Abstract
Voltage-gated sodium channels are responsible for the upstroke of the action potential in most excitable cells, and their fast inactivation is essential for controlling electrical signaling. In addition, a noninactivating, persistent component of sodium current, INaP, has been implicated in integrative functions of neurons including threshold for firing, neuronal bursting, and signal integration. G-protein βγ subunits increase INaP, but the sodium channel subtypes that conduct INaP and the target site(s) on the sodium channel molecule required for modulation by Gβγ are poorly defined. Here, we show that INaP conducted by Nav1.1 and Nav1.2 channels (Nav1.1 > Nav1.2) is modulated by Gβγ; Nav1.4 and Nav1.5 channels produce smaller INaP that is not regulated by Gβγ. These qualitative differences in modulation by Gβγ are determined by the transmembrane body of the sodium channels rather than their cytoplasmic C-terminal domains, which have been implicated previously in modulation by Gβγ. However, the C-terminal domains determine the quantitative extent of modulation of Nav1.2 channels by Gβγ. Studies of chimeric and truncated Nav1.2 channels identify molecular determinants that affect modulation of INaP located between amino acid residue 1890 and the C terminus at residue 2005. The last 28 amino acid residues of the C terminus are sufficient to support modulation by Gβγ when attached to the proximal C-terminal domain. Our results further define the sodium channel subtypes that generate INaP and identify crucial molecular determinants in the C-terminal domain required for modulation by Gβγ when attached to the transmembrane body of a responsive sodium channel.
Keywords: sodium, channels, G-proteins, inactivation, neuromodulation, excitability
Introduction
Voltage-gated Na+ channels are essential for neuronal excitability. They initiate fast action potentials and shape subthreshold electrical properties that contribute to important integrative functions. Voltage-gated Na+ channels are complexes of a poreforming α subunit and auxiliary β subunits (Catterall, 2000). The primary sequence of the α subunit contains four homologous domains (I-IV), each containing six predicted transmembrane α-helices (S1-S6). Five different α subunits are expressed in adult brain: Nav1.1, Nav1.2, Nav1.3, Nav1.5, and Nav1.6 (Goldin et al., 2000).
In addition to the transient rapidly inactivating sodium current (INaT) (Hodgkin and Huxley, 1952), voltage-gated Na+ channels also generate a “persistent” current (INaP), which is maintained during long depolarizations, and whose importance in neurons has become increasingly apparent (Crill, 1996). INaP has been observed in multiple neuron types. It contributes to shaping repetitive firing (Azouz et al., 1997; Mantegazza et al., 1998; Parri and Crunelli, 1998; Brumberg et al., 2000), generating rhythmicity (Alonso and Llinas, 1989; Pennartz et al., 1997; Pape et al., 1998; Taddese and Bean, 2002) and amplifying both IPSPs and EPSPs (Schwindt and Crill, 1995; Stuart and Sakmann, 1995; Stuart, 1999). INaP has also been implicated in the generation of epileptiform activities (Kearney et al., 2001), suggesting that its regulation plays a crucial role in controlling electrical excitability (Schwindt and Crill, 1995; Stuart and Sakmann, 1995; Segal and Douglas, 1997; Stuart, 1999).
Central neuronal INaP is subject to modulation by intracellular signal transduction pathways. It is decreased in parallel with INaT by activation of muscarinic acetylcholine receptors and consequent phosphorylation by protein kinase C in hippocampal neurons (Cantrell et al., 1996). It is increased by hypoxia and nitric oxide in hippocampal neurons (Hammarstrom and Gage, 1998, 1999, 2000). In addition, INaP is increased when G-protein βγ subunits are coexpressed with Nav1.2 channels (Ma et al., 1997). This increase was prevented by a putative Gβγ-binding peptide from the C terminus of the Nav1.2 channel, suggesting that G-protein βγ subunits interact with that C-terminal site during modulation (Ma et al., 1997). Consistent with this idea, G-protein β subunits immunoprecipitate with sodium channel α subunits from cortical neuron preparations (Marin et al., 2001).
In the experiments presented here, we have compared modulation of INaP by G-protein βγ subunits for Nav1.1, Nav1.2, Nav1.4, and Nav1.5 channels. We show that both Nav1.1 and Nav1.2 conduct INaP (Nav1.1 > Nav1.2) that can be modulated by Gβγ, but Nav1.4 and Nav1.5 do not. Analysis of channel chimeras reveals that the transmembrane bodies of Nav1.4 and Nav1.5 channels are responsible for their lack of G-protein modulation rather than their C-terminal domains. However, the quantitative extent of modulation of Nav1.2 channels is critically dependent on the C-terminal domain. Through analysis of deletion mutants, we identify new molecular determinants for modulation in the Nav1.2 channels in the C terminus and show that the final 28 amino acids are necessary for generation of INaP and can substitute for the entire distal half of the C terminus in supporting regulation by Gβγ.
Materials and Methods
Isolation of cDNA for hNav1.1 and generation of cell lines CL1 and CL2. Six overlapping clones spanning the human Nav1.1 coding region were isolated from human cerebellum and medulla cDNA libraries. A full-length cDNA was assembled from these partial clones using natively occurring restriction sites (BamHI, nucleotide 561; BamHI, nucleotide 2433; SphI, nucleotide 2694; NdeI, nucleotide 3786; SapI, nucleotide 5630). This was then inserted upstream of the internal ribosome entry site (IRES) element in the mammalian expression vector pCIN5 (Rees et al., 1996). Cell lines stably expressing hNav1.1 were isolated after transfection of human embryonic kidney (HEK) 293 cells with 2 μg of pCIN5-hNav1.1 and selection for 3-4 weeks in 800 μg/ml Geneticin-G418 (Burbidge et al., 2002). Clonal cell lines CL1 and CL2 were isolated after two rounds of single-cell dilution cloning.
Construction of Nav1.2 and Nav1.5 channel mutants. The mammalian expression plasmids, pCDM8-rH1 encoding the rat cardiac rNav1.5 α subunit and pCDM8-rIIA encoding the rat brain rNav1.2a α subunit, were described previously (Qu et al., 1994; Linford et al., 1998). cDNA encoding the full-length human brain type I Na+ channel hNav1.1 was in pCIN5-hNav1.1 (Clare et al., 2000). cDNA encoding the rat skeletal muscle Na+ channel rNav1.4 (Trimmer et al., 1989) (kind gift from Dr. P. Ruben, Utah State University, Logan, UT) was subcloned into pCDM8. The chimeric Na+ channel rNav1.2/Nav1.5 C terminus (CT) was described previously (Mantegazza et al., 2001). Chimeric Na+ channel Nav1.2/1.4 CT was comprised of the Nav1.4 C-terminal cytoplasmic domain (beginning at amino acid position E1592) appended to Nav1.2a cDNA after residue L1776. In mutant Nav1.2/1.4QxxER, the Nav1.2a sequence was changed from RIQMEER (R1876 to R1882) to KQTMEEK, the equivalent sequence in rat Nav1.4. In Nav1.2/AAMEAA mutant, the rNav1.2a sequence IQMEER beginning at I1875 was converted to AAMEAA. The truncation mutants Nav1.2ΔK1890, Nav1.2ΔA1909, Nav1.2ΔS1929, Nav1.2ΔT1951, Nav1.2ΔS1977, and Nav1.2ΔK1998, which delete segments of C-terminal cytoplasmic region of rat Nav1.2a, have been described previously (Mantegazza et al., 2001). The Nav1.2a deletion mutant Nav1.2/Δ1891-1977 removed an internal segment bounded by amino acid residues V1891 and S1977. Full-length cDNA of bovine G-protein β2 subunit was cloned into a bicistronic mammalian expression plasmid, pIRES-enhanced yellow fluorescent protein (YFP) (Clontech, Palo Alto, CA), which encodes the yellow variant of the green fluorescent protein (GFP). This construct was called pGβ2IRES-YFP. The G-protein γ3 subunit was cloned into a cyan variant of the same bicistronic vector and was named pGγ3IRES-cyan fluorescent protein (CFP). All Na+ channel mutations generated by PCR mutagenesis were sequenced in between the restriction sites used for subcloning to verify that they were free of unintended alteration of sequences.
