Abstract
This study aimed to compare the phytochemicals from coffee and cocoa by-products and their relationship with the potential for reducing markers of inflammation, oxidative stress, adipogenesis, and insulin resistance in vitro. We characterized the phytochemical profile of extracts from coffee husk, coffee silverskin, and cocoa shell and evaluated their in vitro biological activity in RAW264.7 macrophages and 3T3-L1 adipocytes. Pearson correlations and principal component regressions were performed to find the contribution of phytochemicals and underlying mechanisms of action. Coffee husk and silverskin extracts were mainly composed of caffeine and chlorogenic acid. Major components in cocoa shell included theobromine and protocatechuic acid. Both coffee and cocoa by-product extracts effectively reduced inflammatory markers in macrophages and adipocytes (NO, PGE2, TNF-α, MCP-1, and IL-6) and the production of reactive oxygen species (21.5–66.4%). Protocatechuic and chlorogenic acids, together with caffeine, were suggested as main contributors against inflammation and oxidative stress. Furthermore, extracts reduced lipid accumulation (4.1–49.1%) in adipocytes by regulating lipolysis and inducing adipocyte browning. Gallic and chlorogenic acids were associated with reduced adipogenesis, and caffeine with adipocyte browning. Extracts from coffee and cocoa by-products also modulated the phosphorylation of insulin receptor signaling pathway and stimulated GLUT-4 translocation (52.4–72.9%), increasing glucose uptake. The insulin-sensitizing potential of the extracts was mainly associated with protocatechuic acid. For the first time, we identified the phytochemicals from coffee and cocoa by-products and offered new insights into their associations with biomarkers of inflammation, oxidative stress, adipogenesis, and insulin resistance in vitro.
Keywords: phytochemicals, inflammation, oxidative stress, adipogenesis, insulin resistance, coffee by-products, cocoa by-products
1. Introduction
According to the World Health Organization, chronic diseases are responsible for 71% of all deaths worldwide [1]. About half of American adults suffer one or more chronic diseases, including cardiovascular disease, obesity, type 2 diabetes, and cancer. These diseases could be prevented through nutrition and adequate food patterns [2]. The disruption of cellular metabolic processes triggers energy and redox imbalance and induces many pathophysiological conditions in the organism, designated as metabolic disorders [3]. The main hallmarks of metabolic disorders include insulin resistance, glucose intolerance, dyslipidemia, and overweight. Likewise, the established chronic oxidative and inflammatory conditions increase the risk of these diseases [4].
Obesity and the resulting metabolic disorders are linked with chronic low-grade inflammation in the adipose tissue [5]. The adipose tissue is an organ with functions of storage, endocrine signaling, and energy homeostasis. Being mostly composed of adipocytes, it also comprises other cell types. Adipose tissue macrophages have been highly associated with the promotion of inflammation and oxidative stress [6]. The promotion of a pro-inflammatory status and oxidative status in the adipose tissue is influenced by the activation of the innate immune system [7]. The adipose tissue produces and releases hormones and cytokines (adipokines) that display different actions on the whole body. Adipokines can elicit the production of reactive oxygen species (ROS), triggering oxidative stress and, consequently, an exacerbated and irregular secretion of other adipokines [8]. The contribution of oxidative stress and mitochondrial dysfunction in cellular and tissue damage in obesity and type 2 diabetes is a hot research topic. A chronic increase in ROS and cytokine levels in adipocytes results in mitochondrial dysfunction, which has a causative role in the attenuation of insulin signaling [9]. Likewise, excessive production of ROS may prompt an inflammatory response through the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Jun N-terminal kinase (JNK) pathways [10]. Inflammatory cytokines, primarily secreted by the infiltrated macrophages, induce an exacerbated lipolysis in adipocytes. The deregulated release of free fatty acids activates inflammatory signals in both macrophages and adipocytes, creating an inflammatory interplay between both cell types [11].
Epidemiological and clinical studies have evidenced that dietary phytochemicals improve human health by lowering the risk and preventing chronic diseases. Dietary phenolics are one of the most known natural antioxidant compounds and chemopreventive agents found in food. These compounds are associated with lower risks of obesity, diabetes, and other chronic diseases related with inflammation and oxidative stress [12,13,14]. On the other hand, methylxanthines, such as caffeine and theobromine, have been recognized as effective phytochemicals in weight control, and in the prevention of obesity and type 2 diabetes. Caffeine increases the metabolic rate, energy expenditure, lipid oxidation, and basal-lipolytic and thermogenic activities in the adipose tissue [15,16]. Meanwhile, theobromine has been evidenced as a browning agent that improves lipid catabolic metabolism via the β-adrenergic and AMP-activated protein kinase (AMPK) signaling pathway. Theobromine could exert anti-inflammatory effects; it has demonstrated the ability to reduce cytokines in vitro, in adipocytes and macrophages [17].
Coffee and cocoa by-products, mainly coffee husk, coffee silverskin, and cocoa shell, are produced worldwide in large amounts [18,19]. They are commonly discarded and represent a negative impact on the environment [20,21]. Coffee and cocoa are well-recognized for their health-promoting features [22,23,24]. Their by-products could be considered as potential ingredients for the food and pharmaceutical industries [25,26]. Hence, using these by-products as sources of phytochemicals could be a sustainable strategy in the prevention of chronic diseases. We previously evidenced the modulatory activity of phenolics from coffee and cocoa by-products on obesity-related inflammation in adipocytes and the alleviation of mitochondrial dysfunction and insulin resistance [27,28]. Nonetheless, the bioactivity of extracts from coffee and cocoa by-products has not been compared, nor its association with their phytochemical composition. Hence, this research hypothesized that the different phytochemicals in three plant materials from coffee and cocoa by-products would be related to selected bioactivities. We aimed to compare the phytochemicals from coffee and cocoa by-products and their relationship with the in vitro effects for reducing markers of inflammation, oxidative stress, adipogenesis, and insulin resistance.
2. Materials and Methods
2.1. Materials
Dulbecco’s modified Eagle medium (DMEM) was obtained from Corning cellgro® (Manassas, VA, USA). Newborn calf serum (NBS), fetal bovine serum (FBS), antibiotic (penicillin–streptomycin, 100×), and 0.25% trypsin–EDTA were purchased from Gibco Life Technologies (Grand Island, NY, USA). All additional reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.2. Preparation and Characterization of Extracts from Coffee and Cocoa By-Products
The coffee husk was provided by “Las Morenitas” (Nicaragua), the coffee silverskin, from Colombia, by Fortaleza S.A. (Spain), both from the Arabica species, and the cocoa shell by Chocolates Santocildes (Castrocontrigo, León, Spain). According to optimized extraction protocols, aqueous extracts were prepared [29]. After milling and sieving, coffee husk (10 g), coffee silverskin (25 g), or cocoa shell (10 g) were added into 500 mL of boiling water (100 °C) and stirred for 90 min for coffee husk and cocoa shell, and 10 min for coffee silverskin. Coffee husk (CHE), coffee silverskin (CSE), and cocoa shell (CAE) aqueous extracts were filtered and frozen at −20 °C for 24 h. Extracts were freeze-dried and stored at −20 °C until further use. The analysis of targeted phenolic compounds was carried following an ultra-performance liquid chromatography electrospray ionization-tandem mass spectrometry in multiple reactions monitoring mode (UPLC–ESI–MS/MS) previously described [30]. Briefly, dissolved extracts were filtered (0.22 μm), and the internal standard 4-hydroxybenzoic-2,3,5,6-d4 acid solution (Sigma-Aldrich, St Louis, MO) (1.25 mg/mL in formic acid/acetonitrile (1:200, v/v)) was added to the samples in a proportion 1:5 (v/v). The column employed was a BEH-C18, 2.1 × 100 mm and 1.7 μm particle size from Waters. The liquid chromatographic system was a Waters Acquity UPLC (Milford, MA) equipped with a binary pump, an autosampler thermostatted at 10 °C, and a heated column compartment (40 °C). The LC effluent was pumped to an Acquity TQD tandem quadrupole mass spectrometer equipped with a Z-spray electrospray ionization (ESI) source. For quantification purposes, data were collected in the multiple reaction monitoring (MRM) mode, tracking the transition of the parent and product ions specific to each compound. The ESI was operated in the negative ionization mode for the analysis of phenolics and in the positive for the analysis of methylxanthines. All compounds were quantified using the calibration curves of their corresponding standards. Injections were carried out in triplicate (n = 3).
2.3. Cell Cultures
The mouse RAW264.7 macrophages and 3T3-L1 preadipocytes (ATCC, Rockville, MD, USA) cell lines were cultured in DMEM containing 10% (v/v) NBS or FBS, respectively, L-glutamine, sodium pyruvate, and 1% (v/v) antibiotics, and maintained in a humidified atmosphere at 37 °C and 5% CO2.
2.3.1. Adipocyte Differentiation
The 3T3-L1 cells were cultured at a density of 6 × 103 cells cm−2 and differentiated into adipocytes according to the protocol described by Zebisch et al. [31]. After 48 h, cell differentiation was induced by changing the medium to 10% FBS enriched DMEM containing 0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, 1 μg/mL insulin, and 2 μM rosiglitazone. After 48 h, the medium was changed to 10% FBS enriched DMEM containing 1 μg/mL insulin for 48 h. On day 7, the medium was changed to 10% FBS enriched DMEM and the cells were kept in this culture media until they achieve the mature adipocyte phenotype. Cells were fully differentiated at day 10–12 and then employed for the following experiments.
2.3.2. Cell Viability Assay
Cell viability of macrophages, pre-adipocytes, and mature adipocytes treated with extracts (31–500 μg/mL) was performed with the CellTiter® 96 Aqueous One Solution Proliferation assay (Promega Corporation, Madison, WI, USA) following manufacturer’s instructions.
2.4. Anti-Inflammatory Potential
The anti-inflammatory effects of extracts from coffee and cocoa by-products were evaluated in both RAW264.7 macrophages and 3T3-L1 mature adipocytes. Macrophages were seeded at a density of 7 × 104 cells cm−2 and maintained 24 h in DMEM. Then, macrophages were co-treated for 24 h with lipopolysaccharide (LPS) (1 μg/mL) and the aqueous extracts (31–500 μg/mL) simultaneously. The conditioned media (CM) obtained after the stimulations of macrophages with LPS (1 μg/mL) was used to develop inflammatory conditions in adipocytes. Mature adipocytes (after 10–12 days of differentiation) were incubated for 24 h with CM and the extracts (31–500 μg/mL). Cells and their contact media were collected and stored at −80 °C until analyzed.
2.4.1. Determination of Inflammatory Factors in Macrophages
NO concentration in the cell media was determined using the Griess reagent according to the manufacturer’s protocol and using NaNO2 standard curve. Prostaglandin E2 (PGE2), tumor necrosis alpha (TNF-α), and monocyte chemoattractant protein 1 (MCP-1) were quantified in the cell culture media using commercially available kits following the manufacturer’s instructions (PGE2 ELISA from Cayman Chemical (Ann Arbor, MI, USA) and TNF-α and MCP-1 ELISA kits from R&D systems (Minneapolis, MN, USA)).
