Abstract
A new series of triarylpyrazole derivatives having different heterocycle terminal groups have been designed and synthesised. Compounds 1h–j and 1l exhibited the highest mean percentage inhibition against the 58 cancer cell lines at a concentration of 10 μM, and thus were next examined in 5-dose testing mode to detect their IC50 value. The four compounds showed stronger antiproliferative activities upon comparing their results with sorafenib as a reference compound. Among them, compounds 1j and 1l possessing N-ethylpiperazinyl and N-benzylpiperazinyl terminal moiety through ethylene linker showed the greatest values of mean percentage inhibition (97.72 and 107.18%, respectively) over the 58-cell line panel at 10 μM concentration. The IC50 values of compound 1j over several cancer cell lines were in submicromolar scale (0.26 ∼ 0.38 μM). Moreover, the compounds 1j and 1l showed highly inhibitory activities (99.17 and 97.92%) against V600E-B-RAF kinase.
Keywords: Antiproliferative activity, morpholine, substituted piperazine, triarylpyrazole, V600E-B-RAF
Introduction
Cancer is considered as one of the dangerous world health problems. Statistics according to World Health Organization (WHO) report shows that the global cancer burden is about 18.1 million new cases and 9.6 million deaths in 2018, and the number of deaths can increase to more than 13 million deaths in 20301. Despite the remarkable achievements in the diagnostic and therapeutic techniques nowadays, cancer could be assessed as the second leading cause of death globally, about 1 in 6 deaths is due to cancer after cardiovascular diseases2,3. Considering many reports and publications on the synthesis of anti-cancer agents, there is no medicine achieving 100% efficacy and potency. Therefore, there is still a continuous need for more drug inventions leading to more potent and efficient anti-cancer compounds with new structures or novel mechanism of action to overcome the adverse effects associated with present chemotherapeutics in cancer treatment, such as toxicity and drug resistance.
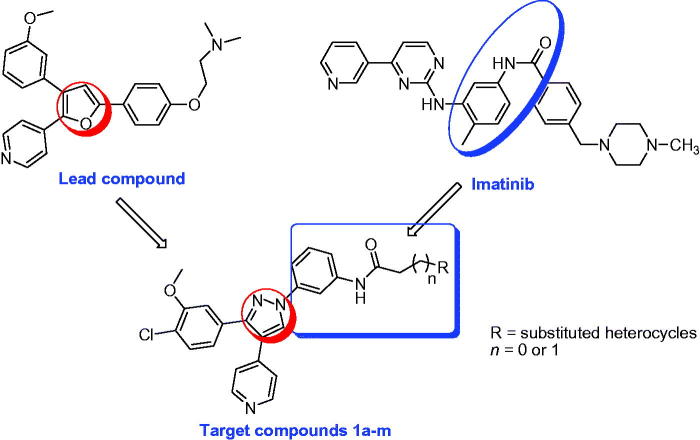
Metastatic melanoma is among the serious types of cancer that could lead to the limited survival time of less than one year4. Melanoma is associated with important pathway RAS–RAF-MEK-ERK5. One mutation that is most frequent in melanoma is mutated BRAF kinase (V600E-B-RAF)6. It is reported also that sorafenib (Nexavar®) targets ERK signal transduction pathway that was also involved in many cancer types including melanoma. Many research articles have nowadays outlined the potential antiproliferative activity against melanoma would be through inhibition of V600E-B-RAF which is so frequent in melanoma7. Imatinib (Gleevec®, Figure 1) as an example of anticancer agents having arylamides8–11 terminal moiety that is used for chronic myeloid leukaemia (CML) treatment with minimised adverse effects12. It has been reported in clinical trials for the management of different diseases like GIST, meningioma, ovarian cancer, non-small cell lung cancer (NSCLC), thyroid cancer, and breast cancer along with other drugs13.
Figure 1.
Structures of the lead compound, imatinib, and the target compounds 1a–m.
Furthermore, much consideration has been directed to the chemistry and biological activities of 1,3,4-triarylpyrazole derivatives. Diverse chemical entities having 1,3,4-triarylpyrazole nucleus have been lately turned up as potent antiproliferative agents14–18.
In the current article, we outline the synthesis of a new series of 1,3,4-triarylpyrazole nucleus bearing meta-amino group possessing heterocyclic terminal moieties linked by methylene or ethylene carbonyl spacer. In this study, we have replaced the furan ring of the lead compounds by its bioisosteric nucleus pyrazole, and we have tried the meta substitution for comparison of the para ones of the lead compounds. Our target compounds were examined against NCI-58 cancer cell line panel of nine different cancer type and their in vitro antiproliferative activities were reported. The most active compounds were examined against V600E-B-RAF and JNK kinases for detecting their possible molecular mechanism of action (Figure 1).
Experimental
General
The synthesised compounds were purified using silica gel chromatography of size (0.040–0.063 mm, 230–400 mesh) and solvents of technical grade. Bruker Avance 400 spectrometer utilising tetramethylsilane as an internal standard was used to record 1H NMR and 13C NMR spectra. Hypodermic syringes were used to transfer Solvents and liquid reagents. All the made compounds were examined by HPLC and found to have purity of >95%. All solvents and reagents were commercially available and used without further purification.
Compounds 3 till 8 were synthesised as reported in the literature15,16.
3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)benzenamine (8)
1H NMR (400 MHz, CDCl3) δ 8.52 (s, 2H), 8.06 (s, 2H), 8.32 (d, 1H, J= 8.0 Hz), 7.23–7.14 (m, 5H), 7.53–7.45 (m, 3H), 7.04 (brs, 2H), 6.98–6.95 (m, 1H), 6.44 (d, 1H, J= 4.8 Hz), 6.31 (d, 1H, J= 7.6 Hz), 4.03 (brs, 2H), 3.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 154.9, 149.9, 147.9, 140.3, 132.3, 130.3, 127.5, 122.8, 122.6, 121.5, 119.8, 113.8, 112.3, 108.7, 105.9, 56.8; MS m/z: 377.2 (M+).