Expression of sodium channels. The fluorescent proteins in the bicistronic vectors or the CD8 receptor in pCD8-neomycin were used as markers of transfected cells (Margolskee et al., 1993). Plasmids were cotransfected into tsA-201 cells by CaPO4 precipitation. Cells were grown to 75% confluence in 35 mm tissue culture dishes (Corning, Acton, MA). After addition of DNA, they were incubated at 37°C in 3% CO2. Twelve hours after transfection, cells were removed from the dishes using 2 mm EDTA in PBS and replated at low density for electrophysiological recordings. HEK293 cell lines that expressed hNav1.1, CL1 and CL2, were transfected using TransFast (Promega, Madison, WI). Positive transfectants were selected visually either by anti-CD8-coated beads (Dynal, Brown Deer, WI) or by their fluorescence using a Nikon (Tokyo, Japan) Eclipse TE300 microscope for epifluorescence. Cells transfected with plasmids encoding green fluorescent proteins were selected visually using excitation/emission cubes for YFP (Chroma 41028; Chroma Technology, Rockingham, VT) and CFP (Chroma 31044v2). The tsA-201 subclone of HEK293 cells and stably transfected HEK cell lines were maintained as described previously (Herlitze et al., 1996).
Electrophysiology and data analysis. Whole-cell patch-clamp recordings were performed at room temperature using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Capacitative currents were minimized by means of the amplifier circuitry. Seventy percent prediction and 90-95% series resistance compensation were used routinely. The remaining capacity transients and leakage currents were eliminated using P/4 subtraction. The intracellular solution contained the following (in mm): 120 Cs-aspartate, 5 NaCl, 2 MgCl2, 10 EGTA, 10 HEPES, pH 7.3 with CsOH. The extracellular solution contained the following (in mm): 140 NaCl, 2 CaCl2, 2 MgCl2, 10 HEPES, pH 7.4 with NaOH. pClamp 6.0.4 software and a Digidata 1200 interface (Axon Instruments) were used to generate the voltage stimuli and to acquire the current signals, which were filtered at 5 or 10 kHz. The data were analyzed using pClamp and Origin 6.0 (OriginLab, Northampton, MA) on a Pentium II-based personal computer (Intel, Santa Clara, CA). Unless indicated, the holding potential for voltage clamp recordings was -70 mV.
Conductance-voltage (G-V) relationships were calculated from the current-voltage (I-V) relationships according to extended Ohm's law GNa = INa/(V - ENa), where INa was the peak Na+ current measured at potential V, GNa was the sodium conductance, and ENa was the calculated Nernst equilibrium potential for Na+. Curves were fit using the Levenberg-Marquardt algorithm. The voltage dependence of activation and voltage dependence of fast inactivation were fitted to Boltzmann relationships of the following form: normalized GNa = ((1 - C)/(1 + exp (V - V½)/k)) + C, where V was the membrane potential, V½ was the voltage of half-maximal activation or inactivation, k was a slope factor, and C was the baseline noninactivating current. For cells with large INaP, a second Boltzmann component was included, as follows: ((1 - C - A2)/(1 + exp ((V - V½,1)/k1))) +(A2/(1 + exp((V - V½, 2)/k2))) + C. The statistical results are given as mean ± SEM. The threshold p value for statistical significance was 0.05.
Sequence alignments were produced by ClustalW (Thompson et al., 1994) followed by manual editing to produce the alignments shown.
Results
INaP conducted by rNav1.2a and hNav1.1 channels
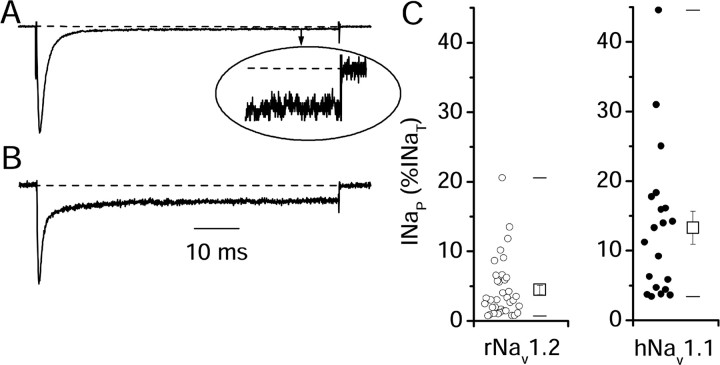
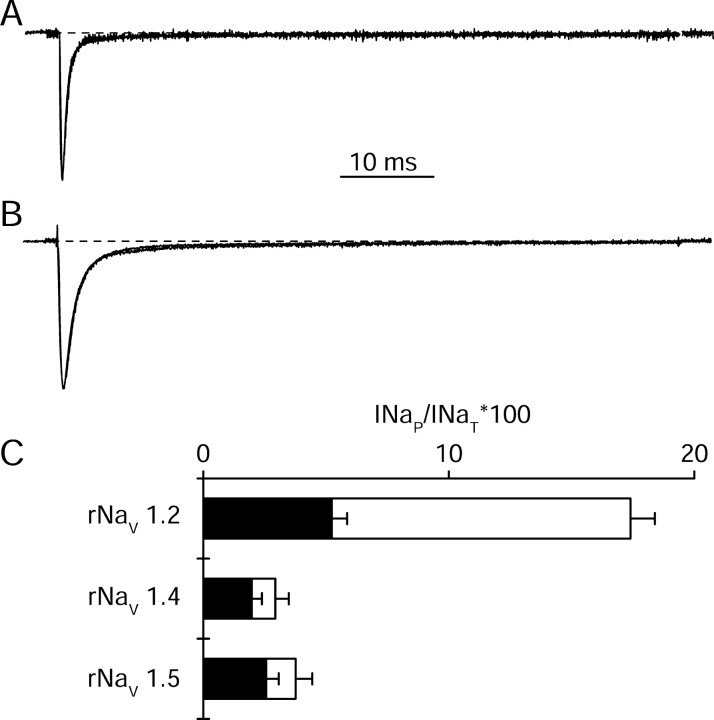
Sodium currents produced by expression of Nav1.2 channels in tsA-201 cells have two components: a large, rapidly inactivating component followed by a persistent component that fails to inactivate by the end of 60 ms depolarizations (Fig. 1A). INaP was quantitated as the mean current remaining between 45 and 55 ms after the beginning of the voltage step and expressed as a percentage of INaT. The level of INaP varied considerably from cell to cell with a mean of 4.5 ± 0.7% of INaT (n = 36) (Fig. 1C). hNav1.1 channels expressed similarly in tsA-201 cells conducted significantly larger INaP than rNav1.2a (mean INaP = 13 ± 2%; n = 20; p <0.01) (Fig. 1 B, C), similar to the results of Smith and Goldin (1998) for expression of rNav1.1 in Xenopus oocytes. The variability of the fraction of INaP versus INaT among single clonal tsA-201 cells transfected with identical cDNA for either rNav1.2a and hNav1.1 channels (Fig. 1C) suggests that cellular signaling processes that differ from cell to cell can control the level of INaP.
Figure 1.
Normalized current traces elicited by a step stimulus to 0 mV from a holding potential of -70 mV from representative tsA-201 cells transfected with rNav1.2a (A) or with hNav1.1 (B). The inset in A (oval) shows the end of the pulse on a 15-fold expanded vertical axis. C, Individual data points (circles) and mean values ± SEM (open squares) of INaP as a percentage of INaT in tsA-201 cells transiently transfected with rNav1.2a (n = 36) or hNav1.1 (n = 20; p < 0.01). The horizontal lines represent the first and 99th percentiles of the range.
