Abstract
Purpose
To develop a volumetric imaging technique with 0.8-mm isotropic resolution and 10-s/volume rate to detect and analyze breast lesions in a bilateral, dynamic, contrast-enhanced MRI exam.
Methods
A local low-rank temporal reconstruction approach that also uses parallel imaging and spatial compressed sensing was designed to create rapid volumetric frame rates during a contrast-enhanced breast exam (vastly undersampled isotropic projection [VIPR] spatial compressed sensing with temporal local low-rank [STELLR]). The dynamic-enhanced data are subtracted in k-space from static mask data to increase sparsity for the local low-rank approach to maximize temporal resolution. A T1-weighted 3D radial trajectory (VIPR iterative decomposition with echo asymmetry and least squares estimation [IDEAL]) was modified to meet the data acquisition requirements of the STELLR approach. Additionally, the unsubtracted enhanced data are reconstructed using compressed sensing and IDEAL to provide high-resolution fat/water separation. The feasibility of the approach and the dual reconstruction methodology is demonstrated using a 16-channel breast coil and a 3T MR scanner in 6 patients.
Results
The STELLR temporal performance of subtracted data matched the expected temporal perfusion enhancement pattern in small and large vascular structures. Differential enhancement within heterogeneous lesions is demonstrated with corroboration from a basic reconstruction using a strict 10-second temporal footprint. Rapid acquisition, reliable fat suppression, and high spatiotemporal resolution are presented, despite significant data undersampling.
Conclusion
The STELLR reconstruction approach of 3D radial sampling with mask subtraction provides a high-performance imaging technique for characterizing enhancing structures within the breast. It is capable of maintaining temporal fidelity, while visualizing breast lesions with high detail over a large FOV to include both breasts.
Keywords: bilateral breast, compressed sensing, dynamic contrast-enhanced MRI, local low-rank, parallel imaging, radial sampling
1 |. INTRODUCTION
Dynamic contrast-enhanced MRI is a highly sensitive imaging technique for detecting breast cancer.1–3 However, breast DCE MRI is performed primarily for screening patients at high risk for breast cancer or in patients already diagnosed with breast cancer.4,5 As a result, relatively few women benefit from this powerful imaging modality. The information obtained from the DCE MRI exam is capable of identifying 14.7 additional cancers per 1000 exams beyond the 11.4 detected by mammography and ultrasound in patients at high risk for breast cancer.6 In contrast, large-scale efforts to supplement mammography with ultrasound screening has netted only an additional 4.2 cancers per 1000 exams.7 Clearly, there is a tremendous opportunity to improve breast cancer screening with MRI, but a significant cost differential contributes to directing screening methods toward ultrasound and other less sensitive technologies. Reducing the length and increasing the simplicity of MRI would provide a path to reduce the cost of breast MRI, thus improving patient access to this powerful imaging tool for the detection of breast cancer.
Current clinical strategies to evaluate lesion perfusion with MRI require long scanning sessions. These clinical strategies rely on 2 methods. The first approach attempts to qualitatively evaluate the change in MRI signal as a result of the wash-in and wash-out of a contrast agent over a long postcontrast acquisition phase (5–9 minutes or longer), and to use the shape of the signal intensity time curve to improve specificity for breast cancer.8 This approach has high sensitivity and thus has been widely used in clinical practice, as the technical demands are limited and it can be achieved in routine practice. The second primary approach is pharmacokinetic modeling of gadolinium contrast dynamics, which is technically demanding in the breast imaging environment and subject to multiple sources of bias and variance, even when using the exact same raw input data.9 Although some studies of pharmacokinetic modeling have been promising for the quantitative evaluation of tumor response, biology assessment and prediction of prognosis and survival, it has not been widely adopted in clinical practice, and is unlikely to add further contributions in the breast cancer screening setting, where a high negative rate is expected.
An abbreviated MR breast protocol that focuses on the initial enhancement phase holds promise for reducing the length of screening breast MR exams.10 For instance, a prospective observational reader study of 443 women maintained sensitivity to cancer detection by subtraction of a single pre-enhancement volume from a post-enhancement volume, showing the potential to reduce acquisition time from 17 to 3 minutes with less than 1 minute average reading time by a radiologist.11 Additionally, promising work has been presented showing the value of determining whether a lesion enhances faster than the rest of the fibroglandular tissue and the lesion’s early contrast uptake rate.12 Moreover, another study that visualized breast perfusion used 7-second frame rates to demonstrate the value of higher temporal resolution during initial enhancement phase by obtaining statistically significant differences between benign and malignant lesions.13,14 However, the high temporal performance in this study required a substantial reduction of spatial resolution. Both of these studies suggest that “loss” of the delayed DCE pattern through abbreviating the full protocol could potentially be balanced by detailed evaluation of the initial enhancement phase. However, the studies suggest 2 quite varying approaches: The former suggests a very low temporal resolution (90 seconds) with modest spatial resolution, whereas the latter suggests a very high temporal resolution (7 seconds) with very poor spatial resolution.
Breast DCE MRI would ideally provide high temporal resolution to depict lesion enhancement over time, while maintaining high spatial resolution to characterize anatomic detail.15 Numerous approaches have been proposed to provide the high temporal and spatial resolution, in combination with high spatial resolution over the large FOV required for breast imaging. Unfortunately, most available techniques are able to only partially improve temporal resolution at the cost of spatial resolution, which is unacceptable to radiologists given the clinical importance of high spatial resolution to characterize lesion morphology. Thus, most clinical MRI exams have prioritized spatial resolution over temporal resolution, as morphologic features have been shown to have greater specificity for breast cancer.16–18 Clinical breast DCE MR exams have temporal resolutions on the order of 60 to 180 seconds.19,20 Currently, most successful MRI efforts have increased temporal resolution using nonconventional acquisition trajectories that range from Cartesian pseudorandom k-space sampling to non-Cartesian trajectories such as spirals21 and radial stack of stars.22 Many proposed techniques use a spatially invariant, view-sharing methodology (i.e., TRICKS,23 TWIST,24 KWIC,25 and DISCO26 for reconstruction with a linear interpolation of adjacent frames to represent the temporal behavior of enhancing structures. However, aggressive view sharing leads to artifacts that misrepresent enhancement of small or heterogeneous features, and thus the achievable acceleration, whereas accurately modeling the temporal behavior is modest.27
More recently, parallel imaging in combination with nonlinear regularization methods have been used to provide higher temporal and spatial performance. The radial XDGRASP28 method has recently been extended to provide frame rates of less than 7 seconds during free breathing, but examples of enhancing breast lesions were provided at 40-second intervals or greater with spatial resolution at or over 1 mm.29 Often, compressed sensing (CS) studies in the breast have demonstrated acceleration by taking a fully sampled data set, artificially removing a fraction of the data, and showing that a similar imaging output was obtained.30–35 Unfortunately, these methods can be affected by significant partial voluming, thus eliminating the capability to visualize key features of differential enhancement in other planes.
We hypothesize that constrained, iterative reconstruction methodologies could provide a platform for simultaneously providing high spatial and temporal information of the early enhancement phase. Such a capability would allow breast researchers to extract all of the diagnostic and lesion characterization data possible out of the early enhancement phase when larger trials of abbreviated breast DCE-MRI screening are likely to be carried out. Such trials could also answer whether the proposed abbreviated protocol is able to maintain or improve specificity. Thus, the purpose of this work was to develop and demonstrate the feasibility of a bilateral DCE breast MRI technique that provides at least 2 strategic components. Primarily, a dynamic reconstruction with 10-second volumetric temporal resolution for evaluation of early tumor perfusion over the first 180 seconds, while maintaining submillimeter isotropic spatial resolution for lesion morphologic assessment. We exploit the sparsity provided by a 3D radial mask–subtracted set (vastly undersampled isotropic projection [VIPR]) through a spatial CS with temporal local low-rank assistances (STELLR) reconstruction. The local low-rank (LLR) component of the approach allows the algorithm to alter the temporal behavior in a spatially variant pattern, effectively allowing the temporal footprint to narrow in heterogeneous areas and widen in more static regions. Second, the method provides a static reconstruction with fat/water separation (VIPR CS+IDEAL [iterative decomposition with echo asymmetry and least squares estimation]) to show the contrast-enhanced breast architecture. Both reconstructions require a total of 6 minutes of acquisition time, significantly less than the standard clinical imaging time. To the best of our knowledge, this is the first implementation of a locally spatial variant temporal constraint in a bilateral DCE breast exam. The results provide a framework for detailed analysis of the information content that can be derived during an abbreviated breast exam.
