Abstract
In this paper, a series of novel 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid bearing different N-(piperazin-1-yl)alkyl side chains were designed, synthesised and evaluated for their in vitro anticancer activities against three human hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B). Among them, compound 10g exhibited the most potent activity against three cancer cell lines with IC50 values of 1.39 ± 0.13, 0.51 ± 0.09 and 0.73 ± 0.08 µM, respectively. In the kinase inhibition assay, compound 10g could significantly inhibit MEK1 kinase activity with IC50 of 0.11 ± 0.02 µM, which was confirmed by western blot analysis and molecular docking study. In addition, compound 10g could elevate the intracellular ROS levels, decrease mitochondrial membrane potential, destroy the cell membrane integrity, and finally lead to the oncosis and apoptosis of HepG2 cells. Therefore, compound 10g could be a potent MEK inhibitor and a promising anticancer agent worthy of further investigations.
KEYWORDS: Dehydroabietic acid, anticancer activity, MEK inhibitor, oncosis, apoptosis
GRAPHICAL ABSTRACT
1. Introduction
Cancer has become the leading cause of human death worldwide and imposed tremendous health problem to human beings. Besides traditional chemotherapy agents, the exploration on signal transduction networks closely related to oncogenesis and cancer development, has led to a lot of targeted cancer therapeutics with prominent therapeutic benefits1. Among them, the mitogen-activated protein kinase (MAPK) pathway plays a central role in controlling mammalian cell functions, including adhesion, migration, differentiation, metabolism and proliferation2.
The MAPK pathway includes a chain of proteins that communicates the signal from a receptor on the cell surface to DNA in the nucleus3. The pathway is activated when an extracellular stimulus binds to its receptor, which results in activation of the membrane-bound GTPase (RAS) and then leads to the recruitment and activation of Raf, a serine-threonine kinase. Subsequently, the signal is transmitted downstream through activated Raf by phosphorylating and activating its main substrates MEK1/2, two dual-specific kinases which also activate their substrates ERK1/2 via phosphorylation of conserved threonine and tyrosine residues in the activation loop4,5. When activated, ERK1/2 in turn phosphorylates and activates several downstream proteins located in cytoplasm or nucleus, leading to a range of cellular events6,7. This pathway is also known as Ras-Raf-MEK-ERK pathway8, which is aberrantly activated in more than 30% of human cancers such as hepatocarcinoma (HCC), prostate carcinoma, non-small cell lung cancer (NSCLC), leukemia and melanoma9. Consequently, the inhibition of signal transduction through MAPK pathway can be a promising strategy for tumour targeted therapy.
As a key node of MAPK pathway, the Ser/Thr kinases MEK1/2 specifically phosphorylate and activate ERK1/2. The inhibition of MEK kinase activity will effectively impede the signal transduction of MAPK pathway. Hence, the interest in MEK1/2 has generated several small molecule inhibitors, e.g. highly specific MEK1/2 inhibitors such as U0126, PD98059, BI-847325, trametinib (GSK1120212), CI-1040 (PD184352), cobimetinib (GDC-0973), selumetinib (AZD6244) and myricetin (Figure 1)10–17. CI-1040 is an ATP non-competitive MEK1/2 inhibitor which directly inhibits MEK1 with a 50% inhibitory concentration (IC50) of 17 nM18. It is the first MEK inhibitor which entered clinical trials for treating a panel of advanced cancers. However, the phase II study results provided little support for further investigation of CI-1040 and the development was terminated19. Selumetimib (AZD6244) is an orally available, selective, ATP-noncompetitive MEK1/2 inhibitor which showed significant antitumour activity in cell lines harboring BRAF or RAS mutations20 and in various xenograft models21. In a phase II trial that compared selumetinib plus docetaxel with matching placebo plus docetaxel in patients with previously treated KRAS-mutant NSCLC, the median overall survival (OS) and progression-free survival (PFS) was significantly longer in the selumetinib plus docetaxel group22. However, in a follow-up randomised phase III study (SELECT-1), the results did not confirm the survival benefit of selumetinib plus docetaxel seen in the phase II trial23. Despite this disappointing result, several studies are underway to investigate combination approaches of selumetinib with a variety of partner drugs24. In addition, trametinib (GSK1120212) is an oral, reversible, potent and selective inhibitor of MEK1/2 with IC50 of 0.7–0.9 nM25. FDA recently approved the combination of dabrafenib and trametinib for the treatment of BRAF-mutant metastatic melanoma, NSCLC and anaplastic thyroid cancer26. These examples have highlighted the potential of MEK inhibitors as potential targeted anticancer drugs. The limitations of present inhibitors on efficacy and/or adverse effects also put forward an urgent need for the discovery of novel MEK inhibitors.
Figure 1.
Examples of MEK kinase inhibitors.
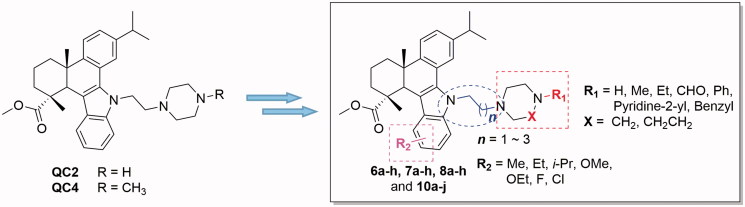
Dehydroabietic acid (DAA) is a natural occurring diterpenic resin acid, which can be easily obtained from Pinus rosin or commercial disproportionated rosin. Recent reports indicate that DAA and its derivatives exhibited a broad spectrum of biological activities, such as antimicrobial, antitumour, antiviral, antiprotozoal, antiulcer, antioxidant, anti-ageing and BK-channel opening activities27–34. Therefore, DAA has proved to be a promising starting material in search of derivatives with potent anticancer activities. In our previous studies, a series of N-substituted 1H-dibenzo[a,c]carbazole derivatives of DAA were synthesised, some of which showed notable antimicrobial activities35. Subsequently, in the in vitro cytotoxic assay, two compounds (QC2 and QC4) (Figure 2) of these derivatives exhibited significant antiproliferative activity against hepatocarcinoma and gastric cancer cell lines with IC50 values at low micromolar level. In pharmacological studies, it was found that QC2 could activate oncosis related protein calpain to induce the damage of cytomembrane and organelles which finally lead to oncosis in hepatocarcinoma cells36. QC4 could also induce the oncosis and apoptosis in gastric cancer cells37. In addition, QC2 showed moderate inhibitory activity in a preliminary screening of in vitro MEK1 inhibitory activity. Based on these findings, the two compounds were subject to further structure modifications at the following sites: (i) the N-substituents on the piperazine moiety of the side chain; (ii) the length of the alkyl linker and (iii) the substituents on the indole benzene ring (Figure 2). Through these strategies, several series of compounds derived from QC2 and QC4 can be designed and synthesised in order to find derivatives with better anticancer activities. Furthermore, the anticancer mechanisms of the active compounds will also be extensively explored.
Figure 2.
The strategy for the structure modification of QC2 and QC4.
2. Experimental
2.1. General
IR spectra were measured on a Nexus 870 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), and the absorption bands are expressed in cm−1. The HRMS spectra were recorded on a high-resolution mass spectrometer equipped with electrospray (ESI) and nanospray sources, and a quadrupole-time of flight hybrid analyzer (Q-TOF Premier/nanoAquity, Waters, Milford, MA). 1H NMR and 13 C NMR spectra were accomplished in CDCl3 on a Bruker AV-300, AV-500 and DRX-600 NMR spectrometer (Billerica, MA, USA) using TMS as internal standard. Reactions and the resulted products were monitored by TLC which was carried out on TLC Silica gel 60 F254 Aluminium sheets from Merck KGaA, Darmstadt, Germany and visualised in UV light (254 nm). Silica gel (3 0 0–400 mesh) for column chromatography was purchased from Qingdao Marine Chemical Factory, China. The reagents (chemicals), all being of A.R. grade, were purchased from Shanghai Chemical Reagent Company (Shanghai, China) and Energy Chemical (Shanghai, China). Disproportionated rosin was provided by Zhongbang Chemicals Co., Ltd. (Zhaoqing, China), from which dehydroabietic acid (97%) was isolated according to the published method38.
2.2. General procedure for the synthesis of compounds 5a-c
To a solution of compound 4 (0.7 g, 1.75 mmol) in benzene (5 mL) were added one kind of dibromoalkane (20 mmol), tetrabutyl ammonium bromide (TBAB) (0.02 g, 0.062 mmol) and 50% NaOH solution (3 mL). The mixture was stirred at room temperature for 12 h. Then the mixture was poured into 100 mL of ice-cold water. The suspension was extracted with CH2Cl2 (3 × 80 mL). The organic layer was combined, washed with water and brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on a silica gel column, eluting with petroleum ether-acetone (100:1, v/v) to give compounds 5a-c.
2.2.1. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-bromoethyl)-1H- dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (5a)
Yield 56%; light yellow resin; 1H NMR (300 MHz, CDCl3): 1.05 (s, 3H), 1.32 (d, J = 7.0 Hz, 3H), 1.35 (d, J = 7.0 Hz, 3H), 1.65 (m, 1H), 1.73 (s, 3H), 1.8 0 ∼ 1.99 (m, 4H), 2.29 (d, J = 13.4 Hz, 1H), 2.98 (m, 1H), 3.59 (m, 1H), 3.62 (s, 3H), 3.69 (m, 1H), 3.72 (s, 1H), 4.80 (m, 2H), 7.07 (t, J = 7.8 Hz, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.30 (d, J = 1.2 Hz, 1H), 7.31 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H); IR (KBr, cm−1): 2963, 2932, 2866, 1717, 1460, 1439, 1381, 1341, 1253, 1220, 1138, 833; HRMS (ESI): m/z [M + H]+ calcd. for C29H35BrNO2: 508.1851; found: 508.1858.
2.2.2. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(3-bromopropyl)-1H- dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (5b)
Yield 48%; light yellow resin; 1H NMR (300 MHz, CDCl3): 1.05 (s, 3H), 1.32 (d, J = 7.0 Hz, 3H), 1.34 (d, J = 7.0 Hz, 3H), 1.66 (m, 1H), 1.73 (s, 3H), 1.80–2.00 (m, 4H), 2.11 (m, 2H), 2.28 (d, J = 13.8 Hz, 1H), 2.98 (m, 1H), 3.52 (m, 2H), 3.62 (s, 3H), 3.71 (s, 1H), 4.58 (m, 2H), 7.05 (t, J = 8.0 Hz, 1H), 7.15 (d, J = 8.0 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.28 (d, J = 7.8 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.36 (s, 1H), 7.38 (d, J = 8.4 Hz, 1H); IR (KBr, cm−1): 2965, 2931, 2869, 1712, 1463, 1435, 1380, 1343, 1248, 1217, 1130, 829; HRMS (ESI): m/z [M + H]+ calcd. for C30H37BrNO2: 522.2008; found: 522.2003.
2.2.3. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(4-bromobutyl)-1H- dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (5c)
Yield 55%; light yellow resin; 1H NMR (300 MHz, CDCl3): 1.06 (s, 3H), 1.32 (d, J = 7.0 Hz, 3H), 1.35 (d, J = 7.1 Hz, 3H), 1.65 (m, 1H), 1.72 (s, 3H), 1.75 (m, 2H), 1.80–2.05 (m, 6H), 2.28 (d, J = 13.8 Hz, 1H), 2.96 (m, 1H), 3.50 (m, 2H), 3.61 (s, 3H), 3.72 (s, 1H), 4.47 (m, 2H), 7.04 (t, J = 7.8 Hz, 1H), 7.15 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.38 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H); IR (KBr, cm−1): 2968, 2932, 2874, 1716, 1461, 1432, 1382, 1351, 1236, 1227, 1118, 837; HRMS (ESI): m/z [M + H]+ calcd. for C31H39BrNO2: 536.2164; found: 536.2170.
2.3. General procedure for the synthesis of compounds 6a-h, 7a-h and 8a-h
To a solution of compound 5a-c (0.5 mmol) in acetonitrile (15 mL) was added anhydrous K2CO3 (0.345 g, 2.5 mmol), KI (0.083 g, 0.5 mmol) and 10 mmol of corresponding N-substituted piperazine. The mixture was refluxed for 8–12 h and monitored by TLC. At the end of reaction, the mixture was poured into cold water, which was extracted by CH2Cl2 (100 mL) for three times. The organic phase was combined, washed with water and brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was subjected to silica gel chromatography, eluting with petroleum ether-acetone (100:1, v/v) to afford compounds 6a-h, 7a-h or 8a-h.
2.3.1. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(piperazin-1-yl)ethyl) -1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6a)
Yellow amorphous solid; Yield: 60%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.32 (d, J = 6.9 Hz, 3H), 1.33 (d, J = 6.9 Hz, 3H), 1.66 (m, 1H), 1.74 (s, 3H), 1.79–2.01 (m, 4H), 2.30 (d, J = 12.7 Hz, 1H), 2.40 (brs, 1H, NH), 2.54 (t, J = 4.4 Hz, 4H), 2.80 (m, 2H), 2.89 (t, J = 4.4 Hz, 4H), 2.99 (m, 1H), 3.62 (s, 3H), 3.72 (s, 1H), 4.60 (m, 2H), 7.05 (t, J = 8.1 Hz, 1H), 7.15 (dd, J = 7.5, 1.3 Hz, 1H), 7.18 (t, J = 7.9 Hz, 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 1.7 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.3, 19.3, 21.2, 24.2, 24.3, 34.1, 36.6, 38.7, 38.8, 43.3, 45.6, 45.7, 46.1, 52.5, 54.3, 58.1, 110.1, 113.9, 119.8, 120.9, 121.1, 121.6, 123.5, 125.4, 126.0, 127.3, 135.6, 139.1, 146.3, 147.1, 180.4; IR (KBr, cm−1): 3042, 2928, 2860, 2811, 1723, 1607, 1495, 1461, 1350, 1255, 1134, 826, 737; HRMS (ESI): m/z [M + H]+ calcd. for C33H44N3O2: 514.3434; found: 514.3439.
2.3.2. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-methylpiperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6b)
Yellow amorphous solid; Yield: 60%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 6.9 Hz, 3H), 1.33 (d, J = 6.9 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.792.01 (m, 4H), 2.30 (d, J = 12.8 Hz, 1H), 2.31 (s, 3H), 2.47 (brs, 4H), 2.61 (brs, 4H), 2.81 (m, 1H), 2.90–3.01 (m, 2H), 3.61 (s, 3H), 3.72 (s, 1H), 4.57 (m, 2H), 7.05 (t, J = 8.0 Hz, 1H), 7.15 (d, J = 8.1, 1H), 7.16 (t, J = 8.1 Hz, 1H), 7.30 (d, J = 8.5 Hz, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.43 (d, J = 8.2 Hz, 1H), 7.47 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.3, 19.3, 21.3, 24.2, 24.3, 34.1, 36.6, 38.7, 38.8, 43.5, 45.7, 46.1, 46.1, 52.5, 53.7, 55.1, 57.5, 110.1, 113.8, 119.8, 120.9, 121.0, 121.7, 123.4, 125.3, 125.9, 127.2, 135.6, 139.2, 146.4, 147.1, 180.4; IR (KBr, cm−1): 3046, 2929, 2862, 2797, 1724, 1604, 1460, 1354, 1252, 1166, 826, 736; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O2 528.3590; found: 528.3587.