Modulation of INaP through rNav1.2a channels by Gβγ
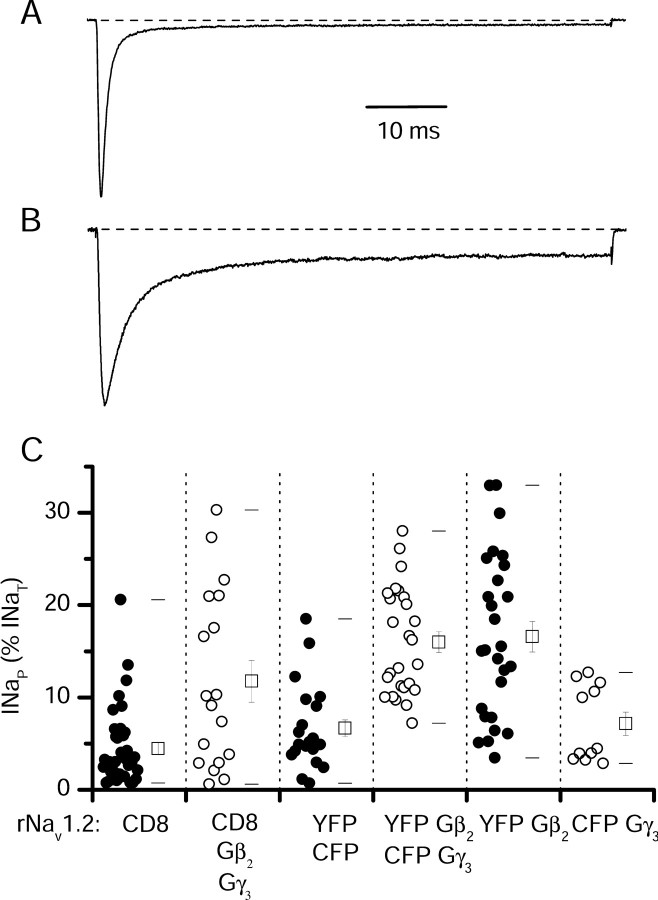
INaP conducted by Nav1.2 channels is increased by coexpression of G-protein βγ subunits (Fig. 2B,C) (Ma et al., 1997). Transfection of Gβ2γ3 increased INaP from 4.5 ± 0.7 to 11.8 ± 2.3% (n = 18) (Fig. 2C). The level of INaP obtained with cotransfection of plasmids encoding G-protein β2 and γ3 subunits was highly variable in different cells (Fig. 2C), and this variability complicated the study of INaP modulation. One possible source of variability was the requirement for cotransfection of cDNAs encoding three different proteins: the Nav1.2 α subunit, the G-protein β2 subunit, and the G-protein γ3 subunit. To ensure expression of both G-protein β and γ subunits in all cells studied, we cloned the cDNAs encoding these G-protein subunits into bicistronic vectors containing an IRES followed by cDNA encoding the yellow or cyan mutants of GFP as reporter genes (Trouet et al., 1997). Control cells transfected with the same fluorescent protein vectors lacking G-protein subunits (Fig. 2C, CFP, YFP) had INaP that was not significantly different from cells transfected with rNav1.2 and CD8 (CD8, mean INaP = 4.5 ± 0.7%, n = 36; CFP, YFP, mean INaP = 6.8 ± 1.0%, n = 20; p = 0.08) (Fig. 2C). When Gβ2-YFP and Gγ3-CFP were cotransfected, and only cells clearly fluorescent for both markers were studied, the data were less variable than without use of bicistronic vectors (mean INaP = 16 ± 1.0%; n = 26) (Fig. 2C, YFP Gβ2,/CFP Gγ3). Variance of the experimental groups decreased from 92 with CD8 as a reporter to 34 with bicistronic Gβγ as reporter (p = 2 × 10-8). As a result of the reduced variability using GFP reporters to monitor expression, smaller numbers of experiments were required to obtain meaningful results when Gβγ coexpression was studied. The IRES vectors were used to verify expression in all additional studies.
Figure 2.
Changes in INaP produced by Nav1.2 induced by cotransfection of G-protein β and γ subunits. A, B, Current traces from representative tsA-201 cells transfected with rNav1.2a alone (A) or cotransfected with Gβ2 and Gγ3 (B) elicited by a depolarization to 0 mV from a holding potential of -70 mV. C, Individual data points (circles) and mean values ± SEM (open squares) of INaP as a percentage of INaT in tsA-201 cells transfected with rNav1.2a and the indicated plasmids. The whiskers represent the first and 99th percentile values. Mean values were the following, for cells transfected with (from left to right): CD8 receptor as reporter gene (INaP = 4.5 ± 0.7%; n = 36); rNav1.2a, Gβ2, Gγ3, and CD8 receptor (INaP = 11.8 ± 2.3%; n = 18); rNav1.2a with YFP and CFP as reporter genes (INaP = 6.8 ± 1.0%; n = 20); rNav1.2a, Gβ2 expressed in a bicistronic vector with YFP and Gγ3 expressed in a bicistronic vector with CFP (INaP = 16 ± 1.0%; n = 26); rNav1.2a and Gβ2 expressed in a bicistronic vector with YFP (INaP = 17 ± 2%; n = 27); rNav1.2a and Gγ3 expressed in a bicistronic vector together with YFP (INaP = 7 ± 1%; n = 11). Because the CD8 control group and the CFP YFP control group did not differ significantly, they were pooled for additional statistical comparisons (mean of pooled data, INaP = 5.3 ± 0.6%; n = 56).
Modulation of INaP through rNav1.2a channels by Gβ2 subunits alone
To better characterize Gβγ modulation, we examined the ability of single G-protein β and γ subunits to increase INaP. Expression of only Gβ2 with rNav1.2a was sufficient to increase INaP comparably to that caused by expression of Gβγ (mean INaP = 17 ± 2%; n = 27; p = 0.00005) (Fig. 2C, YFP, Gβ2). In contrast, expression of Gγ3 alone did not modulate INaP (mean INaP = 7 ± 1%; n = 11; p = 0.8) (Fig. 2C, CFP, Gγ3). Expression of Gβ subunits is also sufficient for G-protein modulation of calcium channels (Herlitze et al., 1996).
Effect of Gβ2γ3 on voltage-dependent gating of rNav1.2
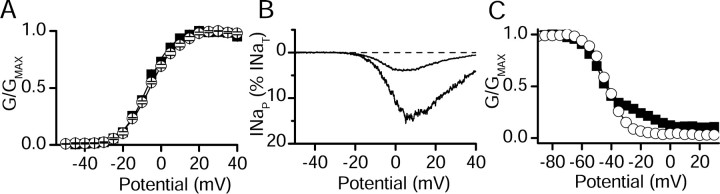
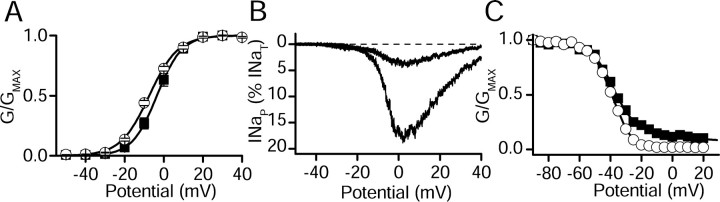
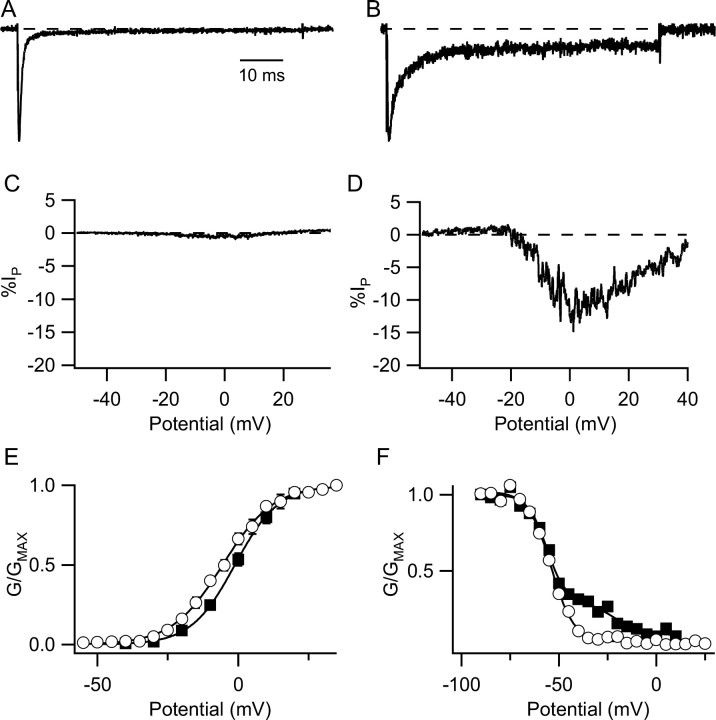
We compared the voltage-dependent gating of INaT and INaP before and after modulation by Gβ2γ3. As in previous work (Ma et al., 1997), the major effect of Gβ2γ3 on the properties of rNav1.2a was the increase in INaP. Little change in the voltage dependence of activation of INaT was observed (control, V½ = -5.9 ± 0.2 mV; Gβ2γ3, V½ =-7.2 ± 0.2 mV) (Fig. 3A). Coexpression of Gβγ strikingly increased the amplitude of INaP as measured in response to slowly rising voltage ramps (Fig. 3B), during which INaT is inactivated by the slow ramp depolarization. This protocol gives the clearest visual illustration of the large effect of Gβγ coexpression on INaP. The presence of INaP resulted in a biphasic inactivation curve, which was fit by the sum of two Boltzmann relationships (Fig. 3C). The component of current that had the more positive voltage dependence of inactivation also had slower inactivation kinetics and corresponded to INaP (data not shown) (Ma et al., 1997). Gβγ caused an increase in the fraction of current inactivating with the more positive voltage dependence with little change in the voltage dependence of the individual components (Fig. 3C).
