Abstract
Forest disturbances leading to replacement of whole tree stands are a cornerstone of forest dynamics, with drivers including fire, wind-throw, biotic outbreaks and harvest. The frequency of disturbances may change over the next century, impacting the age, composition and biomass of forests. However, the variation in disturbance return time, i.e. the mean interval between disturbance events, across the world’s forested biomes remains poorly characterised, hindering quantification of their role in the global carbon cycle. Here we present the global distribution of stand-replacing disturbance return time inferred from satellite-based observations of forest loss. Prescribing this distribution within a vegetation model with a detailed representation of stand structure, we quantify the importance of stand-replacing disturbances for biomass carbon turnover globally over 2001-2014. Return time varied from less than 50 years in heavily-managed temperate ecosystems to over 1000 years in tropical evergreen forests. Stand-replacing disturbances accounted for 12.3% (95% confidence interval, 11.4-13.7%) of annual biomass carbon turnover due to tree mortality globally, and in 44% of forested area biomass stocks are strongly sensitive to changes in disturbance return time. Relatively small shifts in disturbance regimes in these areas would substantially influence the forest carbon sink, that currently limits climate change by offsetting emissions.
The amount of carbon stored in global forest biomass is similar to that in the atmosphere1, and, excluding the effects of land-use change, has been estimated to have grown at ca. 1.5 PgC a-1 over recent decades2. This uptake has significantly slowed the atmospheric growth rate of carbon dioxide and thus the rate of climate change3. The accumulation of carbon in the stems of growing trees results from the balance between the growth rates of vegetation and the average length of time carbon remains in live biomass (hereafter, “biomass”), the turnover time, calculated as the carbon stock divided by the flux of carbon loss through plant and tissue death4. Quantification of this turnover time is crucial because it governs the size of biomass stocks for a given growth rate and it is one of the most significant uncertainties affecting projections of the terrestrial carbon cycle5,6. Large-scale estimates of carbon turnover times for whole ecosystems and for biomass have been recently developed7,8, but offer limited insight into the processes governing biomass stocks because the turnover flux could only be approximated by estimates of net primary productivity (NPP). This conflates turnover of soft tissues, such as leaves and roots, with that of the woody carbon pools that dominate biomass carbon stocks1. To understand forest biomass turnover times globally, large-scale tree mortality rates must be quantified.
Tree death is often the culmination of a prolonged period of physiological stress related to a shortage of essential resources required for the synthesis of basic metabolites, for instance, due to shading by other plants, low water availability, or a shortage of soil nutrients such as N and P in plant-available forms9,10. Alternatively, disturbances such as wind-throw, fire, insect and disease outbreaks, or anthropogenic activities such as wood harvest may constitute the cause of death11–16. Disturbances act on scales ranging from a single tree to whole forest stands or landscapes. Here we investigate stand-replacing disturbances, defined operationally as discrete events resulting in the death of all, or almost all, living tree biomass at a scale of 0.1 ha or larger. Such events affect the average tree age as well as the stature, density and composition of forest stands11,17, in turn impacting carbon storage18. There is evidence that the frequency of disturbances may be changing globally, with continued change likely in the future8,13–15,19,20. Yet, to understand the consequences of future changes, it is first necessary to provide a baseline of current conditions. Such a baseline is lacking for stand-replacing disturbances across global forests.
The frequency of stand-replacing disturbances
We estimated the frequency of stand-replacing disturbances across all global forests at 1° spatial resolution. Drawing on a Landsat-based (2000-2014) forest-loss product21, we performed a space-for-time substitution, calculating the disturbance rotation period, τ, defined as the mean time period for the area disturbed to equal the area of the grid cell11,
| (1) |
where i is a grid-cell index, AT is total forest area in that 1° x 1° grid cell, the annual mean total forest loss over 2000-2014 calculated based on all Landsat-pixels within the grid cell and the annual mean forest loss due to conversion to a non-forest land-use type (Methods). This observation-based τ is referred to as τo. While rare disturbances may be undersampled for individual grid cells, this is less of an issue across a neighbourhood of many grid cells, and the global-scale pattern is expected to be robust (Suppl. Note 1). We take τ as indicative of the typical or average disturbance return time from all causes at any location in a grid cell, reflecting causes such as wood harvest, as well as natural disturbances such as fires, large-scale wind-throws and biotic outbreaks. Whilst the drivers of each of these disturbance agents differ markedly, their consequences for carbon turnover in live biomass are assumed to be similar at large scales. Disturbances associated with the conversion of forests to other land-uses were explicitly excluded (Methods), allowing us to focus on dynamics within closed-canopy forests.
