Abstract
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an under-recognized cause of heart failure (HF) in older adults, resulting from myocardial deposition of misfolded transthyretin (TTR or prealbumin). Characteristic patterns of echocardiography and cardiac magnetic resonance imaging are strongly suggestive but not diagnostic. The diagnosis can be made with non-invasive nuclear imaging when there is no evidence of a monoclonal protein. Amyloid fibril formation results from a destabilizing mutation in hereditary ATTR amyloidosis (hATTR) or from an aging-linked process in wild-type ATTR amyloidosis (wtATTR). Recent studies suggest that up to 10–15% of older adults with HF may harbor unrecognized wtATTR. Associated features, including carpal tunnel syndrome and lumbar spinal stenosis, raise suspicion and may afford a means for early diagnosis. Previously treatable only by organ transplantation, pharmaceutical therapy that slows or halts ATTR-CM progression and favorably affects clinical outcomes is now available. Early recognition remains essential to afford the best treatment efficacy.
Keywords: amyloidosis, transthyretin, cardiomyopathy
Condensed Abstract:
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an increasingly important but under-recognized cause of heart failure in persons over 60 years of age. Both ATTRwt, or less frequently hATTR are possibilities. Advances in diagnostics have identified specific populations in whom ATTR-CM is clinically important. The recent emergence of effective therapies that slow disease progression and improve clinical outcomes promises to render ATTR-CM a treatable disease. For such therapies to be most effective, early identification of affected individuals is critical. This review aims to afford providers the tools required to facilitate earlier diagnosis of ATTR-CM and delineate management strategies.
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an under-recognized cause of heart failure (HF) in older adults. ATTR is one of the systemic amyloidoses – disorders characterized by a misfolded precursor protein that forms cross-β-sheet-rich amyloid fibrils extracelluarly in several tissues (1). While there are over 30 known amyloidogenic proteins, cardiac amyloidosis typically arises from misfolded transthyretin (ATTR) or immunoglobulin light-chain (AL) aggregation (1). AL amyloidosis results from misfolding of light-chains secreted by clonal plasma cells (2,3), involving the heart in 50–75% of cases (4). Once felt to be untreatable, contemporary therapies have improved survival for AL amyloidosis (5,6).
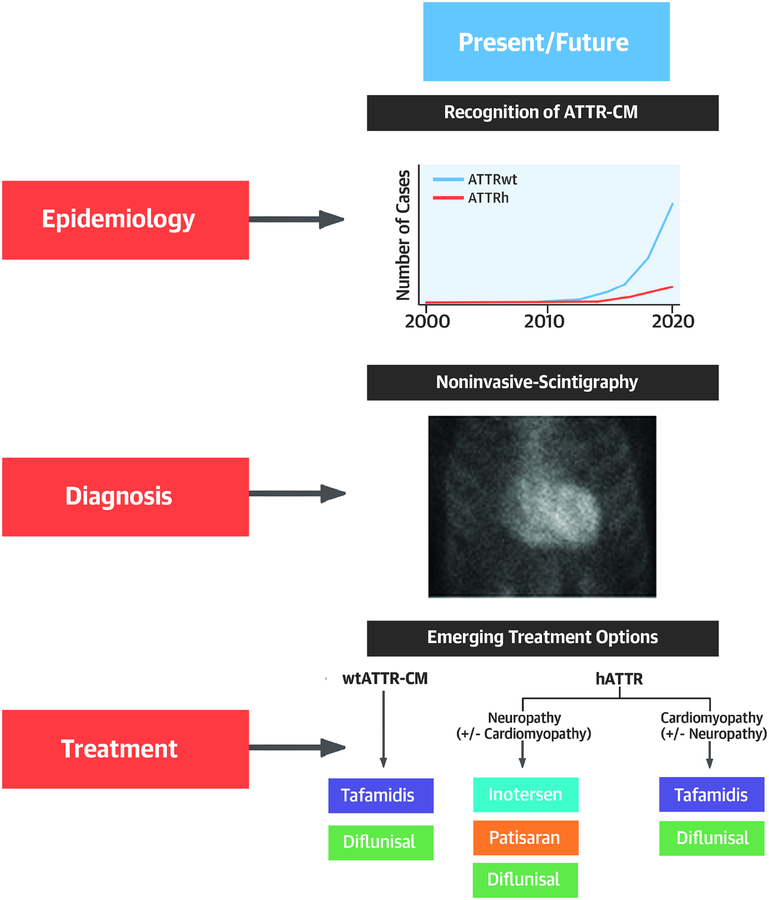
ATTR-CM is increasingly being recognized by the cardiology community. The disease is classified by the sequence of the TTR gene, either wtATTR-CM (no mutation) or hATTR-CM (a mutation is present). Recent studies suggest that the prevalence of wtATTR-CM is substantially higher than previously appreciated in older adults with HF. Further, the most common mutation associated with hATTR (Val122Ile) is present in 3.4% of African Americans, with 1.5 million individuals in the United States being allele carriers (7). Recent advances in nuclear imaging using bone-avid radiotracers permit diagnosis of ATTR-CM without a tissue biopsy (8). Contemporary treatment strategies that suppress expression (9,10) or stabilize TTR (11) have been recently reported to slow or halt disease progression in ATTR polyneuropathy. Additionally, strategies that stabilize TTR improve survival in ATTR-CM (12). Advances in non-invasive diagnosis coupled with concurrent demonstration of efficacy and the anticipated regulatory approval of specific ATTR-CM therapies has shifted ATTR-CM from a rarely encountered and untreatable “zebra,” to a condition that clinicians should consider on a daily basis (Central Illustration).
Central Illustration: Transthyretin Cardiac Amyloidosis:
llustrates the present ad future of ATTR-CM with respect to epidemiology diagnostic approach and treatment.
Pathobiology of ATTR
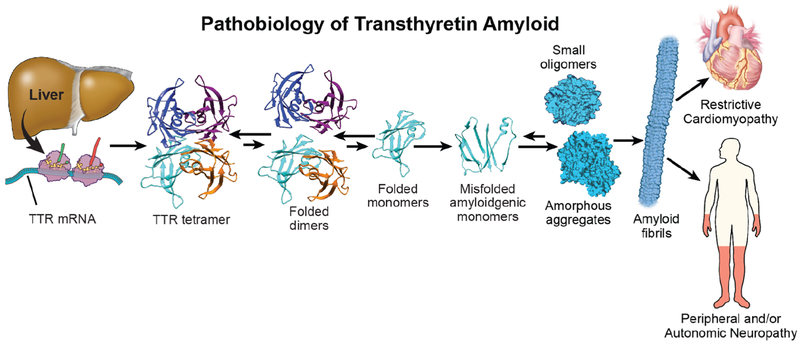
Transthyretin (TTR), formerly named prealbumin, is composed of four b-sheet rich monomers that circulate as a tetramer and function as a carrier protein for thyroxine and holoretinol binding protein (RBP) (13). The native TTR tetramer is secreted from the liver into the blood, with lesser amounts produced by the choroid plexus for the CSF, and retinal pigmented epithelial cells for the vitreous of the eye. Transthyretin misfolding and aggregation in these fluids leads to tissue dysfunction and the clinical phenotypes of the ATTR amyloidoses (Figure 1)(14).
Figure 1. Pathobiology of ATTR:
The mechanism of TTR protein dissociation, misfolding and aggregation as amyloid fibrils is illustred with resultant end-organ dysfunction.
The transthyretin gene is found on chromosome 18. In hATTR, there are single amino acid mutations in the 127 amino-acid sequence that destabilize the heterotetramer, rendering aggregation more efficient. The nomenclature for hATTR places a one- or three-letter abbreviation for the normal amino acid at the postion indicated followed the amino acid substituted (e.g. Val30Met signifies substitution for valine at position 30 by methionine). Notably, while Val30Met is the commonly used literature nomenclature, this will be reported as as pV50M in genetic testing reports including the 20 amino acid signal peptide in the numbering of residues. In wtATTR, the genetic sequence of transthyretin is normal. It is not clear why the wild type protein becomes kinetically unstable and aggregates, however this appears to involve the aging process. Since < 5 % of transthyretin carries thyroid hormone, this ligand does not influence TTR’s aggregation propensity(15). In contrast, holo-RBP does bind and stabilize tetrameric TTR, suggesting that low concentrations of holo-RBP may be a risk factor for ATTR-CM(16).
The rate limiting step of TTR amyloid formation is dissocation of the tetramer into monomers, possibly involving proteolysis. Subsequently, partial monomer denaturation(17) enables misassembly into several aggregate structures, including amyloid fibrils. In ATTR-CM, one consequence of the aggregation process is cardiac infiltration by rigid, space occupying TTR amyloid fibrils leading to stiffness, associated fibrosis, and dysfunction. Non-amyloid aggregates appear to exhibit proteotoxicity in ATTR-CM, as occurs in AL amyloidosis.
Disease Course and Prognosis
The natural history of ATTR-CM includes progressive HF, complicated by arrhythmias and conduction system disease (Table 1) (18). The clinical course is more variable for those with hATTR-CM compared with wtATTR-CM. hATTR can present as a primary cardiomyopathy or a primary peripheral and autonomic neuropathy, sometimes with vitreous opacities. Not uncommonly, there is a mixed phenotype in hATTR with components of both cardiomyopathy and polyneuropathy (19). The natural history, including age of onset, primary phenotype, and clinical course varies with mutation, fibril type (full length versus fragments) and within families(20). The presence and extent of cardiac involvement is a major determinant of outcome. Severe autonomic neuropathy may mask the degree of cardiac involvement due to pooling of blood in the splanchnic bed. Although both peripheral and autonomic neuropathy can sometimes occur in wtATTR, it is less severe than in hATTR. Interestingly, amyloid neuropathy has become manifest post heart transplant in patients with wtATTR-CM(21), perhaps because patients were spared an earlier death from HF. Data also suggest that survival appears worse in hATTR-CM owing to Val122Ile than wtATTR(22–24). While there is clearly variation in survival with respect to genotype in hATTR, most series have reported a median survival in the range of 8–10 years for patients with hATTR and polyneuropathy versus 2.5–3.5 years in those diagnosed with HF (19).