2.4.2. Assessment of Adipokines and Inflammation-Triggered Lipolysis in Adipocytes
The amount of TNF-α, MCP-1, interleukin 6 (IL-6), and adiponectin released to the medium was determined using a Milliplex® MAP Mouse Adipocyte Luminex assay (Millipore, Billerica, MA, USA). The culture media was used for glycerol quantification using a glycerol cell-based assay kit, and the cell lysates were assayed for triglycerides (TAG) and lipase activity using colorimetric assay kits. All the kits were purchased from Cayman Chemical.
2.5. Antioxidant Potential
The antioxidant activity was also evaluated in RAW264.7 macrophages and 3T3-L1 mature adipocytes. Treatments were conducted as described in Section 2.4. In addition, RAW264.7 macrophages (7 × 104 cells cm−2), maintained 24 h in DMEM, were stimulated for 4 h with 100 μM H2O2 and the extracts were added simultaneously.
2.5.1. Detection of Intracellular ROS, Mitochondrial Superoxide, and Mitochondrial Membrane Potential
After treatments, ROS were measured by incubating the cells for 1 h in DMEM with 2′,7′-dichlorofluorescein diacetate (25 μM). After washing with phosphate buffer solution (PBS), the fluorescence was measured at an excitation/emission wavelength at 485 nm/535 nm, respectively. Mitochondrial O2• ‒ was detected in adipocytes with a specific fluorescent dye, Mitosox Red (Invitrogen, Carlsbad, CA, USA) and measuring fluorescence at an excitation/emission wavelength of 510 nm and 580 nm, respectively. Mitochondrial membrane potential (ΔΨm) was determined using the mitochondria-specific fluorescent dye, JC-1 (Thermo Fisher, Skokie, IL, USA), according to manufacturer’s instructions. JC1 aggregates were detected at 550/590 nm (excitation/emission), while JC1 monomers were detected at 485/535 nm (excitation/emission). The JC1 ratio aggregates/monomers was calculated as an indicator of mitochondrial functionality.
2.5.2. Mitochondrial Content and Activity
In adipocytes, mitochondrial content was evaluated using Mitotracker Red (Mitotracker Deep Red FM, Invitrogen) measuring fluorescence intensity at excitation and emission wavelengths of 644 nm and 665 nm, respectively. Citrate synthase (CS) activity and adenosine triphosphate (ATP) content were measured in cell lysates with commercial kits (Cayman Chemical) following the manufacturer’s protocol. Oxygen consumption rate (OCR) was also measured using a kit according to the manufacturer’s instructions (Abcam, Cambridge, UK).
2.6. Anti-Adipogenic Potential
3T3-L1 cells were seeded in 6-well plates and induced to differentiation in the presence of the extracts (31–500 μg/mL) during the differentiation process.
2.6.1. Determination of Cellular Lipid Accumulation
Oil Red O (ORO) lipid staining was carried out as previously described [32]. Lipid staining was performed at day 10–12. ORO stock solution was prepared dissolving 0.35% w/v ORO in isopropanol overnight. Cells were fixed with formalin and a working ORO solution was added to each well. ORO staining was eluted with 100% isopropanol and the absorbance was measured at 500 nm.
2.6.2. Assessment of Lipolysis in Adipocytes
The culture media collected from fully differentiated adipocytes after a 24 h treatment with the extracts (31–500 μg/mL) was tested for glycerol and the cell lysates for TAG and lipase activity as described in Section 2.4.2.
2.6.3. Evaluation of Adipocyte Brown Differentiation
To evaluate adipocyte browning, mature adipocytes differentiated in the presence of extracts were used to measure mitochondrial content, CS activity, and ATP content as described in Section 2.5.2.
2.7. Insulin Sensitizing Potential
Insulin resistance was triggered in adipocytes by CM-stimulation, as described in Section 2.4.
2.7.1. Quantification of Glucose Uptake
The 3T3-L1 cells differentiated in a black 96-well plate with clear bottom were treated with CM and extracts (31–500 μg/mL) as indicated. After 24 h treatment, the cells were incubated for 1 h in glucose-free DMEM with 100 μM 2-deoxy-2-((7-nitro-2,1,3-benzoxadiazol-4-yl)amino)-D-glucose. At that moment, the cells were washed with PBS, and the fluorescence was detected at an excitation/emission wavelength of 485 nm/535 nm.
2.7.2. Determination of GLUT4 Translocation
The 3T3-L1 cells were cultured on 8-well chambers for cell culture and immunofluorescence, differentiated for 12 days, and treated with CM for 24 h, as previously described. Cells were cultured overnight in low-glucose (5.5 mM) serum-free DMEM supplemented with 0.25% FBS and treated along with the extracts (31–500 μg/mL); then, cells were starved for 4 h and stimulated with 100 nM insulin for 30 min. Glucose transporter type 4 (GLUT4) translocation was determined, according to Luna-Vital et al. [32]. Cells were fixed with 4% paraformaldehyde aqueous solution (Electron Microscopy Sciences) and permeabilized with 0.1% Triton X-100 in PBS. Cells were blocked with Image-iT FX Signal Enhancer (Life Technologies) and incubated with GLUT4 antibody overnight. Cells were washed with PBS and incubated with Alexa Fluor 568 Goat Anti-Rabbit IgG (Life Technologies) secondary antibody (1:200). Cells were cured with ProLong Gold antifade reagent with DAPI (Life Technologies). The microscopy chamber plate was stored at 4 °C in the dark until analysis. Samples were imaged using a 63 × /1.4 Oil DIC M27 objective with a Zeiss LSM 880 laser-scanning confocal microscope (Carl Zeiss AG, Germany). To evaluate GLUT4 translocation to the membrane, the fluorescence intensity, or GLUT4 expression, was measured in the membrane of the cells and normalized using the same laser gain (650) for all the samples. For the total GLUT4 measurement, the intensity of the whole picture was taken in consideration and normalized to the nuclear DAPI staining.
2.7.3. Evaluation of Insulin Signaling Pathway Phosphorylation Pattern
After the 24 h treatments incubation, the cells were starved and stimulated with 10 ng/mL insulin for an extra 30 min. Cell lysates were applied to each one of the array slides following manufacturer instructions. An antibody array, including proteins from insulin, PI3K/AKT, AMPK, MAPK, NF-κB signaling pathways, was used following the manufacturer instructions (Insulin Receptor Phospho Antibody Array, Full Moon BioSystems®, Sunnyvale, CA, USA). Results were analyzed as previously described [32]. The arrays were labeled with a Cy3-labeled streptavidin solution (0.5 mg/mL) (Sigma Aldrich, S6402). The slides were scanned on a GenePix4000B scanner and the images were analyzed with GenePix Pro 6.0 (Molecular Devices, Sunnivale, CA). Fluorescence signal of each array spot was quantified; the mean values and the standard deviations of the replicates were calculated.
2.8. Bioinformatic Analysis
Functional enrichment analysis was performed using IPA v9.0 (Ingenuity® Systems). Downstream effect analysis was performed to identify biological functions that are expected to be increased/decreased from the observed protein phosphorylation pattern in each sample; significant predictions were considered when biological functions presented an absolute z-score of ≥ 2 (positive indicate activation, negative indicate inhibition). The differentially phosphorylated proteins were categorized based on the biological process and analyzed for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis by using the KEGG database.
2.9. Statistical Analysis
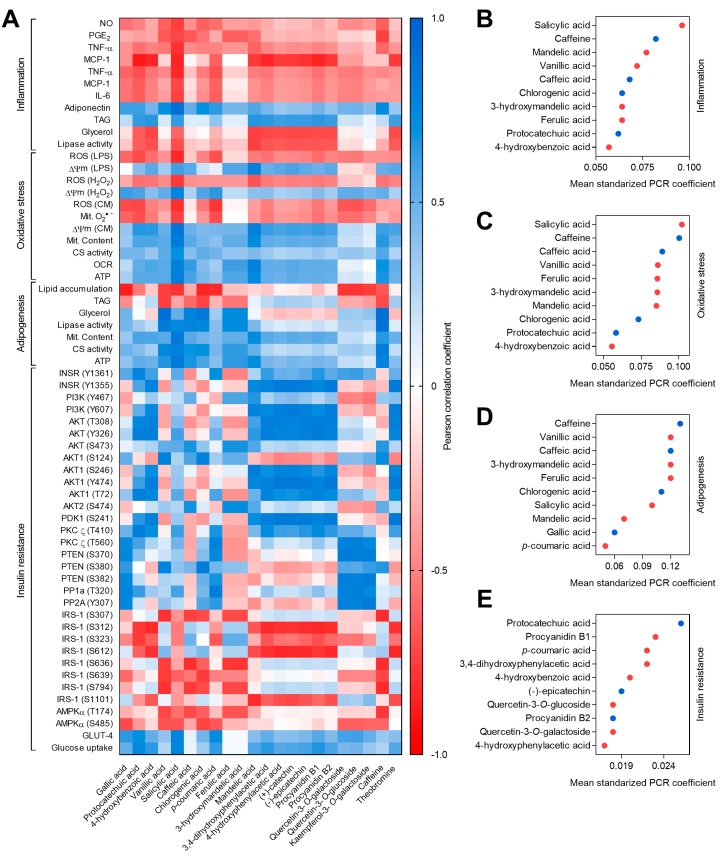
Samples were prepared in triplicate, and determinations were performed in triplicate. Results are presented as the mean ± standard deviation (SD) (n = 3) and were analyzed by one-way analysis of variance (ANOVA) and post hoc Tukey test. Differences were considered significant at p < 0.05 [33,34,35]. The statistical analysis of the results was performed using SPSS 23.0. Non-linear regressions and half maximal effective concentration (EC50), and 30% effective concentration (EC30) values were calculated using GraphPad Prism 7. Bi- and multivariate analyses, including Pearson correlations and principal component regression (PCR) were performed with XLSTAT 2018 for Microsoft Excel 2016. All the results were represented using GraphPad Prism 7.0. Pearson correlation. PCR analysis were carried out using the concentration of each one of the compounds in the cell media after coffee husk, coffee silverskin, and cocoa shell extracts application, as well as the values for each of the biomarkers of inflammation, oxidative stress, adipogenesis, and insulin resistance at the respective concentration. A non-treated cells control was also included and a concentration equal to zero for each of the phytochemicals was assigned. PCR analysis is a regression method in two steps; first, a principal component analysis (PCA) is carried out in the x variables (concentration of phytochemical) to reduce the dimensionality of the data extracting the factors with the higher variation; the second step is to do a linear regression between the sample scores on the most significant factors in x and y axes of the PCA. This method reduced collinearity between the predictors (phytochemicals concentration), since in a multiple linear regression model highly intercorrelated predictors may lead to an overfit model with inflated regression coefficients and p-values. PCR method takes care of the multicollinearity by using the principal components as new predictor variables to explain the observed variability without considering the response variable. Only significant (p < 0.05) Pearson correlation and PCR coefficients were discussed even if all the results were depicted as a heat map (Figure 1A and Figure S2).
