Abstract
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart disease characterized by sudden death in young people and featured by fibro-adipose myocardium replacement, malignant arrhythmias, and heart failure. To date, no etiological therapies are available. Mutations in desmosomal genes cause abnormal mechanical coupling, trigger pro-apoptotic signaling pathways, and induce fibro-adipose replacement. Here, we discuss the hypothesis that the ACM causative mechanism involves a defect in the expression and/or activity of the cardiac Ca2+ handling machinery, focusing on the available data supporting this hypothesis. The Ca2+ toolkit is heavily remodeled in cardiomyocytes derived from a mouse model of ACM defective of the desmosomal protein plakophilin-2. Furthermore, ACM-related mutations were found in genes encoding for proteins involved in excitation‒contraction coupling, e.g., type 2 ryanodine receptor and phospholamban. As a consequence, the sarcoplasmic reticulum becomes more eager to release Ca2+, thereby inducing delayed afterdepolarizations and impairing cardiac contractility. These data are supported by preliminary observations from patient induced pluripotent stem-cell-derived cardiomyocytes. Assessing the involvement of Ca2+ signaling in the pathogenesis of ACM could be beneficial in the treatment of this life-threatening disease.
Keywords: arrhythmogenic cardiomyopathy, desmosomes, plakophilin-2, type 2 ryanodine receptors, phospholamban, Ca2+ sparks
1. Introduction
The heart is a highly specialized machine that requires fine regulation: intracellular changes and cell-to-cell communication disturbances could destroy this balance. Depending on the type of cardiac arrhythmia, a series of events may occur: intracellular dysfunction‒tissue remodeling‒impaired cell‒cell connection–electrical dysfunction. Loss of cardiac tissue integrity could be both a cause and an effect of arrhythmic phenotype. A typical example is arrhythmogenic cardiomyopathy (ACM), a cardiac disorder in which mutations in proteins of the desmosome may be present, causing cell-to-cell discontinuity, arrhythmic events, and myocardial fibro-adipose substitution [1,2,3].
Intracellular calcium (Ca2+) signals drive excitation‒contraction (EC) coupling and are, therefore, necessary for the heart to effectively pump blood into the pulmonary artery and aorta. It is, therefore, not surprising that any defect in the Ca2+ cycling machinery or in the complex network of Ca2+-related proteins that maintain cardiac Ca2+ homeostasis under tight control may severely compromise cardiac function. Alterations or mutations in the Ca2+ handling machinery have been associated with a number of severe cardiac diseases, including cardiac hypertrophy, dilated cardiomyopathy, and heart failure [4,5], and several inherited arrhythmia syndromes (i.e., Timothy syndrome, Brugada syndrome, and early repolarization syndromes) [6,7,8].
Herein, we describe the emerging evidence in favor of Ca2+ dysregulation as a primary (or arrhythmia-causative) event in ACM. We discuss how the Ca2+ cycling machinery could be rewired into a pro-arrhythmogenic phenotype by some of the desmosomal mutations that are known to induce ACM by causing cardiac tissue discontinuity. Furthermore, we illustrate the effect of mutations in ACM causative genes that encode for crucial components of the Ca2+ handling system.
2. Calcium Signaling and Cardiac Arrhythmias: A Tight Connection
Cardiac contraction is triggered by a transient increase in intracellular Ca2+ concentration ([Ca2+]i) induced by membrane depolarization according to a process known as EC coupling [6,7,9]. Briefly, as the action potential, triggered by an influx of sodium (Na+) via the voltage-gated Na+ channels, propagates along the T-tubules, membrane depolarization opens CaV1.2, which is the pore-forming α-subunit of L-type voltage-gated Ca2+ channels (VGCCs), thereby causing the influx of Ca2+ into the dyadic junction, which separates the sarcolemma from the closely apposed (~15 nm) junctional sarcoplasmic reticulum (SR) [10] in ventricular myocytes [9]. Extracellular Ca2+ influx causes a large increase in local [Ca2+]i, which activates a cluster (7–20) of type 2 Ryanodine receptors (RYR2) in a process known as Ca2+-induced Ca2+ release (CICR) and gives rise to significant Ca2+ release events from the junctional SR (called Ca2+ sparks). The temporal summation of these local events by the propagating action potential results in a regenerative Ca2+ transient that is detected by troponin C to initiate the sliding of the thick (myosin) and thin (actin) filaments, cell shortening, and hence pressure development within each ventricle and ejection of blood into the circulation during cardiac systole [6,7,9]. Subsequently, [Ca2+]i drops and mechanical force relaxes quickly to pre-systolic levels during cardiac diastole, which is essential to enable cardiac chambers to refill with blood. The increase in [Ca2+]i is indeed short-lived as cytosolic Ca2+ is extruded across the plasma membrane by the Na+/Ca2+ exchanger (NCX1) and the plasmalemmal Ca2+-ATPase (PMCA) and sequestered back into the SR by the sarco-endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) [6,7,9]. The Ca2+ affinity of SERCA2a is regulated by the phosphoprotein phospholamban (PLN), which increases or reduces the pumping rate of SERCA2a depending on its phosphorylation state. In the dephosphorylated state, PLN binds to SERCA2a and inhibits the Ca2+ transport ability of the pump by reducing its apparent affinity for Ca2+. However, when PLN is phosphorylated, it dissociates from SERCA2a, thereby increasing the pump’s affinity for Ca2+ and favoring relaxation [11]. PLN presents two sites of phosphorylation: Ser16 for protein kinase A (PKA) and Thr17 for Ca2+/Calmodulin-dependent protein kinase II (CaMKII) and protein kinase B (AKT) [12]. Importantly, β-adrenergic stimulation exerts a profound impact on cardiac Ca2+ handling by modulating CaV1.2, RYR2 and SERCA2a activity. Accordingly, stimulation of β-adrenergic receptors, e.g., with isoproterenol, engages PKA and CaMKII. PKA, in turn, phosphorylates CaV1.2 and PLN, thereby increasing the amplitude of L-type voltage-gated Ca2+ currents and boosting SR Ca2+ uptake. Furthermore, Bovo and colleagues recently demonstrated that PKA-dependent phosphorylation also regulates RYR2-mediated SR Ca2+ release independently of PKA effects on SR Ca2+ load [13].
Evidence that has emerged over the last 20 years about inherited mutations in genes encoding for proteins involved in the regulation of Ca2+ homeostasis has clearly showed how defects in intracellular Ca2+ handling are associated with various forms of arrhythmogenic disorders, including catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada syndrome, Timothy syndrome, long and short QT syndrome [6,7,8]. These life-threatening diseases feature distinctive electrocardiogram patterns, ventricular arrhythmias, and a high risk of sudden cardiac death [6,7,8]. The major genes responsible for such inherited arrhythmias are RYR2 (encoding for RYR2), CASQ2 (encoding for calsequestrin-2), and CACNA1C (encoding for CaV1.2) [6,7,8].
RYR2 receptors cluster into functional Ca2+-release units presenting a finite open probability and are, therefore, able to mediate unsynchronized Ca2+ sparks even at diastole, when Ca2+ leaks out of the SR [9,14]. While the Ca2+ leak finely tunes the SR Ca2+ concentration, a pathological increase in the rate of Ca2+ efflux from the SR is detrimental to the heart as it can deplete the SR Ca2+ content, thereby reducing the amplitude of voltage-dependent Ca2+ transients and diminishing force production [9,14]. In addition, the increased SR Ca2+ leak during diastole can increase the frequency of spontaneous Ca2+ sparks, which can coalesce into a sub-sarcolemmal Ca2+ wave cleared by NCX1. This results in an untimely depolarizing (3 Na+ in: 1 Ca2+ out) inward current that triggers delayed afterdepolarizations (DADs) and induces ventricular arrhythmia [9,14]. An increase in RYR2-mediated Ca2+ leak has been observed in failing and aging hearts due to an increase in the extent of CaMKII-dependent phosphorylation [8,14]. Furthermore, a number of gain-of-function mutations in RYR2 increases the SR Ca2+ leak in CPVT1, the autosomal dominant form of CPVT [15]. CPVT is an inherited arrhythmia disorder featuring polymorphic or bidirectional ventricular tachycardia; It arises in response to β-adrenergic stimulation during exercise or acute emotional stress and may lead to syncope and sudden cardiac death [7,8]. Arrhythmias in CPVT are induced by spontaneous RYR2-mediated Ca2+ release, which stimulates DADs to induce extrasystolic beats [8,15].
In turn, the recessive form of this pathology (CPVT2) has been associated with CASQ2 mutations [16,17]. Calsequestrin-2 (CASQ2) is a moderate-affinity, high-capacity SR Ca2+ binding protein that maintains a reservoir of releasable Ca2+ in close proximity of the luminal mouth of RYR2 pore, while preserving low levels of free Ca2+ [8]. In addition, CASQ2 inhibits both Ca2+-induced and spontaneous RYR2 activation, thus acting as a negative regulator of SR Ca2+ release [18]. Consistently, a number of CPVT2-related mutations that increase CASQ2 proteolytic degradation, preventing its Ca2+-buffering activity or impairing its ability to modulate RYR2 opening, were found to induce spontaneous Ca2+ release, DADs, and, ultimately, ventricular arrhythmia during β-adrenergic stimulation [6,7,8].
