Abstract
Endocannabinoids mediate retrograde signaling and modulate synaptic transmission in various regions of the CNS. Depolarization-induced elevation of intracellular Ca2+ concentration causes endocannabinoid-mediated suppression of excitatory/inhibitory synaptic transmission. Activation of Gq/11-coupled receptors including group I metabotropic glutamate receptors (mGluRs) also causes endocannabinoid-mediated suppression of synaptic transmission. However, precise mechanisms of endocannabinoid production initiated by physiologically relevant synaptic activity remain to be determined. To address this problem, we made whole-cell recordings from Purkinje cells (PCs) in mouse cerebellar slices and examined their excitatory synapses arising from climbing fibers (CFs) and parallel fibers (PFs). We first characterized three distinct modes to induce endocannabinoid release by analyzing CF to PC synapses. The first mode is strong activation of mGluR subtype 1 (mGluR1)-phospholipase C (PLC) β4 cascade without detectable Ca2+ elevation. The second mode is Ca2+ elevation to a micromolar range without activation of the mGluR1-PLCβ4 cascade. The third mode is the Ca2+-assisted mGluR1-PLCβ4 cascade that requires weak mGluR1 activation and Ca2+ elevation to a submicromolar range. By analyzing PF to PC synapses, we show that the third mode is essential for effective endocannabinoid release from PCs by excitatory synaptic activity. Furthermore, our biochemical analysis demonstrates that combined weak mGluR1 activation and mild depolarization in PCs effectively produces 2-arachidonoylglycerol (2-AG), a candidate of endocannabinoid, whereas either stimulus alone did not produce detectable 2-AG. Our results strongly suggest that under physiological conditions, excitatory synaptic inputs to PCs activate the Ca2+-assisted mGluR1-PLCβ4 cascade, and thereby produce 2-AG, which retrogradely modulates synaptic transmission to PCs.
Keywords: retrograde signaling, 2-arachidonoylglycerol, metabotropic glutamate receptor type 1, phospholipase Cβ4, calcium, Purkinje cell
Introduction
Cannabinoid receptors are widely distributed throughout the mammalian CNS and are involved in various brain functions, including control of movement, emotion, and memory (Howlett et al., 2002; Piomelli, 2003). Recent studies have revealed that their endogenous ligands (endocannabinoids) work as retrograde messengers at central synapses and contribute to both short-term and long-term modulations of synaptic transmission (Maejima et al., 2001a; Alger, 2002; Gerdeman et al., 2002; Marsicano et al., 2002; Robbe et al., 2002; Wilson and Nicoll, 2002; Gerdeman and Lovinger, 2003). Endocannabinoids are produced on demand in an activity-dependent manner and released from postsynaptic neurons. Released endocannabinoids then activate presynaptic cannabinoid receptors and modify transmitter release. Such endocannabinoid-mediated synaptic modulation was found originally in the cerebellum (Kreitzer and Regehr, 2001a; Maejima et al., 2001b) and the hippocampus (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001) and later in other brain regions (Alger, 2002; Wilson and Nicoll, 2002; Piomelli, 2003).
Two major endocannabinoids, N-arachidonoylethanolamide (AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995), are synthesized from membrane phospholipids through two enzymatic reactions (Sugiura and Waku, 2000; Piomelli, 2003). AEA is produced by N-acyltransferase and phospholipase D (Di Marzo et al., 1994), whereas 2-AG is produced by phospholipase C (PLC) and diacylglycerol (DAG) lipase (Stella et al., 1997). Another route for 2-AG biosynthesis has been suggested (Sugiura and Waku, 2000; Piomelli, 2003). Although several previous reports suggest physiologically relevant pathways for endocannabinoid release, it is not fully determined whether 2-AG or AEA mediates retrograde signal and which synthetic pathway is driven by synaptic activity.
Endocannabinoid release can be triggered by two distinct protocols, depolarization-induced elevation of Ca2+ (Kreitzer and Regehr, 2001a; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001) and activation of Gq-coupled receptors, including group I metabotropic glutamate receptors (mGluRs) (Maejima et al., 2001b; Varma et al., 2001). In the hippocampus, synergic effect of these two protocols is reported. Combining mild receptor activation with weak depolarization prominently enhances endocannabinoid release (Varma et al., 2001; Kim et al., 2002; Ohno-Shosaku et al., 2002; Ohno-Shosaku et al., 2003). Gq-coupled receptors activate PLCβ, one of the five subfamilies of PLC (Rebecchi and Pentyala, 2000; Rhee, 2001; Kouchi et al., 2004). PLCβ consists of four isoforms (PLCβ1-4), and their enzymatic activities are shown to be dependent on Ca2+ levels (Rebecchi and Pentyala, 2000; Rhee, 2001). Using cultured hippocampal neurons, we found recently that the synergic effect of mild receptor activation and weak depolarization results from Ca2+-dependent enhancement of PLCβ1 activation (Hashimotodani et al., 2005). However, it is unclear whether the synergy is a general mechanism for endocannabinoid release that can be driven by excitatory synaptic activity. To address these issues, we examined excitatory synapses onto cerebellar Purkinje cells (PCs) in which synaptic activity is shown to drive endocannabinoid signaling (Maejima et al., 2001b; Brown et al., 2003). Our results strongly suggest that the synergy between the activation of mGluR subtype 1 (mGluR1) to PLCβ4 cascade and mild Ca2+ elevation underlies synaptically induced release of endocannabinoids, presumably 2-AG, in PCs.