Figure 1.
Heat map of the Pearson correlation coefficient established among observed in vitro effects and the concentration of different phytochemicals in coffee and cocoa by-products (A) and the top ten mean standardized principal component regression (PCR) coefficients of the regression constructed among the phytochemicals identified in coffee husk, coffee silverskin, and cocoa shell aqueous extracts and the potential of the extracts in the different biomarkers of inflammation (B), oxidative stress (C), adipogenesis (D), and insulin resistance (E). Blue (●) and red (●) circles indicate major (one of the ten in higher concentration) and minor phytochemicals, respectively.
3. Results and Discussion
3.1. Coffee By-Products were Mainly Composed of Chlorogenic Acid and Caffeine whereas Cocoa Shell Primarily Contained Methylxanthines
The phytochemical composition of coffee and cocoa by-products, namely coffee silverskin, coffee husk, and cocoa shell was studied (Table 1).
Table 1.
Phytochemical composition of coffee husk, coffee silverskin, and cocoa shell aqueous extracts characterized by UPLC–ESI–MS/MS. Values are expressed as mean ± SD (n = 3). Different letters among rows indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test.
| Compound | Concentration (µg/g Extract) | Chemical Structure | ||
|---|---|---|---|---|
| Coffee Husk | Coffee Silverskin | Cocoa Shell | ||
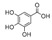
| Hydroxybenzoic acids |
|
|||
| Gallic acid | 87.0 ± 5.5c | 16.9 ± 1.2a | 19.2 ± 0.4b | |
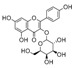
| Protocatechuic acid | 488.4 ± 26.2b | 44.1 ± 3.4a | 761.5 ± 47.6c |
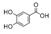
|
| 4-hydroxybenzoic acid | 13.4 ± 1.3b | 3.4 ± 0.3a | 70.1 ± 9.3c |
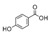
|
| Vanillic acid | 22.90 ± 9.62a | 29.6 ± 0.6a | N.D. |

|
| Salicylic acid | 3.1 ± 0.1b | 2.3 ± 0.2a | 3.3 ± 0.2b |
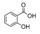
|
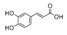
| Hydroxycinnamic acids |
|
|||
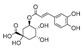
| Caffeic acid | 57.9 ± 2.0b | 538.0 ± 54.3c | 1.9 ± 0.2a | |
| Chlorogenic acid | 3456.8 ± 70.6b | 2791.7 ± 97.3a | N.D. |
|
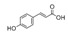
| p-coumaric acid | 8.7 ± 0.2c | 0.9 ± 0.1a | 4.2 ± 0.6b |
|
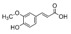
| Ferulic acid | N.D. | 3.8 ± 0.2 | N.D. |
|
| Mandelic acids |
|
|||
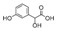
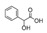
| 3-hydroxymandelic acid | N.D. | 4.4 ± 0.5 | N.D. | |
| Mandelic acid | N.D. | 5.1 ± 0.2a | 11.18 ± 1.2b |
|
| Phenylacetic acids |
|
|||
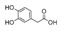
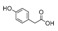
| 3,4-dihydroxyphenylacetic acid | 5.6 ± 2.0a | N.D. | 25.9 ± 2.4b | |
| 4-hydroxyphenylacetic acid | N.D. | N.D. | 48.5 ± 4.3 |
|
| Flavan-3-ols: monomers |

|
|||
| (+)-catechin | 1.7 ± 0.2a | 10.2 ± 1.1b | 200.8 ± 16.0c | |
| (−)-epicatechin | 18.0 ± 2.0a | N.D. | 222.1 ± 13.8b |

|
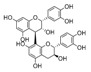
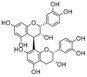
| Flavan-3-ols: dimers |
|
|||
| Procyanidin B1 | 22.3 ± 2.6a | N.D. | 83.6 ± 7.8b | |
| Procyanidin B2 | 11.6 ± 1.8a | N.D. | 219.9 ± 11.4b |
|
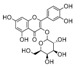
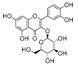
| Flavonols |
|
|||
| Quercetin-3-O-galactoside | 54.7 ± 0.5b | N.D. | 9.3 ± 0.4a | |
| Quercetin-3-O-glucoside | 57.4 ± 3.7b | N.D. | 11.12 ± 0.77a |
|
| Kaempferol-3-O-galactoside | 122.6 ± 3.6 | N.D. | N.D. |
|
| Alkaloids |
|
|||
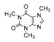
| Caffeine | 9815.5 ± 15.4b | 19,219.2 ± 37.6c | 2433.5 ± 7.8a | |
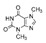
| Theobromine | N.D. | N.D. | 10,035.0 ± 4.5 |
|
N.D. Non-detected.
A total of 20 phenolic compounds and two methylxanthines were identified among the three by-products. The composition of the three by-products was different. Coffee husk and silverskin shared 10 compounds, whereas a total of eight of their phytochemicals was identified in cocoa shell. Coffee husk was mainly composed of caffeine and chlorogenic acid, also containing a significant concentration of protocatechuic acid and flavonols, mainly kaempferol-3-O-galactoside. Previous studies also reported similar concentrations of these compounds in coffee husk, as well as the presence of rutin [36]. Coffee silverskin presented a significantly higher (p < 0.05) concentration of caffeine (19.2 mg/g) and a lower concentration of chlorogenic acid. This coffee by-product has been one of the most characterized by-products, Bresciani et al. [37] demonstrated the presence of 10 different chlorogenic acids, the 3- and 5-caffeoylquinic acids being the most abundant ones. On the other hand, cocoa shell was primarily composed of theobromine and caffeine, the main phenolics being protocatechuic acid and flavan-3-ols, both monomers ((+)-catechin and (−)-epicatechin), and dimers (procyanidin B2). Other authors have recently reported a similar concentration of theobromine in hydroalcoholic acidic aqueous extracts [38]. Additionally, these abovementioned polyphenols were previously shown to have different reduction potentials, thereby exhibiting various properties in modulation of redox signaling pathways [39,40]. Given these results, it was expected that coffee and cocoa by-products, with different phytochemical composition, would present different biological activities.
3.2. Caffeine and Phenolics in Coffee and Cocoa By-Products Reduced Inflammation in Macrophages and Adipocytes
The aqueous extracts of coffee and cocoa by-products did not exert cytotoxicity in macrophages nor pre-adipocytes or mature adipocytes at the tested doses (31–500 µg/mL) (Figure S1). Extracts significantly reduced NO production (23.0–77.5%) and PGE2 (14.1–70.7%) in LPS-stimulated macrophages (Table S1). NO and PGE2 play a key role in the development of inflammatory diseases involved in the immune response produced by cytokine-activated macrophages [41,42]. Besides, TNF-α (14.1–50.1%) and MCP-1 (8.0–99.4%) release were reduced by coffee and cocoa by-product extracts. TNF-α is considered the main inflammatory stimuli in the adipose tissue while MCP-1 is involved in macrophage recruitment in adipose tissue [43]. Together, they are implicated in the establishment of a paracrine inflammatory interaction between macrophages and adipocytes in obese conditions [44]. As observed (Table 2), coffee husk exhibited the lowest EC30/50 for the secretion of NO, while coffee silverskin and cocoa shell did for PGE2 and TNF-α and MCP-1, respectively.
Table 2.
Anti-inflammatory effect of coffee husk, coffee silverskin, and cocoa shell extracts (31–500 μg/mL) regulating NO, PGE2, TNF-α, and MCP-1 release in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages, and TNF-α, MCP-1, IL-6, adiponectin, intracellular triglyceride content, extracellular glycerol release, and lipase activity conditioned media (CM)-stimulated in 3T3-L1 adipocytes. Values are expressed as mean (µg/mL) ± SD (n = 3). Different letters among rows indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test.
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC 30 | EC 50 | EC 30 | EC 50 | EC 30 | EC 50 | |
| RAW264.7 macrophages | ||||||
| NO release | 31.5 ± 5.0a | 73.4 ± 8.9a | 41.8 ± 4.4b | 97.5 ± 6.5b | 40.3 ± 9.8ab | 94.0 ± 16.5ab |
| PGE2 release | 134.0 ± 33.7b | 312.6 ± 48.4b | 58.0 ± 6.4a | 135.2 ± 9.4a | 118.4 ± 13.1b | 276.4 ± 19.2b |
| TNF-α release | 158.8 ± 43.2a | 370.4 ± 61.7a | 146.5 ± 29.5a | 341.8 ± 42.8a | 106.7 ± 35.7a | 498.9 ± 50.3b |
| MCP-1 release | 87.2 ± 12.1b | 203.5 ± 17.7b | 173.9 ± 19.7c | 420.3 ± 25.0a | 32.6 ± 4.7a | 76.1 ± 8.6a |
| 3T3-L1 adipocytes | ||||||
| TNF-α release | 59.8 ± 15.3a | 139.5 ± 21.9a | 138.0 ± 16.5c | 321.8 ± 24.1c | 87.8 ± 10.4b | 204.9 ± 15.2b |
| MCP-1 release | <31.0 | 80.0 ± 16.6a | <31.0 | 179.3 ± 36.8b | <31.0 | 132.5 ± 18.8b |
| IL-6 release | 88.2 ± 7.6a | >500 | 233.5 ± 86.7c | >500 | 112.5 ± 19.8b | >500 |
| Adiponectin release | 96.6 ± 9.7a | 225.4 ± 14.3a | 95.7 ± 9.8a | 232.9 ± 16.5a | 115.2 ± 17.2a | 268.7 ± 25.1b |
| Triglyceride content | <31.0 | 60.3 ± 10.0b | <31.0 | 32.6 ± 5.5a | <31.0 | <31.0 |
| Glycerol release | 77.5 ± 30.2a | 265.7 ± 42.2c | 68.5 ± 23.4a | 187.2 ± 25.7b | <31.0 | 26.7 ± 2.8a |
| Lipase activity | 107.1 ± 20.5b | 249.8 ± 29.7c | 70.5 ± 8.2a | 148.0 ± 12.3b | <31.0 | 30.2 ± 4.4a |
These effects were mainly correlated with the concentration of salicylic acid (r > 0.72, p < 0.001) (Figure 1). In previous studies, salicylic acid has been reported as an anti-inflammatory agent [45,46]; this well-recognized anti-inflammatory compound could reduce TNF-α and IL-6 in RAW264.7 and THP-1 macrophages. Moreover, salicylic acid is the active bioavailable form of the well-known aspirin (acetylsalicylic acid), a common medication used to treat inflammation [47,48].