Figure 3.
Properties of INaT and INaP in tsA-201 cells transfected with Nav1.2 alone or cotransfected with Gβ2γ3. A, Mean voltage dependence of activation for cells transfected with rNav1.2a alone (open circles) and cells cotransfected with rNav1.2a and Gβ2γ3 (filled squares). The solid lines are the Boltzmann fits that gave the following parameters (see Materials and Methods for definitions): Nav1.2 alone, n = 9, V½ = -5.9 ± 0.2 mV, k = -7.3 ± 0.2 mV; Nav1.2a plus Gβ2γ3, n = 13, V½ = -7.2 ± 0.2 mV, k = -6.6 ± 0.2 mV. B, Currents elicited by slow voltage ramps from a representative cell transfected with rNav1.2a alone (smaller current) or cotransfected with Gβ2γ3 (larger current). C, Voltage dependence of inactivation from representative cells with the mean level of INaP determined with 100 ms prepulses to a variable voltage followed by a test depolarization to 0 mV for Nav1.2a alone (open circles) or cotransfected with Gβ2γ3 (filled squares). Normalized test pulse current is plotted as a function of prepulse potential. Mean parameters derived from fits of Boltzmann functions to the data were: rNav1.2a alone, n = 7, V½ = -44.1 ± 0.2, k = 7.3 ± 0.1, C = 0.023 ± 0.002; rNav1.2a plus Gβ2γ3, n = 13 (fit with the sum of 2 Boltzmann components), V½, 1 = -45.1 ± 0.4, k1 = 8.3 ± 0.4, A2 = 0.35, V½, 2 = -10.0 ± 0.7, k2 = 6.0 ± 0.7, C = 0.117 ± 0.003).
Modulation of INaP conducted by hNav1.1
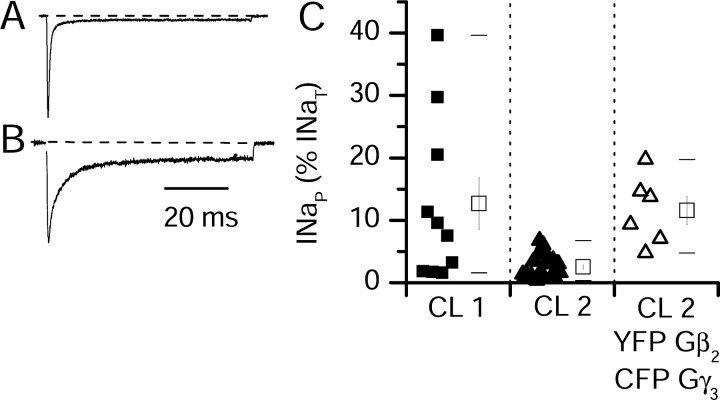
We were concerned that the large variation in INaP among single tsA-201 cells expressing hNav1.1 channels (Fig. 1) might impair measurements of Gβγ modulation, even using our bicistronic vectors and imaging transfected Gβγ. Therefore, we examined the level of INaP in two clonal cell lines, derived by stable transfection of hNav1.1 into HEK293 cells (Clare et al., 2000). In the first cell line tested, CL1, the level of INaP was similar to that observed after transient transfection of hNav1.1 into tsA-201 cells (mean INaP = 13 ± 4%; n = 10) (Fig. 4C). In the second cell line, CL2, lower levels of INaP were observed (mean INaP = 2.5 ± 0.4%; n = 25) (Fig. 4A,C, CL2). These results with two clonal cell lines are consistent with the striking variation of INaP among single cells transfected transiently (Fig. 1C).
Figure 4.
INaP conducted by hNav1.1 in two stable cell lines and its modulation by Gβ2γ3. INaP was measured in two cell lines, CL1 and CL2, stably expressing hNav1.1 and in CL2 transiently transfected with G-protein β2 and γ3 subunits. A, Current traces from representative cells during depolarizations to 0 mV from a holding potential of -70 mV from CL2 alone (A) or CL2 cotransfected with Gβ2 and Gγ3 subunits (B). C, INaP as a percentage of INaT in the indicated cells. Values for individual cells are plotted as small symbols; open squares indicate mean ± SEM. Horizontal bars represent the first and 99th percentiles of the range.
Because of its low level of intrinsic INaP in control, we chose CL2 to study the effect of Gβ2γ3 on INaP. We cotransfected pGβ2IRES-YFP and pGγ3IRES-CFP into CL2 cells stably expressing hNav1.1. INaP with Gβ2γ3 was significantly higher than in untransfected CL2 cells (mean INaP = 11 ± 2%; n = 6; p = 10-7 relative to untransfected CL2) (Fig. 4B,C, CL2, YFP Gβ2, CFP Gγ3). Thus, as for rNav1.2a, INaP conducted by hNav1.1 is also increased by coexpression of Gβ2γ3.
Effect of Gβ2γ3 on voltage-dependent gating of hNav1.1
Cotransfection of hNav1.1 with Gβ2γ3 resulted in a small positive shift in the voltage dependence of activation of INaT (Fig. 5A). Coexpression of Gβ2γ3 also substantially increased INaP measured using slowly rising voltage ramps (Fig. 5B). The increased persistent sodium current in cells expressing Gβ2γ3 caused a positive shift in the overall inactivation curve, and the baseline of noninactivating current at positive potentials was significantly increased (Fig. 5C). However, as a result of the relatively small level of INaP in this cell line, even in the presence of Gβ2γ3, the second Boltzmann component from these cells was not as clearly resolved as for Nav1.2. As described above, the relative size of INaP in tsA-201 cells transiently transfected with hNav1.1 or in cell line CL1, in which hNav1.1 had been stably expressed in HEK293 cells, was much larger than in CL2. However, the voltage-dependent properties of INaT and INaP in these cells were not different from those of CL2 except for the larger amplitude of INaP (data not shown). Thus, for both hNav1.1 and rNav1.2a, coexpression of Gβ2γ3 increases both the amount of INaP relative to INaT and the fraction of current with slowed and positive voltage-dependent inactivation without major effects on the voltage dependence of activation of INaT.
Figure 5.
Properties of transient and persistent current in Nav1.1 alone and cotransfected with Gβ2γ3 as expressed in CL2. A, Mean voltage dependence of activation for CL2 stably expressing hNav1.1 (open circles) and CL2 cells transiently transfected with Gβ2γ3 (filled squares). The solid lines are fits of a Boltzmann equation to the mean data. For CL2 cells expressing Nav1.1 (n = 18), Va = -7.5 ± 0.5 mV and k = -7.3 ± 0.4 mV; for CL2 cells cotransfected with Gβ2γ3 (n = 6), Va = -3.5 ± 0.5 mV and k = -6.4 ± 0.4 mV. B, Currents elicited by slow (70 mV/s) voltage ramps from a representative CL2 cell (smaller current) or a representative CL2 cell cotransfected with Gβ2γ3 (larger current). The ramps shown are from cells with INaP that is somewhat larger than the means of the control and Gβγ-transfected groups to more clearly demonstrate the voltage-dependent properties of INaP. C, Voltage dependence of inactivation from representative cells, selected to have approximately the mean level of INaP, determined with 100 ms prepulses to a variable voltage followed by a test depolarization to 0 mV for CL2 alone (open circles) or CL2 cotransfected with Gβ2γ3 (filled squares). Normalized test pulse current is plotted as a function of prepulse potential. Mean parameters derived from fits of Boltzmann functions to the data were as follows: for CL2, V½ = -37.6 ± 0.1 mV, k = 6.2 ± 0.1 mV, C = 0.029 ± 0.003, n = 11; for CL2 cotransfected with Gβ2γ3, V½ = -34.6 ± 0.2, k = 7.2 ± 0.2, C = 0.063 ± 0.003, n = 4.
