Summary
Kawasaki disease (KD) vasculitis is an acute febrile illness of childhood characterized by systemic vasculitis of unknown origin, and is the most common cause of acquired heart disease among children in the United States. While histological evidence of myocarditis can be found in all patients with acute KD, only a minority of patients are clinically symptomatic and a subset demonstrate echocardiographic evidence of impaired myocardial function, as well as increased left ventricular mass, presumed to be due to myocardial edema and inflammation. Up to a third of KD patients fail to respond to first‐line therapy with intravenous immunoglobulin (IVIG), and the use of interleukin (IL)‐1 receptor antagonist (IL‐1Ra, anakinra) is currently being investigated as an alternative therapeutic approach to treat IVIG‐resistant patients. In this study, we sought to investigate the effect of IL‐1Ra on myocardial dysfunction and its relation to myocarditis development during KD vasculitis. We used the Lactobacillus casei cell‐wall extract (LCWE)‐induced murine model of KD vasculitis and investigated the effect of IL‐1Ra pretreatment on myocardial dysfunction during KD vasculitis by performing histological, magnetic resonance imaging (MRI) and echocardiographic evaluations. IL‐1Ra pretreatment significantly reduced KD‐induced myocardial inflammation and N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) release. Both MRI and echocardiographic studies on LCWE‐injected KD mice demonstrated that IL‐1Ra pretreatment results in an improved ejection fraction and a normalized left ventricular function. These findings further support the potential beneficial effects of IL‐1Ra therapy in preventing the cardiovascular complications in acute KD patients, including the myocarditis and myocardial dysfunction associated with acute KD.
Keywords: anakinra, echocardiogram, IL‐1, IL‐1Ra, Kawasaki disease, MRI, myocarditis, myocardial edema, NT‐proBNP, vasculitis
Introduction
Kawasaki disease (KD) is an acute febrile illness with systemic vasculitis of unknown etiology that afflicts young children, causing coronary artery aneurysms (CAA) or abnormalities in approximately 25% of untreated patients 1. With high‐dose intravenous immunoglobulin (IVIG) treatment, this number falls to ~5% 2, but IVIG resistance is a growing problem, with approximately 25–30% of KD patients IVIG‐resistant and thus at higher risk of developing CAA 3, 4, 5. Therefore, alternative therapies, including blocking interleukin (IL)‐1 signaling, are being investigated 6, 7.
Although KD research and clinical practice has largely focused on the development of CAA during acute KD, and on long‐term complications of coronary artery stenosis and ischemia 8, 9, KD‐induced myocarditis is more prevalent than CAA and occurs in almost all patients 10, 11, 12, 13, 14. Indeed, histological evidence of myocarditis is found in all KD patients during the acute phase, irrespective of the presence of CAA 15, 16, 17, and myocarditis may be a cause of early fatal KD cases 18, 19.
Myocarditis is subclinical in the majority of KD patients, and those who are symptomatic may present with mild clinical, electrocardiographic or echocardiographic signs of ventricular dysfunction. Echocardiographic signs of myocarditis improve after the acute phase, particularly following IVIG therapy. Normalization of systolic function is typically observed over long‐term follow‐up, but more subtle abnormalities may persist and KD myocarditis can result in long‐term sequelae 16, 20.
The Lactobacillus casei cell‐wall extract (LCWE)‐induced murine model of KD vasculitis and coronary arteritis closely phenocopies the important histological and immune pathological features of the cardiovascular lesions seen in human KD vasculitis (i.e. coronary arteritis, coronary stenosis, aortitis, myocarditis and aneurysms) 21, 22, 23. We have previously shown that NLRP3 inflammasome, caspase‐1 activation and IL‐1β are required for vasculitis and coronary arteritis and myocarditis in this model 21, 24, 25, and that pretreatment with the IL‐1 receptor antagonist (IL‐1Ra, anakinra) significantly inhibits the development of coronary vascular inflammation as well as myocarditis 21. These studies, together with human data on the emerging key role of IL‐1β in the pathogenesis of KD, have recently led to two Phase II clinical trials of anakinra therapy in IVIG‐resistant KD patients (NCT02179853, NCT02390596) 6. Indeed, several observations suggested that IL‐1 plays an important role in KD patients 6. The IL‐1 pathway is up‐regulated in children with KD compared to pediatric febrile controls, as demonstrated by increased transcript abundance in acute peripheral blood mononuclear cells (PBMCs) as well as plasma proteins, as reviewed in 6. Elevated levels of IL‐1 have been reported in acute KD patients and have been correlated with vascular endothelial cell damage, and IVIG treatment was associated with a decrease in IL‐1, but levels remain elevated in refractory patients 26. Furthermore, there are several case reports of safe and successful use of IL‐1Ra (anakinra) in otherwise non‐responsive KD patients 27.
However, the features of acute myocardial mechanical dysfunction have not been previously investigated in the LCWE‐induced or any other mouse model of KD vasculitis. Here, we show that similar to what is observed in children with KD, myocardial dysfunction and increased left ventricular mass occur in the LCWE‐induced KD vasculitis murine model, and these features are significantly inhibited by the IL‐1Ra.
