response (Anti-trypsin, Anti-chymotrypsin) (Bots & Medema, 2008), programmed cell death and cell development (Bird, 1998; Kryvalap et al., 2018). Moreover, Serpins display functions such as blood pressure regulation (SERPINA8) (Davisson et al., 1997), hormone transport (SERPINA6, SERPINA7) (Zhou et al., 2006; Klieber et al., 2007), tumor suppression (SERPINB5) (Zhang, Magit & Sager, 1997) as well as molecular chaperone functions (SERPINH1) (Silverman et al., 2010) which are not based on protease inhibition.
In agreement with their functions, serpin disequilibrium is associated to several physiopathologies in humans (Table 1). The expression of α1-AT is altered in patients suffering from inflammatory bowel diseases (IBD) (Karbach, Ewe & Bodenstein, 1983; Grill, Hillemeier & Gryboski, 1984). Hence, the administration of this protein attenuated the intestinal inflammation in mice by reducing the cellular infiltration and the secretion of pro-inflammatory cytokines as well as restoring the epithelial barrier and limiting tissue damage (Collins et al., 2013). Moreover, it was described that SERPINE1 was associated to lung inflammation (Table 1). Serpins are also involved in obesity as demonstrated for vaspin (visceral adipose tissue-derived serpin). Clinical data revealed an increase of vaspin level in adipose tissues from obese and type 2 diabetes patients (Cho, Han & Kang, 2010; Klöting et al., 2011; Zhang et al., 2011; Teshigawara et al., 2012). Furthermore, the administration of vaspin to obese mice improved glucose tolerance and insulin sensitivity (Hida et al., 2005). Such beneficial effect was linked to the inhibition of KLK7 (Kallikrein-Related Peptidase 7) which is up-regulated in obesity-induced insulin resistance patients (Hida et al., 2005; Heiker et al., 2013). In addition to that, it was suggested that blocking serpinB13 might prevent the development of type1diabetes (Table 1). Serpins are also believed to be involved in cardiovascular diseases. In fact, Kallistatin, a protease inhibitor widely distributed in tissues relevant to cardiovascular function (Chai et al., 1993; Chao & Chao, 1995; Chao et al., 1996; Wolf et al., 1999), is significantly reduced in coronary artery disease (Chao, Guo & Chao, 2018). This protein displays many properties including anti-atherosclerotic effects and reduction of infarct size (Chao et al., 2006; Gao et al., 2008; Shen et al., 2010). Besides metabolic and inflammatory disorders, many studies reported the clinical relevance of serpins in cancer. In this context, it was reported that Maspin, a non-inhibitory serpin, is significantly associated to breast and prostate cancers (Cao et al., 2007; Vecchi et al., 2008). Increased level of Maspin was detected in different types of cancer and shown to (i) efficiently promote cancer cell apoptosis, (ii) exhibit anti-angiogenesis activity and (iii) inhibit cancer cell migration (Zou et al., 1994; Zhang et al., 1999; Ngamkitidechakul et al., 2001; Song et al., 2002; Cher et al., 2003; Sopel, Kasprzyk & Berdowska, 2005). In contrast, it was recently demonstrated that Maspin cannot be considered as a tumor suppressor but may be a prognostic indicator (Teoh et al., 2014). In addition to Maspin, SERPINE2 and SERPINF1 are associated to many carcinoma types including lung, prostate, pancreatic and papillary thyroid cancers (Halin et al., 2004; Zhang et al., 2006; Stepień et al., 2017). Based on these findings, serpins appear as attractive therapeutic targets to set out new medical strategies against some human pathologies.
Table 1. Biological functions of serpins and their association to human diseases.
| Clade | Serpin | Biological function | Associated disease | Reference |
|---|---|---|---|---|
| A | α1-Antitrypsin (SERPINA1) | Complement activation, apoptosis | Emphysema, Cirrohosis, IBD, Cancer (liver) | Eriksson, Carlson & Velez (1986), Lomas et al. (1992), Yang et al. (2000), Saunders et al. (2012) and Heit et al. (2013) |
| Antichymotrypsin (SERPINA3) | Complement activation, apoptosis, prohormone conversion | Emphysema, Alzheimer’s disease | Law et al. (2006), Kamboh et al. (2006) and Heit et al. (2013) | |
| Kallistatin (SERPINA4) | Complement activation, angiogenesis, fibrinolysis, apoptosis | Coronary artery, Hypertension, Cardiovascular diseases, Chronic liver diseases | Chao et al. (1996), Heit et al. (2013) and Nallagangula et al. (2017) | |
| Coticosteroid -binding globulin (SERPINA6) | Hormone transport | Chronic fatigue | Torpy et al. (2004) | |
| Thyroxine-binding globulin (SERPINA7) | Hormone transport | Hypothyroidism | Refetoff et al. (1996) and Moeller et al. (2015) | |
| Angiotensinogen (SERPINA8) | Blood pressure regulation, hormone transport | Hypertension | Kim et al. (1995) and Yan et al. (2018) | |
| Protein Z-dependent proteinase inhibitor (SERPINA10) | Inhibition of factor Z and XI | Venous thromboembolic disease | Van de Water et al. (2004) and Law et al. (2006) | |
| Vaspin (SERPINA12) | Insulin-sensitizing adipocytokine | Obesity, Insulin resistance, Diabetes | Hida et al. (2005) and Heiker (2014) | |
| B | Plasminogen activator inhibitor-II (SERPINB2) | Fibrinolysis, elastase inhibitor | Cancer | Medcalf & Stasinopoulos (2005), Su et al. (2015) and Harris et al. (2017) |
| Squamous cell carcinoma antigen-I/II (SERPINB3/B4) | Anti-apoptosis | Respiratory and skin inflammatory diseases | Sun, Sheshadri & Zong (2017) and Izuhara et al. (2018) | |
| Maspin (SERPINB5) | Anti-angiogenesis | Cancer (breast, prostate, colon, bladder) | Zou et al. (1994) and Berardi et al. (2013) | |
| Megsin (SERPINB7) | Renal development, Mesangial cell proliferation | IgA nephropathy | Miyata et al. (1998) | |
| C | Antithrombin (SERPINC1) | Coagulation, angiogenesis | Thrombosis, Lung inflammation | Perry & Carrell (1996) and Ishikawa et al. (2017) |
| D | Heparin cofactor II (SERPIND1) | Coagulation | Thrombosis, Cancer (lung) | He et al. (2002) and Liao et al. (2015) |
| E | Plasminogen activator inhibitor I (SERPINE1) | Angiogenesis, fibrinolysis, anti-apoptosis | Bleeding disorders, Cancer, Septic shock, acute lung inflammation | Law et al. (2006), Placencio et al. (2015), Gupta et al. (2016) and Ozolina et al. (2016) |
| F | PEDF (SERPINF1) | Anti-angiogenesis | Cancer (prostate, melanoma) | Becerra & Notario (2013) |
