Abstract
The arrival of large masses of drifting Sargassum since 2011 has caused changes in the natural dynamics of Caribbean coastal ecosystems. In the summer of 2015, unprecedented and massive mats of S. fluitans and S. natans have been observed throughout the Mexican Caribbean including exceptional accumulations ashore. This study uses stable isotopes to assess the impact of Sargassum blooms on the trophic dynamics of the Diadema antillarum sea urchin, a keystone herbivore on many Caribbean reefs. Bayesian models were used to estimate the variations in the relative proportions of carbon and nitrogen of assimilated algal resources. At three lagoon reef sites, the niche breadth of D. antillarum was analysed and compared under massive influx of drifting Sargassum spp. vs. no influx of Sargassum blooms. The effects of the leachates generated by the decomposition of Sargassum led to hypoxic conditions on these reefs and reduced the taxonomic diversity of macroalgal food sources available to D. antillarum. Our trophic data support the hypothesis that processes of assimilation of carbon and nitrogen were modified under Sargassum effect. Isotopic signatures of macroalgae associated with the reef sites exhibited significantly lower values of δ15N altering the natural herbivory of D. antillarum. The Stable Isotopes Analysis in R (SIAR) indicated that, under the influence of Sargassum blooms, certain algal resources (Dictyota, Halimeda and Udotea) were more assimilated due to a reduction in available algal resources. Despite being an abundant available resource, pelagic Sargassum was a negligible contributor to sea urchin diet. The Stable Isotope Bayesian Ellipses in R (SIBER) analysis displayed differences between sites, and suggests a reduction in trophic niche breadth, particularly in a protected reef lagoon. Our findings reveal that Sargassum blooms caused changes in trophic characteristics of D. antillarum with a negative impact by hypoxic conditions. These dynamics, coupled with the increase in organic matter in an oligotrophic system could lead to reduce coral reef ecosystem function.
Keywords: Echinoids, Pelagic macroalgae, Stable isotopes, Trophic ecology, Coral reefs, Niche breadth, Mexican caribbean
Introduction
The arrival of massive amounts of pelagic Sargassum spp. has caused changes in the natural benthic dynamics of Caribbean coastal ecosystems for the last nine years (Gower, Young & King, 2013; Schell, Goodwin & Siuda, 2015). Pelagic Sargassum is a complex of two species, namely S. fluitans and S. natans (Oyesiku & Egunyomi, 2014). Since 2011, extensive masses of Sargassum appeared in unusual ways in oceanic waters off northern Brazil (De Széchy et al., 2012; Sissini et al., 2017), along the West Indies and Caribbean coasts (Gower, Young & King, 2013) from Trinidad to the Dominican Republic (Rodríguez-Martínez, van Tussenbroek & Jordán-Dahlgren, 2016; van Tussenbroek et al., 2017), and along the west African coast from Sierra Leone to Ghana (Smetacek & Zingone, 2013). Wang et al. (2019) recorded that for June 2018, wet biomass reached more than 20 million tons in the Caribbean Sea and Central Atlantic Ocean.
The Mexican Caribbean shores faced atypical massive mats of pelagic Sargassum in the summer of 2015 (van Tussenbroek et al., 2017; Cuevas, Uribe-Martínez & Liceaga-Correa, 2018; Arellano-Verdejo, Lazcano-Hernandez & Cabanillas-Terán, 2019). There was a subsequent decrease during 2016 and 2017, but for most of 2018 and thus far in 2019 influx has increased again. Several studies revealed that these massive mats of Sargassum have a new possible distribution source different from the historic North Atlantic Recirculation Region (NARR) known as “The Sargasso Sea” (Schell, Goodwin & Siuda, 2015). Instead, the most probable origin of the massive influx on the Caribbean shores is the North Equatorial Recirculation Region (NERR) (Johnson et al., 2013; Schell, Goodwin & Siuda, 2015). High oceanic temperatures and nutrient inputs (Franks, Johnson & Ko, 2016; Wang et al., 2018), among other oceanographic coupled patterns such as changes of surface currents, are the most probable causes of this new region of Sargassum flourishment (Johnson et al., 2013; Gower, Young & King, 2013; Sissini et al., 2017). A recent study by Wang et al. (2019) revealed that increases of pelagic Sargassum are driven by upwelling off West Africa during the boreal winter and by Amazon River discharge during the spring and summer. The authors state that recurrent blooms in the Caribbean Sea and tropical Atlantic are likely, highlighting the importance for understanding their effects on existing ecosystems for future planning.
Changes in habitat structure can directly influence trophic dynamics (Hunter & Price, 1992; Sweatman, Layman & Fourqurean, 2017) and have been shown to cause synergistic effects on coral reefs (Smetacek & Zingone, 2013). For example, harmful macroalgae blooms have been recognized as drivers of degradation in coral reef habitats (Lapointe et al., 2005). This has effects on the diversity of reef biota (Bauman et al., 2010; Louime, Fortune & Gervais, 2017) like variations in the sea urchin populations (Lapointe et al., 2010). The carbon and nitrogen flow by macroalgae blooms likely has adverse effects at different scales. Such disturbances from Sargassum, coupled with pre-existing threats on coral reefs, add to the drivers of Anthropocene reef degradation (Alvarez-Filip et al., 2011; Cramer et al., 2012).
The massive decomposition of Sargassum has negative impacts not only on tourism and local fisheries, but on nearshore ecosystems (Solarin et al., 2014; Louime, Fortune & Gervais, 2017). However, few studies assess the trophic impact of Sargassum blooms on benthic communities. Pelagic Sargassum and their attached epiphytic algae can contribute new organic matter to these communities (Rooker, Turner & Holt, 2006; Wang et al., 2018). Therefore, we consider whether or not these new sources of nitrogen and carbon act in a detrimental manner on the trophic chain of benthic communities. The beaching and decomposing of massive Sargassum mats produce hypoxia in near-shore coral reef communities (Rodríguez-Martínez et al., 2019). This effect coupled with high hydrogen sulfide and ammonium concentrations have been shown to cause faunal mortality in the Mexican Caribbean (Rodríguez-Martínez et al., 2019). As a consequence, the coastal environment becomes even more sensitive to degradation agents. To assess these issues, we included measurements of dissolved oxygen in our study.
Evaluating consumers and resources through a trophic approach by tracking the relationships between consumers and prey provides relevant information on the trophic structure and dynamics of a benthic community (Minagawa & Wada, 1984; Vanderklift, Kendrick & Smit, 2006; Behmer & Joern, 2008). Stable isotopes of carbon (δ13C) and nitrogen (δ15N) have been used in marine ecosystems to determine the feeding habits of species (Peterson & Fry, 1987), nutrient migrations within food webs, trophic position of organisms and their contribution at all trophic levels (Vander Zanden & Rasmussen, 1996). It is also possible to trace the origin and transformation of the ingested organic matter and to detect changes in the trophic positions of organisms that coexist in the same habitat (Hobson, 1999; Vanderklift, Kendrick & Smit, 2006; Rodríguez-Barreras et al., 2016).
Stable carbon and nitrogen isotope ratios provide time-integrated information regarding feeding relationships and energy flow through food webs (DeNiro & Epstein, 1981; Peterson & Fry, 1987; Vander Zanden & Rasmussen, 2001). Moreover, stable isotopes can be used to study the trophic niche breadth of a species (Bearhop et al., 2004; Parnell et al., 2010; Phillips et al., 2014). This is directly influenced by consumers and resource input, providing a quantitative assessment of trophic conditions (Newsome et al., 2007; Boecklen et al., 2011). Stable isotope analyses are useful for assessing the health of ecosystems because it is possible to associate the consumers trophodynamics and niche breadth with habitat disturbances (Layman et al., 2007b; Hamaoka et al., 2010). It is also possible to detect changes in the trophic spectrum from anthropogenic impacts or unusual conditions that cause shifts in ecosystems (Wing et al., 2008; Prado, Alcoverro & Romero, 2010; Tomas, Box & Terrados, 2011). In light of the massive arrival of pelagic macroalgae, sea urchin herbivory is a good model to understand variability in the benthic trophic chain, as sea urchins are considered generalist consumers with a plastic feeding habit (Lawrence, 1975; Vanderklift, Kendrick & Smit, 2006). Echinoids have the capability to modify the community structure through foraging behaviour (Carpenter, 1986; Hay & Fenical, 1988; Sala et al., 1998; Eklöf et al., 2008). Thus, the relative position of δ13C vs. δ15N echinoids displayed in a bi-plot can give insights about organism responses to niche shifts, diet variability and habitat modification (Layman et al., 2007a; Layman et al., 2007b; Layman et al., 2012; Sweatman, Layman & Fourqurean, 2017).
The effect of Sargassum and their leachates on the diet of D. antillarum can improve our understanding on the impact on trophic ecology of one of the most important sea urchins of the Mexican Caribbean. The main reason to focus this study on D. antillarum is that this species is and was the major shallow-hard-bottom grazer in our study sites (Jorgensen, Espinoza-Ávalos & Bahena-Basave, 2008; Jordán-Garza et al., 2008). One of the most dramatic events in the Caribbean resulted from the pathogen-driven reduction in the populations of D. antillarum (Lessios et al., 1984) with detrimental ecological consequences like coral-algal phase-shifts. The southern part of Quintana Roo is not an exception encompassing with the effects of the abrupt coastal development and watershed pollution as key drivers along the Costa Maya (Arias-González et al., 2017).
The overarching aim of this study was to determine variations in the relative proportions of carbon and nitrogen of assimilated algal resources and the niche breadth of D. antillarum under massive influx of drifting Sargassum spp. vs. no influx of Sargassum at back reefs. We also aimed to determine whether pelagic Sargassum was a substantial source of energy for D. antillarum. To do this, we compared δ15N and δ13C values of D. antillarum with and without influx of Sargassum to track changes in this species trophic ecology (diet, trophic position and niche breadth). Ultimately, we tested the hypothesis that an influx in Sargassum in coastal ecosystem creates a significant change in the available algal sources and a shift in the trophic structure.
Material & Methods
Study sites
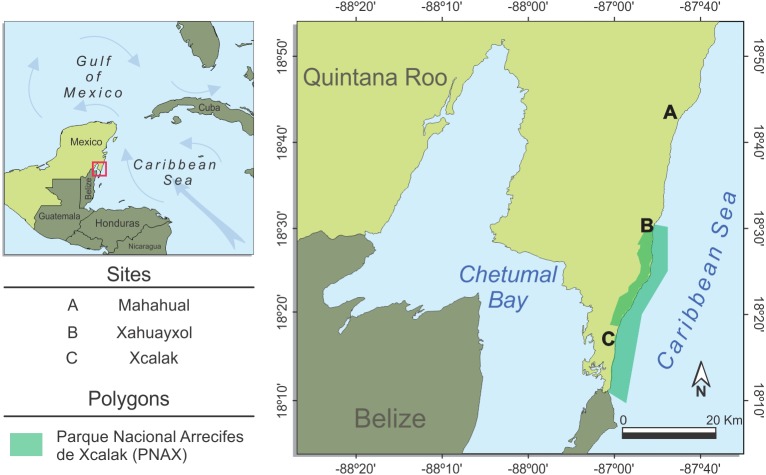
We determined the stable isotopes of carbon and nitrogen for D. antillarum at three reef lagoons (Mahahual, Xahuayxol, and Xcalak) with different distances from the beach to the reef crest (Fig. 1). The main strategy implemented by local authorities at some beaches with the massive arrival of macroalgae included the removal and disposal of Sargassum in the highest part of the beach or in places determined ex profeso. This contributed to a continuous accumulation of Sargassum masses on the beach. However, the Sargassum removal was not quantified and the information regarding removal included here is only preliminary.
Figure 1. Study sites.
Study area and sampling localities at the south coast of Quintana Roo: Mahahual (A), Xahuayxol (B) and Xcalak (C). The green polygon represents the marine protected area Parque Nacional Arrecifes de Xcalak (PNAX). Figure credit: Alejandro A. Aragón-Moreno.
Mahahual (18°42′16.96″N 87°42.619′W) is located in the northern part of the Mesoamerican Barrier Reef System (MBRS) in the state of Quintana Roo. Mahahual is a former fishing village but during the last two decades has undergone reef degradation due to anthropogenic impact (Martínez-Rendis et al., 2016). It has a narrow reef lagoon (230–450 m). Sargassum management in this locality was active through removing it from the beach and ex situ disposition.
Xahuayxol (18°30′21.78″N; 87°45′24.84″W) located south of Mahahual, has a larger reef lagoon measuring 300 to 500 m from the beach to the reef crest. Sargassum was not removed from the beach in any systematic way and remained accumulated on the shore. This reef is the northern limit of the marine protected area Parque Nacional Arrecifes de Xcalak (PNAX) and human activities are less salient than in Mahahual (Schmitter-Soto et al., 2018).
