Abstract
Objective:
To identify the best method for determining freedom from disease after gastrinoma resection and for predicting long-term disease-free status in patients with the Zollinger-Ellison syndrome.
Design:
Prospective study in consecutive patients.
Setting:
Referral-based clinical research center.
Patients:
Eighty-one consecutive patients with the Zollinger-Ellison syndrome who underwent surgical exploration for gastrinoma resection.
Intervention:
Patients were evaluated after gastrinoma resection, before discharge, 3 to 6 months after surgery, and yearly thereafter. Evaluation included secretin provocative testing and fasting serum gastrin determinations. Follow-up examinations after the initial postoperative evaluations included a clinical assessment, acid secretion studies, a calcium provocative test, and various imaging studies.
Measurements and Main Results:
Most patients (96%) had gastrinomas. Freedom from disease was defined by improved symptoms, reduced acid output and antisecretory drug requirements, and a normal gastrin level, normal imaging studies, and negative gastrin provocative studies. Fifty-two percent of patients (n = 42) were disease-free immediately after surgery, 44% at 3 to 6 months, 42% at 1 year, and 35% by 5 years (mean follow-up, 39 months). The secretin provocative test was the first test to become positive in 45% of patients with a recurrence, the serum gastrin determination was the first test to become positive in 36%, and both tests became positive at the same time in 18%. No recurrence was first detected by imaging studies or by calcium provocative testing. Fasting serum gastrin levels and secretin provocative test results at different postoperative times can be used to predict the probability of a patient remaining disease free at 3 years. Patients with a normal gastrin level and a normal secretin provocative test immediately after surgery had a 3-year disease-free probability of 75%, and normal results on both tests at 6 months, 1 year, and 2 years yielded respective probabilities of 88%, 95%, and 100%.
Conclusions:
Both the secretin provocative test and fasting serum gastrin determination are necessary for the early diagnosis of cases of recurrent disease after gastrinoma resection. The calcium provocative test and imaging studies do not detect any recurrences first. Fasting serum gastrin determinations and secretin provocative testing at different postoperative times can be used to predict the probability of a patient remaining disease free at 3 years.
Gastrinoma is the most common functional pancreatic endocrine tumor (1–3). Because of autonomous gastrin release by the gastrinoma, patients develop gastric acid hypersecretion as part of the Zollinger-Ellison syndrome (1, 4). In recent years, effective medical therapies have been developed to control gastric acid hypersecretion, which previously was the leading cause of death in patients with the Zollinger-Ellison syndrome (5–7). Increasingly, the malignant nature of the tumor is becoming the primary determinant of long-term survival (2, 8), and therefore patients with the Zollinger-Ellison syndrome are increasingly being considered for possible gastrinoma resection (1, 2, 9, 10). However, considerable disagreement has arisen about the possible benefit of resection to the patient (11). This difference in opinion has occurred primarily because of widely ranging disease-free rates (4% to 90%) in different series (1, 12–14). Further, even without surgery, many patients have an excellent long-term prognosis because of the slow growth of the gastrinoma (15, 16). Also, many patients experience complications from peptic ulcer disease (for example, perforation) before diagnosis of the syndrome; such complications increase the surgical risk. Thus, the possible long-term benefit of surgery in terms of disease-free survival must be studied to determine whether it outweighs the risk.
Disease-free rates have varied markedly among studies for several reasons, including differences in sample size, follow-up, selection criteria for surgery, and operative approach. For example, surgery in these studies has ranged from a detailed exploration with removal of only gastrinomas identified at surgery to blind proximal pancreaticoduodenectomy in cases in which no tumor was found but selective venous sampling showed gas-trin positivity in this area (1, 12, 14, 17–21). However, the primary reason for the variation in the disease-free rate in different series is the lack of agreement on what evaluations should be routinely done after operation to establish disease-free status. Many studies only deter-mined fasting serum gastrin levels (10, 17, 22–24); however, fasting hypergastrinemia can be caused by achlorhydria, which is frequently seen in patients receiving gastric acid antisecretory drugs, and thus hypergastrine-mia may not always represent a disease recurrence or a failure to render the patient disease free (25). Further, some patients with the Zollinger-Ellison syndrome have a normal fasting serum gastrin level but a positive secretin or calcium provocative test result (26); other patients have been described who only had a positive calcium provocative test or only abnormal imaging studies (27, 28).
To address this problem, we did a study to determine the best method to assess disease-free status after surgery and to predict long-term disease-free status during follow-up.
Methods
Our investigation included 81 consecutive patients who underwent surgical exploration for possible curative gastrinoma resection between December 1980 and December 1991 as part of the ongoing National Institutes of Health prospective study of patients with the Zollinger-EUison syndrome. Thus, many of these patients have been described previously (4, 18, 19, 28–39).
Patients were considered to have a confirmed diagnosis of the Zollinger-Ellison syndrome if they met at least two of the following criteria: an elevated fasting serum gastrin level; a basal acid output of more than 15 mEq/h if the patient had not undergone previous gastric surgery or of more than 5 mEq/h if the patient had undergone previous gastric surgery; an increase in the serum gastrin level of 200 pg/mL after the intravenous administration of secretin; and an increase in the serum gastrin level of 395 pg/mL after an intravenous calcium infusion. The secretin provocative test was done as described previously (28), with Secretin-Kabi (Ferring AB, Malmo, Sweden) given by intravenous bolus injection (2 U/kg body weight). The calcium provocative test was done as described previously (28), with calcium gluconate (10%) (54 mg/kg per hour [5 mg calcium/kg per hour]) given by continuous intravenous infusion for 3 hours. Serum gastrin levels were determined by Bioscience Laboratories (New York, New York). All samples were diluted as necessary to give values that fell on the midportion of the standard curve as described previously (28). Patients with elevated gastrin levels had repeated determinations.