| Alpha-2-antiplasmin (SERPINF2) | Fibrinolysis | Bleeding disorders | Miles et al. (1982) | |
| G | C1 inhibitor (SERPING1) | C1 esterase inhibitor | Angioedema | De Marchi et al. (1973) |
| H | Heat shock protein (SERPINH1) | Chaperone | Osteogenesis imperfecta | Nagata (1996) and Lindert et al. (2015) |
| I | Neuroserpin (PII4) (SERPINI1) | Neutrofic factor | Dementia | Davis et al. (1999) |
Serpins structure
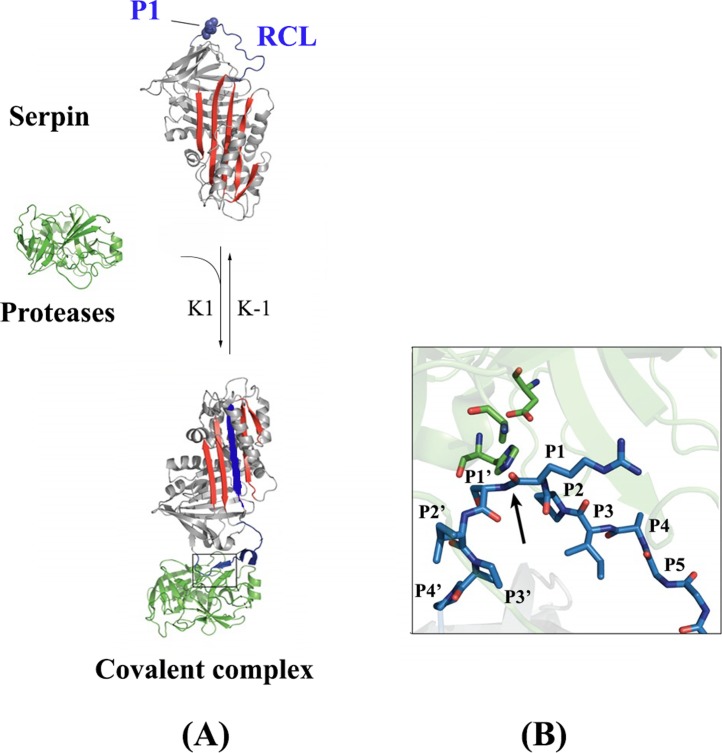
Many structural and biochemical analysis provided a major knowledge progress on serpin family. Serpins display a single domain of 40–60 kDa (PFAM ID PF00079) with an average size of 350–400 amino acids (Stein & Carrell, 1995; Irving et al., 2000; Gettins, 2002). Currently, around 200 three-dimensional structures of serpin and serpin-protease complexes are available in PDB database deriving from both eukaryotes and prokaryotes that display significant structural similarities. Most of these structures (∼90%) belong to eukaryotic species, while only three serpins structure from thermophilic and pathogenic bacteria are solved (Irving et al., 2003; Fulton et al., 2005; Zhang et al., 2007; Goulas et al., 2017). Overall, serpins shared a common fold in spite of their low sequences homology (∼25%) (Huntington, 2011). Serpin architecture is typically composed of 3 β-sheets (A, B and C), 8-9 α-helices (named hA–hI) and a Reactive Center Loop (RCL) (Fig. 1A). The latter is a long and flexible loop (20–25 amino acids linking the β-sheets A and C) that mediates the conformational conversion during the protease docking and inhibition (Gettins, 2002; Law et al., 2006; Huntington, 2011). As a result, RCL plays a critical role in the efficiency and the specificity of serpin inhibition (Huntington, Read & Carrell, 2000; Gettins, 2002). Such mechanism of action was reported for prokaryotic and eukaryotic serpins. Interestingly, serpin family is distinguishable by the fact that the native fold is not the most stable form (Gettins, 2002).
Figure 1. Serpin structure and mechanism of inhibition.
(A) The RCL (Blue) is recognized by a serine protease (green). After cleavage, RCL rapidly inserts into β-sheet and forms a covalent serpin-protease complex. (B) Close-up view of the interaction between the serpin and its target protease (adapted from Song et al., 2011, permission license number 4545950475192).
Mechanism of inhibition
Many studies proved that serpins inhibit their targets by an irreversible substrate-like mechanism (Lawrence et al., 1995; Huntington, 2011; Khan et al., 2011). Upon inhibition, both molecules undergo extreme conformational changes that generate a stable covalent serpin-protease complex (Huntington, Read & Carrell, 2000; Khan et al., 2011). Initially, catalytic serine/cysteine of serine/cysteine peptidases performs a nucleophilic attack on the RCL within the scissile bond P1-P1′. Such hydrolysis reaction generates the cleavage of the peptide bond P1-P1′ and the formation of a covalent acyl-ester linkage between P1 and the catalytic serine (Fig. 1B). Then, the RCL is inserted between the A β-sheets allowing the translocation of the protease on the opposite side of the serpin. Such structural changes strongly distort the protease active site and both proteins are inactivated by this suicide inhibition mechanism (Lawrence et al., 1995; Wilczynska et al., 1995; Huntington, Read & Carrell, 2000) (Fig. 1A). Several studies highlighted serpin structure-function relationships based on mutagenesis and molecular engineering strategies (Seo et al., 2000; Im, Ryu & Yu, 2004). It was demonstrated that the serpin native form is a metastable conformation, which is converted to a more stable state during protease inhibition (Kaslik et al., 1997; Im, Ahn & Yu, 2000). Notably, the inhibition efficiency is modulated by the protein flexibility and mainly the RCL (Huntington et al., 1997; Lee et al., 1998; Zhou, Carrell & Huntington, 2001). Indeed, it was demonstrated that numerous mutations in the RCL increased the protein stability and significantly reduced the inhibition efficiency (Im, Seo & Yu, 1999; Im & Yu, 2000; Seo et al., 2000; Im, Ryu & Yu, 2004; Jung, Na & Im, 2004).
Bacterial serpins
The presence of serpins was believed to be restricted to eukaryotes and virus (Irving et al., 2002; Silverman et al., 2010). Owing to recent advances in sequencing technology and the development of bioinformatic tools, new additional serpins were identified in bacteria, protozoa and fungi. Serpins constitute the most distributed superfamily of protease inhibitors across all major branches of life (Irving et al., 2002; Gettins, 2002; Silverman et al., 2010; Harish & Uppuluri, 2018). Studies on bacterial serpins provided limited data regarding their origin and potential functions. The presence of genes encoding serpins in all life kingdoms suggests that such superfamily firstly appeared in prokaryotes before the divergence of the major domains of life (Irving et al., 2002). The loss of serpin genes by some prokaryotes during evolution can be related to the surrounding environment. However, the sporadic presence of serpins in prokaryotes did not support such hypothesis (Irving et al., 2002; Kantyka, Rawlings & Potempa, 2010). The second hypothesis proposes that serpin-encoding genes appeared first in eukaryotes and were acquired by prokaryotes through horizontal gene transfer (Irving et al., 2002). Such statement is challenged by serpins having a competing microbes and modulating the host immune response including that from gingival crevice (Eckert et al., 2018). Several reports supporting the latter hypothesis were described (Irving et al., 2002; Roberts et al., 2004; Goulas et al., 2017). However, as far as we know no evidence exists to reinforce one hypothesis over another.
Phylogenetic study
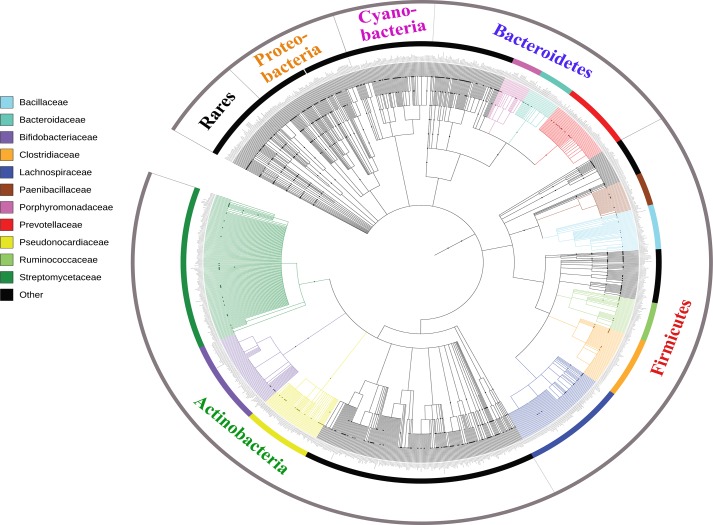
Analysis of serpins available in the public databases (NCBI) demonstrated that these bacterial antiproteases are distributed in different phyla, mainly Actinobacteria, Firmicutes, Bacteroidetes, Cyanobacteria and Proteobacteria (Fig. 2). In order to explore the distribution of these serpins within each phylum, we carried out a phylogenetic study at the family level (Fig. 2). We noted a significant proportion of serpins that were only represented in a small number of species (<50 species) of a given family which we classified as rare.
