Abstract
Background/objective
A prospective cohort study aimed to evaluate the clinical efficacy of a specific vasoactive herbal formula, Huo Xue Tong Luo capsule (HXTL capsule), for the treatment of patients with asymptomatic osteonecrosis of femoral head (ONFH).
Methods
We evaluated a clinical trial of 55 patients (59 hips) with asymptomatic ONFH (no joint collapse) evaluated by Steinberg staging system and necrosis range classification criteria. Then, they were given HXTL capsule under specific protocol. Among them, 39 males and 16 females with an average age of 36.4 ± 10.1 years were followed up for an average of 4.38 years during treatment. The aggravation of clinical and imaging results was assessed by initial pain and joint collapse. The clinical and imaging outcomes of these patients were compared with those of a historical control group from the literature under critical inclusion criteria.
Results
At the latest follow-up, initial pain occurred in five of 59 (8.5%) hips of patients taking HXTL capsule compared with 31 of 81 (38.3%) controls (p < 0.001), and joint collapse occurred in 13 of 59 (22.0%) hips of patients taking HXTL capsule compared with 26 of 81 (32.1%) controls (p < 0.001). There was no association between joint collapse and multiple key factors of ONFH. Only the location of type C2 necrotic lesions (hazard ratio, 4.12; 95% confidence interval, 2.64–18.91) and the extent of large necrotic lesions (hazard ratio, 3.39; 95% confidence interval, 1.43–16.21) predicted joint collapse.
Conclusion
As an agent formulated by vasoactive herbals, HXTL capsule with specific protocol is an effective medicine for relieving hip pain and preventing joint collapse in patients with asymptomatic ONFH.
The translational potential of this article
The translation potential of this prospective cohort study is that the initially officially approved clinical indication for HXTL capsule for treatment of ONFH is due to its possible effect of revascularization on angiogenesis of necrosis. It is has been now proven to be effective for a new clinical application.
Keywords: Clinical progression, Femoral head, HXTL capsule, Osteonecrosis, Radiographic evaluation
Introduction
The progression of patients with osteonecrosis of femoral head (ONFH) with an insidious onset usually deteriorated further and eventually led to joint collapse and hip osteoarthritis [1]. Ischaemia was a significant pathological feature of necrotic lesion in ONFH [2]. It was demonstrated that skeletal angiogenesis and vascularity were interrupted in necrotic tissue, no matter if it is steroid-induced or alcohol-induced one [3]. Femoral head blood flow was greatly reduced once osteonecrosis occurred, and vasoconstriction was usually found on lateral epiphyseal arteries of the femoral head [4]. Currently, the hip preservation surgery is used to provide biomechanical support and, more importantly, induce revascularization or transposition of the necrotic area [5]. Furthermore, in medicine treatment, at an early stage of ONFH, a single-centre study with a 3-year follow-up was conducted. An activating blood and dredging collaterals nourishing herbal formula (multiherbal components) method was clinically tested for its efficacy in alleviating the clinical symptoms and improving the joint function of patients with ONFH with systemic lupus erythematosus [6]. Studies in vivo also demonstrated that this formula had a therapeutic effect on ONFH in an animal model [7]. Huo Xue Tong Luo capsule (HXTL capsule) was one of the effective formulas used in the treatment of ONFH at earlier osteonecrosis stage in our clinical centre. HXTL capsule contained seven vasoactive herbal compounds such as cajan leaf [8], Angelica sinensis [9], red peony [10], Ligusticum wallichii [11], peach kernel [12], Carthamus tinctorius [13], and Radix Rehmanniae [14]. All compounds were discovered previously to be effective in promoting angiogenesis of ischaemic tissues. Hence, HXTL capsule was used as a promising formula to eliminate ischaemia in ONFH and promote tissue regeneration, removing obstruction.
Asymptomatic ONFH, known as “silent hip” or no joint collapse femoral head, usually occurred in bilateral ONFH, in which the more seriously affected hip is the contralateral side [15]. However, until now, the current therapies for asymptomatic ONFH remain controversial. Owing to the stability of clinical symptoms during progression, some proposed that asymptomatic ONFH did not require a systemic treatment such as physical therapy or impact graft implant surgery, especially in some patients with small- or medium-sized necrotic lesions [16]. In contrast, some researchers have proposed that aggressive treatments at an early stage are truly important, on account of the dynamic development of clinical symptoms in the vast majority of patients [17]. An oral vasoactive agent is one of the possible selections and might be taken to be a complemental therapy in the early stage of ONFH as we concerned. Hence, our research aims to explore the function of HXTL capsule on the clinical and radiological progression of asymptomatic ONFH and reveal its potential to leverage of level of evidences for wide clinical application of this vasoactive formula for treatment of asymptomatic ONFH.
Methods
Ethics statement
This study was conducted in agreement with the Declaration of Helsinki and had been approved by the ethics board. Informed consent form was obtained from each patient before evaluation. This study is registered at chictr.org.cn (http://www.chictr.org.cn/showproj.aspx?proj=10829).