Material and methods
Preparation of L. casei cell wall extracts (LCWE)
LCWE was prepared as previously described 24, 28. Briefly, L. casei was grown for 48 h, harvested and washed with phosphate‐buffered saline (PBS). Bacteria were disrupted with 4% sodium dodecyl sulphate (SDS) and cell wall fragments were washed with PBS and sonicated in a dry ice‐ethanol bath for 2 h. Cell wall fragments were then centrifuged for 20 min at 11,000 g, the supernatant collected and subsequently centrifuged for 1 h at 180,000 g and 4°C, and the pellet was discarded. The total rhamnose content of the cell wall extract was measured by colorimetric phenol‐sulfuric assay, as described previously 24, 28.
KD vasculitis mouse model and treatment protocols
All animal experiments were performed under protocols that had been approved by the Institutional Animal Care and Use Committee at Cedars‐Sinai Medical Center and Texas Biomedical Research Institute. Four‐ to 5‐week‐old male C57BL/6 WT mice (Jackson Laboratory, Bar Harbor, ME, USA) were injected intraperitoneally (i.p.) with a single dose of 500 μl LCWE to induce KD vasculitis. PBS was given to control mice. Mice were euthanized at different time‐points depending on the experimental design, perfused with PBS containing heparin. Human IL‐1 receptor antagonist (IL1Ra, anakinra, 25 mg/kg; Swedish Orphan Biovitrum AB, Stockholm, Sweden) was given daily i.p. from day –1 before LCWE injection to day 5. Tissues were harvested at different time‐points, embedded in optimal cutting temperature compound (OCT) for further histopathological analysis, and heart vessel inflammation scoring of the coronary arteries, aortic root vasculitis and myocarditis severity scoring were assessed by a blinded pathologist, as described previously 21.
N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) serum quantification
Serum samples from PBS, LCWE‐injected and LCWE‐injected mice pretreated with IL‐1Ra were collected 2 weeks post‐LCWE injection and used to quantify NT‐proBNP levels with a murine NT‐proBNP enzyme‐linked immunosorbent assay (ELISA) kit (MBS2501591; myBiosource, San Diego, CA, USA), following the manufacturer's instructions. Optical densities were read on a SpectraMax M2 spectrometer with SoftMaxPro version 5.2. software (Molecular Devices, San Jose, CA, USA).
Magnetic resonance imaging (MRI)
Each cohort (days 0, 7, 14 and 35) was scanned via a 9.4T Bruker's Biospin magnetic resonance imaging (MRI) system to assess ejection fraction by a blinded MRI technician. For each scan, a tripilot sequence from the Bruker's paravision system was implemented for the localization of the mouse heart. Next, several IntraGateFLASH sequences were performed for the left‐ventricle imaging. Five to six sequential 1‐mm slices were then analyzed in order to span the entire heart from bottom to top, with clear demonstration of ventricular dilation and contraction with a frame rate of 10 frames/s. To measure cardiac function, left ventricular chamber area outlined by the endocardial border for each slice at end‐diastole and end‐systole was measured. Left ventricular end‐diastolic volume was calculated as (LV‐EDV) = Σ LV volume per slice at end‐diastole and the LV end‐systolic volume (LV‐ESV) = ΣLV volume per slice at end‐systole. Ejection fraction (EF) was calculated as (LV‐EDV−LV‐ESV)/LV‐EEV. End‐point ejection fraction, diastolic volume and systolic volume were obtained at days 0, 7, 14 and 35 after LCWE injection in five mice and another five mice also pretreated with IL‐1Ra for each group.
Echocardiography
Serial echocardiography was performed and interpreted according to standard protocols by a blinded investigator. Briefly, animals were anesthetized with isoflurane. Echocardiographic images were obtained with a Vevo 2100® system (Visualsonics, Inc., Toronto, Canada) equipped with a MS400 linear array transducer (30 MHz). The transducer was positioned in a stationary stand perpendicular to the mouse. A frame rate of > 200 frames per minute was maintained for all B‐ and M‐mode images. Measurements of ejection fraction, shortening fraction, end‐diastolic and ‐systolic volumes, interventricular and left posterior wall thickness, left ventricular internal dimension and left ventricular mass/body weight were obtained at weekly intervals from day 0 until day 42 in male 4‐week‐old mice injected with either PBS, LCWE or LCWE with IL‐1 receptor antagonist anakinra treatment.
Statistical analysis
All data are presented as mean ± standard error of the mean (s.e.m.). No randomization was used in this study. Statistical significance was determined using either one‐way analysis of variance (ANOVA) or two‐way ANOVA with post‐test analysis for multiple group comparison involving one or two independent variables, respectively. Significant differences were defined at a P < 0·05. All statistical analysis were performed with Graphpad Prism software.
Results
Pretreatment with IL‐1Ra prevents coronary, myocardial inflammation and increased serum levels of NT‐proBNP in LCWE‐injected mice
We assessed the development of LCWE‐induced coronary arteritis and myocardial inflammation in mice at days 7, 14 and 35 post‐LCWE injection (Fig. 1a–d). LCWE‐injected mice displayed pronounced coronary and myocardial inflammation associated with acute and chronic cellular infiltration at all time‐points (Fig. 1b–d). Intense inflammation around the coronary arteries was observed, in some cases to the extent of complete coronary occlusion (Fig. 1b). Importantly, the coronary inflammation in LCWE‐injected mice decreased over time (Fig. 1c,d), consistent with the evolution of the disease from an acute to a post‐acute phase with vascular remodeling, similar to human KD 29, 30. Elevated serum levels of N‐terminal prohormone brain natriuretic peptide (NT‐proBNP) are associated with increased intracardiac pressure and myocardial stress 31, as well as with worsening heart failure 32. Accordingly, serum levels of NT‐proBNP were significantly higher in LCWE‐injected KD mice compared with PBS‐injected control mice (Fig. 1e).