Figure 2. Bacterial serpins distribution.
Protein sequences and their taxonomic assignation were retrieved from public database NCBI. Taxonomic lineages are represented in different colors. Phylogenetic tree was built with PhyloT (https://phylot.biobyte.de/) and ITOL.
In addition to rare families, we found that serpins from the Actinobacteria phylum were mainly distributed in three families: Streptomycetaceae, Bifidobacteriaceae and Pseudonocardiaceae. In the Bacteroidetes phylum beside rare families, serpins belong to the Prevotellaceae, Bacteroidaceae and Porphyromonadaceae families. In Firmicutes, serpins were found in five families: Lachnospiraceae, Clostridiaceae, Ruminococcaceae, Bacilliaceae and Paenibacillaceae while in Proteobacteria and Cyanobacteria, serpins are only found in rare families (Fig. 2). However, in the other phyla there is less diversity at family level but with more abundant bacteria encoding for serpins. We propose that the high abundance of serpins in a given bacterial family could be linked to the adaptation of these bacterial groups to their environments.
Ecological niches
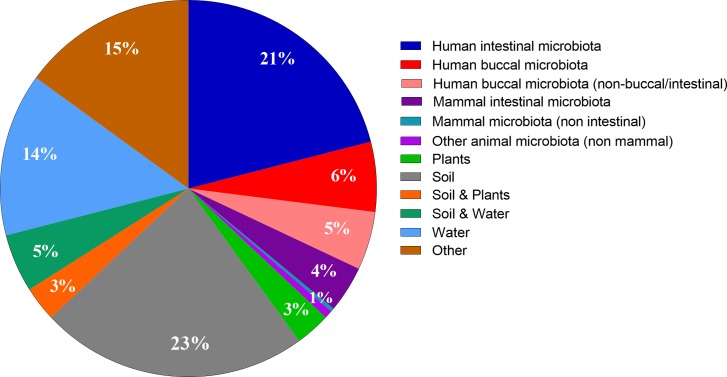
According to current knowledge, the main ecological niches housing bacteria harboring serpins are: human microbiota (32%), soil (23%) and water (14%) (Fig. 3). These results confirm the finding of Kantyka, Rawlings & Potempa (2010) who reported that serpins belong mainly to benign environments (Kantyka, Rawlings & Potempa, 2010).
Figure 3. Distribution of bacterial serpin in ecological niches.
The pie-chart represents the relative percentage of serpins in various ecological niches.
Taking into account the wide distribution of serpins in prokaryotes and the lack of data about their regulation and role, the physiological functions of these protease inhibitors remain elusive. Nevertheless, the variability of the ecological niches of the bacterial species encoding serpins stressed that these inhibitors have evolved to perform key functions.
Thermophilic bacterial serpins
Prokaryotic serpins were initially observed in archaea and some extremophilic bacterial genera (Irving et al., 2002). Sequence analysis of serpins from thermophilic bacteria predicted that these proteins were protease inhibitors (Irving et al., 2002). Indeed, thermopin, a serpin produced by the thermophilic bacterium Thermobifida fusca, was first studied and shown to inhibit chymotrypsin. Such inhibitory function was further confirmed by the formation of a covalent complex with the target protease (Irving et al., 2003). Thermopin was also shown to be stable at 60 °C, at which the α-1-antitrypsin rapidly lost its activity (Irving et al., 2003). Structural analyses revealed that thermopin exhibits a C-terminal extension (amino acid: 363-367) interacting with Glu309 and Arg258 residues in the s5A and s6A β-strands respectively. This takes more importance if we consider that Glu309 and Arg258 residues are highly conserved among serpins and particularly important for the stability of these proteins (Irving et al., 2003).
The serpin from the extremophilic bacterium Thermoanaerobacter tengcondensis was further characterized. This serpin, tengpin, inhibits the human neutrophil elastase and forms a covalent complex typical of inhibitory serpins. Like thermopin, tengpin is distinguishable by a structural feature allowing to operate at extreme temperatures (Zhang et al., 2007). In fact, mutagenesis and X-ray studies demonstrated that this serpin displays an N-terminal extension that is essential to stabilize the native metastable status of tengpin (Zhang et al., 2007).
To better investigate the role of serpins in bacteria, three additional serpins were also characterized from the thermophilic bacterium Clostridium thermocellum (Kang et al., 2006). This strain has a high ability to degrade cellulose using a multi-enzyme complex, the cellulosome, and exhibits three distinct serpins. Clotm-serpin 1 and Clotm-serpin 2 were predicted as cellulosomal proteins while Clotm-3 is a membrane protein. Biochemical characterization revealed that Clotm-serpin 1 inhibits the bacterial subtilisin. As C. thermocellum displays a subtilisin-encoding gene, it was suggested that its serpins are specific inhibitors of bacterial proteases, including its own subtilisin-like protease (Kang et al., 2006). Taking into account these data, bacterial serpins were proposed to protect the cellulosome structure through the regulation of endogenous and exogenous proteases (Kang et al., 2006; Cuív et al., 2013).
Serpins from the human microbiota
To date, only few serpins from the human microbiota were studied (Ivanov et al., 2006; Ksiazek et al., 2015; Mkaouar et al., 2016; Goulas et al., 2017). A novel serpin from Tanerella forsythia, miropin, was characterized and shown to display a broad range of inhibition including serine and cysteine proteases such as neutrophil elastase, cathepsin G, trypsin, and papain (Ksiazek et al., 2015; Goulas et al., 2017). Besides host proteases, miropin inhibits bacterial protease like gingipain and subtilisin (Ksiazek et al., 2015; Goulas et al., 2017). Therefore, it was suggested to act as a virulence factor protecting the bacterium from host and endogenous proteases (Ksiazek et al., 2015). Three serpins from the human gut microbiota were also studied. In fact, the Bifidobacteria genome sequencing revealed the presence of a serpin-encoding gene (Schell et al., 2002; Turroni et al., 2010). Based on transcriptomic studies using Bifidobacterium strain, Turroni et al. (2010) reported the up-regulation of various genes including serpin in presence of proteases (Turroni et al., 2010). Recently, a serpin from B. longum has been characterized and reported to inhibit the human neutrophil elastase (Ivanov et al., 2006). A stable covalent complex serpin-protease was further observed when incubating purified serpin with fecal proteases from mice (Ivanov et al., 2006). This serpin was recently reported to prevent enteric neurons activation by supernatants from irritable bowel syndrome patients (Buhner et al., 2018). Such data stressed the potential key role of bacterial serpins to improve gastrointestinal symptoms. Lately, we reported the biochemical characterization of two putative serpins from the human gut bacterium Eubacterium sireaum and supposed to be secreted in the intestinal lumen (Mkaouar et al., 2016). The analysis of these novel bacterial serpins, called Siropins, revealed that they efficiently inhibit the human neutrophil elastase and proteinase 3. Interestingly, Siropins are the first bacterial serpins that significantly inhibit the human proteinase 3, known to be involved in IBD. Kinetic studies demonstrated that Siropins were highly efficient in comparison to other bacterial serpins including that of B. longum. Furthermore, siropins exhibit a high efficiency to inhibit fecal proteases issued from mice with chemically induced colitis (Mkaouar et al., 2016). This highlights the importance of serpins from the human gut microbiota to inhibit proteases related with human physiopathologies.