All patients had upper gastrointestinal endoscopy, computed tomography (29), ultrasonography (30), and selective angiography (31) that included selective injections of the hepatic, gastroduodenal, splenic, and superior mesenteric arteries before operation. In addition, patients evaluated after 1987 also underwent magnetic resonance imaging (32).
Before surgery, basal and maximal acid outputs were measured as described previously (33, 34). Maximal acid output was measured after pentagastrin stimulation (6 μg/kg subcutaneously). Sufficient oral antisecretory medication was given to reduce gastric acid output to less than 10 mEq/h before the next dose of medication for patients without previous gastric surgery and to less than 5 mEq/h for those who had previous gastric surgery (4, 33). The minimal dose of cimetidine or ranitidine given by continuous infusion or of omeprazole given by intermittent intravenous bolus injection to reduce gastric acid secretion to 10 mEq/h or less was determined in all patients as described previously (35, 36, 39), and this dose was administered at surgery and during the postoperative period to control gastric acid secretion.
At surgery, an extensive exploration, including mobilization of the duodenum, was done as described previously (18, 19). In addition, patients who had surgery after 1986 also underwent transduodenal endoscopic transillumination (37) and duo-denotomy (19) to localize duodenal gastrinomas and intraoperative ultrasonography as described previously (19, 38).
Specific Protocol
After surgery, before discharge from the hospital, fasting serum gastrin concentrations were determined on at least 3 separate days and a secretin provocative test was done.
Each patient was discharged on the same dose of oral antisecretory drug that he or she was taking before operation, as described previously (18, 19). If tumor was resected, patients were reevaluated 3 to 6 months after surgery to determine disease-free status and to assess control of gastric acid hypersecretion. Thereafter, patients who had had tumor resection were reassessed yearly. Patients who did not undergo resection were evaluated yearly. Tumor recurrence after resection was assessed by noninvasive imaging studies (ultrasonography, computed tomography, and magnetic resonance imaging) functional studies, which included determination of fasting serum gastrin levels on 3 different days, and provocative tests with secretin and calcium. Gastric acid antisecretory drugs were withdrawn and gastric acid output was determined to ensure that fasting serum gastrin determinations and secretin provocative tests were done on days when patients were not achlor-hydric to avoid physiologic hypergastrinemia. As shown in Figure 1, elevation of the fasting serum gastrin level may be secondary to drug-induced achlorhydria. Therefore, to adequately assess the importance of either an elevated fasting serum gastrin level or a positive secretin or calcium provocative test, all gastric acid antisecretory agents had to be withdrawn before these studies.
Figure 1. Relation between the fasting serum gastrin concentration and basal acid output in a patient assessed for recurrence 6 months after resection of a gastrinoma.
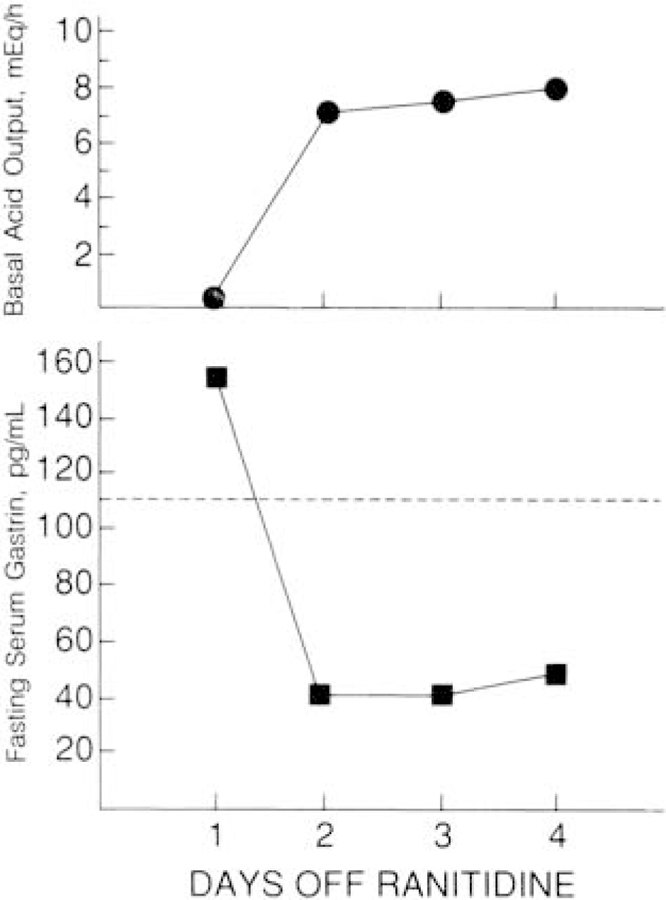
This patient was disease free immediately after surgery. At this 6-month assessment, fasting hypergastrinemia was present 1 day after discontinuing ranitidine therapy, 600 mg every 6 hours, suggesting that the disease had recurred; however, achlorhydria was also present at this time. Two days after discontinuing ranitidine therapy, basal acid output (BAO) increased and the fasting serum gastrin level decreased to within the normal range, showing that the patient was still disease free and had instead developed physiologic hypergastrinemia secondary to ranitidine-induced achlorhydria. The dotted line represents the upper limit of normal for the fasting serum gastrin level. See Methods section for definition of recurrence.
Disease-free status was defined by amelioration of all symptoms of the Zollinger-Ellison syndrome after operation; a significant reduction in gastric acid output at follow-up, no significant increase in basal acid output (that is, <50% increase) at follow-up, and no significant increase in gastric acid antisecretory drug requirements after operation as described previously (34); a normal fasting serum gastrin concentration (<110 pg/mL) (19, 34); negative results on provocative testing with secretin (an increase of less than 200 pg/mL in gastrin level) and calcium (an increase of less than 395 pg/mL in gastrin level) (27); and no evidence of tumor on imaging studies. Further, these criteria were used at all follow-up times so that the persistence of these findings further established disease-free status.