Pro-arrhythmogenic mutations may also target CaV1.2, which is the primary determinant of L-type Ca2+ current (ICa,L) in the ventricular myocardium and drives EC coupling by recruiting RYR2 through the CICR process. A missense mutation was reported at the cytosolic COOH-terminal of CaV1.2, resulting in impaired voltage-dependent inactivation in ICa,L and thereby causing an aberrant increase in [Ca2+]i and DADs in Timothy syndrome [7]. This is a rare (< 30 patients around the world), multisystem disorder characterized by cardiac, hand/foot, facial, and neurodevelopmental features that can cause the patient’s death by inducing 2:1 atrioventricular block, QT prolongation in the ECG, and ventricular tachyarrhythmia [7]. Novel genetic alterations in CACNA1C were also described by Wemhöner and colleagues, causing typical long QT syndrome (LQTS) without any clinical features of Timothy syndrome and leading to a gain-of-function activity of the channel combining different mechanisms [19].
Conversely, a loss-of-function mutation in CACNA1C observed in 10‒15% of patients caused a dramatic reduction in CaV1.2-mediated Ca2+ entry that was associated with ST-segment elevation and increased risk of sudden cardiac death secondary to polymorphic ventricular fibrillation or tachycardia in Brugada syndrome [20]. Likewise, CaV1.2-mediated Ca2+ entry is dramatically reduced in short QT syndrome (SQTS), another rare inherited disease characterized by a short QT interval combined with life-threatening ventricular arrhythmias. The SQTS may arise as a consequence of three mutations that target both the pore-forming α subunit (i.e., CACNA1C) and the ancillary β (i.e., CACNB2B) and δ (i.e., CACN2D1) subunits [20,21].
In addition, other genes related to abnormal Ca2+-machinery functioning can lead to cardiac disorders. In particular, in the case of CPTV, three other genes have been linked to the pathology: CPVT3 to CPVT5. In the case of CPVT3, the mutation is in the TECRL gene encoding for the trans-2,3-enoyl-CoA reductase-like protein. Even though alteration in this gene seems to have overlapping clinical features of both LQTS and CPVT, studies in patient induced pluripotent stem-cell-derived cardiomyocytes (iPSC-CM) revealed smaller [Ca2+]i transient amplitudes, lower SR Ca2+ stores, and elevated diastolic [Ca2+]i, consistent with the presence of a CPVT-related SR Ca2+ leak [22]. CPVT4, on the other hand, is caused by a heterozygous mutation in the calmodulin (CAM) gene (CALM1). In vitro assays have demonstrated that the pathogenic mechanism relies on altered Ca2+-binding and leads to a defective interaction between RYR2 and CAM [23]. Lastly, CPVT5 is caused by a homozygous or compound heterozygous mutation in the triadin gene (TRDN). Triadin is a transmembrane protein that forms a quaternary complex with RYR2, CASQ2, and junction proteins. In vivo expression of the mutant protein by viral transduction in triadin knockout mice led to fragmentation, a reduction in contacts between SR and T-Tubules, and an alteration in Ca2+ release units’ (CRUs) structure, rendering hearts more prone to ventricular arrhythmias [24].
3. Arrhythmogenic Cardiomyopathy
Arrhythmogenic cardiomyopathy (ACM) is a progressive heart disease characterized by fibrofatty substitution of the right (ARVC), left (ALVC), or both cardiac ventricles. Clinical manifestations include ventricular arrhythmias, impaired ventricular systolic function, heart failure, and sudden cardiac death. ACM is a genetic disorder caused by mutations in genes encoding for desmosomal proteins and non-desmosomal mutations, including genes coding for Ca2+ cycling regulators, growth factors, and structural proteins [25,26,27] (Table 1). A causative genetic alteration can be identified in the majority of cases [27], but different triggers, such as inflammation and excessive exercise, can worsen the ACM phenotype. However, treadmill exercise has been shown to partially rescue the remarkable dysregulation in gene expression observed in a mouse model of ACM carrying myocyte-specific desmoplakin haplo-insufficiency [28]. Because of its multiple phenotypes and the variability in the presentation, no single diagnostic test is available for ACM diagnosis. The diagnostic strategy consists of the collection of several parameters, such as genetic, electrocardiographic, arrhythmic, morphological, functional, and histopathologic abnormalities [29,30]. The population prevalence ranges from 1:1000 to 1:5000 and phenotypic expression is more common in males (2:1 to 3:1) [31]. Fibrofatty infiltration and progressive loss of ventricular myocardium represent the typical tissue remodeling in ACM. The infiltration has an epicardial to endocardial progression [32] and mainly involves the so-called ‘triangle of dysplasia’ (right ventricle inflow tract, outflow tract, and apex). The left ACM form is characterized by the involvement of the left ventricular wall with no alterations of right ventricle function [33,34], while the biventricular form involves both ventricles and is characterized by systolic impairment and biventricular dilation, with arrhythmic events that originate from either ventricle [34].
Table 1.
Lists of genes involved in ACM pathogenesis.
| Gene | Coding Protein | Locus | Reference | |
|---|---|---|---|---|
| desmosomal mutations | JUP | Junction Plakoglobin | 17q21.2 | [35] |
| DSP | Desmoplakin | 6p24.3 | [36] | |
| PKP2 | Plakophilin 2 | 12p11.21 | [37] | |
| DSG2 | Desmoglein 2 | 18q12.1 | [38,39] | |
| DSC2 | Desmocollin 2 | 18q12.1 | [40] | |
| non-desmosomal mutations | TMEM43 | Transmembrane protein 43 | 3p25.1 | [41] |
| LMNA | Lamin A/C | 1q22 | [42] | |
| DES | Desmin | 2q35 | [43] | |
| CTNNA3 | Alpha-T-catenin | 10q21.3 | [44] | |
| PLN | Phospholamban | 6q22.31 | [45] | |
| TGFB3 | Transforming growth factor-3 | 14q24.3 | [27,46] | |
| TTN | Titin | 2q31.2 | [47] | |
| SCN5A | Sodium voltage gated channel alpha subunit 5 (NaV 1.5) | 3p22.2 | [48] | |
| CDH2 | Cadherin C | 18q12.1 | [49] | |
| RYR2 | Type 2 ryanodine receptor | 1q42-q43 | [50,51] |
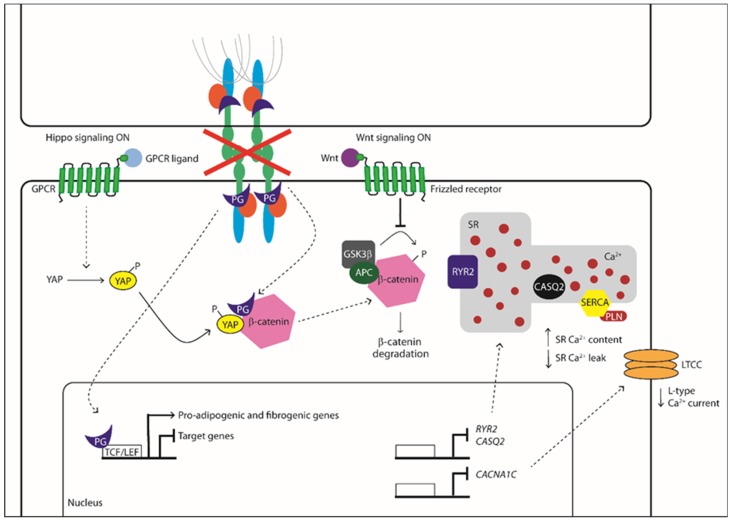
The molecular mechanisms associated with ACM pathogenesis have been linked to dysregulation of the Wnt/β-catenin pathway, which leads to enhanced adipogenesis (Figure 1). The Wnt pathway is involved in cell proliferation, cell polarity, and cell fate determination during embryonic development and tissue homeostasis. The canonical Wnt signaling controls the developmental gene expression programs, acting through the transcriptional co-activator β-catenin. In the absence of Wnt ligands, the cytoplasmic β-catenin protein is constantly degraded; instead, Wnt ligands inhibit β-catenin degradation, leading to its accumulation in the cytoplasm and translocation into the nucleus, where it activates T cell/lymphoid-enhancing binding (Tcf/Lef) transcription factors. The β-catenin stabilization and translocation result in cell specification and differentiation [52]. The desmosomal protein plakoglobin (PG) also plays a key role in ACM pathogenesis because of its high functional and structural homology with β-catenin, including its increased localization in the nuclear compartment in ACM patients’ heart cells [53,54]. In the nucleus, PG may compete with β-catenin activity, leading to the suppression of Wnt signaling and the activation of the adipo-fibro-genetic transcription program [53].
Figure 1.
Molecular mechanisms associated with ACM pathogenesis. GPCR: G protein-coupled receptors, YAP: yes-associated protein, PG: Plakoglobin, TCF/LEF: T-cell factor/lymphoid enhancer-binding factor, Wnt: homologous wingless, APC: adenomatous polyposis coli, RYR2: type 2 ryanodine receptor, CASQ2: calsequestrin 2, CACNA1C: calcium voltage-gated channel subunit alpha1 C, SR: sarcoplasmic reticulum, SERCA: sarco-endoplasmic reticulum Ca2+ ATPase, PLN: phospholamban, LTCC: L-type calcium channel. ( Activation/Phosphorylation,
Activation/Phosphorylation,  Translocation,
Translocation,  Inhibition).
Inhibition).
