Abstract
P2X3 receptors desensitize within 100 ms of channel activation, yet recovery from desensitization requires several minutes. The molecular basis for this slow rate of recovery is unknown. We designed experiments to test the hypothesis that this slow recovery is attributable to the high affinity (< 1 nm) of desensitized P2X3 receptors for agonist. We found that agonist binding to the desensitized state provided a mechanism for potent inhibition of P2X3 current. Sustained applications of 0.5 nm ATP inhibited >50% of current to repetitive applications of P2X3 agonist. Inhibition occurred at 1000-fold lower agonist concentrations than required for channel activation and showed strong use dependence. No inhibition occurred without previous activation and desensitization. Our data are consistent with a model whereby inhibition of P2X3 by nanomolar [agonist] occurs by the rebinding of agonist to desensitized channels before recovery from desensitization. For several ATP analogs, the concentration required to inhibit P2X3 current inversely correlated with the rate of recovery from desensitization. This indicates that the affinity of the desensitized state and recovery rate primarily depend on the rate of agonist unbinding. Consistent with this hypothesis, unbinding of [32P]ATP from desensitized P2X3 receptors mirrored the rate of recovery from desensitization. As expected, disruption of agonist binding by site-directed mutagenesis increased the IC50 for inhibition and increased the rate of recovery.
Keywords: purinergic, desensitization, recovery, ATP, nociceptor, ligand-gated
Introduction
Over the last several decades, research has focused on the mechanisms involved in the desensitization of ligand-gated ion channels (Quick and Lester, 2002). However, recovery from desensitization has received comparatively little attention. P2X receptors represent an attractive model system for the study of desensitization and recovery (North, 2002). For P2X3 receptors, desensitization and recovery differ greatly in kinetic rates. Brief application of micromolar ATP results in a rapid inward current that desensitizes in <30 ms (Pankratov Yu et al., 2001). In contrast, recovery of P2X3 current takes >10 min and is >10,000-fold slower than the rate of desensitization (τdesens) (Cook et al., 1998). The reason for such slow recovery is unknown, but it is likely an intrinsic property of the channel (Cook et al., 1998; Alexander et al., 1999; Fabbretti et al., 2004). Using site-directed mutagenesis and chimeric P2X receptors, researchers have identified several regions that influence the rate at which P2X receptors desensitize (Werner et al., 1996; Fabbretti et al., 2004; Zemkova et al., 2004). However, desensitization is not simply a reversal of recovery because mutations of P2X receptors that affect the rate of desensitization can have little effect on the rate of recovery.
Experiments indicate that P2X recovery from desensitization may be linked to agonist structure. In rat sensory neuron cultures, the rate of P2X recovery from desensitization depends on the agonist used to activate the channel (Sokolova et al., 2004). This finding was interpreted as evidence for multiple desensitized states with more potent agonists favoring entry into longer-lived desensitized states. This scheme is analogous to one proposed for nicotinic acetylcholine receptors (Elenes and Auerbach, 2002; Paradiso and Steinbach, 2003). In this model, the rate-limiting step to recovery from desensitization is a conformational change of the receptor that does not involve agonist dissociation. Alternatively, agonist dependence of recovery could be explained if agonist unbinding is the rate-limiting step to recovery from desensitization. If true, this predicts that the slow recovery of P2X3 receptors is caused by the high affinity for agonist of the desensitized state.
We explored the relationship between agonist binding and recovery from desensitization using human, rat, and mutant P2X3 receptors. Our data are consistent with the hypothesis that P2X3 recovery from desensitization requires two steps. The first step is a slow unbinding of agonist and is rate limiting for recovery. The second step is a rapid conformational change that is independent of agonist. As evidence for this mechanism, we find that a high-affinity binding site on P2X3 is exposed during recovery from desensitization. Agonist binding to this site effectively “traps” the receptor in the desensitized state. As predicted from this hypothesis, the rate of unbinding of ligand from this site, and thus the overall rate of recovery, depends on the affinity of a given ligand for the desensitized receptor.
Materials and Methods
Tissue culture and transfections. Human embryonic kidney 293 (HEK293) cells (American Type Culture Collection, Manassas, VA) were grown in F12/DMEM (Invitrogen, San Diego, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA) at 37°C in 5% CO2/95% air. New thaws were started every 6 weeks. Stable HEK lines containing the human P2X3 receptor (HEK-P2X3) (generously provided by A. Surprenant, Institute of Molecular Physiology, University of Sheffield, Sheffield, UK) were maintained in 300 μg/ml G418 (Sigma, St. Louis, MO). For electrophysiological recording, the cell lines were plated at <10% confluency. Plasmids containing sequences coding for rat wild-type (P2X3-p481; Glaxo Wellcome, Research Triangle Park, NC) and mutant (K65R; constructed by Mark Voigt, St. Louis University) P2X3 receptors were introduced along with a plasmid containing green fluorescent protein (Invitrogen) into HEK293 cells using FuGENE 6 transfection reagent (Roche Diagnostics, Basel, Switzerland) according to manufacturer's instructions. Transfected cells were identified using fluorescent optics on an Olympus (Melville, NY) IX70 inverted microscope. Cells were used for electrophysiology 24 h after transfection.