2-Chloro-N-(3-(3-(4-chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-acetamide (9a) or 3-chloro-N-(3–(3-(4-chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)propanamide (9b)
To a cooled solution of compound 8 (0.5 g, 1.325 mmol) in anhydrous methylene chloride (15 ml) at −10 °C, triethylamine (148 mg, 1.4575 mmol) was added dropwise within 5 min then either chloroacetyl chloride or chloropropionyl chloride were added dropwise keeping the reaction mixture temperature at −10 °C for another 5 min .To the reaction mixture, water (15 ml) was added, then reaction mixture was swirled then the organic layer was separated. The water layer was further extracted with methylene chloride once again. The combined organic layers were combined, washed with brine and dried using anhydrous Na2SO4. Then evaporated under reduced pressure to give the target compounds that were used in the next step without further purification with yield 65%. Compound 9a: 1H NMR (400 MHz, DMSO-d6) δ 8.85 (d, 1H, J= 8.0 Hz), 8.08 (brs, 2H), 7.76–7.53 (m, 6H), 7.29–7.16 (m, 3H), 4.13 (s. 2H), 3.86 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 166.8, 159.5, 148.2, 141.2, 140.4, 139.8, 139.7, 135.1, 131.7, 129.2, 128.8, 127.7, 125.9, 123.1, 120.5, 116.4, 115.1, 111.2, 105.7, 55.6; MS m/z: 453.2 (M+ + 1). Compound 9b: 1H NMR (400 MHz, DMSO-d6) δ 8.85 (d, 1H, J= 8.0 Hz), 8.08 (brs, 2H), 7.76–7.53 (m, 6H), 7.29–7.16 (m, 3H), 4.13 (s. 2H), 3.86 (s, 3H); MS m/z: 466.5 (M+ + 1).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-2-(piperidin-1-yl)acetamide (1a)
To a solution of 9a (100 mg, 0.175 mmol) in anhydrous methylene chloride (5 ml), triethylamine (20 mg, 0.193 mmol) was added at 0 °C, stirred for 15 min, then morpholine (18.3 mg, 0.21 mmol) was added dropwise. The reaction mixture was stirred for 24 h at r.t. Upon completion of the reaction, the mixture was dried and then was partitioned between ethyl acetate and aqueous phase. Followed by separation of the organic layer and extraction of the aqueous layer with organic solvent (3 × 10 ml) was repeated. The collected organic layers were washed with NaCl solution (5%) and the organic solvent was evaporated under vacuum. The residue was purified using column chromatography (silica gel, hexane:ethyl acetate 4:1 v/v) to give the required product. 1H NMR (400 MHz, CDCl3) δ 8.48 (d, 2H, J= 4.8 Hz), 8.14(s, 1H), 7.45 (d, 1H, J= 4.8 Hz), 7.39 (d, 1H, J= 8 Hz), 7.38 (t, 1H, J= 8 Hz), 7.28 (d, 1H, J= 8 Hz), 7.19–7.18 (m, 2H), 7.07 (br s, 1H), 7.01 (dd, 1H, J= 2.0, 8.0 Hz), 3.74 (s, 3H), 3.22 (s, 2H), 2.65 (brs, 4H), 1.67 (t, 4H, J= 5.2 Hz),1.47 (brs, 2H); 13C NMR (100 MHz, CDCl3) δ 170.6, 154.9, 150.0, 139.6, 139.3, 138.9, 132.1, 130.1, 130.1, 127.6, 122.9, 122.8, 121.5, 120.3, 117.9, 114.9, 112.3, 110.2, 62.2, 56.3, 55.2, 25.7, 23.2; MS m/z: 502.43 (M+ + 1).
Compounds 1b–g were synthesised from 9a and appropriate amine derivatives as reported in the synthesis of 1a.
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-2-morpholino-acetamide (1b)
1H NMR (400 MHz, CDCl3) δ 9.33 (s, 1H), 8.56 (d, 2H, J= 4.8 Hz), 8.03(s, 1H), 7.57 (d, 1H, J= 7.6 Hz), 7.51–7.45 (m, 2H), 7.36 (d, 1H, J = 8.4 Hz), 7.29–7.27 (m, 2H), 7.14 (s, 1H), 7.09 (d, 1H, J = 8.0 Hz), 3.80 (m, 7H), 3.23 (s, 2H), 2.69–2.67 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 168.1, 154.6, 150.1, 149.7, 140.8, 139.9, 138.7, 132.2, 130.5, 130.2, 127.6, 122.9, 122.8, 122.2, 117.9, 114.9, 112.3, 110.2, 66.9, 62.4, 56.1, 50.8; MS m/z: 505.1 (M+ + 2).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-2–(4-methyl-piperazin-1-yl)acetamide (1c)
1H NMR (400 MHz, CDCl3) δ 9.28 (s, 1H), 8.58 (d, 2H, J = 8.4 Hz), 8.22 (d, 2H, J= 3.6 Hz), 7.59–7.56 (m, 2H), 7.46 (t, 1H, J= 8.0 Hz), 7.38 (d, 1H, J= 8.0 Hz), 7.29–7.27 (m, 2H), 7.15–7.08 (m, 2H), 3.83 (m, 3H), 3.31 (s, 2H), 2.94–2.90 (m, 8H), 2.63 (s, 3H); MS m/z: 517.42 (M+ + 1).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-2–(4-ethyl-piperazin-1-yl)acetamide (1d)
1H NMR (400 MHz, CDCl3) δ 9.29 (s, 1H), 8.58 (brs, 2H), 8.15 (d, 2H, J= 5.2 Hz), 7.59–7.56 (m, 2H), 7.47 (t, 1H, J = 8.0 Hz), 7.38 (d, 1H, J= 8.0 Hz), 7.16–7.08 (m, 4H), 3.83 (m, 3H), 3.27 (s, 2H), 2.83–2.50 (m, 10H), 1.25 (t, 3H, J= 7.6 Hz); 13C NMR (100 MHz, CDCl3) δ 169.0, 155.0, 150.1, 149.9, 139.9, 138.9, 132.1, 130.3, 130.0, 127.5, 122.3, 121.5, 120.3, 117.9, 114.8, 112.3, 110.2, 61.7, 56.1, 52.4, 52.2, 29.7; MS m/z: 531.0 (M+ + 1).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-2–(4-phenyl-piperazin-1-yl)acetamide (1e)
1H NMR (400 MHz, CDCl3) δ 8.48 (d, 2H, J = 4.8 Hz), 8.13 (brs, 1H), 7.63 (brs, 1H), 7.51–7.28 (m, 3H), 7.28–7.18 (m, 6H), 7.06 (s, 1H), 6.98 (d,1H, J = 8.0 Hz), 6.88–6.83 (m, 3H), 3.71 (s, 3H), 3.25–3.24 (m, 4H), 2.81 (s, 2H), 2.11–1.99 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 170.7, 155.0, 149.6, 139.9, 138.1, 132.1, 130.9, 130.3, 130.0, 129.3, 128.9, 127.7, 123.0, 121.5, 120.3, 117.9, 116.5, 114.9, 112.3, 110.2, 61.7, 56.1, 52.4; MS m/z: 579.9 (M+ + 2).
