Abstract
Two new flavonoids, calquiquelignan M (1), calquiquelignan N (2), along with nine known compounds (3–11), were isolated from the nuts of Areca catechu (Palmae). The new structures, including absolute configurations, were established by a combination of spectroscopic data and electronic circular dichroism (ECD) calculation. The known compounds were identified by comparing their spectroscopic data with reported in the literature. The flavonoids compounds (1–8) were evaluated for their cytotoxicity activities against three human cancer cell lines. Compounds 1 and 2 exhibited a moderate cytotoxic activity against HepG2 cell lines with IC50 values of 49.8 and 53.6 μM, respectively.
Keywords: Areca catechu, flavonoids, absolute configuration, cytotoxicity
1. Introduction
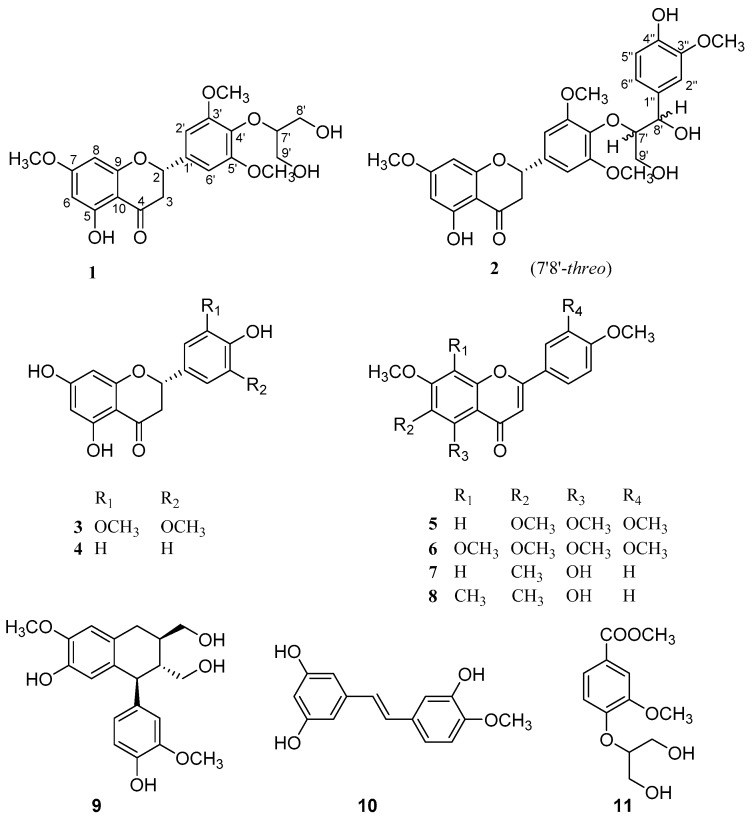
Areca nut is obtained from the fruit of the Areca catechu (Palmae), which is widely distributed in southeast Asia and southern China [1]. Areca nuts are regarded as a traditional Chinese medicine usually used for the treatment of indigestion, liver disorders, and also used as a vermifuge [2,3]. Previous pharmacological studies of areca nuts have demonstrated antibacterial, antioxidant, anti-inflammatory, antifungal, and anthelmintic activities [4,5]. In a search for novel and bioactive constituents from Chinese herbal medicines [6,7,8,9,10], our group had reported three new areca alkaloids from the nuts of A. catechu [11]. In our continuing investigation, two new flavonoids calquiquelignan M (1), calquiquelignan N (2), and nine known compounds naringenin (3) [12], dihydrotricin (4) [13], sinesetin (5) [14], nobiletin (6) [15], 8-demethyleucalyptin (7) [16], eucalyptin (8) [16], (+)-isolariciresinol (9) [17], rhapontigenin (10) [18], glyceryl-2-vanillic acid methyl ester (11) [19], were isolated from the same plant parts as shown in Figure 1. The new structures, including absolute configurations, were elucidated by spectroscopic data and electronic circular dichroism (ECD) calculation, and the known ones were identified by comparison with data in the literature. Herein, we report the isolation, structure elucidation, and cytotoxic activities of these compounds.
