Abstract
The need to search for new, alternative treatments for various diseases has prompted scientists and physicians to focus their attention on regenerative medicine and broadly understood cell therapies. Currently, stem cells are being investigated for their potentially widespread use in therapies for many untreatable diseases. Nowadays modern treatment strategies willingly use mesenchymal stem cells (MSCs) derived from different sources. Researchers are increasingly aware of the nature of MSCs and new possibilities for their use. Due to their properties, especially their ability to self-regenerate, differentiate into several cell lineages and participate in immunomodulation, MSCs have become a promising tool in developing modern and efficient future treatment strategies. The great potential and availability of MSCs allow for their various clinical applications in the treatment of many incurable diseases. In addition to their many advantages and benefits, there are still questions about the use of MSCs. What are the mechanisms of action of MSCs? How do they reach their destination? Is the clinical use of MSCs safe? These are the main questions that arise regarding MSCs when they are considered as therapeutic tools. The diversity of MSCs, their different clinical applications, and their many traits that have not yet been thoroughly investigated are sources of discussions and controversial opinions about these cells. Here, we reviewed the current knowledge about MSCs in terms of their therapeutic potential, clinical effects and safety in clinical applications.
Keywords: mesenchymal stem cells, somatic cell therapy, transplantation, engraftment, immunomodulatory properties
Introduction
In the 1960s, Friedenstein et al. identified a population of fibroblast-like cells that formed clonal colonies in vitro (CFU-F, Colony Forming Unit-Fibroblast)1. Friedenstein’s observations allowed for the discovery of a specific type of cell, currently referred to as mesenchymal stem cells (MSCs). MSCs are primary, non-specialized, nonhematopoietic, plastic adherent cells with great proliferation potential and the capacity for self-renewal and differentation2.
In 2006, the International Society of Cellular Therapy (ISCT) proposed basic criteria for defining human multipotent mesenchymal stromal cells whose name then evolved to MSCs. In addition to their plastic adherent properties under standard culture conditions and trilineage differentiation capacity into osteoblasts, chondrocytes and adipocytes, > 95% of the MSCs population is positive for the three specific surface markers—CD73 (SH3/4), CD90 (Thy-1), and CD105 (SH2)—and do not express CD45, CD34, CD14, CD11b, CD79a, CD19, or major histocompatibility complex (MHC) class II3,4. MSCs also express others markers, including CD9, CD10, CD13, CD29, CD44, CD49, CD51, CD54 (ICAM-1), CD117 (c-kit), CD146 (MCAM), CD166 (ALCAM), and Stro-1, but the expression of specific combinations of the markers appear to be host tissue dependent5. Although a wide range of positive markers describing MSCs has been identified, no single marker has been indicated as specific for MSCs.
It should be also noted that the potential of MSCs for differentiation and proliferation may vary considerably between different MSC sources6,7. It has been suggested that these differences are a result of the direct influence of the specific microenvironments in which they primarily reside8,9.
Despite increasing numbers of reports describing MSCs, numerous controversies have arisen regarding the proper identification of MSCs. It appears that the criteria proposed by the ISCT are not sufficient because MSCs isolated from different tissues represent a relatively heterogeneous group of cells in terms of differentiation, proliferation abilities, and cell surface expression6,10–13.
Mesenchymal Stem Cells—the Main Players in Cell Therapy
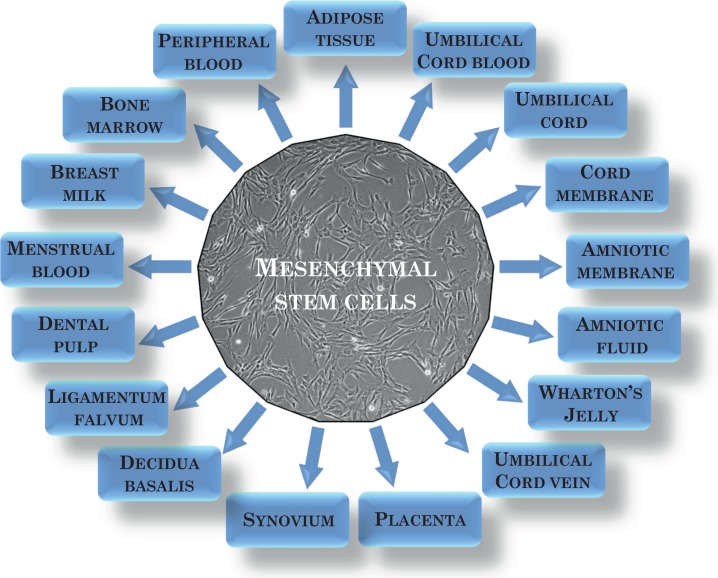
The fact that MSCs can be isolated from numerous sources1,2,6–8,10 (Fig. 1), their relative ease to culture in vitro, their ability to differentiate into several different cell types, and their special immunological properties make MSCs a promising tool for cell therapy and tissue regeneration. The best known and the most commonly used source of MSCs is bone marrow (BM)14. BM is the tissue in which MSCs were first identified. Another easily accessible source of MSCs is adipose tissue15. Obtaining MSCs from these sources requires invasive procedures. Interestingly, rich sources of MSCs include birth-associated tissues that are treated as medical waste, such as placenta, umbilical cord, amniotic fluid, and amniotic membrane. Among those tissues, umbilical cord blood16 is believe to contain MSCs; however, the use of this source is questioned by some researchers because of low efficiency of their isolation17. MSCs derived from Wharton’s jelly of the umbilical cord (WJ-MSCs) appear to have great future clinical utility due to their limited heterogeneity and some unique properties, such as ease of their isolation and culture, availability in several tissues, their immunomodulatory properties, ability to self-regenerate, differentiate into several cell lineages, and the lack of ethical problems resulting from their use18. Moreover, in contrast to BM or adipose tissue, the acquisition and isolation of birth-associated tissues, including WJ-MSCs, do not require invasive surgical procedures; therefore, the isolation process does not pose any risk of complications for the donor, giving them an advantage over other MSC sources. Currently, new sources of MSCs have been proposed. MSCs are found in dental pulp, periodontal ligament, tendon, skin, muscle, and other tissues19 (Fig. 1). However, there are differences in isolation efficiency that are related to the availability, condition, and age of the donor tissue. A very important issue is the age of the donor’s cells20. Cells obtained from younger donors are less susceptible to oxidative damages and changes, they age considerably more slowly in culture, and they have a higher proliferation rate21,22.