Study participants and inclusion and exclusion criteria
Fifty-five patients with asymptomatic ONFH were recruited from June 2005 to January 2013. The inclusion criteria were as follows: (1) patients with asymptomatic ONFH diagnosed by physical examination, magnetic resonance imaging (MRI) and X-ray imaging of the hip performed at our institution following a standardized protocol [18] and (2) patients who had not received systemic treatment before. The exclusion criteria were as follows: (1) patients who had undergone previous hip surgery; (2) patients with trauma to the femoral head and neck; (3) patients with severe congenital malformation of the hip joint and (4) patients with any diseases that affect the hip joint including rheumatoid arthritis, ankylosing spondylitis, joint tuberculosis and pyogenic arthritis. A total of 55 patients were enrolled into our study, including 39 men and 16 women with an average age of 36.4 ± 10.1 years (range, 19–61 years). Four patients had bilateral ONFH (a total of 59 affected hips), and 51 had unilateral ONFH. Twenty-nine patients had steroid-induced ONFH, 9 patients had alcohol-associated ONFH and 17 patients had unknown reasons for developing ONFH. They were followed up until their femoral heads were collapsed at the late stage III of Steinberg staging system, or until they were lost to follow-up, passed away or the end of our study in 2017. All patients were followed up for 2–12 years.
Clinical and radiographic evaluations
At the time of the initial examination, MRI and X-ray imaging of all the patients were evaluated. According to the Steinberg staging system [19], there were 10 hips at stage I disease (MRI evidence of ONFH without radiographic evidence) and 49 hips at stage II disease (radiographic evidence of ONFH without collapse or a crescent sign). Based on the necrosis range classification criteria, there were 7 hips with small lesions (<15%), 27 hips with medium-sized lesions (15%∼30%) and 25 hips with large lesions (>30%). According to the Japanese Investigation Committee classification criteria [20], there were 3 hips with type A, 25 hips with type B, 21 hips with type C1 and 10 hips with type C2 necrotic lesions.
All patients underwent clinical and radiographic follow-up evaluations at three-month intervals for the first 2 years and annually thereafter until the time of collapse or for a minimum of 4 years. In particular, pain scores and radiographic progression of collapse were recorded at each follow-up evaluation.
Treatment
HXTL capsule was formulated for eliminating ischaemia in the femoral head, promoting tissue regeneration and removing obstruction. Seven herbs were used for formulation, including cajan leaf (main chemical ingredient, flavonoids), tails of Angelica sinensis (main chemical ingredient, ferulic acid), red peony root (main chemical ingredients, total paeony glycoside and Radix Paeoniae Rubra), Ligusticum wallichii (main chemical ingredient, tetramethylpyrazine), peach kernel (main chemical ingredient, glycoside), Carthamus tinctorius (main chemical ingredient, flavonoid), Radix Rehmanniae preparata (main chemical ingredient, polysaccharide). All the herbs were decocted and combined before condensing into a thick paste at 30°C and then blended, granulated, dried and finally packed to form an HXTL capsule for oral administration (Institutional approval number: Z20071224).
All patients with asymptomatic ONFH consented to receiving daily oral HXTL capsule at a dosage of one tablet (6 g/day) three times per day. The treatment with our herbal formula was the main part of our protocol, and we evaluated the location of necrosis and bone repair by bilateral hip X-ray in anteroposterior and flog position. To those patients with asymptomatic ONFH, they were permitted to walk at normal weight-bearing, and regular examinations were conducted. To those with painful hip, they were recommended to walk with stick for avoiding weight loading. In our report, there were only five cases (8.5% in 59 hips) suffering with hip pain at their last follow-up and 13 cases (22.0% in 59 hips) with femoral head collapse. In case of pain, these patients were guided to use stick for weight-bearing walking until their pain is relieved.
Control group
We compared the treatment group with an established group of historic controls taken from a peer-reviewed publication with a same baseline [21]. The publication prospectively analysed the fate of untreated asymptomatic ONFH in 81 patients (81 hips) in Chinese population. Glucocorticoids (14.9%), alcohol (48.1%) and idiopathic (37%) were the aetiologic causes of ONFH. The cohort included 81 hips: 55 hips had Steinberg stage I disease, and 26 had stage II; 30 hips had small-sized lesions, 29 had medium-sized lesions and 22 had large-sized lesions; 3 hips had Japanese Investigation Committee type A, 35 had type B, 15 had type C1 and 28 had type C2 necrotic lesions. The mean patient age was 50.5 years, and the mean follow-up was 8.3 years (range, 5–16 years). At the last follow-up, 31 of 81 (38%) patients in the study showed evidence of pain progression, and 26 of 81 (32%) patients had unsuccessful radiographic outcomes.
Statistical analysis
The data were analysed using SPSS, version 18.0 (SPSS Inc., USA). A p-value of less than 0.05 was considered statistically significant. Clinical failure was defined as the occurrence of pain, and radiographic failure was defined as the occurrence of segmental collapse. With collapse of the femoral head as seen on a radiograph as the endpoint, survival for all the enrolled patients was calculated using the Kaplan–Meier method. The differences in the survival distributions were tested with the log-rank test. The presence or absence of pain and radiological progression was recorded as a binary variable. Pain and radiological progression were compared between the two groups using the Chi-square test and Fisher's exact test. We performed a multivariate regression analysis using Cox proportional hazards model to identify the independent factors associated with collapse of the femoral head.