Table 1.
Disease course and prognosis of hereditary and wild-type ATTR-CM
| Hereditary (hATTR-CM) | Wild-type (wtATTR-CM) | |
|---|---|---|
| Age of onset | Variable (30 to 80 years) dependent on the mutation | Average 75 years, usually > 60 years |
| TTR genotype | Abnormal, single nucleotide mutation | Normal |
| Heritability | Autosomal dominant (50% chance of passage to offspring) | Not known to be heritable |
| Predominant countries of origin | Vall22Ile: US, UK, Western Africa Thr60Ala (Appalachian mutation): US, UK (predominately Northern part of Republic of Ireland) Val30Met: Sweden, Portugal, Japan Leu111Met: Denmark Ile68Leu: Italy |
No known geographic disparities |
| Prevalence | Val122Ile genotype: 3.4% of African Americans (ref 7) Thr60Ala genotype: ~1% of Irish Descent (ref 37) |
Up to 25% with wtATTR deposits at autopsy 13% in hospitalized HFpEF with wall thickness > 12 mm 6–16% of patients undergoing AVR possibly 1–3% > 75 years of age |
| Median survival After Diagnosis without treatment | ~2.5 years * (Val122Ile) | ~3.5 years* |
can be further risk stratified with cardiac biomarker staging systems
The phenotype and natural history of wtATTR-CM has been quite consistent in reported series (25–27). The median survival from diagnosis in untreated patients is consistently ~3.5 years, but is dependent on the stage of disease. Cardiac biomarkers can be used to risk stratify ATTR-CM patients. The Mayo Clinic wtATTR-CM staging system uses thresholds of troponin T and NTproBNP (> 0.05ng/ml and > 3000 pg/ml respectively). Three stages are defined as: stage I - both biomarker values below threshold, stage II – one above, and stage III –both above with median survivals of 66, 42 and 20 months, respectively(27). The ATTR staging system from the UK National Amyloidosis Center using NT-proBNP (same threshold of >3000 pg/ml) and estimated GFR (< 45 ml/min) included both wtATTR-CM and hATTR cohorts and reported a median survival for stage II wtATTR patients of 49 months and hATTR (Val122Ile mutation only) of 29 months(23). Both studies found that echocardiographic findings, including wall thickness, left ventricular mass, and diastolic function, were not independent predictors of survival. Although cardiac amyloidosis has been associated with a “preserved” ejection fraction, reduced ejection fraction (<40%) has been reported in 30–50% of wtATTR-CM cases (18,27). Reduced ejection fraction at diagnosis is more common in hATTR-CM than wtATTR-CM, likely reflecting a more advanced stage of disease at diagnosis and perhaps accounting for reduced survival reported in these patients.
In general, ATTR-CM is characterized by years of relative stability despite advanced disease based on imaging, hemodynamics, and reduced functional capacity. This is commonly followed by a significant decline to severe and refractory HF, suggesting that the disease progresses very slowly. Accordingly, wtATTR patients “look much better” clinically than cardiac imaging and invasive hemodynamics would suggest. This is in contrast to AL amyloidosis, in which the imaging findings may be subtle despite rapidly progressive HF(18). The discrepancy has been attributed to a more significant direct cardiotoxicity of circulating free light-chains and pre-fibrillar aggregates in AL compared to ATTR. The discrepancy between imaging findings, especially wall thickness, and clinical course, highlights the fact that cardiac amyloidosis is not a simple infiltrative disorder and is better characterized as a “toxic-infiltrative cardiomyopathy”(4).
In addition to HF, the natural history of ATTR-CM commonly includes both conduction system disease and arrhythmias, which may occur years before the onset of HF (18). Conduction system disease is more common in wtATTR-CM than hATTR-CM with up to a third of patients requiring permanent pacemakers. Atrial arrhythmias are also more common in wtATTR-CM than in hATTR, occurring in 40–60% of patients at diagnosis in recent series (18,25–27) and in almost all patients during the course of the disease. Atrial fibrillation often occurs with a controlled ventricular response because of underlying conduction disease and when present becomes persistent in most patients with wtATTR-CM (28). The risk of intracardiac thrombus is increased in all patients with cardiac amyloidosis and may occur even in sinus rhythm (29). Unfortunately, stroke or systemic embolization is the presentation in some patients, usually due to unrecognized atrial fibrillation.
Affected Populations
Overview of Genotypes
Wild-type ATTR-CM is almost exclusively a disease of older adults with an average age at diagnosis of 74 years, however rare patients are diagnosed in their 40’s(25–27). In most of the studied cohorts and registries, over 90% of patients are male and Caucasian, but whether this relates to a true disease predilection in this population or a referral bias is unknown. In addition to universal cardiac involvement, involvement of soft tissues leads to an increased incidence of bilateral carpal tunnel syndrome, spinal stenosis, or spontaneous biceps tendon rupture (30,31). Although peripheral and/or autonomic neuropathy are uncommon in ATTRwt, neuropathy can be seen in up to 10% of patients (26), but whether this relates to amyloidosis or associated other etiologies also remains undefined.
The delineation between wtATTR and hATTR is critical given clinical differences in phenotype, prognosis, and implications for screening of family members. In hATTR, each amyloidogenic mutation is thought to have arisen from a genetic founder, with population enrichment of the affected allele in certain geographic regions and based on migration patterns. The most common mutation in the United States is Val122Ile (pV142I), which is almost exclusively seen in individuals of west African origin, and occurs in 3.4% of African Americans (7). The phenotype is very similar to wtATTR-CM in that it causes a late-onset restrictive cardiomyopathy with minimal neuropathy at an average age of onset of 69 years (32). Polyneuropathy is uncommon (33), but has implications based on current drug approval indications (see Emerging Therapies). As in wtATTR-CM, there appears to be a male preponderance with only 25% of reported cases being female (34). The true penetrance of this mutation is unknown and clearly relates both to the age of ascertainment and methodology utilized to define disease. While a recent report suggested that penetrance may be as low as 10–20%(35), this study utilized a relatively insensitive echocardiographic wall thickness criterion to define disease presence. Furthermore, this study and others demonstrated an association of Val122Ile and HF incidence with advancing age (35,36) suggesting that more sensitive methods could more accurately demonstrate disease penetrance.
The second most common mutation in the US that causes hATTR-CM is Thr60Ala (pT80A). This variant originates in Northern part of the Republic of Ireland and causes a mixed phenotype with a high rate of carpal tunnel syndrome (up to 70%), often presenting as the first manifestation (37). Disease onset, particularly of neuropathy, can be earlier (4th decade of life) with a male predominance of about 3:1. Val30Met (pV50M) is the most common world-wide mutation and is the prototype for hATTR polyneuropathy, endemic in certain regions of Portugal, Japan and Sweden. Interestingly, Val30Met has a “late onset” variant in non-endemic areas that can present with cardiac symptoms including heart block and HF. The THAOS registry demonstrated that the other important mutations that cause hATTR-CM are Leu111Met and Ile68Leu that occur in Denmark and Italy, respectively (38).
TTR genetic testing should be performed in all patients with ATTR-CM, irrespective of patient age. The results have significant implications for family members at risk and genetic counseling is recommended. The age threshold for testing of offspring of patients with hATTR is an individual decision best addressed through genetic counseling. Once a variant genotype is identified, how to perform surveillance of disease in gene mutation carriers is not yet delineated, though baseline and longitudinal neurologic and cardiac assessments are recommended.
Elderly patients with HF
Wild-type ATTR-CM is undoubtedly the most common type of ATTR-CM, but the true population prevalence of ATTR-CM is unknown. In several autopsy studies, the incidence of wtATTR myocardial deposits increases with age, with a prevalence as high as 20–25% in octogenarians, and 37% of people over the age of 95 yrs(39,40). In an autopsy study of 109 patients with an ante-mortem diagnosis of heart failure with preserved ejection fraction (HFpEF), 17% had wtATTR myocardial deposits with 5% having moderate to severe interstitial deposition indicative of a causative etiology(41). Furthermore, among patients who were > 80 years (n= 35), the incidence of wtATTR deposits was dramatically increased to 40%, with a striking bias in male patients.
With the advent of bone scintigraphy as a diagnostic tool for the diagnosis of ATTR-CM), ~13% of older patients hosptitalized with HFpEF were shown to have ATTR-CM(42). All were subsequently diagnosed with wtATTR-CM at a mean age of 86 years. Interestingly in this active ascertainment approach, 50% were female in contrast to previous studies of wtATTR. Further, a study using scintigraphy demonstrated that among Afro-Carribean patients admitted with HF, hATTR-CM was identified in 10% of cases attributable to the Val122Ile mutation (32).
Aortic stenosis
Patients with ATTR-CM and aortic stenosis (AS) are demographically similar. Retrospective studies report a prevalence of 6–12% of ATTR-CM in patients with severe AS (43,44) undergoing surgical valve replacement. Interestingly, the phenomenon of low-flow, low-gradient severe AS in elderly patients may be in part explained by co-existent ATTR-CM and restrictive physiology (45). Among 151 consecutive patients over 65 years of age referred for transcatheter aortic valve replacement (TAVR), Tc99m-pyrophosphate imaging revealed that 16% overall and 22% of males had uptake consistent with ATTR-CM–of whom 62% met criteria for low-flow, low-gradient severe AS (46).