Modulation of INaP through rNav1.4 and rNav1.5 channels by Gβ2γ3
We tested whether skeletal muscle rNav1.4 and cardiac rNav1.5 channel isoforms were modulated by Gβ2γ3 when cotransfected in tsA-201 cells. When transfected alone, rNav1.4 and rNav1.5 give little INaP (INaP = 1.9 ± 0.4%, n = 7; and 2.6 ± 0.5%, n = 10, respectively) (Fig. 6). When Gβ2γ3 was coexpressed with either Nav1.4 or Nav1.5 channels, no significant increase in INaP was observed (Nav1.4 plus Gβ2γ3, 2.9 ± 0.6%, n = 8; Nav1.5 plus Gβ2γ3, 3.7 ± 0.7%, n = 5) (Fig. 6). Thus, little INaP is produced by these two isoforms, and it is not increased by coexpression of Gβ2γ3.
Figure 6.
A, Normalized superimposed current traces from two representative tsA-201 cells transfected with rNav1.4 alone or cotransfected with Gβ2 and Gγ3. Currents were elicited by a depolarization to 0 mV from a holding potential of -70 mV. B, Normalized superimposed current traces from two representative tsA-201 cells transfected with rNav1.5 alone or cotransfected with Gβ2 and Gγ3. Currents were elicited by a depolarization to -10 mV from a holding potential of -70 mV. C, Bar graph showing INaP as percentage of INaT for cells transfected with the indicated sodium channel α subunit alone (black bars) or in combination with Gβ2 and Gγ3 (open bars). For rNav1.2a: control (pooled data), INaP = 5.3 ± 0.6%, n = 56; plus Gβ2γ3, INaP = 16 ± 1.0%, n = 26. For rNav 1.4: control, INaP = 1.9 ± 0.4%, n = 7; plus Gβ2γ3, 2.9 ± 0.6%, n = 8. For rNav1.5: control, 2.6 ± 0.5%, n = 10; plus Gβ2γ3, 3.7 ± 0.7%, n = 5. Error bars represent SEM.
Modulation of INaP by the CT domains of rNav1.4 and rNav1.5 channels
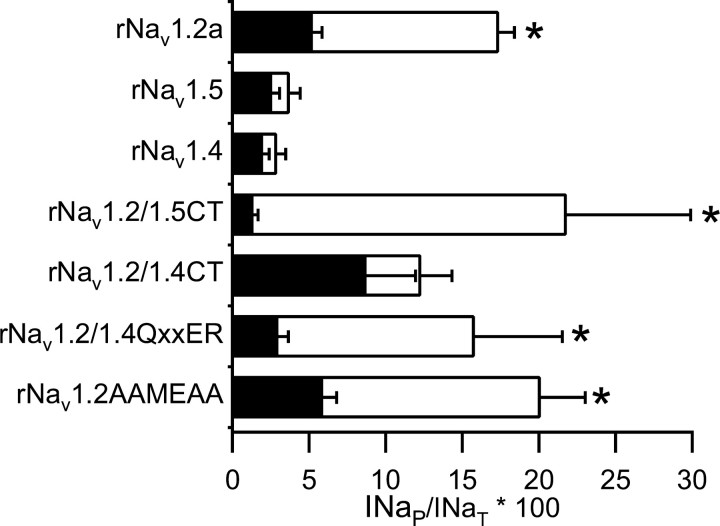
We proposed previously that the CT of rNav1.2a is a domain required to produce INaP in that channel (Ma et al., 1997). To test this, we used the construct Nav1.2/1.5 CT (Mantegazza et al., 2001). In this construct, the rNav1.5 CT beginning with E1755 replaced the CT of rNav1.2a, which had been truncated before E1777. This chimeric mutation did not increase INaP in the absence of modulation by Gβγ (Fig. 7). However, the chimera was strongly modulated by Gβ2γ3 (Fig. 7). Thus, although the CT of Nav1.5 does not produce modulation when attached to its native subunit, it produces strong modulation of rNav1.2a.
Figure 7.
INaP in sodium channels containing mutations affecting the QXXER motif and their modulation by G-protein βγ subunits. Bar graph showing INaP as percentage of INaT for cells transfected with the indicated sodium channel α subunit alone (black bars) or in combination with Gβ2 and Gγ3 (open bars). For rNav1.2a, rNav1.4, and rNav1.5, see Figures 2 and 6 for mean values and errors. For Nav1.2a/1.5CT: control, INaP = 1.4 ± 0.3, n = 7; Gβ2γ3, INaP = 21.8 ± 8.1, n = 5. For Nav1.2a/1.4 CT: control, INaP = 8.7 ± 3.2, n = 8; Gβ2γ3, INaP = 12 ± 2, n = 12. For rNav1.2/1.4QxxER: control, INaP = 3.0 ± 0.7, n = 9; Gβ2γ3, INaP = 16 ± 6, n = 5. For rNav1.2a/AAMEAA: control, INaP = 5.9 ± 0.9, n = 10; Gβ2γ3, INaP = 20 ± 3, n = 7. Error bars represent SEM. Asterisks indicate significant modulation by GB2γ3 (p < 0.05).
This contrasts with the effects observed in chimeras of rNav1.4 and rNav1.2a, in which the CT of rNav1.2a was completely or partially replaced by the analogous sequence from Nav1.4. In Nav1.2/1.4 CT, the rNav1.4 CT beginning with E1592 replaced the CT of rNav1.2a, which had been truncated before E1777. Substitution of the C terminus in Nav1.2/1.4 CT did not significantly change the level of INaP under control conditions. In addition, there was no increase after coexpression of Gβ2γ3 (Fig. 7). Thus, the Nav1.4 C terminus does not support modulation by Gβγ. These findings are consistent with the CT domains of Nav1.2 and Nav1.5 containing molecular determinants required for modulation of INaP by Gβγ that are absent in the Nav1.4 CT.
Requirement for the rNav1.2a CT and its QxxER motif for modulation by Gβγ
A short sequence motif (QxxER) in the Nav1.2a CT was suggested initially to be an important molecular determinants of Gβγ modulation based on our finding that a peptide containing this motif effectively inhibited G-protein modulation (Ma et al., 1997). A QxxER motif is conserved in hNav1.1, partially conserved in rNav1.5, but absent in the CT of rNav1.4. To assess the role of the QxxER region of rNav1.2a in modulation by Gβ2γ3, we constructed two mutant channels. For the first, we used the CT of Nav1.4 to replace the QxxER sequence in the CT of Nav1.2a, because Nav1.4 did not support modulation by Gβγ. In Nav1.2/1.4QxxER, the Nav1.4 CT from K1691 to K1697 replaced the QxxER region in rNav1.2a CT from R1876 and R1882. In the second construct, Nav1.2/AAMEAA, I1875, Q1876, E1879, and R1880 in rNav1.2a were replaced with alanines, thus removing the specific side chains of the critical amino acids in the QxxER motif. Both Nav1.2/1.4QxxER and Nav1.2/AAMEAA were modulated fully by Gβ2γ3 (Fig. 7), indicating that this motif is not necessary for modulation of Nav1.2 by Gβγ. Molecular determinants for modulation of Nav1.2a sodium channel by Gβγ must reside in other regions of the Nav1.2 CT that have amino acid sequence differences from the Nav1.4 CT.
