Abstract
To test the hypothesis that trkA (the high-affinity NGF receptor) is selectively expressed in nociceptive dorsal root ganglion (DRG) neurons, we examined the intensity of trkA immunoreactivity in single dye-injected rat DRG neurons, the sensory receptor properties of which were identified in vivo with mechanical and thermal stimuli. We provide the first evidence in single identified neurons that strong trkA expression in DRGs is restricted to nociceptive neurons, probably accounting for the profound influence of NGF on these neurons. Furthermore, we demonstrate that trkA expression is as high in rapidly conducting (Aα/β) as in more slowly conducting (Aδ and C) nociceptors. All Aα/β low-threshold mechanoreceptors (LTMs) are trkA negative, although weak but detectable trkA is present in some C and Aδ LTMs.
NGF can influence electrophysiological properties of DRG neurons, probably by binding to trkA. We found positive correlations for single identified Aα/β (but not C or Aδ) nociceptors between trkA immunocytochemical intensity and electrophysiological properties typical of nociceptors, namely long action potential and afterhyperpolarization durations and large action potential amplitudes. Furthermore, for Aα/β (notCorAδ) nociceptors, trkA intensity is inversely correlated with conduction velocity. Similar relationships, again only in Aα/β nociceptors, between electrophysiological properties and trkA expression exist for sodium channel Nav1.8 but not Nav1.9 immunoreactivities. These findings suggest that in Aα/β nociceptors, influences of NGF on expression levels of Nav1.8 are related to, and perhaps limited by, expression levels of trkA. This view is supported by a positive correlation between immuno-intensities of trkA and Nav1.8 in A-fiber, but not C-fiber, nociceptors.
Keywords: NGF, pain, dorsal root ganglion (DRG), conduction velocity, action potential, sodium channel
Introduction
Sensory [dorsal root ganglion (DRG)] neurons depend on nerve growth factor (NGF) for survival during development (Goedert et al., 1984) and for maintenance of aspects of their adult nociceptive phenotype (Mendell, 1999). For example, NGF influences action potential (AP) duration in A-fiber nociceptors in rat DRGs early postnatally (Ritter et al., 1991) and in adults (Djouhri et al., 2001). NGF binds to two types of receptor on DRG neurons: the low-affinity receptor p75 (Casaccia-Bonnefil et al., 1999) and the high-affinity receptor tyrosine kinase A (trkA) (Barbacid, 1994), through which NGF exerts its main influence on sensory neurons (Wiesmann and de Vos, 2001). trkA is expressed in a subgroup of DRG neurons that is assumed to be nociceptive, based on indirect evidence including (1) their relatively small size; (2) trkA expression in primary afferent fibers mainly in superficial dorsal horn (Averill et al., 1995; Molliver et al., 1995), a region associated with nociceptive input; and (3) high trkA coexpression with calcitonin gene-related peptide (Averill et al., 1995) and substance P (Kashiba et al., 1996), which are expressed mainly in nociceptive DRG neurons (Lawson et al., 1997, 2002). However, some small-medium sized neurons are low-threshold mechanoreceptors (LTMs), and some medium-large sized neurons are nociceptors (Fang et al., 2002; Djouhri et al., 2003a). We therefore examined whether trkA is present in all nociceptors and/or is limited to nociceptors.
Because NGF exerts its main effects on sensory neurons through trkA, we hypothesized that the influence of NGF on their electrophysiological properties may depend on their trkA expression levels. We therefore examined in rat DRG neurons whether electrophysiological properties typical of nociceptors are related to trkA immunocytochemical intensity and found these to be related only in rapidly conducting (Aα/β) nociceptive neurons that may contribute to sharpness perception, first mechanical pain, and rapid withdrawal responses (for review, see Djouhri and Lawson, 2004).
These relationships could result from the influence of NGF on Na+ channel expression. Two TTX-resistant Na+ channels, Nav1.8 and Nav1.9, are expressed mainly in nociceptors and are associated with their electrophysiological properties: Nav1.8 with high AP amplitude and long AP duration (Renganathan et al., 2001; Djouhri et al., 2003b) and Nav1.9 with AP duration (Dib-Hajj et al., 1998b; Fang et al., 2002). Because NGF (via trkA) regulates Nav1.8 expression (Black et al., 1997; Dib-Hajj et al., 1998a), whereas glial cell line-derived neurotrophic factor (GDNF) regulates both Nav1.8 and Nav1.9 expression in DRG neurons (Fjell et al., 1999; Cummins et al., 2000), and because there is higher Nav1.8 than Nav1.9 immunoreactivity in A-fiber nociceptors (Fang et al., 2002; Djouhri et al., 2003b), we hypothesized that relationships between trkA expression levels and electrophysiological properties in Aα/β nociceptors might result primarily from trkA expression levels limiting the influence of NGF on Nav1.8 expression. If so, relationships between electrophysiological properties and expression of Nav1.8 (but not Nav1.9) should resemble those between electrophysiological properties and trkA intensity in these neurons. We therefore studied Na+ channel expression in nociceptors that were classified separately into Aδ and Aα/β subgroups to determine the relationship between their electrophysiological properties and immunoreactivity.
Materials and Methods
Animal preparation. Experimental procedures complied throughout with Home Office (UK) guidelines. Animal preparation was as described previously (Fang et al., 2002). Briefly, young adult Wistar rats (female; 6-7 weeks of age; 160-180 g) were deeply anesthetized with sodium pentobarbitone (70-80 mg/kg, i.p.) and maintained areflexic throughout. They were tracheotomized and artificially ventilated. The left external jugular vein and carotid artery were cannulated to allow supplementary anesthetic at regular intervals and to monitor blood pressure, respectively. After a laminectomy, the left L3-L6 DRGs were exposed, and a liquid paraffin pool was constructed using silicone dental impression paste Xantopren (Hanau, Germany) VL plus. The dura over the spinal cord was opened, and the dorsal root of the DRG under study was cut close to its entry to the spinal cord and placed over a pair of platinum stimulating electrodes.
Intracellular recording. Just before recording, a muscle relaxant (pancuronium bromide; 0.6 mg/kg, i.v.) was administered, preceded by 10 mg/kg anesthetic. These same doses of muscle relaxant and anesthetic were subsequently given together at regular intervals (approximately hourly). The paraffin pool temperature near the DRG was maintained at ∼30°C (range, 28.5-32°C). Intracellular voltage recordings from DRG neuronal somata were made with sharp glass microelectrodes filled with a fluorescent dye: 50 mg/ml Lucifer yellow CH (Sigma, St. Louis, MO) in 0.1 m LiCl (Stewart, 1978), 6 mg/ml ethidium bromide (Sigma) in 1 m KCl (LePecq and Paoletti, 1967), or 3% cascade blue (Molecular Probes, Eugene, OR) in 0.1 m LiCl (Whitaker et al., 1991). Cell penetrations were as described previously (Lawson et al., 1997). After penetration, the dorsal root was stimulated with a rectangular pulse (0.03 ms for A-fiber neurons, 0.3 ms for C-fiber neurons) up to twice threshold voltage for evoking a somatic AP. Before stimulation of the receptive field, the presence of any spontaneous/ongoing activity was recorded, usually for 1-2 min.
Conduction velocity. Conduction velocity (CV) excluding utilization time was calculated as described previously (Djouhri and Lawson, 2001); the conduction distance was 4-14 mm. Each neuron was classified by its CV, according to dorsal root CV boundaries determined previously in female rats of the same weight (Fang et al., 2002), as C (<0.8 m/s), C/Aδ (0.8-1.5 m/s; included in the Aδ group unless stated otherwise), Aδ (1.5-6.5 m/s), or Aα/β (>6.5 m/s).
Identification of sensory receptive properties. Details of methods used for identifying sensory receptive properties were as described previously (Fang et al., 2002). In brief, stimuli were applied to the left hindlimb and flank. These were first nonnoxious (brush, tapping, light touch, vibration) stimuli. If no response to nonnoxious stimuli was seen, noxious stimuli were applied including high-intensity mechanical stimulation with needle, forceps (fine and/or toothed), and thermal stimulation (cooling by a brief localized spray of ethyl chloride and/or heating with water >50°C).