Materials and Methods
Electrophysiology. Experiments were conducted according to the guidelines of the animal welfare committee of Kanazawa University. Parasagittal slices (250 μm thick) were cut postnatally from the cerebellar vermis of C57BL/6 or mutant mice 9-13 d of age, except for the experiments shown in Figure 6 [postnatal day 23 (P23) to P29]. Slices were superfused with an external solution containing the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose, and 10 μm bicuculline bubbled with 95% O2 and 5% CO2. Whole-cell recordings were made from visually identified PCs using an upright microscope (Axioskop-FS; Zeiss, Thornwood, NY) at 30°C. Patch pipettes (2-4 MΩ) were filled with one of the solutions with the following compositions (in mm): (1) 60 CsCl, 10 Cs d-gluconate, 20 tetraethylammonium (TEA)-Cl, 20 BAPTA, 4 MgCl2, 30 HEPES, 4 Mg-ATP, and 0.4 Na-GTP, pH 7.3, adjusted with CsOH, for the experiments shown in Figure 1; (2) 105 CsMeSO3, 5 CsCl, 20 TEA-Cl, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 10 HEPES, 4 Na-ATP, 0.4 Na-GTP, and 2 N-(2,6-dimethylcarbamoylmethyl) triethylammonium bromide, pH 7.3, for Figure 2 and supplemental Figure S2 (available at www.jneurosci.org as supplemental material); (3) 140 CsCl, 1 EGTA, 0.1 CaCl2, 4.6 MgCl2, 10 HEPES, 4 Na-ATP, and 0.4 Na-GTP, pH 7.3, for Figure 3 and supplemental Figure S1 (available at www.jneurosci.org as supplemental material) (this solution was modified by replacing CsCl and EGTA with 5 or 30 mm BAPTA and appropriate amounts of CaCl2 for the experiments in Figures 4 and 5); (4) 130 K d-gluconate, 6 KCl, 10 NaCl, 10 HEPES, 0.16 CaCl2, 2 MgCl2, 0.5 EGTA, 4 Na-ATP, and 0.4 Na-GTP, pH 7.3, adjusted with KOH, for Figure 6. For buffering postsynaptic calcium in Figure 6C, we replaced K d-gluconate with 30 mm BAPTA. Values of free calcium concentration were calculated with WinMAXC (http://www.stanford.edu/~cpatton/maxc.html). The pipette access resistance was compensated by 70-80%. Glass electrodes filled with the external solution were used to stimulate climbing fibers (CFs) in the granule cell layer or parallel fibers (PFs) in the molecular layer. In most experiments for recording CF-mediated EPSCs at -70 mV, their amplitudes were reduced by adding CNQX (1-2 μm) to the perfusate. Membrane currents or voltages were recorded with an EPC-9/2 amplifier (Heka Elektronik, Lambrecht/Pfalz, Germany). The signals were filtered at 3 kHz and digitized at 20 kHz. The PULSE software (Heka Elektronik) was used for controlling stimulation, data acquisition, and off-line analysis. All agonists and antagonists were purchased from Tocris Cookson (Bristol, UK) except tetrahydrolipstatin (THL), bovine albumin (Sigma Aldrich, St. Louis, MO), and N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole carboxamide (SR14176; a generous gift from Sanofi Recherche, Libourne, France). In the experiments with THL, slices were incubated for 1-2 h in the external solution containing 10-20 μm THL and 0.2 mg/ml bovine albumin and were also superfused continuously with the external solution containing 10 μm THL during recording.
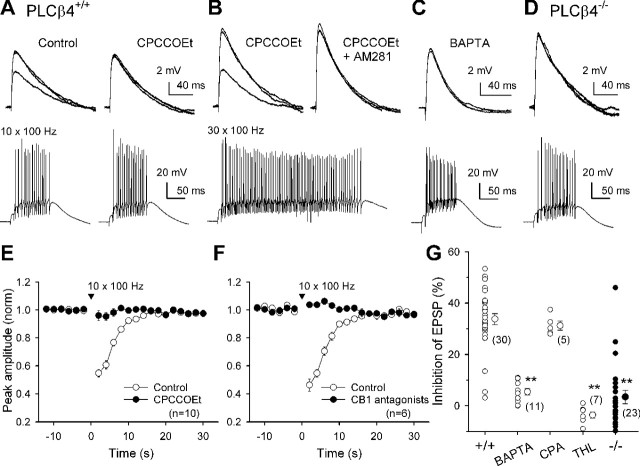
Figure 6.
A physiological range of PF activity can trigger the endocannabinoid signal through the mGluR1-PLCβ4 pathway. Effects of repetitive PF stimulation on PF-EPSPs recorded from PLCβ4+/+ (A-C) and PLCβ4-/- (D) PCs. A-D, Superimposed EPSP traces recorded 2 s before and 2 and 30 s after a PF burst (top) and the corresponding responses of a PC during the burst (bottom). A, A brief PF burst (10 stimuli at 100 Hz) transiently inhibited PF-EPSPs in control (left) but not in the presence of 100 μm CPCCOEt (right). B, Even in the presence of CPCCOEt, a stronger PF burst (30 stimuli at 100 Hz) induced transient inhibition (left), which was blocked by 2 μm AM281 (right). A and B were sequentially obtained. C, D, The brief PF burst failed to induce the inhibition in PCs dialyzed with the solution containing 30 mm BAPTA (C) and in PLCβ4-/- PCs (D). E, F, Effects of repetitive PF stimulation (10 stimuli at 100 Hz) on PF-EPSPs recorded from PCs. Averaged time courses of changes in peak amplitude of PF-EPSPs obtained in the indicated conditions. Data obtained in the presence of CB1 antagonists AM281 (2 μm) or SR141716A (2 μm) were pooled. G, Summary plots showing the magnitude of inhibition induced by a brief PF burst (10 stimuli; 100 Hz) under the indicated conditions. Magnitudes of inhibition were calculated from the mean amplitude of EPSPs obtained 2, 4, and 6 s after PF burst against that obtained before the burst. In each PC, the most effective site of stimulation was selected from several sites and more than three trials were conducted, and the average is plotted. **p < 0.01 versus wild-type control; t test.
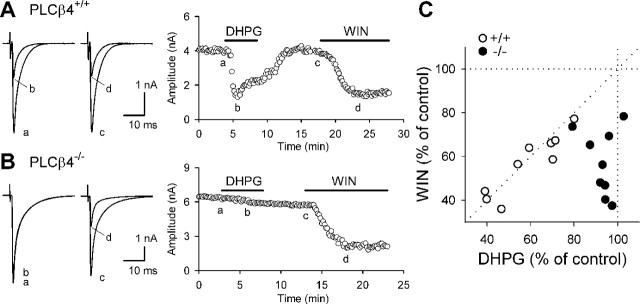
Figure 1.