Results
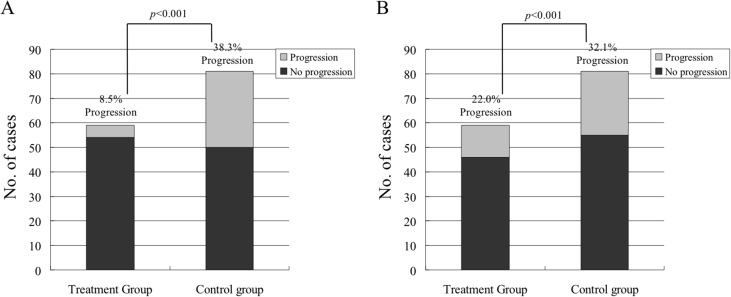
In terms of the study's primary outcome, 5 of 59 hips (8.5%) were symptomatic, and 13 hips (22.0%) had bone collapse at an average follow-up of 4.38 ± 2.38 years. Compared with patients in the control group, patients taking HXTL capsule showed a significantly decreased rate of pain (31 of 81 hips, 38.3% rate of progression vs. 5 of 59, 8.5% rate of progression, p = 0.000; Figure 1A). Compared to patients in the historical control group, patients taking HXTL capsule still showed a decreased rate of radiographic progression (13 of 59 hips taking HXTL capsule vs. 26 of 81 controls, p = 0.000; Figure 1B).
Fig. 1.
Patients taking a Huo Xue Tong Luo capsule (HXTL capsule; treatment group) and historical controls (control group) with any presenting Steinberg stage osteonecrosis of the femoral head (ONFH) after more than 4 years of follow-up. (A) The treatment group exhibited a significantly lower percentage of cases with clinical pain progression of ONFH than the control group. (B) The treatment group exhibited a significantly lower percentage of cases with radiographic progression of ONFH than the control group.
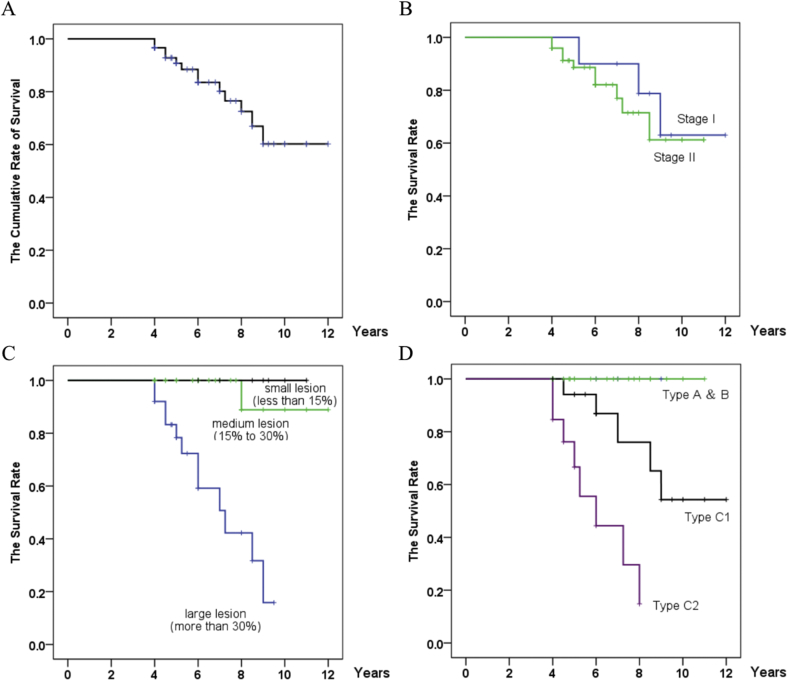
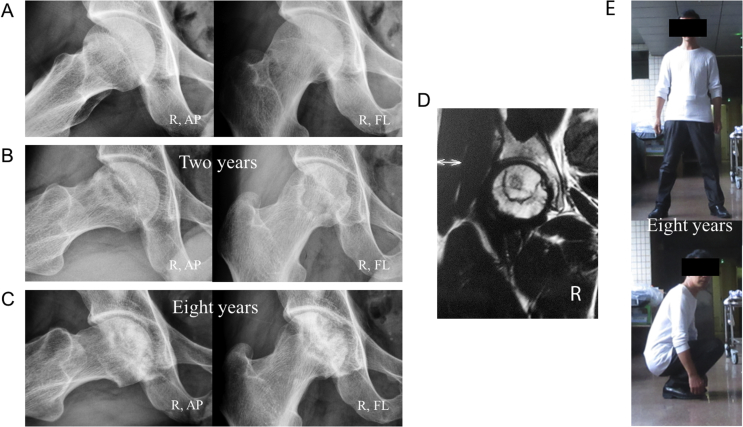
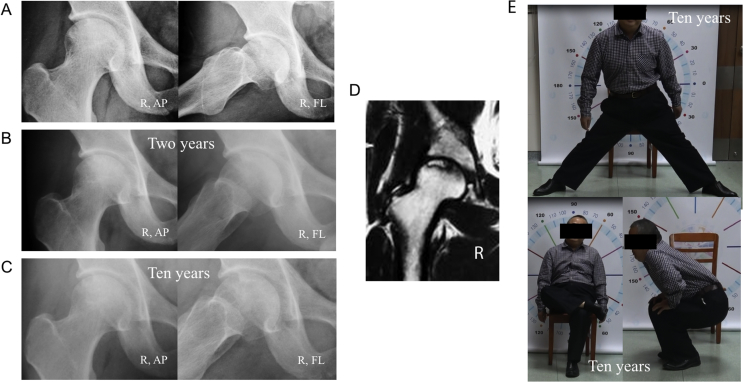
With the occurrence of collapse as the endpoint, the cumulative rates of survival (with 95% confidence intervals) were 96.6% at 4 years, 90.8% at 5 years, 83.5% at 6 years, 80.2% at 7 years, 72.5% at 8 years and 60.2% at 9 years in our group (Figure 2A). All five hips that were symptomatic collapsed. The mean interval between diagnosis and collapse was 1.8 years (range, 0.4–4.3 years). The mean interval between diagnosis and the onset of pain was 1.5 years (range, 0.4–2.2 years). Eight of the 13 hips (61.5%) that had collapsed were not associated with pain (Figure 3). There was no pain or collapse in 43 patients (46 hips) with good function at the last follow-up (Figure 4).