Figure 1.
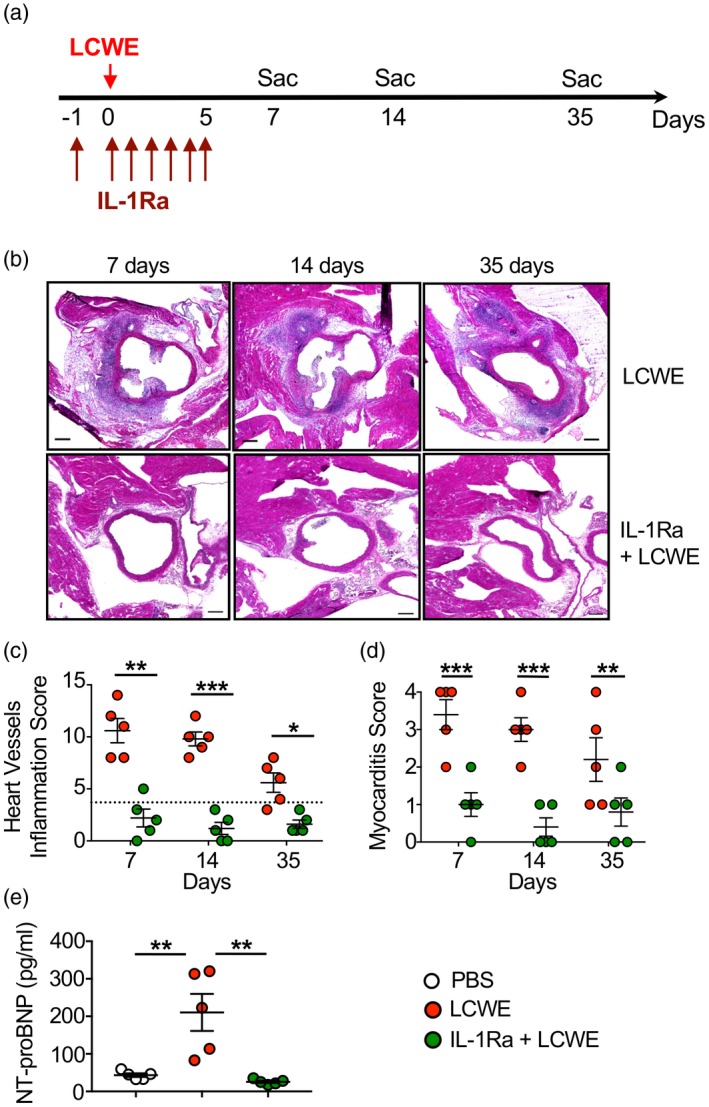
Interleukin (IL)‐1Ra treatment prevents coronary and myocardial inflammation in the Lactobacillus casei cell‐wall extract (LCWE) murine model of Kawasaki disease (KD) vasculitis. (a) Schema of the study experimental design. Mice received either LCWE or LCWE with IL‐1Ra, which was given daily for 6 consecutive days starting 1 day before LCWE injection. Heart tissues were harvested at the indicated time‐points and heart inflammation was evaluated by hematoxylin and eosin (H&E) staining. (b) Representative H&E staining of heart sections from LCWE‐injected mice with and without IL‐1Ra treatment at 7, 14 and 35 days post‐LCWE injection. Scale bars indicate 500 µm. (c,d) Vascular (c) and myocardial (d) inflammation score of untreated and IL‐1Ra pretreated LCWE‐injected mice. (e) Serum N‐terminal‐prohormone B‐type natriuretic peptide (NT‐ProBNP) levels of untreated and IL‐1Ra pretreated LCWE‐injected mice 2 weeks post‐LCWE injection. Data are presented as mean ± standard error of the mean (s.e.m.); n = 5–9 mice per group. *P < 0·05, **P < 0·01 and ***P < 0·001 by one and two‐way analysis of variance (ANOVA) with Tukey's post‐test analysis.
To establish the role of IL‐1 signaling in the development of coronary and myocardial inflammation in this model, we pretreated mice with IL‐1Ra daily for 1 week, beginning the day before LCWE injection (Fig. 1a). In agreement with a previous report 21, IL‐1Ra pretreatment blocked LCWE‐induced KD vasculitis, as demonstrated by a significant decrease in heart and myocardial inflammation (Fig. 1b–d). IL‐1Ra pretreatment also significantly prevented serum increase NT‐proBNP increase in LCWE‐injected mice (Fig. 1e).
