Abstract
Base editors are tools that chemically modify the nucleobases of DNA and RNA in a programmable manner, allowing for genome, epigenome, and transcriptome editing in live cells. These tools can be used to introduce specific base transitions in DNA or RNA, manipulate methylation patterns in the epigenome, and create genetically encoded libraries in target genes. These various functions can be used to modulate every aspect of the central dogma. The efficiency and precision of base editors makes them useful in both basic research and in the development of new therapies. Here we describe currently available base editors and the ways they can be used to better understand and manipulate different aspects of the central dogma.
Graphical Abstract
Introduction
The development of tools that directly chemically modify the nucleobases of DNA and RNA (via enzymatic methylation, deamination, or demethylation) in living cells has opened the door for studying and manipulating the components of the central dogma. These tools have been enabled by an increasing understanding of CRISPR systems and their ability to recognize and bind to specific dsDNA (for Cas9 and Cas12 enzymes)1 and ssRNA (for Cas13 enzymes)2 sequences inside living cells. In these systems, a guide RNA (gRNA) is bound by a Cas enzyme to form a ribonucleoprotein (RNP) complex. The RNP recognizes a target locus (the protospacer) through base pairing between the gRNA and the target nucleic acid sequence. When the target is DNA, this process separates the two DNA strands, creating a DNA-RNA heteroduplex with a small single-stranded DNA (ssDNA) region at the target locus (known as an R-loop).3 Once bound to its target dsDNA sequence, the Cas enzyme cleaves both phosphodiester backbones of the target nucleic acid using either a single (in the case of Cas12 enzymes)4–6 or two distinct (in the case of Cas9)1 endonuclease domains. If the target is ssRNA, the Cas13 enzyme becomes activated upon target binding, and cleaves the phosphodiester backbone of both the target sequence and any neighboring ssRNA molecules in a promiscuous manner.7–9 This RNA-guided endonuclease activity has been utilized extensively for genome editing and RNA degradation purposes.10
The catalytic activity of Cas enzymes can be inactivated by introducing specific mutations into the endonuclease domains to create catalytically dead Cas variants (dCas) that use a gRNA to bind a target DNA or RNA sequence without cleaving the phosphodiester backbone.11 A diverse set of genome, epigenome, and transcriptome editing tools (collectively referred to here as base editors) have been developed from these dCas proteins by physically attaching them to various nucleobase modifying enzymes.12 In this way, the chemical activity of these enzymes can be confined to specific genomic and transcriptomic loci where they chemically modify a canonical nucleobase into either a noncanonical DNA base or a naturally occurring modified base. As such, different base editing tools allow researchers to modify and study the effects of nucleic acid primary sequences, chromatin organization, RNA activity and stability, and DNA damage. In this perspective, we discuss the various base editor tools available for directly chemically modifying target nucleobases in the genome and transcriptome, and their potential for manipulating the composition and expression of target genes.
Cytosine deamination
Base editing tools were first created by fusing cytidine deaminase enzymes to dCas9.13,14 These deaminases recognize cytosine in a ssDNA context and deaminate the nucleobase to form uracil, which has the base pairing properties of thymine (Figure 1). Due to the substrate requirement of ssDNA, the deaminase activity of the tethered enzyme is confined to only the single-stranded portion of the dCas9:gRNA:DNA R-loop. This strategy results in an editing “window” of only five to nine nucleotides (depending on the Cas9 variant used) within the protospacer.13,15,16
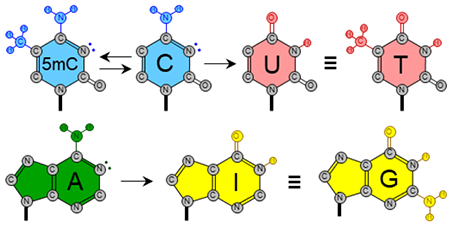
Figure 1.