2 |. METHODS
There are 3 key elements that describe the dynamic component of the abbreviated breast protocol SVIPR STELLR: (1) a high-performance 3D radial sampling acquisition pattern that meets the requirement for CS; (2) a LLR constraint to exploit temporal data redundancy; and (3) a method that concentrates only on the early phase enhancement. The versatility of our acquisition trajectory allows for a static reconstruction to provide multipeak 4-point fat/water separation within the same data set used to produce the dynamic reconstruction. Thus, the advancement in our proposed methodology relies on integrating an acquisition strategy and 2 reconstruction approaches whose capabilities complement each other.
2.1 |. Data acquisition
The modified 3D radial sampling VIPR36 trajectory is based on a previously published 3D radial dual half-echo trajectory (VIPR IDEAL) 37 in T2-weighted volumetric imaging. This trajectory is well suited for constrained reconstruction algorithms, because it combines variable density sampling and pseudorandom undersampling of the periphery of k-space in all directions, extending upon the 2D radial benefits obtained through stack-of-stars approaches. Thus, the aliasing artifact caused by undersampling is not constructive or structured, but rather incoherent.38 Moreover, radial trajectories start sampling right after excitation, which makes the technique rapid and efficient. Consequently, the sampling pattern is very appropriate for a dynamic reconstruction.
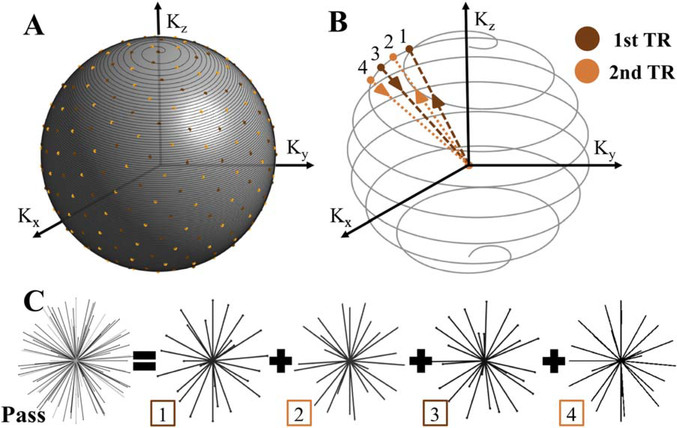
Data are collected with a T1-weighted RF spoiled gradient-echo sequence with a bipolar 3D radial trajectory.39 A spiral-based algorithm is used to distribute at equidistance all radial spokes for the 4 unique echoes through the surface of the sample sphere in k-space (Figure 1A). Four interleaved TEs distributed into 2 TRs, as shown in Figure 1B, are acquired with TEs suitable for fat/water decomposition with an IDEAL.40 A bit-reversal permutation of a sequential sampling of the exterior of the sphere was added to pseudorandomize the order of each group of 4 unique spokes for a dynamic, subsampled reconstruction. The set of all radial spokes is considered a composite sample set or pass (Figure 1C). The radial sampling pattern allows for a unique set of radial lines to be acquired in each echo (as opposed to the same lines acquired in Cartesian sampling), which, when combined with the efficient out and back trajectory and radial undersampling, leads to a 2 × improvement in sampling efficiency.41 In this work, we used 2 identical sample sets to demonstrate the technique. The first sample set or pass before the contrast injection is used as a mask, and the second pass starts as the contrast agent is administered.
FIGURE 1.
T1-weighted vastly undersampled isotropic projection (VIPR) iterative decomposition with echo asymmetry and least squares estimation (IDEAL) sampling pattern. A, Equidistant distribution of radial spokes in k-space. B, Order of sampling echoes within the 2 TRs. C, A full composite set or pass of radial spokes formed by 4 unique echo data sets
The VIPR IDEAL acquisition allows us to achieve comparable TEs for a theoretical optimal combination of echoes for a 3-point method (−π/6, π/2, 7π/6) 42 plus an additional echo to increase the effective number of signal averages. Our IDEAL implementation uses 6 resonant peaks to model fat and accounts for the additional phase accumulated by offresonant spins at each point in the k-space acquisition trajectory.43 Because of the short interval over which data are acquired (TEs: 0.18, 1.032, 1.624, 2.32 ms), the longest TE is expected to have a minimal reduction in contrast-enhanced signal intensity caused by the signal decay.44 Moreover, it is unlikely that enough concentration of gadolinium will accumulate in the breast over the first 3 minutes of postcontrast to generate shortening effects.45 Therefore, no correction was calculated in favor of maximizing SNR. Cramér-Rao bounds46 were used to identify optimal TEs that produced the highest estimated number of signals averaged for our 4-point method, while maintaining a relatively small TR of 4.3 ms.
2.2 |. Dynamic reconstruction
Low rank has been used successfully in various nonbreast MRI applications by assuming that all temporal behavior in the image volume can be represented by a limited number of temporal basis functions.47–49 The idea of breaking up the kernel into considerably smaller local blocks rather than 1 global block promotes higher data redundancy, which significantly reduces the rank of the vector space of temporal signals needed to represent the local temporal behavior.50–53 Algorithms enforcing LLR benefit from the additional sparsity added by k-space subtraction. A local temporal constraint is imposed by subdividing time-gated volumes into small blocks. Subsequently, all pixels within a block across all time-gated volumes are reorganized into a Casorati matrix with all of the pixels as columns and their temporal behavior as rows.54 Singular-value decomposition is performed to provide the principal components by which the image information can be represented or decomposed, followed by singular-value thresholding. The assumption is that there are a few large eigenvalues in the singular-value decomposition, allowing for data representation using fewer components with negligible data (i.e., low rank). In other words, singular-value decomposition provides the reconstruction algorithm the capability of learning a data-specific domain in which the information can be represented with fewer components.55 The location of this block is moved across until the entire volume is covered with 4 overlapping patterns. Consequently, this approach allows us to provide high temporal resolution without compromising spatial resolution.
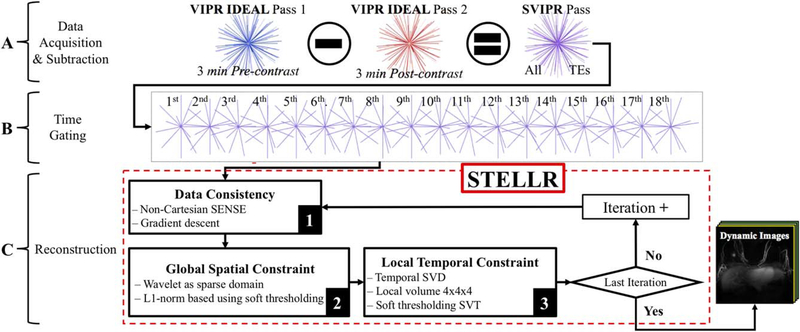
The dynamic reconstruction or SVIPR STELLR was designed based on a projection over convex sets–type algorithm to produce a dual constrained reconstruction.56 The reconstruction is separated into 3 steps: (1) data consistency, (2) spatial constraint, and (3) local temporal constraint (Figure 2C). Such steps are described by the 3 terms inside the summation of the following optimization problem:
| (1) |
where x is the combined image from all coils from the complete subtracted pass (SVIPR); Dt is a subsample operator at temporal volume t; F is Fourier encoding; S represents the coil sensitivities; and dt is the measured k-space data at temporal volume t. The total number of temporally gated volumes in the DCE acquisition for this study was 18 over 180 seconds. The first part of the equation refers to the data consistency portion of the reconstruction in which the benefits of parallel imaging are exploited through a SENSE57 signal model. The second term describes the global spatial constraint using the L1-norm |x|1 of spatially wavelet transformed (Ψ) images. Finally, the third term defines the local temporal constraint part, in which Cb refers to an operator that selects image block of size b (4 × 4 × 4 pixels for this study) throughout all T temporal volumes. The number of blocks by which the volume is subdivided is Nb. |x|* is the nuclear norm of a matrix A.58 This cost function was minimized iteratively using alternating data-consistent gradient descent, iterative soft thresholding, and singular-value decomposition corresponding to each of the 3 components. Variables λS and λLLR are regularization parameters to weigh the relative importance of spatial and temporal constraint, respectively. For the SVIPR STELLR reconstruction, λCS was empirically set to 0.01 and λLLR to 0.05.