Fig. 2.
Kaplan–Meier survivorship curve. (A) The cumulative rates of survival (with 95% confidence intervals) are 96.6% at 4 years, 83.5% at 6 years, 72.5% at 8 years and 60.2% at 9 years with collapse of the femoral head as the endpoint. (B) Survival rates according to initial Steinberg stages of osteonecrosis. There were no differences (p = 0.607, log-rank test) in survival among hips classified at different Steinberg stages. (C) Survival rates according to the initial extent of the osteonecrosis as determined by magnetic resonance imaging (MRI) using the method of Steinberg et al. (Steinberg et al., 1995). The time to collapse was different (p = 0.000, log-rank test) among the three groups. (D) Survival rates according to the location of osteonecrosis. The time to collapse was different (p = 0.000, log-rank test) among the three groups.
Figure 3.
A 34-year-old man who received steroid therapy for nephrotic syndrome. (A) Frog-leg lateral radiographs and anteroposterior radiographs show bilateral asymptomatic hips with stage I disease at his initial diagnosis. (B) After taking Chinese medicine for two years, the patient was free from symptoms and had no collapse of the femoral heads bilaterally on frog-leg lateral radiographs and anteroposterior radiographs four and a half years later. (C) Six and a half years later, the patient was free from symptoms and had no radiographic progression of the left femoral head, but collapse of the right femoral head was seen on a frog-leg lateral radiograph, not on an anteroposterior radiograph. (D) MRI image of the suffered hip obtained at his initial diagnosis. (E) Until eight years later, the patient was free from symptoms and had good function. MRI = magnetic resonance imaging.
Figure 4.
A 38-year-old man who received steroid therapy for brainstem encephalitis. (A–C) Anteroposterior radiograph and frog-leg lateral radiograph showing a right asymptomatic hip with stage II disease at his initial diagnosis. The patient took Chinese medicine for two years, and radiographs taken seven years and ten years later showed no progression to collapse of the femoral head. (D) MRI image of the suffered hip obtained at his initial diagnosis. (E) The patient was free from symptoms and had good function. MRI = magnetic resonance imaging.
No differences were observed in survival (p = 0.607, log-rank test) among hips classified according to Steinberg stages (Figure 2B). The survival rates for stage I and II hips were 60% and 81.6%, respectively (Table 1). Lesion size predicted survival likelihood and duration. The log-rank test showed longer durations (p = 0.000) of survival for hips with small- or medium-sized lesions than for hips with large lesions (Figure 2C). The survival rates for hips with small lesions, medium-sized lesions and large lesions were 100%, 88.9% and 60.0%, respectively (Table 1). The log-rank test showed longer durations (p = 0.000) of survival for hips with type A, B or C1 necrotic lesions than for those with type C2 necrotic lesion (Figure 2D). The survival rate for hips with type A or B necrotic lesions was 100%. The survival rates for hips with type C1 and C2 necrotic lesions were 76.2% and 20.0%, respectively (Table 1).
Table 1.
Fate of asymptomatic ONFH according to Steinberg stage, extent and location of necrotic lesion for HXTL capsule-treated patients.
| Variables | Hips | Pain | Collapse |
|---|---|---|---|
| Total hips | 59 | 5 (8.5%) | 13 (22.0%) |
| Stage | |||
| Stage I | 10 (16.9%) | 2 (20.0%) | 4 (40.0%) |
| Stage II | 49 (83.1%) | 3 (6.1%) | 9 (18.4%) |
| Extent | |||
| Small (less than 15%) | 7 (11.9%) | 0 (0) | 0 (0) |
| Medium (15–30%) | 27 (45.8%) | 2 (7.4%) | 3 (23.1%) |
| Large (greater than 30%) | 25 (42.4%) | 3 (12.0%) | 10 (76.9%) |
| Location | |||
| Type A | 3 (5.1%) | 0 (0) | 0 (0) |
| Type B | 25 (42.4%) | 0 (0) | 0 (0) |
| Type C1 | 21 (35.6%) | 2 (9.5%) | 5 (23.8%) |
| Type C2 | 10 (16.9%) | 3 (30.0%) | 8 (80.0%) |
ONFH = osteonecrosis of the femoral head.
There was no association among bone collapse and patients' age, gender, weight, aetiology of ONFH, length of follow-up or ONFH stage (Table 2). When all the possible factors were analysed together with a Cox model, the extent of large necrotic lesions (hazard ratio, 3.39; 95% confidence interval, 1.43–16.21) and the location of type C2 necrotic lesions (hazard ratio, 4.12; 95% confidence interval, 2.64–18.91) were risk factors for collapse.