Conclusions
In this review, we analyzed human serpins and their functions to maintain homeostasis as well as their involvement in several diseases. Such data stressed the key role of human antiproteases and highlighted their potential to establish innovative therapeutic strategies. In contrast, bacterial serpins remain today poorly studied. The emergence of metagenomics allowed the identification of new bacterial serpins. Phylogenetic study of this protein family demonstrated that bacterial serpins essentially belong to five phyla colonizing benign environments. The distribution of the serpins in ecological niches showed that the human gastrointestinal tract harbors an elevated number of serpins. The relevance of these bacterial proteins was reinforced through (i) the determination of their efficiency to inhibit fecal proteases recovered from mice with chemically induced inflammation and (ii) the inhibition of human proteases involved in IBD. Above all, it will be interesting to characterize more microbial serpins and to further explore their therapeutic potential. Resolution of the structure of serpin-protease complexes will bring useful structural insights to investigate the serpins structure-function relationships that will allow the improvement of their efficiency and specificity through engineering approaches. Such analysis will promote the use of bacterial serpin mainly in biomedical applications including the set out of new therapeutic alternatives against protease-related diseases.
Acknowledgments
The authors would like to express their gratitude to N. Pons for his helpful discussion concerning the analysis of serpin sequences. Our acknowledgments are also addressed to M. Bourgin for proofreading this manuscript.
Funding Statement
This work received funding from the Microbiology and the Food Chain division (MICA) of the INRA institute through the metaprogramme MEM - Meta-omics and microbial ecosystems, two ANR projects SerpinGuTarget and Titan and the CMCU-PHC Utique (n°19G0819) - Campus France (41786NC). This work was supported by the SerpinGuTarget (Contract number ANR-14-CE16-0018) and Titan (Contract number ANR-18-CE18-0019-03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Héla Mkaouar conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Nizar Akermi conceived and designed the experiments, performed the experiments.
Aicha Kriaa and Amin Jablaoui performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables.
Anne-Laure Abraham performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables.
Souha Soussou analyzed the data, prepared figures and/or tables.
Raja Mokdad-Gargouri and Emmanuelle Maguin contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Moez Rhimi conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review and does not have raw data.
References
- Al-Horani (2014).Al-Horani RA. Serpin regulation of fibrinolytic system: implications for therapeutic applications in cardiovascular diseases. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2014;12:91–125. doi: 10.2174/1871525712666141106095927. [DOI] [PubMed] [Google Scholar]
- Bao et al. (2018).Bao J, Pan G, Poncz M, Wei J, Ran M, Zhou Z. Serpin functions in host-pathogen interactions. PeerJ. 2018;6:e4557. doi: 10.7717/peerj.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra & Notario (2013).Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nature Reviews Cancer. 2013;13:258–271. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi et al. (2013).Berardi R, Morgese F, Onofri A, Mazzanti P, Pistelli M, Ballatore Z, Savini A, De Lisa M, Caramanti M, Rinaldi S, Pagliaretta S, Santoni M, Pierantoni C, Cascinu S. Role of maspin in cancer. Clinical and Translational Medicine. 2013;2(1):8. doi: 10.1186/2001-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird (1998).Bird PI. Serpins and regulation of cell death. Results and Problems in Cell Differentiation. 1998;24:63–89. doi: 10.1007/978-3-540-69185-3_4. [DOI] [PubMed] [Google Scholar]
- Bots & Medema (2008).Bots M, Medema JP. Serpins in T cell immunity. Journal of Leukocyte Biology. 2008;84:1238–1247. doi: 10.1189/jlb.0208140. [DOI] [PubMed] [Google Scholar]
- Buhner et al. (2018).Buhner S, Hahne H, Hartwig K, Li Q, Vignali S, Ostertag D, Meng C, Hörmannsperger G, Braak B, Pehl C, Frieling T, Barbara G, De Giorgio R, Demir IE, Ceyhan GO, Zeller F, Boeckxstaens G, Haller D, Kuster B, Schemann M. Protease signaling through protease activated receptor 1 mediate nerve activation by mucosal supernatants from irritable bowel syndrome but not from ulcerative colitis patients. PLOS ONE. 2018;13(3):e0193943. doi: 10.1371/journal.pone.0193943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2007).Cao D, Zhang Q, Wu LS, Salaria SN, Winter JW, Hruban RH, Goggins MS, Abbruzzese JL, Maitra A, Ho L. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: tissue microarray analysis of 223 surgically resected cases. Modern Pathology. 2007;20:570–578. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- Carrell & Read (2017).Carrell RW, Read RJ. How serpins transport hormones and regulate their release. Seminars in Cell & Developmental Biology. 2017;62:133–141. doi: 10.10,16/j.semcdb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Carrell & Travis (1985).Carrell R, Travis J. α1-Antitrypsin and the serpins: variation and countervariation. Trends in Biochemical Sciences. 1985;10:20–24. doi: 10.1016/0968-0004(85)90011-8. [DOI] [Google Scholar]
- Chai et al. (1993).Chai KX, Chen LM, Chao J, Chao L. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution and expression in Escherichia coli. The Journal of Biological Chemistry. 1993;268:24498–24505. [PubMed] [Google Scholar]
- Chao & Chao (1995).Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. The Journal of Biological Chemistry. 1995;376:705–713. [PubMed] [Google Scholar]
- Chao, Guo & Chao (2018).Chao J, Guo Y, Chao L. Protective role of endogenous kallistatin in vascular injury and senescence by inhibiting oxidative stress and inflammation. Oxidative Medicine and Cellular Longevity. 2018;2018:1–8. doi: 10.1155/2018/4138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao et al. (1996).Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. The Journal of Laboratory and Clinical Medicine. 1996;127:612–620. doi: 10.1016/s0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- Chao et al. (2006).Chao J, Yin H, Yao YY, Shen B, Smith RS, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Human Gene Therapy. 2006;17:1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- Cher et al. (2003).Cher ML, Biliran Jr HR, Bhagat S, Meng Y, Che M, Lockett J, Abrams J, Fridman R, Zachareas M, Sheng S. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7847–7852. doi: 10.1073/pnas.1331360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Han & Kang (2010).Cho JK, Han TK, Kang HS. Combined effects of body mass index and cardio/respiratory fitness on serum vaspin concentrations in Korean young men. European Journal of Applied Physiology. 2010;108:347–353. doi: 10.1007/s00421-009-1238-8. [DOI] [PubMed] [Google Scholar]
- Clarke et al. (1991).Clarke EP, Cates GA, Ball EH, Sanwal BD. A collagen-binding protein in the endoplasmic reticulum of myoblasts exhibits relationship with serine protease inhibitors. Journal of Biological Chemistry. 1991;266:17230–17235. [PubMed] [Google Scholar]