FIGURE 2.
Dynamic reconstruction flow digram showing data acquisition of VIPR IDEAL trajectory, mask subtraction in k-space and echo combination to create a subtracted VIPR pass (SVIPR) (A); time gating as a preprocessing step (B); and STELLR reconstruction algorithm containing 3 steps: 1) data consistency, 2) global spatial thresholding, and 3) local temporal thresholding(C). SVD, singular value decomposition; SVT, singular value thresholding
A graphical representation of the algorithm is shown in Figure 2. The dynamic reconstruction quadruples the data sampling performance of VIPR IDEAL by adding all of the echoes in a single data set and increasing sparsity within the image by subtracting the precontrast pass from the postcontrast pass (Figure 2A). The new subtracted VIPR set (SVIPR) is rearranged into sets of 10-second temporal windows. As a result, 18 unique volumes are obtained with dynamic information (Figure 2B). These volumes are then fed into a STELLR algorithm as described by Figure 2C. First, these volumes alternate between radial and Cartesians grids every iteration as a prestep and poststep for a non-Cartesian SENSE algorithm. Gradient descent is used for optimization, by finding the minimum difference between the acquired data and the constrained solution in the SENSE model. Second, a L1-norm minimization of a wavelet transform, in the spatial domain, is used to regularize the data as part of CS. Third, a small local varying window for thresholding is applied—LLR. These 3 consecutive steps are performed for a total of 30 iterations.
2.3 |. Static reconstruction
The optimization problem and signal model for the static reconstruction or VIPR CS1IDEAL59 lacks the temporal constraint of the dynamic model. Therefore, our approach becomes a more traditional CS algorithm. The mathematical expression for this optimization problem can be expressed as
| (2) |
The first term or data consistency portion is similar to Equation 1, where x is the all-coil combined image from a complete pass (VIPR); De is a subsample operator pertaining to echo e; F is a Fourier encoding matrix corresponding to the k-space sampling trajectory; S represents the coil sensitivities; and de is the measured k-space data from echo e. Similarly, a wavelet transform is used as a sparsifying transform Ψ in the second term to enforce a global spatial constraint. Iterative soft thresholding is also used to solve for the L1-norm of the system defined as |x|1. The reconstruction regularization parameter λ was empirically set to threshold 10% of the coefficients per iteration.
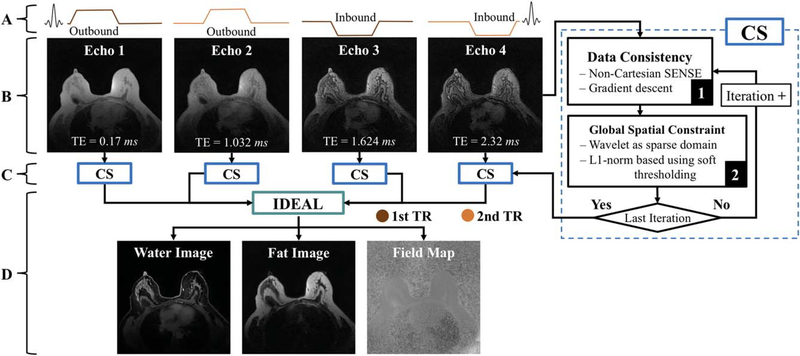
The static reconstruction uses all of the data from the second pass without temporal gating (Figure 3A,B). The reconstruction processes each unique k-space volume collected at each echo time separately using a CS algorithm (Figure 3C). The static reconstruction also takes advantage of parallel imaging during the gradient-descent SENSE step.60 The reconstructed radial echo images are fed into a modified IDEAL fat/water decomposition algorithm to provide the final set of water and fat image volumes (Figure 3D).
FIGURE 3.
Static reconstruction diagram demonstrating the collection of 4 half echoes by the alternating TRs (A); and 2 half echoes are acquired in 2 readouts offset by a 0.85-ms delay to produce 4 gradient echoes that sample 4 unique radial lines (B). Each echo is separately processed with a compressed sensing (CS) approach (C) followed by a modified IDEAL algorithm (D)
2.4 |. Evaluation
Six patient volunteers with a previous clinical breast MRI performed for a standard clinical indication and containing an area of breast enhancement that could be well depicted with time-resolved breast MRI were recruited to participate in this internal review board–approved, HIPAA compliant study. Imaging was performed on a 3T system (Discovery 750, GE Healthcare, Milwaukee, WI) with a 16-channel breast coil (Sentinelle, Invivo, Gainsville, FL) using the following 3 precontrast scans: (1) an investigational version of a commercially available quantitative chemical shift–encoded MR sequence with 6 echoes, referred to as Cartesian IDEAL (IDEAL IQ, GE Healthcare); (2) T1-weighted fast spoiled gradient echo (FSPGR) with 2 TEs and 2-point Dixon reconstruction (VIBRANT FLEX, GE Healthcare); and (3) the 3D radial VIPR IDEAL sequence described previously. Table 1 provides the image acquisition parameters for each of the 3 sequences. The static reconstruction or VIPR CS1IDEAL was compared with the 2 other acquired sequences, Cartesian IDEAL and T1-weighted FSPGR 2-point Dixon, to demonstrate its ability to decompose fat/water.
TABLE 1.
Scan parameters for volumetric fat/water breast imaging methods prior to contrast administration
| Pulse sequence | Scan time (min) | TR (ms) | TE | Resolution (mm) | Voxel volume (mm3) | Matrix size | FOV (cm) |
|---|---|---|---|---|---|---|---|
| Cartesian IDEAL | 4:40 | 7.7 | 6 | 1 × 1 × 3 | 3.0 | 320 × 320 × 66 | 32 |
| T1-weighted FSPGR 2-point Dixon | 1:10 | 4.2 | 2 | 1 × 1 × 1.4 | 1.4 | 320 × 320 × 138 | 32 |
| VIPR IDEAL | 3:00 | 4.3 | 4 | 0.8 × 0.8 × 0.8 | 0.5 | 384 × 384 × 384 | 32 |
Note: The VIPR IDEAL k-space trajectory was repeated as contrast was injected and used for the dynamic study. Although the VIPR IDEAL scan time is 2.5 times longer than the 2-point Dixon method, its voxel volume is 2.8 times smaller than the Dixon method.