PLCβ4 is required for mGluR1-driven endocannabinoid release. CF-EPSCs were recorded from PCs of wild-type mice (PLCβ4+/+) (A) and PLCβ4-deficient mice (PLCβ4-/-) (B). A, B, Representative results showing the effects of bath-applied DHPG (50 μm) and WIN (5 μm) on CF-EPSCs. Sample EPSC traces (left; a, b, c, d) were obtained at the time points indicated in the corresponding graphs (right) showing the time courses of changes in CF-EPSC amplitudes. Each sample trace is the average of six consecutive EPSCs. C, Scatter plots showing the correlation between the effects of 50 μm DHPG and 5 μm WIN on EPSC amplitudes.
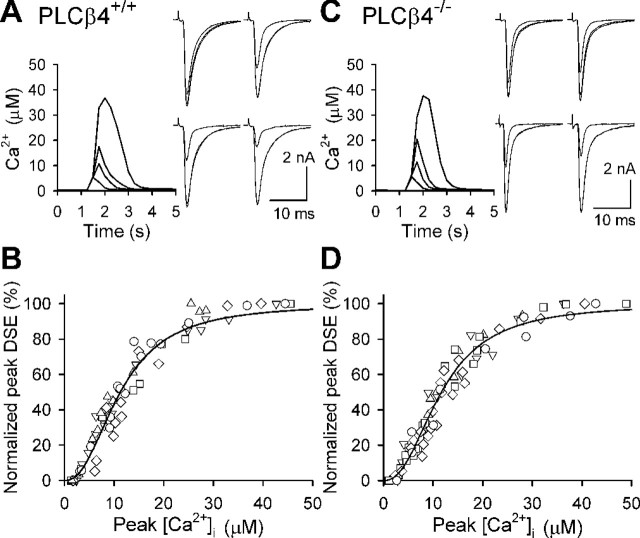
Figure 2.
PLCβ4 is not required for depolarization-induced endocannabinoid release. CF-EPSCs were recorded from PCs of wild-type mice (PLCβ4+/+) (A, B) and PLCβ4-deficient mice (PLCβ4-/-) (C, D). A, C, Examples of simultaneous recordings of Ca2+ transients (left) and DSE induced by PC depolarization with various durations. Ca2+ transients were detected with fura-FF at the proximal dendrites of PCs. The durations of depolarizing voltage steps were 200, 300, 400, and 750 (A) or 1000 ms (C). Three EPSC traces obtained 5 s before and 5 and 60 s after the depolarization are superimposed. B, D, Scatter plots showing the relationship between peak [Ca2+]i and the normalized value of peak DSE. The peak DSE was calculated as [1 - (EPSC2/EPSC1)] × 100%, where EPSC1 is the average baseline EPSC amplitude, and EPSC2 is the amplitude obtained 5 s after depolarization and was normalized to DSEmax in each cell. Data were obtained from five cells for each strain of mice, and each symbol indicates the data from the same cell. Pooled data are fitted with the Hill equation. Half-maximum DSE occurred at 11.2 μm Ca2+ in wild-type mice (B) and at 11.7 μm Ca2+ in PLCβ4-/- mice (D). The Hill coefficient was 2.3 for both strains.
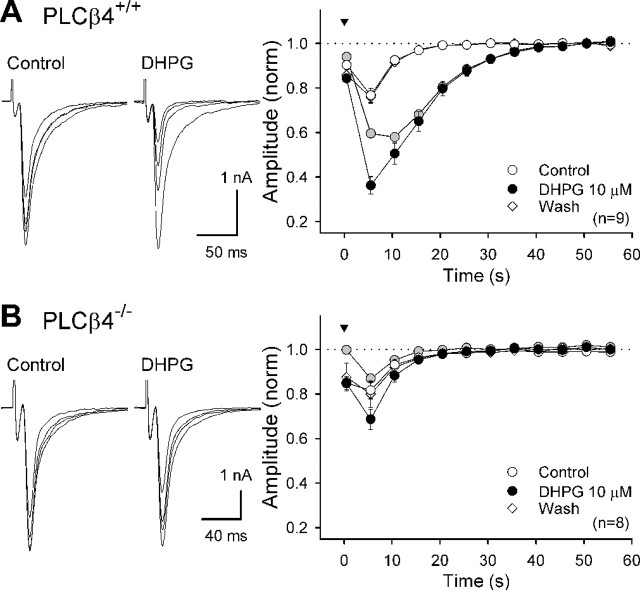
Figure 3.
Lack of DHPG-induced enhancement of DSE in PLCβ4-/- mice. CF-EPSCs were recorded from PLCβ4+/+ (A) and PLCβ4-/- (B) PCs. DSE was induced by a short-voltage step (100 ms; 0 mV; arrowhead). Averaged data for time courses of depolarization-induced changes in EPSC amplitude before (Control), during (DHPG 10 μm), and after (Wash) DHPG application are superimposed. The amplitude was normalized (norm) to the value before depolarization. Gray circles indicate differences between the normalized amplitudes before and during DHPG application. Left, Four CF-EPSCs obtained 5 s before and 5, 10, and 15 s after depolarization are superimposed in the presence or absence of DHPG.
Figure 4.
Endocannabinoid release by conjoint mild mGluR1 activation and Ca2+ elevation to a submicromolar range. The internal solution containing 5 mm BAPTA was used to reduce Ca2+ transients. CF-EPSCs and Ca2+ signals detected by fura-2 were simultaneously recorded. A, B, A representative experiment showing effects of 10 μm DHPG on changes in EPSC amplitudes (A) and Ca2+ transients (B) induced by a 75 ms voltage step. Inset, The Ca2+ signal was measured in the proximal dendrite of a PC and is depicted with a dashed line. Scale bar, 20 μm. C, Averaged time courses of changes in normalized (norm) EPSC amplitude evoked by a 75 or a 100 ms voltage step (0 mV) before (Control), during (DHPG 10 μm), and after (Wash) DHPG application. D, Summary plots showing the results of simultaneous recordings. Peak inhibition of EPSC was calculated from the EPSC amplitude obtained 10 s after depolarization and plotted against peak [Ca2+]i. Data from the same PCs obtained before and during DHPG application are connected with lines.
Figure 5.