- Collins et al. (2013).Collins CB, Aherne CM, Ehrentraut SF, Gerich ME, McNamee EN, McManus MC, Lebsack MD, Jedlicka P, Azam T, De Zoeten EF, Dinarello CA, Rivera-Nieves J. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflammatory Bowel Diseases. 2013;19:1964–1973. doi: 10.1097/MIB.0b013e31829292aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuív et al. (2013).Cuív PÓ, Gupta R, Goswami HP, Morrison M. Extending the cellulosome paradigm: the modular clostridium thermocellum cellulosomal serpin pinA is a broad-spectrum inhibitor of subtilisin-like proteases. Applied and Environmental Microbiology. 2013;79:6173–6175. doi: 10.1128/AEM.01912-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al. (1999).Davis RL, Holohan PD, Shrimpton AE, Tatum AH, Daucher J, Collins GH, Todd R, Bradshaw C, Kent P, Feiglin D, Rosenbaum A, Yerby MS, Shaw CM, Lacbawan F, Lawrence DA. Familial encephalopathy with neuroserpin inclusion bodies. American Journal of Pathology. 1999;155:1901–1913. doi: 10.1016/S0002-9440(10)65510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davisson et al. (1997).Davisson RL, Kim HS, Krege JH, Lager DJ, Smithies O, Sigmund CD. Complementation of reduced survival, hypotension, and renal abnormalities in angiotensinogen-deficient mice by the human renin and human angiotensinogen genes. The Journal of Clinical Investigation. 1997;99:1258–1264. doi: 10.1172/JCI119283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi et al. (1973).De Marchi M, Jacot-Guillarmod H, Ressa TG, Carbonara AO. Hereditary angioedema: report of a large kindred with a rare genetic variant of C1-esterase inhibitor. Clinical Genetics. 1973;4:229–236. doi: 10.1111/j.1399-0004.1973.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Diebold et al. (2008).Diebold I, Kraicun D, Bonello S, Görlach A. The “PAI-1 paradox” in vascular remodeling. Journal of Thrombosis and Haemostasis. 2008;100:984–991. [PubMed] [Google Scholar]
- Eckert et al. (2018).Eckert M, Mizgalska D, Sculean A, Potempa J, Stavropoulos A, Eick S. In vivo expression of proteases and protease inhibitor, a serpin, by periodontal pathogens at teeth and implants. Molecular Oral Microbiology. 2018;33:240–248. doi: 10.1111/omi.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, Carlson & Velez (1986).Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. The New England Journal of Medicine. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- Fulton et al. (2005).Fulton KF, Buckle AM, Cabrita LD, Irving JA, Butcher RE, Smith I, Reeve S, Lesk AM, Bottomley SP, Rossjohn J, Whisstock JC. The high resolution crystal structure of a native thermostable serpin reveals the complex mechanism underpinning the stressed to relaxed transition. The Journal of Biological Chemistry. 2005;280:8435–8442. doi: 10.1074/jbc.M410206200. [DOI] [PubMed] [Google Scholar]
- Gaci et al. (2013).Gaci N, Dobrijevic D, Boudebbouze S, Moumen B, Maguin E, Rhimi M. Patented biotechnological applications of serpin: an update. Recent Patents on DNA & Gene Sequence. 2013;7:137–143. doi: 10.2174/1872215611307020008. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2008).Gao L, Yin H, Smith R, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Laboratory Investigation. 2008;88:1157–1166. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- Gettins (2002).Gettins PG. Serpin structure, mechanism, and function. Chemical Reviews. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Goulas et al. (2017).Goulas T, Ksiazek M, Garcia-Ferrer I, Sochaj-Gregorczyk AM, Waligorska I, Wasylewski M, Potempa J, Gomis-Rüth FX. A structure-derived snap-trap mechanism of a multispecific serpin from the dysbiotic human oral microbiome. The Journal of Biological Chemistry. 2017;292:10883–10898. doi: 10.1074/jbc.M117.786533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, Hillemeier & Gryboski (1984).Grill BB, Hillemeier AC, Gryboski JD. Fecal alpha 1-antitrypsin clearance in patients with inflammatory bowel disease. The Journal of Pediatric Gastroenterology and Nutrition. 1984;3:56–61. doi: 10.1097/00005176-198401000-00013. [DOI] [PubMed] [Google Scholar]
- Gupta et al. (2016).Gupta KK, Xu Z, Castellino FJ, Ploplis VA. Plasminogen activator inhibitor-1 stimulates macrophage activation through Toll-like Receptor-4. Biochemical and Biophysical Research Communications. 2016;477:503–508. doi: 10.1016/j.bbrc.2016.06.065. [DOI] [PubMed] [Google Scholar]
- Halin et al. (2004).Halin S, Wikström P, Rudolfsson SH, Stattin P, Doll JA, Crawford SE, Bergh A. Decreased pigment epithelium-derived factor is associated with metastatic phenotype in human and rat prostate tumors. Cancer Research. 2004;64:5664–5671. doi: 10.1158/0008-5472.CAN-04-0835. [DOI] [PubMed] [Google Scholar]
- Hammond et al. (1987).Hammond GL, Smith CL, Goping IS, Underhill DA, Harley MJ, Reventos J, Musto NA, Gunsalus GL, Bardin CW. Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:5153–5157. doi: 10.1073/pnas.84.15.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish & Uppuluri (2018).Harish BS, Uppuluri KB. Microbial serine protease inhibitors and their therapeutic applications. International Journal of Biological Macromolecules. 2018;107:1373–1387. doi: 10.1016/j.ijbiomac.2017.09.115. [DOI] [PubMed] [Google Scholar]
- Harris et al. (2017).Harris NLE, Vennin C, Conway JRW, Vine KL, Pinese M, Cowley MJ, Shearer RF, Lucas MC, Herrmann D, Allam AH, Pajic M, Morton JP, Australian Pancreatic Cancer Genome Initiative. Biankin AV, Ranson M, Timpson P, Saunders DN. SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene. 2017;36:4288–4298. doi: 10.1038/onc.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2002).He L, Vicente CP, Westrick RJ, Eitzman DT, Tollefsen DM. Heparin cofactor II inhibits arterial thrombosis after endothelial injury. The Journal of Clinical Investigation. 2002;109:213–219. doi: 10.1172/JCI13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiker (2014).Heiker JT. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. Journal of Peptide Science. 2014;20:299–306. doi: 10.1002/psc.2621. [DOI] [PubMed] [Google Scholar]
- Heiker et al. (2013).Heiker JT, Klöting N, Kovacs P, Kuettner EB, Sträter N, Schultz S, Kern M, Stumvoll M, Blüher M, Beck-Sickinger AG. Vaspin inhibits kallikrein 7 by serpin mechanism. Cellular and Molecular Life Sciences. 2013;70:2569–2583. doi: 10.1007/s00018-013-1258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit et al. (2013).Heit C, Jackson BC, McAndrews M, Wright MW, Thompson DC, Silverman GA, Nebert DW, Vasiliou V. Update of the human and mouse SERPIN gene superfamily. Human Genomics. 2013;7:1–14. doi: 10.1186/1479-7364-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner & Karlaftis (2013).Hepner M, Karlaftis V. Antithrombin. Methods in Molecular Biology. 2013;992:355–364. doi: 10.1007/978-1-62703-339-8_28. [DOI] [PubMed] [Google Scholar]
- Hida et al. (2005).Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho et al. (1994).Ho GJ, Smirnova IV, Akaaboune M, Hanta D, Festoff BW. Serine proteases and their serpin inhibitors in Alzheimer’s disease. Biomedicine & Pharmacotherapy. 1994;48:296–304. doi: 10.1016/0753-3322(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Hunt & Dayhoff (1980).Hunt LT, Dayhoff MO. A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochemical and Biophysical Research Communications. 1980;95:864–871. doi: 10.1016/0006-291x(80)90867-0. [DOI] [PubMed] [Google Scholar]