For the DCE portion, a second 3-minute VIPR IDEAL trajectory pass was initiated concurrently with the start of the intravenous contrast injection. The proposed dynamic, masksubtracted reconstruction was compared with a masksubtracted CS reconstruction with a strict, 10-second temporal footprint. Comparison of the 2 reconstructions allowed assessment of the value of the STELLR approach in improving SNR against a method with strict temporal fidelity. Although the 10-second strict temporal fidelity is inherently low in SNR, it provides an easy method to assess the actual ordering of dynamic enhancement within the lesion and elsewhere in the breast. Additionally, our method was compared with a more conventional method using parallel imaging reconstruction (PILS) 61 and view sharing through a tornado filter that expands its temporal footprint from 30 seconds at the k-space center to 90 seconds at the k-space edge (Tornado+PILS).39 Furthermore, a static reconstruction of all combined postcontrast echoes with mask subtraction of the precontrast pass (SVIPR CS) was performed to produce a postcontrast volume similar to those provided in conventional DCE-MRI studies today. Quantitative image comparison between methods was performed using the structural similarity index.62
Image reconstruction was performed in a AMD Opteron central processing unit (Santa Clara, CA) computer node with 32 cores and 120 GB RAM. Reconstruction time for the dynamic SVIPR STELLR reconstruction data set (16 channels, 384 slices, 18 frames, 2333 spokes) was 22:48 hours. The static VIPR IDEAL+CS reconstruction time (16 channels, 384 slices, 4 echoes, 10 500 spokes) was 8:52 hours. The static SVIPR CS reconstruction time (16 channels, 384 slices, 42 000 spokes) was 2:32 hours.
3 |. RESULTS
3.1 |. Dynamic reconstruction
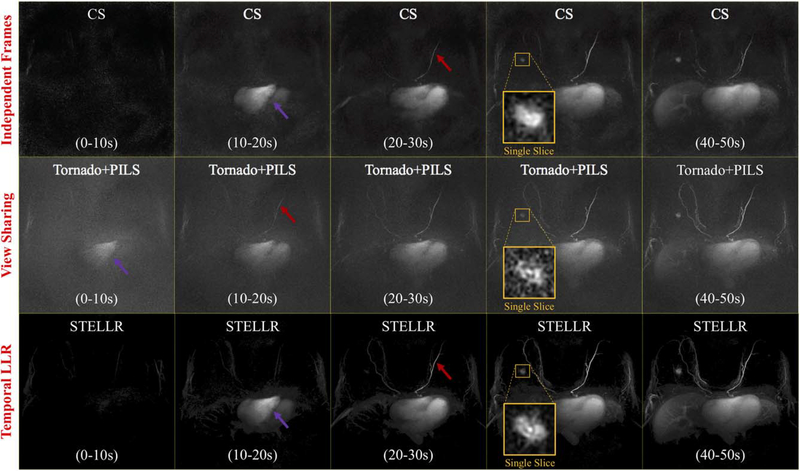
For the evaluation of the dynamic, mask-subtracted study of contrast passage (SVIPR STELLR), maximum intensity projections of each 10-second volumetric frame were generated for each method (Figure 4). The strict 10-second temporal footprint of CS provides a reference to visualize perfusion within the breast. STELLR’s ability to effectively deliver high temporal bandwidth is shown by its ability to capture the enhancement of both large (heart) and small (mammary arteries) structures in the proper time frame as compared with the CS reconstruction (Figure 4). Because of its wide temporal footprint, the Tornado+PILS reconstruction shows premature visualization of the mammary arteries and heart (Figure 4). Furthermore, STELLR provides higher contrast between the mammary arteries and background. Single-slice visualization of the lesion demonstrates the ability of the high spatial resolution (0.8 mm isotropic) provided by the dynamic reconstruction to visualize fine features as compared with the Tornado+PILS and CS reconstruction, despite 45 × data undersampling. STELLR was able to capture the lesion morphologic features, including spiculated margins and an irregular shape, while matching perfusion visualization with the 10-second strict footprint. These qualitative findings are corroborated by the structural similarity index values in Supporting Information Figures S1 and S2.
FIGURE 4.
An SVIPR data set reconstructed using 3 different methods: compressed sensing with strict 10-second temporal footprint (top), Tornado+PILS (parallel imaging reconstruction) reconstruction (middle), and the proposed dynamic reconstruction using spatial compressed sensing with temporal local low-rank (STELLR) (bottom). Targeted maximum intensity projections (MIPs) of each 10-second volumetric frame are shown for the first 5 frames. The heart is indicated in the first frame, where it is visibly enhanced by a purple arrow, while the mammary artery is indicated in the first frame using a red arrow. The tornado filter, with its 30-second base footprint, incorrectly shows the heart enhancing and left mammary artery enhancing in the first and second 10-second time frames, respectively, as a result of its larger temporal footprint. The STELLR method is able to provide consistent perfusion with CS and more lesion detail. Magnification of the lesion over a single slice (30 to 40 seconds) is shown for each method (yellow box) and demonstrates the improved spatial resolution with the STELLR approach
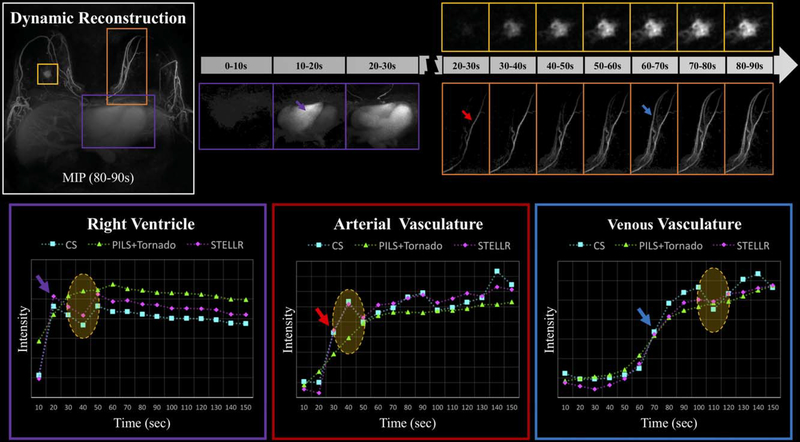
Visualization of the expected physiology is demonstrated by the proposed dynamic reconstruction of the SVIPR set through inspection of axial sequential frames generated at 10-second intervals (Figure 5 and Supporting Information Video S1). The maximum intensity projections demonstrate temporal fidelity throughout the volume in the 3 regions of interest (ROIs). Heart perfusion matches expected physiology (purple box, Figure 5), with the right ventricle enhancing after 10 seconds of contrast administration. Lesion temporal heterogeneity is captured while preserving morphologic features (yellow box, Figure 5). Expected temporal enhancement of the breast arterial-venous vasculature is shown (orange box, Figure 5) despite significant data undersampling and small diameter of the vessels. The temporal performance of SVIPR STELLR with a 10-second volumetric frame rate demonstrated proper visualization of expected physiology, with expected sequential filling of the right ventricle, left ventricle, mammary arteries, and mammary veins. Signal intensity measurements support SVIPR STELLR temporal fidelity when compared against CS.
FIGURE 5.
Top: Demonstration of the dynamic image reconstruction capabilities using the SVIPR STELLR method evaluated over 3 regions, demarcated on the left (purple heart chambers, orange mammary artery and vein, and yellow lesion). The middle MIP over the purple region of interest (ROI) shows the ability to rapidly capture chamber filling with a 10-second frame rate. The MIP over the orange ROI displays rapid transit of arterial (red arrow) blood in mammary artery to nearby venous vasculature (blue arrow); the yellow ROI shows temporal enhancement of complex lesion. Bottom: Signal intensity measurements from 3 ROIs: right ventricle (purple), artery (red), and vein (blue). In all 3 signal intensity measurements, STELLR (magenta) has a similar profile to compressed sensing (cyan), which is the only reconstruction with a strict 10-second temporal resolution. In contrast, Tornado+PILS has the smallest intensity range and low dynamic variation, whereas STELLR and CS show a richer change in signal (yellow ellipse)
Figure 6 demonstrates the capabilities of the proposed dynamic reconstruction. In Figure 6A, a single slice from a volumetric frame is offered as a point of reference. Two ROIs from within the lesion are chosen to measure signal intensities as a function of time. In Figure 6B, comparison of the enhancement curves of the anterior portion (ROI 1) and posterior portion (ROI 2) of the lesion at 10-second intervals demonstrates that the posterior portion enhances more rapidly and more intensely. In Figure 6C, the irregular shape and varied onset of contrast enhancement within the tumor can be appreciated using 3 orthogonal planes. Supporting Information Video S2 shows SVIPR STELLR capabilities over a benign lesion.