Electrical recording. Whole-cell currents were recorded with a patch-clamp amplifier (Multiclamp 700B; Molecular Devices, Union City, CA). Unless indicated, holding and test potential was -60 mV. Normal internal solution contained the following (in mm): 55 KCl, 60 K2SO4, 7 MgCl2, 10 EGTA, 10 HEPES, pH 7.4 with KOH. Control extracellular solutions contained the following (mm): 135 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 glucose, 10 HEPES, pH 7.4 with NaOH. Control and test solutions perfused the vicinity of the cell through 1 or 10 μl pipettes with flow controlled by computer-operated solenoid valves. Solution exchange (10-90%) typically took <20 ms. Low (nanomolar) [ATP] were applied 1-2 s after the activating (30 or 100 μm) concentration of nucleotide and left on for the time indicated. Unless indicated otherwise, the control solution bathed the cells between activating ATP applications. For rapid solution exchange over the entire cell, it was necessary to remove the cell from the bottom of the dish, or P2X3 desensitization rates were significantly slower.
ATP unbinding. To assay the rate of release of ATP from the P2X3 receptor, nontransfected HEK293 cells or transiently transfected HEK-P2X3 cells (both grown to 70% confluence) were incubated at room temperature with 1 ml of extracellular solution containing 1 nm [32P]ATP (specific activity, 6000 Ci/mmol; NEN, Boston, MA), 20 nm GTP, and 5 nm ADP. (ADP and GTP were included to decrease nonspecific binding.) After a 15 min incubation, the unbound [32P]ATP was removed by washing five times with 1 ml of ice-cold extracellular solution containing 10 μm nonradioactive ATP. After incubation, the amount of released [32P]ATP was determined by removal of 100 μl of the bath solution at the times indicated and counting in a scintillation counter (LS 6500; Beckman Instruments, Fullerton, CA). One hundred microliters of fresh bath solution were added to replace the test media. A correction was made in subsequent analysis for the volume of solution removed. At the end of the incubation, 100 μl of a 1% Triton X-100 solution was added to the culture dish. The cells were triturated gently in the detergent solution and the resulting suspension placed in a Microfuge tube. Cell suspensions were spun at 10,000 × g for 10 min, and a sample of the supernatant was removed for protein determination.
Analysis. The equation It = Imax[1 - exp(-t/τ)]2 (Cook et al., 1998) was fit to recovery and unbinding data using the program NFIT (University of Texas Medical Branch, Galveston, TX), a least-squares algorithm. Rates of entry into the desensitized state (τentry) by 3 nm ATP and αβ methyleneATP (αβmeATP) (see Fig. 6) or by 100 μm ATP (see Fig. 1) were determined by fitting with a single-exponential equation. [A second, slower component to desensitization onset (Fig. 1) was detected for all clones, but because of its small size, its contribution to τentry was insignificant.] Calculated Imax was used to normalize the recovery data for multiple agonists. IC50 and EC50 values were determined using the Hill equation. Other data acquisition and analysis used pClamp 8 (Molecular Devices) and Origin 6 (Microcal Software, Northampton, MA). Chemicals were from Sigma.
Figure 6.

Kinetics of onset and offset of inhibition by nanomolar agonist. A, CTP (100 μm) was applied for 0.5 s at 15 s intervals to activate and desensitize hP2X3 current. This protocol results in a stable inward current (first 3 deflections). Application of 3 nm ATP (120 s) decreased current to CTP > 95% within 100 s (n = 12). After washout of ATP, the current to CTP recovered. Onset of inhibition was faster than offset. B, Inhibition by nanomolar ATP was use dependent. Application of 3 nm ATP (120 s) did not inhibit current when applied without CTP-induced channel activation and subsequent desensitization (n = 8). C, The ATP analog αβmeATP (3 nm for 120 s) was less effective and had a slower onset than ATP at inhibiting current (n = 11). Current recovery from αβmeATP-induced inhibition was faster than with ATP.
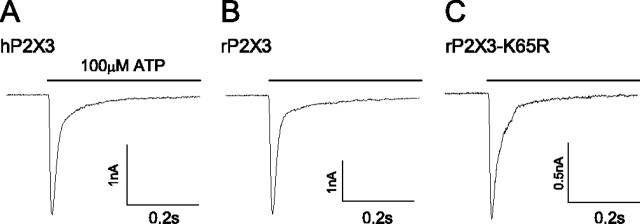
Figure 1.
Representative currents from wild-type and mutant P2X3 receptors. P2X3 receptors from human (A), rat (B), and rat K65R mutation (C) were expressed in HEK cells and currents recorded at -60 mV using whole-cell patch-clamp methods. ATP (100 μm; 0.5 s) application produced a rapidly desensitizing inward current. Mutation of a lysine residue (K65R) slowed the rate of desensitization by twofold (τentry = 26 ± 3 ms; n = 8) when compared with wild-type rat (τentry = 13 ± 1 ms; n = 10) or human (τ = 12 ± 1 ms; n = 8) P2X3 receptors.
Results
P2X3 recovery depends on agonist structure
Human, rat, and mutant P2X3 receptors gave similar currents in response to brief (0.5 s) applications of 100 μm ATP. Current decayed during the ATP application as a result of rapid receptor desensitization (Fig. 1). Desensitization was long lived; reapplication of ATP within 30 s of the first application resulted in no current (Fig. 2). Recovery from desensitization required several minutes. With respect to rates of activation and desensitization, we saw no obvious differences in the wild-type human P2X3 (hP2X3) and rat P2X3 (rP2X3) receptors. However, recovery from desensitization was 1.5-fold slower for rat than for human P2X3 receptors (Table 1). The recovery of cloned rP2X3 was approximately twofold faster than reported for native sensory neurons (Cook et al., 1998). The reason for differences in rate of recovery for species and expression system are not known.