IL‐1Ra treatment prevents myocardial dysfunction and ventricular enlargement in LCWE‐injected KD mice
Myocarditis is ubiquitous in KD patients; myocardial dysfunction and left ventricular enlargement are also often observed 33, 34, 35, and in some instances can lead to severe cardiac dysfunction and cardiogenic shock 36. To determine whether IL‐1Ra pretreatment also prevents myocardial dysfunction and left ventricular enlargement during LCWE‐induced KD vasculitis, we performed MRI on LCWE‐injected mice in the presence or absence of IL‐1Ra pretreatment (Fig. 2a). One week after LCWE injection, mice pretreated with IL‐1Ra showed improved end‐point ejection fraction, which remained significantly elevated compared with the control group for 5 weeks post‐LCWE (Fig. 2b). IL‐1Ra pretreatment also significantly improved end‐diastolic and end‐systolic volume at 35 days post‐LCWE injection (Fig. 2c,d). Lastly, IL‐1Ra pretreatment ameliorated the elevation in heart rate observed acutely following LCWE injection, as assessed by a higher number of beats per minute, which was also reduced with anakinra treatment (Fig. 2e).
Figure 2.
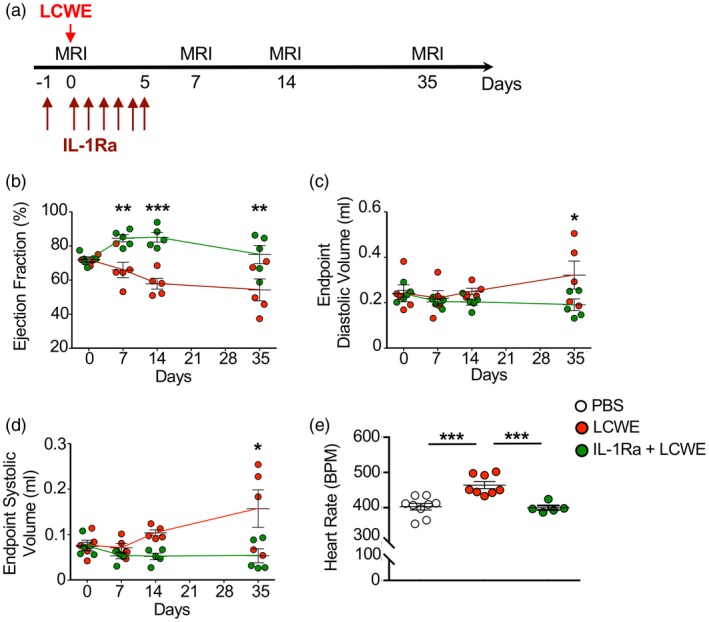
Interleukin (IL)‐1Ra treatment prevents myocardial dysfunction and ventricular enlargement. (a) Schema of the magnetic resonance imaging (MRI) study experimental design. Mice received either Lactobacillus casei cell‐wall extract (LCWE) or LCWE with IL‐1Ra, which was given daily for 6 consecutive days starting 1 day before LCWE injection. MRI analysis was performed at the indicated different time‐points. (b–d) Ventricular ejection fraction (b), end‐point diastolic (c) and ‐systolic (d) volume measured at days 7, 14 and 35 post‐LCWE injection by MRI studies. (e) Heart rate (beats per minute; bpm) measurement in phosphate‐buffered saline (PBS) control mice, LCWE‐injected mice and LCWE‐injected mice pretreated with IL‐1Ra at 7 days post‐LCWE‐injection. Data are presented as mean ± standard error of the mean (s.e.m.); n = 5–9 mice per group. *P < 0·05, **P < 0·01 and ***P < 0·001 by one and two‐way analysis of variance (ANOVA) with Tukey's post‐test analysis.
To confirm that LCWE‐induced KD vasculitis is associated with myocardial dysfunction and left ventricular enlargement, we next performed weekly echocardiographic evaluations on LCWE‐injected mice until day 42 post‐LCWE injection (Fig. 3a). Starting at 3 weeks post‐injection, LCWE‐injected mice demonstrated transient decreases in ejection fraction and shortening fraction compared with PBS control mice, and these changes were ameliorated in LCWE‐injected mice pretreated with IL‐1Ra (Fig. 3b, c). LCWE‐injected KD mice also began to demonstrate indications of increased left ventricular mass during the same time‐frame, which was significantly prevented by IL‐1Ra treatment (Fig. 3d). End‐systolic and end‐diastolic volume were also increased in LCWE‐injected mice compared to PBS control and IL‐1Ra pretreated groups starting at day 14 post‐LCWE injection, with end‐diastolic volume changes being more pronounced and persistent following the acute phase, while end‐systolic volume was elevated within early in the disease process (Fig 3e,f). Notably, in humans, persistently increased end‐diastolic volume has been described in KD patients as much as 12 months following disease onset 37.
Figure 3.
