Abstract
Redox-mediated protein modifications control numerous processes in both normal and disease metabolism. Protein sulfenic acids, formed from the oxidation of protein cysteine residues, play a critical role in thiol-based redox signaling. The reactivity of protein sulfenic acids requires their identification through chemical trapping, and this paper describes the use of the triphenylphosphonium (TPP) ion to direct known sulfenic acid traps to the mitochondria, a verified source of cellular reactive oxygen species. Coupling of the TPP group with the 2,4-(dioxocyclohexyl)propoxy (DCP) unit and the bicyclo[6.1.0]nonyne (BCN) group produces two new probes, DCP-TPP and BCN-TPP. DCP-TPP and BCN-TPP react with C165A AhpC-SOH, a model protein sulfenic acid, to form the expected adducts with second-order rate constants of k = 1.1 M−1 s−1 and k = 5.99 M−1 s−1, respectively, as determined by electrospray ionization time-of-flight mass spectrometry. The TPP group does not alter the rate of DCP-TPP reaction with protein sulfenic acid compared to dimedone but slows the rate of BCN-TPP reaction compared to a non-TPP-containing BCN-OH control by 4.6-fold. The hydrophobic TPP group may interact with the protein, preventing an optimal reaction orientation for BCN-TPP. Unlike BCN-OH, BCN-TPP does not react with the protein persulfide, C165A AhpC-SSH. Extracellular flux measurements using A549 cells show that DCP-TPP and BCN-TPP influence mitochondrial energetics, with BCN-TPP producing a drastic decrease in basal respiration, perhaps due to its faster reaction kinetics with sulfenylated proteins. Further control experiments with BCN-OH, TPP-COOH, and dimedone provide strong evidence for mitochondrial localization and accumulation of DCP-TPP and BCN-TPP. These results reveal the compatibility of the TPP group with reactive sulfenic acid probes as a mitochondrial director and support the use of the TPP group in the design of sulfenic acid traps.
Graphical abstract
INTRODUCTION
Protein oxidation plays important roles in cellular signaling and damage pathways in both normal and pathophysiological conditions. Protein cysteine residues (P-SH) have emerged as a focal site of protein redox chemistry based on the chemical reactivity of the thiol group.1 The direct reaction of hydrogen peroxide (H2O2), formed during normal or pathophysiological metabolism or generated by external sources, such as radiation or toxins, with a cysteine thiol group in proteins forms a protein sulfenic acid (PSOH), a critical initial post-translational modification that provides redox-driven control of enzyme and transcription factor activity.1,2 Other reagents, including HOSCN and HOX (X = Cl, Br, I), generate PSOHs via hydrolysis of the corresponding sulfenyl derivative.3 PSOHs react with thiols or protein backbone amides to form disulfides or sulfenamides, respectively, products that allow reversible activity control.1 PSOHs also react with H2S to yield persulfides (PSSHs), providing a molecular mechanism for redox-coupled H2S signaling.1,4 Further PSOH reaction with excess H2O2 yields protein sulfinic (PSO2H) and sulfonic (PSO3H) acids, generally indicative of oxidative damage.1 These multiple and rapid reactions make the tagging of protein sulfenic acids and the subsequent identification of the protein and site of modification under biological conditions challenging.1,2 A number of probes containing acidic carbon nucleophiles (including the 2,4-(dioxocyclohexyl)propoxy (DCP) unit) or strained cyclic alkynes (including the bicyclo[6.1.0]nonyne (BCN) group) trap PSOHs at rates sufficient to reveal information regarding the site of PSOH formation in various proteins and their role in redox-mediated processes.5–16
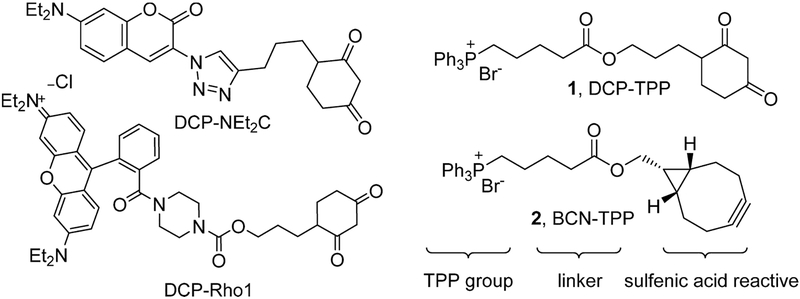
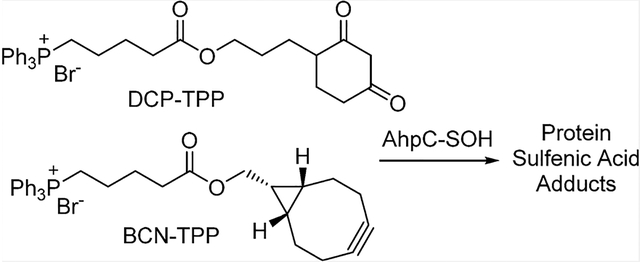
Mitochondria play major roles in cellular energy production through pyruvate metabolism via the tricarboxylic acid cycle, fatty acid oxidation, and ATP synthesis through oxidative phosphorylation. Mitochondria also represent a major source of reactive oxygen species (ROS) in cells through incomplete oxygen reduction in the electron transport chain during oxidative phosphorylation.17,18 Mitochondrial redox dysfunction has been implicated in various conditions, including aging,19,20 cancer,21 diabetes,22 and neurodegenerative disease.23,24 Given mitochondrial ROS production, changes in the thiol redox state of mitochondrial proteins likely accompany both normal and pathophysiological processes and focus attention on mitochondrial PSOHs as important signaling/detoxification intermediates.1 While many agents react with PSOHs, probes that specifically label and identify mitochondrial PSOHs remain limited. We recently published the first examples of mitochondrial-directed PSOH probes that coupled the sulfenic acid reactive DCP group with positively charged dye molecules to target the mitochondria and provide a fluorescent marker (DCP-NEt2C and DCP-Rho1, Chart 1).25 These compounds react with a model PSOH at competent rates, accumulate in the mitochondria, minimally influence mitochondrial function, and show increased mitochondrial protein labeling upon oxidative stress.25 The lipophilic triphenylphosphonium (TPP) group bears a diffuse positive charge and finds extensive use as a mitochondrial director for numerous drugs and antioxidants.26–28 Combination of TPP with known sulfenic acid reactive groups should yield another group of mitochondrial-directed sulfenic acid traps of PSOH, and we report the synthesis of DCP-TPP (1) and BCN-TPP (2, Chart 1), their reactivity and kinetics with a model PSOH, and effects on mitochondrial respiration. These studies expand the group of mitochondria-directed PSOH probes and further define their reactivity and limitations, providing a basis for the development of superior reagents.