Neurons responding to nonnoxious mechanical stimuli were classified as LTMs. Aα/β LTMs included muscle spindle units and cutaneous units: rapidly adapting, slowly adapting, guard hair, or field units. Slowly conducting LTMs included Aδ down hair (D hair) units and C-fiber LTM units (C LTMs). Neurons responding only, or better, to noxious stimuli were classed as nociceptors. Nociceptors included high-threshold mechanoreceptors (HTMs), polymodal, mechano-heat units, and mechano-cold units (see below for properties).
Nociceptors that responded to mechanical stimuli were subdivided into those with superficial (probably epidermal or at the border of epidermis and dermis), dermal, or subcutaneous receptive fields as follows. Neurons that responded to needle pressure and pinch of superficial tissues with fine forceps were classed as having superficial receptive fields. Receptive fields of neurons that did not respond to stimulation of superficial tissues but did respond to squeeze of a fold of skin tissue including dermis were classed as dermal. Receptive fields of neurons that failed to respond to the above stimuli but responded to squeezing across the foot or whole leg or pressure to deeper tissues including deep fascia, muscle, and associated connective tissue and periosteum were said to be subcutaneous. A-fiber nociceptors with dermal or subcutaneous receptive fields were combined and categorized as deep.
HTM units included units with superficial or dermal receptive fields that responded to noxious mechanical stimuli but not to noxious thermal stimuli. Because heat penetration into subcutaneous tissues is poor, nociceptive units with such receptive fields were not tested with thermal stimuli. Furthermore, heat stimuli may not penetrate adequately to the terminals of all dermal units. Nonetheless, subcutaneous and dermal nociceptors that did not respond to noxious heat were included in the HTM group in this paper. Moderate pressure units with superficial receptive fields responded weakly to moderate pressure but much better to both needle and stronger pressure; thus, they encode into the noxious range and are classed as nociceptors (Burgess and Perl, 1967). In the present study, they are classed as a subgroup of the A-fiber HTM units.
C-fiber units that responded to noxious mechanical stimuli and also promptly to a single brief application of noxious heat were called C polymodal units if they had superficial receptive fields and mechano-heat units if they had dermal receptive fields. C- and A-fiber mechano-cold units responded to both noxious mechanical and to cooling stimuli. Although in other studies in this laboratory, small numbers (∼2%) of A-fiber nociceptive units responded to heat (Djouhri and Lawson, 2004), none of those in the present study responded to heat.
Unresponsive units did not respond to any of the above stimuli. They may include some so-called “silent nociceptors” or units with inaccessible receptive fields (Djouhri et al., 2003b). Unresponsive units with C fibers have similar electrophysiological properties to nociceptive units within the same CV range in both guinea pig (Djouhri et al., 1998) and rat (our unpublished observations). In this paper, therefore, the term nociceptor-type units included C-unresponsive units (n = 9) plus all nociceptive units with C fibers (n = 12) and A fibers (Aδ, n = 15; Aα/β, n = 23). Proportions of C-fiber neurons that are unresponsive are hard to assess in this preparation, because it is easy to lose these recordings in the longer process of testing many different stimuli on all tissues. A-fiber unresponsive units were excluded unless specifically mentioned.
Neuronal labeling. Methods for labeling of neuronal somata have been described in detail previously (Fang et al., 2002). For each neuron, after completion of recordings, fluorescent dye was electrophoretically ejected into the soma from the recording electrode by rectangular current pulses (1.0-1.3 nA for 500 ms at 1 Hz) for periods up to 15 min for A-fiber neurons and 8 min for C-fiber neurons. Negative currents were used for dye injection of Lucifer yellow and cascade blue, and positive currents were used for ethidium bromide ejection. Usually five neurons per DRG were dye injected, using Lucifer yellow for one neuron at each end and one in the middle of the DRG and ethidium bromide for two neurons, one proximal and one distal to the middle Lucifer yellow injection site. Sometimes, a neuron lateral to a Lucifer yellow injection site was injected with cascade blue.
Precautions necessary to avoid the problems and pitfalls associated with identification of dye-injected cells (Lawson et al., 1997) were taken. Additionally, after dye injection, the receptive field properties and CV were checked to ensure that the electrode tip was still in the same neuron. Any doubt about the reliability of identity of dye-injected neurons, including weak fluorescent dye labeling, inappropriate position in the DRG, or more than one neuron labeled where one was expected (indicating likely dye leakage) led to rejection of that neuron.
Tissue processing. At the end of the experiment, the rat was given additional anesthetic and perfused with normal saline followed by Zamboni's fixative. DRGs were postfixed for 1 h, left overnight in 30% sucrose buffer at 4°C, and serial 7 μm cryostat sections were cut and stored at -20°C before immunostaining. After block of endogenous peroxidase and biotin-like activity with 2% H2O2 and an avidin-biotin kit (Vector Laboratories, Peterborough, UK), respectively, ABC immunocytochemistry was used to demonstrate trkA-like immunoreactivity or immunoreactivity for one of two TTX-resistant Na+ channel α-subunits, Nav1.8-like immunoreactivity (Djouhri et al., 2003b) and Nav1.9-like immunoreactivity (Fang et al., 2002), as described previously. Only one of these primary antibodies was used per section, but different ones were often used on different sections of the same dye-injected neuron. Sections were incubated with a primary rabbit polyclonal antibody for 48 h at 4°C. These were anti-trkA at 1:7500 (a gift from L. F. Reichardt, University of California, San Francisco, San Francisco, CA), anti-Nav1.8 α-subunit (SNS11) at 1.7 × 10-3 μg/ml, and anti-Nav1.9 α-subunit at 1.7 × 10-3 μg/ml. Characterization of anti-trkA (Clary and Reichardt, 1994), anti-Nav1.8 (Djouhri et al., 2003b), and anti-Nav1.9 (Fjell et al., 2000) antibodies have been described previously. Sections were incubated for 30 min at room temperature with biotinylated secondary antibody (goat anti-rabbit Ig; 1:200; Vector Laboratories). Omission of primary antibody resulted in no staining.
Image analysis. The semiquantitative methods used to measure immunocytochemical intensities for Nav1.8 (Djouhri et al., 2003b) and Nav1.9 (Fang et al., 2002) were described previously and are described below for trkA. The methods used to measure trkA immunocytochemical staining intensity in non-dye-injected and single dye-injected DRG neurons were as follows. To examine the relationship between immunocytochemical intensity for trkA and neuronal size (cross-sectional area) of non-dye-injected DRG neurons (for comparison with that for dye-injected neurons), all neuronal profiles containing a nucleus in all sections at 140 μm intervals from a single L5 DRG from each of three experimental rats were analyzed with image analysis. Images were captured (40× objective, bright-field optics) with a digital camera (Hamamatsu; Digital Pixel, Brighton, UK) and montaged to reproduce the entire section. The neuronal cross-sectional area (including the nucleus) and mean pixel density of immunostaining in the cytoplasm (excluding the nucleus) were determined using Digital Pixel software. For all antibodies, a low level of background staining enabled even negative neurons and their nuclei to be clearly visualized. For each DRG, the 100 and 0% values were calculated by averaging the cytoplasmic density of immunostaining from the five most strongly (100%; b) and the five least strongly (0%; a) stained neuronal profiles, and for each neuron, cytoplasmic density (c) was used to calculate the relative intensity (percentage of maximum) as follows: immunostaining relative intensity (percentage) = 100 × (c - a)/(b - a).
For single dye-injected neurons, immunostaining was subjectively scored from 0 (negative) or 1-5 (positive, 5 being the score of the most strongly stained profile in the section). In addition, relative intensity (cytoplasmic) of each dye-injected neuron was similarly calculated as a percentage of maximum neuronal cytoplasmic intensity (Fang et al., 2002) by comparing its intensity (c) with the intensities of three clearly negative (0%; a) and of the three most strongly labeled neurones (100%; b) in the same section, using the same equation as above.
The terms trkA, Nav1.8, or Nav1.9 intensity or immuno-intensity from this point on mean relative intensity of immunostaining for trkA, Nav1.8, or Nav1.9, respectively.
The methods used to determine the borderline of relative intensity between neurons that were positive and negative for trkA staining were described previously for Nav1.8 (Djouhri et al., 2003b) and Nav1.9 staining (Fang et al., 2002). As for these Na+ channels, calculated trkA intensities were highly positively correlated with subjective scores (linear regression analysis; r2 = 0.97; p < 0.0001), and neurons with intensity ≥20% were consistently judged as positive (subjective score, ≥1), whereas those with intensity <20% were consistently scored as appearing negative (subjective score, <1). Thus, for trkA (as for the Na+ channels), the borderline between negative (trkA-) and positive (trkA+) neurons was defined as 20% intensity. The term negative is used for consistency with the literature and for simplicity of communication, with the caveat that even below 20% intensity, percentage values may indicate real (low) levels of immunoreactivity. Based on the distributions of data (see below), neurons were classed as strongly (≥40% relative intensity) or weakly (20-40%) positive.
