Abstract
Prostate cancer is globally the second most commonly diagnosed cancer type in men. Recent studies suggest that mutations in DNA repair genes are associated with aggressive forms of prostate cancer and castration resistance. Prostate cancer with DNA repair defects may be vulnerable to therapeutic targeting by Poly(ADP-ribose) polymerase (PARP) inhibitors. PARP enzymes modify target proteins with ADP-ribose in a process called PARylation and are in particular involved in single strand break repair. The rationale behind the clinical trials that led to the current use of PARP inhibitors to treat cancer was to target the dependence of BRCA-mutant cancer cells on the PARP-associated repair pathway due to deficiency in homologous recombination. However, recent studies have proposed therapeutic potential for PARP inhibitors in tumors with a variety of vulnerabilities generating dependence on PARP beyond the synthetic lethal targeting of BRCA1/BRCA2 mutated tumors, suggesting a wider potential than initially thought. Importantly, PARP-associated DNA repair pathways are also closely connected to androgen receptor (AR) signaling, which is a key regulator of tumor growth and a central therapeutic target in prostate cancer. In this review, we provide an extensive overview of published and ongoing trials exploring PARP inhibitors in treatment of prostate cancer and discuss the underlying biology. Several clinical trials are currently studying PARP inhibitor mono-and combination therapies in the treatment of prostate cancer. Integration of drugs targeting DNA repair pathways in prostate cancer treatment modalities allows developing of more personalized care taking also into account the genetic makeup of individual tumors.
Keywords: castration resistant prostate cancer, DNA damage repair, PARP inhibitors, precision medicine
1. Introduction
Although advances have been made in early detection and treatment of localized prostate cancer, metastatic prostate cancer is one of the most common causes of male cancer deaths. The standard first line treatment of metastatic prostate cancer is androgen deprivation therapy (ADT). ADT can be combined with docetaxel chemotherapeutic agent or androgen receptor (AR) signaling inhibitors at first line setting or later when castration resistance has developed [1]. However, the majority of patients eventually develop a resistant disease, which does not respond to any current therapies. New clinical targets and therapies are needed to develop better and more personalized treatment options for aggressive and castration resistant prostate cancer (CRPC). In this review, we discuss the significance of targeting DNA damage repair pathways in prostate cancer and focus in particular on covering the biological background and clinical potential of drugs inhibiting Poly(ADP-ribose) polymerase (PARP) enzymes which are also uniquely connected to AR signaling.
2. DNA Damage Response and PARP
In the course of evolution, a complex signaling pathway known as DNA damage response (DDR) developed to handle endogenous threats arising during cellular metabolism and hydrolytic reactions or exogenous insults constantly damaging the DNA. Maintaining the genomic integrity is needed for preventing diseases, such as cancer, which are associated with genomic instability. Genomic instability is an inherent property of most malignancies [2]. In order for cells to proliferate they proceed through cell cycle by entering checkpoints in which they must pass certain signaling requirements. During checkpoints, protein complexes assess the condition of cellular DNA and react on aberrations. DDR is a complicated system with three phases–detection, accumulation of repair factors and physical repair. Within DDR simultaneous pathways exist that can accommodate shortcomings of parallel mechanisms. DDR monitors integrity of the DNA and in the case of lesion, it activates cell cycle arrest and DNA repair machinery [3].
Poly(ADP-ribose) polymerase (PARP) family has a significant role in DNA repair. PARP protein family is composed of 17 members, but however, only PARP1 and PARP2 are related to DDR [4] PARP1 is a highly conserved multifunctional enzyme and it binds to DNA breaks and recruits DNA repair proteins to the damaged site. It is involved in regulation of chromatin structure and DNA metabolism, and it is a survival factor that plays a role in the maintenance of genomic integrity [5,6,7]. It has been estimated that the catalytic activity of PARP1 is stimulated 500 fold by single strand or double strand DNA breaks [4]. Upon detection of a DNA strand break, PARP1 binds to the DNA through its DNA-binding domain, cleaves nicotinamide adenine dinucleotide (NAD+), and then transfers the resulting ADP-ribose onto itself or other target proteins by formation of a covalent bond between the protein and the ADP-ribose or subsequent polymers of ADP-ribose [8,9]. This reaction called PolyADP-ribosylation (PARylation), is a post-translational modification that auto-activates PARP and acts as a signal for other DNA-repairing enzymes and DNA repair [4,8].
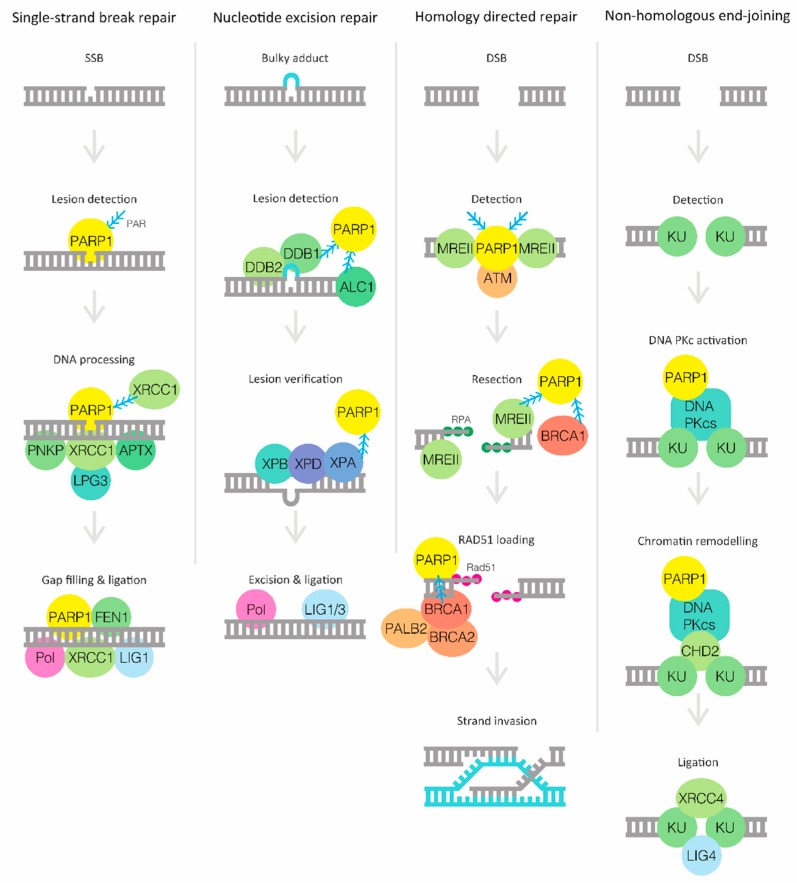
DNA damage may occur as single strand breaks (SSB) or double strand breaks (DSB) in response to a variety of DNA damaging agents and conditions. The most common type of DNA damage are single base modifications and nucleotide damage caused by reactive endogenous metabolites and by spontaneous base modifications, such as oxidations of bases or generation of abasic sites [10]. This type of damage is repaired by excision repair mechanisms, including single strand break repair (SSBR), base excision repair (BER) and nucleotide excision repair (NER) (Figure 1). PARP1 is essential especially in SSBR by detecting and binding to SSBs followed by addition of PAR to PARP1 itself leading to its activation [11]. Active PARP1 then recruits X-ray repair cross-complementing protein 1 (XRCC1), a core repair factor that acts as a scaffold for polynucleotide kinase 3’-phosphatase (PNKP), aparataxin (APTX) and DNA ligase 3 (LIG3) to process the SSB. This is followed by a gap filling step carried out by DNA polymerase δ (Pol δ), Pol ε and Pol β, in addition to PARP1 that promotes the activity of flap endonuclease 1 (FEN1). The final DNA ligation is performed by LIG1 [12].
Figure 1.
Basic principles and components of major DNA damage response (DDR) pathways in which Poly(ADP-ribose) polymerase 1 (PARP1) has a fundamental role. PARP1 detects the DNA lesion in single strand break repair (SSBR) and in homologous recombination (HR), which is the most common form of homology directed repair [11,20]. PARP1 generally takes part in recruiting repair factors to the lesion site and later interacts with or promotes activity of enzymes during physical repair stage of DDR [12,13,14,15,26].