Figure 1.
Chemical structures of 1 to 11.
2. Results and Discussion
Structural Elucidation
Calquiquelignan M (1) was isolated as a yellow powder. The molecular formula of 1 was assigned as C21H24O9 based on its HRESIMS at m/z 443.1310 [M + Na]+ (calcd for C21H24O9Na, 443.1313), indicating 10 degrees of unsaturation. The IR absorption showed the characteristic absorptions for hydroxyl (3477 cm−1), carbonyl (1660 cm−1) groups, and aromatic ring (1535 and 1450 cm−1). The UV spectrum exhibited absorption maxima at 287 and 208 nm. The 1H-NMR spectrum indicated the presence of four aromatic protons [δH 6.71 (2H, s, H-2′/6′), 6.09 (1H, s, H-8), 6.08 (1H, s, H-6)]; two methines [δH 5.36 (1H, dd, J = 13.0, 3.0 Hz, H-2), 4.08 (1H, m, H-7′)]; three methylenes [δH 3.05 (1H, dd, J = 17.0, 13.0, H-3α), 2.82 (1H, dd, J = 17.0, 3.0, H-3β), 3.77 (2H, d, J = 3.4 Hz, H-8′), 3.85 (2H, d, J = 3.4 Hz, H-9′)], and three methoxyls [δH 3.91 (6H, s), 3.82 (3H, s)]. The 13C-NMR spectrum displayed twenty-one carbon signals, including a carbonyl, eight quaternary carbons, six methines, three methylenes, and three methoxyls. With the aid of 1H–1H COSY, HSQC, and HMBC experiments, all of the 1H- and 13C-NMR signals of 1 were assigned as shown in Table 1.
Table 1.
1H- and 13C-NMR data of 1 and 2 (δ in ppm, J in Hz).
| Position | 1 a | 2 b | ||||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | |||
| 2 | 5.36 dd (13.0, 3.0) | 79.4 | 5.45 dd (11.0, 6.0) | 80.7 | ||
| 3 | α β |
3.05 dd (17.0, 13.0) 2.82 dd (17.0, 3.0) |
43.8 | 3.18 dd (16.5, 11.0) 2.81 dd (16.5, 6.0) |
44.4 | |
| 4 | - | 195.5 | - | 197.8 | ||
| 5 | - | 164.4 | - | 165.3 | ||
| 6 | 6.08 s | 95.4 | 6.08 s | 95.9 | ||
| 7 | - | 168.2 | - | 169.6 | ||
| 8 | 6.09 s | 94.6 | 6.12 s | 95.1 | ||
| 9 | - | 162.6 | - | 164.4 | ||
| 10 | - | 103.2 | - | 104.1 | ||
| 1′ | - | 135.0 | - | 136.5 | ||
| 2′/6′ | 6.71 s | 103.4 | 6.87 s | 104.9 | ||
| 3′/5′ | - | 153.6 | - | 154.4 | ||
| 4′ | - | 135.7 | - | 137.4 | ||
| 7′ | 4.08 m | 85.0 | 4.14 m | 88.9 | ||
| 8′ | 3.85 d (3.4) | 62.7 | 5.02 d (6.7) | 74.5 | ||
| 9′ | α b |
3.77 d (3.4) | 62.7 | 3.79 m 3.37 m |
61.8 | |
| 1′′ | - | - | - | 133.5 | ||
| 2′′ | - | - | 7.03 s | 111.7 | ||
| 3′′ | - | - | - | 148.7 | ||
| 4′′ | - | - | - | 147.2 | ||
| 5′′ | - | - | 6.77 d (8.0) | 115.8 | ||
| 6′′ | - | 6.89 d (8.0) | 120.8 | |||
| 7-OCH3 | 3.82 s | 55.9 | 3.85 s | 56.4 | ||
| 3′-OCH3 | 3.91 s | 56.5 | 3.89 s | 56.8 | ||
| 5′-OCH3 | 3.91 s | 56.5 | 3.89 s | 56.8 | ||
| 3′′-OCH3 | - | - | 3.84 s | 56.3 | ||
a Measured in CDCl3. b Measured in CD3OD.