2-(4-Benzylpiperazin-1-yl)-N-(3–(3-(4-chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)acetamide (1f)
1H NMR (400 MHz, CDCl3) δ 8.57 (d, 2H, J= 5.6 Hz), 7.78–7.75 (m, 4H), 7.59–7.53 (m, 4H), 7.45 (t, 2H, J= 4.0 Hz), 7.40 (d, 1H, J= 8.4 Hz), 7.14–7.07 (m, 2H), 3.84 (s, 3H), 3.31 (s, 2H), 3.10 (brs, 4H), 2.36 (brs, 4 H), 2.32 (brs, 2H); MS m/z: 593.9 (M+ + 2).
Compounds 1 g–m were prepared from 9 b and appropriate acid chloride as reported in the synthesis of 1a.
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-3-(piperidin-1-yl)propanamide (1 g)
1H NMR (400 MHz, CDCl3) δ 8.59 (brs, 1H), 8.36 (s, 1H), 8.19 (s, 1H), 7.57 (d, 1H, J= 8.0 Hz), 7.44 (t, 1H, J= 8.0 Hz), 7.36 (d, 2H, J= 8.0 Hz), 7.32–7.29 (m, 3H), 7.17 (s, 1H), 7.10 (d, 1H, J= 7.6 Hz), 3.83 (s, 3H), 3.22 (s, 2H), 2.84–2.67 (m, 6H), 1.79–1.28 (m, 8H); 13C NMR (100 MHz, CDCl3) δ 170.7, 154.9, 150.1, 149.9, 149.8, 140.1, 139.9, 132.6, 130.5, 130.2, 129.6, 127.6, 122.7, 120.5, 120.2, 117.7, 114.9, 112.2, 110.2, 56.1, 54.1, 53.6, 32.6, 25.7; MS m/z: 517.21 (M+ + 2).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-3-morpholino-propanamide (1 h)
1H NMR (400 MHz, CDCl3) δ 9.33 (s, 1H), 8.56 (d, 2H, J= 4.8 Hz), 8.03(s, 1H), 7.57 (d, 1H, J= 7.6 Hz), 7.51–7.45 (m, 2H), 7.46 (d, 1H, J= 8.4 Hz), 7.29–7.27 (m, 2H), 7.16 (s, 1H), 7.07 (d, 1H, J= 8.0 Hz), 3.75 (m, 9H), 3.23 (s, 2H), 2.69–2.67 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 168.1, 154.6, 150.1, 149.7, 140.8, 139.9, 138.7, 132.2, 130.5, 130.2, 127.6, 122.9, 122.8, 122.2, 117.9, 114.9, 112.3, 110.2, 66.9, 62.4, 56.1, 50.8, 25.2; MS m/z: 518.43 (M+ + 1).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-3-(4-methyl-piperazin-1-yl)propanamide (1i)
1H NMR (400 MHz, CDCl3) δ 8.49 (d, 2H, J= 6.4 Hz), 8.29 (s, 1H), 8.15 (s, 1H), 7.49 (dd, 8H, J= 1.2, 8.0 Hz), 7.36 (t, 1H, J= 8.0 Hz), 7.28 (d, 1H, J= 4.8 Hz), 7.21 (d, 2H, J= 6.4 Hz), 7.07 (s, 1H), 7.03 (dd, 1H, J= 1.6, 8.0 Hz), 3.73 (s, 3H), 2.73–2.51 (m, 12H), 2.31 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 154.6, 149.9, 149.6, 140.6, 139.9, 139.8, 132.3, 130.1, 129.6, 127.6, 122.9, 122.6, 121.4, 120.1, 117.6, 114.1, 112.3, 110.2, 56.0, 55.1, 53.4, 53.1, 45.8, 32.5; MS m/z: 529.9 (M+ + 1).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-3-(4-ethyl-piperazin-1-yl)propanamide (1j)
1H NMR (400 MHz, CDCl3) δ 8.56 (d, 2H, J= 6.4 Hz), 8.29 (s, 1H), 8.15 (s, 1H), 7.49(dd, 8H, J= 1.2, 8.0 Hz), 7.36 (t, 1H, J= 8.0 Hz), 7.28 (d, 1H, J= 4.8 Hz), 7.21 (d, 2H, J= 6.4 Hz), 7.07 (s, 1H), 7.03 (dd, 1H, J= 1.6, 8.0 Hz), 3.73 (s, 3H), 2.73–2.51 (m, 12H), 2.31 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 154.6, 149.9, 149.6, 140.6, 139.9, 139.8, 132.3, 130.1, 129.6, 127.6, 122.9, 122.6, 121.4, 120.1, 117.6, 114.1, 112.3, 110.2, 56.0, 55; MS m/z: 545.9 (M+ + 2).
N-(3-(3-(4-Chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)-3-(4-phenyl-piperazin-1-yl)propanamide (1k)
1H NMR (400 MHz, CDCl3) δ 8.58 (d, 2H, J= 6.0 Hz), 8.34 (s, 1H), 8.21 (s, 1H), 7.58 (d, 1H, J= 7.2 Hz), 7.43 (t, 1H, J= 8.0 Hz), 7.35–7.28 (m, 2H), 7.14 (s, 1H), 7.08 (dd, 1H, J= 2.0, 8.0 Hz), 6.96–6.93 (m, 6H), 3.79 (s, 3H), 2.96–2.65 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 169.7, 155.1, 151.9, 150.9, 150.8, 146.9, 142.9, 140.0, 132.0, 130.3, 129.3, 127.5, 123.1, 122.4, 121.5, 120.5, 120.3, 117.6, 117.2, 116.4, 114.5, 112.2, 110.2, 56.1, 53.1, 52.4, 32.5; MS m/z: 593.43 (M+ + 1).