Xcalak (18°14′7.68″N; 87°50′1.46″W), at the southern limit of the Mexican Caribbean, is part of PNAX since 2000. It is also part of the MBRS (Hoffman, 2009). It has a wide reef lagoon (950–1,200 m), and Sargassum was accumulated along the shore in large amounts. There was active but less intense Sargassum management in place at Xcalak, where final disposal was in situ on the highest part of beach.
At all sampled sites, the dominant forcing mechanism was reef lagoon circulation from wave action (Mariño-Tapia et al., 2010). In our study area, during the period from June to August has the wave orbital velocity over the threshold of motion (Maldonado-Sánchez et al., 2019), indicating active circulation in the reef lagoons.
Collecting and processing data
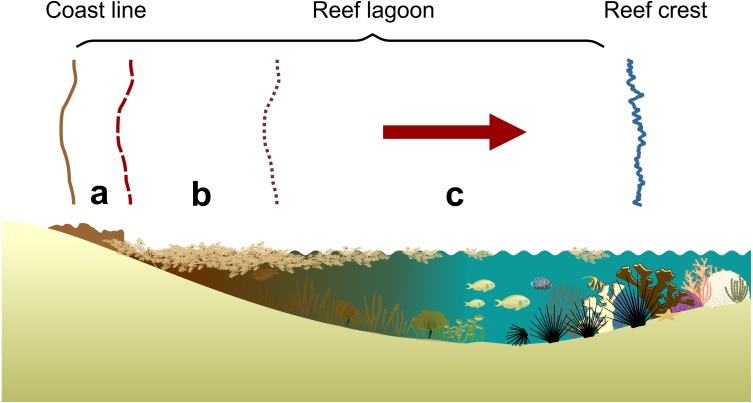
This study covers two periods: Under Sargassum effect (USE) during the months of July–August 2015 and without Sargassum effect (WSE) in July–August 2016. USE sampling for stable isotope analysis included drifting Sargassum (mixture of S. fluitans and S. natans), turf associated pelagic Sargassum, benthic macroalgae, local turf and 19 individuals of D. antillarum. WSE sampling included benthic macroalgae, local turf and 15 individuals of D. antillarum (see sampling details ST1). Samples sizes were based on previous studies to obtain sufficient data for statistical analysis (Rodríguez, 2003; Tomas et al., 2006; Wing et al., 2008; Rodríguez-Barreras et al., 2016). The sampling sites were at coastal lagoons in the back reef zone (section c, Fig. 2), zone with no visible presence of Sargassum leachates (van Tussenbroek et al., 2017) and where D. antillarum is distributed (Steneck & Lang, 2003; Jorgensen, Espinoza-Ávalos & Bahena-Basave, 2008; Jordán-Garza et al., 2008; Maldonado-Sánchez, 2018).
Figure 2. Lagoon reef-scape showing the sections with Sargassum blooms.
Lagoon reef-scape showing the sections: a: decomposing Sargassum spp., Section b, leachates (dark brown water) and section c, back reef, areas without visible leachates. Based on van Tussenbroek et al. (2017).
Under Sargassum effect (USE) measurements
USE included measurements of dissolved oxygen (mg l−1) recorded with a calibrated Multi-parameter water quality checker HORIBA 50 at Mahahual, Xahuayxol and Xcalak. Measurements of dissolved oxygen were made at points distributed in three sections from areas with decomposing Sargassum (section a), leachates (section b -dark brown water-) and reef lagoon areas without Sargassum leachates (section c) (Fig. 2).
Pelagic Sargassum spp., turf (benthic turf and the associated turf to pelagic Sargassum) and macroalgae samples were collected in coral reef patches of section c (back reef zone) for each sampling site.
Under and without Sargassum effect (USE and WSE) measurements
We collected algal samples to obtain biomass, and for stable isotope analysis using nine quadrats (50 × 50 cm) per site. Pelagic Sargassum biomass was calculated based on sunken thalli and overlaid on reef substrates inside the quadrats. The quadrats were located randomly within the sea urchin habitat (radius of 15 m from collected echinoids). The substrate inside each quadrat was scrapped, carefully removed, collected in bags, and frozen for later analysis.
Macroalgae were identified according to Littler & Littler (2000). Analyses were performed to genus level. For biomass estimates samples were dried for 48 h in an oven at 60 °C. Samples were weighed with a digital balance (standard error = 0.0001 g). To determine D. antillarum differential algae assimilation considering USE and WSE, algae samples were pooled per site. The sampled echinoids and algal species for this study are not threatened. The collection permit was obtained from the Comisión Nacional de Acuacultura y Pesca (CONAPESCA, PPF/DGOPA-002/17).
The collected individuals of D. antillarum were at the same depth range (1.5–2.5 m) and only individuals greater than 5.0 cm in test diameter were collected to avoid any ontogenic effect. Samples were frozen shortly after collection and processed later at the laboratory. The muscles of Aristotle’s lanterns were carefully removed and washed from the stomach contents to estimate algal assimilation by D. antillarum because this tissue offers a time-integrated measure of carbon and nitrogen assimilated sources (Polunin et al., 2001; Ben-David & Schell, 2001; Phillips & Koch, 2002).
Macroalgae and local turf, pelagic Sargassum species (S. fluitans and S. natans), turf associated to pelagic Sargassum, and echinoids muscle samples were rinsed with filtered water, dried at 50 °C during 48 h, grounded to a fine powder and placed in glass vial for isotope analyses. To remove carbonates from some algal species (eg., Halimeda spp. Penicillus spp., etc.), the samples were washed with diluted HCl at 1 N prior to drying to avoid disturbance in the mass spectrometer reading.
A subsample of each algae and muscle (1mg) was taken to evaluate the 13C/12C and 15N/14N ratios using a Delta V Plus Mass Spectrometer. Catalyzers silvered cobaltous/cobaltic oxide and chromium oxide were used. Carbon and nitrogen samples were analysed in a dual isotope mode at the Centro Interdisciplinario de Ciencias Marinas from Instituto Politécnico Nacional. Isotope samples were loaded into tin-capsules and placed in a 50-position automated Zero Blank sample carousel on a COSTECH 4020 elemental analyzer. The carbon and nitrogen isotopic results were expressed in standard delta notation relative to Vienna Pee Dee Belemnite (VPDB) and to atmospheric air.
and
The standard deviations of δ13C and δ15N replicate analyses were estimated; the precision values were 0.2‰ for carbon and nitrogen isotope measurements. In addition, we calculated the trophic level (TL) according to Hobson & Welch (1992) for every individual of D. antillarum in each site, expressed as:
Where Nm is the mean δ15N ratio of each sea urchin, Nb is average basis δ15N value of the algal community, and TEF is the given value for the trophic enrichment factor (TEF). We assumed a TEF of 2.4 following Moore & Semmens (2008).
Data analysis
Dissolved oxygen data were summarized to obtain average values (± standard error) by section (sections a, b, c in Fig. 2) and reef lagoons (Mahahual, Xahuayxol, and Xcalak). We evaluated differences among sections and at the reef lagoons (sections a, b, c, in Fig. 2). We plotted raw data of dissolved oxygen as a function of distance to coast to visualize the low to high values gradient related to that distances in every reef lagoon.
The relative contribution of algae to the diet of the sea urchins D. antillarum was estimated with a Bayesian isotopic mixing model (SIAR Parnell & Jackson, 2013), which included the isotopic signatures, fractionation and variability to estimate the probability distribution of the contribution of the food source to a mixture. This procedure supplied accurate information about the contribution of algal species to the sea urchin tissues, as it provided the proportion for every source and recognized the main sources as important components of the diet (Peterson, 1999; Fry, 2006; Wing et al., 2008) at three different sites, and under and without Sargassum effect. To run the model, the isotopic discrimination factor values used were 2.4 ± 1.6‰ (mean ± SD) for δ15N, and 0.4 ± 1.3‰ (mean ± SD) for δ13C (Minagawa & Wada, 1984; Fry & Sherr, 1989; Moore & Semmens, 2008; Cabanillas-Terán et al., 2016).
The following algal taxa/groups were considered for the mixing models analyses: Caulerpa, Codium, Dictyota, Halimeda, Laurencia, Lobophora, Padina, Penicillus, Sargassum polyceratum, Stypopodium, turf, and Udotea. The sources for the model were selected following the theoretical geometric assumptions of the mixing model according to Phillips et al. (2014) and Rodríguez-Barreras et al. (2015) to ensure reliable resources. Samples of D. antillarum did not require lipid extraction since C:N ratios of Aristotle lantern’s muscle were lower than 3.5 (Post et al., 2007).
We performed a comparison USE and WSE between the niche width and overlap for D. antillarum by using Stable Isotope Bayesian Ellipses in R (SIBER) (Jackson et al., 2011) from the SIAR package (Parnell & Jackson, 2013). This procedure performs metrics based on ellipses and provides the standard ellipse corrected area (SEAc) used as the trophic niche breadth and the overlap between ellipses, presuming that values close to 1 exhibit a higher trophic overlap. Models were run with 200,000 iterations and a burn in of 50,000.
Homogeneity and normality of variance were tested by performing a Kolmogorov–Smirnov and a Cochran’s test (Zar, 1999). Nitrogen data followed the premises of parametric analysis, but the carbon, dissolved oxygen and biomass data required a power transformation for reaching normality and homogeneity of variance (Box & Cox, 1964). We ran two-way ANOVA to evaluate dissolved oxygen data differences among sections in the reef lagoons and we performed a post hoc comparison using Tukey-HSD test. The functions aov and glm from the Gaussian family were used to test the differences in isotopic ratios of carbon and nitrogen values to compare the effect (WSE and USE) between sites and their interaction. Statistics were performed with α < 0.05 (R Core Team, 1.0.153, 2017).
Results
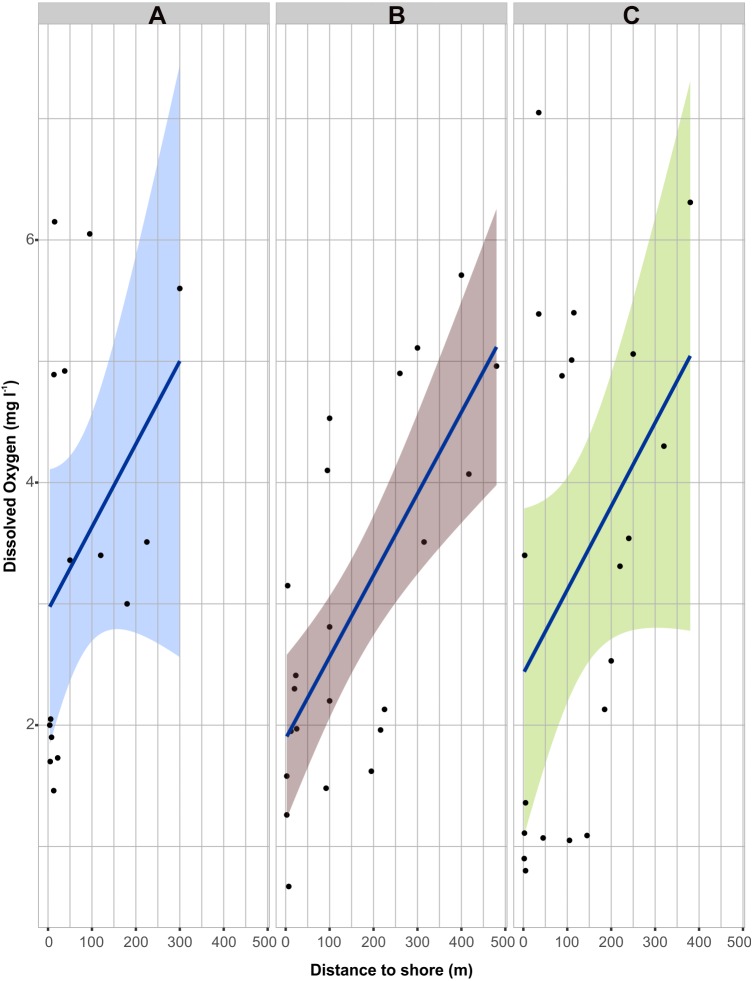
The dissolved oxygen values USE indicated that the effects of the leachates generated by the decomposition process, together with the organic material carried in their vegetal structures, reduced the values of dissolved oxygen in the reef lagoon water. The decomposing Sargassum area (section a, Fig. 2) showed an average range from 1.01 (S.E. ± 0.30) mg l−1at Xcalak to 1.88 (S.E. ± 0.37) mg l−1 at Mahahual. The leachates area (section b, Fig. 2) showed an average range from 2.42 (S.E. ± 0.32) mg l−1 at Xahuayxol to 3.66 (S.E. ± 0.42) mg l−1at Mahahual. The back reef area (section c, Fig. 2) showed an average range from 4.1 (S.E. ± 0.34) mg l−1at Mahahual to 4.8 (S.E. ± 0.22) mg l−1at Xcalak. The two-way ANOVA indicated significant differences between reef lagoons (p < 0.05) and sections (p < 0.01); Mahahual was significantly different to Xcalak, but Mahahual and Xcalak were not significantly different to Xahuayxol (Post-hoc HSD of Tukey test, 95% confidence). The three sections at the three reefs were significantly different, except the sections b and c of Mahahual (Post-hoc HSD of Tukey test, 95% confidence). Therefore dissolved oxygen data showed a gradient significantly different between sections. The overall values of dissolved oxygen displayed the lowest concentrations for section a, near the shoreline and higher values beyond the back reef section c (Fig. 3).