Nucleobase chemistries facilitated by current base editors. Cytosines can be methylated to 5-methylcytosines by DNMT3A-derived base editors. Likewise, 5-methylcytosines can be demethylated by TET1-containing base editors. These two types of tools can be used to control epigenetic patterns. Cytosine and adenine can be deaminated to uracil and inosine, respectively. These chemical transformations are catalyzed by base editors comprised of APOBEC or AID in the case of uracil, and TadA or ADAR in the case of inosine in DNA or RNA, respectively. Uracil is further converted to thymine and inosine is further converted to guanine by cellular replication or repair processes due to the new hydrogen-bonding properties of these base editing intermediates. Uracil is efficiently excised by the base excision repair protein UDG to form an abasic site, which is mutagenized to all four canonical DNA bases (dashed lines). The details of this process are still relatively unknown and the outcome is largely uncontrollable and unpredictable.
Several tools have since been created that link a cytidine deaminase to a DNA targeting enzyme such as dCas913,14,17,18 or dCas12a.15 Though each tool may have slight variations, they each use the Cas protein to bring the cytidine deaminase to a target locus and convert cytosines to uracils. The final outcome following uracil incorporation, however, depends on the tool being used. Mutagenesis tools such as Targeted AID-mediated mutagenesis (TAM)19 and CRISPR-X18 rely on the cell’s efficient excision of uracil from genomic DNA by the base excision repair pathway (BER). Excision of uracil by uracil-DNA glycosylase (UDG) creates an abasic site which can be permanently converted to any of the four canonical DNA bases through an as-yet-unknown mechanism that potentially involves error-prone DNA polymerases (Figure 1).20
A particularly intriguing feature of these mutagenesis tools is their ability to generate genetically-encoded libraries. Within living cells, a library of gRNAs can be used to target these tools across an entire gene to introduce mutations. When combined with an appropriate selection or screen, these libraries can be used to identify mutations that give rise to specific phenotypes. Both TAM and CRISPR-X have been used to identify protein variants that give rise to chemotherapeutic resistance.18,19 The ability to rapidly identify mutations that cause drug resistance (or another specific phenotype of interest) in a cellular context is a powerful tool that holds great promise in the areas of personalized medicine and reverse genetics.
Cytidine deaminase-derived base editing tools such as BE1-413,21 and Target-AID14 can also be used to create predictable C•G to C•A mutations by manipulating cellular DNA repair pathways. Specifically, the bacteriophage protein uracil glycosylase inhibitor (UGI) can be physically linked22 to or co-expressed23 with the base editor. UGI protects the uracil intermediate from excision by reversibly binding to UDG24 and significantly decreases C•G to non-T•A mutation rates.21 Alternatively, the uracil intermediate can be avoided entirely by directly deaminating a methylated cytosine, which results in a direct conversion to thymine.25 To manipulate DNA repair pathways, the catalytic activity of a single endonuclease domain in dCas9 can be restored to create a Cas9 nickase (Cas9n).26 During base editing, Cas9n creates a nick in the DNA strand opposite the uracil. This nick marks the strand as “newly synthesized”, resulting in the mismatch repair pathway (MMR) repairing the U•G mismatch using the uracil-containing strand as a template.27 By manipulating the MMR pathway in this way, the guanine of the original C•G base pair is replaced with an adenine, solidifying the desired edit before uracil can be excised. These features can be combined (as in BE3, BE4, and Target-AID) to maximize C•G to T•A base editing efficiency.
The ability to precisely introduce single or multiple uracil lesions at a pre-defined location of the genome of living cells makes base editors valuable tools for both therapeutic and research purposes. Beyond using cytidine deaminase base editors to correct disease-causing point mutations and create genetically-encoded libraries, these tools have also unearthed surprising new properties of uracil repair. For example, it was discovered that single G•U lesions are repaired through a different, more error-prone mechanism than multiple clustered G•U lesions.21 Though the mechanism of this differential processing is still not fully understood, its elucidation will likely be aided by using cytidine deaminase base editors as precision DNA damaging tools. Likewise, base editors capable of installing other types of DNA damage will help further our understanding of other mechanisms of DNA repair.