It is important to note that the Wnt signaling does not work as an isolated pathway but is highly interconnected to other signaling pathways, including YAP/TAZ signaling, that have also been linked to ACM [55]. YAP and TAZ are two effectors of Hippo signaling, involved in cardiac development and regeneration [56]. YAP has been shown to act as an inhibitor of transforming growth factor beta (TGF-β) signaling by interacting with Smad7 and repressing the nuclear signaling [57]. Furthermore, during the Wnt inactive state, YAP and TAZ are associated with β-catenin into a destruction complex, thereby facilitating its ubiquitin-mediated-degradation. Wnt stimulation induces the YAP and TAZ dissociation from the destruction complex, resulting in β-catenin release [58] (Figure 1).
Based on their coexistence in the same macromolecular complex, the physical interaction between plakophilin-2 (PKP2) and the gap junction protein connexin 43 (Cx43) has been reported to highlight an electrical pathogenic mechanism for ACM [59]. Thereafter, it has been described that the voltage-gated Na+ channel Nav1.5 is also present in this interaction network, called a ‘connexome’ [29]. Notably, there is a reciprocal regulation between the components forming this structure. The Cx43 is responsible for expression [60,61] and cell membrane localization of Nav1.5 [62]. Furthermore, a loss of PKP2 causes Cx43 remodeling, leading to an alteration of intercalated disc structures [59] and reduced Nav1.5 functionality, which results in a decreased Na+ current and slower conduction velocity [63].
Besides contributing to desmosome formation and cell signaling [64], PKP2 is also a regulator of RhoA activity in desmosomal plaque assembles [65]. RhoA is a member of the GTPases family and regulates gene transcription during cardiomyocyte differentiation through its downstream effector kinases, ROCKs [66]. RhoA activity is often enhanced in the myocardial tissue of ACM patients and correlates with PKP2/connexin43 regulation [67]. Moreover, in PKP2-mutated iPSC-CM, RhoA signaling is suppressed, leading to the overexpression of target genes such as serum response factor (SRF) and myocardin-related transcription factor (MRTF) and subsequent enhanced differentiation into adipocytes [66].
Increasing evidence indicates a correlation between the expression level of specific miRNAs and the regulation of cellular signaling involved in ACM pathogenesis. The miRNA profile of ACM and normal control heart samples showed that miR-21-5p and miR-135b are differently expressed in the two groups. MiR-21-5p has been linked to hypertrophic cardiomyopathy and fibrosis, dilated cardiomyopathy [68], and heart failure [69]. It was also demonstrated that miR-21-5p inhibition leads to reduced cardiomyocyte fibrosis and dysfunction in regulating the ERK/MAP kinase signaling pathway [70]. Both miR-21-5p and miR-135b are involved in Wnt and Hippo signaling pathways [71,72], and regulate the same target genes: BMPR2, associated with adipogenesis [73], and TGFBR2, which contributes to the extracellular matrix production. Transcriptome analysis of knockdown PKP2 HL-1 cells revealed that miR-184, whose expression is progressively reduced in cardiac myocytes during cardiac development, has a low expression in ACM. The downregulation of miR-184 is associated with the upregulation of its target gene, peroxisome proliferator-activated receptor gamma [74], leading to enhanced adipogenesis. Furthermore, the transcriptome characterization of ACM cardiac stromal cells showed that miR-29b and miR-1183 [75], already associated with ACM and other cardiac diseases, are upregulated compared to control cells. Notably, miR-29b plays a role in controlling the cardiac expression of collagen, fibrillin, and elastin genes [76], and also act as a regulator of Desmocollin 2 (DSC2) [77].
Despite all the abovementioned pathways having been linked to ACM, a full understanding of the molecular mechanisms leading to ACM pathogenesis is still missing, and other molecular players are likely involved. These include the dysregulation of the Ca2+ handling machinery that may occur as a consequence of mutations that affect desmosomal genes (i.e., PKP2) or some of the genes encoding for crucial components of the Ca2+ cycling machinery (i.e., PLN and RYR2).
4. Arrhythmogenic Cardiomyopathy as Adhesion Disorder: Ca2+-Dependent Desmosomes’ Stability
Cell to cell junctions are essential to confer stability to tissues, especially those undergoing continuous mechanical stretch, such as the heart and skin. The myocardium tissue integrity is based on desmosomes, anchoring cell junctions that are assembled in strong and highly specialized complexes. Loss or mutations of cell junctions are associated with human genetic diseases and ACM [39,53,78,79]. Desmosomes consist of specific cadherins, i.e., DSC2 and desmoglein-2 (DSG2), which act like a bridge to join the lateral edges of neighboring cells. The desmosomal cadherins bind proteins of the armadillo family, PG and PKP2, that are anchored to desmoplakin (DSP), the main intracellular component responsible for the adhesion to the intermediate filament network [80].
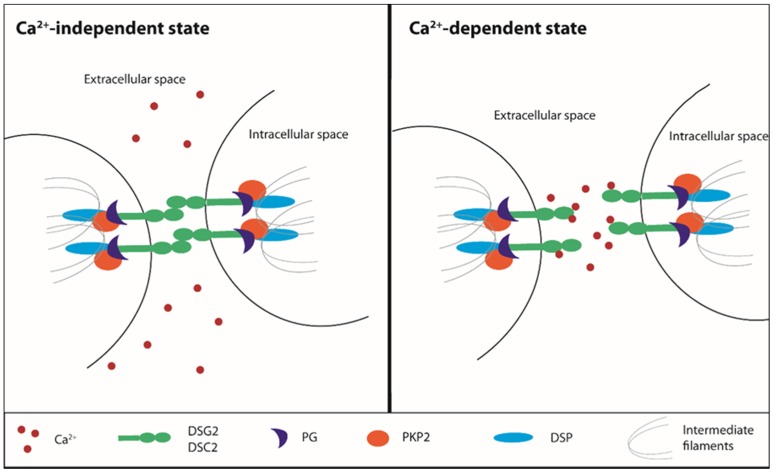
Desmosomes undergo a transition from hyper- to low-adhesion states depending on extracellular Ca2+ levels. The Ca2+-independent adhesive state of desmosomes is referred to as hyper-adhesion-state since it represents a higher-affinity and more stable binding ability both for desmosomes and adherens junctions. During tissue remodeling, wounding, and cell migration, a lower-affinity adhesive state is required and desmosomes adopt the Ca2+-dependent state, losing their organized structure [81,82,83] (Figure 2). This transition does not involve protein composition rearrangement, but it can influence the cadherin packing. Based on the nature of molecules underlying the desmosomal adhesion, both the formation and disruption are Ca2+-dependent mechanisms [84,85,86]. The desmosomal cadherins, DSC2 and DSG2, and the classical cadherin, E-cadherin, represent the Ca2+-dependent components of desmosomes and adherens junctions, respectively [87]. In vitro, the Ca2+-dependent mechanism is linked to the culture confluence state: in a confluent cell monolayer, desmosomes are Ca2+-independent until the confluence is destroyed by wounding the cell sheet, thereby resulting in the propagation of Ca2+ dependence through the whole monolayer [81].
Figure 2.
Ca2+-dependent desmosomes stability. Ca2+: calcium, DSG2: desmoglein 2, DSC2: desmocollin 2, PG: plakoglobin, PKP2: plakophilin 2, DSP: desmoplakin.
5. Desmosomal Mutations and Arrhythmogenic Cardiomyopathy: Derangement of the Ca2+ Toolkit as a Consequence Rather than a Pathogenic Defect
The mechanism that induces the reduction of adhesion during tissue remodeling is not completely clear, but desmosomes can be totally internalized by cells. After the internalization, they are degraded or disassembled in order to recycle their component proteins [88,89]. During the Ca2+-dependence state, the desmosomes undergo a conformational rearrangement of the cadherin extracellular domains that induces a less organized structure and a more easily adhesive binding disruption [90]. The molecular basis of the adhesion between desmosomes involves the activation of protein kinase C (PKC) or inhibition of protein phosphatases [82,83]. The treatment with a low-Ca2+ medium induces loss of intercellular adhesion and the formation of half-desmosomes: the broken desmosomes are internalized by a PKC-dependent mechanism and transported to the centrosome, where they undergo proteasomal and lysosomal degradation [89]. The central role of PKCα is supported by experiments performed on a mouse model lacking or overexpressing this kinase. Both in absence and following inhibition of PKCα, the switch to Ca2+-dependence is blocked and hyperadhesive desmosomes lock the cells together, preventing cell motility and epithelial migration. On the contrary, in mice overexpressing a constitutively active PKCα, a rapid transition to a Ca2+ dependence state make desmosomes weakly adhesive, thus facilitating cell mobilization and promoting re-epithelialization [91].
Desmosomal mutations have been identified as a crucial determinant of ACM since the late 1980s [92]. A homozygous truncating mutation in the JUP gene, encoding for PG, was first identified through genetic linkage analysis as a typical syndromic form of right-sided ACM, known as Naxos disease [93]. Subsequently, genetic analysis revealed that another desmosomal gene, DSP, encoding for desmoplakin, harbored a homozygous truncating mutation in ACM in patients with a cardiocutaneous syndrome, named Carvajal syndrome [93,94]. These earlier discoveries sparked the search for causative mutations in additional desmosome genes in ACM patients and led to the identification of either missense or truncating mutations in the following desmosome genes: PKP2 (encoding for plakophilin 2), DSC2 (encoding desmocollin 2), and DSG2 (encoding for desmoglein 2) (Table 1) [93]. It has been estimated that mutations in desmosomal genes are present in approximately half of ACM patients, with PKP2 being the most commonly affected gene in adults [93]. The molecular mechanisms whereby PKP2 mutations cause ACM are yet to be fully clarified, but no change in the Ca2+-sensitivity of the desmosomal junctions has been reported [93,95]. Conversely, desmosomal mutations can affect the electrical activity of the heart by compromising the expression, localization, and/or function of other components of the ‘connexome’, such as NaV1.5, the α subunit of voltage-gated Na+ channels, and Cx43 (see above). Intriguingly, a recent report demonstrated that the Ca2+ cycling machinery is defective in a conditional Pkp2 knockout mouse model [95].