Requirement for the last 28 amino acids of rNav1.2a CT for modulation by Gβγ
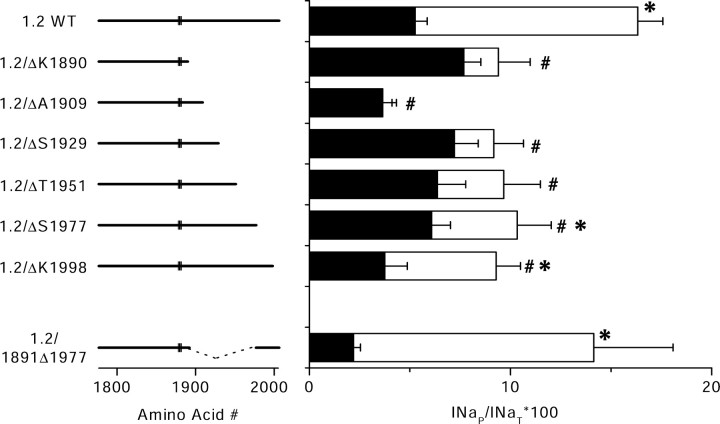
To localize the molecular determinants in the CT responsible for INaP and its modulation by Gβγ, we used a series of rNav1.2a mutants in which the CT was truncated at different positions, as described previously (Mantegazza et al., 2001). Mutants that were truncated on the N-terminal side of K1890 did not express measurable sodium current or gave rise to currents that were too small to study (Mantegazza et al., 2001). Therefore, we only tested mutants in which the truncation was on the C-terminal side of K1890. Although the mean level of INaP in the absence of transfected Gβγ subunits varied among the truncation mutants, the differences from wild type (WT) were not significant (p > 0.05) (Fig. 8). However, the effects of cotransfected Gβγ on these mutant channels differed significantly. Coexpression of Gβ2γ3 did not significantly increase INaP when the channel was shortened beyond T1951 in mutants ΔΚ1890, ΔΚ1909, ΔΚ1929, and ΔT1951 (Fig. 8, bars lacking asterisks). In constructs with longer C termini, including ΔS1977 and ΔK1998, INaP was increased significantly by Gβ2γ3 coexpression (Fig. 8, bars with asterisks). However, the increase of INaP caused by Gβ2γ3 coexpression with ΔS1977 and ΔK1998 was not as large as for WT rNav1.2, as denoted by the bars labeled with #. The pattern of decreased modulation by Gβγ in these deletion constructs suggests that the amino acid residues between positions 1951 and 1977 and between position 1977 and the C terminus of the protein at position 2005 are important for modulation by Gβγ.
Figure 8.
INaP in truncated Nav1.2a channels and its modulation by G-protein β2γ3 subunits. Left, Diagram of the CT of the truncated channel constructs. The rectangle indicates the position of the QXXER motif. Right, Bar graph showing INaP as percentage of INaT for cells transfected with the indicated sodium channel α subunit construct alone (black bars) or in combination with Gβ2 and Gγ3 (open bars). For rNav1.2/K1890: control, INaP = 7.7 ± 0.8%, n = 9; plus Gβ2γ3, INaP = 9 ± 2%, n = 6. For rNav1.2/A1909: control, INaP = 3.6 ± 0.7%, n = 16; plus Gβ2γ3, INaP = 3.5 ± 0.6%, n = 8. For rNav1.2/S1929: control, INaP = 7 ± 1%, n = 8; plus Gβ2γ3, INaP = 9 ± 1%, n = 10. For rNav1.2/T1951: control, INaP = 6 ± 1%, n = 10; plus Gβ2γ3, INaP = 10 ± 2%, n = 9. For rNav1.2/S1977: control, INaP = 6.1 ± 0.9%, n = 21; plus Gβ2γ3, INaP = 10 ± 2%, n = 10. For rNav1.2/K1998: control, INaP = 4 ± 1%, n = 7; plus Gβ2γ3, 9 ± 1%, n = 13. For rNav1.2/1891Δ1977: control, INaP = 2.2 ± 0.3%, n = 14; plus Gβ2γ3, INaP = 14 ± 4%, n = 8. Asterisks indicate significant modulation of a construct by Gβγ (p < 0.05); # indicates modulation by Gβγ that is significantly reduced when compared with modulation of the full-length channel (p < 0.05). Error bars represent SEM.
To test the functional role of the final 28 amino acid residues directly, we constructed the mutant Nav1.2a/Δ1891-1977, in which amino acid residues from V1891 to S1977 were deleted. This is equivalent to Nav1.2a/ΔK1890 having the last 28 amino acids of the C terminal added directly to it. Modulation of this construct by Nav1.2a/Δ1891-1977 was similar to modulation of the WT Nav1.2a channel (Fig. 8). Thus, the last 28 amino acids of the CT are necessary for modulation by Gβγ and are sufficient to replace the requirement for the remainder of the distal half of the C-terminal domain.
Voltage-dependent gating and modulation of Nav1.2Δ1891-1977
We analyzed the functional properties of Nav1.2Δ1891-1977 more completely and compared them with WT. Coexpression of Gβ2γ3 increased INaP during single depolarizations (Fig. 9A,B) or during slow voltage ramps (Fig. 9C,D). The voltage dependence of activation of INaT conducted by Nav1.2a/Δ1891-1977 was unchanged from that of wild-type Nav1.2a, and, as for WT, cotransfection of Gβγ caused little change in the voltage dependence of activation (Fig. 9E). The voltage dependence of inactivation was shifted ∼10 mV more negative than for WT Nav1.2a. This is consistent with previous findings implicating the C-terminal region of the sodium channel in modulating inactivation (Deschenes et al., 2001; Mantegazza et al., 2001; Cormier et al., 2002; Motoike et al., 2004). Cotransfection of Gβ2γ3 subunits with Nav1.2a/Δ1891-1977 did not appreciably change the negative component of the steady-state inactivation curve but increased the amplitude of a more positive component of inactivation (Fig. 9F, closed squares), similar to Gβγ modulation of WT. Thus, although the deletion in Nav1.2a/Δ1891-1977 causes a negative shift in the voltage dependence of inactivation of INaT under control conditions, INaP is modulated essentially normally by cotransfection of Gβ2γ3.
Figure 9.
Modulation of rNav1.2a/1891Δ1977 channels by G-protein βγ subunits. A, B, Examples of representative current traces during depolarizations to 0 mV from a holding potential of -70 mV from a cell expressing rNav1.2a/1891Δ1977 channels alone (A) and from a cell coexpressing rNav1.2a/1891Δ1977 and Gβ2γ3 (B). C, D, Currents in response to voltage ramps at 70 mV/s from -60 to +40 mV from the same cells shown in A and B, respectively. E, Mean voltage dependence of activation for cells transfected with rNav1.2a/1891Δ1977 channels alone (open circles) and cotransfected with Gβ2γ3 (filled squares). The solid lines are fits of a Boltzmann equation to the mean data. For rNav1.2a/1891Δ1977: control, V½= -5.6 ± 1.10 mV, k = -8.6 ± 0.47 mV, n = 9; plus Gβ2γ3, V½ = -0.1 ± 1.55 mV and k = -8.5 ± 0.48 mV, n = 7. F, Voltage dependence of inactivation from a representative cell transfected with rNav1.2a/1891Δ1977 channels alone (open circles) and cotransfected with Gβ2γ3 (filled squares). Representative cells were chosen to have approximately the mean level of INaP. Mean parameters derived from fits of Boltzmann functions to the data were for rNav1.2a/1891Δ1977: control, V½= -54.5 ± 0.15 mV, k = 6.3 ± 0.13 mV, C = 0.04 ± 0.002, n = 8; plus Gβ2γ3 (fit with 2 Boltzmann components), V½, 1 = -54.5 ± 0.52 mV, k1 = 4.0 ± 0.80 mV, A2 = 0.43, V½, 2 = -50.8 ± 2.89, k2 = 15.7 ± 2.67 mV, C = 0.03 ± 0.008, n = 3.
Comparison of the amino acid sequences of the distal C-terminal domains of Nav1 channels
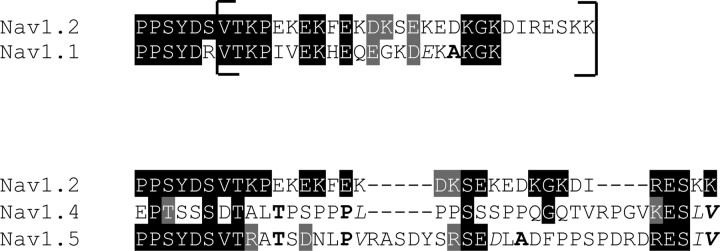
Because the distal CT of Nav1.1 and Nav1.2 can support modulation by Gβγ, but the CT of Nav1.4 cannot, it is interesting to compare the amino acid sequences of the distal CT showing the identities (black blocks) and similarities (gray blocks) to Nav1.2a, as follows:
Scheme 1.
Nav1.1 is quite similar to Nav1.2 but is seven amino acids shorter. Among the 21 aligned amino acid residues, 10 are identical, two are similar, and there are no gaps. In contrast, Nav1.4 has only five identical and one similar residues, and a four-residue gap in Nav1.2 is required for alignment. Nav1.5 has seven identical and three similar residues but requires gaps of four and five residues in Nav1.2 for alignment. Thus, the similarities of the Nav1.1 and Nav1.2 sequences support a common function for their distal C-terminal segments, whereas the differences in sequence of Nav1.5 and especially Nav1.4 C-terminal sequences suggest different functions. Additional experiments will be required to determine whether these segments play important roles in some aspect of G-protein modulation of cardiac and skeletal muscle sodium channels.