2.3.3. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-ethylpiperazin -1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6c)
Yellow amorphous solid; Yield: 50%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.10 (t, J = 7.0 Hz, 3H), 1.31 (d, J = 6.6 Hz, 3H), 1.33 (d, J = 6.5 Hz, 3H), 1.66 (m, 1H), 1.74 (s, 3H), 1.75–2.10 (m, 4H), 2.29 (d, J = 11.1 Hz, 1H), 2.44 (q, J = 7.1 Hz, 2H), 2.50 (brs, 4H), 2.63 (brs, 4H), 2.84 (m, 1H), 2.94 (m, 2H), 3.61 (s, 3H), 3.72 (s, 1H), 4.45 (m, 2H), 7.05 (t, J = 7.6 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.17 (t, J = 7.8 Hz, 1H), 7.31 (d, J = 9.3 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.44 (d, J = 8.1 Hz, 1H), 7.48 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 11.8, 18.2, 19.2, 21.2, 24.1, 24.3, 34.0, 36.5, 38.6, 38.7, 43.4, 45.6, 46.0, 52.3, 52.4, 52.6, 53.5, 57.5, 110.1, 113.7, 119.7, 120.8, 121.0, 121.6, 123.4, 125.2, 125.9, 127.2, 135.5, 139.1, 146.3, 147.0, 180.3; IR (KBr, cm−1): 3029, 2924, 2856, 2806, 1716, 1603, 1453, 1336, 1160, 1128, 730; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O2: 542.3747; found: 542.3753.
2.3.4. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(1,4-diazepan-1-yl) ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6d)
Yellow amorphous solid; Yield: 32%; 1H NMR (500 MHz, CDCl3) δ: 1.05 (s, 3H), 1.30 (d, J = 7.2 Hz, 3H), 1.32 (d, J = 6.9 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.80–2.10 (m, 6H), 2.29 (d, J = 11.5 Hz, 1H), 2.78–2.82 (m, 5H), 2.85–3.05 (m, 5H), 3.05 (t, J = 5.5 Hz, 2H), 3.61 (s, 3H), 3.71 (s, 1H), 4.56 (m, 2H), 7.04 (t, J = 7.5 Hz, 1H), 7.15 (d, J = 8.2 Hz, 1H), 7.16 (t, J = 9.3 Hz, 1H), 7.30 (d, J = 8.1 Hz, 1H), 7.33 (d, J = 8.3 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.44 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.3, 19.3, 21.2, 22.8, 24.2, 24.3, 27.4, 34.1, 36.6, 38.8, 38.8, 44.1, 45.7, 46.0, 46.1, 47.8, 52.5, 54.9, 57.4, 110.1, 114.2, 119.9, 121.0, 121.2, 121.4, 123.6, 125.5, 126.1, 127.4, 135.6, 139.4, 146.4, 147.1, 180.4; IR (KBr, cm−1): 3380, 3046, 2955, 2928, 2851, 2806, 1729, 1653, 1454, 1359, 1241, 1110, 730; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O2: 528.3590; found: 528.3582.
2.3.5. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-formylpiperazin -1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6e)
Yellow amorphous solid; Yield: 61%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.30 (d, J = 6.9 Hz, 3H), 1.32 (d, J = 6.9 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.76–2.08 (m, 4H), 2.29 (d, J = 12.2 Hz, 1H), 2.47 (brs, 4H), 2.82 (m, 2H), 2.96 (m, 1H), 3.26 (m, 2H), 3.44 (m, 2H), 3.62 (s, 3H), 3.71 (s, 1H), 4.61 (t, J = 6.8 Hz, 2H), 7.05 (t, J = 7.7 Hz, 1H), 7.15 (d, J = 7.5, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.31 (d, J = 8.7 Hz, 1H), 7.34 (d, J = 9.0 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.45 (s, 1H), 7.98 (s, 1H, CHO); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.3, 21.3, 24.2, 24.3, 34.1, 36.6, 38.7, 39.8, 43.2, 45.5, 45.6, 46.0, 52.5, 52.8, 54.1, 57.5, 110.0, 114.1, 119.9, 121.0, 121.1, 121.4, 123.6, 125.5, 126.1, 127.3, 135.7, 139.1, 146.3, 147.1, 160.8, 180.4; IR (KBr, cm−1): 3010, 2923, 2851, 1719, 1677, 1460, 1218, 1134, 997, 739; HRMS (ESI): m/z [M + H]+ calcd. for C34H44N3O3: 542.3383; found: 542.3389.
2.3.6. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-phenylpiperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6f)
Yellow amorphous solid; Yield: 45%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.32 (d, J = 6.9 Hz, 3H), 1.33 (d, J = 6.4 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.80–2.10 (m, 4H), 2.28 (d, J = 9.3 Hz, 1H), 2.65 (m, 2H), 2.69 (m, 4H), 2.97 (m, 1H), 3.16 (m, 4H), 3.61 (s, 3H), 3.72 (s, 1H), 4.61 (m, 2H), 6.84 (t, J = 7.3 Hz, 1H), 6.90 (d, J = 8.2 Hz, 2H), 7.05 (t, J = 7.8 Hz, 1H), 7.14 (d, J = 7.1 Hz, 1H), 7.16 (t, J = 7.3 Hz, 1H), 7.24 (t, J = 7.8 Hz, 2H), 7.29 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 7.45 (d, J = 8.2 Hz, 1H), 7.50 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.3, 19.3, 21.3, 24.2, 24.4, 34.1, 36.6, 38.8, 38.8, 43.5, 45.7, 46.1, 49.1, 52.5, 53.8, 57.6, 110.2, 113.9, 116.2, 119.8, 119.9, 120.9, 121.1, 121.6, 123.5, 125.4, 126.0, 127.3, 129.2, 135.7, 139.2, 146.4, 147.1, 151.4, 180.4; IR (KBr, cm−1): 3011, 2953, 2924, 2851, 1724, 1600, 1497, 1463, 1383, 1233, 1139, 730; HRMS (ESI): m/z [M + H]+ calcd. for C39H48N3O2: 590.3747; found: 590.3753.
2.3.7. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-(pyridine-2-yl) piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6g)
Yellow amorphous solid; Yield: 50%; 1H NMR (500 MHz, CDCl3) δ: 1.05 (s, 3H), 1.32 (d, J = 6.7 Hz, 3H), 1.33 (d, J = 6.7 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.8 0–2.10 (m, 4H), 2.28 (d, J = 12.3 Hz, 1H), 2.64 (t, J = 5.0 Hz, 4H), 2.84 (m, 1H), 2.90–3.00 (m, 2H), 3.50 (m, 4H), 3.61 (s, 3H), 3.72 (s, 1H), 4.63 (m, 2H), 6.60 (t, J = 4.0 Hz, 1H), 6.62 (s, 1H), 7.05 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.3 Hz, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 7.45 (d, J = 8.2 Hz, 1H), 7.46 (m, 1H), 7.50 (s, 1H), 8.18 (d, J = 3.8 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.3, 19.3, 21.3, 24.2, 24.4, 34.1, 36.6, 38.8, 38.8, 43.5, 45.3, 45.7, 46.1, 52.5, 53.6, 57.7, 107.2, 110.2, 113.5, 114.0, 119.8, 120.9, 121.1, 121.6, 123.5, 125.4, 126.0, 127.3, 135.7, 137.6, 139.2, 146.4, 147.1, 148.1, 159.6, 180.4; IR (KBr, cm−1): 3010, 2924, 2851, 1723, 1593, 1480, 1382, 1243, 1126, 732; HRMS (ESI): m/z [M + H]+ calcd. for C38H47N4O2: 591.3699; found: 591.3706.
2.3.8. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-benzylpiperazin -1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (6h)
Yellow amorphous solid; Yield: 64%; 1H NMR (300 MHz, CDCl3) δ: 1.04 (s, 3H), 1.30 (d, J = 6.4 Hz, 3H), 1.32 (d, J = 6.2 Hz, 3H), 1.64 (m, 1H), 1.73 (s, 3H), 1.76–2.10 (m, 4H), 2.28 (d, J = 11.8 Hz, 1H), 2.48 (brs, 4H), 2.60 (brs, 4H), 2.90 (m, 2H), 2.96 (m, 1H), 3.52 (s, 2H), 3.61 (s, 3H), 3.71 (s, 1H), 4.56 (m, 2H), 7.04 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.7, 1H), 7.16 (t, J = 7.0 Hz, 1H), 7.29 (d, J = 7.5 Hz, 1H), 7.31 (s, 5H), 7.32 (d, J = 10.2 Hz, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.47 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.3, 21.2, 24.1, 24.3, 34.0, 36.5, 38.6, 38.7, 43.5, 45.6, 46.0, 52.4, 52.9, 53.7, 57.5, 63.1, 110.1, 113.7, 119.7, 120.8, 121.0, 121.6, 123.4, 125.3, 125.9, 127.2, 127.2, 128.3, 129.3, 135.5, 138.0, 139.1, 146.3, 147.0, 180.4; IR (KBr, cm−1): 3028, 2924, 2851, 2809, 1722, 1457, 1350, 1251, 1140, 734, 697; HRMS (ESI): m/z [M + H]+ calcd. for C40H50N3O2: 604.3903; found: 604.3898.
2.3.9. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(piperazin-1-yl) propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7a)
Yellow amorphous solid; Yield: 67%; 1H NMR (300 MHz, CDCl3) δ: 1.04 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.32 (d, J = 6.7 Hz, 3H), 1.64 (m, 1H), 1.73 (s, 3H), 1.75–2.15 (m, 5H), 2.1 8 ∼ 2.40 (m, 4H), 2.43 (brs, 1H, NH), 2.50 (m, 4H), 2.95 (m, 1H), 3.02 (m, 4H), 3.61 (s, 3H), 3.70 (s, 1H), 4.51 (m, 2H), 7.03 (t, J = 7.5 Hz, 1H), 7.13 (d, J = 7.7 Hz, 1H), 7.15 (t, J = 7.7 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H), 7.39 (d, J = 9.4 Hz, 1H), 7.40 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.0, 19.1, 20.9, 24.0, 24.1, 27.2, 33.7, 36.3, 38.4, 38.5, 43.5, 45.4, 45.9, 49.5, 52.3, 54.5, 55.3, 110.2, 113.7, 119.5, 120.6, 120.8, 121.1, 123.3, 125.2, 125.7, 127.1, 135.2, 139.1, 146.0, 146.7, 180.1; IR (KBr, cm−1): 3486, 3041, 2946, 2929, 2858, 1722, 1608, 1462, 1361, 1249, 1137, 732; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O2: 528.3590; found: 528.3593.
2.3.10. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(3-(4-methyl piperazin-1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7b)
Yellow amorphous solid; Yield: 49%; 1H NMR (300 MHz, CDCl3): δ 1.04 (s, 3H), 1.31 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 6.8 Hz, 3H), 1.65 (m, 1H), 1.73 (s, 3H), 1.75–2.15 (m, 6H), 2.28 (d, J = 12.1 Hz, 1H), 2.30 (s, 3H), 2.36 (t, J = 7.2 Hz, 2H), 2.45 (brs, 8H), 2.95 (m, 1H), 3.61 (s, 3H), 3.71 (s, 1H), 4.49 (t, J = 7.4 Hz, 2H), 7.03 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.15 (t, J = 7.8 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.41 (s, 1H), 7.42 (d, J = 9.7 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.0, 19.1, 21.0, 24.0, 24.1, 27.4, 33.7, 36.3, 38.4, 38.5, 43.3, 45.4, 45.9, 52.2, 53.0, 55.0, 55.4, 110.2, 113.7, 119.4, 120.5, 120.7, 121.2, 123.2, 125.2, 125.6, 127.2, 135.2, 139.0, 146.0, 146.7, 180.1; IR (KBr, cm−1): 3045, 2933, 2871, 2794, 1722, 1609, 1460, 1359, 1283, 1164, 734; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O2: 542.3747; found: 542.3741.
2.3.11. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-ethylpiperazin -1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7c)
Yellow amorphous solid; Yield: 38%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.09 (t, J = 7.2 Hz, 3H), 1.31 (d, J = 6.2 Hz, 3H), 1.33 (d, J = 6.5 Hz, 3H), 1.65 (m, 1H), 1.75 (s, 3H), 1.80–2.10 (m, 6H), 2.28 (d, J = 12.1 Hz, 1H), 2.36 (t, J = 6.5 Hz, 2H), 2.42 (q, J = 7.2 Hz, 2H), 2.47 (brs, 8H), 2.96 (m, 1H), 3.61 (s, 3H), 3.72 (s, 1H), 4.50 (t, J = 7.6 Hz, 2H), 7.04 (t, J = 7.5 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.15 (t, J = 8.3 Hz, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 7.42 (s, 1H), 7.43 (d, J = 8.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 11.9, 18.2, 19.2, 21.1, 24.1, 24.2, 27.6, 33.9, 36.5, 38.6, 38.7, 43.5, 45.6, 46.1, 52.3, 52.4, 52.8, 53.2, 55.6, 110.3, 113.9, 119.6, 120.7, 120.9, 121.4, 123.3, 125.3, 125.8, 127.4, 135.4, 139.2, 146.2, 147.0, 180.3; IR (KBr, cm−1): 3049, 2962, 2924, 2852, 1725, 1604, 1463, 1380, 1336, 1248, 1190, 908; HRMS (ESI): m/z [M + H]+ calcd. for C36H50N3O2: 556.3903; found: 556.3909.
2.3.12. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(1,4-diazepan-1-yl) propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7d)
Yellow amorphous solid; Yield: 41%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 6.6 Hz, 3H), 1.32 (d, J = 6.5 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.76–2.10 (m, 8H), 2.29 (d, J = 10.6 Hz, 1H), 2.3 5 ∼ 2.55 (m, 5H), 2.63 (m, 4H), 2.95 (m, 1H), 2.99 (m, 2H), 3.61 (s, 3H), 3.71 (s, 1H), 4.52 (t, J = 7.1 Hz, 2H), 7.03 (t, J = 7.7 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.15 (t, J = 7.8 Hz, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.42 (s, 1H), 7.43 (d, J = 7.0 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.2, 21.1, 24.2, 24.2, 25.2, 26.4, 28.1, 33.9, 36.5, 38.6, 38.7, 43.0, 45.6, 46.0, 48.0, 51.4, 52.5, 54.2, 55.0, 110.4, 114.1, 119.7, 121.0, 121.2, 121.3, 123.4, 125.5, 125.7, 127.3, 135.3, 139.2, 146.2, 146.9, 180.4; IR (KBr, cm−1): 3421, 3037, 2949, 2928, 2861, 1720, 1609, 1462, 1382, 1359, 1248, 1110, 733; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O2: 542.3747; found: 542.3742.