Hypertrophic cardiomyopathy misdiagnosis
Cardiac amyloidosis can appear phenotypically as hypertrophic cardiomyopathy (HCM). Rare patients referred for surgical myectomy are diagnosed with ATTR-CM histologically(47). More commonly, ATTR-CM is confused with non-obstructive HCM. Among 298 patients with unexplained LV hypertrophy initially diagnosed as HCM, TTR genetic testing revealed that 5% harbored a TTR mutation with clinical evidence of hATTR-CM using bone scintigraphy and cardiac MRI (48). This study did not assess for the presence of wtATTR-CM, which is likely the most common phenocopy of HCM in older adults. Finally, asymmetrically increased septal wall thickness can occur in up to 20–25% of wtATTR-CM, further confounding diagnosis based on wall thickness (49).
Carpal tunnel syndrome
ATTR amyloidosis can lead to deposits in the soft tissues causing nerve entrapment syndromes, the most common being carpal tunnel syndrome (CTS). Deposits in the flexor retinaculum and tenosynovial tissue within the carpal tunnel occur more often with ATTR than AL amyloidosis and classically present with bilateral symptoms (40). The presence of CTS among referred patients with wtATTR-CM is approximately 50% (25,26). The symptoms of CTS often precede overt ATTR-CM by an average of 5–10 years and are a common initial manifestation (50).
In an effort to facilitate early diagnosis of cardiac amyloidosis, Sperry et al conducted a prospective study of 98 patients (male ≥50 years and females ≥60 years) undergoing CTS release surgery for idiopathic CTS and examined a small sample of tenosynovium pathologically for amyloid deposits, finding that 10 (10.2%) were positive(51). While the majority had ATTRwt deposits, there were two patients diagnosed with hATTR and two with AL. One patient with wtATTR deposits was found to have ATTR-CM based upon diagnostic uptake on Tc99m-pyrophosphate scintigraphy.
Lumbar Spinal Stenosis and other orthopedic manifestations
Lumbar spinal stenosis is associated principally with wtATTR-CM. Amyloid deposition causes thickening of the ligamentum flavum leading to compression and narrowing of the spinal canal(30). Amyloid deposition in the ligamentum flavum of older patients undergoing spinal stenosis surgery occurs in 45–96%, with an increasing incidence with age(52). Spontaneous rupture of the distal biceps tendon has been reported in 33% of patients with wtATTR-CM(31). A study of patients undergoing other orthopedic surgery for rotator cuff repair found that 38% of tissue samples removed were found to have ATTRwt deposits(53) and total knee and hip arthroplasty was 3–5 times more common among patients with ATTR amyloidosis than age and gender matched controls(54).
Diagnosis
The diagnosis of ATTR-CM poses a challenge to the clinician for a number of reasons. First, the clinical phenotype of wall thickening and HF may be attributed to other common diseases such as hypertensive heart disease, aortic stenosis, or HCM. Second, there is a perceived rarity of ATTR-CM related to confusion with the AL type. Third, clinicians are unfamiliar with the appropriate diagnostic algorithm to follow. Finally, the disease was previously thought to be untreatable resulting in therapeutic nihilism.
A number of important clinical clues to the presence of ATTR-CM have been described. One clue is a “natural cure” of hypertension: the need for down-titration or discontinuation of anti-hypertensive therapy. Likewise, the intolerance of beta blockade in newly diagnosed HF should prompt consideration of amyloidosis. A history of HF with carpal tunnel syndrome, lumbar spinal stenosis, and biceps tendon rupture should be actively ascertained. The presence of unexplained peripheral or autonomic neuropathy suggests the possibility of hATTR amyloidosis, but can occur in AL, and occasionally in wtATTR.
The presence of increased left ventricular wall thickness in the presence of a low-voltage ECG pattern can differentiate ATTR-CM from hypertensive or hypertrophic cardiomyopathy. However, only 25–40% of patients with ATTR-CM meet low voltage criteria (18) and in one series of hATTR-CM due to Val122Ile, 25% met criteria for LVH(55). There should be heightened clinical suspicion in any patient presenting with low-flow low-gradient AS, or unexplained increased left or right ventricular wall thickness. The currently accepted echocardiographic diagnostic threshold for cardiac amyloidosis is an interventricular septal wall thickness > 12 mm, but this does not adjust for sex and is insensitive (56).
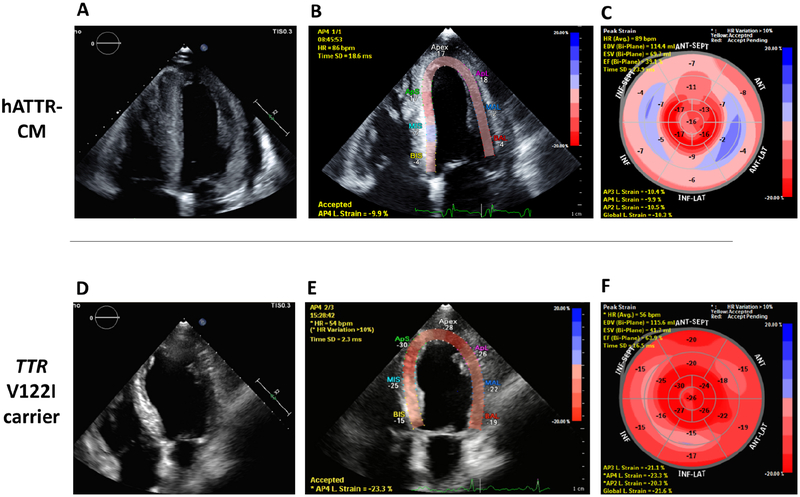
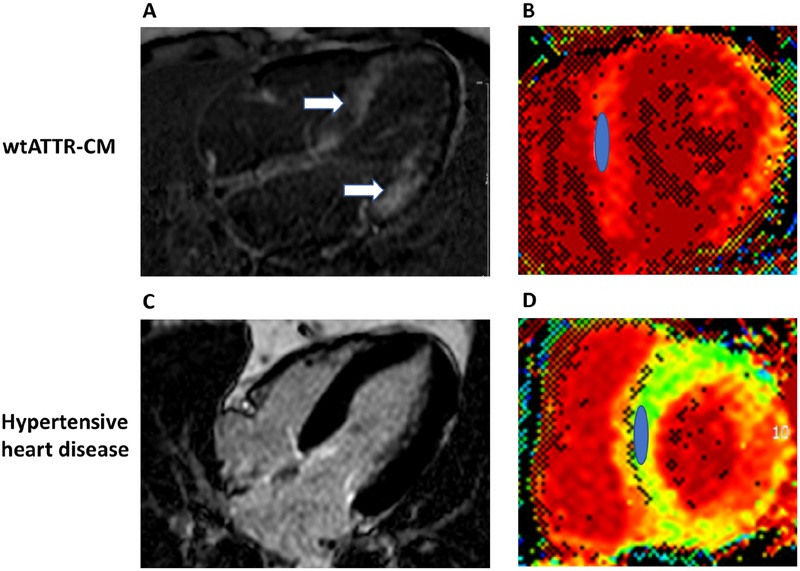
Persistent elevation in cardiac biomarkers are commonly observed in ATTR-CM. While useful for staging of disease and prognosis in wtATTR-CM(27) and hATTR-CM(23), they are also useful for raising suspicion and, although not specific, values are usually higher in the compensated state than in the average HFpEF patient. Unlike AL amyloidosis, there are no specific circulating biomarkers of ATTR-CM, though the endogenous TTR ligand RBP4 has shown promise(16). Echocardiographic analyses of cardiac deformation, specifically reduced longitudinal systolic strain, is useful in ATTR-CM. There is a distinctive pattern of “apical sparing” in which the left ventricular apical region shows more normal strain compared to progressively worse values at the mid and basal regions (Figure 2). Quantification of abnormal ratios of apical to basal strain, or apical to basal plus mid-ventricular strain, have good diagnostic accuracy (57) for differentiating amyloid heart disease from other etiologies. Similarly, with late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR), an inability to suppress or “null” the myocardial signal or the presence of diffuse sub-endocardial or transmural enhancement patterns suggests amyloidosis(58) with a sensitivity and specificity that approach 85–90% (Figure 3) (59). CMR parametric imaging using T1 mapping to determine native (non-contrast) myocardial T1 and extracellular volume fraction (ECV) have emerged as even more sensitive and quantitative measures of amyloid deposition in ATTR-CM, with values that are elevated as compared to other myocardial processes (58). While useful for differentiating amyloidosis from non-amyloid diseases, neither echocardiography nor CMR is able to reliably differentiate ATTR-CM from AL amyloidosis(60,61). All of the above observations merely suggest the presence of amyloidosis, necessitating further diagnostic testing for confirmation.
Figure 2. Echocardiography in ATTR-CM:
Two patients genopositive for TTR Val122Ile : 73-year-old male patient in Panels A, B, and C. Panel A is a 4-chamber view with the corresponding longitudinal strain map in panel B. Panel C shows the map of all myocardial segments. Note the reduced global longitudinal strain (GLS) at −10.3% and apical sparing (> 2:1 apical:basal ratio or “cherry on top”) pattern. A 65-year-old male with Val122Ile genotype and hypertension (Tc99m-PYP scan showing grade 1 uptake) is illustrated in Panels C, D, and E. the bullseye map shows the absence of apical sparing and GLS is normal at −21.6%.
Figure 3. Cardiac MRI of ATTR-CM:
An 82 year old female with wtATTR-CM is illustrated in panels A and B and a 76 year old male with hypertension is illustrated in panels C and D. Phase sensitive inversion recovery (PSIR) late gadolinium enhancement (LGE) images taken in the 4-chamber plane are seen in panels A and C. Diffuse LGE (arrows) in ATTR-CM (panel A), but absence of LGE and normal myocardial signal suppression in HTN (panel C). Panels B and D show corresponding short axis extracellular volume fraction (ECV) maps taken at the mid-ventricle with regions of interest drawn over the septum. The ECV in panel B was 60% consistent with ATTR-CM, whereas the ECV in panel D was 27% (normal).