Chart 1.
Structures of Mitochondrial-Directed Sulfenic Acid Probes
EXPERIMENTAL PROCEDURES
General.
All chemicals were purchased from commercial vendors and used as received. Thin-layer chromatography (TLC) was performed on Sorbent polyester-backed Silica G plates with a UV254 indicator, and visualization was accomplished with UV light unless otherwise indicated. Solvents for extraction and purification were of technical grade and used as received. Liquid chromatography–mass spectrometry (LC-MS) solvents were HPLC grade. 1H and 13C NMR spectra were recorded using a Bruker Avance 300 or 500 MHz NMR spectrometer. Chemical shifts are given in ppm (δ); multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). The data were collected by TOPSPIN software, and the NMR spectra were generated by MestReNova software. UV–vis spectroscopy was performed on a Cary 50 UV–vis spectrophotometer.
Synthesis.
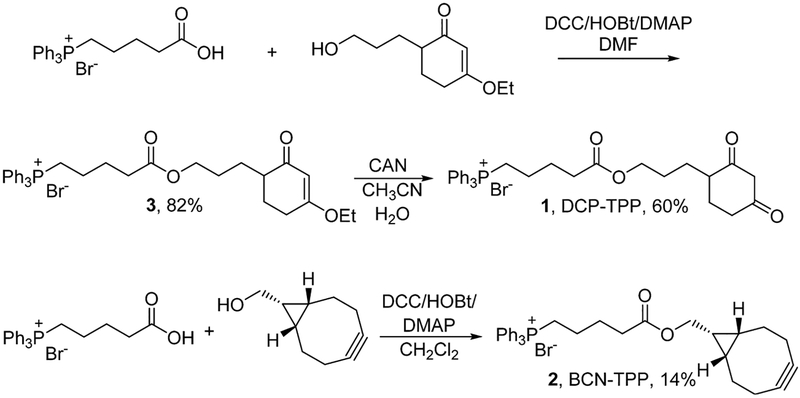
(5-(3-(4-Ethoxy-2-oxocyclohex-3-en-1-yl)propoxy)-5-oxopentyl)triphenylphosphonium Bromide (3).
A solution of 4-(carboxybutyl)triphenylphosphonium bromide (TCP-COOH, 0.443 g, 1.0 mmol), N,N′-dicyclohexylcarbodiimide (DCC, 0.226 g, 1.1 mmol), and 1-hydroxybenzotriazole hydrate (HOBt, 28 mg, 0.18 mmol) in anhydrous DMF (10 mL) was stirred in an oven-dried 50 mL round-bottom flask charged with 4 Å molecule sieves (~1 g, baked in a microwave oven on high power mode for 2 min) under argon for 3 h at room temperature. 3-Ethoxy-6-(3-hydroxypropyl)-cyclohex-2-ene (0.204 g, 1.1 mmol) and 4-dimethylaminopyridine (DMAP, 13 mg, 0.11 mmol) were added to this solution, and the resulting mixture was stirred for 2 days at room temperature. At this time, the mixture was gravity filtered and the solid residue washed with CH2Cl2:methanol (1:1, 20 mL). The solvent was removed under vacuum and the crude product chromatographed on silica gel (10% methanol in CH2Cl2) to give a white solid (0.513 g, 0.82 mmol, 82% yield). 1H NMR (300 MHz, CDCl3, TMS): δ 7.89–7.65 (m, 15H), 5.31–5.38 (m, 1H), 3.99 (t, J = 6.0 Hz, 2H), 3.92–3.81 (m, 4H), 2.46–2.37 (m, 3H), 2.20−1.55 (m, 12H), 1.36 (t, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3, TMS): δ 201.04, 176.98, 173.12, 135.01 (d, J = 3.0 Hz), 133.73 (d, J = 10.5 Hz), 130.48 (d, J = 12.0 Hz), 118.28 (d, J = 84.7 Hz), 102.11, 64.35 (d, J = 7.5 Hz), 44.71, 33.30, 28.19, 26.41, 26.13, 25.99, 25.43 (d, J = 16.5 Hz), 22.89, 22.22, 21.92 (d, J = 3.75 Hz), 14.15; 31P NMR (121 MHz, CDCl3): δ 24.35. ESI-MS calcd for C34H40O4P [M]+: 543.27; found 543.32 (Supporting Information, Figures S1–S4).
(5-(3-(2,4-Dioxocyclohexyl)propoxy)-5-oxopentyl)-triphenylphosphonium Bromide (DCP-TPP, 1).
Ceric ammonium nitrate (CAN, 20 mg, 0.036 mmol) was added to a solution of 3 (155 mg, 0.25 mmol) in water and acetonitrile (1:1, 6 mL) and the solution heated at 70 °C for 5 h. After the solution cooled to room temperature, the solvent was removed under vacuum and the crude product purified by C-18 reverse-phase chromatography (acetonitrile in water, 3% to 50%) to give DCP-TPP (90 mg, 0.15 mmol, 60% yield) as a powder after lyophilization. 1H NMR (300 MHz, CDCl3, TMS): δ 7.84–7.67 (m, 15H), 5.00 (s, 1H), 4.08–3.96 (m, 2H), 3.61–3.51 (m, 2H), 3.02 (s, 3H), 2.36 (t, J = 6.0 Hz, 2H), 2.32–2.09 (m, 2H), 1.99–1.80 (m, 5H), 1.73–1.46 (m, 5H), 1.23–1.13 (m, 1H); 13C NMR (75 MHz, CDCl3, TMS): δ 196.56, 193.94, 172.79, 135.15 (d, J = 3.0 Hz), 133.66 (d, J = 9.8 Hz), 130.55 (d, J = 12.0 Hz), 117.87 (d, J = 85.5 Hz), 102.12, 77.27, 64.52, 43.18, 34.00 (d, J = 29.2 Hz), 26.82 (d, J = 21.0 Hz), 26.52, 26.18 (d, J = 18.0 Hz), 22.52, 21.92, 21.91 (d, J = 10.5 Hz); 31P NMR (121 MHz, CDCl3): δ 24.20. ESI-MS calcd for C32H36O4P [M]+: 515.23; found 515.15 (Supporting Information, Figures S5–S8).
(5-((Bicyclo[6.1.0]non-4-yn-9-yl)methoxy)-5-oxopentyl)-triphenylphosphonium Bromide (BCN-TPP, 2).