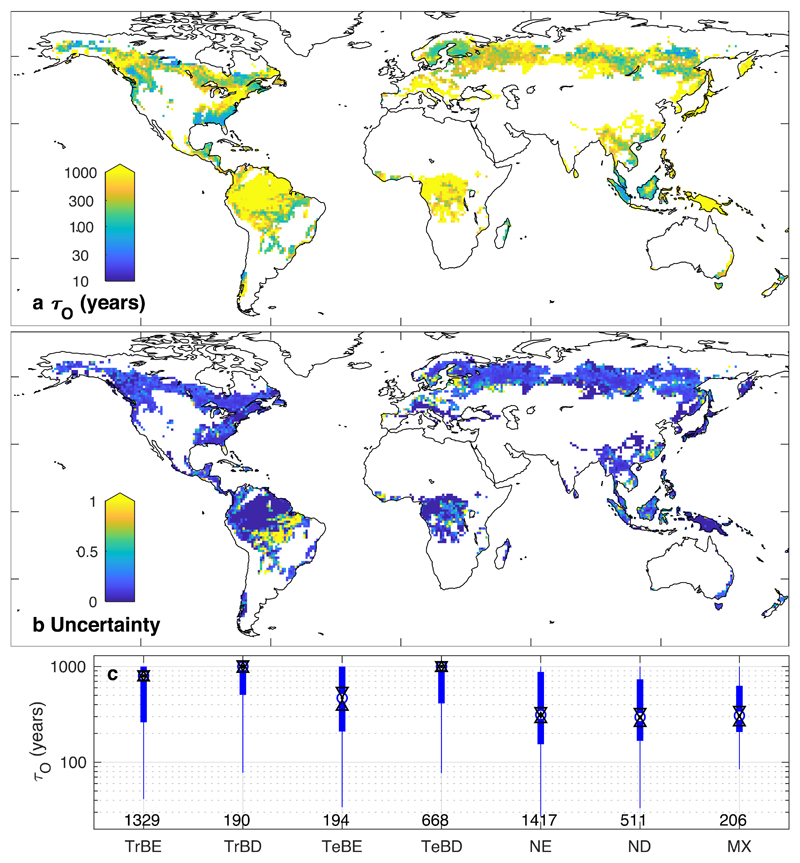
Disturbance return time varies substantially across the global forest area (Fig. 1a). The stand-replacing disturbances quantified here are more common in needleleaf and mixed forests (median τo of ca. 300 years) than they are in temperate or tropical broadleaf forests (median τo of 830 to more than 1000 years), however forest type emerges as a poor predictor of the spatial distribution of τo (Fig. 1c). In large areas of forest, stand-replacing disturbances are actually very rare events; 35% of forest area experienced stand-replacing disturbances with an average frequency equivalent to less than once every thousand years. In these forests the vast majority of tree mortality must thus be non-stand-replacing. The 95% confidence intervals for τo typically span a range less than one third of the absolute value of τo, except in areas of substantial land-use change (Fig. 1b, Suppl. Fig. 7).
Figure 1. Forest disturbance rotation periods.
a, τo calculated over 2000-2014. b, Uncertainty in τo, displayed as the difference between the 95% confidence intervals divided by the central estimate. Uncertainty values of zero reflect 95% confidence that τo is over 1000 years. c, Boxplot of τo grouped by forest type (see Methods for codes). Circles show the median value, black triangles the 95% confidence limits of the median, thick lines the interquartile range and whiskers extend to a maximum of 1.5 times the interquartile range. Numbers indicate the number of grid cells for each forest type.
We compared our results against an inventory-based compilation of forest stand age (GFADv1.122). Despite the different scale and characteristics of inventory data we found qualitative consistency in tropical evergreen and boreal forests, as well as some regions under intensive forest management, but also suggestions of a substantial amount of disturbance in some temperate forests below the scale captured in the Landsat data and of legacies of past afforestation (Suppl. Note 2, Suppl. Fig. 1). We also found consistency between our results and previous studies of disturbance frequencies in the tropics17,23,24 and Canada25 (Suppl. Note 3; Suppl. Fig. 2), and biotic outbreak disturbances in the U.S.A.26 (Suppl. Note 3; Suppl. Fig. 3).
Influence of stand-replacing disturbances on the carbon cycle
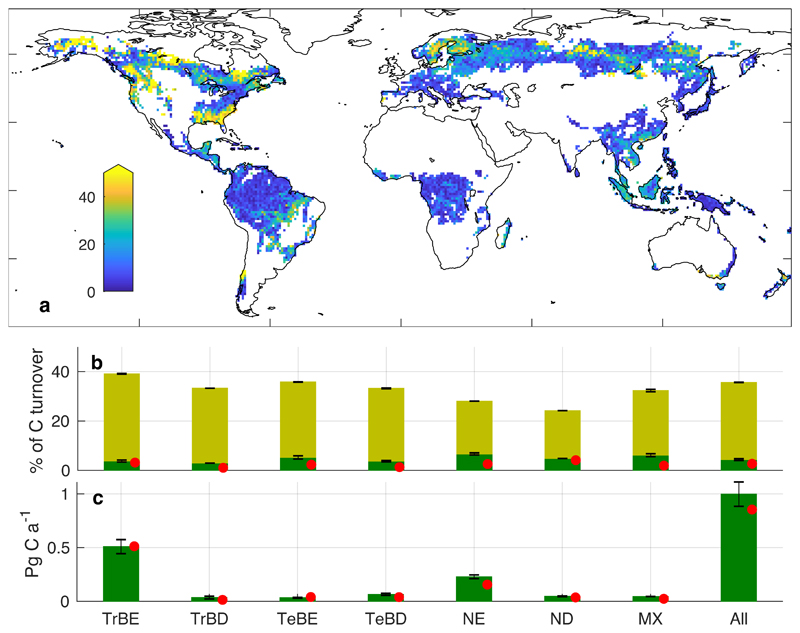
We apply the gridded estimates of τo within a dynamic global vegetation model (DGVM) with an explicit representation of forest stand structural development. τ was kept constant in each grid cell for the entire model simulation, calculating the pseudoequilibrium effect of τo on forest dynamics. Stand-replacing disturbances are simulated to dominate overall tree mortality, and associated carbon turnover, across large areas of the mid-latitude and boreal forests, accounting for over 60% in some locations, but are not the dominant cause of mortality in most tropical forests (Fig. 2a,b).
Figure 2. Carbon turnover fluxes from closed-canopy forest for 2001-2014.
a, Fraction of carbon turnover fluxes resulting from vegetation mortality due to stand-replacing disturbances (colour scale capped at 50%), calculated using τo to drive LPJ-GUESS. Breakdown by forest type of: b, fraction of carbon turnover fluxes resulting from vegetation mortality (whole bars) and from stand-replacing disturbances (darker shading); c, total turnover flux of vegetation carbon due to stand-replacing disturbance. Error bars show the range of simulations driven by the 95% confidence intervals of τo. Red dots show results from an observationally-based cross-check method (Methods). Forest types as in Fig. 1.