Figure 3. Dissolved Oxygen values under Sargassum effect (USE).
Dissolved oxygen (mgl−1) values along the distance to shoreline at (A) Mahahual (blue), (B) Xahuayxol (purple) and (C) Xcalak (green) considering the sections depicted in Fig. 2: dissolved oxygen <2 mgl−1: decomposing Sargassum spp; dissolved oxygen between 2–4 mgl−1: leachates (dark brown water) and dissolved oxygen > 4 mgl−1: back reef, areas without visible leachates.
Biomass, δ15N and δ13C of macroalgae
Biomass data for benthic taxa displayed no significant differences between USE and WSE, but significant differences were found among localities (ANOVA, df = 2, F = 8.24, p < 0.0001). Mahahual had the highest mean benthic biomass values (55.2 dry weight m−2) followed by Xahuayxol with (38.8 dry weight m−2) and Xcalak (16 dry weight m−2 ±). WSE biomass average values for local benthic algae ranged from 3.01 dry weight m−2 ±0.95 (Codium spp. at Xcalak) to 133.50 dry weight m−2 ±30.29 (Halimeda spp. at Mahahual). USE values ranged from 7.75 dry weight m−2 ±5.4 (Caulerpa at Xcalak) to 145.99 dry weight m−2 ± 36.21 (Halimeda spp. at Mahahual, Table 1). Genus-level biomass of pelagic taxa showed no significant differences per site neither at genus level, however Sargassum fluitans displayed the highest biomass values.
Table 1. Algal biomass values.
Mean ± standard deviation values of algal biomass (grams dry weight m−2) at Mahahual, Xahuayxol and Xcalak. Genus considered for the mixing models analysis. Data below the grey line belongs to pelagic taxa.
| Mahahual | Xahuayxol | Xcalak | ||||||
|---|---|---|---|---|---|---|---|---|
| Genus | WSE | USE | Genus | WSE | USE | Genus | WSE | USE |
| Caulerpa | 39.49 ± 20.79 | 19.82 ± 6.48 | Caulerpa | 5.38 ± 0.93 | Caulerpa | 7.97 ± 3.51 | 7.75 ± 5.4 | |
| Dictyota | 19.92 ± 11.69 | 20.40 ± 5.41 | Dictyota | 6.61 ± 2.49 | 20.36 ± 5.96 | Codium | 3.01 ± 0.95 | |
| Halimeda | 133.50 ± 30.29 | 145.99 ± 36.21 | Halimeda | 118.07 ± 29.43 | 89.18 ± 9.998 | Dictyota | 21.99 ± 5.99 | 17.76 ± 2.34 |
| Laurencia | 14.73 ± 22.15 | Laurencia | 8.49 ± 4.10 | Lobophora | 26.96 ± 4.30 | |||
| Stypopodium | 95.41 ± 66.10 | Lobophora | 19.933 ±11.50 | Padina | 12.62 ± 4.30 | |||
| Turf | 24.69 ± 9.17 | 19.042 ± 6.045 | Penicillus | 12.88 ± 3.94 | Penicillus | 27.49 ± 3.51 | 26.23 ± 2.45 | |
| Udotea | 59.79 ± 45.74 | Sargassum | 14.26 ± 4.42 | Sargassum | 15.01 ± 4.30 | |||
| Stypopodium | 10.06 ± 12.13 | Turf | 12.00 ± 3.51 | 11.40 ± 4.21 | ||||
| Turf | 5.886 ± 2.83 | 14.26 ± 7.84 | ||||||
| Udotea | 39.13 ± 14.76 | 34.02 ± 16.54 | ||||||
| S. fluitans | 12.39 ± 8.33 | S. fluitans | 11.86 ± 2.75 | S. fluitans | 13.00 ± 6.99 | |||
| S. natans | 4.92 ± 3.14 | S. natans | 7.07 ± 3.26 | S. natans | 10.03 ± 7.94 | |||
| Sargassum’s associated turf | 3.10 ± 1.21 | Sargassum’s associated turf | 3.23 ± 1.28 | Sargassum’s associated turf | 1.98 ± 1.29 | |||
Under and without Sargassum effect values revealed significant differences in overall benthic algae values of δ15N (ANOVA, df = 1, F = 20.27, p < 0.0001). Specifically under Sargassum blooms most of the algae exhibited isotopic signatures with significantly depleted δ15N like Dictyota and turf across the lagoon reef sites (Table 2). The overall macroalgal δ15N under Sargassum fluctuated from 0.023 to 2.08‰. At Xcalak Caulerpa displayed the highest mean values of nitrogen with 2.02 ± 0.08‰. Local Turf USE displayed negative values and overall turf values fluctuated from −0.97‰ to 0.42‰. Xahuayxol displayed the most negative δ15N mean value of local turf (−0.51 ± 0.02‰). Without Sargassum effect the mean algal genus δ15N fluctuated from 0.06 ± 0.08 with Penicillus at Xcalak, and Xahuayxol displayed the highest mean value of δ15N with Caulerpa (5.68 ± 0.01‰) (Table 2).
Table 2. Mean ± standard deviation values of δ13C and δ15N of algal genus considered in the mixing model analysis taken from Mahahual, Xahuayxol and Xcalak, the asterisks represent the sources under Sargassum effect.
| Mahahual | Xahuayxol | Xcalak | ||||||
|---|---|---|---|---|---|---|---|---|
| Genus | δ13C | δ15N | Genus | δ13C | δ15N | Genus | δ13C | δ15N |
| Caulerpa | −9.89± 0.15 | 2.22± 0.01 | Caulerpa | −8.86± 0.19 | 5.68± 0.01 | Caulerpa | −12.60± 0.04 | 1.00± 0.10 |
| Caulerpa* | −16.22± 0.55 | 0.93±0.08 | Dictyota | −15.71± 0.90 | 2.29± 0.41 | Caulerpa* | −9.63± 0.02 | 2.02± 0.08 |
| Dictyota | −16.38± 1.23 | 1.56±1.37 | Dictyota* | −16.31± 0.95 | 0.71± 0.02 | Codium | −12.17 ± 0.07 | 1.25 ± 0.07 |
| Dictyota* | −15.95 ± 0.04 | 0.82 ± 0.04 | Halimeda* | −12.61 ± 1.70 | 0.88 ± 0.01 | Dictyota | −15.47 ± 0.68 | 0.67 ± 0.03 |
| Halimeda | −7.01 ± 1.25 | 0.29 ± 0.43 | Laurencia | −14.81 ± 0.23 | 1.36 ± 0.71 | Dictyota* | −15.69 ± 0.20 | 0.04 ± 0.06 |
| Halimeda* | −8.39 ± 0.69 | 0.68 ± 0.12 | Lobophora* | −10.49 ± 1.35 | 0.33 ± 0.64 | Lobophora | −14.15 ± 0.53 | 0.77 ± 0.33 |
| Laurencia | −16.16 ± 0.90 | 2.61 ± 1.41 | Penicillus | −11.51 ± 8.28 | 1.84 ± 0.30 | Padina | −10.18 ± 0.18 | 0.25 ± 0.19 |
| Stypopodium | −11.33 ± 0.52 | 0.67 ± 0.05 | Sargassum | −14.65 ± 1.82 | 3.21 ± 0.23 | Penicillus | −14.50 ± 0.08 | 0.06 ± 0.08 |
| Turf | −13.44 ± 0.00 | 3.03 ± 0.02 | Stypopodium | −16.80 ± 1.40 | 1.47 ± 0.56 | Penicillus* | −9.75 ± 0.14 | 1.98 ± 0.04 |
| Turf* | −16.54 ± 0.22 | −0.51 ± 0.02 | Turf | −16.43 ± 1.32 | 1.84 ± 0.30 | Sargassum* | −14.76 ± 0.87 | 0.37 ± 0.08 |
| Udotea | −12.86 ± 0.42 | 2.19 ± 0.03 | Turf* | −18.56 ± 0.04 | −0.89 ± 0.11 | Turf | −17.44 ± 0.48 | 4.59 ± 0.64 |
| Udotea | −11.62 ± 1.34 | 2.42 ± 1.12 | Turf* | −21.98 ± 0.10 | 0.41 ± 0.01 | |||
| Udotea * | −12.65 ± 0.20 | 2.65 ± 0.77 | ||||||
| S. fluitans | −16.03 ± 0.99 | −0.53 ± 0.26 | S. fluitans | −16.36 ± 0.15 | −1.74 ± 0.38 | S. fluitans | −16.26 ± 0.17 | −2.51 ± 0.52 |
| S. natans | −17.44 ± 0.71 | −1.59 ± 0.70 | S. natans | −16.82 ± 0.73 | −1.49 ± 0.42 | S. natans | −17.28 ± 0.81 | −1.62 ± 0.55 |
| Sargassum’s associated turf | −18.29 ± 0.51 | −1.13 ± 0.05 | Sargassum’s associated turf | −15.93 ± 0.79 | −0.47 ± 0.07 | Sargassum’s associated turf | −16.27 ± 0.63 | −0.96 ± 0.01 |
As for δ13C USE ratios fluctuated from −21.98 to −9.23‰ and WSE from −20.90 to −5.65‰. Considering only the algae presented in both sampling periods (WSE and USE) there was no significant difference in δ13C among sites (ANOVA, df = 2, F = 0.55, p > 0.05) neither was significant difference analysing the effect (ANOVA, df = 1, F = 1.14, p > 0.05) and their interaction (ANOVA, df = 2, F = 0.86, p > 0.05).
Overall USE pelagic Sargassum δ13C values fluctuated from −17.95‰ to −15.24‰. S. natans exhibited the most negative mean values of δ13C (−17.44 ± 0.71‰) at Mahahual (Table 2). There was no difference in δ13C among sites (ANOVA, df = 2, F = 0.05, p > 0.05) but there were significant differences δ13C between species (ANOVA, df = 2, F = 7.57, p = 0.01). Sargassum’s associated turf δ13C values fluctuated from −18.65‰ to −15.37‰. The most negative δ13C mean value was displayed at Mahahual (−18.3 ± 0.5‰) for Sargassum’s associated turf.
Overall pelagic Sargassum δ15N values ranged from −2.87‰ to −0.30‰. The less negative mean value was exhibited at Mahahual (−0.53 ± 0.26‰) for S. fluitans. There was no significant difference for δ15N among sites (ANOVA, df = 2, F = 3.90, p = 0.05), but there was a remarkable trend to depleted δ15N at Xcalak where S. fluitans displayed the lowest mean values of δ15N (−2.51 ± 0.52‰). Turf associated to floating Sargassum δ15N values fluctuated from −0.42‰ to −1.17‰. The most depleted δ15N was exhibited at Mahahual (−1.13 ± 0.05‰) and the less negative mean value was displayed in Xahuayxol (−0.47 ± 0.07‰).
Sea urchins
There were significant differences δ15N among sites (ANOVA df = 2, F = 6.473, p = 0.005) and the interaction between site*effect (USE and WSE) showed significant differences (ANOVA, df = 2, F = 7.321, p = 0.003).
D. antillarum exhibited no differences among sites for δ13C values p > 0.05. However, we found significant differences analysing the USE and WSE effect (ANOVA df = 1, F = 5.301, p =0.03). The isotopic ratios of D. antillarum (USE) varied from 3.83‰ to 6.13‰ for δ15N, while δ13C ranged from −9.41‰ to −13.62‰. Mahahual was the site with the highest average values for δ15N 5.80 ± 0.30‰, while Xcalak displayed the lowest average value 4.38 ± 0.29‰. The isotopic ratios of D. antillarum (WSE) ranged from 4.69‰ to 6.16 for δ15N, while δ13C fluctuated from −8.83‰ to −13.42‰. We found significant differences for δ15N for sea urchins between sites (USE, ANOVA, df = 2, F = 6.47, p < 0.005).-Xcalak showed particularly low values under Sargassum effect (average value 4.38 ± 0.29‰ versus WSE average value 5.44 ± 0.36‰). Nevertheless, δ13C exhibited no significant differences although we noticed a negative trend in the values of δ13C under Sargassum effect (USE).