Cytosine methylation and 5-methylcytosine oxidative demethylation
Methylation of cytosines at the 5 position results in 5-methylcytosine (5mC), a naturally occurring modified DNA base (Figure 1). The presence of 5mC in promoter regions (where it occurs mostly at CpG sites) has a significant influence on gene expression at the transcriptional level. As a general rule, hypermethylation of these regions results in gene silencing.28,29 The natural methylation of cytosines and demethylation of 5mC’s are facilitated by DNA methyltransferase (DNMT) and ten-eleven translocation methylcytosine dioxygenase (TET) enzymes, respectively (Figure 1). Modulation of 5mC levels is particularly essential during development as shifting methylation levels control cell differentiation.30 Inappropriate silencing due to hypermethylation can have devastating effects, as observed in several cancers and fragile X syndrome.31 Therefore, tools that manipulate methylation levels at target regions are greatly beneficial for studying and developing treatments for a variety of diseases.
Two types of base editors have been created that allow researchers to modulate 5mC levels in target regions of the genome.32–39 A DNMT3A-dCas9 fusion protein was developed as a tool to increase methylation levels. DNMT3A recognizes cytosines in dsDNA and uses S-Adenosyl methionine (SAM) as a methyl donor to create 5mC (Figure 1). To create a tool that decreases methylation levels, the enzymatic domain of TET1 was fused to dCas9. TET1 recognizes 5mC’s and uses α-ketoglutarate to oxidize the methyl group to form 5-hydroxymethylcytosine as the first step of cytosine demethylation. 5-hydroxymethylcytosine is then further oxidized and subsequently excised by thymine DNA glycosylase to ultimately yield unmethylated cytosine (Figure 1).40 As the natural substrates of these enzymes are dsDNA, the activity of these epigenome editors is not constrained within the ssDNA region of the R-loop. This allows the enzymes to access and modify a large window of nucleobases surrounding the binding site of the editor. This window is further widened due to the tight coiling of chromatin, which can allow the enzyme to access genomic DNA that is potentially thousands of base pairs away from its target site, but spatially very close.
These epigenome editors are quite valuable for studying the effects of methylation in untranslated regions (UTR) of the genome. For example, they have been instrumental in understanding methylation effects in fragile X syndrome.41 This genetic disease is caused by a CGG trinucleotide repeat expansion in the 5’ UTR of the FMR1 gene, which codes for fragile X mental retardation protein (FMRP).42 Hypermethylation of this UTR leads to silencing of FMR1 and loss of FMRP expression. By targeting the dCas9-TET1 editor to this region, demethylation was observed and FMRP expression was recovered, leading to alleviation of the phenotype in post-mitotic neurons.
Adenine deamination
Cytosine is unique among the DNA bases as it is modified by several naturally occurring enzymes. While this facilitated the creation of the first base editors, the development of base editors that modify DNA bases other than cytosine is complicated by the lack of natural enzymes that can be repurposed to perform this chemistry. Fortunately, RNA nucleobases are extensively post-transcriptionally modified by natural enzymes.43 By evolving one of these RNA-modifying enzymes to accept ssDNA as a substrate, an adenine base editor (ABE) that deaminates target adenines to inosines was engineered.17 Adenosine deamination substitutes the amino group for a keto group and alters the base pairing properties of the nucleobase to match those of guanine.44 As such, ABE catalyzes an overall A•C to G•C edit in genomic DNA at a locus programmed by the gRNA (Figure 1).