Fig. 1.
Mesenchymal stem cells sources.
Currently, many studies focus on the use of MSCs in cell therapy. MSCs are used as a tool to treat degenerative changes in joints and to reconstruct bones and cartilage, and are used in plastic surgeries, aesthetic medicine, cardiovascular diseases, endocrine and nervous system diseases, cell transplantation, and in the repair of damaged musculoskeletal tissues23. Due to the special properties of these cells, such as their rapid proliferation, high differentiation capacity, and the ability to migrate into the site of damage, new clinical applications are being tested.
BM-MSCs are the most frequently used in clinical settings24. BM-MSCs were also first to be registed by the Food and Drug Administration as a drug against Graft versus Host Disease called “Prochymal”25. Recently, “Alofisel” has been registered by the European Medicines Agency to treat complex perianal fistulas. The drug is based on expanded adipose-derived stem cells26. In both cases the drugs are allogeneic, which provides strong advantage other autologous products due to possibility of detailed testing regarding both safety and potency before release. Nowadays other sources of MSCs are also used for clinical therapies. Our research group used MSCs isolated from Wharton Jelly to treat patients with acute myocardial infarction, showing the safety and feasibility of such therapy27. Currently, we are conducting phase II/III randomized, double-blinded clinical trials with the use of the product “CardioCell” that is based on WJ-MSCs in three indications: acute myocardial infarction (AMI-Study, EudraCT Number: 2016-004662-25), chronic ischemic heart failure (CIHF-Study, EudraCT Number: 2016-004683-19), and non-option critical limb ischemia (N-O CLI-Study, EudraCT Number: 2016-004684-40).
However, it should be noted that although we possess great knowledge about their in vitro characteristics, we still know much less about the in vivo behaviors of MSCs. They can act both directly—due to their ability to differentiate28—and indirectly, by producing and secreting many factors that enhance the endogenous regeneration potential of injured tissue19.
The new approach in stem cell therapy is the use of extracellular vesicles (EVs), which can be used as a substitute for MSCs29. EVs as a therapeutic vector have the paracrine effect without the direct involvement of the cells. They are released from stem cells and they supply many components such as mRNA, DNA, and proteins to the target site30. This approach is described in many recent studies31,32 but a thorough understanding of the mechanism of action of EVs is still required.
Migration and Homing of Mesenchymal Stem Cells
The therapeutic effect of MSCs depends on their ability to reach the injured site, which is possible due to their ability to migrate, adhere, and engraft into a target tissue. Several factors affect the therapeutic efficacy of MSCs’ homing. Among them, culture conditions, the number of passages, donor age, delivery method, and host receptibility play important roles33–36. It has been shown that freshly isolated cells compared with in vitro-cultured cells have a higher engraftment efficiency37, which can be a result of the aging/differentiation process that cells undergo in in vitro culture conditions38,39. Culture conditions also have a significant impact on homing capacity, as they can modify the expression of the surface markers involved in this process. As an example, CXCR4, a chemokine receptor, is involved in the migration of MSCs. It has been shown that CXCR4 expression is lost on BM-MSCs during culture37,40,41, whereas the presence of cytokines (e.g., HGF, IL-6), hypoxic conditions, or direct introduction using viral vectors allow for restoration of its expression42–44.
In addition, MSCs isolated from older donors show altered compositions and functions of membrane glycerophospholipids45. All of these aspects affect MSCs’ ability to migrate, home, and engraft into a site of injury.
The efficacy of cell therapy largely depends on the delivery method. The most common method of administration of MSCs is intravenous infusion46–48. However, before the cells reach their target, the majority are trapped within capillaries of various organs, especially in the lungs46,49–52. This attrition can be explained by the fact that MSCs are relatively large cells and express various adhesion molecules. Despite the fact that MSCs can become trapped in the lungs, numerous pieces of evidence suggest that they are able to home to injured tissue50,53. Interestingly, recent data also suggest that despite the problems associated with intravenous infusions, this route results in similar efficacy as other modes of delivery of MSCs54. In some instances, intra-arterial injection seems to be a more effective route. It has been shown that delivery of MSCs through the internal carotid artery more effectively facilitates their migration and homing into injured brain compared with administration via the femoral vein. The risk associated with this route of delivery includes occlusions, which can arise in microvessels53. When the MSCs were delivered directly to the heart, near the damaged area, the number of cells that reached the peri-infarct region was much higher55.
