Abstract
Background:
Substance use disorder is driven by complex gene-environment interactions. Epigenetic histone regulation is a significant contributor to several behavioral phenotypes of drug abuse. The primary epigenetic mechanisms that drive drug-taking and drug-seeking are still being investigated, and it is unclear how environmental conditions alter epigenetic histone acetylation to change behaviors geared toward drug reward.
Objective:
This study examined the effects of environmental condition on amphetamine self-administration, and whether drug-taking and drug-seeking behaviors could be influenced through inhibition of an epigenetic regulator, histone deacetylase (HDAC).
Method:
Male rats reared for 30 days in enriched (EC), isolated (IC), or standard conditions (SC) prior to amphetamine (0.03, 0.05, 0.1 mg/kg/infusion, i.v.) self-administration, extinction, and reinstatement sessions. The HDAC inhibitor, Trichostatin A (TsA; 0.3 mg/kg, i.v.), was injected 30 min prior to operant sessions. After amphetamine-induced reinstatement (0.25 mg/kg, s.c.), tissue was extracted for Western blot analyses of acetylated histone H3 lysine 9 (acH3K9) in the nucleus accumbens (NAc) and dorsal striatum (DSt).
Results:
While TsA did not significantly affect amphetamine self-administration or extinction, TsA decreased cue-, but not drug-induced reinstatement in IC rats only. In the DSt, but not in the NAc, IC rats exhibited significantly less acH3K9 expression than EC and SC rats, irrespective of TsA treatment.
Conclusions:
HDAC inhibition decreases cue-induced reinstatement of amphetamine seeking in IC rats. While IC rats exhibit less acH3K9 expression in the DSt, future studies are needed to elucidate the critical epigenetic factors that drive substance abuse, particularly in vulnerable populations.
Keywords: Amphetamine self-administration, Environmental enrichment, Histone deacetylase inhibition, Extinction, Reinstatement
Introduction
Persistent drug taking and relapse stem from multidimensional genetic and environmental factors (McQuown & Wood, 2010; Schwarz et al., 2011). The field of epigenetics, at the nexus of gene-environment interactions, examines how environmental stimuli influence transcription and gene expression. Drugs of abuse alter gene expression, and epigenetic mechanisms have been investigated in preclinical models of drug abuse due to their ability to modulate the tendency to seek drug reward (Renthal et al., 2007; Robison & Nestler, 2011; Mason et al., 2018).
Early life experiences, such as social and environmental stress, affect epigenetic mechanisms that influence the propensity to develop substance use disorders (Gudsnuk & Champagne, 2012; Ajonijebu et al., 2017). The differential rearing paradigm (i.e., rearing rats in enriched or isolated environments) models social and environmental factors that drive both healthy and maladaptive behaviors (Renner & Rosenzweig, 1987). Rats reared in an enriched condition self-administer less amphetamine at low unit doses compared to rats reared in isolated conditions (Bardo et al., 2001; Green et al., 2002). Enriched rats also display less amphetamine-induced hyperactivity (Arndt et al., 2014; Bardo et al., 1995) and decreased vulnerability to psychomotor stimulants (Stairs et al., 2011).
One epigenetic mechanism, histone acetylation and deacetylation, has become a possible target for mediating both the rewarding properties of drugs of abuse (Romieu et al., 2008; 2011) and the environmentally-induced changes in learning and memory (Sweatt, 2009). Histone deacetylase (HDAC) removes acetyl groups from the lysine groups of histone proteins to allow for tighter coiling of DNA and reduced gene expression (Ito & Adcock, 2002). Histone marks on acetylated histone 3 lysine 9 (acH3K9) co-localize on active gene promoters and are associated with active transcription (Gates et al., 2017). Early life stress has been shown to decrease mRNA levels for multiple HDACs in the cortex (Levine et al., 2012; Lewis & Olive, 2014), and inhibiting HDAC mechanisms may normalize deficits in acH3K9 during alcohol withdrawal (You et al., 2018).
Acute administration of morphine, ethanol, and cocaine modulates HDAC activity in the dorsal striatum (DSt; Adachi & Monteggia, 2009; Sanchis-Segura, Lopez-Atalaya, & Barco, 2009), a key region in the brain that alters conditioned responses to rewarding visual stimuli and drug seeking maintained by drug cues (Malenka et al., 2009; Taylor et al., 2013; Di Ciano et al., 2008; Vanderschuren et al., 2005). Chronic psychostimulant-induced histone hyperacetylation in the nucleus accumbens (NAc) significantly increases the motivation to self-administer cocaine, and daily HDAC infusions into the NAc results in vertical shifts in the cocaine dose-response function, indicative of a highly vulnerable phenotype (Wang et al., 2010). Previous studies have also shown that HDAC inhibition prevents or reverses amphetamine-induced locomotor behavior (Frey et al., 2006; Schroeder et al., 2013) and attenuates the long-lasting maintenance of amphetamine-induced behavioral sensitization (Kalda et al., 2007).
Trichostatin A (TsA) is an HDAC inhibitor that attenuates cocaine intake while having no effect on sucrose consumption in operant self-administration tasks (Romieu et al., 2008; 2011). While other studies have suggested rearing-induced changes to the epigenome (Covington et al., 2011; Dash, Orsi, & Moore, 2009; Fischer et al., 2007; Tsankova et al., 2006), it is unknown if a chronic environmental manipulation such as differential rearing interacts with HDAC inhibition to alter amphetamine-taking, amphetamine-seeking, or downstream expression of acH3K9 in the NAc or DSt.
The primary objective of this study was to determine if different environmental conditions and HDAC inhibition via TsA alter amphetamine intake and seeking. Two experiments were conducted. The first experiment investigated the role of differential rearing and TsA on the acquisition and maintenance of intravenous amphetamine self-administration. The second experiment investigated differential rearing and TsA on the extinction and reinstatement of amphetamine-seeking. Protein expression of acetylated histone H3 lysine 9 (acH3K9) was assessed in the nucleus accumbens (NAc) and dorsal striatum (DSt). We hypothesized that TsA would generally attenuate drug-taking and drug-seeking for amphetamine. We further hypothesized that isolated rats would exhibit deficiencies in acH3K9 in the NAc and DSt which would be restored by TsA to reduce amphetamine seeking following extinction of amphetamine self-administration.