A solution of TPP-COOH (50.1 mg, 0.113 mmol), DCC 24.2 mg, 0.117 mmol), and HOBt (2.3 mg, 0.015 mmol) in anhydrous CH2Cl2 (5 mL) was stirred in an oven-dried 50 mL round-bottom flask charged with 4 Å molecule sieves (~1 g, baked in a microwave oven on high power for 2 min) under argon for 5 h at room temperature. To this solution were added bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN-OH, 14.9 mg, 0.0993 mmol) and DMAP (1.3 mg, 0.011 mmol), and the resulting mixture was stirred for 3 days at room temperature. At that time, the solid was removed by gravity filtration and the residue washed thoroughly with methanol (3 × 5 mL). The solvent was removed under vacuum, and the crude product was purified by silica gel chromatography (methanol in CH2Cl2, 3% to 60%) and C-18 reverse-phase chromatography (methanol in water, 3% to 60%) to give BCN-TPP as an off-white solid (8 mg, 0.00139 mmol, 14% yield) after lyophilization. 1H NMR (300 MHz, CDCl3, TMS): δ 7.88–7.66 (m, 15H), 4.08 (d, J = 9.0 Hz, 2H), 3.95–3.85 (m, 2H), 2.42 (t, J = 9.0 Hz, 2H), 2.21–2.17 (m, 5H), 2.06–1.97 (m, 4H), 1.80–1.67 (m, 3H), 1.51–1.47 (m, 3H), 1.29–1.26 (m, 2H), 0.94–0.88 (m, 2H); 13C NMR (75 MHz, CDCl3, TMS): δ 173.31, 134.99 (d, J = 3.0 Hz), 133.74 (d, J = 9.8 Hz), 130.46 (d, J = 12.8 Hz), 118.37 (d, J = 85.5 Hz), 98.79, 62.33, 33.41, 29.05, 25.48 (d, J = 17.25 Hz), 22.90, 22.12 (d, J = 15.7 Hz), 21.39, 20.12, 17.35; 31P NMR (121 MHz, CDCl3): δ 24.42. ESI-MS calcd for C33H36O2P [M]+: 495.24; found 495.19 (Supporting Information, Figures S9–S12).
Generation of C165A AhpC-SOH and -SSH.
Salmonella typhimurium AhpC protein C165A mutant was overexpressed and purified in E. coli as previously described.29,30 An aliquot was first reduced by incubation with DTT (10 mM) for 1 h at room temperature and then desalted by passing through a bio-gel P6 spin column pre-equilibrated with ammonium bicarbonate (ABC, 50 mM). Protein concentration was determined from the solution absorbance at 280 nm (ε = 24 300 M−1 cm−1). Oxidation to sulfenic acid was achieved by addition of H2O2 to 1.2 mol equiv for 30–45 s and the reaction quenched by passing through a bio-gel P6 spin column pre-equilibrated with ABC. The protein was then exchanged into pH 7.2 assay buffer (50 mM ammonium acetate adjusted to pH 7.2 with 50 mM ABC) using a pre-equilibrated bio-gel P6 spin column. Formation of C165A AhpC-SOH was confirmed by electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS). To generate C165A AhpC-SSH, freshly prepared C165A AhpC-SOH was incubated with 2 mol equiv of Na2S for 15 min at room temperature. The reaction was quenched by passing through a bio-gel P6 spin column pre-equilibrated with ABC (50 mM) and then exchanged into pH 7.2 assay buffer (50 mM ammonium acetate adjusted to pH 7.2 with 50 mM ABC) using a pre-equilibrated bio-gel P6 spin column. Formation of the persulfide species was confirmed by ESI-TOF-MS, which is distinguishable from -SO2H due to its reaction with the thiol alkylating reagent iodoacetamide.
Reaction of C165A AhpC-SOH and -SSH with Chemical Probes.
Freshly prepared AhpC-SOH or -SSH (30 μM) was reacted with each chemical probe in pH 7.2 assay buffer (50 mM ammonium acetate adjusted to pH 7.2 using 50 mM ABC) at 25 °C with 750 rpm shaking. The concentration of the chemical probe was varied to give an observed exponential increase in labeled protein over an appropriate time frame. At a single concentration, samples were taken at set time points and quenched by passing through a bio-gel P6 spin column pre-equilibrated with 0.1% formic acid in water for ESI-TOF-MS analysis.
ESI-TOF-MS Analysis.
Analysis of intact AhpC proteins was performed on an Agilent 6120 MSD-TOF system operating in positive ion mode with the following settings: capillary voltage of 3.5 kV, nebulizer gas pressure of 30 psig, drying gas flow of 5 L/min, fragmentor voltage of 175 V, skimmer voltage of 65 V, and drying gas temperature of 325 °C. Samples were introduced via direct infusion at a flow rate of 20 μL/min using a syringe pump. Mass spectra were acquired over the range of 600–3200 m/z and then averaged and deconvoluted, and ion abundance was quantified using Agilent MassHunter Workstation software v B.02.00. Relative ion abundances were used to determine the reaction progress, and the reaction rates were then determined by fitting to the exponential equation in GraphPad Prism 7.0.
Mitochondrial Respirometry Analysis.
The impact of probes on mitochondrial respiration was measured using the Seahorse Mito Stress Test following the manufacturer’s protocol. Briefly, the day before the planned studies, 1.5 × 104 A549 cells/well were plated on a Seahorse plate and incubated overnight. The next day the assay media and compounds (to final concentrations of 1 μM for oligomycin (Fisher Scientific), 1 μM for carbonyl cyanide p-(trifluoromethoxyphenyl)hydrazine (FCCP, Cayman Chemical), and 1 μM antimycin (Abcam)/rotenone (Sigma-Aldrich)) were prepared according to Seahorse protocols. The cells were washed twice with assay media and incubated at 37 °C without CO2 for 1 h, after which the analysis was run using a Seahorse XF 24 Flux Analyzer (Agilent Technologies). After the analysis, the cells were lysed with modified RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% NP40; 0.25% sodium deoxycholate; 15 mM NaCl; 1 mM EDTA; 1 mM NaF; supplemented with Roche protease inhibitor tablets), and protein concentration was measured with BCA (Thermo Scientific) for data normalization. The data were analyzed with Wave software (Agilent Technologies).
RESULTS
Synthesis.
Scheme 1 depicts the synthesis of DCP-TPP (1) and BCN-TPP (2). Coupling of commercially available (5-carboxybutyl)triphenylphosphonium bromide (TCP-COOH) with the previously described alcohol containing a protected 1,3-carbonyl group gives the ester 3 in 82% yield (Scheme 1).31 Oxidative deprotection with CAN produces DCP-TPP in 60% yield. Similarly, direct coupling of bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN-OH) with TCP-COOH gives BCN-TPP in 14% yield (Scheme 1).6 Both DCP-TPP and BCN-TPP were purified by reverse-phase (C18) chromatography and characterized by 1H, 13C, and 31P NMR spectroscopy and MS (Supporting Information).
Scheme 1.
