Summary
Nearly every tissue in the body undergoes routine turnover of cells as part of normal healthy living. The majority of these cells undergoing turnover die via apoptosis, and then are rapidly removed by phagocytes by the process of efferocytosis that is anti-inflammatory. However, a number of pathologies have recently been linked to defective clearance of apoptotic cells. Perturbed clearance arises for many reasons, including overwhelming of the clearance machinery, disruptions at different stages of efferocytosis, and/or responses of phagocytes during efferocytosis, all of which can alter the homeostatic tissue environment. This review covers linkages of molecules involved in the different phases of efferocytosis to disease pathologies that can arise due to their loss or altered function.
A number of inflammatory pathologies have recently been linked to defective clearance of apoptotic cells. Perturbed clearance arises for many reasons, and alter the homeostatic tissue environment. Ravichandran and colleagues review the linkage of specific molecules involved in the different phases of efferocytosis to disease states.
It is estimated that we turnover roughly 200–300 billion cells in the various tissues of the body every day, as part of homeostasis (Arandjelovic and Ravichandran, 2015; Elliott and Ravichandran, 2016). These include: excess cells generated during development, where only a subset are selected to mature and progress; ‘aged cells’ that have come to the end of their lifespan that need to be replaced; tissue remodeling; and damaged cells that arise due to various insults (Henson and Hume, 2006; Poon et al., 2014b). With respect to apoptosis and efferocytosis at the developmental stage, two relevant immunological tissues are the thymus and bone marrow, where many excess immature T and B cells are produced, and through a highly orchestrated selection process, only a subset of cells are deemed fit to progress beyond the key checkpoints; the others undergo apoptosis and are removed by phagocytes (Cao et al., 2004; Dogusan et al., 2004; Kawane et al., 2003). Similarly, many excess neurons are produced daily as a part of adult neurogenesis in the brain, of which only a subset turns into mature neurons, with the rest undergoing apoptosis and being cleared (Sierra et al., 2010). The best examples of aged cells and their removal are neutrophils that usually have a 24-hour lifespan, and red blood cells that usually have a 4-month lifespan (Antonelou et al., 2010; Newman et al., 1982). An example of tissue remodeling, where a significant number of cells undergo apoptosis and have to be removed, includes the involution of the mammary gland after lactation/weaning, where significant a number of mammary epithelial cells become apoptotic (Akhtar et al., 2016; Stanford et al., 2014). The damage associated cell turnover as part of homeostasis is exemplified in the retina of the eye, where the outer segments of photoreceptors (rods and cones) that undergo photo-damage during the day are removed daily around dawn by the retinal pigmented epithelial cells (RPE); although these are segments of photoreceptors (not whole cells), they appear to be cleared by a process analogous to traditional efferocytosis (Burstyn-Cohen et al., 2012; Penberthy et al., 2018). Thus, homeostatic turnover of cells occurs in every tissue in the body, and is fundamentally important for organ function and health.
One of the beautiful aspects of homeostatic cell turnover is that it is immunologically non-inflammatory (see below) and frequently anti-inflammatory, whereas disturbances in the routine process can lead to inflammation and various pathologies. To better appreciate altered or defective clearance leading to disease, one needs to consider several aspects of the efferocytosis process. This includes what programmed cell death process is normally seen in tissues as part of routine turnover (e.g. apoptosis, pyroptosis, necroptosis, etc.), the tissue specific context and environment, who are the phagocytes and how are the dying cells recognized, and what tissue factors control the uptake process and the subsequent response of the phagocytes. We discuss each of these points below.
Apoptosis as the dominant modality of homeostatic turnover:
Different types of cell death in target cells could trigger different immune responses by phagocytes (Pasparakis and Vandenabeele, 2015). Caspase-mediated apoptosis results in DNA fragmentation, membrane blebbing, and engulfment of such apoptotic cells elicits an anti-inflammatory response from the engulfing phagocytes. Necroptosis (mediated by receptor interacting protein kinase (RIPK) 1–RIPK3–MLKL) and pyroptosis (mediated by inflammatory caspases such as caspase-1 and 11 and gasdermin D (GSDMD) result in membrane rupture and the release of damage-associated molecular patterns (DAMPs) including DNA, RNA, high-mobility group box 1 (HMGB1), and IL-1β. These DAMPs are sensed by pattern recognition receptors (PRRs) on the phagocyte, resulting in a pro-inflammatory response (Kolb et al., 2017). What is the modality of death during homeostatic cell turnover? Although this has not been conclusive (due to lack of a clear approach to track all dying cells and their subsequent removal), and is dependent on tissue contexts, current evidence suggests that the majority of cells that are turned over die via the process of caspase-dependent apoptosis (Pellettieri and Sanchez Alvarado, 2007). This is based on several observations. First, apoptosis can be selectively induced in normal cells due to lack of growth factors or survival signals (e.g. lymphoid progenitors, activated B and T cells, neuronal progenitor cells, spontaneous neutrophil apoptosis) (Hildeman et al., 2007; Kristiansen and Ham, 2014), lack of a proper selection/survival signal (e.g. thymic selection of T cells) (Hernandez et al., 2010; Surh and Sprent, 1994), or actively (such as Fas-induced apoptosis) (Nagata, 1999). Second, when multiple pro-apoptotic genes are mutated and thereby apoptosis is dampened, less than 1% of these animals survive (Ke et al., 2018). Third, the other cell death processes, such as pyroptosis and necroptosis, are best seen under conditions of infection or following inflammatory stimuli, or when apoptosis itself is blocked, rather than as part of homeostasis (Man et al., 2017; Mihaly et al., 2014; Pasparakis and Vandenabeele, 2015; Weinlich et al., 2017). This does not discount the role of pyroptosis, necroptosis, ferroptosis, and other recently recognized forms of cell death in homeostasis; rather, it indicates that the contribution of caspase-dependent apoptotic cell death appears to be the dominant mode for homeostatic cell turnover. Therefore, in this review, we have predominantly focused on the clearance of apoptotic cells by efferocytosis as the primary modality during homeostatic cell turnover.
Phagocytic capacity, and the ratio of apoptotic cells versus phagocytes
In most tissues, a cell undergoing apoptosis can be cleared by either professional or non-professional phagocytes. The professional phagocytes often include tissue-resident macrophages, and immature dendritic cells (Arandjelovic and Ravichandran, 2015; Elliott and Ravichandran, 2016; Poon et al., 2014b; Steinman et al., 2000). The term ‘professional’ comes from observations that macrophages have the capacity to ingest and process multiple corpses in succession, and the rate of uptake is quite rapid. In contrast, non-professional phagocytes generally eat with slower kinetics, and frequently have lower capacity for taking up multiple corpses in succession. The non-professional phagocytes can be epithelial cells, fibroblasts, endothelial cells, or other stromal cells. There are also specialized phagocytes that mediate engulfment in specific tissues contexts, such as the Sertoli cells of the testes or retinal pigment epithelial cells (RPE) of the eye (Burstyn-Cohen et al., 2012; Elliott and Ravichandran, 2010; Park et al., 2011; Penberthy et al., 2018). Even in tissues with a high turnover of cells, such as the thymus (where millions of immature thymocytes undergo apoptosis), and the bone marrow (where an enormous number of leukocytes undergo developmental death or are clared at the end of their lifespan), only very few apoptotic cells are detectable at steady state (Arandjelovic and Ravichandran, 2015; Elliott and Ravichandran, 2016). This has led to the generally accepted notion that the capacity of phagocytes to engulf apoptotic cells is enormous: Contrary to the efficient clearance of apoptotic cells during homeostasis, there are many diseases where uncleared apoptotic cells are seen in the diseased tissue, suggesting that the capacity for apoptotic cell clearance is normally held in balance, and that the process is readily sensitive to disruption (Arandjelovic and Ravichandran, 2015; Elliott and Ravichandran, 2016; Poon et al., 2014b). Inherent within this ‘phagocytic capacity’ within a tissue or an organism is the numbers game between apoptotic cells that arise and the numbers and efficiency of phagocytes that can engulf these emerging corpses.