Algal source contributions (SIAR)
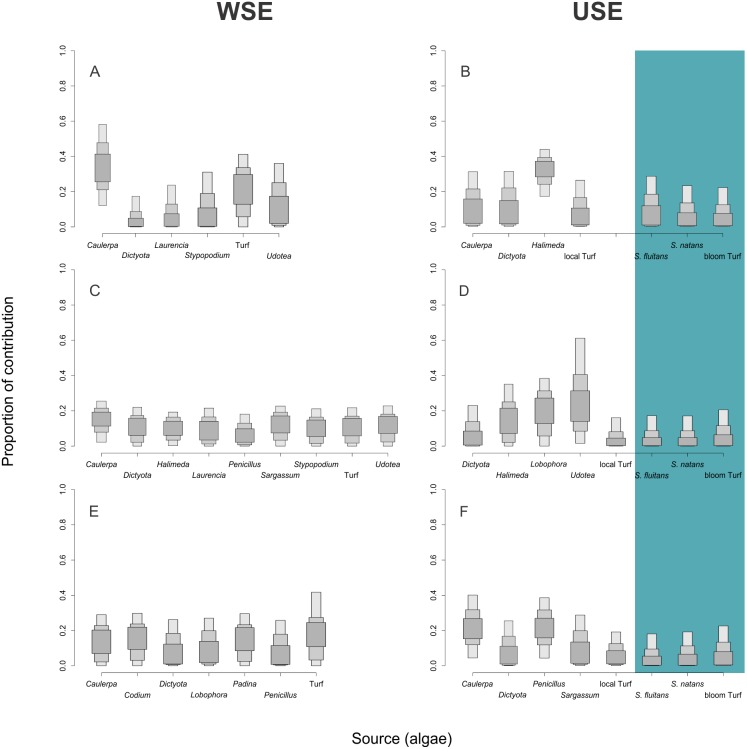
Mixing models provided evidence for the contribution of different algal resources for three sites USE and WSE (Table 3). SIAR analysis showed that D. antillarum behaved as an opportunistic grazer under the Sargassum effect, it is important to note that pelagic Sargassum, despite being one of the most abundant available resources, was not the most assimilated resource (Fig. 4). Relatedely, there was a reduction in benthic food sources USE (Fig. 4). Without Sargassum effect D. antillarum consumed, Laurencia, Stypopodium and Udotea (12–15% in average) at Mahahual; Caulerpa, Laurencia, Penicillus, Sargassum and Stypopodium (8–14% in average) at Xahuayxol; and Codium, Lobophora and Padina (13–15% in average) at Xcalak. Nevertheless, those resources were absent in the diet of D. antillarum under Sargassum effect (Table 3). Hence, the species displayed differential resource assimilation and Caulerpa was the most important resource for D. antillarum in Mahahual WSE (up to 37%), followed by Turf (up to 34%) and Halimeda and Udotea (up to 29% for both). USE the most important resource was Halimeda (up to 44%) followed by Caulerpa and Dictyota (both up to 31% of contribution). S. fluitans and S. natan s were no important sources (0–28% and 0–23% respectively), and turf associated to Sargassum blooms was the lesser assimilated resources by D. antillarum from 0 up to 22% (Table 3).
Table 3. Average percentage (%) contribution of algal genus to the diet of the sea urchins D. antillarum considering the effect of Sargassum: without Sargassum effect (WSE) and under Sargassum effect (USE) at Mahahual, Xahyayxol and Xcalak produced by the SIAR model using isotope values from algae.
Minimum and maximum values for each algae are shown in parentheses.
| Mahahual | Xahuayxol | Xcalak | ||||||
|---|---|---|---|---|---|---|---|---|
| Genus | WSE | USE | Genus | WSE | USE | Genus | WSE | USE |
| Caulerpa | 19 (1–37) | 14 (0–31) | Caulerpa | 14 (2–25 ) | – | Caulerpa | 14 (0–29) | 22 (0–40) |
| Dictyota | 9 (0–22) | 14 (0–31) | Dictyota | 11 (0–22) | 9 (0–23) | Codium | 15 (0–30) | – |
| Halimeda | 16 (1–29) | 31 (17–44) | Halimeda | 10 (0–19) | 17 (0–35) | Dictyota | 12 (0–26) | 11 (0–26) |
| Laurencia | 12 (0–25) | – | Laurencia | 11 (0–21) | – | Lobophora | 13 (0–27) | – |
| Stypopodium | 12 (0–25) | – | Lobophora | – | 20 (0–38) | Padina | 15 (0–29) | – |
| Turf | 17 (0–34) | 11(0–26) | Penicillus | 8 (0–18) | – | Penicillus | 12 (0–26) | 22 (4–39) |
| Udotea | 15 (0–29) | – | Sargassum | 12 (0–23) | – | Turf | 19 (0–45) | 8 (0–19) |
| S. fluitans | – | 12(0–28) | Stypopodium | 11 (0–21) | – | Sargassum | – | 13 (0–29) |
| S. natans | – | 9(0–23) | Turf | 11 (0–22) | 6 (0–16) | S. fluitans | – | 7 (0–18) |
| Sargassum’s associated turf | – | 9 (0–22) | Udotea | 12 (0–23) | 28 (2–61) | S. natans | – | 8 (0–19) |
| S. fluitans | – | 6 (0–17) | Sargassum’s associated turf | – | 9 (0–23) | |||
| S. natans | – | 6 (0–17) | ||||||
| Sargassum’s associated turf | – | 8 (0–21) | ||||||
Figure 4. Algal resources proportions consumed by Diadema antillarum.
Contribution rates of algae to the diet of Diadema antillarum in the two scenarios (WSE and USE). Results are shown as 25% (light error bars), 75% (grey error bars) and 95% (dark error bars) of credibility intervals. (A) Represents the contribution for D. antillarum at Mahahual without Sargassum effect (WSE), (B) represents D. antillarum at Mahahual under Sargassum effect (USE); (C) represents D. antillarum in Xahuayxol WSE, (D) represents D. antillarum in Xahuayxol USE; (E) represents D. antillarum in Xcalak WSE and (F) represents D. antillarum in Xcalak USE. Bloom turf is the Sargassum’s associated turf. The blue bar represents the pelagic sources USE.
At Xahuayxol WSE D. antillarum showed Caulerpa was the most important resource for D. antillarum (from 2 up to 25%) and for the rest of algae there were very similar algal contribution (from 0 up to 23%). The main macroalgal contributor of USE was Udotea with up to 61%, followed by Halimeda and Lobophora (with up to 35% and 38% respectively) as secondary resources. Sargassum’s associated turf showed evidence of low contribution (from 0 up to 21%) and S. fluitans, S. natan s had negligible contribution to D. antillarum diet with a maximum of 17% of the proportional contribution (Table 3).
Turf was the main algal resources for D. antillarum in Xcalak WSE (up to 45%) followed by Caulerpa, Codium and Padina as secondary resources (close to 30% maximum of contribution); contrasting USE the main macroalgal contributors in Xcalak were Penicillus and Caulerpa with up to 39% and 40% respectively. Likewise Dictyota and Sargassum polyceratium ( benthic Sargassum) were secondary resources up to 26% and 29%, respectively. The pelagic components in the other reef lagoons were negligible contributors for D. antillarum diet with just 18–23% of maximum contribution (Table 3, Fig. 4).
Trophic Levels
The overall trophic level data for D. antillarum (TL) ranged from 1.97 to 3.22. The species exhibited significant differences among sites (ANOVA df = 2, F = 10.63, p = 0.0004), and exhibited significant differences between WSE and USE (ANOVA, df = 1, F = 17.7, p = 0.0003). Likewise, calculating the interaction between site*effect (USE and WSE) revealed significant differences (ANOVA, df = 2, F = 12.65, p = 0.0001). The highest TL values were reported for Mahahual USE, while the lowest one was recorded in Xahauayxol WSE. At Mahahual, the TL mean value of D. antillarum was 2.35 ± 0.18 WSE and 3.08 ± 0.13 USE; at Xahuayxol, the TL mean value was 2.13 ± 0.30 WSE and 2.49 ± 0.27 USE, and at Xcalak TL mean value was 2.62 ± 0.15 WSE and 2.45 ± 0.12 USE (Table 4).
Table 4. Trophic level of D. antillarum.
Mean Trophic level (TL), and δ15N and δ13C ± standard deviation of D. antillarum without Sargassum effect (WSE) and under Sargassum effect (USE) at Mahahual, Xahuayxol and Xcalak.
| Site | TL WSE | TL USE | δ15N WSE | δ15N USE | δ13C WSE | δ13C USE |
|---|---|---|---|---|---|---|
| Mahahual | 2.35 ± 0.18 | 3.08 ± 0.13 | 5.22 ± 0.43 | 5.8 ± 0.3 | −10.46 ± 0.6 | −12.32 ± 0.95 |
| Xahuayxol | 2.13 ± 0.3 | 2.49 ± 0.27 | 5.09 ± 0.71 | 4.9 ± 0.24 | −11.5 ± 0.81 | −11.21 ± 1.48 |
| Xcalak | 2.62 ± 0.15 | 2.45 ± 0.12 | 5.44 ± 0.18 | 4.38 ± 0.29 | −10.58 ± 2.01 | −12.02 ± 0.89 |
Isotopic Niches
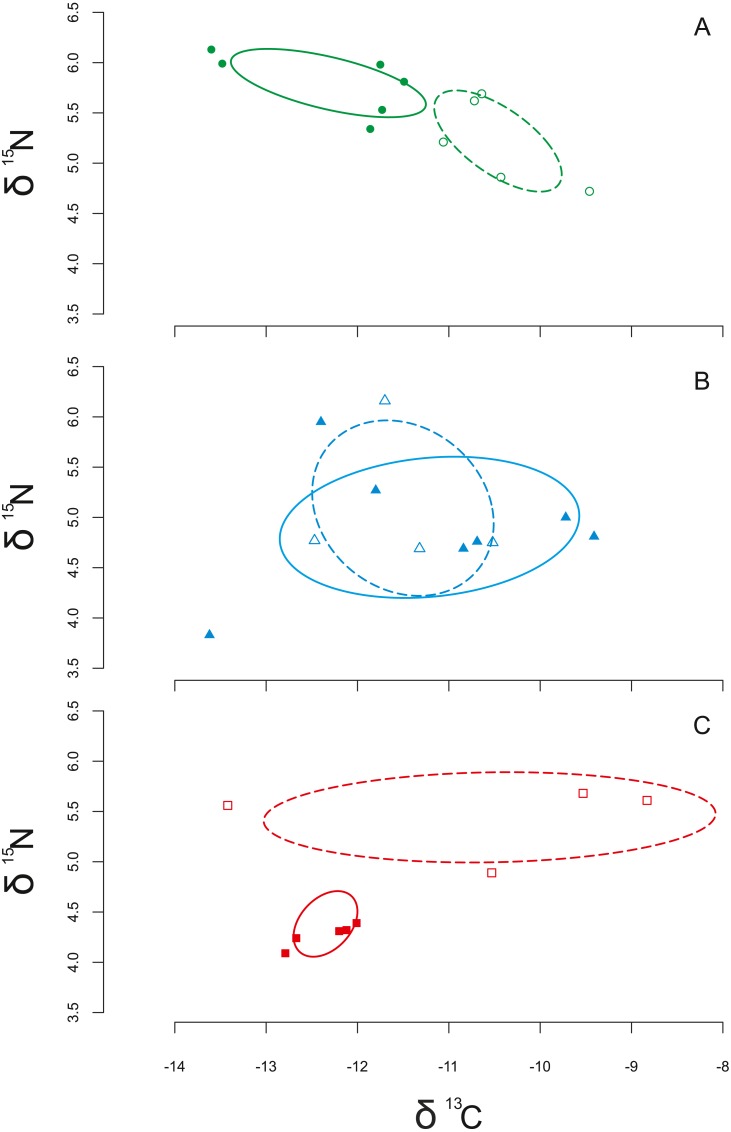
Table 5 shows data on isotopic niche breadth as measured by the corrected standard ellipse area (SEAc). The Stable Isotope Bayesian Ellipses in R (SIBER) analysis suggested a reduction in trophic niche particularly in Xcalak. This site showed the main difference in the trophic niche breadth with SEAc of 3.48 and 0.14 (WSE and USE respectively). An overlap of isotopic niches between WSE and USE was only found in Xahuayxol (Fig. 5). SEAc was higher USE in this site with 3.57 versus 2.68 SEAc WSE (Fig. 5).