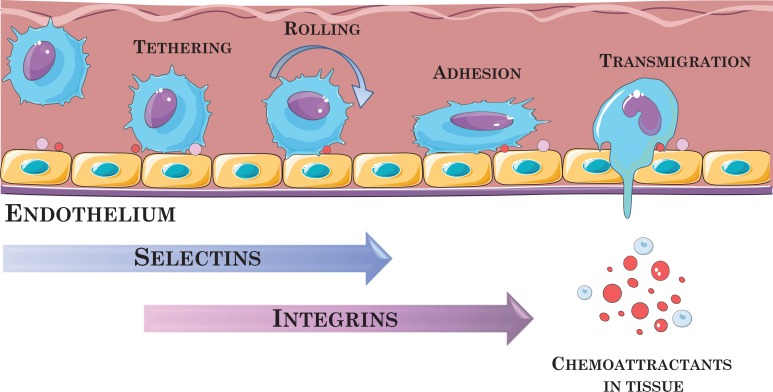
As has already been mentioned, the necessary condition for effective MSC-based therapy is for the cells to reach the site of injury and home to the affected tissue. There is no doubt that specific receptors and adhesion molecules and interactions with endothelial cells play crucial roles in this migration and homing. Cell adhesion proteins are expressed in the plasma membrane, such as integrins, which are involved in cell adhesion to extracellular matrix proteins (EMC), such as collagen, fibronectin, and laminin38,56–60. In vivo studies have shown that MSCs exhibit chemotactic properties and, after intravenous injections, are able to attach to endothelium and migrate between endothelial cells toward injured tissue in response to factors that are upregulated under the inflammatory conditions61–64. However, the detailed mechanisms of their transendothelial migration (TEM), diapedesis, and homing to sites of injury and inflammation have not yet been explained in detail. It is presumed that this mechanism may be similar to that of leukocytes (Fig. 2)65–67 but is performed with the participation of different adhesion molecules. To date, many chemokines and growth factors have been identified (e.g., EGF, VEGF-A, FGF, PDGF-AB, HGF, TGF-b1, TNF-α, SDF-1α, IL-6, IL-8, IGF-1), including their receptors, adhesion molecules, and metalloproteinases, that are involved in the MSCs migration process (e.g., CXCL-12, CCL-2, CCL-3, CCR4, CXCR4, VCAM, ICAM)55,59,65,68–71. Many reports suggest that damaged tissue expresses specific factors that act as chemoattractants to facilitate the migration, adhesion, and infiltration of MSCs to sites of injury, as in the case of leukocytes trafficking to sites of inflammation. However, although the leukocyte recruitment process (i.e., binding to endothelial cells, rolling, adhesion, and TEM) is well understood, the mechanism of the interaction between MSCs and endothelial cells will require more detailed investigations. Studies by Rüster et al. showed that the ability of MSCs to bind and roll on endothelial cells was derived from human umbilical cord vein cells. Once the MSCs adhere to endothelium, they become shaped like protrusions and roll. The molecules involved in this process have been identified and include P-selectin and VLA-4 expressed on MSCs and VCAM-1 on endothelial cells (VLA-4/VCAM-1 interaction)65. It has also been confirmed that a vital role in the homing and migration of MSCs is played by the proteolytic enzymes matrix metalloproteinases (MMPs)37,41.
Fig. 2.
Schematic of leukocyte transmigration through the endothelium. It is supposed that MSCs migration occurs in a similar manner. The graphic was prepared using modified art elements from Servier Medical Art, found at https://smart.servier.com.
Immunological Properties of Mesenchymal Stem Cells
It is generally accepted that MSCs do not display immunogenic properties, so they can be transplanted to an allogenic host without need for immunosuppression. The mechanism of their action is based on their immunomodulatory properties as well as immunosuppressive activity. They are able to suppress proliferation and activation of different cells of the immune system. These interactions may occure directly (i.e., cell–cell interaction) and indirectly (via soluble factors), and this pathway of suppression is independent of MHC matching between MSCs and T cells 39,72,73. The immunomodulating effect of MSCs is reflected in many T-cell properties, such as activation and proliferation, and in this way they efficiently suppress an immune response73. The MSCs suppress the proliferation of activated T cells by secreting substances, such as indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2)74–76. They also suppress the development of pro-inflammatory Th17 cells and stimulate regulatory T cells by secreting immunosuppressive cytokines including IL-6, IL-8, IL-10, TGF-β, and HGF. In addition, the nonclassical HLA class I molecules (HLA-G) expressed by MSCs exert immunosuppressive effects on various immune cells; that is, they inhibit T-cell proliferation and cytotoxic T lymphocyte-mediated cytolysis, and they also induce the development of tolerogenic dendritic cells and inhibit natural killer cell cytolytic functions77–80. It has been shown that HLA-G contributes to decrease graft rejection81. MSCs also participate in regulation of Th1/Th2 balance (T helper cells) by affecting the level of interleukin-4 (IL-4) and interferon (IFN)-γ in effector T cells. MSCs disturb maturation, differentiation, and functions (i.e. cytokine secretion) of dendritic cells (DCs), which play a crucial role in antigen presentation. There is much evidence that MSCs inhibit the proliferation, differentiation, and chemotaxis of B cells75,82,83. They also prevent monocyte differentiation into DCs. Because of their immunoregulatory properties, they are protected against cell lysis and the cytotoxic effects of the host’s immune system.