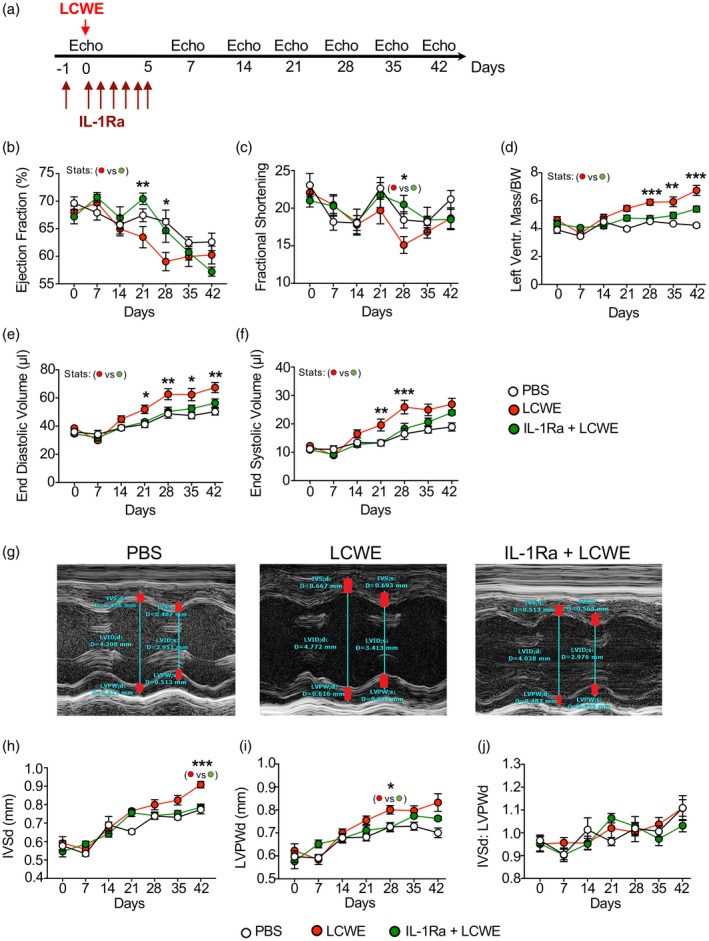
Echocardiogram analysis reveals that interleukin (IL)‐1Ra anakinra treatment prevents Lactobacillus casei cell‐wall extract (LCWE)‐induced myocardial dysfunction and ventricular enlargement. (a) Schema of the echocardiogram experimental design. Mice received either phosphate‐buffered saline (PBS), LCWE alone or LCWE with IL‐1Ra which was given daily for 5 consecutive days starting 1 day before LCWE injection. Echocardiogram analysis was performed at days 7, 14, 21, 28, 35 and 42 post‐LCWE injection. (b–e) Ejection fraction (b), fractional shortening (c), left ventricular mass/body weight (d), end‐diastolic volume (e) and end‐systolic volume (f) measured in PBS control mice, LCWE‐injected mice and LCWE‐injected mice pretreated with IL‐1Ra. (g) M‐mode measurements (red bars) of interventricular septum (IVS), left ventricular internal dimensions (LVID) and left ventricular posterior wall (LVPW) in PBS control mice, LCWE injected mice and LCWE‐injected mice pretreated with IL‐1Ra. (h,i) IVS (h) and LVPW (i) quantification during diastole in PBS control mice, LCWE‐injected mice and LCWE‐injected mice pretreated with IL‐1Ra. (j) Ratio of the IVS to LVPW in PBS control mice, LCWE‐injected mice and LCWE‐injected mice pretreated with IL‐1Ra. Data represent mean ± standard error of the mean (s.e.m.), where n = 8 mice per group. Statistics are comparing LCWE‐injected versus LCWE‐injected IL‐1Ra pretreated mice. *P < 0·05, **P < 0·01 and ***P < 0·001 by two‐way analysis of variance (ANOVA) with Tukey's post‐test analysis.
To further investigate the degree and nature of the left ventricular mass increase, we evaluated the changes in interventricular septum (IVS) and left ventricular posterior wall (LVPW) individually, as well as the ratio of the IVS to the LVPW (Fig. 3g–j). An abnormal IVS/LVPW ratio can be seen in hypertropic cardiomyopathy and also can be seen after myocardial infarct 38, whereas a preserved ratio indicates diffuse thickening as may be seen in edema. Here, we found that in LCWE‐injected mice, both IVS and LVPW demonstrated increase in thickness, but the ratio of IVS to LVPW was preserved (Fig. 3g–j). However, we did not specifically look at histological analysis of tissue edema.
Our results demonstrate that, similar to human KD, LCWE‐induced KD vasculitis in mice is also associated with myocarditis and myocardial dysfunction with ventricular enlargement. We also show by using both MRI and echocardiographic techniques that pretreatment with the IL‐1Ra not only prevents coronary and myocardial inflammation during the acute phase of LCWE‐induced KD vasculitis, but also blocks the acute and long‐term myocardial dysfunction and ventricular enlargement in this model.
Discussion
The dramatic nature of CAA in KD has traditionally overshadowed the potential importance of the concomitant myocarditis that is present in almost all patients 39. However, KD‐induced myocarditis and its potential long‐term complications are now attracting increasing attention by clinicians and researchers 16, 20, 40, 41. The development of myocarditis during the acute phase of KD is a universal histological finding, and can occur in the absence of coronary artery abnormalities. The presence of myocarditis in KD acute phase is supported by pathology, biopsy, nuclear imaging, echocardiography, magnetic resonance and serum biomarker studies.
While only a minority of patients are clinically symptomatic 16, in many children with acute KD echocardiography studies show depressed shortening fraction, increased end‐systolic and end‐diastolic dimension strain abnormalities as well as diastolic dysfunction 33, 42. Increased left ventricular mass, believed to indicate cardiac edema, is also seen in patients with acute KD diagnosis prior to IVIG treatment 34, 35.
Following IVIG treatment, most patients do not demonstrate significant mechanical dysfunction, although subclinical dysfunction can be detected which may persist for 1–3 months 43, and there is now growing evidence that ongoing myocardial abnormalities may occur 14. Children with subclinical myocardial complications during acute KD display long‐term cardiovascular complications, such as lower myocardial flow reserve and higher total coronary resistance 41, 44, 45, and potentially both long‐term subclinical diastolic dysfunction 16 and a persistently increased end‐diastolic volume many months after acute KD 37. However, despite these descriptions of diastolic dysfunction, conclusive evidence of long‐term changes in shortening or ejection fraction is lacking, and physicians are not directed to follow these indices in KD patients 46, 47.