Cell size. The largest cross-sectional area of all the sections of each dye-injected neuron was taken as a measure of its size; if the largest section was not available, that neuron was excluded from size analyses. As defined previously in relation to the shape of the size distributions of neurons (Fang et al., 2002), those within the small cell peak are called small (cross-sectional area up to 400 μm2; diameter, 23 μm), those above 800 μm2 area (32 μm diameter) are called large, because this includes only the extreme right-hand end of the large light cell distribution (Lawson et al., 1984), and those with areas of 400-800 μm2 (23-32 μm diameter) were classified as medium-sized.
Action potential variables and acceptance criteria. Analyzed somatic AP variables included AP: duration at base, rise time, fall time, overshoot and height, plus afterhyperpolarization duration to 80% recovery [Djouhri et al. (1998), their Fig. 1]. These variables were measured only in neurons with resting membrane potentials that were equal to, or more negative than, -40 mV with one exception: -39 mV in one C nociceptor. Apart from AP size (overshoot and height), all other AP variables were measured only in neurons with a positive AP overshoot. AP overshoot and AP height were measured in neurons with an AP height of at least 35 mV and an AP peak that reached to at least -20 mV, because it has been shown that some DRG neurons (mainly fast conducting LTMs) do not have overshooting APs (Djouhri and Lawson, 2001). For CV only, all neurons were included regardless of AP size and resting membrane potential.
Na+ channel data. To increase the number of data points, some previously published Nav1.8 and Nav1.9 data (Fang et al., 2002; Djouhri et al., 2003b) were added to the novel data (for n values, see Results). These combined data were analyzed, enabling direct comparisons between the correlations for Nav1.8, Nav1.9, and trkA immuno-intensities with electrophysiological properties.
Statistics. Linear regression analysis (see Figs. 2, 3, 4) and nonparametric Mann-Whitney U tests and Kruskal-Wallis tests on data shown in Figures 1 and 2 were performed with Prism 3 (GraphPad Software, San Diego, CA).
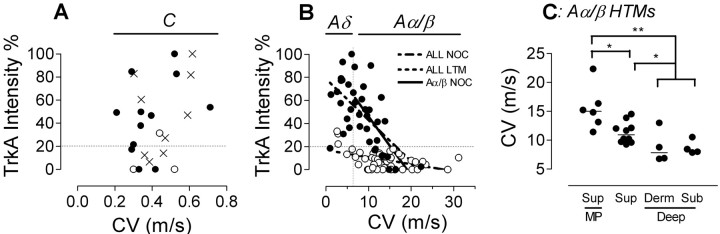
Figure 2.
trkA intensity plotted against dorsal root CVs for all C-fiber (A) and all A-fiber neurons (B) and comparison of CVs in subgroups of Aα/β moderate pressure (MP) units with other Aα/β HTMs with receptive fields at different tissue depths (C). In B, p and r2 values for regression lines for all A-fiber nociceptors, for all A-fiber LTMs, and Aα/β nociceptors are specified in Results. The vertical dotted line in B indicates the upper border of CV for Aδ fibers (6.5 m/s). In A and B, the horizontal dotted line indicates the 20% borderline between trkA+ and trkA-. In C, MP units had significantly faster CVs than other Aα/β HTMs with superficial or deeper (dermal and subcutaneous together) receptive fields, and Aα/β HTMs with superficial receptive fields had lower CVs than those with deep receptive fields (Mann-Whitney tests; *p < 0.05; **p < 0.01). NOC, Nociceptive; Sup, superficial; Derm, dermal; Sub, subcutaneous. Symbols and abbreviations are as in Figure 1.
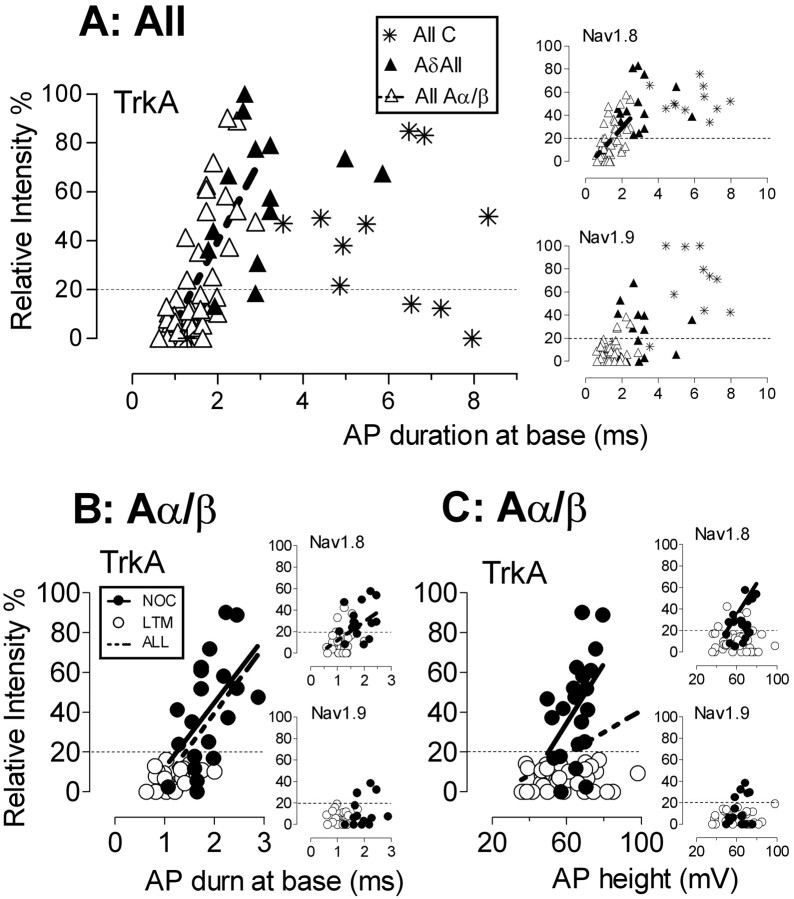
Figure 3.
Intensity of trkA, Nav1.8, and Nav1.9 immunostaining versus AP duration and height. A, AP duration in all neurons subdivided into C, Aδ, and Aα/β. B, AP duration in Aα/β neurons. C, AP height in Aα/β neurons. Units in B and C are subdivided into nociceptive and LTM units. A-fiber unresponsive neurons are excluded. In A and B, only neurons with resting membrane potential greater than or equal to -40 mV (with 1 exception: -39 mV in 1 C nociceptor) and overshooting somatic APs are included. In C, all Aα/β neurons with AP overshoot more than or equal to -20 mV and AP height ≥35 mV are included. Symbols in B relate to B and C. Regression lines are given for significant linear correlation, and p and r2 values are given in Table 1. NOC, Nociceptive.
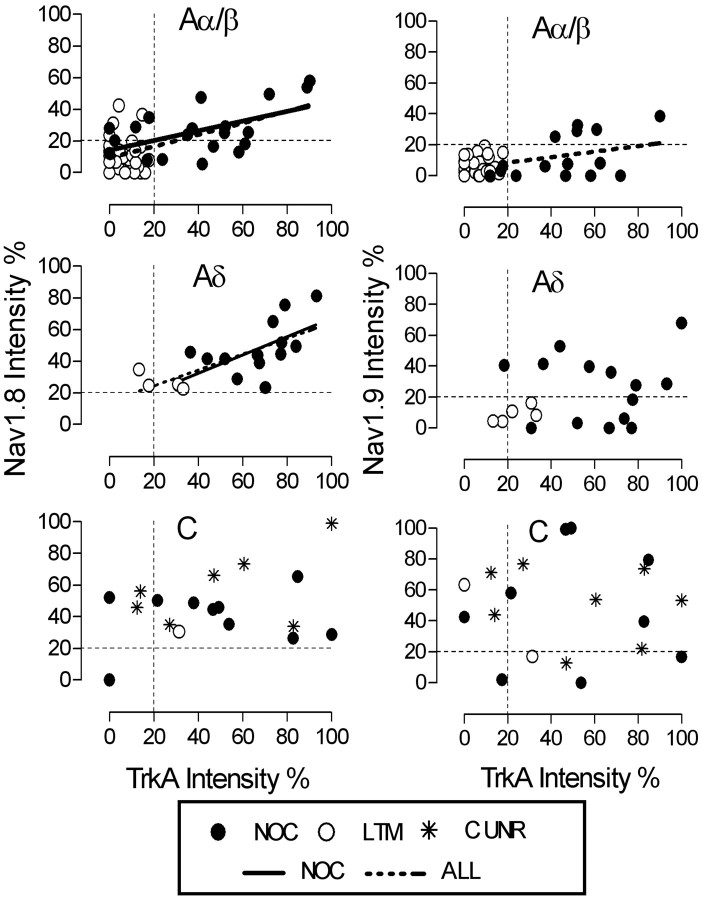
Figure 4.