Discussion
Persistent sodium current in neurons
INaP is implicated in many aspects of neuronal function, including shaping bursting behavior, controlling firing frequency, integrating synaptic input, and generating epileptiform hyperexcitability (see Introduction). INaP is thought to be conducted by the same sodium channels that generate INaT in some neurons (Alzheimer et al., 1993; Taddese and Bean, 2002), but the sodium channel subtypes that generate INaP and the mechanisms that regulate INaT versus INaP in neurons are unknown. Here, we have addressed this gap in knowledge by analyzing the level of INaP generated by different sodium channel subtypes, demonstrating subtype-selective effects of Gβγ subunits on INaP and identifying an essential molecular determinant of Gβγ modulation of Nav1.2 channels.
Different levels of persistent sodium current conducted by sodium channel subtypes
Our results show that different sodium channel α subunits intrinsically generate different levels of INaP. Nav1.1 and Nav1.2 both conduct substantial INaP, but when expressed transiently in tsA-201 cells, Nav1.1 produces significantly more INaP than Nav1.2. Conversely, Nav1.4 and Nav1.5 conduct little INaP. Although the CT has been implicated in the modulation of INaP by Gβγ (Ma et al., 1997; this work) and in setting the different inactivation properties of Nav1.5 relative to Nav1.2a or Nav1.4 channels (Mantegazza et al., 2001; Deschenes et al., 2001; Cormier et al., 2002; Motoike et al., 2004), the CT of Nav1.4 did not reduce INaP produced by the Nav1.2a in chimera Nav1.2/1.4 CT. Thus, the intrinsic level of INaP is determined by the pre-CT portion of the channel rather than by the CT. However, the CT alters modulation of INaP when attached to a sodium channel that conducts substantial INaP.
Regulation of persistent sodium currents by G-proteins
Although INaP is important physiologically, few experimental conditions have been identified that alter INaP relative to INaT. We showed previously that coexpression of G-protein βγ subunits can substantially increase INaP, relative to INaT, and we implicated the CT of the Nav1.2 channel α subunit in those effects by demonstrating block of INaP by a competing CT peptide (Ma et al., 1997). Our present results extend those findings in three important respects. First, using bicistronic vectors and fluorescence to verify expression of Gβ and Gγ, we substantially reduced cell-to-cell variability in Gβγ modulation of INaP, thereby providing stronger evidence that Gβγ subunits are important regulators of INaP. Second, with this improved assay, we identified a novel subtype specificity for Gβγ modulation of INaP.Gβγ subunits can regulate INaP of Nav1.1 and Nav1.2 channels but not of Nav1.4 or Nav1.5 channels. However, the CT of Nav1.5, but not that of Nav1.4, can support modulation by Gβ2γ3 when attached to Nav1.2a. The ability of the Nav1.5 CT to support modulation when incorporated in a chimera with Nav1.2a but not in Nav1.5 highlights the importance of the pre-CT portion of the channel in permitting modulation by Gβγ acting via the CT. Finally, our improved methods allowed analysis of deletion mutants to directly identify the site of Gβγ action in the CT. Surprisingly, these results implicate the final 28 amino acids of Nav1.2 in Gβγ binding and regulation. We propose that this short segment forms part of the Gβγ interaction site and plays an important role in modulation of channel gating.
Gβγ subunits do not greatly alter the voltage dependence of activation or inactivation of INaT. In contrast, the voltage dependence of inactivation of INaP is shifted positively with respect to INaT. These results are consistent with a mechanism in which Gβγ generates INaP by destabilizing fast inactivation and switching a fraction of sodium channels to a noninactivating gating mode with slowed and positively shifted inactivation (Ma et al., 1997). The molecular basis for this might be Gβγ-induced destabilization of binding of the inactivation gate to its receptor.
Molecular determinants of Gβγ modulation in the C terminus
Ma et al. (1997) proposed that Gβγ bound to the CT of the Nav1.2 channel to modulate INaP, because its effect was blocked by a peptide containing the QMEER sequence of the CT. Recent biochemical evidence shows that Gβγ indeed binds to Nav1.2 sodium channel α subunits (Marin et al., 2001) and to the CT of the Nav1.3 α subunit (Lenkowski et al., 2004). These findings are consistent with an essential role for the CT in Gβγ modulation of INaP. Peptides containing a similar QxxER motif reduced Gβγ modulation of other target proteins, including calcium channels (Chen et al., 1995; Herlitze et al., 1997; Zamponi et al., 1997). Such peptides are expected to bind Gβγ subunits and inhibit their action. However, we show that the QxxER motif is unnecessary for modulation of Nav1.2 by coexpressed Gβγ. Perhaps the QxxER motif tethers and localizes G-protein βγ subunits near their site of action in high local concentration, enhancing Gβγ effects at low levels of G-protein activation.
Our results show that the last 28 amino acids of rNav1.2 are necessary for Gβγ modulation of Na+ channels. Modulation was lost when the CT of rNav1.2a was replaced with that of rNav1.4 or when the CT of Nav1.2 was truncated at K1890. Addition of only the last 28 amino acids of the rNav1.2a CT to this mutant restored Gβγ modulation. Thus, these 28 residues are sufficient for modulation of rNav1.2a by Gβγ, and the intervening residues between K1890 and S1977 are unnecessary.
The distal CTs of sodium channels contain other motifs of potential importance in regulation of channel function, localization, and degradation. An IQ domain starting at position 1912 in Nav1.2 has been implicated in interactions of calmodulin with several sodium channel isoforms (Deschenes et al., 2002; Tan et al., 2002; Herzog et al., 2003; Kim et al., 2004). Just N-terminal to the site of G-protein regulation, a sequence (PPSY) is implicated in interaction of Nedd4 ubiquitin ligase and is present in Nav1.1, Nav1.2, and Nav1.5 but not in Nav1.4 (van Bemmelen et al., 2004). In addition, the final three amino acids of Nav1.4 and Nav1.5 are potential interaction sites for PDZ (postsynaptic density-95/Discs large/zona occludens-1) proteins such as the syntrophin family of dystrophin-associated proteins, which may contribute to channel localization in cardiac and skeletal muscle (Gee et al., 1998). These processes could potentially interact with regulation by G-proteins.
Comparison of regulation of INaP in transfected cells and neurons
INaP in neurons is generally smaller than INaP in transfected tsA-201 cells, even without modulation by Gβγ. The ratio of INaP to INaT reported here would probably be incompatible with normal neuronal function in vivo (Kearney et al., 2001). Evidently, other regulation in neurons limits INaP. Nevertheless, INaP in neurons resembles INaP produced by Gβγ in transfected cells in several respects. Activation of INaP of sodium channels expressed in tsA-201 cells is shifted negatively relative to INaT (data not shown), as it is in neurons (Brown et al., 1994; Mantegazza et al., 1998; Taddese and Bean, 2002). In addition, INaP in both neurons and tsA cells has positively shifted voltage dependence of inactivation relative to INaT. Our results with transfection of single sodium channel isoforms demonstrate that INaP can result from modulation of the same sodium channels that produce INaT (Ma et al., 1997). Thus, our working hypothesis is that Gβγ modulation is an important regulatory influence on INaP in neurons.
The persistent current in a neuron is probably produced by a mixture of sodium channel subtypes with individual propensities for producing INaP. The sodium channel α subunit(s) responsible for INaP has not been identified unequivocally in any central neuron, but it has been proposed that Nav1.6 is the primary contributor to INaP in cerebellar Purkinje neurons and in prefrontal cortex pyramidal neurons (Raman et al., 1997; Maurice et al., 2001). In addition to Nav1.6, the other primary brain Na+ channel α subunit isoforms, Nav1.1, Nav1.2, and Nav1.3, all can produce INaP. Their relative contributions could be modulated according to cell expression pattern and/or degree of neuromodulation of individual channel subtypes.
