Abstract
Objectives:
Placental insufficiency contributes to altered maternal-fetal amino acid transfer, and thereby to poor fetal growth. An important placental function is the uptake of tryptophan and its metabolism to serotonin (5-HT) and kynurenine metabolites, which are essential for fetal development. We hypothesised that placental 5-HT content will be increased in pregnancies affected with fetal growth restriction (FGR).
Methods:
The components of the 5-HT synthetic pathway were determined in chorionic villus samples (CVS) from small-for gestation (SGA) and matched control collected at 10–12 weeks of human pregnancy; and in placentae from third trimester FGR and gestation-matched control pregnancies using the Fluidigm Biomarker array for mRNA expression, the activity of the enzyme TPH and 5-HT concentrations using an ELISA.
Results:
Gene expression for the rate limiting enzymes, TPH1 and TPH2; 5-HT transporter, SLC6A4; and 5-HT receptors HTR5A, HTR5B, HTR1D and HTR1E were detected in all CVS and third trimester placentae. No significant difference in mRNA was observed in SGA compared with control. Although there was no significant change in TPH1 mRNA, the mRNA of TPH2 and SLC6A4 was significantly decreased in FGR placentae (p < 0.05), while 5-HT receptor mRNA was significantly increased in FGR compared with control (p < 0.01). Placental TPH enzyme activity was significantly increased with a concomitant increase in the total placental 5-HT concentrations in FGR compared with control.
Conclusion:
This study reports differential expression and activity of the key components of the 5-HT synthetic pathway associated with the pathogenesis of FGR. Further studies are required to elucidate the functional consequences of increased placental 5-HT in FGR pregnancies.
1. Introduction
Fetal growth restriction (FGR), defined as defined as a failure of the fetus to reach its full growth potential, is a common and significant obstetric complication, affecting approximately 5–10% of all pregnancies worldwide [1–3]. The second leading cause of perinatal mortality after prematurity, FGR remains the major cause of late pregnancy stillbirth, a leading cause of morbidity and a major contributor to lifelong impairment including neurological disorders, cerebral palsy, cardiovascular disease, systemic hypertension, stroke and non-insulin-dependent diabetes mellitus in adulthood [1,4]. Identifying high-risk pregnancies for FGR is key in order to prevent or alleviate the consequences of poor fetal growth. Clinically, the ability to predict FGR is limited and current strategies are largely restricted to prenatal screening based on ultrasonographic biometrical measurements of the fetus and Doppler velocimetry for placental perfusion, for evidence of fetal decompensation secondary to hypoxia [5,6]. This allows clinicians to identify FGR from otherwise healthy small for gestational age (SGA) infants [5]. FGR is managed based on careful monitoring of fetal growth and biophysical profile. Premature delivery is often the only solution when fetal growth is significantly impaired, contributing to the risk of perinatal mortality and morbidity [7]. Therefore, understanding the mechanisms implicated in the development of FGR is necessary to develop novel strategies to prevent or limit FGR and its consequences.
There are multiple aetiologies of FGR, including those of maternal (hypertension, gestational diabetes mellitus), fetal (chromosomal abnormalities) or placental (infarcts) origins. Although the mechanisms implicated in the development of FGR are poorly understood, it is widely accepted that the origins of FGR lie within a functionally insufficient placenta [8]. Therefore, understanding the molecular mechanisms of placental insufficiency associated with FGR is of increasing importance. Typically, placentae from FGR-affected pregnancies are smaller than gestation-matched control pregnancies and may exhibit a number of morphological and functional defects [9–12]. For example, aberrant fusion of the villous cytotrophoblasts (VCT), with an associated increase in apoptosis, reduces transfer of nutrients and growth factors to the fetus, thereby restricting fetal growth [13]. Another significant feature of FGR pathogenesis is uteroplacental ischemia, due to failure of the extravillous trophoblast cells (EVCT) to proliferate, migrate, invade, and adequately transform and remodel spiral arterioles in the placental bed [2,10,14]. In addition to this cytotrophoblastic dys-function, various studies suggest that the resulting hypoxia, oxidative stress or both may have a significant effect on key placental metabolic pathways [13,15].
One such metabolic pathway that plays a prominent role in feto-placental growth is the metabolism of the amino acid tryptophan. During pregnancy, the essential amino acid tryptophan is actively transported to the fetus by the placenta [16]. Besides being utilised for protein synthesis by the placenta and fetus, another fate of tryptophan is the formation of serotonin (5-HT) and kynurenine (KYN) metabolites [16]. In a recent study, we reported that the expression and activity of the KYN pathway is present in the human placenta from early gestation, and is down-regulated by hypoxia and in FGR pregnancies [17]. However, the expression of key metabolic components of the 5-HT synthetic pathway in early and late pregnancy placental tissues is largely unknown, particularly in cases of impaired placental function observed in FGR, where the activity of several placental nutrient transporters is dysregulated [18].
Conversion of tryptophan to 5-HT occurs through the hydroxylation of precursor tryptophan and decarboxylation of the intermediate product - 5-hydroxytryptophan (5-HTP) [16]. The rate-limiting enzymes in this process are tryptophan hydroxylase 1 (TPH1) and TPH2 which, in addition to the brain, are expressed in peripheral tissues including the placenta [19]. Some 5-HT of maternal origin may also be transported into the placenta by 5-HT transporter SERT/SLC6A4, which is localised to the syncytiotrophoblast [20], although most maternal 5-HT appears to be metabolised by monoamine oxidase enzymes in the cytotropho-blast cells, possibly to prevent vasoconstriction of spiral arterioles [21–23].
As FGR is associated with decreased placental KYN synthesis [17], a greater availability of tryptophan may lead to an increased placental synthesis of 5-HT, with an associated increase in expression of components of the 5-HT synthetic pathway in FGR pregnancies. In this study, we hypothesized that altered expression of the components of the placental 5-HT synthetic pathway contributes to the aetiology of FGR. Small for gestational age (SGA), defined as a birth weight below the 10th percentile of a birth weight curve, is often considered as a surrogate for FGR. Therefore, our overall aim was to determine the relationship between the mRNA levels of TPH1, TPH2, SLCA64 and receptors of 5-HT in first-trimester (10–12 weeks’ gestation) chorionic villous samples (CVS) collected from women who went on to develop SGA later in pregnancies and gestation-matched uncomplicated control pregnancies; and in placentae from third-trimester FGR and gestation-matched uncomplicated control pregnancies. Placental 5-HT concentrations and localisation of 5-HT receptors were determined in third trimester FGR and gestation-matched control pregnancies.