3-(4-Benzylpiperazin-1-yl)-N-(3–(3-(4-chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl)propanamide (1 l)
1H NMR (400 MHz, CDCl3) δ 8.57 (d, 2H, J= 6.0 Hz), 8.36 (s, 1H), 8.21 (s, 1H), 7.58 (dd, 1H, J= 1.2, 9.2 Hz), 7.43 (t, 1H, J= 8.0 Hz), 7.33–7.29 (m, 5H), 7.16–7.11 (m, 2H), 3.79 (s, 3H), 3.58 (s, 2H), 2.81–2.58 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 170.9, 156.9, 150.9, 150.8, 146.9, 142.9, 140.0, 137.6, 132.5, 130.3, 129.1, 127.6, 123.0, 121.5, 120.2, 117.6, 114.3, 112.3, 110.2, 62.9, 56.1, 53.1, 52.3, 32.6; MS m/z: 608.1 (M+ + 1).
3-(4-(4-Fluorobenzyl)piperazin-1-yl)-N-(3–(3-(4-chloro-3-methoxyphenyl)-4-(pyridin-4-yl)-1H-pyrazol-1-yl)phenyl) propanamide (1 m)
1H NMR (400 MHz, CDCl3) δ 8.49 (d, 2H, J= 6.0 Hz), 8.26 (s, 1H), 8.09 (s, 1H), 7.48 (d, 1H, J= 8.0 Hz), 7.36 (t, 1H, J= 8.0 Hz), 7.26 (d, 1H, J= 8.0 Hz), 7.19–7.17 (m, 5H), 7.05–7.01 (m, 2H), 6.92 (t, 3H, J= 8.8 Hz), 3.71 (s, 3H), 3.44 (s, 2H), 2.74–2.51 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 170.7, 154.9, 149.9, 149.8, 140.6, 140.0, 133.2, 132.2, 130.6, 130.3, 127.1, 123.0, 122.7, 121.5, 120.3, 117.5, 115.3, 114.3, 112.3, 110.2, 61.9, 56.1, 53.4, 53.2, 32.5; MS m/z: 626.0 (M+ + 2).
Cancer cell line screening at the NCI
Screening against the cancer cell lines was carried out at the National Cancer Institute (NCI), Bethesda, MD, applying the standard protocol of the NCI19.
Antiproliferative assay against A375 human melanoma cell line
Kinase profiling
Reaction Biology Corp. Kinase HotSpotSM service was used for screening of compounds 1i, 1j, and 1l according to the reported assay protocol23.
Results and discussion
Chemistry
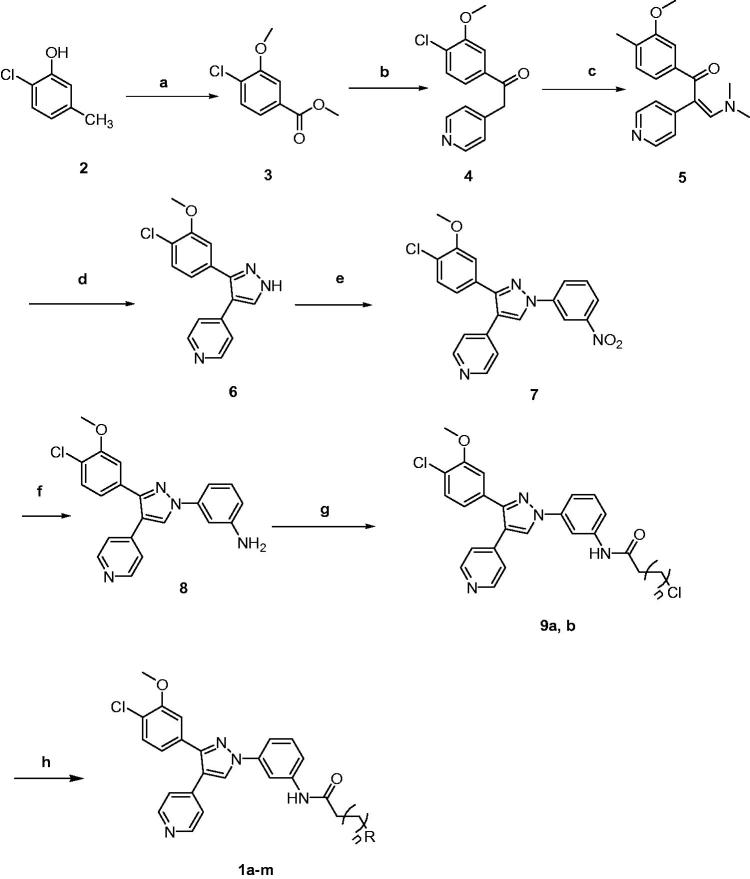
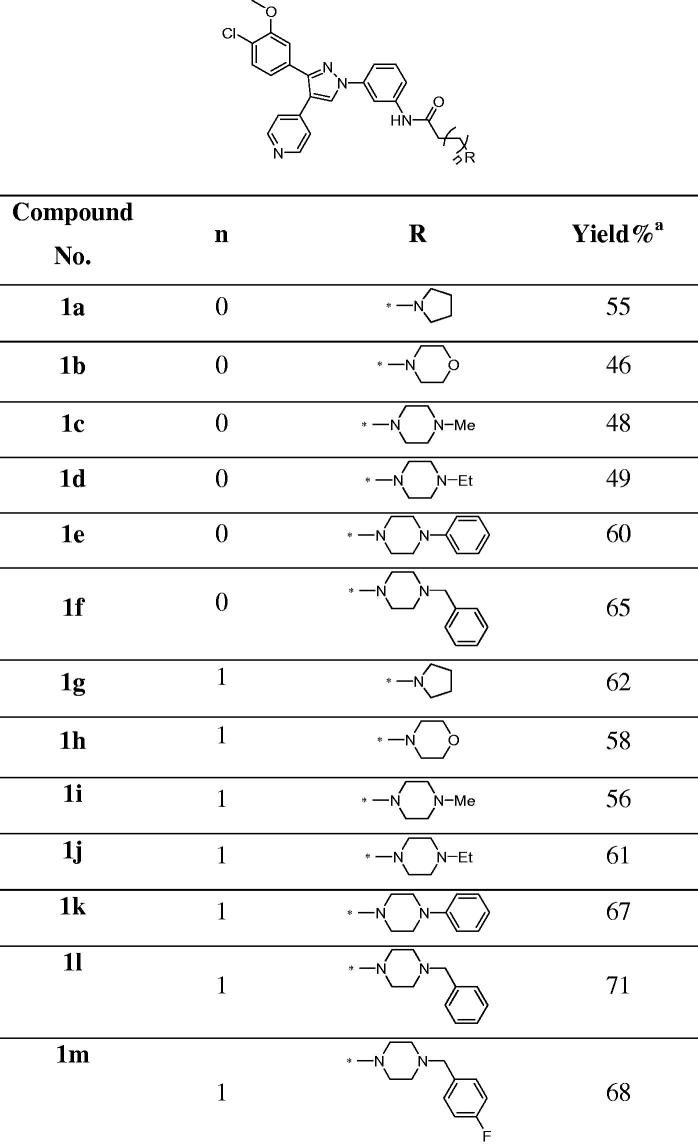
Synthesis of the designed compounds 1a–m was carried out using the reactions adopted in Scheme 1. The phenolic starting material 2 was methylated using dimethyl sulfate in the presence of potassium carbonate to yield the methoxy analogue, which was further oxidised using potassium carbonate to 3-methoxy-4-chlorobenzoic acid. Esterification of the 3-methoxy-4-chlorobenzoic acid with methanol in the presence of acetyl chloride gave the corresponding methyl ester 3. Activation of the ester with strong base as lithium bis(trimethylsilyl)amide (LiHMDS) then dropwise addition of 4-picoline produced the pyridyl intermediate 4. Cyclisation to the pyrazole compound 6 was carried out by treatment of 4 with dimethylformamide dimethyl acetal (DMF-DMA) to produce compound 5, and subsequent addition of hydrazine monohydrate. Heating of 3,4-driarylpyazole compound 6 with 3-iodonitrobenzene at 90 °C in DMSO