Figure 2.
P2X3 recovery from desensitization depends on agonist structure and is speeded by a binding-site mutation. Saturating (30 μm) ATP (filled circles), αβmeATP (open circles), ATPγS (squares), or CTP (100 μm; triangles) were applied to human (left), rat (middle), or the mutant P2X3-K65R (right) P2X3 receptors to induce desensitization. Current recovery was monitored by reapplication of agonist at the indicated intervals. Note change of time scale for P2X3-K65R curve (right). Data are expressed as a percentage of maximal recovery determined by a fit of the data (see Materials and Methods; n = 4-10 cells for each data point). Error bars represent SEM.
Table 1.
EC50 values and recovery rates for native and mutant P2X3 receptors
|
|
ATP |
αβmeATP |
ATPγS |
CTP |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
EC50 (μm) |
τrecovery (S) |
EC50 (μm) |
τrecovery (S) |
EC50 (μm) |
τrecovery (S) |
EC50 (μm) |
τrecovery (S) |
||||
| Human P2X3 | 1.6 ± 0.1 | 149 ± 4 | 2.4 ± 0.2 | 45 ± 3 | 2.3 ± 0.1 | 16 ± 1 | 17.3 ± 0.3 | 4 ± 1 | ||||
| Rat P2X3 | 2.6 ± 0.9 | 224 ± 10 | 2.7 ± 0.1 | 62 ± 3 | 2.3 ± 0.2 | 16 ± 1 | ND | ND | ||||
| Rat P2X3-K65R |
3.6 ± 0.4 |
18 ± 1 |
17 ± 0.8 |
19 ± 1 |
15 ± 1 |
7 ± 1 |
ND |
ND |
||||
EC50 values were determined in HEK cells transfected with DNA coding for wild-type human P2X3 receptors, rat P2X3 receptors, or single-site mutant (P2X3-K65R) rat receptors. To calculate EC50 values, agonists were varied from 0.3 to 1000 μm. EC50 was defined as the concentration of agonist required to evoke half-maximal peak current. EC50 and recovery rate values were calculated by fitting the data as described in Materials and Methods (n = 5-12 determinations for each). Error is expressed as the SD of a least squares fit to data. ND, Not determined.
The dependence of desensitization on agonist structure was tested using three other P2X3 agonists (CTP, αβmeATP, and ATPγS) (Table 1, Fig. 2). All agonists gave similar maximal currents and indistinguishable rates of desensitization (τdesens < 20 ms; data not shown). However, recovery from desensitization depended strongly on agonist structure and suggested that agonist affinity limited recovery. Human P2X3 recovered 3.3-, 9.3-, and 37-fold faster with αβmeATP, ATPγS, and CTP, respectively, than when activated by similar [ATP] (Table 1). If differences in agonist affinity are dominated by differences in agonist off-rate, then we might expect that agonists that promote faster recovery should have higher EC50 values for activation. CTP promoted the fastest recovery and had a 10-fold higher EC50 than ATP. However, despite the large differences in recovery rates, EC50 values for ATP, αβmeATP, and ATPγS did not differ significantly. These results demonstrated that the efficacy of activation and the rate of desensitization were not strictly correlated with the rate of recovery from desensitization.
A binding-site mutation speeds recovery
A different approach was used to test the hypothesis that agonist binding influences recovery from desensitization. A lysine residue at position 65 was changed to an arginine (P2X3-K65R) in rP2X3 with the goal of disrupting the agonist binding site. This mutation corresponds to a residue that forms the ATP binding site of other P2X receptor subtypes (Jiang et al., 2000; Roberts and Evans, 2004). With 100 μm ATP as agonist, peak current from P2X3-K65R was similar in onset kinetics to current of the wild-type receptor and, like wild-type P2X3, yielded several nanoamps of current (Fig. 1). The average current for wild-type rP2X3 and P2X3-K65R when fully recovered was 9.1 ± 1.2 nA (n = 18) and 10.0 ± 2.3 nA (n = 10), respectively. P2X3-K65R current decayed twofold slower than wild-type P2X3 (τdesens = 13 ± 1 and 26 ± 3 ms for wild-type and P2X3-K65R, respectively; p < 0.001; n = 8 and 10 cells). In contrast to the mild effects P2X3-K65R had on the rates of current activation and desensitization, the mutation strongly altered recovery from desensitization (Fig. 2, bottom). P2X3-K65R recovered 12-fold faster than the wild-type rat P2X3 receptor with ATP as agonist. However, the mutation had no significant effect on the EC50 for activation, consistent with a lack of direct correlation between EC50 and recovery rate. The mutation also increased to a lesser extent the recovery rate when αβmeATP and ATPγS were used as agonists. P2X3-K65R recovered 3.3-fold and 2.3-fold faster than the wild-type receptor when desensitized with αβmeATP and ATPγS, respectively. In contrast to the lack of effect on EC50 for ATP, the EC50 for αβmeATP and ATPγS increased approximately sixfold for P2X3-K65R. These data illustrate that mutation near the proposed binding site for ATP can decrease the potency of agonists for channel activation and speed the rate of recovery from desensitization, yet the data also illustrate a complex relationship between these two properties.