At present, our knowledge of the estimates ratio of apoptotic cells to phagocytes within tissues is rather vague. This is in part because of the differences in the types of phagocytes, and the difficultly in tracking the many cell types that turnover within a given tissue. Here, we attempted to gain a better insight using two cell types that we know from significant body of work are routinely turned over daily in our body: neutrophils, and red blood cells. When one considers neutrophils, ~100 billion neutrophils are produced per day in an average human adult at about 0.85–1.6 × 109 neutrophils per kg body weight (Summers et al., 2010). Since the overall number of neutrophils remains constant during homeostasis, a similar number of neutrophils needs to be removed daily. Although a huge proportion of the neutrophils re-enter the bone marrow (Wang et al., 2017a), elegant parabiosis experiments have shown that neutrophils also infiltrate other tissues, and are subsequently cleared by tissue resident macrophages (Casanova-Acebes et al., 2018) and a significant proportion leaves the body and gets removed via saliva, urine or stool (Summers et al., 2010). Another layer of complexity comes from the unknown number of total number of phagocytes that are actually involved in the clearance of neutrophils – for example, it is quite difficult to assess the numbers of macrophages lodged within the marrow of long bones that are linked to significant clearance of aged neutrophils (Heideveld and van den Akker, 2017). Thus, as we delved deeper in determining the ratio of neutrophils that turned over versus phagocyte numbers, quantitatively assessing this is nearly impossible.
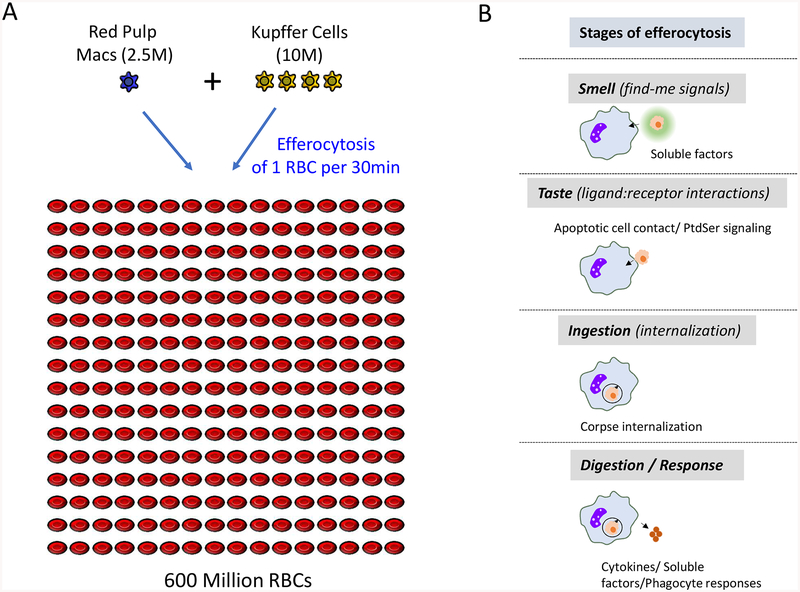
Perhaps a better cell type to possibly get a gauge of the targets to phagocyte ratio in vivo is the red blood cell (RBC) (Figure 1). RBCs, which are produced in the bone marrow in the adult, have a typical life span of about 120 days in humans and 40 days in mice. The ‘aged RBCs’ are mainly cleared by the red pulp macrophages (RPM) of the spleen, although Kupffer cells (KC) in the liver may also perform this function in certain conditions (Theurl et al., 2016; Youssef et al., 2018). It is estimated that in mice, 7000 RBC are cleared every second (Higgins, 2015). As for the phagocytes, there are ~2.5 × 106 red pulp macrophages in the murine spleen, and ~1 × 107 Kupffer cells in the murine liver (Crofton et al., 1978). To make an estimation, we assumed that the removal of aged RBCs is equivalently performed by both the red pulp macrophages and the Kupffer cells. With these conderations, each phagocyte would have to ingest at least 48 RBCs every day, or one RBC every 30 min. If one were to consider only one of the two phagocytes performing the RBC removal, then the red pulp macrophage would have to eat an RBC every 6 min and a Kupffer cell would have to ingest an RBC every 24 min. This estimation gives the perspective on the target load per individual phagocyte, even just considering one cell type! Thus, it is often assumed that there are fewer phagocytes than apoptotic cells, and that when the number of apoptotic cells vastly outnumbers those of the phagocytes (e.g. unregulated dying cells during tissue injury or infection), the phagocytes may be overwhelmed, resulting in the accumulation of uncleared apoptotic cells and the associated pathologies. Although overwhelming of the phagocytic machinery within a tissue is plausible, precise understanding of this concept requires better tools to directly address the number of cells undergoing apoptosis and the ability to track these apoptotic cells being engulfed in vivo. Along with the observation that, in many instances, the phagocytic receptors and the engulfment machinery components themselves are upregulated during efferocytosis (A-Gonzalez et al., 2017; Morioka et al., 2018), we posit that like most biological processes, efferocytosis capacity is tightly controlled and linked to the numbers of cells undergoing apoptosis within a tissue. The number of phagocytes, their appetite, and the rate of clearance have to be regulated under homeostatic conditions. Thus, the homeostatic efferocytosis can be considered as a delicate balance, or ‘living on the edge’, and that when this balance is disturbed, it can be a predisposing factor for various inflammatory diseaseses.
Figure 1.
A game of numbers – erythrocyte clearance in mice and the phases of efferocytosis. (A) Every symbol represents 2.5 × 106 cells of the respective population. Red blood cells (RBC) are depicted in red, Kupffer cells (KC) are depicted in yellow and red pulp macrophages (RPM) are depicted in blue. Every day, 600 × 106 RBC are cleared, resulting in a clearance rate of 7000 RBC per second. This task is performed by either 10 × 106 KC, 2.5×106 macrophages or both, resulting in the necessity of each phagocyte to engulf one RBC on average every 24 min, 6min, or 30 min, respectively.
(B) Efferocytosis is carried out via four distinct steps, and potential disturbance in one or more of these steps could result in defective apoptotic cell clearance and the associated pathologies. The first step in engulfment of apoptotic cells is the ‘Smell phase’ that involves communication of dying cells with nearby phagocytes through find-me signals that are released in the early stages of apoptosis. The second step is the ‘Taste phase’ that involves recognition of the ligands of apoptotic cells, termed ‘eat-me signals’ by the phagocytes through specific efferocytotic receptors. The third step of the process is the ‘Ingestion phase’, where signaling in the phagocyte downstream of engagement of apoptotic cells by phagocytic receptors leads to corpse internalization. The fourth step is the ‘Digestion/response phase’ that involves processing of the corpses and the production of anti-inflammatory mediators by the efferocytic phagocytes.