to get the 3-nitrophenyl derivative 7. The nitro derivative 7 underwent reduction with Pd/C giving the amine compound 8. Compound 8 was reacted with either chloroacetyl chloride or chloropropionyl chloride giving chloroacetamide or chloropropionamide derivatives 9a,b, respectively. The reaction of the terminal alkyl halide moiety of compounds 9a,b with substituted or unsubstituted aliphatic cyclic amines yielded the target compounds 1a–m. Structures of the target compounds and their yield percentages are illustrated in Table 1.
Scheme 1.
Reagents and conditions: (a) i) (CH3)2SO4, K2CO3, acetone, reflux, 1 h; ii) KMnO4, C5H5N, H2O, 50 °C, 24 h, then rt, 13 h; iii) acetyl chloride, CH3OH, rt, 15 h; (b) 4-picoline, LiHMDS, THF, rt, overnight; (c) DMF-DMA, rt, 18 h; (d) hydrazine monohydrate, C2H5OH, rt, overnight; (e) 1-iodo-4-nitrobenzene, K2CO3, CuI, L-proline, DMSO, 90 °C, 8 h; (f) H2, Pd/C, THF, rt, 2 h; (g) chloroacetyl chloride, or chloropropionyl chloride, TEA, CH2Cl2, −10 °C, 15 min; (h) appropriate amine derivative, TEA, CH2Cl2, rt, 1 h.
Table 1.
Structures of the target amide compounds 1a–m and their yield percent.
Yield of the last step.
In vitro antiproliferative activity
Single-dose testing
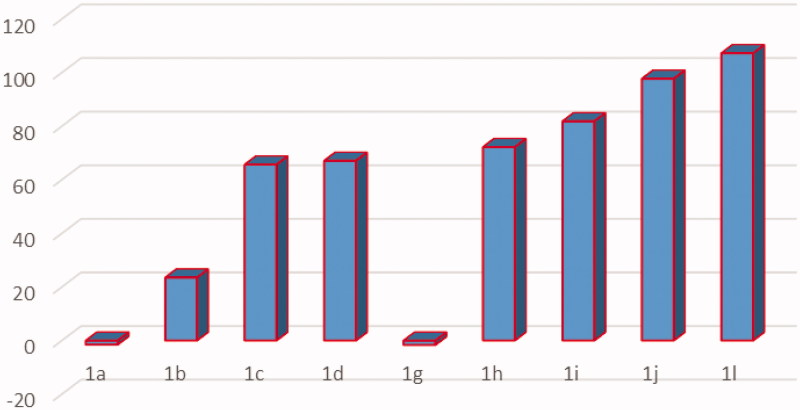
The designed compounds’ structures were submitted to the National Cancer Institute (NCI), Bethesda, MD, and nine of them were chosen for testing their antineoplastic activity. The selected compounds shown in Figure 2 were in vitro tested over 58 cancer cell lines of nine different tissues (blood, lung, colon, CNS, skin, ovary, kidney, prostate, and breast). They were examined at a single-dose concentration of 10 μM, for detecting growth inhibition percentages over the 58 tested cell lines. Figure 2 illustrates the mean percentage inhibition of each tested compound against the NCI-58 cancer cell line panel. The NCI results as well as representative 1H NMR, 13C NMR, and MS spectra are depicted in the Supplementary File.
Figure 2.
Mean percentage inhibition of target compounds against NCI-58 cancer cell line panel.
The outcome data expressed that the 1,3,4-triarylpyrazole derivatives possessing ethylene spacer (compounds 1g–j and 1l) showed better activity than those derivatives with methylene spacer in compounds 1a–d. They also exhibited higher mean % inhibition values than those of methylene. This could be attributed to better compound fitting and stronger affinity at the receptor site, or by even increasing its lipophilicity. These effects would enable optimal drug interaction in the enzyme active site, and therefore better antiproliferative activity.