2.3.13. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-formylpiperazin-1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7e)
Yellow amorphous solid; Yield: 52%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.32 (d, J = 6.8 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.75–2.12 (m, 6H), 2.2 0 ∼ 2.40 (m, 7H), 2.96 (m, 1H), 3.32 (brs, 2H), 3.50 (brs, 2H), 3.62 (s, 3H), 3.71 (s, 1H), 4.56 (m, 2H), 7.04 (t, J = 7.8 Hz, 1H), 7.15 (d, J = 7.9 Hz, 1H), 7.16 (t, J = 7.9 Hz, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.41 (s, 1H), 7.42 (d, J = 7.4 Hz, 1H), 7.99 (s, 1H, CHO); 13C NMR (125 MHz, CDCl3) δ: 18.2, 19.2, 21.1, 24.1, 24.2, 27.3, 33.9, 36.5, 38.6, 38.7, 39.9, 43.1, 45.6, 46.0, 52.4, 52.5, 53.6, 55.3, 110.3, 114.1, 119.7, 120.8, 120.9, 121.3, 123.4, 125.4, 125.9, 127.3, 135.4, 139.2, 146.1, 146.9, 160.7, 180.3; IR (KBr, cm−1): 3041, 2958, 2930, 2861, 2808, 2773, 1722, 1679, 1608, 1436, 1398, 1260, 1108, 733; HRMS (ESI): m/z [M + H]+ calcd. for C35H46N3O3: 556.3539; found: 556.3534.
2.3.14. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-phenyl piperazin-1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7f)
Yellow amorphous solid; Yield: 46%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.30 (d, J = 7.7 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.64 (m, 1H), 1.74 (s, 3H), 1.80–2.10 (m, 6H), 2.27 (d, J = 10.8 Hz, 1H), 2.35 (brs, 2H), 2.51 (m, 4H), 2.94 (m, 1H), 3.15 (brs, 4H), 3.58 (s, 3H), 3.72 (s, 1H), 4.53 (brs, 2H), 6.81 (t, J = 6.8 Hz, 1H), 6.87 (d, J = 7.6 Hz, 2H), 7.03 (t, J = 7.2 Hz, 1H), 7.12 (d, J = 6.1 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 7.21 (t, J = 7.0 Hz, 2H), 7.28 (d, J = 7.7 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.42 (s, 1H), 7.43 (d, J = 7.4 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.2, 19.3, 21.1, 24.2, 24.3, 27.5, 33.9, 36.6, 38.6, 38.8, 43.4, 45.6, 46.1, 49.1, 52.4, 53.3, 55.5, 110.3, 114.0, 116.1, 119.7, 119.8, 120.8, 120.9, 121.4, 123.4, 125.4, 125.9, 127.4, 129.1, 135.4, 139.3, 146.2, 147.0, 151.3, 180.3; IR (KBr, cm−1): 3041, 2949, 2920, 2850, 1721, 1600, 1497, 1462, 1382, 1231, 1139, 733; HRMS (ESI): m/z [M + H]+ calcd. for C40H50N3O2: 604.3903; found: 604.3910.
2.3.15. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-(pyridine-2-yl) piperazin-1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7g)
Yellow amorphous solid; Yield: 52%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 6.7 Hz, 3H), 1.32 (d, J = 6.7 Hz, 3H), 1.66 (m, 1H), 1.74 (s, 3H), 1.78–2.20 (m, 6H), 2.29 (d, J = 11.5 Hz, 1H), 2.40 (brs, 2H), 2.49 (brs, 4H), 2.97 (m, 1H), 3.55 (brs, 4H), 3.61 (s, 3H), 3.72 (s, 1H), 4.58 (brs, 2H), 6.62 (d, J = 8.0 Hz, 1H), 6.63 (t, J = 8.0 Hz, 1H), 7.04 (t, J = 7.7 Hz, 1H), 7.15 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.33 (d, J = 8.9 Hz, 1H), 7.4 0 ∼ 7.50 (m, 3H), 8.18 (d, J = 3.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.2, 19.3, 21.2, 24.2, 24.3, 27.5, 34.0, 36.6, 38.7, 38.8, 43.4, 45.3, 45.7, 46.1, 52.5, 53.1, 55.6, 107.2, 110.3, 113.5, 114.0, 119.7, 120.8, 121.0, 121.4, 123.4, 125.4, 125.9, 127.4, 135.5, 137.6, 139.2, 146.2, 147.0, 148.1, 159.6, 180.4; IR (KBr, cm−1): 3050, 2954, 2866, 2808, 1718, 1595, 1480, 1432, 1379, 1265, 1115, 733; HRMS (ESI): m/z [M + H]+ calcd. for C39H49N4O2: 605.3856; found: 605.3852.
2.3.16. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-benzylpiperazin-1-yl)propyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (7h)
Yellow amorphous solid; Yield: 47%; 1H NMR (300 MHz, CDCl3) δ: 1.04 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.65 (m, 1H), 1.74 (s, 3H), 1.7 6 ∼ 2.15 (m, 6H), 2.28 (d, J = 12.1 Hz, 1H), 2.36 (t, J = 7.0 Hz, 2H), 2.46 (brs, 8H), 2.95 (m, 1H), 3.50 (s, 2H), 3.61 (s, 3H), 3.71 (s, 1H), 4.48 (t, J = 7.5 Hz, 2H), 7.03 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 8.0 Hz, 1H), 7.14 (t, J = 8.0 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.30 (s, 5H), 7.33 (d, J = 9.5 Hz, 1H), 7.40 (s, 1H), 7.42 (d, J = 10.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.1, 19.2, 21.1, 24.1, 24.2, 27.5, 33.9, 36.5, 38.5, 38.7, 43.5, 45.5, 46.0, 52.4, 53.1, 53.2, 55.6, 63.1, 110.3, 113.7, 119.6, 120.6, 120.8, 121.3, 123.3, 125.3, 125.7, 127.0, 127.3, 128.2, 129.2, 135.3, 138.0, 139.1, 146.1, 146.9, 180.3; IR (KBr, cm−1): 3028, 2954, 2929, 2870, 2809, 1721, 1607, 1458, 1383, 1345, 1248, 1138, 1012, 734; HRMS (ESI): m/z [M + H]+ calcd. for C41H52N3O2: 618.4060; found: 618.4066.
2.3.17. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(piperazin-1-yl) butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8a)
Yellow amorphous solid; Yield: 62%; 1H NMR (300 MHz, CDCl3) δ: 1.04 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.64 (m, 1H), 1.73 (s, 3H), 1.7 5 ∼ 2.10 (m, 6H), 2.28 (d, J = 10.6 Hz, 1H), 2.38 (m, 2H), 2.42–2.75 (m, 7H), 2.95 (m, 1H), 3.10 (m, 4H), 3.61 (s, 3H), 3.71 (s, 1H), 4.45 (m, 2H), 7.04 (t, J = 7.6 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.16 (t, J = 7.3 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.37 (s, 1H), 7.40 (d, J = 9.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.1, 21.0, 23.8, 24.1, 24.2, 27.5, 33.8, 36.4, 38.5, 38.6, 43.5, 45.0, 45.5, 46.0, 49.5, 52.4, 57.2, 110.1, 113.7, 119.6, 120.7, 120.9, 121.2, 123.4, 125.4, 125.7, 127.2, 135.4, 138.9, 146.1, 146.9, 180.3; IR (KBr, cm−1): 3396, 3046, 2956, 2928, 2867, 2813, 1722, 1609, 1460, 1382, 1360, 1250, 1133, 734; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O2: 542.3747; found: 542.3741.
2.3.18. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(3-(4-methyl piperazin-1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8b)
Yellow amorphous solid; Yield: 63%; 1H NMR (300 MHz, CDCl3): δ 1.04 (s, 3H), 1.30 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 6.3 Hz, 3H), 1.5 0–1.70 (m, 3H), 1.74 (s, 3H), 1.80–2.10 (m, 6H), 2.29 (d, J = 11.4 Hz, 1H), 2.31 (s, 3H), 2.40 (t, J = 7.4 Hz, 2H), 2.48 (brs, 8H), 2.95 (m, 1H), 3.61 (s, 3H), 3.72 (s, 1H), 4.43 (m, 2H), 7.04 (t, J = 7.3 Hz, 1H), 7.14 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.4 Hz, 1H), 7.30 (d, J = 8.7 Hz, 1H), 7.33 (d, J = 8.9 Hz, 1H), 7.38 (s, 1H), 7.42 (d, J = 8.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.1, 21.0, 24.1, 24.1, 24.1, 28.0, 33.8, 36.4, 38.5, 38.6, 45.2, 45.5, 45.8, 46.0, 52.3, 52.8, 54.9, 57.7, 110.2, 113.5, 119.5, 120.6, 120.8, 121.2, 123.3, 125.3, 125.7, 127.2, 135.3, 138.9, 146.1, 146.9, 180.3; IR (KBr, cm−1): 3037, 2932, 2869, 2797, 1724, 1604, 1458, 1358, 1252, 1164, 742; HRMS (ESI): m/z [M + H]+ calcd. for C36H50N3O2: 556.3903; found: 556.3910.
2.3.19. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-ethylpiperazin -1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8c)
Yellow amorphous solid; Yield: 49%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.09 (t, J = 7.5 Hz, 3H), 1.31 (d, J = 6.7 Hz, 3H), 1.32 (d, J = 6.8 Hz, 3H), 1.50–1.70 (m, 3H), 1.74 (s, 3H), 1.80–2.15 (m, 6H), 2.29 (d, J = 11.9 Hz, 1H), 2.35–2.44 (m, 4H), 2.48 (brs, 8H), 2.95 (m, 1H), 3.61 (s, 3H), 3.72 (s, 1H), 4.42 (m, 2H), 7.04 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.7 Hz, 1H), 7.30 (d, J = 9.2 Hz, 1H), 7.33 (d, J = 9.0 Hz, 1H), 7.38 (s, 1H), 7.43 (d, J = 8.1 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 11.8, 18.1, 19.1, 21.0, 24.1, 24.1, 24.1, 28.0, 33.8, 36.4, 38.5, 38.6, 45.2, 45.5, 46.0, 52.2, 52.3, 52.6, 52.9, 57.8, 110.1, 113.4, 119.5, 120.6, 120.8, 121.2, 123.3, 125.3, 125.6, 127.2, 135.3, 138.9, 146.0, 146.9, 180.2; IR (KBr, cm−1): 3050, 2958, 2927, 2854, 2810, 1724, 1608, 1463, 1347, 1252, 1165, 733; HRMS (ESI): m/z [M + H]+ calcd. for C37H52N3O2: 570.4060; found: 570.4064.
2.3.20. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(1,4-diazepan-1-yl) butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8d)
Yellow amorphous solid; Yield: 39%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.30 (d, J = 6.3 Hz, 3H), 1.32 (d, J = 6.6 Hz, 3H), 1.40–1.70 (m, 3H), 1.73 (s, 3H), 1.77–2.05 (m, 8H), 2.29 (d, J = 11.4 Hz, 1H), 2.50 (t, J = 7.2 Hz, 2H), 2.58–2.70 (m, 5H), 2.95 (m, 1H), 3.08 (m, 2H), 3.17 (m, 2H), 3.61 (s, 3H), 3.71 (s, 1H), 4.45 (t, J = 7.7 Hz, 2H), 7.04 (t, J = 8.0 Hz, 1H), 7.15 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 7.8 Hz, 1H), 7.38 (s, 1H), 7.39 (d, J = 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 16.6, 18.2, 19.2, 21.1, 22.8, 24.2, 24.2, 27.6, 33.9, 36.5, 38.5, 38.6, 38.7, 44.5, 45.0, 45.6, 46.0, 47.7, 52.5, 53.7, 57.5, 110.3, 114.2, 119.7, 120.8, 121.1, 121.3, 123.5, 125.5, 125.9, 127.3, 135.5, 139.0, 146.2, 147.0, 180.4; IR (KBr, cm−1): 3394, 3041, 2958, 2930, 2864, 1721, 1603, 1462, 1382, 1361, 1248, 1127, 735; HRMS (ESI): m/z [M + H]+ calcd. for C36H50N3O2: 556.3903; found: 556.3909.
2.3.21. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-formylpiperazin-1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8e)
Yellow amorphous solid; Yield: 53%; 1H NMR (300 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 6.6 Hz, 3H), 1.33 (d, J = 6.7 Hz, 3H), 1.50–1.72 (m, 3H), 1.74 (s, 3H), 1.76–2.10 (m, 6H), 2.25–2.45 (m, 7H), 2.96 (m, 1H), 3.33 (brs, 2H), 3.52 (brs, 2H), 3.62 (s, 3H), 3.71 (s, 1H), 4.46 (m, 2H), 7.04 (t, J = 7.6 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.16 (t, J = 7.8 Hz, 1H), 7.31 (d, J = 8.2 Hz, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.39 (s, 1H), 7.41 (d, J = 10.3 Hz, 1H), 8.00 (s, 1H, CHO); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.1, 21.0, 23.9, 24.1, 27.7, 33.8, 36.4, 38.5, 38.6, 39.9, 45.1, 45.5, 45.9, 52.2, 52.3, 53.5, 57.6, 110.1, 113.6, 119.5, 120.7, 120.8, 121.2, 123.3, 125.4, 125.7, 127.2, 135.4, 138.9, 146.0, 146.9, 160.6, 180.2; IR (KBr, cm−1): 3045, 2949, 2925, 2855, 2817, 1721, 1679, 1466, 1360, 1219, 1137, 735; HRMS (ESI): m/z [M + H]+ calcd. for C36H48N3O3: 570.3696; found: 570.3689.