Tissue context and phagocytes
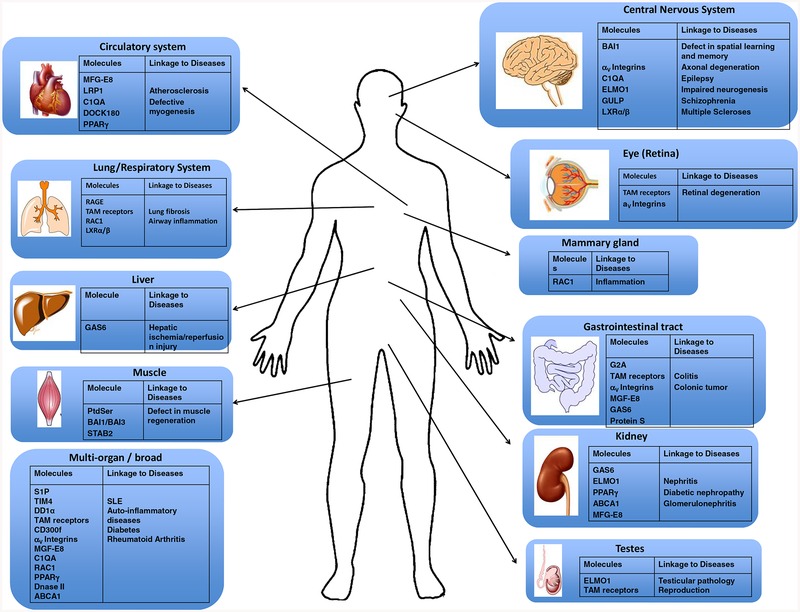
Molecules involved in efferocytosis have been reported to be involved in various diseases in different organs (Figure 2). While tissue-resident macrophages are common among most tissues, the population of other myeloid cells (such as dendritic cells) and their access to emerging apoptotic corpses within a tissue can significantly differ between tissues (Cummings et al., 2016). In the brain, in addition to microglia, neuronal progenitor cells, and likely astrocytes, can also participate in the removal of dying cells and debris (Chung et al., 2013; Mazaheri et al., 2014; Penberthy et al., 2018; Sierra et al., 2010). In addition, specialized phagocytes are the main clearance modality in two contexts. In the testes, the Sertoli cells mediate the uptake of the apoptotic germ cells, in addition to providing nurse function for other developing germ cells. In the retina, the retinal pigment epithelial cells are the primary phagocytes removing the used/damaged photoreceptor outer segments (Burstyn-Cohen et al., 2012; Penberthy et al., 2018). Deletion of particular engulfment components either in the Sertoli cells or the RPE lead to specific defects in germ cell development (testes) or retinal integrity (eye) (Burstyn-Cohen et al., 2012; Lu et al., 1999). In addition, there is also communication between phagocytes that can alter the ability of specific phagocytes to perform efferocytosis. For example, macrophages of the lung influence the engulfment of apoptotic corpses by airway epithelial cells through IGF-1 (Han et al., 2016). Defective clearance leads to accumulation of uncleared dead cells, with the cells releasing pro-inflammatory molecules leading to excessive inflammation, suggesting that clearance of apoptotic cells is indespensable to homeostasis. Thus, alterations in the ability of individual phagocytes (either professional or non-professional) to ingest apoptotic cells, depending on the tissue type, can affect tissue homeostasis and promote inflammation and/or developmental pathologies.
Figure 2.
Molecules involved in efferocytosis and their disease linkage in different tissues. In many tissues of the body, clearance of apoptotic cells is performed by the professional phagocytes and non-professional phagocytes. Pathologies associated with either natural or induced deletions or mutations of the specific molecules and their linkage t0 diseases or the different tissues are shown.
Disturbance in different steps of efferocytosis
Works from a number of laboratories have now established certain distinguishable steps in efferocytosis (Figure 1) (Chekeni et al., 2010; Elliott et al., 2010; Flannagan et al., 2014; Mazaheri et al., 2014; Miyanishi et al., 2007; Park et al., 2007; Scott et al., 2001), and potential disturbance in one or more of these steps could result in defective apoptotic cell clearance and the associated pathologies (Table 1).
Table 1.
Molecules and Pathologies Linked to Impaired Efferocytosis
| Relevant molecules |
Mouse (M) or human (H) disease connections | References |
|---|---|---|
| Smell Phase Associated Molecules and Disease Connections | ||
| Pannexin-1 |
|
(Billaud et al., 2015; Good et al., 2018; Yamaguchi et al., 2014) |
| P2Y2 |
|
(Müller et al., 2010) |
| CX3CR1 |
|
(Combadière et al., 2003) |
| G2A |
|
(Frasch et al., 2016) |
| S1P |
|
(Luo et al., 2016) |
| Taste Phase Associated Molecules and Disease Connections | ||
| Direct PtdSer Receptors | ||
| BAI1 |
|
(Fond et al., 2015; Hochreiter-Hufford et al., 2013; Lee et al., 2016; Zhu et al., 2015) |
| TIM-4 |
|
(Ji et al., 2014; Miyanishi et al., 2012; Wong et al., 2010) |
| Stabilin-1 |
|
(Karikoski et al., 2014; Schledzewski et al., 2011) |
| Stabilin-2 |
|
(Schledzewski et al., 2011) |
| RAGE |
|
(Englert et al., 2008; Englert et al., 2011; He et al., 2007; He et al., 2011) |
| CD300f |
|
(Tian et al., 2014) |
| TREM-2 |
|
(Poliani et al., 2015; Wang et al., 2015) |
| DD1α |
|
(Yoon et al., 2015) |
| Indirect PtdSer Receptors | ||
| TAM receptors |
|
(Bosurgi et al., 2013; D’Cruz et al., 2000; Duncan et al., 2003; Felton et al., 2018; Lu et al., 1999; Lu and Lemke, 2001; Neher et al., 2013; Prasad et al., 2006; Scott et al., 2001; Weinger et al., 2011) |
| Integrins |
|
(Acharya et al., 2010; Lacy-Hulbert et al., 2007; McCarty et al., 2008; McCarty et al., 2005) |
| Bridging Molecules | ||
| MFG-E8 |
|
(Ait-Oufella et al., 2007; Kusunoki et al., 2015; Miyanishi et al., 2012; Peng and Elkon, 2011) |
| Gas6 |
|
(Akitake-Kawano et al., 2013; Binder et al., 2011; Llacuna et al., 2010; Yanagita et al., 2002) |
| Protein S |
|
(Carrera Silva et al., 2013) |
| Other Receptors and Molecules | ||
| CD36 |
|
(Cai et al., 2012; Greenberg et al., 2006; Parks et al., 2013) |
| LRP1 |
|
(Yancey et al., 2010; Yancey et al., 2011) |
| SCARF-1 |
|
(Ramirez-Ortiz et al., 2013) |
| C1qa |
|
(Bhatia et al., 2007; Bossi et al., 2014; Botto et al., 1998; Chu et al., 2010; Stevens et al., 2007) |
| SLC2A1 |
|
(Freemerman et al., 2019; Morioka et al., 2018) |
| Ingestion Phase Associated Molecules and Disease Connections | ||
| ELMO1 |
|
(Elliott and Ravichandran, 2010; Elliott et al., 2010; Hathaway et al., 2016; Lu et al., 2011) |
| DOCK180 |
|
(Laurin et al., 2008; Sanematsu et al., 2010) |
| GULP |
|
(Qingchun et al., 2008) |
| RAC1 |
|
(Abreu et al., 2010; Akhtar et al., 2016; Juncadella et al., 2013) |
| Digestion Phase Associated Molecules and Disease Connections | ||
| LXRα, LXRβ |
|
(Bischoff et al., 2010; Jeon et al., 2014; Yang et al., 2014) |
| PPAR |
|
(Babaev et al., 2005; Mukundan et al., 2009; Roszer et al., 2011; Vasheghani et al., 2013) |
| DNase II |
|
(Kawane et al., 2003; Kawane et al., 2006) |
| ABCA1 |
|
(Christiansen-Weber et al., 2000) |
| Drp1 |
|
(Wang et al., 2017b) |
| Rubicon and NOX2 |
|
(Martinez et al., 2016; Martinez et al., 2015) |
| UCP2 |
|
(Blanc et al., 2003) |
| SLC16A1 |
|
(Merezhinskaya et al., 2000) |
The first step in engulfment of apoptotic cells is the ‘Smell phase’ that involves dying cells announcing their presence to nearby phagocytes through find-me signals that are released in the early phases of apoptosis. These find-me signals provide an attraction cue for the resident or recruited phagocytes to seek the apoptotic cells. The find-me signals come in different flavors, such as proteins (fractalkine), nucleotides (ATP, UTP, AMP), lipids (lysophosphatidylcholine) and lipid products (sphingosine 1-phosphate). While some modes of regulated release of find-me signals have been identified (e.g. ATP through pannexin channels), other release mechanisms and comprehensive identification of find-me signals are needed (Elliott et al., 2009; Medina and Ravichandran, 2016). These find-me signal cues are then sensed by receptors on phagocytes that initiate their movement to the proximity of the apoptotic meal. Most of the find-me signals have been delineated using in vitro assays, with some by in vivo observations, and it is not known what the ‘tissue range’ is, i.e., how far such apoptotic cell-derived find-me signal gradients can propagate in a tissue to attract phagocytes, or how quickly some of these find-me signals may be degraded (e.g. nucleotides by extracellular enzymes that degrade nucleotides). Thus, defects in the efficiency of find-me signal release (e.g. mutations in PANX1 channels or altered PANX1 function), defects in the sensing of find-me signals, or degradation of find-me signals could all contribute to defective apoptotic cell recognition and clearance.
