Abstract
Agrobacterium-mediated transient expression and virus-induced gene silencing (VIGS) are very useful in functional genomics in plants. However, whether these methods are effective in cassava (Manihot esculenta), one of the most important tropical crops, remains elusive. In this study, we used green fluorescent protein (GFP) and β-glucuronidase (GUS) as reporter genes in a transient expression assay. GFP or GUS could be detected in the infiltrated leaves at 2 days postinfiltration (dpi) and were evidenced by visual GFP and GUS assays, reverse-transcription PCR, and Western blot. In addition, phytoene desaturase (PDS) was used to show the silencing effect in a VIGS system. Both Agrobacterium GV3101 and AGL-1 with tobacco rattle virus (TRV)-MePDS-infiltrated distal leaves showed an albino phenotype at 20 dpi; in particular, the AGL-1-infiltrated plants showed an obvious albino area in the most distal leaves. Moreover, the silencing effect was validated by molecular identification. Notably, compared with the obvious cassava mosaic disease symptom infiltrated by African-cassava-mosaic-virus-based VIGS systems in previous studies, TRV-based VIGS-system-infiltrated cassava plants did not show obvious virus-induced disease symptoms, suggesting a significant advantage. Taken together, these methods could promote functional genomics in cassava.
Keywords: agrobacterium, transient expression, virus-induced gene silencing (VIGS), tobacco rattle virus (TRV), cassava (Manihot esculenta)
1. Introduction
Aiming at analyzing genomic sequences and functions, functional genomics has rapidly developed as a result of sequencing projects of different species, especially of important crops. Cassava (Manihot esculenta), a kind of tuber crop, is widely cultivated in the tropics and some subtropical areas. Because of its low planting cost and high efficiency, it has become one of the most important industrial crops in the world [1]. Although cassava sequencing and resequencing were accomplished years ago, cassava functional genomics has developed slowly due to the time-consuming nature and low transformation rate of obtaining stable transgenic cassava plants [2]. Therefore, it is necessary to find another way to promote rapid gene function studies of cassava.
Agrobacterium-mediated transient overexpression is widely used in gene function studies in plants [3]. The most important advantages of this method are its rapid and simple process and that it does not require complex equipment, unlike other overexpression assays [4]. The simple protocol is accomplished by creating an overexpression vector, transforming it into Agrobacterium, and infiltrating the Agrobacterium into plant culture [5]. Green fluorescent protein (GFP) and β-glucuronidase (GUS) are two convenient reporter genes in biology which can be observed by fluorescent microscopy or direct staining [6,7]. Normally, they can continue to express, reaching their peak at 3 days postinfiltration (dpi), and exert obvious expression for at least one week [4,8]. In addition, they can be efficiently expressed in a wide range of plant species. So far, transient overexpression assays have been successfully used in many species, such as Arabidopsis spp. [4], tobacco [5], Mimulus lewisii [6], Piper colubrinum [7], rose [9], soybean [10], Theobroma cacao L. [11], cotton [12], Brassica juncea [13], potato [14], tomato [15], and lettuce [15]. However, its effectiveness in cassava requires further investigation.
Agrobacterium-mediated virus-induced gene silencing (VIGS) is a widely used, efficient technique in gene function studies [16]. VIGS is mostly based on RNA viruses, such as tobacco mosaic virus (TMV), potato virus X (PVX), barley stripe mosaic virus (BSMV), cucumber mosaic virus (CMV), and tobacco rattle virus (TRV) [17,18,19,20,21,22,23]. Among these viruses, TRV is widely used because of its high silencing effect, long silencing duration, wide range of infiltration host species, and the mild virus-induced disease symptoms [24,25]. Presently, TRV has been used in Arabidopsis [26], tobacco [27], strawberry [27,28], cotton [29], piper [30], wheat [31], maize [31], and so on. For the silencing effect, most of the abovementioned infiltrated species show the phenotype at two weeks postinfiltration and the most obvious changes around the third week [27,29,32].
In this study, Agrobacterium-mediated transient overexpression and VIGS were established in cassava. The phenotype and examination of these assays illustrated the feasibility of the protocols, which should help promote the rapid analysis of functional genomics in cassava.
2. Results
2.1. The Effect of the Transient Expression Assay in Cassava Leaves
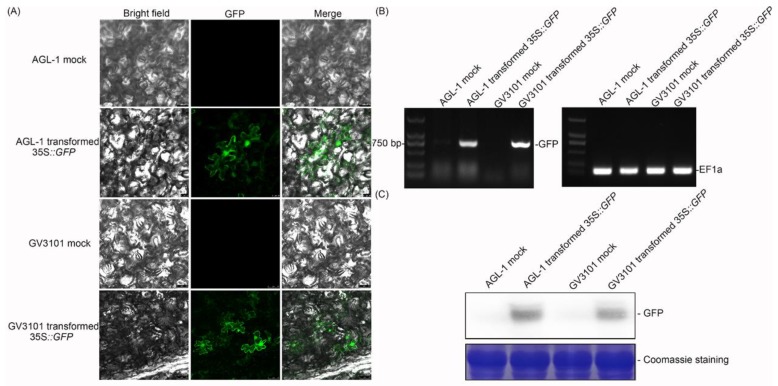
To investigate the effect of GFP transient expression, the 35S::GFP vector [33] was transformed into Agrobacterium AGL-1 and GV3101, and the transformed strains were infiltrated into cassava leaves, respectively (Figure 1). At 2 dpi, the infiltrated leaves were harvested and examined by confocal laser-scanning microscopy. Agrobacterium with 35S::GFP-infiltrated leaves showed clear green fluorescence in both the cytoplasm and nucleus, while the empty Agrobacterium-infiltrated leaves showed no fluorescence (Figure 2A). Reverse-transcription PCR was performed to clarify the effect of transient expression. Agrobacterium with the 35S::GFP-infiltrated sample showed a bright band by PCR, indicating that GFP was expressed in the cassava leaves (Figure 2B). Moreover, GFP was also detected by Western blot, which was consistent with confocal observation (Figure 2C).