The immunophenotype of MSCs is generally described as: MHC I+, MHC II-. They also do not express the costimulatory molecules (CD40, CD80, CD86) and hematopoietic markers CD45, CD34, CD14, CD11, CD19, and CD18 (LFA-1; leukocyte function-associated antigen-1), which makes them non-immunogenic. MHC class I may activate T cells, but with the absence of costimulatory molecules, the T cells are non-reactive84–88.
Safety of Mesenchymal Stem Cell Therapies
Many studies have been conducted thus far to investigate the safety of MSC-based therapies. Clinical trials show that in vitro-cultured human MSCs are less susceptible to adverse changes.
A Canadian group analyzed clinical trials in which BM-MSCs were used. After a thorough analysis of 36 studies, they found that there was no relationship between the use of MSCs and tumorigenic potential, and no serious side effects of the therapy were reported89. The safety and impact of MSCs therapy were also investigated by Karussis et al. in patients with multiple sclerosis and amyotrophic lateral sclerosis90. In 34 examined patients, during a study lasting 25 months, no serious adverse effects resulting from the therapy were observed. In addition, 20 patients were examined 1 year after transplantation, and the MRI results did not show any disturbing changes90. However, more long-term studies and observations regarding the safety of using MSCs therapies will be required.
However, one study reported that the use of autologous adipose tissue-derived MSCs (AT-MSCs) in a patient with chronic kidney disease resulted not only in the improvement of renal function but also in fibrosis of the interstitial tissue and atrophy of the tubules, which could suggest nephrotoxicity of the applied MSCs91. Another group investigated the efficacy of the allogeneic treatment of MSCs administered to the aortas of patients with acute kidney injury after cardiac surgery. No differences were observed between the treated group and the control group in terms of improvement of renal function or in the occurrence of adverse events92.
Tatsumi et al. demonstrated in an in vivo model that the administration of AT-MSCs may result in thrombus formation around the cells through a coagulation mechanism, which can also cause pulmonary embolism due to the accumulation of cells in the lung region93. This finding was confirmed by other studies performed using umbilical cord MSCs, which showed the procoagulant properties of these cells after peripheral vein injection94. Many researchers currently focus on thromboinflammation, also known as the instant blood-mediated inflammatory reaction, which can occur after transplantation of MSCs95,96. Taking into account all of these issues, it is clear that more long-term studies and observations regarding the safety of using MSCs are required.
Despite the many cons for using MSCs in clinical settings, there are still a few issues that need to be resolved for the successful application of MSCs. One of them involves obtaining sufficient numbers of the cells. Unfortunately, during in vitro culture, cells at higher passages age due to decreased telomerase activity97. In addition, during long-term culture, MSCs lose their potential to differentiate and begin to exhibit morphological changes98. Even more importantly, long-term culture might lead to the increased probability of malignant transformation99,100. Certain components of the culture medium and growth factors may predispose the cells to such processes. There is also a risk of viral and prion transmission after administration of the cells101.
The Dark Side of Mesenchymal Stem Cell Biology
When using stem cell-based therapies, all possible undesirable effects should be considered. The risk associated with tumorigenesis after stem cell transplantation is widely discussed in the literature. In a certain sense, stem cells can be compared to tumor cells because of their ability to proliferation for a long period of time, high viability, and resistance to apoptosis102. Many components may affect the potential tumorigenesis after MSCs transplantation, including the donor’s age, host tissue, growth regulators expressed by recipient tissue, and mechanisms that control the behavior of the MSCs at the target site103–105. Also, manipulations and long-term in vitro cultures of MSCs can cause genetic instability and chromosomal abberations105. Many cumulative factors can give a response in the form of a spontaneous tumor transformation. Patients who are transplanted with stem cells often undergo long-term chemotherapy or radiotherapy, so their immune system does not work properly, which may also be associated with the risk of tumorigenesis106.
Protumorigenic Effect of Mesenchymal Stem Cells
The direct role of MSCs in promoting tumorigenesis has been investigated by several research groups in animal models. Results obtained for BM-MSCs show that the cells can engraft and home to many different types of solid tumors107–111. MSCs have been injected simultaneously with tumor cells in vivo. BM-MSCs promoted tumor growth in a colon cancer model109 and in breast cancer108, colorectal cancer112, ovarian113, prostate114, lung107, and gastric carcinoma115.
A highly complex tumorigenesis process involves many factors that promote tumor growth, one of which is hypoxia116. The published data indicate that BM-MSCs can be associated with tumor progression by the secretion of proangiogenic factors107.
MSCs have also been examined in the tumorigenic context due to the identification of carcinoma-associated fibroblast (CAF) cells, tumor-associated fibroblast (TAF) cells, and other tumor-associated cells, such as endothelial and pericyte-like cells, since MSCs can differentiate into these cell types under appropriate conditions117. In vitro and in vivo studies have shown that BM-MSCs cultured together with tumor cells may adopt the CAF-like phenotype, and the tumor microenvironment predisposes the transformation of these cells into α-smooth muscle actin (α-SMA)-expressing myofibroblasts118. Depending on the research model used, the percentage of MSCs taking part in this phenomenon varies. In an ovarian cancer model, it was found that the percentage of MSC-derived CAF cells ranged from 60 to 70%, whereas in the pancreatic cancer model, the percentage was only approximately 20%.