2. Materials and methods
2.1. Patient details and tissue sampling
2.1.1. Third trimester FGR and control placental tissues
Human placentae from pregnancies complicated by FGR (n = 23) and healthy control pregnancies (n = 42) were obtained following informed patient consent and approval from the Research and Ethics Committee of the Royal Women’s Hospital, Melbourne. Patient characteristics are shown in Table 1; previous studies have demonstrated consistent differences in the expression of various genes in the placentae from these cohorts of patients [17,24]. Briefly, the inclusion criteria for FGR samples was defined as a birth weight less than 10th percentile for gestational age using Australian growth charts [25] and any one of the following criteria on antenatal ultrasound: oligohydramnios (amniotic fluid index < 7); abnormal umbilical artery Doppler velocimetry; or asymmetric growth (head circumference to abdominal circumference ratio > 1.2 for gestational age). Uncomplicated control samples were matched to FGR cases based on gestational age, determined using last menstrual period dates and confirmed by early pregnancy ultrasound. Both caesarean sections and spontaneous and induced vaginal deliveries were included in the sample collection. The following characteristics were excluded in the selection of both control and FGR samples: prolonged rupture of membranes beyond 24 h and/or evidence of placental abruption; maternal chemical dependency; underlying maternal diseases including preeclampsia, maternal hypertension, gestational diabetes, type 1 and 2 diabetes; fetal congenital anomalies; chromosomal abnormalities; pregnancies with multiple fetuses; and suspected intrauterine infection. All control mothers gave birth to normally formed babies with birth weights appropriate for gestational age. Control placentae were normal in appearance with no observed pathology. Following delivery of the placentae, the decidua was removed and placental tissue pieces were excised from the periphery and from the central cotyledons and pooled and rinsed in buffered saline. Samples were processed within 20 min of delivery of the placenta, and snap frozen and stored at −80 °C for RNA and protein analyses; some samples were fixed in 10% formalin for immunohistochemical analysis.
Table 1.
Clinical characteristics of third trimester pregnancies used in this study.
| Control n = 42 | FGR n = 23 | Significance (p-value) |
|
|---|---|---|---|
| Maternal Age (years) | 32.37 ± 5.85 | 32.53 ± 6.33 | p = 0.9305 |
| Gravidity: n (%) | 5 (20.00%) | 7 (35.00%) | p = 0.2582 |
| = 1 | 20 (80.00%) | 13 (65.00%) | |
| > 1 | |||
| Parity: n (%) | |||
| ≤ 1 | 12 (28.57%) | 12 (52.17%) | p = 0.0594 |
| > 1 | 30 (71.43%) | 11 (47.83%) | |
| Gestation (weeks) (Mean ± SD) | 35.48 ± 3.92 | 35.83 ± 3.55 | p = 0.7284 |
| Mode of delivery: (%) | |||
| VD | 12 (28.57%) | 9 (39.13%) | p = 0.6547 |
| CSIL | 3 (7.14%) | 1 (4.35%) | |
| CSNIL | 27 (64.29%) | 13 (56.52%) | |
| Birth weight (g) (Mean ± SD) | 2731 ± 870.5 | 1921 ± 708.9 | p = 0.0003* |
| Birth weight percentile: n (%) | |||
| < 3rd | 10 (47.62%) | N/A | |
| 3rd-4th | 6 (28.57%) | ||
| 5th-9th | 5 (23.81%) | ||
| ≥10th | 42 (100%) | ||
| Additional FGR parameters: n (%) | |||
| Asymmetry | 14 (66.67%) | N/A | |
| AFI ≤ 7 | 14 (66.67%) | ||
| Abnormal Doppler | 13 (59.09%) | ||
|
Placental weight(Mean ± SD) |
540.3 ± 147.4 | 388.5 ± 121.9 | p = 0.0001* |
| Newborn gender: n (%) | |||
| Male | 23 (54.76%) | 10 (47.83%) | p = 0.5924 |
| Female | 19 (45.24%) | 12 (52.17%) |
Statistically significant difference in characteristic between two groups.
For continuous non-parametric data, the unpaired independent t-test was used to calculate p-values.
For discrete data, the chi-square test was used to calculate p-values.
2.1.2. First trimester CVS samples
Human first trimester villous tissues (n = 52 control, n = 28 SGA) were obtained as surplus tissue from chorionic villus sampling (CVS) performed at the University Medical Centre of Groningen, The Netherlands. These CVS samples were snap frozen and stored at −80 °C for gene expression analysis. Patient characteristics are shown in Table 2 for the SGA and matched control samples used in this study. SGA was used as a surrogate for FGR, as has been done in previous studies [26,27]. Briefly, CVS was performed vaginally between 10 and 12 weeks’ gestation, for aneuploidy risk due to increased maternal age or serum screening results. The tissues were collected after obtaining informed written patient consent and ethics approval from the Federation of Dutch Medical Scientific Societies. Patient identification was removed prior to receiving the samples. Patient demographic details were obtained through questionnaires completed by each patient postpartum and included details of the pregnancy outcome. Pregnancies that were complicated by SGA, defined as a birth weight less than 10th percentile (as per Dutch population charts, Stichting Perinatale Registratie, Nederland) were then matched to uncomplicated control pregnancies, based on maternal age, gestational age, parity and crown-rump-length at the time of sampling. Exclusion criteria for both control and SGA samples were gestational diabetes, pregnancies associated with multiple fetuses, fetal congenital anomalies and chromosomal abnormalities. Control patients did not have clinical evidence of preeclampsia or maternal hypertension. All control mothers gave birth to infants with birth weights appropriate for gestational age.
Table 2.
Clinical Characteristics of first trimester SGA and control pregnancies.
| Control n = 52 | SGA n = 28 | Significance (p-value) | |
|---|---|---|---|
| Maternal Age (years) (Mean ± SD) | 38.35 ± 3.14 | 37.96 ± 4.25 | p = 0.6363 |
| Gravidity: n (%) | |||
| = 1 | 7 (13.46%) | 6 (21.43%) | p = 0.3569 |
| > 1 | 45 (86.54%) | 22 (78.57%) | |
| Parity: n (%) | p = 0.9812 | ||
| ≤ 1 | 28 (53.85%) | 15 (53.57%) | |
| >1 | 24 (46.15%) | 13 (46.43%) | |
| Gestation (weeks) (Mean ± SD) | 39.32 ± 2.44 | p = 0.0999 | |
| Birth weight (g) (Mean ± SD) | 2575 ± 548.2 | p < 0.0001* | |
| Birth weight percentile: | N/A | ||
| < 3rd | 9 (32.14%) | ||
| 3rd-4th | 11 (39.29%) | ||
| 5th-9th | 8 (28.57%) | ||
| 10th-49th | 31 (59.62%) | ||
| ≥50th | 21 (40.38%) | ||
| Additional pathology: n (%) | |||
| Mild PE | 1 (3.57%) | N/A | |
| Mild HTN | 4 (14.29%) | ||
|
Newborn gender:
n (%) |
|||
| Male | 31 (59.62%) | 15 (53.57%) | p = 0.6020 |
| Female | 21 (40.38%) | 13 (46.43%) |
2.1.3. First trimester placental tissues
Human placentae from 8 to 14 weeks’ gestation (n = 6) were obtained from elective terminations. First-trimester placentae collection and processing for immunohistochemical analyses were performed with the approval of the University Hospital ethics committees at the University Hospital, Grenoble, France, and with the informed written consent of each patient.