In patients who were considered disease-free at any postoperative visit, recurrence was defined by the development of an elevated fasting serum gastrin level (>110 pg/mL) when achlorhydria was not present, a positive result on a calcium or secretin provocative test, abnormal imaging studies, or a significant increase in basal acid output during follow-up, increased drug requirements, or clinical symptoms of Zollinger-Ellison syndrome that persisted during subsequent visits. Patients who had a recurrence (n = 11) were divided into two groups: One group included eight patients who had a recurrence within 36 months after surgery (early recurrence), and the other group included three patients who had a recurrence 36 to 72 months after surgery (late recurrence).
Statistics
The proportion of patients remaining disease free after surgery was plotted using the Kaplan-Meier method (40), and this curve was used to estimate the probability that a patient would remain disease free (41). The sensitivity, specificity, and predictive values (42) for the fasting serum gastrin determination and the secretin provocative test were calculated using the results at 3-year follow-up for the 46 patients who remained in the study to that point. To compare differences in recurrence rates, the McNemar test was used (40). We used Rothman’s method (41) to calculate 95% CIs for the probability of being disease free at 3 years and Brown and Hollander’s (42) method to calculate sensitivity, specificity, and predictive values.
Results
As shown in Table 1, the clinical and laboratory char-acteristics of our patients with the Zollinger-Ellison syndrome were similar to those of patients in other surgical series (1, 6, 12–14). Immediately after surgery, 52% of patients were disease free (Figure 2). At 6 months after surgery, 44% were disease free; at 1 year, 42%; at 3 years, 40%; at 5 years, 37%; and at 7 years, 25% (see Figure 2). The mean follow-up time was 39 months. All 42 patients who were initially disease free were followed for 6 months: Thirty of these 42 patients (71%) were followed for 12 months; 29 (29%), for 24 months; 27 (64%), for 36 months; 21 (50%), for 48 months; 12 (21%), for 60 months; and 8 (19%), for 72 months. During the follow-up period, 11 patients (25%) who were initially disease free had a relapse.
Table 1.
Preoperative Clinical and Laboratory Charac-teristics and Tumor Location at Surgery
| Characteristic | Value |
|---|---|
| Patients, n | 81 |
| Male sex, % | 69 |
| Mean age (range), y* | 47 ± 1 (23–69) |
| Previous gastric surgery, n(%) | 10 (12) |
| Mean disease interval before surgery (range), y | 4.7 ± 0.7 (0.1–22) |
| Mean BAO (range), mEq/h | 43.6 ± 2.8 (7–159) |
| Mean fasting serum gastrin level (range), pg/mL | 1373 ± 209 (268–7000) |
| Positive secretin test, n(%) | 70 (86) |
| Positive calcium infusion test, n(%) | 59 (73) |
| Positive imaging studies, n(%) | 58 (72) |
| Primary tumor location, n(%)† | |
| Duodenum | 27 (33) |
| Pancreatic head | 22 (27) |
| Pancreatic body | 4 (5) |
| Pancreatic tail | 5 (6) |
| No tumor found | 3 (4) |
| Ovary | 1 (1) |
| Lymph node only | 19 (24) |
| Liver metastases, n(%) | 5 (6) |
Means are expressed ± SE. BAO = basal acid output.
Tumor location was determined at surgery in all patients.
Figure 2. Proportion of patients remaining disease free at different follow-up times.
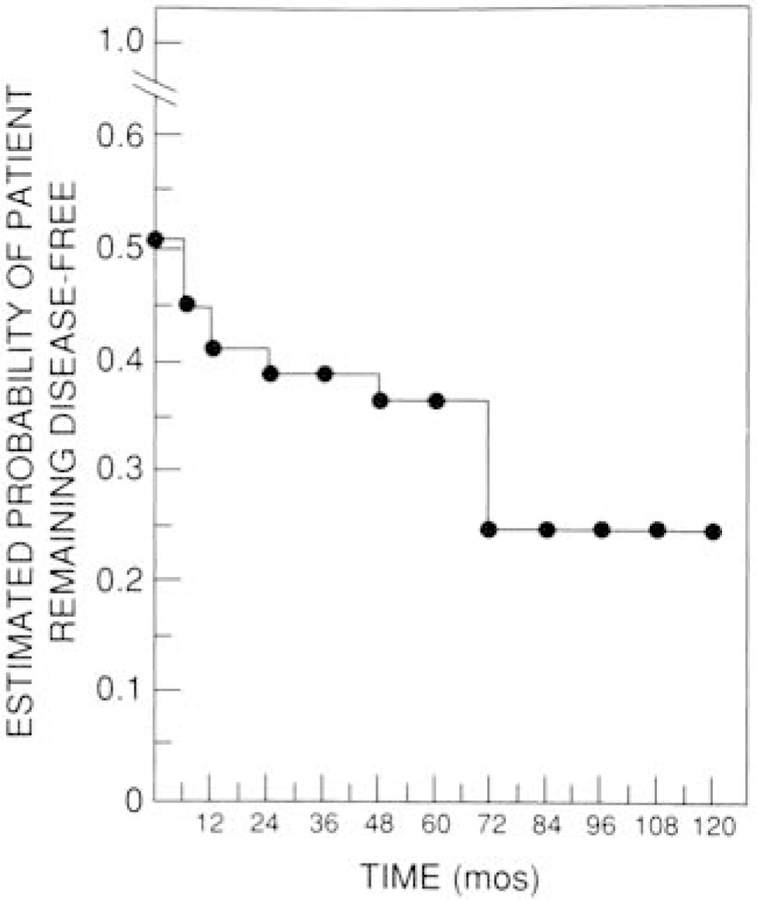
Forty-two patients were disease free immediately after surgery. The proportion of patients remain-ing disease free is shown as a Kaplan-Meier plot. Eleven patients had disease recurrence during a follow-up of up to 72 months.