2.3.22. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-phenyl piperazin-1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8f)
Yellow amorphous solid; Yield: 49%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.32 (d, J = 7.6 Hz, 3H), 1.33 (d, J = 7.5 Hz, 3H), 1.60–1.70 (m, 3H), 1.75 (s, 3H), 1.80–2.10 (m, 6H), 2.29 (d, J = 11.8 Hz, 1H), 2.43 (t, J = 7.4 Hz, 2H), 2.55 (m, 4H), 2.97 (m, 1H), 3.18 (m, 4H), 3.61 (s, 3H), 3.72 (s, 1H), 4.46 (m, 2H), 6.84 (t, J = 7.3 Hz, 1H), 6.91 (d, J = 8.2 Hz, 2H), 7.04 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 7.15 (t, J = 7.4 Hz, 1H), 7.25 (t, J = 7.8 Hz, 2H), 7.30 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 7.40 (s, 1H), 7.43 (d, J = 8.2 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.3, 19.3, 21.2, 24.2, 24.3, 24.3, 28.1, 34.0, 36.6, 38.7, 38.8, 45.4, 45.7, 46.1, 49.3, 52.5, 53.3, 58.0, 110.3, 113.7, 116.2, 119.7, 119.8, 120.9, 121.0, 121.4, 123.4, 125.5, 125.9, 127.5, 129.2, 135.6, 139.2, 146.2, 147.1, 151.5, 180.4; IR (KBr, cm−1): 3041, 2949, 2930, 2818, 1720, 1600, 1501, 1454, 1357, 1235, 1149, 757; HRMS (ESI): m/z [M + H]+ calcd. for C41H52N3O2: 618.4060; found: 618.4052.
2.3.23. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-(pyridine-2-yl) piperazin-1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8g)
Yellow amorphous solid; Yield: 56%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.30 (d, J = 6.0 Hz, 3H), 1.33 (d, J = 6.0 Hz, 3H), 1.55–1.72 (m, 3H), 1.74 (s, 3H), 1.78–2.15 (m, 6H), 2.29 (d, J = 11.4 Hz, 1H), 2.43 (t, J = 7.3 Hz, 2H), 2.51 (brs, 4H), 2.96 (m, 1H), 3.53 (brs, 4H), 3.61 (s, 3H), 3.72 (s, 1H), 4.46 (brs, 2H), 6.62 (t, J = 8.0 Hz, 1H), 6.63 (d, J = 8.0 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 7.15 (d, J = 7.7 Hz, 1H), 7.16 (t, J = 7.7 Hz, 1H), 7.30 (d, J = 8.9 Hz, 1H), 7.33 (d, J = 9.0 Hz, 1H), 7.40 (s, 1H), 7.43 (d, J = 8.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 8.19 (d, J = 4.0 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.3, 21.1, 24.1, 24.2, 24.3, 28.1, 33.9, 36.5, 38.6, 38.7, 45.2, 45.3, 45.6, 46.1, 52.5, 53.0, 58.0, 107.1, 110.2, 113.4, 113.7, 119.6, 120.8, 120.9, 121.4, 123.4, 125.5, 125.8, 127.4, 135.5, 137.5, 139.1, 146.2, 147.0, 148.0, 159.6, 180.4; IR (KBr, cm−1): 3046, 2962, 2926, 2853, 1722, 1593, 1436, 1380, 1247, 1140, 734; HRMS (ESI): m/z [M + H]+ calcd. for C40H51N4O2: 619.4012; found: 619.4018.
2.3.24. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a-dimethyl-9–(2-(4-benzylpiperazin -1-yl)butyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (8h)
Yellow amorphous solid; Yield: 54%; 1H NMR (300 MHz, CDCl3) δ: 1.04 (s, 3H), 1.30 (d, J = 6.7 Hz, 3H), 1.32 (d, J = 6.7 Hz, 3H), 1.50–1.70 (m, 3H), 1.74 (s, 3H), 1.76–2.10 (m, 6H), 2.29 (d, J = 12.4 Hz, 1H), 2.40 (t, J = 7.6 Hz, 2H), 2.48 (brs, 8H), 2.95 (m, 1H), 3.53 (s, 2H), 3.61 (s, 3H), 3.72 (s, 1H), 4.42 (m, 2H), 7.04 (t, J = 7.8 Hz, 1H), 7.14 (d, J = 8.0 Hz, 1H), 7.15 (t, J = 7.0 Hz, 1H), 7.30 (d, J = 8.5 Hz, 1H), 7.31 (s, 5H), 7.32 (d, J = 8.3 Hz, 1H), 7.38 (s, 1H), 7.42 (d, J = 8.3 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.2, 21.1, 24.1, 24.2, 24.2, 28.0, 33.8, 36.4, 38.5, 38.6, 45.2, 45.5, 46.0, 51.4, 53.0, 53.5, 57.7, 63.1, 110.3, 113.4, 119.6, 120.6, 120.9, 121.2, 123.3, 125.4, 125.7, 127.0, 127.2, 128.2, 129.2, 135.3, 138.1, 139.0, 146.0, 146.8, 180.1; IR (KBr, cm−1): 3024, 2932, 2872, 2806, 1722, 1603, 1455, 1348, 1283, 1162, 1009, 738; HRMS (ESI): m/z [M + H]+ calcd. for C42H54N3O2: 632.4216; found: 632.4207.
2.4. General procedure for the synthesis of compounds 10a-j
To a solution of compound 3 (1.8 g, 5.5 mmol) in 20 mL of EtOH was added 12 mmol of substituted phenylhydrazine hydrochloride and 2 mL of concentrated HCl. The mixture was refluxed for 3 h. After cooling, the mixture was poured into 100 mL of ice-cold water and extracted with CH2Cl2 (3 × 80 mL). The organic layer was combined, washed with saturated NaHCO3 solution and brine, dried over anhydrous Na2SO4 and concentrated to give a crude product, which was subject to a silica gel column chromatography (petroleum ethereacetone 50:1, v/v) to afford compound 4a-j. Subsequently, to a solution of compound 4a-j (1.75 mmol) in benzene (5 mL) were added 1,2-dibromoalkane (3.76 g, 20 mmol), tetrabutyl ammonium bromide (TBAB) (0.02 g, 0.062 mmol) and 50% NaOH solution (3 mL). The mixture was stirred at room temperature for 12 h. Then the mixture was poured into 100 mL of ice-cold water. The suspension was extracted with CH2Cl2 (3 × 80 mL). The organic layer was combined, washed with water and brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on a silica gel column, eluting with petroleum ether-acetone (100:1, v/v) to give compounds 9a-j (Yield: 58–69%). Further, to a solution of compound 9a-j (0.5 mmol) in acetonitrile (15 mL) was added anhydrous K2CO3 (0.345 g, 2.5 mmol), KI (0.083 g, 0.5 mmol) and 10 mmol of anhydrous piperazine. The mixture was refluxed for 8–12 h and monitored by TLC. At the end of reaction, the mixture was poured into cold water, which was extracted by CH2Cl2 (100 mL) for three times. The organic phase was combined, washed with water and brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was subjected to silica gel chromatography, eluting with petroleum ether-acetone (100:1, v/v) to afford compounds 10a-j.
2.4.1. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a,12-trimethyl-9–(2-(piperazin-1-yl) ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10a)
Yellow amorphous solid; Yield: 51%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.30 (d, J = 6.2 Hz, 3H), 1.32 (d, J = 6.2 Hz, 3H), 1.64 (m, 1H), 1.73 (s, 3H), 1.8 1–2.00 (m, 4H), 2.28 (d, J = 11.7 Hz, 1H), 2.42 (s, 3H), 2.58 (brs, 4H), 2.79 (m, 2H), 2.90 (m, 4H), 3.01 (m, 1H), 3.13 (brs, 1H, NH), 3.64 (s, 3H), 3.68 (s, 1H), 4.55 (m, 2H), 6.99 (d, J = 8.2 Hz, 1H), 7.12 (s, 1H), 7.13 (d, J = 9.0 Hz, 1H), 7.29 (d, J = 8.2 Hz, 2H), 7.43 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.4, 21.3, 21.9, 24.2, 24.3, 34.0, 36.5, 38.6, 38.7, 43.0, 43.4, 45.6, 46.0, 50.1, 52.4, 57.1, 109.7, 113.9, 120.9, 121.1, 122.7, 123.7, 125.4, 126.4, 127.4, 128.9, 135.9, 137.5, 146.2, 147.0, 180.2; IR (KBr, cm−1): 2948, 2929, 2868, 1724, 1678, 1498, 1457, 1363, 1253, 1190, 1000, 826, 736; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O2: 528.3590; found: 528.3595.
2.4.2. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-1,4a,10-trimethyl-9–(2-(piperazin-1-yl) ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10b)
Yellow amorphous solid; Yield: 45%; 1H NMR (300 MHz, CDCl3) δ: 1.14 (s, 3H), 1.30 (d, J = 6.4 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.63 (m, 1H), 1.76 (s, 3H), 1.8 0–2.10 (m, 4H), 2.22 (d, J = 13.7 Hz, 1H), 2.31 (brs, 4H), 2.35 (m, 2H), 2.61 (brs, 1H, NH), 2.73 (s, 3H), 2.78 (m, 4H), 2.96 (m, 1H), 3.58 (s, 3H), 3.66 (s, 1H), 4.75 (m, 2H), 6.90 (d, J = 6.7 Hz, 1H), 6.98 (t, J = 7.5 Hz, 1H), 7.13 (d, J = 7.4 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.38 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.1, 19.4, 21.0, 21.2, 24.1, 24.2, 33.9, 36.5, 38.4, 38.8, 44.2, 45.5, 46.1, 46.2, 52.2, 52.4, 57.8, 116.6, 119.0, 120.5, 121.9, 122.1, 123.4, 124.9, 125.2, 126.9, 128.5, 138.4, 139.8, 146.2, 146.9, 180.3; IR (KBr, cm−1): 2953, 2926, 2855, 1724, 1680, 1493, 1459, 1382, 1253, 1188, 1081, 965, 741; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O2: 528.3590; found: 528.3583.
2.4.3. 2,3,4,4a,9,13c-Hexahydro-12-ethyl-7-isopropyl-1,4a-dimethyl-9–(2-(piperazin -1-yl) ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10c)
Yellow amorphous solid; Yield: 58%; 1H NMR (600 MHz, CDCl3) δ: 1.05 (s, 3H), 1.25–1.31 (m, 9H), 1.64 (m, 1H), 1.74 (s, 3H), 1.82–1.98 (m, 4H), 2.28 (d, J = 11.5 Hz, 1H), 2.63 (m, 4H), 2.71 (q, J = 6.2 Hz, 2H), 2.76 (m, 1H), 2.85 (m, 1H), 2.92 (m, 4H), 2.99 (m, 1H), 3.46 (brs, 1H, NH), 3.63 (s, 3H), 3.69 (s, 1H), 4.54 (m, 2H), 7.02 (d, J = 8.4 Hz, 1H), 7.13 (dd, J = 8.0, 1.6 Hz, 1H), 7.16 (s, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 1.3 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 16.4, 18.2, 19.3, 21.3, 24.2, 24.2, 29.8, 33.9, 36.5, 38.6, 38.7, 43.4, 45.6, 45.9, 46.0, 50.2, 52.4, 57.2, 109.6, 113.9, 119.5, 121.1, 121.8, 123.6, 125.3, 126.3, 127.4, 135.6, 135.8, 137.6, 146.2, 147.0, 180.2; IR (KBr, cm−1): 2949, 2928, 2855, 1720, 1615, 1498, 1469, 1384, 1217, 1052, 824, 737; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O2: 542.3747; found: 542.3753.
2.4.4. 2,3,4,4a,9,13c-Hexahydro-7,12-diisopropyl-1,4a-dimethyl-9–(2-(piperazin-1-yl) ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10d)
Yellow amorphous solid; Yield: 42%; 1H NMR (600 MHz, CDCl3) δ: 1.05 (s, 3H), 1.29 (d, J = 6.8 Hz, 6H), 1.30 (d, J = 6.5 Hz, 3H), 1.31 (d, J = 6.9 Hz, 3H), 1.64 (m, 1H), 1.74 (s, 3H), 1.8 2 ∼ 1.98 (m, 4H), 2.28 (d, J = 11.9 Hz, 1H), 2.6 0 ∼ 2.70 (m, 5H), 2.75 (m, 1H), 2.85 (m, 1H), 2.9 0 ∼ 2.98 (m, 6H), 3.62 (s, 3H), 3.69 (s, 1H), 4.53 (m, 2H), 7.05 (dd, J = 8.5, 1.3 Hz,1H), 7.13 (dd, J = 8.0, 1.6 Hz, 1H), 7.21 (s, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 1.6 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.3, 21.3, 24.2, 24.7, 24.9, 34.0, 34.5, 36.5, 38.7, 38.8, 43.0, 43.4, 45.6, 45.9, 50.2, 52.5, 57.2, 109.5, 114.0, 118.0, 120.7, 121.0, 123.6, 125.3, 126.1, 127.4, 135.7, 137.6, 140.4, 146.2, 147.0, 180.3; IR (KBr, cm−1): 2951, 2928, 2854, 1723, 1609, 1498, 1459, 1381, 1362, 1250, 1130, 824, 736; HRMS (ESI): m/z [M + H]+ calcd. for C36H50N3O2: 556.3903; found: 556.3911.
2.4.5. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-12-methoxy-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10e)
Yellow amorphous solid; Yield: 55%; 1H NMR (300 MHz, CDCl3) δ: 1.06 (s, 3H), 1.30 (d, J = 7.0 Hz, 3H), 1.32 (d, J = 6.2 Hz, 3H), 1.67 (m, 1H), 1.75 (s, 3H), 1.80–2.00 (m, 4H), 2.30 (d, J = 11.8 Hz, 1H), 2.50–2.55 (m, 5H), 2.72–3.00 (m, 7H), 3.62 (s, 3H), 3.70 (s, 1H), 3.81 (s, 3H), 4.54 (m, 2H), 6.83 (d, J = 9.4 Hz, 1H), 6.85 (s, 1H), 7.14 (d, J = 8.1 Hz, 1H), 7.30 (d, J = 7.9 Hz, 2H), 7.44 (s, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.2, 19.3, 21.3, 24.2, 24.2, 34.0, 36.4, 38.7, 38.7, 43.0, 43.3, 45.7, 45.9, 50.1, 52.6, 55.8, 57.2, 102.8, 110.5, 111.6, 114.0, 121.1, 123.7, 125.5, 126.3, 127.3, 134.3, 136.3, 146.2, 147.0, 154.2, 180.5; IR (KBr, cm−1): 2957, 2929, 2855, 1719, 1677, 1616, 1499, 1453, 1363, 1223, 1049, 826, 734; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O3: 544.3539; found: 544.3531.