The role of PARP1 in BER is still unclear; however, PARP1 plays an important role in NER during initial steps of damage recognition as well as during recruitment of subsequent repair proteins (Figure 1). The DNA-damage-binding protein 1 (DDB1)-DDB2 complex is recruited to autoPARylated PARP1 at the DNA lesion, followed by the recruitment of amplified in liver cancer protein 1 (ALC1) resulting in chromatin decondensation [13,14]. This is followed by recruitment of xeroderma pigmentosum group C-complementing protein (XPC) and RAD23B leading to lesion verification involving binding of XPB and XPD through interaction with XPA and PARP1. Finally, the damaged site is excised by nucleases, excision repair cross-complementing group 1 protein (ERCC1) and XPG, and the gap is filled by Pol δ, Pol ε and Pol κ, and ligated by LIG1 and LIG3 [15].
DSBs are formed upon exposure to DNA damaging agents, such as ionizing irradiation, endogenously during programmed genome arrangements or from replication fork collapse [16]. The two main DSB repair pathways in eukaryotic cells are homologous recombination (HR) and non-homologous end joining (NHEJ) (Figure 1) [17]. HR requires a DNA template for error-free repair and occurs therefore after replication in S and G2 phases of the cell cycle while the error-prone NHEJ is the predominant repair pathway during G1 phase. In addition to detecting single strand breaks, PARP1 also recognizes DSBs [18]. The key kinases involved in the repair of DSBs belong mainly to phosphatidylinositol 3-kinase-like protein kinase (PIKKs) family [3]. These proteins include atypical serine-threonine kinases ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3 related (ATR) and DNA-dependent protein kinases (DNA-PKs) [19]. After detecting and binding to a DSB, PARylated PARP1 promotes recruitment of ATM and the Mre11-Rad50-Nbs1 (MRN) complex through binding of meiotic recombination 11 (Mre11) to activate HR [20]. While ATM activates checkpoint and repair pathway proteins by phosphorylation, the MRN complex initiates DNA end resection to enable binding of replication protein A (RPA) to the resulting single stranded DNA. For the next step in repairing the lesion, PARP1 is involved in recruiting breast cancer type 1 susceptibility protein (BRCA1) to the damaged site in complex with BRCA2 and partner and localizer of BRCA2 (PALB2) [21]. BRCA2 delivers RAD51 to the damaged site, which is required to initiate strand invasion and finally repair through D-loop formation and DNA synthesis [22,23,24]. Chk1 and Chk2 are serine-threonine kinases acting downstream of ATM and ATR that facilitate the DNA damage response by initiating cell cycle arrest thereby allowing time for repair. Chk1 is phosphorylated by ATR, primarily in response to replication stress, while ATM activates Chk2 following double strand breaks. Both reactions lead to phosphorylation of CDC25 resulting in its degradation preventing release from cell cycle checkpoints [25]. When repair by NHEJ is preferred, the DSB is bound by KU70-KU80 dimers, which activate DNA-PKcs. PARP1 interacts with DNA-PK, stimulating its activity, and is also involved in subsequent steps in the repair process by facilitating the recruitment of chromodomain helicase DNA-binding protein 2 (CHD2) [14,26]. CHD2 is crucial in recruiting XRCC4 and LIG4 to the site for final DNA ligation [12].
3. Targeting DNA Repair Defects in Cancer with PARP Inhibitors
The development of PARP inhibitors is mainly based on two approaches: in the first strategy PARP inhibition is combined with DNA damaging therapy; and in the second, the aim is to target cells that are already genetically predisposed to die in the absence of PARP activity [27]. In 2005, two benchmark studies proved that BRCA1 or BRCA2 DNA repair defect causing mutations sensitize cells to PARP inhibition, which results in chromosomal instability, cell cycle arrest and subsequent apoptosis [28,29]. Mechanistically, a cell with defects in HR becomes reliant on an alternative DDR system that uses PARP as a catalyst for single stranded DNA repair (Figure 1). A PARP system allows cells with HR deficiency to stay viable but at the same time creates a pharmaceutical target as cells that lose both HR and PARP-assisted SSBR face cell death through synthetic lethality. In the synthetic lethality concept combined effects lead to cell death when present simultaneously. In the pioneering clinical trials PARP inhibitors (PARPi) were used to treat HR deficient patients with germline BRCA1/BRCA2 mutations (gBRCA1/2m) [30]. Remarkable recent results in newly diagnosed ovarian cancer patients with gBRCAm further support the proof-of-concept for synthetic lethal targeting of BRCAm patients with PARPi [31].
There are two different aspects to PARP inhibitor mechanism of action–the inhibitions of catalysis and trapping of PARP enzymes at the site of DNA damage (Figure 2) [32]. According to current knowledge, all PARPi suppress the catalytic activity of both PARP1 and PARP2 [27]. This prevents the NAD+ binding and eventually inhibits the attachment of ADP-ribose polymers on targets [33]. Without PARylation cells cannot progress through G2 phase and enter M phase [34]. In addition, PARP inhibition has been linked to increased rates in apoptosis [35]. PARPi also traps both PARP1 and PARP2 at the site of SSB, preventing PARP from detaching from DNA. These PARP-DNA complexes are more cytotoxic than SSBs caused by deficient PARP enzymes [36]. Both PARP1 and PARP2 are parts of larger protein complexes and the meeting of the ongoing replication fork with the PARP-DNA complex eventually leads to double strand ends and replication fork collapse [32,37,38]. The potency of trapping PARP enzymes differs significantly between inhibitors. Talazoparib is more potent than niraparib, which is more potent than olaparib and rucaparib, which again are more potent than veliparib when considering the ability to trap PARP at the site of DNA damage (trapping efficiency: talazoparib >> niraparib > olaparib = rucaparib >> veliparib) [36,39]. Although some PARP inhibitors can equally efficiently target both PARP1 and PARP2, PARP1 may be the major target for them in human cells, since the expression levels of PARP1 are much higher than PARP2 [4,40].
Figure 2.
PARP inhibitors (PARPi) reduce the catalytic activity of PARPs. In addition, PARPi trap PARP at the site of DNA damage by preventing PARP from detaching from DNA. Cytotoxic PARP-DNA complexes prevent replication fork from progressing and lead to cell death unless damage is repaired [32,36].
4. Germline Mutations in DNA Damage Repair Genes in Prostate cancer
Multiple studies have reported association of frequent germline deleterious mutations in DDR genes with advanced prostate cancer, which defines the basis for the use of PARP inhibitors to treat prostate cancer. Specifically, germline BRCA1/2 mutations associate with increased risk and with more aggressive prostate cancer (Gleason ≥ 8), higher risk of nodal involvement, and distant metastasis at diagnosis [41]. Subgroup analyses confirmed the poor outcomes in particular in BRCA2 patients, whereas the role of BRCA1 was not well defined due to the limited size and follow-up in this subgroup [41]. This was strengthened by Leongamornlert et al., who described frequent germline deleterious mutations in DNA repair genes and discovered 14 new loss-of-function (LOF) mutations in 7.3% of familial prostate cancer patients. These germline mutations were more frequently associated with nodal involvement, metastasis or T4 tumor stage [42].
In a prospective multicenter cohort study, the prevalence of germline mutations were screened in 107 DDR-associated genes. 16.2% of patients were found to be carriers (3.3% BRCA2, 1.9% ATM, 0.96% BRCA1). Although the impact of ATM/BRCA1/BRCA2 germline mutations on cause-specific survival (CSS) was not statistically significant, the CSS was halved in germline BRCA2 carriers. Germline BRCA2 mutations were identified as an independent prognostic factor for CSS. It was concluded that germline BRCA2 mutations have a deleterious impact on metastatic CRPC outcomes that may be affected by the first line of treatment used [43].
With respect to germline mutations in DDR genes, a recent study found the incidence of inherited DNA repair gene alterations in metastatic prostate cancer to be significantly higher (11.8%) than in men with localized prostate cancer (4.6%) and in the general population (2.7%) [44] Specifically, mutations in BRCA2 (5.3%), CHEK2 (1.9%), ATM (1.6%), BRCA1 (0.9%), PALB2 (0.4%), RAD51D (0.4%), were significantly enriched in patients with metastatic prostate cancer compared to the general population. These findings suggest that this subset of men with germline mutations in DDR genes are more likely to develop metastatic prostate cancer and may potentially benefit from PARPi therapy [44].