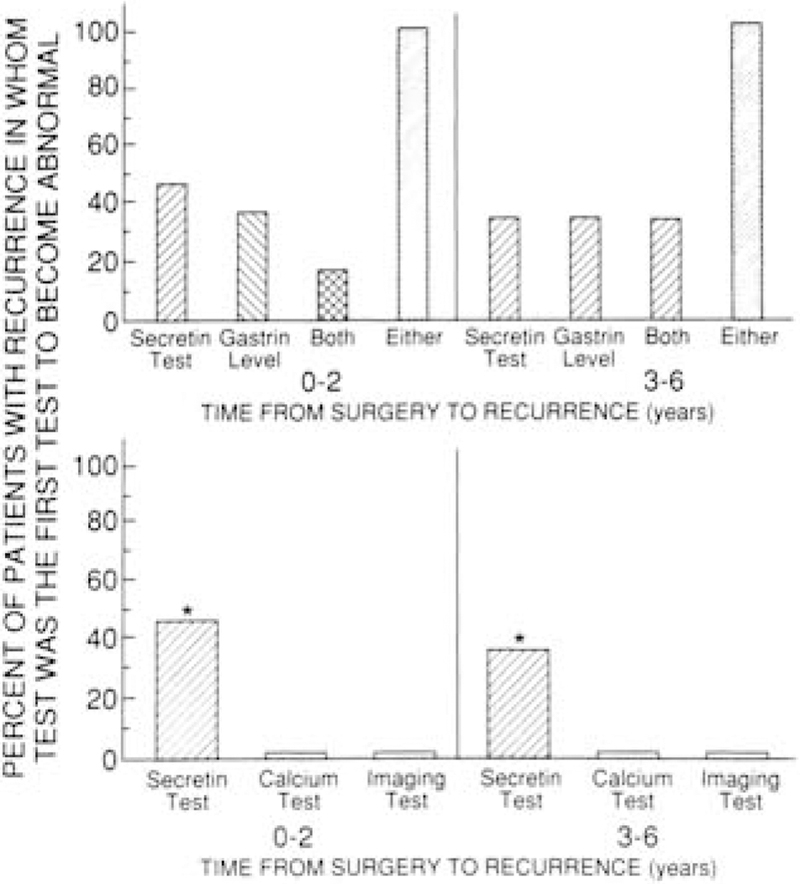
The secretin provocative test was the only test initially positive in 45% of patients who developed early recurrence (<3 years) after surgery, and an elevated fasting gastrin level was the only initial abnormality in 36% of patients (P > 0.05). Both of these tests were more sensitive (P < 0.05) than either the calcium provocative test or tumor imaging studies, both of which were positive initially in 0% of patients at the time of the recurrence (Figure 3). Both the secretin provocative test and the fasting serum gastrin determination were positive at the same time in 18% of patients with early recurrence (see Figure 3). No patient initially manifested recurrence by increased acid output, clinical symptoms, or increased antisecretory drug requirements at follow-up visits.
Figure 3. Comparison of the ability of the fasting serum gastrin level, secretin provocative test, calcium provocative test, and imaging studies to first detect recurrence of the Zollinger-EUison syndrome.
Twenty-seven patients who were disease free immediately after surgery were followed for 36 months, during which time 8 developed recurrence. Seventeen patients who were disease free immediately after surgery were followed from 36 months to 72 months, during which period 3 had a recurrence of disease.
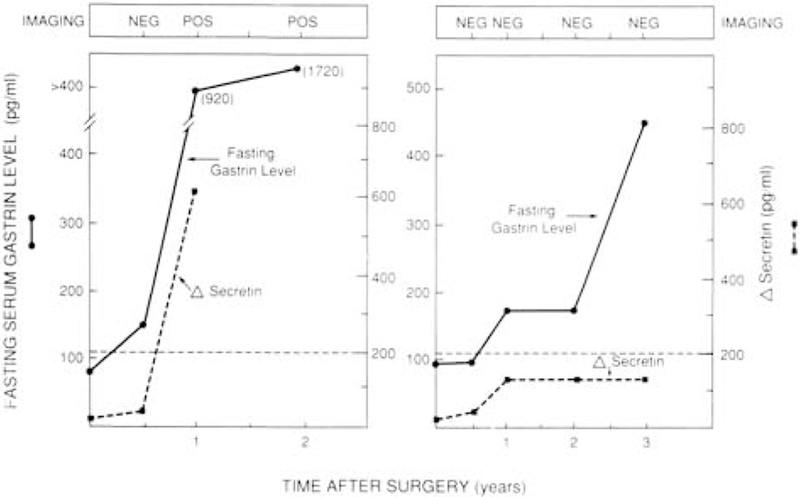
The pattern of the time course for developing an elevated fasting serum gastrin concentration, a positive secretin provocative test, or positive imaging studies differed among patients whose disease recurred. In general, the recurrence followed three different patterns (Figures 4 and 5). Forty-five percent of patients initially had a positive secretin provocative test only and later developed an elevated fasting serum gastrin level with or without abnormal tumor imaging results (see Figure 4, top panel). Eighteen percent of patients developed elevated fasting serum gastrin levels and a positive secretin test at the same time but had normal tumor imaging studies (see Figure 4, bottom panel). Thirty-six percent of patients developed an elevated fasting serum gastrin level as the initial indication of recurrence, either with (18%; see Figure 5, left panel) or without (18%; see Figure 5, right panel) subsequently developing a positive secretin provocative test or abnormal tumor imaging studies.
Figure 4. Changes over time in fasting serum gastrin level, secretin provocative test results, and imaging studies in two patients who developed recurrent disease. Top Panel.
The time course of changes in this patient is typical of the 45% of patients in whom recurrence was detected initially by a positive secretin provocative test alone and who only later developed elevated fasting serum gastrin levels in association with either positive (9%) (as shown in this patient) or negative (36%) imaging studies. Bottom Panel. The time course of changes in this patient is typical of the 18% of patients who developed both an abnormal fasting serum gastrin level and secretin provocative test as the first sign of recurrence and in whom imaging studies continued to be normal. Dotted lines represent the upper limits of normal for the fasting serum gastrin level and secretin provocative test.