Ca2+ dependence of endocannabinoid release induced by mild mGluR1 activation. CF-EPSCs were recorded from PCs dialyzed with pipette solutions containing various concentrations of CaCl2 and 30 mm BAPTA. A, B, Representative results showing the effects of bath application of 10 or 50 μm DHPG on CF-EPSCs. Sample EPSC traces (left; a, b, c) were obtained at the time points indicated in the corresponding graphs (right) showing the time courses of changes in CF-EPSC amplitudes. A, B, The calculated free Ca2+ concentration of the pipette solution was 1 nm (A) and 1 μm (B). C, Summary bar graph showing DHPG-induced suppression of EPSCs with the pipette solutions containing nominal [Ca2+] of 1 nm (n = 3), 10 nm (n = 6), 300 nm (n = 5), and 1000 nm (n = 6). Because the data obtained with 1 and 10 nm Ca2+ were identical, and those obtained with 300 and 1000 nm Ca2+ were not different, they were collectively shown as [Ca2+] of 1-10 nm (n = 9) and of 0.3-1 μm (n = 11), respectively.
Ca2+ imaging. For measuring intracellular Ca2+, PCs were loaded via dialysis for at least 25 min through a patch pipette with the internal solution containing a Ca2+ indicator, 500 μm Magnesium Green, 500 μm fura-FF (Molecular Probes, Eugene, OR), or 250 μm fura-2 (Dojindo, Kumamoto, Japan). Magnesium Green (500 μm) was excited at 488 nm with a monochromator system (Polychrome II; TILL Photonics, Martinsried, Germany). Fluorescence images were acquired with 10 ms of exposures at 30 Hz by using a cooled CCD camera system (IMAGO; TILL Photonics). The Ca2+-dependent fluorescence signals from selected regions of interest were background corrected and expressed as the ratio of the increase in fluorescence and the prestimulus fluorescence value (ΔF/F0) using Igor Pro software (WaveMetrics, Lake Oswego, OR). Ca2+ measurements with fura-FF (500 μm) and fura-2 (250 μm) followed the methods previously described in detail (Brenowitz and Regehr, 2003). Fura-FF and fura-2 were excited at 380 and 355 nm and 380 and 360 nm, respectively. The set of two images was collected at 4 Hz for 10 s. Exposure times were 20 ms for fura-FF and 30 ms for fura-2. To convert fluorescence ratios to [Ca2+]i, the following formula (Grynkiewicz et al., 1985) was used: [Ca2+]i = Keff*(R - Rmin)/(Rmax - R), where R is the ratio value, and Rmin and Rmax are the values obtained at zero and saturating levels of Ca2+, respectively. We used the Rmin values measured at the experimental imaging system with 30 μm path length cuvette filled with the Ca2+ free internal solutions plus the indicators. The Rmax values were measured at the proximal dendrite of PCs either by applying ionomycin (20 μm) for fura-FF or by long depolarization (>2 s) for fura-2 in the presence of 10 mm external Ca2+. For the measurement with fura-FF, the indicator dissociation constant obtained in vitro measurement was assigned to the Keff based on the previous experiment (Brenowitz and Regehr, 2003). For fura-2, the Keff was determined from the ratio value obtained in the proximal dendrite close to the soma of the PC dialyzed with internal solutions containing 300 nm free Ca2+.
Measurement of 2-AG. Eight parasagittal cerebellar slices were prepared from each P11-P13 mouse as in electrophysiological experiments and were pooled as one sample. Slices were incubated for 1-2 h in a reservoir chamber filled with the normal external solution at room temperature. Then, slices were transferred to a glass sample bottle filled with the external solution containing 1 μm tetrodotoxin to prevent synaptic transmission and were kept at 22-24°C for 10-20 min. The reaction was performed by adding one of the reagents for 30 s and then stopped by adding dry ice-cooled Bligh-Dyer extraction mixture including 0.002% butylated hydroxytoluene to prevent lipid peroxidation. Frozen slices were homogenized quickly using a blender (Polytoron; Kinematica, Littau, Switzerland). The mixture was supplemented with acetic acid against liquid alkalization to keep 2-monoacylglycerols from converting to 1(3)-monoacylglycerols and was then kept in cold storage for at least 1 h. After total lipids were extracted, monoacylglycerols were purified and analyzed as described previously (Kondo et al., 1998b; Sugiura et al., 2000). Briefly, monoacylglycerols were separated by TLC and converted to their 1-dianthroyl derivatives for detecting the fluorescence. The purified derivatives were analyzed with an HPLC system (Shimadzu, Tokyo, Japan) equipped with a reverse-phase column (Shiseido, Tokyo, Japan) and a fluorescence detector. 2-AG amount was normalized with the amount of lipid phosphorus measured from each total of lipids extracts. To compare with mutant mice of the same age in the consecutive experiments, C57BL/6 mice were used as wild-type control mice.
Measurement of AEA. For measuring AEA, rat cerebellar slices were used to obtain a larger amount of extracted lipids. AEA was analyzed as described previously (Kondo et al., 1998a). A total of lipids, with 0.1 nmol of N-heptadecanoylethanolamine added as an internal standard, was fractionated by TLC, with development with chloroform:methanol:25% NH4OH (80:20:2, v/v), in a tank sealed with N2 gas. The area corresponding to standard N-acylethanolamine was scraped off the TLC plate and extracted from the silica gel by the method of Bligh and Dyer. The extraction was conducted in the presence of butylated hydroxytoluene (0.001%) in an N2 gas-sealed tube. N-acylethanolamine was further purified by two-dimensional TLC, with development first with petroleum ether:diethyl ether:acetone:acetic acid (30:40:20:1, v/v) and then with an organic layer of ethyl acetate:petroleum ether:acetic acid:water (100:50:20:100, v/v). The purified N-acylethanolamine was converted to its 1-anthroyl derivative using 1-anthroyl cyanide and quinuclidine and then purified by TLC, with development with petroleum ether:diethyl ether:acetic acid (40:60:1, v/v). The purified 1-anthroyl derivative of N-acylethanolamine was analyzed with an HPLC system (Shimadzu) equipped with a reverse-phase column (Shiseido) and a fluorescence detector.
PLCβ4-deficient mice and mGluR1-rescue mice. PLCβ4-deficient (PLCβ4-/-) mice were generated with a CJ7 ES cell clone derived from 129sv mice and were back crossed with C57BL/6 mice (Jiang et al., 1996; Kano et al., 1998). Knock-out mice of F4 or later generations and the corresponding wild-type littermates (PLCβ4+/+) were used for electrophysiological studies. The mGluR1-rescue mice used in the present study were homozygous for L7-mGluR1α transgene, which expressed mGluR1α ∼1/40 of the wild-type mice (Ichise et al., 2000).
