Abstract
Intranigral injection of the transient receptor potential vanilloid subtype 1 (TRPV1; also known as VR1) agonist capsaicin (CAP) into the rat brain, or treatment of rat mesencephalic cultures with CAP, resulted in cell death of dopaminergic (DA) neurons, as visualized by immunocytochemistry. This in vivo and in vitro effect was ameliorated by the TRPV1 antagonist capsazepine (CZP) or iodo-resiniferatoxin, suggesting the direct involvement of TRPV1 in neurotoxicity. In cultures, both CAP and anandamide (AEA), an endogenous ligand for both TRPV1 and cannabinoid type 1 (CB1) receptors, induced degeneration of DA neurons, increases in intracellular Ca2+ ([Ca2+]i), and mitochondrial damage, which were inhibited by CZP, the CB1 antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) or the intracellular Ca2+ chelator BAPTA/AM. We also found that CAP or AEA increased mitochondrial cytochrome c release as well as immunoreactivity to cleaved caspase-3 and that the caspase-3 inhibitor z-Asp-Glu-Val-Asp-fmk protected DA neurons from CAP- or AEA-induced neurotoxicity. Additional studies demonstrated that treatment of mesencephalic cultures with CB1 receptor agonist (6aR)-trans 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d] pyran-9-methanol (HU210) also produced degeneration of DA neurons and increases in [Ca2+]i, which were inhibited by AM251 and BAPTA/AM. The CAP-, AEA-, or HU210-induced increases in [Ca2+]i were dependent on extracellular Ca2+, with significantly different patterns of Ca2+ influx. Surprisingly, CZP and AM251 reversed HU210- or CAP-induced neurotoxicity by inhibiting Ca2+ influx, respectively, suggesting the existence of functional cross talk between TRPV1 and CB1 receptors. To our knowledge, this study is the first to demonstrate that the activation of TRPV1 and/or CB1 receptors mediates cell death of DA neurons. Our findings suggest that these two types of receptors, TRPV1 and CB1, may contribute to neurodegeneration in response to endogenous ligands such as AEA.
Keywords: transient receptor potential vanilloid 1, capsaicin, capsazepine, anandamide, cannabinoid type-1 receptor, dopaminergic neurons
Introduction
Transient receptor potential vanilloid subtype 1 (TRPV1) is a nonselective cation channel activated by the vanilloids (Caterina and Julius, 2001; Neubert et al., 2003; Cortright and Szallasi, 2004; Ferrer-Montiel et al., 2004; van der Stelt and Di Marzo, 2004) such as capsaicin (CAP), by its endogenous ligands such as anandamide (AEA) or N-arachidonoyl-dopamine (Di Marzo et al., 2001a; Huang et al., 2002), and by products of lipoxygenases (Hwang et al., 2000). Activation of TRPV1 excites sensory neurons, causes pain (Shin et al., 2002; Trevisani et al., 2002), and induces the accumulation of intracellular Ca2+ ([Ca2+]i) (Di Marzo et al., 2001a; Shin et al., 2003). Excessive mitochondrial Ca2+ load resulting from the treatment of sensory neurons with TRPV1 agonists has been shown to cause mitochondrial disruption, resulting in cell death (Olah et al., 2001; Shin et al., 2003).
The widespread distribution of TRPV1 in the brain has suggested that this receptor plays a significant role in the CNS. This hypothesis is supported by recent evidence showing TRPV1-mediated activities in several regions of the rat brain, including the hypothalamus (Sasamura et al., 1998), locus ceruleus (Marinelli et al., 2002), and hippocampus (Huang et al., 2002). Moreover, CAP has been reported to induce increased glutamate release from nigral slices (Marinelli et al., 2003), to enhance motor behavior (Hajos et al., 1988), and to produce hypokinesia in parallel to decrease in the activity of nigrostriatal dopaminergic (DA) neurons (Di Marzo et al., 2001b; de Lago et al., 2004), suggesting that TRPV1 has a functional role in the substantia nigra (SN). In particular, AEA has been found to activate both TRPV1 and the cannabinoid type 1 (CB1) receptors (Hermann et al., 2003), both of which are expressed in areas of the brain involved in the control of motor behavior, including the SN (Mezey et al., 2000; Szallasi and Di Marzo, 2000) and striatum (Tsou et al., 1998; Ploner et al., 2002). This has suggested that the endovanilloid and/or endocannabinoid systems may be implicated in neurodegenerative disorders, including Parkinson's disease (PD) (Di Marzo et al., 2000; Gubellini et al., 2002) and Huntington's disease (Lastres-Becker et al., 2003). We therefore determined whether TRPV1 mediates neurotoxicity against mesencephalic neurons in vivo and in vitro, and we assayed whether this neurotoxicity was accompanied by increased [Ca2+]i via influx through TRPV1, mitochondrial disruption, and release of cytochrome c and activation of caspase-3 and whether these activities were also mediated by CB1 receptor in mesencephalic cultures.
Materials and Methods
Chemicals. CAP, BAPTA/AM, and iodo-resiniferatoxin (iodo-RTX) were purchased from Sigma (St. Louis, MO), and AEA, capsazepine (CZP), N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251), and (6aR)-trans 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d] pyran-9-methanol (HU210) were purchased from Tocris Cookson (Ellisville, MO). The Live/Dead Viability/Cytotoxicity kit, fura-2/AM, and Mito-Tracker were obtained from Molecular Probes (Eugene, OR), and the caspase-3 inhibitor z-DEVD-fmk was purchased from Biochemicals (San Diego, CA). CAP was dissolved in sterile PBS containing 14% ethanol for stereotaxic injection into the brain.
Neuron-enriched mesencephalic cell cultures. Rat ventral mesencephalons were isolated from embryonic day 14 (E14) fetal brains of Sprague Dawley (SD) rats, and the cells were dissociated as described previously (Chung et al., 2001; Choi et al., 2003b). Dissociated cells were plated on 25 mm round glass coverslips precoated with poly-d-lysine and laminin in 35 mm culture dishes at a density of 4.0 × 105 cells/coverslip for measurement of cytosolic Ca2+ and for observation of mitochondria. To suppress the proliferation of glial cells, cultures at 2 d in vitro were incubated with chemically defined serum-free medium.
Immunohistochemistry. Cultures and brain tissue were prepared for immunostaining as described previously (Choi et al., 2003a,b). The primary antibodies included those directed against neuron-specific nuclear protein (NeuN; Chemicon, Temecula, CA), tyrosine hydroxylase (TH; Pel-freez, Brown Deer, WI), glutamic acid decarboxylase (GAD; Sigma), GABA (Sigma), TRPV1 (Sensory Research Center, Seoul National University, Seoul, Korea) (Jung et al., 2002; Shin et al., 2003), CB1 receptor (Sigma), cytochrome c (Promega, Madison, WI), and cleaved caspase-3 (Cell Signaling Technology, Beverly, MA). For Nissl staining, some of the SN tissue samples were stained in 0.5% cresyl violet. Stained cells were viewed and analyzed under a brightfield microscope (Nikon, Tokyo, Japan) or viewed with a confocal laser-scanning microscope (Olympus, Tokyo, Japan).
Live and dead cell assay. Mesencephalic neurons seeded on 25 mm coverslips were treated with CAP (50 μm), and the cultures were stained 24 h later with 2 μm calcein-acetoxymethyl ester and 4 μm ethidium homodimer-1 (Yang et al., 2002). The calcein-positive live cells (green) and ethidium-positive dead cells (red) were visualized using a confocal laser-scanning microscope.
Lactate dehydrogenase release assay. As described previously (Koh and Choi, 1987), lactate dehydrogenase (LDH) release into the bathing medium was measured after exposure of cells to CAP or AEA. A volume of 50 μl of culture medium was transferred to a 96-well microplate, to which 100 μl of 0.1 m phosphate buffer (PB) containing 0.3 mg/ml β-nicotinamide adenine dinucleotide phosphate was added, followed 1 min later by 50 μl of 0.1 m PB containing 2.7 mm pyruvic acid. Increases in optical density at 340 nm were measured at 10 sec intervals for 2 min using a MaXsoft microplate reader (Bio-Tek Instruments, Winooski, VT). The percentage of neuronal cell death was normalized to the mean LDH values released after exposing cells for 24 h to 1% Triton X-100, which was defined as 100% neuronal death.