Figure 5. Changes over time in fasting serum gastrin levels, secretin provocative test results, and imaging studies in two patients who developed recurrent disease.
The time course of changes in these two patients is typical of the 36% of patients in whom recurrence was first detected by an abnormal fasting serum gastrin level and who either subsequently had a positive secretin provocative test result (18%) (left panel) or continued to have a negative secretin provocative test result throughout the entire follow-up period (18%) (right panel). Positive imaging study results were found later in some patients (9%) (left panel).
The probability that a patient with a normal fasting serum gastrin level and a negative secretin provocative test at different postoperative times would remain free of disease at 3 years was assessed. Of the 42 patients presumed to be disease free immediately after surgery, 27 patients were followed for at least 3 years, with 8 experiencing recurrence. These 42 patients had a 75% likelihood of being disease free at 3 years (CI, 57% to 87%). If both tests remained negative at 6 months, 1 year, and 2 years after surgery, the likelihood of the patient being disease free at 3 years was 88% (CI, 70% to 96%), 95% (CI, 78% to 99%), and 100% (CI, 82% to 100%), respectively (Table 2).
Table 2.
Probability of Being Disease Free at 3 Years after Resection Based on a Normal Fasting Serum Gastrin Level and a Negative Secretin Test at Different Post-operative Times*
| Time after Surgery | Probability of Being Disease Free at 3 Years (95% CI) |
|---|---|
| 1 Week† | 75% (57% to 85%) |
| 6 Month | 88% (70% to 96%) |
| 12 Month | 95% (78% to 99%) |
| 24 Month | 100% (82% to 100%) |
Twenty-seven patients were followed for at least 3 years: During this time, 8 patients developed recurrence. The probability of remaining disease free was estimated for 42 patients using the Kaplan-Meier method (see Figure 2), and 95% CIs were derived using Rothman’s method (see reference 41).
Calculated after excluding three patients who were achlorhydric.
The sensitivity, specificity, and predictive values for fasting serum gastrin determinations, the secretin provocative test, or both as predictors of disease-free status at different postoperative times are shown in Table 3. For the individual tests, sensitivity and negative predictive value approach 100%, suggesting that either a normal fasting serum gastrin level or a negative secretin test can accurately establish disease-free status. However, the specificity and positive predictive value for individual tests are lower, suggesting that some patients who are not rendered disease free may appear to be disease free if only one test is used to define freedom from disease. By using a combination of tests, specificity can be increased to more than 80% and the positive predictive value to more than 75% (see Table 3).
Table 3.
Sensitivity, Specificity, and Predictive Values for the Fasting Serum Gastrin Determination and the Secretin Provocative Test as Predictors of Disease-free Status after Gastrinoma Resection*
| Time after Surgery |
Sensitivity (95% CI) |
Specificity (95% CI) |
Positive Predictive Value (95% CI) |
Negative Predictive Value (95% CI) |
|---|---|---|---|---|
| Fasting serum gastrin | ||||
| 1 week† | 100 (79 to 100) | 78 (58 to 91) | 73 (50 to 89) | 100 (84 to 100) |
| 6 months | 100 (82 to 100) | 85 (66 to 96) | 83 (61 to 95) | 100 (85 to 95) |
| 12 months | 100 (82 to 100) | 89 (71 to 98) | 86 (65 to 97) | 100 (86 to 100) |
| 24 months | 100 (82 to 100) | 96 (81 to 100) | 95 (75 to 100) | 100 (87 to 100) |
| Secretin test | ||||
| 1 week† | 100 (81 to 100) | 59 (39 to 78) | 62 (42 to 79) | 100 (79 to 100) |
| 6 months | 100 (82 to 100) | 67 (46 to 83) | 68 (48 to 84) | 100 (81 to 100) |
| 12 months | 100 (82 to 100) | 85 (66 to 96) | 83 (61 to 96) | 100 (85 to 100) |
| 24 months | 100 (82 to 100) | 89 (71 to 98) | 86 (65 to 97) | 100 (86 to 100) |
| Combination of both tests | ||||
| 1 week† | 100 (79 to 100) | 82 (62 to 94) | 76 (53 to 92) | 100 (85 to 100) |
| 6 months | 100 (82 to 100) | 93 (76 to 99) | 90 (70 to 99) | 100 (86 to 100) |
| 12 months | 100 (82 to 100) | 93 (76 to 99) | 90 (70 to 99) | 100 (86 to 100) |
| 24 months | 100 (82 to 100) | 100 (87 to 100) | 100 (82 to 100) | 100 (87 to 100) |
Data were analyzed for 46 patients who were followed for at least 3 years. Nineteen patients were disease free at 3 years. Sensitivity, specificity, and predictive values for being disease free were determined at the indicated postoperative times (see reference 42). For the purpose of this analysis, a test is called positive (for being disease free) if it is normal. The combination of both tests is called positive if both tests are normal, and negative if either or both tests are abnormal.
Calculated after excluding three patients who were achlorhydric.