Intriguingly, A•C to G•C editing with ABE exhibits far lower rates of random mutagenesis than uracil-derived base editors, suggesting significant differences in cellular excision efficiencies of inosine (by the DNA-3-methyladenine glycosylase enzyme, AAG, and homologs)45,46 as compared to uracil. As spontaneous adenosine deamination is 50-times slower than cytosine deamination,47 the cellular repair machinery for uracil must be more efficient than that of inosine to preserve the integrity of the genome. Consequently, ABE-installed inosines are excised less efficiently than uracils, resulting in a more consistent mutagenic outcome for ABE. This highlights another interesting feature of DNA repair discovered through the use of base editors, and suggests that less common types of DNA damage can be used to create genome editing tools with more predictable outcomes.
While making modifications to DNA nucleobases can create a permanent mutation or modification in a cell, transiently mutating or modifying the transcriptome may be desirable in certain situations. RNA Base editing can be achieved using SNAP-ADAR,48 λN-ADAR,49 and RNA Editing for Programmable A to I Replacement (REPAIR).50 All three of these technologies rely on a fusion complex of the adenosine deaminase acting on RNA 2 (ADAR2) enzyme with a gRNA molecule (SNAP-ADAR and λN-ADAR) or the dCas13b, which uses a gRNA to bind to target RNA sequences. The gRNA is designed to base pair with a target mRNA sequence and form a C•A mismatch (with the adenosine on the mRNA strand). ADAR2 deaminates the mismatched adenosine to inosine more efficiently51 than any well-matched adenosines in the protospacer, providing single nucleotide resolution. The resulting inosine exhibits the base pairing properties of guanine during translation, and thus RNA base editing can be used for the transient expression of mutant proteins.
Along with transient base editing, SNAP-ADAR, λN-ADAR, and REPAIR open up the opportunity for better understanding the roles of RNA modifications. RNA modifications occur across all domains of life and affect the activity, localization, and stability of RNA. Over 100 types of modifications have been identified, and they have been found to exist in all types of RNAs.43 Tools that allow researchers to install these modifications throughout the transcriptome will significantly aid in illuminating their functions and mechanisms.
Conclusions and Future Outlook
Most importantly, base editing has been shown to work in a variety of in vivo contexts. Successful C•G to T•A base editing has been accomplished in plants, including rice, wheat, maize, and tomato,52–54 as well as a variety of animals including silkworms,55 zebrafish,56,57 mice,56,58 and human embryos. ABE has been used in rats,59 mice,60 rice,61–63 and wheat.61 Furthermore, base editing has been performed in post-mitotic sensory cells, showing that this technology does not require cellular replication.64 These experiments show that base editing is compatible with a large variety of cell types and organisms, making it valuable in many different areas of research.
ABE and cytosine deamination base editors can be used to study protein mutants and disease-associated intronic and non-coding mutations in an endogenous manner. Current strategies to study protein mutants involve knocking-out or silencing the corresponding gene, followed by overexpressing a variant to observe phenotypic effects. This method often results in protein expression levels that are significantly different from the endogenous system. Additionally, this strategy is difficult to apply to the study of point mutations that occur in intronic and non-coding regions of the genome. While using wtCas9 and a donor template can be used to edit endogenous genomic loci,65 base editors can introduce point mutations with higher efficiency and fewer genome editing byproducts than DSB-reliant methods. This allows for the more rapid study of multiple variants in parallel, and presents the potential to efficiently introduce mutations at different sites throughout the genome in a given cell. Furthermore, base editors can be used to knock out genes of interest by introducing early stop codons via a method termed CRISPR-STOP.66 This method has advantages over traditional, DSB-reliant methods for gene knock out, including less cytotoxic genome editing intermediates and more predictable genome editing outcomes. Finally, base editors can be used to mutate conserved splice acceptor sites within introns, in effect facilitating exon skipping, in a method called CRISPR-SKIP.67 This method has the potential to modulate expression of different protein isoforms and skip mutation-containing exons such as in Duchenne muscular dystrophy and Huntingon’s disease.68
Base editors provide a potentially less cytotoxic and more efficient method of point mutation introduction than DSB-reliant methods. Nevertheless, this technology faces several limitations as it continues to grow. For example, the current deaminating base editors are only able to facilitate C•G to T•A and A•T to G•C base pair conversions. To be more universally applicable, new base editors must be developed that catalyze additional point mutation changes. Effective intermediates for future base editors will likely be found in less common naturally occurring DNA modifications, as demonstrated by the more predicable editing outcomes observed with base editing using inosine intermediates compared to uracil.17 Off-target effects are a large concern with genome editing agents in general, and indeed off-target editing is observed with current base editors.69 The majority of off-target base editing sites overlap with those of wild-type Cas9. Consequently, base editors derived from Cas9 variants with increased specificity have been shown to alleviate unwanted editing at these sites.56 However, off-target sites unique to BE3 have been identified, indicating that creative new solutions are needed in the future to increase base editing specificity. The base editors described here allow for the direct chemical modification of target nucleobases in a programmable manner.