Prevalence of germline BRCA2 mutations with known pathogenic annotation was reported to be significantly higher in men with advanced and metastatic prostate cancer (3.1%) compared to organ-confined disease (0.7%) [45] Racial variation was also observed: African American patients carried more frequently BRCA1/2 variants of unknown significance (VUS) when compared to Caucasian Americans (4.6 vs. 1.6%, respectively). However, the prevalence of pathogenic mutations was similar across the races.
5. Somatic Mutations in DNA Damage Repair Genes in Prostate Cancer
Multiple candidate driver mutations in DDR and collaborating AR signaling pathway genes have been identified through exome sequencing of lethal, heavily pre-treated mCRPC autopsy samples [46]. Two deleterious somatic mutations in PRKDC (I1137 frame shift and E640 non-sense) encoding the catalytic subunit of the DNA-PK were reported in a patient with extremely aggressive localized prostate cancer. In addition, high level focal deletions of genes involved in DNA repair in hypermutated CRPC samples were found. For example, somatic, focal homozygous deletion in the mismatch repair gene MSH2, and a somatic homozygous deletion of a ~2 MB region on chr13 harboring BRCA2 [46].
Further discoveries of genomic landscape of mCRPC showed that approximately 23% of patients harbor somatic DNA repair pathway aberrations [47]. Of these, BRCA2, BRCA1, and ATM account for 19.3% overall and they were substantially more frequent in mCRPC compared to those in primary prostate cancers. 12.7% of cases had loss of BRCA2, of which 90% exhibited biallelic loss. Of note, 5.3% of mCRPC patients harbored pathogenic germline BRCA2 mutations with a subsequent somatic event that resulted in biallelic loss, revealing a high frequency relative to primary prostate cancer. In addition, mutational events were noted in CDK12, FANCA, RAD51B and RAD51C [47]. Also other studies have showed similar results finding approximately 12% and 8% of prostate cancer patients carrying a BRCA2 or ATM mutation, respectively [48]. Somatic BRCA2 mutations have been suggested to arise early in tumors from patients who eventually develop metastatic disease, while ATM alterations seem to enrich in CRPC [49].
6. Crosstalk between AR Signaling and DNA Damage Response
AR signaling is one of the major growth promoting pathways in prostate cancer, and ADT is the corner stone of prostate cancer treatment. Several lines of evidence suggest that prostate tumor cells are uniquely connected to DDR through AR signaling. AR signaling inhibitors seem to down-regulate DDR gene expression and increase DNA damage [50]. ADT results in the state of BRCAness, a phenotype with treatment susceptibility analogous to that of BRCA dysfunctional cancer, including HR deficiency and increased PARP activation, leading to sensitivity of prostate cancer to PARP inhibition in combination with AR signaling inhibitors in preclinical setting [51]. In prostate cancer xenograft experiments, a superior effect was observed when enzalutamide was administered prior to olaparib when compared to olaparib or enzalutamide monotherapy [52]. PARP1 expression is significantly up-regulated in several cancers including breast and ovarian cancer, although up-regulation is less striking in prostate cancer [53]. Moreover, PARP may promote the transcriptional activity of AR in prostate cancer [54]. Thus, PARPs may have a role as drug targets in prostate cancer beyond being targeted as a part of the concept of synthetic lethality in tumors with DDR gene mutations. Increased PARP1 activity correlates also with more advanced disease and poor outcome in prostate cancer [55]. However, changes in PARP1 activity appear to be unaffected by DSBs or increased PARP1 expression [55]. In addition, in genetic mouse models, loss of PARP activity has been linked to increase prostate tumorigenesis, suggesting that the role of PARP in prostate cancer is complex [56]. Interestingly, also combination of AR signaling inhibitors with inhibitors of Chk1 promotes DNA damage and eventually leads to cell death, supporting the close relationship between AR signaling and DDR [57].
7. Clinical Development of PARP Inhibitors in Prostate Cancer
Four PARP inhibitors currently have an FDA approval and an indication in treating ovarian and breast cancer (Table 1). A few early phase studies have already been completed in the treatment of prostate cancer with PARP inhibitors (Table 2). Several ongoing clinical trials are currently studying different PARP inhibitors as monotherapy or in combination with other treatments ranging from chemotherapy, AR signaling inhibitors, radical prostatectomy, radiation therapy, immune therapy and different targeted agents (Table 3).
Table 1.
PARP inhibitor drugs approved by the FDA.
| Compound | Company | Indications | Date of Approval | Stage of Development for PCa |
|---|---|---|---|---|
| Olaparib (Lynparza ®) | AstraZeneca Pharmaceuticals LP (Cambridge, UK) | gBRCA-mutated advanced ovarian cancer | December 2014 | III |
| Maintenance treatment of recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer | August 2017 | |||
| gBRCA-mutated HER2-negative metastatic breast cancer | January 2018 | |||
| Maintenance treatment of gBRCA- or sBRCA-mutated advanced epithelial ovarian, fallopian tube or primary peritoneal cancer | December 2018 | |||
| Rucaparib (Rubraca ®) | Clovis Oncology, Inc. (Boulder, CO, USA) | gBRCA- or sBRCA-mutated advanced ovarian cancer | December 2016 | III |
| Maintenance treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer | April 2018 | |||
| Niraparib (Zejula ®) | Tesaro, Inc., (Waltham, MA, USA) | Maintenance treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer. | March 2017 | III |
| Talazoparib (Talzenna ®) | Pfizer, Inc. (New York, NY, USA) | gBRCA-mutated HER2-negative locally advanced or metastatic breast cancer | October 2018 | III |
Pca: Prostate cancer; germline BRCA (gBRCA) or somatic BRCA (sBRCA) mutation.
Table 2.
Efficacy results of PARP inhibitor treatment in prostate cancer.
| Treatment Regimen | Phase | Number of Pca Patients | Biomarkers | CR (%) | PR (%) | SD (%) | PD (%) | OS (Months) | PFS (Months) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Niraparib | I | 21 | BRCAm 5 % (n = 1) | 0 | 43 | [58] | ||||
| Olaparib | I | 3 | BRCA2m 33 % (n = 1) | [30] | ||||||
| Olaparib or niraparib | I | 4 | BRCA2m 100 % (n = 4) | 0 | 25 | 25 | 50 | [59] | ||
| Olaparib | II | 8 | gBRCA2m 87.5% (n = 7), gBRCA1m 12.5 % (n = 1) | 0 | 50 | 25 | 25 | 18.4 | 7.2 | [60] |
| Olaparib (TOPARP-A) | II | 50 | Overall 33 % (n = 16), BRCA2m (n = 7), ATMm (n = 5), BRCA1m or CHEK2m with FANCAm (n = 3), PALB2m (n =1), HDAC2m (n = 1) | 0 | 19 | 6 | 13.8 vs 7.5 * | 9.8 vs 2.7 * | [61] | |
| Abiraterone and prednisolone with or without olaparib | II | 71 vs 71 † | HRR mutation 15 vs 14 % † | 0 | 27 vs 32 †, ns | 48 vs 21 † | 21vs 47 † | 22.7 vs 20.9 †, ns | 13.8 vs 8.2 † | [62] |
| Olaparib and durvalumab | II | 17 | DDRm 77% of responders‡, gBRCA2m 33%, sBRCA2m 22%, gNBNm 11%, PMS2m 11% |
24 | 16.1 | [63] | ||||
| Talazoparib | I | 4 | [64] | |||||||
| Veliparib and temozolomide | I | 26 | 0 | 0§ | 9.2 | 2.1 | [65] | |||
| Veliparib | I | 3 | BRCA2m 100% | 0 | 66 | 33 | [66] | |||
| Veliparib, carboplatin and gemcitabine | I | 1 | BRCAm 100 % (n = 1) | 100 | [67] | |||||
| Abiraterone and prednisolone with or without veliparib | II | 79 vs 74 † | Overall DDRm 31% (n = 25) of 80 analyzed, BRCA2m (n = 15), ATMm (n = 4), BRCA1m (n = 4), RAD51Bm (n = 1), RAD51Cm (n = 1), PALB2m (n = 1), FANCAm (n =1) | 0 vs 3 † | 52 vs 45 † | 26 vs 35 † | 17 vs 20 † | 32.3 vs 30.6 † | 11 vs 10.1 † | [68] |
* Biomarker-positive group vs biomarker-negative group. † PARPi vs control. ‡ Subjects with PSA decline of ≥50%. §None of the measurable disease achieved an objective response according to RECIST. CR: Complete response; HRR: Homologous recombination repair; OS: Overall survival;; PFS: Progression-free survival; PD: Progressive disease; PR: Partial response; SD: Stable disease; ns= not significant. Information was compiled by searching the Pubmed and Web of Science databases. The search terms included ‘Prostate cancer’, ‘Olaparib’, ‘AZD2281’, ‘KU-0059436’, ‘Rucaparib’, ‘Niraparib’, ‘MK-4827’, ‘Talazoparib’, ‘BMN 637’, ‘MDV3800’, ‘Veliparib’, ‘CEP-9722’, ‘Pamiparib’ and ‘BGB-290’.