Statistics. Data are represented as means ± SEM, and numbers of experiments are indicated in parentheses. Statistical significance was assessed by Student's t test. One and two asterisks indicate p < 0.05 and p < 0.01, respectively.
Results
mGluR1-driven endocannabinoid release requires PLCβ4
Activation of postsynaptic mGluR1 retrogradely suppresses CF to PC excitatory transmission through endocannabinoids under the condition preventing elevation of the intracellular Ca2+ concentration ([Ca2+]i) (Maejima et al., 2001b). First, we examined whether deficiency of PLCβ4 affects this endocannabinoid-mediated retrograde signaling with PLCβ4-/- mice. We made whole-cell recordings from PCs in the rostral portion of parasagittal cerebellar slices (lobules 1-5 and the rostral half of lobule 6). In this portion, PLCβ4 is the major isoform of PCs (Kano et al., 1998). We recorded CF-EPSCs in monoinnervated PCs or the largest CF-EPSCs in multiply-innervated PCs (Hashimoto and Kano, 2003) with an internal solution containing 20 mm BAPTA. Although PLCβ4-/- mice have persistent multiple CF innervations, basic electrophysiological properties of CF-EPSCs are normal (Kano et al., 1998; Hashimoto et al., 2001; Miyata et al., 2001). In the wild-type (PLCβ4+/+) mice, bath application of a group I mGluR agonist, R,S-3,5-dihydroxyphenylglycine (DHPG) (50 μm), readily caused reversible suppression of CF-EPSCs (amplitude; 59 ± 5% of control; n = 9) (Fig. 1A). After the recovery of CF-EPSC, subsequent application of a cannabinoid receptor agonist, WIN55,212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate] (WIN) (5 μm), caused inhibition with a similar extent to that induced by DHPG (57 ± 5% of control; n = 9). The extent of suppression by DHPG strongly correlated with that by WIN in individual PCs (Fig. 1C). In contrast, DHPG (50 μm) little affected CF-EPSCs in PLCβ4-/- mice (93 ± 2% of control; n = 9), whereas WIN (5 μm) effectively suppressed CF-EPSCs to an extent similar to that for PLCβ4+/+ mice (57 ± 5% of control; n = 9) (Fig. 1B,C). These results clearly indicate that PLCβ4 is involved in the endocannabinoid release induced by mGluR1 activation.
Depolarization-induced endocannabinoid release is normal in PLCβ4-/- mice
We next examined whether PLCβ4 is involved in depolarization-induced endocannabinoid release. Depolarization of PCs induces transient suppression of excitatory (Kreitzer and Regehr, 2001a) and inhibitory (Llano et al., 1991) synaptic transmission, phenomena termed depolarization-induced suppression of excitation (DSE) and depolarization-induced suppression of inhibition (DSI). DSE/DSI are mediated by endocannabinoids produced by depolarization-induced transient elevation of [Ca2+]i without mGluR activation (Kreitzer and Regehr, 2001a,b; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001; Diana et al., 2002; Yoshida et al., 2002) and requires Ca2+ elevation to a micromolar range (Wang and Zucker, 2001; Brenowitz and Regehr, 2003). It is reported that the extent of DSE/DSI is strongly dependent on the peak [Ca2+]i level (Wang and Zucker, 2001; Brenowitz and Regehr, 2003). To compare the Ca2+ dependence of DSE at CF-PC synapses between wild-type and PLCβ4-/- mice, we performed the experimental procedure previously used in rat PCs (Brenowitz and Regehr, 2003). PCs were loaded with a low-affinity Ca2+ indicator dye, fura-FF (500 μm). After recording eight control CF-EPSCs evoked at 0.2 Hz, we applied a depolarizing voltage pulse to the soma. We measured the evoked [Ca2+]i in proximal dendritic regions of PCs and simultaneously recorded the resultant changes of CF-EPSCs (Fig. 2A,C). We repeated several trials of depolarization in the same PC varying duration of voltage steps from 100-1000 ms, which yielded Ca2+ transients with various peak levels. Then we plotted peak DSE magnitude against peak [Ca2+]i and fitted the data with the Hill equation (Fig. 2B,D). The mean [Ca2+]i for half-maximal DSE, Hill coefficient, and maximum peak DSE (DSEmax) were 11.3 ± 0.5 μm, 2.4 ± 0.1, and 75 ± 3% for PLCβ4+/+ PCs (n = 5), and 11.5 ± 0.3 μm, 2.3 ± 0.1, and 68 ± 5% for PLCβ4-/- PCs (n = 5). The relationship between the extent of DSE and the peak [Ca2+]i was almost the same for PLCβ4+/+ and PLCβ4-/- PCs, indicating that PLCβ4 is dispensable for DSE in PCs evoked by large Ca2+ elevation to a micromolar range. Thus, in the absence of Gq/11-coupled receptor activation, large Ca2+ elevation to a micromolar range is required for the endocannabinoid release (Wang and Zucker, 2001; Brenowitz and Regehr, 2003), which we term solely Ca2+-driven endocannabinoid release (CaER). Conversely, activation of Gq/11-coupled receptors can induce endocannabinoid release without detectable Ca2+ elevation as reported previously (Maejima et al., 2001b), which we term receptor-driven endocannabinoid release (RER).