These tools hold tremendous potential for regulating the central dogma. Cytosine and adenosine deamination DNA base editors facilitate the efficient installation of point mutations throughout the genome, allowing researchers to alter the identity or expression levels of proteins and functional RNAs. Cytosine methylation and demethylation DNA base editors allow researchers to modulate gene transcription levels. Adenosine deamination RNA base editors open the door for the targeted modification of RNAs which can ultimately modulate all aspects of transcription and translation. As base editing technologies continue to develop, so will our ability to manipulate the contents of the cell.
Acknowledgements
B.L.R. is supported by the Chemistry-Biology Interface (CBI) Training Program (NIGMS, 5T32GM112584).
Footnotes
Competing Financial Interests
A.C.K. is a consultant of Pairwise Plants and Beam Therapeutics, companies that are developing and utilizing base editing technologies.
References
- (1).Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E (2012) A Programmable Dual-RNA – Guided DNA Endocnuclease in Adaptive Bacterial Immunity. Science 337, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DBT, Kellner MJ, Regev A, Lander ES, Voytas DF, Ting AY, and Zhang F (2017) RNA targeting with CRISPR-Cas13. Nature 550, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K, Thompson AJ, Nogales E, and Doudna JA (2016) Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science 351, 867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Swarts DC, van der Oost J, and Jinek M (2017) Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol. Cell 66, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, Van Der Oost J, Regev A, Koonin EV, and Zhang F (2015) Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 163, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K, Zhang F, and Koonin EV (2015) Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 60, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DBT, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, and Zhang F (2016) C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, and Hsu PD (2018) Transcriptome Engineering with RNA-Targeting Type Vl-D CRISPR Effectors. Cell 173, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Yan WX, Chong S, Zhang H, Makarova KS, Koonin EV, Cheng DR, and Scott DA (2018) Cas13d Is a Compact RNA-Targeting Type VI CRISPR Effector Positively Modulated by a WYL-Domain-Containing Accessory Protein. Mol. Cell 70, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Adli M (2018) The CRISPR tool kit for genome editing and beyond. Nat. Commun 9, 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, and Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hess GT, Tycko J, Yao D, and Bassik MC (2017) Methods and Applications of CRISPR-Mediated Base Editing in Eukaryotic Genomes. Mol. Cell 68, 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Komor AC, Kim YB, Packer MS, Zuris JA, and Liu DR (2016) Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, and Kondo A (2016) Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729.. [DOI] [PubMed] [Google Scholar]
- (15).Li X, Wang Y, Liu Y, Yang B, Wang X, Wei J, Lu Z, Zhang Y, Wu J, Huang X, Yang L, and Chen J (2018) Base editing with a Cpf1-cytidine deaminase fusion. Nat. Biotechnol 36, 324–327. [DOI] [PubMed] [Google Scholar]