Table 2.
Relationship between the final status of femoral head and various clinical and radiographic parameters for patients with asymptomatic ONFH.
| Patient characteristics | No collapse | Collapse | p |
|---|---|---|---|
| Number of hips: 59 | 46 hips (78%) | 13 hips (22%) | — |
| Age (years) | 36.2 ± 10.7 | 36.8 ± 8.1 | 0.852 |
| Males:females (no.) | 31:12 | 8:4 | 0.714 |
| Weight (kg) | 62.4 ± 11.5 | 60.8 ± 9.9 | 0.628 |
| Aetiology (steroid:alcohol:idiopathic) (no.) | 22:9:15 | 10:1:2 | 0.177 |
| Follow-up period (years) | 6.8 ± 2.2 | 6.1 ± 1.7 | 0.297 |
| Stage (I:II) (no.) | 6:40 | 4:9 | 0.505 |
| Extent (small:medium:large) (no.) | 7:24:15 | 0:3:10 | 0.014 |
| Location (Type A:B:C1:C2) (no.) | 3:25:16:2 | 0:0:5:8 | 0.000 |
ONFH = osteonecrosis of the femoral head.
Discussion
Currently, there is controversy regarding the pivotal issue of whether a trial of conservative treatment or hip preserving surgery should be instituted for patients with asymptomatic ONFH [22], [23]. There is also considerable disagreement about which type of preservation treatment is appropriate for asymptomatic ONFH with no joint collapse [17], [24]. One prospective study proposed that there is no significant difference between the failure rates of simultaneous bilateral core decompression and bone grafting for the asymptomatic side and the contralateral symptomatic side of each patient [22]. These findings demonstrate that both kinds of surgical treatment for asymptomatic ONFH are unpredictable, and conservative therapies seem to be more favourable. Our view is in accordance with previous studies that considered that preventive intervention for asymptomatic ONFH was a necessary action [25]. Owing to the required indications for surgery, it is more reliable and feasible to select conservative treatment under the precondition of indeterminate indications.
Conservative treatment mainly includes weight-bearing restriction, extracorporeal shock wave (ESW) therapy and drug therapy [23]. First, weight-bearing restriction as a stand-alone therapy is inadequate for preventing ONFH progression, but it might be more useful if combined with medical management or surgery [26]. Second, as a safe and noninvasive method of biophysical stimulation, ESW therapy has been shown to promote bone repair in necrotic femoral heads of rabbits through the proliferation and activation of osteoblasts in histomorphometric analysis [27], [28]. Clinically, ESW improves motor function and relieves pain in patients with early-stage ONFH but has limited effects for healing necrosis. Third, previous studies have demonstrated that antiresorptive drugs such as alendronate effectively prevent early collapse of the femoral head in patients with ONFH and delay the time until total hip replacement [29]. In contrast, one randomized controlled trial and one meta-analysis demonstrated that alendronate had no obvious effects on relieving clinical symptoms, reducing disease progression or preventing the necessity for total hip arthroplasty [30], [31]. Similarly, a recent randomized controlled trial illustrated that zoledronate did not prevent collapse of the femoral head during early-stage ONFH or reduce the need for total hip arthroplasty [32]. Only teriparatide was discovered to have greater pharmacological effects than alendronate for treating ONFH and preventing bone collapse [33].
By comparison, Chinese medicine has been long and widely utilized in Asia to improve bone homoeostasis by relieving pain and controlling disease progression in ONFH [34], [35]. As one kind of particular therapy, Chinese medicine with vasoactive function has strong potential to clarify the healing capacity of necrotic bone by promoting angiogenesis in tissues. As mentioned, ischaemia in osteonecrosis is the main feature throughout the whole dynamic pathological process, and a vasoactive agent is efficient for promoting revascularization [36]. Specifically, a vasoactive agent is able to rebuild or promote vascular channel, trigger the active of angiogenesis in necrosis area and improve the potential for bone repair thought the cross-talk of endothelial cells and osteocytes [30]. Hence, a vasoactive agent is one kind of important therapy for ONFH. Here, we explored the administration of a vasoactive herbal formula HXTL capsule with seven powerful herbal compounds, which is a preparation produced by our hospital, and assessed its medical effects on the progression of ONFH.
Our prospective study showed the potential of HXTL capsule to prevent clinical symptoms and the progression of bone collapse progression in treated patients compared with those patients in the historical cohort. We found that most patients treated with daily HXTL capsule did not have worse clinical or radiographic progression of ONFH during short-term to midterm follow-up. The failure rates of clinical and radiographic progression were both lower than those reported in previous studies (Table 3). In our study, only 5 out of 59 (8.5%) hips were symptomatic, and 13 hips (22.0%) had bone collapse. The rates of clinical and radiographic improvement in the patients in this study were markedly enhanced compared with those of the controls in the historical cohort [21]. In terms of the Steinberg stage classification, one other study suggested that pain occurred in 88% of patients and bone collapse occurred in 73% of patients with asymptomatic hips with Steinberg stage I disease [37]. Jergesen et al [38] reported that 73.7% of asymptomatic hips with Steinberg stage II disease had clinical progression and that 42.1% had bone collapse. In our report, only 20% of hips with Steinberg stage I disease were associated with pain syndrome, and 40% had bone collapse. Approximately 6.1% of patients with stage II disease became symptomatic, and 18.4% had collapse.