Table 5. Trophic niche breadth of sea urchins without Sargassum effect (WSE) and under Sargassum effect (USE) at Mahahual, Xahuayxol and Xcalak calculated by SIBER analysis of muscle values.
SEAc, corrected standard ellipse area.
| Niche breadth | Mahahual | Xahuayxol | Xcalak | |||
|---|---|---|---|---|---|---|
| WSE | USE | WSE | USE | WSE | USE | |
| SEA | 0.62 | 0.71 | 1.79 | 2.97 | 2.32 | 2.32 |
| SEAc | 0.83 | 0.89 | 2.68 | 3.57 | 3.48 | 0.14 |
Figure 5. Isotope niche breadth of the sea urchin Diadema antillarum.
Isotope niche breadth of the sea urchin Diadema antillarum at Mahahual (A), Xahuayxol (B) and Xcalak (C). Dotted lines are without Sargassum effect (WSE) and solid lines under Sargassum effect (USE).
Discussion
Our results provide evidence of the detrimental effect of Sargassum blooms on the physicochemical water properties and ecological processes in near-shore coral reef communities as recently has been identified in our study area (Rodríguez-Martínez, van Tussenbroek & Jordán-Dahlgren, 2016; van Tussenbroek et al., 2017; Cuevas, Uribe-Martínez & Liceaga-Correa, 2018). Particularly, the results provide evidence for the input of external carbon and nitrogen resulting from Sargassum blooms on benthic communities that alter the nutrient inputs and trophic niche for D. antillarum. These findings contribute to the growing recognition of the role of exogenous nutrient enrichment in modifying natural sources in a food web. Hence the organic matter inputs from Sargassum coupled with hypoxia leads to modification of natural algal resources for D. antillarum. Considering the detrimental effects this likely represents a nutrient limitation to sea urchin herbivory.
Onshore Sargassum exhibits physical processes of fragmentation, decomposition and remineralization by bacteria, meiofauna and grazers (Colombini & Chelazzi, 2003). The algae-derived organic matter, product of that decomposition, has an effect on in situ oxygen availability (Haas et al., 2010). Sargassum blooms clearly showed a negative impact hypoxic conditions found at the three studied reef lagoons (Fig. 3). This could ultimately drive the success of the communities’ nitrogen fixation, evidenced by depleted values of δ15N as reported by Dorado et al. (2012) and France (1995).
The dissolved oxygen values in the back reefs of our study areas were lower than the standard values for coral reefs dominated by algae (7.9 ± 0.5 mg l−1) according to Haas et al. (2010) and values reported by Camacho-Cruz et al. (2019) for Xahuayxol and Mahahual. This supports ideas from Kendrick et al. (2000) and Haas et al. (2010), who argue that benthic communities linked to reef lagoons are very susceptible to environmental degradation. Some benthic algae play an important play in the transfer of energy and can be catalyzers of oxygen dynamics in reefs due to coral reef associated algae-derived organic matter (Wild et al., 2010).
Isotopic variations in the algal resources
We found that the composition of benthic macroalgae assemblages were different under Sargassum and without Sargassum effect. USE showed a reduction in the taxonomic diversity of macroalgal food sources available to D. antillarum and isotope values presented substantially lower δ15N values (Table 2). The fact that there were fewer available algal sources in the USE condition implies that the trophic chain becomes less complex as the interaction of primary consumers with their resources is reduced (Phillips & Gregg, 2003).
Overall δ13C values ranged from −21.98 to −5.65‰ are similar to ranges reported by Fry & Sherr (1984) and Morillo-Velarde, Briones-Fourzán & Álvarez Filip (2018). Those authors reviewed the δ13C data of benthic algae, noting that values ranged between −30 and 5‰. δ15N overall algae values fluctuated from 0.02 to 5.68‰. Despite these values agree with the variation reported in other studies like Owens (1987) and France (1995), we found USE very low, ergo according to Lapointe et al. (2005) and France et al. (1998). These low 15N:14N ratios can be indicative of macroalgae living in oligotrophic reefs which experience nitrogen fixation (Montoya, Carpenter & Capone, 2002). In the presence of the leachates of decomposing Sargassum, it is possible that anaerobic bacteria gained significance over other benthic groups (Table 2), (Carpenter & Cox, 1974; Rooker, Turner & Holt, 2006), and could be the cause of the low macroalgal isotopic signatures. On the other hand, high values of δ15N in macroalgae are linked to land-based N enrichment sources, being a good indicator of anthropogenic nitrogen inputs (Umezawa et al., 2002) such as sewage discharges (Risk et al., 2009; Lapointe et al., 2011).
France (1995) reported nitrogen ranges of marine macroalgae from −3 to 18‰. The inconsistencies in this pattern with values of δ15N close to atmospheric signature of 0% suggest a fixation of nitrogen. Dorado et al. (2012) associated the depleted values of δ15N with nitrogen fixation and its impact on the trophic position of consumers. So, temporal difference between values in this study WSE and USE might be explained by the influence of organic input derived from floating Sargassum dragged components. We considered that it is likely that the Sargassum effect modifies organic matter dynamics. These modifications stem from changes in the oxygen levels, which were consistently reflected in the low δ15N values we recorded of for the primary producers.
Status of Diadema antillarum in the Mexican Caribbean
It is important to note that we focused our study on the most abundant species at the three localities and the most important shallow-bottom herbivore on Caribbean reefs (Carpenter, 1981; Hughes, 1994; Aronson & Precht, 2006; Kissling et al., 2014). For the Mexican Caribbean, there has been considerable variation in D. antillarum population data. Jordán-Garza et al. (2008) showed a high presence of D. antillarum with densities of more than 7 ind m−2 in several areas, including our study area. Jorgensen, Espinoza-Ávalos & Bahena-Basave (2008) reported densities of 12.6 ind m−2 after hurricane Dean. According to Maldonado-Sánchez (2018) population density of D. antillarum displayed <1 ind m−2 for five different habitats of the Parque Nacional Arrecifes de Xcalak (PNAX) reef lagoon (back reef, seagrasses, sandy bottoms and reef patches) and the fore reef. The back reef exhibited the highest abundance with an average of 0.5 ind m−2. However for Mahahual, we registered an average density of 0.6 ind m−2 (N Cabanillas-Terán, pers. obs., 2017), because of the broad variability exhibited in D. antillarum populations from the back reef.
Trophic parameters of D. antillarum
Our results support the evidence that Sargassum blooms impacted δ15N differentially among sites, as the ratios of δ15N and δ13C are determined by their resources (Phillips & Gregg, 2003). It was conspicuous that D. antillarum showed higher δ15N values USE at Mahahual.
Although some available resources (e.g., Dictyota and turf) were present in both conditions (WSE and USE), measuring the contribution of algae to the sea urchin tissues can display key information about how consumers assimilate habitat resources and this could reveal information on the degree of disturbance (Layman et al., 2007b). Therefore, it is possible that the ecological role of D. antillarum was different in each site and could be explained by the variation in the number of available resources and a differential assimilation (Table 3). The higher δ15N values USE in the muscle of D. antillarum were a result of the synergistic effect determined by resource availability and disturbance condition.
Pelagic sources may provide new sources of food and the possible nitrogen fixation carried out by turf attached to pelagic Sargassum undoubtedly brought a new source of organic matter to basal trophic levels (Rooker, Turner & Holt, 2006). However, those sources were not major contributors for D. antillarum and appear to avoid the invasive pelagic macroalgae. This is consistent with the feeding ecology by marine generalist herbivores (Boudouresque & Verlaque, 2001) and such feeding response is in line with evidence from other sea urchin species in the face of other invasive resources. The experiments carried out by Tomas, Box & Terrados (2011) provide evidence that some seaweed invaders were strongly avoided by Paracentrotus lividus and therefore escape enemy control by reducing herbivore preference.
The trophic level metric is very useful because the classical discrete trophic level definitions ignore the value of food web connections, omnivory, and diet changes (Polis & Strong, 1996; Vanderklift, Kendrick & Smit, 2006). Generally the sea urchin D. antillarum has been considered as a generalist herbivore (Ogden & Lobel, 1978; Sammarco, 1980; Solandt & Campbell, 2001; Weil, Torres & Ashton, 2005). Morillo-Velarde, Briones-Fourzán & Álvarez Filip (2018) found that for the North of Quintana Roo Mexico D. antillarum occupied an herbivorous trophic position. However, invertebrate samples have been found in the stomach contents this species in the Caribbean, suggesting omnivorous behaviour (Rotjan & Lewis, 2008; Rodríguez-Barreras et al., 2015; Rodríguez-Barreras et al., 2016).
The mean trophic level for D. antillarum exhibited at Mahahual was 2.35 ± 0.18 WSE up to 3.08 ± 0.13 USE. Hence, WSE supported the idea that this species occupies an herbivorous position. However USE D. antillarum revealed that the species can occupy different trophic niches when faced with resource limitation. Under Sargassum blooms, D. antillarum displayed a position more in line with omnivorous conditions, suggesting trophic level indicative of herbivorous behaviour tending towards omnivory, according to Vander Zanden & Rasmussen (1999). These authors stated that primary consumers have a trophic position of 2.0 (strictly herbivorous); but if organisms assimilate primary consumers, they are considered to be a trophic level of 3.0. The results for Mahahual are consistent with Andrew (1989) who argued that sea urchins could take advantage of ecosystem changes through omnivory if variation exists in the availability of resources. Our results suggest that D. antillarum behave as a facultative omnivore depending on patterns of nutrient availability. δ15N signatures for D. antillarum in Mahahual suggest a different carbon source USE. These signatures are also likely the result of anthropogenic nitrogen inputs, as this site has a high eutrophication, being an area with elevated touristic demand (Martínez-Rendis et al., 2016; Arias-González et al., 2017). Furthermore, possible nitrogen fixation by anaerobic bacteria as an important factor in the variation of available sources of food.
Regarding the TL values exhibited for D. antillarum in Mahahual USE 3.08 ± 0.13 versus 2.35 ± 0.18 for WSE would place D. antillarum in an omnivorous position tending towards carnivory. Similar values were obtained from Mediterranean sea urchins as a strategy to avoid exclusion by sympatric species (Wangensteen et al., 2011). However, we cannot state that D. antillarum is carnivorous in Mahahual. This would require a more complete temporal study, and an adjustment of a new δ15N baseline for primary producers, considering that 15N/14N ratios can vary spatially and temporally (Jennings et al., 1997; Vanderklift, Kendrick & Smit, 2006).
The results for Xahuayxol showed also a trend towards higher δ15N. However by analyzing the condition of D. antillarum in Xahuayxol no significant differences were observed. We can assume that this locality was least changed in its foraging behavior position against the nutrients modification and the species occupied a lower trophic level WSE. Meanwhile, Xcalak displayed the opposite trend compared to Mahahual and Xahuayxol and USE D. antillarum trophic level was lower than WSE. Our results suggest that for Xcalak the effect of Sargassum blooms completely modified and reduced the possibility for finding available resources, displaying a trophic level around 2.5 between the two scenarios of Sargassum blooms. This corresponds to a predominantly herbivorous to omnivorous condition. Moreover this was confirmed with the isotopic niche breadth data where a reduced niche was observed for Xcalak (Fig. 3).
The rank found for D. antillarum in this study is consistent with the study conducted by Rodríguez-Barreras et al. (2015) in Puerto Rico where microinvertebrates were used as source of organic matter by the sea urchin. Finally, TL values support the premise that echinoids are able to modify their foraging behaviour depending on the availability of resources (Randall, Schroeder & Starck, 1964; Muthiga & McClanahan, 2007), and in this case under Sargassum blooms condition was not only determined by macroalgae availability, but for unusual conditions that caused a shift in the ecosystem (Cabanillas-Terán et al., 2016).
Isotopic niche breadth
The ellipses provide integrated information on the relationship between the availability of sources and the niche width. The results of Mahahual indicated that in USE. D. antillarum consumes different carbon and nitrogen sources (Fig. 4).
Several studies (Lawrence, 1975; Carpenter, 1981; Sammarco, 1982; Hay & Fenical, 1988) noted that echinoids have the ability to adapt their foraging behavior depending on algae availability as well as their population density and site characteristics (Bak, Carpay & De Ruyter Van Steveninck, 1984; Bak, 1994; Alvarado et al., 2016). We observed at Mahahual that USE D. antillarum exhibited a broader trophic niche than WSE. Despite the limited resources this could lead to trophic overlap and stronger habitat degradation. SIAR results showed a resource shift and this could be explained in terms of omnivory as stated by France et al. (1998) “omnivory is a prevalent attribute of aquatic food webs”.