PKP2
PKP2, encoding for plakophilin-2, is the most frequently mutated gene in ACM patients [37]. It has also been linked to other inherited cardiac arrhythmia syndromes, such as Brugada syndrome [96], idiopathic ventricular fibrillation, hypertrophic cardiomyopathy, and dilated cardiomyopathy, even if with a low frequency [97]. PKP2 is expressed in both myocytes and non-myocytes [54]. The main function of PKP2 is to guarantee mechanical stability during the desmosomal intermediate filament assembly required for cell-to-cell contact. PKP2 is part of the so-called ‘connexome’ [98]. Different studies highlighted the consequent novel role for PKP2 in the intracellular signaling regulation, electrophysiological and trafficking regulation, and the control of transcription processes [95].
Data from a cardiomyocyte-specific tamoxifen-activated Pkp2 homozygous knockout (Pkp2-cKO) mouse model correlated the lack of PKP2 with transcriptional alteration of Ca2+ homeostasis [95]. This investigation reported the development of a cardiomyopathy of RV predominance that become evident at 21 days and then progressed into biventricular cardiomyopathy and HF. Transcriptome analysis showed that the transcripts of proteins involved in maintaining the intracellular Ca2+ concentration were downregulated in Pkp2-cKO hearts. The low level of transcripts relevant to Ca2+ cycling, i.e., Ryr2, Ank2, Cacna1c, and Trdn, accompanied a decreased expression of corresponding proteins and the impairment of the EC mechanism [95]. Accordingly, the downregulation of AnkB (encoded by Ank2) and triadin (encoded by Trdn), which contribute to maintaining the structural integrity of dyadic junctions [99,100], significantly reduced the distance between CaV1.2 and RYR2 [95]. In addition, Pkp2-cKO-derived cardiomyocytes showed decreased ICa,L density and a slower rate of current inactivation, which is consistent with the reduced expression of Cacna1c [95]. It should, however, be pointed out that, although the peak Ca2+ current was decreased, the total Ca2+ charge (i.e., the total amount of Ca2+ entering the cell upon membrane depolarization) was unaltered as compared to wild-type cardiomyocytes because of the slower inactivation rate [95]. Notably, the loss of PKP2 was also correlated with a reduction in the SR Ca2+ leak due to the downregulation of both Ryr2 and Casq2 [95] (Figure 1). As a consequence, the SR Ca2+ content was remarkably increased in Pkp2-cKO cardiomyocytes, which exhibited an increase in the amplitude and frequency of spontaneous Ca2+ release events (due to RYR2 sensitivity to intraluminal Ca2+ levels) and were therefore more prone to release SR Ca2+ during the EC coupling [95]. Accordingly, when Pkp2-cKO cardiomyocytes were paced at increasing rates, they displayed both early and delayed after-transients, which were sufficient to generate ventricular arrhythmogenic events during β-adrenergic stimulation with isoproterenol [95]. A more recent investigation focused on the early events driving the remodeling of the Ca2+ handling machinery in RV-derived PKP2-cKO (Pkp2-cKO-RV) cardiomyocytes isolated 14 days after tamoxifen injection [101], i.e., when cardiomyopathy was not evident yet. This report revealed an increase in RyR2-dependent Ca2+ release and RyR2-mediated Ca2+ sparks due to the remarkable elevation in the SR Ca2+ load that was caused by a Cx43-dependent increase in membrane permeability [101]. Notably, uncoupled Cx43 hemichannels may provide an alternative pathway for extracellular Ca2+ influx [102,103,104] and may, therefore, contribute to refilling the SR Ca2+ store in a SERCA2A-dependent manner. In addition, RyR2’s eagerness to release Ca2+ may be boosted by the observed phosphorylation in Thr2809, an amino acid residue near the consensus sequence for CaMKII and PKA [101]. These alterations were not detected in PKP2-cKO LV cardiomyocytes, thereby suggesting that this asymmetric dysregulation of the Ca2+ handling machinery precedes overt ultrastructural alterations and manifestations of ACM.
The relationship between PKP2 and Ca2+ machinery has also been highlighted by a recent bioinformatic approach that took advantage of a database containing transcriptomic information from human hearts, searching for coordinated transcription networks that are subjected to variations based on PKP2 abundance. The results were then validated with the information deriving from Pkp2-cKO murine hearts, thereby confirming the downregulation of RYR2, ANK2, and CACNA1C [105]. The results of the combined data supported the idea of a correlation between the PKP2 expression and the abundance of transcripts related to intracellular Ca2+ homeostasis. In this context, mathematical modeling confirmed that the PKP2-dependent downregulation of RYR2 and CASQ2 proteins is sufficient to cause the decrease in SR Ca2+ leak, which results in enhanced SR Ca2+ loading and EC coupling [95]. Accordingly, studies carried out on human iPSC-CM from a PKP2-mutated ACM patient showed that they present an abnormal Ca2+ handling capacity [106]. Of note, a recent investigation in iPSC-CM suggested that the Ca2+ handling machinery could also be affected by DSG2 mutations. Accordingly, although the systolic and diastolic Ca2+ levels were similar, human ACM iPSC-CM exhibited spontaneous SR Ca2+ release and DADs in both the absence and the presence of β-adrenergic stimulation [107]. This investigation did not evaluate the molecular expression of Ca2+-related proteins, but further supports the notion that desmosomal mutations may affect the cardiac Ca2+ toolkit in ACM.
6. Non-Desmosomal Mutations and Altered Ca2+ Handling
As described in Section 2, mutations in Ca2+-related proteins may induce life-threatening inherited arrhythmogenic diseases, such as CPVT. It is, therefore, not surprising that, besides desmosomal mutations, ACM may also involve mutations that directly affect genes encoding for the Ca2+ cycling machinery, as suggested for RYR2 and PLN [12].
6.1. RYR2
Despite RYR2 being mentioned in most ACM databases as associated with this disease, not all the scientific community agrees in considering it an ACM-causative gene. The phenotypic overlapping with CPVT leaves doubts about the proper clinical classification of patients in whom mutations were detected [108,109].
The RYR2 gene encodes for a 565-kDa monomer that forms a homo-tetrameric structure associated with FK506-binding proteins that stabilize the channel in a closed state and are important for cooperative interactions among the RYR2 subunits. As previously mentioned, RYR2 is mostly known for its involvement in EC coupling, releasing Ca2+ from the SR and thus driving muscle contraction and heart beating [9,110,111]. Mutations in the RYR2 gene have been reported in a family with an autosomal dominant form of ACM, associated with polymorphic ventricular tachycardia (induced by exercise stress testing) and a risk of sudden death. This condition was first described in 1988 and classified as arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2): a clinically different form because of its particular effort-induced ventricular arrhythmias, high penetrance, and 1:1 male: female ratio among the affected subjects [112]. Four missense mutations of RYR2 were detected in ARVD2 patients [51] resulting in substitutions of highly conserved amino acids in the cytosolic portion of the molecule involving two regions also associated with malignant hyperthermia or central core disease and clustered in the corresponding skeletal muscle isoform, RYR1 [113]. The mutations occur in a regulatory domain of RYR2 known to be involved in the interaction with FKBP12.6 and therefore in the stabilization of a tetrameric structure [114]. A functional characterization of these mutations is yet to be provided at the single-channel level, but the R176Q substitution responsible for ARVD2 corresponds to the Arg163Cys substitution reported in RYR1, which dramatically increases its sensitivity to halothane in malignant hyperthermia [115]. It is, therefore, predictable that the RYR2 mutations observed in ARVD2 patients enhance Ca2+ leakage and increase spontaneous SR Ca2+ release, thereby leading to DADs and ventricular arrhythmia [51]. In agreement with this hypothesis, transgenic mice carrying the R176Q mutation underwent ventricular tachycardia, which was associated with the appearance of spontaneous SR Ca2+ release events in both paced and non-paced isolated cardiomyocytes [116]. Notably, a recent structural investigation suggested that the R176Q mutation interferes with the Ca2+-induced molecular rearrangement of RYR2, thereby increasing the fractional Ca2+ release [117]. Besides being pro-arrhythmogenic, these mutations could promote mitochondrial Ca2+ overload and stimulate apoptosis [51], which has actually been observed in ARVD [118].
Six further rare missense variants have been identified in RYR2, mapping the locus to chromosome 1q42‒q43 [50,51] in four independent families with the same clinical manifestations and close to CPVT based on exercise-induced ventricular arrhythmias, high penetrance, and a 1:1 sex ratio. The described variants affect different RYR2 domains [119]. Several mutations were found in the cytoplasmic region of the protein (NH2-terminal), whose alterations are responsible for the gain of function of RYR2 and are associated with hypertrophic cardiomyopathy and unexplained sudden cardiac death, as described elsewhere [120,121,122]. Missense variants mapped to the transmembrane domain of the protein and involved in the pore formation of the Ca2+ channel have been associated so far with CPVT or sudden death [119].