Synthesis of DCP-TPP and BCN-TPP
BCN-TPP reacts with Fries acid, a stable anthraquinonederived sulfenic acid, to yield diastereomeric sulfoxide products. Monitoring the decrease in absorbance at 453 nm by UV–vis spectroscopy as a function of time provides kinetic information for the reaction of 2 and Fries acid (Supporting Information, Figure S13). Kinetic analysis of this reaction under pseudo-first-order conditions in acetonitrile gives a second-order rate constant of 10.9 M−1 s−1, about one-half the rate of ~25 M−1 s−1 previously reported for the reaction of Fries acid and BCN-OH.6 Fries acid does not react efficiently with dimedone, likely due to competitive acid–base chemistry, making this method unsuitable for determining the reaction kinetics of 1 with this small-molecule sulfenic acid.6
MS Labeling and Kinetic Studies of Chemical Probes with Oxidized Proteins.
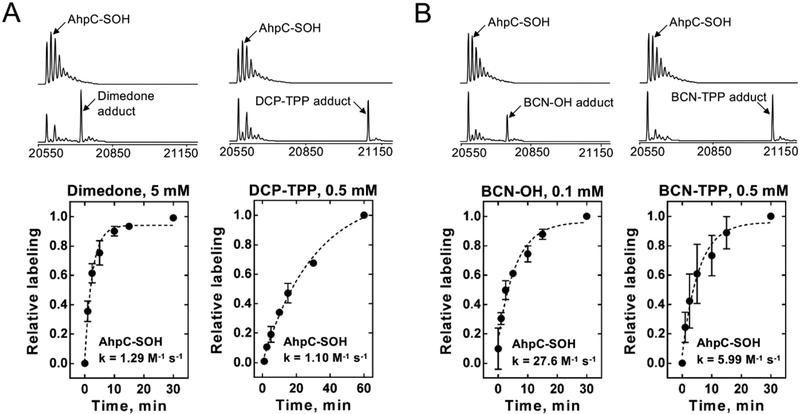
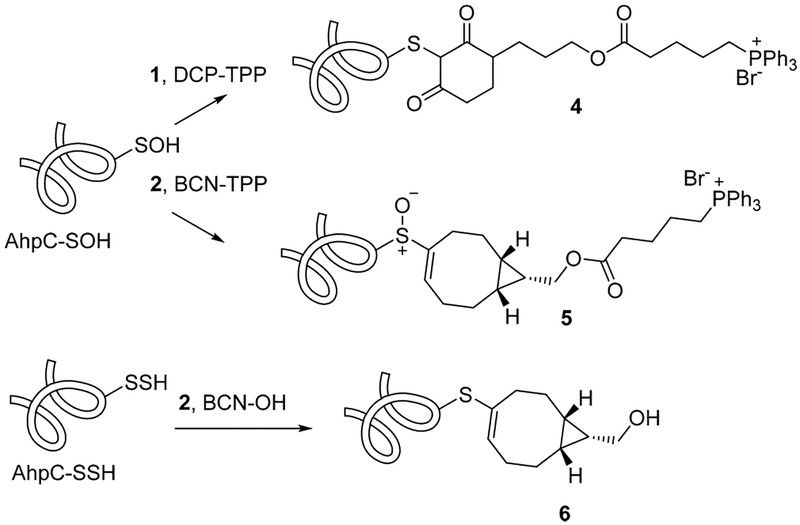
The labeling and comparative kinetics of 1 and 2 with protein sulfenyl and persulfide species were performed using S. typhimurium peroxiredoxin AhpC. In the wild-type enzyme, the peroxidatic C46 residue reacts with a peroxide substrate, resulting in formation of a protein sulfenic acid (C46-SOH), which reacts rapidly with the resolving C165 residue to form a disulfide bond.32 Mutation of the C165 residue to alanine or serine allows for generation of a stabilized AhpC-SOH species at C46, a useful tool for evaluation of sulfenic acid probes.5,6,25,33–35 Generation of C165A AhpC-SOH (20 600 Da) followed by reaction with DCP-TPP or BCN-TPP forms adducts 4 or 5, respectively, as determined by ESI-TOF-MS (Figure 1A,B and Scheme 2). Reaction of C165A AhpC-SOH with dimedone and BCN-OH as controls also produces the expected adducts (Figure 1A,B). Further reaction of C165A AhpC-SOH with Na2S generates the persulfide (-SSH) species4 (AhpC-SSH) that reacts with BCN- OH to yield the vinyl thioether adduct 6 (Scheme 2 and Supporting Information, Figure S14). Under these conditions, BCN-TPP did not react with C165A AhpC-SSH to form a product, even after an extended incubation period.
Figure 1.
Reaction kinetics of DCP-TPP (A) and BCN-TPP (B) probes with C165A AhpC-SOH. Oxidized protein was reacted with each probe, and at set time points samples were taken and desalted and their species abundance determined using ESI-TOF-MS. To account for differences in batches of AhpC-SOH, the plateau of each reaction is normalized to a value of 1, and all data points are expressed relative to the plateau.
Scheme 2.
Reactions of DCP-TPP (1), BCN-TPP (2), and BCN-OH with C165A AhpC-SOH and AhpC-SSH
Mass spectrometric analysis of the formation of 4 and 5 over time upon reaction of DCP-TPP (1) or BCN-TPP (2) with C165A AhpC-SOH provides kinetic information regarding these reactions. The addition of the TPP moiety only slightly alters the kinetics of the dimedone reaction with C165A AhpC-SOH (k = 1.29 M−1 s−1) compared with DCP-TPP (1, k = 1.1 M−1 s−1, Figure 1A). However, the combination of the TPP group with BCN significantly reduces (4.6×) the rate of the BCN-OH reaction (k = 27.6 M−1 s−1) with C165A AhpC-SOH compared with BCN-TPP (2, k = 5.99 M−1 s−1, Figure 1B). Consistent with previous data, BCN-OH reacts much faster with C165A AhpC-SOH (>20×) than with dimedone.6
Similar experiments reveal the kinetics of the reaction of BCN-OH and BCN-TPP (2) with C165A AhpC-SSH, generated by reacting C165A AhpC-SOH with Na2S.4 MS analysis distinguishes the reaction products of BCN-OH or BCN-TPP with either the -SOH or -SSH on the basis of the +16 Da mass difference between the vinyl sulfoxide that forms in the -SOH reactions and the vinyl thioether product that arises from -SSH.6,36 As noted for the reaction of BCN-TPP with C165A AhpC-SOH, the TPP group significantly impacts the reaction of BCN-TPP (2) compared to BCN-OH with C165A AhpC-SSH (BCN-OH, k = 2.60 M−1 s−1; BCN-TPP (2), no reaction observed by ESI-TOF-MS; Supporting Information, Figure S14). The reaction of BCN-OH with C165A AhpC-SSH gives a smaller rate constant than that found with C165A AhpC-SOH (approximately 10× smaller).