Discussion
Our study was designed to identify the best method for determining freedom from disease after operation and for predicting long-term disease free status during follow-up in patients with the Zollinger-Ellison syndrome after gastrinoma resection. Several recent developments have enhanced the clinical importance of this question. As recently as the early 1980s (5, 11), it was recommended that patients with the Zollinger-Ellison syndrome not undergo routine surgical exploration because of the low possibility of cure. However, in the last few years, the approach has changed (1–3, 9, 10, 14, 19): Many authorities now recommend that all patients with the sporadic form of the Zollinger-Ellison syndrome without metastatic disease and without an accompanying serious medical condition should be considered for surgical exploration. Several factors are responsible for the change in management approach. Because of the development over the last 5 years of potent gastric acid antisecretory agents such as the H+/K+-ATPase (adenosinetriphosphatase) inhibitors omeprazole or lansoprazole (4, 8, 43) and potent H2 receptor antagonists such as ranitidine and famotidine (1, 44), gastric acid hypersecretion can now be controlled medically in almost every patient. Thus, complications of gastric acid hypersecretion are no longer the principal cause of death in patients with the Zollinger-Ellison syndrome; rather, the natural history of the gastrinoma itself (that is, its malignant potential) has become the most important determinant of long-term survival (1–3, 9). Early studies found that 60% to 90% of all gastrinomas are malignant (6, 8, 45), and recent studies have shown that once metastatic spread to the liver occurs, the prognosis is poorer than previously thought, suggesting that early surgical resection should be attempted (8, 46). Further, the ability to control gastric acid hypersecretion medically gives physicians time to do detailed preoperative imaging studies to attempt to localize the primary tumor (8, 29, 31). In addition, imaging studies done before surgery identify about 95% of patients with metastatic disease to the liver who would not benefit from attempted surgical resection (8, 29, 31). The chances for successful surgical resection have also been improved by the increased general awareness of the early symptoms of the Zollinger-Ellison syndrome and by its earlier diagnosis; because of this increased recognition, fewer than one third of patients have metastatic disease at the time of diagnosis (8, 24). Last, recent studies suggest that the 20% of patients with the Zollinger-Ellison syndrome as part of the multiple endocrine neoplasia type 1 syndrome (8) may also benefit from surgical exploration and be curable (9, 47), whereas early studies suggested that these patients were never cured by simple tumor excision and thus should not undergo routine surgical exploration (5).
Although there are compelling reasons why patients with the Zollinger-Ellison syndrome may increasingly benefit from surgical resection, several factors limit the ability to predict confidently whether surgery in fact does have obvious benefits. First, in view of the changing natural history of the Zollinger-Ellison syndrome, it is difficult to determine whether survival is improving because of curative surgery alone, the influence of medical therapy for gastric acid hypersecretion alone, or both factors. Currently, control of acid output is the major determinant of short-term (<5 years) outcome. With further follow-up, this issue will become clearer as more patients who are not cured develop metastatic disease. Second, in earlier studies, almost all patients underwent total gastrectomy with, in many cases, tumor resection (1, 6, 7, 15, 16, 20). Therefore, few patients who did not have surgery exist to clearly define the natural history of the disease. Now that potent gastric acid antisecretory agents are available, it will be possible to follow patients without any surgery, as is being increasingly done. Last, a further difficulty in assessing the possible benefit-to-risk ratio is that no general agreement has been reached about what percentage of patients will be rendered disease free in the long term or about how to define disease-free status (1, 12, 13, 15, 16, 19, 23, 48). Some groups suggest that provocative tests should be done periodically to assess postoperative disease-free status (10, 12, 14, 19); however, these tests involve added expense, and it is unclear whether only the secretin provocative test needs to be done or whether the calcium provocative test should be done as well. Finally, imaging studies may also be important because some patients with recurrent disease after gastrinoma resection have had normal fasting serum gastrin levels and negative provocative tests but abnormal imaging studies (27).
In our study, we found that it was necessary to do both fasting serum gastrin determinations and the secretin provocative test to initially detect all disease recurrence. In patients with recurrent disease, the secretin provocative test was the only initially positive test in 45%, whereas the fasting serum gastrin determination was the only initially positive study in 36%. Both the secretin provocative test and the fasting serum gastrin level were initially abnormal in 18% of patients with recurrent disease. Our results show that previous investigators who reported freedom from disease based only on normal fasting serum gastrin levels after resection (10, 17, 22–24) overestimated the actual disease-free rate, because the secretin provocative test must also be done to provide an accurate assessment. In our study, the addition of the calcium provocative test or imaging studies to the secretin provocative test and the fasting serum gastrin level determination did not increase the likelihood of detecting the initial recurrence. A recent study (27) suggests, because of the recurrence pattern in a single case, that routine postoperative imaging should be done in all patients with the Zollinger-Ellison syn-drome who are thought to be disease free (27). This approach would involve considerable expense because multiple imaging tests (computed tomography, magnetic resonance imaging, and ultrasonography) are required because of the low sensitivity of these studies individually for detecting small tumors (29, 32). Our results suggest that routine imaging studies are not necessary. All patients with recurrent disease had an abnormal functional study result (fasting serum gastrin level or secretin provocative test, or both) first. Imaging test results only became abnormal later and in only one third of patients.
In contrast to patients with insulinomas (49), several factors make it difficult to predict which patients with the Zollinger-Ellison syndrome will remain disease free after gastrinoma resection. Insulinomas are benign in 90% of cases, and in more than 90% of cases a single adenoma is found that is almost always within the pancreas; therefore, resection almost always results in disease-free status (49). In contrast, patients with the Zollinger-Ellison syndrome frequently have multiple tumors (6, 8), and in 60% to 90% of cases tumors are malignant so that unresected synchronous primary tumors or associated lymph node or hepatic metastases may remain after resection (45). Further, gastrinomas are often found only in lymph nodes, removal of which in some cases results in long-term disease-free status, leading to the suggestion that, in certain cases, gastrinomas may originate in lymph nodes (1, 3, 50). Therefore, it may remain unknown for a variable time period after resection whether a metastatic focus or additional primary tumor was missed at surgery. Our study provides for the first time guidelines that can be used to predict long-term disease-free status. Specifically, 75% of the patients with a normal fasting serum gastrin level and a negative secretin provocative test result immediately after surgery will remain disease free at 3 years. If both test results are normal at 6 months, 88% of patients will remain disease free at 3 years, and, if both tests are normal at 1 year, 95% of patients will remain disease free at 3 years. Both a determination of the fasting serum gastrin level and a secretin provocative test need to be done at each follow-up. The results of these two tests at these times after operation can be used to predict the likelihood of remaining free from disease and to plan the proper follow-up approach. Based on our results, we suggest the following protocol for evaluating patients with the Zollinger-Ellison syndrome after possible curative gastrinoma resection: After surgery and before discharge from the hospital, a secretin provocative test and two fasting serum gastrin determinations should be done. If both are normal, the patient has an 80% chance of being disease free at 1 year and a 75% chance at 3 years. Preoperative gastric acid antisecretory therapy should be maintained for 3 to 6 months after surgery to allow complete recovery after resection and then discontinued before performing a repeated secretin provocative test and fasting serum gastrin determinations. If both are normal, antisecretory drugs can be markedly reduced or withdrawn and these two screening tests can be done yearly. If either test becomes abnormal, imaging studies should be done and then repeated yearly. At present we are not attempting reoperation unless the tumor mass is clearly seen on repeated imaging studies. However, we do not yet have sufficient data to suggest that repeated surgery is justified in patients who relapse, regardless of the results of imaging studies. Patients who remain disease free should not have routine imaging studies or a calcium provocative test.