The second step is the ‘Taste phase’ that involves recognition of the ligands of apoptotic cells, termed ‘eat-me signals’ by the phagocytes through specific receptors. The eat-me signals can also come in different flavors, such as lipids (phosphatidylserine), proteins (ICAM-3) (Moffatt et al., 1999), or modified carbohydrates. The best studied eat-me signal is phosphatidylserine (PtdSer) and this appears to be conserved through evolution. PtdSer, which is normally present on the inner leaflet of the plasma membrane, gets exposed on the outer leaflet during apoptosis (due to altered functionality of scramblases and flippases), thereby providing a marker of apoptotic cells (Segawa et al., 2014; Suzuki et al., 2013). This exposed PtdSer on the apoptotic cells can be recognized by phagocytes through several different PtdSer receptors, either directly (via TIM-4, BAI1, Stabilin-2) (Miyanishi et al., 2007; Park et al., 2007; Park et al., 2008) or indirectly through bridging molecules (such as Gas6, Protein S, MFGE8) (Borisenko et al., 2004; Nakano et al., 1997) that bind both the PtdSer on the apoptotic cells and receptors on phagocytes (such as MERTK and integrins) (Lemke and Burstyn-Cohen, 2010). It is clear from the current body of work from a number of laboratories that not all receptors are expressed on all phagocytes, nor is this necessary. However, different phagocytes express different subsets of efferocytic receptors, and some are also further increased in expression during different stages of efferocytosis. Thus, defects in either expression or function of specific engulfment receptors have been linked to specific pathologies, depending on the tissue types. In addition, we need to consider don’t eat-me signals that are expressed on live cells that repel phagocytes from efferocytosis. Among these, a number of recent studies have identified a clear role for CD47 as a key don’t eat-me signal on live cells, and the Cd47 engagement of SIRPα on macrophages as an important inhibitory signal (Chao et al., 2012; Kojima et al., 2017; Veillette and Chen, 2018; Weiskopf et al., 2013). Specifically, blocking CD47 recognition promotes uptake of tumor cells (contextdependent and requiring Fc portion of the antibody), and also in the context of atherosclerosis (Kojima et al., 2016). At this point, precisely how the SIRPα signaling in macrophages is coordinated with the engulfment receptors is still under investigation.
The third step of the process is the ‘ingestion phase’, where signaling in the phagocyte downstream of engagement of apoptotic cells by phagocytic receptors leads to corpse internalization. For example, the binding of PtdSer by BAI1 leads to signaling via the ELMO/Dock180/Rac pathway to promote cytoskeletal reorganization and corpse uptake (Fond et al., 2015; Lee et al., 2016; Mazaheri et al., 2014; Park et al., 2007). Additionally, TIM-4, along with either integrins, TAM receptors or fibronectin 1, leads to signaling within phagocytes to facilitate corpse internalization (Flannagan et al., 2014; Lee et al., 2018). Similarly, apoptotic cell engagement by the TYRO3, AXL, MERTK (TAM) family of receptors leads to downstream signaling (Lemke and Burstyn-Cohen, 2010). Defects in signaling downstream of BAI1, MERTK, or TIM-4 have been linked to pathologies of the testes, the eye, lung, and the liver (Bosurgi et al., 2017; Poon et al., 2014b; Rothlin et al., 2015).
The fourth step is the ‘Digestion/response phase’ that involves processing of the corpses and the production of anti-inflammatory mediators. Elegant studies have now shown that efferocytic phagocytes release a number of anti-inflammatory mediators including PGE2, TGFβ IL-10, and lactate, among others (Morioka et al., 2018). Defects in corpse processing and/or production of anti-inflammatory mediators can lead to pathologies and chronic inflammatory diseas such as atherosclerosis, colitis, and lupus (Blanc et al., 2003; Kawane et al., 2006; Martinez et al., 2016).
In vivo, the recognition and removal of apoptotic corpses usually occurs extremely rapidly. For example, if one injects millions of apoptotic cells into the peritoneum of a mouse, in as little as 15–30 minutes, most of these apoptotic cells reside within phagocytes (in some cases, in as little as a few min) (Morioka et al., 2018). While apoptotic cells themselves can have beneficial effects (see below), the failure to clear them promptly results in secondary necrosis and either the contents of these necrotic cells or their interaction with other cells in the tissue appear to promote inflammation. Thus, failed apoptotic cell clearance is often associated with local tissue inflammation that can become chronic, if resolution does not take place, and the potential release of self-antigens can lead to autoimmunity in some cases (Henson and Hume, 2006). Below, we have provided specific examples of defects in the different steps of apoptotic cell clearance that can lead to pathological sequelae.
Pathologies linked to molecules in the ‘Smell phase’ of efferocytosis
As the find-me signals during efferocytosis are soluble factors, the potential pathologies linked to defective clearance can arise from the failure to generate these factors or in sensing of these cues. It has been revealed that there are a number of different associations with disease in mice (and in some cases human) when the receptors for specific find-me signals are deficient, such as the CX3CR1 receptor that engages fraktalkine/CD3CL1, or the P2Y2 receptor important for ATP recognition (Truman et al., 2008). It is worth noting that the knockout mouse studies did not directly link the inflammation state to failed clearance, although the loss of P2Y2 does lead to accumulation of uncleared apoptotic cells in the thymus (Elliott et al., 2009). However, the release mechanism by which the find-me signals get out of the apoptotic cells is yet to be defined, except for nucleotides, which occurs via pannexin channels (Medina and Ravichandran, 2016;Yamaguchi et al., 2014). Caspase-dependent cleavage of the C-terminal tail of the pannexin 1 channels leads to release of nucleotides (and likely other molecules less than 1 kDa) and these can either act as find-me signals (such as ATP or UTP) or as anti-inflammatory mediators (such as AMP that gets converted to adenosine) (Chekeni et al., 2010; Sandilos et al., 2012). Pannexin channels also have other physiological roles, including blood pressure regulation, neuropathic pain propagation, and metastasis (Weaver et al., 2017). Cells lacking Pannexin 1 die in a fragmented fashion (displaying features of apoptopodia) and in a pro-inflammatory fashion, with a potential linkage to trovafloxacininduced toxicity in humans (Poon et al., 2014a).
Pathologies associated with molecules in the ‘Taste phase’ of efferocytosis
Given the many molecules that are exposed on the apoptotic cells, and the many phagocytic receptors that engage these receptors, the potential disturbances and the linkages to diseases are numerous. Among the ligands on apoptotic cells, the best known is PtdSer and this is recognized either directly via specific receptors or indirectly through intermediary molecules that bridge PtdSer on the apoptotic cells to the receptors on phagocytes. Mice with knockout of specific PtdSer receptors have shown a striking or mild defect depending on the receptor, tissue type involved, and whether the phenotypes were assessed in the steady state or after specific insults. Mice with a global loss of a single receptor often display mild phenotypes (except for a few exceptions, such as MERTK loss in the retina)(Burstyn-Cohen et al., 2012), and in vitro, the loss of a single receptor often leads to only a partial reduction in apoptotic cell uptake. These observations have led to the current notion that there are redundancies among engulfment receptors. Clearly, engagement of a polyvalent eat-me signal such as PtdSer by multiple PtdSer receptors (with either direct or indirect recognition) is complicated, and this is not easily manifested by in vitro or in vivo assessments. However, we posit that this redundancy is not a blanket phenomenon, and rather context-dependent. For example, transgenic overexpression of the PtdSer BAI1 could substitute for MERTK loss in the Sertoli cell-mediated clearance of apoptotic germ cells, while BAI1 could not rescue the striking retinal degeneration phenotype seen with MERTK deficiency (even when being expressed in the right location in the retina and in apparently sufficient densities) (Burstyn-Cohen et al., 2012; Penberthy et al., 2017). This is consistent with an elegant earlier study which determined that MERTK and its ligands (either Gas6 or Protein S) are necessary for normal photoreceptor clearance and prevention of retinal degeneration (Burstyn-Cohen et al., 2012). MERTK may also signal uniquely in the retinal pigmented epithelial cells, and this may also contribute to the retinal degeneration, as RNAseq comparison of RPE with and without MERTK expression displayed a unique gene signature (independent of photoreceptor uptake) (Penberthy et al., 2017). Thus, the engulfment receptors may provide specificity both at the uptake level and also engage unique ligand specificities and downstream signaling within specific cells and tissues (Zagorska et al., 2014).