Measurement of intracellular Ca2+. Changes in intracellular Ca2+ concentration were assayed as described previously with modifications (Pita et al., 1999). Briefly, mesencephalic neurons seeded on 25 mm coverslips were preloaded with 5 μm fura-2 dye plus 2% Pluronic F-127. After incubation for 30 min at 37°C, the cells were washed three times in HBSS (HBSS containing no CaCl2) supplemented with 145 mm NaCl, 2.5 mm KCl, 1.0 mm MgCl2, and 20 mm HEPES, pH 7.4, to remove excess dye. The cells were selected by fluorescence microphotometry. [Ca2+]i was determined on the basis of the ratio of fura-2 fluorescence (R) at 340 and 380 nm, which were measured at 10 sec intervals with a Zeiss (Thornwood, NY) inverted microscope and CCD camera and analyzed using an Ion Application (Empix, Cheektowaga, NY).
Mitochondrial morphology assay. To label mitochondria in mesencephalic neurons seeded on 25 mm coverslips, the cells were incubated with 250 nm Mito-Tracker dye for 30 min, washed three times in HBSS, and examined under a confocal laser-scanning microscope at 10 sec intervals with a He-Ne green laser.
Western immunoblot analysis. Mitochondrial fractions from cultured mesencephalic neurons treated with CAP or AEA were prepared as described previously with some modifications (Rajgopal et al., 2003). Briefly, cells were homogenized with buffer containing 20 mm HEPES, pH 7.5, 250 mm sucrose, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 0.1 mm PMSF, and a protease inhibitor mixture. The homogenates were centrifuged at 500 × g for 10 min at 4°C, and the supernatant was centrifuged at 100,000 × g for 1 h at 4°C in an ultracentrifuge (Beckman, Fullerton, CA). The supernatant from this centrifugation was considered the cytosolic fraction, and the pellet was considered the membrane and mitochondria-rich fraction. Proteins were separated by 12% SDS-PAGE gels, transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) using an electrophoretic transfer system (Bio-Rad, Hercules, CA). The membranes were immunoblotted with mouse anti-cytochrome c (Pharmingen, San Diego, CA), and proteins were visualized using the ECL kit (Amersham Biosciences, Piscataway, NJ).
Stereotaxic injection of capsaicin and anandamide. After anesthetization with chloral hydrate, female SD rats (∼230-250 gm) each received a unilateral injection of 3 μl of CAP, AEA or CZP at a rate of 0.5 μl/min into the right SN (anteroposterior, -5.3; mediolateral, -2.3; dorsoventral, -7.6 mm from bregma), according to the atlas of Paxinos and Watson (1998), using a 26 gauge Hamilton syringe attached to an automated microinjector (Buwon, Seoul, Korea).
Stereological estimation. As described previously (Choi et al., 2003a,b), the total number of TH-positive neurons in the SN was counted in the various groups of animals at 7 d after vehicle, CAP, or CZP injection using the optical fractionator method using the Olympus Computer Assisted Stereological Toolbox system version 2.1.4 (Olympus). This is an unbiased stereological method of cell counting that is not affected by either the volume of reference (SNpc) or the size of the counted elements (neurons) (West et al., 1991).
Statistical analysis. All data are presented as mean ± SEM. The statistical significance of differences was assessed using ANOVA (GraphPad software; GraphPad, San Diego, CA), followed by Student-NewmanKeuls multiple comparison tests. All calculations were performed using the standard version of SPSS for Windows (SPSS, Chicago, IL).
Results
Expression of TRPV1 and CB1 receptors in neuron-enriched mesencephalic cultures
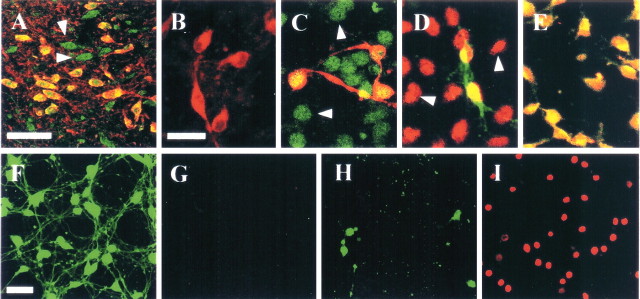
Double immunostaining using well-characterized antibodies to TRPV1 (Jung et al., 2002; Shin et al., 2003) and TH showed that TRPV1 expression was localized to TH-immunopositive (i.p.) neurons in the SN (Fig. 1A) as well as in neuron-enriched mesencephalic cultures (Fig. 1C). TRPV1 was also expressed in non-TH-i.p. neurons in vivo and in vitro (Fig. 1A,C). Additional double immunostaining showed that CB1 receptors were localized to both TH-i.p. and non-TH-i.p. neurons in mesencephalic cultures (Fig. 1D), although CB1 immunostaining was observed in DA neurons in the ventral tegmental area (VTA), but not the SN, in rat midbrain (Wenger et al., 2003). These results are in good agreement with previous findings, showing the presence of CB1 receptors in DA neurons in rat mesencephalic cultures (Hernández et al., 2000) but their absence from the corresponding neurons in the adult brain (Pettit et al., 1998; Julian et al., 2003). Moreover, double immunostaining using antibodies to TRPV1 and CB1 receptor showed that TRPV1 expression was localized to neurons expressing CB1 receptors in neuron-enriched mesencephalic cultures (Fig. 1E).
Figure 1.
A, Rat SN sections were immunostained simultaneously with antibodies to TRPV1 (green) and TH (red), and the two were merged (yellow). TRPV1 was detected in TH-i.p. neurons as well as non-TH-i.p. neurons (arrowheads). Cells in neuron-enriched mesencephalic cultures were immunostained with antibody to TH alone (B; red) or together with antibody to TRPV1 (C; green) or CB1 (D; red). Both TRPV1 and CB1 were detected in TH-i.p. and non-TH-i.p. neurons (arrowheads). E, The colocalization of TRPV1 and CB1 receptors in neuron-enriched mesencephalic cultures. F-I, Cultures were treated with vehicle (F, G) or 50 μm capsaicin (H, I) for 24 h and stained with calcein-acetoxymethyl ester (green for live cells; F, H) or ethidium homodimer-1 (red for dead cells; G, I). Scale bars: A, 100 μm; B-E, 30 μm; F-I, 50 μm.
TRPV1-mediated neurotoxicity in mesencephalic cultures
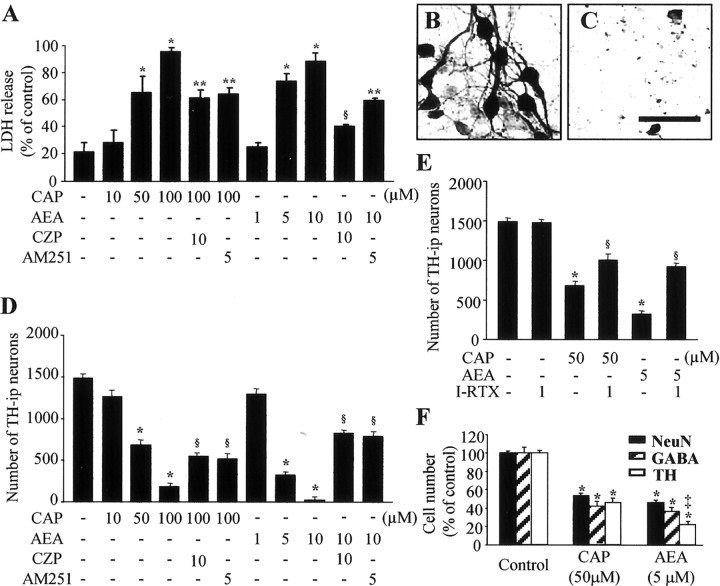
Treatment of mesencephalic cultures with 50 μm CAP dramatically increased the number of ethidium homodimer-1-positive dead cells (Fig. 1I), which was accompanied by a decrease in the number of calcein-acetoxymethyl ester-positive live cells (Fig. 1H), when compared with vehicle-treated controls (Fig. 1F,G). Similar results were obtained in cultures treated with 5 μm AEA (data not shown). When we assayed neuronal cell death by measuring the level of LDH, we found that treatment with 10 μm CAP or 1 μm AEA had no effect, but treatment with 50-100 μm CAP or 5-10 μm AEA produced three-fold to fourfold increases in LDH compared with nontreated control cultures (Fig. 2A) (p < 0.001). Pretreatment with 10 μm of the TRPV1 antagonist CZP (Hwang et al., 2000) partially attenuated the level of LDH induced by 100 μm CAP (33%; p < 0.05) or 10 μm AEA (48%; p < 0.05).