trkA intensity versus Nav1.8 (left) or Nav1.9 (right) intensity in different sections from the same identified neurons. A-fiber unresponsive neurons are excluded. Left, A linear correlation between trkA intensity and Nav1.8 intensity exists in all Aα/β units (p < 0.0001; r2 = 0.36); Aα/β nociceptors (p < 0.005; r2 = 0.29); all Aδ units (p < 0.005; r2 = 0.48); Aδ nociceptors (p < 0.005; r2 = 0.38). No such correlation is in C-fiber units (p > 0.05). Right, There is a linear correlation between trkA intensity and Nav1.9 intensity only in all Aα/β units (p < 0.005; r2 = 0.17). The vertical and horizontal dotted lines from y-axis and from x-axis indicate the borderlines between positive and negative for trkA and for Nav1.8 or Nav1.9, respectively. NOC, Nociceptive; C UNR, C-fiber unresponsive. For p, n, and r2 values, see Results.
Figure 1.
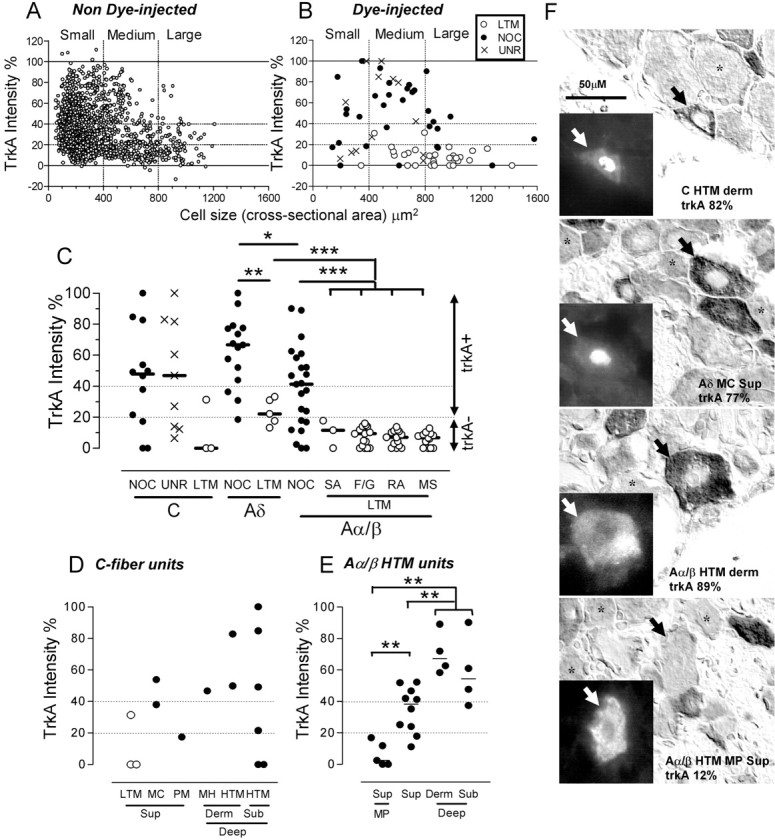
A, B, Sizes of all non-dye-injected neuronal profiles with nuclei in sections of one L5 DRG from each of three rats (A) compared with those of identified dye-injected neurons (B). Vertical dotted lines indicate boundaries between small, medium, and large neurons. Horizontal dotted lines indicate borderlines between negative and positive neurons and between weakly positive (20-40%) and strongly positive (≥40%) neurons. C, trkA intensities of physiologically identified DRG neurons; a Kruskal-Wallis test comparing medians of the three groups of nociceptors shows a significant difference between Aδ and Aα/β neurons and no differences between the four groups of Aα/β LTMs; Mann-Whitney U tests show significant differences between Aδ nociceptors and Aδ LTM neurons, between Aα/β nociceptors and LTMs, and between Aδ and all Aα/β LTMs. CLTM neurons are excluded, because n = 3. D, trkA intensity in CLTMs and populations of C nociceptive neurons. E, Comparisons of trkA intensities of Aα/β-fiber HTM subgroups. Moderate pressure (MP) units had significantly lower trkA intensities than other HTMs with superficial or deeper (dermal and subcutaneous together) receptive fields, and HTMs with superficial receptive fields had significantly lower intensities than units with deep receptive fields (Mann-Whitney tests). F, Photomicrographs show fluorescent images of representative dye-injected neurons in insets to left: dyes were Lucifer yellow, cascade blue, and for the two lowest cells ethidium bromide; note the typically nuclear fluorescence with cascade blue and Lucifer yellow. The same neurons stained for trkA, under interference contrast optics, are indicated by arrows (right). Asterisks indicate examples of trkA- neurons. The top three injected neurons are strongly trkA+, and the last is a trkA-MP unit. The scale bar (50 μm; top left image) applies to all photomicrographs, including insets. For C and E, *p < 0.05, **p < 0.01, ***p < 0.001. NOC, Nociceptive; UNR, C-fiber unresponsive; SA, slowly adapting; F/G, field or guard hair; RA, rapidly adapting; MS, muscle spindle; MC, mechano-cold; PM, polymodal; MH, mechano-heat. Depth in tissues: Sup, superficial; Derm, dermal; Sub, subcutaneous.
Possible sources of error or sampling bias. Because intracellular penetrations and recording stability are easier in large neurons, and because Aα/β LTMs were rejected in some experiments, and because unresponsive neurons are harder to categorize, the proportions of different types of dye-labeled neurons (e.g., A-fiber neurons compared with C-fiber neurons, or C-fiber nociceptive compared with C-fiber unresponsive) do not necessarily represent their numerical distribution within the DRG. However, the percentages of immunopositive neurons within a particular group (e.g., Aδ nociceptors, Aδ D hair units, Aα/β nociceptors) are considered representative for that group.
Results
Comparison of the relationship between cell size and trkA intensity for neurons that were not dye injected (Fig. 1A) and neurons that were dye injected (Fig. 1B) shows a similar distribution pattern in both cases, indicating that the trkA immunoreactivity was not discernibly altered by recording and dye injection.
Overall, in non-dye-injected neurons (Fig. 1A), 75% (830 of 1098) of small, 58% (205 of 353) of medium-sized, and 43% (40 of 94) of large neurons were trkA positive; overall, 70% (1075 of 1545) of all neurons were classed as trkA+, including 37% (573 of 1545) that were strongly positive (intensity, ≥40%). Most (77%; 830 of 1075) of the trkA+ neurons were small (Fig. 1A). The small-medium size of somata with strong trkA staining is consistent with previously published data (Averill et al., 1995; Bennett et al., 1996). The similarity in previously published percentages of positive neurons (35-40%) (Averill et al., 1995; Kashiba et al., 1995) to our strongly stained neurons (37%) suggests that these investigators used criteria for defining trkA+ staining that are close to 40% intensity (strongly positive in our study), a level that we find is selective for nociceptive somatic afferent neurons (see below). Thus, neurons in the present study with weak but positive trkA staining may not be classed as positive by other groups. Our finding that some A-fiber neurons are trkA+ (Fig. 1C) is consistent with trkA expression in some medium-large sized DRG neurons (Fig. 1B) and with observations (Averill et al., 1995; Molliver et al., 1995) that some trkA+ neurons are neurofilament rich, indicative of having myelinated fibers (Lawson and Waddell, 1991).
trkA intensity and sensory receptive properties
In total, 122 neurons were successfully dye injected with fluorescent dye, found in the appropriate location, and stained for trkA. These included 62 neurons labeled with Lucifer yellow dye, 48 with ethidium bromide dye, and 12 with cascade blue dye. These were classified as 50 nociceptive (12 C, 2 C/Aδ, 13 Aδ, 23 Aα/β), 53 LTM (3 C, 5 Aδ, 45 Aα/β), and 19 unresponsive neurons (9 C, 1 C/Aδ, 2 Aδ, 7 Aα/β).