Table 3.
Review of the literature regarding outcomes in patients with untreated asymptomatic ONFH.
| Authors | Year | Number of hips | Length of follow-up (years) | Symptomatic progression (%) | Collapse (%) |
|---|---|---|---|---|---|
| Kang et al | 2013 | 68 | 2.27 | 55.9 | Not reported |
| Min et al | 2008 | 81 | 8.3 | 38.3 | 32.1 |
| Nam et al | 2008 | 105 | 8.58 | 59.0 | Not reported |
| Hernigou et al | 2006 | 121 | 14 | 91.0 | 77.0 |
| Hernigou et al | 2004 | 40 | 11.5 | 88.0 | 73.0 |
| Yoshida et al | 2002 | 24 | 4.25 | 29.0 | 4.0 |
| Jergessen et al | 1997 | 19 | 2.96 | 74.0 | 42.0 |
| Sugano et al | 1994 | 149 | 5.2 | Not reported | 80.5 |
| Takatori et al | 1993 | 32 | 0.9 | Not reported | 44.0 |
| Ohzono et al | 1992 | 115 | 5.25 | Not reported | 67.8 |
| Our data | 2017 | 59 | 4.38 | 8.5 | 22.0 |
ONFH = osteonecrosis of the femoral head.
In terms of the possible factors affecting the outcomes of our study, multiple studies have concluded that bone collapse usually occurs in lesions with a larger area of necrosis and a more lateral location of the weight-bearing surface of the femoral head [15], [39]. In Steinberg stage I disease, it has been reported that the risk of bone collapse increases with an increasing necrotic lesion size. The development of pain is also positively related to a 5% area of necrosis for small lesions (<30% of the area of the femoral head), 46% for medium-sized lesions (30%–50% of the area) and 83% for large lesions (>50% of the area) [40]. In our study, we measured this relationship by using the Steinberg stage classification [19]. We found that the incidence of pain was low to 0% in patients with small lesions (<15% of the area of the femoral head), 7.4% for those with medium-sized lesions (15%–30%) and only 12% for those with large lesions (>30%). While various classification schemes and intervention methods may be used by different researchers, the comparison of results emphasizes the distinguishing use of HXTL capsule for preventing asymptomatic ONFH of any lesion size.
Lesion location is another critical factor and potential indicator of outcome during ONFH treatment [39]. After Sugano first proposed the revised classification system of necrotic lesion location in 2001, most researchers adopted his scheme for evaluating lesion location. If the lesion occupies the lateral portion of the femoral head, it is likely predictive of deterioration of ONFH. Min et al [21] reported that the development of pain in 81 asymptomatic hips was 0% for patients with type A disease, 3% for those with type B, 13% for those with type C1 and 100% for those with type C2, and the incidence of collapse was 0% for patients with type A disease, 0% for those with type B, 13% for those with type C1 and 86% for those with type C2. Our data showed that all patients with type A and type B hips did not develop pain and collapse under treatment with HXTL capsule. Furthermore, the risk of pain and collapse was lower in type C1 lesions than in type C2 lesions (9.5% vs. 30.0% and 23.8% vs. 80.0%, respectively).
Hence, we further confirmed that the extent and location of necrotic lesions are important prognostic factors after the treatment of HXTL capsule. As reported, bone collapse was more likely to occur in hips with large necrotic lesions, but poor outcomes may also be found in patients with medium-sized lesions if the necrotic area involves the lateral portion of the femoral head. Furthermore, we found that in five of 59 asymptomatic hips in patients with ONFH, initial pain was associated with bone collapse within several days. This suggests that the initial pain syndrome in an asymptomatic hip may be suggestive of the risk of bone collapse, even though the mechanism still needs to be elucidated. In these cases, we recommend that physicians evaluate patients immediately after the onset of pain and provide an appropriate Traditional Chinese Medicine treatment to prevent further collapse.
Taken together, our results highlight that the administration of the vasoactive herbal formulas HXTL capsule for patients with the early stages of asymptomatic ONFH can significantly suppress pain and necrosis progression towards joint collapse in short term to midterm. The vast majority of patients who received oral HXTL capsule remained asymptomatic for more than four years. It should also be noted that HXTL capsule administration did not perform well for large lesions with a lateral location. In addition, multicentre prospective studies with long-term follow-up are necessary to elucidate the potential benefits of HXTL capsule treatment in asymptomatic ONFH.
Conflicts of interest
The authors have no conflicts of interest to declare.
Declaration
This study was conducted in agreement with the Declaration of Helsinki and had been approved by the ethics board of the first affiliated hospital of Guangzhou University of Chinese Medicine (No: 20140307). Informed consent form was obtained from each patient before evaluation. This study is registered at chictr.org.cn, ChiCTR-RPC-15006290 (http://www.chictr.org.cn/showproj.aspx?proj=10829).