- (16).Kim YB, Komor AC, Levy JM, Packer MS, Zhao KT, and Liu DR (2017) Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol 35, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, and Liu DR (2017) Programmable base editing of A•C to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hess GT, Fresard L, Han K, Lee CH, Li A, Cimprich KA, Montgomery SB, and Bassik MC (2016) Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells. Nat. Methods 13, 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ma Y, Zhang J, Yin W, Zhang Z, Song Y, and Chang X (2016) Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods 13, 1029–1035. [DOI] [PubMed] [Google Scholar]
- (20).Odegard VH, and Schatz DG (2006) Targeting of somatic hypermutation. Nat. Rev. Immunol 6, 573–583. [DOI] [PubMed] [Google Scholar]
- (21).Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, Kim YB, Badran AH, and Liu DR (2017) Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv 3, eaao4774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jiang W, Feng S, Huang S, Yu W, Li G, Yang G, Liu Y, Zhang Y, Zhang L, Hou Y, Chen J, Chen J, and Huang X (2018) BE-PLUS: a new base editing tool with broadened editing window and enhanced fidelity. Cell Res. 28, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang L, Xue W, Yan L, Li X, Wei J, Chen M, Wu J, Yang B, Yang L, and Chen J (2017) Enhanced base editing by co-expression of free uracil DNA glycosylase inhibitor. Cell Res. 21, 1289–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bennett SE, and Mosbaughs DW (1992) Characterization of the Escherichia coli Uracil-DNA Glycosylase Inhibitor Protein Complex. J. Biol. Chem 267, 22512–22521. [PubMed] [Google Scholar]
- (25).Wang X, Li J, Wang Y, Yang B, Wei J, Wu J, Wang R, Huang X, Chen J, and Yang L (2018) Efficient base editing in methylated regions with a human APOBEC3A-Cas9 fusion. Nat. Biotechnol 36, 946–949. [DOI] [PubMed] [Google Scholar]
- (26).Gasiunas G, Barrangou R, Horvath P, and Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci 109, E2579–E2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, and Modrich P (2010) PCNA function in the activation and strand direction of MutL endonuclease in mismatch repair. Proc. Natl. Acad. Sci 107, 16066–16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jones PA, and Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat. Rev. Genet 3, 415–28. [DOI] [PubMed] [Google Scholar]
- (29).Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21. [DOI] [PubMed] [Google Scholar]
- (30).Jaenisch R, and Bird A (2003) Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet 33, 245–254. [DOI] [PubMed] [Google Scholar]
- (31).Robertson KD (2005) DNA methylation and human disease. Nat. Rev. Genet 6, 597–610. [DOI] [PubMed] [Google Scholar]
- (32).Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, and Jaenisch R (2016) Editing DNA Methylation in the Mammalian Genome. Cell 167, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Vojta A, Dobrinic P, Tadic V, Bockor L, Korac P, Julg B, Klasic M, and Zoldos V (2016) Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 44, 5615–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Xu X, Tao Y, Gao X, Zhang L, Li X, Zou W, Ruan K, Wang F, Xu G, and Hu R (2016) A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Huang Y-H, Su J, Lei Y, Brunetti L, Gundry MC, Zhang X, Jeong M, Li W, and Goodell MA (2017) DNA epigenome editing using CRISPR-Cas SunTag-directed DNMT3A. Genome Biol. 18, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pflueger C, Tan D, Swain T, Nguyen T, Pflueger J, Nefzger C, Polo JM, Ford E, and Lister R (2018) A modular dCas9-SunTag DNMT3A epigenome editing system overcomes pervasive off-target activity of direct fusion dCas9-DNMT3A constructs. Genome Res. 28, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Stepper