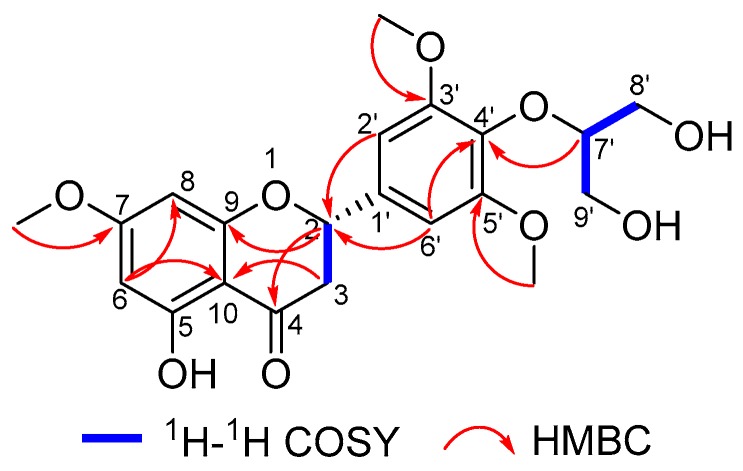
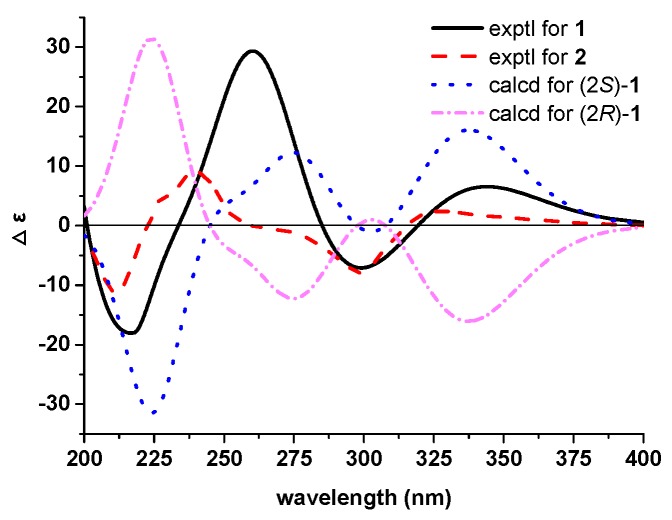
The above data of 1 resembled those of dihydrotricin (3) [12], except for the presence of one methoxy and one glycerin unit carbon signals. The 1H–1H COSY correlations between δH 3.85 (H2-8′) –4.08 (H-7′)–3.77 (H2-9′) confirmed the glycerin unit existence, and the HMBC correlations between H-7′ and C-4′ allowed the linkage between dihydroflavone fragment and glycerin fragment through the C-4′–O–C-7′ bond. The HMBC correlations between δH 3.82 (methoxy) and C-7 led to the increased methoxy substitution position at C-7 (Figure 2). Finally, the absolute configuration of 1 was deduced using the computational calculation method. The experimental ECD spectrum of 1 showed positive Cotton effects at 342 (Δε + 6.6), 260 (Δε + 29.3) nm and negative Cotton effects at 299 (Δε − 7.0), 216 (Δε − 18.2) nm, which were similar to those in the quantum chemical ECD calculation in Gaussian 09 software (Figure 3) [20]. Accordingly, the absolute configuration of 1 was determined as 2S.
Figure 2.
1H–1H COSY and key HMBC correlations of 1.
Figure 3.
Experimental electronic circular dichroism (ECD) spectra for 1 and 2; and calculated for 1.