FIGURE 6.
The SVIPR STELLR reconstruction of a volunteer patient with a 1.6-cm-diameter malignant tumor. A, Axial single slice of the subject 50 seconds after contrast injection shows 2 areas of contrast enhancement (actual in small yellow box and magnified in larger yellow box). B, Signal intensity average for 2 ROIs within the tumor during the first 180 seconds of perfusion at 10-second intervals shows more rapid enhancement in the more posterior aspect of the tumor (ROI 2). C, Single-slice (0.8 mm) visualization of the entire tumor enhancement pattern through 4 sequential 10-second frames in 3 orthogonal axes shows complex spatial and temporal behavior
3.2 |. Static reconstruction
3.2.1 |. Static reconstruction of pass 1
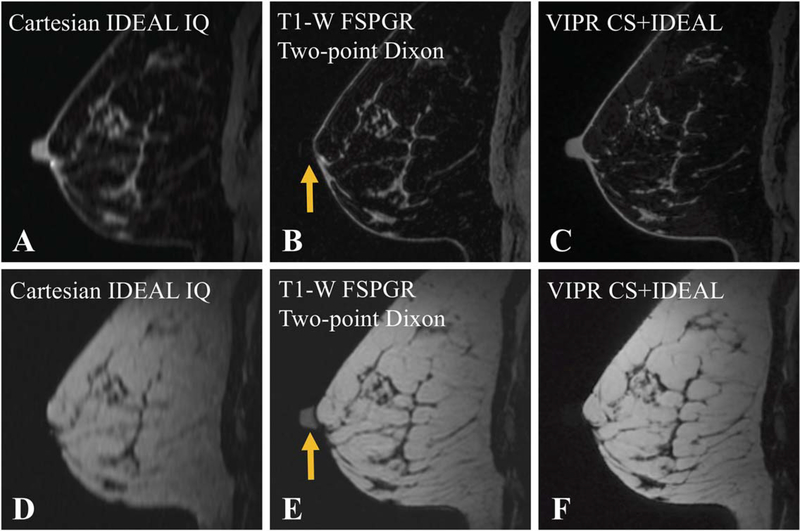
In the fat/water decomposition, all 3 sequences (Table 1) prescribed in the axial plane were reformatted into sagittal views. Cartesian IDEAL provides reliable fat/water separation but redundant acquisition of the same k-space lines at all 3 TEs, forcing the slice thickness to be 3 times the in-plane resolution. Consequently, image blurring is clearly visible in the sagittal reformat (Figure 7D). For the 2-point Dixon T1-weighted FSPGR method, the 2 TEs and reconstruction kernel size fail to assign the nipple to the water channel (Figure 7B), and instead placed it in the fat channel (Figure 7E). In comparison, VIPR CS+IDEAL correctly decomposed water in the nipple, demonstrating its ability to provide the needed inputs for a robust multipeak fat model while estimating and correcting for B0 inhomogeneities in a challenging location. Furthermore, the 0.8-mm isotropic resolution better depicts fibroglandular detail when reconstructed into orthogonal planes, as shown when comparing Figure 7D–F.
FIGURE 7.
Reformatted sagittal views of 3 fat/water decomposition methods sampled before contrast injection was applied with water volumes shown on top and fat on the bottom. Cartesian IDEAL (A and D) has limited resolution. The 2-point approach in T1-weighted fast spoiled gradient echo (FSPGR) (B and E) fails to keep the nipple in the water channel (yellow arrows). The VIPR CS+IDEAL method (C and F) maintains high resolution while robustly decouplingfat and water
3.2.2 |. Static reconstruction of pass 2
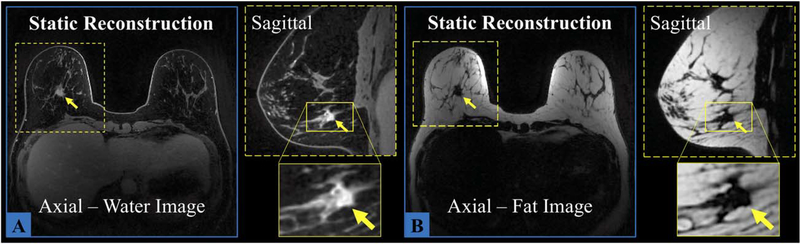
As previously described, the entire pass of the enhanced VIPR CS+IDEAL can be reconstructed without mask subtraction to depict the postcontrast pass (Figure 8). Water images depict high level of morphologic detail in both planes of a known breast cancer, including spiculated margin and irregular shape, as a result of the 0.8-mm isotropic resolution. Fat images provide a negative contrast reference of the architecture of the breast. The implemented trajectory phase correction diminished the blurring effect caused by fat offresonance at 3 T.
FIGURE 8.
Demonstration of static reconstruction in the prescribed axial plane and the reformatted sagittal plane using the postcontrast pass (0 to 180 seconds). In both water (A) and fat (B) images, the spiculated margins of a malignant lesion, identified by yellow arrows, are shown with the proposed method
3.2.3 |. Compressed sensing reconstruction of SVIPR pass
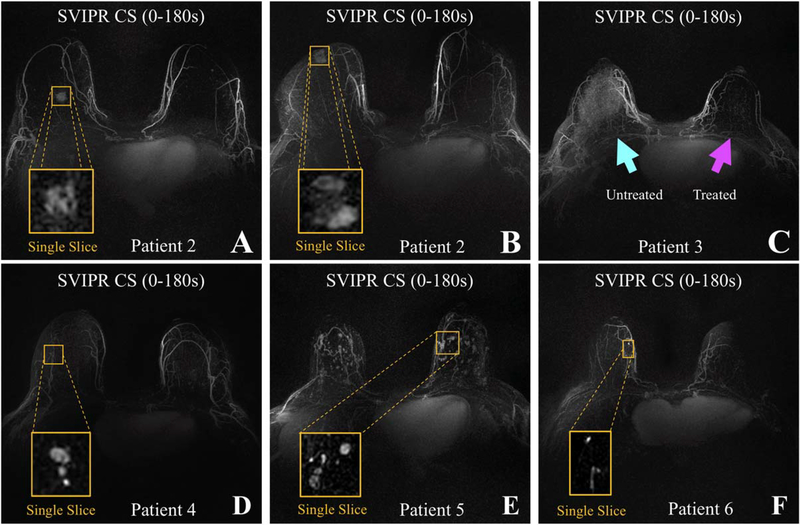
Maximum intensity projections of the entire postcontrast pass (180 seconds) with mask subtraction (Figure 9) provide a rapid overview of the regions of increased permeability, akin to the capability shown in Kuhl’s rapid screening technique.11 In the case of the first 2 subjects, the abnormalities (known malignancies) in the breasts can be easily detected, as shown by the yellow boxes in Figure 9A,B. Single-slice magnification shows morphologic features such as shape, size, and structure. Subject 3 (Figure 9C), whose left breast had undergone radiation treatment, does not show any parenchymal enhancement in the radiated breast, which is consistent with the expected treatment effect.
FIGURE 9.