Recent insights gained from clinical and transcriptome data from KD patients, as well as experimental data from the LCWE‐induced mouse model of KD vasculitis, have provided strong evidence for the critical role of the IL‐1β signaling pathway in the development of cardiovascular pathologies induced by KD 6. We have shown that pretreatment with IL1Ra prevents coronary arteritis and vasculitis, as well as myocarditis, in the LCWE‐induced KD vasculitis mouse model 21. A limitation of the present study is the preventive administration of IL‐1Ra, which was given 1 day before LCWE injection. Importantly, IL‐1Ra has been shown to decrease LCWE‐induced coronary lesions when administered up to 3 days after LCWE injection 21. Therefore, it is tempting to suggest that if IL‐1Ra was injected during the first 3 days after LCWE injection, it may also block the long‐term cardiovascular consequences of KD by inhibiting acute KD myocarditis and cardiac dysfunction. Blocking tumor necrosis factor (TNF)‐α in the LCWE‐injected mice significantly decreases vascular inflammation and incidence of coronary arteritis, but had modest effects on preventing myocarditis development 21, 48. Importantly, IL‐1Ra treatment was more efficient in limiting both coronary artery and myocarditis development 21, 25, 49. The significance of myocarditis during acute KD for post‐inflammatory myocardial fibrosis has not been adequately studied 16. Therefore, it was of great interest to observe that IL‐1Ra was able to reduce both coronary arterial and myocardial inflammation in the experimental KD vasculitis mouse model. This is particularly important, as recent studies have established the beneficial role of IL‐1 blockade to treat myocarditis and heart failure 50, 51, 52, as well as dilated cardiomyopathy 53 and pericarditis secondary to Erdheim–Chester disease 54.
Here, we showed that the myocarditis present in this experimental KD model is also associated with cardiac dysfunction, including decreased ejection fraction and increased left ventricular mass, similar to features reported in children with KD 34, 55, 56. In the present study, we show for the first time, to our knowledge, that IL‐1Ra is able to significantly improve the myocardial mechanical dysfunction as measured by both MRI and echocardiography. The efficacy of IL‐1Ra treatment for cardiac dysfunction of KD is expected, given the correlation of myocardial dysfunction with myocarditis 57 and the beneficial effect of IL‐1Ra on LCWE‐induced KD myocarditis 21.
Rarely, children present with Kawasaki shock syndrome and hemodynamic instability as a result of decreased systolic function and vasoplegia. These cases are often partially refractory to inotropic support and demonstrate impaired systolic and diastolic mechanical function with significantly decreased ejection fraction 16, 33, 36. These patients have an increased degree of systemic inflammation, a greater incidence of valvulitis (manifesting as valve regurgitation), a significantly higher rate of IVIG resistance and overall a greater incidence of coronary abnormalities 36. It is tempting to speculate that this subset of patients may have significantly higher circulating levels of IL‐1β levels, which may contribute to the depressed myocardial function leading to shock as well as to IVIG resistance, and future studies should investigate the role of IL‐1Ra in these KD patients.
The finding of increased thickness of IVS and LVPW and preserved IVS/LVPW ratio are notable in this experimental model, as these features are also seen in human patients with KD 55. While the changes in LVM described in other human studies are calculated from IVS and LVPW, Lee et al. 55 evaluated school‐aged children with past Kawasaki disease and provided a detailed review of all parameters, showing increases in both IVS and LVPW in a similar fashion as we see in the mouse model.
In summary, while IL‐1Ra is now in two Phase II clinical trials in IVIG‐non‐responsive acute KD patients, our findings indicate a potential benefit of IL‐1 receptor blockade on the long‐term cardiovascular consequences of KD by inhibiting acute KD myocarditis and cardiac dysfunction. These observations further support that interventions blocking IL‐1β and its receptor could be more broadly utilized as a front‐line agent to treat KD, a concept that is now readily entertained 6, 7.
Disclosures
The authors declare no conflicts of interest.
Author contributions
M. G., G. J. A., M. A., Y. L., M. N. R. and M. Arditi conceived the project and designed the experiments. M. G., Y. L., T. A., J. P., S. C., M. N. R. and M. A. performed the experiments. T. R. C., M. N. R., M. G. and M. A. wrote the manuscript with input from all authors.
Acknowledgements
M. G. was supported by the Max and Minnie Tomerlin Voelcker Fund Young Investigator Award and the San Antonio Medical Foundation Collaborative Grant and the Vasculitis Foundation Pilot Grant; G. J. A. was supported by St Baldrick's Foundation Scholar: Career Development Award, the Turn it Gold Foundation, the Pablove Foundation Childhood Cancer Research Seed Grant and the San Antonio Medical Foundation grant. M. N. R. was supported by the NIH R01 HL139766 grant; S. C. was supported by the NIH HL111483‐01 grant. The MRI analysis was supported by the Cedars‐Sinai Biomedical Imaging Research Institute Seed Grant to S.C. The research was supported by NIH grant R01 AI072726 to M. Arditi.