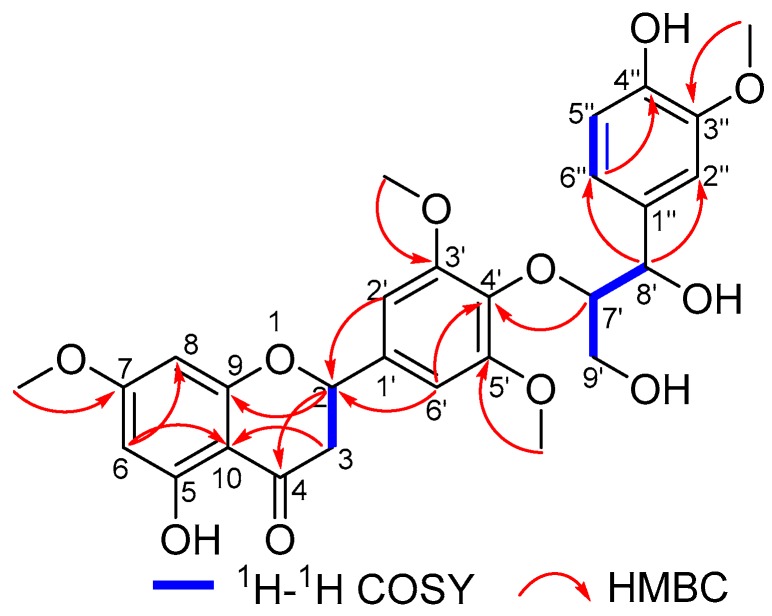
Calquiquelignan N (2) was obtained as a yellow powder, possessed a molecular formula C28H30O11 as established by its HR-ESI-MS at m/z 565.1685 [M + Na]+ (calcd for C28H30O11Na, 565.1680). The IR absorption revealed the presence of hydroxyl (3443 cm−1), carbonyl (1651 cm−1) groups, as well as an aromatic ring (1511 and 1429 cm−1). The UV spectrum showed the characteristic absorption maxima at 287, 230, and 208 nm. The 1H-NMR spectrum indicated the presence of seven aromatic protons [δH 7.03 (1H, s, H-2′′), 6.89 (1H, d, J = 8.0 Hz, H-6′′), 6.87 (2H, s, H-2′/6′), 6.77 (1H, d, J = 8.0 Hz, H-5′′), 6.12 (1H, s, H-8), 6.08 (1H, s, H-6)]; three methines [δH 5.45 (1H, dd, J = 11.0, 6.0 Hz, H-2), 5.02 (1H, d, J = 6.7, Hz, H-8′), 4.14 (1H, m, H-7′)]; two methylenes [δH 3.18 (1H, dd, J = 16.5, 11.0, H-3α), 2.81 (1H, dd, J = 16.5, 6.0, H-3β), 3.79 (1H, m, H-9′a), 3.37 (1H, m, H-9′b)], and four methoxyls [δH 3.89 (6H, s), 3.85 (3H, s), 3.84 (3H, s)]. The 13C-NMR spectrum displayed 28 carbon signals, including a chelated phenolic ketone carbon, eighteen olefinic carbons, three methines, two methylenes, and four methoxyls (Table 1). The above-NMR of 2 resembled those of 1, except for the absence of a methylene, and the present of a 1′′,3′′,4′′-trisubstitution phenyl ring and a methine at (δC 74.5). The signal at δC 74.5 was assigned to C-8′ indicated the 1′′,3′′,4′′-trisubstitution phenyl ring substituted there, which was supported by the HMBC correlations between H-8′ and C-2′′/C-6′′. Furthermore, the HMBC correlations between H-7′ and C-4′ confirmed the C-4′–O–C-7′ bond existence, and correlations between methoxy (δH 3.84) and C-3′′, led to the methoxy substitution position at C-3′′ (Figure 4). Thus, the planar structure of 2 was determined, as shown in Figure 4.
Figure 4.
1H–1H COSY and key HMBC correlations of 2.
Generally, adjacent protons of the erythro type have been reported to have smaller coupling constants (2.8–5.6 Hz) than those of the threo type (6.0–8.6 Hz) in different d-solvents [21,22,23]. Likewise, compound 2 was identified as a threo-configuration due to the 6.7 Hz coupling constants between H-7′ and H-8′ in CD3OD. Furthermore, the agreement of the ECD spectrum of 2 with those of 1 allowed the absolute configuration of 2 to be determined as 2S (Figure 3).