P, Kungulovski G, Jurkowska RZ, Chandra T, Krueger F, Reinhardt R, Reik W, Jeltsch A, and Jurkowski TP (2017) Efficient targeted DNA methylation with chimeric dCas9-Dnmt3a-Dnmt3L methyltransferase. Nucleic Acids Res. 45, 1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).McDonald JI, Celik H, Rois LE, Fishberger G, Fowler T, Rees R, Kramer A, Martens A, Edwards JR, and Challen GA (2016) Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol. Open 5, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Xiong T, Meister GE, Workman RE, Kato NC, Spellberg MJ, Turker F, Timp W, Ostermeier M, and Novina CD (2017) Targeted DNA methylation in human cells using engineered dCas9-methyltransferases. Sci. Rep 1, 6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, Abramowitz LK, Bartolomei MS, Rambow F, Bassi MR, Bruno T, Fanciulli M, Renner C, Klein-Szanto AJ, Matsumoto Y, Kobi D, Davidson I, Alberti C, Larue L, and Bellacosa A (2011) Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 146, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Liu XS, Wu H, Krzisch M, Wu X, Graef J, Muffat J, Hnisz D, Li CH, Yuan B, Xu C, Li Y, Vershkov D, Cacace A, Young RA, and Jaenisch R (2018) Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 172, 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Avitzour M, Mor-Shaked H, Yanovsky-Dagan S, Aharoni S, Altarescu G, Renbaum P, Eldar-Geva T, Schonberger O, Levy-Lahad E, Epsztejn-Litman S, and Eiges R (2014) FMR1 epigenetic silencing commonly occurs in undifferentiated fragile X-affected embryonic stem cells. Stem Cell Reports 3, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Roundtree IA, Evans ME, Pan T, and He C (2017) Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Yasui M, Suenaga E, Koyama N, Masutani C, Hanaoka F, Gruz P, Shibutani S, Nohmi T, Hayashi M, and Honma M (2008) Miscoding Properties of 2’-Deoxyinosine, a Nitric Oxide-Derived DNA Adduct, during Translesion Synthesis Catalyzed by Human DNA Polymerases. J. Mol. Biol 371, 1015–1023. [DOI] [PubMed] [Google Scholar]
- (45).Saparbaev M, and Laval J (1994) Excision of hypoxanthine from DNA containing dIMP residues by the Escherichia coli, yeast, rat, and human alkylpurine DNA glycosylases. Proc. Natl. Acad. Sci 91, 5873–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Alseth I, Dalhus B, and Bjoras M (2014) Inosine in DNA and RNA. Curr. Opin. Genet. Dev 26, 116–123. [DOI] [PubMed] [Google Scholar]
- (47).Karran P, and Lindahl T (1980) Hypoxanthine in Deoxyribonucleic Acid: Generation by Heat-Induced Hydrolysis of Adenine Residues and Release in Free Form by a Deoxyribonucleic Acid Glycosylase from Calf Thymus. Biochemistry 19, 6005–6011. [DOI] [PubMed] [Google Scholar]
- (48).Stafforst T, and Schneider MF (2012) An RNA-deaminase conjugate selectively repairs point mutations. Angew. Chemie - Int. Ed 51, 11166–11169. [DOI] [PubMed] [Google Scholar]
- (49).Montiel-Gonzalez MF, Vallecillo-Viejo I, Yudowski GA, and Rosenthal JJC (2013) Correction of mutations within the cystic fibrosis transmembrane conductance regulator by site-directed RNA editing. Proc. Natl. Acad. Sci 110, 18285–18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, and Zhang F (2017) RNA editing with CRISPR-Cas13. Science 358, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wong SK, Sato S, Lazinski DW, Wong SKEE, Sato S, and Lazinski DW (2001) Substrate recognition by ADAR1 and ADAR2 . RNA 1, 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Ciu JL, Wang D, and Gao C (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol 35, 438–440. [DOI] [PubMed] [Google Scholar]