2.1.4. Isolation of villous cytotrophoblast cells
Isolation and purification of primary villous trophoblast cells was performed from placentae collected from uncomplicated term pregnancies (n = 6). These cells were used for the quantitation of TPH enzyme activity and also to determine the synthesis of 5-HT in vitro, as detailed below. Placental villous cytotrophoblasts were isolated as previously described [28] by DNase/trypsin digestion and purified by separation on a Percoll gradient. Briefly, placental villous tissue (~25 g) was dissected and washed in saline and then digested three times in a HEPES-buffered salt solution containing 0.25% trypsin and 0.2 mg/ml DNAse. The digested villous tissue was shaken at 37 °C for 30 min. The cytotrophoblast cells were separated on a Percoll gradient and resuspended in standard cell culture medium containing 5.5 mM glucose, 44.5% DMEM, 44.5% Ham’s-F12, supplemented with 10% fetal calf serum and antibiotics. The cells were plated on 24-well plates at a density of 5 × 105 cells per well. The cells were cultured at 37 °C in 8% O 2, 5% CO2 atmosphere following treatment as described below for activity assays. Trophoblast cell purity was confirmed by high protein expression of cytokeratin-7 (epithelial cell marker), absence of vimentin (fibroblast cell marker) expression, and secretion of hCG (measure of biochemical differentiation) (data not shown).
2.1.5. Quantitation of TPH activity
Placental tissues obtained from third trimester FGR and control pregnancies (n = 6) were homogenised using the extraction buffer composed of 0.05 M Tris (pH 7.5), containing 1 mM dithiothreitol and 1 mM EGTA and then assayed for TPH1/2 activity as previously described [29]. For the isolated trophoblasts, following 48 h of differentiation, villous trophoblasts were pre-treated with a serum-free medium: the fetal bovine serum (FBS) was replaced by 1× SITE-3 (Sigma-Aldrich) to avoid traces of exogenous 5-HT in the FBS. Cultured trophoblasts and the placental extracts (20 μl of supernatant) were treated with 80 μl of reaction or control buffer to give a final concentration of: 0.05 M Tris buffer (pH 7.5), 1 mM EGTA, 50 g/ml catalase, 200 M L-tryptophan (TRP), 100 M ammonium iron (II) sulphate, and 100 M tetrahydrobiopterin (BH4; a cofactor required for TPH1 and TPH2 activity). Control buffers did not receive TRP or BH4. Tubes were incubated for 30 min at 37 °C and reactions were terminated through protein denaturation by adding 100 l of 0.2 M percholoric acid with 100 M EDTA. Samples were stored on ice for 15 min to allow for complete protein denaturation and then centrifuged for 15 min at 21,000 g at 4 °C. TRP metabolism was determined by measuring the 5-HT concentration using an immunoassay as described below. Protein concentration of the supernatants was determined using a detergent-compatible protein assay (Bio-Rad) and enzymatic activity was quantified by the measurement of 5-HT and represented as nmol 5-HT/μg of protein as described below.
2.1.6. Quantitation of 5-HT concentration
5-HT concentrations in term placental tissues (n = 6) and villous trophoblast culture media (n = 6), prepared as described above, were measured using an enzyme-linked immunosorbent 5-HT assay (ENZO Life Sciences, New York, USA) according to the manufacturer’s instructions and the absorbance was recorded at 490 nm using an ELISA plate reader (SpectraMax i3) as previously described [30]. Concentrations of 5-HT in the trophoblast culture media and in the placental tissues obtained from FGR and uncomplicated control pregnancies are represented as nmol 5-HT/μg protein.
2.1.7. Total RNA extraction and cDNA preparation
Total RNA from third trimester human placental tissues was extracted using the RNeasy Midi kit as previously described [31]. Total placental RNA from surplus CVS tissues was extracted and purified using the Macherey-Nagel NucleoSpin® RNA kit according to the manufacturer’s instructions (Macherey-Nagel Inc. Bethlehem, USA). RNA purity was determined using NanoDropTM spectrophotometer (ThermoScientific, USA). cDNA was prepared from 2 μg total RNA reverse-transcribed using Superscript III ribonuclease H-reverse transcriptase (Invitrogen, Australia) in a two-step reaction also as previously described [31].
2.1.8. Fluidigm Dynamic array
The mRNA expression of key metabolic components of the placental 5-HT pathway was quantitated using the Fluidigm Dynamic array (BioMark™ HD System) at the Monash Health Translation Precinct (MHTP) Medical Genomics Facility, Clayton, Australia. Gene expression was determined using validated assays that consisted of a TaqMan® FAM™ labelled MGB probes (list gene names and their catalogue # 18S rRNA Hs99999901_s1; YWHAZ (Hs01122445_g1); TBP (Hs00427620_ m1); SLC6A4 Hs00984349_m1; TPH1 Hs00188220_m1; TPH2 Hs00542783_m1; HTR5A Hs04194553_s1; HTR5B Hs00168362_m1; HTR1D Hs00704742_s1 and HTR1E Hs00704779_s1, Thermo Fisher Scientific, USA). Gene expression relative to 18S rRNA was calculated according to the 2−ΔΔCT method [32]. Further validation of gene expression relative to geometric averaging of the three endogenous control genes 18S rRNA, YWHAZ and TBP as previously described [33].