Effects of Probes on Mitochondria Respiration.
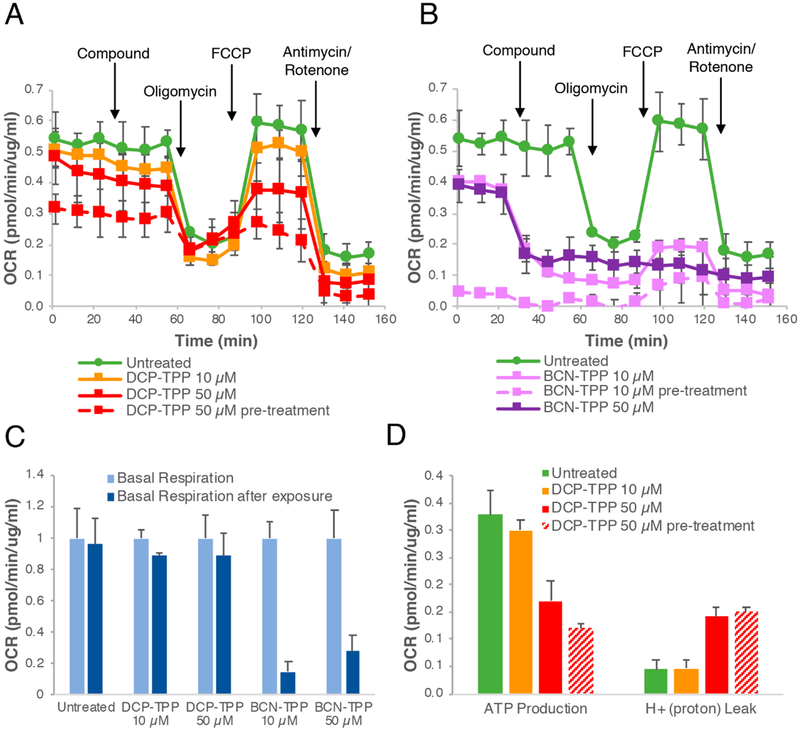
The Seahorse Mito Stress Test (Experimental Procedures) provides insight into the effects of the protein sulfenylation probes at different concentrations and treatment regimens on mitochondrial respiration in A549 cells (Figure 2A,B).37 Both DCP-TPP and BCN-TPP alter mitochondrial respiration and decrease basal respiration (Figure 2C). The ATP production was decreased and proton leak increased at 50 μM for DCP-TPP (Figure 2D). BCN-TPP, on the other hand, decreased the mitochondrial respiration rapidly, reaching the level of non-mitochondrial respiration (Figure 2B). BCN-TPP produces a larger decrease in basal respiration compared with DCP-TPP, possibly due to its faster reaction kinetics (Figure 1A). Pre-treatment (1 h) of the cells with DCP-TPP results in a further decrease in the oxygen consumption rate (OCR) and ATP production and an increase in proton leak, similar to the effects observed with BCN-TPP-treated cells in the previous experiments (Figure 2A–D). Pre-treatment of cells with the BCN-TPP results in almost complete shutdown of mitochondrial respiration (Figure 2B), which suggests the rapid reaction of the strained alkyne group of BCN-TPP with PSOH and/or other species severely disrupts mitochondrial respiration.
Figure 2.
Effects of mitochondria-targeted dimedone and BCN derivatives (DCP-TPP and BCN-TPP) on mitochondrial respiration. (A) Seahorse Mito Stress test for DCP-TPP. Pre-treatment with the compound was started 1 h before the analysis. (B) Seahorse Mito Stress test for BCN-TPP. Pre-treatment with the compound was started 1 h before the analysis. (IC) Quantification of DCP-TPP and BCN-TPP effects on the mitochondrial basal respiration; values are presented relative to the basal respiration before compound exposure. (D) Quantification of DCP-TPP effects on ATP production and proton leak. Error bars represent the standard error of mean.
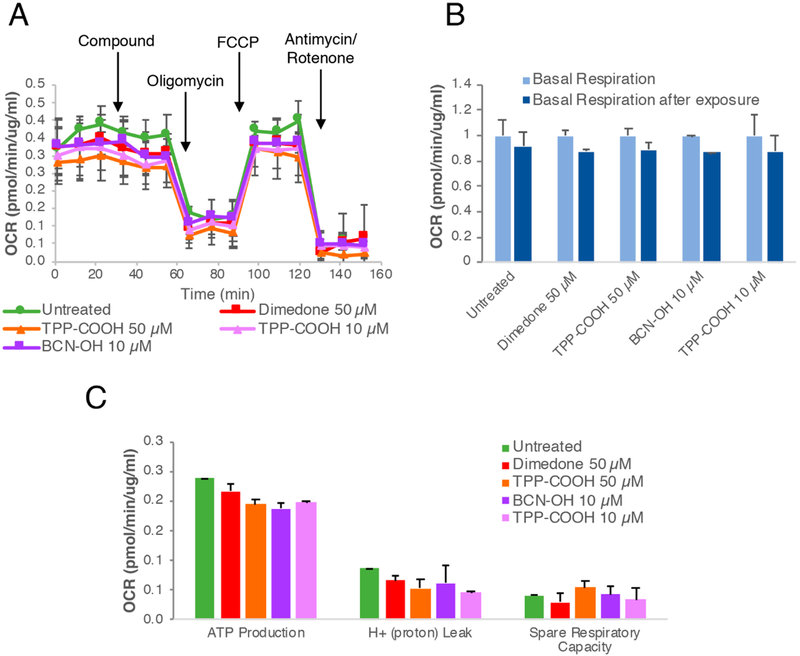
These changes in the mitochondrial respiration appear specific to the mitochondria-targeted sulfenic acid probes DCP-TPP and BCN-TPP. Mitochondrial extracellular flux analysis with the basic components of these probes (dimedone, BCN-OH, and TPP-COOH) reveals no significant changes in mitochondrial respiration, as judged by basal respiration, ATP production, proton leak, and spare respiratory capacity (Figure 3). The observed activity/toxicity of DCP-TPP and BCN-TPP relative to their component parts suggests 1 and 2 gain access at relevant concentrations to the mitochondrial intermembrane space in close proximity to the components of the respiratory chain.
Figure 3.
Control studies to evaluate the effects of dimedone, BCN-OH, and TPP-COOH on mitochondrial respiration. (A) Seahorse Mito Stress test for dimedone, BCN-OH, and TPP-COOH. (B) Quantification of effects on mitochondrial basal respiration; values are presented relative to the basal respiration before compound exposure. (C) Quantification of effects on ATP production, proton leak, and respiratory capacity. Error bars represent the standard error of mean.