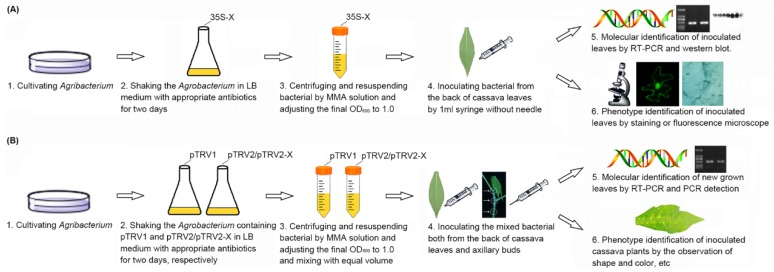
Figure 1.
Schematic views of Agrobacterium-mediated gene transient expression and TRV based gene silencing systems in cassava. (A) The schematic view of Agrobacterium-mediated gene transient expression assay. (B) The schematic view of Agrobacterium-mediated TRV based VIGS assay. The white arrows and circles pointed the axillary buds. The figure was drew by using ChemDraw (https://chemdrawdirect.perkinelmer.cloud/js/sample/index.html) and Microsoft Office PowerPoint 2003 (Microsoft, Redmond, WA, USA).
Figure 2.
Analysis of transient expression of green fluorescent protein (GFP) in cassava leaves. (A) At 2 days postinfiltration (dpi), Agrobacterium with no plasmid was used as the mock, and Agrobacterium containing 35S::GFP was used as the sample. Bar = 25 μm. (B) The relative transcript level of GFP is shown by reverse-transcription PCR, and elongation factor 1 (EF1) was used as a reference gene. (C) GFP was detected by Western blot assay, 10 μg total protein extracted from infiltrated leaves were loaded onto SDS-PAGE gel, and Coomassie staining shows the equal protein content.
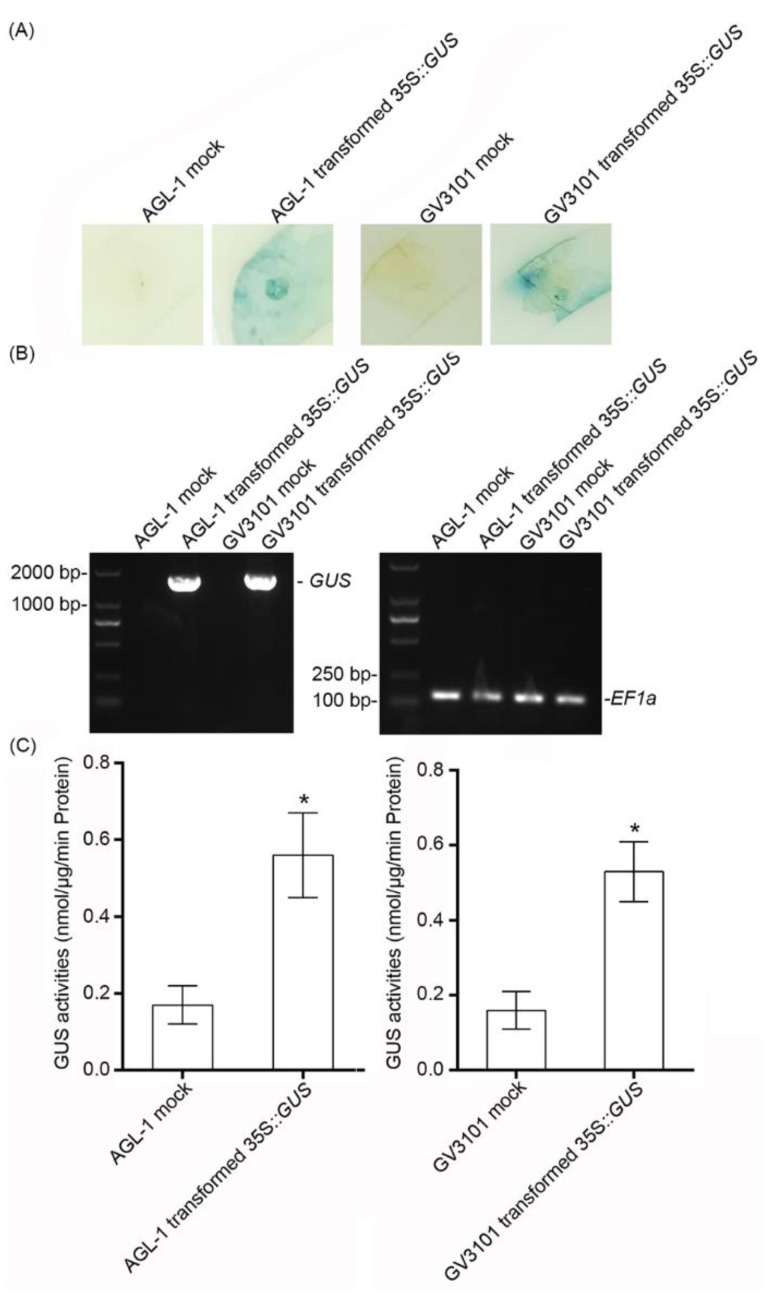
To further confirm the effect of transient expression, GUS was used as the second marker. After GUS staining, Agrobacterium with the 35S::GUS-infiltrated area became light blue (Figure 3A), indicating that GUS was expressed at the protein level. In addition, reverse-transcription PCR showed that the expression of 35S::GUS could be examined at the transcript level (Figure 3B), with significant GUS activity (Figure 3C). These results suggested that GFP and GUS were expressed with biological activity in the transient expression assay.
Figure 3.
Analysis of transient expression of β-glucuronidase (GUS) in cassava leaves. (A) At 2 dpi, Agrobacterium with no plasmid was used as the mock, and Agrobacterium containing 35S::GUS was used as the sample. The leaves were stained using GUS staining solution. Bar = 1 cm. (B) The relative transcript level of GUS is shown by reverse-transcription PCR, and EF1 was used as a reference gene. (C) The GUS activities of infiltrated leaves. Statistical tests were performed using IBM SPSS (v21). Briefly, the Kolmogorov–Smirnov test and Levene’s test were performed to check the normality of the data distribution and the homogeneity of variance of the data, respectively. The statistical analysis was performed using Student’s t-test and the Tukey–Kramer test. Asterisk symbol (*) indicates significant difference at p < 0.05. n = 10 per group. All data are expressed as mean ± SD of three independent experiments.