Principal component regressions (PCR) were calculated to obtain a less biased quantitative value (standardized PCR coefficients) of the effect of phytochemicals on the observed bioactivities (Figure S2). Thus, we found an association of caffeine with NO, PGE2, and TNF-α release, and correlation of caffeine and protocatechuic acid with MCP-1 production. Chlorogenic and caffeic acid also showed a high contribution to the anti-inflammatory effects of coffee and cocoa by-products extracts. Caffeine previously demonstrated anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages, reducing inducible nitric oxide synthase (iNOS), NO, cyclooxygenase-2 (COX-2), and IL-6 via NF-κB and p38 MAPK pathway inhibition [49]. Chlorogenic and caffeic acids have also evidenced anti-inflammatory potential in LPS-stimulated RAW264.7 macrophages decreasing iNOS, NO, COX-2, PGE2, and inflammatory cytokines, including IL-6, TNF-α, and MCP-1 [28,50].
Along with obesity development, the progression of adipocyte hypertrophy and adipose tissue dysfunction evokes an exacerbated release of fatty acids, hormones, and pro-inflammatory cytokines [51]. Here we observed that in 3T3-L1 adipocytes, the extracts abrogated the secretion of TNF-α (8.0–66.7%), MCP-1 (37.1–71.2), IL-6 (21.0–40.1), and restored the production of adiponectin (1.0–75.5%). Additionally, they diminished inflammation-derived lipolysis, maintaining the content of TAG (23.3–134.3%), and reducing glycerol release (7.1–99.9%) through the inhibition of lipases activity (8.9–99.7%). Coffee husk presented the lowest EC30/50 values for TNF-α, MCP-1, and IL-6, whereas cocoa shell was the most potent inhibition inflammation-derived lipolysis (Table 2). Salicylic and protocatechuic acids also correlated with the secretion of adipokines; caffeine correlated with adiponectin production (r = 0.727, p < 0.01), and lipolysis markers were better associated with protocatechuic acid. PCR coefficients demonstrated the contribution of p-coumaric in diminishing MCP-1 and IL-6 releases. Chlorogenic acid exhibited a high contribution to the promotion of adiponectin release. Salicylic acid has been shown to decrease the inflammatory interplay of macrophages–adipocytes in CM-treated 3T3-L1 adipocytes [48]. It reduced TNF-α and IL-6 secretion and promoted adiponectin release. Furthermore, protocatechuic acid has shown to decrease TNF-α, MCP-1, and IL-6 in the adipose tissue both in vitro (3T3-L1 cells) and in vivo (humans) [27,52] Meanwhile, p-coumaric acid have exhibited anti-inflammatory properties in RAW264.7 cells suppressing COX-2, iNOS, and TNF-α expression via inhibition of NF-κB and p-38 MAPK pathways [53].
The top ten phytochemicals analyzed contributing to the anti-inflammatory properties of coffee and cocoa by-products are shown in Figure 1B. Caffeine, chlorogenic, and caffeic acids were the 3 major compounds showing anti-inflammatory potential, whereas salicylic, mandelic, and vanillic acids were the minor compounds with the highest contribution.
3.3. Phenolic Compounds Reduced Oxidative Stress in Macrophages and Adipocytes and Caffeine Preserved Adipocyte Mitochondrial Function
Obesity has been linked not only to metabolic dysfunction but also to oxidative stress. This stress derives from inflammation, as part of the immune response of macrophages, and from mitochondrial dysfunction, due to the excess of substrates to be oxidized [54]. Thus, together with the inflammatory cascade triggered by LPS-stimulation, an increase of oxidative state, and a deficient mitochondrial function were observed in macrophages. Coffee and cocoa by-products aqueous extracts significantly (p < 0.05) scavenged ROS produced by both LPS (36.0–99.5%) and H2O2 (30.8–86.9%) stimuli (Table S2), maintaining the ΔΨm. Cocoa shell exhibited the highest antioxidant capacity (lowest values of EC30/50) (Table 3). LPS-derived ROS was correlated with protocatechuic acid concentration (r = −0.710, p < 0.01) as well as H2O2-derived ROS (r = −0.571, p < 0.05). The recovery of ΔΨm upon both stimuli was significantly associated with mandelic and p-coumaric acid concentration (r = 0.802, p < 0.001 and r = 0.644, p < 0.01, respectively). PCR coefficient denoted the contribution of caffeic and chlorogenic acids on the prevention of ΔΨm loss with LPS and H2O2 treatments, respectively. Furthermore, in adipocytes, the extracts reduced ROS (21.5–66.4%) and particularly mitochondrial O2• ‒ (18.1–52.6%). The loss of ΔΨm was arrested by 11.0–96.6%. Coffee husk showed the lowest EC30/50 values for ROS production in adipocytes (Table 3).
Table 3.
Protective effect of coffee husk, coffee silverskin, and cocoa shell extracts (31–500 μg/mL) against oxidative stress and mitochondrial dysfunction regulating ROS and ΔΨm in RAW264.7 macrophages and ROS, mitochondrial O2• ‒, ΔΨm, mitochondrial content, citrate synthase (CS) activity, oxygen consumption rate (OCR), and ATP content in 3T3-L1 adipocytes. Values are expressed as mean (µg/mL) ± SD (n = 3). Different letters among rows indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test.
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC 30 | EC 50 | EC 30 | EC 50 | EC 30 | EC 50 | |
| RAW264.7 macrophages | ||||||
| ROS (LPS) | <31.0 | 48.2 ± 2.4b | <31.0 | 69.5 ± 6.6c | <31.0 | 34.7 ± 2.7a |
| ΔΨm (LPS) | >500 | >500 | 167.7 ± 22.6a | 391.3 ± 33.0a | 143.5 ± 36.9a | 334.9 ± 52.8a |
| ROS (H2O2) | 51.7 ± 9.3 | 120.7 ± 13.5c | <31.0 | 71.7 ± 3.2b | <31.0 | 46.6 ± 3.5a |
| ΔΨm (H2O2) | <31.0 | 33.6 ± 3.7a | 32.4 ± 3.5a | 75.6 ± 6.6b | 41.9 ± 8.6a | 97.8 ± 12.4c |
| 3T3-L1 adipocytes | ||||||
| ROS | 54.8 ± 10.5a | 128.1 ± 15.2a | 150.2 ± 39.7c | 350.6 ± 56.9b | 82.4 ± 16.1b | 192.3 ± 23.4a |
| Mitochondrial O2• ‒ | 86.4 ± 24.5a | 201.6 ± 35.0a | 207.8 ± 57.2b | >500 | 94.8 ± 28.5a | 221.2 ± 40.4a |
| ΔΨm | 140.9 ± 14.8c | 336.0 ± 12.5b | 58.0 ± 6.3a | 135.3 ± 9.2a | 94.8 ± 18.5b | 161.5 ± 22.9a |
| Mitochondrial content | 40.2 ± 7.6 | 96.0 ± 8.6b | <31.0 | 43.2 ± 6.8a | <31.0 | 74.9 ± 12.1b |
| CS activity | <31.0 | <31.0 | <31.0 | <31.0 | <31.0 | <31.0 |
| OCR | 399.0 ± 66.1b | >500 | 125.01 ± 23.5a | 291.8 ± 34.0a | 158.11 ± 24.6a | 368.8 ± 35.9b |
| ATP content | 80.7 ± 11.3b | 188.2 ± 16.5c | <31.0 | 59.4 ± 7.6a | 39.4 ± 8.3a | 91.9 ± 14.3b |
ROS inhibition was correlated with protocatechuic and p-coumaric acids (r = −0.716, p < 0.01 and r = −0.779, p < 0.001, respectively). Mitochondrial O2• ‒ followed the same behavior. ΔΨm was correlated with the concentration of mandelic acids (r = −0.787, p < 0.001). Moreover, PCR showed a high contribution of p-coumaric and gallic acid in the attenuation of ROS and O2• ‒ in adipocytes, whereas ΔΨm was associated with the concentration of caffeine and caffeic acid. Protocatechuic acid has evidenced its potent antioxidant properties in vitro and in vivo [55]. In macrophages, protocatechuic acid up-regulated enzymatic antioxidant defenses through JNK-mediated Nrf2 activation [56]. Likewise, caffeic acid previously has exhibited protective effects against mitochondrial oxidative stress, scavenging H2O2 [57], and preserving glutathione levels upon tert-butyl hydroperoxide-induced oxidative stress in HepG2 cells [58]. Additionally, caffeine has demonstrated to possess ROS scavenging properties and capacity to maintain ΔΨm [59].
The loss of mitochondrial function derived from an inflammatory and oxidative environment has been associated with increased insulin resistance in the adipose tissue [60]. Here, treating adipocytes with coffee and cocoa by-products preserved mitochondrial content (12.1–144.8%) and activity, measured as CS activity (75.6–150.8%), OCR (0.6–66.5%), and the production of ATP (11.4–108.3%) (Table S2). Coffee silverskin was the significantly (p < 0.05) most potent treatment in the conservation of mitochondrial content and activity, exhibiting EC30/50 values lower than 31 µg/mL except for OCR (125 µg/mL) (Table 3). These effects were associated with the concentration of caffeine (r ≥ 0.563, p < 0.05). Protocatechuic acid was linked to the protection of mitochondrial content (r = 0.571, p < 0.05); caffeic acid was associated with OCR (r = 0.626, p < 0.01); and mandelic acid with OCR and ATP content (r ≥ 0.746, p < 0.01). PCR highlighted the contribution of caffeic acid and caffeine in the preservation of mitochondrial content and activity (CS, activity, OCR, and ATP content). Protocatechuic acid has proved potential on mitochondrial function in vitro and in vivo, preventing derived cell apoptosis [61,62]. Caffeic acid has displayed protective effects on mitochondrial function under oxidative stress conditions via activation of Nrf2 [58]. Furthermore, the effects of caffeine have been investigated; this methylxanthine exhibited the ability to increase OCR and ATP production in mice brain [59].
The top ten compounds analyzed ordered by contribution to the preventive properties of coffee and cocoa by-products against oxidative stress and mitochondrial dysfunction are shown in Figure 1C. Again, caffeine, and chlorogenic and caffeic acids were the three compounds identified in a higher concentration that exhibited the best antioxidant properties. On the other hand, salicylic, vanillic, and ferulic acids were the minor compounds with the highest contribution.