Similarly, the requirement for certain signaling molecules downstream of efferocytic receptors can also be tissue specific. For example, loss of Elmo1 leads to a defect in Sertoli cell-mediated clearance of apoptotic germ cells, but has little effect on macrophage uptake, likely because Elm02 expression is increased in most tissues when Elmo1 is deficient, but not in the Sertoli cells (Elliott et al., 2010). Thus, either context dependent expression, signaling, or redundancy may dictate the specific pathologies. Table 1 details some of the many different mouse and human pathologies or consequences associated with the loss of various engulfment receptors and downstream signaling molecules. A cautionary note in these disease linkages is that in most cases, even though uncleared apoptotic cells are seen when a particular receptor(s) is deleted or lost, it has not been clearly established whether the observed phenotype/pathology is solely due to the efferocytic defect or whether additional tissue specific functions of these molecules contributed to the phenotype (Table 1).
Pathologies linked to multiple phases of efferocytosis
Efferocytosis has multiple stages and these phases operate in a continuum, with both feed-forward and feedback regulation. For example, a defect in proper regulation of specific downstream molecules (see below) can lead to defective dying cell uptake. Subsequently, the release of pro-inflammatory factors from the uncleared secondarily necrotic cells, and the lack of proper anti-inflammatory mediators from the efferocytic phagocytes can lead to induction of local inflammation. Further, a failed initial clearance step leading to local inflammation and recruitment of other immune cells such as neutrophils, can exacerbate this situation by contributing to the apoptotic cell burden due to their short life span (e.g. ~24 hours for neutrophils) after which they have to be cleared as well. Thus, an initial defect could be accentuated beyond the first step. Often, characterization of a phenotype as an efferocytotic defect is initially noticed as an increased number of TUNEL or Annexin V positive cells in a tissue context. A major distinction that has to be considered is whether this is due to increased apoptosis (by existing or recruited cells) versus a defect in efferocytosis. Below we discuss two disease states where the failed/defective efferocytosis has been very well linked to disease progression.
Atherosclerosis:
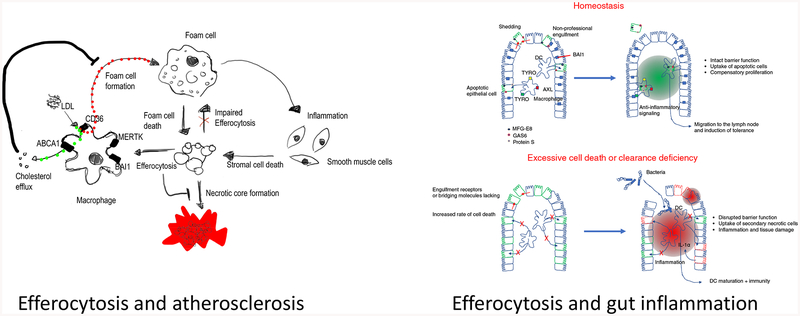
Cardiovascular disease linked to formation of atherosclerotic plaques in the vessel walls is the major cause of human mortality worldwide (for excellent reviews, please see: (Libby, 2002; Moore and Tabas, 2011)). It is now established that formation and propagation of the atherosclerotic plaques is associated with defects in proper efferocytosis, and the associated chronic inflammation (Morioka et al., 2018; Wang et al., 2017b) (Figure 3A). Most atherosclerotic plaques in the later stages of disease are characterized by accumulation of apoptotic cells as well as a necrotic core. Macrophages play a major role within the plaques at several levels. First, the infiltration of monocytes and their differentiation to macrophages, and the subsequent inflammatory responses of these macrophages, significantly contribute to the initiation and propagation of the pathology. Further, a fraction of macrophages in the plaques are also cholesterol-laden and these macrophages display defective efferocytosis. These macrophages also undergo apoptosis and have to be removed by either newly recruited or plaque-associated macrophages (Kojima et al., 2017; Moore and Tabas, 2011; Tabas, 2010). Thus, any changes in the recognition of the apoptotic cells within the plaques, the ability of the macrophages to uptake cargo, the processing of the cargo, or the subsequent responses of the phagocytes can all contribute to the disease progression and severity. In terms of the apoptotic cell recognition phase during development of atherosclerosis, the receptor MERTK has been linked to clearance of apoptotic cells by macrophages within the plaques. Mice deficient in MERTK expression have larger necrotic plaque areas in mouse models of atherosclerosis. Interestingly, proteolytic cleavage of the extracellular region of MERTK dampens efferocytosis, and this occurs within both human and murine atherosclerotic plaques (Cai et al., 2017). In elegant studies, Tabas and colleagues engineered protease-resistant MERTK bone marrow chimera mice that have less plaque necrosis during experimental atherosclerosis (Cai et al., 2017). In addition, signaling within the phagocytes during corpse processing also seems important – for example, aerobic glycolysis is a key aspect of efferocytosis and the successive uptake of more than one corpse, and this depends on the glucose transporter Glut1/Slc2A1 and glucose uptake (Morioka et al., 2018); further, mitochondrial fission through Drp1 and Ucp2 regulates macrophages to continue to engulf multiple corpses (Blanc et al., 2003; Park et al., 2011; Wang et al., 2017b). Moreover, macrophages are remarkably capable of reverse cholesterol transport where they release the cholesterol via ABCA1 to form HDL particles that then get cleared by the liver (Fond et al., 2015). During efferocytosis of normal apoptotic cells or cholesterol-laden macrophages that are dying, this problem is further accentuated. Interestingly, during efferocytosis, ABCA1 gets upregulated, and this in turn promotes cholesterol efflux. It now appears that one modality for ABCA1 upregulation is through the BAI1/ELMO1/Rac1 pathway. Another critical step of efferocytosis is the production of anti-inflammatory mediators by engulfing phagocytes, which is important for dampening tissue inflammation. Recent work suggests that duringthe resolution phase of inflammation, Treg cells can communicate with macrophages (through IL-13) and, in turn, via IL-10, can improve efferocytosis (Proto et al., 2018). Thus, multiple phases of efferocytosis are linked to atherosclerosis and therefore targeting the efferocytotic pathway via small molecules may be a potential therapeutic approach.
Figure 3.
Apoptotic cell clearance in atherosclerosis and the intestine. (A) Monocyte-derived macrophages in atherosclerotic plaques take up LDL via CD36, and the free cholesterol if often released via ABCA1 to HDL (part of reverse cholesterol transport to the liver). The macrophages express the phosphatidylserine receptors such as MERTK and BAI1, limiting the accumulation of dead cells and necrotic core formation. Further, BAI1-mediated efferocytosis increases capacity of macrophages to perform reverse cholesterol efflux via upregulation of ABCA1. If the load of LDL is too high, efferocytosis or reverse cholesterol efflux is impaired, then macrophages accumulate cholesterol and develop into foam cells. Foam cells show inflammatory characteristics, impaired efferocytosis, and are poised to undergo cell death. Dying foam cells and inflammation-induced cell death of plaque-associated cells contribute to necrotic core formation.