Two common single nucleotide polymorphisms in exon 37 of the human RYR2 gene, causing non-conservative amino acid exchanges, i.e., G1885E and G1886S, have been associated with arrhythmogenic right ventricular cardiomyopathy (ARVC) [123]. These polymorphisms, found in a subgroup of patients in whom no known RYR2 mutations have been detected, are responsible for creating a putative protein kinase C phosphorylation site known to be involved in cardiac contractility and propensity to heart failure [124]. Genetic analysis and single-channel measurement of RYR2 activity revealed that the combination of these polymorphisms in a single individual led to a leaky SR channel under diastolic conditions, thereby triggering arrhythmia and sudden cardiac death.
6.2. PLN
PLN is one of the main regulators of Ca2+ cycling and an effector of β-adrenergic stimulation. PLN is sensitive to PKA-dependent phosphorylation and PLN expression levels and its phosphorylation state is a key to fine-tuning SERCA activity. The de-phosphorylated PLN interacts with SERCA, resulting in a low Ca2+ affinity state that limits Ca2+ sequestration into SR lumen; when it is phosphorylated, the inhibitory activity on SERCA is abolished and the affinity of SERCA to Ca2+ is restored, so that a larger amount of Ca2+ is pumped back into the SR [9].Therefore, PLN is a crucial modulator of SR Ca2+ content and the major determinant of cardiac contractility and relaxation, depending on its phosphorylation levels: increased phosphorylation improves contractility; Vice versa, the de-phosphorylation state promotes heart failure [125].
Mutations in the human PLN gene were firstly associated with dilated cardiomyopathy (DCM) [126] Patients, but have also been reported for patients diagnosed with ACM [45], as for the PLNArg14Del mutation. This is a deletion of Arg-14 in the coding region and has been identified in a family in which heterozygous carriers exhibited inherited DCM and died by middle age. Notably, a mouse model with cardiac-specific expression of the heterozygous PLNArg14Del mutant recapitulates the human phenotype, such as depressed cardiac function, fibrosis, and premature death [126]. At molecular level, the PLNArg14Del mutation prevents the phosphorylated PLN from relieving SERCA inhibition, thereby causing a reduction in SR Ca2+ concentration [12]. Accordingly, when SERCA2 was co-expressed with the PLNArg14Del mutant in HEK-293 cells, Ca2+ uptake into microsomal vesicles was severely impaired [126]. Likewise, SERCA2a-mediated Ca2+ transport in cardiac homogenates isolated from transgenic mice expressing the PLNArg14Del mutation was impaired as compared to wild-type samples [126]. Immunohistochemistry on samples derived from patients clinically diagnosed with DCM or ACM and carrying the PLNArg14Del mutation further revealed that plakoglobin is downregulated at cell junctions, mostly in ACM samples [45]. Moreover, a comparative analysis performed on myocardial tissue from ACM and DCM patients showed that, in ACM patients, PLN total mRNA, total and phosphorylated protein levels are significantly increased compared to healthy controls and patients with DCM [127]. This finding suggests a role of PLN in the pathogenesis of ARVC and indicates the existence of overlapping pathways with DCM [45].
The pathological mechanisms of the PLN mutation underlying ACM are still unclear, but, in general, the PLNArg14Del mutation is likely to result in reduced SR Ca2+ content. In agreement with this hypothesis, a recent investigation revealed that the intracellular Ca2+ dynamics were perturbed in human iPSC-CM deriving from a patient affected by DCM and bearing the PLNArg14Del mutation [128]. Indeed, although the releasable SR Ca2+ pool was significantly reduced, there was an increase in the diastolic Ca2+ levels, beating rate, and frequency of irregular Ca2+ waves [128]. Interestingly, when the PLNArg14Del mutant was introduced into a PLN null mouse model, it did not associate with SERCA2a, but translocated to the plasma membrane to physically interact with the Na+/K+ ATPase [129]. As a consequence, cardiac contractility became insensitive to β-adrenergic stimulation [126]. The causative relationship between the PLNArg14Del mutation and ACM could be better interpreted by considering another DCM-associated PLN mutation, i.e., R25C [130]. This mutation renders the heart susceptible to ventricular arrhythmia because of a dramatic remodeling of the Ca2+ cycling machinery. Indeed, when R25C-PLN was overexpressed in rat adult cardiomyocytes, it suppressed SERCA2a activity, thereby reducing SR Ca2+ content and the amplitude of voltage-dependent Ca2+ transients, with impairment of contractile activity following [130]. The resulting increase in diastolic Ca2+ levels was found to recruit CaMKII, which in turn phosphorylated RYR2 to induce an increase in SR Ca2+ leakage, spontaneous Ca2+ sparks, and resultant arrhythmias [130]. Compared to desmosomal mutation carriers, PLN mutation carriers more often showed T-wave inversion and are associated with additional left ventricle (LV) involvement and low-voltage electrocardiogram. However, as reported for desmosomal mutation carriers, PLN mutation carriers also showed RV disease, fibrofatty replacement, and arrhythmogenic phenotype [45,131]. In addition, PLN mutation carriers are unlikely to show downregulation of synapse-associated protein 97, which is commonly reduced in the ventricular sarcomeres of ACM patients, while they do not present redistribution of glycogen synthase kinase-3 beta to the intercalated disks, as reported in a desmosomal ACM cohort [132].
7. Conclusions
ACM is classified as a genetically determined cardiac disease that is clinically characterized by arrhythmic events. Different pathways have been associated with ACM and were mostly correlated with enhanced adipogenesis and electrical dysfunction. Despite the identification of different molecular mechanisms, a full understanding of the ACM pathogenesis is still lacking. Ca2+ dysregulation can be considered as a possible causative event based on its involvement in the cells’ adhesive property and its pro-arrhythmic ability. The desmosomes’ adhesive state is Ca2+-dependent and their structural stability consists of a PKC-mediated mechanism. Moreover, defects in the Ca2+ cycling machinery, also identified among the ACM-causative genetic mutations, are responsible for dysregulated Ca2+ homeostasis, leading to cardiac arrhythmia. Of note, a loss of PKP2, most frequently mutated in ACM patients, correlates with an alteration of Ca2+ machinery [95]. These observations raise the possibility that the ACM-causative mechanism may involve a defect in the expression and/or activity of the cardiac Ca2+ handling machinery, not only limited to myocytes, ultimately leading to arrhythmias, but also in the stromal compartment, which is actively involved in the pathological remodeling [54].
To date, pharmacological treatments generically targeting Ca2+ homeostasis, such as calcium antagonists and flecainide, are used to treat arrhythmias. However, confirming the involvement of Ca2+ signaling and understanding the molecular underpinnings of Ca2+ signaling in the pathogenesis of ACM could be pivotal to finding targeted therapies (cell-specific and/or molecule-specific) that could be beneficial for the treatment of this life-threatening disease.