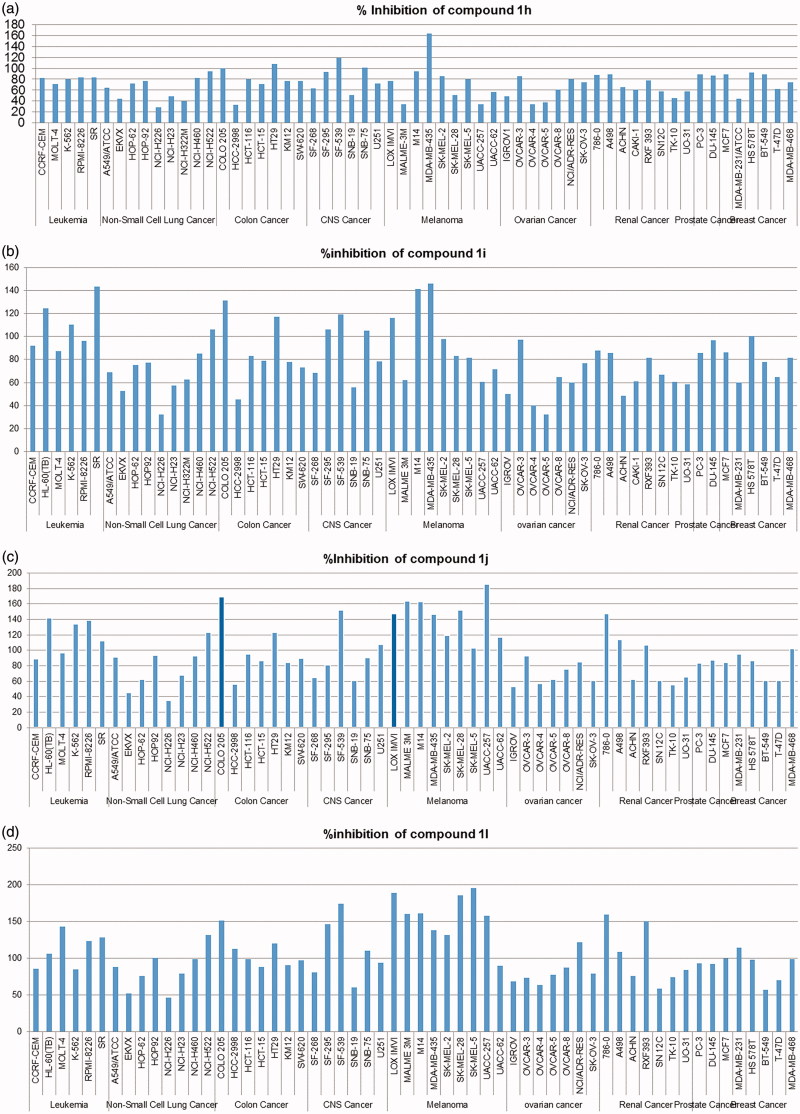
Regarding the effect of terminal moiety on the activity, it was found that substitution with morpholine (1b,h) achieved better mean % inhibition than that of piperidinyl derivatives (1a,g). This may be due to the presence of the oxygen atom making more hydrogen bond or enhancing the hydrophilic/aqueous solubility properties hence more compound fitting inside the receptor. It was also found that replacing morpholine with N-substituted piperazinyl derivatives as methyl, ethyl, phenyl, and benzyl with both shorter and longer spacer had made great enhancement of activity which may be caused by nitrogen atom instead of oxygen and protrusion of the substituent group; methyl, ethyl, phenyl, or benzyl exhibited higher mean percentage inhibition than those analogues without protrusion with lipophilic moiety leading to either more fitting of the compound within the receptor site or through enhancing its lipophilicity resulting in extra cell perforation and so, better cytotoxic activity (for methylene linker compounds 1c,d gave higher mean percentage inhibition than compounds 1a,b) and (for ethylene linker compounds 1i–m gave higher mean percentage inhibition than 1g and 1h. So it could be concluded that ethylene spacer and substituted piperazine derivatives at the terminal position of the aminophenyl ring of the pyrazole are optimum for activity. As expressed in Figure 2, we can see compound 1l exhibited 107.21% mean inhibition percentage at 10 μM concentration and compounds as 1h–j showed 72, 81, and 97%, respectively, as mean percentage inhibition. Moreover, they exerted greater than 100% inhibition over many cancer cell lines. For example, compound 1j exhibited 185.46, 169.25, and 163.6% against UACC-257, COLO 205, and MALME-3M cancer cell lines, respectively. Other compounds have also achieved broad-spectrum activity and better inhibition values of more than 100% inhibition over various cancer cell lines as shown in Figure 3.
Figure 3.
Inhibitory effects of compounds 1h–j and 1l against the NCI-58 cancer cell line panel. (a) 1h, (b) 1i, (c) 1j, and (d) 1l.
Five dose testing
Compounds 1h–j and 1l that have shown encouraging results in single-dose testing were further selected for a five-dose testing mode, to detect their IC50 values over 58 different cancer cells. Table 2 illustrates the mean IC50 values of these 4 compounds over the nine cancer types.
Table 2.
Mean IC50 values (μM) of the tested compounds over in vitro subpanel cancer cell linesa.
| Subpanel cancer lineb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | |
| No. of cell lines in each subpanel | 5 | 8 | 7 | 6 | 9 | 7 | 8 | 2 | 6 |
| 1h | 2.25 | 3.12 | 2.35 | 2.48 | 1.44 | 2.81 | 2.97 | 2.11 | 2.25 |
| 1i | 1.15 | 1.67 | 1.00 | 1.29 | 0.94 | 2.41 | 1.67 | 0.99 | 2.32 |
| 1j | 0.41 | 0.85 | 0.54 | 0.55 | 0.71 | 1.16 | 0.80 | 0.44 | 0.74 |
| 1l | 0.72 | 1.48 | 0.68 | 1.58 | 1.03 | 2.02 | 1.51 | 1.13 | 2.06 |
| Sorafenib | 2.43 | 2.25 | 2.19 | 2.33 | 1.87 | 2.88 | 2.94 | 2.58 | 2.17 |
Mean IC50 values were calculated by dividing the summation of IC50 values of the compound over cell lines of the same cancer type by the number of cell lines in the subpanel. bI: Leukemia; II: non-small cell lung cancer; III: colon cancer; IV: CNS Cancer; V: melanoma VII: ovarian cancer; VII: renal cancer; VIII: prostate cancer; IX: breast cancer. Bold figures indicate superior potency than the reference drug, Sorafenib.
The tested compounds exerted high activity with sub-micromolar to one-digit micromolar IC50 scale against all the nine cancer subpanels. Compounds 1i,j, and 1l exerted superior potencies compared with sorafenib (Table 2). Table 3 illustrates the 4 tested compounds’ IC50 data in five-dose testing mode over the most perceptive cell line of each subpanel. Compounds 1h–j and 1l exerted superior potencies against the most perceptive cell line of each cancer subpanel as contrasted with sorafenib. Most of their IC50 values were in the sub-micromolar range. Among them, compound 1j possessing ethylene spacer and N-ethylpiperazinyl terminal moiety was the most potent.
Table 3.