Based on the above results of GFP and GUS overexpression, the effects of transient overexpression showed no significant difference in GFP expression and GUS activity between AGL-1 and GV3101 (Figure 2 and Figure 3, Table 1).
Table 1.
Effects of Agrobacterium-mediated gene transient expression.
| Report Gene | Bacterial Strain | Number | The Positive Rate of Reporter Gene |
|---|---|---|---|
| GFP | GV3101 | 20 | 60.00% |
| GUS | GV3101 | 20 | 75.00% |
| GFP | AGL-1 | 20 | 65.00% |
| GUS | AGL-1 | 20 | 75.00% |
2.2. Silencing of MePDS in Cassava
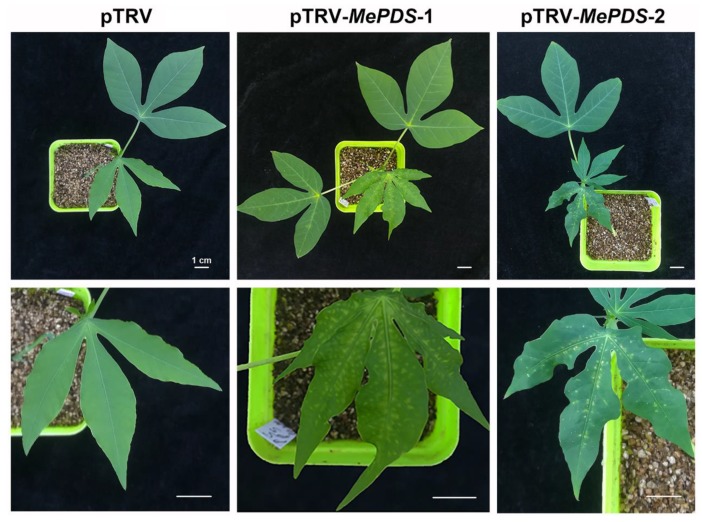
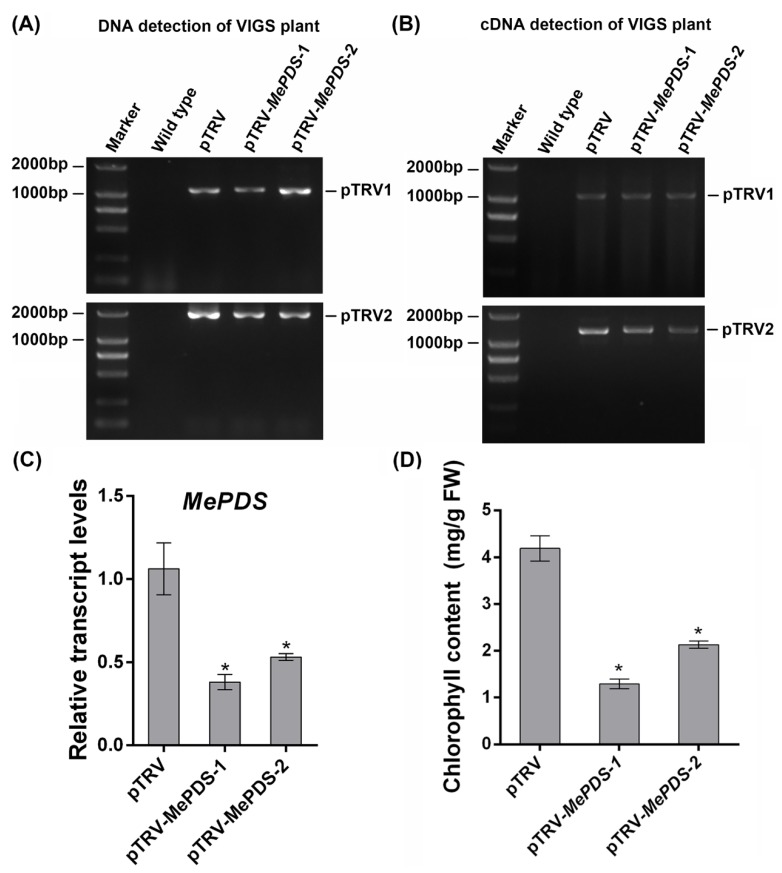
To reveal the effect of TRV-based VIGS on cassava, phytoene desaturase (PDS) was selected as a reporter gene. A 370 bp sequence of MePDS was amplified and inserted into the multiple cloning site of the pTRV2 vector. The pTRV1, pTRV2, and pTRV2-MePDS vectors were transformed into Agrobacterium, respectively. The cultivated Agrobacterium solution was infiltrated into cassava local leaves and axillary buds (Figure 1). At 20 dpi, AGL-1-infiltrated cassava showed an obvious albino area in the distal leaves, especially for the area around the main vein (Figure 4).
Figure 4.
Phenotypes of the infiltrated albino cassava plants. The cassava plants were infiltrated with Agrobacterium strain AGL-1 containing the pTRV1 and pTRV2 vectors, which were used as the mock, pTRV1 and pTRV2-MePDS, which were used to test the albino effect. In the photos, the upper line shows the whole cassava plants and the underline shows the magnified leaves, both at 20 dpi. Bar = 1 cm.
To further investigate the level of MePDS silencing, DNA was extracted from distal leaves and sequences of pTRV1, pTRV2, or pTRV2-MePDS were detected by PCR. The result indicated that the sequences of pTRV vectors could be detected in the distal leaves of all AGL-1-infitrated cassava plants but not in the wild-type (WT) cassava (Figure 5A). In addition, the transcript levels of pTRV1 and pTRV2 sequences in the distal leaves were analyzed. Both the transcripts of pTRV1 and pTRV2 could be obviously examined in the pTRV and pTRV-MePDS cassava plants but showed no PCR band in WT cassava (Figure 5B). Notably, the pTRV-MePDS cassava leaves showed an obvious albino area around the main vein. The transcript level of MePDS in the distal leaves of MePDS-VIGS plants was 37.9% and 53.1% of that in the mock (Figure 5C), displaying significantly lower chlorophyll content than the mock (Figure 5D). Based on these results, we could conclude that the gene silencing of MePDS in the distal leaves of MePDS-VIGS plants was caused by VIGS, and the albino phenotype in the distal leaves was derived by VIGS but not the infection-induced effects, which resulted in no significant difference in local leaves.