DISCUSSION
The ability of mitochondria to generate ROS directs focus toward this organelle in terms of specific protein oxidation processes and redox regulation of cellular function. We recently reported two compounds, a coumarin-based reagent (DCP-NEt2C) and a rhodamine-based reagent (DCP-Rho1), that react with protein sulfenic acids at competent rates and accumulate in the mitochondria.25 DCP-NEt2C and DCP-Rho1 have little effect on mitochondrial function at 10 μM or lower concentration.25 Both compounds label mitochondrial proteins in a time- and dose-dependent manner under different conditions of oxidative stress (serum starvation and treatment with silver nanoparticles).25 While DCP-NEt2C and DCP-Rho1 target and label mitochondrial proteins, their lack of a biotin or other separation/identification tag limits their ability to identify specific mitochondrial PSOHs. During the preparation and evaluation of DCP-NEt2C and DCP-Rho1, we simultaneously explored hybrids of the TPP group and known sulfenic acid traps to design the first generation of TPP-directed mitochondrial PSOH traps, DCP-TPP (1) and BCN-TPP (2, Chart 1). Scheme 1 depicts the straightforward synthesis of 1 and 2 through coupling of the corresponding alcohols with commercially available TPP-COOH. Preparative reverse-phase (C18) chromatography facilitates the purification and characterization of DCP-TPP and BCN-TPP that contain the permanently charged TPP group.
ESI-TOF-MS experiments show that both DCP-TPP and BCN-TPP react with the model PSOH, C165A AhpC-SOH, to give the expected thioether (4) and vinyl sulfoxide (5) adducts (Scheme 2 and Figure 1). ESI-TOF-MS kinetic measurements provide information regarding the rates of these reactions. The combination of the TPP group with DCP to give DCP-TPP did not alter the second-order rate constant of the reaction with C165A AhpC-SOH compared to dimedone (k = 1.10 M−1 s−1 for 1 vs k = 1.29 M−1 s−1 for dimedone, Figure 1A). DCP-NEt2C and DCP-Rho1 also react with C165A AhpC-SOH at very similar rates as compared to dimedone (k = 0.11 M−1 s−1 and k = 0.41 M−1 s−1 for DCP-NEt2C and DCP-Rho1, respectively, vs k = 0.13 M−1 s−1 for dimedone).25 While the current values show a ~10-fold increase in rate from previous measurements,25 possibly due to differences in the independent preparation of the reactive PSOH species, the major observation remains that each of these DCP-derived mitochondria-directed PSOH traps reacts at rates similar to dimedone. Detailed kinetic analysis of the reaction of a number of structurally varied protein sulfenylation probes with both a dipeptide-derived sulfenamide and other PSOHs shows that structural modification greatly influences the reaction rate, permitting the development of more reactive and selective reagents.38–40
Kinetic UV–vis experiments show that BCN-TPP reacts with Fries acid, a stable organic sulfenic acid, in organic solvent with a second-order rate constant of 10.9 M−1 s−1, about one-half the rate of ~25 M−1 s−1 previously reported for the reaction of Fries acid and BCN-OH.6 These results suggest that the addition of the TPP group to BCN does not dramatically alter its organic reaction kinetics. BCN-TPP reacts slightly slower (~4.6-fold slower) with C165A AhpC-SOH as compared to BCN-OH (second-order rate constants of k = 5.99 M−1 s−1 for 2 vs k = 27.6 M−1 s−1 for BCN-OH, Figure 1B) as determined by ESI-TOF-MS. A biotin-tagged BCN derivative (BCN-BIO) reacts with C165A AhpC-SOH at nearly the same rate as BCN-OH and faster than BCN-TPP (second-order rate constants of k = 16.7 M−1 s−1 for BCN-BIO vs k = 13.3 M−1 s−1 for BCN-OH in these experiments) suggesting that the addition of TPP to the BCN group inhibits reactivity with C165A Ahp-SOH.6 Hydrophobic interactions of the TPP group with the protein that results in an unfavorable reaction conformation or alignment of the probe and the sulfenic acid group may prevent optimal orientation of the reacting groups. Based on this idea, the differences in the reaction mechanism between the DCP-based probes (nucleophilic) and the BCN-based probes (electrophilic, ene-like reaction) could account for the lower than expected reactivity of BCN-TPP (compared to BCN-OH) with PSOHs, while DCP-TPP reacts at a rate similar to that of dimedone. In general, the kinetic results with DCP-TPP and BCN-TPP follow the general trend that BCN-derived probes react faster with C165A AhpC-SOH than DCP-derived probes.
Previous work shows that BCN-OH reacts with trityl persulfide to yield a vinyl thioether with a second-order rate constant of k = 18.8 M−1 s−1, similar to ~25 M−1 s−1 for Fries acid in organic solvent.36 BCN-OH reacts with C165A AhpC-SSH, a protein persulfide, to yield a vinyl thioether adduct (6, Scheme 2; Supporting Information, Figure S13) with a second-order rate constant of k = 2.60 M−1 s−1 (7.2 times slower than the rate for the reaction of BCN-OH with trityl persulfide). Solvent effects (aqueous vs organic) and the structural change in persulfide (small molecule vs protein) may contribute to this large change in observed rate. However, BCN-TPP does not form a PSSH adduct as judged by ESI-TOF-MS supporting the idea that the TPP group of BCN-TPP may hinder the electrophilic reaction required for adduct formation by interfering with the proper alignment of the reactive groups, and further work will be required to test this hypothesis. BCN-OH reacts with the protein persulfide of bovine serum albumin, indicating that cycloalkyne reagents trap PSSHs but differences between these proteins may alter the reactivity or stability of the persulfides.36 The ability of mass spectrometric analysis to distinguish PSOH and PSSH cycloalkyne adducts (ΔDa = 16) provides a means of differentiating PSOHs from PSSHs.