Although changes in EC50 by amino acid mutation could result from altered conformational transitions that do not involve ligand binding (e.g., gating, desensitization, or recovery), the effects of the P2X3-K65R mutation were consistent with binding site disruption. For example, the P2X3-K65R mutation caused a 6.5-fold increase in the EC50 for activation with ATPγS and αβmeATP but caused no significant increase in EC50 for ATP (Table 1). Likewise, the change in recovery rate was more pronounced for ATP (>12-fold) than ATPγS (less than threefold). If the P2X3-K65R mutation affected conformational transitions such as gating or recovery, one might expect similar changes for all agonists, because these steps do not involve agonist binding and unbinding. Moreover, the differential effects of P2X3-K65R mutation on ATP analogs with phosphate group modifications is consistent with this amino acid residue contributing to phosphate recognition (Roberts and Evans, 2004). Our data provide no evidence for global effects caused by the P2X3-K65R mutation, because the desensitization rate and current expressed were similar in mutant and wild-type receptors (Fig. 1).
Agonist unbinds slowly from P2X3 receptor
A possible explanation for agonist-dependent recovery is that agonist remains bound to the desensitized receptor. To test this hypothesis, cells expressing the hP2X3 receptor were grown in culture and 1 nm [32P]ATP was applied for 15 min to label high-affinity binding sites (Fig. 3). Such long applications of agonist can induce P2X desensitization at nanomolar [agonist] (Rettinger and Schmalzing, 2003). Unbound and low-affinity binding of [32P]ATP was avoided by brief wash with unlabeled nucleotide. The rate at which [32P]ATP was released from the hP2X3-expressing cells (τunbinding = 213 ± 15 s) was similar to the rate of recovery from desensitization as determined by electrophysiological methods (Fig. 2). Cells that did not express P2X3 bound little [32P]ATP. The slow unbinding of [32P]ATP from the P2X3 receptor provides strong evidence that agonist remains bound to the receptor while it is desensitized. Furthermore, the similarity of the kinetics of unbinding and recovery suggest that these two processes are mechanistically linked.
Figure 3.
The dissociation rate of ATP from the hP2X3 receptor follows the rate of recovery. HEK cells expressing the hP2X3 receptor (filled symbols) or nontranfected HEK cells (open symbols) were incubated with 1 nm [32P]ATP. Unbound [32P]ATP was washed away, and the amount of released [32P]ATP was determined by sampling the bath solution at the times indicated. Released ATP was expressed as a function of total [protein]. The time course of [32P]ATP release was fit with the same equation as recovery from desensitization (see Materials and Methods) and followed a similar time course. Nontransfected cells released little [32P]ATP. Each point represents the mean ± SEM for four independent experiments.
P2X3 inhibition by nanomolar agonist
A large increase in agonist affinity at the ATP-binding site during desensitization could explain the weak correlation between EC50 for activation and recovery rate. If this hypothesis is correct, the desensitized receptor should respond to [ATP] that are far lower than the EC50 for activation. To test this hypothesis, we desensitized P2X3 receptors by brief applications of a saturating [ATP] (30 μm for 0.5 s) at 1 min intervals (Fig. 4A). As a result of slow recovery from desensitization, this protocol gives a reproducible current that is ∼6% of the maximal current to ATP (average current at 1 min interval, 560 ± 60 pA; n = 9) (Fig. 2). During the entire interval (≈60 s) between ATP applications, 0.5-10 nm ATP was applied. This low [ATP] suppressed the activity of P2X3, with preincubation for 60 s of 0.5 nm ATP inhibiting >50% of P2X3 current. No additional decrease in current was seen during the next 60 s of ATP. Although able to fully suppress subsequent P2X3 current, 10 nm [ATP] promoted no detectible current and thus little simultaneous channel opening (Fig. 4B). These results indicate the presence of a high-affinity site for ATP on the desensitized P2X3 receptor.
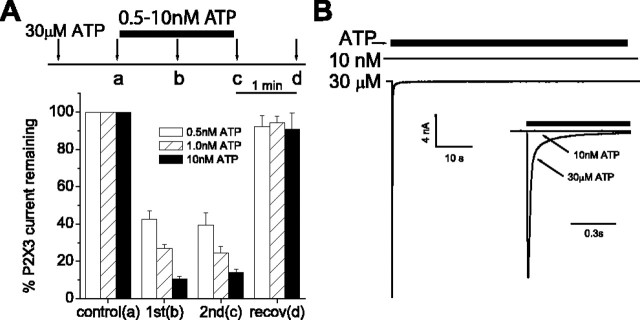
Figure 4.
Nanomolar [ATP] inhibit the desensitized, but not recovered, P2X3 receptor. A, Saturating [ATP] (30 μm for 0.5 s; arrows) was applied at 1 min intervals. During the 2 min interval between a and c, a low [ATP] (0.5-10 nm) was washed onto the cell. This treatment decreased the current to a similar extent at 1 min (b) and 2 min (c) after the nm ATP wash. Current recovered (recov) completely from inhibition when ATP was removed from the wash during the interval between c and d. B, ATP (solid bar) at 10 nm produced no current when applied for 60 s to a cell expressing hP2X3 (top trace). Immediately after the cessation of 10 nm ATP, the cell was washed for 30 s with an ATP-free solution. ATP (30 μm) applied after washout produced a large inward current (bottom trace), indicating that the cell was responsive to higher [ATP]. The two currents were superimposed on a shorter time scale (inset). Data are expressed as mean peak currents ± SEM (n = 10-12).