Nociceptive neurons
Most C-, Aδ-, and Aα/β-nociceptive and C-unresponsive neurons (75, 93, 70, and 68%, respectively) were trkA+ (intensity, ≥20%). Overall, 61% of nociceptors were strongly trkA+, and an additional 16% were weakly trkA+, making 78% of all nociceptive neurons trkA+ in total. Higher percentages of Aδ nociceptors (80%) than of C (58%) and Aα/β (52%) nociceptors and C-unresponsive (56%) neurons were strongly trkA+ (i.e., intensity, ≥40%). Surprisingly, percentages of nociceptive neurons that were trkA+ or strongly trkA+ were as high for Aα/β as for C nociceptors. The only neurons that were strongly trkA+ (intensity, ≥40%) were nociceptor type (Fig. 1C, nociceptive or C-unresponsive). Two C/Aδ nociceptors included in the Aδ group had trkA intensities of 65 and 19%. Finally, some C- (25%), Aδ- (7%), and Aα/β- (30%) nociceptive neurons were not trkA+.
trkA median intensities tended to be higher for Aδ nociceptors (median intensity 67%) than for C nociceptors (48%), C unresponsive (47%), and Aα/β-fiber nociceptors (41%) (Fig. 1C); this difference was significant between Aδ- and Aα/β-fiber nociceptors (p < 0.05). Examples of trkA immunoreactivity in nociceptors with C, Aδ, and Aα/β fibers are shown in Figure 1F.
C nociceptors
These ranged in trkA intensity from 0 to 100% (Fig. 1C,D). Examination of subdivisions of C nociceptors (Fig. 1D) showed that: (1) most (five of eight) C HTM units were strongly trkA+, an additional unit was weakly trkA+; (2) two of two C mechano-cold units were trkA+; both fired spontaneously at room temperature; (3) one of two C heat responsive (polymodal/mechano-heat) unit was trkA+, and the remaining unit was trkA- but with an intensity only just below 20%; (4) of the trkA+ C nociceptors with superficial receptive fields that were tested with noxious heat, zero of two responded to heat; (5) all three C nociceptors with trkA intensities >80% projected to deep (dermal or subcutaneous) tissues, although two other C nociceptors projecting to subcutaneous tissues were trkA-.
Aδ nociceptors
Most (>90%) Aδ nociceptors were trkA+, and most (80%) were strongly trkA+ (intensity, ≥40%) (Fig. 1C). Two Aδ mechano-cold nociceptors were strongly trkA+ (intensity, 77 and 79%). There was no significant difference in median intensity between Aδ HTM units with receptive fields in superficial (median, 59%; n = 5) and deep tissues (dermal and subcutaneous; median, 67%; n = 8; Mann-Whitney U test; data not shown).
Aα/β nociceptors
These were all classed as HTM units (n = 16, 7 for glabrous skin and hairy skin, respectively) and were subdivided as follows: moderate pressure units with superficial receptive fields (n = 5, 0) and other HTM units with superficial (n = 5, 5), dermal (n = 2, 2), and subcutaneous (n = 4, 0) receptive fields. Comparisons of median trkA intensities were made with Mann-Whitney U tests for glabrous and hairy skin units combined. trkA intensity was not significantly different between units with dermal (54%) and those with subcutaneous (67%) receptive fields, and these were pooled as deep units for statistical analysis (Fig. 1E). In contrast, moderate pressure units (all trkA-) (Fig. 1F) had a significantly lower median intensity (2.5%) than other Aα/β HTM units, both HTM units with superficial (median, 38%; p < 0.01) and those with deep receptive fields (median, 62%; p < 0.01). Furthermore, even if moderate pressure units were excluded, HTMs with superficial receptive fields were less intensely labeled for trkA than those with deep receptive fields (p < 0.01) (Fig. 1E). These differences and levels of significance (p < 0.01) were also found for glabrous units alone (data not shown), but there were too few units with receptive fields in hairy skin for statistical analysis on their own.
Unresponsive units
The C-fiber unresponsive units had trkA intensities as high as the C-fiber nociceptors (Fig. 1C). This high trkA level is therefore additional evidence of a nociceptor-type phenotype, supporting previous evidence that such units express phenotypic properties similar to those of nociceptors and consistent with the view that at some of these may be silent nociceptors with very high sensory thresholds (Djouhri et al., 1998, 2003b). In addition, three Aδ unresponsive units had strong trkA intensities (80-100%), suggesting that these also have properties akin to nociceptors, whereas only one of seven Aα/β-unresponsive units was trkA+. The tendency of Aα/β-unresponsive units to be trkA- is presumably because most (80%) Aα/β units are LTMs (our unpublished data); there is therefore a higher chance of Aα/β-unresponsive units being LTMs with inaccessible receptive fields than in C and Aδ groups in which the proportion of LTMs is much lower.
LTM neurons
In contrast to nociceptors, most LTMs were trkA- (all Aα/β, two of five Aδ, and two of three C LTMs) or only weakly trkA+ (one of three C and three of five Aδ LTMs) (Fig. 1C). The few C LTMs (too few for statistic comparisons) tended to have much lower trkA intensities than C nociceptors or unresponsive units (Fig. 1C). Similarly, LTMs had lower intensities than nociceptors in both the Aδ and Aα/β CV ranges (Mann-Whitney tests; p < 0.01 and 0.001, respectively). Aδ LTM D hair units had a significantly higher (22%) median trkA intensity than that for all Aα/β LTMs grouped together (7%; n = 45; p < 0.001), but all Aα/β cutaneous LTMs (8%) and Aα/β muscle spindle LTMs (7.8%) had similar low values (Fig. 1C).
trkA intensity and dorsal root CVs
Given the known trkA expression in small DRG neurons, the negative correlation between trkA intensity and CV for all neurons (p < 0.0001; r2 = 0.26; data not shown) was expected. Furthermore, the lack of correlation within Aα/β LTMs was to be expected, because they were all trkA- (Fig. 2B). However, a correlation for all A-fiber LTMs (p < 0.001; r2 = 0.22) (Fig. 2B) appears to result from the higher trkA in Aδ than in Aα/β LTM units.
In contrast to the lack of correlation in C-fiber neurons regardless of sensory properties (Fig. 2A), there were clear correlations between CV and trkA intensity (Fig. 2B) for all A-fiber neurons (p < 0.0001; r2 = 0.35; line not shown), for all Aα/β neurons (p < 0.0005; r2 = 0.21; line not shown), for A-fiber nociceptors (p < 0.0001; r2 = 0.37), and for Aα/β nociceptors (p < 0.0005; r2 = 0.46) (Fig. 2B). The clearest correlation was for Aα/β nociceptors, and this appears to be responsible for correlations seen within the entire population of A-fiber nociceptors and for all Aα/β neurons. Furthermore, the lower relative intensity of trkA in Aα/β than Aδ nociceptors (Fig. 1C) results from the lower intensity of trkA in the more rapidly conducting Aα/β units (Fig. 2B).
To determine whether there were differences in CV in the subgroups of Aα/β nociceptors that may be related to the differences in trkA intensity in Figure 1E, we plotted CVs for each of these subgroups (Fig. 2C). The results were consistent with a possible causal relationship between expression of trkA and CV, with greater trkA (Fig. 1E) and slower CVs (Fig. 2C) in deeper Aα/β nociceptors, with lower trkA and faster CVs in superficially projecting neurons, and also with even lower trkA and even faster CVs in the moderate pressure units compared with others that projected superficially (compare Figs. 1E, 2C).
trkA intensity and AP configuration
We examined whether trkA intensity was correlated with any of the following: somatic AP duration at base, AP rise time, AP fall time, AP overshoot, AP height, and AP afterhyperpolarization duration to 80% recovery. Linear correlations for each of these against trkA intensity were examined for: (1) all neurons (excluding A-fiber unresponsive neurons), all nociceptors, and all LTMs; (2) for all C, all Aδ, and all Aα/β neurons; (3) for nociceptors separately for each CV group; and (4) for Aα/β LTMs (too few C and Aδ LTMs met the criteria for analysis). In all neurons, trkA intensity was weakly positively correlated with all the above variables (data not shown); in all nociceptive units, it was correlated with none of them. In all LTM units, it was weakly positively correlated only with AP rise time.