Extracellular flux metabolic measurements provide information regarding the influence of DCP-TPP and BCN-TPP on mitochondrial respiration and overall mitochondrial function. These experiments measure real-time oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in the absence (basal) and presence of compounds that alter mitochondrial function.37 These agents include oligomycin, which inhibits ATP synthase, FCCP, which uncouples the electron transport chain from ATP synthesis, and a mixture of roteneone and antimycin, which block the electron transport chain. The comparison of test compounds to these standards provides insight regarding potential mechanisms of action on mitochondrial function. OCR was evaluated by the Mito Stress Test with a Seahorse Extracellular Flux Analyzer (Agilent) using A549 cells. Both DCP-TPP and BCN-TPP immediately decrease the observed OCR (Figure 2A) and respond in an expected way to oligomycin, FCCP, and rotenone/antimycin (BCN-TPP at 50 μM does not). Further analysis based on parameters derived during the assay shows that DCP-TPP decreases basal respiration and ATP production and increases proton leak (Figure 2C,D). BCN-TPP produces a larger decrease in basal respiration compared with DCP-TPP, which demonstrates a profile similar to DCP-NEt2C.25 The increased rate of reaction of C165A AhpC-SOH with BCN-TPP compared to DCP-TPP (Figure 1A,B) may result in the more profound effect on basal respiration. To examine this hypothesis, pre-treatment of the cells with DCP-TPP for 1 h before analysis further decreases OCR similar to that observed with the BCN-TPP-treated cells, suggesting that the additional incubation time led for further disruption of mitochondrial respiration (Figure 2A,B). Similar pre-treatment of cells with the BCN-TPP results in almost complete shutdown of OCR, indicative of complete blockage of the electron transport chain and mitochondrial respiration, suggesting that the rapid reactions of the strained alkyne group (with protein sulfenic acids and other targets) lead to toxicity. Indeed, BCN-BIO demonstrates more toxicity in SCC-61 cells than dimedone (IC50 = 1.46 ± 0.12 mM for dimedone vs 199.3 ± 27.3 μM for BCN-BIO).6 These results highlight the value of extracellular flux analyses on mitochondrial-directed probes to evaluate toxicity and mechanism.
Further control experiments reveal these changes in the mitochondrial respiration appear specific to the addition of the TPP group in DCP-TPP and BCN-TPP. Extracellular flux analysis with the constituents of DCP-TPP and BCN-TPP (dimedone, BCN-OH, and TPP-COOH) reveal no significant changes in mitochondrial respiration as judged by basal respiration, ATP production, proton leak, and spared respiratory capacity (Figure 3). The failure of these components to alter mitochondrial function provides strong evidence of the entry, accumulation, and activity of DCP-TPP and BCN-TPP in the mitochondria at concentrations sufficient to produce effects and shows that the simple sulfenic acid traps dimedone and BCN-OH do not interfere with mitochondrial respiration.
In summary, DCP-TPP and BCN-TPP, formed by the combination of the TPP group with known sulfenic acid traps, react with C165A AhpC-SOH, a model PSOH, to form the expected adducts. Kinetically, DCP-TPP reacts at rates similar to dimedone and other DCP-derived traps, indicating that the TPP group does not influence the rate of reaction. BCN-TPP reacts 4.6-fold slower with C165A AhpC-SOH than BCN-OH indicating the TPP group hinders the reaction in some way. BCN-TPP also does not react with the protein persulfide C165A AhpC-SSH casting doubt as to the overall generality of BCN-derived probes as PSSH labeling agents. Extracellular flux measurements show that DCP-TPP and BCN-TPP influence mitochondrial energetics, providing strong evidence for mitochondrial localization and accumulation. Similar to DCP-NEt2C and DCP-Rho1, the absence of biotin or other purification tags in DCP-TPP and BCN-TPP makes the isolation and identification of specifically modified mitochondrial proteins difficult, and experiments to label and identify oxidized mitochondrial proteins with these tags were not attempted given these limitations. Taken together, these results reveal the compatibility of the TPP group with sulfenic acid trapping and indicate the feasibility of using the TPP group to direct sulfenic acid probes to the mitochondria. These results support current efforts to combine sulfenic acid traps, mitochondria directors, and identification tags to determine specific mitochondrial protein sulfenic acid formation.
Supplementary Material
Funding
This work was supported by National Cancer Institute and National Institute of Environmental Health Sciences under award numbers R33 CA177461 (C.M.F., L.B.P., S.B.K.) and R21/33 ES025645 (C.M.F., S.B.K., L.B.P.). We would also like to acknowledge the Comprehensive Cancer Center of Wake Forest University NCI CCSG P30CA012197 grant for support of shared resource facilities, the Center for Redox Biology and Medicine (pilot funds to S.B.K.) and the Kimbrell family for the support of high-end mass spectrometry instrumentation in C.M.F.’s laboratory.
ABBREVIATIONS
- ABC
ammonium bicarbonate buffer
- BCA
bicinchonic acid assay
- BCN
bicyclo[6.1.0]nonyne
- CAN
ceric ammonium nitrate
- DCC
N,N′-dicyclohexylcarbodiimide
- DCP
2,4-(dioxocyclohexyl)propoxy unit
- DMAP
N,N-dimethylaminopyridine
- DTT
- ECAR
extracellular acidification rate
- ESI-TOF-MS
electrospray ionization time-of-flight mass spectrometry
- FCCP
carbonyl cyanide p-(trifluoromethoxyphenyl)hydrazine
- HOBt
hydroxy benzotriazole
- OCR
oxygen consumption rate
- PSOH
protein sulfenic acid
- PSSH
protein persulfide
- RIPA
radioimmunoprecipitation assay
- ROS
reactive oxygen species
- TPP
triphenylphosphonium group
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemres-tox.8b00385.