The total turnover of biomass carbon as a result of stand-replacing disturbances at pseudo-equilibrium in our simulations is 1.00 (95% confidence interval, 0.91-1.11) PgC a-1, equivalent to 4.4 (4.0-4.9) % of total biomass carbon turnover in closed-canopy forests (i.e. including soft-tissue turnover) (Fig. 2b,c). These numbers are supported by an empirical cross-check based on satellite-derived NPP and biomass estimates which combined satellite LIDAR and radar observations with ground-based reference plots (red dots in Fig. 2b,c; Methods). The fraction of biomass carbon turnover due to mortality must be taken with caution, however, until biomass turnover rates from other forms of mortality can be fully constrained. Likewise the total turnover flux is dependent on accurate calculation of global biomass stocks, which remain uncertain1.
The tropical broadleaved evergreen forest type provides the largest contribution to global biomass carbon turnover from stand-replacing disturbances, followed by needleleaved evergreen forest (Fig. 2c). Although stand-replacing disturbances are infrequent in tropical evergreen forest, the disturbance-related flux is significant, as the amount of biomass in these forests is very high compared to elsewhere27–30. Conversely, low τ will tend to suppress biomass stocks, limiting the turnover flux generated in each disturbance event. Our estimates of biomass carbon losses for tropical evergreen forest will tend towards the upper limit of uncertainty because τo was capped at 1000 years for reasons of sampling (Methods); disturbance return times could in fact be even longer in some parts of the tropics31. However, as demonstrated below, the sensitivity of biomass to very high τ is low. Different disturbance agents cannot be distinguished in our data, but carbon emissions from wildfire taken from the GFED dataset32 summed over the same global forest area give a mean of 0.12 Pg C a-1 over 2000-2014 (Methods), suggesting that fires are globally a relatively minor driver of stand-replacing disturbances in closed-canopy forests.
Sensitivity of forest biomass to disturbance return time
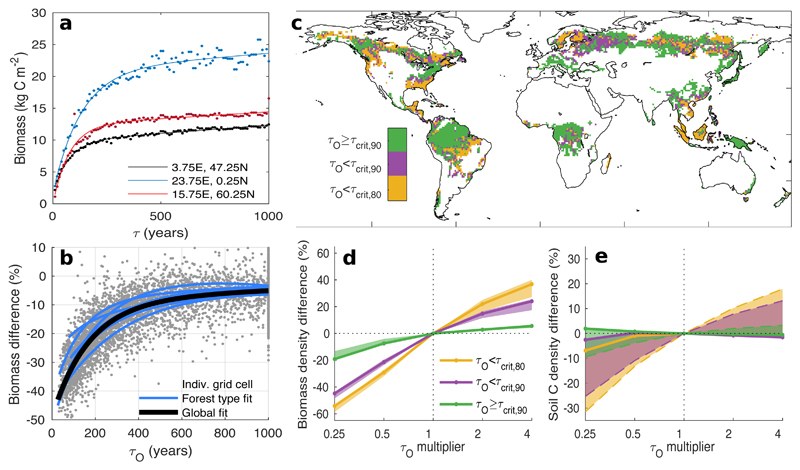
We ascertain the influence on ecosystem properties of possible changes in τ, or errors in its determination, for a randomly-selected grid cell from each of the tropical, temperate and boreal zones. For each grid cell we run 100 individual simulations varying τ sequentially within a plausible range of 10 to 1000 years. The resultant range in pseudo-equilibrium carbon stocks reflects variation in τ alone, independent of other environmental conditions or vegetation attributes (in particular that the resilience of vegetation to disturbance does not change with τ). The resulting curve of biomass carbon versus τ shows two distinct regimes (Fig. 3a); a regime of strong sensitivity of biomass to τ when τ is low, and a weak sensitivity regime when τ is high. These regimes result from shifts in the primary cause of dominant tree death. With low τ the majority of trees die from stand-replacing disturbance before they get old enough to die from another cause. Thus, τ emerges as the primary limit on simulated stand biomass across almost all stands. In contrast, when τ is large most canopy trees die from causes other than stand-replacing disturbances. In this case, τ is not a primary limit on simulated stand maximum biomass and changes in τ will only affect a subset of stands across the landscape at any time.
Figure 3. Sensitivity of biomass to changes in τ.
a, Simulated biomass versus τ for three random forested locations. Dots show individual simulations and lines a fitted exponential function. b, Sensitivity to τo of difference in simulated biomass between simulations with τo and 0.5τo. c, Sensitivity of biomass carbon stocks to changes in τ. Shading indicates the sensitivity regime. d, e, Effect of multiplicative perturbation in τ on vegetation and soil carbon density averaged across the different sensitivity classes. Shaded areas show range of sensitivity simulations testing assumptions on the type of disturbance assumed (solid lines for standard simulation) (Methods).
To map the sensitivity of forests to changes in τ globally we propose a new metric based on a fractional reduction of τo. Plotting τo against the difference in simulated biomass between global simulations run with τo and 0.5τo reveals a similar curve to the site-based simulations (Fig. 3b). We categorise global forests into two classes of sensitivity to disturbances: where the biomass under 0.5τo is less than 90% of that under τo, the forest is classified as having strong sensitivity to changes in τ (i.e. stand-replacing disturbance frequency is a strong control on biomass), with other areas having weak sensitivity (i.e. other forms of mortality control biomass). The τ0 at which this sensitivity threshold, τcrit,90, is crossed varies with forest type, with a global average of 444 (429-457) years. This implies a mean recovery time of 222 years (i.e. 0.5τcrit,90) to 90% of biomass stocks under τo. This is substantially longer than the 66 years reported for regain 90% of old-growth biomass in individual tropical forest stands33 and follows because our simulations take account of succession and also scale one stage further to the net landscape change in biomass. I.e. individual stands may recover rapidly, but across the landscape more stands are in a recently-disturbed state. Overall, 44 (39-49) % of global forest falls into the strongly sensitive category, with 23 (20-27) % falling into a very strong sensitivity category in which halving τo leads to biomass dropping below 80% of that under τo (τcrit,80). Forests in weak-sensitivity τ regimes are particularly located in tropical and temperate zones.(Fig. 3c).