Method and Materials
Animals & Environmental Conditions
90 male Sprague-Dawley rats (Charles River, Portage, MI, USA) arrived in the lab at 21 days of age and were randomly assigned to rear in one of the three environmental conditions: enriched (EC), isolated (IC), or standard (SC) (Arndt et al., 2015; Bardo et al., 2001; Simpson & Kelly, 2011; Stairs & Bardo, 2009). Enriched rats lived with several other cohorts and were housed in a large metal cage (60 × 120 × 45 cm) lined with paper bedding. To provide further enrichment, experimenters gently handled each rat for approximately one minute daily during the rearing phase. Fourteen objects were regularly rotated and replaced inside the EC cage. Because the items are replaced and cleaned regularly, we maintain a large stock of items to be used in the enrichment caging. All items are commercially available and are purchased at local discount stores, garage sales, or donated. Items include plastic children’s toys including an assortment of different types of vehicles, wheel barrels, bowling pins, and castles; various sizes and configurations of PVC pipe; plastic sand toys including buckets and shovels; and plastic kitchenware including larger cups, measuring cups, and strainers. Isolated rats reared individually in hanging cages (17 × 24 × 20 cm) with wire mesh on the front and bottom, with solid stainless-steel sides and rear. The IC rats were not handled throughout the 30-day rearing phase and were not exposed to novel objects or paper bedding. Standard-housed rats were housed in pairs in standard shoebox cages (20 × 43 × 20 cm). SC rats were exposed to the same bedding as EC rats but did not have any novel objects in their cage and were only handled during the weekly cage change. The inclusion of the SC group of rats was not intended to control for differences between EC and IC rats, but rather to provide a known laboratory standard for comparison. Rats remained in their respective environmental conditions for the entire duration of the experiment. All rats were given free access to food and water except during lever press training. The colony room was on a 12-hr light-dark cycle and was maintained at approximately 22° C, with humidity ranging from approximately 30–45%. All behavioral tests and procedures were conducted during the light portion of the cycle and were in accordance with the Institutional Animal Care and Use Committee at Kansas State University and NIH guidelines and standards (Institute for Laboratory Animal Research, 2011).
Apparatus: Operant Chambers
Lever press training and amphetamine self-administration sessions were conducted inside operant conditioning chambers (ENV-001, Med Associates, St. Albans, VT). Each chamber was enclosed in a sound-attenuating compartment and was operated via computer interface. Two metal levers were located on either side of the food tray 7.3 cm above the metal grid floor. A 28-V, 3-cm diameter white cue light was centered above each lever. A house light was equipped in each operant box which was always on during lever press training. The house light also illuminated during the ‘timeout’ period following drug infusion in Experiment II. The same active lever (left or right) was maintained for each rat for both the sucrose training and amphetamine self-administration phases. For Experiment II, an 80 dB, 3000-Hz tone generator was utilized to accompany the cue light during the FR-1 amphetamine training phase and the cue-, but not drug-induced reinstatement test.
Lever Press Training
Temporary food restriction to 85% of rats’ free-feeding weight was maintained only during lever press training and ensured rats were motivated to learn lever pressing. After each active lever press during training, a 0.1 ml, 20% sucrose solution was presented to the rat in a recessed food receptacle inside a magazine. Rats experienced four additional, daily 30-min sessions of FR-1 responding for 20% sucrose.
Surgery and Self-Administration
After returning to free-feeding weight, rats were anesthetized with ketamine (80 mg/kg; 1 mg/ml, i.p.) and diazepam (5 mg/kg; 1 mg/ml, i.p.) prior to jugular catheter implantation. Polyurethane catheters measured approximately 12 cm in length and 0.2 mm in internal diameter (SAI Infusion Technologies) and were inserted through an incision on the rat’s back that led up under the skin and around into the rat’s left jugular vein. Catheter tubing from the jugular vein was connected subcutaneously to a 22-gauge back-mounted cannula (Plastics One; Roanoke, VA) secured and sutured to surgical mesh (Biomedical Structures; Warwick, RI). A stainless-steel bolt covered the cannula cap to prevent damage to the back mount. To maintain patency and to protect against infection, catheters were flushed daily with infusions of heparinized saline (10–30 IU/ml; 0.1 ml, i.v., before self-administration and 0.1 ml, i.v., after self-administration) and cefazolin (50 mg/ml; 0.1 ml, i.v., after self-administration).
Amphetamine infusions were administered via a syringe pump (PHM-100, Med Associates) plunging a 10-ml syringe. Amphetamine was infused through a leash and swivel arrangement comprised of a polyethylene supply tube encased in vinyl tubing with a captive collar to secure the unit to the cannula (Plastics One; Roanoke, VA). Each amphetamine infusion lasted 5.9 sec at a dose of 0.03, 0.05, or 0.1 mg/kg/infusion at a volume of 100-μl. An active lever press resulted in an amphetamine infusion and concurrent illumination of both cue lights (a tone was added to Experiment II). The stimuli remained on for 20 sec to signal a timeout period. Active lever presses during timeout and inactive lever presses had no programmed consequence.
Drugs: Self-Administration Testing
Trichostatin A (TsA; 0.3 mg/kg; 1.0 mg/ml; ApexBio) was dissolved in sterile saline and 10% DMSO. Sterile saline with 10% DMSO was injected as the vehicle control. TsA was stored in frozen (−20° C) 2.0 ml centrifuge tubes. TsA was thawed and injected intravenously (i.v.) through the indwelling jugular catheters thirty minutes prior to amphetamine self-administration, extinction, or reinstatement test sessions (Romieu et al., 2008; 2011).
D-amphetamine (Sigma Aldrich, MO, USA) was dissolved in 0.9% sterile saline and was self-administered intravenously (0.03, 0.05, or 0.1 mg/kg/infusion). Brevital (10 mg/ml; 0.1–0.15 ml, i.v.) was infused to assess loss of righting reflex to verify catheter patency.
Experiment I Behavioral Procedures: Acquisition and Maintenance
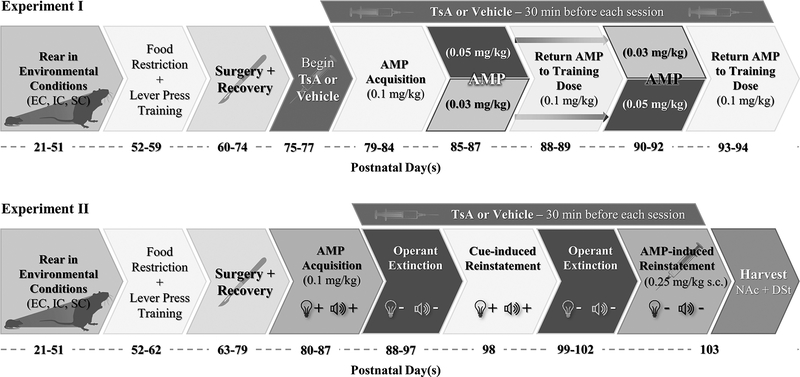
Following surgery recovery, patency checks were conducted and only patent rats were randomly assigned to receive TsA or vehicle pretreatment for the duration of experiment. Non-patent rats were excluded from the study. Repeated i.v. injections of TsA at 0.3 mg/kg for at least four days have been shown to result in significant decreases of HDAC activity in the NAcc, at levels of approximately 40% below their vehicle counterparts (Romieu et al., 2008). Therefore, daily TsA (0.3 mg/kg, i.v.) or vehicle pretreatment started three days before the start of amphetamine self-administration sessions and continued for the duration of the experiment 30-min prior to 1-hour drug-taking sessions (Host et al., 2010; Romieu et al., 2008; 2011). Rats trained to lever press for amphetamine at 0.1 mg/kg/infusion for 10 days and were randomly assigned to receive amphetamine at either 0.03 or 0.05 mg/kg/infusion during the first set of lower-unit amphetamine dose sessions for 3 days. After 2 additional days of amphetamine at 0.1 mg/kg/infusion for all rats, 3 more days of lower-unit dose sessions occurred with the original 0.03 or 0.05 mg/kg/infusion groups receiving the dose not received during the first set of lower-unit dose sessions to counterbalance. The dose then returned to 0.1 mg/kg/infusion again for the final two days (Figure 1) and catheter patency was confirmed (final Experiment I N = 42; Table 1).