Figure 5.
Detection of VIGS cassava plants. (A) PCR detection of pTRV1 and pTRV2 sequences from the genomic DNA of VIGS cassava plants. (B) Reverse-transcription PCR detection of pTRV1 and pTRV2 sequences from the cDNA of VIGS cassava plants. (C) The relative transcript levels of MePDS of the infiltrated cassava plants. MeEF1 was used as internal control. (D) The chlorophyll contents in the infiltrated cassava plants. Statistical tests were performed using IBM SPSS (v21). Briefly, the Kolmogorov–Smirnov test and Levene’s test were performed to check the normality of the data distribution and the homogeneity of variance of the data, respectively. The statistical analysis was performed using Student’s t-test and the Tukey–Kramer test. Asterisk symbol (*) indicates significant difference at p < 0.05. All data are expressed as mean ± SD of three independent experiments.
Compared with the phenotype of Agrobacterium AGL-1-infiltrated distal leaves, GV3101-infiltrated distal leaves showed the albino phenotype in the part around the petiole (Supplementary Figure S1), with slightly weaker PCR detection bands, a lower MePDS transcript level, and lower chlorophyll content than the mock (Supplementary Figure S2). For AGL-1, 75.00% of cassava plants showed less than 60% transcript level of MePDS in the distal leaves, and only 37.50% of the plants exhibited the albino phenotype (Table 2). For GV3101, 62.50% of cassava plants showed a lower transcript level of MePDS in the distal leaves, and only 12.50% of plants showed the albino phenotype (Table 2). These results suggested that both AGL-1 and GV3101 with TRV could induce MePDS silencing and regulate the color of leaves in cassava, with better effects in AGL-1-transformed TRV-based VIGS.
Table 2.
Effects of Agrobacterium-mediated TRV-based gene silencing systems in cassava.
| Bacterial Strain | Number | The Positive Rate of Relative Transcript Level (<60%) | The Positive Rate of Albino Phenotype |
|---|---|---|---|
| AGL-1 | 8 | 75.00% | 37.50% |
| GV3101 | 8 | 62.50% | 12.50% |
3. Discussion
Although genome sequencing of cassava has been completed for several years now, little progress has been made in determining the functional genomics of cassava [34]. In order to address this question and promote functional genomics in cassava, this study investigated Agrobacterium-mediated gene transient overexpression and TRV-based VIGS.
The Agrobacterium-mediated transient overexpression assay is a powerful tool for analyzing gene function in vivo [35]. In this study, Agrobacterium AGL-1 and GV3101 with GFP or GUS overexpressing plasmids were used for transient overexpression. In a previous study, GV3101 containing the 35S::GUS vector was used in MCOL2215 and 60,444 cassava varieties to test the transient expression effect, but no GUS was detected. However, AGL with the 35S::GUS vector showed a positive result [36]. In this study, both GFP and GUS were expressed by the GV3101 strain in SC124 and could be detected at the transcript (Figure 2 and Figure 3) and protein (Figure 2 and Figure 3) levels, indicating that they could be efficiently expressed in cassava leaves. Generally, the GV3101- and AGL-1-based transient overexpression assays had relatively high success rates (Table 1), while the negative results might have been due to the RNA cosilence process [37,38], the morphology, or the structure of the plants [39]. Therefore, whether the target genes are overexpressed in the transient overexpression assay should be analyzed first.
In addition, Agrobacterium-mediated VIGS is another powerful tool to investigate gene function in vivo. Different Agrobacterium strains (GV3101 and AGL-1) with pTRV vectors were used to silence the MePDS gene. AGL-1 with the transformation of pTRV1 and pTRV2-MePDS vector-infiltrated distal leaves showed obvious albino phenotypes at 20 dpi (Figure 4). Interestingly, the albino phenotype only showed in distal leaves and the albino area was mainly located around the leaf vein, similar to the results in California poppy [40] and Solanum pseudocapsicum [41]. From the albino leaf blade, we found that the area near the petiole became white, while the area distant from the petiole was still green (Figure 4), indicating that TRV-based MePDS silence might spread from the bottom to the tip of the blade in cassava. Additionally, TRV-based Agrobacterium-mediated VIGS was examined by genome DNA or cDNA to ensure the Agrobacterium-mediated transformation and expression of pTRV1 and pTRV2 or pTRV2-X [41,42]. PCR results indicated that Agrobacterium-mediated pTRV1 and pTRV2 or pTRV2-MePDS could be successfully transformed and expressed in the distal leaves of cassava plants, with a 37.9%–53.1% transcript level of MePDS and 58%–76.5% of chlorophyll content in the distal plant leaves (Figure 5). Because the relative transcript level of MePDS of the albino cassava plants was 71% of the mock and the detected bands were weak, GV3101 with pTRV1 and pTRV2-MePDS infiltrated cassava plants showed less obvious change, and only the part around the petiole became albino (Supplementary Figures S1 and S2). VIGS is a post-transcriptional gene silencing method [24,25]. In a VIGS system, the expressed levels of silenced marker genes were 30%–40% of control in Solanum pseudocapsicum L. [41], 37% of control in columbine [43], 41%–60% of control in barley [44], 28%–38% of control in tomato [45], and 30%–70% of control in rose [27]. Although these genes could not silenced completely, the albino phenotype was obvious. Consistently, the transcript level of MePDS in MePDS-VIGS plants were 37.9%–53.1% of that in the mock, with an obvious albino phenotype and a 58%–76.5% level of chlorophyll. Compared with GV3101, Agrobacterium AGL-1 showed better effects in the VIGS system (Table 2), which might have been due to the specific hypervirulence of AGL-1 [46]. AGL-1 is derived from pTiBo542, which has a high induction of the vir gene [47], which is necessary for T-DNA transfer [48].