IC50 values (μM) of the tested compounds over the most sensitive cell line of each subpanel.
| Cancer cell line | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound no. | SRa | HOP-92b | NCI-H522b | HCT-29c | SW-620c | SNB-75d | SK-MEL-5e | OVCAR-3f | UO-31g | PC-3h | MDA-MB-468i |
| 1h | 0.68 | 2.06 | 0.44 | 1.90 | 1.56 | 0.82 | 0.62 | 0.64 | 1.69 | 1.74 | 0.63 |
| 1i | 0.47 | 0.67 | 0.28 | 0.37 | 0.44 | 0.59 | 0.53 | 0.36 | 1.38 | 1.00 | 0.35 |
| 1j | 0.43 | 0.78 | 0.27 | 0.38 | 0.34 | 0.39 | 0.51 | 0.35 | 0.92 | 0.45 | 0.27 |
| 1l | 0.39 | 0.79 | 0.36 | 0.32 | 0.49 | 1.55 | 1.10 | 0.39 | 0.89 | 0.71 | 0.39 |
| Sorafenib | 3.16 | 1.58 | 1.99 | 1.99 | 2.51 | 3.16 | 1.58 | 2.51 | 2.51 | 1.99 | 1.99 |
Leukemia cell line; bnon-small cell lung cancer cell line; ccolon cancer cell line; dCNS cancer cell line; emelanoma cell line; fovarian cancer cell line; grenal cancer cell line; hprostate cancer cell line; ibreast cancer cell line. Bold figures indicate superior potency than the reference compound, Sorafenib.
A375 melanoma cell line screening
In addition to the 58 cancer cell lines, 10 of our target compounds that were promising were tested also against A375 human melanoma cell line. The IC50 values are summarised in Table 4. The most potent compound of the previous series with para substitution was also reported for comparison20. Sorafenib was used as a reference standard. From Table 4, we can see that compounds exerted better activity against sorafenib. They all exhibited lower IC50 values than that of sorafenib. Compounds with ethylene spacer (compounds 1h–m) were more active than those with methylene linker (compounds 1b–d and 1f). It was also found that substituted piperazinyl derivatives with both methylene and ethylene spacer compound 1c–g and 1i–m were active than morpholino derivatives 1b,h, respectively, which may be attributed to presence of aliphatic or aromatic substitution on piperazinyl moiety that may make more fitting in the receptor through lipophilic interaction within it or even add to the compound’s lipophilicity so it may penetrate better through the cell reaching its molecular target(s). We have also found that compounds with N-methyl, ethyl, or benzyl piperazinyl moieties showed better IC50 on A375 melanoma cell line than that of morpholino, phenyl, and fluorobenzyl piperazinyl moieties. Compound 1c,d with methylene spacer are more potent than 1f,b and compounds 1i,j, and 1l with ethylene spacer are more potent than compounds 1k,m. It was also found that the most potent compound of meta series 1j exerted higher activity with IC50 value of 0.82 μM relative to that the most potent one of para series with IC50 1.82 μM against A375 human melanoma cancer cell line.
Table 4.
IC50 values of the tested compounds against A375 human melanoma cell line.
| Compound no. | IC50 (µM)a |
|---|---|
| 1b | 4.46 ± 0.35 |
| 1c | 4.24 ± 0.25 |
| 1d | 4.02 ± 0.26 |
| 1f | 4.37 ± 0.36 |
| 1h | 2.62 ± 1.21 |
| 1i | 0.96 ± 0.13 |
| 1j | 0.82 ± 0.15 |
| 1k | 1.64 ± 0.23 |
| 1l | 1.24 ± 0.38 |
| 1m | 1.66 ± 0.31 |
 b b
|
1.82 ± 0.13 |
| Sorafenib | 5.60 ± 0.52 |
The IC50 values are expressed as means of triplicate assays ± SEM. bThe most potent compound of para series.
In vitro kinase screening
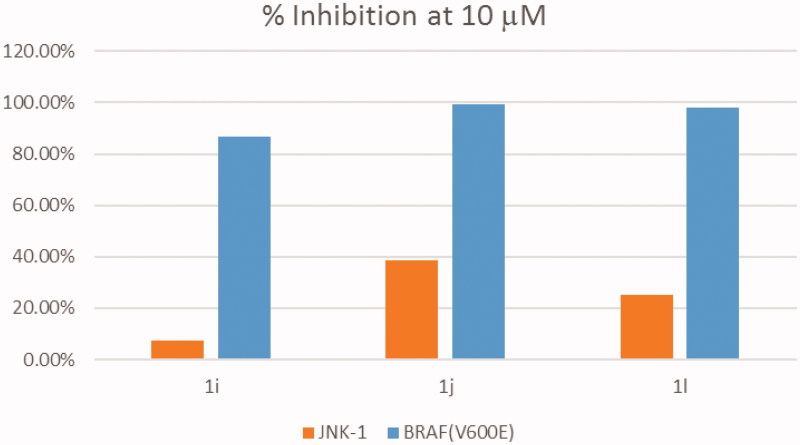
In order to investigate the mechanism of action of these target compounds at molecular level that showed promising results against the cancer cell lines, the most potent compounds 1i, 1j, and 1l were chosen to be examined at a single-dose concentration of 10 µM over two types of kinases V600E-B-RAF which is over-expressed in A37520 and some other cell lines from NCI panel such as SK-MEL-5, and JNK kinases which is not overexpressed in such cell lines trying to deduce their possible mechanism of action. As shown in Figure 4, the three compounds showed stronger inhibitory effect over V600E-B-RAF kinase than JNK-1 kinase at 10 µM. Compound 1j exhibited almost 100% inhibition against V600E-B-RAF kinase. The two other compounds gave 86.57 and 97.92% against V600E-B-RAF, but 7.57 and 25.04% against JNK-1 kinase. This indicates that there is about 3 folds more selectivity against V600E-B-RAF kinase than that corresponding to JNK-1 kinase. Compound 1j showed high activity over several cancer cell lines in which V600E-B-RAF is over-expressed such as COLO 205, HT 29 colon cancer cell lines, and SK-MEL-5 melanoma cell line20. As explained previously, V600E-B-RAF is over-expressed in diverse cancer types. So we can deduce from the inhibitory effect of compound 1j on V600E-B-RAF kinases is, at least in part, considered as a possible mechanism of its antiproliferative effect.
Figure 4.
Inhibitory effect of compounds 1i, 1j, and 1l on JNK-1 and V600E-B-RAF kinases.