(B) During homeostasis, apoptotic cells in the epithelial lining are taken up by nearby healthy epithelial cells, by professional phagocytes of the lamina propria (such as dendritic cells), or shed into the lumen. Under these circumstances, the epithelial barrier function is maintained, and cell clearance establishes a tolerogenic, anti-inflammatory environment. If apoptotic cell clearance is impaired or cell death exceeds the clearance capacity of the intestine, apoptotic cells progress into secondary necrosis. Secondary necrotic cells either release pro-inflammatory mediators themselves or induce an inflammatory signature in the phagocytes. The disruptions of epithelial lining allow bacterial passage through the barrier, further contributing to the overall inflammatory environment associated with increased cell death and clearance deficiency
Gut inflammation:
Both ulcerative colitis (UC) and inflammatory bowel disease (IBD) affect millions of people worldwide with the current therapies largely targeting molecules that promote inflammation (such as IL-1 or TNFa) without directly addressing the cause. It has been known for a long time that both UC and IBD are chronic inflammatory conditions and that there are often many uncleared apoptotic corpses in these inflammatory tissues. It is unclear at this point whether this is due to increased apoptosis (due to the inflammatory milieu) or defective clearance by phagocytes, but recent studies in mouse models suggest that impaired efferocytosis might be part of the cause (Figure 3B). There might be at least two types of phagocytes one needs to consider. First, dendritic cells residing in the lamina propria can mediate uptake of the dying epithelial cells, and the gene programs induced within these dendritic cells suggest that they can influence the inflammatory state (Cummings et al., 2016). Further, epithelial cells themselves seem capable of eating their dying brethren. Although it is often assumed that epithelial cells of the gut can simply be extruded into the gut lumen, there are both physical limitations and the necessity of maintaining epithelial barrier integrity. In support of the notion that epithelial cells can eat their dying neighbors and that it can be beneficial, transgenic overexpression of an engulfment receptor BAI1 (and its signaling downstream to the engulfment machinery) was able to dampen inflammatory colitis in a murinemodel of the disease (Lee et al., 2016). Thus, targeting the efferocytosis process either by the myeloid cells or the epithelial neighbors, and the potential antiinflammatory mediators produced during uptake, may be of benefit in reducing inflammation during chronic gut inflammation.
Other issues, and where do we go from here?
Efferocytosis is a complex process.
Through exciting discoveries from multiple investigators approaching efferocytosis from different angles, we know that efferocytosis occurs in many tissue contexts, and the field has made remarkable progress in recent years. As we understand more of this process, both during homeostasis and in various disease pathologies, we recognize that this process is both intricate and multi-faceted, and there are a number of key areas in which we still have rather limited knowledge. We have highlighted a few of these below. When a phagocyte ingests an apoptotic cell (often the same size as itself!) its volume is likely to double or more than double if the phagocyte were to take up multiple corpses in succession. Yet, remarkably, phagocytes maintain their cell volume beautifully, and the molecular players involved in cell volume regulation are not understood. Understanding phagocyte volume regulation could be highly relevant in tissues with high cellular turnover, or in atherosclerotic plaques, where one macrophage attempts to eat another cholesterol-laden macrophage. Similarly, how a phagocyte manages the excess metabolic cargo load from the ingested cells is another area that is not well deciphered and could be hugely important for homeostatic cell turnover and pathologies that arise. Recent studies on the solute carrier (SLC) family gene program initiated during efferocytosis suggests that some of the cargo management may be mediated through these SLCs (Morioka et al., 2018). Further, we know very little about how a macrophage handles various stresses, including oxidative stress and ER stress, how it manages the multiplication of many cellular contentsproteins, carbohydrates, lipids, and nucleotides to name a few. This could again be highly relevant for rapid cell turnover, and under metabolic disturbances, such as obesity. It is known that adipocyte death is a feature of obesity in inflamed fat tissue, and macrophages in a crown-like structure localize around adipocytes, which are many fold bigger and cannot be ingested by a single macrophage (Alkhouri et al., 2010; Haka et al., 2016). How the macrophages manage the issues of volume, metabolic load, and anti-inflammatory responses would be highly useful in interpreting the disease progression and in designing approaches to therapeutically intervene in these metabolic disorders from an efferocytosis perspective. Another dimension of homeostatic efferocytosis that requires attention in different tissues is the circadian aspects of cell clearance. Elegant studies from Hidalgo and colleagues in the context of neutrophil clearance already suggests that this is highly regulated over the circadian cycle (A-Gonzalez et al., 2017; Casanova-Acebes et al., 2013). Similarly, clearance of photoreceptor outer segments by the RPE is also circadian regulated. How the death itself is regulated during different points in the circadian cycle, how this may match with the competency of the cell clearance machinery, are fascinating avenues for future pursuit. Also, we need better tools to monitor both apoptosis and efferocytosis in vivo and to be able to track dying and engulfing cells for further analysis. Thus, defining efferocytosis during homeostatic cell turnover is at an exciting phase and insights into potential points of therapeutic intervention could have significant implications for many human diseases.
Acknowledgements:
This work is supported by grants to K.S.R. from the NIGMS (GM064709), NHLBI (P01HL120840), Odysseus Award from the FWO (Belgium), and the EOS-DECODE award from the FWO (Belgium). S.M. is supported by grants from the Mishima-Kaiun Memorial Foundation and The Kanae Foundation for the Promotion of Medical Science. C.M. is supported by a postdoctoral research fellowship from the German Research Foundation (DFG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A-Gonzalez N, Quintana JA, Garcia-Silva S, Mazariegos M, Gonzalez de la Aleja A, Nicolas-Avila JA, Walter W, Adrover JM, Crainiciuc G, Kuchroo VK, et al. (2017). Phagocytosis imprints heterogeneity in tissue-resident macrophages. J Exp Med 214, 128–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu JR, Dontje W, Krausz S, de Launay D, van Hennik PB, van Stalborch AM, Ten Klooster JP, Sanders ME, Reedquist KA, Vervoordeldonk MJ, et al. (2010). A Rac1 inhibitory peptide suppresses antibody production and paw swelling in the murine collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther 12, R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, and Lacy-Hulbert A (2010). alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest 120, 4445–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, et al. (2007). Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115, 2168–2177. [DOI] [PubMed] [Google Scholar]
- Akhtar N, Li W, Mironov A, and Streuli CH (2016). Rac1 Controls Both the Secretory Function of the Mammary Gland and Its Remodeling for Successive Gestations. Dev Cell 38, 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akitake-Kawano R, Seno H, Nakatsuji M, Kimura Y, Nakanishi Y, Yoshioka T, Kanda K, Kawada M, Kawada K, Sakai Y, et al. (2013). Inhibitory role of Gas6 in intestinal tumorigenesis. Carcinogenesis 34, 1567–1574. [DOI] [PubMed] [Google Scholar]
- Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, and Feldstein AE (2010). Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 285, 3428–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelou MH, Kriebardis AG, and Papassideri IS (2010). Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus 8 Suppl 3, s39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic S, and Ravichandran KS (2015). Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, and Linton MF (2005). Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 25, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, and Haskard DO (2007). Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol 170, 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, et al. (2015). A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal 8, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Xiao J, Kemper D, Ma GZ, Murray SS, and Kilpatrick TJ (2011). Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PLoS One 6, e17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, and Schulman IG (2010). Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res 51, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, and Mallat Z (2003). Protective role of uncoupling protein 2 in atherosclerosis. Circulation 107, 388–390. [DOI] [PubMed] [Google Scholar]
- Borisenko GG, Iverson SL, Ahlberg S, Kagan VE, and Fadeel B (2004). Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: implications for macrophage clearance of apoptotic cells. Cell Death Differ 11, 943–945. [DOI] [PubMed] [Google Scholar]
- Bossi F, Tripodo C, Rizzi L, Bulla R, Agostinis C, Guarnotta C, Munaut C, Baldassarre G, Papa G, Zorzet S, et al. (2014). C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci U S A 111, 4209–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, Booth CJ, Ghosh S, and Rothlin CV (2013). Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci U S A 110, 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, and Walport MJ (1998). Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature genetics 19, 56–59. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, and Lemke G (2012). Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 76, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G, and Tabas I (2017). MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest 127, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Wang Z, Ji A, Meyer JM, and van der Westhuyzen DR (2012). Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One 7, e36785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WM, Murao K, Imachi H, Hiramine C, Abe H, Yu X, Dobashi H, Wong NC, Takahara J, and Ishida T (2004). Phosphatidylserine receptor cooperates with high-density lipoprotein receptor in recognition of apoptotic cells by thymic nurse cells. J Mol Endocrinol 32, 497–505. [DOI] [PubMed] [Google Scholar]