NMR and MS characterization of synthetic intermediates and 1–3; UV–vis kinetic analysis of the reaction of 2 with Fries acid; ESI-TOF-MS data for the reaction of BCN-OH with AhpC-SSH (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Devarie-Baez NO, Silva Lopez EI, and Furdui CM (2016) Biological chemistry and functionality of protein sulfenic acids and related thiol modifications. Free Radical Res 50 (2), 172–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Forman HJ, Davies MJ, Krämer AC, Miotto G, Zaccarin M, Zhang H, and Ursini F (2017) Protein cysteine oxidation in redox signaling: Caveats on sulfenic acid detection and quantification. Arch. Biochem. Biophys 617, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Barrett TJ, Pattison DI, Leonard SE, Carroll KS, Davies MJ, and Hawkins CL (2012) Inactivation of thiol-dependent enzymes by hypothiocyanous acid: role of sulfenyl thiocyanate and sulfenic acid intermediates. Free Radical Biol. Med 52 (6), 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cuevasanta E, Lange M, Bonanata J, Coitino EL, Ferrer-Sueta G, Filipovic MR, and Alvarez B (2015) Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem 290 (45), 26866–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Klomsiri C, Nelson KJ, Bechtold E, Soito L, Johnson LC, Lowther WT, Ryu SE, King SB, Furdui CM, and Poole LB (2010) Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol 473, 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Poole TH, Reisz JA, Zhao W, Poole LB, Furdui CM, and King SB (2014) Strained cycloalkynes as new protein sulfenic acid traps. J. Am. Chem. Soc 136 (17), 6167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kaplan N, Urao N, Furuta E, Kim SJ, Razvi M, Nakamura Y, McKinney RD, Poole LB, Fukai T, and Ushio-Fukai M (2011) Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free Radical Res 45 (10), 1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Klomsiri C, Rogers LC, Soito L, McCauley AK, King SB, Nelson KJ, Poole LB, and Daniel LW (2014) Endosomal H2O2 production leads to localized cysteine sulfenic acid formation on proteins during lysophosphatidic acid-mediated cell signaling. Free Radical Biol. Med 71, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mauney CH, Rogers LC, Harris RS, Daniel LW, Devarie-Baez NO, Wu H, Furdui CM, Poole LB, Perrino FW, and Hollis T (2017) The SAMHD1 dNTP Triphosphohydrolase Is Controlled by a Redox Switch. Antioxid. Redox Signaling 27, 1317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Truong TH, Ung PM, Palde PB, Paulsen CE, Schlessinger A, and Carroll KS (2016) Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase. Cell Chem. Biol 23 (7), 837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wani R, Qian J, Yin L, Bechtold E, King SB, Poole LB, Paek E, Tsang AW, and Furdui CM (2011) Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A 108 (26), 10550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, and Komander D (2013) Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat. Commun 4, 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Garcia FJ, and Carroll KS (2016) An immunochemical approach to detect oxidized protein tyrosine phosphatases using a selective C-nucleophile tag. Mol. BioSyst 12 (6), 1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gupta V, Yang J, Liebler DC, and Carroll KS (2017) Diverse Redoxome Reactivity Profiles of Carbon Nucleophiles. J. Am. Chem. Soc 139 (15), 5588–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wu J, Cheng Z, Reddie K, Carroll K, Hammad LA, Karty JA, and Bauer CE (2013) RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid. J. Biol. Chem 288 (7), 4755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yang J, Gupta V, Tallman KA, Porter NA, Carroll KS, and Liebler DC (2015) Global, in situ, site-specific analysis of protein S-sulfenylation. Nat. Protoc 10 (7), 1022–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Idelchik M, Begley U, Begley TJ, and Melendez JA (2017) Mitochondrial ROS control of cancer. Semin. Cancer Biol 47, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Diebold L, and Chandel NS (2016) Mitochondrial ROS regulation of proliferating cells. Free Radical Biol. Med 100, 86–93. [DOI] [PubMed] [Google Scholar]
- (19).Bharadwaj MS, Tyrrell DJ, Leng I, Demons JL, Lyles MF, Carr JJ, Nicklas BJ, and Molina AJ (2015) Relationships between mitochondrial content and bioenergetics with obesity, body composition and fat distribution in healthy older adults. BMC Obes 2, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, and Molina AJ (2015) Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J. Gerontol., Ser. A 70 (11), 1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wallace DC (2012) Mitochondria and cancer. Nat. Rev. Cancer 12 (10), 685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sivitz WI, and Yorek MA (2010) Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signaling 12 (4), 537–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Arun S, Liu L, and Donmez G (2016) Mitochondrial Biology and Neurological Diseases. Curr. Neuropharmacol 14 (2), 143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Luo Y, Hoffer A, Hoffer B, and Qi X (2015) Mitochondria: A Therapeutic Target for Parkinson’s Disease? Int. J. Mol. Sci 16 (9), 20704–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Holmila RJ, Vance SA, Chen X, Wu H, Shukla K, Bharadwaj MS, Mims J, Wary Z, Marrs G, Singh R, Molina AJ, Poole LB, King SB, and Furdui CM (2018) Mitochondria-targeted Probes for Imaging Protein Sulfenylation. Sci. Rep 8, 24493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Smith RAJ, Hartley RC, and Murphy MP (2011) Mitochondria-Targeted Small Molecule Therapeutics and Probes. Antioxid. Redox Signaling 15 (12), 3021–3038. [DOI] [PubMed] [Google Scholar]
- (27).Arias-Mayenco I, Gonzalez-Rodriguez P, Torres-Torrelo H, Gao L, Fernandez-Aguera MC, Bonilla-Henao V, Ortega-Saenz P, and Lopez-Barneo J (2018) Acute O2 Sensing: Role of Coenzyme QH2/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab 28 (1), 145–58. [DOI] [PubMed] [Google Scholar]
- (28).Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, and Murphy MP (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem 276 (7), 4588–96. [DOI] [PubMed] [Google Scholar]
- (29).Poole LB, and Ellis HR (1996) Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry 35 (1), 56–64. [DOI] [PubMed] [Google Scholar]
- (30).Ellis HR, and Poole LB (1997) Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry 36 (43), 13349–56. [DOI] [PubMed] [Google Scholar]
- (31).Poole LB, Zeng BB, Knaggs SA, Yakubu M, and King SB (2005) Synthesis of chemical probes to map sulfenic acid modifications on proteins. Bioconjugate Chem 16 (6), 1624–8. [DOI] [PubMed] [Google Scholar]
- (32).Parsonage D, Nelson KJ, Ferrer-Sueta G, Alley S, Karplus PA, Furdui CM, and Poole LB (2015) Dissecting peroxiredoxin catalysis: separating binding, peroxidation, and resolution for a bacterial AhpC. Biochemistry 54 (7), 1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chen X, Wu H, Park CM, Poole TH, Keceli G, Devarie-Baez NO, Tsang AW, Lowther WT, Poole LB, King SB, Xian M, and Furdui CM (2017) Discovery of Heteroaromatic Sulfones As a New Class of Biologically Compatible Thiol-Selective Reagents. ACS Chem. Biol 12 (8), 2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Qian J, Klomsiri C, Wright MW, King SB, Tsang AW, Poole LB, and Furdui CM (2011) Simple synthesis of 1,3-cyclopentanedione derived probes for labeling sulfenic acid proteins. Chem. Commun. (Cambridge, U. K.) 47 (32), 9203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Poole LB, Klomsiri C, Knaggs SA, Furdui CM, Nelson KJ, Thomas MJ, Fetrow JS, Daniel LW, and King SB (2007) Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjugate Chem 18 (6), 2004–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Galardon E, and Padovani D (2015) Reactivity of persulfides toward strained bicyclo[6.1.0]nonyne derivatives: relevance to chemical tagging of proteins. Bioconjugate Chem 26 (6), 1013–6. [DOI] [PubMed] [Google Scholar]
- (37).Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, and Jastroch M (2014) Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 547, 309–54. [DOI] [PubMed] [Google Scholar]
- (38).Gupta V, and Carroll KS (2016) Profiling the Reactivity of Cyclic C-Nucleophiles towards Electrophilic Sulfur in Cysteine Sulfenic Acid. Chem. Sci 7 (1), 400–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gupta V, and Carroll KS (2016) Rational design of reversible and irreversible cysteine sulfenic acid-targeted linear C-nucleophiles. Chem. Commun. (Cambridge, U. K.) 52 (16), 3414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Gupta V, Paritala H, and Carroll KS (2016) Reactivity, Selectivity, and Stability in Sulfenic Acid Detection: A Comparative Study of Nucleophilic and Electrophilic Probes. Bioconjugate Chem 27 (5), 1411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.