Figure 2.
Neurotoxicity induced by CAP or AEA in neuron-enriched mesencephalic cultures. Cultures were treated with 10-100 μm CAP or 1-10 μm AEA for 24 h. Where indicated, cells were pretreated with 10 μm CZP or 5 μm AM251 for 5 min before treatment with 100 μm CAP or 10 μm AEA for 24 h. In all cultures, cell death was assessed as LDH released into the bathing media (A) and by counting the number of TH-i.p. cells (D). TH immunostaining of cultures treated with vehicle (B) or 50 μm CAP (C) is shown. Scale bar: B, C, 100 μm. E, Number of TH-i.p. neurons in neuron-enriched mesencephalic cultures treated with CAP or AEA in either the absence or presence of TRPV1 antagonist iodo-RTX. Cultures were pretreated with 1 μm iodo-RTX for 5 min before treatment with 50 μm CAP or 5 μm AEA for 24 h. F, Reduction in the number of NeuN-i.p., GABA-i.p., and TH-i.p. neurons in cultures treated with 50 μm CAP or 5 μm AEA for 24 h. All values represent the mean ± SEM of triplicate cultures in four separate platings. *p < 0.001 compared with control; **p < 0.05 and §p < 0.001 compared with cells treated with CAP or AEA; ‡p < 0.001 compared with cells treated with 50 μm CAP.
We next examined whether activation of TRPV1 induced the death of DA neurons in neuron-enriched mesencephalic cultures. TH immunocytochemical staining showed that healthy, large TH-i.p. neurons with long and branched neuritic processes were present in vehicle-treated control cultures (Fig. 2B). In contrast, the addition of 50 μm CAP (Fig. 2C) or 5 μm AEA (data not shown) produced a significant loss of TH-i.p. neurons. This neurotoxicity against DA neurons occurred in a dose-dependent manner (Fig. 2D). Application of 10 μm CAP or 1 μm AEA had no effect. However, when quantified and expressed as a percentage of nontreated control values, treatment with 50-100 μm CAP reduced the number of TH-i.p. neurons by 54-88% (p < 0.001), whereas treatment with 5-10 μm AEA reduced number of TH-i.p. neurons by 78-98% (p < 0.001) (Fig. 2D). Pretreatment of these cultures with 10 μm CZP partially attenuated the effects of 100 μm CAP (25%; p < 0.001) and 10 μm AEA (54%; p < 0.001), indicating TRPV1-mediated neurotoxicity. Because the neuroprotective action of CZP against oxygen glucose deprivation model of cell death in organotypic hippocampal slice cultures does not result from an interaction with TRPV1 (Ray et al., 2003), additional experiments were performed to corroborate TRPV1-mediated neurotoxicity by using another TRPV1 antagonist, iodo-RTX (Rigoni et al., 2003), and showed similar results. The data show that pretreatment of these cultures with 1 μm iodo-RTX partially attenuated the death of TH-i.p. neurons in mesencephalic cultures treated with 50 μm CAP (22%; p < 0.001) or 5 μm AEA (39%; p < 0.001) (Fig. 2E), further indicative of TRPV1-mediated neurotoxicity.
We also immunostained these cultures with antibodies to NeuN, to detect general neurons, and GABA, to detect GABAergic neurons. In cultures treated with 50 μm CAP, there was a significant reduction in the number of NeuN-i.p. (47%; p < 0.01), GABA-i.p. (59%; p < 0.001), and TH-i.p. (54%; p < 0.01) neurons (Fig. 2F) when compared with untreated cultures. Similarly, 5 μm AEA significantly attenuated the number of NeuN-i.p. (54%; p < 0.01), GABA-i.p. (64%; p < 0.001), and TH-i.p. (78%; p < 0.01) neurons. Interestingly, TH-i.p. neurons were more significantly vulnerable than GABA-i.p. neurons to AEA treatment than to CAP treatment (Fig. 2F) (p < 0.001).
Ca2+ influx through TRPV1 and subsequent mitochondrial disruption contribute to degeneration of DA neurons in vitro
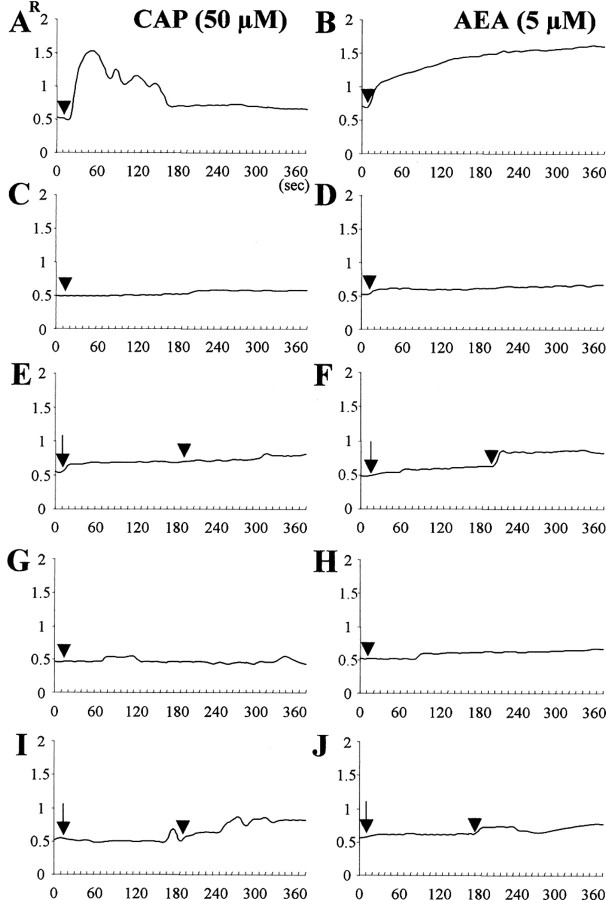
We hypothesized that increases in [Ca2+]i via influx through TRPV1 may account for the neurotoxicity induced by CAP and AEA (Shin et al., 2003). To test this, we measured changes in [Ca2+]i by tracing the intensity of fura-2 fluorescent images. We found that administration of CAP (Fig. 3A) or AEA (Fig. 3B) increased fluorescence intensity in cultured mesencephalic neurons, indicative of [Ca2+]i elevation. This increase in [Ca2+]i was completely abolished in cultures treated with CAP or AEA in the presence of 10 μm CZP (Fig. 3E,F) and in Ca2+-free extracellular solution (Fig. 3C,D), indicating influx of extracellular Ca2+ through TRPV1. To further determine the source of the [Ca2+]i increase, we treated these cultures with thapsigargin, an endoplasmic reticulum Ca2+ pump inhibitor (Marshall et al., 2003). We found that this reagent had no effect on the Ca2+ influx induced by CAP or AEA in the presence of extracellular Ca2+ (Fig. 3A,B). Moreover, treatment with BAPTA/AM completely blocked the increases in [Ca2+]i, induced by CAP (Fig. 3G) or AEA (Fig. 3H) as well as rescuing TH-i.p. neurons, indicating that cell death was associated with increases in [Ca2+]i. When quantified and expressed as a percentage of control values, treatment with 5 μm BAPTA/AM plus 50-100 μm CAP increased the number of TH-i.p. neurons by 31-36% (p < 0.001), whereas treatment with 5 μm BAPTA/AM plus 10 μm AEA increased the number of TH-i.p. neurons by 31% (p < 0.001), compared with cultures treated with CAP or AEA alone (Table 1).