2.4.6. 2,3,4,4a,9,13c-Hexahydro-7-isopropyl-10-methoxy-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10f)
Yellow amorphous solid; Yield: 51%; 1H NMR (600 MHz, CDCl3) δ: 1.08 (s, 3H), 1.29 (d, J = 6.6 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H), 1.61 (m, 1H), 1.73 (s, 3H), 1.80–2.00 (m, 4H), 2.28 (d, J = 12.0 Hz, 1H), 2.50–2.65 (m, 6H), 2.80–3.00 (m, 6H), 3.58 (s, 3H), 3.64 (s, 1H), 3.94 (s, 3H), 4.75 (m, 1H), 4.97 (m, 1H), 6.61 (d, J = 7.1 Hz, 1H), 6.92 (d, J = 8.1 Hz, 1H), 6.96 (d, J = 7.6 Hz, 1H), 7.12 (dd, J = 8.0, 1.6 Hz, 1H), 7.28 (d, J = 8.2 Hz, 1H), 7.39 (d, J = 1.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.3, 21.2, 24.1, 24.2, 33.8, 36.5, 38.5, 38.7, 43.2, 44.6, 45.5, 46.0, 49.9, 52.4, 55.4, 58.5, 102.6, 113.9, 115.3, 120.2, 121.7, 123.5, 125.3, 127.0, 128.7, 129.1, 137.1, 146.1, 146.9, 147.7, 180.3; IR (KBr, cm−1): 2957, 2929, 2855, 1722, 1678, 1608, 1568, 1458, 1365, 1259, 1046, 825, 732; HRMS (ESI): m/z [M + H]+ calcd. for C34H46N3O3: 544.3539; found: 544.3543.
2.4.7. 2,3,4,4a,9,13c-Hexahydro-12-ethoxy-7-isopropyl-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10g)
Yellow amorphous solid; Yield: 53%; 1H NMR (500 MHz, CDCl3) δ: 1.11 (s, 3H), 1.30 (d, J = 7.6 Hz, 3H), 1.31 (d, J = 7.8 Hz, 3H), 1.52 (t, J = 7.0 Hz, 3H), 1.63 (m, 1H), 1.74 (s, 3H), 1.80–2.05 (m, 4H), 2.29 (d, J = 11.6 Hz, 1H), 2.41 (brs, 4H), 2.50–2.60 (m, 4H), 2.80–2.90 (m, 3H), 2.94 (m, 1H), 3.59 (s, 3H), 3.66 (s, 1H), 4.20 (q, J = 7.0 Hz, 2H), 4.80 (m, 1H), 5.07 (m, 1H), 6.60 (d, J = 5.7 Hz, 1H), 6.92 (s, 1H), 6.93 (d, J = 6.1 Hz, 1H), 7.12 (d, J = 7.8 Hz, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.42 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 15.1, 18.1, 19.3, 21.2, 24.0, 24.2, 33.8, 36.5, 38.5, 38.7, 43.4, 45.4, 45.5, 46.0, 50.4, 52.3, 58.6, 63.8, 103.4, 113.8, 115.3, 120.2, 121.8, 123.4, 125.2, 127.1, 128.9, 129.1, 137.2, 146.0, 146.9, 147.0, 180.2; IR (KBr, cm−1): 2945, 2928, 2855, 1720, 1615, 1498, 1469, 1383, 1217, 1052, 824, 737; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O3: 558.3696; found: 558.3701.
2.4.8. 2,3,4,4a,9,13c-Hexahydro-10-ethoxy-7-isopropyl-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10h)
Yellow amorphous solid; Yield: 58%; 1H NMR (500 MHz, CDCl3) δ: 1.06 (s, 3H), 1.31 (d, J = 7.8 Hz, 3H), 1.33 (d, J = 7.3 Hz, 3H), 1.44 (t, J = 7.0 Hz, 3H), 1.67 (dt, J = 12.9, 2.0 Hz, 1H), 1.75 (s, 3H), 1.8 0 ∼ 2.05 (m, 4H), 2.29 (d, J = 11.9 Hz, 1H), 2.52 (brs, 4H), 2.7 0 ∼ 2.95 (m, 7H), 2.96 (m, 1H), 3.61 (s, 3H), 3.70 (s, 1H), 4.03 (m, 2H), 4.53 (m, 2H), 6.84 (dd, J = 8.2, 1.8 Hz, 1H), 6.85 (s, 1H), 7.14 (d, J = 8.0 Hz, 1H), 7.28–7.32 (m, 2H), 7.45 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 15.2, 18.3, 19.3, 21.3, 24.2, 24.3, 34.1, 36.6, 38.8, 38.8, 43.4, 45.6, 45.8, 46.0, 52.5, 54.5, 58.2, 64.1, 103.7, 110.6, 112.3, 113.6, 121.5, 123.5, 125.3, 126.1, 127.4, 134.5, 136.2, 146.3, 147.0, 153.4, 180.6; IR (KBr, cm−1): 2951, 2927, 2852, 1717, 1612, 1493, 1460, 1381, 1221, 1051, 812, 758; HRMS (ESI): m/z [M + H]+ calcd. for C35H48N3O3: 558.3696; found: 558.3690.
2.4.9. 2,3,4,4a,9,13c-Hexahydro-12-fluoro-7-isopropyl-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10i)
Yellow amorphous solid; Yield: 49%; 1H NMR (500 MHz, CDCl3) δ: 1.05 (s, 3H), 1.31 (d, J = 7.8 Hz, 3H), 1.33 (d, J = 7.6 Hz, 3H), 1.65 (t, J = 13.0 Hz, 1H), 1.72 (s, 3H), 1.80–2.00 (m, 4H), 2.20 (brs, 1H, NH), 2.29 (d, J = 11.7 Hz, 1H), 2.50 (m, 4H), 2.80 (m, 2H), 2.86 (m, 4H), 2.96 (m, 1H), 3.67 (s, 3H), 3.68 (s, 1H), 4.55 (m, 2H), 6.90 (dt, J = 8.8, 1.8 Hz, 1H), 7.01 (dd, J = 10.2, 1.5 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.33 (dd, J = 8.8, 4.6 Hz, 1H), 7.46 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 18.2, 19.1, 21.3, 24.1, 24.3, 34.1, 36.5, 38.7, 43.6, 45.5, 45.9, 46.0, 52.5, 55.0, 58.2, 105.8 (d, J = 25.4 Hz), 109.2 (d, J = 25.9 Hz), 110.5 (d, J = 9.7 Hz), 113.7 (d, J = 4.6 Hz), 121.7, 123.5, 125.7, 126.0 (d, J = 9.8 Hz), 127.0, 135.9, 137.3, 146.4, 147.2, 157.9 (d, J = 231.6 Hz), 180.0; IR (KBr, cm−1): 2948, 2930, 2869, 1723, 1679, 1616, 1497, 1456, 1382, 1243, 1125, 1037, 823; HRMS (ESI): m/z [M + H]+ calcd. for C33H43FN3O2: 532.3339; found: 532.3345.
2.4.10. 2,3,4,4a,9,13c-Hexahydro-12-chloro-7-isopropyl-1,4a-dimethyl-9–(2- (piperazin-1-yl)ethyl)-1H-dibenzo[a,c]carbazole-1-carboxylic acid methyl ester (10j)
Yellow amorphous solid; Yield: 58%; 1H NMR (600 MHz, CDCl3) δ: 1.05 (s, 3H), 1.27 (d, J = 7.0 Hz, 3H), 1.29 (d, J = 6.8 Hz, 3H), 1.63 (m, 1H), 1.71 (s, 3H), 1.80–1.97 (m, 4H), 2.27 (d, J = 12.7 Hz, 1H), 2.64 (m, 4H), 2.7 0 ∼ 2.80 (m, 3H), 2.91 (m, 4H), 2.98 (m, 1H), 3.65 (s, 1H), 3.71 (s, 3H), 4.51 (m, 1H), 4.62 (m, 1H), 7.10 (dd, J = 8.7, 1.9 Hz, 1H), 7.16 (dd, J = 8.1, 1.6 Hz, 1H), 7.25 (s, 1H), 7.28 (d, J = 1.9 Hz, 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.37 (d, J = 1.5 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 18.1, 19.0, 21.2, 24.1, 24.2, 33.9, 36.4, 38.5, 43.3, 45.2, 45.4, 45.9, 52.4, 53.7, 57.8, 110.9, 113.3, 120.1, 121.0, 121.5, 123.5, 125.4, 125.8, 126.8, 137.0, 137.4, 146.3, 147.1, 162.5, 179.7; IR (KBr, cm−1): 2952, 2929, 2856, 1725, 1670, 1607, 1495, 1455, 1360, 1262, 1111, 968, 737; HRMS (ESI): m/z [M + H]+ calcd. for C33H43ClN3O2: 548.3044; found: 548.3038.
2.5. Biological evaluation
2.5.1. Cell lines and culture
Three human hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B) and normal hepatocyte cell line (QSG-7701) were maintained in Dulbecco Modified Eagle Medium (DMEM) containing 4.0 mM L-Glutamine and 4500 mg/l Glucose supplemented with 10% (v/v) foetalbovine serum (FBS) and 100 unites/mL penicillin/streptomycin at 37 °C in humidified atmosphere of 5% CO2 and 95% air.
2.5.2. Cytotoxic assay
The in vitro cytotoxic activities of the carbazole derivatives of DHA were evaluated against three human hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B) and a normal human hepatocyte cell line (QSG-7701) via the MTT colorimetric method39. Briefly, SMMC-7721, HepG2, Hep3B and QSG-7701 cells were harvested at log phase of growth and seeded in 96-well plates (100 µL/well at a density of 2 × 105 cells/mL). After 24 h incubation at 37 °C and 5% CO2 to allow cell attachment, cultures were exposed to various concentrations of the isolated compounds for 48 h. Finally, MTT solution (2.5 mg/mL in PBS) was added (40 µL/well). Plates were further incubated for 4 h at 37 °C, and the formazan crystals formed were dissolved by adding 150 µL/well of DMSO. Absorption at 570 nm was measured with an ELISA plate reader. The results were expressed as IC50 values (mean, n = 3), which was defined as the concentration at which 50% survival of cells was discerned. Doxorubicin was co-assayed as positive control.
2.5.3. In vitro MEK1 inhibition assay
An in vitro kinase assay of MEK1 was performed using ADP-Glo kinase assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Briefly, the kinase reaction was conducted in a 5 µL mixture [25 mM Tris-HCl (pH 7.5), 25 mH MgCl2, 2 mM dithiothretol, 10 µM ATP, 0.02% Triton X-100, 200 ng of recombinant GST-MEK1 protein (Active) and 200 ng of GST-ERK2 (Inactive) protein (Carna Biosciences, Japan)] with or without various concentrations of tested compounds at 22 °C for 30 min. Reactions were stopped by adding 5 µL of ADP-Glo reagent to each well. After incubating at 22 °C for 40 min, 10 µL of the kinase detection reagent was added and the plates were incubated for another 30 min at 22 °C in the dark. The reaction mixture was analysed by EnSpire (PerkinElmer, Waltham, MA, USA). U0126 was used as the positive control for MEK1 inhibition.
2.5.4. Molecular docking
The molecular modelling of compound 10g was performed with Schrödinger Suite 2015–1 (Schrödinger LLC., New York, NY, USA). The crystal structure of the MEK1 (PDB ID: 3EQF) was downloaded from Protein Data Bank (PDB) and prepared using the Protein Preparation Wizard workflow from Schrödinger Suite, including the optimisation of hydrogen bond network and a short energy minimisation with position restraints on heavy atoms using OPLS_2005 force field. The docking grid was generated according to the initial ligand K252A. Then the target compounds were freely docked into the designated binding site using the standard protocol implemented in Maestro v 10.1 (Schrödinger LLC, Cambridge, MA, USA). Van der Waals (vdW) scaling of 0.8 and partial cut-off of 0.15 were set to soften the potential for non-polar sites, and no constraints were specified. The best docked pose ranked by Glide Score value was recorded, and saved for each ligand. The structures of complexes were analysed for interaction modes, and the binding pose of compound 10g with MEK1 kinase was displayed using Discovery studio 3.5 client.
2.5.5. Cell cycle analysis
Cell cycle distributions in HepG2 cells were determined through propidium iodide (PI) staining and analysed by flow cytometry. HepG2 cells were seeded into a six-well plate at 5 × 105 cell/mL and treated with different concentrations of compound 10g for 48 h. After treatment, cells were detached with 0.25% trypsin, harvested by centrifugation, washed twice with ice-cold PBS and then fixed and permeabilised with ice-cold 70% ethanol at 4 °C overnight. Ethanol was removed and the cells were washed twice with ice-cold PBS. After this, the cells were treated with 100 µL of RNase (100 µg/mL) at 37 °C for 30 min, followed by incubation with 400 µl of DNA staining solution (PI) (1 mg/mL) in the dark at 4 °C for 30 min. The samples were analyzed by a flow cytometer (Becton-Dickinson FACSCalibur, NJ, USA) and data were analysed using the FlowJo software (Becton-Dickinson & Co, Totowa, NJ, USA).
2.5.6. Annexin V-FITC/PI dual staining assay
The extent of apoptosis was quantitatively measured using Annexin V-FITC/PI dual staining assay. HepG2 cells were seeded into a six-well plate at 5 × 105 cells per well in 10% foetal calf serum (FBS)-DMEM into six-well plates and treated with different concentrations of the indicated compound 10g for 48 h. The cells were detached with 0.25% trypsin, washed with ice-cold PBS for twice and then resuspended in 1 × Binding buffer (0.1 M Hepes/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2). The cells were stained with 5 µL of Annexin V-FITC and 5 µL of PI (propidium iodide) to each tube. The cells were gently vortexed and incubated in the dark at room temperature for 15 min and then keep them at 4 °C. The samples were analysed by a flow cytometer (Becton-Dickinson FACSCalibur, NJ, USA) and data were analysed using the FlowJo software (Becton-Dickinson & Co, Totowa, NJ, USA).
2.5.7. ROS generation assay
Reactive oxygen species (ROS) generation assay was performed by using the reactive oxygen species assay kit (Beyotime Biotech., China). Intracellular ROS generation was tested through dichlorodihydro fluorescein diacetate (DCFH-DA) assay. DCFH-DA is taken up by HepG2 cells, and then activated by esterase-mediated cleavage of acetate to form dichlorodihydro fluorescein (DCFH), which is trapped in the cells. DCFH is converted to fluorescein DCF in the presence of ROS. HepG2 cells were seeded in six-well plates and incubated with different concentrations of compound 10g for 24 h. After removing the compound solution, cells were treated with 10 µM of DCFH-DA at 37 °C for 20 min. Subsequently, the cells were washed with PBS for three times and then exposed to light. Immediately after light exposure, cell images were acquired through an inverted fluorescence microscope (Olympus 1X71 Inverted System Microscope, Olympus, Tokyo, Japan).