3.4. Caffeine and Phenolics in Coffee and Cocoa By-Products Attenuated Adipogenesis and Promoted Adipocyte Browning
Besides favoring inflammatory and oxidative processes, the adipose tissue continues expanding in energy imbalance conditions (high caloric diets and reduced physical activity). Reducing lipid accumulation and promoting lipolysis in the first stages of adipose tissue expansion can be considered to be anti-adipogenic strategies [63]. Extracts from coffee and cocoa by-products reduced lipid accumulation (4.1–44.5%) (Table S3). The lowest (p < 0.05) EC30/50 value was that of coffee husk (Table 4). This lipid-reducing effect may be either owing to cell proliferation or the inhibition of the preadipocyte differentiation (adipogenesis) [63]. This effect mainly correlated with the concentration of gallic, chlorogenic, and p-coumaric acids (r = −0.870, −0.843, and −0.843, respectively, p < 0.0001). PCR analysis supported those results. Gallic acid proved to have anti-adipogenic activity in 3T3-L1 adipocytes by inhibiting Pparγ, Scd-1, and Fasn expression [64]. Besides exerting these effects, p-coumaric acid was able to down-regulate the expression of the transcription factor CEBPα, Lpl, AdipoQ, and Fabp4. Additionally, chlorogenic acid reduced lipid accumulation via Plin1 and Srebp1 down-regulation. The impact of the coffee and cocoa by-products extracts on lipogenesis/lipolysis in mature adipocytes was further assessed, and the results are shown in Table 4 and Table S3. Extracts reduced TAG content by 11.9–63.0% via lipolysis stimulation, enhancing glycerol release (0.4 to 3.2-fold) and lipase activity (6.5–45.6%). Coffee silverskin was found to be the most effective treatment exhibiting the lowest EC30/50 values. The effect in glycerol significantly correlated with the content of vanillic and chlorogenic acids (r = 0.908 and 0.845, respectively, p < 0.0001) and the concentration of caffeine (r = 0.899, p < 0.0001); similarly, these compounds correlated with the activation of lipases activity. Both the Pearson correlation and PCR analysis revealed the contribution of caffeic acid. Vanillic acid presented a strong effect reducing TAG content in 3T3-L1 adipocytes through the reduction of AdipoQ, Plin1, and Fabp4 expression [64]. Chlorogenic acid has shown to increase lipolysis and up-regulate the expression of lipases (Hsl and Dgat1) [65,66]. Similarly, caffeine has exhibited potential to up-regulate HSL and AGTL and increase lipolysis, reducing TAG content in 3T3-L1 cells [67,68].
Table 4.
Anti-adipogenic effect of coffee husk, coffee silverskin, and cocoa shell extracts (31–500 μg/mL) modulating lipid accumulation, intracellular triglyceride content, extracellular glycerol release, lipase activity, mitochondrial content, citrate synthase (CS) activity, and ATP content in 3T3-L1 adipocytes. Values are expressed as mean (µg/mL) ± SD (n = 3). Different letters among rows indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test.
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC 30 | EC 50 | EC 30 | EC 50 | EC 30 | EC 50 | |
| Lipid accumulation | 132.7 ± 18.1a | 309.6 ± 23.8a | 220.8 ± 35.1b | 515.3 ± 46.1b | 388.1 ± 67.4c | 905.5 ± 88.4c |
| Triglyceride content | 64.9 ± 12.4a | 151.5 ± 30.5b | 49.6 ± 17.5a | 115.7 ± 22.3a | 353.1 ± 64.5b | 823.8 ± 146.7c |
| Glycerol release | <31.0 | <31.0 | <31.0 | <31.0 | 36.8 ± 8.4 | 85.9 ± 4.3 |
| Lipase activity | 357.3 ± 73.8b | 833.6 ± 106.7b | 210.9 ± 33.4a | 492.1 ± 48.6a | 422.2 ± 57.6b | 985.2 ± 142.9b |
| Mitochondrial content | >500 | >500 | >500 | >500 | >500 | >500 |
| CS activity | 227.6 ± 43.1b | 531.1 ± 62.4b | 140.9 ± 22.8a | 328.9 ± 33.2a | 247.4 ± 51.4b | 577.2 ± 74.3b |
| ATP content | >500 | >500 | 251.8 ± 24.0a | 587.5 ± 35.2a | 312.4 ± 44.6b | 728.9 ± 65.1b |
Furthermore, it has been shown that during differentiation, adipocytes can acquire a brown-like or beige phenotype, a process known as adipocyte browning. These beige adipocytes exhibit higher mitochondrial content and activity [69,70]. Thus, coffee and cocoa by-products increased (1.0–26.6%) the mitochondrial content as well as increased mitochondrial citrate synthase activity by 9.0–54.5%; the production of ATP was also increased (2.9–48.0%). The lowest EC30/50 values were shown for coffee silverskin treatment (Table 4). The increase in mitochondrial content was associated with the concentration of salicylic and mandelic acids in the extracts (r = 0.831 and 0.735, respectively, p < 0.001). However, the promotion of mitochondrial CS activity and the production of ATP better correlated with the content of salicylic acid and caffeine (r = 0.789 and 0.774, respectively, p < 0.001). Nonetheless, the contribution of caffeic acid, beyond caffeine, was demonstrated by PCR analysis. The browning effects of salicylic acid has been associated with its capacity to increase Ucp1, Pparα, and Elovl3 expression [71]. The effects of caffeine on adipocyte browning have been intensely studied. Caffeine prompts mitochondrial biogenesis and UCP1 expression, increasing the oxidative capacity of mitochondria and ATP production, via PGC-1α pathway activation, and evidencing enhanced thermogenesis in humans [72,73]. Likewise, caffeic acid has been suggested to be able to increase mitochondrial content and prompt its oxidative activity in adipocytes [28]. The use of nutritional interventions, natural products, and phytochemicals to stimulate mitochondrial biogenesis and bioenergetics and attenuate oxidative stress are current strategies for the prevention and treatments of chronic metabolic disorders [74].
The ten compounds with the highest potential to inhibit adipogenesis and elicit adipocyte browning are described in Figure 1D. Caffeine, chlorogenic, and caffeic acids were the main contributor among those phytochemicals identified in higher concentration in coffee and cocoa by-products. Vanillic, 3-hydroxymandelic, and ferulic acids were the minor compounds exhibiting the highest contribution.
3.5. Phenolic Compounds Counteracted Insulin Resistance through the Modulation of Insulin Signaling and the Promotion of GLUT4 Translocation
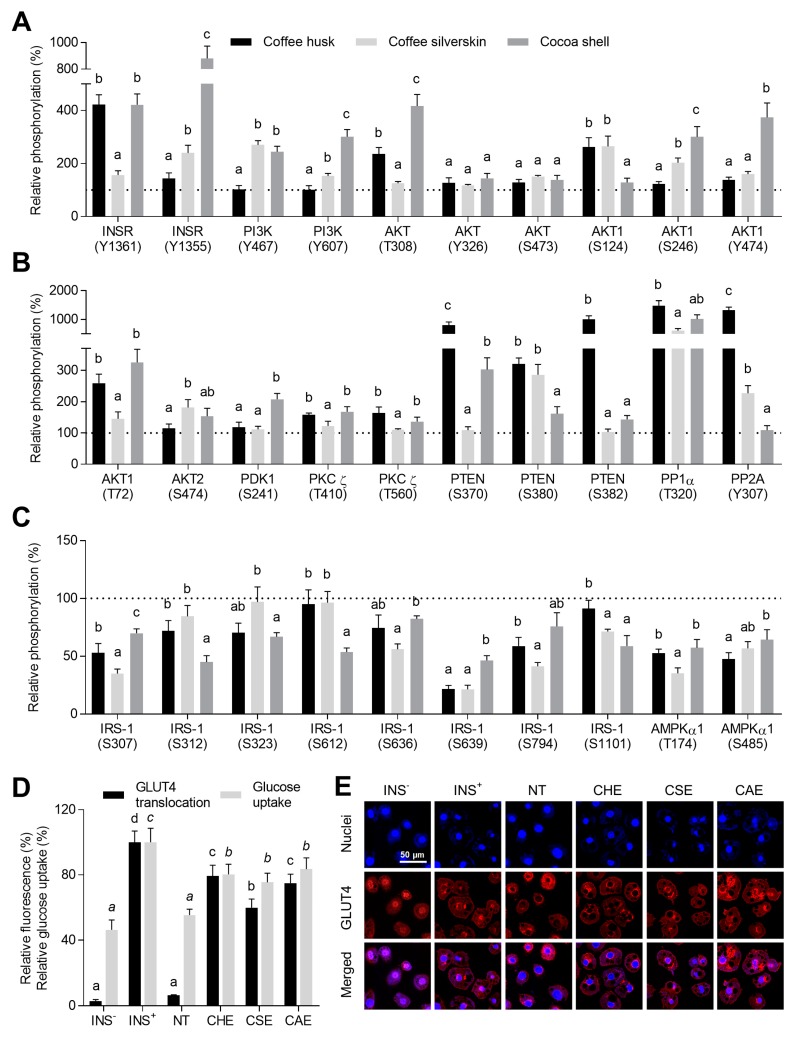
Upon insulin binding with its receptor, a sequence of reactions conduces to GLUT4 translocation into the membrane and the subsequent glucose uptake. In obese conditions, inflammation derives in insulin resistance, which is associated with the development of type 2 diabetes [75,76]. The extracts from coffee and cocoa by-products counteracted the inhibition of insulin signal transduction via modulation of the phosphorylation of key proteins in the insulin pathway (Figure 2). From a total of 30 protein involved in the insulin receptor signaling pathway, 20 protein were up-phosphorylated and 10 down-phosphorylated. CHE and CAE promoted INSR tyrosine phosphorylation. CSE and CAE potentiated PI3K phosphorylation (52.8–201.2%). That resulted in augmented AKT phosphorylation (from 16.9 to 316.5%). Lately, extracts prompted PKC ζ phosphorylation (9.9–67.8%), which would result in GLUT 4 translocation into the cell membrane [77]. Furthermore, extracts inhibit the action of diverse phosphatases (PTEN, PP1α, and PP2A) through their phosphorylation (from 1.4 to 14.8-fold). IRS-1 was down-phosphorylated by at least one of the extracts in six different serine residues, which promoted insulin signaling. As a result, GLUT4 was significantly (p < 0.05) more translocated to the membrane (59.8–79.3%) and glucose uptake stimulated (75.4–80.4%) in comparison to the non-treated control. INRS phosphorylation was associated with the content of protocatechuic acid and theobromine (r = 0.763 and 0.833, respectively, p < 0.001). PCR denoted the contribution of (+)-catechin. Likewise, the phosphorylation of PI3K correlated with the concentration of (+)-catechin and theobromine (r = 0.818 and 0.817, respectively, p < 0.001). AKT phosphorylation, in its different residues, correlated with protocatechuic acid and procyanidin B1 (T308), theobromine and caffeine (S326 and S473, respectively), chlorogenic acid and caffeine (S124), theobromine (S246), (+)-catechin, (−)-epicatechin, procyanidin B2 (Y474), and protocatechuic acid (T72). PCR exhibited a significant contribution in the phosphorylation of AKT of protocatechuic acid and procyanidin B1 (Y326) and corroborated the results obtained using Pearson correlations. PKC ζ phosphorylation was associated with protocatechuic and p-coumaric acids (T410 and T560). PTEN exhibited an association with chlorogenic (S380), gallic acid, and kaempferol-3-O-galactoside (S382). Similar behavior demonstrated PP1α and PP2A. Moreover, the results from PCR analysis gave similar information on the contribution of those phytochemicals. The down-phosphorylation of IRS-1 mainly correlated with the concentration of protocatechuic and caffeic acids, caffeine, and theobromine. GLUT4 translocation correlated with the concentration of protocatechuic and gallic acids (r = 0.699 and 0.642, respectively, p < 0.01). Glucose uptake was associated with the concentration of protocatechuic acid (r = 0.746, p < 0.001). The contribution of p-coumaric acid was also significant, as observed in both the Pearson correlation and PCR coefficients.