The biomass content of weak-sensitivity forests would still be vulnerable to very large reductions in τ, for instance through a catastrophic shift to an entirely new disturbance regime34 or introduction of a new disturbance type35, but is robust to moderate changes in τ. This is demonstrated in additional simulations using τo adjusted up or down by a factor of up to 4; weak-sensitivity forests show large biomass changes only with substantial reductions in τ, whereas high-sensitivity forests show a steep relationship with τ (Fig. 3d). These results were robust to assumptions on the type of disturbance (shaded areas in Fig. 3d), although we note that selective effects of disturbance type on species composition35,36, and thus potentially on biomass and turnover37, could only be treated crudely within the plant functional type classes used for global simulation and there may be non-linear shifts not accounted for in the model38. The time taken for forest biomass to approach a new pseudo-equilibrium state will depend on the new value of τ to which the ecosystem is subjected. In general, changes in τ will only be fully reflected in carbon fluxes over the next century if that new τ is of the order of 100 years or less.
Unlike the influence of τ on biomass (Fig. 3d), its influence on soil carbon stocks is strongly sensitive to the rate of decompostion of the resulting litter and soil organic matter, and also depends on biomass removals, for instance in conjunction with wood harvest (Fig. 3e). When disturbed biomass is transferred to the litter, disturbance only has notable negative consequences when τ becomes very low, reducing the fraction of longer-lived woody biomass entering the litter. However, harvest removals or burning of biomass substantially reduce the input rate of carbon to the soil, leading to a strong positive relationship between soil carbon density and τ. This strong sensitivity of soil carbon storage to the type, as well as the frequency, of disturbance, underlines the need for improved discrimination of different disturbance types at the global scale39. Response times for soil will lag those for vegetation, and be influenced by the form of necromass left after disturbance40, another area of high process uncertainty. Summing over both vegetation and soil, a widespread shift in disturbance regimes equivalent to a halving of τo across all closed-canopy forests would ultimately release 47-80 Pg C, depending on the form of that disturbance, while an increase in time between disturbances could promote carbon uptake (Suppl. Fig. 10).
Concluding remarks
The results of this study allow us to partition one important component of overall biomass turnover rates in global forests1,8. Although stand-replacing disturbances constitute a relatively small portion of the overall global biomass turnover flux, small changes in τ would exert a strong influence on biomass stocks in almost half of the world's forests. DGVMs and land-surface models currently incorporating explicit representations of forest demography41 must properly account for stand-replacing disturbances to avoid biases in net carbon uptake or erroneous calibration of processes to account for these biases. Our study highlights the importance of accounting for variability in forest disturbance regimes, yet constitutes only a first step; 88% of global carbon turnover due to tree mortality is not explained by stand-replacing disturbances. It thus remains crucial to constrain other causes of mortality, including disturbances below stand-scale23,42, drought43, and demography. High-resolution data from satellites, along with forest inventories, will be key in this regard.
Our results provide a snapshot of a global stand-replacing disturbance pattern that may be undergoing rapid change13–15. Drivers of such change, whether climate, management or otherwise are uncertain and likely highly region-specific13–16,19,20. Future work must consider how carbon emissions through changes in τ are likely to interact with other aspects of environmental change, such as the fertilising effects of rising CO2 concentrations, which may reduce vulnerability to disturbance44, as well as seeking to close the feedback loops between disturbances, climate and vegetation properties. Changing disturbances could both augment and offset carbon loading of the atmosphere caused by anthropogenic carbon emissions3; better understanding the role of forest disturbances in the carbon cycle is therefore highly relevant to the assessment of emissions reductions necessary to meet climate targets.
Methods
Calculation of τo
τo was calculated as defined in Eq. 1. We first created a forest mask by aggregating year 2000 forest canopy cover data at 0.00025° (ca. 30 m) resolution21 to 0.01° resolution. Grid cells with at least 50% canopy cover at 0.01° resolution were assigned as closed-canopy forest. Further aggregation then provided the fractional coverage of closed-canopy forest at 1° resolution (AT). Across each 1° grid cell we then summed up the total area of 0.00025° pixels which underwent forest loss during 2000-2014 and were located within the 0.01° grid cells assigned as closed-canopy forest. A grid cell could only be counted as lost once during the period. Dividing this sum by the length of the 14 year observation period provided A threshold of 25% forest cover at the 1° grid cell level was used throughout this study in order to provide sufficient statistical power for calculation of τo. The total forested area meeting these conditions is 2.71 × 107 km2. Fig. 1c was calculated from the gridded τo estimate using the “boxplot” function of Matlab® 2014b.
This above definition provides a calculation of τ as a function of forest area. An alternative definition of τ would be to define it relative to canopy area. In this case AT would be the total canopy area within the 0.01° grid cells designated as closed canopy forest, making use of the fractional canopy cover metric provided by Hansen et al. 21, and would be the sum of pixels undergoing forest loss multiplied by the fractional canopy cover of those pixels before disturbance. Using this canopy-area definition slightly reduces our estimates of τo in most locations (Suppl. Fig. 4), but the forest-area definition is preferred as it recognises that whilst disturbances reduce canopy cover, they do not reduce the area of forest unless associated with a land-use change.
Forest losses due to land-use change, were calculated for the period 2000-2014 using the ESA CCI landcover product v2.0.7 (accessed 29th June 2017). ESA CCI landcover classes were simplified into forested (classes 50, 60, 61, 62, 70, 71, 72, 80, 81, 82, 90, 100, 160, 170) and non-forested (classes 10, 11, 20, 30, 110, 130, 190) classes, the latter corresponding to cropland, grassland and urban land uses. Then the area of 0.0028° pixels which were classified as forested in 2000 but non-forested in 2014 was calculated. The forest loss due to land-use change calculated from this dataset shows excellent consistency with the total forest loss dataset based on Hansen et al. 21, with only very few locations where the loss due to land-use change is reported to be larger than the total (Suppl. Fig. 5).