- Huntington (2011).Huntington JA. Serpin structure, function and dysfunction. Journal of Thrombosis and Haemostasis. 2011;9:26–34. doi: 10.1111/j.1538-7836.2011.04360.x. [DOI] [PubMed] [Google Scholar]
- Huntington et al. (1997).Huntington JA, Fan B, Karlsson KE, Deinum J, Lawrence DA, Gettins PGW. Serpin conformational change in ovalbumin. Enhanced reactive center loop insertion through hinge region mutation. Biochemistry. 1997;6:5432–5440. doi: 10.1021/bi9702142. [DOI] [PubMed] [Google Scholar]
- Huntington, Read & Carrell (2000).Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- Im, Ahn & Yu (2000).Im H, Ahn HY, Yu MH. Bypassing the kinetic trap of serpin protein folding by loop extension. Protein Science: a Publication of the Protein Society. 2000;9:1497–1502. doi: 10.1110/ps.9.8.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, Ryu & Yu (2004).Im H, Ryu MJ, Yu MH. Engineering thermostability in serine protease inhibitors. Protein Engineering, Design & Selection. 2004;17:325–331. doi: 10.1093/protein/gzh036. [DOI] [PubMed] [Google Scholar]
- Im, Seo & Yu (1999).Im H, Seo EJ, Yu MH. Metastability in the inhibitory mechanism of human α1-antitrypsin. The Journal of Biological Chemistry. 1999;274:11072–11077. doi: 10.1074/jbc.274.16.11072. [DOI] [PubMed] [Google Scholar]
- Im & Yu (2000).Im H, Yu MH. Role of Lys335 in the metastability and function of inhibitory serpins. Protein Science: a Publication of The Protein Society. 2000;9:934–941. doi: 10.1110/ps.9.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving et al. (2003).Irving JA, Cabrita LD, Rossjohn J, Pike RN, Bottomley SP, Whisstock JC. The 1.5 Å crystal structure of a prokaryote serpin: controlling conformational change in a heated environment. Structure. 2003;11:387–397. doi: 10.1016/s0969-2126(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Irving et al. (2000).Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Research. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- Irving et al. (2002).Irving JA, Steenbakkers PJM, Lesk AM, Op den Camp HJM, Pike RN, Whisstock JC. Serpins in prokaryotes. Molecular Biology and Evolution. 2002;19:1881–1890. doi: 10.1093/oxfordjournals.molbev.a004012. [DOI] [PubMed] [Google Scholar]
- Ishikawa et al. (2017).Ishikawa M, Yamashita H, Oka N, Ueda T, Kohama K, Nakao A, Kotani J. Antithrombin III improved neutrophil extracellular traps in lung after the onset of endotoxemia. The Journal of Surgical Research. 2017;208:140–150. doi: 10.1016/j.jss.2016.09.041. [DOI] [PubMed] [Google Scholar]
- Ivanov et al. (2006).Ivanov D, Emonet C, Foata F, Affolter M, Delley M, Fisseha M, Blum-Sperisen S, Kochhar S, Arigoni F. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. The Journal of Biological Chemistry. 2006;281:17246–17252. doi: 10.1074/jbc.M601678200. [DOI] [PubMed] [Google Scholar]
- Izuhara et al. (2018).Izuhara K, Yamaguchi Y, Ohta S, Nunomura S, Nanri Y, Azuma Y, Nomura N, Noguchi Y, Aihara M. Squamous cell carcinoma antigen 2 (SCCA2, SERPINB4): an emerging biomarker for skin inflammatory diseases. International Journal of Molecular Sciences. 2018;19(4):E1102. doi: 10.3390/ijms19041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, Na & Im (2004).Jung CH, Na YR, Im H. Retarded protein folding of deficient human α1-antitrypsin D256V and L41P variants. Protein Science: a Publication of The Protein Society. 2004;13:694–702. doi: 10.1110/ps.03356604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh et al. (2006).Kamboh MI, Minster RL, Kenney M, Ozturk A, Desai PP, Kammerer CM, DeKosky ST. Alpha-1-antichymotrypsin (ACT or SERPINA3) polymorphism may affect age-at-onset and disease duration of Alzheimer’s disease. Neurobiology of Aging. 2006;27:1435–1439. doi: 10.1016/j.neurobiolaging.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2006).Kang S, Barak Y, Lamed R, Bayer EA, Morrison M. The functional repertoire of prokaryote cellulosomes includes the serpin superfamily of serine proteinase inhibitors. Molecular Microbiology. 2006;60:1344–1354. doi: 10.1111/j.1365-2958.2006.05182.x. [DOI] [PubMed] [Google Scholar]
- Kantyka, Rawlings & Potempa (2010).Kantyka T, Rawlings ND, Potempa J. Prokaryote-derived protein inhibitors of peptidases: a sketchy occurrence and mostly unknown function. Biochimie. 2010;92:1644–1656. doi: 10.1016/j.biochi.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach, Ewe & Bodenstein (1983).Karbach U, Ewe K, Bodenstein H. Alpha 1-antitrypsin, a reliable endogenous marker for intestinal protein loss and its application in patients with Crohn’s disease. Gut. 1983;24:718–723. doi: 10.1136/gut.24.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslik et al. (1997).Kaslik G, Kardos J, Szabó E, Szilágyi L, Závodszky P, Westler WM, Markley JL, Gráf L. Effects of serpin binding on the target proteinase: global stabilization localized increased structural flexibility, and conserved hydrogen bonding at the active site. Biochemistry. 1997;36:5455–5464. doi: 10.1021/bi962931m. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2011).Khan MS, Singh P, Azhar A, Naseem A, Rashid Q, Kabir MA, Jairajpuri MA. Serpin inhibition mechanism: a delicate balance between native metastable state and polymerization. Journal of Amino Acids. 2011;2011:1–10. doi: 10.4061/2011/606797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (1995).Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieber et al. (2007).Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. The Journal of Biological Chemistry. 2007;282:29594–29603. doi: 10.1074/jbc.M705014200. [DOI] [PubMed] [Google Scholar]
- Klöting et al. (2011).Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 2011;54:1819–1823. doi: 10.1007/s00125-011-2137-1. [DOI] [PubMed] [Google Scholar]
- Kryvalap et al. (2018).Kryvalap Y, Jiang ML, Kryvalap N, Mueller KA, Czyzyk J. Serpin B13 plays a role in beta-cell development and progression to insulin-dependent diabetes . Abstract 51Diabetes. 2018;67(Supplement 1) [Google Scholar]
- Ksiazek et al. (2015).Ksiazek M, Mizgalska D, Enghild JJ, Scavenius C, Thogersen IB, Potempa J. Miropin, a novel bacterial serpin from the periodontopathogen Tannerella forsythia, inhibits a broad range of proteases by using different peptide bonds within the reactive center loop. The Journal of Biological Chemistry. 2015;290:658–670. doi: 10.1074/jbc.M114.601716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law et al. (2006).Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome Biology. 2006;7(5):216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence et al. (1995).Lawrence DA, Ginsburg D, Day DE, Berkenpas MB, Verhamme IM, Kvassman JO, Shore JD. Serpin-protease complexes are trapped as stable acyl-enzyme intermediates. The Journal of Biological Chemistry. 1995;270:25309–25312. doi: 10.1074/jbc.270.43.25309. [DOI] [PubMed] [Google Scholar]
- Lee et al. (1998).Lee KN, Im H, Kang SW, Yu MH. Characterization of a human alpha1-antitrypsin variant that is as stable as ovalbumin. The Journal of Biological Chemistry. 1998;273:2509–2516. doi: 10.1074/jbc.273.5.2509. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2015).Liao WY, Ho CC, Hou HH, Hsu TH, Tsai MF, Chen KY, Chen HY, Lee YC, Yu CJ, Lee CH, Yang PC. Heparin co-factor II enhances cell motility and promotes metastasis in non-small cell lung cancer. The Journal of Pathology. 2015;235:50–64. doi: 10.1002/path.4421. [DOI] [PubMed] [Google Scholar]