Endomyocardial biopsy remains the gold-standard for ATTR-CM diagnosis and is nearly 100% sensitive and specific if biopsy specimens are collected from multiple sites (4 or more recommended) and tested for amyloid deposits by Congo red staining(62). Definitive identification of the misfolded precursor protein must be determined by either immunohistochemistry (in experienced pathology laboratories) or by the gold-standard, laser dissection, tandem mass spectrometry analysis (Figure 4) (63). Other tissue biopsies, such as gastrointestinal or abdominal fat aspirate have varying sensitivity for ATTR-CM, and in the case of wild-type disease, fat aspirate has a sensitivity of only 15% (64).
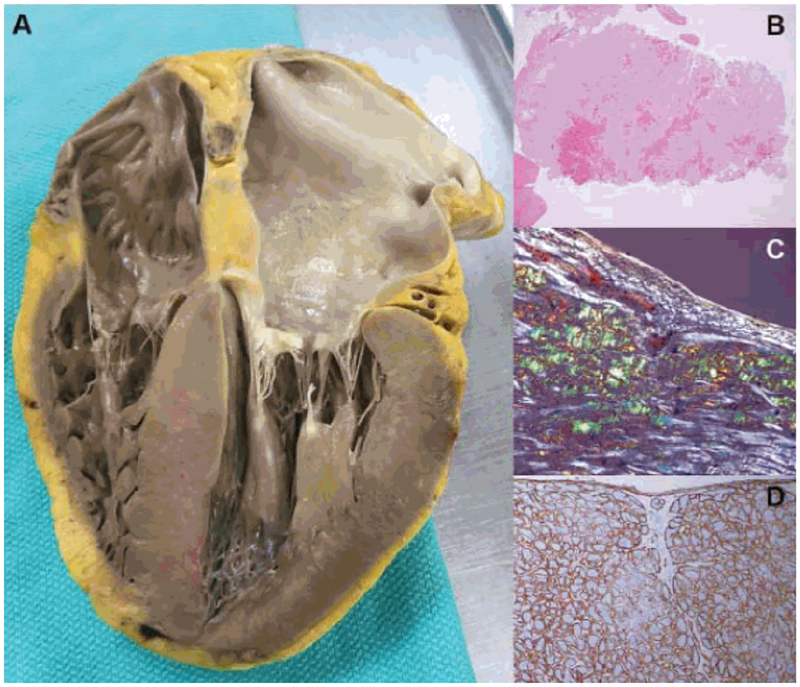
Figure 4. Gross and histopathology of ATTR-CM:
Autopsy specimen reveals biventricular thickening, biatrial dilatation, and thickening of both mitral and tricuspid valves (A), hemotoxylin and eosin staining showing diffuse amyloid deposition (B), characteristic “apple green” birefringence on polarized light microscopy (C), and immunohistochemistry for typing of amyloid (D). Mass spectrometry (not illustrated) can also be peformed for typing and is considered the gold-standard. Reproduced with permission by the author.
The only imaging modality that can accurately diagnose ATTR-CM without the need for invasive cardiac biopsy is nuclear scintigraphy using bone-avid radiotracers. Three technetium labeled radiotracers have been evaluated clinically for ATTR-CM identification. These include: 99mTc-pyrophospate (PYP) available in the US and Tc99m-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) or Tc99m-hydroxymethylene diphosphonate (HMDP) available in Europe. Interest in these radiotracers for amyloidosis identification was rekindled approximately 10–15 years ago with initial work showing the capacity of Tc99m-DPD to identify cardiac amyloidosis(65,66). The mechanism underlying the myocardial retention of these tracers is unknown but has been attributaed to the presence of micro-calcifications which are more common in ATTR than AL cardiac tissue (20,67). Cardiac tracer uptake is compared to bone uptake of the rib with a simple, semi-quantitative scheme developed from grade 0 (no uptake) to grade 3 (cardiac uptake that exceeds rib). Subsequently, studies using 99mTc-PYP demonstrated that AL and ATTR-CM could be readily differentiated using the quantitative refinement of a heart to contralateral chest ratio uptake measurement that exceeds 1.5(68) (Figure 5) and that the test could be reproducibly performed at multiple sites with high accuracy(69). An international collaboration with a large cohort of endomyocardial biopsy proven cases of ATTR-CM concluded that these bone avid tracers conferred 100% specificity for ATTR-CM when grade 2 or 3 uptake is seen in the absence of a monoclonal protein by serum and urine testing(8) in patients with HF and typical echocardiographic or CMR findings of amyloidosis. Consequently, the advent of nuclear imaging for non-biopsy diagnosis of ATTR-CM has affected a sea change in the clinical approach to this disease, figuring prominently in proposed diagnostic algorithms.
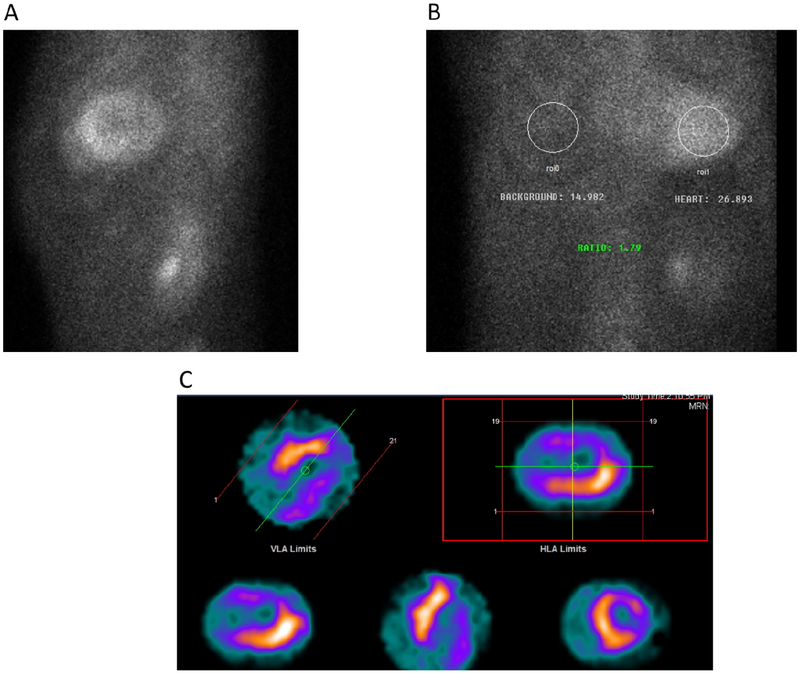
Figure 5. Nuclear imaging of ATTR-CM with bone avid tracers:
A typical patient with ATTR-CM is depicted with grade 3 tracer uptake on planar imaging (A), increased heart to contralateral chest ratio of 1.79 (B), and multiplanar single photon emission computed tomography (SPECT) showing myocardial (and not blood pool) tracer uptake, with some heterogeneity in uptake intensity. Coronary artery disease was excluded by angiography.
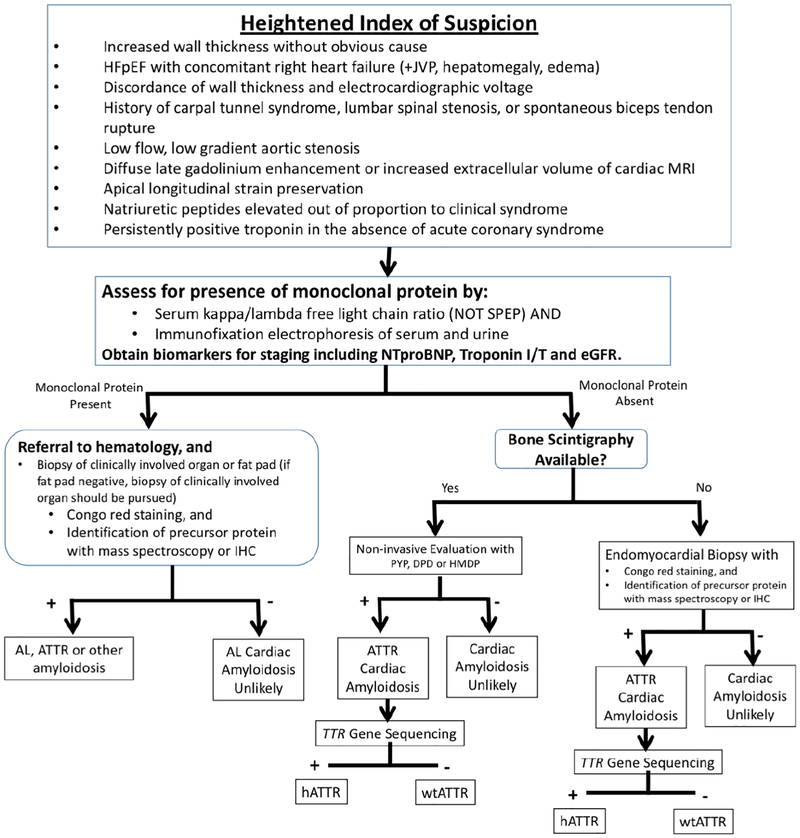
A proposed diagnostic algorithm can be found in Figure 6. Here 99mTc-PYP or Tc99m-DPD scintigraphy is combined with serum and urine assessment of light-chain amyloidosis. There are a number of important caveats. First, AL amyloidosis cannot be diagnosed without a tissue biopsy showing light-chain amyloid deposits from some organ or site (not necessarily the heart). Second, the historically used screening method to test for AL amyloidosis with serum and urine protein electorphoresis (SPEP and UPEP) is insensitive, inappropriate, and should be avoided. Serum free light-chains and immunofixation of the serum and urine is the required testing. Third, it is important to note that occasionally AL cardiac amyloidosis can lead to grade 1 and even higher grades of cardiac uptake on bone scintigraphy. In addition, the co-existence of an unrelated monoclonal gammopathy is common in ATTR-CM (up to 40–50% of cases)(70). Thus, the nuclear scan result, irrespective of uptake grade, cannot be interpreted as to exclude AL amyloidosis; screening for monoclonal protein must always accompany nuclear scintigraphy in the diagnostic evaluation. In instances where a patient has grade 2 or 3 cardiac uptake on bone scintigraphy but is also found to have an abnormal light-chain ratio or an M protein found on serum and/or urine immunofixation, hematology consultation is required and cardiac biopsy may be necessary. It should be noted that in renal insufficiency, a mildly elevated kappa to lambda ratio may occur which, in the absence of an M protein on serum/urine immunofixation, may not be significant; nonetheless this finding should be discussed with a hematologist. Fourth, low intensity uptake (grade 1 or sometimes even grade 2) can often be confused for blood-pool signal from the radiotracer, thus acquisition of confirmatory single photon emission computed tomography (SPECT) along with additional repeat scanning an hour (or 2 hours) later is recommended to demonstrate that uptake seen is indeed cardiac.