Uncertainties in τo due to the sample sizes in the forest loss data were estimated through bootstrapping. In each 1° grid cell 1000 samples of were created by resampling with replacement the 0.01° grid cells designated as closed-canopy forest. Uncertainties in result from classification accuracy and scaling differences between the Hansen et al.21 and ESA CCI datasets. Producer’s accuracy for the forest and non-forest classification in ESA CCI v2.0.7 is 92% and 78% respectively, whilst the corresponding user’s accuracy is 78% and 85%45. However, because we count the whole area of the pixel when an ESA CCI pixel changes from forest to non-forest, and the CCI pixel area is ca. 100 times that of Landsat, a scaling inaccuracy is induced, whereby the fraction of forest conversion within the grid cell may be enough to cause a land-cover classification switch, but substantially less than complete deforestation of the ESA pixel. To conservatively account for classification and scaling errors we thus assume a 95% confidence interval of +- 50% in the forest conversion area values. For each 1° grid cell, 1000 samples of were taken from a normal distribution defined by this confidence interval. We crossed these 1000 samples of with those from to create a matrix of 1 x 106 estimates of the denominator in Eq. 1. The 2.5th and 97.5th percentiles of this matrix were then used to estimate the 95% confidence limits of τo. This resampling of the forest loss areas within the 1° pixel addresses the uncertainty induced when the forest area in the pixel is relatively small, in which case confidence in the fidelity of the space-for-time swap would be reduced. It also accounts for classification errors if those errors are not correlated across the grid cell. Hansen et al. 21 report a tendency to underestimate forest loss by ca. 4% in the tropics and overestimate it by ca. 6% in the temperate and boreal regions. These classification biases are not captured in our uncertainty estimate, nor are potential biases from for which global quantification was not available. Based on the available information, these biases are expected to be small and focused in regions where the uncertainty is already assessed as being large (Suppl. Note 4; Suppl. Fig. 8). Note that the capping of τo at 1000 years often leads to very low uncertainty for these grid cells, i.e. there is very high certainty that τo > 1000 years. Calculated τo is robust to subsampling of the 14 year observational period, especially when the data series exceeds 10 years (Suppl. Fig. 6).
The resolution of ESA CCI landcover means it will have limited sensitivity to very small-scale land-use conversions, such as have been recently reported in the Amazon46. However, given that our τo values in the tropical evergreen forests are very high, even in absence of the land-use correction (Suppl. Fig. 7) we expect the influence on our results to be minimal.
The use of a 1° aggregation resolution represents a compromise between spatial detail and sufficient area to make an effective space-for-time substitution. Following the simplifying assumption that disturbance events are equally likely in all locations in the grid cell, the maximum τ that we can expect to reliably quantify, τm, for a given disturbance size, D, can be calculated as:
| (2) |
where t is the total number of years sampled. The largest disturbance events are generally fires, especially in the Canadian boreal region, for which the typical large fire size is 6000 ha47. Assuming a grid-cell area of 628 000 ha (60° latitude), a forest coverage of 25% of grid-cell area (i.e. AT = 157 000 ha) and a 14-year sampling period, τm is 350 years at this scale. For smaller disturbances much larger values of τ can be expected to be reliably captured. Substantial undersampling of large rare events at 1° resolution would be expected to induce scatter in our results, but Fig. 1 shows spatial coherence in variation of τ, suggesting any such under-sampling to have minimal effects. τo was capped at 1000 years to avoid spuriously large values in grid cells with very infrequent disturbance. The influence of this capping on simulated forest biomass is very small (e.g. Fig. 3b).
Forest type classification
Forest types were classified based on ESA CCI landcover v2.0.7. The mapping of landcover classes to the forest types used in this analysis in shown in Suppl. Table 2. A map of these forest types is shown in Suppl. Fig. 11. There is a small fraction of forest area that is not assigned to any of these major forest classes, but is included in the global totals. Note that open canopy forests (<50% canopy cover at 0.01° scale, see above) are not included in any of the calculations herein. Forest type codes are: Tropical broadleaved evergreen (TrBE), tropical broadleaved deciduous (TrBD), temperate broadleaved evergreen (TeBE), temperate broadleaved deciduous (TeBD), needleleaved evergreen (NE), needleleaved deciduous (ND), broadleaved-needleleaved mixed forest (MX).
Forest age dataset
For cross comparison of spatial patterns in our results, we used the Global Forest Age Dataset (GFAD v1.1)22, a forest stand age dataset developed as part of the EU FP7 GEOCARBON project. It provides a distribution of stand age and associated uncertainties in 10-year age bins up to an age of 140 years from a base year of 2010 on a 0.5° grid. The salient features are summarised here and described in more detail in Pugh et al.48. It combines datasets of forest age distributions from the following forest inventories: United States Forest Inventory and Analysis (v 5.1, state summaries, representative for the 2000s), IIASA Russian Forests and Forestry Database (late 2000s), Canadian Forest Inventory (CanFI, state summaries, 2001-2006), EFISCEN (Europe, 32 countries, 2000s), 6th National Forest Inventory (China, 1999-2003), and the national forest inventories of Kazakhstan (2000s), New Zealand (2000s), Mongolia (2000s) and Japan (2005). GFAD estimates forest age in tropical regions, where widespread inventories are not available, by applying plant-functional-type-specific biomass-age curves49 to a large-scale forest biomass dataset50.
Forest modelling
The LPJ-GUESS DGVM v4.051 was used to calculate the effects of τ on forest structure, dynamics and carbon cycling. LPJ-GUESS explicitly simulates forest stand development and canopy structure divided among age cohorts of trees co-occurring in patches representative of a wider landscape. Leaf area to sapwood area ratio and maximum crown area for tropical evergreen tree types were set to 10 000 and 130 m2 respectively, in accordance with estimates for tropical forests52,53. Mortality and establishment are stochastic, with replicate 1000 m2 patches simulated to capture the distribution of stands of different time-since-last-disturbance across each grid cell. Stand-replacing disturbances are simulated by clearing all trees in a patch and transferring their biomass stocks to litter or out of the ecosystem (see below). We introduced a spatially-varying stochastic disturbance frequency with an annual probability defined by 1/τ. In order to allow LPJ-GUESS to simulate the closed-canopy forest area unrestricted by the 25% cover threshold used to calculate τo, the nearest-neighbour rule was used to assign τ values to grid cells with less than 25% forest coverage. All forest-type-level and global numbers are presented based on a 5% minimum forest coverage mask at the grid-cell level to avoid overextrapolation of τo to regions with very low forest cover. The map in Fig. 2 is presented with a 25% closed-canopy forest cover map for consistency with Fig. 1. Inclusion of τo in LPJ-GUESS improves the simulation of biomass compared to the disturbance settings in the standard version of the model (Suppl. Fig. 12).