PLCβ4 is required for mGluR1-mediated enhancement of DSE
Previous experiments have shown that weak activation of group I mGluRs (Varma et al., 2001; Ohno-Shosaku et al., 2002) or M1/M3 muscarinic acetylcholine receptors (Kim et al., 2002; Ohno-Shosaku et al., 2003; Fukudome et al., 2004) markedly enhances DSI in the hippocampus. As detailed in supplemental Figure S1 (available at www.jneurosci.org as supplemental material), we confirmed that weak activation of postsynaptic mGluR1 enhances DSE of CF-EPSCs in PCs. We then examined whether PLCβ4 is required for the DHPG-induced enhancement of DSE by using PLCβ4-/- mice. In PLCβ4+/+ mice, bath application of a low dose (10 μm) of DHPG, which by itself had no effect on CF-EPSCs (amplitude; 100 ± 2% of control; n = 9), markedly enhanced DSE induced by a short-voltage step (100 ms; 0 mV) (Fig. 3A). In contrast, DHPG (10 μm) failed to enhance DSE in PLCβ4-/- mice (Fig. 3B), although the magnitude and time course of control DSE were similar to those of PLCβ4+/+ mice (Fig. 3B). Furthermore, three repetitive voltage steps (at 1 Hz) normally induced large DSE in these PLCβ4-/- mice (peak DSE; 60 ± 4%, n = 7 for PLCβ4-/-;62 ± 4%, n = 5 for PLCβ4+/+). These results indicate that PLCβ4 is required for DHPG-induced enhancement of DSE but is dispensable for CaER.
Ca2+-assisted receptor-driven endocannabinoid release
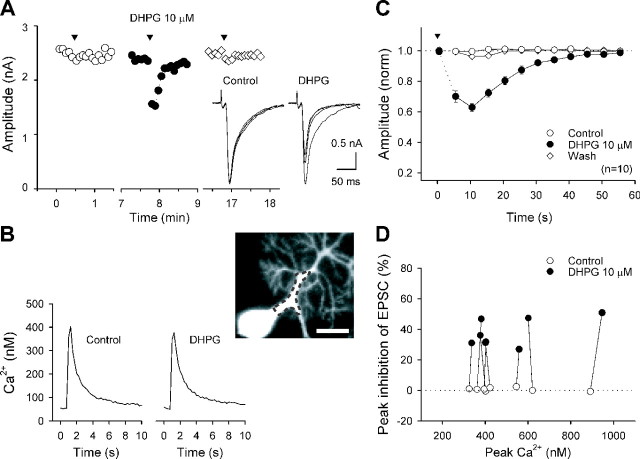
Our recent study on cultured hippocampal neurons (Hashimotodani et al., 2005) has demonstrated that the enhancement of DSI by muscarinic or group I mGluR activation results from potentiation of receptor-driven PLCβ1 activation by Ca2+ elevation to a submicromolar range rather than potentiation of DSI mechanism itself. This study suggests that the mGluR1-driven enhancement of cerebellar DSE may result from Ca2+-dependent upregulation of PLCβ, presumably PLCβ4. Therefore, we examined whether Ca2+ transients in a submicromolar range, which were too small to induce CaER, could suppress CF-EPSCs when the mGluR1 cascade is activated simultaneously. We recorded CF-EPSCs using an internal solution containing 5 mm BAPTA to reduce the amplitude of Ca2+ transient. A short depolarizing pulse alone (75 or 100 ms) failed to suppress CF-EPSCs under this condition (Fig. 4A,C). In the presence of a low dose (10 μm) of DHPG, which did not suppress CF-EPSCs (amplitude; 96 ± 2% of control; n = 10), the same voltage pulse induced a clear suppression of CF-EPSCs (Fig. 4A,C). We measured the [Ca2+]i in the proximal dendrite of PCs using fura-2 (250 μm), a high-affinity Ca2+ indicator while simultaneously recording CF-EPSCs (Fig. 4B). The peak values of Ca2+ transients were not influenced by DHPG (496 ± 66 nm in control; 501 ± 72 nm in DHPG; n = 8). The peak [Ca2+]i was within a submicromolar range and much lower than that required for CaER (Fig. 4D). The time course and the extent of suppression (Fig. 4C) were very similar to those of the enhanced component of DSE by DHPG (i.e., the differences of DSE before and during DHPG application) (Fig. 3A, gray symbols, and supplemental Fig. S1B, gray symbols, available at www.jneurosci.org as supplemental material). These results suggest that the DSE enhancement by DHPG is caused by endocannabinoid release triggered by weak mGluR1 activation combined with mild Ca2+ elevation to a submicromolar range, which we term Ca2+-assisted receptor-driven endocannabinoid release (Ca-RER).
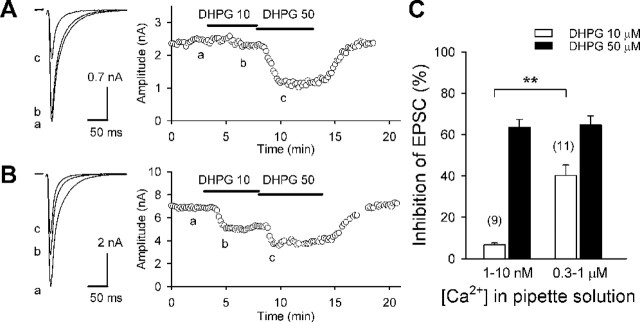
Ca-RER requires [Ca2+]i in a submicromolar range
In the experiments shown in Figures 3 and 4 and supplemental Figure S1 (available at www.jneurosci.org as supplemental material), we used the pipette solutions with an estimated free Ca2+ concentration of ∼10 nm. In this condition, a low dose (5 or 10 μm) of DHPG by itself did not cause significant suppression of CF-EPSCs. To further examine the Ca2+ dependence of mGluR1-induced endocannabinoid release that underlies Ca-RER, we used pipette solutions with estimated free Ca2+ concentrations of 1, 10, 300, and 1000 nm prepared with 30 mm BAPTA plus appropriate amounts of CaCl2. After membrane rupture, we waited at least 20 min before recording EPSCs to allow the pipette solution to diffuse into the soma and dendrite of PCs. A low dose (10 μm) of DHPG caused a clear suppression of CF-EPSCs with the solution containing 0.3-1 μm Ca2+ (Fig. 5B), whereas the same dose of DHPG little affected CF-EPSCs with the solution containing 1-10 nm Ca2+ (Fig. 5A). In contrast, a higher dose (50 μm) of DHPG induced marked suppression of CF-EPSCs regardless of the Ca2+ concentration of the pipette solution. The clear difference between low Ca2+ (1-10 nm) and higher Ca2+ (0.3-1 μm) (Fig. 5C) indicates that the effect of 10 μm DHPG on endocannabinoid release is strongly dependent on the intracellular free Ca2+ level. We checked the possibility that endocannabinoids might be released constitutively when the intracellular Ca2+ level was high. The addition of an antagonist of CB1 cannabinoid receptor, SR141716 (1 μm), had no effect on CF-EPSCs (amplitude; 100 ± 1% of control; n = 4) with the pipette solution containing 1 μm Ca2+, suggesting that constitutive endocannabinoid release did not occur under these conditions. These results suggest that Ca-RER results from enhancement of mGluR1-induced endocannabinoid release by elevation of the intracellular free Ca2+ level to several hundred nanomolar. In contrast, CaER required elevation of Ca2+ to much higher levels (Fig. 2) (Wang and Zucker, 2001; Brenowitz and Regehr, 2003).