In this study, an Agrobacterium-mediated transient overexpression system was established in cassava. The successful examination of the transcription, translation, and biological activity of expressed proteins suggests its application in subcellular localization experiments, bimolecular fluorescence complementation (BIFC), co-immunoprecipitation (Co-IP), enzyme activity detection, and so on. In addition, a TRV-based Agrobacterium-mediated VIGS system was also successfully verified in cassava (Figure 1B), as shown by the expressions of pTRV and the corresponding gene, as well as the albino phenotype. Previous studies have found that cassava could be silenced by an African-cassava-mosaic-virus-based VIGS system; however, this could lead to serious malformation of newly grown leaves, thereby resulting in interference in the assay of plant disease resistance [49,50,51]. On the contrary, TRV-based VIGS-system-infiltrated cassava plants only showed mild virus-induced disease symptoms and might be more appropriate for gene function analysis. In conclusion, this study provided two valid and efficient methods for the characterization of gene function, so as to promote functional genomics in cassava.
4. Materials and Methods
4.1. Plant Materials
The cassava plants South China 124 (SC124) were kindly provided by Dr. Wei Hu (Institute of Tropical Bioscience and Biotechnology, Haikou, China). Two-week-old tissue culture cassava plants (SC124) were transferred to small pots and grown in a chamber for two weeks until the experiment at 25 ℃ under a 16 h light/8 h dark cycle.
4.2. Vectors and Vector Construction
The pEGAD and pBI121 vectors were used to express GFP and GUS, respectively. The full-length sequences of GFP and GUS were driven by the 35S promoter. The VIGS assay used pTRV1 and pTRV2 vectors, which have been described previously [52]. The partial sequence of the MePDS gene was cloned into the multiple cloning site of the pTRV2 vector, and the primers are listed in Supplementary Table S1.
4.3. Agrobacterium Infiltration of Cassava
Agrobacterium strains GV3101 and AGL-1 were transformed by different plasmids: pEGAD, pBI121, pTRV1, pTRV2, and pTRV2-MePDS, respectively. GV3101 was cultivated in liquid LB medium containing 50 mg/L kanamycin, 20 mg/L rifampicin, and 50 mg/L gentamycin, while AGL-1 was cultivated in LB medium with 50 mg/L kanamycin, 20 mg/L rifampicin, and 50 mg/L carbenicillin. Both were then shook at 28 ℃ at 200 rpm for 2 days. The bacteria was centrifuged at 4000 rpm for 10 min, then the supernatant was discarded. The remaining bacteria was washed with double-distilled water, then centrifuged, and the supernatant was discarded again. The bacterial sediment was resuspended in MMA solution (10 mM MgCl, 10 mM MES, and 150 μM acetosyringone), the OD600 was adjusted to 1, and then the resuspended bacterial solution was placed in the dark for 3 h. For the transient expression assay, the standing bacterial solution was infiltrated into the second and third leaves from the top of the cassava by a 1 mL needle. For the VIGS assay, the bacterial solutions containing pTRV1 or pTRV2-MePDS were mixed with the same volume, and the mixture of pTRV1 or pTRV2 was used as the mock. The mixed solution was infiltrated into both the leaves and axillary buds of cassava plants to keep the silencing effect [48]. After infiltration, the cassava plants were removed to the chamber at the same light and temperature.
4.4. DNA and RNA Extraction
DNA was extracted by the Plant Genomic DNA Extraction Kit (DP305, TIANGEN, Beijing, China). Total RNA was extracted by the RNAprep Pure Plant Kit (Polysaccharides & Polyphenolics-rich) (DP441, TIANGEN, Beijing, China). The remaining DNA from the extracted RNA was digested by RNase-free DNase I (EN0521, Thermo, Waltham, MA, USA). Then, the quality and concentration of DNA and RNA were examined by Nano Drop 2000 (Thermo, Waltham, MA, USA).
4.5. Reverse-Transcription PCR and Quantitative Real-Time PCR
The first strand cDNA was synthesized by the RevertAid First Strand cDNA Synthesis Kit (K1621, Thermo, Waltham, MA, USA). Then, the cDNA was adjusted to an equal concentration. Reverse-transcription PCR was performed by EasyTaq PCR SuperMix (AS111, TRANS, Beijing, China) with a PCR program of (1) 94 ℃ for 3 min; (2) 29 cycles of 94 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 2 min; and (3) 72 ℃ for 10 min and 16 ℃ for storage.
Quantitative real-time PCR was performed using TransStart Tip Green qPCR SuperMix (AQ141, TRANS, Beijing, China) in LightCycler® 96 (Roche, Basel, Switzerland) with a PCR program of (1) 94 ℃ for 30 s; (2) 45 cycles of 94 ℃ for 5 s, 55 ℃ for 15 s, and 72 ℃ for 15 s; and (3) 95 ℃ for 10 s, 65 ℃ for 60 s, and 97 ℃ for 1 s as the melting curve. Elongation factor 1 (EF1) was used as the reference gene, and the primers used in reverse-transcription PCR and quantitative real-time PCR are listed in Supplementary Tables S2 and S3.
4.6. Confocal Microscopy Scanning
At 2 dpi, the infiltrated area of cassava leaves was harvested, cut into small pieces, and then observed by confocal laser-scanning microscopy (TCS SP8, Leica, Heidelberg, Germany).
4.7. Protein Extraction and Western Blot
Cassava leaves were harvested and ground by liquid nitrogen and phosphate buffer solution (pH 7.4). The extracted solution was centrifuged at 12,000 rpm and 4 ℃ for 10 min. The supernatant was boiled with SDS-PAGE sample loading buffer (P0015, Biyotime, Shanghai, China) for 5 min. After centrifugation for 10 min, the supernatant could be used in Western blot. The Western blot assay was performed according to a previous study [53]. Briefly, the protein samples were loaded into 12% polyacrylamide gel and separated by electrophoresis. The polyacrylamide gel was transferred to a PVDF membrane (475855-1R, Millpore, Massachusetts, America) by a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (1703940, Bio-rad, Hercules, America). Then, the PVDF membrane was blocked and incubated in 5% skim milk with anti-GFP antibody (AG281, Biyotime, Haimen, China).