State dependence of inhibition
The previous experiment does not differentiate whether nanomolar ATP suppresses recovery from the desensitized state or promotes desensitization after channel recovery. If the latter hypothesis is true, P2X3 current should be reduced by nanomolar ATP when recovered receptors are most abundant. To ensure maximal numbers of recovered P2X3 receptors, we applied 10 nm ATP to cells that had not been exposed previously to 30 μm ATP (data not shown). We saw no significant inhibition of current with this treatment. Mean current was 6.7 ± 1.0 nA without ATP pretreatment and 5.6 ± 0.6 nA with a 60 s, 10 nm ATP pretreatment (n = 10). In contrast, 10 nm ATP suppressed >90% of P2X3 current when applied for the same duration to P2X3 receptors that were desensitized with repetitive P2X3 activation (Fig. 4A). These findings demonstrate that recovered P2X3 receptors do not desensitize on this time scale when exposed to nanomolar ATP and suggest that the high-affinity binding site is only available after P2X3 desensitization.
To further test the state dependence of ATP sensitivity, we examined the inhibition of P2X3 receptors by nanomolar ATP during and after recovery. This experiment took advantage of the agonist dependence of recovery rate. P2X3 channels were activated at 60 s intervals by a saturating concentration (100 μm) of ATPγS (Fig. 5). Channels recover from ATPγS desensitization quickly; 72% recover during the first 30 s of the interval (Fig. 2). By changing the timing of the nanomolar ATP applications, ATP should act on mostly desensitized (nanomolar ATP applied during first 30 s) or mostly recovered (nanomolar ATP during second 30 s) P2X3 receptors. Because only 2% of channels desensitized with ATP recover in 30 s (estimated from Fig. 2), any channel that binds ATP should remain desensitized and result in decreased current to subsequent ATPγS application. The amount of inhibition should reflect the sensitivity of recovered and desensitized states. Figure 5C shows that P2X3 receptors are >10-fold more sensitive to ATP in the first 30 s of the interval when compared with the last 30 s. Only when [ATP] is ≥30 nm does significant inhibition of current occur during the second 30 s. This temporal sensitivity of P2X3 to low [ATP] suggests that the high-affinity site is preferentially exposed in the desensitized state.
Figure 5.
Potency of P2X3 inhibition diminishes as channels recover from desensitization. A, 100 μm ATPγS (arrows; first application not shown) was applied for 0.5 s at 60 s intervals to HEK cells that expressed the hP2X3 receptor. During the first or second half of the interval between applications b and c, 1-100 nm ATP was applied for 30 s. Inhibition was monitored as the fraction of current induced after the test ATP treatment (current at c/current at b). B shows a representative experiment in which 10 nm ATP was applied “early” in the interval. The current to subsequent 100 μm ATPγS (c) was diminished. (Current time scale is expanded from A to visualize P2X3 current; axis break, 59 s.) C, Summary of experiments as in B. Application of ATP during the first 30 s of the interval (early; triangles) was more effective at inhibiting P2X3 current than when applied for 30 s immediately before the test ATPγS application (“late”; squares). Data are expressed as average peak current ratios ± SEM (n = 6-11 cells for each data point).
Kinetics and “use dependence” of inhibition
The rapid recovery of P2X3 receptors when activated by CTP (τ = 4 s) allowed us to examine the kinetics of inhibition by ATP binding to the desensitized state (Fig. 6A). A saturating [CTP] was applied at 15 s intervals to fully activate and desensitize the P2X3 receptors. Because CTP promotes almost complete recovery within 15 s (96% of maximal recovery, estimated from Fig. 2), each CTP-induced current reflects opening of nearly all of the P2X3 channels of the cell. Application of 3 nm ATP caused nearly complete inhibition of the CTP-induced current (1.4 ± 0.3% of the initial current remained 90 s after start of ATP application; n = 12) (Fig. 6A). Recovery from inhibition by 3 nm ATP followed a time course similar to recovery after desensitization by 30 μm ATP (Fig. 2). This suggests that recovery originates from the same desensitized state whether desensitization is achieved by binding and trapping in the desensitized state at nanomolar ATP or after channel opening at micromolar ATP (Fig. 2). Although the extent of inhibition was always complete at 3 nm ATP, the current did not always recover to its original level and may reflect current rundown. Inhibition by 3 nm ATP depended on channel activity. If CTP was not applied during the 3 nm ATP application, then we saw no inhibition of current (97 ± 5% of the initial current remained 130 s after start of ATP application; n = 8) (Fig. 6B). The strong use dependence of inhibition is consistent with our hypothesis that the desensitized receptor, but not the recovered receptor, binds nanomolar ATP. If ATP was replaced by 3 nm αβmeATP, inhibition was incomplete and developed more slowly (48.5 ± 3.2% of the initial current remained 150 s after start of αβmeATP application; n = 11; τentry = 25 ± 2 s for ATP; τentry = 48 ± 6 s for αβmeATP) (Fig. 6C). This result indicates that the desensitized state binds some agonists better than others.