In the standard simulation setting, all cleared biomass is transferred to the litter pools. For sensitivity simulations underlying ranges in Fig. 3d,e and Suppl. Fig. 10 two further setting types were employed to test the effect of the fate of disturbed material. In the harvest sensitivity simulations fine root and leaf biomass, along with 34% of woody biomass, are transferred to the litter, with the remaining woody biomass being removed from the ecosystem, emulating product extraction. The fire sensitivity simulations employ the interactive fire sub-model51,54 with a local probability of fire occurrence (burnt area fraction) set to 1/τ, resulting in most biomass carbon and some litter carbon being transferred to the atmosphere. Stochastic processes use the same seed to ensure replication between simulations. Simulations covered 1901-2014 using climate, atmospheric CO2 mixing ratio and N deposition as described in Le Quéré et al.55. All model outputs shown are means for 2001-2014. The standard simulations with τo and 0.5τo used 100 replicate patches per grid cell. Simulations testing additional multiplicative perturbations of τ (0.25, 2, 4) and using the confidence intervals of τo used 10 replicate patches. Differences at forest-type level were negligible between simulations with 10 and 100 patches.
Simulations used to create Fig. 3a used the standard model setup described above, but the model was only run for the specified three grid cells. 100 simulations were carried out for each grid cell using levels of τ from 10 years to 1000 years. A second-order exponential equation of the form B = aebτ + cedτ was fitted to these simulations using the “fit” function of Matlab® 2014b.
Sensitivity metric
The metric is based upon differencing biomass between the τo and 0.5τo simulations. The choice of a halving of τo for the sensitivity metric was informed by recent disturbance trends in Europe14, and is also similar to changes in background mortality rates in the western U.S.A.56. It thus represents a reasonable sensitivity test. The sensitivity threshold τcrit (Suppl. Table 1) was estimated by first plotting against τo the difference between biomass simulated with τ = τo and that simulated with τ = 0.5τo, (Fig. 3b, Suppl. Fig. 13). A second-order exponential function was fitted to the data as for Fig. 3a. These fits were carried out both globally and for individual forest types. 95% confidence intervals for the fits were calculated using 1000 bootstrapped samples of the modelled grid cells. τ crit,90 and τ crit,80 were taken as the intersection of the fitted line with a difference of -10% and -20% biomass respectively (Suppl. Fig. 13), with confidence intervals for τ crit estimated using the confidence intervals of the fitted lines. Scatter in the results is caused by the stochastic nature of the LPJ-GUESS model, as well as variation in climate across the domain. The 90% biomass threshold is consistent with recent work on the recovery of forest biomass33 and with the character of the curve in Fig. 3b.
The area of forest in each sensitivity regime (Fig. 3c) was created by comparing τo for each grid cell with the τcrit for the forest type to which that grid cell was assigned. Uncertainty in the areas of the regimes (Main text) was calculated based on the 95% confidence intervals of τ O. For forest grid cells not classified by one of the seven forest types, not enough data points existed to make a reliable fit to calculate τ crit. Therefore the global mean τcrit was used to determine the sensitivity regime. Fig. 3d,e shows the difference in biomass and soil carbon density between model sensitivity simulations with different multiplicative factors of τ (see above) averaged across the area of forest allocated to each sensitivity class. Variation in response across the vulnerability classes is much less than that between them (Suppl. Fig. 9).
Empirical cross-checks
For cross-checking of biomass carbon turnover flux due to disturbance (Fd) we used the GEOCARBON global biomass dataset27–29, in which biomass values are based on linking satellite-based LIDAR and radar observations with ground-based forest plot data. We replaced values for northern forests with those of Thurner et al.30 due to the latter’s more sophisticated approach to linking satellite-based radar observations with above- and below-ground biomass in these regions. Below-ground biomass for the GEOCARBON dataset was estimated following Saatchi et al.50 and a biomass to carbon conversion factor of 0.5 was assumed. We then multiplied the carbon content of this observationally-based total biomass dataset by 1/τo to calculate Fd. Cross-checking the fraction of total turnover due to disturbance (Tfrac) involved making the assumption that NPP and turnover fluxes are not drastically out of equilibrium, and therefore NPP must be broadly equal to the turnover flux of biomass carbon in the multi-annual mean. Annual mean NPP over the period 2001-2010 was calculated from Zhao and Running57. Tfrac was then approximated as Fd/NPP. Fire emissions from the GFED dataset32 were calculated by summing the boreal, temperate and tropical forest wildfire emissions, excluding the savannah category, which does not fit our definition of closed-canopy forest. The mask of at least 5% forest cover per grid cell was applied to all these cross-check calculations as above.
Supplementary Material
Acknowledgements
TAMP acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 758873, TreeMort). TAMP, AA and MK acknowledge support from EU FP7 grant LUC4C (grant no. 603542), and the Helmholtz Association in its ATMO programme and its impulse and networking fund. This is paper number 36 of the Birmingham Institute of Forest Research. BS acknowledges funding from the Swedish Research Council FORMAS, the Strategic Research Area BECC and the Lund University Centre for Studies of Carbon Cycle and Climate Interactions (LUCCI). BP was supported by the NASA Terrestrial Ecology program. Stijn Hantson, Sally Archibald, Jon Sadler, Tom Matthews and Sergei Petrovskii are thanked for discussions which helped improve the manuscript, as are Mike Wulder for providing the Canadian ecozones mask, Emma Ferranti for help with file conversion and Veiko Lehsten for assistance with the data deposition.