Modulation of persistent sodium current in neurons
Dynamic modulation of INaP would dramatically modify integrative properties of neurons. Activation of muscarinic acetylcholine receptors in hippocampal neurons reduces both INaP and INaT to comparable extents via protein kinase C signaling (Cantrell et al., 1996). There have been few reports of positive modulation of INaP in neurons (see Introduction). Increase of INaP by dopamine has been observed in prefrontal cortex (Gorelova and Yang, 2000) (but see Maurice et al., 2001). Modulation by free Gβγ in neurons might occur locally in membrane microdomains (Galbiati et al., 2001) strategically positioned to affect action potential generation in the axon hillock, action potential back-propagation at dendritic branches, or other localized electrical events. Such tightly delimited modulation might be critically important for integrative properties of the neuron but would make little contribution to whole-cell sodium current. Additional studies of sodium channel modulation by G-proteins in vitro may provide methods to probe the mechanism and significance of this form of modulation in intact neurons more incisively.
Footnotes
This work was supported by National Institutes of Health (NIH) Research Grant NS34801 to T.S., a Human Frontiers Science Program Fellowship to M.M., a grant from the Fondazione Pierfranco e Luisa Mariani to M.M., and NIH Research Grant NS25704 to W.A.C. We thank Dr. Mel Simon for the kind gift of plasmids encoding G-protein subunits.
Correspondence should be addressed to Dr. Todd Scheuer, Department of Pharmacology, Mailstop 357280, University of Washington School of Medicine, Seattle, WA 98195-7280. E-mail: scheuer@u.washington.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253341-09$15.00/0
M.M. and F.H.Y. contributed equally to this work.
References
- Alonso A, Llinas RR (1989) Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342: 175-177. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE (1993) Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci 13: 660-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Alroy G, Yaari Y (1997) Modulation of endogenous firing patterns by osmolarity in rat hippocampal neurones. J Physiol (Lond) 502: 175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Schwindt PC, Crill WE (1994) Different voltage dependence of transient and persistent Na+ currents is compatible with modal-gating hypothesis for sodium channels. J Neurophysiol 71: 2562-2565. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA (2000) Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci 20: 4829-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WR, Xie XM, Romanos MA, Clare JJ (2002) Molecular cloning, distribution and functional analysis of the Nav1.6. Voltage-gated sodium channel from human brain. Brain Res Mol Brain Res 103: 80-90. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Ma JY, Scheuer T, Catterall WA (1996) Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron 16: 1019-1026. [DOI] [PubMed] [Google Scholar]
- Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13-25. [DOI] [PubMed] [Google Scholar]
- Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty DJ, Blank JL, Exton JH, Stoffel RH, Inglese J, Lefkowitz RJ, Logothetis DE, Hildebrandt JD, Iyengar R (1995) A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits. Science 268: 1166-1169. [DOI] [PubMed] [Google Scholar]
- Clare JJ, Tate SN, Nobbs M, Romanos MA (2000) Voltage-gated sodium channels as therapeutic targets. Drug Discov Today 5: 506-520. [DOI] [PubMed] [Google Scholar]
- Cormier JW, Rivolta I, Tateyama M, Yang A-S, Kass RS (2002) Secondary structure of the human cardiac Na+ channel carboxy terminus: evidence for a role of helical structures in modulation of channel inactivation. J Biol Chem 277: 9233-9241. [DOI] [PubMed] [Google Scholar]
- Crill WE (1996) Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349-362. [DOI] [PubMed] [Google Scholar]
- Deschenes I, Trottier E, Chahine M (2001) Implication of the C-terminal region of the α-subunit of voltage-gated sodium channels in fast inactivation. J Membr Biol 183: 103-114. [DOI] [PubMed] [Google Scholar]
- Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF (2002) Isoform-specific modulation of voltage-gated Na+ channels by calmodulin. Circ Res 90: E49-E57. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP (2001) Emerging themes in lipid rafts and caveolae. Cell 106: 403-411. [DOI] [PubMed] [Google Scholar]
- Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC (1998) Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci 18: 128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA (2000) Nomenclature of voltage-gated sodium channels. Neuron 28: 365-368. [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR (2000) Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol 84: 75-87. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW (1998) Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol (Lond) 510: 735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW (1999) Nitric oxide increases persistent sodium current in rat hippocampal neurons. J Physiol (Lond) 520: 451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW (2000) Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol (Lond) 529: 107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA (1996) Modulation of Ca2+ channels by G-protein βγ subunits. Nature 380: 258-262. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Hockerman GH, Scheuer T, Catterall WA (1997) Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel α1A subunit. Proc Natl Acad Sci USA 94: 1512-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Liu C, Waxman SG, Cummins TR (2003) Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J Neurosci 23: 8261-8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117: 500-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JA, Plummer NW, Smith MR, Kapur J, Cummins TR, Waxman SG, Goldin AL, Meisler MH (2001) A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience 102: 307-317. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS (2004) Calmodulin mediates Ca2+ sensitivity of sodium channels. J Biol Chem 279: 45004-45012. [DOI] [PubMed] [Google Scholar]
- Lenkowski PW, Stevens EB, Yusaf SP, Patel MK (2004) G-protein β1γ2 subunits directly bind to the C-terminus of Nav1.3 and modulate channel properties. Biophys J 87a: 881. [Google Scholar]
- Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA (1998) Interaction of batrachatoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci USA 95: 13947-13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Catterall WA, Scheuer T (1997) Persistent sodium currents through brain sodium channels induced by G protein βγ subunits. Neuron 19: 443-452. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Franceschetti S, Avanzini G (1998) Anemone toxin (ATX II)-induced increase in persistent sodium current: effects on the firing properties of rat neocortical pyramidal neurones. J Physiol (Lond) 507: 105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Catterall WA, Scheuer T (2001) Role of the C-terminal domain in inactivation of brain and cardiac sodium channels. Proc Natl Acad Sci USA 98: 15348-15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, McHendry-Rinde B, Horn R (1993) Panning transfected cells for electrophysiological studies. Biotechniques 15: 906-911. [PubMed] [Google Scholar]
- Marin P, Fagni L, Torrens Y, Alcaraz G, Couraud F, Bockaert J, Glowinski J, Premont J (2001) AMPA receptor activation induces association of G-beta protein with the alpha subunit of the sodium channel in neurons. Eur J Neurosci 14: 1953-1960. [DOI] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ (2001) D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci 21: 2268-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoike HK, Liu H, Glaaser IW, Yang AS, Tateyama M, Kass RS (2004) The Na+ channel inactivation gate is a molecular complex: a novel role of the COOH-terminal domain. J Gen Physiol 123: 155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D, Driesang RB (1998) Two types of intrinsic oscillations in neurons of the lateral and basolateral nuclei of the amygdala. J Neurophysiol 79: 205-216. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V (1998) Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci 18: 854-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Bierlaagh MA, Geurtsen AM (1997) Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. J Neurophysiol 78: 1811-1825. [DOI] [PubMed] [Google Scholar]
- Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA (1994) Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci USA 91: 3289-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP (1997) Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19: 881-891. [DOI] [PubMed] [Google Scholar]
- Rees S, Coote J, Stables J, Goodson S, Harris S, Lee MG (1996) Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques 20: 102-112. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE (1995) Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol 74: 2220-2224. [DOI] [PubMed] [Google Scholar]
- Segal MM, Douglas AF (1997) Late sodium channel openings underlying epileptiform activity are preferentially diminished by the anticonvulsant phenytoin. J Neurophysiol 77: 3021-3034. [DOI] [PubMed] [Google Scholar]
- Smith RD, Goldin AL (1998) Functional analysis of the rat I sodium channel in Xenopus oocytes. J Neurosci 18: 811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G (1999) Voltage-activated sodium channels amplify inhibition in neocortical pyramidal neurons. Nat Neurosci 2: 144-150. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B (1995) Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron 15: 1065-1076. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP (2002) Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 33: 587-600. [DOI] [PubMed] [Google Scholar]
- Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, Anderson ME, Balser JR (2002) A calcium sensor in the sodium channel modulates cardiac excitability. Nature 415: 442-447. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4623-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Cooperman SS, Tomiko SA, Zhou JY, Crean SM, Boyle MB, Kallen RG, Sheng ZH, Barchi RL, Sigworth FJ (1989) Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron 3: 33-49. [DOI] [PubMed] [Google Scholar]
- Trouet D, Nilius B, Voets T, Droogmans G, Eggermont J (1997) Use of a bicistronic GFP-expression vector to characterise ion channels after transfection in mammalian cells. Pflügers Arch 434: 632-638. [DOI] [PubMed] [Google Scholar]
- van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA, Kass RS, Staub O, Abriel H (2004) Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2 mediated ubiquitination. Circ Res 95: 284-291. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP (1997) Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature 385: 442-446. [DOI] [PubMed] [Google Scholar]