Figure 3.
Changes of R (F340/380) fluorescence in fura-2-loaded cultured mesencephalic neurons. [Ca2+]i was measured in cultures treated with 50 μm CAP (left panel, arrowhead) or 5 μm AEA (right panel, arrowhead) in the presence (A, B) or absence (C, D) of 1.8 mm extracellular calcium. E-J, Response to 50 μm CAP or 5 μm AEA (arrowhead) of cultures pretreated with 10 μm CZP (E, F, arrow) or 5 μm AM251 (I, J, arrow) in the presence of 1.8 mm extracellular calcium, or cultures treated with 5 μm BAPTA/AM plus 50 μm CAP (G) or 5 μm AEA (H). Data were averaged from 20-25 randomly selected cells for each condition.
Table 1.
Effects of the calcium chelator BAPTA/AM
|
Treatment |
Number of TH-i.p. neurons |
Percentage of control |
|---|---|---|
| Control (nontreated) | 1486 ± 51 | 100 ± 3 |
| Capsaicin (50 μm) | 685 ± 56a | 46 ± 4a |
| plus BAPTA (5 μm) | 1145 ± 40b | 77 ± 3b |
| Capsaicin (100 μm) | 181 ± 41a | 12 ± 4a |
| plus BAPTA (5 μm) | 710 ± 57b | 48 ± 4b |
| Anandamide (10 μm) | 32 ± 11a | 2 ± 1a |
| plus BAPTA (5 μm) |
487 ± 41b
|
33 ± 4b
|
Cell cultures were prepared as described in Figure 2, exposed to CAP or AEA for 24 hr in the absence or presence of BAPTA, and immunostained with antibody to IH. The number of TH-i.p. neurons was counted in mesencephalic cultures in microscopic fields on the coverslip. Untreated cultures were used as controls. All values represent mean ± SEM of triplicate cultures from four separate platings.
p <0.001 compared with control.
p <0.001 compared with treatment with CAP or AEA.
We next determined whether increases in [Ca2+]i could contribute to mitochondrial disruption in live cells and whether this mitochondrial damage resulted in the release of cytochrome c. In untreated controls, cells showed intact mitochondrial structure, as determined by Mito-Tracker fluorescence (Fig. 4A). In contrast, mitochondrial disruption was noted as early as 3 min after addition of 50 μm CAP (Fig. 4B) and was more pronounced after 10 min (Fig. 4C). Consistent with the clearance of cytosolic Ca2+, CZP (Fig. 4E) and BAPTA/AM (Fig. 4G) prevented the CAP-induced mitochondrial damage. Similarly, 5 μm AEA induced mitochondrial disruption (data not shown) but required approximately twice as much time as CAP to reach a similar level of mitochondrial damage. Double immunofluorescence staining with Mito-Tracker and cytochrome c antibodies revealed that, in untreated controls, cytochrome c was localized to the mitochondria (Fig. 4H), whereas, in cells treated with 50 μm CAP or 5 μm AEA, there was a redistribution of cytochrome c into the cytosol, indicating that this protein had been released from the mitochondria (Fig. 4I,J). This was confirmed by Western blot analysis (Fig. 4K).
Figure 4.
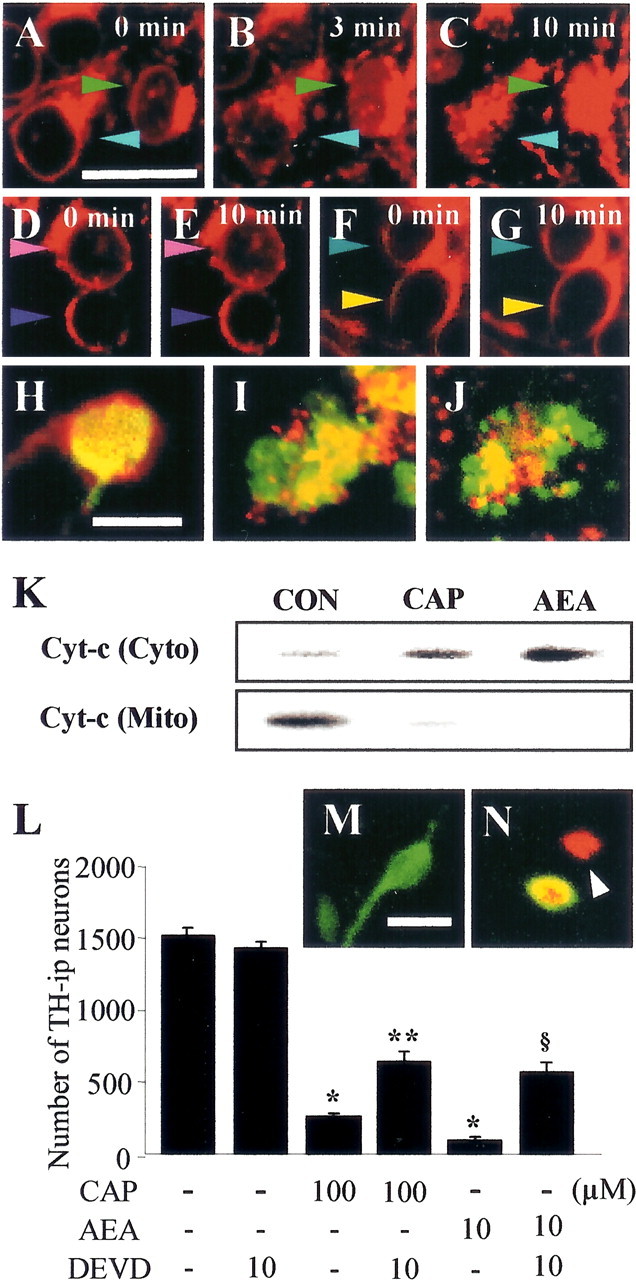
A-C, Mitochondrial disruption in neuron-enriched mesencephalic cultures treated with 50 μm CAP in the presence of 1.8 mm extracellular calcium. D-G, Inhibition of CAP-induced mitochondrial disruption by pretreatment with 10 μm CZP (D, E) or cotreatment with 5 μm BAPTA/AM (F, G). Each colored arrow indicates the same cells. H-J, Localization of cytochrome c (green) immunoreactivity and Mito-Tracker (red) in untreated cells (H) and cells treated with 50 μm CAP (I) or 5 μm AEA (J) for 12 h. K, Western blot analysis of cytochrome c (Cyto-c) levels after treatment of cells for 6 h with CAP or AEA. Con, Nontreated controls; Cyto, cytosolic fraction; Mito, mitochondrial fraction. L, z-DEVD-fmk attenuates CAP- or AEA-induced cell death. M, N, Immunochemical localization of cleaved caspase-3 (red) in TH-i.p. (green) and non-TH-i.p. (arrowheads) neurons in untreated cultures (M) and cultures treated with CAP or AEA (N). *p < 0.001 compared with untreated controls; **p < 0.01 and §p < 0.001 compared with culture treated with 100 μm CAP or 10 μm AEA. Scale bars: (in A) A-G, 40 μm; (in H) H-J, 20 μm; (in M) M, N, 30 μm.
Effects of caspase-3 inhibitor
AEA induced cell death of human neuroblastoma and lymphoma cell lines through activation of caspase-3 via cytochrome c release by Ca2+ influx via activation of TRPV1 (Maccarrone et al., 2000). We also found that activation of TRPV1 resulted in the release of cytochrome c from mitochondria, leading to degeneration of DA neurons, and caspase-3 has been also shown to contribute to the death of DA neurons in human PD and in vivo and in vitro models of this disease (Cutillas et al., 1999; Hartmann et al., 2000; Turmel et al., 2001). Therefore, we next determined whether caspase-3 is involved in CAP- or AEA-induced death of DA neurons. Cultures were treated with the caspase-3 inhibitor z-DEVD-fmk, together with CAP or AEA. Treatment with 10 μm z-DEVD-fmk significantly reduced the death of TH-i.p. neurons induced by 100 μm CAP (25%; p < 0.01) or 10 μm AEA (32%; p < 0.001) compared with their respective controls (Fig. 4L), whereas 10 μm z-DEVD-fmk alone had no effect. Double immunofluorescence staining with antibodies to cleaved caspase-3 and TH showed that activated caspase-3 was present in both DA and non-DA neurons (Fig. 4M,N).