Recovery rate depends on affinity for desensitized state
The most straightforward explanation for these data are that the channel has one site (or multiple equivalent sites) that triggers channel opening and that, during desensitization, this site increases its affinity for ligand. Recovery depends on the rate of agonist unbinding from this high-affinity desensitized state. Differences in recovery rate with different agonists reflect differences in agonist affinity for the desensitized state. This model predicts that an agonist with lower affinity for the desensitized state would recover more quickly and require higher concentrations to trap the receptor in the desensitized state. To test this hypothesis, we compared the sensitivity of desensitized receptors to different agonists (Fig. 7A). Channels were activated with 0.5 s of 100 μm ATPγS at 60 s intervals. (More than 95% of the channels should recover in this interval.) This yielded a baseline current. During the entire interval between baseline and test ATPγS applications, the P2X3 receptors were exposed to 0.3-300 nm ATP, αβmeATP, ATPγS, or CTP. This exposure inhibited the subsequent current to ATPγS. When compared with ATP, significantly higher concentrations of CTP, ATPγS, and αβmeATP were required for inhibition. A fit of the concentration-response data for inhibition of P2X3 current to the Hill equation gave values of 0.9 ± 0.1 nm (nhill = 1.0 ± 0.2), 1.8 ± 0.1 nm (nhill = 1.2 ± 0.1), 7.5 ± 0.8 nm (nhill = 1.2 ± 0.1), and 49 ± 4 nm (nhill = 1.6 ± 0.2) for ATP, αβmeATP, ATPγS, and CTP, respectively. The rank order potency of the four agonists was inversely related to the rate of recovery from desensitization (Fig. 7B) and was consistent with the hypothesis that the recovery from desensitization is determined by the affinity of the agonist for the desensitized receptor. A similar analysis of the K65R mutant shows a decrease in the potency of ATP for desensitization and a higher EC50 for activation. These results are expected from a disruption of the agonist-binding site and more rapid ATP unbinding.
Figure 7.
Agonist that promote faster recovery are less potent inhibitors of P2X3 current. A, Half-maximal inhibition occurs at >400-fold lower concentrations than activation. For inhibition curves (filled symbols), 100 μm ATPγS was applied at 60 s intervals to evoke P2X3 current. After a stable current is established, the indicated concentration of nucleotide, ATP (•), αβmeATP (▴), ATPγS(▪), or CTP (▾), was applied for 60 s. The decrease in 100 μm ATPγS-induced current immediately after the treatment interval is plotted against agonist concentration and fitted with the Hill equation (curves). Dose-response for activation of P2X3 current is plotted on the same graph (open symbols). Dose-response data were collected at 60 s intervals for all agonists except ATP (120 s interval). Experiment was repeated using the binding site mutant P2X3-K65R and ATP as desensitizing (•) or activating (○) agonist (dashed lines). B, IC50 values for inhibition of current positively correlate to the rate of recovery from desensitization. Like low-potency agonists, the K65R mutation increases rate of recovery and decreases ability to promote desensitization. Error bars represent SEM.
Discussion
Agonist affinity at the ATP-binding site of P2X receptors has been estimated by comparing efficacies of various ATP analogs for current activation. However, affinity estimates yield values that are 100-fold to 1000-fold lower than estimates based on equilibrium binding assays (Michel et al., 1996; Lachnit et al., 2000; North and Surprenant, 2000). Our results provide a possible explanation for this inconsistency and suggest that agonist binds to at least two classes of binding sites: (1) a low-affinity (micromolar ATP) activator site on the recovered receptor and (2) a high-affinity (nanomolar ATP) inhibitory site that becomes available after desensitization and when occupied traps the receptor in the desensitized state. The slow unbinding of agonist from this high-affinity site determines the unusually slow rate of recovery of P2X3 from desensitization.
Desensitization by low [agonist] is a common feature of ligand-gated ion channels and supports the existence of a high-affinity desensitized state (Rang and Ritter, 1970a). Nicotinic acetylcholine receptors (nAChR), GABAA receptors, 5-hydroxytryptamine receptors, and P2X1 receptors are desensitized by concentrations of agonist that are lower than those required for channel activation (Katz and Theseleff, 1957; Bartrup and Newberry, 1996; Newell and Dunn, 2002; Paradiso and Steinbach, 2003). We found that 60 s of 0.5 nm ATP inhibited >50% of P2X3 current after activation by high [agonist] (Fig. 4A). This concentration was >1000-fold lower than the EC50 for activation of P2X3 (Table 1) and indicates that exposure to high [agonist] increases ATP affinity.
Without previous exposure to high [agonist], the recovered P2X3 receptor was insensitive to low [ATP] (Figs. 4, 5, 6). This suggests that few desensitized P2X3 receptors exist in the absence of ligand. In contrast, low [ligand] desensitize nAChRs by binding to the significant fraction of receptors that exist in a high-affinity desensitized state in the absence of agonist (Changeux, 1990; Quick and Lester, 2002). Higher [ATP] increase the availability of high-affinity sites by promoting P2X3 desensitization. This creates a use dependence for P2X3 inhibition (Fig. 6B) similar to that seen in other channels (Bartrup and Newberry, 1996; Newell and Dunn, 2002).