Figure 1.
Study design: Rats arrived in lab at postnatal day 21 (PND 21) and were differentially-reared in enriched (EC), isolated (IC), or standard (SC) conditions. Trichostatin A (TsA; 0.3 mg/kg, i.v.) or vehicle (10% DMSO in saline) injections were administered 30 minutes prior to operant sessions and continued through the final day of experimentation.
Abbreviations: AMP, amphetamine; DMSO, dimethyl sulfoxide; DSt, dorsal striatum; EC, enriched condition; IC, isolated condition; NAc, nucleus accumbens; SC, standard condition; TsA, Trichostatin A
Table 1.
Final patent rats included in all data analyses. Catheter patency checks were routinely conducted throughout the experiments to ensure rats were properly receiving i.v. TsA or vehicle pretreatment, and to ensure adequate i.v. amphetamine self-administration. Non-patent rats were excluded from behavioral procedures and all data analyses. Abbreviations: TsA, Trichostatin A
| Experiment I - Acquisition and Low-Dose Tests |
Experiment II - Extinction and Reinstatement |
|||
|---|---|---|---|---|
| Vehicle | TsA | Vehicle | TsA | |
| Enriched (EC) | 5 | 8 | 5 | 7 |
| Isolated (IC) | 7 | 8 | 4 | 6 |
| Standard (SC) | 6 | 8 | 5 | 7 |
Experiment II Behavioral Procedures: Extinction and Reinstatement
Rats experienced identical procedures and methods as Experiment I through the surgery recovery phase. Following surgery recovery, rats were trained to lever press for amphetamine at 0.1 mg/kg/infusion. These sessions included both a cue light and tone that turned on during the 5.9 second infusion after each active lever press. The light and tone turned off and the house light illuminated during the 20-second time out period in which no amphetamine infusion was possible. Rats exhibiting ten or more active lever presses per session and a greater than 2:1 active: inactive ratio of lever presses were then moved to the extinction phase. Patency checks were administered after the last day of amphetamine training and non-patent rats were excluded from extinction testing and analyses.
Hour-long extinction sessions began after eight FR-1 amphetamine sessions. All previously associated drug cues (light, tone, house light, and pump) were off during extinction. The levers remained in the chamber and lever presses resulted in no programmed consequence. TsA or vehicle injections began on Day 1 of extinction and occurred 30 min prior to operant sessions (Figure 1). After ten extinction sessions, all rats exhibited less than or equal to 20% of their peak response rate (Bastle et al., 2012) and were moved to the cue-induced reinstatement phase.
For the cue-induced reinstatement test, rats continued to receive a TsA or vehicle injection 30 min prior to the session. Reinstatement sessions began with a 3-sec non-contingent cue presentation (80 dB, 3000-Hz tone accompanied with cue lights). After this cue presentation, subsequent active lever presses were paired with presentation of the cues, but amphetamine was never infused. Rats then experienced another round of extinction sessions prior to the amphetamine (drug)-induced reinstatement test. During this second extinction phase, active lever responses returned to the previously set extinction criteria (Bastle et al., 2012). For the amphetamine-induced reinstatement test, 0.25 mg/kg amphetamine, s.c., was injected 15 min prior to the 1 hr reinstatement test with TsA or vehicle pretreatment identical to previous sessions. Catheter patency checks were conducted after the drug-induced reinstatement test and non-patent rats were excluded from data analyses (final Experiment II N = 34; Table 1).
Tissue Preparation and Western Blot
Immediately following the drug-induced reinstatement test, rats were briefly anesthetized with 4% isoflurane, sacrificed and brains were rapidly extracted. Bilateral samples of NAc and DSt were dissected from 1 mm coronal slices using a 1 mm biopsy punch (Sprow et al., 2014). Tissue from both hemispheres of each rat were combined and manually homogenized in cold NP-40 lysis buffer with HALT protease and phosphatase inhibitor (Thermo Scientific) added fresh. Tissue was spun at 10× g for 5 min. The supernatant was taken immediately after the spin to remove any unlysed material from the whole-cell lysate to be analyzed. The samples were kept frozen at −80 °C until the Pierce BCA assay (Thermo Scientific) to determine total protein concentration.
20μg of protein from each animal and region of interest were suspended in 2× Laemmli buffer with added 2.5% SDS solution (Sprow et al., 2014). Samples were loaded in a counterbalanced fashion into 4–20% polyacrylamide gels (Bio-Rad), separated in Tris-Glycine-SDS buffer, and wet transferred onto a PVDF membrane in a 20% methanol Tris-Glycine buffer. Membranes were blocked with 5% non-fat dry milk in TBST. Primary antibodies, acetylated histone K9 (acH3K9; Abcam ab10812; 1:2000) and β-actin (Abcam, ab8227; 1:5,000,000), were suspended in blocking solution. Membranes were incubated with primary antibodies separately at 4 °C and rinsed with TBST. Subsequent incubation with secondary antibody (HRP; ab97051) occurred at room temperature at 1:4000 (for acH3K9) and 1:50,000 (for β-actin) in blocking solution. Signal was developed via the ECL method (Bio-Rad), using the default settings on the imager (Kodak Image Station 4000) and software (Carestream MI SE). Densitometry was performed using ImageJ. Signal from acH3K9 was expressed as the percent of β-actin signal normalized to the SC-Saline group.
Data Analyses
Self-Administration
Mixed-factorial ANOVAs including the between-groups factors environmental condition (EC/IC/SC) and drug pretreatment (TsA/Vehicle), and the within-groups factor, self-administration session, were conducted on rats’ active and inactive lever presses. In Experiment 1, the data for the lower dose test sessions were averaged across all 3 sessions. The alpha-level was set at 0.05 for all analyses and Bonferroni corrected post-hoc analyses were used to probe significant main effects and interactions.
Western Blots
Expression of acH3K9 (arbitrary units) were analyzed using separate factorial ANOVAs for each region of interest (NAc & DSt) with the full factorial effects of environment (EC/IC/SC) and drug treatment (TsA/Vehicle) as between-subjects factors. The alpha-level was set at 0.05 for all analyses and Bonferroni corrected post-hoc analyses were used to probe significant main effects and interactions.