Effects of CB1 receptors
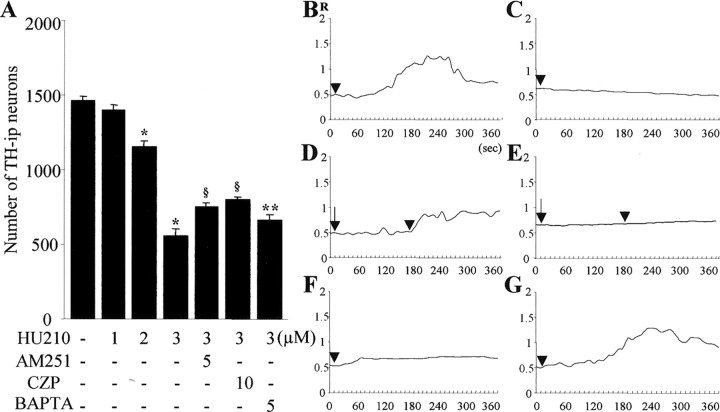
AEA has been reported to activate both TRPV1 and CB1 receptors (Hermann et al., 2003). Because CB1 receptors were also expressed in our cultures (Fig. 1D,E), we hypothesized that activation of CB1 receptors by AEA could contribute to neurodegeneration in mesencephalic cultures. To test this, we investigated whether CB1 receptor antagonist AM251 (Campbell, 2001; Downer et al., 2001) altered the effects of AEA on mesencephalic cultures. We found that pretreatment with 5 μm AM251 before treatment with AEA reduced the level of LDH by 29% (Fig. 2A) (p < 0.05) and increased the number of TH-i.p. neurons by 52% (Fig. 2B)(p < 0.001) when compared with cultures treated with 10 μm AEA alone. Pretreatment with 5 μm AM251 also completely inhibited AEA-induced Ca2+ influx (Fig. 3J) and mitochondrial damage (data not shown). To further verify CB1 receptor-mediated neurotoxicity, we determined whether CB1 receptor agonist HU210 (Hillard et al., 1999) could induce cell death of TH-i.p. neurons and increase in [Ca2+]i in mesencephalic cultures. The data show that HU210-induced neurotoxicity against DA neurons occurred in a dose-dependent manner (Fig. 5A). Application of 1 μm HU210 had no effect. However, when quantified and expressed as a percentage of nontreated control values, treatment with 2-3 μm HU210 reduced the number of TH-i.p. neurons by 22-62% (p < 0.001). This neurotoxicity was partially inhibited by pretreatment with 5 μm AM251 (p < 0.001) or cotreatment with 5 μm BAPTA/AM (p < 0.05). We also found that administration of HU210 increased the intensity of fura-2 fluorescence in cultured mesencephalic neurons (Fig. 5B), indicative of [Ca2+]i elevation. This increase in [Ca2+]i was completely abolished in cultures treated with HU210 in Ca2+-free extracellular solution (Fig. 5C) and in the presence of 5 μm AM251 (Fig. 5D). Treatment with BAPTA/AM completely blocked the increases in [Ca2+]i, induced by HU210 (Fig. 5F). To further determine the source of the [Ca2+]i increase, we treated these cultures with thapsigargin. This reagent had no effect on the Ca2+ influx induced by HU210 in the presence of extracellular Ca2+ (Fig. 5G). These findings suggest that HU210 induces an influx of extracellular Ca2+ in cultured mesencephalic neurons in a CB1 receptor-dependent manner. Surprisingly, CAP-induced effects on LDH (Fig. 2A), TH-i.p. neurons (Fig. 2B), and Ca2+ influx (Fig. 3I) were reduced by AM251. Moreover, HU210-induced effects on TH-i.p. neurons (Fig. 5A) and Ca2+ influx (Fig. 5E) were also inhibited by CZP.
Figure 5.
A, Neurotoxicity induced by HU210 on TH-i.p. neurons in neuron-enriched mesencephalic cultures. Cultures were treated with 1-3 μm HU210 for 24 h. Where indicated, cells were pretreated with 10 μm CZP or 5 μm AM251 for 5 min before treatment with 3 μm HU210 or cotreated with 5 μm BAPTA/AM for 24 h. Death of TH-i.p. neurons was assessed by counting the number of TH-i.p. cells. All values represent the mean ± SEM of triplicate cultures in four separate platings. *p < 0.001 compared with control; **p < 0.05 and §p < 0.001 compared with cells treated with 3 μm HU210. B-G, Changes of R (F340/380) fluorescence in fura-2-loaded cultured mesencephalic neurons. [Ca2+]i was measured in cultures treated with 3 μm HU210 (arrowhead) in the presence (B) or absence (C) of 1.8 mm extracellular calcium. Changes of R in cultures pretreated with 5 μm AM251 (D, arrow) or 10 μm CZP (E, arrow) or cotreated with 5 μm BAPTA/AM with 3 μm HU210 in the presence of 1.8 mm extracellular calcium (F) are shown. G, The effect of thapsigargin on the Ca2+ influx induced by HU210 in the presence of extracellular Ca2+. Data were averaged from 20-25 randomly selected cells for each condition.
TRPV1-mediated neurotoxicity in the SN in vivo
Seven days after intranigral injection of CAP (500 pmol/3 μl), we observed dramatic reductions in the number of Nissl-stained (Fig. 6C,D), NeuN-i.p. (Fig. 6G,H), and TH-i.p. cells (Fig. 6O,P) in the SN compared with vehicle-treated controls (Fig. 6A, B, E, F, M, N). Consistent with in vitro data, immunostaining showed that CAP induced a decrease in the number of GAD-i.p. cells (GABAergic neurons) in the substantia nigra reticulate (SNr) (Fig. 6K,L), compared with vehicle-treated SN (Fig. 6I,J). We also found that AEA (30 nmol/3 μl) mimicked the effects of CAP (data not shown). Intranigral coinjection of CZP partially prevented the CAP-induced death of DA neurons in the SN (Fig. 6, compare Q and R with O and P). When quantified and expressed as a percentage of neurons on the ipsilateral side compared with the contralateral side, 500 pmol of CAP reduced the number of TH-i.p. neurons by 34% (Fig. 6U) (p < 0.01). However, coinjection of CZP with CAP was found to increase the number of TH-i.p. neurons by 22% (Fig. 6U) (p < 0.01), suggesting the involvement of TRPV1, but CZP alone had no effect (Fig. 6S,T).
Figure 6.