References
- 1.Jensen RT, Maton PN. In: Gustavsson S, Kumar D, Graham DY, eds. The Stomach London: Churchill Livingstone; 1991:341–74. [Google Scholar]
- 2.Andersen DK. Current diagnosis and management of Zollinger-Ellison syndrome. Ann Surg 1989;210:685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe MM, Jensen RT. Zollinger-Ellison syndrome. Current concepts in diagnosis and management. N Engl J Med 1987;317:1200–9. [DOI] [PubMed] [Google Scholar]
- 4.Maton PN, Gardner JD, Jensen RT. Recent advances in the man-agement of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterol Clin North Am 1989;18:847–63. [PubMed] [Google Scholar]
- 5.Jensen RT, Gardner JD, Raufman JP, Pandol SJ, Doppmann JL, Collen MJ, et al. Zollinger-Ellison syndrome. Current concepts and management. Ann Intern Med 1983;98:59–75. [DOI] [PubMed] [Google Scholar]
- 6.Ellison EH, Wilson SD. The Zollinger-Ellison syndrome: re-appraisal and evaluation of 260 registered cases. Ann Surg 1964;160:512–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox PS, Hofmann JW, Wilson SD, DeCosse JJ. Surgical management of the Zollinger-Ellison syndrome. Surg Clin North Am 1974;54: 395–407. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT, Doppman JL, Gardner JD. In: Brooks F, DiMagno E, Gardner JD, Go VL, Lebenthal E, Scheele G, eds. The Exocrine Pancreas: Biology, Pathobiology, and Diseases New York: Raven Press; 1986;727–44. [Google Scholar]
- 9.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with Zollinger-Ellison syndrome. World J Surg 1990;15:151–9. [DOI] [PubMed] [Google Scholar]
- 10.Bonfils S, Jensen RT, Malagelada J, Stadil F. Zollinger-Ellison syndrome management: a protocol for strategy. Gastroenterol Internatl 1989;2:9–15. [Google Scholar]
- 11.McCarthy DM. The place of surgery in the Zollinger-Ellison syndrome. N Engl J Med 1980;302; 1344–7. [DOI] [PubMed] [Google Scholar]
- 12.Howard TJ, Zinner MJ, Stabile BE, Passaro E Jr. Gastrinoma excision for cure. A prospective analysis. Ann Surg 1990;211:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadil F, Stage JG. The Zollinger-Ellison syndrome. Clin Endocrinol Metab 1979;9:433–46. [DOI] [PubMed] [Google Scholar]
- 14.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg 1989;209:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JC, Reeder DD, Villar HV, Fender HR. Natural history and experience with diagnosis and treatment of the Zollinger-Ellison syndrome. Surg Gynecol Obstet 1975;140:721–39. [PubMed] [Google Scholar]
- 16.Mignon M, Ruszniewski R, Haffar S, Riganud, D, Rene E, Bonfils S. Current approach to the management of tumoral process in patients with gastrinoma. World J Surg 1986;10:702–9. [DOI] [PubMed] [Google Scholar]
- 17.Imamura M, Takashi MP, Isobe Y, Hattori Y, Satomura K, Tobe T. Curative resection of multiple gastrinomas aided by selective arterial secretin injection and intraoperative secretin test. Ann Surg 1989;210:710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton JA, Doppman JL, Collen MJ, Harmon JW, Maton PN, Gardner JD, et al. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg 1986;204:468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome. Results of a 10-year study. Ann Surg 1992;215:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roche A, Raisonnier A, Gillon-Savouret MC. Pancreatic venous sampling and arteriography in localizing insulinomas and gastrinomas: procedure and results in 55 cases. Radiology 1982;145:621–7. [DOI] [PubMed] [Google Scholar]
- 21.Bardram L, Stadil F. The place of surgery in the treatment of APUDomas. In: Buchanan KD, ed. Gastrointestinal APUDomas London: Royal Society of Medicine Services; 1988. [Google Scholar]
- 22.Stabile BE, Morrow DJ, Passaro E Jr. The gastrinoma triangle: operative implications. Am J Surg 1984;147:25–32. [DOI] [PubMed] [Google Scholar]
- 23.Deveney CW, Deveney KE, Stark D, Moss A, Stein S, Way LW. Resection of gastrinomas. Ann Surg 1983;198:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellison EC, Carey LC, Sparks J, O’Dorisio TM, Men jian HS, Fromkes JJ, et al. Early surgical treatment of gastrinoma. Am J Med 1987;82 (Suppl 5B):17–24. [DOI] [PubMed] [Google Scholar]
- 25.Feldman M, Schiller LR, Walsh JH, Fordtran JS, Richardson CT. Positive intravenous secretin test in patients with achlorhydria-related hypergastrinemia. Gastroenterology 1987;93:59–62. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe MM, Jain DK, Edgerton JR. Zollinger-Ellison syndrome associated with persistently normal fasting serum gastrin concentrations. Ann Intern Med 1985;103:215–7. [DOI] [PubMed] [Google Scholar]
- 27.Diepstraten A, Driessen WM, Jansen JBM, Lamers CB. Fallability of gastrin level as an indicator of complete excision of gastrinoma. Br J Surg 1990;77:1403–5. [DOI] [PubMed] [Google Scholar]