2.1.9. Immunohistochemistry
Spatio-temporal distribution and localisation of TPH1, SLC6A4 and HTR2A protein in first trimester and third trimester FGR and control placental tissue sections was performed by immunohistochemistry as previously described [34]. Briefly, 5 μm thick, paraffin-embedded sections were dewaxed in xylene, rehydrated in graded ethanol (100%–50% ethanol), and non-specific antibody binding was blocked with 1% bovine serum albumin (BSA) prepared in PBS. Tissue sections were then incubated overnight at 4 °C with the primary antibody, either rabbit anti-human TPH1 (ab52954) or anti-human SLCA64 (ab181034) or anti-human HTR5A (ab61002), (Abcam, Cambridge, MA, USA), at a concentration of 0.01 μg/μL in 1% BSA/PBS. Control sections were incubated with 0.02 μg/μL non-immune rabbit IgG (Sigma-Aldrich, St Louis, MO, USA) prepared in 1% BSA/PBS (DAKO, Copenhagen, Denmark). Chromogen detection was performed using 0.05% (w/v) 3,3′-Diaminobenzidine (DAB) and 0.015% (v/v) hydrogen peroxide (Sigma-Aldrich, St Louis, MO, USA) and colour development was monitored under a light microscope. Slides were washed in distilled water, counterstained with hematoxylin, rehydrated in alcohol and Histoclear, and mounted in DPX (Sigma-Aldrich, St Louis, MO, USA) for imaging with a light microscope. All tissue sections were incubated with DAB for the same length of time so that comparisons could be made between individual samples, and all slides were stained in a single run to eliminate between-run variations in staining intensity.
2.1.10. Data analyses
Patient characteristics were compared using the unpaired t-test and chi-square test for continuous and categorical variables respectively. Levels of mRNA expression relative to the housekeeping gene 18S rRNA/geometrical average of three house-keeping genes YWHAZ, 18S rRNA and TBP were determined using the 2−ΔΔCT method [32,33]. All data analyses including 5-HT concentration and TPH activity assays were performed using the Mann-Whitney U test. Data is presented as mean ± SEM unless otherwise stated and P < 0.05 was accepted as statistically significant.
3. Results
Gestation, maternal age, mode of delivery and the sex of the newborn did not differ significantly between the FGR and the un-complicated control groups. Mean birthweight (p = 0.04) and mean placental weight (p = 0.03) were significantly lower in the FGR group compared with the control.
Patient characteristics of first trimester SGA and control pregnancies used in this study are shown in Table 2. There were no statistically significant differences between both groups for maternal age, gravidity, parity and newborn gender. However, there was a statistically significant difference in birth weight (p < 0.0001), with SGA newborns having a lower mean birth weight (2575 g) than control newborns (3534 g).
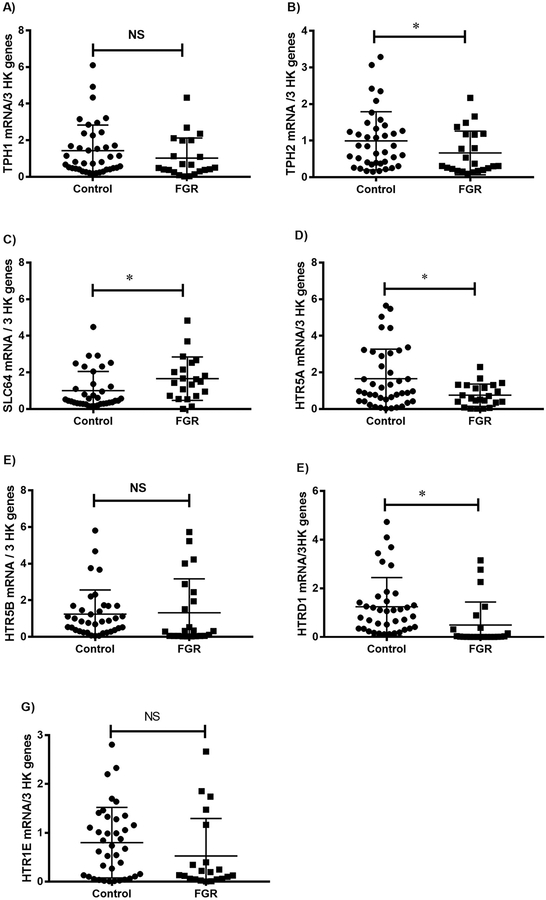
Gene expression of the components of the 5-HT synthetic pathway in third trimester placental tissue is shown in Fig. 1. As shown in Fig. 1A, although there was no significant difference in placental TPH1 mRNA relative to 18S rRNA, a significant decrease in TPH2 mRNA (Fig. 1B) as well as in SLC6A4 mRNA (Fig. 1C) was observed in FGR compared with gestation-matched control pregnancies. Furthermore, gene expression analyses of the 5-HT receptors demonstrated a significantly increased mRNA expression of HTR5A (Fig. 1D), HTR1D (Fig. 1E) and HTR1E (Fig. 1F) in FGR placentae compared with control placentae (p < 0.005).
Fig. 1.
Gene expression of the components of the 5-HT synthetic pathway in third trimester FGR placentae compared with gestation-matched control placentae. Placental mRNA of TPH1 (Fig. 1A); TPH2 (Fig. 1B); SLC6A4 (Fig. 1C); and 5-HT receptors HTR5A (Fig. 1D), HTR1D (Fig. 1E) and HTR1E (Fig. 1F) relative to the geometrical averaging of the three housekeeping genes 18S rRNA, YWHAZ and TBP were determined using the 2−ΔΔCT method [32,33] and analysed using the Mann-Whitney U test.
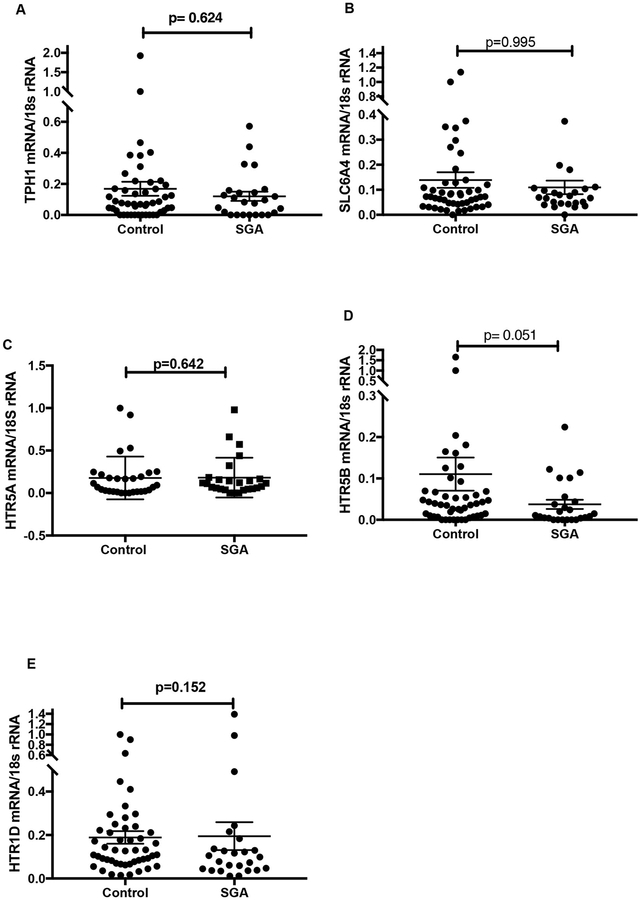
Fig. 2 illustrates mRNA expression of TPH1 (Fig. 2A), SLC6A4 (Fig. 2B), HTR5A (Fig. 2C), HTR5B (Fig. 2D) and HTR1D (Fig. 2E) relative to 18S rRNA in first trimester CVS samples. As depicted, there were no statistically significant differences observed between first trimester SGA and matched control CVS tissues. TPH2 and HTR1E mRNA were undetectable in both SGA and control CVS tissues.