2.5.8. Mitochondrial membrane potential assay
The JC-1 mitochondrial membrane potential assay kit (Keygene Biotech., China) was employed to measure mitochondrial depolarization in HepG2 cells. Briefly, cells cultured in six-well plates after indicated treatments were incubated with an equal volume of JC-1 staining solution (5 µg/mL) at 37 °C for 20 min and rinsed twice with PBS. Mitochondrial membrane potentials were monitored by determining the relative amounts of dual emissions from mitochondrial JC-1 monomers or aggregates using flow cytometry (Becton-Dickinson FACSCalibur, New York, USA). Mitochondrial depolarization is indicated by an increase in the pencentage of cells with low ΔΨm (green fluorescence, lower right quadrant) compared with cells with high ΔΨm (red fluorescence, upper right quadrant).
2.5.9. Lactate dehydrogenase (LDH) leakage assay
The cell membrane integrity was determined by LDH leakage assay by using a LDH assay kit (Beyotime, China). In brief, HepG2 cells were plated on 96-well plates at the density of 5 × 103 cells per well and allowed to attach overnight. After being incubated with compound 10g for 24 h, the supernatants were collected and centrifuged at the speed of 1000 rpm and were subjected to LDH detection as the description in the manual. The absorbance at 490 nm was measured by a Cytation 3 Cell Imaging Multi-Mode Reader (BioTek Instruments, Inc., Winooski, VT, USA).
2.5.10. Western blot analysis
HepG2 cells were seeded at a density of 5 × 106 cells per well and attached for 8 h, and then treated with different concentrations of compound 10g for 48 h. After the treatment, the cells were harvested and washed twice with PBS. The harvested cells were lysed with radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotech., Nantong, China) with 1% cocktail (Sigma-Aldrich, USA). Whole-cell protein lysates were prepared and centrifuged at 12,000 rpm for 10 min at 4 °C. The total proteins were determined using Bradford reagent (Bio-Rad Laboratories, Inc., USA). Exactly 40 µg of protein per lane was separated through sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membranes were incubated with each antibody and detected through immunoblot analysis. All of the antibodies were purchased from Cell signaling Technology, Inc. (Boston, MA, USA) and diluted in accordance with the manufacturer’s instruction. Proteins were visualized using a C-Digit® imaging system (LI-COR Biosciences, Lincoln, NE, USA).
3. Results and discussion
3.1. Chemistry
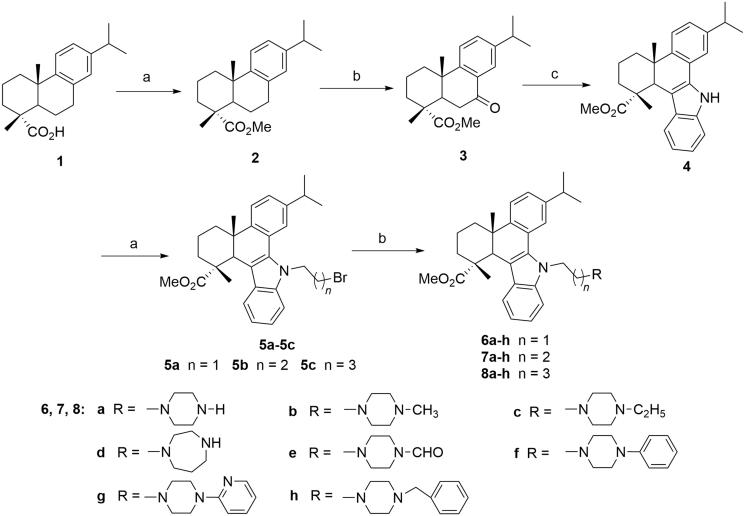
The reaction sequences employed for the synthesis of the target compounds (6a-h, 7a-h, 8a-h and 10a-j) was outlined in Schemes 1 and 2, according to the previous studies35,40. Initially, methyl 7-oxo-dehydroabietate (3) was synthesised from the starting material dehydroabietic acid (1) through methyl esterification and oxidation by CrO3. Then compound 3 was converted to the carbazole derivative (4) by reacting with phenylhydrazine through Fisher indole reaction. Subsequently, the intermediate 4 was treated with 1,2-, 1,3- or 1,4-dibromoalkanes, NaOH and TBAB to give the N-bromoalkyl derivatives 5a-c (Scheme 1, n = 1–3), which were further reacted with different N-substituted piperazines and homopiperazine in the presence of K2CO3, KI in acetonitrile to yield three series of N-substituted carbazole derivatives of 6a-h, 7a-h and 8a-h with different linkers and N-containing heterocyclic moieties (Scheme 1).
Scheme 1.
Synthetic route of target compounds 6a-h, 7a-h and 8a-h from dehydroabietic acid. (a) (i) SOCl2, benzene, reflux, 3 h, (ii) MeOH, reflux, 2 h; (b) CrO3, AcOH, Ac2O, 0 °C to rt, 12 h; (c) phenylhydrazine hydrochloride, EtOH, conc. HCl, reflux, 3 h; (d) 1,2-dibromoethane, 1,3-dibromopropane or 1,4-dibromobutane, TBAB, NaOH, benzene, H2O, rt, 12 h; (e) N-substituted piperazine, K2CO3, KI, MeCN, reflux, 8 ∼ 12 h.
Scheme 2.
Synthetic route of target compounds 10a-j from the intermediate 3. (a) Substituted phenylhydrazine hydrochloride, EtOH, conc. HCl, reflux, 3 h; (b) 1,2-dibromoethane, TBAB, NaOH, benzene, H2O, rt, 12 h; (c) piperazine, K2CO3, KI, MeCN, reflux, 8 ∼ 12 h.
Subsequently, to explore the relationships between the substituent on indole moiety and anticancer activity, compounds 10a-j with different substituents on the indole benzene rings were also synthesised according to Scheme 2. Briefly, compound 3 was reacted with different substituted phenylhydrazines to afford a series of carbazole derivatives with different substituents on indole moieties (4a-j), which were converted to the corresponding N-bromoethyl derivatives 9a-j and then N-(piperazin-1-yl)ethyl derivatives 10a-j through similar two-step procedures. The structures of all the synthesised compounds were characterized by their IR, ESI-MS, 1H NMR and 13C NMR spectral data analysis (Supplementary Figures S1–S68).
3.2. In vitro cytotoxic activity
The in vitro cytotoxic activities of all the target compounds were evaluated by MTT assay against three human hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B) and a normal human hepatocyte cell line (QSG-7701). Doxorubicin was co-assayed as the positive control. The results expressed as IC50 values for these compounds were summarised in Tables 1 and 2.
Table 1.
IC50 values of compounds 6a-t, 7 m-t and 8 m-t against two hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B) and normal hepatocyte cell line (QSG-7701).
| Compound | IC50 value (μM) |
|||
|---|---|---|---|---|
| SMMC-7721 | HepG2 | Hep3B | QSG-7701 | |
| 6a | 5.20 ± 0.21 | 2.28 ± 0.19 | 0.82 ± 0.08 | 8.75 ± 0.65 |
| 6b | 13.2 ± 0.76 | 11.7 ± 0.58 | 6.78 ± 0.42 | 27.53 ± 1.87 |
| 6c | 14.00 ± 2.10 | 17.40 ± 1.73 | 10.97 ± 0.65 | 41.05 ± 3.69 |
| 6d | 7.01 ± 0.74 | 5.87 ± 0.42 | 6.31 ± 0.33 | 22.1 ± 2.75 |
| 6e | 17.27 ± 1.78 | 15.65 ± 1.61 | 11.42 ± 0.53 | >50 |
| 6f | >50 | >50 | >50 | NT |
| 6g | >50 | >50 | >50 | NT |
| 6h | >50 | >50 | >50 | NT |
| 7a | 5.10 ± 0.18 | 3.10 ± 0.45 | 1.37 ± 0.20 | 17.22 ± 1.89 |
| 7b | 6.80 ± 0.72 | 29.00 ± 1.29 | 5.98 ± 0.39 | >50 |
| 7c | >50 | >50 | 40.72 ± 2.62 | NT |
| 7d | 10.34 ± 1.02 | 8.68 ± 1.13 | 12.83 ± 0.67 | 32.79 ± 3.22 |
| 7e | 23.34 ± 2.35 | 16.85 ± 1.58 | 20.03 ± 1.22 | >50 |
| 7f | >50 | >50 | >50 | NT |
| 7g | >50 | >50 | >50 | NT |
| 7h | >50 | >50 | >50 | NT |
| 8a | 6.10 ± 0.47 | 4.80 ± 0.32 | 2.89 ± 0.27 | 23.38 ± 2.97 |
| 8b | 34.90 ± 3.35 | 19.60 ± 2.91 | 18.34 ± 1.02 | >50 |
| 8c | >50 | >50 | >50 | NT |
| 8d | 12.77 ± 0.84 | 11.52 ± 1.23 | 14.38 ± 0.77 | 43.65 ± 4.03 |
| 8e | 32.12 ± 2.78 | 41.17 ± 3.56 | 38.02 ± 1.75 | >50 |
| 8f | >50 | >50 | >50 | NT |
| 8g | >50 | >50 | >50 | NT |
| 8h | >50 | >50 | >50 | NT |
| Doxorubicin | 1.13 ± 0.11 | 2.38 ± 0.29 | 1.02 ± 0.09 | 13.78 ± 0.53 |
The results are expressed as mean value ± SD.
NT: Not tested.
Table 2.
IC50 values of compounds 10a-j against two hepatocarcinoma cell lines (SMMC-7721, HepG2, and Hep3B) and normal hepatocyte cell line (QSG-7701).
| Compound | IC50 value (μM) |
|||
|---|---|---|---|---|
| SMMC-7721 | HepG2 | Hep3B | QSG-7701 | |
| 10a | 1.73 ± 0.22 | 3.05 ± 0.37 | 1.17 ± 0.12 | 16.32 ± 1.56 |
| 10b | 5.52 ± 0.47 | 6.11 ± 0.38 | 5.03 ± 0.31 | 21.89 ± 1.72 |
| 10c | 6.56 ± 0.19 | 7.27 ± 0.51 | 5.84 ± 0.36 | 22.19 ± 1.81 |
| 10d | 10.51 ± 0.48 | 8.42 ± 0.39 | 8.85 ± 0.42 | 30.98 ± 2.39 |
| 10e | 3.02 ± 0.21 | 3.73 ± 0.19 | 4.38 ± 0.35 | 15.32 ± 1.13 |
| 10f | 2.03 ± 0.15 | 3.15 ± 0.13 | 2.23 ± 0.16 | 17.29 ± 0.78 |
| 10g | 1.39 ± 0.13 | 0.51 ± 0.09 | 0.73 ± 0.08 | 12.52 ± 0.58 |
| 10h | 2.21 ± 0.17 | 4.87 ± 0.48 | 1.78 ± 0.12 | 18.87 ± 1.09 |
| 10i | 4.32 ± 0.27 | 3.91 ± 0.34 | 3.32 ± 0.20 | 23.67 ± 1.53 |
| 10j | 2.49 ± 0.18 | 2.88 ± 0.23 | 3.75 ± 0.34 | 12.23 ± 1.01 |
| Doxorubicin | 1.13 ± 0.11 | 2.38 ± 0.29 | 1.02 ± 0.09 | 13.78 ± 0.53 |
The results are expressed as mean value ± SD.
As shown in Table 1, compounds 6a-h, 7a-h and 8a-h displayed variable cytotoxic activities against three cancer cells. Among them, compounds 6a, 7a and 8a with piperazine moieties, 6b and 7b with N-methylpiperazine moieties, 6d and 7d containing homopiperazine moieties revealed strong inhibitory activities with IC50 < 10 µM against at least one hepatocarcinoma cell line. Specially, compound 6a with N-(piperazin-1-yl)ethyl substituent emerged as the most potent cytotoxic agent against SMMC-7721, HepG2 and Hep3B cells with IC50 values of 5.20 ± 0.21, 2.28 ± 0.19 and 0.82 ± 0.08 µM, respectively, equipotent to those of doxorubicin (IC50: 1.13 ± 0.11, 2.38 ± 0.29 and 1.02 ± 0.09 µM, respectively). Notably, the compound was substantially less cytotoxic to normal hepatocyte cells QSG-7701 (8.75 ± 0.65 µM). In addition, its analog 7a and 8a with 3 C and 4 C chain linker also showed promising cytotoxic activities (IC50: 1.37–5.10 µM and 2.89–6.10 µM, respectively) compared with compound 6a. Further, N-formylpiperazine derivatives (6e, 7e and 8e) displayed moderate inhibitions to three cancer cell lines. Compound 6c bearing N-ethylpiperazine also showed moderate activity while its analogs 7c and 8c displayed weak or no inhibitions to three cancer cell lines. One the other hand, all the derivatives with N-phenyl, N-pyridinyl and N-benzyl piperazine moieties (6f-8f, 6 g-8g and 6 h-8h) appeared to be inactive against three cancer cells (IC50 >50 µM).
From the results, it could be indicated that the cytotoxic activities of these derivatives were significantly affected by the piperazine moieties introduced to the side chain. For compounds 6a-h, the order of cytotoxicities of these derivatives could be generally expressed as: piperazine > homopiperazine > N-methylpiperazine > N-ethyl, N-formylpiperazine > N-phenyl, N-pyridinyl-, and N-benzylpiperazine derivatives. Similar relationships could also be observed for compounds 7a-h and 8a-h. These results indicated that the introduction of alkyl, acyl or aryl substituents, especially bulky aryl groups on the nitrogen atom of piperazine moiety will significantly reduce the anticancer activity. On the other hand, the length of alkyl side chain also substantially affected the cytotoxicity. In general, the cytotoxic activities of compounds 6a-h with 2 C linkers appeared to be stronger than those of compounds 7a-h with 3 C linkers, which were markedly stronger than 8a-h with 4 C linkers. These results suggested that the N-(piperazin-1-yl)ethyl side chain with piperazine heterocycle and ethyl linker proved to be most beneficial to the cytotoxic activity, and compound 6a (QC2) was still chosen for further structural modification.
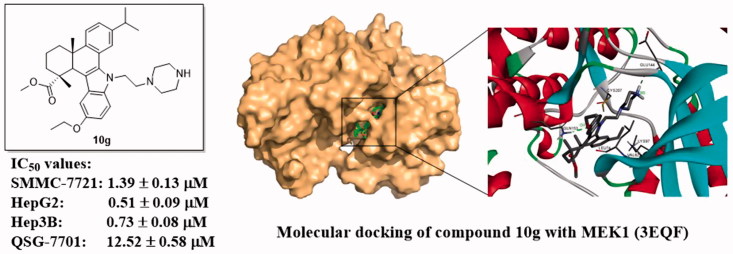
The effects of substituents on the indole benzene ring on the cytotoxicity were also explored. Compounds 10a-j with different substituted indole moieties were synthesised and screened for their in vitro anticancer activities against SMMC-7721, HepG2 and Hep3B cells. As shown in Table 2, compounds 10a-j all exhibited strong cytotoxic activities with IC50 values below 10 µM. Among them, compounds 10a, 10e, 10f, 10g, 10h and 10j displayed relatively higher anticancer potency than other derivatives, which indicated that methyl, methoxyl, ethoxyl and chloro groups anchored on the indole moiety were more beneficial to the anticancer activity. In addition, the derivatives (10a and 10g) containing 12-Me and 12-OEt generally showed greater cytotoxic activities than their analogs (10b and 10h) with same substituents at C-10, while compound 10f with 10-OMe substituent was relatively more active than compound 10e with 12-OMe. Especially, compound 10g with 12-OEt substituent exhibited the most potent anticancer activity against SMMC-7721, HepG2 and Hep3B cells with IC50 values of 1.39 ± 0.13, 0.51 ± 0.09 and 0.73 ± 0.08 µM, respectively. Compared with lead compound 6a (QC2) and the positive control doxorubicin, it exhibited considerably more potent anticancer activities against three cancer cells and lower cytotoxicity to normal hepatocyte cell line QSG-7701 (IC50: 12.52 ± 0.58 µM). Because of its significant anticancer property, compound 10g was selected for further inverstigations on its anticancer mechanisms.