- 28.Frucht H, Howard JM, Slaff JE, Wank SA, McCarthy DM, Maton PN, et al. Secretin and calcium provocative tests in patients with Zollinger-Ellison syndrome: a prospective study. Ann Intern Med 1989;111:713–22. [DOI] [PubMed] [Google Scholar]
- 29.Wank SA, Doppman JL, Miller DL, Collen MJ, Maton PN, Vinayek R, et al. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology 1987;92:905–12. [DOI] [PubMed] [Google Scholar]
- 30.London JF, Shawker TH, Doppman JL, Frucht HH, Vinayek R, Stark HA, et al. Zollinger-Ellison syndrome: prospective assessment of abdominal US in the localization of gastrinomas. Radiology 1991;178:763–7. [DOI] [PubMed] [Google Scholar]
- 31.Maton PN, Miller DL, Doppman JL, Collen MJ, Norton JA, Vinayek R, et al. Role of selective angiography in the management of Zollinger-Ellison syndrome. Gastroenterology 1987;92:913–9. [DOI] [PubMed] [Google Scholar]
- 32.Frucht H, Doppman JL, Norton JA, Miller DL, Dwyer AJ, Frank JA, et al. Gastrinomas: comparison of MR imaging with computed tomography, angiography and US. Radiology 1989;171:713–7. [DOI] [PubMed] [Google Scholar]
- 33.Raufman JP, Collins SM, Pandol S, Korman LY, Collen MJ, Cornelius MJ, et al. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology 1983;84:108–13. [PubMed] [Google Scholar]
- 34.Pisegna JR, Norton JA, Slimak GG, Metz DC, Maton PN, Gardner JD, et al. Effects of curative gastrinoma resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology 1992;102:767–78. [DOI] [PubMed] [Google Scholar]
- 35.Saeed ZA, Norton JA, Frank W, Young MD, Maton PN, Gardner JD, et al. Parenteral antisecretory drug therapy in patients with Zollinger-Ellison syndrome. Gastroenterology 1989;96:1393–402. [DOI] [PubMed] [Google Scholar]
- 36.Hahne WF, Vinayek R, Euler A, Jensen RT. Parenteral control of gastric acid output in patients with Zollinger-Ellison syndrome [abstract]. Gastroenterology 1991; 100: A79. [DOI] [PubMed] [Google Scholar]
- 37.Frucht H, Norton JA, London JF, Vinayek R, Doppman JL, Gardner JD, et al. Detection of duodenal gastrinomas by operative transillumination. A prospective study. Gastroenterology 1990;99:1622–7. [DOI] [PubMed] [Google Scholar]
- 38.Norton JA, Cromack DT, Shawker TH, Doppman JL, Comi R, Gorden P, et al. Intraoperative ultrasonographic localization of islet cell tumors: A prospective comparison to palpation. Ann Surg 1988;207:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinayek R, Frucht H, London JF, Miller LS, Stark HA, Norton JA, et al. Intravenous omeprazole in patients with Zollinger-Ellison syndrome undergoing surgery. Gastroenterology 1990;99:10–6. [DOI] [PubMed] [Google Scholar]
- 40.Dawson-Sanders B, Trap RG. Basic and Clinical Biostatics Appleton & Lange Press, 1990. [Google Scholar]
- 41.Rothman K. Estimation of confidence limits for the cumulative probability of survival in life table analysis. J Chronic Dis 1978;31: 557–60. [DOI] [PubMed] [Google Scholar]
- 42.Brown WB, Hollander M. Statistics: A Biomedical Introduction New York: John Wiley & Sons; 1977; 150–7. [Google Scholar]
- 43.Frucht H, Maton P, Jensen RT. The use of omeprazole in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1991;36:405–8. [DOI] [PubMed] [Google Scholar]
- 44.Vinayek R, Howard JM, Maton PN, Wank SA, Slaff JI, Gardner JD, et al. Famotidine in the therapy of gastric hypersecretory states. Am J Med 1986;81 (Suppl 4B):49–59. [DOI] [PubMed] [Google Scholar]
- 45.Creutzfeldt W, Arnold R, Creutzfeldt C, Track NS. Pathomorpho-logic, biochemical, and diagnostic aspects of gastrinomas (Zollinger-Ellison syndrome). Hum Pathol 1975;6:47–76. [DOI] [PubMed] [Google Scholar]
- 46.Norton JA, Sugarbaker PH, Doppman JL, Wesley RA, Maton PN, Gardner JD, et al. Aggressive resection of metastatic disease in selected patients with malignant gastrinomas. Ann Surg 1986;203: 352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, et al. Gastrinomas in the duodenum of patients with multiple endocrine neoplasia type I and the Zollinger-Ellison syndrome. N Engl J Med 1990;332:723–7. [DOI] [PubMed] [Google Scholar]
- 48.Zollinger RM, Ellison EC, Fabri PJ, Johnson J, Sparks J, Carey LC. Primary peptic ulceration of the jejunum associated with islet cell tumors. Twenty-five year appraisal. Ann Surg 1980; 192:422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen RT, Norton JA. Endocrine neoplasms of the pancreas. In: Yamada T, Alpers DH, Owyang C, Powell DW, Silverstein FE, eds. Textbook of Gastroenterology Philadelphia: JB Lippincott; 1991:1912–37. [Google Scholar]
- 50.Delcore R Jr, Cheung LY, Friesen SR. Outcome of lymph node involvement in patients with Zollinger-Ellison syndrome. Ann Surg 1988;206:291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]