- (53).Li J, Sun Y, Du J, Zhao Y, and Xia L (2017) Generation of Targeted Point Mutations in Rice by a Modified CRISPR/Cas9 System. Mol. Plant 10, 526–529. [DOI] [PubMed] [Google Scholar]
- (54).Shimatani Z, Kashojiya S, Takayama M, Terada R, Arazoe T, Ishii H, Teramura H, Yamamoto T, Komatsu H, Miura K, Ezura H, Nishida K, Ariizumi T, and Kondo A (2017) Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol 35, 441–443. [DOI] [PubMed] [Google Scholar]
- (55).Li Y, Ma S, Sun L, Zhang T, Chang J, Lu W, Chen X, Liu Y, Wang X, Shi R, Zhao P, and Xia Q (2018) Programmable Single and Multiplex Base-Editing in Bombyx mori Using RNA-Guided Cytidine Deaminases. G3 8, 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Rees HA, Komor AC, Yeh W-H, Caetano-Lopes J, Warman M, Edge ASB, and Liu DR (2017) Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun 8, 15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Zhang Y, Qin W, Lu X, Xu J, Huang H, Bai H, Li S, and Lin S (2017) Programmable base editing of zebrafish genome using a modified CRISPR-Cas9 system. Nat. Commun 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Kim K, Ryu SM, Kim ST, Baek G, Kim D, Lim K, Chung E, Kim S, and Kim JS (2017) Highly efficient RNA-guided base editing in mouse embryos. Nat. Biotechnol 35, 435–437. [DOI] [PubMed] [Google Scholar]
- (59).Ma Y, Yu L, Zhang X, Xin C, Huang S, Bai L, Chen W, Gao R, Li J, Pan S, Qi X, Huang X, and Zhang L (2018) Highly efficient and precise base editing by engineered dCas9-guide tRNA adenosine deaminase in rats. Cell Discov. 4, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ryu S-M, Koo T, Kim K, Lim K, Baek G, Kim S-T, Kim HS, Kim D, Lee H, Chung E, and Kim J-S (2018) Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol 36, 536–539. [DOI] [PubMed] [Google Scholar]
- (61).Li C, Zong Y, Wang Y, Jin S, Zhang D, Song Q, Zhang R, and Gao C (2018) Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 19, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yan F, Kuang Y, Ren B, Wang J, Zhang D, Lin H, Yang B, Zhou X, and Zhou H (2018) Highly Efficient A·T to G·C Base Editing by Cas9n-Guided tRNA Adenosine Deaminase in Rice. Mol. Plant 11, 631–634. [DOI] [PubMed] [Google Scholar]
- (63).Hua K, Tao X, Yuan F, Wang D, and Zhu JK (2018) Precise A·T to G·C Base Editing in the Rice Genome. Mol. Plant 11, 627–630. [DOI] [PubMed] [Google Scholar]
- (64).Yeh W-H, Chiang H, Rees HA, Edge ASB, and Liu DR (2018) In vivo base editing of post-mitotic sensory cells. Nat. Commun 9, 2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc 8, 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Kuscu C, Parlak M, Tufan T, Yang J, Szlachta K, Wei X, Mammadov R, and Adli M (2017) CRISPR-STOP: Gene silencing through base-editing-induced nonsense mutations. Nat. Methods 14, 710–712. [DOI] [PubMed] [Google Scholar]
- (67).Gapinske M, Luu A, Winter J, Woods WS, Kostan KA, Shiva N, Song JS, and Perez-Pinera P (2018) CRISPR-SKIP: Programmable gene splicing with single base editors. Genome Biol. 19, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Evers MM, Tran H-D, Zalachoras I, Meijer OC, den Dunnen JT, van Ommen G-JB, Aartsma-Rus A, and van Roon-Mom WMC (2014) Preventing Formation of Toxic N-Terminal Huntingtin Fragments Through Antisense Oligonucleotide-Mediated Protein Modification. Nucleic Acid Ther. 24, 4–12. [DOI] [PubMed] [Google Scholar]
- (69).Kim D, Lim K, Kim ST, Yoon SH, Kim K, Ryu SM, and Kim JS (2017) Genome-wide target specificities of CRISPR RNA-guided programmable deaminases. Nat. Biotechnol 35, 475–480. [DOI] [PubMed] [Google Scholar]