In studies by Karnoub et al., mice were used to graft non-metastatic breast cancer cells together with MSCs (BM-MSCs)108. The results of this study showed that, compared with mice injected only with cancer cells, the mix of MSCs and cancer cells increased the metastasis potential. The special engraftment properties and specific tropism of injected GFP + BM-MSCs into a mouse tumor model were also shown by Ren et al.119. Interestingly, it has been shown that the actions of stem cells (including nonhematopoietic and hematopoietic stem cells) in combination with different tumor cells can vary in vitro and in vivo. In vitro, MSCs cells showed antiproliferative activity, stopping in the G1 phase, in contrast to in vivo studies, where MSCs caused faster tumor growth120.
The Bright Side of Mesenchymal Stem Cell Biology
MSCs display a dualistic nature in relation to their tumorigenicity. Some studies have also shown their anti-tumorigenic effects. Factors secreted by MSCs may have antitumor properties. Clarke et al. showed that breast cancer cells cultured in MSC-conditioned medium exhibit significant migratory inhibition compared with cells cultured in a standard medium. The tumorigenesis effect of MSCs may be exerted by the secretion of the proteins TIMP-1 and TIMP-2, which inhibit the activity of the MMPs that are involved in migration processes121.
The inhibition of tumor cell growth was also shown by Bruno et al.122. A human hepatocellular carcinoma cell line (HepG2), a human ovarian cancer cell line (Skov-3), and Kaposi’s sarcoma cell lines co-cultured in the presence of BM-MSCs exhibited reduced in vitro growth. In addition, microvesicles (MVs) isolated from MSCs caused significant decreases in tumor cell proliferation through inhibiting cell cycle progression and inducing apoptosis and necrosis of the tumor cells. These observations were confirmed by in vivo studies in which tumor growth was slowed down by the administration of BM-MSC-derived MVs122.
Similar data were obtained with MVs derived from human WJ-MSCs. Wu et al. observed that WJ-MSC-derived MVs down-regulated the phosphorylation of Akt protein kinase and activated p53/p21 in bladder tumor cell lines123. Oxidative stress, which occurs in damaged tissues, is a natural process after the occurrence of damage. Therapies that use stem cells mainly focus on the regeneration of damaged tissues. Thus, the enhanced apoptotic resistance of MSCs, which is the result of regulation of the apoptosis process through complex cellular pathways, is highly desirable in the regeneration process that is the result of MSC therapy102,124.
There is no unambiguous answer regarding the potential of MSCs in tumorigenesis. In fact, the effect of MSCs depends not only on the tumor model used but also on the method of culture, cell heterogeneity, dose, secreted molecules, and many other factors that have not yet been fully understood.
Other Restrictions Related to the Application of Mesenchymal Stem Cells
Many studies (both preclinical and clinical trials) show increasing evidence of the therapeutic effectiveness of MSCs. However, many studies also provide evidence of low engraftment of MSCs due to their short-lived viability after injection125,126. It has also been demonstrated that after MSCs are transplanted, many of them are trapped in the lungs, resulting in a reduction in the population of cells that occupy the target site127. However, portions of MSCs populations reach damaged tissue, such as infarcted myocardium, traumatically injured brain, fibrotic liver, and various types of tumors125. The method by which the cells are administered may be an important factor in their reaching their intended destination. The advantage of the targeted application of these cells versus systemic administration is reductions in cell losses during delivery and cell migration128.
The low immunogenicity of the MSCs makes cell transplantation well tolerated by the recipient organism, reducing the likelihood of rejection of the transplantation. However, differentiated MSCs may exhibit low or no therapeutic effects. Huang et al. demonstrated that differentiated MSCs have increased immunogenicity due to MHC-I and MHC-II expression129.
Most of the studies conducted show that a single transplantation of MSCs is safe and does not induce an immune response. However, repeated administration of MSCs may result in the production of allo-antibodies130. Moreover, the fetal bovine serum (FBS) used in the MSC culture medium may cause an immune response in patients who have received such cells. Von Bonin et al. showed that the transplantation of MSCs that had been in contact with FBS induced the production of antibodies against FBS in the recipient’s blood131.
Concluding Remarks
Stem cells are undoubtedly a great hope for the treatment of many diseases. Since they occur in many adult tissues and do not raise ethical issues, they have great advantages over embryonic stem cells. Due to their unique features, such as their ease of isolation and culture, availability in many tissues, their immunomodulatory properties, and the lack of ethical problems resulting from their use, we believe that they can be used in both autologous and allogeneic transplantations. Despite numerous in vitro and in vivo studies, the mechanisms underlying MSCs transmigration and homing require further detailed examination. Nevertheless, there is no doubt that the cells can migrate and home to injured tissues. More research is emerging regarding the potential long-term risks associated with MSCs therapy. Long-term studies and observations will be necessary to investigate the long-term effects of MSCs therapies, including the negative effects. Based on our data, allogeneic clinical use of the MSCs seems to be promising tool in regenerative medicine.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grant STRATEGMED2/265761/10/NCBR/2015 form the National Center for Research and Development.