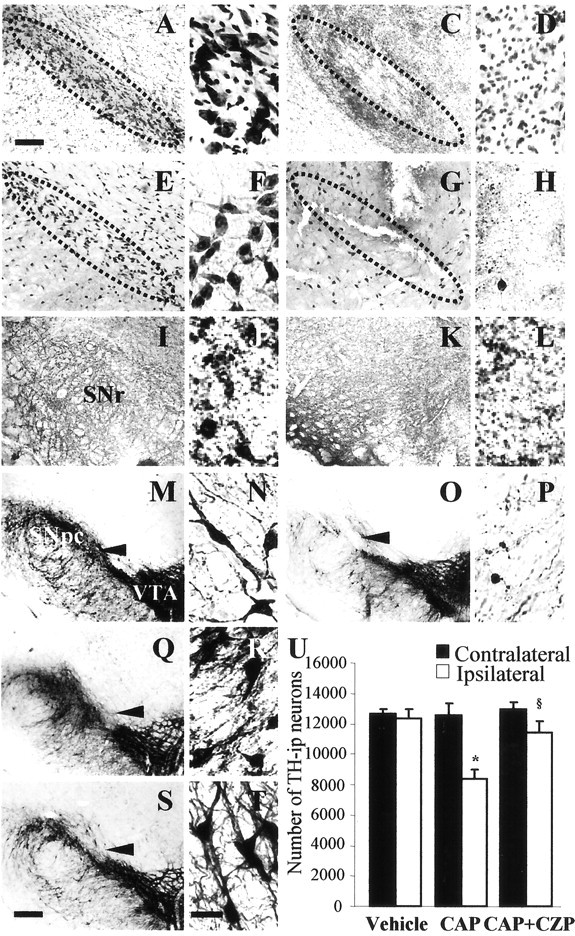
CAP-induced neurotoxicity in the SN of rat brains. Animals were administered a unilateral injection of 500 pmol of CAP in 3 μl of PBS containing 14% ethanol (C, D, G, H, K, L, O, P) or vehicle (A, B, E, F, I, J, M, N) into the SN and killed 7 d later. Brain tissues were stained with cresyl violet (A-D) and immunostained with antibody to NeuN (E-H), GAD (I-L), or TH (M-P). Q-T, CZP alters the effect of CAP on TH-i.p. neurons in the SN. Coinjection of 500 pmol of CAP plus 500 pmol of CZP rescued TH-i.p. neurons, as evidenced by TH immunocytochemistry (Q, R), whereas CZP alone had no effect (S, T). The arrowhead indicates syringe track. Dotted lines indicate SNpc (where dopaminergic neurons were degenerated). Scale bars: (in S) M, O, Q, S, 300 μm; (in A) A, C, E, G, I, K, 200 μm; (in T) B, D, F, H, J, L, N, P, R, T, 50 μm. U, Number of TH-i.p. neurons in the SN treated with CAP in the absence or presence of CZP. Animals receiving intranigral CAP injection (500 pmol) with or without administration of CZP (500 pmol) were killed 7 d after injection. Brain tissues were cut, and every sixth serial section was immunocytochemically stained with antibodies against TH. TH-i.p. neurons were counted using a stereological technique in the whole SN. Six to eight animals were used for each experimental group. *p < 0.01, significantly different from contralateral side; §p < 0.01, significantly different from ipsilateral side treated with CAP only.
Discussion
TRPV1 mediates cell death of dopaminergic neurons in vivo and in vitro
Although TRPV1-mediated cell death has been demonstrated in a variety of cell types, including human neuroblastoma and lymphoma cell lines (Maccarrone et al., 2000), Jurkat cells (Jambrina et al., 2003), vanilloid receptor subtype 1 (VR1)-transfected human kidney cells (Shin et al., 2003), and sensory neurons (Olah et al., 2001), little is known about TRPV1-mediated neurotoxicity in the CNS. We have shown here that treatment with CAP or AEA revealed degeneration of mesencephalic DA neurons both in vivo and in vitro. To our knowledge, this study is the first to show TRPV1-mediated neurotoxicity on DA neurons and supports the hypothesis that CAP or AEA exerts neurotoxic effects by activating TRPV1 (Mezey et al., 2000; Szallasi and Di Marzo, 2000; Szöke et al., 2000; Cernak et al., 2004). However, our results do not rule out the possibility that TRPV1-independent cell death may also be involved, because CZP, a TRPV1 antagonist, was ineffective in preventing the AEA-induced cell death of PC12 cells, which constitutively express endogenous TRPV1 (Sarker and Maruyama, 2003).
Contrary to our present results, TRPV1-mediated neuroprotection in vivo excitotoxicity has been reported recently (Veldhuis et al., 2003). This study shows that arvanil, a synthetic AEA analog, attenuated excitotoxic brain injury and cotreatment of arvanil with CZP reduced the neuroprotective effect of arvanil, indicative of TRPV1-mediated neuroprotection. Dib and Falchi (1996) proposed that these results are probably attributable to stimulation of CB1 receptors and desensitization of TRPV1 by its agonist and resultant inhibition of desensitization by an agonist in the presence of an antagonist (CZP) (Jung et al., 1999). Thus, collective action of arvanil may attenuate neuronal degeneration elicited by excitotoxin. However, it is worthy to note that Veldhuis et al. (2003) also showed that CZP alone reduced brain injury caused by excitotoxicity. This neuroprotective action of CZP may imply TRPV1-mediated neurotoxicity, arguing against a TRPV1-mediated neuroprotection, although the neuroprotection of CZP is not always via an action on TRPV1 (Ray et al., 2003). Alternatively, this apparent discrepancy (neurotoxicity versus neuroprotection) is probably a result of the use of different experimental conditions. Here, we showed the direct actions of TRPV1 by applying its agonists (CAP and AEA) and antagonists (CZP and iodo-RTX), whereas in studies by Veldhuis et al. (2003), TRPV1 actions were evaluated during excitotoxic injury, which might compromise physiological functions of TRPV1 for preventing the secondary damage after excitotoxic insult.
Ca2+ influx via TRPV1 contributes to cell death of dopaminergic neurons in mesencephalic cultures
After the application of either CAP or AEA, there is a large increase in [Ca2+]i via influx through TRPV1 in sensory neurons (Caterina and Julius, 2001; Shin et al., 2003) and human neuroblastoma and lymphoma cell lines (Maccarrone et al., 2000), leading to the induction of mitochondrial damage and cell death. The results shown here are consistent with these previous findings, in that treatment of mesencephalic neurons with CAP or AEA induced an elevation of [Ca2+]i, mitochondrial damage, and eventually cell death, which were blocked by CZP or BAPTA/AM. We also found that activation of TRPV1 resulted in the release of cytochrome c from mitochondria. However, in Jurkat cells, although activation of TRPV1 induced Ca2+ overload on mitochondria sufficient for mitochondrial damage, it induced cell death without release of cytochrome c (Jambrina et al., 2003), suggesting that the latter step is not directly involved in TRPV1-mediated cell death.
Activation of casapse-3 is involved in TRPV1-mediated cell death of dopaminergic neurons
Among the several factors released from the mitochondria in response to various stimuli, cytochrome c is well known for playing a critical role in the initiation of cell death (Liu et al., 1996; Li et al., 1997; Zou et al., 1999). Although we did not provide direct evidence for apoptotic protease activating factor 1 (Apaf-1) and caspase-9 activation, it is possible to speculate that cytochrome c and Apaf-1 allow for recruitment of procaspase-9 into the apoptosome complex, leading to caspase-9 activation, which in turn activates caspase-3, leading to eventual cell death (Hengartner, 2000). Alternatively, it seems noteworthy to mention that cell surface death receptors like the Fas receptor could induce activation of caspases, including caspase-8 and caspase-10, leading to eventual activation of caspase-3 and cell death without cytochrome c release (Hengartner, 2000; Rieux-Laucat et al., 2003). Several studies indicated that caspase-3 activation is required for TRPV1-mediated cell death of human endothelial (Yamaji et al., 2003) and human neuroblastoma and lymphoma cells (Maccarrone et al., 2000). Caspase-3 has also been shown to contribute to the death of DA neurons in human PD and in vivo and in vitro model of this disease (Cutillas et al., 1999; Hartmann et al., 2000; Turmel et al., 2001). In the present study, we found cytochrome c release from mitochondria and localization of caspase-3 immunoreactivity within DA neurons in CAP- or AEA-treated cultures and that treatment with a caspase-3 inhibitor reduced the death of DA neurons induced by CAP or AEA. This is in line with previous findings showing that cytochrome c release and caspase-3 activation resulted in cell death of human neuroblastoma and lymphoma cells via AEA action on TRPV1 (Maccarrone et al., 2000). Similarly, DA neuronal cell death in mesencephalic cultures treated with 6-hydroxydopamine, but not 1-methyl-4-phenylpyridinium, caused by cytochrome c release and caspase-3 activation has been reported previously (Han et al., 2003). These data collectively support the hypothesis that caspase-3 is an important effector of DA neuronal cell death mediated by TRPV1.