Results
Experiment I – Acquisition and Maintenance of Amphetamine Self-Administration
Across 10 sessions, acquisition of amphetamine (0.1 mg/kg/infusion) self-administration was not affected by either environmental condition (F(2, 36) = 1.00, p = .378, η2 = .05) or HDAC inhibition (F(1, 36) = 0.55, p = .464, η2 =.02), data not shown. Consistent with previous research (Arndt et al., 2015; Bardo et al., 2001), differential rearing did not alter self-administration of a high dose of amphetamine and therefore it is not surprising that TsA did not differentially alter responding.
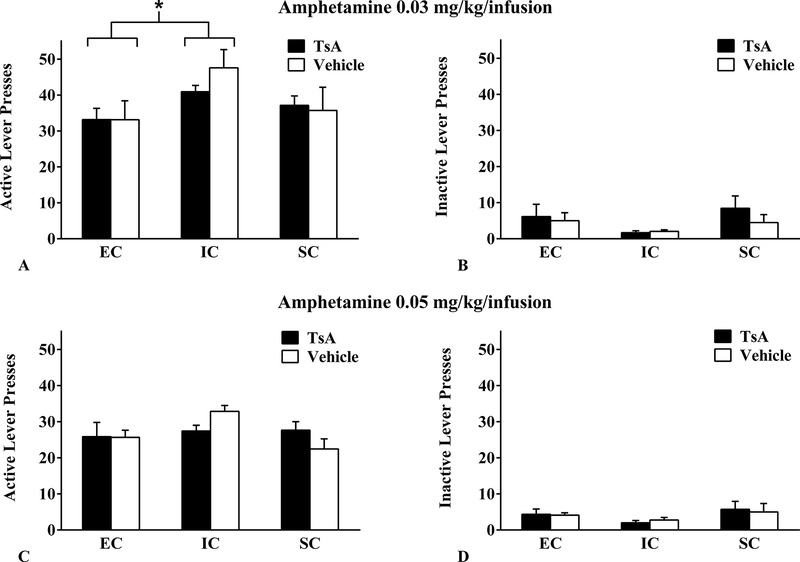
For self-administration of 0.03 mg/kg/infusion amphetamine, analysis of the average active lever from all 3 sessions of 0.03mg/kg/infusion revealed a significant main effect of environmental condition (F(2, 36) = 4.06, p = .026, η2 = .18), such that while SC rats did not differ from the other environmental groups, EC rats exhibited significantly fewer active lever responses for amphetamine than IC rats (p < .05). No significant differences were observed for drug pretreatment (F(1, 36) = 0.28, p = .602, η2 =.01) or environmental condition × drug pretreatment (F(2, 36) = 0.60, p = .554, η2 = .03; Figure 2 A–B). Similar null effects of TsA and environmental condition were observed for 0.05 mg/kg/infusion amphetamine (Figure 2 C–D). There was no significant main effect of environmental condition (F(2, 36) = 2.26, p = .119, η2 =.11), no significant main effect of drug pretreatment (F(1, 36) < 0.01, p = .999, η2 < .01), and no significant environmental condition × drug pretreatment interaction (F(2, 36) = 2.12, p = .135, η2 = .11). This suggests that while environmental enrichment attenuated low unit dose amphetamine self-administration, HDAC inhibition does not appear to differentially attenuate amphetamine self-administration at the low (0.03 mg/kg/infusion) or moderate (0.05 mg/kg/infusion) unit dose. Neither HDAC inhibition nor environmental condition had a significant effect on inactive lever presses (all ps > .05).
Figure 2.
Mean ± SEM (A & C) active and (B & D) inactive lever presses for amphetamine 0.03 mg/kg/infusion, i.v. (upper panels) and 0.05 mg/kg/infusion, i.v. (lower panels) between EC, IC, and SC rats following Trichostatin A (TsA; 0.3 mg/kg, i.v.) or Vehicle (10% DMSO in saline) pretreatment. These data reflect the average active lever presses of all three of the 0.03 and 0.05 mg/kg/infusion amphetamine sessions. EC rats exhibited significantly fewer active lever responses for amphetamine at 0.03 mg/kg/infusion than IC rats (*=p < .05), but there were no significant differences between EC, IC and SC rats at the 0.05 mg/kg/infusion dose. There were no significant differences between any of the drug pretreatment conditions and there were no significant differences in inactive lever responding between EC, IC, or SC rats in any treatment condition or at any dose.
Abbreviations: DMSO, dimethyl sulfoxide; EC, enriched condition; IC, isolated condition; SC, standard condition; TsA, Trichostatin A
Experiment II – Extinction and Reinstatement of Amphetamine Self-Administration
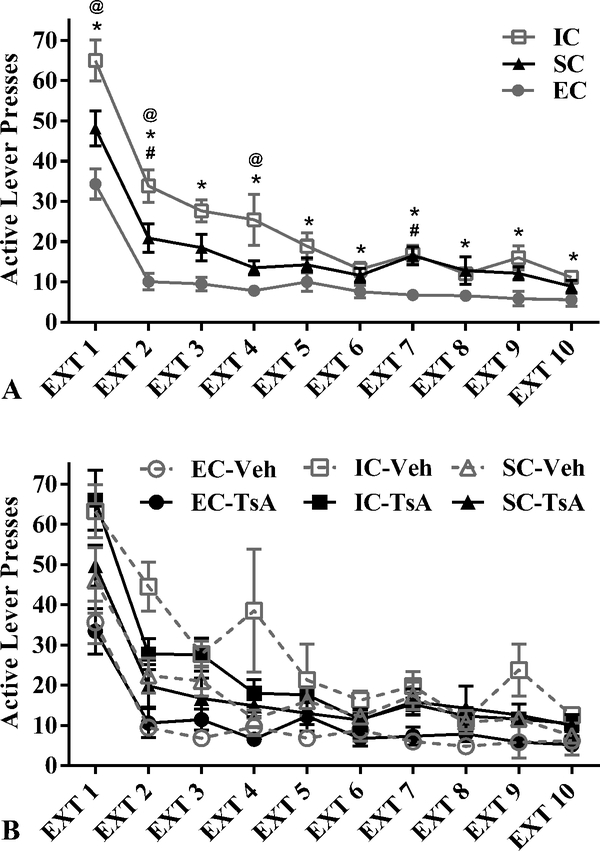
There were no differences between EC, IC, or SC rats in the acquisition of amphetamine (0.1 mg/kg/infusion) self-administration prior to extinction sessions (data not shown). There was a main effect of environmental condition in extinction, F(2, 29) = 18.51, p < .001, η2 = .56. During extinction, IC rats exhibited significantly more responses on the previously active lever than EC rats (Fs(1, 21) = 5.10–32.45, ps < .05). There was no significant main effect of TsA pretreatment on rats’ active lever responding over the ten extinction sessions (F(1, 29) = 0.81, p = .375, η2 =.03). There was also no significant interaction between environmental condition and TsA pretreatment (F(2, 29) = 1.24, p = .303, η2 = .08), and no significant interaction between extinction session and TsA pretreatment (F(9, 261) = 1.23, p = .278, η2 = .04); Figure 3. SC rats did not differ between groups of drug treatment. These results suggest that drug pretreatment (TsA or vehicle) did not appear to play a significant role in the extinction of active lever pressing for any one of the three environmental conditions across the ten extinction sessions.