Table 3.
Ongoing clinical trials using PARP inhibitors to treat prostate cancer.
| Treatment Regimen | Status | Allocation | HRD Selection | Estimated Enrollment | Phase | CTID |
|---|---|---|---|---|---|---|
| PARPi monotherapies | ||||||
| Niraparib | Recruiting | Yes | 301 | II | NCT02854436 | |
| Olaparib | Recruiting | No | 89 | II | NCT01682772 | |
| Olaparib | Active, not recruiting | Randomized | Yes | 340 | III | NCT02987543 |
| Olaparib | Recruiting | Yes * | 50 | II | NCT03047135 | |
| Olaparib | Recruiting | Randomized | No | 96 | II | NCT03263650 |
| Olaparib | Recruiting | Yes | 27 | II | NCT03434158 | |
| Pamiparib | Recruiting | Yes | 100 | II | NCT03712930 | |
| Rucaparib | Recruiting | Yes | 360 | II | NCT02952534 | |
| Rucaparib | Recruiting | Yes | 30 | II | NCT03413995 | |
| Rucaparib | Recruiting | Yes | 29 | II | NCT03533946 | |
| Talazoparib | Recruiting | Yes | 100 | II | NCT03148795 | |
| PARPi + AR signaling inhibitors | ||||||
| Niraparib and Abiraterone and Prednisolone | Recruiting | Randomized | Yes | 1000 | III | NCT03748641 |
| Olaparib or Olaparib and Abiraterone and Prednisone | Recruiting | Randomized | Yes | 70 | II | NCT03012321 |
| Olaparib and Abiraterone and Prednisolone | Recruiting | Randomized | No | 720 | III | NCT03732820 |
| Rucaparib and Abiraterone, Enzalutamide or Docetaxel | Recruiting | Randomized | Yes | 400 | III | NCT02975934 |
| Niraparib and Apalutamide or Abiraterone and Prednisolone | Active, not recruiting | No | 34 | I | NCT02924766 | |
| Niraparib and Enzalutamide | Terminated (Suspended by funder) | No | 2 | I | NCT02500901 | |
| Talazoparib and Enzalutamide | Recruiting | Randomized | Yes† | 872 | III | NCT03395197 |
| PARPi + immune checkpoint inhibitors | ||||||
| Talazoparib and Avelumab | Recruiting | Non-Randomized | No | 242 | Ib/II | NCT03330405 |
| Olaparib and Durvalumab | Recruiting | Yes | 32 | II | NCT03810105 | |
| Niraparib and JNJ-63723283 or Abiraterone and Prednisolone | Recruiting | Non-Randomized | Yes | 150 | Ib–II | NCT03431350 |
| Rucaparib and Nivolumab | Recruiting | Non-Randomized | No | 330 | II | NCT03338790 |
| Rucaparib or Rucaparib and Nivolumab | Recruiting | Randomized | No | 60 | Ib/Iia | NCT03572478 |
| Olaparib and Pembrolizumab | Recruiting | Non-Randomized | No | 400 | I | NCT02861573 |
| Olaparib and Pembrolizumab | Not yet recruiting | Randomized | No | 780 | III | NCT03834519 |
| PARPi + chemotherapy agents | ||||||
| Rucaparib, Docetaxel and Carboplatin | Recruiting | Yes | 20 | II | NCT03442556 | |
| Pamiparib and Temozolomide | Recruiting | Non-Randomized | Yes | 150 | I | NCT03150810 |
| PARPi + radionuclide therapies | ||||||
| Niraparib and Radium Ra 223 Dichloride | Recruiting | No | 6 | I | NCT03076203 | |
| Olaparib and Radium Ra 223 Dichloride | Recruiting | Randomized | No | 112 | II | NCT03317392 |
| Olaparib and 177Lu-PSMA | Not yet recruiting | No | 52 | I | NCT03874884 | |
| PARPi + surgical procedures | ||||||
| Olaparib and RP | Recruiting | Yes | 13 | II | NCT03432897 | |
| Olaparib and RP | Recruiting | Yes | 15 | II | NCT03570476 | |
| PARPi + VEGF RTK inhibitors | ||||||
| Olaparib and Cediranib | Active, not recruiting | Randomized | No | 90 | II | NCT02893917 |
| PARPi + AKT inhibitors | ||||||
| Rucaparib and Ipatasertib | Not yet recruiting | Non-Randomized | No | 54 | Ib | NCT03840200 |
| PARPi + androgens | ||||||
| Olaparib and Testosterone Enanthate or Cypionate | Recruiting | Yes | 30 | II | NCT03516812 | |
| PARPi + ATR inhibitors | ||||||
| Olaparib and AZD6738 | Not yet recruiting | Non-Randomized | No | 47 | II | NCT03787680 |
| PARPi + GnRH antagonists | ||||||
| Olaparib and Degarelix | Recruiting | Randomized | No | 20 | I | NCT02324998 |
| PARPi + nanoparticle conjugate | ||||||
| Olaparib and CRLX101 | Recruiting | Non-Randomized | No | 123 | I/II | NCT02769962 |
| Personalized medicine approach | ||||||
| SMMART therapy | Not yet recruiting | No | 52 | I | NCT03878524 | |
| PARPi + radiation treatment | ||||||
| Olaparib and RT | Recruiting | Randomized | No | 112 | I/II | NCT03317392 |
* Two-stage design study will conduct enrichment of study population before entering stage 2 if the original population has less than desired number of confirmed HRD associated gene aberrations. † Part 1 of the study confirms the starting dose of talazoparib in combination with enzalutamide in genetically unselected population. CTID: Clinical trials identifier; HRD Selection: prescreening of homologous recombination deficiency associated mutations and preselection prior to treatment; RP: Radical prostatectomy; RT: Radiation therapy.Information was compiled by searching the ClinicalTrials.gov. The search was conducted under ‘Condition or disease’ of ‘Prostate cancer’ and additional search terms included ‘Olaparib’, ‘AZD2281’, ‘KU-0059436’, ‘Rucaparib’, ‘Niraparib’, ‘MK-4827’, ‘Talazoparib’, ‘BMN 637’, ‘MDV3800’, ‘Veliparib’, ‘CEP-9722’, ‘Pamiparib’ and ‘BGB-290’.
A phase II study evaluating the efficacy of olaparib for preselected gBRCA1m or gBRCA2m solid tumors showed 50% tumor response rates in CRPC patients [60] In a phase II TOPARP study, treatment with olaparib in CRPC patients with germline or somatic DDR gene defect also led to a high response rate (88%). Homozygous deletions, deleterious mutations, or both in DDR genes, including BRCA1/2, ATM, CHEK2, and PALB2, were detected in 33% of mCRPC patients enrolled in the study. Importantly, all patients with BRCA2 loss, and 4 of 5 patients with ATM aberrations, had a response to olaparib [61] Only a few responses (6 %) were detected in patients without DDR gene mutations [69] The ongoing phase 3 trials will give further insight if patients with DDR mutations benefit from PARPi. In the PROfound phase 3 study (NCT02987543) evaluating the efficacy of olaparib vs. physicians choice of either entzalutamide or abiraterone in metastatic CRPC, patients that are included should have an aberration in one of 15 genes analyzed by an HR repair assay (Foundation Medicine, Inc., Cambridge, MA, USA). Cohort A (n = 240 approx.) includes patients with mutations in BRCA1, BRCA2 or ATM, while patients with a mutation in 12 other HR genes will be assigned to Cohort B (n = 100 approx) [70] TRITON3 is an ongoing trial (NCT02975934) evaluating rucaparib monotherapy vs. physicians choice of either enzalutamide, abiraterone or docetaxel in patients with metastatic CRPC and a deleterious germline or somatic BRCA1, BRCA2, or ATM mutation identified by prior local testing or central testing during screening [71].