The trophic niche of Xahuayxol reflects that there was no difference in the use of carbon and nitrogen sources. It is noteworthy that for the case of Xcalak, the resulting isotopic niche of D. antillarum was significantly smaller under Sargassum effect. This is consistent with the metric that associates smaller niche amplitude with disturbed ecosystems (Layman et al., 2007b).
Limitations of the study
To assess the effect of differential management of Sargassum and to effectively evaluate the effect of disposal management, quantitative information on beach disposal would be necessary.
From our results, it is clear that algae communities were modified due to Sargassum. However, due to the structuring role of sea urchins, and, considering that algae respond to temporal variability naturally, it would be necessary to study changing gradients at different time scales. Such a temporal study would provide more conclusive information about the effect of Sargassum spp. on benthic communities.
It is necessary to strengthen the sampling effort to evaluate current population status. A more comprehensive discussion would need to include the interactions with other herbivorous/omnivorous species, that coexist at each site and whether, or how they carry out resource partitioning.
The metrics used in this study allowed us to evaluate the variation of the isotopic signatures that formed the trophic spectrum of D. antillarum under two different scenarios. Metric values based on an instantaneous characterization of a single food web provide a limited view of the food web. Therefore, to evaluate the trophic structure and consequently its functional structure, the most promising evaluations would have to include a comparison of multiple gradients, and, to examine the same food web on a longer temporal perspective.
The deposited biomass regarding to S. fluitans and S. natans did not include a measurement of the total arrived Sargassum blooms. However, our results established a baseline for the amounts that were more available for the echinoids that inhabit the back section of the Caribbean shallow reefs.
It would be challenging to evaluate the ecological role of other coexisting species (Echinometra viridis, E. lucunter and Eucidaris tribuloides), and to include samples of micro-invertebrates. However, this could offer new clues to the connectivity between sympatric species, including trophic loops and successional states of algal communities (Camus, Daroch & Opazo, 2008) within the benthic communities of coral reefs.
Conclusions
The present study provides an initial review of how trophic parameters of D. antillarum were modified by the impact of pelagic Sargassum blooms in the Mexican Caribbean. The results indicated that the effects of the leachates generated by the decomposition process, the input of organic material and deposition in its vegetal structures modify the organic matter in the environment and hence the isotopic signatures. This has negative consequences in the benthic trophic structure, limiting the natural herbivory of D. antillarum. The source of available carbon and nitrogen was modified, and the isotopic signatures of macroalgae associated with the reef sites exhibited significantly lower values of δ15N. Consequently, the trophic niches were changed and in the case of Xcalak, significantly reduced.
Supplemental Information
Values of algal biomass (grams dry weight m−2) at Mahahual, Xahuayxol and Xcalak.
Raw data of δ13C and δ15N of algal genus considered in the mixing model analysis taken from Mahahual, Xahayxol and Xcalak, the asterisks represent the sources under Sargassum effect.
Confidential supplemental file. We based our Figure 2 on this Figure by van Tussenbroek et al., 2017.
Permission Letter written by Alejandro A. Aragón-Moreno
Acknowledgments
We acknowledge Katie Cramer, Gerald Islebe and two anonymous reviewers for their valuable and helpful comments on the manuscript. We thank Comisión Nacional de Áreas Naturales Protegidas (CONANP) for logistic support and Alberto de Jesus-Navarrete for lending the multi-parameter water quality checker. The first author is grateful to María Alfaro-Padilla, Roberto Herrera-Pavón and James Boon for their logistic help during fieldwork and laboratory work, to Alejandro Aragón for his help in editing Fig. 1 and helping to modify Fig. 2, and Rebecca Friedel is acknowledged for improving the English of the manuscript.
Funding Statement
This work was funded by Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT) through the project: OGRMIS- DAC-UCR #001/2015 ECOSUR/SEMARNAT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Nancy Cabanillas-Terán, Email: ncabanillas@ecosur.mx.
Héctor A. Hernández-Arana, Email: hhernand@ecosur.mx.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Nancy Cabanillas-Terán conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Héctor A. Hernández-Arana conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Miguel-Ángel Ruiz-Zárate and Alejandro Vega-Zepeda performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Alberto Sanchez-Gonzalez analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The collection permit (PPF/DGOPA-002/17) was obtained from the Comisión Nacional de Acuacultura y Pesca (CONAPESCA).
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Alvarado et al. (2016).Alvarado JJ, Cortés J, Guzman H, Reyes-Bonilla H. Bioerosion by the sea urchin Diadema mexicanum along Eastern Tropical Pacific coral reefs. Marine Ecology. 2016;37:1088–1102. doi: 10.1111/maec.12372. [DOI] [Google Scholar]
- Alvarez-Filip et al. (2011).Alvarez-Filip L, Gill JA, Dulvy NK, Perry AL, Watkinson AR, Côté IM. Drivers of region-wide declines in architectural complexity on Caribbean reefs. Coral Reefs. 2011;30:1051–1060. doi: 10.1007/s00338-011-0795-6. [DOI] [Google Scholar]
- Andrew (1989).Andrew NL. Contrasting ecological implications of food limitation in sea urchins and herbivorous gastropods. Marine Ecology Progress Series. 1989;51:189–193. doi: 10.3354/meps051189. [DOI] [Google Scholar]
- Arellano-Verdejo, Lazcano-Hernandez & Cabanillas-Terán (2019).Arellano-Verdejo J, Lazcano-Hernandez HE, Cabanillas-Terán N. ERISNet: deep neural network for Sargassum detection along the coastline of the Mexican Caribbean. PeerJ. 2019;7:e6842. doi: 10.7717/peerj.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-González et al. (2017).Arias-González JE, Fung T, Seymour RM, Garza-Pérez JR, Acosta-González G, Bozec Y-M, Johnson CR. A coral-algal phase shift in Mesoamerica not driven by changes in herbivorous fish abundance. PLOS ONE. 2017;12(4):e0174855. doi: 10.1371/journal.pone.0174855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson & Precht (2006).Aronson RB, Precht WF. Conservation, precaution, and Caribbean reefs. Coral Reefs. 2006;25:441–450. doi: 10.1007/s00338-006-0122-9. [DOI] [Google Scholar]
- Bak (1994).Bak RPM. Sea urchin bioerosion on coral reefs: place in the carbonate budget and relevant variables. Coral Reefs. 1994;13:99–103. doi: 10.1007/BF00300768. [DOI] [Google Scholar]
- Bak, Carpay & De Ruyter Van Steveninck (1984).Bak RPM, Carpay M, De Ruyter Van Steveninck ED. Densities of the sea urchin Diadema antillarum before and after mass mortalities on the coral reefs of Curacao. Marine Ecology Progress Series. 1984;17:105–108. [Google Scholar]
- Bauman et al. (2010).Bauman AG, Burt JA, Feary DA, Marquis E, Usseglio P. Tropical harmful algal blooms: an emerging threat to coral reef communities? Marine Pollution Bulletin. 2010;60:2117–2122. doi: 10.1016/j.marpolbul.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Bearhop et al. (2004).Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H. Determining trophic niche width: a novel approach using stable isotope analysis. The Journal of Animal Ecology. 2004;73:1007–1012. doi: 10.1111/j.0021-8790.2004.00861.x. [DOI] [Google Scholar]
- Behmer & Joern (2008).Behmer ST, Joern A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1977–1982. doi: 10.1073/pnas.0711870105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David & Schell (2001).Ben-David M, Schell DM. Mixing models in analyses of diet using multiple stable isotopes: a response. Oecologia. 2001;127:180–184. doi: 10.1007/s004420000570. [DOI] [PubMed] [Google Scholar]
- Boecklen et al. (2011).Boecklen WJ, Yarnes CT, Cook BA, James AC. On the use of stable isotopes in trophic ecology. Annual Review of Ecology, Evolution, and Systematics. 2011;42:411–440. doi: 10.1146/annurev-ecolsys-102209-144726. [DOI] [Google Scholar]
- Boudouresque & Verlaque (2001).Boudouresque CF, Verlaque M. Ecology of Paracentrotus lividus. Developments in Aquaculture and Fisheries Science. 2001;32:177–216. doi: 10.1016/S0167-9309(01)80013-2. [DOI] [Google Scholar]
- Box & Cox (1964).Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 1964;26:211–243. [Google Scholar]
- Cabanillas-Terán et al. (2016).Cabanillas-Terán N, Loor-Andrade P, Rodríguez-Barreras R, Cortés J. Trophic ecology of sea urchins in coral-rocky reef systems, Ecuador. PeerJ. 2016;4:e1578. doi: 10.7717/peerj.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Cruz et al. (2019).Camacho-Cruz KA, Ortiz-Hernández MC, Sánchez A, Carrillo L, De Jesús Navarrete A. Water quality in the eastern karst region of the Yucatan Peninsula: nutrients and stable nitrogen isotopes in turtle grass, Thalassia testudinum. Environmental Science and Pollution Research. 2019:1–17. doi: 10.1007/s11356-019-04757-3. [DOI] [PubMed] [Google Scholar]
- Camus, Daroch & Opazo (2008).Camus PA, Daroch K, Opazo LF. Potential for omnivory and apparent intraguild predation in rocky intertidal herbivore assemblages from northern Chile. Marine Ecology Progress Series. 2008;361:35–45. doi: 10.3354/meps07421. [DOI] [Google Scholar]
- Carpenter (1981).Carpenter RC. Grazing by Diadema antillarum (Philippi) and its effects on the benthic algal community. Journal of Marine Research. 1981;39:749–765. [Google Scholar]
- Carpenter (1986).Carpenter RC. Partitioning herbivory and its effects on coral reef algal communities. Ecological Monographs. 1986;56:345–364. doi: 10.2307/1942551. [DOI] [Google Scholar]
- Carpenter & Cox (1974).Carpenter EJ, Cox JL. Production of pelagic Sargassum and a blue–green epiphyte in the western Sargasso Sea. Limnology and Oceanography. 1974;19:429–436. doi: 10.4319/lo.1974.19.3.0429. [DOI] [Google Scholar]
- Colombini & Chelazzi (2003).Colombini I, Chelazzi L. Influence of marine allochthonous input on sandy beach communities. Oceanography and Marine Biology: An Annual Review. 2003;41:115–159. [Google Scholar]
- Cramer et al. (2012).Cramer KL, Jackson JBC, Angioletti CV, Leonard-Pingel J, Guilderson TP. Anthropogenic mortality on coral reefs in Caribbean Panama predates coral disease and bleaching. Ecology Letters. 2012;15:561–567. doi: 10.1111/j.1461-0248.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- Cuevas, Uribe-Martínez & Liceaga-Correa (2018).Cuevas E, Uribe-Martínez A, Liceaga-Correa M De LÁ. A satellite remote-sensing multi-index approach to discriminate pelagic Sargassum in the waters of the Yucatan Peninsula, Mexico. International Journal of Remote Sensing. 2018;39:3608–3627. doi: 10.1080/01431161.2018.1447162. [DOI] [Google Scholar]
- DeNiro & Epstein (1981).DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta. 1981;45:341–351. doi: 10.1016/0016-7037(81)90244-1. [DOI] [Google Scholar]
- De Széchy et al. (2012).De Széchy MTM, Guedes PM, Baeta-Neves MH, Oliveira EN. Verification of Sargassum natans (Linnaeus) Gaillon (Heterokontophyta: Phaeophyceae) from the Sargasso Sea off the coast of Brazil, western Atlantic Ocean. Check List. 2012;8:638–641. doi: 10.15560/8.4.638. [DOI] [Google Scholar]