Funding
A.S.M., G.P., E.S., and M.B. acknowledge financial support from the Transnational Research Projects on Cardiovascular Diseases (ACM-HF JTC2016_FP-40-021). F.L. and F.M. acknowledge financial support from the EU Horizon 2020 FETOPEN-2018-2020 Program under Grant Agreement N. 828984 (LION-HEARTED).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thiene G., Nava A., Corrado D., Rossi L., Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G., Basso C. Arrhythmogenic right ventricular cardiomyopathy: An update. Cardiovasc. Pathol. 2001;10:109–117. doi: 10.1016/S1054-8807(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D., Basso C., Judge D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017;121:784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- 4.Bartoli F., Sabourin J. Cardiac Remodeling and Disease: Current Understanding of STIM1/Orai1-Mediated Store-Operated Ca2+ Entry in Cardiac Function and Pathology. Adv. Exp. Med. Biol. 2017;993:523–534. doi: 10.1007/978-3-319-57732-6_26. [DOI] [PubMed] [Google Scholar]
- 5.Xie W., Santulli G., Guo X., Gao M., Chen B.X., Marks A.R. Imaging atrial arrhythmic intracellular calcium in intact heart. J. Mol. Cell. Cardiol. 2013;64:120–123. doi: 10.1016/j.yjmcc.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landstrom A.P., Dobrev D., Wehrens X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017;120:1969–1993. doi: 10.1161/CIRCRESAHA.117.310083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ter Keurs H.E., Boyden P.A. Calcium and arrhythmogenesis. Physiol. Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venetucci L., Denegri M., Napolitano C., Priori S.G. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat. Rev. Cardiol. 2012;9:561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 9.Eisner D.A., Caldwell J.L., Kistamas K., Trafford A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KC S., Nair R.S., Banerji A., Somasundaram V., Srinivas P. Structure activity relationship of plumbagin in BRCA1 related cancer cells. Mol. Carcinog. 2013;52:392–403. doi: 10.1002/mc.21877. [DOI] [PubMed] [Google Scholar]
- 11.Vangheluwe P., Sipido K.R., Raeymaekers L., Wuytack F. New perspectives on the role of SERCA2’s Ca2+ affinity in cardiac function. Biochim. Et Biophys. Acta. 2006;1763:1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 12.van Opbergen C.J., Delmar M., van Veen T.A. Potential new mechanisms of pro-arrhythmia in arrhythmogenic cardiomyopathy: Focus on calcium sensitive pathways. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2017;25:157–169. doi: 10.1007/s12471-017-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bovo E., Huke S., Blatter L.A., Zima A.V. The effect of PKA-mediated phosphorylation of ryanodine receptor on SR Ca2+ leak in ventricular myocytes. J. Mol. Cell. Cardiol. 2017;104:9–16. doi: 10.1016/j.yjmcc.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton S., Terentyev D. Altered Intracellular Calcium Homeostasis and Arrhythmogenesis in the Aged Heart. Int. J. Mol. Sci. 2019;20:2386. doi: 10.3390/ijms20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priori S.G., Chen S.R. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzi N., Liu N., Napolitano C., Nori A., Turcato F., Colombi B., Bicciato S., Arcelli D., Spedito A., Scelsi M., et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: A complex arrhythmogenic cascade in a knock in mouse model. Circ. Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 17.De la Fuente S., Van Langen I.M., Postma A.V., Bikker H., Meijer A. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin. Electrophysiol. 2008;31:916–919. doi: 10.1111/j.1540-8159.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 18.Faggioni M., Knollmann B.C. Calsequestrin 2 and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1250–H1260. doi: 10.1152/ajpheart.00779.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wemhoner K., Friedrich C., Stallmeyer B., Coffey A.J., Grace A., Zumhagen S., Seebohm G., Ortiz-Bonnin B., Rinne S., Sachse F.B., et al. Gain-of-function mutations in the calcium channel CACNA1C (Cav1.2) cause non-syndromic long-QT but not Timothy syndrome. J. Mol. Cell. Cardiol. 2015;80:186–195. doi: 10.1016/j.yjmcc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Burashnikov E., Pfeiffer R., Barajas-Martinez H., Delpon E., Hu D., Desai M., Borggrefe M., Haissaguerre M., Kanter R., Pollevick G.D., et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templin C., Ghadri J.R., Rougier J.S., Baumer A., Kaplan V., Albesa M., Sticht H., Rauch A., Puleo C., Hu D., et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur. Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devalla H.D., Gelinas R., Aburawi E.H., Beqqali A., Goyette P., Freund C., Chaix M.A., Tadros R., Jiang H., Le Bechec A., et al. TECRL, a new life-threatening inherited arrhythmia gene associated with overlapping clinical features of both LQTS and CPVT. Embo Mol. Med. 2016;8:1390–1408. doi: 10.15252/emmm.201505719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyegaard M., Overgaard M.T., Sondergaard M.T., Vranas M., Behr E.R., Hildebrandt L.L., Lund J., Hedley P.L., Camm A.J., Wettrell G., et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra N., Yang T., Asghari P., Moore E.D., Huke S., Akin B., Cattolica R.A., Perez C.F., Hlaing T., Knollmann-Ritschel B.E., et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrado D., Basso C., Thiene G., McKenna W.J., Davies M.J., Fontaliran F., Nava A., Silvestri F., Blomstrom-Lundqvist C., Wlodarska E.K., et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: A multicenter study. J. Am. Coll. Cardiol. 1997;30:1512–1520. doi: 10.1016/S0735-1097(97)00332-X. [DOI] [PubMed] [Google Scholar]
- 26.Dalal D., Nasir K., Bomma C., Prakasa K., Tandri H., Piccini J., Roguin A., Tichnell C., James C., Russell S.D., et al. Arrhythmogenic right ventricular dysplasia: A United States experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 27.Marcus F.I., Fontaine G.H., Guiraudon G., Frank R., Laurenceau J.L., Malergue C., Grosgogeat Y. Right ventricular dysplasia: A report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.CIR.65.2.384. [DOI] [PubMed] [Google Scholar]
- 28.Cheedipudi S.M., Hu J., Fan S., Yuan P., Karmouch J., Czernuszewicz G., Robertson M.J., Coarfa C., Hong K., Yao Y., et al. Exercise Restores Dysregulated Gene Expression in a Mouse Model of Arrhythmogenic Cardiomyopathy. Cardiovasc. Res. 2019 doi: 10.1093/cvr/cvz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agullo-Pascual E., Cerrone M., Delmar M. Arrhythmogenic cardiomyopathy and Brugada syndrome: Diseases of the connexome. Febs Lett. 2014;588:1322–1330. doi: 10.1016/j.febslet.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur. Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalal D., Tandri H., Judge D.P., Amat N., Macedo R., Jain R., Tichnell C., Daly A., James C., Russell S.D., et al. Morphologic variants of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy a genetics-magnetic resonance imaging correlation study. J. Am. Coll. Cardiol. 2009;53:1289–1299. doi: 10.1016/j.jacc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 32.Corrado D., Link M.S., Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017;376:1489–1490. doi: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Ortega M., Rodriguez-Vita J., Sanchez-Lopez E., Carvajal G., Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Akdis D., Brunckhorst C., Duru F., Saguner A.M. Arrhythmogenic Cardiomyopathy: Electrical and Structural Phenotypes. Arrhythmia Electrophysiol. Rev. 2016;5:90–101. doi: 10.15420/AER.2016.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asimaki A., Syrris P., Wichter T., Matthias P., Saffitz J.E., McKenna W.J. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rampazzo A., Nava A., Malacrida S., Beffagna G., Bauce B., Rossi V., Zimbello R., Simionati B., Basso C., Thiene G., et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerull B., Heuser A., Wichter T., Paul M., Basson C.T., McDermott D.A., Lerman B.B., Markowitz S.M., Ellinor P.T., MacRae C.A., et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 38.Awad M.M., Dalal D., Cho E., Amat-Alarcon N., James C., Tichnell C., Tucker A., Russell S.D., Bluemke D.A., Dietz H.C., et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Hum. Genet. 2006;79:136–142. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilichou K., Nava A., Basso C., Beffagna G., Bauce B., Lorenzon A., Frigo G., Vettori A., Valente M., Towbin J., et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 40.Syrris P., Ward D., Evans A., Asimaki A., Gandjbakhch E., Sen-Chowdhry S., McKenna W.J. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am. J. Hum. Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merner N.D., Hodgkinson K.A., Haywood A.F., Connors S., French V.M., Drenckhahn J.D., Kupprion C., Ramadanova K., Thierfelder L., McKenna W., et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 2008;82:809–821. doi: 10.1016/j.ajhg.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quarta G., Syrris P., Ashworth M., Jenkins S., Zuborne Alapi K., Morgan J., Muir A., Pantazis A., McKenna W.J., Elliott P.M. Mutations in the Lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2012;33:1128–1136. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
- 43.Klauke B., Kossmann S., Gaertner A., Brand K., Stork I., Brodehl A., Dieding M., Walhorn V., Anselmetti D., Gerdes D., et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum. Mol. Genet. 2010;19:4595–4607. doi: 10.1093/hmg/ddq387. [DOI] [PubMed] [Google Scholar]
- 44.van Hengel J., Calore M., Bauce B., Dazzo E., Mazzotti E., De Bortoli M., Lorenzon A., Li Mura I.E., Beffagna G., Rigato I., et al. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2013;34:201–210. doi: 10.1093/eurheartj/ehs373. [DOI] [PubMed] [Google Scholar]
- 45.van der Zwaag P.A., van Rijsingen I.A., Asimaki A., Jongbloed J.D., van Veldhuisen D.J., Wiesfeld A.C., Cox M.G., van Lochem L.T., de Boer R.A., Hofstra R.M., et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: Evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur. J. Heart Fail. 2012;14:1199–1207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rampazzo A., Nava A., Danieli G.A., Buja G., Daliento L., Fasoli G., Scognamiglio R., Corrado D., Thiene G. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum. Mol. Genet. 1994;3:959–962. doi: 10.1093/hmg/3.6.959. [DOI] [PubMed] [Google Scholar]