Footnotes
Author contributions
TP conceived and designed the study with contributions from AA and BS. BP and MK contributed data. TP carried out the model simulations. TP led the analysis and wrote the paper with contributions from all authors.
Competing financial interests
The authors declare no competing financial interests.
Data availability
Calculations of τo, data from the model simulations and the forest mask used are available from https://dataguru.lu.se/app#PughDisturbance2019 (dois: 10.18161/disturbance_tauo.201905, 10.18161/disturbance_lpj-guess.201905, 10.18161/disturbance_forestmask.201905). GFAD v1.1 was obtained from PANGAEA22, and the Global Forest Change 2000-2014 v1.2 forest loss product from https://earthenginepartners.appspot.com/science-2013-global-forest/download_v1.2.html. The ESA CCI Landcover v2.0.7 was obtained from http://maps.elie.ucl.ac.be/CCI/viewer/.
Code availability
Matlab code for the data analysis herein is available from GitHub, https://github.com/pughtam/GlobalDist. Source code for LPJ-GUESS v4.0 can be obtained on request through Lund University, see web.nateko.lu.se/lpj-guess.
References
- 1.Erb K, et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature. 2018;553:73–76. doi: 10.1038/nature25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y, et al. A large and persistent carbon sink in the world’s forests. Science. 2011;333:988–93. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 3.Le Quéré C, et al. Global Carbon Budget 2017. Earth Syst Sci Data. 2018;10:405–448. [Google Scholar]
- 4.Sierra CA, Müller M, Metzler H, Manzoni S, Trumbore SE. The muddle of ages, turnover, transit, and residence times in the carbon cycle. Glob Chang Biol. 2017;23:1763–1773. doi: 10.1111/gcb.13556. [DOI] [PubMed] [Google Scholar]
- 5.Friend AD, et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc Natl Acad Sci U S A. 2014;111:3280–3285. doi: 10.1073/pnas.1222477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlström A, Xia J, Arneth A, Luo Y, Smith B. Importance of vegetation dynamics for future terrestrial carbon cycling. Environ Res Lett. 2015;10:054019. [Google Scholar]
- 7.Carvalhais N, et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature. 2014;514:213–217. doi: 10.1038/nature13731. [DOI] [PubMed] [Google Scholar]
- 8.Erb K-H. Biomass turnover time in terrestrial ecosystems halved by land use. Nat Geosci. 2016;9:674–678. [Google Scholar]
- 9.Waring RH. Characteristics of Trees Predisposed to Die. BioSicence. 1987;37:569–574. [Google Scholar]
- 10.McDowell NG, et al. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol. 2011;26:523–532. doi: 10.1016/j.tree.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Pickett STA, White PS. The ecology of natural disturbances and patch dynamics. Academic Press Inc; Orlando: 1985. [Google Scholar]
- 12.Frolking S, et al. Forest disturbance and recovery: A general review in the context of spaceborne remote sensing of impacts on aboveground biomass and canopy structure. J Geophys Res. 2009;114:G00E02. [Google Scholar]
- 13.Kurz W, Stinson G, Rampley G, Dymond C, Neilson E. Risk of natural disturbances makes future contribution of Canada’s forests to the global carbon cycle highly uncertain. Proc Natl Acad Sci U S A. 2008;105:1551–1555. doi: 10.1073/pnas.0708133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Chang. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannigan M, Stocks B, Turetsky M, Wotton M. Impacts of climate change on fire activity and fire management in the circumboreal forest. Glob Chang Biol. 2009;15:549–560. [Google Scholar]
- 16.Hurtt GC, et al. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim Change. 2011;109:117–161. [Google Scholar]
- 17.Cole LES, Bhagwat SA, Willis KJ. Recovery and resilience of tropical forests after disturbance. Nat Commun. 2014;5:1–7. doi: 10.1038/ncomms4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pregitzer KS, Euskirchen ES. Carbon cycling and storage in world forests: biome patterns related to forest age. Glob Chang Biol. 2014;10:2052–2077. [Google Scholar]
- 19.Seidl R, et al. Forest disturbances under climate change. Nat Clim Chang. 2017;7:395–402. doi: 10.1038/nclimate3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyer CPO, et al. Are forest disturbances amplifying or canceling out climate change-induced productivity changes in European forests? Environ Res Lett. 2017;12:034027. doi: 10.1088/1748-9326/aa5ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen MC, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 22.Poulter B, et al. The global forest age dataset and its uncertainties (GFADv1.1) 2019 doi: 10.1594/PANGAEA.897392. [DOI] [Google Scholar]
- 23.Espírito-Santo FDB, et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat Commun. 2014;5 doi: 10.1038/ncomms4434. 3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers JQ, et al. The steady-state mosaic of disturbance and succession across an old-growth Central Amazon forest landscape. Proc Natl Acad Sci U S A. 2013;110:3949–3964. doi: 10.1073/pnas.1202894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White JC, Wulder MA, Hermosilla T, Coops NC, Hobart GW. A nationwide annual characterization of 25 years of forest disturbance and recovery for Canada using Landsat time series. Remote Sens Environ. 2017;194:303–321. [Google Scholar]
- 26.Kautz M, Meddens AJH, Hall RJ, Arneth A. Biotic disturbances in Northern Hemisphere forests – a synthesis of recent data, uncertainties and implications for forest monitoring and modelling. Glob Ecol Biogeogr. 2017;26:533–552. [Google Scholar]
- 27.Avitabile V, et al. An integrated pan-tropical biomass map using multiple reference datasets. Glob Chang Biol. 2016;22:1406–1420. doi: 10.1111/gcb.13139. [DOI] [PubMed] [Google Scholar]