Contributor Information
M. Gorelik, Email: mark.gorelik@bcm.edu
M. Arditi, Email: moshe.arditi@cshs.org.
References
- 1. McCrindle BW, Rowley AH, Newburger JW et al Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–e999. [DOI] [PubMed] [Google Scholar]
- 2. Newburger JW, Takahashi M, Burns JC et al The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 1986; 315:341–7. [DOI] [PubMed] [Google Scholar]
- 3. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma‐globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study. Group. Pediatr Infect Dis J 1998; 17:1144–8. [DOI] [PubMed] [Google Scholar]
- 4. Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re‐treatment in Kawasaki disease. J Pediatr 1993; 123:657–9. [DOI] [PubMed] [Google Scholar]
- 5. Tremoulet AH, Jain S, Kim S et al Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp Clin Trials 2016; 48:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns JC, Kone‐Paut I, Kuijpers T, Shimizu C, Tremoulet A, Arditi M. Review: Found in translation: international initiatives pursuing interleukin‐1 blockade for treatment of acute Kawasaki disease. Arthritis Rheumatol 2017; 69:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dusser P, Kone‐Paut I. IL‐1 Inhibition may have an important role in treating refractory Kawasaki disease. Front Pharmacol 2017; 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki A, Miyagawa‐Tomita S, Komatsu K et al Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation 2000; 101:2935–41. [DOI] [PubMed] [Google Scholar]
- 9. Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol 1996; 28:253–7. [DOI] [PubMed] [Google Scholar]
- 10. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997–2007. Pediatr Infect Dis J 2010; 29:483–8. [DOI] [PubMed] [Google Scholar]
- 11. Burns JC. Kawasaki disease update. Indian J Pediatr 2009; 76:71–6. [DOI] [PubMed] [Google Scholar]
- 12. Newburger JW, Takahashi M, Gerber MA et al, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease Council on Cardiovascular Disease in the Young, American Heart Association. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004; 114:1708–33. [DOI] [PubMed] [Google Scholar]
- 13. Burns JC, Glode MP. Kawasaki syndrome. Lancet 2004; 364:533–44. [DOI] [PubMed] [Google Scholar]
- 14. Orenstein JM, Shulman ST, Fox LM et al Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLOS ONE 2012; 7:e38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu AM, Ghazizadeh M, Onouchi Z, Asano G. Ultrastructural characteristics of myocardial and coronary microvascular lesions in Kawasaki disease. Microvasc Res 1999; 58:10–27. [DOI] [PubMed] [Google Scholar]
- 16. Dionne A, Dahdah N. Myocarditis and Kawasaki disease. Int J Rheum Dis 2018; 21:45–9. [DOI] [PubMed] [Google Scholar]
- 17. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics 1978; 61:100–7. [PubMed] [Google Scholar]
- 18. Ayusawa MAO, Miyashita T. The study of death cases in acute phase Kawasaki disease over the last 10 years based on a National Survey [in Japanese]. Pediatr Cardiol Cardiovasc Surg 2000; 20:245. [Google Scholar]
- 19. Takahashi M. Myocarditis in Kawasaki syndrome. A minor villain? Circulation 1989; 79:1398–400. [DOI] [PubMed] [Google Scholar]
- 20. Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol 2009; 54:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee Y, Schulte DJ, Shimada K et al Interleukin‐1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 2012; 125:1542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wakita D, Kurashima Y, Crother TR et al Role of interleukin‐1 signaling in a mouse model of Kawasaki disease‐associated abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2016; 36:886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noval Rivas M, Lee Y, Wakita D et al CD8+ T cells contribute to the development of coronary arteritis in the Lactobacillus casei cell wall extract‐induced murine model of Kawasaki disease. Arthritis Rheumatol 2017; 69:410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenkranz ME, Schulte DJ, Agle LM et al TLR2 and MyD88 contribute to Lactobacillus casei extract‐induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation 2005; 112:2966–73. [DOI] [PubMed] [Google Scholar]
- 25. Lee Y, Wakita D, Dagvadorj J et al IL‐1 signaling is critically required in stromal cells in Kawasaki disease vasculitis mouse model: role of both IL‐1alpha and IL‐1beta. Arterioscler Thromb Vasc Biol 2015; 35:2605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung DY, Cotran RS, Kurt‐Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin‐1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 1989; 2:1298–302. [DOI] [PubMed] [Google Scholar]
- 27. Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL‐1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis 2012; 71:2059–61. [DOI] [PubMed] [Google Scholar]
- 28. Lehman TJ, Walker SM, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum 1985; 28:652–9. [DOI] [PubMed] [Google Scholar]
- 29. Amano S, Hazama F, Kubagawa H, Tasaka K, Haebara H, Hamashima Y. General pathology of Kawasaki disease. On the morphological alterations corresponding to the clinical manifestations. Acta Pathol Jpn 1980; 30:681–94. [PubMed] [Google Scholar]
- 30. Hoshino S, Tsuda E, Yamada O. Characteristics and Fate of Systemic Artery Aneurysm after Kawasaki Disease. J Pediatr 2015; 167:108–12 e1–2. [DOI] [PubMed] [Google Scholar]