- Dorado et al. (2012).Dorado S, Rooker JR, Wissel B, Quigg A. Isotope baseline shifts in pelagic food webs of the Gulf of Mexico. Marine Ecology Progress Series. 2012;464:37–49. doi: 10.3354/meps09854. [DOI] [Google Scholar]
- Eklöf et al. (2008).Eklöf JS, De la Torre-Castro M, Gullström M, Uku J, Muthiga N, Lyimo T, Bandeira SO. Sea urchin overgrazing of seagrasses: a review of current knowledge on causes, consequences, and management. Estuarine, Coastal and Shelf Science. 2008;79:569–580. doi: 10.1016/j.ecss.2008.05.005. [DOI] [Google Scholar]
- France (1995).France RL. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnology and Oceanography. 1995;40:1310–1313. doi: 10.4319/lo.1995.40.7.1310. [DOI] [Google Scholar]
- France et al. (1998).France R, Holmquist J, Chandler M, Cattaneo A. δ15N evidence for nitrogen fixation associated with macroalgae from a seagrass-mangrove-coral reef system. Marine Ecology Progress Series. 1998;167:297–299. doi: 10.3354/meps167297. [DOI] [Google Scholar]
- Franks, Johnson & Ko (2016).Franks JS, Johnson DR, Ko DS. Pelagic Sargassum in the tropical North Atlantic. Gulf and Caribbean Research. 2016;27:SC6–SC11. [Google Scholar]
- Fry (2006).Fry B. Stable isotope ecology. Springer; New York: 2006. [DOI] [Google Scholar]
- Fry & Sherr (1984).Fry B, Sherr EB. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions to Marine Science. 1984;27:13–47. [Google Scholar]
- Fry & Sherr (1989).Fry B, Sherr EB. δ13C Measurements as Indicators of Carbon Flow in Marine and Freshwater Ecosystems. In: Rundel PW, Ehleringer JR, Nagy KA, editors. Stable isotopes in ecological research. Springer; New York: 1989. pp. 196–229. [DOI] [Google Scholar]
- Gower, Young & King (2013).Gower J, Young E, King S. Satellite images suggest a new Sargassum source region in 2011. Remote Sensing Letters. 2013;4:764–773. doi: 10.1080/2150704X.2013.796433. [DOI] [Google Scholar]
- Haas et al. (2010).Haas AF, Jantzen C, Naumann MS, Iglesias-Prieto R, Wild C. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Marine Ecology Progress Series. 2010;409:27–39. doi: 10.3354/meps08631. [DOI] [Google Scholar]
- Hamaoka et al. (2010).Hamaoka H, Okuda N, Fukumoto T, Miyasaka H, Omori K. Seasonal dynamics of a coastal food web: stable isotope analysis of a higher consumer. In: Ohkouchi N, Tayasu I, Koba K, editors. Earth, life, and isotopes. Kyoto University Press; Kyoto: 2010. pp. 161–181. [Google Scholar]
- Hay & Fenical (1988).Hay ME, Fenical W. Marine plant-herbivore interactions: the ecology of chemical defense. Annual Review of Ecology and Systematics. 1988;19:111–145. doi: 10.1146/annurev.es.19.110188.000551. [DOI] [Google Scholar]
- Hobson (1999).Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- Hobson & Welch (1992).Hobson KA, Welch HE. Determination of trophic relationships within a high Arctic marine food web using δ 13 C and δ 15 N analysis. Marine Ecology Progress Series. 1992;84:9–18. doi: 10.3354/meps084009. [DOI] [Google Scholar]
- Hoffman (2009).Hoffman DM. Institutional legitimacy and co-management of a marine protected area: implementation lessons from the case of Xcalak Reefs National Park, Mexico. Human Organization. 2009;68:39–54. doi: 10.17730/humo.68.1.28gw1106u131143h. [DOI] [Google Scholar]
- Hughes (1994).Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Hunter & Price (1992).Hunter MD, Price PW. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology. 1992;73:724–732. [Google Scholar]
- Jackson et al. (2011).Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. The Journal of Animal Ecology. 2011;80:595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Jennings et al. (1997).Jennings S, Reñones O, Morales-Nin B, Polunin NV, Moranta J, Coll J. Spatial variation in the 15N and 13C stable isotope composition of plants, invertebrates and fishes on Mediterranean reefs: implications for the study of trophic pathways. Marine Ecology Progress Series. 1997;146:109–116. doi: 10.3354/meps146109. [DOI] [Google Scholar]
- Johnson et al. (2013).Johnson DR, Ko DS, Franks JS, Moreno P, Sanchez-Rubio G. The Sargassum Invasion of the Eastern Caribbean and Dynamics of the Equatorial North Atlantic Invasión de Sargazo en el Caribe Oriental y la Dinámica en la Zona Ecuatorial del Atlántico Norte L’Invasion de Sargasse dans les Caraïbes Orientales et leur Dynamique dans la. Proceedings of the 65th gulf and caribbean fisheries institute.2013. pp. 102–103. [Google Scholar]
- Jordán-Garza et al.(2008).Jordán-Garza AG, Maldonado MA, Baker DM, Rodríguez-Martínez RE. High abundance of Diadema antillarum on a Mexican reef. Coral Reefs. 2008;27:295–295. doi: 10.1007/s00338-007-0338-3. [DOI] [Google Scholar]
- Jorgensen, Espinoza-Ávalos & Bahena-Basave (2008).Jorgensen P, Espinoza-Ávalos J, Bahena-Basave H. High population density survival of the sea urchin Diadema antillarum (Philippi 1845) to a category 5 hurricane in southern Mexican Caribbean. Hidrobiológica. 2008;18:257–260. [Google Scholar]
- Kendrick et al. (2000).Kendrick GA, Hegge BJ, Wyllie A, Davidson A, Lord DA. Changes in seagrass cover on success and Parmelia Banks, Western Australia Between 1965 and 1995. Estuarine, Coastal and Shelf Science. 2000;50:341–353. doi: 10.1006/ecss.1999.0569. [DOI] [Google Scholar]
- Kissling et al. (2014).Kissling DL, Precht WF, Miller SL, Chiappone M. Historical reconstruction of population density of the echinoid Diadema antillarum on Florida Keys shallow bank-barrier reefs. Bulletin of Marine Science. 2014;90:665–679. doi: 10.5343/bms.2013.1022. [DOI] [Google Scholar]
- Lapointe et al. (2005).Lapointe BE, Barile PJ, Littler MM, Littler DS. Macroalgal blooms on southeast Florida coral reefs: II. Cross-shelf discrimination of nitrogen sources indicates widespread assimilation of sewage nitrogen. Harmful Algae. 2005;4:1106–1122. doi: 10.1016/j.hal.2005.06.002. [DOI] [Google Scholar]
- Lapointe et al. (2010).Lapointe BE, Langton R, Bedford BJ, Potts AC, Day O, Hu C. Land-based nutrient enrichment of the Buccoo Reef Complex and fringing coral reefs of Tobago, West Indies. Marine Pollution Bulletin. 2010;60:334–343. doi: 10.1016/j.marpolbul.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lapointe et al. (2011).Lapointe BE, Thacker K, Hanson C, Getten L. Sewage pollution in Negril, Jamaica: effects on nutrition and ecology of coral reef macroalgae. Chinese Journal of Oceanology and Limnology. 2011;29:775–789. doi: 10.1007/s00343-011-0506-8. [DOI] [Google Scholar]
- Lawrence (1975).Lawrence JM. On the relationships between marine plants and sea urchins. Oceanography and Marine Biology: An Annual Review. 1975;13:213–286. [Google Scholar]
- Layman et al. (2012).Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological reviews of the Cambridge Philosophical Society. 2012;87:545–562. doi: 10.1111/j.1469-185X.2011.00208.x. [DOI] [PubMed] [Google Scholar]
- Layman et al. (2007a).Layman CA, Arrington DA, Montaña CG, Post DM. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology. 2007a;88:42–48. doi: 10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Layman et al. (2007b).Layman CA, Quattrochi JP, Peyer CM, Allgeier JE. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecology Letters. 2007b;10:937–944. doi: 10.1111/j.1461-0248.2007.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessios et al. (1984).Lessios HA, Cubit JD, Robertson DR, Shulman MJ, Parker MR, Garrity SD, Levings SC. Mass mortality of Diadema antillarum on the Caribbean coast of Panama. Coral Reefs. 1984;3:173–182. doi: 10.1007/BF00288252. [DOI] [Google Scholar]
- Littler & Littler (2000).Littler DS, Littler MM. Caribbean reef plants. OffShore Graphics; Washington: 2000. [Google Scholar]
- Louime, Fortune & Gervais (2017).Louime C, Fortune J, Gervais G. Sargassum invasion of coastal environments: a growing concern. American Journal of Environmental Sciences. 2017;13:58–64. doi: 10.3844/ajessp.2017.58.64. [DOI] [Google Scholar]
- Maldonado-Sánchez (2018).Maldonado-Sánchez MA. PhD thesis. 2018. Influencia ecológica del erizo de mar Diadema antillarum (Philippi, 1845) sobre la estructuración de la comunidad bentónica de la laguna arrecifal de Xcalak, Quintana Roo. [Google Scholar]
- Maldonado-Sánchez et al. (2019).Maldonado-Sánchez J, Mariño Tapia I, Teresa Herrera-Dorantes M, Ardisson P-L. Hydrodynamic conditions that favor the settlement of Diadema antillarum to a western Caribbean coral reef. Bulletin of Marine Science. 2019;95:251–264. doi: 10.5343/bms.2018.0001. [DOI] [Google Scholar]
- Mariño-Tapia et al. (2010).Mariño-Tapia I, Silva R, Enriquez C, Mendoza-Baldwin E, Escalante-Mancera E, Ruiz-Rentería F. Wave transformation and wave-driven circulation on natural reefs under extreme hurricane conditions. Coastal Engineering Proceedings. 2010;32 Article 28. [Google Scholar]
- Martínez-Rendis et al. (2016).Martínez-Rendis M, Acosta-González G, Hernández-Stefanoni JL, Arias González JE. Quantifying the reefscape transformation of a coastal Caribbean coral reef during a phase shift and the associated coastal landscape change. Marine Ecology. 2016;37:697–710. doi: 10.1111/maec.12334. [DOI] [Google Scholar]
- Minagawa & Wada (1984).Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta. 1984;48:1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- Montoya, Carpenter & Capone (2002).Montoya JP, Carpenter EJ, Capone DG. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnology and Oceanography. 2002;47:1617–1628. doi: 10.4319/lo.2002.47.6.1617. [DOI] [Google Scholar]
- Moore & Semmens (2008).Moore JW, Semmens BX. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters. 2008;11:470–480. doi: 10.1111/j.1461-0248.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- Morillo-Velarde, Briones-Fourzán & Álvarez Filip (2018).Morillo-Velarde PS, Briones-Fourzán P, Álvarez Filip L. Habitat degradation alters trophic pathways but not food chain length on shallow Caribbean coral reefs. Scientific Reports. 2018;8:4109. doi: 10.1038/s41598-018-22463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthiga & McClanahan (2007).Muthiga NA, McClanahan TR. Developments in aquaculture and Fisheries Science. Elsevier; Amsterdam: 2007. Ecology of Diadema; pp. 205–225. [Google Scholar]
- Newsome et al. (2007).Newsome SD, Martinez del Rio C, Bearhop S, Phillips DL. A niche for isotopic ecology. Frontiers in Ecology and the Environment. 2007;5:429–436. doi: 10.1890/060150.1. [DOI] [Google Scholar]
- Ogden & Lobel (1978).Ogden JC, Lobel PS. The role of herbivorous fishes and urchins in coral reef communities. Environmental Biology of Fishes. 1978;3:49–63. doi: 10.1007/BF00006308. [DOI] [Google Scholar]
- Owens (1987).Owens NJP. Natural Variations in 15N in the Marine Environment. In: Blaxter JHS, Southward AJ, editors. Advances in marine biology. Academic Press; New York: 1987. pp. 389–451. [DOI] [Google Scholar]
- Oyesiku & Egunyomi (2014).Oyesiku OO, Egunyomi A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. African Journal of Biotechnology. 2014;13:1188–1193. doi: 10.5897/AJB2013.12335. [DOI] [Google Scholar]
- Parnell et al. (2010).Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: coping with too much variation. PLOS ONE. 2010;5(3):e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell & Jackson (2013).Parnell A, Jackson A. siar: stable isotope analysis in R. R package version 4.2. http://CRAN.R-project.org/package=siar. 2013 (23 March 2014) [Links]
- Peterson (1999).Peterson BJ. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecologica. 1999;20:479–487. doi: 10.1016/S1146-609X(99)00120-4. [DOI] [Google Scholar]
- Peterson & Fry (1987).Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics. 1987;18:293–320. doi: 10.1146/annurev.es.18.110187.001453. [DOI] [Google Scholar]