When neurons were divided into different CV groups, surprisingly there were no correlations of trkA intensity with AP variables for all C or all Aδ neurons, or for nociceptors or LTMs separately in either of these CV groups, with the exception of a weakly positive correlation with AP rise time in all C neurons (n = 12; r2 = 0.39; p < 0.05; data not shown). The lack of correlation of trkA intensity with AP duration for both C and Aδ neurons can be seen in Figure 3A.
In contrast, for Aα/β neurons, there were many significant positive correlations with trkA intensity including: all variables for all Aα/β neurons, all variables except AP overshoot for Aα/β nociceptors, and only AP rise time for Aα/β LTMs. This last correlation is interesting, because all Aα/β LTMs were trkA-, and it may indicate that even below 20% intensity, low levels of functional trkA may be present. These correlations are shown in Table 1. To illustrate these correlations, the relationships between trkA intensity and AP duration at base and AP height are shown in Figure 3. Figure 3A shows that for all Aα/β (but not C and Aδ) neurons, trkA intensity is clearly correlated with AP duration. Figure 3, B and C, shows that within the Aα/β group, there was a significant correlation for nociceptors, but not LTMs, between trkA intensity and both AP duration at base and AP height.
Table 1.
Aα/β neurons: trkA, TTX-resistant Na+ channel intensities versus AP variables
|
|
AP duration |
Rise time |
Fall time |
AHP80 duration |
AP overshoot |
AP height |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
p
|
r2
|
n
|
p
|
r2
|
n
|
p
|
r2
|
n
|
p
|
r2
|
n
|
p
|
r2
|
n
|
p
|
r2
|
n
|
||||||||||||
| trkA versus | ||||||||||||||||||||||||||||||
| LTM | NS | 25 | * | 0.2 | 25 | NS | 25 | NS | 25 | NS | 36 | NS | 36 | |||||||||||||||||
| NOC | * | 0.29 | 20 | * | 0.28 | 20 | * | 0.25 | 20 | * | 0.25 | 20 | NS | 21 | * | 0.22 | 21 | |||||||||||||
| AII | **** | 0.49 | 45 | **** | 0.51 | 45 | **** | 0.41 | 45 | *** | 0.25 | 45 | *** | 0.19 | 57 | * | 0.09 | 57 | ||||||||||||
| Nav1.8 versus | ||||||||||||||||||||||||||||||
| LTM | NS | 27 | * | 0.15 | 27 | NS | 27 | NS | 26 | NS | 37 | NS | 37 | |||||||||||||||||
| NOC | NS | 17 | NS | 17 | NS | 17 | * | 0.28 | 17 | * | 0.22 | 19 | * | 0.23 | 19 | |||||||||||||||
| AII | **** | 0.31 | 44 | *** | 0.30 | 44 | *** | 0.27 | 44 | ** | 0.16 | 43 | ** | 0.14 | 56 | NS | 56 | |||||||||||||
| Nav1.9 versus | ||||||||||||||||||||||||||||||
| LTM | NS | 23 | NS | 23 | NS | 23 | NS | 23 | NS | 29 | NS | 29 | ||||||||||||||||||
| NOC | NS | 13 | NS | 13 | NS | 13 | (*) | 0.28 | 13 | NS | 16 | NS | 16 | |||||||||||||||||
| AII |
(*) |
0.09 |
36 |
NS |
|
36 |
(*) |
0.09 |
36 |
NS |
|
36 |
*
|
0.09 |
45 |
NS |
|
45 |
||||||||||||
Linear correlation analysis of trkA intensity and Nav1.8 or Nav1.9 intensity with AP duration at base, AP rise time, AP fall time, AP afterhyperpolarization duration to 80% recovery (AHP80 duration in the table), AP overshoot, and AP height in Aα/β neurons was performed. Unresponsive neurons are excluded. All neurons had resting membrane potential equal to or more negative than −40 mV. All neurons analyzed for AP duration, rise time, fall time, and AHP80 durations had overshooting APs. All neurons analyzed for AP overshoot and height had AP overshoot greater than or equal to −20 mV and height ≥35 mV. In each case where p < 0.1, r2 and n values are given. NS, p > 0.05; (*) 0.05 < p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. NOC, Nociceptive neurons. C- and Aδ-fiber neurons were excluded from this table, because their trkA, Nav1.8, and Nav1.9 intensities were not correlated with any of these variables except for positive correlations of trkA and Nav1.9 with AP rise time in all C-fiber neurons (see Results).
TTX-resistant Na+ channels and AP configuration
A number of studies (Black et al. 1997; Dib-Hajj et al., 1998a) indicate that NGF upregulates expression of the Na+ channel Nav1.8 within DRG neurons. This raises the possibility that trkA may be linked to electrophysiological properties via its regulation of NGF uptake and thus Nav1.8 expression. We therefore examined correlations of both Nav1.8 and, for comparison, Nav1.9 expression with electrophysiological properties to compare them with those for trkA described above. Because there were too few A-fiber nociceptors in previously published studies (Fang et al., 2002; Djouhri et al., 2003b) for separate analysis of Aδ and Aα/β nociceptors, more data were required for this comparison. We therefore performed additional studies of Nav1.8 and Nav1.9 expression in nociceptors and also included the units in those previous studies. The final numbers of nociceptors in each CV group that meet the electrophysiological criteria for analysis of most AP variables (AP overshoot and membrane potential of at least -40 mV; see Materials and Methods) are given below with, in parentheses, the percentage of each total that was contributed by units in the previous study. Note that for AP height, numbers in some cases were higher (see Materials and Methods). For Nav1.8, numbers of nociceptive-type units were: Aα/β, n = 17 (41%); Aδ, n = 13 (23%); and C, n = 11 (81%) [percentages relate to units from Djouhri et al. (2003b)]. For Nav1.9, units were: Aα/β, n = 14 (42%); Aδ, n = 13 (30%); and C, n = 10 (70%) [percentages relate to units from Fang et al. (2002)]. In all groups, the numbers of A-fiber nociceptors examined in the present study for these Na+ channels were thus doubled or tripled compared with the previously published data (Fang et al., 2002; Djouhri et al., 2003b). For both Nav1.8 and Nav1.9, smaller numbers (up to 30%) of LTMs were also added in the present study. The pooled data were analyzed and linear regression analyses performed exactly as described for the trkA data.
Nav1.8 expression showed correlations with electrophysiological properties that were remarkably similar to those for trkA in the following respects. (1) There was no linear correlation between Nav1.8 intensity and any AP variables in subgroups of C and Aδ units (Fig. 3, AP duration). (2) In all Aα/β neurons, Nav1.8 intensity showed a significant linear correlation with all AP variables exactly as seen for trkA (Table 1), with the exception of AP height but even for trkA this correlation with AP height was weak (r2 = 0.09) (Fig. 3, Table 1). (3) In Aα/β nociceptors, as for trkA, Nav1.8 was correlated with AP afterhyperpolarization duration to 80% recovery, and AP height with similar r2 values to those for trkA (Table 1). (4) Again as for trkA, there was a significant correlation with similar r2 values between Nav1.8 intensity and AP rise time for Aα/β LTMs, despite the fact that a few Aα/β LTMs were Nav1.8 positive, but none were positive for trkA. Furthermore, in Aα/β nociceptors, XY plots of Nav1.8 against AP duration, AP rise time, and fall time variables, although they were not significantly correlated, tended to show similar patterns to those for trkA (Fig. 3, AP duration). The above observations demonstrate similar relationships of electrophysiological properties with both Nav1.8 and trkA intensities in Aα/β nociceptors, suggesting a functional link between the two. Interestingly, similar patterns for Aα/β LTMs, with correlations between AP rise time and both trkA and Nav1.8, may support the view that even low levels (below 20% intensity) of trkA may be enough to influence Nav1.8 expression (see below).
In contrast to Nav1.8, relationships of Nav1.9 with electrophysiological properties did not resemble those for trkA (Fig. 3). There was no significant correlation between Nav1.9 intensity and AP variables in C, Aδ, or Aα/β neurons (Fig. 3A, AP duration), with the exception of AP rise time in all C-fiber nociceptortype neurons (n = 10; p < 0.01; r2 = 0.70; data not shown) and in all C-fiber neurons (n = 11; p = 0.0004; r2 = 0.77; data not shown) and AP overshoot in all Aα/β neurons (Table 1). Thus, the relationships between immunoreactivity for trkA and AP variables resembled much more closely the relationships for Nav1.8 than for Nav1.9.