Figure 2.
Insulin-sensitizing effects of coffee husk, coffee silverskin, and cocoa shell aqueous extracts (125 μg/mL) on adipocyte treated with the conditioned media from macrophages. Up-phosphorylated proteins (A,B) and down-phosphorylated proteins (C) from the insulin signaling pathway. GLUT4 translocation into the cell membrane measured as the relative fluorescence intensity between GLUT4 and the nucleus and glucose uptake (D), and confocal laser scanning microscopy representative images (E). INS: insulin; NT: non-treated cells; CHE: coffee husk extract; CSE: coffee silverskin extract; CAE: cocoa shell extract.
Protocatechuic acid possesses insulin-mimicking both in vitro and in vivo. Engaging the INSR and including a reversion of INS-1 serine phosphorylation, and activation of insulin/PI3K/AKT and AMPK signaling pathways, protocatechuic acid triggers a positive regulation of and glucose uptake through GLUT4 translocation [78]. Likewise, gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells via PKC ζ activation [79]. Insulin resistance is strongly associated with the pathogenesis of type 2 diabetes and obesity and results from a sustained low-grade inflammatory status and the loss of the mitochondrial function [80]. Hence, the alleviation of these conditions and the modulation of insulin sensitivity are needed in the prevention of the development and exacerbation of obesity and diabetes outcomes.
The top ten phytochemicals analyzed from coffee and cocoa by-products with the highest potential to prevent insulin resistance development are included in Figure 1E. Protocatechuic acid, (−)-epicatechin, and procyanidin B2 were the major compounds contributing to these effects. Procyanidin B1, p-coumaric and 3,4-dihydroxyphenilacetic acids, in turn, the minor compounds exhibiting the highest contribution.
3.6. Phytochemicals from Coffee and Cocoa By-Products Regulated Protein Phosphorylation thereby Preventing Inflammation, Oxidative Stress, and Insulin Resistance
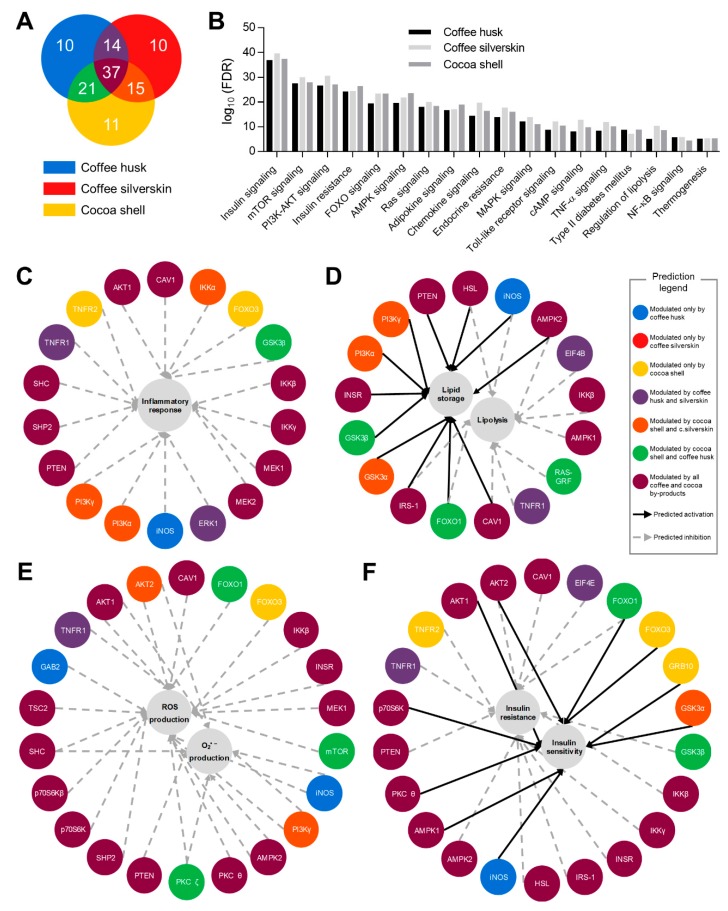
Beyond modifying the phosphorylation of proteins in the insulin receptor pathway, coffee and cocoa by-product aqueous extracts modulated the phosphorylation state of proteins associated with pathways related to inflammation and oxidative stress (Figure 3). Coffee husk significantly (p < 0.05) modulated the phosphorylation of 82 out of 122 (67%) phosphorylation sites evaluated, whereas coffee silverskin modified 76 and cocoa shell 84 (62 and 69%, respectively) (Figure 3A; Table S3). Among the three treatments, the phosphorylation pattern was different. They shared 51–58 phosphorylation sites when comparing among pairs, and only 37 phosphorylation sites were modulated by all the treatments. Among the top pathways accounting with modified proteins, the most significant ones were insulin, mTOR, PI3K-AKT, FOXO, and AMPK signaling pathways (Figure 3B).
Figure 3.
Venn diagram showing the overlap of differentially phosphorylated proteins in adipocytes upon the treatment with coffee husk, coffee silverskin, and cocoa shell aqueous extracts (31–500 μg/mL) (A). KEGG pathways associated with the differentially phosphorylated proteins (B). Downstream effect analysis of coffee and cocoa extracts on inflammatory response (C), lipid storage and lipolysis (D), oxidative stress (E), and insulin resistance (F).
Downstream effect analysis validated the effects analyzed in adipocytes for inflammation, oxidative stress, and insulin resistance (Figure 3C–F). The inflammatory response in adipocytes was mediated by the phosphorylation of proteins from the TNF-α (TNFR1, TNFR 2), NF-κB (IKKβ, IKKγ), and MAPK (MEK1, MEK2, ERK1) pathways, among others (Figure 3C). Lipid accumulation and lipolysis in inflammatory conditions were regulated by the modulation of the phosphorylation of proteins related to the insulin (INSR, IRS-1, PI3K, AKT, FOXO) and the TNF-α (TNFR, IKKβ, iNOS) signaling pathways and HSL (Figure 3D). Inflammation in the adipose tissue causes lipolysis and the release of free fatty acids, serving as ligands for toll-like receptor-4 (TLR4) and further activating NF-κB and JNK, leading mediators of inflammation [81]. Thus, the reduction of inflammation in adipocytes results in lower lipolysis (higher accumulation of lipid) through the maintaining of insulin sensitivity. As observed in Figure 2B, the main compounds identified in coffee and cocoa by-products responsible for the anti-inflammatory properties were caffeine, caffeic, chlorogenic, and protocatechuic acids.
Furthermore, the phosphorylation of proteins from insulin (INSR, PI3K, AKT), PKC (PKC ζ, PKC θ), and mTOR (mTOR, p70S6K) pathways was predicted to be affecting the production of ROS and specifically the production of mitochondrial superoxide (Figure 3E). The main phytochemicals contributing to these effects were also caffeine, caffeic, chlorogenic, and protocatechuic acids. It has been reported that mTOR, a downstream of the insulin/PI3K/AKT pathway, depending upon PKC, can be conducive to defective ROS production [82]. Most of these proteins were modulated by both coffee and cocoa by-products. Therefore, the production of ROS and mitochondrial superoxide was repressed.
On the other hand, the most important compounds contributing to the anti-adipogenic properties of coffee and cocoa by-products were caffeine, and chlorogenic and gallic acids. Finally, the modification of the phosphorylation state of proteins in insulin (INSR, IRS-1, AKT), AMPK (PKA), TNF-α (TNFR1, TNFR 2), and NF-κB (IKKβ, IKKγ) signaling pathways triggered an increase in insulin sensitivity in adipocytes in inflammatory conditions (Figure 3F). Among the pro-inflammatory stimuli secreted by macrophages, TNF-α presence proved evidence of conducting insulin resistance through the IRS-1 serine phosphorylation, and inactivation, via activation of NF-κB, ERK, JNK, mTOR, and PKC θ [83]. We might attribute these effects to protocatechuic acid, (−)-epicatechin, and procyanidin B2.
Hence, coffee husk, coffee silverskin, and cocoa shell mediated alleviation of inflammatory processes could also reverse inflammation-triggered oxidative stress, mitochondrial dysfunction, and insulin resistance.
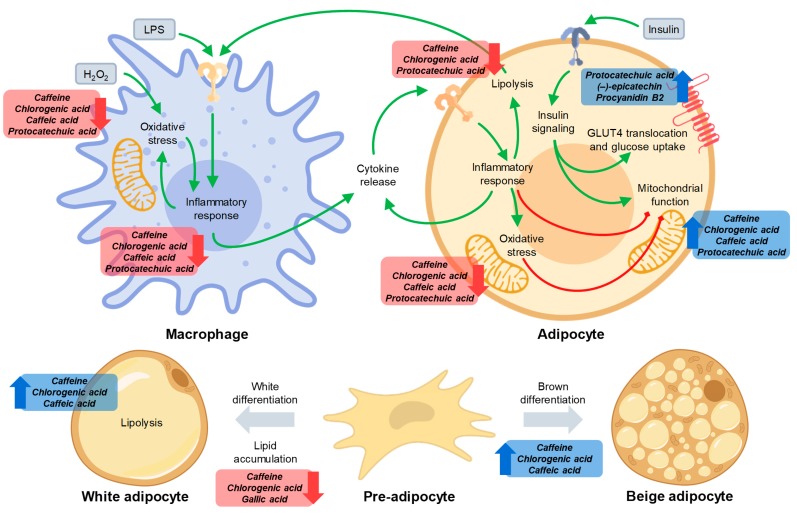
A diagram illustrating the mechanism and main actors of the effects in macrophages and adipocytes is shown in Figure 4. As described, the phytochemicals exerting most of the biological effects associated with inflammation, oxidative stress, adipogenesis, and insulin resistance were caffeine, chlorogenic, and protocatechuic acids.
Figure 4.
Integrative diagram illustrating the underlying molecular mechanism of the phytochemicals from coffee husk, coffee silverskin, and cocoa shell in inflammation, oxidative stress, adipogenesis, and insulin resistance in macrophages and adipocytes.