The pharmacological study of flavonoids and their derivatives showed that they possess cytotoxic activity [24]. Thus, the flavonoid compounds (1–8) were tested for their cytotoxic activity against three human cancer cells MCF-7, HepG2, and A-549 using MTT assay. Compounds 1 and 2 exhibited a moderate cytotoxic activity against HepG2 cell lines with IC50 values of 49.8 and 53.6 μM, respectively (Table 2).
Table 2.
Cytotoxicity of compounds 1–8 (IC50, μM).
| Compound | MCF-7 | A-549 | HepG2 |
|---|---|---|---|
| 1 | >100 | >100 | 49.8 |
| 2 | >100 | >100 | 53.6 |
| 3 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | >100 | >100 | >100 |
| 6 | >100 | >100 | 89.6 |
| 7 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 |
| cisplatin | 19.8 | 15.3 | 17.6 |
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotation was carried out using a Jasco P-1020 digital polarimeter (JASCO, Tokyo, Japan). UV spectra were obtained on a Jasco V-550 UV/VIS spectrometer (JASCO, Tokyo, Japan), and IR spectra on a Jasco FT/IR-480 plus infrared spectrometer (JASCO, Tokyo, Japan) with KBr discs. HR-ESI-MS data were detected on an Agilent 6210 ESI/TOF mass spectrometer (Agilent, Palo Alto, CA, USA). NMR spectra were recorded on Bruker AV-500 spectrometer (Bruker, Karlsruhe, Germany). Column chromatography (CC) was performed on silica gel (80~100 and 200~300 mesh; Qingdao Marine Chemical Inc., Qingdao, China), ODS (YMC, Kyoto, Japan) and Sephadex LH-20 (Pharmacia Biotech AB). Preparative HPLC was carried out on an Agilent 1260 system (G1311 pump and G1315D photodiode array detector) with a C18 reversed-phase column (20 × 250 mm, 5 μm, Shiseido Fine Chemicals Ltd., Osaka, Japan).
3.2. Plant Material
The areca nuts were collected in Sanya, Hainan province, P. R. China, in October 2014, and identified by Professor Guang-Xiong Zhou (College of Pharmacy, Jinan University). A voucher specimen (No. CP2014101403) was deposited at the herbarium of the College of Pharmacy, Jinan University, Guangzhou, P. R. China.
3.3. Extraction and Isolation
The air-dried and powdered areca nuts (30.0 kg) were extracted at room temperature with 95% EtOH to afford a residue (1.1 kg), which was then suspended in H2O and treated with 0.5% hydrochloric acid to adjust the pH to 2 to 3. After extraction with CHCl3, the CHCl3 extract (60.0 g) was subjected to silica gel column chromatography and eluted with CHCl3–CH3OH (100:0 → 0:100) to afford 8 fractions (Fr. A–H). Fr. D (2.8 g) was chromatographed on Sephadex LH-20 (CHCl3–CH3OH, 1:1) and HPLC (CH3OH–H2O, 35:65) to afford 1 (7.2 mg, tR 14.3 min), 2 (6.8 mg, tR 28.7 min), 3 (10.4 mg, tR 37.6 min). Fr. E (5.9 g) was separated by Sephadex LH-20 (CHCl3–CH3OH, 1:1) and further HPLC (CH3OH–H2O, 50:50) to afford 9 (35.8 mg, tR 17.8 min) and 11 (26.3 mg, tR 29.8 min). Fr. F (2.3 g) was subjected to ODS CC (CH3OH–H2O, 1:9 → 7:3) and HPLC (CH3OH–H2O, 40:60) to yield 4 (9.2 mg, tR 40.4 min), 7 (6.7 mg, tR 25.8 min), 8 (8.3 g, tR 33.8 min), 10 (12.3 g, tR 19.6 min). Fr. G (1.9 g) was separated by Sephadex LH-20 (CHCl3–CH3OH, 1:1) and further HPLC (CH3OH–H2O, 40:60) to afford 5 (12.2 mg, tR 24.1 min) and 6 (10.5 mg, tR 35.8 min).