The MIPs of the complete SVIPR pass using a CS reconstruction in 6 patient volunteers. Breast lesions are enlarged and displayed as single slice (yellow boxes). Top: Volunteers with known malignant cancer (A-C). Minimal background parenchymal enhancement (expected postradiation therapy, as in this patient) is indicated in the third patient (magenta arrow) compared with the untreated breast with moderate benign background parenchymal enhancement (C). Bottom: Three volunteers with known benign enhancing lesions (D-F)
4 |. DISCUSSION
Over the past 15 years, the clinical breast MR imaging community has become increasingly accustomed to examining the entire dynamic passage of an intravenous contrast injection with submillimeter resolution in at least 2 spatial dimensions. However, this is achieved in routine clinical practice at the expense of temporal resolution. We present the results of a novel, constrained, iterative reconstruction methodology to provide a platform for simultaneously providing high spatial and temporal information of the early enhancement phase for an abbreviated MRI screening exam. Our technique maintains the submillimeter spatial resolution that is important clinically for lesions’ morphologic assessment, while providing a platform to shorten the total exam time to improve patient comfort, throughput, and patient access.
Our results show preliminary experience with an ultrafast volumetric bilateral breast MRI exam with submillimeter isotropic resolution, surpassing the clinical standard while providing temporal resolutions 6-to-15-fold faster than typical clinical protocols. Although a judicious combination of CS and view sharing can produce high spatiotemporal resolution in various DCE MRI applications,63 the imaging performance of these methods is object dependent.64 The local spatially variant temporal constraint is able to further exploit data redundancy by learning and modeling temporal behavior rather than the assumption of consistency over a period of time.65,66 The method relies on the assumption that there is more data redundancy with a local subvolume compared with the entire volume. The innovation in our proposed method is derived from interleaving echoes from a 3D radial acquisition and then exploiting mutually compatible technologies: parallel imaging, compressed sensing that exploits image space sparsity (generated with mask subtraction), and a temporal constraint (LLR). In addition, our approach allows for volumetric, unsubtracted fat/water separated structural volumes using an iterative algorithm (VIPR CS+IDEAL) in a challenging B0 environment.
Undersampling artifacts and low SNR are 2 of the most prominent challenges when dealing with very small temporal windows in a reconstruction. Three-dimensional radial sampling assists in reducing these problems, but it is especially beneficial in breast imaging where large volumes need to be acquired and the temporal behavior of small or heterogeneous features with varying dynamic enhancement patterns needs to be resolved. The VIPR IDEAL trajectory with the use of other technologies such as high-count channel receiver coils and iterative reconstruction provide an effective alternative to overcome these 2 problems and accomplish high spatiotemporal resolution in a bilateral breast DCE exam.
The dynamic reconstruction approach learns spatially varying models of temporal behaviors through an LLR constraint that effectively permits the temporal footprint to widen in regions of low temporal bandwidth, while narrowing in regions where signal intensity is changing rapidly. Widening the temporal footprint in temporally sparse regions provides the SNR for high temporal performance in regions of dynamic signal changes. However, if the spatial and/or temporal bandwidth of the data set was sufficiently low, a very simple view sharing or temporal interpolation strategy would have worked just as well. Equivalent approaches have validated their specified temporal resolution through displaying a series of noncontinuous frames with large lesions that cover up to half the breast and ROI measurements through time.29 Because of the lack of a gold standard for high temporal resolution dynamic reconstructions, definitive validation is challenging. As one cannot determine the spatial and/or temporal bandwidth of lesions in each patient prior to examination, and often even after examination, assessing the clinical capabilities of the VIPR IDEAL acquisition and STELLR reconstruction methodologies is difficult. The use of a digital phantom would allow for quantitative measurements to find the limits of our acquisition and reconstruction technique and validate them.
Our validation image set, with an example shown previously in the top row of Figure 4, was restricted to a strict 10-second temporal footprint method (VIPR CS) that shared no data between volumetric frames. Although low in SNR, the order of enhancement can be discerned easily for many structures and thus provided a ground truth for enhancement patterns. The proposed method (SVIPR STELLR) created temporal frames whose enhancement order agreed with the strict 10-second frames, while producing better SNR. Tornado+PILS could not produce the same effect.
The static reconstruction in volumetric T1-weighted imaging demonstrated robust fat/water separation, which becomes an additional exam component at no additional scan time. The VIPR CS+IDEAL technique is able to provide comparable fat/water separation to Cartesian IDEAL with 6-times smaller voxel size and a similar imaging performance compared with the T1-weighted FSPGR 2-point Dixon technique. The T1-weighted FSPGR 2-point Dixon technique allowed for thinner slices in comparison with Cartesian IDEAL, but the 2-point method is still constrained to in-phase and outphase TEs. Both reconstructions described in this work exploit the 3D radial trajectory to achieve 0.8-mm isotropic spatial resolution and fulfill regularization requirements while taking full advantage of a 16-channel breast coil. In our experience, the 16-channel system was essential to creating the performance shown here relative to an 8-channel coil.67 We have successfully demonstrated the benefits of adding the sampling characteristics of VIPR in conjunction with IDEAL to obtain high spatial resolution and flexible echo selection at 3 T with a T1-weighted contrast.
The versatile characteristics of the VIPR IDEAL trajectory enable image reconstruction in various arrangements, increasing the amount of information that can be derived from a single scan. For instance, the mask-subtracted, SVIPR CS reconstruction of the postcontrast pass as a single volume can be used as a screening tool to establish the absence of breast cancer. In case of findings, the same data can be reformatted and reconstructed to provide the dynamic imaging volumes depicting enhancement at 10-second frame rates (SVIPR STELLR). Moreover, additional k-space passes can be acquired to study the late perfusion in the breast, if desired. The static reconstruction of the precontrast or postcontrast pass (VIPR CS+IDEAL) provides a volumetric, T1-weighted map of the breast at the cost of additional computing time.
The presented work creates a foundation for a robust ultrafast imaging technique with the possibility of expansion to multiple applications outside the realm of screening. In other words, this new technology could be expanded to study the heterogeneity of tumor response during therapy, assess tumor biology, and ultimately predict prognosis and survival. However, we first plan to evaluate it for abbreviated breast screening applications, where we believe it can most quickly benefit the greatest number of women. There are additional limitations to our results. The B0 field maps obtained from IDEAL have the potential to be incorporated into the dynamic model to further correct B0 field inhomogeneity, but were not used because of random-access memory limitations and the need to reduce computing time. Nevertheless, reconstruction times can be dramatically reduced by computing the nonuniform fast Fourier transform on a graphics processing unit.68 Finally, further validation to demonstrate the feasibility of our high-performance sequence for an abbreviated breast MRI is required.
5 |. CONCLUSION
We demonstrated a 3D radial breast MRI data acquisition with a dual (dynamic and static) reconstruction methodology to provide (1) 10-second volumetric frame rate with 0.8-mm isotropic spatial resolution; and (2) a static, postcontrast, fat/water separated breast architecture map over a FOV large enough to image both breasts simultaneously (32 cm). Together, this dual-reconstruction approach combines capabilities for high-performance acquisition, consistent fat suppression, and overcomes significant data undersampling. This combination allows assessment of lesion morphology and early-phase perfusion in a total scan time of only 6 minutes. It is the joint implementation of a high-performance data sampling and 2 constrained reconstructions that makes this capability possible. Upon further validation, this new methodology may translate to high-performance, rapid breast cancer screening with MRI.