Figure 6. Diagnostic algorithm for evaluation of suspected ATTR-CM:
A proposed flow diagram is illustrated demonstrating the critical requirement to exclude light-chain amyloidosis by serum/urine testing and concomitant use of of nuclear scintigraphy to identify the presence of ATTR-CM. It is emphasized that serum free light-chains and serum/urine immunofixation electrophoresis are the appropriate tests to exclude a monoclonal gammopathy, which also may be present in patients with ATTR-CM. Nuclear imaging can also be performed concurrent to light-chain assessment, even in the case of a detected monoclonal gammopathy, for additive information. IHC - immunohistochemistry; PYP, DPD, HMDP - Tc99m associated tracers.
Amyloid-specific molecular imaging agents such as 18F-florbetapir (Amyvid, Eli Lilly) and the 11C-Pittsburgh-B compound that have been developed for imaging of Alzheimer’s dementia can identify cardiac amyloidosis, but are not specific for amyloid type(71,72). While these agents have potential to similarly diagnose ATTR-CM, presently none are reimbursable by the Centers for Medical and Medicaid Services nor third party payors.
Clinical Management
Medical therapy of HF
Maintenance of euvolemia is central to management in ATTR-CM with sodium restriction and diuretics. This is challenging because ventricular capacitance is markedly reduced (73), which when coupled with age-related changes in the vascular system and concomitant autonomic dysfunction, enhances load lability. Thus, with dietary indiscretion volume overload rapidly ensues and overly aggressive diuresis is associated with symptomatic hypotension and worsening renal perfusion. Given circulatory congestion and gut wall edema, loop diuretics with higher bioavailibity (torsemide and bumetanide) are preferred and often used in combination with aldosterone antagonists. Non-dihydropyridine calcium channel blockers (e.g. verapamil) should be avoided in patients with ATTR-CM given previous case reports in patients with AL amyloidosis(74) of high degree heart block and shock.
There are no guideline-based recommendations for ACE, ARBs or ARNi therapies in cardiac amyloidosis and such medications may not be well tolerated owing to hypotension. Similarly, beta blockers, especially at higher dosages and those with accompanying alpha blocking properties, are not well tolerated by patients with cardiac amyloidosis. As cardiac amyloidosis progresses, decline in ventricular capacitance and altered ventricular vascular coupling results in decrements in stroke volume, cardiac output and hence, blood pressure(73). In order to maintain adequate organ perfusion, heart rate increases steadily. Accordingly, beta blockers can blunt the compensatory increase in heart rate necessary to maintain adequate cardiac output. Unfortunately, as the disease progresses, declines in blood pressure and cardio-renal syndrome ensue. Compression stockings and midodrine may be useful in these advanced stages.
Arrhythmia Management and Prevention
Management of atrial fibrillation is often very challenging in patients with cardiac amyloidosis who have a narrow window of “optimal” heart rate. Extreme tachycardia is poorly tolerated and bradycardia is similarly dangerous due to low stroke volume resulting from severe restrictive hemodynamics. Maintaining sinus rhythm and “atrial kick” in cardiac amyloidosis may be overemphasized as most patients have significantly reduced atrial mechanical function, making the atrial contribution to ventricular filling minimal or absent. Data on the outcomes of catheter ablation in ATTR-CM are limited. In early stage patients, ablation may help maintain sinus rhythm, especially in the setting of atrial flutter(19). However, long-term success of ablation therapies, other than AV node ablation, is likely less than in non-amyloid patients. While anti-arrhythmic treatment and cardioversion may be considered in selected patients, it is crucial to exclude intracardiac thrombus, even in patients receiving therapeutic anticoagulation. A recent study found left atrial appendage thrombus in 33% of patients with ATTR-CM presenting for TEE cardioversion, with most receiving anticoagulant therapy(75). Amiodarone is the preferred anti-arrhythmic agent given its favorable safety profile in cardiomyopathy, and limited data suggesting its safety in ATTR-CM(28). Long-term anticoagulation is preferred once atrial fibrillation is detected in ATTR-CM, irrespective of CHADs-VASc score.
Deposits of TTR amyloid fibrils infiltrate the conduction system with a significant percentage requiring permanent pacing(18,42). In hATTR, conduction disturbances do not seem to be related to wall thickness and may be related to other factors such as loss of autonomic nervous control of cardiac function. Due to the high incidence of conduction disturbances, Holter monitoring should be considered when symptoms of syncope, pre-syncope, or palpitations are reported. Indications for permanent pacing should follow the ACC/AHA/HRS guidelines (76), with common indications for pacing including sinus node dysfunction, AV block, and atrial fibrillation with a slow ventricular response.
Patients with ATTR-CM can have markedly reduced stroke volumes and often are pacemaker dependent. Accordingly, concern has been raised that chronic right ventricular apical pacing can result in left ventricular dyssynchrony leading to a further reduction in stroke volume and hence cardiac output, leading some centers to favor the use of biventricular pacing when a pacing indication is present. When patients develop worsening HF over time, the lower rate limit on the pacemaker can be increased to help maintain cardiac output. The routine use of implanted automatic implanted cardio-defibrillators (ICDs) in patients with ATTR-CM is debatable. If anticipated survival is <1 year, then practice guidelines do not recommend ICD placement for the primary prevention of sudden cardiac death (SCD)(76). Additionally, SCD in patients with AL cardiac amyloidosis has often been attributed to electromechanical dissociation rather than to a primary arrhythmic cause (77), further arguing against ICD in this population. While several small series have reported successful defibrillation in individual patients with ICDs(78,79), an overall survival benefit from ICD therapy has not been demonstrated. Thus, there are no clear indications for primary prevention ICD implantation in ATTR-CM at this time. Secondary prevention is reasonable as per ACC/AHA/HRS guidelines. Careful risk/benefit analysis is recommended for patients who might benefit from an ICD, which include those with outpatient telemetry monitoring demonstrating a high burden of NSVT or sustained VT, and patients listed for heart transplantation (78). The decision to implant a secondary prevention ICD should be individualized.
Organ transplantation
Although liver transplantation has been used to treat hATTR by removing mutant TTR from blood, progression of disease may occur as a result of wild-type TTR deposition on preexisting mutant ATTR deposits (80). Liver transplantation to treat hATTR has dramatically decreased with the advent of TTR stabilizers and is not indicated for wtATTR-CM. For patients with advanced ATTR-CM, both hereditary and wild-type, orthotopic heart transplantation transplantation (with combined liver transplantation in some variants of hATTR) is an option in selected patients(81). However, heart transplantation is not a viable option for most patients with ATTR-CM given the shortage of donor organs, the advanced age of most affected individuals, and other factors.
Available and Emerging TTR-specific therapies
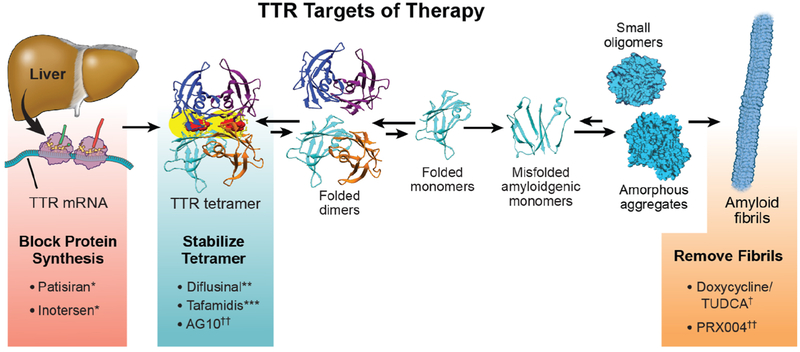
There are numerous pharmacologic strategies under development (Figure 7) to ameliorate the ATTR amyloidoses, including: 1.) TTR mRNA knockdown/”silencing,” 2.) TTR stabilization, 3.) TTR amyloid fibril disruption/extraction. The first of these strategies has led to development of the FDA approved drugs patisiran (Alnylam) and inotersen (Akcea) for hATTR polyneuropathy but not ATTR-CM. The stabilizer tafamidis (Pfizer) is undergoing accelerated review for ATTR-CM.
Figure 7. Therapies available or in development to treat ATTR-CM:
Therapeutic strategies for ATTR-CM are illustrated with agents either presently approved, under review, or in development. * TTR silencers patisiran and inotersen are not presently approved for ATTR-CM but rather for hATTR polyneuropathy (with or without cardiomyopathy). ***The agent tafamidis is undergoing FDA review for an ATTR-CM indication. **Diflunisal may be used off-label in selected patients with ATTR-CM but only with careful monitoring. †Combination of doxycycline and tauro-deoxycholic acid can be used in conjunction with other strategies and is being evaluated in clinical trial. †† AG10, a TTR stabilizer, and PRX004, a monoclonal antibody that binds and potentially removes ATTR deposits, are both in development.
Presently under regulatory review for ATTR-CM
Tafamidis.
Tafamidis binds the thyroxine-binding sites of TTR with high affinity and selectivity, slowing dissociation of TTR tetramers into monomers, therefore inhibiting aggregation. Tafamidis was shown to slow the progression of peripheral neurologic impairment in ATTR polyneuropathy(82), leading to its approval for the treatment of stage I and 2 hATTR polyneuropathy in numerous countries.