Calquiquelignan M (1): yellow powder; − 8.9 (c 0.36, CH3OH); UV (CH3OH) λmax (log ε): 208 (4.14), 287 (3.88) nm; CD (CH3OH, Δε) λmax: 342 (+6.6), 299 (−7.0), 260 (+29.3), 216 (−18.2) nm; IR (KBr) νmax 3477, 1660, 1621, 1535, 1450, 1304, 1158, 1114 cm−1; 1H- and 13C-NMR spectral data, see Table 1; HR-ESI-MS: m/z [M + Na]+, calcd for C21H24O9Na: 443.1313, found: 443.1310.
Calquiquelignan N (2): yellow powder; − 13.5 (c 0.47, CH3OH); UV (CH3OH) λmax (log ε): 208 (4.45), 230 (4.02), 287 (4.05) nm; CD (CH3OH, Δε) λmax: 324 (+2.5), 299 (−7.9), 250 (+3.4), 211 (−11.3) nm; IR (KBr) νmax 3443, 1651, 1511, 1429, 1362, 1032, 826 cm−1; 1H- and 13C-NMR spectral data, see Table 1; HR-ESI-MS: m/z [M + Na]+ calcd for C28H30O11Na: 565.1680, found: 565.1685.
1H- and 13C-NMR spectra of these compounds are available in the Supplementary Materials.
3.4. Computational Calculation
Conformational searches were performed in the Sybyl 8.1 software by using the MMFF94S molecular force field, which afforded 12 conformers for 1, with an energy cutoff of 10 kcal/mol. The ECD calculation for the optimized conformers was carried out using time-dependent DFT (TDDFT) methods at the B3LYP/6-31+G(d) level in the gas phase by using Gaussian 09 software. The overall ECD curves of 1 were weighted by Boltzmann distribution of each conformer (with a half-bandwidth of 0.3 eV). The calculated ECD spectra of 1 were subsequently compared with the experimental ones. The ECD curves were produced by SpecDis 1.6 software (University of Wuerzburg, Bavaria, Germany).
3.5. Cytotoxicity Assay
The MTT assay for the determination of the cytotoxicity was performed as described previously [25]. Briefly, cancer cells were plated into 96-well plates. After 48 h of preculture, the cells were treated with compounds at various concentrations for 72 h and then stained with MTT. Absorbance at 570 nm was measured on a microplate reader.
4. Conclusions
In summary, we isolated two new flavonoids, calquiquelignan M and N (1–2) and nine known compounds naringenin (3), dihydrotricin (4), sinesetin (5), nobiletin (6), 8-demethyleucalyptin (7), eucalyptin (8), (+)-isolariciresinol (9), rhapontigenin (10), glyceryl-2-vanillic acid methyl ester (11) from the nuts of A. catechu. The new compounds were elucidated by spectroscopic analyses, and computational calculation, and the known ones were identified by comparing their spectroscopic data with reported in the literature. Moreover, all of the flavonoids (1–8) were evaluated for their cytotoxicity activities against three human cancer cell lines. Compounds 1 and 2 showed moderate cytotoxicity against HepG2 cell lines with IC50 values of 49.8 and 53.6 μM, respectively.
Supplementary Materials
The following are available online, Computational data of 1. Cytotoxicity assay. Figures S1–S9: HRESIMS, UV, IR, 1D, and 2D-NMR spectra of compound 1. Figures S10–S17: HRESIMS, UV, IR, 1D, and 2D-NMR spectra of compound 2.
Author Contributions
M.Y., Y.A., and J.X. isolated and identified the structure of the compounds. N.Y. and D.Z carried out the cytotoxicity assay. J.Z. and X.Z. designed and supervised the study, and wrote and revised the manuscript. W.Y. revised the manuscript.
Funding
This research was funded by the National Key R&D Program of China (No. 2017YFC1703802), the National Natural Science Foundation of China (Nos. U1801287, 81630095, and 81803377), the Science and Technology Planning Project of Guangdong Province (No. 2016B030301004 and 2018B020207008), the Guangdong Medical Products Administration (No. 2018TDZ21), the Natural Science Foundation of Guangdong Province (NO. 2018A030310640).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–11 are available from the authors.