Supplementary Material
FIGURE S1 Structural similarity index (SSIM) analysis of a single time interval (30 to 40 seconds) over only the breast tissue provides a measure of similarity among 4 methods and STELLR reconstruction. Columns 1 and 4 show MIPs and single-slice renderings of each reconstruction, respectively. Columns 2 and 5 contain SSIM maps of the structural coefficient and its decomposed SSIM index number. Columns 3 and 5 provide a complete SSIM measurement of all 3 components (luminance, contrast, and structure) and its combined maps. The highest SSIM similarity scores were obtained when comparing STELLR against CS, which is the reference method for temporal resolution. Both methods scored even higher when computing only the structural index
FIGURE S2 Top: Visual comparison of 4 methods over a single time interval (30 to 40 seconds). Bottom: SSIM index plot of each method using STELLR as reference over the first 15 time points. Consistent SSIM similarity scores can be seen over time at the MIP and single-slice level. Qualitative observations match quantitative measurements. Compressed sensing obtains the highest similarity measure, whereas PILS obtains the lowest. The PILS+Tornado performance fluctuates at the beginning, as a result of the temporal filter, but stabilizes as it moves forward. The SSIM indexes of MIP measurements obtained higher similarity overall than single slice, as MIPs are less affected by pseudo-noise caused by undersampling
VIDEO S1 Temporal performance of SVIPR STELLR at the 10-second volumetric frame rate. The MIPs enable visualization of breast perfusion with sequential filling of the right ventricle, arterial and venous system, and lesion enhancement. Single-slice visualization provides depicture of morphologic detail, including a spiculated margin and irregular shape of a known breast cancer with 0.8-mm isotropic spatial resolution
VIDEO S2 Visualization of SVIPR STELLR performance on a patient with multiple enhancing features. Overall breast perfusion can be appreciated through MIP images, whereas single-slice renderings capture spatial detail of a known benign lesion and allow observation of its temporal enhancing behavior
ACKNOWLEDGMENT
Research supported by NIH R25GM083252, K24 DK102595, T32CA009206, and F31CA217160, RSNA Research & Education Foundation, the Department of Radiology R & D Fund at the University of Wisconsin, Wisconsin Women’s Health Foundation, the Science and Medicine Graduate Research Scholars and GE Healthcare.
Funding information
Research supported by NIHR25 GM083252, K24DK102595, T32CA009206, and F31CA217160, RSNA Research & Education Foundation, the Department of Radiology R & D Fund at the University of Wisconsin, Wisconsin Women’s Health Foundation, the Science and Medicine Graduate Research Scholars, and GE Healthcare
Footnotes
CONFLICT OF INTEREST
Walter F. Block has an ownership interest in TherVoyant, a company aiming to advance MR image-guided surgery.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the supporting information tab for this article.
REFERENCES
- [1].DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:143–150. [DOI] [PubMed] [Google Scholar]
- [2].Kuhl C The current status of breast mr imaging. I, Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. [DOI] [PubMed] [Google Scholar]
- [3].Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244: 381–388. [DOI] [PubMed] [Google Scholar]
- [4].Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: Recommendations from the eusoma working group. Eur J Cancer. 2010;46:1296–1316. [DOI] [PubMed] [Google Scholar]
- [5].Lohrke J, Frenzel T, Endrikat J, et al. 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther. 2016;33:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening mri to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang LC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. MRI-detected suspicious breast lesions: predictive values of kinetic features measured by computer-aided evaluation. AJR Am J Roentgenol. 2009;193:826–831. [DOI] [PubMed] [Google Scholar]
- [9].Huang W, Li X, Chen Y, et al. Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl Oncol. 2014;7:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mango VL, Morris EA, David Dershaw D, et al. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84:65–70. [DOI] [PubMed] [Google Scholar]
- [11].Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–2310. [DOI] [PubMed] [Google Scholar]
- [12].Kuhl CK, Gold GE, Trzasko JD. High-throughput: The 5 minute MR scan (breast imaging). In Proceedings of the 24th Annual Meeting of ISMRM; 2016; Singapore. [Google Scholar]
- [13].Abe H, Mori N, Tsuchiya K, et al. , Kinetic analysis of the ultra early phase on breast MRI: comparison between benign and malignant lesions using ultrafast dynamic contrast enhanced MRI. In Proceedings of the Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA); 2015; Chicago IL. [Google Scholar]
- [14].Pineda F, Tsuchiya K, Abe H, et al. , Characterization of breast lesion kinetics with accelerated DCE-MRI using conventional sampling methods. In Proceedings of the Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA); 2015; Chicago IL. [Google Scholar]
- [15].Moon M, Cornfeld D, Weinreb J. Dynamic contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am. 2009;17: 351–362. [DOI] [PubMed] [Google Scholar]
- [16].Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology. 2005;236:789–800. [DOI] [PubMed] [Google Scholar]
- [17].Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced mri: a sensitivity and specificity study. Magn Reson Med. 2008;59:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuhl CK. Current status of breast MR imaging. II, Clinical applications. Radiology. 2007;244:672–691. [DOI] [PubMed] [Google Scholar]
- [19].Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS magnetic resonance imaging. ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- [20].Gutierrez RL, Strigel RM, Partridge SC, et al. Dynamic breast MRI: Does lower temporal resolution negatively affect clinical kinetic analysis? AJR Am J Roentgenol. 2012;199:703–708. [DOI] [PubMed] [Google Scholar]
- [21].Han M, Daniel BL, Hargreaves BA. Accelerated bilateral dynamic contrast-enhanced 3D spiral breast MRI using TSENSE. J Magn Reson Imaging. 2008;28:1425–1434. [DOI] [PubMed] [Google Scholar]
- [22].Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36:345–351. [DOI] [PubMed] [Google Scholar]
- [24].Ramsay E, Causer P, Hill K, Plewes D. Adaptive bilateral breast MRI using projection reconstruction time-resolved imaging of contrast kinetics. J Magn Reson Imaging. 2006;24:617–624. [DOI] [PubMed] [Google Scholar]
- [25].Dougherty L, Isaac G, Rosen MA, et al. High frame-rate simultaneous bilateral breast DCE-MRI. Magn Reson Med. 2007;57: 220–225. [DOI] [PubMed] [Google Scholar]
- [26].Saranathan M, Rettmann D, Hargreaves BA, Lipson J, Daniel BL. High spatio-temporal resoltuion breast dynamic contrast enhanced MRI at 3T. In Proceedings of the 20th Annual Meeting of ISMRM; 2012; Melbourne, Australia p. 456. [Google Scholar]
- [27].Henderson E, Rutt BK, Lee TY. Temporal sampling requirements for the tracer kinetics modeling of breast disease. Magn Reson Imaging. 1998;16:1057–1073. [DOI] [PubMed] [Google Scholar]
- [28].Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016;75:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med. 2017;78:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schnall MD, Ikeda DM. Lesion diagnosis working group report. J Magn Reson Imaging. 1999;10:982–990. [PubMed] [Google Scholar]
- [31].Ji J, Lang T. Dynamic MRI with compressed sensing imaging using temporal correlations. In Proceedings of the 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro; 2008; Paris p. 1613–1616. [Google Scholar]
- [32].Wang H, Miao Y, Zhou K, et al. Feasibility of high temporal resolution breast DCE-MRI using compressed sensing theory. Med Phys. 2010;37:4971–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen L, Schabel MC, DiBella EV. Reconstruction of dynamic contrast enhanced magnetic resonance imaging of the breast with temporal constraints. Magn Reson Imaging. 2010;28:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adluru G, Tasdizen T, Schabel MC, DiBella EV. Reconstruction of 3D dynamic contrast-enhanced magnetic resonance imaging using nonlocal means. J Magn Reson Imaging. 2010;32:1217–1227. [DOI] [PubMed] [Google Scholar]