Phase 2 data showed that tafamidis meglumine (20 mg daily) stabilized transthyretin in ATTR-CM(83). The 30-month ATTR-CM phase 3 Clinical Trial (ATTR-ACT)(84), compared tafamidis meglumine (20 or 80 mg) to placebo in 441 subjects with ATTR-CM, due to ATTRwt or hATTR(12). The primary analysis employed the Finkelstein-Schoenfeld method(85), which is a hierarchical rank sum in which all-cause mortality was first assessed followed by the rates of cardiovascular hospitalizations. The primary endpoint was achieved with a win ratio (the number of pairs of treated-patient “wins” divided by number of pairs of placebo patient “wins”) of 1.70 (95% CI, 1.26 to 2.29; p value of 0.0006). In more traditional time to first event analyses, tafamidis treatment resulted in lower all-cause mortality than placebo with a 13.4% absolute difference in mortality and a 32% relative risk reduction in cardiovascular hospitalizations. This translated into a number needed to treat of 7.5 to prevent one death after 2.5 years of treatment. Tafamidis treatment resulted in a lower rate of decline in the 6-minute walk test (P<0.001) and in the Kansas City Cardiomyopathy Questionnaire (KCCQ-OS) score (P<0.001). Tafamidis was very well tolerated with the incidence and types of adverse events not differing from placebo. Across 11 pre-specified subgroups, the point estimates for the hazard ratios favored tafamidis over placebo, except for the subjects with NYHA class III at baseline, for whom the rates of cardiovascular-related hospitalizations were higher among patients receiving tafamidis. These data highlight the importance of early diagnosis to optimize the benefit from tafamidis therapy. Tafamidis was awarded Breakthrough designation by the FDA in May 2018 and approval for ATTR-CM is anticipated by July 2019; however, cost may prove be a significant obstacle to widespread use.
Available for off-label use, effective in ATTR polyneuropathy, with limited data in ATTR-CM
Diflunisal:
Diflunisal is an NSAID that has been repurposed as a TTR kinetic stabilizer, binding within the two thyroxine binding sites. In a phase 3, randomized, placebo controlled trial of patients with hATTR polyneuropathy resulting from a diverse number of mutations, diflunisal (250 mg orally bid) was well tolerated. While there was significant attrition of subjects, which required multiple imputation, data nonetheless demonstrated that diflunisal improved symptoms of neuropathy vs. placebo (11).
The experience with diflunisal in ATTR-CM has been limited to open label single center studies(86–89). Diflunisal (250 mg orally bid) was generally well tolerated with side effects in a minority of subjects including thrombocytopenia and renal dysfunction. Diflunisal was associated with a survival benefit similar to tafamidis in one non-randomized ATTR-CM study(89). Despite its application in patients with concomitant use of oral anticoagulants, significant bleeding has not been reported, although studies have included highly selected patients. The dose administered for TTR stabilization is lower than the dose for anti-inflammatory benefits, perhaps explaining the low toxicity observed. Given the encouraging safety profile, coupled with the potential efficacy and low cost, especially in comparison to other disease modifying agents, use of diflunisal could be considered for selected patients with ATTR-CM. In general, we restrict its use to subjects without significant renal dysfunction (e.g. eGFR>45 ml/min/1.73m2), who do not have evidence of thrombocytopenia, are not volume overloaded nor on high dose diuretic, and who have no evidence of recent renal or hemodynamic instability. We also advise discontinuation of other NSAIDs and recommend administration of a proton pump inhibitor. Significant toxicity can be avoided with careful attention to renal function, volume status, and monitoring for gastro-intestinal bleeding.
Therapies presently approved for ATTR polyneuropathy, with limited data in ATTR-CM
Patisiran:
Patisiran is a small interfering RNA (siRNA) specficially targeting TTR messenger RNA (mRNA) leading to its degradation and lowering of TTR protein levels. Phase I and Phase II studies of patisiran in healthy volunteers and in patients with hATTR polyneuropathy showed a dose dependent and robust mean reduction in serum TTR levels of up to 90%(90). The APOLLO trial successfully tested the hypothesis that by reducing the precursor protein in hATTR amyloidosis, improvement in the modified Neuropathy Impairment Score (mNIS+7), would be achieved(9). The mNIS+7 is an aggregate score that combines a combination of sensory, motor, and autonomic neuropathy measurements. A total of 225 patients with hATTR polyneuropathy (43% of whom had the Val30Met mutation), underwent randomization in a 2:1 fashion to receive patisiran (n=148) or placebo (n=77) at a dose of 0.3 mg/kg every 3 weeks for 18 months, along with premedication to minimize the risk of infusion-related reactions. The study excluded patients with NYHA class III or IV HF. Patisiran therapy was effective, with a highly significant difference in the change in the mNIS+7 after 18 months. In addition, the Norfolk Quality of Life–Diabetic Neuropathy (QOL-DN score) showed a significant benefit with patisiran. Mild or moderate infusion-related reactions occurred in approximately 20% of the patients who received patisiran and 10% of those who received placebo, with similar other adverse events incidence and severity(9). These data led to the FDA approval of patisiran in August 2018 for the treatment of hATTR polyneuropathy.
In a pre-specified cardiac subpopulation of the APOLLO trial comprising patients with a baseline left ventricular (LV) wall thickness ≥13 mm without a history of hypertension or aortic valve disease (n=126, 56% of the overall population), patisiran reduced mean LV wall thickness, increased end-diastolic volume, improved global longitudinal strain (particularly at the base), and increased cardiac output at month 18 compared to with placebo(91). While the myocardial effects took 18 months to be observed, the lowering of NT-proBNP occurred as early as 9 months and persisted during 18 months of therapy. In a post-hoc exploratory analysis, the exposure-adjusted rates of cardiac hospitalizations and/or all-cause death were lower with patisiran than placebo. Of the subjects who died, 7 deaths in the patisiran group (4.7%) were possibly related to HF (cause characterized as sudden cardiac death or HF), whereas there was only one such death in the placebo group (with 2:1 randomization). Also, AV block requiring pacemaker support was observed in 4 of 148 patients in the patisiran group (2.7%) versus 0 of 77 patients in the placebo group (91).
Inotersen:
Inotersen is a 2′-O-methoxyethyl–modified antisense oligodeoxynucleotide (ASO) that lowers hepatic production of TTR. NEURO-TTR was a phase 3 trial in adults with stage 1 or 2 hATTR polyneuropathy, who received inotersen (300 mg subcutaneously weekly) or placebo in a 2:1 randomization. The primary end point included the change in the mNIS+7 and a co-primary endpoint of the change in the Norfolk QOL-DN questionnaire. Both primary efficacy assessments favored inotersen. Five deaths occurred in the inotersen group and none in the placebo group. The most frequent serious adverse events in the inotersen group were glomerulonephritis (3%) and thrombocytopenia (3%), with one death associated with thrombocytopenia(10). Inotersen was approved by the FDA in October 2018 for hATTR polyneuropathy with a Risk Evaluation and Mitigation Strategy (REMS) that includes weekly monitoring of platelet counts and every two week monitoring of renal function and urinary protein.
An open label study of inotersen in 22 patients with ATTR-CM, 15 of whom had completed 12 months of follow-up, demonstrated stabilization of disease as measured by left ventricular wall thickness, left ventricular mass (LVM), 6-min walk test (6MWT), and echocardiographic global systolic strain (92).
Interestingly, both knockdown therapies (ASO and RNAi) demonstrated cardiac effects after 15–18 months of therapy, later than the 8–9 months when neurologic improvement was observed. The safety and efficacy of patisiran, inotersen, and other TTR “silencing” therapies in patients with ATTR-CM are the focus of upcoming clinical trials.
Role and Cost Effectiveness of Therapies.
Both patisiran and inotersen are approved for hATTR polyneuropathy with or without cardiac involvement but not for ATTR-CM without polyneuropathy. The Institute for Clinical and Economic Review (ICER) conducted a clinical evidence review using the PICOTS framework(93) and applied its framework for an ultra-rare disease, with the assumption that <10,000 individuals will be eligible for treatment with these drugs. ICER’s report found that both inotersen and patisiran provide a substantial net health benefit when compared to best supportive care alone, but current pricing far exceeds commonly cited thresholds for cost-effectiveness. They note that at the net price of $345,000 per year, both therapies exceed commonly cited thresholds for cost-effectiveness of $50,000–$150,000 per quality-adjusted life year (QALY) gained. Further, they note that the both agents would need to be discounted by 90% or greater to reach threshold prices. While the cost of diflunisal is minimal in comparison to silencers, the cost of tafamidis is unknown as it has yet to be approved.
Therapeutic Choices and Combination therapy.
Clinicians may soon have the enviable dilemma of choosing among several disease-modifying therapies for ATTR-CM. While selection of a particular therapy for an individual patient will always remain complex and best addressed through a process of shared decision making, there are differences in available treatments (see Table 3) that may guide choices. First, therapies shown to be effective have been tested in patients with symptomatic disease and thus are not indicated for asymptomatic carriers of TTR mutations. For such patients, many amyloidosis experts have been offering asymptomatic carriers diflunisal when they approach an age at which disease is likely to penetrate or if they demonstrate tissue evidence of amyloidosis by biopsy or myocardial retention of a bone scintigraphy agent.