References
- 1.Srimany A., George C., Naik H.R., Pinto D.G., Chandrakumar N., Pradeep T. Development patterning and segregation of alkaloids in arece nut (seed of Areca catechu) revealed by magnetic resonance and mass spectrometry imaging. Phytochemistry. 2016;125:35–42. doi: 10.1016/j.phytochem.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Tsai C.C., Kao C.T., Hsu C.T., Lin C.C., Lin J.G. Evaluation of four prescriptions of traditional Chinese medicine: Syh-mo-yiin, guizhi-fuling-wan, shieh-qing-wan and syh-nih-sann on experimental acute liver damage in rats. J. Ethnopharmacol. 1997;55:213–222. doi: 10.1016/S0378-8741(96)01503-6. [DOI] [PubMed] [Google Scholar]
- 3.Lin Q.H., Jia Z., Xu X.F., Xu S.Y., Han T., Gao Y., Zhang Y., Zhang H., Liu H., Li J., et al. Sub-chronic toxicity study of areca semen aqueous extract in Wistar rats. J. Ethnopharmacol. 2018;215:176–183. doi: 10.1016/j.jep.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Pithayanukul P., Nithitanakool S., Bavovada R. Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules. 2009;14:4987–5000. doi: 10.3390/molecules14124987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjali S., Rao A.R. Modulatory influence of arecanut on antioxidant 2(3)-tert-butyl-4-hydroxy anisole-induced hepatic detoxification system and antioxidant defense mechanism in mice. Cancer Lett. 1995;91:107–114. doi: 10.1016/0304-3835(95)03727-e. [DOI] [PubMed] [Google Scholar]
- 6.Shao M., Wang Y., Jian Y.Q., Huang X.J., Zhang D.M., Tang Q.F., Jiang R.W., Sun X.G., Lv Z.P., Zhang X.Q., et al. Guadial A and psiguadials C and D, three unusual meroterpenoids from Psidium guajava. Org. Lett. 2012;14:5262–5265. doi: 10.1021/ol302423b. [DOI] [PubMed] [Google Scholar]
- 7.Tang B.Q., Wang W.J., Huang X.J., Li G.Q., Wang L., Jiang R.W., Yang T.T., Shi L., Zhang X.Q., Ye W.C. Iboga-type Alkaloids from Ervatamia hainanensis. J. Nat. Prod. 2014;77:1839–1846. doi: 10.1021/np500240b. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Ding Y., Huang X.J., Jiang R.W., Wang Y., Sun P.H., Fan R.Z., Zhang X.Q., Ye W.C. Melohemsines A-I, melodius-type alkaloids from Melodinus hemsleyanus. RSC Adv. 2016;6:92218–92224. doi: 10.1039/C6RA16762D. [DOI] [Google Scholar]
- 9.Liu Z.W., Zhang J., Li S.T., Liu M.Q., Huang X.J., Ao Y.L., Fan C.L., Zhang D.M., Zhang Q.W., Ye W.C., et al. Ervadivamines A and B, two unusual trimeric monoterpenoid indole alkaloids from Ervatamia divaricate. J. Org. Chem. 2018;83:10613–10618. doi: 10.1021/acs.joc.8b01371. [DOI] [PubMed] [Google Scholar]
- 10.Kuok C.F., Zhang J., Fan C.L., Zhang Q.W., Fan R.Z., Zhang D.M., Zhang X.Q., Ye W.C. Meloslines A and B, two novel indole alkaloids from Alstonia scholaris. Tetrahedron Lett. 2017;58:2740–2742. doi: 10.1016/j.tetlet.2017.05.094. [DOI] [Google Scholar]
- 11.Tang S.N., Zhang J., Liu D., Liu Z.W., Zhang X.Q., Ye W.C. Three new areca alkaloids from the nuts of Areca catechu. J. Asian Nat. Prod. Res. 2017;19:1155–1159. doi: 10.1080/10286020.2017.1307187. [DOI] [PubMed] [Google Scholar]
- 12.Smejkal K., Grycova L., Marek R., Lemiere F., Jankovska D., Forejtnikova H., Vanco J., Suchy V. C-Geranyl compounds from Paulownia tomentosa fruits. J. Nat. Prod. 2007;70:1244–1248. doi: 10.1021/np070063w. [DOI] [PubMed] [Google Scholar]