- 31. de Lemos JA, McGuire DK, Drazner MH. B‐type natriuretic peptide in cardiovascular disease. Lancet 2003; 362:316–22. [DOI] [PubMed] [Google Scholar]
- 32. Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi P. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J 2008; 155:527–33. [DOI] [PubMed] [Google Scholar]
- 33. Frank B, Davidson J, Tong S et al Myocardial strain and strain rate in Kawasaki disease: range, recovery, and relationship to systemic inflammation/coronary artery dilation. J Clin Exp Cardiolog 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu JJ, Choi HS, Kim YB et al Analyses of left ventricular myocardial deformation by speckle‐tracking imaging during the acute phase of Kawasaki disease. Pediatr Cardiol 2010; 31:807–12. [DOI] [PubMed] [Google Scholar]
- 35. Yu JJ, Kwak BO, Jeon YH et al Elevation of the index of left ventricular mass during the acute and subacute phase of Kawasaki disease, and its association with indexes of diastolic function. Cardiol Young 2009; 19:64–9. [DOI] [PubMed] [Google Scholar]
- 36. Kanegaye JT, Wilder MS, Molkara D et al Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009; 123:e783–e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson TM, Meyer RA, Kaplan S. Long‐term echocardiographic evaluation of cardiac size and function in patients with Kawasaki disease. Am Heart J 1985; 110:107–15. [DOI] [PubMed] [Google Scholar]
- 38. Williams LK, Frenneaux MP. Steeds RP. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur J Echocardiogr 2009; 10:iii9‐14. [DOI] [PubMed] [Google Scholar]
- 39. Yutani C, Go S, Kamiya T et al Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med 1981; 105:470–3. [PubMed] [Google Scholar]
- 40. Gordon JB, Daniels LB, Kahn AM et al The spectrum of cardiovascular lesions requiring intervention in adults after Kawasaki disease. JACC Cardiovasc Interv 2016; 9:687–96. [DOI] [PubMed] [Google Scholar]
- 41. Dahdah N. Not just coronary arteritis, Kawasaki disease is a myocarditis, too. J Am Coll Cardiol 2010; 55:1507; author reply ‐8. [DOI] [PubMed] [Google Scholar]
- 42. Printz BF, Sleeper LA, Newburger JW et al Pediatric Heart Network I. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J Am Coll Cardiol 2011; 57:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moran AM, Newburger JW, Sanders SP et al Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma‐globulin. Am Heart J 2000; 139:217–23. [DOI] [PubMed] [Google Scholar]
- 44. Crystal MA, Syan SK, Yeung RS, Dipchand AI, McCrindle BW. Echocardiographic and electrocardiographic trends in children with acute Kawasaki disease. Can J Cardiol 2008; 24:776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tuan SH, Su HT, Chen CH et al Analysis of exercise capacity of children with Kawasaki disease by a coronary artery z score model (ZSP version 4) derived by the Lambda‐Mu‐Sigma method. J Pediatr 2018; 201:128–33. [DOI] [PubMed] [Google Scholar]
- 46. Tacke CE, Romeih S, Kuipers IM, Spijkerboer AM, Groenink M, Kuijpers TW. Evaluation of cardiac function by magnetic resonance imaging during the follow‐up of patients with Kawasaki disease. Circ Cardiovasc Imaging 2013; 6:67–73. [DOI] [PubMed] [Google Scholar]
- 47. McCrindle BW, Rowley AH, Newburger JW et al, American Heart Association Rheumatic Fever, Endocarditis, Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Epidemiology and Prevention. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–e999. [DOI] [PubMed] [Google Scholar]
- 48. Hui‐Yuen JS, Duong TT, Yeung RS. TNF‐alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J Immunol 2006; 176:6294–301. [DOI] [PubMed] [Google Scholar]
- 49. Burns JC. Of mice and children: lessons from a Kawasaki mouse model. Circulation 2012; 125:1480–1. [DOI] [PubMed] [Google Scholar]
- 50. Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M, Dagna L. Interleukin‐1 receptor blockade rescues myocarditis‐associated end‐stage heart failure. Front Immunol 2017; 8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Tassell BW, Buckley LF, Carbone S et al Interleukin‐1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D‐HART2). Clin Cardiol 2017; 40:626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Luca G, Cavalli G, Campochiaro C, Tresoldi M, Dagna L. Myocarditis: an interleukin‐1‐mediated disease? Front Immunol 2018; 9:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Luca G, Campochiaro C, Dinarello CA, Dagna L, Cavalli G. Treatment of dilated cardiomyopathy with interleukin‐1 inhibition. Ann Intern Med 2018; 169:819–20. [DOI] [PubMed] [Google Scholar]
- 54. Tomelleri A, Cavalli G, De Luca G et al Treating heart Inflammation with interleukin‐1 blockade in a case of Erdheim–Chester disease. Front Immunol 2018; 9:1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee H, Shin J, Eun L. Myocardial assessment in school‐aged children with past Kawasaki disease. J Korean Med Sci 2017; 32:1835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Newburger JW, Sanders SP, Burns JC, Parness IA, Beiser AS, Colan SD. Left ventricular contractility and function in Kawasaki syndrome. Effect of intravenous gamma‐globulin. Circulation 1989; 79:1237–46. [DOI] [PubMed] [Google Scholar]
- 57. Gaur L, Waloff K, Schiller O, Sable CA, Frank LH. Noncoronary inflammation in Kawasaki disease is associated with abnormal myocardial deformation in the acute phase. J Am Soc Echocardiogr 2014; 27:1329–35. [DOI] [PubMed] [Google Scholar]