Funding/support statement
This work was supported by grants from the project of the National Natural Science Foundation of China (grant nos. 81473697, 81573996 and 81873327), the special scientific research project from Guangdong Provincial Department of Science and Technology and Guangdong Provincial Academy of Traditional Chinese Medicine (2016A020226028) and Guangdong Province Natural Science Fund Project (2017A030313698).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2018.11.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Wang C., Peng J., Lu S. Summary of the various treatments for osteonecrosis of the femoral head by mechanism: a review. Exp Ther Med. 2014;8(3):700–706. doi: 10.3892/etm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chughtai M., Piuzzi N.S., Khlopas A., Jones L.C., Goodman S.B., Mont M.A. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J. 2017;99-B(10):1267–1279. doi: 10.1302/0301-620X.99B10.BJJ-2017-0233.R2. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein R.S., Wan C., Liu Q., Wang Y., Almeida M., O'Brien C.A. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9(2):147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Zhao D., Wang W.M., Wang B.J., Zhang Y., Li Z.G. Hemodynamic changes in osteonecrosis treatment of the femoral head with iliac bone flaps pedicled with the lateral femoral circumflex artery ascending branch: a 10-year report. Technol Health Care. 2016;24(Suppl. 2):S493–S498. doi: 10.3233/THC-161173. [DOI] [PubMed] [Google Scholar]
- 5.Kang P., Xie X., Tan Z., Yang J., Shen B., Zhou Z. Repairing defect and preventing collapse of femoral head in a steroid-induced osteonecrotic of femoral head animal model using strontium-doped calcium polyphosphate combined BM-MNCs. J Mater Sci Mater Med. 2015;26(2):80. doi: 10.1007/s10856-015-5402-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang W., Yu J.N., Tao X.J. Systemic lupus erythematosus complicated with femoral head ischemic necrosis treated by Chinese medicine therapy for activating blood and dredging collaterals method. Chin J Integr Med. 2011;17(2):105–110. doi: 10.1007/s11655-011-0637-y. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y., Liu C., Chen W., Wang H., Wang C., Lin N. Tetramethylpyrazine enhances vascularization and prevents osteonecrosis in steroid-treated rats. Biomed Res Int. 2015;2015:315850. doi: 10.1155/2015/315850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi D., Sun Y., Yin J., Fan X., Duan H., Liu N. Cajan leaf combined with bone marrow-derived mesenchymal stem cells for the treatment of osteonecrosis of the femoral head. Exp Ther Med. 2014;7(6):1471–1475. doi: 10.3892/etm.2014.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Guo J., Tang Y., Wang H., Huang M., Qian D. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of Angelica sinensis, Ligusticum chuanxiong and their combination. Int J Mol Sci. 2012;13(3):3583–3597. doi: 10.3390/ijms13033583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y.F., Wu K.J., Wood W.G. Paeonia lactiflora extract attenuating cerebral ischemia and arterial intimal hyperplasia is mediated by paeoniflorin via modulation of VSMC migration and Ras/MEK/ERK signaling pathway. Evid Based Complement Alternat Med. 2013;2013:482428. doi: 10.1155/2013/482428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zengyong Q., Jiangwei M., Huajin L. Effect of Ligusticum wallichii aqueous extract on oxidative injury and immunity activity in myocardial ischemic reperfusion rats. Int J Mol Sci. 2011;12(3):1991–2006. doi: 10.3390/ijms12031991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E.Y., Kim J.H., Rhyu M.R. Endothelium-independent vasorelaxation by Ligusticum wallichii in isolated rat aorta: comparison of a butanolic fraction and tetramethylpyrazine, the main active component of Ligusticum wallichii. Biol Pharm Bull. 2010;33(8):1360–1363. doi: 10.1248/bpb.33.1360. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y., Lu P., Han C., Yu C., Chen M., He F. Hydroxysafflor yellow A (HSYA) from flowers of Carthamus tinctorius L. and its vasodilatation effects on pulmonary artery. Molecules. 2012;17(12):14918–14927. doi: 10.3390/molecules171214918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C.L., Tam J.C., Sanders A.J., Ko C.H., Fung K.P., Leung P.C. Molecular angiogenic events of a two-herb wound healing formula involving MAPK and Akt signaling pathways in human vascular endothelial cells. Wound Repair Regen. 2013;21(4):579–587. doi: 10.1111/wrr.12055. [DOI] [PubMed] [Google Scholar]
- 15.Mont M.A., Zywiel M.G., Marker D.R., McGrath M.S., Delanois R.E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92(12):2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 16.Liu L.H., Zhang Q.Y., Sun W., Li Z.R., Gao F.Q. Corticosteroid-induced osteonecrosis of the femoral head: detection, diagnosis, and treatment in earlier stages. Chin Med J (Engl) 2017;130(21):2601–2607. doi: 10.4103/0366-6999.217094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang J.S., Moon K.H., Kwon D.G., Shin B.K., Woo M.S. The natural history of asymptomatic osteonecrosis of the femoral head. Int Orthop. 2013;37(3):379–384. doi: 10.1007/s00264-013-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L.B., Huang Z.G., Wei H.Y., Wang W., Ren A., Xu Y.Y. Osteonecrosis of the femoral head: using CT, MRI and gross specimen to characterize the location, shape and size of the lesion. Br J Radiol. 2015;88(1046):20140508. doi: 10.1259/bjr.20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77(1):34–41. [PubMed] [Google Scholar]