Fig. 2.
Gene expression of the components of the 5-HT synthetic pathway in first trimester SGA and matched control villus tissues. Placental mRNA of TPH1 (Fig. 2A), SLC6A4 (Fig. 2B), HTR5A (2C), HTR5B (2D) and HTR1D (2E) relative to the housekeeping gene 18S rRNA were determined using the 2−ΔΔCT method [32] and analysed using the Mann-Whitney U test.
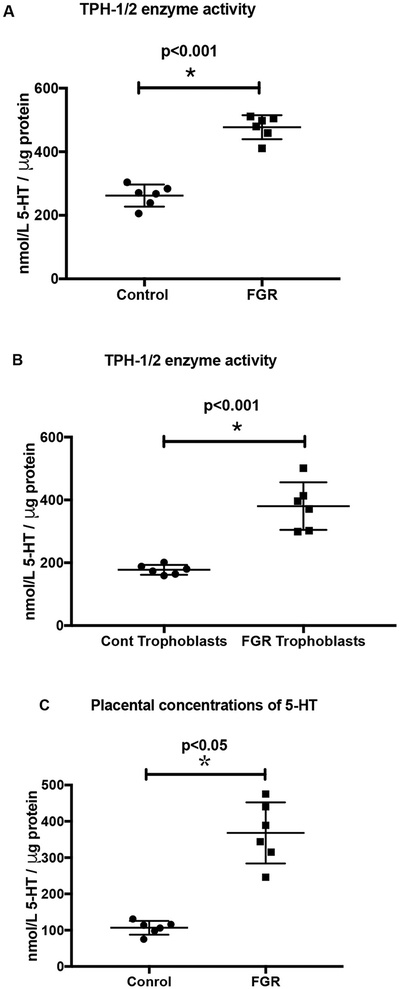
To further correlate the mRNA levels of TPH1/2, the activity of the enzymes TPH1/2 was measured in cultured trophoblasts derived from FGR and control placental tissues. A shown in Fig. 3A, significantly increased TPH1/2 activity was observed in the placental tissues and in the cultured trophoblasts obtained from third trimester FGR pregnancies compared with gestation-matched control pregnancies (Fig. 3B). This increase in TPH1/2 activity was further substantiated by the concomittant increase in total placental 5-HT content in placental tissues from FGR pregnancies compared with gestation-matched control (Fig. 3C).
Fig. 3.
Quantitation of TPH1/2 enzyme activity. As described in the methods section, the rate limiting enzyme, TPH (1 and 2) activity was measured in the placental tissues (Fig. 3A) and in the cultured trophoblasts (Fig. 3B) obtained from third trimester FGR pregnancies compared with gestation-matched control pregnancies. Fig. 3C depicts the total placental 5-HT content in FGR pregnancies compared with gestation-matched control. Concentrations of 5-HT are represented as nmol 5-HT/μg protein.
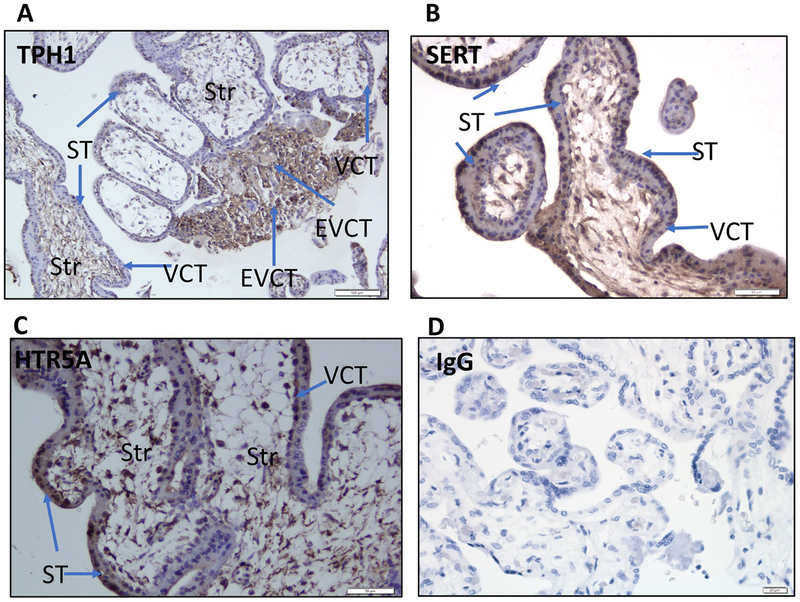
Immunohistochemical localisation of components of the 5-HT synthetic pathway, including TPH1, HTR5A and SERT protein (SLC6A4), was performed in placental tissues obtained from first trimester control placentae and in third-trimester FGR and gestation-matched control pregnancies. In the first trimester placental tissues, immunoreactivity for TPH1 (Fig. 4A) was localized to the villous cytotrophoblasts (VCT), syncytiotrophoblast (ST) and extravillous cytotrophoblasts (EVCT), and immunoreactivity for SLC6A4 (Fig. 4B) and HTR5A (Fig. 4C) was localised to VCT, ST and in some stromal cells. Absence of immunoreactivity was observed in the IgG negative control sections (Fig. 4D).
Fig. 4.
Immunohistochemical localisation of TPH1, SERT (SLC6A4) and HTR5A protein in first trimester control placental tissues was performed as described in the methods section. Representative images are depicted in Fig. 5. Arrows indicate immunoreactivity for TPH1 (Fig. 4A) in the villous cytotrophoblasts (VCT), syncytiotrophoblast (ST) and in the extravillous cytotrophoblasts (EVCT), while immunoreactivity for SERT (Fig. 4B) and HTR5A (Fig. 4C) localisaed in ST, endothelial cells (EC) and in some stromal cells (Str). Immunoreactivity to IgG was used as a negative control (Fig. 4D).