Conclusions
In this investigation, a series of new 1,3,4-triarylpyrazole analogues having morpholino and substituted piperazinyl terminal moieties were synthesised. Out of the thirteen submitted compounds, nine were examined at single dose in vitro antiproliferative test over NCI-58 cancer cell line panel of nine different cancer tissues, and four promising compounds were selected for five dose testing mode. The compounds were also tested against A375 human melanoma cell line. These four compounds 1h–j and 1l with ethylene linker and N-substituted piperazinyl showed higher mean percentage inhibition of 72.18, 81.8, 97.68, and 107.21%, respectively, and compound 1j possessing N-ethylpiperazinyl moiety attached to 1,3,4-triarylpyrazole ring through ethylene linker showed the most promising results at five-dose testing. It was also found that many compounds with meta substitution of this nucleus has exerted higher activity against A375 than that of para series. It showed elevated potency and broad-spectrum antiproliferative activities over several different cell lines of different cancer types higher than sorafenib. It has also exerted tremendous percentage inhibition against V600E mutant B-RAF kinase of 99.17%. So N-substituted piperazinyl moiety, ethylene spacer, and meta-disubstitued phenyl at N1 of the core pyrazole ring are optimal for activity of these compounds. They could express antiproliferative activity through inhibition of V600E-B-RAF. Further modifications of this series in order to optimise the scaffold and to improve their anticancer activities are in progress.
Supplementary Material
Funding Statement
This work was held by the support of Korea Institute of Science and Technology (KIST), KIST Project (2E27930).
Acknowledgements
We would like to thank the National Cancer Institute (NCI), Bethesda, Maryland, USA, for their help in performing the in vitro anticancer testing over the cell lines.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.World Health Organization , Media Centre, Cancer. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/ [last accessed 16 March 2019].
- 2.Belpomme D, Irigaray P, Sasco AJ, et al. The growing incidence of cancer: role of lifestyle and screening detection. Int J Oncol 2007;30:1037–49. [DOI] [PubMed] [Google Scholar]
- 3.Frankish H. 15 million new cancer cases per year by 2020, says WHO. Lancet 2003;361:1278. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncology (Williston Park) 1995;9:1149–58. [PubMed] [Google Scholar]
- 5.Smith RA, Dumas J, Adnane L, et al. Recent advances in the research and development of RAF kinase inhibitors. Curr Top Med Chem 2006;6:1071–89. [DOI] [PubMed] [Google Scholar]
- 6.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997–7000. [PubMed] [Google Scholar]
- 7.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109. [DOI] [PubMed] [Google Scholar]
- 8.El-Gamal MI, Abdel-Maksoud MS, Gamal El-Din MM, et al. Cell-based biological evaluation of a new bisamide FMS kinase inhibitor possessing pyrrolo[3,2-c]pyridine scaffold. Arch Pharm Chem Life Sci 2014;347:635–41. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA, El-Gamal MI, Abdel-Maksoud MS, et al. Broad-spectrum antiproliferative activity of diarylureas and diarylamides possessing pyrrolo[3,2-c]pyridine scaffold. J Pharm Pharmacol 2014;2:157–69. [Google Scholar]
- 10.El-Gamal MI, Khan MA, Abdel-Maksoud MS, et al. A new series of diarylamides possessing quinoline nucleus: synthesis, in vitro anticancer activities, and kinase inhibitory effect. Eur J Med Chem 2014;87:484–92. [DOI] [PubMed] [Google Scholar]
- 11.Gamal El-Din MM, El-Gamal MI, Abdel-Maksoud MS, et al. Synthesis and broad-spectrum antiproliferative activity of diarylamides and diarylureas possessing 1,3,4-oxadiazole derivatives. Bioorg Med Chem Lett 2015;25:1692–9. [DOI] [PubMed] [Google Scholar]
- 12.Capdeville R, Buchdunger E, Zimmermann J, et al. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 2002;1:493–502. [DOI] [PubMed] [Google Scholar]
- 13.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 2002;1:117–23. [DOI] [PubMed] [Google Scholar]
- 14.Choi WK, Oh CH. Synthesis and antiproliferative activities of 1-substituted-3-(3-chloro-5-methoxyphenyl)-4-pyridinylpyrazole derivatives against melanoma cell line. Bull Korean Chem Soc 2009;30:2027–31. [Google Scholar]
- 15.Choi WK, El-Gamal MI, Choi WK, et al. New diarylureas and diarylamides containing 1,3,4-triarylpyrazole scaffold: synthesis, antiproliferative evaluation against melanoma cell lines, ERK kinase inhibition, and molecular docking studies. Eur J Med Chem 2011;46:5754–62. [DOI] [PubMed] [Google Scholar]
- 16.El-Gamal MI, Park YS, Chi DY, et al. New triarylpyrazoles as broad-spectrum anticancer agents: design, synthesis, and biological evaluation. Eur J Med Chem 2013;65:315–22. [DOI] [PubMed] [Google Scholar]
- 17.El-Gamal MI, Choi HS, Cho HG, et al. Design, synthesis, and antiproliferative activity of 3,4-diarylpyrazole-1-carboxamide derivatives against, melanoma cell line. Arch Pharm Chem Life Sci 2011;344:745–54. [DOI] [PubMed] [Google Scholar]
- 18.El-Gamal MI, Choi HS, Yoo KH, et al. Antiproliferative diarylpyrazole derivatives as dual inhibitors of the ERK pathway and COX-2. Chem Biol Drug Des 2013;82:336–47. [DOI] [PubMed] [Google Scholar]
- 19.DTP Human Tumor Cell Line Screen Process: Available from: http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html [last accessed 16 March 2019].
- 20.Khan MA, El-Gamal MI, Tarazi H, et al. Design and synthesis of a new series of highly potent RAF kinase-inhibiting triarylpyrazole derivatives possessing antiproliferative activity against melanoma. Future Med Chem 2016;8:2197–211. [DOI] [PubMed] [Google Scholar]
- 21.El-Gamal MI, Sim TB, Hong JH, et al. Synthesis of 1H-pyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Arch Pharm Chem Life Sci 2011;344:197–204. [DOI] [PubMed] [Google Scholar]
- 22.El-Gamal MI, Oh CH. Design and synthesis of 3-(3-chloro-4-substituted phenyl)-4-(pyridin-4-yl)-1Hpyrazole-1-carboxamide derivatives and their antiproliferative activity against melanoma cell line. Bull Korean Chem Soc 2011;32:821–8. [Google Scholar]
- 23.Reaction Biology + ProQinase = Your Partner for Drug Discovery Available from http://www.reactionbiology.com [last accessed 2 August 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.