- 13.Jeon S.H., Chun W., Choi Y.J., Kwon Y.S. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharm. Res. 2008;31:978–982. doi: 10.1007/s12272-001-1255-9. [DOI] [PubMed] [Google Scholar]
- 14.Okuno Y., Miyazawa M. Microbial O-demethylation of sinesetin and antimutagenic activity of the metabolite. J. Chem. Technol. Biot. 2006;81:29–33. doi: 10.1002/jctb.1353. [DOI] [Google Scholar]
- 15.Han S., Kim H.M., Lee J.M., Mok S.Y., Lee S. Isolation and identification of polymethoxyflavones from the Hybrid citrus Hallabong. J. Agric. Food Chem. 2010;58:9488–9491. doi: 10.1021/jf102730b. [DOI] [PubMed] [Google Scholar]
- 16.Park S.Y., Lim J.Y., Jeong W., Hong S.S., Yang T.T., Hwang B.Y., Lee D. C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Aβ-induced toxicity. Planta Med. 2010;76:863–868. doi: 10.1055/s-0029-1240801. [DOI] [PubMed] [Google Scholar]
- 17.Ruan J.Y., Li Z., Yan J.Q., Huang P.J., Yu H.Y., Han L.F., Zhang Y., Wang T. Bioactive constituents from the aerial parts of Pluchea indica less. Molecules. 2018;23:2104. doi: 10.3390/molecules23092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D., Park S., Choi S., Kim S.H., Kang K.S. In vitro estrogenic and breast cancer inhibitory activities of chemical constituents isolated from Rheum undulatum L. Molecules. 2018;23:1215. doi: 10.3390/molecules23051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama T., Nakatsubo F., Higuchi T. Degradation of arylglycerol-b-aryl ethers, lignin substructure models, by Fusarium solani. Arch. Microbiol. 1981;130:198–203. doi: 10.1007/BF00459519. [DOI] [Google Scholar]
- 20.Li X.C., Ferreira D., Ding Y.Q. Determination of absolute configuration of natural products: Theoretical calculation of electronic circular dichroism as a tool. Curr. Org. Chem. 2010;14:1678–1697. doi: 10.2174/138527210792927717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouaziz M., Veitch N.C., Grayer R.J., Simmonds M.S.J., Damak M. Flavonolignans from Hyparrhenia hirta. Phytochemistry. 2002;60:515–520. doi: 10.1016/S0031-9422(02)00145-0. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima Y., Yun Y.S., Kunugi A. Six new flavonolignans from Sasa veitchii, (Carr.) Rehder. Tetrahedron. 2003;59:8011–8015. doi: 10.1016/j.tet.2003.08.026. [DOI] [Google Scholar]
- 23.Gan M.L., Zhang Y.L., Lin S., Liu M.Y., Song W.X., Zi J.C., Yang Y.C., Fan X.N., Shi J.G., Hu J.F., et al. Glycosides from the root of Iodes cirrhosa. J. Nat. Prod. 2008;71:647–654. doi: 10.1021/np7007329. [DOI] [PubMed] [Google Scholar]
- 24.Taleghani A., Tayarani-Najaran Z. Potent cytotoxic natural flavonoids: The limits of perspective. Curr. Pharm. Des. 2018;24:5555–5579. doi: 10.2174/1381612825666190222142537. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K., Kuwahara A., Yoshida H., Fujita S., Nishikiori T., Nakagawa T. NF00659A1, A2, A3, B1 and B2, novel antitumor antibiotics produced by Aspergillus sp. NF 00659. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1997;50:314–317. doi: 10.7164/antibiotics.50.314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.