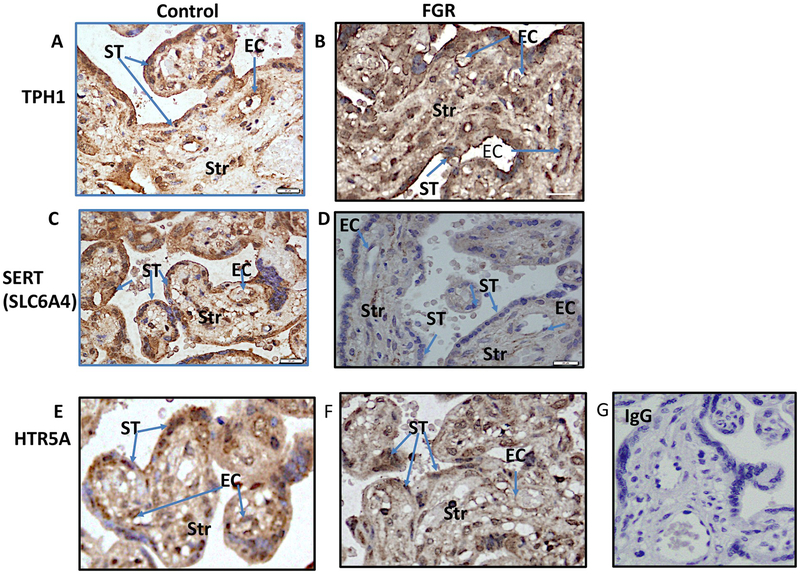
In the third trimester placental tissues (Fig. 5), immunoreactivity for TPH1 (Fig. 5A-Control; Fig. 5B-FGR); SLCA64 (Fig. 5C-Control; Fig. 5DFGR) and HTR5A (Fig. 5E-Control; Fig. 5F-FGR) was present in the syncytiotrophoblast (ST), endothelial cells (EC) and in some stromal cells (Str). No specific immunoreactivity was observed in the IgG negative control (Fig. 5G).
Fig. 5.
Immunolocalisation for the components of 5-HT synthetic pathway was performed in the third trimester FGR and in gestation-matched control placentae as described in the methods section. Representative images are depicted in Fig. 5. Arrows indicate immunoreactivity for TPH1 (Fig. 5A-Control; Fig. 5B-FGR); SLCA64 (Fig. 5C-Control; Fig. 5D-FGR) and HTR5A (Fig. 5E-Control; Fig. 5F-FGR) in the syncytiotrophoblast (ST), endothelial cells (EC) and in some stromal cells (Str). Immunoreactivity to IgG was used as a negative control (Fig. 5G).
4. Discussion
This study demonstrated the presence of genes associated with 5-HT synthesis in CVS samples collected in the first trimester from pregnancies that went on to develop either SGA or uncomplicated pregnancy outcome at delivery. Our data, showing expression of TPH1, SLC6A4 and 5-HT receptors in first trimester placentae, strengthens the hypothesis that 5-HT could be involved in the early stages of pregnancy, including placentation. Previous studies have reported that the placental synthesis of 5-HT may be particularly important for successful blastocyst implantation as well as overall placental development [35]. Indeed, animal model studies have demonstrated the significant role of 5-HT in implantation, placentation and also in decidualisation [36,37]. In a recent study, Laurent et al. (2018) described that components of the 5-HT synthetic pathway, particularly the rate limiting enzymes TPH1 and TPH2, are co-expressed in the VCT, ST, EC lining the fetal capillaries, EVCT, and decidual cells in first trimester placental tissues. In the same study, the authors reported that mRNA and protein levels of both TPHs were detected in human primary trophoblasts isolated from first trimester and term placental tissues, and demonstrated the de novo synthesis of 5-HT in cultured VCT [38]. Although there were no significant differences in mRNA expression between first-trimester SGA and uncomplicated control CVS samples, our study further substantiates the crucial role that placental production of 5-HT may have in successful fetal development and pregnancy outcome.
Although we report the presence of TPH1, TPH2, SLC6A4 and 5-HTR receptor mRNA expression in third trimester uncomplicated control and FGR pregnancies, our study demonstrated no significant difference in TPH1 mRNA expression in placentae from FGR compared with control pregnancies. However, placental TPH2 mRNA was significantly decreased in FGR compared with control. Furthermore, TPH2 mRNA was expressed at a much lower level compared to TPH1 in both term un-complicated and in FGR placentae. The examination of enzyme activity is vital, considering the complex regulatory mechanisms for gene expression that occur at both post-transcriptional and post-translational levels. Therefore, in this study we further measured the activity of the TPH1/2 enzymes in third trimester placental tissues obtained from FGR and gestation-matched control pregnancies. Our study reported significantly enhanced activity of the enzyme TPH1/2 present in the FGR placentae compared with control placentae. This is consistent with previous reports by Correa et al. (2009) of increased expression of TPH protein in human placental tissues from pregnancies complicated by intrauterine stress [39].
This is the first study to report on increased activity of the rate limiting enzymes TPH1/2 in placentae from FGR compared to un-complicated control pregnancies. Theoretically, TPH activity directly corresponds de novo 5-HT synthesis [40]. Maternal 5-HT levels have been thought not to significantly contribute to placental and fetal 5-HT levels, with most maternal 5-HT expected to be degraded in the placenta [21–23,41]. However, recent evidence using a knockout mouse model has found that variations in the maternal SLC6A4 genotype affect placental 5-HT levels and fetal neurodevelopment [42].
Placental insufficiency in FGR-affected pregnancies is associated with the altered placental transport of many substances [43]. In this context, down-regulation of placental transporters in FGR may correspond to a primary event or cause in the onset of this pathology, and up-regulation may be secondary or compensatory for growth restriction [44]. Our results demonstrated that SLC6A4 was detectable in placental tissues collected from first trimester; and its expression was unchanged in first trimester SGA and matched uncomplicated control pregnancies. However, in third trimester placental tissues, its expression at both mRNA and protein levels was significantly decreased in the placentae from FGR-affected pregnancies compared with that of the un-complicated control pregnancies. The presence of SERT protein in VCTs of human term placenta [45,46], and also in the brush-border membrane vesicles of VCTs isolated from human term placentae, is shown to control 5-HT concentrations in the maternal bloodstream, maintaining stable transplacental blood flow and nourishing the developing embryo [47–49]. Although, reduced placental SLC6A4 expression has been implicated in the aetiology of gestational-diabetes affected pregnancies [20], in vitro studies on the functional consequences of reduced SLC6A4 on trophoblast-derived cell lines have linked it with placental fibrosis, necrosis and increased trophoblast cell death [50], which are characteristic of the placental pathogenesis of FGR pregnancies.