- Lindert et al. (2015).Lindert U, Weis MA, Rai J, Seeliger F, Hausser I, Leeb T, Eyre D, Rohrbach M, Giunta C. Molecular consequences of the SERPINH1/HSP47 mutation in the dachshund natural model of osteogenesis imperfecta. The Journal of Biological Chemistry. 2015;290:17679–17689. doi: 10.1074/jbc.M115.661025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas et al. (1992).Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- Lucas et al. (2018).Lucas A, Yaron JR, Zhang L, Ambadapadi S. Overview of serpins and their roles in biological systems. Methods in Molecular Biology. 2018;1826:1–7. doi: 10.1007/978-1-4939-8645-3_1. [DOI] [PubMed] [Google Scholar]
- Medcalf & Stasinopoulos (2005).Medcalf RL, Stasinopoulos SJ. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. The FEBS Journal. 2005;272:4858–4867. doi: 10.1111/j.1742-4658.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- Miles et al. (1982).Miles LA, Plow EF, Donnelly KJ, Hougie C, Griffin JH. A bleeding disorder due to deficiency of alpha 2-antiplasmin. Blood. 1982;59:1246–1251. [PubMed] [Google Scholar]
- Miyata et al. (1998).Miyata T, Nangaku M, Suzuki D, Inagi R, Uragami K, Sakai H, Kurokawa K. A mesangium-predominant gene, megsin, is a new serpin upregulated in IgA nephropathy. The Journal of Clinical Investigation. 1998;102:828–836. doi: 10.1172/JCI2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkaouar et al. (2016).Mkaouar H, Akermi N, Mariaule V, Boudebbouze S, Gaci N, Szukala F, Pons N, Marquez J, Gargouri A, Maguin E, Rhimi M. Siropins, novel serine protease inhibitors from gut microbiota acting on human proteases involved in inflammatory bowel diseases. Microbial Cell Factories. 2016;15(1) doi: 10.1186/s12934-016-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller et al. (2015).Moeller LC, Appiagyei-Dankah Y, Köhler B, Biebermann H, Janssen OE, Führer D. Two novel mutations in the Serpina7 gene are associated with complete deficiency of thyroxine-binding globulin. European Thyroid Journal. 2015;4:108–112. doi: 10.1159/000381093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata (1996).Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends in Biochemical Sciences. 1996;21:22–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- Nallagangula et al. (2017).Nallagangula KS, Shashidhar KN, Lakshmaiah V, Muninarayana C. Cirrhosis of liver: interference of serpins in quantification of SERPINA4—a preliminary study. Practical Laboratory Medicine. 2017;9:53–57. doi: 10.1016/j.plabm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamkitidechakul et al. (2001).Ngamkitidechakul C, Burke JM, O’Brien WJ, Twining SS. Maspin: synthesis by human cornea and regulation of in vitro stromal cell adhesion to extracellular matrix. Investigative Ophthalmology & Visual Science. 2001;42:3135–3141. [PubMed] [Google Scholar]
- Olson & Gettins (2011).Olson ST, Gettins PGW. Regulation of proteases by protein inhibitors of the serpin superfamily. Progress in Molecular Biology and Translational Science. 2011;99:185–240. doi: 10.1016/B978-0-12-385504-6.00005-1. [DOI] [PubMed] [Google Scholar]
- Ozolina et al. (2016).Ozolina A, Sarkele M, Sabelnikovs O, Skesters A, Jaunalksne I, Serova J, Ievins T, Bjertnaes LJ, Vanags I. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Frontiers in Medicine. 2016;3:64. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry & Carrell (1996).Perry DJ, Carrell RW. Molecular genetics of human antithrombin deficiency. Human Mutation. 1996;7:7–22. doi: 10.1002/(SICI)1098-1004(1996)7:1<7::AID-HUMU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Pickering & Hewitt (1922).Pickering JW, Hewitt JA. Studies of the coagulation of the blood: part II. Thrombin and antithrombins. Biochemical Journal. 1922;16:587–598. doi: 10.1042/bj0160587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike et al. (2005).Pike RN, Buckle AM, Le Bonniec BF, Church FC. Control of the coagulation system by serpins. Getting by with a little help from glycosaminoglycans. The Federation of European Biochemical Societies. 2005;272:4842–4851. doi: 10.1111/j.1742-4658.2005.04880.x. [DOI] [PubMed] [Google Scholar]
- Placencio et al. (2015).Placencio VR, Ichimura A, Miyata T, DeClerck YA. Small molecule inhibitors of plasminogen activator inhibitor-1 elicit anti-tumorigenic and anti-angiogenic activity. PLOS ONE. 2015;10(7):e0133786. doi: 10.1371/journal.pone.0133786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinsey et al. (2004).Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike RN. Antithrombin: in control of coagulation. The International Journal of Biochemistry & Cell Biology. 2004;36:386–389. doi: 10.1016/s1357-2725(03)00244-9. [DOI] [PubMed] [Google Scholar]
- Rau et al. (2007).Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. Journal of Thrombosis and Haemostasis. 2007;5:102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff et al. (1996).Refetoff S, Murata Y, Mori Y, Janssen OE, Takeda K, Hayashi Y. Thyroxine-binding globulin: organization of the gene and variants. Hormone Research. 1996;45:128–138. doi: 10.1159/000184775. [DOI] [PubMed] [Google Scholar]
- Richardson, Viswanathan & Lucas (2006).Richardson J, Viswanathan K, Lucas A. Serpins, the vasculature, and viral therapeutics. Frontiers in Bioscience. 2006;11:1042–1056. doi: 10.2741/1862. [DOI] [PubMed] [Google Scholar]
- Roberts et al. (2004).Roberts TH, Hejgaard J, Saunders NFW, Cavicchioli R, Curmi PMG. Serpins in unicellular Eukarya, Archaea, and Bacteria: sequence analysis and evolution. Journal of Molecular Evolution. 2004;59:437–447. doi: 10.1007/s00239-004-2635-6. [DOI] [PubMed] [Google Scholar]
- Sanrattana, Maas & De Maat (2019).Sanrattana W, Maas C, De Maat S. SERPINs-from trap to treatment. Frontiers in Medicine. 2019;6:25. doi: 10.3389/fmed.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders et al. (2012).Saunders DN, Tindall EA, Shearer RF, Roberson J, Decker A, Wilson JA, Hayes VM. A novel SERPINA1 mutation causing serum alpha(1)-antitrypsin deficiency. PLOS ONE. 2012;7(12):e51762. doi: 10.1371/journal.pone.0051762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell et al. (2002).Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick et al. (1998).Schick C, Pemberton PA, Shi GP, Kamachi Y, Cataltepe S, Bartuski AJ, Gornstein ER, Brömme D, Chapman HA, Silverman GA. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry. 1998;37:5258–5266. doi: 10.1021/bi972521d. [DOI] [PubMed] [Google Scholar]
- Seo et al. (2000).Seo EJ, Im H, Maeng JS, Kim KE, Yu MH. Distribution of the native strain in human alpha 1-antitrypsin and its association with protease inhibitor function. The Journal of Biological Chemistry. 2000;275:16904–16909. doi: 10.1074/jbc.M001006200. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2010).Shen B, Gao L, Hsu YT, Bledsoe G, Hagiwara M, Chao L, Chao J. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. American Journal of Physiology Heart and Circulatory. Physiology. 2010;299:1419–1427. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman et al. (2010).Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird P. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. The Journal of Biological Chemistry. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2011).Song J, Matthews AY, Reboul CF, Kaiserman D, Pike RN, Bird PI, Whisstock JC. Predicting serpin/protease interactions. Methods in Enzymology. 2011;501:237–273. doi: 10.1016/B978-0-12-385950-1.00012-2. [DOI] [PubMed] [Google Scholar]