3.3. MEK1 inhibitory activity
The inhibitory activities of selected compounds (6a and 10a-j) against MEK1 were evaluated by the Raf/MEK/ERK cascade kinase assay using recombinant proteins. The potent MEK1 inhibitor AZD6244 was co-assayed as positive control. The results were summarised in Table 3. It can be found that these compounds exhibited diverse inhibitory activities. Specifically, the lead compound 6a showed weak MEK1 inhibitory activity, and compounds 10a-d and 10i-k displayed mild or even no activities. In contrast, compounds 10e-h with OMe and OEt substituents demonstrated strong inhibitory activities. Among them, compound 10g showed the most potent inhibitory activity with IC50 of 0.11 ± 0.02 µM, near to the positive control AZD6244 with IC50 of 0.029 ± 0.003 µM. These results indicated that the derivatives with potent antiproliferative activities generally showed significant MEK1 inhibitory activities in this assay. Therefore, the antiproliferative effects of these derivatives were probably correlated with their MEK1 kinase inhibitory activity.
Table 3.
MEK1 inhibitory activities of compounds 6a and 10a-j. The results are expressed as mean value ± SD.
| Compound | IC50 value (μM) |
|---|---|
| 6a | 13.21 ± 0.73 |
| 10a | 6.36 ± 0.51 |
| 10b | >20 |
| 10c | >20 |
| 10d | >20 |
| 10e | 1.28 ± 0.06 |
| 10f | 0.23 ± 0.03 |
| 10g | 0.11 ± 0.02 |
| 10h | 1.63 ± 0.12 |
| 10i | >20 |
| 10j | 5.62 ± 0.48 |
| 10k | >20 |
| AZD6244 | 0.029 ± 0.003 |
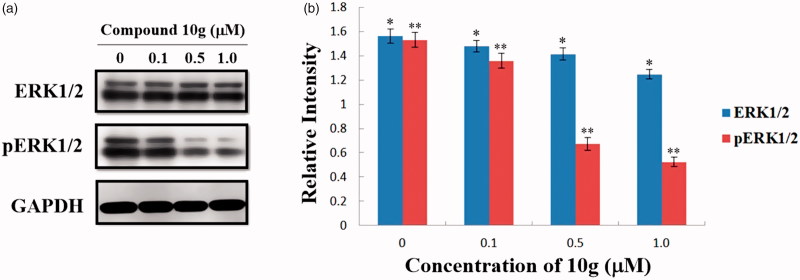
Subsequently, to evaluate the MEK inhibition of compound 10g in HepG2 cells, western blot analyses were also carried out to test the change of levels of ERK and phosphorylated ERK (pERK) in compound 10g-treated HepG2 cells. As shown in Figure 3, the expression levels of ERK1/2 decreased slightly, while the levels of pERK1/2 were significantly downregulated by compound 10g in a dose-dependent manner. After treatment with different concentrations of 10g (0.1, 0.5 and 1.0 µM) for 48 h, the expression levels of pERK1/2 were reduced to 88.8%, 43.8% and 34.2% of the control group, respectively. As a result, the immunoblot analyses demonstrated that compound 10g could significantly inhibit MEK catalytic activity in HepG2 cells, therefore could suppress the phosphorylation and activation of the downstream ERK proteins.
Figure 3.
(a) Effects of compound 10g on the expression of ERK and pERK in HepG2 cells. HepG2 cells were treated with compound 10g (0, 0.1, 0.5 and 1.0 μM) for 48 h; (b) The expression level of ERK1/2 and pERK1/2 in HepG2 cells. *p < 0.001; **p < 0.001.
3.4. Molecular docking
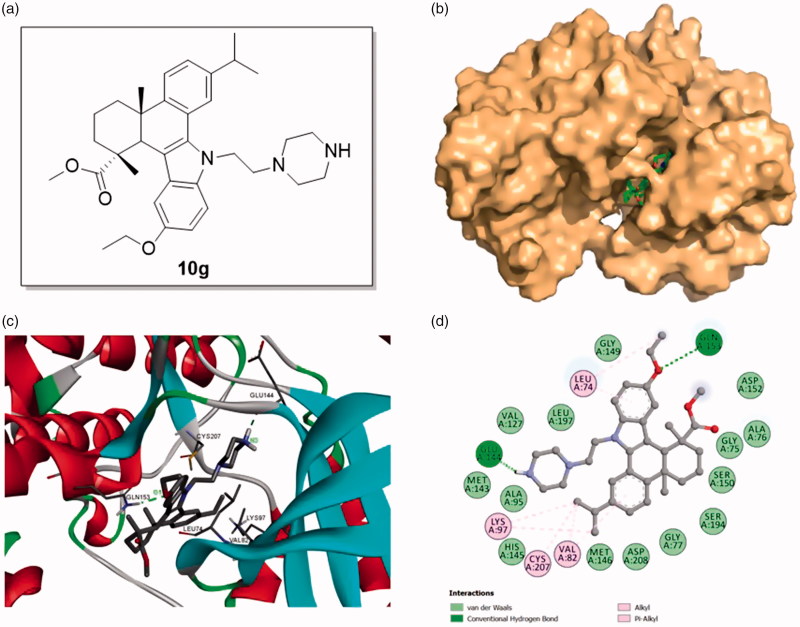
To gain more understanding of the interaction between target compounds and MEK, we explored their binding modes by molecular docking based on the reported MEK-1/inhibitor complex structure (PDB code: 3EQF). The docking studies were performed by using GLIDE docking module of Schrodinger suite 2015–1 and the docking results were analyzed and visualized by Discovery Studio 2016 Client. The binding models of compound 10g with MEK1 protein were shown in Figure 4.
Figure 4.
Binding mode of compound 10g at MEK1 kinase domain (PDB: 3EQF). (a) Molecular structure of compound 10g; (b) Space filling model of MEK1 protein with compound 10g embedded in the binding pocket; (c) Binding pose of compound 10g within the MEK1 kinase domain. Ligand and key residues are presented as stick models and colored by atom type, whereas the proteins are represented as ribbons. The dash lines exhibit the hydrogen bond interactions; (d) 2D projection drawing of compound 10g docked into MEK1 active site.
It was observed that compound 10g could be suitably docked into the binding site of MEK1 protein (Figure 4(b,c)), affording a significant docking score (–7.518), comparable to the docking score of AZD6244 (–7.401). Specifically, the (piperazin-1-yl)ethyl side chain was deeply inserted into the binding pocket of MEK1 structure, and a hydrogen bond was established between N3 atom of 10g and Glu 144 (N3–H⋅⋅⋅O/Glu 144, angle N – H⋅⋅⋅O = 125.1°, distance = 1.90 Å). On the other hand, the ethoxyl group on the indole benzene ring also played an important role in the interaction. The ethoxyl group formed a hydrogen bond with Gln 153 (O1⋅⋅⋅H – N/Gln 153, angle N – H⋅⋅⋅O = 150.0°, distance = 2.08 Å) and an alkyl hydrophobic interaction with Leu 74. Other alkyl hydrophobic interactions were also detected between the isopropyl group on C12 and Lys 97, Cys 207, Val 82 and π-alkyl hydrophobic interactions formed between two benzene rings and Val 82, Leu74. In addition, the molecule also formed van der Waals interactions with residues Met 146, His 145, Ala 95, Met 143, Val 127, Leu 197, Gly 149, Asp 152, Ala 76, Gly 75, Ser 150, Ser 194, Gly 77 and Asp 208 (Figure 4(d)). Taken together, the molecular docking results in combination with the biological assay data indicated that compound 10g could be a promising MEK inhibitor worthy of further investigation.
3.5. Cell cycle analysis
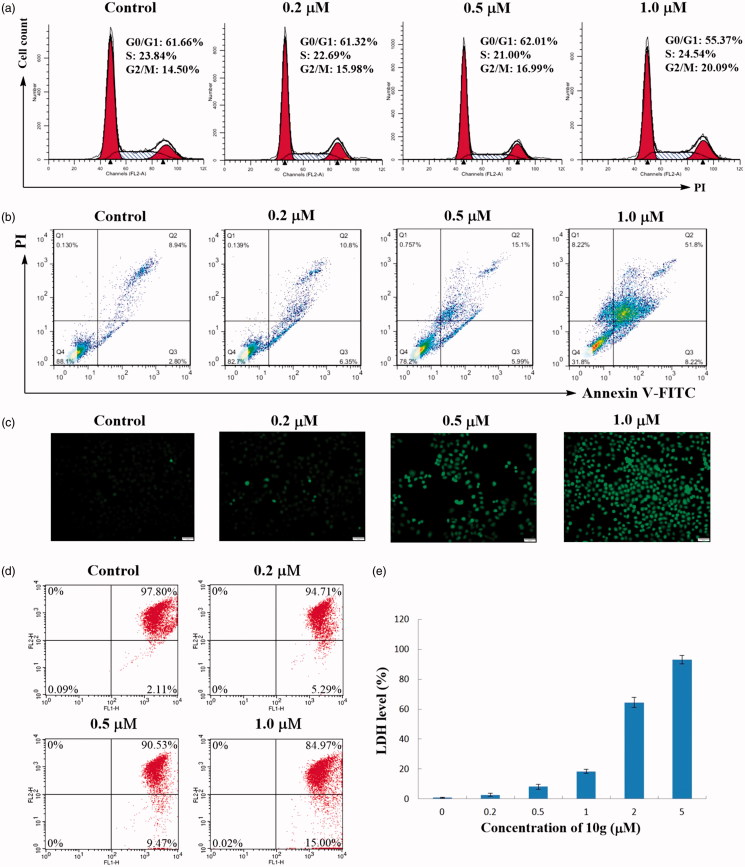
To determine whether the inhibition of cancer cell growth by compound 10g was correlated with cell cycle arrest, HepG2 cells were treated with different concentrations of compound 10g (0, 0.2, 0.5 and 1.0 µM) for 48 h. After staining with propidium iodide (PI), the cell cycle distribution of the treated cells was analysed by flow cytometry method. As shown in Figure 5(a), the percentage of cells in G2/M phase gradually increased from 14.50% (0 µM) to 20.09% (2 µM), while the G0/G1 phase cells decreased from 61.66% (0 µM) to 55.37% (2 µM). These results indicated that compound 10g could dose-dependently arrest the cell cycle of HepG2 cells at G2/M phase.
Figure 5.
(a) Cell cycle assay. HepG2 cells were treated with different concentrations of compound 10g (0, 0.2, 0.5, 1.0 μM) for 48 h, stained with propidium iodide (PI) and analysed using flow cytometer. (b) Annexin V-FITC/PI dual staining assay. HepG2 cells were treated with different concentrations of compound 10g (0, 0.2, 0.5, 1.0 μM) for 48 h, stained with Annexin V-FITC/PI and analysed for apoptosis using flow cytometer. (c) ROS generation assay. HepG2 cells were treated with different concentrations of compound 10g (0, 0.2, 0.5, 1.0 μM) for 48 h, stained with DCFH-DA and analysed using flow cytometer. (d) Mitochondrial membrane potential assay. HeLa cells were treated with compound 4d (0, 0.2, 0.5, 1.0 μM) for 24 h, incubated with JC-1 and analysed using flow cytometry. (e) LDH release assay of HepG2 cells treated with different concentrations of compound 10g (0, 0.2, 0.5, 1, 2 and 5 μM).
3.6. Annexin V-FITC/PI dual staining assay
In order to investigate whether compound 10g could induce apoptosis, HepG2 cells were treated with different concentrations of compound 10g (0, 0.2, 0.5 and 1.0 µM) for 48 h. Then the treated cells were subjected to Annexin V-FITC/PI dual staining followed by flow cytometry assay. As shown in Figure 5(b), the percentage of early and late apoptotic cells (lower right quadrant, AV+/PI– and upper right quadrant, AV+/PI+, respectively) significantly increased from 11.74% (0 µM) to 60.02 (2 µM). The results suggested that compound 10g could induce the cell death of HepG2 cells in a dose-dependent manner.
3.7. ROS generation assay
Reactive oxygen species (ROS) are chemically reactive chemical species containing oxygen, which can exert oxidative stress to cells and result in severe damage to organelles. Excessive ROS generation renders cells vulnerable to apoptosis41. To determine whether compound 10g could trigger ROS generation in HepG2 cells to induce cell death, the cells were treated with different concentrations of compound 10g for 48 h, and the ROS generation was assayed using the fluorescent probe 2,7-dichlorofluorescein diacetate (DCF-DA) by fluorescence microscopy. As shown in Figure 5(c), the treated cells exhibited significant green fluorescence in a dose-dependent manner, indicating that compound 10g could remarkably induce ROS generation in HepG2 cells.
3.8. Mitochondrial membrane potential (MMP) assay
It has been widely believed that ROS accumulation could decrease mitochondrial membrane potential (ΔΨm) and promote apoptosis. The disruption of mitochondrial function is considered as one of the most important apoptotic pathways, which has been recognized as an attractive antitumour target42. To investigate the correlation between MMP and cell death induced by compound 10g, the measurement of MMP were carried out by JC-1 assay kit on the instructions of the manual. As shown in Figure 5(d), the percentage of cells with low ΔΨm (Lower right quadrant) increased from 2.11% (Control) to 5.29% (0.2 µM), 9.47% (0.5 µM) and 15.00% (1.0 µM), which implied that compound 10g could result in the decrease of mitochondrial membrane potential in a concentration dependent manner, and thus the mitochondrial apoptotic pathway was probably involved in the cell death induced by the title compound.