CB1 receptor mediates cell death of dopaminergic neurons in mesencephalic cultures
There is evidence that AEA, the endogenous agonist of both TRPV1 and the CB1 receptors, protects the brain against ouabain-induced in vivo excitotoxicity, an effect mediated by the CB1 receptor but not by TRPV1 (van der Stelt et al., 2001; Veldhuis et al., 2003). CB1 receptor was also found to mediate the neuroprotective effect of the CB1 receptor agonist Δ9-tetrahydrocannabinol (THC) against NMDA-induced retinal neurotoxicity (El-Remessy et al., 2003). In contrast, AEA induces apoptosis via activation of CB1 receptor in bone marrow-derived dendritic cells (Do et al., 2004). THC was also found to induce the degeneration of cultured hippocampal (Chan et al., 1998) and cortical (Campbell, 2001; Downer et al., 2001; Roberts et al., 2002) neurons, accompanied by cytochrome c release and/or caspase-3 activation and blocked by the CB1 receptor antagonist AM251 (Roberts et al., 2002). Our findings are in agreement with these results, in that we showed that AEA induced cytochrome c release and casapse-3 activation, leading to degeneration of TH-i.p. neurons, and that these effects were blocked by AM251. Moreover, we also found that CB1 receptor agonist HU210 produced cell death of DA neurons, which was ameliorated by AM251. Together with TRPV1-mediated neurotoxicity, CB1 receptors may contribute to, at least in part, the death of DA neurons in mesencephalic cultures.
Interactions of TRPV1 and CB1 receptors and neurotoxicity
Similar to our recent report (Shin et al., 2003), we have shown here that in mesencephalic cultures, the effects of CAP, AEA, or HU210 on [Ca2+]i were not observed in the absence of extracellular Ca2+ or in the presence of the TRPV1 receptor antagonist CZP or the CB1 receptor antagonist AM215. In cultured hippocampal neurons (Chan et al., 1998) and resting T cells (Rao et al., 2004), THC-induced increases in [Ca2+]i were not observed in the absence of extracellular Ca2+ or in the presence of the CB1 receptor antagonist SR141716A. Thus, these results collectively suggest that the increases in [Ca2+]i mediated by TRPV1 and/or CB1 receptors are dependent on extracellular Ca2+. This is also supported by the current finding showing that an endoplasmic reticulum Ca2+ pump inhibitor, thapsigargin (Marshall et al., 2003), had no effect on the Ca2+ influx induced by CAP, AEA, or HU210 in the presence of extracellular Ca2+. The patterns of Ca2+ influx, however, differ in cultures treated with CAP, AEA, or HU210. In agreement with previous reports (Chan et al., 1998; Szöke et al., 2000; Shin et al., 2003), we have shown here that application of CAP evoked an initial fast rise of [Ca2+]i, peaking at 25-30 sec, followed by a slow recovery of [Ca2+]i over the next 2 min. In contrast, application of AEA evoked a delayed increase in [Ca2+]i, peaking at 160-170 sec and remaining at the same elevated level for >6 min. This may explain our observation that the time required for AEA-induced mitochondrial damage was at least twice as long as that required for CAP-induced damage. Addition of HU210 also caused a delayed increase in [Ca2+]i that reached a maximum at 220-240 sec and returned to basal levels at 320 sec. Although the underlying mechanism of this apparent discrepancy remains undetermined, it is likely that AEA can activate both TRPV1 and CB1 receptors, and that their interaction modulates Ca2+ influx. This is supported by findings that AEA-induced Ca2+ influx was significantly higher in cells coexpressing TRPV1 and CB1 receptors than in cells expressing TRPV1 alone and that this effect was abolished by pretreatment with SR141716A, a CB1 receptor antagonist (Hermann et al., 2003). Surprisingly, we also found that CAP- and HU210-induced neurotoxicity was inhibited by pretreatment with AM251 (CB1 receptor antagonist) and CZP (TRPV1 antagonist), respectively. These unexpected results are supported by the previous findings that TRPV1 stimulation with CAP, via the subsequent Ca2+ influx, led to biosynthesis of AEA, which can activate both TRPV1 and CB1 receptors (Di Marzo et al., 1994, 2001b). This would explain why CAP-induced neurotoxicity is inhibited by AM251. It is therefore likely that Ca2+ influx via stimulation of CB1 receptors could contribute to the formation of AEA, thus explaining why HU210-induced neurotoxicity is blocked by CZP, although we did not test the effect of HU210 on AEA formation. Because TRPV1 and CB1 receptors are coexpressed in our mesencephalic cultures, our findings suggest that in addition to the direct neurotoxicity via activation of each receptor, the functional interaction of these two receptors in response to Ca2+ influx may also contribute to cell death of cultured mesencephalic neurons, including DA neurons.
It seems noteworthy that these results might be irrelevant for protective effects of CB1 receptors against TRPV1-mediated neurotoxicity (Maccarrone et al., 2000). This study showed that CB1 antagonist SR141716 enhanced AEA-induced death of rat C6 glioma cells, which coexpressed TRPV1 and CB1 receptors. Maccarrone et al. (2000) suggested that this effect is probably because AEA-activated CB1 receptors inhibit TRPV1-mediated toxic events including increases in [Ca2+]i, mitochondrial damage, and cytochrome c release from mitochondria. In contrast, we found that AM251 almost completely abolished AEA-activated increases in [Ca2+]i (Fig. 3J), which might lead to inhibition of mitochondrial damage and cytochrome c release from mitochondria, indicating the opposite effect of AEA-activated CB1 receptors in C6 glioma cells. Thus, data from the present study may indicate that the effect of AEA on calcium is entirely mediated by TRPV1 and that its costimulation of CB1 receptors modulates the kinetics of this effect. This is also supported by our current data showing that AM251 inhibits neurotoxicity (Fig. 5A) and increases in [Ca2+]i (Fig. 5B,D) induced by HU210. Alternatively, this apparent discrepancy in experimental results between two studies is probably a result of different cell types (neurons vs glioma cells) used for AEA treatment.
The principal significance of the findings reported here is that activation of TRPV1 and/or CB1 receptors could lead to degeneration of mesencephalic neurons, including DA and GABAergic neurons. Therefore, it is likely that these two receptor types may contribute to neurodegeneration in the CNS in response to the local release of endogenous ligands such as AEA.
Footnotes
This work was supported by a grant from the Korea Science and Engineering Foundation/Brain Disease Research Center and from the Brain Research Center of the 21st Century Frontier Research Program of the Korea Ministry of Science (B.K.J.).