AR pathway’s distinct role in CRPC and especially its linkage to DDR explored in preclinical trials suggests unique opportunities for combining PARPi with AR signaling inhibitors and castration [72]. The efficacy of olaparib in combination with abiraterone was assessed in a randomized, placebo-controlled phase II study [62]. Significantly longer radiographic progression-free survival (rPFS) was reported in favor of olaparib plus abiraterone (13.8 vs 8.2 months) [62]. Surprisingly, rPFS was significantly better in favor of olaparib arm in patient subpopulation without HR defect and no evidence suggested that DDR gene mutations would sensitize to PARPi in this context [62]. Veliparib plus abiraterone combination showed also promising efficacy (Table 2) [68]. Patients were selected based on ETS transcription factor gene fusions, which did not predict response. However, patients with biallelic DDR mutations had significantly more responses and better median PFS [68]. The ongoing PROPEL phase 3 study (NCT03732820) is investigating olaparib in the combination with abiraterone in the first line setting for metastatic CRPC without prescreening patients for HR aberrations [73]. Talapro-2 in turn is an ongoing 2-part phase 3 trial (NCT03395197) analyzing the efficacy of the combination of enzalutamide plus talazoparib vs. enzalutamide plus placebo in both unselected patients and in patients with DDR mutations [74]. In the phase 3 MAGNITUDE study (NCT03748641) comparing abiraterone plus placebo to abiraterone plus niraparib during the prescreening phase participants will be evaluated for DDR gene defects identified by the sponsor’s required assays and then will be assigned to one of the two cohorts, the other containing only patients tested negative for and the other positive for DDR deficiency [75].
PARPi has also been studied in combination with DNA damaging chemotherapy. According to one phase I study, veliparib was well tolerated in combination with temozolomide but combination had only modest efficacy in CRPC [65]. One ongoing phase II study is evaluating the efficacy of PARPi in combination with docetaxel and carboplatin (Table 3).
Several lines of preclinical evidence suggest synergy between PARPi and immune checkpoint inhibitors. For example, double strand break repair pathway has been suggested to regulate PD-L1 expression in cancer [76]. Interestingly, PARP inhibition has also been shown to increase PD-L1 expression in cells depleted of BRCA2 [76]. Several checkpoint inhibitor therapy targeting immune checkpoints, including PD1 blockers nivolumab, pembrolizumab and JNJ-63723283, and PDL1 blockers avelumab and durvalumab, are combined with PARPi in the current trials in prostate cancer (Table 3). Interestingly, olaparib plus durvalumab combination demonstrated efficacy in prostate cancer patients with DDR mutations in an early phase study (Table 2) [63]. An ongoing phase 3 trial KEYLYNK-010 (NCT03834519) evaluates olaparib in the combination with pembrolizumab vs. abiraterone and prednisolone or enzalutamide in genetically unselected patients [77].
Cell signaling pathways such as PI3K-Akt pathway have been implicated in regulation of BRCAness and sensitivity to PARPi [78,79]. Preclinical evidence suggests efficacy for the combination of PARPi and Pi3K pathway inhibition in prostate cancer [80]. PARP inhibitors are currently explored in the ongoing trials also in prostate cancer in the combination with drugs targeting cell signaling pathways, such as an AKT inhibitor ipatasertib, and a VEGFR inhibitor cediranib. Moreover, PARPi are evaluated in the combination with other DDR components than PARP, such as ATR inhibitor AZD6738 (Table 3).
Adverse Events and Tolerability
PARPi are in general relatively well tolerated as monotherapy, and toxicity can often be managed with dose reductions. Typical adverse effects are myelotoxicity, in particularly anemia, fatigue and gastrointestinal symptoms [61]. However, combining PARPi with other drugs may increase likelihood of toxicity. For example, in a phase II trial of PARPi combined with abiraterone typical grade 1–2 adverse events included nausea, constipation and fatigue, and grade 3 included anemia [62]. Significantly more serious adverse events were reported in patients treated with olaparib and abiraterone (34%) when compared to abiraterone alone (18%), including myocardial infarctions and one treatment-related death due to pneumonitis [62]. Veliparib plus abiraterone combination was relatively well tolerated [68]. 24% of patients (n = 19) had grade 3 treatment-related adverse effects compared to 20% in abiraterone alone arm. One patient had grade 4 thrombocytopenia, and one patient had grade 5 cardiac arrest possibly treatment-related in veliparib plus abiraterone arm [68]. In a phase I trial of PARPi combined with temozolomide (n = 26) serious treatment–emergent adverse effects were reported in 26.9% subjects, including colitis (n = 2), hepatorenal syndrome, hyperglycemia, bone pain, mental status change, hematuria, urinary tract obstruction, epistaxis and deep vein thrombosis (all n = 1) [65]. Olaparib plus durvalumab showed acceptable toxicity profile in an early phase study [63]. The ongoing phase 3 trials will give further information regarding tolerability in prostate cancer patients in different settings (Table 3).
8. Predictive Markers of Response to PARP Inhibitors
Initial studies showed that HR deficiency due to BRCAm predicts response to PARPi [28,29,30]. Preclinical evidence suggests that tumors with mutations in other DDR genes, such as ATM, and HR regulators are also sensitive to PARPi [81,82,83]. Several potential predictive markers of sensitivity have been suggested in preclinical setting, including phosphatases and several kinases regulating DNA damage response and cell cycle [84,85]. In prostate cancer, high response rates have been reported in tumors carrying either germline or somatic mutations in DDR genes [60,61]. However, tumors with BRCA mutations appear to be more sensitive to PARPi olaparib than tumors with ATM mutations in CRPC [86]. Since the functional consequence of a mutation is not always known and the factors resulting in HR deficiency are complex, it is important to assess the functional status of HR in patient samples. Functional HR assays, including ex vivo RAD51 foci assays, have been proven beneficial and have additional value to gene sequencing in identifying patients that may benefit of PARPi in breast and ovarian cancer [87,88]. The ongoing phase 3 trials are expected to shed more light on the validity of the synthetic lethality concept in prostate cancer context.
Responses to PARPi have been detected in combination with androgen receptor inhibitors in prostate cancer also in patients without obvious mutations causing HR defect in tumors [62], suggesting that blocking AR signaling may induce clinically relevant state of BRCAness to tumors [51]. Hypoxic tumors in particular are suggested to be sensitive to PARPi [89].
9. Mechanisms of Intrinsic and Acquired Resistance to PARP Inhibitors
The major clinically described form of PARPi resistance is restoration of HR competency by a reversing secondary mutation of a HR gene [90]. For example, sequencing cell-free DNA from ovarian carcinoma patients treated with rucaparib identified BRCA reversion mutations in gBRCAm and somatic BRCAm carriers, and reversion mutations eventually associated with disease progression [91]. A HR–independent mechanism protecting the stalled replication forks can sustain viability in HR deficient cells in presence of PARPi. It is proposed that if cells survive the initial BRCA-null crisis because the replication forks are transiently protected by a defect in MRE11 recruitment, their survival is no longer dependent of PARP inhibition. Loss of PTIP or CHD4 have been observed to associate fork protection with resistance to PARPi [92,93]. In prostate cancer patients, BRCA2 reversion mutations have been associated with PARPi resistance [94]. Loss of factors regulating DSB end resection can cause PARPi resistance. In BRCA1 mutated cell lines, 53BP1 suppresses resection in HR, promoting NHEJ instead. Loss of 53BP1 function allows HR to occur and 53BP1 loss has been shown to confer resistance to PARPi [95,96]. Deletion of RIF1, a factor cooperating with 53BP1 in 5’ end resection, reduced cytotoxicity of PARPi in mice [97]. Downstream of 53BP1, REV7 was also found to coordinate repair pathway choices and to affect PARPi resistance in preclinical experiments [98]. Similarly loss of CST complex, functioning as a resection antagonist, leads to restoration of HR independently of BRCA1, causing PARPi resistance [99]. Poly(ADP-ribose) glycohydrolase (PARG) is an enzyme capable of removing PAR chains. PARG suppression was found to partially restore PARP1 signaling in the presence of PARPi in mouse Brca2 mutant cell lines and organoids [100]. Sensitivity and resistance mechanisms in combination therapy settings of prostate cancer are likely more complex due to signaling cross talk or other factors that can affect HR competency, as suggested in combination therapy trial with abiraterone and olaparib [62]. Using in vitro and mouse xenograft models Dréan et al. established that exposing tumor cell populations to platinum salt or PARPi therapy produces secondary mutated clones in a Darwinian fashion. Created PARP resistant tumor populations were; however, found to be sensitive to a WEE1 kinase inhibitor AZD-1775, encouraging the possibility of subsequent treatment options after acquired resistance [101].