- 47.Taylor M., Graw S., Sinagra G., Barnes C., Slavov D., Brun F., Pinamonti B., Salcedo E.E., Sauer W., Pyxaras S., et al. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erkapic D., Neumann T., Schmitt J., Sperzel J., Berkowitsch A., Kuniss M., Hamm C.W., Pitschner H.F. Electrical storm in a patient with arrhythmogenic right ventricular cardiomyopathy and SCN5A mutation. Europace. 2008;10:884–887. doi: 10.1093/europace/eun065. [DOI] [PubMed] [Google Scholar]
- 49.Mayosi B.M., Fish M., Shaboodien G., Mastantuono E., Kraus S., Wieland T., Kotta M.C., Chin A., Laing N., Ntusi N.B., et al. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2017;10:e001605. doi: 10.1161/CIRCGENETICS.116.001605. [DOI] [PubMed] [Google Scholar]
- 50.Rampazzo A., Nava A., Erne P., Eberhard M., Vian E., Slomp P., Tiso N., Thiene G., Danieli G.A. A new locus for arrhythmogenic right ventricular cardiomyopathy (ARVD2) maps to chromosome 1q42-q43. Hum. Mol. Genet. 1995;4:2151–2154. doi: 10.1093/hmg/4.11.2151. [DOI] [PubMed] [Google Scholar]
- 51.Tiso N., Stephan D.A., Nava A., Bagattin A., Devaney J.M., Stanchi F., Larderet G., Brahmbhatt B., Brown K., Bauce B., et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum. Mol. Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 52.Buikema J.W., Mady A.S., Mittal N.V., Atmanli A., Caron L., Doevendans P.A., Sluijter J.P., Domian I.J. Wnt/beta-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development. 2013;140:4165–4176. doi: 10.1242/dev.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Gras E., Lombardi R., Giocondo M.J., Willerson J.T., Schneider M.D., Khoury D.S., Marian A.J. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investig. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sommariva E., Brambilla S., Carbucicchio C., Gambini E., Meraviglia V., Dello Russo A., Farina F.M., Casella M., Catto V., Pontone G., et al. Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur. Heart J. 2016;37:1835–1846. doi: 10.1093/eurheartj/ehv579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S.N., Gurha P., Lombardi R., Ruggiero A., Willerson J.T., Marian A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao Y., Leach J., Wang J., Martin J.F. Hippo/Yap Signaling in Cardiac Development and Regeneration. Curr. Treat. Options Cardiovasc. Med. 2016;18:38. doi: 10.1007/s11936-016-0461-y. [DOI] [PubMed] [Google Scholar]
- 57.Piersma B., Bank R.A., Boersema M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Oxford E.M., Musa H., Maass K., Coombs W., Taffet S.M., Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ. Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 60.Jansen J.A., Noorman M., Musa H., Stein M., de Jong S., van der Nagel R., Hund T.J., Mohler P.J., Vos M.A., van Veen T.A., et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9:600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cerrone M., Delmar M. Desmosomes and the sodium channel complex: Implications for arrhythmogenic cardiomyopathy and Brugada syndrome. Trends Cardiovasc. Med. 2014;24:184–190. doi: 10.1016/j.tcm.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agullo-Pascual E., Lin X., Leo-Macias A., Zhang M., Liang F.X., Li Z., Pfenniger A., Lubkemeier I., Keegan S., Fenyo D., et al. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc. Res. 2014;104:371–381. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato P.Y., Musa H., Coombs W., Guerrero-Serna G., Patino G.A., Taffet S.M., Isom L.L., Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ. Res. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X., Bonne S., Hatzfeld M., van Roy F., Green K.J. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta -catenin signaling. J. Biol. Chem. 2002;277:10512–10522. doi: 10.1074/jbc.M108765200. [DOI] [PubMed] [Google Scholar]
- 65.Godsel L.M., Dubash A.D., Bass-Zubek A.E., Amargo E.V., Klessner J.L., Hobbs R.P., Chen X., Green K.J. Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol. Biol. Cell. 2010;21:2844–2859. doi: 10.1091/mbc.e10-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorn T., Kornherr J., Parrotta E.I., Zawada D., Ayetey H., Santamaria G., Iop L., Mastantuono E., Sinnecker D., Goedel A., et al. Interplay of cell-cell contacts and RhoA/MRTF-A signaling regulates cardiomyocyte identity. EMBO J. 2018;37 doi: 10.15252/embj.201798133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L., Liu S., Zhang H., Hu S., Wei Y. RhoA activity increased in myocardium of arrhythmogenic cardiomyopathy patients and affected connexin 43 protein expression in HL-1 cells. Int. J. Clin. Exp. Med. 2015;8:12906–12913. [PMC free article] [PubMed] [Google Scholar]
- 68.Xu H.F., Ding Y.J., Zhang Z.X., Wang Z.F., Luo C.L., Li B.X., Shen Y.W., Tao L.Y., Zhao Z.Q. MicroRNA21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Mol. Med. Rep. 2014;10:161–168. doi: 10.3892/mmr.2014.2205. [DOI] [PubMed] [Google Scholar]
- 69.Vogel B., Keller A., Frese K.S., Leidinger P., Sedaghat-Hamedani F., Kayvanpour E., Kloos W., Backe C., Thanaraj A., Brefort T., et al. Multivariate miRNA signatures as biomarkers for non-ischaemic systolic heart failure. Eur. Heart J. 2013;34:2812–2822. doi: 10.1093/eurheartj/eht256. [DOI] [PubMed] [Google Scholar]
- 70.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 71.Kawakita A., Yanamoto S., Yamada S., Naruse T., Takahashi H., Kawasaki G., Umeda M. MicroRNA-21 promotes oral cancer invasion via the Wnt/beta-catenin pathway by targeting DKK2. Pathol. Oncol. Res. Por. 2014;20:253–261. doi: 10.1007/s12253-013-9689-y. [DOI] [PubMed] [Google Scholar]
- 72.Lin C.W., Chang Y.L., Chang Y.C., Lin J.C., Chen C.C., Pan S.H., Wu C.T., Chen H.Y., Yang S.C., Hong T.M., et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013;4:1877. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 73.Skillington J., Choy L., Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J. Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurha P., Chen X., Lombardi R., Willerson J.T., Marian A.J. Knockdown of Plakophilin 2 Downregulates miR-184 Through CpG Hypermethylation and Suppression of the E2F1 Pathway and Leads to Enhanced Adipogenesis In Vitro. Circ. Res. 2016;119:731–750. doi: 10.1161/CIRCRESAHA.116.308422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rainer J., Meraviglia V., Blankenburg H., Piubelli C., Pramstaller P.P., Paolin A., Cogliati E., Pompilio G., Sommariva E., Domingues F.S., et al. The arrhythmogenic cardiomyopathy-specific coding and non-coding transcriptome in human cardiac stromal cells. BMC Genom. 2018;19:491. doi: 10.1186/s12864-018-4876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S., Hill J.A., Olson E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurinna S., Schafer M., Ostano P., Karouzakis E., Chiorino G., Bloch W., Bachmann A., Gay S., Garrod D., Lefort K., et al. A novel Nrf2-miR-29-desmocollin-2 axis regulates desmosome function in keratinocytes. Nat. Commun. 2014;5:5099. doi: 10.1038/ncomms6099. [DOI] [PubMed] [Google Scholar]
- 78.Kostetskii I., Li J., Xiong Y., Zhou R., Ferrari V.A., Patel V.V., Molkentin J.D., Radice G.L. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 79.Li J., Levin M.D., Xiong Y., Petrenko N., Patel V.V., Radice G.L. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J. Mol. Cell. Cardiol. 2008;44:597–606. doi: 10.1016/j.yjmcc.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheikh F., Ross R.S., Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc. Med. 2009;19:182–190. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrod D.R., Berika M.Y., Bardsley W.F., Holmes D., Tabernero L. Hyper-adhesion in desmosomes: Its regulation in wound healing and possible relationship to cadherin crystal structure. J. Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- 82.Kimura T.E., Merritt A.J., Garrod D.R. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J. Investig. Dermatol. 2007;127:775–781. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- 83.Wallis S., Lloyd S., Wise I., Ireland G., Fleming T.P., Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol. Biol. Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hennings H., Holbrook K.A. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp. Cell Res. 1983;143:127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- 85.Kartenbeck J., Schmid E., Franke W.W., Geiger B. Different modes of internalization of proteins associated with adhaerens junctions and desmosomes: Experimental separation of lateral contacts induces endocytosis of desmosomal plaque material. Embo J. 1982;1:725–732. doi: 10.1002/j.1460-2075.1982.tb01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattey D.L., Garrod D.R. Splitting and internalization of the desmosomes of cultured kidney epithelial cells by reduction in calcium concentration. J. Cell Sci. 1986;85:113–124. doi: 10.1242/jcs.85.1.113. [DOI] [PubMed] [Google Scholar]
- 87.Nollet F., Kools P., van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 88.Allen T.D., Potten C.S. Desmosomal form, fate, and function in mammalian epidermis. J. Ultrastruct. Res. 1975;51:94–105. doi: 10.1016/S0022-5320(75)80011-6. [DOI] [PubMed] [Google Scholar]
- 89.McHarg S., Hopkins G., Lim L., Garrod D. Down-regulation of desmosomes in cultured cells: The roles of PKC, microtubules and lysosomal/proteasomal degradation. PLoS ONE. 2014;9:e108570. doi: 10.1371/journal.pone.0108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He W., Cowin P., Stokes D.L. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- 91.Thomason H.A., Cooper N.H., Ansell D.M., Chiu M., Merrit A.J., Hardman M.J., Garrod D.R. Direct evidence that PKCalpha positively regulates wound re-epithelialization: Correlation with changes in desmosomal adhesiveness. J. Pathol. 2012;227:346–356. doi: 10.1002/path.4016. [DOI] [PubMed] [Google Scholar]