Figure 3.
Mean ± SEM active lever presses during Extinction sessions 1–10 for (A) EC, IC, and SC, rats and (B) EC, IC, and SC rats following Trichostatin A (TsA; 0.3 mg/kg, i.v.) or Vehicle (10% DMSO in saline) pretreatment. (A) Asterisks (*) indicate that IC rats displayed greater active lever presses than EC rats (all ps < .05). The at symbols (@) indicate that IC rats displayed greater active lever presses than SC rats (all ps < .05), and the pound signs (#) indicate that SC rats exhibited significantly greater active lever presses than EC rats (all ps < .05). (B) There were no significant differences in active lever pressing between TsA-pretreated rats and vehicle-pretreated rats within any of the environmental conditions across the ten extinction sessions.
Abbreviations: DMSO, dimethyl sulfoxide; EC, enriched condition; IC, isolated condition; SC, standard condition; TsA, Trichostatin A
Cue-Induced Reinstatement
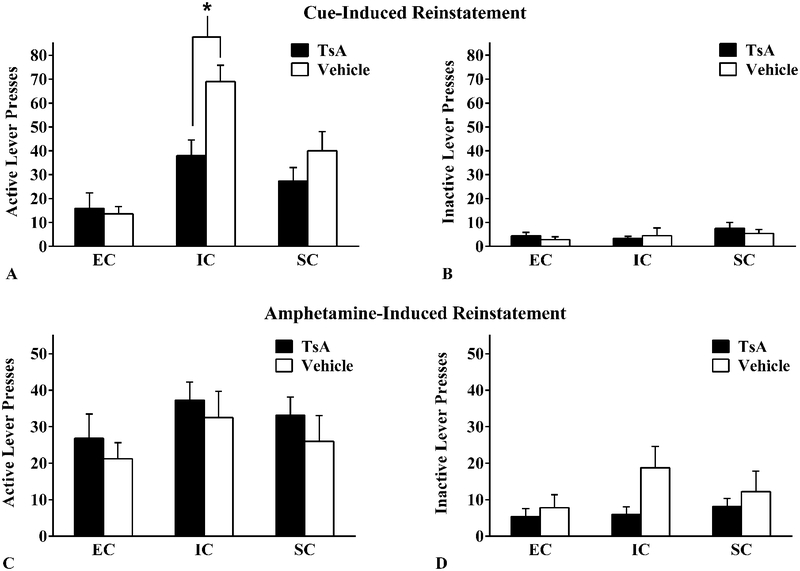
Active lever responding significantly increased from the last extinction session to the cue-induced reinstatement test (F(1,28) = 104.71, p<.001, η2 = .79) and there were significant main effects of environmental condition and drug pretreatment. In addition, the interactions between session and environmental group, session and drug pretreatment, and the three-way interaction between session, environmental condition, and drug pretreatment (F(2, 28) = 3.43, p <.05, η2 =.20) were all significant. Probing the three-way interaction revealed that EC rats did not display cue-induced reinstatement either following TsA or vehicle pretreatment. IC and SC rats did display cue-induced reinstatement when compared to the last extinction session in both the TsA and vehicle conditions. When examining the effects of TsA within the cue-induced reinstatement session itself, there was a significant main effect of environmental condition on rats’ active lever pressing (F(2, 28) = 17.10, p < .001, η2 = .55), and a significant main effect of drug pretreatment on rats’ active lever presses (F(1, 28) = 6.72, p = .015, η2 = .19), indicating a general TsA-induced decrease in cue-induced drug-seeking. An environmental condition × drug pretreatment interaction neared statistical significance (F(2, 28) = 3.14, p = .059, η2 = .18). Given our a-priori hypotheses, the significant main effects, and the marginally significant interaction, we probed the interaction to determine if differential rearing altered the effects of HDAC inhibition. IC-TsA rats exhibited significantly less active lever presses than IC-Vehicle rats (F(1, 28) = 9.94, p < .05, η2 = .56; Figure 4A). There were no differences in active lever presses between EC-TsA and SC-TsA rats compared to their vehicle counterparts (all ps > .05). No significant differences between EC, IC, and SC rats’ inactive lever presses were observed (all ps > .05; Figure 4B). This suggests that HDAC inhibition attenuates cue-induced reinstatement in IC rats only.
Figure 4.
Mean ± SEM active and inactive lever presses during the cue-induced reinstatement test (A&B) and during the amphetamine-induced reinstatement test (C&D) between EC, IC, and SC rats following Trichostatin A (TsA; 0.3 mg/kg, i.v.) or Vehicle (10% DMSO in saline) pretreatment. (A) Asterisk (*) indicates that IC-TsA rats exhibited significantly fewer active lever presses than IC-vehicle rats (p < .05). Abbreviations: DMSO, dimethyl sulfoxide; EC, enriched condition; IC, isolated condition; SC, standard condition; TsA, Trichostatin A
Amphetamine-Induced Reinstatement
Amphetamine pretreatment reinstated amphetamine seeking in all rearing groups when compared to the previous extinction session (F(1,29 = 84.68, p <.001, η2 = .75). There were no main effects or interactions with environmental condition or TsA treatment, indicating that neither environmental group nor drug pretreatment impacted amphetamine-induced reinstatement. No significant effects were found for either environmental condition or TsA on active or inactive lever presses (all ps > .05; Figure 4C–D).
Acetylated acH3K9 Expression
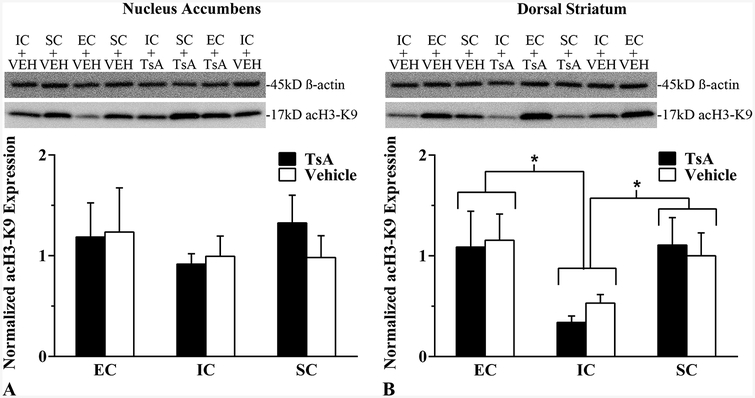
Western blot analyses in the NAc determined that neither TsA nor environmental condition affected acH3K9 expression (Figure 5A). In the DSt, however, there was a main effect of environmental condition, F(2,23) = 5.18, p = .017, η2 = .36. EC and SC rats exhibited greater expression of acH3K9 than IC rats, Fs(1,18) = 6.94–8.53, ps<.05, η2s = .24-.29 (Figure 5B). This suggests isolation rearing reduces acH3K9 expression in the DSt but not the NAc.