10. Future Perspectives
During recent years, advances have been made in understanding the underlying genetic events and biology in different steps of prostate cancer development, but yet no genetic subtypes have been established with prognostic and/or predictive value for clinical use. Recent data suggests that high proportion of prostate cancer patients carry mutations in DNA damage repair genes. These patients may represent a new subgroup of prostate cancer patients that benefit from therapeutics targeting DNA damage response pathways, such as PARP inhibitors. DNA damage response involves hundreds of proteins and many of them are currently investigated as drug targets, in addition to PARPs [102]. Many of these emerging DDR modifiers being developed, such as DNA-PK inhibitors studied preclinically, have promising potential in prostate cancer [103,104]. This opens opportunities to develop more personalized therapeutic modalities for prostate cancer patients taking into account their tumor genomes in the future. However, deeper understanding of DDR biology in prostate cancer context and in conjunction with AR signaling is needed to fully exploit the potential of DDR targeting drugs for the benefit of patients. The first currently ongoing large phase 3 trials will tell if PARP inhibitors enter to the treatment options for CRPC in the near future. More work is defeinitely needed to understand what the best combinations and treatment modalities with PARPi might be.
Acknowledgments
We thank Mervi Toriseva for valuable comments regarding the manuscript.
Author Contributions
All authors participated in conceptualizing, writing, revising and editing; all authors proofread and approved the manuscript.
Funding
The laboratory of MS has been supported by research grants from the Academy of Finland, Sigrid Juselius Foundation, Finnish Medical Foundation, Turku University Foundation, Paulo Foundation, Instrumentarium Science Foundation and Hospital District of Southwest Finland.
Conflicts of Interest
MS has been supported by Pfizer, Novartis, Celgene, MSD, Lilly, BMS, Pierre Fabre and Roche for conference participation costs, and received a consultant fee from Roche and Ipsen. VV, KP, JA, RV and CS declared no conflicts of interest.
References
- 1.Sartor O., de Bono J.S. Metastatic prostate cancer. N. Eng. J. Med. 2018;378:645–657. doi: 10.1056/NEJMra1701695. [DOI] [PubMed] [Google Scholar]
- 2.Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L.F., Kolettas E., Niforou K., Zoumpourlis V.C., et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 3.Ciccia A., Elledge S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber V., Dantzer F., Ame J., Murcia G.D. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 5.Murcia J.M.D., Ricoul M., Tartier L., Niedergang C., Huber A., Dantzer F., Schreiber V., Amé J., Dierich A., LeMeur M., et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Stingl L., Morrison C., Jantsch M., Los M., Schulze-Osthoff K., Wagner E.F. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masutani M., Nozaki T., Nishiyama E., Shimokawa T., Tachi Y., Suzuki H., Nakagama H., Wakabayashi K., Sugimura T. Function of poly(ADP-ribose) polymerase in response to DNA damage: Gene-disruption study in mice. Mol. Cell Biochem. 1999;193:149–152. doi: 10.1023/A:1006941016799. [DOI] [PubMed] [Google Scholar]
- 8.Bürkle A., Virág L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol. Aspects Med. 2013;34:1046–1065. doi: 10.1016/j.mam.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Langelier M., Elsemann T., Riccio A.A., Pascal J.M. PARP family enzymes: Regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 2018;53:187–198. doi: 10.1016/j.sbi.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldecott K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M.S., Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri A.R., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pines A., Vrouwe M., Marteijn J., Typas D., Luijsterburg M., Cansoy M., Hensbergen P., Deelder A., Groot A., Matsumoto S., et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012;199:235–249. doi: 10.1083/jcb.201112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luijsterburg M., de Krijger I., Wiegant W., Shah G., Shah R., Smeenk G., de Groot A.L., Pines A., Vertegaal A.O., Jacobs J.L., et al. PARP1 links CHD2-mediated chromatin expansion and H3.3 deposition to DNA repair by non-homologous end-joining. Mol. Cell. 2016;61:547–562. doi: 10.1016/j.molcel.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marteijn J.A., Lans H., Vermeulen W., Hoeijmakers J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014;15:465–481. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 16.Mehta A., Haber J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014;6:a016428. doi: 10.1101/cshperspect.a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman J., Taylor M.G., Boulton S. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Ali A.A.E., Timinszky G., Arribas-bosacoma R., Kozlowski M., Hassa P.O., Hassler M., Ladurner A.G., Pearl L.H., Olive A.W. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat. Struct. Mol. Biol. 2012;19:685–692. doi: 10.1038/nsmb.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meek K., Van Dang V.D., Lees-Miller S.P. DNA-PK: The means to justify the ends? Adv. Immunol. 2008;99:33–58. doi: 10.1016/S0065-277600602-0. [DOI] [PubMed] [Google Scholar]
- 20.Haince J.F., McDonald D., Rodrigue A., Déry U., Masson J.Y., Hendzel M.J., Poirier G.G. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 2008;283:1197–1208. doi: 10.1074/jbc.M706734200. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Yu X. Function of BRCA1 in the DNA damage response is mediated by ADP-ribosylation. Cancer Cell. 2013;23:693–704. doi: 10.1016/j.ccr.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buisson R., Dion-Côté A., Coulombe Y., Launay H., Cai H., Stasiak A.Z., Stasiak A., Xia B., Masson J. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat. Struct. Mol. Biol. 2010;17:1247. doi: 10.1038/nsmb.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen R.B., Carreira A., Kowalczykowski S.C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467:678. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahid T., Soroka J., Kong E.H., Malivert L., McIlwraith M.J., Pape T., Zhang X. Structure and mechanism of action of the BRCA2 breast cancer tumor suppressor. Nat. Struct. Mol. Biol. 2014;21:962–968. doi: 10.1038/nsmb.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou B.S., Elledge S.J. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 26.Ruscetti T., Lehnert B.E., Halbrook J., Le Trong H., Hoekstra M.F., Chen D.J., Peterson S.R. Stimulation of the DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J. Biol. Chem. 1998;273:14461–14467. doi: 10.1074/jbc.273.23.14461. [DOI] [PubMed] [Google Scholar]
- 27.Rouleau M., Patel A., Hendzel M.J., Kaufmann S.H., Poirier G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer H., McCabe N., Lord C.J., Tutt A.N., Johnson D.A., Richardson T.B., Martin N.M. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 29.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 30.Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M., Ashworth A. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Eng. J. Med. 2009;361 doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 31.Moore K., Colombo N., Scambia G., Kim B., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Eng. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 32.Pommier Y., O’Connor M.J., de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016;8:362ps17. doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 33.Livraghi L., Garber J.E. PARP inhibitors in the management of breast cancer: Current data and future prospects. BMC Med. 2015;13:188. doi: 10.1186/s12916-015-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson E.L., Smith J.Y., Wielckens K., Hilz H., Jacobson M.K. Cellular recovery of dividing and confluent C3H10T1/2 cells from N-methyl-N′-nitro-N-nitrosoguanidine in the presence of ADP ribosylation inhibitors. Carcinogenesis. 1985;6:715–718. doi: 10.1093/carcin/6.5.715. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber V., Hunting D., Trucco C., Gowans B., Grunwald D., De Murcia G., De Murcia J.M. A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis, and sister chromatid exchange following DNA damage. Proc. Natl. Acad. Sci. USA. 1995;92:4753–4757. doi: 10.1073/pnas.92.11.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murai J., Huang S.N., Das B.B., Renaud A., Zhang Y., Doroshow J.H., Ji J., Takeda S., Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahel D., Hořejší Z., Wiechens N., Polo S.E., Garcia-Wilson E., Ahel I., Owen-Hughes T. Poly(ADP-ribose)–dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325:1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottschalk A.J., Trivedi R.D., Conaway J.W., Conaway R.C. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1.PARP1.nucleosome intermediate. J. Biol. Chem. 2012;287:43527–43532. doi: 10.1074/jbc.M112.401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murai J., Huang SY N., Renaud A., Zhang Y., Ji J., Takeda S., Pommier Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amé J., Rolli V., Schreiber V., Niedergang C., Apiou F., Decker P., Muller S., Höger T., Ménissier-de Murcia J., de Murcia G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 41.Castro E., Goh C., Olmos D., Saunders E., Leongamornlert D., Tymrakiewicz M., Mahmud N., Dadaev T., Govindasami K., Guy M., et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. JCO. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leongamornlert D., Saunders E., Dadaev T., Tymrakiewicz M., Goh C., Jugurnauth-Little S., Kozarewa I., Fenwick K., Assiotis I., Barrowdale D., et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer. 2014;110:1663–1672. doi: 10.1038/bjc.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro E., Romero-Laorden N., del Pozo A., Lozano R., Medina A., Puente J., Piulats J.M., Lorente D., Saez M.I., Morales-Barrera R., et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. JCO. 2019;37:490–503. doi: 10.1200/JCO.18.00358. [DOI] [PubMed] [Google Scholar]