- 20.Sugano N., Atsumi T., Ohzono K., Kubo T., Hotokebuchi T., Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7(5):601–605. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 21.Min B.W., Song K.S., Cho C.H., Lee S.M., Lee K.J. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466(5):1087–1092. doi: 10.1007/s11999-008-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu J.E., Wihbey T., Shah R.P., Garino J.P., Lee G.C. Prophylactic decompression and bone grafting for small asymptomatic osteonecrotic lesions of the femoral head. Hip Int. 2011;21(6):672–677. doi: 10.5301/HIP.2011.8760. [DOI] [PubMed] [Google Scholar]
- 23.Klumpp R., Trevisan C. Aseptic osteonecrosis of the hip in the adult: current evidence on conservative treatment. Clin Cases Miner Bone Metab. 2015;12(Suppl. 1):39–42. doi: 10.11138/ccmbm/2015.12.3s.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomaru Y., Yoshioka T., Sugaya H., Aoto K., Wada H., Akaogi H. Hip preserving surgery with concentrated autologous bone marrow aspirate transplantation for the treatment of asymptomatic osteonecrosis of the femoral head: retrospective review of clinical and radiological outcomes at 6 years postoperatively. BMC Musculoskelet Disord. 2017;18(1):292. doi: 10.1186/s12891-017-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X., Zhang D., Chen X., Yang J., Shi L., Pang Q. Effectiveness of various hip preservation treatments for non-traumatic osteonecrosis of the femoral head: a network meta-analysis of randomized controlled trials. J Orthop Sci. 2018;23(2):356–364. doi: 10.1016/j.jos.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.K., Aruwajoye O., Du J., Kamiya N. Local administration of bone morphogenetic protein-2 and bisphosphonate during non-weight-bearing treatment of ischemic osteonecrosis of the femoral head: an experimental investigation in immature pigs. J Bone Joint Surg Am. 2014;96(18):1515–1524. doi: 10.2106/JBJS.M.01361. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Liu L., Sun W., Gao F., Cheng L., Li Z. Extracorporeal shockwave therapy in osteonecrosis of femoral head: a systematic review of now available clinical evidences. Medicine (Baltim) 2017;96(4):e5897. doi: 10.1097/MD.0000000000005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H.Z., Zhou D.S., Li D., Zhang W., Zeng B.F. A histomorphometric study of necrotic femoral head in rabbits treated with extracorporeal shock waves. J Phys Ther Sci. 2017;29(1):24–28. doi: 10.1589/jpts.29.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwala S., Shah S.B. Ten-year follow-up of avascular necrosis of femoral head treated with alendronate for 3 years. J Arthroplasty. 2011;26(7):1128–1134. doi: 10.1016/j.arth.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Chen C.H., Chang J.K., Lai K.A., Hou S.M., Chang C.H., Wang G.J. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(5):1572–1578. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 31.Hong Y.C., Luo R.B., Lin T., Zhong H.M., Shi J.B. Efficacy of alendronate for preventing collapse of femoral head in adult patients with nontraumatic osteonecrosis. Biomed Res Int. 2014;2014:716538. doi: 10.1155/2014/716538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y.K., Ha Y.C., Cho Y.J., Suh K.T., Kim S.Y., Won Y.Y. Does zoledronate prevent femoral head collapse from osteonecrosis? A prospective, randomized, open-label, multicenter study. J Bone Joint Surg Am. 2015;97(14):1142–1148. doi: 10.2106/JBJS.N.01157. [DOI] [PubMed] [Google Scholar]
- 33.Arai R., Takahashi D., Inoue M., Irie T., Asano T., Konno T. Efficacy of teriparatide in the treatment of nontraumatic osteonecrosis of the femoral head: a retrospective comparative study with alendronate. BMC Musculoskelet Disord. 2017;18(1):24. doi: 10.1186/s12891-016-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C.G., Shen L., Yang Y.P., Xu X.J., Shuai B., Ma C. Effects of Modified Qing'e Pill on expression of adiponectin, bone morphogenetic protein 2 and coagulation-related factors in patients with nontraumatic osteonecrosis of femoral head. Chin J Integr Med. 2017;23(3):183–189. doi: 10.1007/s11655-016-2407-3. [DOI] [PubMed] [Google Scholar]
- 35.Li Z.R., Cheng L.M., Wang K.Z., Yang N.P., Yang S.H., He W. Herbal Fufang Xian Ling Gu Bao prevents corticosteroid-induced osteonecrosis of the femoral head-A first multicentre, randomised, double-blind, placebo-controlled clinical trial. J Orthop Translat. 2018;12:36–44. doi: 10.1016/j.jot.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu T., Zhang Z., Xie L., Ke X., Liu Y. The influence of traditional Chinese medicine constitutions on the potential repair capacity after osteonecrosis of the femoral head. Complement Ther Med. 2016;29:89–93. doi: 10.1016/j.ctim.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Hernigou P., Poignard A., Nogier A., Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86-A(12):2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Jergesen H.E., Khan A.S. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1997;79(3):359–363. doi: 10.2106/00004623-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sun W., Li Z.R., Wang B.L., Liu B.L., Zhang Q.D., Guo W.S. Relationship between preservation of the lateral pillar and collapse of the femoral head in patients with osteonecrosis. Orthopedics. 2014;37(1):e24–e28. doi: 10.3928/01477447-20131219-12. [DOI] [PubMed] [Google Scholar]
- 40.Nam K.W., Kim Y.L., Yoo J.J., Koo K.H., Yoon K.S., Kim H.J. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(3):477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.