Although our study did not quantitate immunoreactive TPH1 protein in the VCT and ST using immunohistochemistry, Laurent et al. (2017) reported a decrease in the level of TPH2 protein seen in ST compared to VCT, and no difference in TPH1 level [38], suggesting that differentiation into term ST may reduce the level of TPH2. High levels of free tryptophan in third trimester placenta, as reported by Badawy (2014) (reviewed [51]), may stabilize TPH1 protein by decreasing its turnover, as reported in in vitro studies [52,53]. The results of our study further confirm the presence of HTR5B and HTR1E in both first and third trimester placentae. Interestingly, although HTR5A and HTR1D were expressed at low levels in first trimester SGA and control CVS tissues, immunohistochemical analysis localized the presence of HTR5A in VCT and ST in first trimester placental tissues, compared to additional localisation of HTR5A in the endothelial cells lining fetal capillaries in third trimester placental tissues. The increase in 5-HT concentrations observed in FGR placental tissues, together with the increased expression of HTR5A in the endothelium of third trimester tissues, suggests a potential role of 5-HT in the regulation of arterial contraction linked to hypertension [54] and placental dysfunction [55]. These are important research questions for future investigation.
5. Strengths and limitations
The primary strength of this study is the use of a highly sensitive assay with a dynamic range of at least 6–7 logs for gene expression analysis on a microfluidic real-time PCR [56]. Another significant strength is the use of a well-defined cohort of third trimester FGR pregnancies that were clinically characterised for placental insufficiency. We have also made use of a unique resource of first trimester tissues obtained via chorionic villus sampling during the first trimester, to investigate the relationship between altered gene expression in the 5-HT synthetic pathway and any subsequent development of SGA. Although SGA has been used as a proxy for FGR in several gene expression studies [26,57], the lack of Doppler velocimetry measurements in CVS sample pregnancies was a major limitation to our study, as the sample set may have included fetuses and neonates who were constitutionally small and at low risk for adverse outcomes [58]. Additionally, a proportion of SGA CVS samples was from mothers who smoked cigarettes (32.14%), had mild pre-eclampsia (3.57%) or mild hypertension (14.29%). There were also mothers who had smoked cigarettes up until conception or first antenatal visit in the third trimester FGR and uncomplicated control pregnancies. The effect of this, if any, on our results is unclear, as these factors may contribute to different phenotypes of FGR.
6. Conclusion
This study demonstrates significant associations between FGR pregnancies in the third trimester and altered mRNA expression of various components of the placental 5-HT pathway and an enhanced TPH enzyme activity with an increased placental 5-HT content. Further studies are required to elucidate the functional consequences of increased placental 5-HT in contributing to the placental pathogenesis in FGR pregnancies.
Acknowledgment
This work was supported by an award to PM and PRE from the Australian Institute of Musculoskeletal Science, Western Health, Victoria, Australia.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.placenta.2019.05.012.
References
- [1].Clausson B, Cnattingius S, Axelsson O, Outcomes of post-term births: the role of fetal growth restriction and malformations, Obstet. Gynecol 94 (5) (1999) 758–762. [DOI] [PubMed] [Google Scholar]
- [2].Nardozza LMM, et al. , Fetal Growth Restriction: Current Knowledge, Archives of Gynecology and Obstetrics, 2017, pp. 1–17. [DOI] [PubMed] [Google Scholar]
- [3].Miller SL, Huppi PS, Mallard C, The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome, J. Physiol 594 (4) (2016) 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frøen JF, et al. , Restricted fetal growth in sudden intrauterine unexplained death, Acta Obstet. Gynecol. Scand 83 (9) (2004) 801–807. [DOI] [PubMed] [Google Scholar]
- [5].Figueras F, Gratacós E, Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol, Fetal Diagn. Ther 36 (2) (2014) 86–98. [DOI] [PubMed] [Google Scholar]
- [6].Mari G, et al. , Staging of intrauterine growth-restricted fetuses, J. Ultrasound Med 26 (11) (2007) 1469–1477 quiz 1479. [DOI] [PubMed] [Google Scholar]
- [7].Miller J, Turan S, Baschat AA, Fetal growth restriction, Semin. Perinatol 32 (4) (2008) 274–280. [DOI] [PubMed] [Google Scholar]
- [8].Krishna U, Bhalerao S, Placental insufficiency and fetal growth restriction, J. Obstet. Gynaecol. India 61 (5) (2011) 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roberts DJ, Post MD, The placenta in pre-eclampsia and intrauterine growth restriction, J. Clin. Pathol 61 (12) (2008) 1254–1260. [DOI] [PubMed] [Google Scholar]
- [10].Chen CP, Bajoria R, Aplin JD, Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms, Am. J. Obstet. Gynecol 187 (3) (2002) 764–769. [DOI] [PubMed] [Google Scholar]
- [11].Jackson MR, et al. , Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms, Am. J. Obstet. Gynecol 172 (2 Pt 1) (1995) 518–525. [DOI] [PubMed] [Google Scholar]
- [12].Kingdom J, et al. , Development of the placental villous tree and its consequences for fetal growth, Eur. J. Obstet. Gynecol. Reprod. Biol 92 (1) (2000) 35–43. [DOI] [PubMed] [Google Scholar]
- [13].Scifres CM, Nelson DM, Intrauterine growth restriction, human placental development and trophoblast cell death, J. Physiol 587 (14) (2009) 3453–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crocker IP, et al. , Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction, Am. J. Pathol 162 (2) (2003) 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roberts Md JM, Pathophysiology of ischemic placental disease, Semin. Perinatol 38 (3) (2014) 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Badawy AA, Tryptophan metabolism, disposition and utilization in pregnancy, Biosci. Rep 35 (5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murthi P, Wallace EM, Walker DW, Altered placental tryptophan metabolic pathway in human fetal growth restriction, Placenta 52 (2017) 62–70. [DOI] [PubMed] [Google Scholar]
- [18].Gaccioli F, Lager S, Placental nutrient transport and intrauterine growth restriction, Front. Physiol 7 (2016) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Patrick RP, Ames BN, Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism, FASEB (Fed. Am. Soc. Exp. Biol.) J 28 (6) (2014) 2398–2413. [DOI] [PubMed] [Google Scholar]
- [20].Viau M, Lafond J, Vaillancourt C, Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies, Reprod. Biomed. Online 19 (2) (2009) 207–215. [DOI] [PubMed] [Google Scholar]