In this comparative study, we have observed that the differential composition of coffee and cocoa by-products is associated with their in vitro biological properties. Both major and minor components of coffee husk, coffee silverskin, and cocoa shell presented a relationship with the potential against inflammation, oxidative stress, adipogenesis, and insulin resistance. Comparing the effects of coffee husk, coffee silverskin, and cocoa shell, we observed (Figure S3) that the best extract for preventing inflammation was that of cocoa shell. Results suggest that oxidative stress was prevented by cocoa shell and coffee silverskin, whereas the anti-adipogenic potential was stronger for coffee silverskin. The ability to counteract insulin resistance was higher for coffee husk. Despite the potential biological activity of phytochemicals identified in coffee and cocoa by-products, phenolic compound bioefficacy is conditioned by their low bioavailability. These phytochemicals are only partially absorbed in the gastrointestinal tract. After being metabolized by the microbiota or in the liver (methylation, sulfation, and glucuronidation), they reach target tissues at low concentrations [71,72]. Future in vivo studies with coffee and cocoa by-products will allow us to ascertain their pharmacokinetic behavior and their efficacy in delaying the development of metabolic disorders related to inflammation and oxidative stress.
4. Conclusions
The use of coffee and cocoa by-products as a source of phytochemicals is proposed as a strategy in the prevention of chronic diseases related to inflammation and oxidative stress, such as obesity and diabetes. This study presents an evaluation of the chemical composition of coffee husk, coffee silverskin, and cocoa shell in the inhibition of inflammation, oxidative stress, adipogenesis, and insulin resistance using RAW264.7 macrophages and 3T3-L1 adipocytes. Taken together, our results support the notion that phenolic compounds and methylxanthines from coffee and cocoa by-products can trigger an attenuation of inflammation and oxidative stress in macrophages and adipocytes, counteracting the consequent loss of mitochondrial function, as well as reduce adipogenesis and induce browning in adipocytes, and potentiating insulin sensitivity. Furthermore, we provide new knowledge on the underlying mechanism of action and contribution of the phytochemicals composing coffee husk, coffee silverskin, and cocoa shell. This research revalorizes by-products from coffee and cocoa, therefore, increasing their potential utilization and their conversion into food ingredients, with the added-value potential to prevent chronic metabolic diseases. Additionally, in the future, in vivo studies will be accomplished to validate the safety and efficacy of coffee and cocoa by-products as new ingredients. In conclusion, we established, for the first time, the relationship of the composition of different phytochemicals among coffee and cocoa by-products and their in vitro potential to reduce markers of inflammation, oxidative stress, adipogenesis, and insulin resistance.
Supplementary Materials
The following are available on line at https://www.mdpi.com/2076-3921/8/8/279/s1, Figure S1: Impact of treatments on cell viability of (A) RAW264.7 macrophages, (B) 3T3-L1 preadipocytes, and (C) 3T3-L1 differentiated adipocytes. Cells were treated in the absence (NT) or presence of coffee husk, coffee silverskin, or cocoa shell aqueous extracts (31–500 μg/mL) for 24 h, after which the cell viability was determined using an MTS colorimetric assay. The results are expressed as mean ± SD (n = 3). NT: non-treated cells; Figure S2: Heat map including standardized principal component regression (PCR) coefficient of the regression constructed among the phytochemicals identified in coffee husk, coffee silverskin, and cocoa shell aqueous extracts and the potential of the extracts in the different biomarkers of inflammation, oxidative stress, adipogenesis, and insulin resistance; Figure S3: Dendrograms of hierarchical cluster analysis of the treatments of coffee husk (CHE), coffee silverskin (CSE), and cocoa shell (CAE) on the different in vitro models od inflammation, oxidative stress, adipogenesis, and insulin resistance. All the variables in each group of biological activity were included. NT: non-treated cells; Table S1: Anti-inflammatory effect of coffee husk, coffee silverskin, and cocoa shell extracts regulating NO, PGE2, TNF-α, and MCP-1 release in LPS-stimulated RAW264.7 macrophages, and TNF-α, MCP-1, IL-6, adiponectin, intracellular triglyceride content, extracellular glycerol release, and lipase activity CM-stimulated in 3T3-L1 adipocytes. Values are expressed as mean ± SD (n = 3). Different letters among rows (same concentration) indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test; Table S2: Protective effect of coffee husk, coffee silverskin, and cocoa shell extracts against oxidative stress and mitochondrial dysfunction regulating ROS and ΔΨm in RAW264.7 macrophages and ROS, mitochondrial O2• ‒, ΔΨm, mitochondrial content, citrate synthase activity, OCR, and ATP content in 3T3-L1 adipocytes. Values are expressed as mean ± SD (n = 3). Different letters among rows (same concentration) indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test; Table S3: Anti-adipogenic effect of coffee husk, coffee silverskin, and cocoa shell extracts modulating lipid accumulation, intracellular triglyceride content, extracellular glycerol release, lipase activity, mitochondrial content, citrate synthase activity, and ATP content in 3T3-L1 adipocytes. Values are expressed as mean ± SD (n = 3). Different letters among rows indicate significant differences (p < 0.05) according to ANOVA and Tukey’s multiple range test; Table S4: Significantly (p < 0.05) up- and down-phosphorylated proteins, expressed as log2 (Fold Change), in insulin-resistant 3T3-L1 cells in response to treatment with extracts from coffee husk, coffee silverskin, and cocoa shell.
Author Contributions
Conceptualization, M.R.H. and E.G.D.M.; Data curation, M.R.H.; Formal analysis, M.R.H. and Q.Z.Z.; Funding acquisition, M.M.C. and E.G.D.M.; Investigation, M.R.H., Q.Z.Z., and E.G.D.M.; Methodology, M.R.H., Y.A., and Q.Z.Z.; Project administration, E.G.D.M.; Resources, M.M.C. and E.G.D.M.; Supervision, M.M.C. and E.G.D.M.; Validation, M.R.H.; Visualization, E.G.D.M.; Writing—original draft, M.R.H.; Writing—review and editing, Y.A., Q.Z.Z., M.M.C., and E.G.D.M.
Funding
This research was founded by the USDA–NIFA–HATCH project to E.G.D.M. (1014457) and UAM-Santander (2017/EEUU/01). M. Rebollo-Hernanz received funding from the FPU program of the Ministry of Science, Innovation, and Universities for his predoctoral fellowship (FPU15/04238) and the support for the international research stays at the University of Illinois, Urbana–Champaign (EST17/00823, EST18/0064).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all compounds are available from the authors for possible research projects in cooperation.
References
- 1.World Health Organization Noncommunicable Diseases. [(accessed on 20 May 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- 2.Schulze M.B., Martínez-González M.A., Fung T.T., Lichtenstein A.H., Forouhi N.G. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Guarner V., Rubio-Ruiz M.E. Aging and Health-A Systems Biology Perspective. Interdiscipl Top Gerontol. Basel, Karger. 2015;40:99–106. doi: 10.1159/000364934. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro R., Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancuso P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016;5:47. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y., Sun Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González A., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Aguilera A., Rull A., Rodríguez-Gallego E., Riera-Borrull M., Luciano-Mateo F., Camps J., Menéndez J.A., Joven J. Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediat. Inflamm. 2013;2013:135698. doi: 10.1155/2013/135698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suganami T., Nishida J., Ogawa Y. A Paracrine Loop Between Adipocytes and Macrophages Aggravates Inflammatory Changes. Arterioscler. Thromb. Vasc. Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 12.Aguilera Y., Martin-Cabrejas M.A., González de Mejia E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016;15:405–423. doi: 10.1007/s11101-015-9443-z. [DOI] [Google Scholar]
- 13.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 14.Cooper K.A., Donovan J.L., Waterhouse A.L., Williamson G. Cocoa and health: A decade of research. Br. J. Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 15.Harpaz E., Tamir S., Weinstein A., Weinstein Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017;28:1–10. doi: 10.1515/jbcpp-2016-0090. [DOI] [PubMed] [Google Scholar]
- 16.Clark K.S., Coleman C., Shelton R., Heemstra L.A., Novak C.M. Caffeine enhances activity thermogenesis and energy expenditure in rats. Clin. Exp. Pharmacol. Physiol. 2019;46:475–482. doi: 10.1111/1440-1681.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuggetta M.P., Zonfrillo M., Villivà C., Bonmassar E., Ravagnan G. Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediators Inflamm. 2019;2019:1–10. doi: 10.1155/2019/1515621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez R., Torres P., Meneses M.A., Figueroa J.G., Pérez-Álvarez J.A., Viuda-Martos M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012;49:39–45. doi: 10.1016/j.foodres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Janissen B., Huynh T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018;128:110–117. doi: 10.1016/j.resconrec.2017.10.001. [DOI] [Google Scholar]
- 20.Alves R.C., Rodrigues F., Nunes M.A., Vinha A.F., Oliveira M.B.P.P. Handbook of Coffee Processing By-Products. Academic Press; London, UK: 2017. Chapter 1—State of the art in coffee processing by-products; pp. 1–26. [Google Scholar]
- 21.Oddoye E.O.K., Agyente-Badu C.K., Gyedu-Akoto E. Chocolate in Health and Nutrition. Humana Press; Totowa, NJ, USA: 2013. Cocoa and Its By-Products: Identification and Utilization; pp. 23–37. [Google Scholar]
- 22.Donovan J.L., Crespy V., Oliveira M., Cooper K.A., Gibson B.B., Williamson G. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006;40:1029–1034. doi: 10.1080/10715760600868545. [DOI] [PubMed] [Google Scholar]
- 23.Ellam S., Williamson G. Cocoa and human health. Annu. Rev. Nutr. 2013;33:105–128. doi: 10.1146/annurev-nutr-071811-150642. [DOI] [PubMed] [Google Scholar]
- 24.Higdon J.V., Frei B. Coffee and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 25.Iriondo-DeHond A., Aparicio García N., Fernandez-Gomez B., Guisantes-Batan E., Velázquez Escobar F., Blanch G.P., San Andres M.I., Sanchez-Fortun S., del Castillo M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019;51:194–204. doi: 10.1016/j.ifset.2018.06.010. [DOI] [Google Scholar]
- 26.Panak Balentić J., Ačkar Đ., Jokić S., Jozinović A., Babić J., Miličević B., Šubarić D., Pavlović N., Panak Balentić J., Ačkar Đ., et al. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules. 2018;23:1404. doi: 10.3390/molecules23061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebollo-Hernanz M., Zhang Q., Aguilera Y., Martin-Cabrejas M.A., Gonzalez de Mejia E. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation Between Macrophages and Adipocytes in vitro. Mol. Nutr. Food Res. 2019;63:1801413. doi: 10.1002/mnfr.201801413. [DOI] [PubMed] [Google Scholar]