Because CV is known to be related to Na+ channel expression, similar relationships between CV and both trkA and Nav1.8, but not Nav1.9, intensity also support a possible functional connection between trkA and Nav1.8. These patterns include (1) correlations for all Aα/β neurons between CV and both trkA (this paper) and Nav1.8 (p = 0.0002; n = 65; this paper) that are not seen for Nav1.9 (Fang et al., 2002) plus (2) a lack of correlation between CV and both trkA (this paper) and Nav1.8 intensities (Djouhri et al., 2003b) in C-fiber neurons, although there is a clear correlation between CV and Nav1.9 intensity in these neurons (Fang et al., 2002).
Colocalization of trkA with TTX-resistant Na+ channel subunits in DRG neurons
To determine whether the expression levels of trkA and Nav1.8 were related in individual neurons, we compared the intensities of trkA and Nav1.8 (n = 97; excluding A-fiber unresponsive neurons) and, for comparison, also of trkA and Nav1.9 (n = 96; excluding A-fiber unresponsive units) in different sections from the same identified neurons. Linear regression analysis was performed on all neurons, all C, all Aδ, and all Aα/β neurons, and on nociceptive neurons of each CV group (Fig. 4).
There was a correlation between trkA and Nav1.8 intensities for all neurons (n = 97; r2 = 0.40; p < 0.0001; data not shown) and for all nociceptor-type neurons (n = 51; r2 = 0.14; p < 0.01; data not shown). Of the other groups tested, the only significant correlations were for all Aα/β neurons (n = 61; p < 0.0001; r2 = 0.36), for Aα/β nociceptors (n = 20; p < 0.05; r2 = 0.29), for all Aδ units/neurons (n = 18; p < 0.005; r2 = 0.48), and for all Aδ nociceptors (n = 14; p < 0.005; r2 = 0.38) (Fig. 4). For trkA and Nav1.9 intensities, there were fewer correlations, with lower r2 values, than for Nav1.8; these correlations were only observed for all Aα/β neurons together (n = 56; p < 0.005; r2 = 0.17) (Fig. 4) and all neurons together (n = 96; p = 0.0001; r2 = 0.15) (data not shown).
Discussion
This is the first study to determine directly the sensory properties of somatic afferent DRG neurons that express trkA in normal adult rats. It demonstrates that intense trkA staining is confined exclusively to nociceptors and that trkA expression is higher in nociceptive than LTM DRG neurons in all CV ranges, although some slowly conducting LTMs are weakly trkA+. Additional novel findings include: (1) trkA intensity as intense in Aα/β nociceptors as in C and Aδ nociceptors; (2) trkA intensity correlated negatively with CV and positively with AP duration in Aα/β, but not Aδ and C, nociceptors; (3) similar correlations of both trkA and Nav1.8 intensities with several electrophysiological properties in Aα/β nociceptors; and (4) direct correlations between intensities of trkA and Nav1.8 in A-, not C-, fiber nociceptors.
trkA expression in nociceptive neurons
Expression of trkA by nociceptors in all CV ranges is consistent with an influence of NGF on their physiological properties, as reported previously for A-fiber nociceptors (Ritter et al., 1991). Some C-fiber nociceptors are strongly trkA+, and others are trkA-. From immunocytochemical and electrophysiological studies, it seems likely that many of the latter should bind isolectin B4 (IB4) and that some or all of these are also nociceptive (Averill et al., 1995; Gerke and Plenderleith, 2001) (our unpublished data). These IB4-binding/trkA- small DRG neurons are dependent on GDNF (Zwick et al., 2002). The high trkA intensity in some Aα/β nociceptors (indicating a likely NGF dependence) was unexpected, mainly because little is known about these neurons (Lawson, 2002; Djouhri and Lawson, 2004).
trkA expression in LTM neurons
The weak positive labeling for trkA in some C LTMs and Aδ D hair LTMs and the correlation with AP rise time in Aα/β LTMs suggest a possible (perhaps weak) influence of NGF on these neurons. This may be consistent with the proposed dependence on postnatal NGF availability for the development of Aδ cutaneous afferent precursors into nociceptors or D hair units (Lewin et al., 1992).
trkA expression and receptive field location
Our findings that both the C and Aα/β nociceptors with the greatest trkA intensity tend to project to deep tissues, whereas those with lower trkA project more superficially, are consistent with previous findings in rat glabrous skin that more trkA+ neurons project to deep than to superficial (epidermal) tissues (Lu et al., 2001). Because expression of substance P and calcitonin gene-related peptide (CGRP) in DRG neurons is NGF dependent (Verge et al., 1995), the present findings are also consistent with the greater substance P and CGRP immunoreactivity in dermally than in more superficially projecting C nociceptors in guinea pig (Lawson et al., 1997, 2002). trkA expression in DRG neurons may be partially influenced by NGF availability in target tissues (Li et al., 2000). It is therefore interesting that the superficial/low trkA and deep/higher trkA relationships are similar for C- and A-fiber nociceptors.
The lower trkA intensities of Aα/β nociceptors with faster CVs that tend to project to superficial (epidermal) rather than deeper tissues may be related to expression of a less extreme nociceptive phenotype, because this group includes moderate pressure units with mechanical nociceptive thresholds that are intermediate between those of other HTMs and LTMs.
trkA expression, NGF, and electrophysiology of Aα/β nociceptors
The finding that Aα/β (but not Aδ and C) nociceptors with higher trkA expression have broader APs, greater AP heights, and slower CVs indicates that the extent of their nociceptive electrophysiological phenotype may be regulated mainly by NGF/trkA in these nociceptors. The extent to which their nociceptive phenotype might be altered by changes in the NGF/trkA pathway in disease states remains to be determined.
Possible mechanisms underlying correlation between trkA and electrophysiology in Aα/β nociceptors
Mechanisms underlying the correlation of trkA intensity with CV can only be speculated on but presumably involve NGF mediated influence/s. For example, NGF can influence (1) the process of myelination/internodal length (Urschel and Hulsebosch, 1990), (2) fiber diameter (Gold et al., 1991), and (3) expression or properties of ion channels (Black et al., 1997; Fjell et al., 1999) (see below).
In Aα/β neurons, the remarkable similarity in the relationships of trkA and Nav1.8 expression with AP variables is consistent with the hypothesis that trkA availability limits NGF uptake and thus limits the influence of NGF on Nav1.8 expression, which in turn influences AP variables. This view is supported by previous reports of (1) a contribution of Nav1.8 to AP overshoot/height and AP duration in nociceptive DRG neurons (Renganathan et al., 2001; Djouhri et al., 2003b), (2) modulation of Nav1.8 expression by NGF (Black et al., 1997; Dib-Hajj et al., 1998a), and (3) reduction of the influence of NGF on Nav1.8 mRNA expression by trkA antagonists (Fjell et al., 1999). The present correlation of Nav1.8 intensity with trkA intensity in individual Aα/β nociceptors also supports this hypothesis. Other Na+ channel subtypes could possibly contribute to trkA influences on electrophysiological properties in Aα/β nociceptors. Nav1.9 is likely to be less important than Nav1.8, because Nav1.9 expression levels in these neurons is low (Dib-Hajj et al., 1998b; Fang et al., 2002), and, unlike Nav1.8, its intensity is not correlated with AP duration, AP height, or AP overshoot in Aα/β neurons (this paper). The lack of correlation of Nav1.9 intensity with trkA intensity in these neurons is consistent with the view that NGF does not strongly modulate Nav1.9 expression (Fjell et al., 1999). Nav1.7 is also regulated by NGF (Gould et al., 2000); in guinea pigs, Nav1.7 intensity is correlated with AP duration in Aα/β neurons, but its expression is relatively low in Aα/β nociceptors (Djouhri et al., 2003a). Thus, although we cannot exclude a contribution of other NGF-regulated Na+ channels, the similarities in expression patterns between Nav1.8 and trkA are indicative of an important role for Nav1.8 in the trkA-related electrophysiological properties of Aα/β nociceptors.