- 44.Pritchard C.C., Mateo J., Walsh M.F., De Sarkar N., Abida W., Beltran H., Garofalo A., Gulati R., Carreira S., Eeles R., et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Eng. J. Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrovics G., Price D.K., Lou H., Chen Y., Garland L., Bass S., Jones K., Kohaar I., Ali A., Ravindranath L., et al. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis. 2018;1 doi: 10.1038/s41391-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grasso C.S., Wu Y.M., Robinson D.R., Cao X., Dhanasekaran S.M., Khan A.P., Asangani I.A. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson D., Van Allen E.M., Wu Y., Schultz N., Lonigro R.J., Mosquera J., Montgomery B., Taplin M., Pritchard C.C., Attard G., et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beltran H., Yelensky R., Frampton G.M., Park K., Downing S.R., MacDonald T.Y., Jarosz M., Lipson D., Tagawa S.T., Nanus D.M., et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2012;63:920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abida W., Armenia J., Gopalan A., Brennan R., Walsh M., Barron D., Danila D., Rathkopf D., Morris M., Slovin S., et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. 2017;2017 doi: 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polkinghorn W.R., Parker J.S., Lee M.X., Kass E.M., Spratt D.E., Iaquinta P.J., Arora V.K., Yen W., Cai L., Zheng D., et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asim M., Tarish F., Zecchini H.I., Sanjiv K., Gelali E., Massie C.E., Baridi A., Warren A.Y., Zhao W., Ogris C., et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017;8:374. doi: 10.1038/s41467-017-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L., Karanika S., Yang G., Wang J., Park S., Broom B.M., Manyam G.C., Wu W., Luo Y., Basourakos S., et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ossovskaya V., Koo I.C., Kaldjian E.P., Alvares C., Sherman B.M. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer. 2010;1:812–821. doi: 10.1177/1947601910383418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiewer M.J., Goodwin J.F., Han S., Brenner J.C., Augello M.A., Dean J.L., Liu F., Planck J.L., Ravindranathan P., Chinnaiyan A.M., et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiewer M.J., Mandigo A.C., Gordon N., Huang F., Gaur S., de Leeuw R., Zhao S.G., Evans J., Han S., Parsons T., et al. PARP-1 regulates DNA repair factor availability. EMBO Mol. Med. 2018:e8816. doi: 10.15252/emmm.201708816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pu H., Horbinski C., Hensley P.J., Matuszak E.A., Atkinson T., Kyprianou N. PARP-1 regulates epithelial–mesenchymal transition (EMT) in prostate tumorigenesis. Carcinogenesis. 2014;35:2592–2601. doi: 10.1093/carcin/bgu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karanika S., Karantanos T., Li L., Wang J., Park S., Yang G., Zuo X., Song J.H., Maity S.N., Manyam G.C., et al. Targeting DNA damage response in prostate cancer by inhibiting androgen receptor-CDC6-ATR-Chk1 signaling. Cell Rep. 2017;18:1970–1981. doi: 10.1016/j.celrep.2017.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandhu S.K., Schelman W.R., Wilding G., Moreno V., Baird R.D., Miranda S., Hylands L., Riisnaes R., Forster M., Omlin A., et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 59.Sandhu S.K., Omlin A., Hylands L., Miranda S., Barber L.J., Riisnaes R., Reid A.H., Attard G., Chen L., Kozarewa I., et al. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Annal. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:1416–1418. doi: 10.1093/annonc/mdt074. [DOI] [PubMed] [Google Scholar]
- 60.Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Friedlander M., Balmaña J., Mitchell G., Fried G., Stemmer S.M., Hubert A., et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. JCO. 2014;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mateo J., Carreira S., Sandhu S., Miranda S., Mossop H., Perez-Lopez R., Nava Rodrigues D., Robinson D., Omlin A., Tunariu N., et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Eng. J. Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke N., Wiechno P., Alekseev B., Sala N., Jones R., Kocak I., Chiuri V.E., Jassem J., Fléchon A., Redfern C., et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 63.Karzai F., VanderWeele D., Madan R.A., Owens H., Cordes L.M., Hankin A., Couvillon A., Nichols E., Bilusic M., Beshiri M.L., et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer. 2018;6:141. doi: 10.1186/s40425-018-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bono J., Ramanathan R.K., Mina L., Chugh R., Glaspy J., Rafii S., Kaye S., Sachdev J., Heymach J., Smith D.C., et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7:620–629. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussain M., Carducci M., Slovin S., Cetnar J., Qian J., McKeegan E., Refici-Buhr M., Chyla B., Shepherd S., Giranda V., et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest. N. Drugs. 2014;32:904–912. doi: 10.1007/s10637-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pahuja S., Appleman L.J., Belani C.P., Chen A., Chu E., Beumer J.H., Puhalla S. Preliminary activity of veliparib (V) in BRCA2-mutated metastatic castration-resistant prostate cancer (mCRPC) JCO. 2015;33(Suppl. 7):170. doi: 10.1200/jco.2015.33.7_suppl.170. [DOI] [Google Scholar]
- 67.Gray H.J., Bell-McGuinn K., Fleming G.F., Cristea M., Xiong H., Sullivan D., Luo Y., McKee M.D., Munasinghe W., Martin L.P. Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecol. Oncol. 2018;148:507–514. doi: 10.1016/j.ygyno.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 68.Hussain M., Daignault-Newton S., Twardowski P.W., Albany C., Stein M.N., Kunju L.P., Siddiqui J., Wu Y., Robinson D., Lonigro R.J., et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: Results from NCI 9012. J. Clin. Oncol. 2017 doi: 10.1200/JCO.2017.75.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mateo J., Porta N., McGovern U.B., Elliott T., Jones R.J., Syndikus I., Ralph C., Jain S., Varughese M.A., Parikh O., et al. TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. JCO. 2019;37(Suppl. 15):5005. doi: 10.1200/JCO.2019.37.15_suppl.5005. [DOI] [Google Scholar]
- 70.De Bono J.S., Hussain M., Thiery-Vuillemin A., Mateo J., Sartor A.O., Chi K.N., Fizazi K., Twardowski P., Agarwal N., Sandhu S.K., et al. PROfound: A randomized phase III trial evaluating olaparib in patients with metastatic castration-resistant prostate cancer and a deleterious homologous recombination DNA repair aberration. JCO. 2017;35(Suppl. 15):TPS5091. doi: 10.1200/JCO.2017.35.15_suppl.TPS5091. [DOI] [Google Scholar]
- 71.Ryan C.J., Abida W., Bryce A.H., Balar A.V., Dumbadze I., Given R.W., Morris D., Petrylak D.P., Redfern C.H., Scher H.I., et al. TRITON3: An international, randomized, open-label, phase III study of the PARP inhibitor rucaparib vs. physician’s choice of therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD) JCO. 2018;36(Suppl. 6):TPS389. doi: 10.1200/JCO.2018.36.6_suppl.TPS389. [DOI] [Google Scholar]
- 72.Karanika S., Karantanos T., Li L., Corn P.G., Thompson T.C. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene. 2015;34:2815. doi: 10.1038/onc.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke N.W., Armstrong A.J., Thiery-Vuillemin A., Oya M., Ye D., Mateo J., Goessl C.D., Kang J., Liu S., Saad F. PROPEL: A randomized, phase III trial evaluating the efficacy and safety of olaparib combined with abiraterone as first-line therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) JCO. 2019;37(Suppl. 7):TPS340. doi: 10.1200/JCO.2019.37.7_suppl.234. [DOI] [Google Scholar]
- 74.Agarwal N., Azad A., Fay A., Carles J., Shore N.D., Nordquist L.T., Karsh L.I., Dunshee C., Ponnathapura Nandakumar S., Sullivan B., et al. Talapro-2: A 2-part, placebo-controlled phase 3 study of talazoparib (TALA) with background enzalutamide (ENZA) in metastatic castration-resistant prostate cancer (mCRPC) with DNA damage repair deficiencies. JCO. 2018;36(Suppl. 15):TPS5091. doi: 10.1200/JCO.2018.36.15_suppl.TPS5091. [DOI] [Google Scholar]
- 75.Janssen Research & Development, LLC A Study of Niraparib in Combination with Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone for Treatment of Participants with Metastatic Prostate Cancer (MAGNITUDE) [(accessed on 30 May 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03748641.