- 28.Rebollo-Hernanz M., Zhang Q., Aguilera Y., Martin-Cabrejas M.A., Gonzalez de Mejia E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019;132:110672. doi: 10.1016/j.fct.2019.110672. [DOI] [PubMed] [Google Scholar]
- 29.Aguilera Y., Rebollo-Hernanz M., Cañas S., Taladrid D., Martin-Cabrejas M. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019 doi: 10.1039/C9FO00544G. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Patán F., Monagas M., Moreno-Arribas M.V., Bartolomé B. Determination of Microbial Phenolic Acids in Human Faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011;59:2241–2247. doi: 10.1021/jf104574z. [DOI] [PubMed] [Google Scholar]
- 31.Zebisch K., Voigt V., Wabitsch M., Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012;425:88–90. doi: 10.1016/j.ab.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Luna-Vital D., Weiss M., Gonzalez de Mejia E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-α-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017;61:1700362. doi: 10.1002/mnfr.201700362. [DOI] [PubMed] [Google Scholar]
- 33.Frattaruolo L., Carullo G., Brindisi M., Mazzotta S., Bellissimo L., Rago V., Curcio R., Dolce V., Aiello F., Cappello A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants. 2019;8:186. doi: 10.3390/antiox8060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon D., Cha H.-J., Lee H., Hong S.-H., Park C., Park S.-H., Kim G.-Y., Kim S., Kim H.-S., Hwang H.-J., et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants. 2019;8:82. doi: 10.3390/antiox8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi Y.-H., Lee O.-H., Zheng Y., Kang I.-J. Erigeron annuus (L.) Pers. Extract Inhibits Reactive Oxygen Species (ROS) Production and Fat Accumulation in 3T3-L1 Cells by Activating an AMP-Dependent Kinase Signaling Pathway. Antioxidants. 2019;8:139. doi: 10.3390/antiox8050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heeger A., Kosińska-Cagnazzo A., Cantergiani E., Andlauer W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017;221:969–975. doi: 10.1016/j.foodchem.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 37.Bresciani L., Calani L., Bruni R., Brighenti F., Del Rio D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 2014;61:196–201. doi: 10.1016/j.foodres.2013.10.047. [DOI] [Google Scholar]
- 38.Hernández-Hernández C., Viera-Alcaide I., Morales-Sillero A.M., Fernández-Bolaños J., Rodríguez-Gutiérrez G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018;240:831–839. doi: 10.1016/j.foodchem.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Pulido R., Bravo L., Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Sharma J.N., Al-Omran A., Parvathy S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 42.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engin A.B. Advances in Experimental Medicine and Biology. Volume 960. Springer; Berlin, Germany: 2017. Adipocyte-Macrophage Cross-Talk in Obesity; pp. 327–343. [DOI] [PubMed] [Google Scholar]
- 45.Vane J.R., Botting R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:2S–8S. doi: 10.1016/S0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 46.Yin M.-J., Yamamoto Y., Gaynor R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 47.Bao W., Luo Y., Wang D., Li J., Wu X., Mei W. Sodium salicylate modulates inflammatory responses through AMP-activated protein kinase activation in LPS-stimulated THP-1 cells. J. Cell. Biochem. 2018;119:850–860. doi: 10.1002/jcb.26249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An Y., Liu K., Zhou Y., Liu B. Salicylate Inhibits Macrophage-Secreted Factors Induced Adipocyte Inflammation and Changes of Adipokines in 3T3-L1 Adipocytes. Inflammation. 2009;32:296–303. doi: 10.1007/s10753-009-9135-1. [DOI] [PubMed] [Google Scholar]
- 49.Hwang J.-H., Kim K.-J., Ryu S.-J., Lee B.-Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Biol. Interact. 2016;248:1–7. doi: 10.1016/j.cbi.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.-H., Park S.-Y., Park Y.-L., Myung D.-S., Rew J.-S., Joo Y.-E. Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Mol. Med. Rep. 2017;16:9224–9232. doi: 10.3892/mmr.2017.7686. [DOI] [PubMed] [Google Scholar]
- 51.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Ormazabal P., Scazzocchio B., Varì R., Santangelo C., D’Archivio M., Silecchia G., Iacovelli A., Giovannini C., Masella R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018;42:2012–2021. doi: 10.1038/s41366-018-0075-4. [DOI] [PubMed] [Google Scholar]
- 53.Li X., Zhao J., Zhao Y., Liu J., Liu C., Zeng X. Anti-Inflammatory Effects of p-coumaric acid in LPS-stimulated RAW264.7 cells: Involvement of NF-κB and MAPKs pathways. Med. Chem. (Los Angeles). 2016;6:327. [Google Scholar]
- 54.Salmon A.B. Beyond Diabetes: Does Obesity-Induced Oxidative Stress Drive the Aging Process? Antioxidants. 2016;5:24. doi: 10.3390/antiox5030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semaming Y., Pannengpetch P., Chattipakorn S.C., Chattipakorn N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evidence-Based Complement. Altern. Med. 2015;2015:1–11. doi: 10.1155/2015/593902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varì R., D’Archivio M., Filesi C., Carotenuto S., Scazzocchio B., Santangelo C., Giovannini C., Masella R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011;22:409–417. doi: 10.1016/j.jnutbio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Li J., He D., Wang B., Zhang L., Li K., Xie Q., Zheng L. Synthesis of hydroxycinnamic acid derivatives as mitochondria-targeted antioxidants and cytotoxic agents. Acta Pharm. Sin. B. 2017;7:106–115. doi: 10.1016/j.apsb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai T.-H., Yu C.-H., Chang Y.-P., Lin Y.-T., Huang C.-J., Kuo Y.-H., Tsai P.-J., Tsai T.-H., Yu C.-H., Chang Y.-P., et al. Protective Effect of Caffeic Acid Derivatives on tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells. Molecules. 2017;22:702. doi: 10.3390/molecules22050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dragicevic N., Delic V., Cao C., Copes N., Lin X., Mamcarz M., Wang L., Arendash G.W., Bradshaw P.C. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer’s mice and cells. Neuropharmacology. 2012;63:1368–1379. doi: 10.1016/j.neuropharm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Wang C.-H., Wang C.-C., Huang H.-C., Wei Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013;280:1039–1050. doi: 10.1111/febs.12096. [DOI] [PubMed] [Google Scholar]
- 61.Tarozzi A., Morroni F., Hrelia S., Angeloni C., Marchesi A., Cantelli-Forti G., Hrelia P. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci. Lett. 2007;424:36–40. doi: 10.1016/j.neulet.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Guan S., Jiang B., Bao Y.M., An L.J. Protocatechuic acid suppresses MPP+-induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food Chem. Toxicol. 2006;44:1659–1666. doi: 10.1016/j.fct.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 64.Aranaz P., Navarro-Herrera D., Zabala M., Miguéliz I., Romo-Hualde A., López-Yoldi M., Martínez J., Vizmanos J., Milagro F., González-Navarro C. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARγ. Molecules. 2019;24:1045. doi: 10.3390/molecules24061045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng S., Pang Y., Zhu Q., Kang J., Liu M., Wang Z. Chlorogenic Acid Functions as a Novel Agonist of PPAR γ 2 during the Differentiation of Mouse 3T3-L1 Preadipocytes. Biomed. Res. Int. 2018;2018:1–14. doi: 10.1155/2018/8594767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duangjai A., Nuengchamnong N., Suphrom N., Trisat K., Limpeanchob N., Saokaew S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe, J. Med. Sci. 2018;64:E84–E92. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu X., Yang L., Xu F., Lin L., Zheng G. Combination therapy with catechins and caffeine inhibits fat accumulation in 3T3-L1 cells. Exp. Ther. Med. 2017;13:688–694. doi: 10.3892/etm.2016.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murosaki S., Lee T.R., Muroyama K., Shin E.S., Cho S.Y., Yamamoto Y., Lee S.J. A Combination of Caffeine, Arginine, Soy Isoflavones, and l-Carnitine Enhances Both Lipolysis and Fatty Acid Oxidation in 3T3-L1 and HepG2 Cells in Vitro and in KK Mice in Vivo. J. Nutr. 2007;137:2252–2257. doi: 10.1093/jn/137.10.2252. [DOI] [PubMed] [Google Scholar]
- 69.Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nat. Publ. Gr. 2013;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 70.Ikeda K., Maretich P., Kajimura S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018;29:191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Dam A.D., Nahon K.J., Kooijman S., van den Berg S.M., Kanhai A.A., Kikuchi T., Heemskerk M.M., van Harmelen V., Lombès M., van den Hoek A.M., et al. Salsalate activates brown adipose tissue in mice. Diabetes. 2015;64:1544–1554. doi: 10.2337/db14-1125. [DOI] [PubMed] [Google Scholar]
- 72.Velickovic K., Wayne D., Leija H.A.L., Bloor I., Morris D.E., Law J., Budge H., Sacks H., Symonds M.E., Sottile V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Sci. Rep. 2019;9:9104. doi: 10.1038/s41598-019-45540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoneshiro T., Matsushita M., Hibi M., Tone H., Takeshita M., Yasunaga K., Katsuragi Y., Kameya T., Sugie H., Saito M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am. J. Clin. Nutr. 2017;105:873–881. doi: 10.3945/ajcn.116.144972. [DOI] [PubMed] [Google Scholar]
- 74.Anusree S.S., Nisha V.M., Priyanka A., Raghu K.G. Insulin resistance by TNF-α is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol. Cell. Endocrinol. 2015;413:120–128. doi: 10.1016/j.mce.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lumeng C.N., Deyoung S.M., Saltiel A.R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Meyts P. The Insulin Receptor and Its Signal. Transduction Network. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J.M., Kaltsas G., Koch C., Kopp P., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [PubMed] [Google Scholar]
- 78.Scazzocchio B., Varì R., Filesi C., Del Gaudio I., D’Archivio M., Santangelo C., Iacovelli A., Galvano F., Pluchinotta F.R., Giovannini C., et al. Protocatechuic acid activates key components of insulin signaling pathway mimicking insulin activity. Mol. Nutr. Food Res. 2015;59:1472–1481. doi: 10.1002/mnfr.201400816. [DOI] [PubMed] [Google Scholar]
- 79.Vishnu Prasad C.N., Anjana T., Banerji A., Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010;584:531–536. doi: 10.1016/j.febslet.2009.11.092. [DOI] [PubMed] [Google Scholar]
- 80.Ye J. Mechanisms of insulin resistance in obesity. Front. Med. 2013;7:14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stafeev I.S., Vorotnikov A.V., Ratner E.I., Menshikov M.Y., Parfyonova Y.V. Latent Inflammation and Insulin Resistance in Adipose Tissue. Int. J. Endocrinol. 2017;2017:1–12. doi: 10.1155/2017/5076732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volpe C.M.O., Villar-Delfino P.H., dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Copps K.D., White M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.