Lack of correlations of trkA with C-fiber neuron electrophysiology
The absence of correlations between trkA expression and electrophysiological properties in C-fiber nociceptors could reflect the presence of other influences, which may dilute the observable effects of NGF on relevant pathways in these neurons. GDNF is likely to be one such influence. The generally greater expression of GDNF receptor components in small than large DRG neurons (Bennett et al., 1998) suggests a greater GDNF influence. This GDNF influence is reported in small DRG neurons with strong IB4 binding but not (or less) in those with strong trkA expression, which are NGF dependent (Bennett et al., 1998). After axotomy, Nav1.8 [which is expressed in both these small neuron populations (Benn et al., 2001)] can be upregulated by both NGF and GDNF, whereas Nav1.9 (expressed mainly in IB4-binding neurons) is upregulated mainly by GDNF; thus, the influences of NGF and GDNF on Na+ channel expression and therefore electrophysiological properties are likely to differ in different populations of small DRG neurons (Fjell et al., 1999; Cummins et al., 2000).
To be functional, Nav1.8 needs to be inserted into the membrane. The density of functional Nav1.8 channels in the membrane is increased by annexin11/p11, which is upregulated by NGF-trkA stimulation (Okuse et al., 2002), and is decreased by clathrin-associated protein-1A (CAP-1A), a selective linker between Nav1.8 and clathrin, which enables removal of the channel from the membrane (Liu et al., 2005). Because both p11 mRNA and CAP-1A protein are more highly expressed in small DRG neurons (Okuse et al., 2002; Liu et al., 2005), their influence may be greater in small than large DRG neurons.
Thus, in small DRG neurons with C fibers, the likely influence of factors other than NGF, such as GDNF, on Na+ channel expression, including Nav1.8, in different subgroups may contribute to the lack of correlation between trkA and Nav1.8 levels; furthermore, different (perhaps more complex or over-riding) influences of other regulatory proteins in small neurons may contribute to the lack of correlation of Nav1.8 expression with electrophysiological properties in C-fiber neurons. In contrast, in Aα/β nociceptors, there may be a relative lack of influences from trophic factors other than NGF on these electrophysiological properties that allows the influence of trkA levels on such properties to be detectable.
Conclusions
In conclusion, most somatic afferent nociceptors in DRGs, including those with Aα/β fibers, show strong trkA expression. This should help with discrimination of nociceptors from other DRG neurons. In Aα/β nociceptive neurons, relationships between the nociceptive electrophysiological phenotype, trkA, and Nav1.8 expression levels are consistent with their electrophysiological properties being influenced by Nav1.8 expression that is in turn regulated by NGF, the levels of which are controlled/limited in each neuron by the amount of available trkA.
Footnotes
This work was supported by Biotechnology and Biological Sciences Research Council and Wellcome grants to S.N.L. and by a Bristol University Studentship to X.F. We thank L. F. Reichardt for the gift of anti-trkA antibody and B. Carruthers for technical assistance.
Correspondence should be addressed to Prof. Sally N. Lawson, Department of Physiology, Medical School, University of Bristol, University Walk, Bristol BS8 1TD, UK. E-mail: Sally.Lawson@bristol.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/254868-11$15.00/0
References
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV (1995) Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci 7: 1484-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M (1994) The Trk family of neurotrophin receptors. J Neurobiol 25: 1386-1403. [DOI] [PubMed] [Google Scholar]
- Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ (2001) Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci 21: 6077-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB (1996) trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett 206: 33-36. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV (1998) A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 18: 3059-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Langworthy K, Hinson AW, Dib-Hajj SD, Waxman SG (1997) NGF has opposing effects on Na+ channel III and SNS gene expression in spinal sensory neurons. NeuroReport 8: 2331-2335. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER (1967) Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol (Lond) 190: 541-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Gu C, Khursigara G, Chao MV (1999) p75 neurotrophin receptor as a modulator of survival and death decisions. Microsc Res Tech 45: 217-224. [DOI] [PubMed] [Google Scholar]
- Clary DO, Reichardt LF (1994) An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc Natl Acad Sci USA 91: 11133-11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Black JA, Dib-Hajj SD, Waxman SG (2000) Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci 20: 8754-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG (1998a) Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol 79: 2668-2676. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG (1998b) NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA 95: 8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN (2001) Increased conduction velocity of nociceptive primary afferent neurons during unilateral hindlimb inflammation in the anaesthetised guinea-pig. Neuroscience 102: 669-679. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN (2004) Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 46: 131-145. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN (1998) Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol (Lond) 513: 857-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Dawbarn D, Robertson A, Newton R, Lawson SN (2001) Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci 21: 8722-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Newton R, Levinson SR, Berry CM, Carruthers B, Lawson SN (2003a) Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Na(v)1.7 (PN1) Na(+) channel alpha subunit protein. J Physiol (Lond) 546: 565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson S (2003b) The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol (Lond) 550: 739-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN (2002) The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci 22: 7425-7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG (1999) Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res 67: 267-282. [DOI] [PubMed] [Google Scholar]
- Fjell J, Hjelmstrom P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA (2000) Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. NeuroReport 11: 199-202. [DOI] [PubMed] [Google Scholar]
- Gerke MB, Plenderleith MB (2001) Binding sites for the plant lectin Bandeiraea simplicifolia I-isolectin B(4) are expressed by nociceptive primary sensory neurones. Brain Res 911: 101-104. [DOI] [PubMed] [Google Scholar]
- Goedert M, Otten U, Hunt SP, Bond A, Chapman D, Schlumpf M, Lichtensteiger W (1984) Biochemical and anatomical effects of antibodies against nerve growth factor on developing rat sensory ganglia. Proc Natl Acad Sci USA 81: 1580-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BG, Mobley WC, Matheson SF (1991) Regulation of axonal caliber, neurofilament content, and nuclear localization in mature sensory neurons by nerve growth factor. J Neurosci 11: 943-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould III HJ, Gould TN, England JD, Paul D, Liu ZP, Levinson SR (2000) A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res 854: 19-29. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Noguchi K, Ueda Y, Senba E (1995) Coexpression of trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res Mol Brain Res 30: 158-164. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Ueda Y, Senba E (1996) Coexpression of preprotachykinin-A, alpha-calcitonin gene-related peptide, somatostatin, and neurotrophin receptor family messenger RNAs in rat dorsal root ganglion neurons. Neuroscience 70: 179-189. [DOI] [PubMed] [Google Scholar]
- Lawson SN (2002) Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol 87: 239-244. [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ (1991) Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol (Lond) 435: 41-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH (1984) A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol 228: 263-272. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER (1997) Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol (Lond) 505: 177-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER (2002) Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol (Lond) 540: 989-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq JB, Paoletti C (1967) A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol 27: 87-106. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM (1992) On the role of nerve growth factor in the development of myelinated nociceptors. J Neurosci 12: 1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Deng YS, Zhou XF (2000) Downregulation of TrkA expression in primary sensory neurons after unilateral lumbar spinal nerve transection and some rescuing effects of nerve growth factor infusion. Neurosci Res 38: 183-191. [DOI] [PubMed] [Google Scholar]
- Liu C, Cummins TR, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD (2005) CAP-1A is a novel linker that binds clathrin and the voltage-gated sodium channel Na(v)1.8. Mol Cell Neurosci 28: 636-649. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhou XF, Rush RA (2001) Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res 41: 355-363. [DOI] [PubMed] [Google Scholar]
- Mendell LM (1999) Neurotrophin action on sensory neurons in adults: an extension of the neurotrophic hypothesis. Pain Suppl 6: S127-S132. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD (1995) Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol 361: 404-416. [DOI] [PubMed] [Google Scholar]
- Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN (2002) Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 417: 653-656. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG (2001) Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629-640. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Lewin GR, Kremer NE, Mendell LM (1991) Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature 350: 500-502. [DOI] [PubMed] [Google Scholar]
- Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent napthalimide tracer. Cell 14: 741-759. [DOI] [PubMed] [Google Scholar]
- Urschel BA, Hulsebosch CE (1990) Schwann cell-neuronal interactions in the rat involve nerve growth factor. J Comp Neurol 296: 114-122. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T (1995) Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci 15: 2081-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker JE, Haugland RP, Moore PL, Hewitt PC, Reese M, Haugland RP (1991) Cascade blue derivatives: water soluble, reactive, blue emission dyes evaluated as fluorescent labels and tracers. Anal Biochem 198: 119-130. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, de Vos AM (2001) Nerve growth factor: structure and function. Cell Mol Life Sci 58: 748-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM (2002) Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci 22: 4057-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]