- Phillips & Gregg (2003).Phillips DL, Gregg JW. Source partitioning using stable isotopes: coping with too many sources. Oecologia. 2003;136:261–269. doi: 10.1007/s00442-003-1218-3. [DOI] [PubMed] [Google Scholar]
- Phillips et al. (2014).Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology. 2014;92:823–835. doi: 10.1139/cjz-2014-0127. [DOI] [Google Scholar]
- Phillips & Koch (2002).Phillips DL, Koch PL. Incorporating concentration dependence in stable isotope mixing models. Oecologia. 2002;130:114–125. doi: 10.1007/s004420100786. [DOI] [PubMed] [Google Scholar]
- Polis & Strong (1996).Polis GA, Strong DR. Food web complexity and community dynamics. The American Naturalist. 1996;147:813–846. doi: 10.1086/285880. [DOI] [Google Scholar]
- Polunin et al. (2001).Polunin NVC, Morales-Nin B, Pawsey WE, Cartes JE, Pinnegar JK, Moranta J. Feeding relationships in Mediterranean bathyal assemblages elucidated by stable nitrogen and carbon isotope data. Marine Ecology Progress Series. 2001;220:13–23. doi: 10.3354/meps220013. [DOI] [Google Scholar]
- Post et al. (2007).Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007;152:179–189. doi: 10.1007/s00442-006-0630-x. [DOI] [PubMed] [Google Scholar]
- Prado, Alcoverro & Romero (2010).Prado P, Alcoverro T, Romero J. Influence of nutrients in the feeding ecology of seagrass (Posidonia oceanica L.) consumers: a stable isotopes approach. Marine Biology. 2010;157:715–724. doi: 10.1007/s00227-009-1355-2. [DOI] [Google Scholar]
- Randall, Schroeder & Starck (1964).Randall JE, Schroeder RE, Starck WA. Notes on the biology of the echinoid Diadema antillarum. Caribbean Journal of Science. 1964;4:421–433. [Google Scholar]
- Risk et al. (2009).Risk MJ, Lapointe BE, Sherwood OA, Bedford BJ. The use of δ15N in assessing sewage stress on coral reefs. Marine Pollution Bulletin. 2009;58:793–802. doi: 10.1016/j.marpolbul.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Rodríguez (2003).Rodríguez SR. Consumption of drift kelp by intertidal populations of the sea urchin Tetrapygus niger on the central Chilean coast: possible consequences at different ecological levels. Marine Ecology Progress Series. 2003;251:141–151. doi: 10.3354/meps251141. [DOI] [Google Scholar]
- Rodríguez-Barreras et al. (2016).Rodríguez-Barreras R, Cuevas E, Cabanillas-Terán N, Branoff B. Understanding trophic relationships among Caribbean sea urchins. Revista de Biología Tropical. 2016;64:837–848. doi: 10.15517/rbt.v64i2.19366. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barreras et al. (2015).Rodríguez-Barreras R, Cuevas E, Cabanillas-Terán N, Sabat AM. Potential omnivory in the sea urchin Diadema antillarum? Regional Studies in Marine Science. 2015;2:11–18. doi: 10.1016/j.rsma.2015.08.005. [DOI] [Google Scholar]
- Rodríguez-Martínez et al. (2019).Rodríguez-Martínez RE, Medina-Valmaseda AE, Blanchon P, Monroy-Velázquez LV, Almazán-Becerril A, Delgado-Pech B, Vásquez-Yeomans L, Francisco V, García-Rivas MC. Faunal mortality associated with massive beaching and decomposition of pelagic Sargassum. Marine Pollution Bulletin. 2019;146:201–205. doi: 10.1016/j.marpolbul.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez, van Tussenbroek & Jordán-Dahlgren (2016).Rodríguez-Martínez RE, van Tussenbroek B, Jordán-Dahlgren E. Afluencia masiva de sargazo pelágico a la costa del Caribe mexicano (2014-2015) In: García-Mendoza E, Quijano-Scheggia SI, Olivos-Ortiz A, Núñez-Vázquez EJ, editors. Florecimientos Algales Nocivos en México. CICESE; Ensenada: 2016. pp. 352–365. [Google Scholar]
- Rooker, Turner & Holt (2006).Rooker JR, Turner JP, Holt SA. Trophic ecology of Sargassum-associated fishes in the Gulf of Mexico determined from stable isotopes and fatty acids. Marine Ecology Progress Series. 2006;313:249–259. doi: 10.3354/meps313249. [DOI] [Google Scholar]
- Rotjan & Lewis (2008).Rotjan RD, Lewis SM. Impact of coral predators on tropical reefs. Marine Ecology Progress Series. 2008;367:73–91. doi: 10.3354/meps07531. [DOI] [Google Scholar]
- Sala et al. (1998).Sala E, Ribes M, Hereu B, Zabala M, Alvà V, Coma R, Garrabou J. Temporal variability in abundance of the sea urchins Paracentrotus lividus and Arbacia lixula in the northwestern Mediterranean: comparison between a marine reserve and an unprotected area. Marine Ecology Progress Series. 1998;168:135–145. doi: 10.3354/meps168135. [DOI] [Google Scholar]
- Sammarco (1980).Sammarco PW. Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance. Journal of Experimental Marine Biology and Ecology. 1980;45:245–272. doi: 10.1016/0022-0981(80)90061-1. [DOI] [Google Scholar]
- Sammarco (1982).Sammarco PW. Echinoid grazing as a structuring force in coral communities: whole reef manipulations. Journal of Experimental Marine Biology and Ecology. 1982;61:31–55. doi: 10.1016/0022-0981(82)90020-X. [DOI] [Google Scholar]
- Schell, Goodwin & Siuda (2015).Schell JM, Goodwin DS, Siuda ANS. Recent Sargassum inundation events in the Caribbean: shipboard observations reveal dominance of a previously rare form. Oceanography. 2015;28:8–11. [Google Scholar]
- Schmitter-Soto et al. (2018).Schmitter-Soto JJ, Aguilar-Perera A, Cruz-Martínez A, Herrera-Pavón RL, Morales-Aranda AA, Cobián-Rojas D. Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biodiversity and Conservation. 2018;27:459–474. doi: 10.1007/s10531-017-1446-1. [DOI] [Google Scholar]
- Sissini et al. (2017).Sissini MN, De Barros Barreto MBB, Széchy MTM, De Lucena MB, Oliveira MC, Gower J, Liu G, De Oliveira Bastos E, Milstein D, Gusmão F, Martinelli-Filho JE, Alves-Lima C, Colepicolo P, Ameka G, De Graft-Johnson K, Gouvea L, Torrano-Silva B, Nauer F, Marcos de Castro Nunes J, Barufi JB, Rörig L, Riosmena-Rodríguez R, Mello TJ, Lotufo LVC, Horta PA. The floating Sargassum (Phaeophyceae) of the South Atlantic Ocean—likely scenarios. Phycologia. 2017;56:321–328. doi: 10.2216/16-92.1. [DOI] [Google Scholar]
- Smetacek & Zingone (2013).Smetacek V, Zingone A. Green and golden seaweed tides on the rise. Nature. 2013;504:84–88. doi: 10.1038/nature12860. [DOI] [PubMed] [Google Scholar]
- Solandt & Campbell (2001).Solandt JL, Campbell AC. Macroalgal feeding characteristics of the sea urchin Diadema antillarum Philippi at Discovery Bay, Jamaica. Caribbean Journal of Science. 2001;37:227–238. [Google Scholar]
- Solarin et al. (2014).Solarin BB, Bolaji DA, Fakayode OS, Akinnigbagbe RO. Impacts of an invasive seaweed Sargassum hystrix var. fluitans (borgesen 1914) on the fisheries and other economic implications for the nigerian coastal waters. IOSR Journal of Agriculture and Veterinary Science. 2014;7:1–6. [Google Scholar]
- Steneck & Lang (2003).Steneck RS, Lang JC. Mexico. Rapid assessment of Mexico’s Yucatan reef in 1997 and 1999: pre-and post-1998 mass bleaching and Hurricane Mitch (Stony Corals, Algae and Fishes) Atoll Research Bulletin. 2003;496(17):294–317. doi: 10.5479/si.00775630.496-17.294. [DOI] [Google Scholar]
- Sweatman, Layman & Fourqurean (2017).Sweatman JL, Layman CA, Fourqurean JW. Habitat fragmentation has some impacts on aspects of ecosystem functioning in a sub-tropical seagrass bed. Marine Environmental Research. 2017;126:95–108. doi: 10.1016/j.marenvres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Tomas et al. (2006).Tomas F, Alvarez-Cascos D, Turon X, Romero J. Differential element assimilation by sea urchins Paracentrotus lividus in seagrass beds: implications for trophic interactions. Marine Ecology Progress Series. 2006;306:125–131. doi: 10.3354/meps306125. [DOI] [Google Scholar]
- Tomas, Box & Terrados (2011).Tomas F, Box A, Terrados J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biological Invasions. 2011;13:1559–1570. doi: 10.1007/s10530-010-9913-6. [DOI] [Google Scholar]
- Umezawa et al. (2002).Umezawa Y, Miyajima T, Yamamuro M, Kayanne H, Koike I. Fine-scale mapping of land-derived nitrogen in coral reefs by δ15N in macroalgae. Limnology and Oceanography. 2002;47:1405–1416. doi: 10.4319/lo.2002.47.5.1405. [DOI] [Google Scholar]
- van Tussenbroek et al. (2017).van Tussenbroek BI, Hernández-Arana HA, Rodríguez-Martínez RE, Espinoza-Avalos J, Canizales-Flores HM, González-Godoy CE, Barba-Santos MG, Vega-Zepeda A, Collado-Vides L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Marine Pollution Bulletin. 2017;122:272–281. doi: 10.1016/j.marpolbul.2017.06.057. [DOI] [PubMed] [Google Scholar]
- Vander Zanden & Rasmussen (1996).Vander Zanden MJ, Rasmussen JB. A trophic position model of pelagic food webs: impact on contaminant bioaccumulation in Lake Trout. Ecological Monographs. 1996;66:451–477. doi: 10.2307/2963490. [DOI] [Google Scholar]
- Vander Zanden & Rasmussen (1999).Vander Zanden M, Rasmussen JB. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology. 1999;80:1395–1404. doi: 10.1890/0012-9658(1999)080[1395:PCCANA]2.0.CO;2. [DOI] [Google Scholar]
- Vander Zanden & Rasmussen (2001).Vander Zanden M, Rasmussen JB. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography. 2001;46:2061–2066. doi: 10.4319/lo.2001.46.8.2061. [DOI] [Google Scholar]
- Vanderklift, Kendrick & Smit (2006).Vanderklift MA, Kendrick GA, Smit AJ. Differences in trophic position among sympatric sea urchin species. Estuarine, Coastal and Shelf Science. 2006;66:291–297. doi: 10.1016/j.ecss.2005.09.004. [DOI] [Google Scholar]
- Wang et al. (2019).Wang M, Hu C, Barnes BB, Mitchum G, Lapointe B, Montoya JP. The great Atlantic Sargassum belt. Science. 2019;365:83–87. doi: 10.1126/science.aaw7912. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang M, Hu C, Cannizzaro J, English D, Han X, Naar D, Lapointe B, Brewton R, Hernandez F. Remote sensing of Sargassum biomass, nutrients, and pigments. Geophysical Research Letters. 2018;45:12–359. doi: 10.1029/2018GL078858. [DOI] [Google Scholar]
- Wangensteen et al. (2011).Wangensteen OS, Turon X, García-Cisneros A, Recasens M, Romero J, Palacín C. A wolf in sheep’s clothing: carnivory in dominant sea urchins in the Mediterranean. Marine Ecology Progress Series. 2011;441:117–128. [Google Scholar]
- Weil, Torres & Ashton (2005).Weil E, Torres JL, Ashton M. Population characteristics of the sea urchin Diadema antillarum in La Parguera, Puerto Rico, 17 years after the mass mortality event. Revista de Biologia Tropical. 2005;53(Suppl 3):219–231. [PubMed] [Google Scholar]
- Wild et al. (2010).Wild C, Haas A, Naumann M, Mayr C, El-Zibdah M. Comparative investigation of organic matter release by corals and benthic reef algae–implications for pelagic and benthic microbial metabolism. Coral reefs in a time of change–case studies to understand potential biogeochemical consequences of phase shifts from corals to benthic algae. Proceedings of the 11th international coral reef symposium, vol. 121; 2010. pp. 1319–1323. [Google Scholar]
- Wing et al. (2008).Wing SR, McLeod RJ, Clark KL, Frew RD. Plasticity in the diet of two echinoderm species across an ecotone: microbial recycling of forest litter and bottom-up forcing of population structure. Marine Ecology Progress Series. 2008;360:115–123. doi: 10.3354/meps07434. [DOI] [Google Scholar]
- Zar (1999).Zar JH. Biostatistical analysis. Pearson Education; London: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values of algal biomass (grams dry weight m−2) at Mahahual, Xahuayxol and Xcalak.
Raw data of δ13C and δ15N of algal genus considered in the mixing model analysis taken from Mahahual, Xahayxol and Xcalak, the asterisks represent the sources under Sargassum effect.
Confidential supplemental file. We based our Figure 2 on this Figure by van Tussenbroek et al., 2017.
Permission Letter written by Alejandro A. Aragón-Moreno
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.