- Carrera Silva EA, Chan PY, Joannas L, Errasti AE, Gagliani N, Bosurgi L, Jabbour M, Perry A, Smith-Chakmakova F, Mucida D, et al. (2013). T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M, Nicolas-Avila JA, Li JL, Garcia-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, N AG, et al. (2018). Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med 215, 2778–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, N AG, Kunisaki Y, Zhang D,van Rooijen N, Silberstein LE, et al. (2013). Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Weissman IL, and Majeti R (2012). The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol 24, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. (2010). Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen-Weber TA, Voland JR, Wu Y, Ngo K, Roland BL, Nguyen S, Peterson PA, and Fung-Leung WP (2000). Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol 157, 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, and Prince DA (2010). Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A 107, 7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, and Mallat Z (2003). Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107, 1009–1016. [DOI] [PubMed] [Google Scholar]
- Crofton RW, Diesselhoff-den Dulk MM, and van Furth R (1978). The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med 148, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, and Blander JM (2016). Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, and Vollrath D (2000). Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9, 645–651. [DOI] [PubMed] [Google Scholar]
- Dogusan Z, Montecino-Rodriguez E, and Dorshkind K (2004). Macrophages and stromal cells phagocytose apoptotic bone marrow-derived B lineage cells. J Immunol 172, 4717–4723. [DOI] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, et al. (2003). An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 44, 826–838. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, and Ravichandran KS (2010). ELMO1 signaling in apoptotic germ cell clearance and spermatogenesis. Ann N Y Acad Sci 1209, 30–36. [DOI] [PubMed] [Google Scholar]
- Elliott MR, and Ravichandran KS (2016). The Dynamics of Apoptotic Cell Clearance. Dev Cell 38, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, and Ravichandran KS (2010). Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 467, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, et al. (2008). A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol 172, 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, and Oury TD (2011). Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. International journal of clinical and experimental pathology 4, 241–254. [PMC free article] [PubMed] [Google Scholar]
- Felton JM, Lucas CD, Dorward DA, Duffin R, Kipari T, Vermeren S, Robb CT, MacLeod KG, Serrels B, Schwarze J, et al. (2018). Mer-mediated eosinophil efferocytosis regulates resolution of allergic airway inflammation. J Allergy Clin Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Canton J, Furuya W, Glogauer M, and Grinstein S (2014). The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol Biol Cell 25, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond AM, Lee CS, Schulman IG, Kiss RS, and Ravichandran KS (2015). Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J Clin Invest 125, 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch SC, McNamee EN, Kominsky D, Jedlicka P, Jakubzick C, Zemski Berry K, Mack M, Furuta GT, Lee JJ, Henson PM, et al. (2016). G2A Signaling Dampens Colitic Inflammation via Production of IFN-gamma. J Immunol 197, 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemerman AJ, Zhao L, Pingili AK, Teng B, Cozzo AJ, Fuller AM, Johnson AR, Milner JJ, Lim MF, Galanko JA, et al. (2019). Myeloid Slc2a1-Deficient Murine Model Revealed Macrophage Activation and Metabolic Phenotype Are Fueled by GLUT1. J Immunol 202, 1265–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, et al. (2018). Pannexin 1 Channels as an Unexpected New Target of the AntiHypertensive Drug Spironolactone. Circ Res 122, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, and Hazen SL (2006). Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203, 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, and Maxfield FR (2016). Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res 57, 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CZ, Juncadella IJ, Kinchen JM, Buckley MW, Klibanov AL, Dryden K, Onengut-Gumuscu S, Erdbrugger U, Turner SD, Shim YM, et al. (2016). Macrophages redirect phagocytosis by nonprofessional phagocytes and influence inflammation. Nature 539, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway CK, Chang AS, Grant R, Kim HS, Madden VJ, Bagnell CR Jr., Jennette JC, Smithies O, and Kakoki M (2016). High Elmo1 expression aggravates and low Elmo1 expression prevents diabetic nephropathy. Proc Natl Acad Sci U S A 113, 2218–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, and Yamaya M (2007). The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 293, L1427–1436. [DOI] [PubMed] [Google Scholar]
- He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, et al. (2011). Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep 12, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideveld E, and van den Akker E (2017). Digesting the role of bone marrow macrophages on hematopoiesis. Immunobiology 222, 814–822. [DOI] [PubMed] [Google Scholar]
- Henson PM, and Hume DA (2006). Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 27, 244–250. [DOI] [PubMed] [Google Scholar]
- Hernandez JB, Newton RH, and Walsh CM (2010). Life and death in the thymus--cell death signaling during T cell development. Curr Opin Cell Biol 22, 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JM (2015). Red blood cell population dynamics. Clin Lab Med 35, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman D, Jorgensen T, Kappler J, and Marrack P (2007). Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol 19, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, and Ravichandran KS (2013). Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JY, Nam JY, Kim HA, Park YB, Bae SC, and Suh CH (2014). Liver X receptors alpha gene (NR1H3) promoter polymorphisms are associated with systemic lupus erythematosus in Koreans. Arthritis Res Ther 16, R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Liu Y, Zhang Y, Shen XD, Gao F, Busuttil RW, Kuchroo VK, and Kupiec-Weglinski JW (2014). T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemiareperfusion injury. Hepatology 60, 2052–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, and Ravichandran KS (2013). Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmen M, Kelkka T, Gerke H, Huovinen V, Irjala H, Holmdahl R, et al. (2014). Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res 20, 6452–6464. [DOI] [PubMed] [Google Scholar]
- Kawane K, Fukuyama H, Yoshida H, Nagase H, Ohsawa Y, Uchiyama Y, Okada K, Iida T, and Nagata S (2003). Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol 4, 138–144. [DOI] [PubMed] [Google Scholar]
- Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, and Nagata S (2006). Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443, 998–1002. [DOI] [PubMed] [Google Scholar]
- Ke FFS, Vanyai HK, Cowan AD, Delbridge ARD, Whitehead L, Grabow S, Czabotar PE, Voss AK, and Strasser A (2018). Embryogenesis and Adult Life in the Absence of Intrinsic Apoptosis Effectors BAX, BAK, and BOK. Cell 173, 1217–1230 e1217. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, et al. (2016). CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Weissman IL, and Leeper NJ (2017). The Role of Efferocytosis in Atherosclerosis. Circulation 135, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb JP, Oguin TH 3rd, Oberst A, and Martinez J (2017). Programmed Cell Death and Inflammation: Winter Is Coming. Trends Immunol 38, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, and Ham J (2014). Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ 21, 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki R, Ishihara S, Tada Y, Oka A, Sonoyama H, Fukuba N, Oshima N, Moriyama I, Yuki T, Kawashima K, et al. (2015). Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. Journal of gastroenterology. [DOI] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, and Hynes RO (2007). Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A 104, 15823–15828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin M, Fradet N, Blangy A, Hall A, Vuori K, and Cote JF (2008). The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci U S A 105, 15446–15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Penberthy KK, Wheeler KM, Juncadella IJ, Vandenabeele P, Lysiak JJ, and Ravichandran KS (2016). Boosting Apoptotic Cell Clearance by Colonic Epithelial Cells Attenuates Inflammation In Vivo. Immunity 44, 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park B, Moon B, Park J, Moon H, Kim K, Lee SA, Kim D, Min C, Lee DH, et al. (2018). A scaffold for signaling of Tim-4-mediated efferocytosis is formed by fibronectin. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, and Burstyn-Cohen T (2010). TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci 1209, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P (2002). Inflammation in atherosclerosis. Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- Llacuna L, Barcena C, Bellido-Martin L, Fernandez L, Stefanovic M, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Garcia de Frutos P, and Morales A (2010). Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology 52, 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, et al. (1999). Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398, 723–728. [DOI] [PubMed] [Google Scholar]
- Lu Q, and Lemke G (2001). Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293, 306–311. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, and Kipnis J (2011). Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol 13, 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, Liu Y, Liu Y, Jiang M, Zhang Z, et al. (2016). Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity 44, 287–302. [DOI] [PubMed] [Google Scholar]
- Man SM, Karki R, and Kanneganti TD (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li QZ, Yan M, Janke L, Guy C, et al. (2016). Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 533, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, et al. (2015). Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 17, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mazaheri F, Breus O, Durdu S, Haas P, Wittbrodt J, Gilmour D, and Peri F (2014). Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat Commun 5, 4046. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Barry M, Crowley D, Bronson RT, Lacy-Hulbert A, and Hynes RO (2008). Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am J Pathol 172, 1740–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, and Hynes RO (2005). Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132, 165–176. [DOI] [PubMed] [Google Scholar]