- 92.Ohno S. The genetic background of arrhythmogenic right ventricular cardiomyopathy. J. Arrhythmia. 2016;32:398–403. doi: 10.1016/j.joa.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Austin K.M., Trembley M.A., Chandler S.F., Sanders S.P., Saffitz J.E., Abrams D.J., Pu W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019 doi: 10.1038/s41569-019-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norgett E.E., Hatsell S.J., Carvajal-Huerta L., Cabezas J.C., Common J., Purkis P.E., Whittock N., Leigh I.M., Stevens H.P., Kelsell D.P. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 95.Cerrone M., Montnach J., Lin X., Zhao Y.T., Zhang M., Agullo-Pascual E., Leo-Macias A., Alvarado F.J., Dolgalev I., Karathanos T.V., et al. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat. Commun. 2017;8:106. doi: 10.1038/s41467-017-00127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Campuzano O., Fernandez-Falgueras A., Iglesias A., Brugada R. Brugada Syndrome and PKP2: Evidences and uncertainties. Int. J. Cardiol. 2016;214:403–405. doi: 10.1016/j.ijcard.2016.03.194. [DOI] [PubMed] [Google Scholar]
- 97.Novelli V., Malkani K., Cerrone M. Pleiotropic Phenotypes Associated With PKP2 Variants. Front. Cardiovasc. Med. 2018;5:184. doi: 10.3389/fcvm.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agullo-Pascual E., Reid D.A., Keegan S., Sidhu M., Fenyo D., Rothenberg E., Delmar M. Super-resolution fluorescence microscopy of the cardiac connexome reveals plakophilin-2 inside the connexin43 plaque. Cardiovasc. Res. 2013;100:231–240. doi: 10.1093/cvr/cvt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camors E., Mohler P.J., Bers D.M., Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J. Mol. Cell. Cardiol. 2012;52:1240–1248. doi: 10.1016/j.yjmcc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chopra N., Knollmann B.C. Triadin regulates cardiac muscle couplon structure and microdomain Ca2+ signalling: A path towards ventricular arrhythmias. Cardiovasc. Res. 2013;98:187–191. doi: 10.1093/cvr/cvt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J.C., Perez-Hernandez Duran M., Alvarado F.J., Maurya S.R., Montnach J., Yin Y., Zhang M., Lin X., Vasquez C., Heguy A., et al. Disruption of Ca2+i Homeostasis and Cx43 Hemichannel Function in the Right Ventricle Precedes Overt Arrhythmogenic Cardiomyopathy in PKP2-Deficient Mice. Circulation. 2019 doi: 10.1161/CIRCULATIONAHA.119.039710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Bock M., Wang N., Bol M., Decrock E., Ponsaerts R., Bultynck G., Dupont G., Leybaert L. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J. Biol. Chem. 2012;287:12250–12266. doi: 10.1074/jbc.M111.299610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berra-Romani R., Raqeeb A., Avelino-Cruz J.E., Moccia F., Oldani A., Speroni F., Taglietti V., Tanzi F. Ca2+ signaling in injured in situ endothelium of rat aorta. Cell Calcium. 2008;44:298–309. doi: 10.1016/j.ceca.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Moccia F., Tanzi F., Munaron L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014;14:457–480. doi: 10.2174/1566524013666131118113410. [DOI] [PubMed] [Google Scholar]
- 105.Montnach J., Agullo-Pascual E., Tadros R., Bezzina C.R., Delmar M. Bioinformatic analysis of a plakophilin-2-dependent transcription network: Implications for the mechanisms of arrhythmogenic right ventricular cardiomyopathy in humans and in boxer dogs. EP. Eur. 2018;20:iii125–iii132. doi: 10.1093/europace/euy238. [DOI] [PubMed] [Google Scholar]
- 106.Kim C., Wong J., Wen J., Wang S., Wang C., Spiering S., Kan N.G., Forcales S., Puri P.L., Leone T.C., et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Battrawy I., Zhao Z., Lan H., Cyganek L., Tombers C., Li X., Buljubasic F., Lang S., Tiburcy M., Zimmermann W.H., et al. Electrical dysfunctions in human-induced pluripotent stem cell-derived cardiomyocytes from a patient with an arrhythmogenic right ventricular cardiomyopathy. EP Eur. 2018;20:f46–f56. doi: 10.1093/europace/euy042. [DOI] [PubMed] [Google Scholar]
- 108.Lombardi R., Marian A.J. Arrhythmogenic right ventricular cardiomyopathy is a disease of cardiac stem cells. Curr. Opin. Cardiol. 2010;25:222–228. doi: 10.1097/HCO.0b013e3283376daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stadiotti I., Pompilio G., Maione A.S., Pilato C.A., D’Alessandra Y., Sommariva E. Arrhythmogenic cardiomyopathy: What blood can reveal? Heart Rhythm. 2019;16:470–477. doi: 10.1016/j.hrthm.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 110.Laitinen P.J., Brown K.M., Piippo K., Swan H., Devaney J.M., Brahmbhatt B., Donarum E.A., Marino M., Tiso N., Viitasalo M., et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.CIR.103.4.485. [DOI] [PubMed] [Google Scholar]
- 111.Priori S.G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V., Danieli G.A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.CIR.103.2.196. [DOI] [PubMed] [Google Scholar]
- 112.Nava A., Canciani B., Daliento L., Miraglia G., Buja G., Fasoli G., Martini B., Scognamiglio R., Thiene G. Juvenile sudden death and effort ventricular tachycardias in a family with right ventricular cardiomyopathy. Int. J. Cardiol. 1988;21:111–126. doi: 10.1016/0167-5273(88)90212-4. [DOI] [PubMed] [Google Scholar]
- 113.McCarthy T.V., Quane K.A., Lynch P.J. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 2000;15:410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 114.Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 115.Censier K., Urwyler A., Zorzato F., Treves S. Intracellular calcium homeostasis in human primary muscle cells from malignant hyperthermia-susceptible and normal individuals. Effect Of overexpression of recombinant wild-type and Arg163Cys mutated ryanodine receptors. J. Clin. Investig. 1998;101:1233–1242. doi: 10.1172/JCI993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kannankeril P.J., Mitchell B.M., Goonasekera S.A., Chelu M.G., Zhang W., Sood S., Kearney D.L., Danila C.I., De Biasi M., Wehrens X.H., et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amador F.J., Stathopulos P.B., Enomoto M., Ikura M. Ryanodine receptor calcium release channels: Lessons from structure-function studies. Febs J. 2013;280:5456–5470. doi: 10.1111/febs.12194. [DOI] [PubMed] [Google Scholar]
- 118.Mallat Z., Tedgui A., Fontaliran F., Frank R., Durigon M., Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N. Engl. J. Med. 1996;335:1190–1196. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- 119.Roux-Buisson N., Gandjbakhch E., Donal E., Probst V., Deharo J.C., Chevalier P., Klug D., Mansencal N., Delacretaz E., Cosnay P., et al. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Results of a systematic screening. Heart Rhythm. 2014;11:1999–2009. doi: 10.1016/j.hrthm.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 120.Larsen M.K., Berge K.E., Leren T.P., Nissen P.H., Hansen J., Kristensen I.B., Banner J., Jensen H.K. Postmortem genetic testing of the ryanodine receptor 2 (RYR2) gene in a cohort of sudden unexplained death cases. Int. J. Leg. Med. 2013;127:139–144. doi: 10.1007/s00414-011-0658-2. [DOI] [PubMed] [Google Scholar]
- 121.Thomas N.L., George C.H., Williams A.J., Lai F.A. Ryanodine receptor mutations in arrhythmias: Advances in understanding the mechanisms of channel dysfunction. Biochem. Soc. Trans. 2007;35:946–951. doi: 10.1042/BST0350946. [DOI] [PubMed] [Google Scholar]
- 122.Okudaira N., Kuwahara M., Hirata Y., Oku Y., Nishio H. A knock-in mouse model of N-terminal R420W mutation of cardiac ryanodine receptor exhibits arrhythmogenesis with abnormal calcium dynamics in cardiomyocytes. Biochem. Biophys. Res. Commun. 2014;452:665–668. doi: 10.1016/j.bbrc.2014.08.132. [DOI] [PubMed] [Google Scholar]
- 123.Milting H., Lukas N., Klauke B., Korfer R., Perrot A., Osterziel K.J., Vogt J., Peters S., Thieleczek R., Varsanyi M. Composite polymorphisms in the ryanodine receptor 2 gene associated with arrhythmogenic right ventricular cardiomyopathy. Cardiovasc. Res. 2006;71:496–505. doi: 10.1016/j.cardiores.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Braz J.C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T.F., Lorenz J.N., Nairn A.C., Liggett S.B., et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat. Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 125.del Monte F., Harding S.E., Dec G.W., Gwathmey J.K., Hajjar R.J. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haghighi K., Kolokathis F., Gramolini A.O., Waggoner J.R., Pater L., Lynch R.A., Fan G.C., Tsiapras D., Parekh R.R., Dorn G.W., 2nd, et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akdis D., Medeiros-Domingo A., Gaertner-Rommel A., Kast J.I., Enseleit F., Bode P., Klingel K., Kandolf R., Renois F., Andreoletti L., et al. Myocardial expression profiles of candidate molecules in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia compared to those with dilated cardiomyopathy and healthy controls. Heart Rhythm. 2016;13:731–741. doi: 10.1016/j.hrthm.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Karakikes I., Stillitano F., Nonnenmacher M., Tzimas C., Sanoudou D., Termglinchan V., Kong C.W., Rushing S., Hansen J., Ceholski D., et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat. Commun. 2015;6:6955. doi: 10.1038/ncomms7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raja S.B., Rajendiran V., Kasinathan N.K., Amrithalakshmi P., Venkatabalasubramanian S., Murali M.R., Devaraj H., Devaraj S.N. Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem. Toxicol. 2017;106:92–106. doi: 10.1016/j.fct.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Liu G.S., Morales A., Vafiadaki E., Lam C.K., Cai W.F., Haghighi K., Adly G., Hershberger R.E., Kranias E.G. A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc. Res. 2015;107:164–174. doi: 10.1093/cvr/cvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Groeneweg J.A., van der Zwaag P.A., Olde Nordkamp L.R., Bikker H., Jongbloed J.D., Jongbloed R., Wiesfeld A.C., Cox M.G., van der Heijden J.F., Atsma D.E., et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy according to revised 2010 task force criteria with inclusion of non-desmosomal phospholamban mutation carriers. Am. J. Cardiol. 2013;112:1197–1206. doi: 10.1016/j.amjcard.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 132.Te Rijdt W.P., Asimaki A., Jongbloed J.D.H., Hoorntje E.T., Lazzarini E., van der Zwaag P.A., de Boer R.A., van Tintelen J.P., Saffitz J.E., van den Berg M.P., et al. Distinct molecular signature of phospholamban p.Arg14del arrhythmogenic cardiomyopathy. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2019;40:2–6. doi: 10.1016/j.carpath.2018.12.006. [DOI] [PubMed] [Google Scholar]