- Song et al. (2002).Song SY, Lee SK, Kim DH, Son HJ, Kim HJ, Lim YJ, Lee WY, Chun HK, Rhee JC. Expression of maspin in colon cancers: its relationship with p53 expression and microvessel density. Digestive Diseases and Sciences. 2002;47:1831–1835. doi: 10.1023/a:1016409031562. [DOI] [PubMed] [Google Scholar]
- Sopel, Kasprzyk & Berdowska (2005).Sopel M, Kasprzyk I, Berdowska I. Maspin and c-erbB-2 expression in correlation with microvessel density in invasive ductal breast cancer. Folia histochemica et cytobiologica. 2005;43:109–116. [PubMed] [Google Scholar]
- Stein & Carrell (1995).Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nature Structural & Molecular Biology. 1995;2:96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- Stepień et al. (2017).Stepień T, Brozyna M, Kuzdak K, Motylewska E, Komorowski J, Stepień H, Ławnicka H. Elevated concentrations of SERPINE2/Protease Nexin-1 and secretory leukocyte protease inhibitor in the serum of patients with papillary thyroid cancer. Disease Markers. 2017;2017:1–5. doi: 10.1155/2017/4962137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su et al. (2015).Su CY, Liu YP, Yang CJ, Lin YF, Chiou J, Chi LH, Lee JJ, Wu AT, Lu PJ, Huang MS, Hsiao M. Plasminogen activator inhibitor-2 plays a leading prognostic role among protease families in non-small cell lung cancer. PLOS ONE. 2015;10(7):e0133411. doi: 10.1371/journal.pone.0133411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Sheshadri & Zong (2017).Sun Y, Sheshadri N, Zong WX. SERPINB3 and B4: From biochemistry to biology. Seminars in Cell & Developmental Biology. 2017;62:170–177. doi: 10.1016/j.semcdb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh et al. (2014).Teoh SS, Vieusseux J, Prakash M, Berkowicz S, Luu J, Bird CH, Law RH, Rosado C, Price JT, Whisstock JC, Bird PI. Maspin is not required for embryonic development or tumour suppression. Nature Communications. 2014;5:1–9. doi: 10.1038/ncomms4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara et al. (2012).Teshigawara S, Wada J, Hida K, Nakatsuka A, Eguchi J, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Iseda I, Matsushita Y, Miyatake N, McDonald JF, Hotta K, Makino H. Serum vaspin concentrations are closely related to insulin resistance, and rs77060950 at SERPINA12 genetically defines distinct group with higher serum levels in Japanese population. The Journal of Clinical Endocrinology & Metabolism. 2012;97:1202–1207. doi: 10.1210/jc.2011-3297. [DOI] [PubMed] [Google Scholar]
- Torpy et al. (2004).Torpy DJ, Bachmann AW, Gartside M, Grice JE, Harris JM, Clifton P, Easteal S, Jackson RV, Whitworth JA. Association between chronic fatigue syndrome and the corticosteroid-binding globulin gene ALA SER224 polymorphism. Endocrine Research. 2004;30:417–429. doi: 10.1081/erc-200035599. [DOI] [PubMed] [Google Scholar]
- Turroni et al. (2010).Turroni F, Foroni E, O’Connell Motherway M, Bottacini F, Giubellini V, Zomer A, Ferrarini A, Delledonne M, Zhang Z, Van Sinderen D, Ventura M. Characterization of the serpin-encoding gene of bifidobacterium breve 210B. Applied and Environmental Microbiology. 2010;76:3206–3219. doi: 10.1128/AEM.02938-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water et al. (2004).Van de Water N, Tan T, Ashton F, O’Grady A, Day T, Browett P, Ockelford P, Harper P. Mutations within the protein Z-dependent protease inhibitor gene are associated with venous thromboembolic disease: a new form of thrombophilia. British Journal of Haematology. 2004;127:190–194. doi: 10.1111/j.1365-2141.2004.05189.x. [DOI] [PubMed] [Google Scholar]
- Vecchi et al. (2008).Vecchi M, Confalonieri S, Nuciforo P, Viganò MA, Capra M, Bianchi M, Nicosia D, Bianchi F, Galimberti V, Viale G, Palermo G, Riccardi A, Campanini R, Daidone MG, Pierotti MA, Pece S, Di Fiore P. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene. 2008;27:2148–2158. doi: 10.1038/sj.onc.1210858. [DOI] [PubMed] [Google Scholar]
- Wilczynska et al. (1995).Wilczynska M, Fa M, Ohlsson PI, Ny T. The inhibition mechanism of serpins. Evidence that the mobile reactive center loop is cleaved in the native protease-inhibitor complex. The Journal of Biological Chemistry. 1995;270:29652–29655. doi: 10.1074/jbc.270.50.29652. [DOI] [PubMed] [Google Scholar]
- Wolf et al. (1999).Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. Journal of Histochemistry & Cytochemistry. 1999;47:221–228. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2018).Yan Y, Zhou A, Carrell RW, Read RJ. Structural basis for the specificity of renin-mediated angiotensinogen cleavage. Journal of Biological Chemistry. 2018;294:1–28. doi: 10.1074/jbc.RA118.006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2000).Yang P, Tremaine WJ, Meyer RL, Prakash UB. Alpha1-antitrypsin deficiency and inflammatory bowel diseases. Mayo Clinic Proceedings. 2000;75:450–455. doi: 10.4065/75.5.450. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2007).Zhang Q, Buckle AM, Law RH, Pearce MC, Cabrita LD, Lloyd GJ, Irving JA, Smith AI, Ruzyla K, Rossjohn J, Bottomley SP, Whisstock JC. The N terminus of the serpin, tengpin, functions to trap the metastable native state. EMBO Reports. 2007;8:658–663. doi: 10.1038/sj.embor.7400986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2006).Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. International Journal of Molecular Medicine. 2006;17:937–944. doi: 10.3892/ijmm.17.5.937. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang L, Li L, Yang M, Liu H, Yang G. Elevated circulating vaspin levels were decreased by rosiglitazone therapy in T2DM patients with poor glycemic control on metformin alone. Cytokine. 2011;56:399–402. doi: 10.1016/j.cyto.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (1999).Zhang M, Magit D, Botteri F, Shi HY, He K, Li M, Furth P, Sager R. Maspin plays an important role in mammary gland development. Developmental Biology. 1999;215:278–287. doi: 10.1006/dbio.1999.9442. [DOI] [PubMed] [Google Scholar]
- Zhang, Magit & Sager (1997).Zhang M, Magit D, Sager R. Expression of maspin in prostate cells is regulated by a positive ets element and a negative hormonal responsive element site recognized by androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5673–5678. doi: 10.1073/pnas.94.11.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2013).Zheng D, Chen H, Davids J, Bryant M, Lucas A. Serpins for diagnosis and therapy in cancer. Cardiovascular & Hematological Disorders-Drug Targets. 2013;13:123–132. doi: 10.2174/1871529X11313020005. [DOI] [PubMed] [Google Scholar]
- Zhou, Carrell & Huntington (2001).Zhou A, Carrell RW, Huntington JA. The serpin inhibitory mechanism is critically dependent on the length of the reactive center loop. The Journal of Biological Chemistry. 2001;276:27541–27547. doi: 10.1074/jbc.M102594200. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2006).Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13321–13326. doi: 10.1073/pnas.0604080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (1994).Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review and does not have raw data.