- 76.Sato H., Niimi A., Yasuhara T., Permata T.B.M., Hagiwara Y., Isono M., Nuryadi E., Sekine R., Oike T., Kakoti S., et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017;8:1751. doi: 10.1038/s41467-017-01883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Metastatic Castration-Resistant Prostate Cancer (mCRPC) (MK-7339-010/KEYLYNK-010) [(accessed on 30 May 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03834519.
- 78.Juvekar A., Burga L.N., Hu H., Lunsford E.P., Ibrahim Y.H., Balmañà J., Rajendran A., Papa A., Spencer K., Lyssiotis C.A., et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibrahim Y.H., García-García C., Serra V., He L., Torres-Lockhart K., Prat A., Anton P., Cozar P., Guzmán M., Grueso J., et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Billalabeitia E., Seitzer N., Song S.J., Song M.S., Patnaik A., Liu X., Epping M.T., Papa A., Hobbs R.M., Chen M., et al. Vulnerabilities of PTEN–TP53-deficient prostate cancers to compound PARP–PI3K inhibition. Cancer Discov. 2014;4:896–904. doi: 10.1158/2159-8290.CD-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bryant H.E., Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34:1685–1691. doi: 10.1093/nar/gkl108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng Y., Liao Q., Tan W., Peng C., Hu Z., Chen Y., Li Z., Li J., Zhen B., Zhu W., et al. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat. Commun. 2019;10:1224. doi: 10.1038/s41467-019-09232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCabe N., Turner N.C., Lord C.J., Kluzek K., Bialkowska A., Swift S., Giavara S., OConnor M.J., Tutt A.N., Zdzienicka M.Z., et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PubMed] [Google Scholar]
- 84.Kalev P., Simicek M., Vazquez I., Munck S., Chen L., Soin T., Danda N., Chen W., Sablina A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res. 2012;72:6414–6424. doi: 10.1158/0008-5472.CAN-12-1667. [DOI] [PubMed] [Google Scholar]
- 85.Turner N.C., Lord C.J., Iorns E., Brough R., Swift S., Elliott R., Rayter S., Tutt A.N., Ashworth A. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27:1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall C.H., Sokolova A.O., McNatty A.L., Cheng H.H., Eisenberger M.A., Bryce A.H., Schweizer M.T., Antonarakis E.S. Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur.Urol. 2019 doi: 10.1016/j.eururo.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davies H., Glodzik D., Morganella S., Yates L.R., Staaf J., Zou X., Ramakrishna M., Martin S., Boyault S., Sieuwerts A.M., et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017;23:517–525. doi: 10.1038/nm.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naipal K.A.T., Verkaik N.S., Ameziane N., van Deurzen C.H., ter Brugge P., Meijers M., Sieuwerts A.M., Martens J.W., O’Connor M.J., Vrieling H., et al. Functional ex vivo assay to select homologous Recombination–Deficient breast tumors for PARP inhibitor treatment. Clin. Cancer Res. 2014;20:4816–4826. doi: 10.1158/1078-0432.CCR-14-0571. [DOI] [PubMed] [Google Scholar]
- 89.Chan N., Pires I.M., Bencokova Z., Coackley C., Luoto K.R., Bhogal N., Lakshman M., Gottipati P., Oliver F.J., Helleday T., et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 2010;70:8045–8054. doi: 10.1158/0008-5472.CAN-10-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pettitt S.J., Lord C.J. Dissecting PARP inhibitor resistance with functional genomics. Curr. Opin. Genet. Dev. 2019;54:55–63. doi: 10.1016/j.gde.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Lin K.K., Harrell M.I., Oza A.M., Oaknin A., Ray-Coquard I., Tinker A.V., Helman E., Radke M.R., Say C., Vo L., et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9:210–219. doi: 10.1158/2159-8290.CD-18-0715. [DOI] [PubMed] [Google Scholar]
- 92.Ding X., Chaudhuri A.R., Callen E., Pang Y., Biswas K., Klarmann K.D., Martin B.K., Burkett S., Cleveland L., Stauffer S., et al. Synthetic viability by BRCA2 and PARP1/ARTD1 deficiencies. Nat. Commun. 2016;7 doi: 10.1038/ncomms12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng W., Jasin M. Homologous recombination and replication fork protection: BRCA2 and more! Cold Spring Harb. Symp. Quant. Biol. 2017;82:329–338. doi: 10.1101/sqb.2017.82.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quigley D., Alumkal J.J., Wyatt A.W., Kothari V., Foye A., Lloyd P., Aggarwal R., Kim W., Lu E., Schwartzman J., et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7:999–1005. doi: 10.1158/2159-8290.CD-17-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oplustilova L., Wolanin K., Mistrik M., Korinkova G., Simkova D., Bouchal J., Lenobel R., Bartkova J., Lau A., OO’Connor M.J., et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle. 2012;11:3837–3850. doi: 10.4161/cc.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaspers J.E., Kersbergen A., Boon U., Sol W., van Deemter L., Zander S.A., Drost R., Wientjens E., Ji J., Aly A., et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3:68–81. doi: 10.1158/2159-8290.CD-12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chapman J.R., Barral P., Vannier J., Borel V., Steger M., Tomas-Loba A., Sartori A.A., Adams I.R., Batista F.D., Boulton S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu G., Chapman J.R., Brandsma I., Yuan J., Mistrik M., Bouwman P., Bartkova J., Gogola E., Warmerdam D., Barazas M., et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature. 2015;521:541–544. doi: 10.1038/nature14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barazas M., Annunziato S., Pettitt S.J., Krijger I.d., Ghezraoui H., Roobol S.J., Lutz C., Frankum J., Song F.F., Brough R., et al. The CST complex mediates end protection at double-strand breaks and promotes PARP inhibitor sensitivity in BRCA1-deficient cells. Cell Rep. 2018;23:2107–2118. doi: 10.1016/j.celrep.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gogola E., Duarte A.A., de Ruiter J.R., Wiegant W.W., Schmid J.A., de Bruijn R., James D.I., Guerrero Llobet S., Vis D.J., Annunziato S., et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell. 2018;33:1078–1093.e12. doi: 10.1016/j.ccell.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Dréan A., Williamson C.T., Brough R., Brandsma I., Menon M., Konde A., Garcia-Murillas I., Pemberton H.N., Frankum J., Rafiq R., et al. Modeling therapy resistance in BRCA1/2-mutant cancers. Mol. Cancer Ther. 2017;16:2022–2034. doi: 10.1158/1535-7163.MCT-17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gourley C., Balmaña J., Ledermann J.A., Serra V., Dent R., Loibl S., Pujade-Lauraine E., Boulton S.J. Moving from poly (ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. JCO. 2019;18:02050. doi: 10.1200/JCO.18.02050. [DOI] [PubMed] [Google Scholar]
- 103.Dylgjeri E., McNair C., Goodwin J.F., Raymon H.K., McCue P.A., Shafi A.A., Leiby B., de Leeuw R., Kothari V., McCann J., et al. Pleiotropic impact of DNA-PK in cancer and implications for therapeutic strategies. Clin. Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-18-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kothari V., Goodwin J.F., Zhao S.G., Drake J.M., Yin Y., Chang S.L., Evans J.R., Wilder-Romans K., Gabbara K., Dylgjeri E., et al. DNA-dependent protein kinase drives prostate cancer progression through transcriptional regulation of the wnt signaling pathway. Clin. Cancer Res. 2019;2387:2018. doi: 10.1158/1078-0432.CCR-18-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]