For α7-nAChR and P2X1 receptors, a strict correlation exists between channel activity and the extent of desensitization at low [ligand] (Mike et al., 2000; Rettinger and Schmalzing, 2003). These data suggest that channels must open before desensitization. However, not all ligand-gated channels behave in this manner; for α4β2-nAChRs, desensitization at high [ligand] may not require channel opening (Paradiso and Steinbach, 2003). For P2X3, a 30 s exposure to 30 nm ATP (well below the EC50 for activation) desensitized the recovered receptor without inducing macroscopic current (Fig. 5C). Although this suggests that desensitization occurs without opening, the rapid desensitization of P2X3 and slow recovery may obscure opening with long agonist exposures.
It is assumed that the low-affinity activating site transitions to a high-affinity site during desensitization (Newell and Dunn, 2002). Therefore, both forms of the binding site may share similar properties. Agonists that activated P2X3 current inhibited recovery, whereas ADP, a poor agonist at P2X3, produced only a small inhibition (<10% at 10 nm using protocol indicated in Fig. 7). Current activation and ATP-induced trapping were altered by a binding site mutation (Figs. 2, 7) and inhibited by the competitive antagonist TNP-ATP (data not shown). This suggests that the gating site and the high-affinity inhibitory site are physically inseparable.
Our data are consistent with a model whereby recovery from desensitization occurs in two steps. First, agonist unbinds from the desensitized receptor. Second, the receptor recovers from desensitization. The dependence of recovery rate on agonist structure (Fig. 2) and the equivalent time course of recovery and ATP unbinding (Fig. 3) indicates that the first step is rate limiting. Recovery from desensitization followed a sigmoidal time course with a clear initial lag. This lag probably reflects an intrinsic property of the channel as a biexponential equation best fits recovery data with >10-fold differences in time constants (Fig. 2). Sigmoidal recovery occurs for several ligand-gated channels and could result from recovery requiring the release of multiple agonist molecules from interacting binding sites (Mike et al., 2000; Robert and Howe, 2003; Sokolova et al., 2004). This hypothesis is supported by evidence for multiple ATP binding events during P2X channel activation (Chen et al., 1995).
The rate of P2X3 recovery from desensitization is speeded by increased extracellular [Ca2+] or [Gd3+] (Cook et al., 1998; Fabbretti et al., 2004). The increase in recovery rate by Ca2+ was similar to the effect of ATP analogs on P2X3 recovery. It is possible that Ca2+ acts by speeding agonist unbinding from desensitized P2X3.
The agonist-independent recovery step (e.g., conformational change) must be fast relative to the unbinding step and should set an upper limit for the recovery rate (Fig. 7). Thus, it must be faster than the overall recovery observed with CTP (rate, 0.25 s-1) or IC50 and recovery should not correlate (Fig. 7B). (The curvature observed in Fig. 7B could indicate a more significant contribution of this agonist-independent recovery step as the unbinding rate increases.) However, the rate must be slow enough to allow for rebinding of agonist before the conformational change into the low-affinity recovered state, or we would not see inhibition. Separate unbinding and recovery steps can be distinguished in GABA receptor channels in which both steps occur with similar time constants (Chang et al., 2002). For such channels, the relative rates of the unbinding and recovery steps determine whether overall recovery rate depends on agonist structure. For receptors with slower unbinding, such as P2X3, recovery is agonist dependent, whereas receptors that unbind more rapidly than they recover show little agonist dependence.
Recently, agonist-dependent recovery was demonstrated for P2X3 current of rat dorsal root ganglia (DRG) sensory neurons (Sokolova et al., 2004). Current recovery was speeded twofold by αβmeATP versus ATP. We saw a 3.5-fold increase using αβmeATP as agonist for human and rat P2X3 receptors (Table 1). Our model explains the ability of αβmeATP to “protect” DRG P2X3 receptors from ATP-induced desensitization, because ATP exposure should not promote desensitization when the high-affinity site is occupied by αβmeATP (Sokolova et al., 2004).
The agonist dependence of recovery for DRG P2X3 receptors was interpreted as evidence for multiple desensitized states in which ligands that promote slower recovery bind more tightly to longer-lived desensitized states (Sokolova et al., 2004). Indeed, for nAChRs, strong evidence exists for multiple desensitized states (Reitstetter et al., 1999; Mike et al., 2000; Elenes and Auerbach, 2002; Paradiso and Steinbach, 2003). Our data favor a simpler model for P2X3, whereby different recovery rates arise from differences in affinity for a single desensitized state. As evidence for this model, the rate of release of agonist from the desensitized receptor follows the time course of current recovery (Fig. 3). Moreover, recovery rate and IC50 for inhibition correlate for several agonists and for the K65R mutant (Fig. 7B). This correlation is expected if the affinity differences (IC50) reflect differences in off-rates (assuming similar on-rates) from the desensitized receptor. Thus, a decrease in affinity either by changing the binding site (K65R) or by changing the agonist (ATPγS) has equivalent effects on desensitization.
Nicotinic AChR recovery depends on the duration of agonist application (Reitstetter et al., 1999). Longer applications of agonist induce slower recovery and provide evidence for entry into distinct long-lived desensitized states. In contrast, we found no change in the recovery rate when 30 μm ATP was applied for 0.3-30 s (data not shown). In addition, the recovery rate of P2X3 was unaffected by a 10,000-fold difference in [ATP] used to desensitize the receptor. Recovery rate of current after a 3 min application of 3 nm ATP (to recently desensitized receptors) (Fig. 6B) was indistinguishable from recovery after a 0.5 s application of 30 μm ATP (Fig. 2). Similarly, a 10-fold increase in [agonist] had little effect on recovery rate of P2X current from DRG (Sokolova et al., 2004). These data show that we were unable to induce kinetically distinguishable desensitized states by changing either the exposure duration or concentration of agonist.