Figure 5.
Mean ± SEM normalized acetylated histone H3 lysine 9 (acH3K9) expression (arbitrary units) in the (A) nucleus accumbens and (B) dorsal striatum between EC, IC, and SC rats following Trichostatin A (TsA; 0.3 mg/kg, i.v.) or Vehicle (10% DMSO in saline) pretreatment. Tissue was harvested immediately after drug-induced reinstatement for western blot analysis. *p < .05 for EC vs IC and SC vs IC.
Discussion
These studies indicate that in IC rats, HDAC inhibition is more likely to attenuate cue-rather than drug-induced reinstatement. Western blot analyses indicate that while TsA treatment does not alter acH3K9 expression in the DSt and NAc, isolation housing reduces acH3K9 expression in the DSt but not in the NAc following amphetamine exposure. We provide evidence that broad HDAC inhibition selectively affects behavior motivated by conditioned visual and auditory cues previously paired with drug reward in IC rats.
We replicate prior work in that enriched (EC) rats self-administer less low-unit dose amphetamine but did not differ from IC and SC rats for moderate or high doses (Arndt et al., 2015; Bardo et al., 2001; Garcia, et al., 2019; Green et al., 2002). In addition, we replicate prior work that shows EC rats respond less than IC and SC rats during extinction (Alvers et al., 2012; Green et al., 2010; Stairs, Klein, & Bardo, 2006). Surprisingly, we did not observe cue-induced reinstatement in the EC rats. Environmental enrichment typically reduces cue-induced reinstatement for psychostimulants and other reinforcers (Chauvet et al., 2009; Galaj et al., 2016; Grimm et al., 2008; Hofford et al., 2014). Our lack of cue-induced reinstatement in the EC rats may be due to procedural differences in our experiment (amount of enrichment, number of extinction sessions, and/or cue duration).
Previous studies suggest that modifying the epigenome through HDAC inhibition and environmental enrichment may similarly improve learning and memory mechanisms (Fischer et al., 2007; Gräff & Tsai, 2013; Sweatt, 2009), and acute psychostimulant administration can increase HDAC expression in the NAc (Martin et al., 2012) and DSt (Jayanthi et al., 2014), increasing the probability of histone acetylation. However, we did not observe robust interactions between environmental condition and HDAC inhibition in altering behaviors during drug-taking and drug-seeking. Our lack of robust interaction between environmental conditions and HDAC inhibition may be due to the different approaches used in the previous experiments including enrichment or isolation for differing lengths of time or during different periods of development, differences in the number of animals per cage, differences in handling, and/or enrichment in mice versus rats. Small changes in husbandry, including handling, can have significant impacts on behavior. For example, brief handling after housing in enriched or isolated conditions between PND 21 and PND 85 has been shown to decrease anxiety in isolated rats and increase anxiety in enriched rats (Pritchard, Van Kempen, & Zimmerberg, 2013). Brief handling can alter the hyperactivity effects commonly observed with isolation (Holson, Scallet, Ali, & Turner, 1991) and can alter the accumbal dopaminergic changes that result from isolation (Hall et al., 1998). Because the amount of complexity present in the rearing condition is correlated with neuronal and behavioral changes such that environments with fewer cohorts, less contact with novel objects, or less handling produce fewer behavioral and neurobiological outcomes (Renner and Rosenzweig, 1987), we used enrichment variables (i.e. handling, novel objects for enriched rats, etc.) that when combined, result in enrichment’s effects (Greenough, Black, & Wallace, 1987; Renner & Rosenzweig, 1987). In contrast, our isolated condition was designed to ensure the maximal effect of social isolation, including starting the isolation at PND 21, use of wire mesh floors, no handling, and no contact with conspecifics (Fone & Porkess, 2008; Heidbreder, et al., 2000; Holson, Scallet, Ali, & Turner, 1991).
To our knowledge this is the first experiment to test the effects of HDAC inhibition in differentially reared rats on cue- and drug-induced reinstatement. Romieu et al. (2011) found that TsA pretreatment can attenuate cocaine reinstatement, but they did not include an extinction period. Rather, they implemented a withdrawal period of three weeks prior to TsA pretreatment and reinstatement. Additionally, Romieu et al. (2011) utilized a simultaneous cue+drug-induced reinstatement test for cocaine and did not conduct the reinstatement tests separately following extinction. Our findings extend those from Romieu et al. (2011) and highlight that between the two parameters (cue- vs. drug-induced reinstatement), HDAC inhibition may affect cue-induced reinstatement more so than drug-induced reinstatement. While TsA is a broad-spectrum Class I and Class II HDAC inhibitor, more targeted HDAC inhibition has resulted in findings similar to the current report. Administration of the HDAC3 inhibitor RGFP966 after extinction accelerated extinction in a conditioned place preference paradigm (CPP; Malvaez, et al., 2013). HDAC3 inhibition has also recently been examined in a self-administration paradigm. The HDAC3 inhibitor RGFP966 administered prior to extinction alters cue-, but not cocaine-induced reinstatement. While RGFP966 pretreatment accelerated early extinction learning, it had no effect on cocaine self-administration using a fixed-ratio or progressive ratio schedule (Hitchcock et al., 2018). We support and extend this previous work and suggest a role of HDAC inhibition in decreasing the conditioned and rewarding effects of psychostimulants that are facilitated by associative learning when TsA is administered prior to the extinction and reinstatement sessions. Taken together with our current results and previous work, it appears HDACs may contribute significantly to learning about a cue paired with a psychostimulant and contribute less toward the psychostimulant effects themselves.
The current study’s results cannot discern between an effect of TsA on cue-induced reinstatement itself or an enhancement in extinction learning that decreased cue-induced reinstatement but did not attenuate extinction responding itself. Previous work suggests that specific HDAC3 inhibition via RGFP966 administered prior to or after the session accelerates extinction learning (Hitchcock et al., 2018; Malvaez, et al., 2010; 2013) and we did not replicate that effect with a broad-spectrum HDAC inhibitor. Therefore, it is not clear if our lack of effect is due to differing mechanisms of action and pharmacokinetic profiles between TsA and RGFP966, and/or something unique to the extinction of amphetamine self-administration. Importantly, the amphetamine and/or the reinstatement behavior themselves may have created an acetylation ceiling effect, confounding some of the acH3K9 expression observed. In either case, IC rats maintain a lower ceiling level of acetylation, at least partially supporting the hypothesis that IC rats are in a state of hypoacetylation. However, if amphetamine induced acetylation to ceiling levels, it would explain why there is an effect of TsA during cue-induced reinstatement but no effect of TsA on acH3K9 expression after the amphetamine-induced reinstatement session. Future work examining cue-reinstatement alone and amphetamine-induced reinstatement alone in relation to acetylation is needed to draw further conclusions.