References
- 1. Sarukhan A, Zanotti L, Viola A. Mesenchymal stem cells: myths and reality. Swiss Med Wkly. 2015;145:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells -current trends and future prospective. Biosci Rep Biosci Reports. 2015;35(2):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 4. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells - the international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–1061. [DOI] [PubMed] [Google Scholar]
- 5. Giai Via A, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2(3):154–162. [PMC free article] [PubMed] [Google Scholar]
- 6. Pevsner-Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev Rep. 2011;7(3):560–568. [DOI] [PubMed] [Google Scholar]
- 7. Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Mag’Ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. [DOI] [PubMed] [Google Scholar]
- 11. Bianco P, Cao X, Frenette P, Mao J, Robey P, Simmons P, Wang C. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Im G I, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil. 2005;13(10):845–853. [DOI] [PubMed] [Google Scholar]
- 13. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–395. [DOI] [PubMed] [Google Scholar]
- 14. De Souza Fernandez T, De C, Fernandez S. Mesenchymal stem cells: biological characteristics and potential clinical applications for haematopoietic stem cell transplantation. In: M. Tomizawa M (Ed.), pluripotent stem cells - from the bench to the clinic. IntechOpen. 2016;3:495–519. [Google Scholar]
- 15. L Ramos T, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G, Hernández-Hernández Á, Redondo A, Ortega R, Rodríguez C, Sánchez-Guijo F, del Cañizo C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szabó GV, Kövesd Z, Cserepes J, Daróczy J, Belkin M, Acsády G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease-results of the short- and long-term follow-up. Cytotherapy. 2013;15(10):1245–1252. [DOI] [PubMed] [Google Scholar]
- 17. Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26(1):146–150. [DOI] [PubMed] [Google Scholar]
- 18. Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9(2):226–240. [DOI] [PubMed] [Google Scholar]
- 19. Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71(8):1353–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL, Hoyland JA, Mobasheri A. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. [DOI] [PubMed] [Google Scholar]
- 21. Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. Plos One. 2009;4(6):e5846, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–173. [DOI] [PubMed] [Google Scholar]
- 23. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trias. J Biomed Sci. 2016;23(1):76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17(4):534–541. [DOI] [PubMed] [Google Scholar]
- 26. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Diez MC, Tagarro I, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154(5):1334–1342. [DOI] [PubMed] [Google Scholar]
- 27. Musialek P, Mazurek A, Jarocha D, Tekieli L, Szot W, Kostkiewicz M, Banys RP, Urbanczyk M, Kadzielski A, Trystula M, Kijowski J, Zmudka K, Podolec P. Myocardial regeneration strategy using Wharton’s jelly mesenchymal stem cells as an offthe-shelf “unlimited” therapeutic agent: results from the acute myocardial infarction first-in-man study. Adv Intervent Cardiol. 2015;11:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata JI, Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288(1):51–59. [DOI] [PubMed] [Google Scholar]
- 29. La Greca A, Solari C, Furmento V, Lombardi A, Biani MC, Aban C, Moro L, García M, Guberman AS, Sevlever GE, Miriuka SG, Luzzani C. Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp Mol Med. 2018;50(9):119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sabin K, Kikyo N. Microvesicles as mediators of tissue regeneration. Transl Res. 2014;163(4):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bobis-Wozowicz S, Kmiotek K, Kania K, Karnas E, Labedz-Maslowska A, Sekula M, Kedracka-Krok S, Kolcz J, Boruczkowski D, Madeja Z, Zuba-Surma EK. Diverse impact of xeno-free conditions on biological and regenerative properties of hUC-MSCs and their extracellular vesicles. J Mol Med. 2017;95(2):205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vonk LA, van Dooremalen SF, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8(4):906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pezzi A, Amorin B, Laureano Á, Valim V, Dahmer A, Zambonato B, Sehn F, Wilke I, Bruschi L, Lima da Silva MA, Filippi-Chiela E, Silla L. Effects of hypoxiain long term in vitro expansion of human bone marrow derived mesenchymal stem cells. J Cell Biochem. 2017;118(10):3072–3079. [DOI] [PubMed] [Google Scholar]
- 34. Stubbendorff M, Neofytou E, Deuse T, Mattutat D, Lange C, Reichenspurner H, Robbins RC, Beygui RE, Volk HD, Schrepfer S. Impact of donor age on biological and immunogenic propertiesof mesenchymal stroma cells. Arch Med Res. 2015;3:1–24. [Google Scholar]
- 35. Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff K, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Medicine. 2013;11:146–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33(7):2177–2186. [DOI] [PubMed] [Google Scholar]
- 37. Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109(9):4055–4063. [DOI] [PubMed] [Google Scholar]
- 38. Rombouts WJC, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–170. [DOI] [PubMed] [Google Scholar]
- 39. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. [DOI] [PubMed] [Google Scholar]
- 40. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells. 2007;25(11):2896–2902. [DOI] [PubMed] [Google Scholar]
- 41. De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92(4):440–449. [DOI] [PubMed] [Google Scholar]
- 42. Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. [DOI] [PubMed] [Google Scholar]
- 43. Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bobis-Wozowicz S, Miekus K, Wybieralska E, Jarocha D, Zawisz A, Madeja Z, Majka M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp Hematol. 2011;39(6):686–696.e4. [DOI] [PubMed] [Google Scholar]