The high affinity of the desensitized P2X3 receptor for ATP sets an upper limit on the extracellular [ATP], which allows ongoing channel activity. For undamaged tissue, the extracellular [ATP] has been estimated from high picomolar to low micromolar (Lazarowski et al., 2003). In our experiments, this range of ATP strongly inhibited P2X3 recovery from desensitization. Although we found that agents with a high affinity for the desensitized state were also effective activators of P2X3 current, this may not be true for all compounds. Such “metaphilic” compounds could provide a welcome addition to the paucity of potent P2X antagonists (Rang and Ritter, 1970b).
Footnotes
We thank Drs. Dan McGehee and Edwin McCleskey for their helpful comments on this manuscript.
Correspondence should be addressed to Sean P. Cook, Molecular Neurology Department, Merck Research Laboratories, WP26A-2000, West Point, PA 19486. E-mail: sean_cook@merck.com.
Copyright © 2005 Society for Neuroscience 0270-6474/05/257359-•$15.00/0
References
- Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, van Biesen T (1999) Allosteric modulation and accelerated resensitization of human P2X(3) receptors by cibacron blue. J Pharmacol Exp Ther 291: 1135-1142. [PubMed] [Google Scholar]
- Bartrup JT, Newberry NR (1996) Electrophysiological consequences of ligand binding to the desensitized 5-HT3 receptor in mammalian NG108-15 cells. J Physiol (Lond) 490: 679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Ghansah E, Chen Y, Ye J, Weiss DS (2002) Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J Neurosci 22: 7982-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP (1990) The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol Sci 11: 485-492. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428-431. [DOI] [PubMed] [Google Scholar]
- Cook SP, Rodland KD, McCleskey EW (1998) A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci 18: 9238-9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenes S, Auerbach A (2002) Desensitization of diliganded mouse muscle nicotinic acetylcholine receptor channels. J Physiol (Lond) 541: 367-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti E, Sokolova E, Masten L, D'Arco M, Fabbro A, Nistri A, Giniatullin R (2004) Identification of negative residues in the P2X3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+ sensing modulatory sites. J Biol Chem 279: 53109-53115. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA (2000) Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J Biol Chem 275: 34190-34196. [DOI] [PubMed] [Google Scholar]
- Katz B, Theseleff S (1957) A study of the `desensitization' produced by acetylcholine at the motor end-plate. J Physiol (Lond) 138: 63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit WG, Oglesby IB, Gever JR, Gever M, Huang C, Li XC, Jin H, McGivern JG, Ford AP (2000) Regulated expression of the rat recombinant P2X(3) receptor in stably transfected CHO-K1 tTA cells. J Auton Nerv Syst 81: 75-81. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785-795. [DOI] [PubMed] [Google Scholar]
- Michel AD, Lundstrom K, Buell GN, Surprenant A, Valera S, Humphrey PP (1996) A comparison of the binding characteristics of recombinant P2X1 and P2X2 purinoceptors. Br J Pharmacol 118: 1806-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX (2000) Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res 882: 155-168. [DOI] [PubMed] [Google Scholar]
- Newell JG, Dunn SM (2002) Functional consequences of the loss of high affinity agonist binding to gamma-aminobutyric acid type A receptors. Implications for receptor desensitization. J Biol Chem 277: 21423-21430. [DOI] [PubMed] [Google Scholar]
- North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82: 1013-1067. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A (2000) Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563-580. [DOI] [PubMed] [Google Scholar]
- Pankratov Yu V, Lalo UV, Dashkin AN, Krishtal A (2001) Heterogeneity of the functional expression of P2X3 and P2X2/3 receptors in the primary nociceptive neurons of rat. Neurochem Res 26: 993-1000. [DOI] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH (2003) Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. J Physiol (Lond) 553: 857-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA (2002) Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457-478. [DOI] [PubMed] [Google Scholar]
- Rang HP, Ritter JM (1970a) On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol 6: 357-382. [PubMed] [Google Scholar]
- Rang HP, Ritter JM (1970b) The relationship between desensitization and the metaphilic effect at cholinergic receptors. Mol Pharmacol 6: 383-390. [PubMed] [Google Scholar]
- Reitstetter R, Lukas RJ, Gruener R (1999) Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289: 656-660. [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G (2003) Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol 121: 451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR (2003) How AMPA receptor desensitization depends on receptor occupancy. J Neurosci 23: 847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ (2004) ATP binding at human P2X1 receptors. Contribution of aromatic and basic amino acids revealed using mutagenesis and partial agonists. J Biol Chem 279: 9043-9055. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Skorinkin A, Fabbretti E, Masten L, Nistri A, Giniatullin R (2004) Agonist-dependence of recovery from desensitization of P2X(3) receptors provides a novel and sensitive approach for their rapid up or downregulation. Br J Pharmacol 141: 1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Seward EP, Buell GN, North RA (1996) Domains of P2X receptors involved in desensitization. Proc Natl Acad Sci USA 93: 15485-15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, He ML, Koshimizu TA, Stojilkovic SS (2004) Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J Neurosci 24: 6968-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]