3.9. Lactate dehydrogenase (LDH) leakage assay
As an enzyme existing in cytoplasm, LDH will be released into the medium when the cell membrane integrity is destructed. Hence, the destroyed cell membrane can be confirmed by LDH leakage assay37. HepG2 cells were incubated with different concentrations of compound 10g for 24 h, and then the extent of LDH leakage was detected using a LDH assay kit as the description of the manual. As shown in Figure 5(e), the relative LDH leakage level increased significantly from 0.80% (Control) to 93.03% (5 µM) in a dose-dependent manner. The results indicated that the treatment of compound 10g would markedly destroy the cell membrane integrity of HepG2 cells.
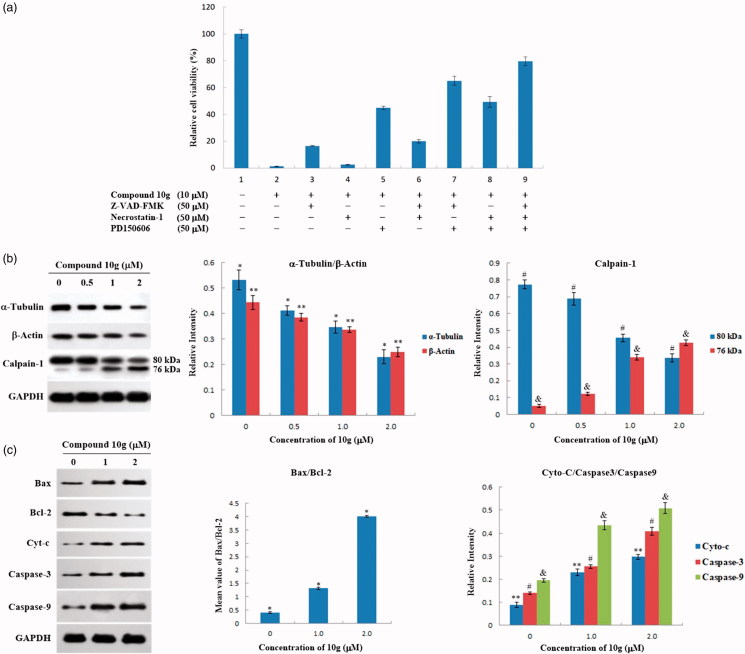
3.10. Cell death inhibition assay
After confirming the cytotoxic effect of compound 10g on hepatocarcinoma cells, we sought to clarify the detailed type of cell death caused by 10g. In this effort, necrosis inhibitor Necrostatin-1, apoptosis inhibitor Z-VAD-FMK and oncosis inhibitor PD150606 were utilised to reverse compound 10g-inducing cell death. As shown in Figure 6(a), the relative cell viability of HepG2 cells treated with 10g (10 µM) was only 1.22%. However, PD150606 (50 µM) could significantly reverse compound 10g-induced cell death with the relative cell viability increased to 44.87%. Z-VAD-FMK could also increase the relative cell viability to 16.53%. On the contrary, Necrostatin-1 had no apparent effect on cell death caused by compound 10g. Based on these results, we speculated that compound 10g could induce cell death of HepG2 cells through oncosis and apoptosis.
Figure 6.
Compound 10g induced oncosis and apoptosis of HepG2 cells. (a) PD150606 and Z-VAD-FMK partially reversed compound 10g-induced cell death. HepG2 cells were incubated with PD150606, Z-VAD-FMK or Necrostatin-1 at indicated concentration for 24 h alone or in combination with compound 10g at indicated concentration for 24 h. (b) Compound 10g induced the degradation of α-tubulin and β-actin, and activated oncosis marker calpain-1 autolysis from the 80 kDa event to 76 kDa event in western blot analysis. *, p < 0.01; **, p < 0.01; #, p < 0.01; &, p < 0.001. (c) Compound 10g induced the upregulation of apoptotic protein Bax, caspase-3, caspase-9 and plasmic cytochrome c levels, and the downregulation of antiapoptotic protein Bcl-2 level. *, p < 0.001; **, p < 0.01; #, p < 0.01; and p < 0.01.
3.11. Western blot analysis
To further explore whether compound 10g could induce the oncosis and apoptosis of HepG2 cells, a number of key protein markers involved in oncosis and apoptosis pathway were examined through Western blot analysis. Because calpains were reported to function in the process of oncotic cell death43, we further detected the expression level of calpain-1 in compound 10g-treated HepG2 cells. It has been reported that the activation of calpain at the membrane included the dissociation of calpain subunits and two successive autolytic events (80 kDa and 76 kDa)44, we found that calpain-1 autolysed from 80 kDa event to 76 kDa event in a dose-dependent manner when treated with compound 10g, which might imply the activation of this protein during the oncotic cell death (Figure 6(b)). Moreover, excessively active calpain can break down molecules in the cytoskeleton, and α-tubulin and β-actin has been identified as calpain substrates during oncosis process43,45. Therefore, we evaluated the expression levels of α-tubulin and β-actin in compound 10g-treated HepG2 cells. As shown in Figure 6(b), the level of α-tubulin and β-actin suffered a significant decrease in a dose-dependent manner. These results further indicated that compound 10g could induce oncotic cell death in HepG2 cells.
In addition, because the apoptosis inhibitor Z-VAD-FMK could partially reverse cell death, the expression of several apoptosis-related proteins was also detected in compound 10g-treated cells. Two important members of Bcl-2 family, the pro-apototic protein Bax and the anti-apoptotic protein Bcl-2 are key regulators of apoptosis46. With the dysfunction of mitochondrial membrane, cytochrome c is released to cytoplasma, which participates in the activation of downstream caspases. Caspases are a family of cysteinyl aspartate specific proteases involved in apoptosis, which can be divided into groups of initiators (caspase 8, 9 and 10) and executioners (caspase 3, 6 and 7)47,48. Therefore, the expression of cytochrome c, caspase-3 and caspase-9 were also detected. As shown in Figure 6(c), the ratio of Bax/Bcl-2 increased significantly with the treatment of compound 10g. In addition, the level of cytochrome c, caspase-3 and caspase-9 also increased dose-dependently in compound 10g-treated HepG2 cells. These data suggested that compound 10g could also induce the apoptosis of HepG2 cells through mitochondrial signalling pathway.
4. Conclusion
A series of novel 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid with different N-(piperazin-1-yl)alkyl side chains were designed, synthesised and evaluated for their in vitro antiproliferative activities against three human hepatocarcinoma cell lines (SMMC-7721, HepG2 and Hep3B) and a normal human hepatocyte cell line (QSG-7701). Several derivatives displayed considerable in vitro antiproliferative activities in MTT assay. Especially, compound 10g exhibited the most potent inhibitory activity against all the cancer cell lines and significantly lower cytotoxicity to human normal hepatocyte cells QSG-7701. In vitro pharmacological studies demonstrated that compound 10g could significantly inhibit MEK1 kinase activity and thus impede the MAPK signaling pathway. In addition, it could arrest cell cycle of HepG2 cells at G2/M phase, induce intracellular ROS generation, decrease mitochondrial membrane potential, destroy the membrane integrity and finally lead to the oncosis and apoptosis of HepG2 cells. All these results highlight the potential of this class of derivatives as promising candidates for the discovery of new targeted anticancer agents.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China (31770616), the Natural Science Foundation for Colleges and Universities in Jiangsu Province (17KJA220002) and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015C221).
Acknowledgement
The authors are grateful to the Advanced Analysis & Testing Center of Nanjing Forestry University for the measurements of spectroscopic data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Tsai CJ, Nussinov R. The molecular basis of targeting protein kinases in cancer therapeutics. Semin Cancer Biol 2013;23:235–42. [DOI] [PubMed] [Google Scholar]
- 2.Schirmer A, Kennedy J, Murli S, et al. Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc Natl Acad Sci USA 2006;103:4234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010;1802:396–405. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279–90. [DOI] [PubMed] [Google Scholar]
- 5.Ji DZ, Zhang LZ, Zhu QH, et al. Discovery of potent, orally bioavailable ERK1/2 inhibitors with isoindolin-1-one structure by structure-based drug design. Eur J Med Chem 2019;164:334–41. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 7.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21–44. [DOI] [PubMed] [Google Scholar]
- 8.Leicht DT, Balan V, Kaplun A, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta 2007;1773:1196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JN, Wang XF, Li T, et al. Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem 2016;107:12–25. [DOI] [PubMed] [Google Scholar]
- 10.Yap JL, Worlikar S, MacKerell AD, et al. Small-molecule inhibitors of the ERK signaling pathway: towards novel anticancer therapeutics. ChemMedChem 2011;6:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 2004;4:937–47. [DOI] [PubMed] [Google Scholar]
- 12.Sini P, Gurtler U, Zahn SK, et al. Pharmacological profile of BI 847325, an orally bioavailable, ATP-competitive inhibitor of MEK and Aurora kinases. Mol Cancer Ther 2016;15:2388–98. [DOI] [PubMed] [Google Scholar]
- 13.Abe H, Kikuchi S, Hayakawa K, et al. Discovery of a highly potent and selective MEK inhibitor: GSK1120212 (JTP-74057 DMSO solvate). ACS Med Chem Lett 2011;2:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fremin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol 2010;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res 2012;72:210–9. [DOI] [PubMed] [Google Scholar]
- 16.Carretero J, Shimamura T, Rikova K, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell 2010;17:547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KW, Kang NJ, Rogozin EA, et al. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis 2007;28:1918–27. [DOI] [PubMed] [Google Scholar]
- 18.Haasbach E, Müller C, Ehrhardt C, et al. The MEK-inhibitor CI-1040 displays a broad anti-influenza virus activity in vitro and provides a prolonged treatment window compared to standard of care in vivo. Antivir Res 2017;142:178–84. [DOI] [PubMed] [Google Scholar]
- 19.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004;22:4456–62. [DOI] [PubMed] [Google Scholar]
- 20.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 2007;6:2209–19. [DOI] [PubMed] [Google Scholar]
- 21.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res 2007;13:1576–83. [DOI] [PubMed] [Google Scholar]
- 22.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38–47. [DOI] [PubMed] [Google Scholar]
- 23.Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. J Am Med Assoc 2017;317:1844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C, Giaccone G. MEK inhibitors under development for treatment of non-small-cell lung cancer. Expert Opin Investig Drugs 2018;27:17–30. [DOI] [PubMed] [Google Scholar]
- 25.Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res 2011;17:989–1000. [DOI] [PubMed] [Google Scholar]
- 26.Resources for Information on Approved Drugs Trametinib and Dabrafenib [Internet]. Silver Spring (MD): US Food & Drug Administration (FDA); 2017. Available from http://www.fda.gov/drugs/informationondrugs/approveddrugs [last accessed 20 Feb 2019].
- 27.Savluchinske-Feio S, Curto MJM, Gigante B, et al. Antimicrobial activity of resin acid derivatives. Appl Microbiol Biotechnol 2006;72:430–6. [DOI] [PubMed] [Google Scholar]
- 28.Fei BL, Tu SY, Wei ZZ, et al. Optically pure chiral copper(II) complexes of rosin derivative as attractive anticancer agents with potential anti-metastatic and anti-angiogenic activities. Eur J Med Chem 2019;176:175–86. [DOI] [PubMed] [Google Scholar]
- 29.Roa-Linares VC, Brand YM, Agudelo-Gomez LS, et al. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur J Med Chem 2016;108:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahermo M, Krogerus S, Nasereddin A, et al. Antiprotozoal activity of dehydroabietic acid derivatives against Leishmania donovani and Trypanosoma cruzi. Med Chem Comm 2016;7:457–63. [Google Scholar]
- 31.Wada H, Kodato S, Kawamori M, et al. Antiulcer activity of dehydroabietic acid derivatives. Chem Pharm Bull 1985;33:1472–87. [DOI] [PubMed] [Google Scholar]
- 32.Lee WS, Kim JR, Han JM, et al. Antioxidant activities of abietane diterpenoids isolated from Torreya nucifera leaves. J Agric Food Chem 2006;54:5369–74. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Kang YG, Lee JY, et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol Cell Endocrinol 2015;412:216–25. [DOI] [PubMed] [Google Scholar]
- 34.Cui YM, Liu XL, Zhang WM, et al. The synthesis and BK channel-opening activity of N-acylaminoalkyloxime derivatives of dehydroabietic acid. Bioorg Med Chem Lett 2016;26:283–7. [DOI] [PubMed] [Google Scholar]
- 35.Gu W, Qiao C, Wang SF, et al. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg Med Chem Lett 2014;24:328–31. [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, Jiang CP, Wang ZX, et al. Dehydroabietic acid derivative QC2 induces oncosis in hepatocellular carcinoma cells. BioMed Res Int 2014;2014:682197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo DJ, Ni Q, Ji AL, et al. Dehydroabietic acid derivative QC4 induces gastric cancer cell death via oncosis and apoptosis. BioMed Res Int 2016;2016:2581061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halbrook NJ, Lawrence RV. The isolation of dehydroabietic acid from disproportionated rosin. J Org Chem 1966;31:4246. [Google Scholar]
- 39.Wang CJ, Delcros JG, Biggerstaff J, et al. Synthesis and biological evaluation of N1-(Anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J Med Chem 2003;46:2663–71. [DOI] [PubMed] [Google Scholar]
- 40.Gu W, Wang SF. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur J Med Chem 2010;45:4692–6. [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Chen CP, Liu J, et al. Design, synthesis, and biological evaluation of (2E)-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)acetate derivatives as anti-proliferative agents through ROS-induced cell apoptosis. Eur J Med Chem 2016;124:809–19. [DOI] [PubMed] [Google Scholar]
- 42.Wang JQ, Wang XB, Wang Y, et al. Novel curcumin analogue hybrids: synthesis and anticancer activity. Eur J Med Chem 2018;156:493–509. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol 2004;44:349–70. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Sorimachi H. A novel aspect of calpain activation. FEBS Lett 1998;433:1–4. [DOI] [PubMed] [Google Scholar]
- 45.Cao X, Zhang Y, Zou L, et al. Persistent oxygen-glucose deprivation induces astrocytic death through two different pathways and calpain-mediated proteolysis of cytoskeletal proteins during astrocytic oncosis. Neurosci Lett 2010;479:118–22. [DOI] [PubMed] [Google Scholar]
- 46.Zhang SS, Nie SP, Huang DF, et al. A novel polysaccharide from Ganoderma atrum exerts antitumor activity by activating mitochondria-mediated apoptotic pathway and boosting the immune system. J Agric Food Chem 2014;62:1581–9. [DOI] [PubMed] [Google Scholar]
- 47.Liu DZ, Tian Z, Yan ZH, et al. Design, synthesis and evaluation of 1,2-benzisothiazol-3-one derivatives as potent caspase-3 inhibitors. Bioorg Med Chem 2013;21:2960–7. [DOI] [PubMed] [Google Scholar]
- 48.Vickers CJ, Gonzalez-Paez GE, Wolan DW. Selective detection and inhibition of active caspase-3 in cells with optimized peptides. J Am Chem Soc 2013;135:12869–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.