- 45. Kilpinen L, Tigistu-Sahle F, Oja S, Greco D, Parmar A, Saavalainen P, Nikkilä J, Korhonen M, Lehenkari P, Käkelä R, Laitinen S. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J Lipid Res. 2013;54(3):622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci. 1998;95(3):1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39(3):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.v. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136(1):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. [DOI] [PubMed] [Google Scholar]
- 51. Erkers T, Kaipe H, Nava S, Mollde´n P, Gustafsson B, Axelsson R, Ringden O. Treatment of severe chronic Graft-Versus-Host disease with decidual stroma cells and tracking with 111Indium radiolabeling. Stem Cells Dev. 2015;24(2):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961–967. [DOI] [PubMed] [Google Scholar]
- 53. Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, Van Zijl PCM, Huang J, Bulte JWM. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39(5):1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wysoczynki M, Khan A, Bolli R. New paradigms in cell therapy. Circ Res. 2018;123(2):138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44(2):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Segers VFM, Van Riet I, Andries LJ, Lemmens K, Demolder MJ, Becker AJML De, Kockx MM, Keulenaer GW, De Vincent FM, Riet I Van, Andries LJ, Demolder MJ, Becker AJML, De Mark M, De Keulenaer GW. Regulation and function of stem cells in the cardiovascular system. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2005;290:1370–1377. [DOI] [PubMed] [Google Scholar]
- 57. Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. Alternative names and criteria. J Cell Mol Med. 2007;11(1):21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Publ Gr. 2010;11(9):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haemat. 2007;137(4):491–502. [DOI] [PubMed] [Google Scholar]
- 60. Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol. 2010;42(10):1622–1633. [DOI] [PubMed] [Google Scholar]
- 61. Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Natsu K, Ochi M, Mochizuki Y, Hachisuka H, Yanada S, Yasunaga Y. Allogeneic bone marrow-derived mesenchymal stromal cells promote the regeneration of injured skeletal muscle without differentiation into myofibers. Tissue Eng. 2004;10(7–8):1093–1112. [DOI] [PubMed] [Google Scholar]
- 63. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15(10):730–738. [DOI] [PubMed] [Google Scholar]
- 65. Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. [DOI] [PubMed] [Google Scholar]
- 66. Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18(6):1754–1764. [DOI] [PubMed] [Google Scholar]
- 67. Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol Mech Dis. 2011;6(1):323–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Labrousse AM, Zappaterra MD, Rube DA, Bliek V Der, Cell M, Sesaki H, Jensen RE, Biol JC, Arimura S, Tsutsumi N, Natl P, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. [DOI] [PubMed] [Google Scholar]
- 69. Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–1041. [DOI] [PubMed] [Google Scholar]
- 70. Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–3801. [DOI] [PubMed] [Google Scholar]
- 72. Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Stem Cells. 2003;101(9):3722–3729. [DOI] [PubMed] [Google Scholar]
- 73. Nicola MD, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. [DOI] [PubMed] [Google Scholar]
- 74. De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574–591. [DOI] [PubMed] [Google Scholar]
- 75. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. [DOI] [PubMed] [Google Scholar]
- 76. Meisel R, Zibert A, Laryea M, Göbel M, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. [DOI] [PubMed] [Google Scholar]
- 77. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. [DOI] [PubMed] [Google Scholar]
- 78. Ding DC, Chou HL, Chang YH, Hung WT, Liu HW, Chu TY. Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stromaderived stem cells. Cell Transplant. 2016;25(2):217–228. [DOI] [PubMed] [Google Scholar]
- 79. Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35(4):1133–1142. [DOI] [PubMed] [Google Scholar]
- 80. Kim JH, Jo CH, Kim HR, Hwang Y. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int. 2018;2018:8429042, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sheshgiri R, Rouas-Freiss N, Rao V, Butany J, Ramzy D, Krawice-Radanne I, Ross HJ, Carosella ED, Delgado DH. Myocardial HLA-G reliably indicates a low risk of acute cellular rejection in heart transplant recipients. J Heart Lung Transplant. 2008;27(5):522–527. [DOI] [PubMed] [Google Scholar]
- 82. Lanza F, Campioni D, Mauro E, Pasini A, Rizzo R. Immunosuppressive properties of mesenchymal stromal cells. Adv Stem Cell Res Chapt. 12. Ed: The Springer 2012;12:281–301. [Google Scholar]
- 83. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Mult Scler. 2006;107(1):367–372. [DOI] [PubMed] [Google Scholar]
- 84. Kovacsovics-Bankowski M, Streeter PR, Mauch KA, Frey MR, Raber A, van t Hof W, Deans R, Maziarz RT. Clinical scale expanded adult pluripotent stem cells prevent graft-versus-host disease. Cell Immunol. 2009;255(1–2):55–60. [DOI] [PubMed] [Google Scholar]
- 85. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Rachmilewitz J, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2010;105(5):2214–2219. [DOI] [PubMed] [Google Scholar]
- 86. Ramasamy R, Tong CK, Seow HF, Vidyadaran S, Dazzi F. The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol. 2008;251(2):131–136. [DOI] [PubMed] [Google Scholar]
- 87. Dazzi F, Marelli-Berg FM. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur J Immunol. 2008;38(6):1479–1482. [DOI] [PubMed] [Google Scholar]
- 88. Tse W, Pendleton J, Beyer W, Egalka M, Guinan E. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;172(5):389–397. [DOI] [PubMed] [Google Scholar]
- 89. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (safecell): a systematic review and meta-analysis of clinical trials. Plos One. 2012;7(10):e47559, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects pf mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2011;67(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim JS, Lee JH, Kwon O, Cho JH, Choi JY, Park SH, Kim CD, Kim YJ, Kim YL. Rapid deterioration of preexisting renal insufficiency after autologous mesenchymal stem cell therapy. Kidney Res Clin Pract. 2017;36(2):200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, Wald R, Verma S, Mazer CD; ACT-AKI investigators. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2017;29(1):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tatsumi K, Ohashi K, Matsubara Y, Kohori A, Ohno T, Kakidachi H, Horii A, Kanegae K, Utoh R, Iwata T, Okano T. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431(2):203–209. [DOI] [PubMed] [Google Scholar]