- 28.Santoro M, et al. Remote Sensing of Environment Forest growing stock volume of the northern hemisphere: Spatially explicit estimates for 2010 derived from Envisat ASAR. Remote Sens Environ. 2015;168:316–334. [Google Scholar]
- 29.Avitabile V, et al. Comparative analysis and fusion for improved global biomass mapping. Global Vegetation Monitoring and Modeling; 3 – 7 February 2014; Avignon (France). 2014. [Google Scholar]
- 30.Thurner M, et al. Carbon stock and density of northern boreal and temperate forests. Glob Ecol Biogeogr. 2014;23:297–310. [Google Scholar]
- 31.Espírito-Santo FDB, et al. Storm intensity and old-growth forest disturbances in the Amazon region. Geophys Res Lett. 2010;37:L11403. [Google Scholar]
- 32.van der Werf GR, et al. Global fire emissions estimates during 1997-2016. Earth Syst Sci Data. 2017;9:697–720. [Google Scholar]
- 33.Poorter L, et al. Biomass resilience of Neotropical secondary forests. Nature. 2016;530:211–214. doi: 10.1038/nature16512. [DOI] [PubMed] [Google Scholar]
- 34.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–6. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone JF, et al. Changing disturbance regimes, ecological memory, and forest resilience. Front Ecol Environ. 2016;14:369–378. [Google Scholar]
- 36.Marra DM, et al. Large-scale wind disturbances promote tree diversity in a Central Amazon forest. PLoS One. 2014;9:e103711. doi: 10.1371/journal.pone.0103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra DM, et al. Predicting biomass of hyperdiverse and structurally complex central Amazonian forests – a virtual approach using extensive field data. Biogeosciences. 2016;13:1553–1570. [Google Scholar]
- 38.Marra DM, et al. Windthrows control biomass patterns and functional composition of Amazon forests. Glob Chang Biol. 2018;24:5867–5881. doi: 10.1111/gcb.14457. [DOI] [PubMed] [Google Scholar]
- 39.McDowell NG, et al. Global satellite monitoring of climate-induced vegetation disturbances. Trends Plant Sci. 2015;20:114–123. doi: 10.1016/j.tplants.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Renninger HJ, Carlo N, Clark KL, Schäfer KVR. Modeling respiration from snags and coarse woody debris before and after an invasive gypsy moth disturbance. J Geophys Res Biogeosciences. 2014;119:630–644. [Google Scholar]
- 41.Fisher RA, et al. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob Chang Biol. 2018;24:35–54. doi: 10.1111/gcb.13910. [DOI] [PubMed] [Google Scholar]
- 42.Marvin DC, Asner GP. Branchfall dominates annual carbon flux across lowland Amazonian forests. Environ Res Lett. 2016;11:094027. [Google Scholar]
- 43.Allen CD, Breshears DD, McDowell NG. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 2015;6:129. [Google Scholar]
- 44.Dolan KA, et al. Disturbance Distance: quantifying forests’ vulnerability to disturbance under current and future conditions. Environ Res Lett. 2017;12:114015. [Google Scholar]
- 45.ESA. Land Cover CCI Product User Guide Version 2.0. ESA; 2017. http://maps.elie.ucl.ac.be/CCI/viewer/download/ESACCI-LC-Ph2-PUGv2_2.0.pdf. [Google Scholar]
- 46.Kalamandeen M, et al. Pervasive Rise of Small-scale Deforestation in Amazonia. Sci Rep. 2018;8 doi: 10.1038/s41598-018-19358-2. 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Groot WJ, et al. A comparison of Canadian and Russian boreal forest fire regimes. For Ecol Manage. 2013;294:23–34. [Google Scholar]
- 48.Pugh TAM, et al. Role of forest regrowth in global carbon sink dynamics. Proc Natl Acad Sci U S A. 2019;116:4382–4387. doi: 10.1073/pnas.1810512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marin-Spiotta E, Cusack DF, Ostertag R, Silver WL. Trends in above and belowground carbon with forest regrowth after agricultural abandonment in the neotropics. In: Myster RW, editor. Post-agricultural succession in the Neotropics. Springer; New York: 2008. [Google Scholar]
- 50.Saatchi SS, et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Natl Acad Sci. 2011;108:9899–9904. doi: 10.1073/pnas.1019576108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith B, et al. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences. 2014;11:2027–2054. [Google Scholar]
- 52.Herwitz S, Slye R. Erwitz STRH, Lye ROES: Long-term survivorship and crown area dynamics of tropical rain forest canopy trees. Ecology. 2000;81:585–597. [Google Scholar]
- 53.Calvo-Alvarado JC, McDowell NG, Waring RH. Allometric relationships predicting foliar biomass and leaf area:sapwood area ratio from tree height in five Costa Rican rain forest species. Tree Physiol. 2008;28:1601–1608. doi: 10.1093/treephys/28.11.1601. [DOI] [PubMed] [Google Scholar]
- 54.Thonicke K, Venevsky S, Sitch S, Cramer W. The role of fire disturbance for global vegetation dynamics: coupling fire into a Dynamic Global Vegetation Model. Glob Ecol Biogeogr. 2001;10:661–677. [Google Scholar]
- 55.Le Quéré C, et al. Global Carbon Budget 2016. Earth Syst Sci Data. 2016;8:605–649. [Google Scholar]
- 56.van Mantgem PJ, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- 57.Zhao M, Running SW. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 Through 2009. Science. 2010;329:940–944. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Calculations of τo, data from the model simulations and the forest mask used are available from https://dataguru.lu.se/app#PughDisturbance2019 (dois: 10.18161/disturbance_tauo.201905, 10.18161/disturbance_lpj-guess.201905, 10.18161/disturbance_forestmask.201905). GFAD v1.1 was obtained from PANGAEA22, and the Global Forest Change 2000-2014 v1.2 forest loss product from https://earthenginepartners.appspot.com/science-2013-global-forest/download_v1.2.html. The ESA CCI Landcover v2.0.7 was obtained from http://maps.elie.ucl.ac.be/CCI/viewer/.
Code availability
Matlab code for the data analysis herein is available from GitHub, https://github.com/pughtam/GlobalDist. Source code for LPJ-GUESS v4.0 can be obtained on request through Lund University, see web.nateko.lu.se/lpj-guess.