Inhibiting HDAC function is a theoretical drug target but is challenged by off target effects that affect acetylation and phosphoacetylation since any cellular process affected by acetylation could be affected. Thus, the pharmacokinetic and pharmacodynamic profile of various HDAC inhibitors should be considered when investigating their role in models of drug reward. TsA inhibits a broad range of HDACs (i.e., HDAC 1, 3, 4, 6 and 10). Differences in binding affinity and intrinsic pharmacological efficacy between the HDAC inhibitors may differentially affect drug taking or drug seeking. Indeed, valproic acid, sodium butyrate, RGFP966, or TsA can yield different behavioral outcomes to psychostimulants depending on the frequency of administration and specific model being utilized (Godino, Jayanthi, & Cadet, 2015; Raybuck et al., 2013).
HDAC inhibitors improve learning and memory (Alarcón et al., 2004; Korzus et al., 2004) and reduce phenotypic drug-seeking behavior (Nestler et al., 2014; Walker et al., 2018). Amphetamine, a psychomotor stimulant with abuse liability, is a Schedule II drug indicated for attention deficit hyperactivity disorder (Teva Pharmaceuticals, 2017). By itself, amphetamine may promote acetylation or promote the precursors that allow the acetylation of critical histones that regulate learning and memory. While amphetamine has abuse potential, it is unclear if the mechanisms of action promote similar histone modification when compared to other psychostimulants.
Isolated rats exhibit profound neurochemical differences in reward regions in the brain (Fone & Porkess, 2008). Future work should focus on the dynamic role of acH3K9 expression and how it may differ across different brain regions in the reward pathway, including the medial prefrontal cortex. Amphetamine acts at multiple levels to alter genetic expression, and both acute and chronic amphetamine exposure has been associated with gene changes that drive the maladaptive behaviors indicative of substance use disorder (McCowan et al., 2015). Thus, the differences we observed in IC-induced acH3K9 expression in the DSt may have been the result of environmental condition, prior amphetamine exposure, or a combination of amphetamine, rearing condition, extinction, or reinstatement. This makes it difficult to determine which of these manipulations had a greater influence on acH3K9 within the DSt.
Conclusions
Broad HDAC inhibition via TsA reduces cue-induced reinstatement of amphetamine seeking in IC rats with no effect on amphetamine self-administration or extinction. Isolated rearing decreases acH3K9 expression in the DSt. However, acH3K9 expression in both the DSt and NAc appear unrelated to the ability of environmental enrichment to reduce the acquisition, maintenance, extinction, and reinstatement of low-dose amphetamine self-administration. Continued research is necessary to elucidate the factors that drive substance use disorder in the context of epigenetics.
Acknowledgements
This research was supported by the American Psychological Association Basic Psychological, Science Research Grant. DLA was supported by DA035435. EJG was supported by, DA035435-S1. The western blot results were collected at the Molecular Biology Core supported, by Kansas State University College of Veterinary Medicine.
Prior dissemination of a portion of the behavioral and Western blot data appearing in this manuscript occurred via poster presentation by TJW at the 2018 Society for Neuroscience (SfN) Annual Conference in San Diego, CA.
Footnotes
Disclosures
None
References
- Adachi M, & Monteggia L (2009). Synergistic interactions between histone deacetylase inhibitors and drugs of abuse. Neuropsychopharmacology, 34(13), 2619–2620. doi: 10.1038/npp.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajonijebu DC, Abboussi O, Russell VA, Mabandla MV, & Daniels WM (2017). Epigenetics: a link between addiction and social environment. Cellular and Molecular Life Sciences, 74(15), 2735–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, & Barco A (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: A model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron, 42(6), 947–959. [DOI] [PubMed] [Google Scholar]
- Alvers K, Marusich J, Gipson C, Beckmann J, & Bardo M (2012). Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behavioural Pharmacology, 23(7), 650–657. doi: 10.1097/fbp.0b013e3283584765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt DL, Arnold J, & Cain M (2014). The effects of mGluR2/3 activation on acute and repeated amphetamine-induced locomotor activity in differentially reared male rats. Experimental and Clinical Psychopharmacology, 22(3), 257. doi: 10.1037/a0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt DL, Johns KC, Dietz ZK, & Cain ME (2015). Environmental condition alters amphetamine self-administration: role of the MGluR5 receptor and schedule of reinforcement. Psychopharmacology, 232(20), 3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, & Dwoskin LP (1995). Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacology, biochemistry, and behavior, 51(2–3), 397–405. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, & Deaton C (2001). Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology, 155(3), 278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bastle RM, Kufahl PR, Turk MN, Weber SM, Pentkowski NS, Thiel KJ, & Neisewander JL (2012). Novel cues reinstate cocaine-seeking behavior and induce fos protein expression as effectively as conditioned cues. Neuropsychopharmacology, 37(9), 2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington H, Vialou V, LaPlant Q, Ohnishi Y, & Nestler E (2011). Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neuroscience Letters, 493(3), 122–126. doi: 10.1016/j.neulet.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, & Solinas M, (2009). Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology, 34(13), 2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, & Moore AN (2009). Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience, 163(1), 1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, & Everitt BJ (2008). Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology, 33(6), 1413. [DOI] [PubMed] [Google Scholar]
- Einon DF, & Morgan MJ (1977). A critical period for social isolation in the rat. Developmental Psychobiology, 10(2), 123–132. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, & Tsai LH (2007). Recovery of learning and memory is associated with chromatin remodelling. Nature, 447(7141), 178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fone K, & Porkess M (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neuroscience & Biobehavioral Reviews, 32(6), 1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Ceresér KMM, Martins MR, Valvassori SS, Réus GZ, Quevedo J, Kapczinski F (2006). Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sciences, 79(3), 281–286. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Galaj E, Manuszak M, & Ranaldi R (2016), Environmental enrichment as a potential intervention for heroin seeking. Drug and Alcohol Dependence, 163, 195–201. doi: 10.1016/j.drugalcdep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Garcia EJ, # Arndt DL, & Cain ME (2019). Dynamic Interactions of Ceftriaxone and Environmental Variables Suppress Amphetamine Seeking. Brain Research, pii: S0006–8993(19)30068-X. doi: 10.1016/j.brainres.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates LA, Shi J, Rohira AD, Feng Q, Zhu B, Bedford MT, Sagum CA, Jung SY, Qin J, Tsai M, Tsai SY, Li W, Foulds CE, & O’Malley BW (2017). Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. Journal of Biological Chemistry, jbc-M117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino A, Jayanthi S, & Cadet JL (2015). Epigenetic landscape of amphetamine and methamphetamine addiction in rodents. Epigenetics, 10(7), 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, & Tsai LH (2013). The potential of HDAC inhibitors as cognitive enhancers. Annual Review of Pharmacology and Toxicology, 53, 311–330. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 58(3), 539–59. [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, & Nestler EJ (2010). Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biological Psychiatry, 67(1), 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Gehrke B, & Bardo M (2002). Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology, 162(4), 373378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, & Harkness J (2008). Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behavioural Pharmacology. 19(8), 777–85. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudsnuk K, & Champagne FA (2012). Epigenetic influence of stress and the social environment. ILAR journal, 53(3–4), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, … Nelson P (2000). Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience, 100(4), 749–768. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, & Robbins TW (1998). Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems, Pharmacology Biochemistry and Behavior, 59(4), 859–872. [DOI] [PubMed] [Google Scholar]