A short PF burst induces Ca-RER involving the mGluR1-PLCβ4 pathway
Previous studies have shown that both mGluR1-induced suppression and DSE occur at PF to PC synapses (Conquet et al., 1994; Kreitzer and Regehr, 2001a; Maejima et al., 2001b; Neale et al., 2001; Brenowitz and Regehr, 2003; Brown et al., 2003). It is also shown that repetitive stimulation of PFs can induce endocannabinoid-mediated transient inhibition of PF synaptic inputs to PC (Brown et al., 2003). We therefore examined PF synaptic transmission to investigate how the mGluR1-PLCβ4 signaling cascade contributes to endocannabinoid release by synaptic activation. We first confirmed that this cascade contributes to endocannabinoid release at PF synapses in a similar manner to that at CF synapses (detailed in supplemental Fig. S2, available at www.jneurosci.org as supplemental material). We next confirmed that PF bursts in a physiological frequency range evokes the endocannabinoid-mediated retrograde inhibition of PF-PC synaptic transmission in mice as shown previously in rats (Brown et al., 2003). We recorded EPSPs in response to PF stimulation at 0.5 Hz using potassium-based internal solution under the current-clamp mode. We found that a train of 10 stimuli at 100 Hz transiently reduced subsequent PF-EPSPs (Fig. 6A,E-G). This inhibition was completely blocked by a selective mGluR1 antagonist, 7-(hidroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt; 100 μm) (Fig. 6A,E) and also by CB1 antagonists SR141716 or 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM281) (2 μm) (Fig. 6F), suggesting that this transient suppression resulted from mGluR1-dependent endocannabinoid release. However, evoking larger PF-EPSPs and increasing the number of pulses per train to >30 resulted in a transient suppression of PF-EPSPs that was resistant to CPCCOEt (n = 8) (Fig. 6B). This suppression was totally blocked by AM281 (Fig. 6B), indicating that it was mediated by endocannabinoids. Because repetitive PF stimulation is known to cause local depolarization in PC dendrites and a Ca2+ transient (Takechi et al., 1998; Brown et al., 2003), the mGluR1 antagonist-resistant suppression is most likely to result from CaER.
We determined whether the mGluR1-dependent component of suppression is caused by Ca-RER by examining the requirement of Ca2+ elevation and PLCβ4. PF-EPSPs were examined after PCs were dialyzed with an internal solution containing 30 mm BAPTA for at least 15 min. A short PF burst (10 stimuli at 100 Hz) occasionally induced prolonged depolarization longer than 200 ms accompanying action potential trains, presumably because intracellular BAPTA blocked Ca2+-activated K+ conductance. To avoid the influence of the prolonged depolarization, we analyzed suppression of PF-EPSPs in 11 PCs in which such prolonged depolarization was not elicited. In these cells, a short PF burst failed to induce clear suppression of PF-EPSPs (Fig. 6C,G). In PLCβ4-/- mice, a short PF burst did not induce transient suppression of PF-EPSP in the majority of rostral PCs (Fig. 6D,G). In contrast, a longer PF tetanus (100 Hz, 20-50 pulses) induced clear suppression (30.6 ± 3.7% of control; n = 11) in PLCβ4-/- mice, which was not affected by the mGluR1 antagonist CPCCOEt (36.4 ± 2.5% in control; 35.7 ± 3.5% in CPCCOEt; n = 3). This result suggests that suppression of PF-EPSPs through CaER is intact in PLCβ4-/- mice.
The mGluR1-PLCβ4-dependent suppression of PF-EPSP was not affected by the treatment with cyclopiazonic acid (30 μm) (Fig. 6G), a blocker of ATP-driven uptake of Ca2+ into the endoplasmic reticulum, which blocks mGluR1-mediated local Ca2+ signals in PCs (Takechi et al., 1998). Therefore, inositol (1,4,5)-trisphosphate-mediated Ca2+ release from internal stores had little contribution to the Ca2+ elevation during the PF train, at least in our recording condition. We assume that Ca2+ influx through voltage-gated Ca2+ channels may be the major source of Ca2+ elevation during a PF train. Furthermore, we found that the lipase inhibitor THL (10 μm), which blocks the enzyme DAG lipase (Bisogno et al., 2003), eliminated the suppression of PF-EPSPs by a short PF burst (Fig. 6G). Because 2-AG is synthesized from phosphoinositides by PLC and DAG lipase (Stella et al., 1997), this result suggests that 2-AG is produced during a short PF burst via mGluR1-PLCβ4 cascade and mediates transient suppression of PF-EPSPs. Thus, Ca-RER that can be elicited by a physiological range of synaptic activity appears to function at PF-PC synapses in vivo.