4.8. GUS Staining and Activity Detection
GUS staining and GUS activity assay were carried out according to a previous study with slight modifications [48]. The infiltrated cassava leaves were harvested and immersed into GUS staining solution (50 mM NaH2PO4, 50 mM Na2HPO4, 10 mM EDTA-Na2, 0.5 mM K4[Fe(CN)6], 0.5 mM K3[Fe(CN)6], 0.1% Triton-X100, and 2 mM X-Gluc; pH 7.0) with vacuum infiltration for 0.5 h in the dark, and then the plant leaves were incubated in the dark at 37 ℃ for at least 12 h. After staining, the leaves were immersed into 70% alcohol to remove chlorophyll.
The infiltrated leaves were harvested and ground by liquid nitrogen and phosphate buffer solution (pH 7.4). The extracted solution was centrifuged (12,000 rpm, 10 min, 4 ℃) and the supernatant was used for further assay. The extraction was incubated in 1 mM 4-Methylumbelliferyl-b-D-glucuronide (4-MUG) at 37 ℃, the reaction mixture was taken out per 5 min, and Na2CO3 was added to stop the reaction. The samples were detected by a microplate system (Infinite M200 Pro, TECAN, Hombrechtikon, Switzerland) with 365 nm excitation and 455 nm emission. The GUS activity was calculated by the standard curve made by different concentrations of 4-methylumbelliferone (4-MU).
4.9. PCR Detection of Cassava Plants
The transient expressed cassava plants were detected by PCR using genomic DNA and cDNA, with a PCR program of (1) 94 ℃ for 3 min; (2) 29 cycles of 94 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 2 min; and (3) 72 ℃ for 10 min and 16 ℃ for storage. The primers are listed in Supplementary Table S4.
4.10. Chlorophyll Content of Cassava Leaves
The chlorophyll content of the cassava leaves was quantified as previously described [54]. The harvested leaves were ground in 80% acetone. After centrifugation, the absorbance of OD647 and OD665 of the supernatant solution was detected by a microplate system (Infinite M200 Pro, TECAN, Hombrechtikon, Switzerland). The total chlorophyll content was 17.90 × OD647 + 8.08 × OD665.
4.11. Statistical Analysis
All data are expressed as mean ± SD of three independent experiments. Statistical tests were performed using IBM SPSS (v21). Briefly, the Kolmogorov–Smirnov test and Levene’s test were used to check the normality of the data distribution and the homogeneity of variance of the data, respectively. Then, Student’s t-test and the Tukey–Kramer test were used for statistical analysis. The asterisk symbol (*) indicates significant difference at p < 0.05.
Acknowledgments
We thank Chris R. Somerville and Jie Zhou for sharing the vectors.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/16/3976/s1.
Author Contributions
Conceptualization, H.Z.; Data curation, H.Z.; Formal analysis, H.Z.; Funding acquisition, H.Z., Y.X., and H.S.; Investigation, H.Z., Y.X., G.L., and Y.W.; Methodology, H.Z.; Project administration, H.S.; Supervision, W.H. and H.S.; Writing—original draft, H.Z.; Writing—review and editing, W.H. and H.S.
Funding
This research was supported by National Key R&D Program of China (No. 2018YFD1000500), the National Natural Science Foundation of China (No. 31760067), the Startup Funding and Scientific Research Foundation of Hainan University (No. kyqd1531), and the Crop Science Postgraduate Innovation Project of Hainan University Tropical Agriculture and Forestry College (College of Tropical Crops) (Nos. ZWCX2018005 and ZWCX2018018).
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Wilson M.C., Mutka A.M., Hummel A.W., Berry J., Chauhan R.D., Vijayaraghavan A., Taylor N.J., Voytas D.F., Chitwood D.H., Bart R.S. Gene expression atlas for the food security crop cassava. New Phytol. 2017;213:1632–1641. doi: 10.1111/nph.14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyaboga E., Njiru J., Nguu E., Gruissem W., Vanderschuren H. Unlocking the potential of tropical root crop biotechnology in east Africa by establishing a genetic transformation platform for local farmer-preferred cassava cultivars. Front. Plant SCI. 2013;4:526. doi: 10.3389/fpls.2013.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wydro M., Kozubek E., Lehmann P. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta. Biochimica. Pol. 2006;53:289–298. [PubMed] [Google Scholar]
- 4.Kim M.J., Baek K., Park C.M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28:1159–1167. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 5.Ma L., Lukasik E., Gawehns F., Takken F.L. The Use of Agroinfiltration for Transient Expression of Plant Resistance and Fungal Effector Proteins in Nicotiana benthamiana Leaves. Methods Mol. Biol. 2012;835:61. doi: 10.1007/978-1-61779-501-5_4. [DOI] [PubMed] [Google Scholar]
- 6.Ding B.Q., Yuan Y.W. Testing the utility of fluorescent proteins in Mimulus lewisii by an Agrobacterium-mediated transient assay. Plant Cell Rep. 2016;35:771–777. doi: 10.1007/s00299-015-1919-1. [DOI] [PubMed] [Google Scholar]
- 7.Mani T., Manjula S. Optimization of Agrobacterium-mediated transient gene expression and endogenous gene silencing in Piper colubrinum by vacuum infiltration. Plant Cell Tiss. Org. 2011;105:113–119. [Google Scholar]
- 8.Rakouský S., Kocábek T., Vincenciová R., Ondřej M. Transient β-glucuronidase activity after infiltration of Arabidopsis thaliana by Agrobacterium tumefaciens. Biol. Plant. 1997;40:33–41. doi: 10.1023/A:1000988316206. [DOI] [Google Scholar]