- Hitchcock LN, Raybuck JD, Wood MA, & Lattal KM (2018). Effects of a histone deacetylase 3 inhibitor on extinction and reinstatement of cocaine self-administration in rats. Psychopharmacology, 1–13. doi: 10.1007/s00213-018-5122-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford R, Darna M, Wilmouth C, Dwoskin L, & Bardo M (2014). Environmental enrichment reduces methamphetamine cue-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behavioural Brain Research, 270, 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, & Turner BB (1991). “Isolation stress” revisited: Isolation rearing effects depend on animal care methods, Physiology and Behavior, 49(6), 1107–1118. [DOI] [PubMed] [Google Scholar]
- Host L, Anglard P, Romieu P, Thibault C, Dembele D, Aunis D, & Zwiller J (2010). Inhibition of histone deacetylases in rats self‐administering cocaine regulates lissencephaly gene-1 and reelin gene expression, as revealed by microarray technique. Journal of Neurochemistry, 113(1), 236–247. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research (2011). Guide for the care and use of laboratory animals (8th ed.). Washington, DC: National Academies Press; Retrieved from http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf [Google Scholar]
- Ito K, & Adcock IM (2002). Histone acetylation and histone deacetylation. Molecular biotechnology, 20(1), 99–106. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A, & Cadet JL (2014). Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biological Psychiatry, 76(1), 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalda A, Heidmets L-T, Shen H-Y, Zharkovsky A, & Chen J-F (2007). Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behavioural Brain Research, 181(1), 76–84. doi: 10.1016/j.bbr.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, & Bae SC (2011). Histone deacetylase inhibitors: Molecular mechanisms of action and clinical trials as anti-cancer drugs. American Journal of Translational Research, 3(2), 166–179. [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, & Mayford M (2004). CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron, 42(6), 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Worrell TR, Zimnisky R, & Schmauss C (2012). Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiology of disease, 45(1), 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, & Olive MF (2014). Early life stress interactions with the epigenome: potential mechanisms driving vulnerability towards psychiatric illness. Behavioural pharmacology, 25(5 0 6), 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nestler EJ, & Hyman SE (2009). Chapter 6: widely projecting systems: monoamines, acetylcholine, and orexin. Molecular neuropharmacology: A foundation for clinical neuroscience, 147–157. [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, & Wood MA (2010). Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biological Psychiatry, 67(1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, … & Wood MA (2013). HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proceedings of the National Academy of Sciences, 110(7), 2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, & Cadet JL (2012). Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLOS ONE, 7(3), e34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason et al. (2018). Neurodevelopmental Epigenetics of Substance Abuse. Journal of Drug and Alcohol Research, 2018. [Google Scholar]
- McCowan TJ, Dhasarathy A, & Carvelli L (2015). The epigenetic mechanisms of amphetamine. Journal of addiction & prevention, 2015 (Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, & Wood MA (2010). Epigenetic regulation in substance use disorders. Current Psychiatry Reports, 12(2), 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2014). Epigenetic mechanisms of drug addiction. Neuropharmacology, 76, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LM, Van Kempen TA, & Zimmerberg B (2013). Behavioral effects of repeated handling differ in rats reared in social isolation and environmental enrichment. Neuroscience Letters, 536, 47–51. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, McCleery EJ, Cunningham CL, Wood MA, & Lattal KM (2013). The histone deacetylase inhibitor sodium butyrate modulates acquisition and extinction of cocaine-induced conditioned place preference. Pharmacology Biochemistry and Behavior, 106, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ, & Rosenzweig MR (1987). Enriched and impoverished environments: Effects on brain and behavior. New York: Springer-Verlag. [Google Scholar]
- Renthal W, Maze I, Krishnan V, H. III, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, & Nestler E (2007). Histone Deacetylase 5 Epigenetically Controls Behavioral Adaptations to Chronic Emotional Stimuli. Neuron, 56(3). doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Robison AJ, & Nestler EJ (2011). Transcriptional and epigenetic mechanisms of addiction. Nature Reviews Neuroscience, 12(11), 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, & Zwiller J (2011). The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Current Neuropharmacology, 9(1), 21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, & Zwiller J (2008). Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. The Journal of Neuroscience, 28(38), 9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya J, & Barco A (2009). Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology, 34(13), 2642–2654. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lewis MC, Fass DM, Wagner FF, Zhang Y-L, Hennig KM, Gale J, Zhao W-N, Reis S, Barker DD, Berry-Scott E, Kim SW, Clore EL, Hooker JM, Holson EB, Haggarty SJ, Petryshen TL (2013). A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests. PLOS ONE, 8(8). doi: 10.1371/journal.pone.0071323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, & Bilbo SD (2011). Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. Journal of Neuroscience, 31(49), 17835–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, & Kelly JP (2011). The impact of environmental enrichment in laboratory rats-Behavioural and neurochemical aspects. Behavioural Brain Research, 222(1), 246–264. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, & Thiele TE (2014). Histone Acetylation in the Nucleus Accumbens Shell Modulates Ethanol‐Induced Locomotor Activity in DBA/2 J Mice. Alcoholism: Clinical and Experimental Research, 38(9), 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Klein ED, & Bardo MT (2006). Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behavioural Pharmacology, 17(7), 597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, & Bardo MT (2009). Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacology Biochemistry & Behavior, 92(3), 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, & Bardo MT (2011). Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology, 218(1), 293–301. doi: 10.1007/s00213-011-2448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD (2009). Experience-dependent epigenetic modifications in the central nervous system. Biological Psychiatry, 65(3), 191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Lewis CR, & Olive MF (2013). The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Substance abuse and rehabilitation, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teva Pharmaceuticals USA. (2017). Adderall® CII (dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate and amphetamine sulfate tablets) [Package insert]. [Google Scholar]
- Tsankova N, Berton O, Renthal W, Kumar A, Neve R, & Nestler E (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature Neuroscience, 9(4), 519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, & Everitt BJ (2005). Involvement of the dorsal striatum in cue-controlled cocaine seeking. Journal of Neuroscience, 25(38), 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, & Nestler EJ (2018). Neuroepigenetics and addiction. Handbook of clinical neurology, 148, 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, & Ma L (2010). Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIα in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology, 35(4), 913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Vandegrift BJ, Zhang H, Lasek AW, Pandey SC, & Brodie MS (2018). Histone Deacetylase Inhibitor Suberanilohydroxamic Acid Treatment Reverses Hyposensitivity to γ-Aminobutyric Acid in the Ventral Tegmental Area During Ethanol Withdrawal. Alcoholism: Clinical and Experimental Research, 42(11), 2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]