- 94. Wu Z, Zhang S, Zhou L, Cai J, Tan J, Gao X, Zeng Z, Li D. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. Transplant Proc. 2017;49(7):1656–1658. [DOI] [PubMed] [Google Scholar]
- 95. Asif S, Ekdahl KN, Fromell K, Gustafson E, Barbu A, Le Blanc K, Nilsson B, Teramura Y. Heparinization of cell surfaces with short peptide-conjugated PEG-lipid regulates thromboinflammation in transplantation of human MSCs and hepatocytes. Acta Biomater. 2016;35:194–205. [DOI] [PubMed] [Google Scholar]
- 96. Fiedler T, Rabe M, Mundkowski RG, Oehmcke-Hecht S, Peters K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int J Biochem Cell Biol. 2018;100:49–53. [DOI] [PubMed] [Google Scholar]
- 97. Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004;6(4):369–374. [DOI] [PubMed] [Google Scholar]
- 98. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. [DOI] [PubMed] [Google Scholar]
- 100. Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371–379. [DOI] [PubMed] [Google Scholar]
- 101. Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren Y, Zhu L. Monitoring the biology stability of human umbilical cord-derived mesenchymal stem cells during long-term culture in serum-free medium. Cell Tissue Bank. 2014;15(4):513–521. [DOI] [PubMed] [Google Scholar]
- 102. Bellagamba BC, Abreu BR, Grivicich I, Markarian CF, Chem E, Camassola M, Nardi NB, Dihl RR. Human mesenchymal stem cells are resistant to cytotoxic and genotoxic effects of cisplatin in vitro. Genet Mol Biol. 2016;39(1):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. Plos One. 2017;12(4):e0175449: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hatzistergos KE, Blum A, Ince T, Grichnik J, Hare JM. What is the oncologic risk of stem cell treatment for heart disease? Circ. Res. 2012;108(11):1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, Büscher D, Fibbe W, Foussat A, Kwa M, Lantz O, Mačiulaitis R, Palomäki T, Schneider CK, Sensebé L, Tachdjian G, Tarte K, Tosca L, Salmikangas P. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15(7):753–759. [DOI] [PubMed] [Google Scholar]
- 106. Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9(29):1479–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17(7–8):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. [DOI] [PubMed] [Google Scholar]
- 109. Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127(10):2323–2333. [DOI] [PubMed] [Google Scholar]
- 110. Mathew E, Brannon AL, Del Vecchio AC, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, di Magliano MP. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia. 2016;18(3):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Komarova S, Roth J, Alvarez R, Curiel DT, Pereboeva L. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. J Ovarian Res. 2010;3(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G, Gespach C, Bracke M, de Wever O. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut. 2013;62(4):550–560. [DOI] [PubMed] [Google Scholar]
- 113. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. Plos One. 2009;4(4): e4992: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumors promotes metastasis. Nat Commun. 2013;4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Quante M, Tu SP, Tomita H, Gonda T, Sophie SW, Takashi S, Baik GH, Shibata W, Diprete B, Kelly S, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the growth MSC niche and promote tumour growth. Cancer Cell. 2012;19(2):257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2–3):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29(2):249–261. [DOI] [PubMed] [Google Scholar]
- 118. Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–8495. [DOI] [PubMed] [Google Scholar]
- 119. Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan Z, Roberts AI, Zhang L, Zheng B, Wen T, Rabson AB, Tischfield JA, Shao C, Shi Y. CCR2-dependent recruitment of macrophages by tumor educated mesenchymal stromal cells promotes tumor development and is mimicked by TNF-alpha. Cell Stem Cell. 2013;11(6):812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ramasamy R, Lam EWF, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304–310. [DOI] [PubMed] [Google Scholar]
- 121. Clarke MR, Imhoff FM, Baird SK. Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol Carcinog. 2015;54(10):1214–1219. [DOI] [PubMed] [Google Scholar]
- 122. Bruno S, Collino F, Iavello A, Camussi G. Effects of mesenchymal stromal cell-derived extracellular vesicles on tumor growth. Front Immunol. 2014;5:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wu S, Ju GQ, Du T, Zhu YJ, Liu GH. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. Plos One. 2013;8(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ertaş G, Ural E, Ural D, Aksoy A, Kozdağ G, Gacar G, Karaöz E. Comparative analysis of apoptotic resistance of mesenchymal stem cells isolated from human bone marrow and adipose tissue. Sci World J. 2012;2012:105698:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. [DOI] [PubMed] [Google Scholar]
- 126. Von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–1578. [DOI] [PubMed] [Google Scholar]
- 127. Mäkelä T, Takalo R, Arvola O, Haapanen H, Yannopoulos F, Blanco R, Ahvenjrvi L, Kiviluoma K, Kerkel E, Nystedt J, Juvonen T, Lehenkari P. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392–402. [DOI] [PubMed] [Google Scholar]
- 128. Kim I, Bang SI, Lee SK, Park SY, Kim M, Ha H. Clinical implication of allogenic implantation of adipogenic differentiated adipose-derived stem cells. Stem Cells Transl Med. 2014;3(11):1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Huang XP, Sun Z, Miyagi Y, Kinkaid HM, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122(23):2419–2429. [DOI] [PubMed] [Google Scholar]
- 130. Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111(1):430–439. [DOI] [PubMed] [Google Scholar]
- 131. von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhäuser M. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43(3):245–251. [DOI] [PubMed] [Google Scholar]