Table 3:
Emerging therapies for ATTR-CM
| Drug Name | Mechanism of Action | Indication | Route of Administration | Dose | Common, Serious or Potential Side Effects | Concomitant Therapy |
Monitoring | Approval |
|---|---|---|---|---|---|---|---|---|
| Patisiran | Silencer | Neuropathy | Intravenous | 0.3 mg/kg q3weeks up to 30 mg |
|
With IV infusion:
|
None | Approved in US and Europe |
| Inotersen | Silencer | Neuropathy | Subcutaneous | 284 mg q week |
|
Daily Vitamin A supplements | Weekly platelet counts. Every 2 weeks measures of serum creatinine, eGFR urinalysis, and UPCR. | Approved in US and Europe |
| Cardiomyopathy | 20 mg or 80 mg | Side effects were less common than with placebo in cardiomyopathy | None | None | Anticipated 2019 | |||
| Tafamidis free salt | Stabilizer | Cardiomyopathy | Oral | 61 mg | Unknown | None | None | Anticipated 2019 |
| Diflunisal | Stabilizer | Neuropathy Cardiomyopathy | Oral | 250 mg PO BID | Related to NSAID properties:
|
Proton pump inhibitor | Monitor renal function, platelet count, hemoglobin 1 week after initiation and then every 3–6 months | Approved in US and Europe, off label usage |
UPCR: urine protein to creatinine ratio, NSAID: Non-steroidal anti-inflammatory drug
Second, TTR silencers including patisiran and inotersen have been not been approved for hATTR-CM without neuropathy. As noted, although patients with coexistent cardiac involvement were included in these trials, NYHA class III and IV patients were excluded. Thus, for the vast majority of subjects with ATTR-CM whom cardiovascular clinicians encounter, including wtATTR-CM and hATTR-CM owing to Val122Ile, these agents are not approved and given their current high cost, may not be reimbursed by third party payers. In the less common instance of hATTR polyneuropathy with cardiomyopathy, the choice between which “silencer” therapy to initiate is difficult to make in the absence of data directly comparing these agents. Finally, with the emergence of these effective therapies, critically unanswered questions arise regarding utility of therapeutic change in non-responders, or the role of combination silencer and stabilizer therapy.
Investigational approaches
Stabilization:
AG-10 is a potent and selective kinetic stabilizer of TTR(94) that has been shown in a phase 2 study of 49 subjects with ATTR-CM to stabilize TTR at 28 days (95). A phase III study of AG10 in ATTR-CM is expected to initiate soon. The cathecol-O-methyltransferase (COMT) inhibitor tolcopone, an FDA approved Parkinson’s disease therapeutic, also functions as a TTR protein stabilizer and is presently under investigation in ATTR amyloidosis (96).
Resorption/Disrpution:
Antibody-mediated removal of amyloid deposits is an area of active development(97), however thus far clinical data using this approach have resulted in cessation of product development owing to futility or toxicity. An antibody that targets TTR residues 89–97 (PRX-004, Prothena Biosciences)(98), has entered into phase I trials in patients with hATTR amyloidosis.
Gene editing/seeding inhibitors:
CRISPR/Cas9 technology is in preclinical development to silence expression of TTR, as are TTR fibril capping agents (99).
Conclusions
ATTR-CM affects a growing population of patients encountered in clinical practice. With the advent of contemporary non-invasive imaging techniques, providers now have the tools required to facilitate earlier diagnosis of ATTR-CM. The emergence of effective therapies for ATTR-CM will likely translate into improved outcomes, but for such therapies to be most effective, early identification of affected individuals is critical.
Table 2.
Non-invasive testing features suggestive of ATTR-CM
| Suggestive features | |
|---|---|
| Electrocardiography | Low voltages in context of increased echocardiographic wall thickness Caution: low voltage seen in < 50% of cases with ATTR-CM (ref18) |
| Echocardiography |
|
| Cardiac magnetic resonance imaging (CMR) |
|
| Nuclear imaging with bone avid tracers |
|
Highlights:
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an under-diagnosed condition.
Diagnosing ATTR-CM requires a high index of suspicion and can be made non-invasively with nuclear scintigraphy when there is no evidence of a monoclonal protein.
Emerging therapies that stabilize TTR have been shown to improve outcomes for patients with ATTR-CM and TTR silencers are entering late phase clinical trials.
Early diagnosis will be critical to afford the best efficacy of therapies.
Disclosures:
Ruberg – Grant support from NIH HL139671–01 and AG 050206–02, and Eidos therapeutics. Consulting income from Pfizer and GlaxoSmithKline. Grogan – Clinical trials for Alnylam, Eidos, Pfizer and Prothena. Consulting: Alnylam, Eidos, Pfizer, Prothena and Akcea. Kelly – Grant Support from NIH DK46335. Consulting, Equity and Royalty income from Pfizer linked to Tafamidis sales. Hanna – Clinical trials for Pfizer, Alnylam, and Akcea. Consulting: Pfizer, Alnylam, Akcea, and Eidos. Maurer – Grant support from NIH R01HL139671–01, R21AG058348 and K24AG036778, consulting income from Pfizer, GSK, EIdos, Prothena, Akcea and Alnylam, and institution received clinical trial funding from Pfizer, Prothena, Eidos and Alnylam.
Abbreviations:
- TTR
Transthyretin or prealbmin
- AL
Light-chain amyloidosis
- ATTR
Transthyretin amyloidosis
- wtATTR
wild-type (genetically normal) transthyretin amyloidosis
- hATTR
hereditary (genetically abnormal) transthyretin amyloidosis
- ATTR-CM
Transthyretin amyloid cardiomyopathy
- RBP
Retinol binding protein 4
- Tc99m-PYP
Technicium pyrophosphate scintigrapy
- Tc99m-DPD
Technicium 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy
- HF
Heart failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet 2016;387:2641–2654. [DOI] [PubMed] [Google Scholar]
- 2.Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv 2018;2:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Linos A, Beard CM et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992;79:1817–1822. [PubMed] [Google Scholar]
- 4.Falk RH, Alexander KM, Liao R, Dorbala S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol 2016;68:1323–41. [DOI] [PubMed] [Google Scholar]
- 5.Muchtar E, Gertz MA, Kumar SK et al. Improved outcomes for newly diagnosed AL amyloidosis over the years 2000–2014: cracking the glass ceiling of early death. Blood 2017;129:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 2019;133:215–223. [DOI] [PubMed] [Google Scholar]
- 7.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med 2017;19:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillmore JD, Maurer MS, Falk RH et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016;133:2404–+. [DOI] [PubMed] [Google Scholar]
- 9.Adams D, Gonzalez-Duarte A, O’Riordan WD et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 10.Benson MD, Waddington-Cruz M, Berk JL et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. New England Journal of Medicine 2018;379:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berk JL, Suhr OB, Obici L et al. Repurposing Diflunisal for Familial Amyloid Polyneuropathy A Randomized Clinical Trial. Jama-Journal of the American Medical Association 2013;310:2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer MS, Schwartz JH, Gundapaneni B et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JW, Colon W, Lai Z et al. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem 1997;50:161–81. [DOI] [PubMed] [Google Scholar]
- 14.Westermark P, Sletten K, Johansson B, Cornwell GG. Fibril In Senile Systemic Amyloidosis Is Derived From Normal Transthyretin. Proceedings of the National Academy of Sciences of the United States of America 1990;87:2843–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purkey HE, Dorrell MI, Kelly JW. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors in blood plasma. Proceedings of the National Academy of Sciences of the United States of America 2001;98:5566–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvanitis M, Simon S, Chan G et al. Retinol binding protein 4 (RBP4) concentration identifies V122I transthyretin cardiac amyloidosis. Amyloid-Journal of Protein Folding Disorders 2017;24:120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc Natl Acad Sci U S A 2001;98:14943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapezzi C, Merlini G, Quarta CC et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203–12. [DOI] [PubMed] [Google Scholar]
- 19.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilebro B, Suhr OB, Naslund U, Westermark P, Lindqvist P, Sundstrom T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci 2016;121:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum AN, AbouEzzeddine OF, Grogan M et al. Outcomes After Cardiac Transplant for Wild Type Transthyretin Amyloidosis. Transplantation 2018;102:1909–1913. [DOI] [PubMed] [Google Scholar]
- 22.Ruberg FL. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J 2012;164:222–228.e1. [DOI] [PubMed] [Google Scholar]
- 23.Gillmore JD, Damy T, Fontana M et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018;39:2799–2806. [DOI] [PubMed] [Google Scholar]
- 24.Higaki JN, Chakrabartty A, Galant NJ et al. Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid 2016;23:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinney JH, Whelan CJ, Petrie A et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013;2:e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connors LH, Sam F, Skinner M et al. Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study. Circulation 2016;133:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grogan M, Scott CG, Kyle RA et al. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol 2016;68:1014–20. [DOI] [PubMed] [Google Scholar]
- 28.Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail 2018;5:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng D, Edwards WD, Oh JK et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007;116:2420–6. [DOI] [PubMed] [Google Scholar]
- 30.Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci 2014;119:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller HI, Singh A, Alexander KM, Mirto TM, Falk RH. Association Between Ruptured Distal Biceps Tendon and Wild-Type Transthyretin Cardiac Amyloidosis. JAMA 2017;318:962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dungu JN, Papadopoulou SA, Wykes K et al. Afro-Caribbean Heart Failure in the United Kingdom: Cause, Outcomes, and ATTR V122I Cardiac Amyloidosis. Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 33.Connors LH, Prokaeva T, Lim A et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J 2009;158:607–14. [DOI] [PubMed] [Google Scholar]
- 34.Maurer MS, Hanna M, Grogan M et al. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quarta CC, Buxbaum JN, Shah AM et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 2015;372:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buxbaum J, Jacobson DR, Tagoe C et al. Transthyretin V122I in African Americans with congestive heart failure. J Am Coll Cardiol 2006;47:1724–5. [DOI] [PubMed] [Google Scholar]