- [21].Bonnin A, et al. , A transient placental source of serotonin for the fetal forebrain, Nature 472 (7343) (2011) 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hansson SR, et al. , Monoamine transporters in human endometrium and decidua, Hum. Reprod. Update 15 (2) (2009) 249–260. [DOI] [PubMed] [Google Scholar]
- [23].Yavarone MS, et al. , Serotonin uptake in the ectoplacental cone and placenta of the mouse, Placenta 14 (2) (1993) 149–161. [DOI] [PubMed] [Google Scholar]
- [24].Chui A, et al. , Placental syndecan expression is altered in human idiopathic fetal growth restriction, Am. J. Pathol 180 (2) (2012) 693–702. [DOI] [PubMed] [Google Scholar]
- [25].Guaran RL, et al. , Update of growth percentiles for infants born in an Australian population, Aust. N. Z. J. Obstet. Gynaecol 34 (1) (1994) 39–50. [DOI] [PubMed] [Google Scholar]
- [26].Murthi P, et al. , Decorin expression is decreased in first trimester placental tissue from pregnancies with small for gestation age infants at birth, Placenta 45 (2016) 58–62. [DOI] [PubMed] [Google Scholar]
- [27].Chui A, et al. , Expression of biglycan in first trimester chorionic villous sampling placental samples and altered function in telomerase-immortalized microvascular endothelial cells, Arterioscler. Thromb. Vasc. Biol 37 (6) (2017) 1168–1179. [DOI] [PubMed] [Google Scholar]
- [28].Murthi P, et al. , Endocan expression is increased in the placenta from obese women with gestational diabetes mellitus, Placenta 48 (2016) 38–48. [DOI] [PubMed] [Google Scholar]
- [29].Goeden N, et al. , Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain, J. Neurosci 36 (22) (2016) 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Murthi P, et al. , Maternal 25-hydroxyvitamin D is inversely correlated with foetal serotonin, Clin. Endocrinol 86 (3) (2017) 401–409. [DOI] [PubMed] [Google Scholar]
- [31].Murthi P, et al. , Homeobox gene HLX1Expression is decreased in idiopathic human fetal growth restriction, Am. J. Pathol 168 (2) (2006) 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25 (4) (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [33].Murthi P, et al. , GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction, Placenta 29 (9) (2008) 798–801. [DOI] [PubMed] [Google Scholar]
- [34].Murthi P, et al. , Role of the placental vitamin D receptor in modulating feto-placental growth in fetal growth restriction and preeclampsia-affected pregnancies, Front. Physiol 7 (2016) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hobel CJ, et al. , Enzymes for epinephrine synthesis and metabolism in the myometrium, endometrium, red blood cells, and plasma of pregnant human subjects, Am. J. Obstet. Gynecol 141 (8) (1981) 1009–1018. [DOI] [PubMed] [Google Scholar]
- [36].Mitchell JA, Hammer RE, Goldman H, Serotonin-induced disruption of implantation in the rat: II. Suppression of decidualization, Biol. Reprod 29 (1) (1983) 151–156. [DOI] [PubMed] [Google Scholar]
- [37].Mitchell JA, Hammer RE, Serotonin-induced disruption of implantation in the rat: I. Serum progesterone, implantation site blood flow, and intrauterine pO2, Biol. Reprod 28 (4) (1983) 830–835. [DOI] [PubMed] [Google Scholar]
- [38].Laurent L, et al. , Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase, Biochimie 140 (2017) 159–165. [DOI] [PubMed] [Google Scholar]
- [39].Correa RR, et al. , Expression of the melatonin receptor and tryptophan hydro-xylase in placentas of the fetus with intra-uterine stress, Eur. J. Obstet. Gynecol. Reprod. Biol 147 (2) (2009) 234–236. [DOI] [PubMed] [Google Scholar]
- [40].Welford RWD, et al. , Serotonin biosynthesis as a predictive marker of serotonin pharmacodynamics and disease-induced dysregulation, Sci. Rep 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Prasad PD, et al. , Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells, Placenta 17 (4) (1996) 201–207. [DOI] [PubMed] [Google Scholar]
- [42].Muller CL, et al. , Impact of maternal serotonin transporter genotype on placental serotonin, fetal forebrain serotonin, and neurodevelopment, Neuropsychopharmacology 42 (2) (2017) 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang X, et al. , Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia, BMC Genomics 19 (1) (2018) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cetin I, et al. , Fetal growth restriction: a workshop report, Placenta 25 (8–9) (2004) 753–757. [DOI] [PubMed] [Google Scholar]
- [45].Prasad PD, et al. , Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells, Placenta 17 (4) (1996) 201–207. [DOI] [PubMed] [Google Scholar]
- [46].Bottalico B, et al. , Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies, Placenta 25 (6) (2004) 518–529. [DOI] [PubMed] [Google Scholar]
- [47].Balkovetz DF, et al. , Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membrane, J. Biol. Chem 264 (4) (1989) 2195–2198. [PubMed] [Google Scholar]
- [48].Cool DR, Leibach FH, Ganapathy V, High-affinity paroxetine binding to the human placental serotonin transporter, Am. J. Physiol 259 (2 Pt 1) (1990) C196–C204. [DOI] [PubMed] [Google Scholar]
- [49].Cool DR, Liebach FH, Ganapathy V, Interaction of fluoxetine with the human placental serotonin transporter, Biochem. Pharmacol 40 (9) (1990) 2161–2167. [DOI] [PubMed] [Google Scholar]
- [50].Hadden C, et al. , Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway, J. Cell. Physiol 232 (12) (2017) 3520–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Badawy AA, The tryptophan utilization concept in pregnancy, Obstet. Gynecol. Sci 57 (4) (2014) 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McKinney J, Knappskog PM, Haavik J, Different properties of the central and peripheral forms of human tryptophan hydroxylase, J. Neurochem 92 (2) (2005) 311–320. [DOI] [PubMed] [Google Scholar]
- [53].Iida Y, et al. , Proteasome-driven turnover of tryptophan hydroxylase is triggered by phosphorylation in RBL2H3 cells, a serotonin producing mast cell line, Eur. J. Biochem 269 (19) (2002) 4780–4788. [DOI] [PubMed] [Google Scholar]
- [54].Watts SW, 5-HT in systemic hypertension: foe, friend or fantasy? Clin. Sci 108 (5) (2005) 399–412. [DOI] [PubMed] [Google Scholar]
- [55].Cruz MA, et al. , Serotonin-induced vasoconstriction is mediated by thromboxane release and action in the human fetal-placental circulation, Placenta 18 (2–3) (1997) 197–204. [DOI] [PubMed] [Google Scholar]
- [56].Spurgeon SL, Jones RC, Ramakrishnan R, High throughput gene expression measurement with real time PCR in a microfluidic dynamic array, PLoS One 3 (2) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Leeuwerke M, et al. , DNA methylation and expression patterns of selected genes in first-trimester placental tissue from pregnancies with small-for-gestational-age infants at birth, Biol. Reprod 94 (2) (2016) 37. [DOI] [PubMed] [Google Scholar]
- [58].R.C.o.O.a Gynaecologists, The Investigation and Management of the Small-For-Gestational-Age Fetus [Internet], RCOG Green Top Guidelines, 2013. Available at: www.rcog.org.uk/clinical-guidance2013. [Google Scholar]