- Medina CB, and Ravichandran KS (2016). Do not let death do us part: ‘find-me’ signals in communication between dying cells and the phagocytes. Cell Death Differ 23, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merezhinskaya N, Fishbein WN, Davis JI, and Foellmer JW (2000). Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 23, 90–97. [DOI] [PubMed] [Google Scholar]
- Mihaly SR, Ninomiya-Tsuji J, and Morioka S (2014). TAK1 control of cell death. Cell Death Differ 21, 1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi M, Segawa K, and Nagata S (2012). Synergistic effect of Tim4 and MFG-E8 null mutations on the development of autoimmunity. International immunology 24, 551–559. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, and Nagata S (2007). Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. [DOI] [PubMed] [Google Scholar]
- Moffatt OD, Devitt A, Bell ED, Simmons DL, and Gregory CD (1999). Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol 162, 6800–6810. [PubMed] [Google Scholar]
- Moore KJ, and Tabas I (2011). Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, et al. (2018). Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, et al. (2009). PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 15, 1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, et al. (2010). The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 65, 1545–1553. [DOI] [PubMed] [Google Scholar]
- Nagata S (1999). Fas ligand-induced apoptosis. Annu Rev Genet 33, 29–55. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, and Arita H (1997). Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 272, 29411–29414. [DOI] [PubMed] [Google Scholar]
- Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, and Brown GC (2013). Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 110, E4098–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SL, Henson JE, and Henson PM (1982). Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med 156, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, Collins S, Lysiak JJ, Hoehn KL, and Ravichandran KS (2011). Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature 477, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, and Ravichandran KS (2007). BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434. [DOI] [PubMed] [Google Scholar]
- Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, and Kim IS (2008). Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ 15, 192–201. [DOI] [PubMed] [Google Scholar]
- Parks BW, Black LL, Zimmerman KA, Metz AE, Steele C, Murphy-Ullrich JE, and Kabarowski JH (2013). CD36, but not G2A, modulates efferocytosis, inflammation, and fibrosis following bleomycininduced lung injury. J Lipid Res 54, 1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, and Vandenabeele P (2015). Necroptosis and its role in inflammation. Nature 517, 311–320. [DOI] [PubMed] [Google Scholar]
- Pellettieri J, and Sanchez Alvarado A (2007). Cell turnover and adult tissue homeostasis: from humans to planarians. Annu Rev Genet 41, 83–105. [DOI] [PubMed] [Google Scholar]
- Penberthy KK, Lysiak JJ, and Ravichandran KS (2018). Rethinking Phagocytes: Clues from the Retina and Testes. Trends Cell Biol 28, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy KK, Rival C, Shankman LS, Raymond MH, Zhang J, Perry JSA, Lee CS, Han CZ, OnengutGumuscu S, Palczewski K, et al. (2017). Context-dependent compensation among phosphatidylserinerecognition receptors. Sci Rep 7, 14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, and Elkon KB (2011). Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest 121, 2221–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliani PL, Wang Y, Fontana E, Robinette ML, Yamanishi Y, Gilfillan S, and Colonna M (2015). TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest 125, 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, and Ravichandran KS (2014a). Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IK, Lucas CD, Rossi AG, and Ravichandran KS (2014b). Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14, 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, and Lemke G (2006). TAM receptor function in the retinal pigment epithelium. Molecular and cellular neurosciences 33, 96–108. [DOI] [PubMed] [Google Scholar]
- Proto JD, Doran AC, Gusarova G, Yurdagul A Jr., Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, et al. (2018). Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 49, 666–677 e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingchun H, Runyue H, LiGang J, Yongliang C, Song W, and Shujing Z (2008). Comparison of the expression profile of apoptosis-associated genes in rheumatoid arthritis and osteoarthritis. Rheumatol Int 28, 697–701. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Pendergraft WF 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, Luster AD, Hacohen N, El Khoury J, and Means TK (2013). The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol 14, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T, Menendez-Gutierrez MP, Lefterova MI, Alameda D, Nunez V, Lazar MA, Fischer T, and Ricote M (2011). Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J Immunol 186, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Carrera-Silva EA, Bosurgi L, and Ghosh S (2015). TAM receptor signaling in immune homeostasis. Annu Rev Immunol 33, 355–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, and Bayliss DA (2012). Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated Cterminal autoinhibitory region. J Biol Chem 287, 11303–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, Ding G, Noda M, Murata Y, Tanaka Y, et al. (2010). DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res 107, 1102–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schledzewski K, Geraud C, Arnold B, Wang S, Grone HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, et al. (2011). Deficiency of liver sinusoidal scavenger receptors stabilin-1 and −2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest 121, 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, and Matsushima GK (2001). Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211. [DOI] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, and Nagata S (2014). Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, and Maletic-Savatic M (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JC, Young C, Hicks D, Owens P, Williams A, Vaught DB, Morrison MM, Lim J, Williams M, Brantley-Sieders DM, et al. (2014). Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. J Clin Invest 124, 4737–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, and Inaba K (2000). The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 191, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, and Chilvers ER (2010). Neutrophil kinetics in health and disease. Trends Immunol 31, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, and Sprent J (1994). T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372, 100–103. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, and Nagata S (2013). Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406. [DOI] [PubMed] [Google Scholar]
- Tabas I (2010). Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 10, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert M, et al. (2016). On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med 22, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Choi SC, Murakami Y, Allen J, Morse HC 3rd, Qi CF, Krzewski K, and Coligan JE (2014). p85alpha recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat Commun 5, 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, et al. (2008). CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112, 5026–5036. [DOI] [PubMed] [Google Scholar]
- Vasheghani F, Monemdjou R, Fahmi H, Zhang Y, Perez G, Blati M, St-Arnaud R, Pelletier JP, Beier F, Martel-Pelletier J, et al. (2013). Adult cartilage-specific peroxisome proliferator-activated receptor gamma knockout mice exhibit the spontaneous osteoarthritis phenotype. Am J Pathol 182, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Veillette A, and Chen J (2018). SIRPalpha-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol 39, 173–184. [DOI] [PubMed] [Google Scholar]
- Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, and Kubes P (2017a). Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subramanian M, Yurdagul A Jr., Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, Ryan TA, Nomura M, Maxfield FR, and Tabas I (2017b). Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell 171, 331–345 e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JL, Arandjelovic S, Brown G, S KM, M SS, Buckley MW, Chiu YH, Shu S, Kim JK, Chung J, et al. (2017). Hematopoietic pannexin 1 function is critical for neuropathic pain. Sci Rep 7, 42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Brosnan CF, Loudig O, Goldberg MF, Macian F, Arnett HA, Prieto AL, Tsiperson V, and Shafit-Zagardo B (2011). Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. Journal of neuroinflammation 8, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich R, Oberst A, Beere HM, and Green DR (2017). Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18, 127–136. [DOI] [PubMed] [Google Scholar]
- Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, et al. (2013). Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Valdez PA, Tan C, Yeh S, Hongo JA, and Ouyang W (2010). Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci U S A 107, 8712–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Maruyama T, Urade Y, and Nagata S (2014). Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. Elife 3, e02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M, Ishimoto Y, Arai H, Nagai K, Ito T, Nakano T, Salant DJ, Fukatsu A, Doi T, and Kita T (2002). Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest 110, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, and Fazio S (2010). Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol 30, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF, and Fazio S (2011). Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation 124, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zheng S, Qiu Y, Yang Y, Wang C, Yang P, Li Q, and Lei B (2014). Activation of liver X receptor alleviates ocular inflammation in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 55, 2795–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Byun S, Kwon E, Hwang SY, Chu K, Hiraki M, Jo SH, Weins A, Hakroush S, Cebulla A, et al. (2015). Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 349, 1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef LA, Rebbaa A, Pampou S, Weisberg SP, Stockwell BR, Hod EA, and Spitalnik SL (2018). Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood 131, 2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorska A, Traves PG, Lew ED, Dransfield I, and Lemke G (2014). Diversification of TAM receptor tyrosine kinase function. Nat Immunol 15, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Li C, Swanson AM, Villalba RM, Guo J, Zhang Z, Matheny S, Murakami T, Stephenson JR, Daniel S, et al. (2015). BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J Clin Invest 125, 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]