- 37.Reilly MM, Staunton H, Harding AE. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: a clinical, genetic, and epidemiological study. Journal of neurology, neurosurgery, and psychiatry 1995;59:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurer M, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Planté-Bordeneuve V, Coelho T, Mundayat RV, Suhr OB, Waddington Cruz M and Rapezzi C On behalf of THAOS Investigators. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis in the United States: TheTransthyretin Amyloid Outcome Survey (THAOS). J Am Coll Cardiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanskanen M, Peuralinna T, Polvikoski T et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med 2008;40:232–9. [DOI] [PubMed] [Google Scholar]
- 40.Cornwell GG 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkanen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med 1983;75:618–23. [DOI] [PubMed] [Google Scholar]
- 41.Mohammed SF, Mirzoyev SA, Edwards WD et al. Left Ventricular Amyloid Deposition in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart failure 2014;2:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–94. [DOI] [PubMed] [Google Scholar]
- 43.Treibel TA, Fontana M, Gilbertson JA et al. Occult Transthyretin Cardiac Amyloid in Severe Calcific Aortic Stenosis: Prevalence and Prognosis in Patients Undergoing Surgical Aortic Valve Replacement. Circ Cardiovasc Imaging 2016;9. [DOI] [PubMed] [Google Scholar]
- 44.Longhi S, Guidalotti PL, Quarta CC et al. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging 2014;7:531–2. [DOI] [PubMed] [Google Scholar]
- 45.Cavalcante JL, Rijal S, Abdelkarim I et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson 2017;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castano A, Narotsky DL, Hamid N et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helder MR, Schaff HV, Nishimura RA et al. Impact of incidental amyloidosis on the prognosis of patients with hypertrophic cardiomyopathy undergoing septal myectomy for left ventricular outflow tract obstruction. Am J Cardiol 2014;114:1396–9. [DOI] [PubMed] [Google Scholar]
- 48.Damy T, Costes B, Hagege AA et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J 2015;37:1826–1834. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Lopez E, Gagliardi C, Dominguez F et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J 2017;38:1895–1904. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa M, Sekijima Y, Yazaki M et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016;23:58–63. [DOI] [PubMed] [Google Scholar]
- 51.Sperry B, Reyes B, Ikram A et al. Screening for amyloidosis at the time of carpal tunnel release and identification of cardiac involvement. J Am Coll Cardiol 2018. [Google Scholar]
- 52.Yanagisawa A, Ueda M, Sueyoshi T et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol 2015;28:201–7. [DOI] [PubMed] [Google Scholar]
- 53.Sueyoshi T, Ueda M, Jono H et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol 2011;42:1259–64. [DOI] [PubMed] [Google Scholar]
- 54.Rubin J, Alvarez J, Teruya S et al. Hip and knee arthroplasty are common among patients with transthyretin cardiac amyloidosis, occurring years before cardiac amyloid diagnosis: can we identify affected patients earlier? Amyloid 2017;24:226–230. [DOI] [PubMed] [Google Scholar]
- 55.Dungu J, Sattianayagam PT, Whelan CJ et al. The electrocardiographic features associated with cardiac amyloidosis of variant transthyretin isoleucine 122 type in Afro-Caribbean patients. Am Heart J 2012;164:72–9. [DOI] [PubMed] [Google Scholar]
- 56.Syed IS, Glockner JF, Feng D et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 2010;3:155–64. [DOI] [PubMed] [Google Scholar]
- 57.Pagourelias ED, Mirea O, Duchenne J et al. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ Cardiovasc Imaging 2017;10:e005588. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Naharro A, Treibel TA, Abdel-Gadir A et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol 2017;70:466–477. [DOI] [PubMed] [Google Scholar]
- 59.Zhao L, Tian Z, Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc Disord 2016;16:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dungu JN, Valencia O, Pinney JH et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging 2014;7:133–42. [DOI] [PubMed] [Google Scholar]
- 61.Quarta CC, Solomon SD, Uraizee I et al. Left Ventricular Structure and Function in TTR-Related versus AL Cardiac Amyloidosis. Circulation 2014. [DOI] [PubMed] [Google Scholar]
- 62.Pellikka PA, Holmes DR Jr., Edwards WD, Nishimura RA, Tajik AJ, Kyle RA. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med 1988;148:662–6. [PubMed] [Google Scholar]
- 63.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009;114:4957–9. [DOI] [PubMed] [Google Scholar]
- 64.Quarta CC, Gonzalez-Lopez E, Gilbertson JA et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J 2017;38:1905–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perugini E, Guidalotti PL, Salvi F et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. Journal of the American College of Cardiology 2005;46:1076–1084. [DOI] [PubMed] [Google Scholar]
- 66.Rapezzi C, Quarta CC, Guidalotti PL et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659–70. [DOI] [PubMed] [Google Scholar]
- 67.Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol 2016;25:413–7. [DOI] [PubMed] [Google Scholar]
- 68.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castano A, Haq M, Narotsky DL et al. Multicenter Study of Planar Technetium 99m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA Cardiol 2016;1:880–889. [DOI] [PubMed] [Google Scholar]
- 70.Phull P, Sanchorawala V, Connors LH et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid 2018;25:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park MA, Padera RF, Belanger A et al. 18F-Florbetapir Binds Specifically to Myocardial Light Chain and Transthyretin Amyloid Deposits: Autoradiography Study. Circ Cardiovasc Imaging 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SP, Lee ES, Choi H et al. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging 2015;8:50–9. [DOI] [PubMed] [Google Scholar]
- 73.Bhuiyan T, Helmke S, Patel AR et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ Heart Fail 2011;4:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollak A, Falk RH. Left ventricular systolic dysfunction precipitated by verapamil in cardiac amyloidosis. Chest 1993;104:618–20. [DOI] [PubMed] [Google Scholar]
- 75.El-Am EA, Dispenzieri A, Melduni RM et al. Direct Current Cardioversion of Atrial Arrhythmias in Adults With Cardiac Amyloidosis. Journal of the American College of Cardiology 2019;73:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Epstein AE, DiMarco JP, Ellenbogen KA et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:e1–62. [DOI] [PubMed] [Google Scholar]
- 77.Sayed RH, Rogers D, Khan F et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J 2015;36:1098–105. [DOI] [PubMed] [Google Scholar]
- 78.Varr BC, Zarafshar S, Coakley T et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014;11:158–62. [DOI] [PubMed] [Google Scholar]
- 79.Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2013;24:793–8. [DOI] [PubMed] [Google Scholar]
- 80.Vollmar J, Schmid JC, Hoppe-Lotichius M et al. Progression of transthyretin (TTR) amyloidosis in donors and recipients after domino liver transplantation-a prospective single-center cohort study. Transpl Int 2018;31:1207–1215. [DOI] [PubMed] [Google Scholar]
- 81.Sousa M, Monohan G, Rajagopalan N, Grigorian A, Guglin M. Heart transplantation in cardiac amyloidosis. Heart Fail Rev 2017;22:317–327. [DOI] [PubMed] [Google Scholar]
- 82.Coelho T, Maia LF, da Silva AM et al. Tafamidis for transthyretin familial amyloid polyneuropathy A randomized, controlled trial. Neurology 2012;79:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maurer MS, Grogan DR, Judge DP et al. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail 2015;8:519–26. [DOI] [PubMed] [Google Scholar]
- 84.Maurer MS, Elliott P, Merlini G et al. Design and Rationale of the Phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- 85.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med 1999;18:1341–54. [DOI] [PubMed] [Google Scholar]
- 86.Castano A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail 2012;18:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid 2015;22:79–83. [DOI] [PubMed] [Google Scholar]
- 88.Ikram A, Donnelly JP, Sperry BW, Samaras C, Valent J, Hanna M. Diflunisal tolerability in transthyretin cardiac amyloidosis: a single center’s experience. Amyloid 2018;25:197–202. [DOI] [PubMed] [Google Scholar]
- 89.Rosenblum H, Castano A, Alvarez J, Goldsmith J, Helmke S, Maurer MS. TTR (Transthyretin) Stabilizers Are Associated With Improved Survival in Patients With TTR Cardiac Amyloidosis. Circulation-Heart Failure 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suhr OB, Coelho T, Buades J et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis 2015;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scott D. Solomon D A, Arnt Kristen, Martha Grogan, Alejandra González-Duarte, Mathew S. Maurer, Giampaolo Merlini, Thibaud Damy, Michel S. Slama, Thomas H Brannagan III, Angela Dispenzieri, John L Berk, Amil M Shah, Pushkal Garg, Akshay Vaishnaw, Verena Karsten, Jihong Chen, Jared Gollob, John Vest, and Ole Suhr Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients with Hereditary Transthyretin-Mediated Amyloidosis: An Analysis of the APOLLO Study. Circulation 2018. [Google Scholar]
- 92.Benson MD, Dasgupta NR, Rissing SM, Smith J, Feigenbaum H. Safety and efficacy of a TTR specific antisense oligonucleotide in patients with transthyretin amyloid cardiomyopathy. Amyloid 2017;24:219–225. [DOI] [PubMed] [Google Scholar]
- 93.Inotersen and Patisiran for Hereditary Transthyretin Amyloidosis: Effectiveness and Value Evidence Report. Institute for Cliinical and Economic Review (ICER), August 29, 2018. [Google Scholar]
- 94.Penchala SC, Connelly S, Wang Y et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci U S A 2013;110:9992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Judge DP, Falk RH, Maurer MS et al. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 96.Sant’Anna R, Gallego P, Robinson LZ et al. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat Commun 2016;7:10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richards DB, Cookson LM, Berges AC et al. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med 2015;373:1106–14. [DOI] [PubMed] [Google Scholar]
- 98.Galant NJ, Bugyei-Twum A, Rakhit R et al. Substoichiometric inhibition of transthyretin misfolding by immune-targeting sparsely populated misfolding intermediates: a potential diagnostic and therapeutic for TTR amyloidoses. Sci Rep 2016;6:25080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saelices L, Chung K, Lee JH et al. Amyloid seeding of transthyretin by ex vivo cardiac fibrils and its inhibition. Proc Natl Acad Sci U S A 2018;115:E6741–E6750. [DOI] [PMC free article] [PubMed] [Google Scholar]