Correspondence should be addressed to Byung K. Jin, Brain Disease Research Center, Ajou University School of Medicine, Suwon 442-749, Korea. E-mail: bkjin@ajou.ac.kr.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250662-10$15.00/0
References
- Campbell VA (2001) Tetrahydrocannabinol-induced apoptosis of cultured cortical neurones is associated with cytochrome c release and caspase-3 activation. Neuropharmacology 40: 702-709. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487-517. [DOI] [PubMed] [Google Scholar]
- Cernak I, Vink R, Natale J, Stoica B, Lea IVPM, Movsesyan V, Ahmed F, Knoblach SM, Fricke ST, Faden AI (2004) The “dark side” of endocannabinoids: a neurotoxic role for anandamide. J Cereb Blood Flow Metab 24: 564-578. [DOI] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR (1998) Hippocampal neurotoxicity of Δ9-tetrahydrocannabinol. J Neurosci 18: 5322-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Ryu JK, Kim J, Joe EH, Jin BK (2003a) Thrombin induces nigral dopaminergic neurodegeneration in vivo by altering expression of death-related proteins. Neurobiol Dis 14: 181-193. [DOI] [PubMed] [Google Scholar]
- Choi SH, Joe EH, Kim SU, Jin BK (2003b) Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo J Neurosci 23: 5877-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Joe EH, Ryu JK, Kim J, Lee YB, Cho KG, Oh YJ, Maeng SH, Baik HH, Kim SU, Jin BK (2001) GT1b ganglioside induces death of dopaminergic neurons in rat mesencephalic cultures. NeuroReport 12: 611-614. [DOI] [PubMed] [Google Scholar]
- Cortright D, Szallasi A (2004) Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem 271: 1814-1819. [DOI] [PubMed] [Google Scholar]
- Cutillas B, Espejo M, Gil J, Ferrer I, Ambrosio S (1999) Caspase inhibition protects nigral neurons against 6-OHDA-induced retrograde degeneration. NeuroReport 10: 2605-2608. [DOI] [PubMed] [Google Scholar]
- de Lago E, de Miguel R, Lastres-Becker I, Ramos JA, Fernandez-Ruiz J (2004) Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res 1007: 152-159. [DOI] [PubMed] [Google Scholar]
- Dib B, Falchi M (1996) Convulsions and death induced in rats by Tween 80 are prevented by capsaicin. Int J Tissue React 18: 27-31. [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686-691. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM (2000) Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J 14: 1432-1438. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L (2001a) Anandamide: some like it hot. Trends Pharmacol Sci 22: 346-349. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Lastres-Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB, Fernandez-Ruiz JJ (2001b) Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol 420: 123-131. [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS (2004) Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol 173: 2373-2382. [DOI] [PubMed] [Google Scholar]
- Downer E, Boland B, Fogarty M, Campbell V (2001) Delta 9-tetrahydrocannabinol induces the apoptotic pathway in cultured cortical neurones via activation of the CB1 receptor. NeuroReport 12: 3973-3978. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI (2003) Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-d-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol 163: 1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Montiel A, Garcia-Martinez C, Morenilla-Palao C, Garcia-Sanz N, Fernández-Carvajal A, Fernández-Ballester, Planells-Cases (2004) Molecular architecture of the vanilloid receptor. Insight for drug. Eur J Biochem 271: 1820-1826. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M (2002) Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22: 6900-6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Engberg G, Nissbrandt H, Magnusson T, Carlsson A (1988) Capsaicin-sensitive vasodilatatory mechanisms in the rat substantia nigra and striatum. J Neural Transm 74: 129-139. [DOI] [PubMed] [Google Scholar]
- Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ (2003) Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci 23: 5069-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA 97: 2875-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770-776. [DOI] [PubMed] [Google Scholar]
- Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, Di Marzo V (2003) Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci 60: 607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Berrendero F, Suárez I, García-Gil L, Cebeira M, Mackie K, Ramos JA, Fernández-Ruiz J (2000) Cannabinoid CB(1) receptors colocalize with tyrosine hydroxylase in cultured fetal mesencephalic neurons and their activation increases the levels of this enzyme. Brain Res 857: 56-65. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Muthian S, Kearn CS (1999) Effects of CB(1) cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS Lett 459: 277-281. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99: 8400-8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambrina E, Alonso R, Alcalde M, del Carmen Rodriguez M, Serrano A, Martinez-AC, Garcia-Sancho J, Izquierdo M (2003) Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J Biol Chem 278: 14134-14145. [DOI] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM (2003) Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience 119: 309-318. [DOI] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U (2002) Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem 277: 44448-44454. [DOI] [PubMed] [Google Scholar]
- Jung YS, Cho TS, Moon CH, Lee B, Lee SM, Shin HS (1999) Systemically administered capsazepine prevents the capsaicin-induced functional desensitization and loss of substance P-like immunoreactivity (SP-LI) in guinea-pig bronchi. Life Sci 64: 173-177. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW (1987) Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods 20: 83-90. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, de Miguel R, De Petrocellis L, Makriyannis A, Di Marzo V, Fernández-Ruiz J (2003) Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington's disease. J Neurochem 84: 1097-1109. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479-489. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c Cell 86: 147-157. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agro A (2000) Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J Biol Chem 275: 31938-31945. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Christie MJ, Connor M (2002) Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro J Physiol (Lond) 543: 531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB (2003) Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci 23: 3136-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall IC, Owen DE, Cripps TV, Davis JB, McNulty S, Smart D (2003) Activation of vanilloid receptor 1 by resiniferatoxin mobilizes calcium from inositol 1,4,5-trisphosphate-sensitive stores. Br J Pharmacol 138: 172-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA 97: 3655-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ (2003) Peripherally induced resiniferatoxin analgesia. Pain 104: 219-228. [DOI] [PubMed] [Google Scholar]
- Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ (2001) Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem 276: 11021-11030. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. San Diego: Academic.
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA (1998) Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res 51: 391-402. [DOI] [PubMed] [Google Scholar]
- Pita I, Jelaso AM, Ide CF (1999) IL-1beta increases intracellular calcium through an IL-1 type 1 receptor mediated mechanism in C6 astrocytic cells. Int J Dev Neurosci 17: 813-820. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Tschirch A, Ostendorf F, Dick S, Gaymard BM, Rivaud-Pechoux S, Sporkert F, Pragst F, Stadelmann AM (2002) Oculomotor effects of delta-9-tetrahydrocannabinol in humans: implications for the functional neuroanatomy of the brain cannabinoid system. Cereb Cortex 12: 1016-1023. [DOI] [PubMed] [Google Scholar]
- Rajgopal Y, Chetty CS, Vemuri MC (2003) Differential modulation of apoptosis-associated proteins by ethanol in rat cerebral cortex and cerebellum. Eur J Pharmacol 470: 117-124. [DOI] [PubMed] [Google Scholar]
- Rao GK, Zhang W, Kaminski NE (2004) Cannabinoid receptor-mediated regulation of intracellular calcium by delta(9)-tetrahydrocannabinol in resting T cells. J Leukoc Biol 75: 884-892. [DOI] [PubMed] [Google Scholar]
- Ray AM, Benham CD, Roberts JC, Gill CH, Lanneau C, Gitterman DP, Harries M, Davis JB, Davies CH (2003) Capsazepine protects against neuronal injury caused by oxygen glucose deprivation by inhibiting Ih J Neurosci 23: 10146-10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux-Laucat F, Fischer A, Deist FL (2003) Cell-death signaling and human disease. Curr Opin Immunol 15: 325-331. [DOI] [PubMed] [Google Scholar]
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, Harrison S (2003) Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol 138: 977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M (2002) Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol 137: 421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker KP, Maruyama I (2003) Anandamide induces cell death independently of cannabinoid receptors or vanilloid receptor 1: possible involvement of lipid rafts. Cell Mol Life Sci 60: 1200-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura T, Sasaki M, Tohda C, Kuraishi Y (1998) Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. NeuroReport 9: 2045-2048. [DOI] [PubMed] [Google Scholar]
- Shin CY, Shin J, Kim BM, Wang MH, Jang JH, Surh YJ, Oh U (2003) Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol Cell Neurosci 24: 57-68. [DOI] [PubMed] [Google Scholar]
- Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U (2002) Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150-10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Di Marzo V (2000) New perspectives on enigmatic vanilloid receptors. Trends Neurosci 23: 491-497. [DOI] [PubMed] [Google Scholar]
- Szöke Ė, Balla Z, Csernoch L, Czéh G, Szolcsányi J (2000) Interacting effects of capsaicin and anandamide on intracellular calcium in sensory neurons. NeuroReport 11: 1949-1952. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P (2002) Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 5: 546-551. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393-411. [DOI] [PubMed] [Google Scholar]
- Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, Agid Y, Hirsch EC (2001) Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord 16: 185-189. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V (2004) Endovanilloids. Putative endogenous ligands for transient receptor potential vanilloid 1 channels. Eur J Biochem 271: 1827-1834. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Veldhuis WB, van Haaften GW, Fezza F, Bisogno T, Bar PR, Veldink GA, Vliegenthart JF, Di Marzo V, Nicolay K (2001) Exogenous anandamide protects rat brain against acute neuronal injury in vivo J Neurosci 21: 8765-8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bar PR, Nicolay K, Di Marzo V (2003) Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci 23: 4127-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S (2003) Neuromorphological background of cannabis addiction. Brain Res Bull 61: 125-128. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231: 482-497. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Sarker KP, Kawahara K, Iino S, Yamakuchi M, Abeyama K, Hashiguchi T, Maruyama I (2003) Anandamide induces apoptosis in human endothelial cells: its regulation system and clinical implications. Thromb Haemost 89: 875-884. [PubMed] [Google Scholar]
- Yang MS, Park EJ, Sohn S, Kwon HJ, Shin WH, Pyo HK, Jin B, Choi KS, Jou I, Joe EH (2002) Interleukin-13 and-4 induce death of activated microglia. Glia 38: 273-280. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X (1999) An APAF-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274: 11549-11556. [DOI] [PubMed] [Google Scholar]