- 9.Lu J., Bai M., Ren H. An efficient transient expression system for gene function analysis in rose. Plant Methods. 2017;13:116. doi: 10.1186/s13007-017-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X.F., Yu X.Q., Zhou Z., Ma W.J., Tang G.X. High-efficiency Agrobacterium tumefaciens mediated transformation system using cotyledonary node as explants in soybean (Glycine max L.) Acta. Physiol. Plant. 2016;38:60. [Google Scholar]
- 11.Fister A.S., Shi Z., Zhang Y.F., Helliwell E.E., Maximova S.N., Guiltinan M.J. Protocol: Transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods. 2016;12:19. doi: 10.1186/s13007-016-0119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H.P., Li K., Guo Y.T., Guo J.D., Miao K.T., Botella J.R., Song C.P., Miao Y.C. A transient transformation system for gene characterization in upland cotton (Gossypium hirsutum) Plant Methods. 2018;14:50. doi: 10.1186/s13007-018-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamboj D., Yadav R.C., Singh A., Yadav N.R., Singh D. Plant regeneration and Agrobacterium-mediated transformation in Indian mustard (Brassica juncea) J. Oilseed Brassica. 2016;6:191–197. [Google Scholar]
- 14.Bhaskar P.B., Venkateshwaran M., Wu L., Ané J.M., Jiang J. Agrobacterium-Mediated Transient Gene Expression and Silencing: A Rapid Tool for Functional Gene Assay in Potato. PLoS ONE. 2009;4:e5812. doi: 10.1371/journal.pone.0005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wroblewski T., Tomczak A., Michelmore R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 2005;3:259–273. doi: 10.1111/j.1467-7652.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Lange M., Yellina A.L., Orashakova S., Becker A. Virus-induced gene silencing (VIGS) in plants: An overview of target species and the virus-derived vector systems. Methods Mol. Biol. 2013;975:1–14. doi: 10.1007/978-1-62703-278-0_1. [DOI] [PubMed] [Google Scholar]
- 17.Hiriart J.B., Aro E.M., Lehto K. Dynamics of the VIGS-mediated chimeric silencing of the Nicotiana benthamiana ChlH gene and of the tobacco mosaic virus vector. Mol. Plant Microbe Interact. 2003;16:99–106. doi: 10.1094/MPMI.2003.16.2.99. [DOI] [PubMed] [Google Scholar]
- 18.Lacomme C., Chapman S. Use of potato virus X (PVX)-based vectors for gene expression and virus-induced gene silencing (VIGS) Curr. Protoc. Microbiol. 2008;16:1. doi: 10.1002/9780471729259.mc16i01s8. [DOI] [PubMed] [Google Scholar]
- 19.Zhao D., Zhao J.R., Huang X., Li N., Liu Y., Huang Z.J., Zhang Z.Y. Functional Analysis of TNBL1 Gene in Wheat Defense Response to Barley yellow dwarf virus Using BSMV-VIGS Technique. Acta. Agronomica. Sinica. 2011;37:2106–2110. doi: 10.3724/SP.J.1006.2011.02106. [DOI] [Google Scholar]
- 20.Zhou T., Liu X., Fan Z. Use of a Virus Gene Silencing Vector for Maize Functional Genomics Research. Methods Mol. Biol. 2018;1676:141–150. doi: 10.1007/978-1-4939-7315-6_8. [DOI] [PubMed] [Google Scholar]
- 21.Liu X.B., Liu N., Li F.K., Wu L.Z., Zhang J., Wang D.M. Establishment of TRV-mediated Transient Gene-Silencing System in Soybean. Sci. Agri. Sin. 2015:1212. [Google Scholar]
- 22.Fu D.Q., Zhu B.Z., Zhu H.L., Jiang W.B., Luo Y.B. The application of TRV-mediated VIGS technique in the study of gene function in fruits and vegetables. Plant Physiol. J. 2017;43:299–308. [Google Scholar]
- 23.Unver T., Budak H. Virus-Induced Gene Silencing, a Post Transcriptional Gene Silencing Method. Int. J. Plant Genomics. 2009:198680. doi: 10.1155/2009/198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratcliff F., Martin-Hernandez A.M., Baulcombe D.C. Technical Advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2001;25:237–245. doi: 10.1046/j.0960-7412.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X.F., Sun J.D., Zhao Z., Lv J., Wei X.W., Cai R., Xu H.W. The Feasibility Analysis of PVX and TRV Vectors as the VIGS Tool for Studying the Gene Function. Physics. Procedia. 2002;23:46–54. doi: 10.1016/j.phpro.2012.05.029. [DOI] [Google Scholar]
- 26.Wang C.C., Cai X.Z., Wang X.M., Zheng Z. Optimisation of tobacco rattle virus-induced gene silencing in Arabidopsis. Funct. Plant Biol. 2006;33:347–355. doi: 10.1071/FP05096. [DOI] [PubMed] [Google Scholar]
- 27.Tian J., Pei H.X., Zhang S., Chen J.W., Chen W., Yang R.Y., Meng Y., You J., Gao J., Ma N. TRV-GFP: A modified Tobacco rattle virus vector for efficient and visualizable analysis of gene function. J. Exp. Bot. 2014;65:311–322. doi: 10.1093/jxb/ert381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J., Cheng L., Han Z.Y., Yao Y.C. Tobacco rattle virus mediated gene silencing in strawberry plants. Plant Cell Tiss. Org. 2015;120:1131–1138. [Google Scholar]
- 29.Wang X.Y., LÜ K., Cai C.P., Xu J., Guo W.Z. Establishment and Application of TRV-Mediated Virus-Induced Gene Silencing in Cotton. Acta. Agronomica. Sin. 2014;40:1356. doi: 10.3724/SP.J.1006.2014.01356. [DOI] [Google Scholar]
- 30.Anu K., Jessymol K.K., Chidambareswaren M., Gayathri G.S., Manjula S. Down-regulation of osmotin (PR5) gene by virus-induced gene silencing (VIGS) leads to susceptibility of resistant Piper colubrinum to the oomycete pathogen Phytophthora capsici Leonian. Indian, J. Exp. Biol. 2015;53:329–334. [PubMed] [Google Scholar]