- [35].Smith DS, Welch EB, Li X, et al. Quantitative effects of using compressed sensing in dynamic contrast enhanced MRI. Phys Med Biol. 2011;56:4933–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Block WF, Barger AV, Mistretta CA. Vastly undersampled isotropic projection imaging. In Proceedings of the 8th Annual Meeting of ISMRM; 2000; Denver, CO; 161. [Google Scholar]
- [37].Lu A, Brodsky E, Grist TM, Block WF. Rapid fat-suppressed isotropic steady-state free precession imaging using true 3D multiple-half-echo projection reconstruction. Magn Reson Med. 2005;53:692–699. [DOI] [PubMed] [Google Scholar]
- [38].Chan RW, Ramsay EA, Cheung EY, Plewes DB. The influence of radial undersampling schemes on compressed sensing reconstruction in breast MRI. Magn Reson Med. 2012;67:363–377. [DOI] [PubMed] [Google Scholar]
- [39].Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med. 2012;48:297–305. [DOI] [PubMed] [Google Scholar]
- [40].Reeder SB, Pineda AR, Wen Z, et al. 2005. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (ideal): application with fast spin-echo imaging. Magn Reson Med 2005;54:636–644. [DOI] [PubMed] [Google Scholar]
- [41].Moran CJ, Brodsky EK, Bancroft LH, et al. High-resolution 3D radial bSSFP with IDEAL. Magn Reson Med. 2014;71:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reeder SB, McKenzie CA, Pineda AR, et al. Water-fat separation with ideal gradient-echo imaging. J Magn Reson Imaging. 2007;25:644–652. [DOI] [PubMed] [Google Scholar]
- [43].Brodsky EK, Holmes JH, Yu H, Reeder SB. Generalized k-space decomposition with chemical shift correction for non-cartesian water-fat imaging. Magn Reson Med. 2008;59:1151–1164. [DOI] [PubMed] [Google Scholar]
- [44].Kuperman VY, Alley MT. Differentiation between the effects of T1 and T2* shortening in contrast-enhanced mri of the breast. J Magn Reson Imaging. 1999;9:172–176. [DOI] [PubMed] [Google Scholar]
- [45].Ramadan S, Mulkern RV. Comment on ADC reductions in postcontrast breast tumors. J Magn Reson Imaging. 2010;31:262; author reply 263–264. [DOI] [PubMed] [Google Scholar]
- [46].Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med. 2005;54:625–635. [DOI] [PubMed] [Google Scholar]
- [47].Majumdar A, Ward RK. Exploiting rank deficiency and transform domain sparsity for MR image reconstruction. Magn Reson Imaging. 2012;30:9–18. [DOI] [PubMed] [Google Scholar]
- [48].Zhang T, Alley MT, Lustig M, Li X, Pauly J, Vasanawala SS. Fast 3D DCE-MRI with sparsity and low-rank enhanced spirit (SLR-SPIRiT). In Proceedings of the 21st Annual Meeting of ISMRM; 2013; Salt Lake City, UT Abstract 2624. [Google Scholar]
- [49].Otazo R, Candes E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med. 2015; 73:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Trzasko J, Manduca A. Local versus global low-rank promotion in dynamic MRI series reconstruction. In Proceedings of the 19th Annual Meeting of ISMRM; 2011; Montreal, Canada; 4371. [Google Scholar]
- [51].Zhang T, Pauly JM, Levesque IR. Accelerating parameter mapping with a locally low rank constraint. Magn Reson Med. 2015; 73:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang T, Cheng JY, Potnick AG, et al. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. J Magn Reson Imaging. 2015;41:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lugauer F, Nickel D, Wetzl J, Kiefer B, Hornegger J, Maier A. Accelerating multi-echo water-fat MRI with a joint locally lowrank and spatial sparsity-promoting reconstruction. MAGMA. 2017;30:189–202. [DOI] [PubMed] [Google Scholar]
- [54].Liang ZP. Spatiotemporal imaging with partially separable functions. In Proceedings of the 4th International Symposium on Biomedical Imaging: From Nano to Macro; 2007; Arlington, VA; 988–991. [Google Scholar]
- [55].McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans Med Imaging. 2014;33:2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jimenez JE, Strigel RM, Johnson KM, Bancroft LCH, Reeder SB, Block WF. 10 second temporal resolution of early enhancement visualization: framework for fast breast MRI screening. In Proceedings of the 25th Annual Meeting of ISMRM; 2017; Honolulu, HI Abstract 2107. [Google Scholar]
- [57].Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. Sense: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- [58].Cai J-F, Candès EJ, Shen Z. A singular value thresholding algorithm for matrix completion. SIAM J Optimiz. 2010;20:1956–1982. [Google Scholar]
- [59].Jimenez JE, Bancroft LCH, Strigel RM, Johnson KM, Reeder SB, Block WF. Non-cartesian compressed sensing with fat/water decomposition: feasibility study for high performance breast DCE-MRI. In Proceedings from the Annual Meeting of ISMRM; 2015; Toronto, CA Abstract 1078. [Google Scholar]
- [60].Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-cartesian parallel imaging reconstruction. J Magn Reson Imaging. 2014;40:1022–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Griswold MA, Jakob PM, Nittka M, Goldfarb JW, Haase A. Partially parallel imaging with localized sensitivities (PILS). Magn Reson Med. 2000;44:602–609. [DOI] [PubMed] [Google Scholar]
- [62].Wang Z, Bovik AC, Sheikh HR, Simoncelli EP. 2004. Image quality assessment: from error visibility to structural similarity. IEEE Trans Image Process. 2004;13:600–612. [DOI] [PubMed] [Google Scholar]
- [63].Levine E, Daniel B, Vasanawala S, Hargreaves B, Saranathan M. 3D cartesian MRI with compressed sensing and variable view sharing using complementary poisson-disc sampling. Magn Reson Med. 2017;77:1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Graff CG, Sidky EY. Compressive sensing in medical imaging. Appl Opt. 2015;54:C23–C44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhao B, Haldar JP, Brinegar C, Liang ZP. Low rank matrix recovery for real-time cardiac MRI. In Proceedings from the IEEE International Symposium on Biomedical Imaging: From Nano to Macro; 2010; Rotterdam, Netherlands p. 996–999. [Google Scholar]
- [66].Haldar JP, Liang ZP. Low-rank approximations for dynamic imaging. In Proceedings of the IEEE International Symposium on Biomedical Imaging: From Nano to Macro; 2011; Chicago, IL p. 1052–1055. [Google Scholar]
- [67].Jimenez JE, Johnson KM, Henze Bancroft LC, et al. High performance volumetric 3T breast acquisition: a foundation for multi-parametric imaging. In Proceedings of the 24th Annual Meeting of ISMRM; 2016; Singapore Abstract 3263. [Google Scholar]
- [68].Baron CA, Dwork N, Pauly JM, Nishimura DG. Rapid compressed sensing reconstruction of 3D non-Cartesian MRI. Magn Reson Med. 2017. 10.1002/mrm.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Structural similarity index (SSIM) analysis of a single time interval (30 to 40 seconds) over only the breast tissue provides a measure of similarity among 4 methods and STELLR reconstruction. Columns 1 and 4 show MIPs and single-slice renderings of each reconstruction, respectively. Columns 2 and 5 contain SSIM maps of the structural coefficient and its decomposed SSIM index number. Columns 3 and 5 provide a complete SSIM measurement of all 3 components (luminance, contrast, and structure) and its combined maps. The highest SSIM similarity scores were obtained when comparing STELLR against CS, which is the reference method for temporal resolution. Both methods scored even higher when computing only the structural index
FIGURE S2 Top: Visual comparison of 4 methods over a single time interval (30 to 40 seconds). Bottom: SSIM index plot of each method using STELLR as reference over the first 15 time points. Consistent SSIM similarity scores can be seen over time at the MIP and single-slice level. Qualitative observations match quantitative measurements. Compressed sensing obtains the highest similarity measure, whereas PILS obtains the lowest. The PILS+Tornado performance fluctuates at the beginning, as a result of the temporal filter, but stabilizes as it moves forward. The SSIM indexes of MIP measurements obtained higher similarity overall than single slice, as MIPs are less affected by pseudo-noise caused by undersampling
VIDEO S1 Temporal performance of SVIPR STELLR at the 10-second volumetric frame rate. The MIPs enable visualization of breast perfusion with sequential filling of the right ventricle, arterial and venous system, and lesion enhancement. Single-slice visualization provides depicture of morphologic detail, including a spiculated margin and irregular shape of a known breast cancer with 0.8-mm isotropic spatial resolution
VIDEO S2 Visualization of SVIPR STELLR performance on a patient with multiple enhancing features. Overall breast perfusion can be appreciated through MIP images, whereas single-slice renderings capture spatial detail of a known benign lesion and allow observation of its temporal enhancing behavior