- 31.Zhang J., Yu D., Zhang Y., Liu K., Xu K., Zhang F., Wang J., Tan G.X., Nie X.H., Ji Q.H., et al. Vacuum and Co-cultivation Agroinfiltration of (Germinated) Seeds Results in Tobacco Rattle Virus (TRV) Mediated Whole-Plant Virus-Induced Gene Silencing (VIGS) in Wheat and Maize. Front. Plant Sci. 2017;8:393. doi: 10.3389/fpls.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartl M., Merker H., Schmidt D.D., Baldwin I.T. Optimized virus-induced gene silencing in Solanum nigrum reveals the defensive function of leucine aminopeptidase against herbivores and the shortcomings of empty vector controls. New Phytol. 2010;179:356–365. doi: 10.1111/j.1469-8137.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- 33.Cutler S.R., Ehrhardt D.W., Griffitts J.S., Somerville C.R. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA. 2000;97:3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apio H.B. Production of friable embryogenic callus and regeneration of Ugandan farmer-preferred cassava genotypes. Afr. J. Biotechnol. 2015;14:1854–1864. [Google Scholar]
- 35.Tsuda K., Qi Y., Nguyen L.V., Bethke G., Tsuda Y., Glazebrook J., Katagiri F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012;69:713–719. doi: 10.1111/j.1365-313X.2011.04819.x. [DOI] [PubMed] [Google Scholar]
- 36.Díaz T.P., Bernal G.A., Camilo L.C. Transient gus gene expression in cassava (manihot esculenta crantz) using agrobacterium tumefaciens leaf infiltration. Revista. Mvz. Cordoba. 2014;19:4338–4349. [Google Scholar]
- 37.Olivier V., Susana R., Pere M., David B. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 38.Johansen L.K., Carrington J.C. Silencing on the Spot. Induction and Suppression of RNA Silencing in the Agrobacterium-Mediated Transient Expression System. Plant Physiol. 2001;126:930–938. doi: 10.1104/pp.126.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrieu A., Breitler J.C., Siré C., Meynard D., Gantet P., Guiderdoni E. Anin planta, agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (oryza sativa L.) leaves. Rice. 2012;5:23. doi: 10.1186/1939-8433-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wege S., Scholz A., Gleissberg S., Becker A. Highly Efficient Virus-induced Gene Silencing (VIGS) in California Poppy (Eschscholzia californica): An Evaluation of VIGS as a Strategy to Obtain Functional Data from Non-model Plants. Ann. Bot. 2007;100:641–649. doi: 10.1093/aob/mcm118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H., Xu L.F., Yang P., Cao Y., Tang Y., He G., Yuan S.X., Ming J. Tobacco rattle virus-induced PHYTOENE DESATURASE (PDS) and Mg-chelatase H subunit (ChlH) gene silencing in Solanum pseudocapsicum L. PeerJ. 2018;6:e4424. doi: 10.7717/peerj.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai W., Wang M., Gong X., Liu J.H. The transcription factor FcWRKY40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS2 and P5CS1 homologs. New Phytol. 2018;219:972–989. doi: 10.1111/nph.15240. [DOI] [PubMed] [Google Scholar]
- 43.Gould B., Kramer E.M. Virus-induced gene silencing as a tool for functional analyses in the emerging model plant Aquilegia (columbine, Ranunculaceae) Plant Methods. 2007;3:6. doi: 10.1186/1746-4811-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruun-Rasmussen M., Madsen C.T., Jessing S., Albrechtsen M. Stability of Barley stripe mosaic virus-induced gene silencing in barley. Molecular plant-microbe interactions. MPMI. 2007;20:1323. doi: 10.1094/MPMI-20-11-1323. [DOI] [PubMed] [Google Scholar]
- 45.Rotenberg D., Thompson T.S., German T.L., Willis D.K. Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. J. Virol. Methods. 2006;138:49–59. doi: 10.1016/j.jviromet.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Uçarlı C., Tufan F., Gürel F. Expression and genomic integration of transgenes after Agrobacterium-mediated transformation of mature barley embryos. Genet. Mol. Res. 2015;14:1096–1105. doi: 10.4238/2015.February.6.13. [DOI] [PubMed] [Google Scholar]
- 47.Lazo G.R., Stein P.A., Ludwig R.A. A DNA transformation-competent arabidopsis genomic library in Agrobacterium. Bio/Technology. 1991;9:963–967. doi: 10.1038/nbt1091-963. [DOI] [PubMed] [Google Scholar]
- 48.Chabaud M., Carvalho-Niebel F.D., Barker D.G. Efficient transformation of Medicago truncatula cv. jemalong using the hypervirulent Agrobacterium tumefaciens strain AGL1. Plant Cell Rep. 2003;22:46–51. doi: 10.1007/s00299-003-0649-y. [DOI] [PubMed] [Google Scholar]
- 49.Fofana I.B., Sangaré A., Collier R., Taylor C., Fauquet C.M. A geminivirus-induced gene silencing system for gene function validation in cassava. Plant Mol Biol. 2004;56:613–624. doi: 10.1007/s11103-004-0161-y. [DOI] [PubMed] [Google Scholar]
- 50.Beyene G., Chauhan R.D., Taylor N.J. A rapid virus-induced gene silencing (VIGS) method for assessing resistance and susceptibility to cassava mosaic disease. Virol. J. 2017;14:47. doi: 10.1186/s12985-017-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matias L.E., Joel-Elias K. Cassava germinivirus agroclones for virus-induced gene silencing in cassava leaves and roots. Plant Methods. 2018 doi: 10.1186/s13007-018-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Schiff M., Dinesh-Kumar S.P. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313X.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 53.Cai J., Qin G., Chen T., Tian S. The mode of action of remorin1 in regulating fruit ripening at transcriptional and post-transcriptional levels. New Phytol. 2018;219:1406–1420. doi: 10.1111/nph.15264. [DOI] [PubMed] [Google Scholar]
- 54.Inskeep W.P., Bloom P.R. Extinction coefficients of chlorophyll a and B in N, N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.