Activation of mGluR1-PLCβ4 pathway produces 2-AG in PCs
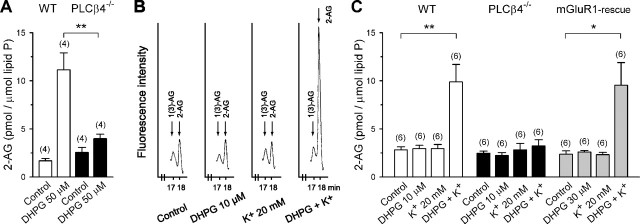
Our electrophysiological data suggest that Ca2+-assisted mGluR1-PLCβ4 cascade is crucial for the release of endocannabinoid, presumably 2-AG, by synaptic activity of the PC. We then measured 2-AG levels directly in cerebellar slices after mGluR1 stimulation with or without depolarization. We first examined the effects of a high dose (50 μm) of DHPG. We found that DHPG markedly increased the level of 2-AG by 6.6 times against sham-operated control in slices from wild-type (C57BL/6) mice (Fig. 7A). In contrast, DHPG only slightly increased the level of 2-AG by 1.6 times that of control in PLCβ4-/- slices. We next tested the effects of a low dose (10 μm) of DHPG and 20 mm K+-induced depolarization on 2-AG levels. In wild-type mice, neither 20 mm K+ nor 10 μm DHPG increased the 2-AG level over control (Fig. 7B,C). However, their combination markedly increased the 2-AG level by >3.5 times that of control. In contrast, none of the three treatments elevated the level of 2-AG over control in PLCβ4-/- mice (Fig. 7C). These results indicate that PLCβ4 is crucial for 2-AG production in the cerebellum. Because mGluR1 is densely expressed in the dendritic membrane of the PC (Martin et al., 1992), it is conceivable that 2-AG elevation by DHPG mainly originated from PCs. To further confirm that PCs generate 2-AG under these conditions, we examined the mGluR1-rescue mice that express mGluR1 only in PCs (Ichise et al., 2000). Neither 30 μm DHPG alone nor 20 mm K+ alone was effective, whereas a combination of the two markedly increased the level of 2-AG by approximately four times that of control (Fig. 7C). A higher dose (30 μm) of DHPG was required to elicit a clear change in the level of 2-AG in the mGluR1-rescue mice, presumably because their mGluR1 expression was ∼1/40 of the wild-type mice (Ichise et al., 2000). Nevertheless, these results clearly indicate that 2-AG is produced in PCs after activation of mGluR1-PLCβ4 cascade with mild depolarization. In marked contrast, we could not detect production of AEA, the other endocannabinoid candidate, in any conditions in which we detected 2-AG production (data not shown). Together, our present results strongly suggest that 2-AG is released from PCs by synaptic activity and mediate retrograde modulation of synaptic transmission to PC.
Figure 7.
PLCβ4 is required for 2-AG production induced by mGluR1 activation with or without mild depolarization. Cerebellar slices were stimulated by adding the reagents for 30 s in the presence of tetrodotoxin (1 μm). Amounts of 2-AG were normalized with the amount of lipid phosphorus (lipid P) measured from each total of lipids extracts. A, Summary bar graph showing the effects of a high dose of DHPG (50 μm) on 2-AG contents of wild-type (WT) and PLCβ4-/- slices. B, Specimen HPLC traces showing the parts of 2-AG and 1(3)-AG retention time from whole-separation profiles of various species of standard monoacylglycerols. The data set was obtained from consecutive measurements of four samples from wild-type mice after the treatments as indicated below the traces. C, Summary bar graph showing the effects of lower doses of DHPG (10 or 30 μm), 20 mm K+, and their combination on 2-AG contents of WT, PLCβ4-/-, and mGluR1-rescue slices.
Discussion
The present study demonstrates three modes of endocannabinoid-mediated retrograde suppression in PCs. Activation of mGluR1 can induce endocannabinoid-mediated retrograde suppression of excitatory synaptic transmission through PLCβ4 without significant elevation of [Ca2+]i (RER). Depolarization-induced [Ca2+]i elevation to a micromolar range can induce endocannabinoid release, which does not require PLCβ4 (CaER). In addition, endocannabinoid release is induced by weak activation of mGluR1 to PLCβ4 cascade combined with mild Ca2+ elevation to a submicromolar range (Ca-RER). A short burst of PF stimulation induces endocannabinoid-mediated transient suppression of PF to PC excitatory transmission, which is blocked by an mGluR1 antagonist and intracellular infusion of BAPTA, and is absent in PLCβ4-/- mice. These data indicate that Ca-RER is essential for effective endocannabinoid release from PCs by PF synaptic activity. Our biochemical experiments demonstrate that combined weak mGluR1 activation in PCs and mild depolarization effectively produce 2-AG, whereas either stimulus alone is insufficient to produce detectable 2-AG. These data strongly suggest that under physiological conditions, excitatory synaptic activity triggers Ca-RER in PCs, and the released 2-AG retrogradely suppresses excitatory synaptic transmission to PCs. The putative cascade driven by synaptic activity is illustrated schematically in supplemental Figure S3 (available at www.jneurosci.org as supplemental material).
PLCβ4 mediates mGluR1-induced endocannabinoid release in PCs
We have shown previously that PLCβ4 deficiency did not cause any perceptive abnormalities in gross cerebellar anatomy, PC morphology, PF synapse formation, synaptic transmission, or voltage-gated Ca2+ channel currents (Kano et al., 1998; Hashimoto et al., 2001; Miyata et al., 2001). Importantly, expression of mGluR1a was normal in PLCβ4-/- mice (T. Miyazaki and M. Watanabe, unpublished data). Moreover, we did not find any difference in cannabinoid sensitivity of CF and PF synapses between wild-type and PLCβ4-/- mice. These results indicate that the lack of mGluR1-induced retrograde signaling and 2-AG formation in PLCβ4-/- mice results from impaired intracellular signaling involving PLCβ4 but not from other morphological or physiological abnormalities. In PLCβ4-/- mice, mGluR1-induced Ca2+ release is deficient in the rostral cerebellum (Miyata et al., 2001). However, this is not the cause of the defect of mGluR1-induced endocannabinoid release, because mGluR1-induced retrograde inhibition occurred in the presence of intracellular BAPTA or under blockade of Ca2+ release in wild-type mice. Therefore, we conclude that the defect of mGluR1-induced endocannabinoid release in PLCβ4-/- mice results from the lack of enzymatic activity of PLCβ to produce DAG, the precursor of 2-AG.
Ca-RER in the cerebellum and hippocampus
Our present results strongly suggest that endocannabinoids are produced through the mGluR1-PLCβ4 cascade that is potentiated by submicromolar Ca2+. In the hippocampus, we reported recently that endocannabinoid release driven by Gq/11-coupled receptors requires PLCβ1 (Hashimotodani et al., 2005). This PLCβ1-mediated endocannabinoid release is strongly dependent on the basal level of [Ca2+]i and is augmented by depolarization-induced transient elevation of [Ca2+]i, indicating that Ca-RER is also seen in the hippocampus. Because PLCβ1 and PLCβ4 are rich in the CNS and display complementary expression in the forebrain and hindbrain, respectively (Watanabe et al., 1998), each central neuron has either of the two PLCβ isoforms. Thus, Ca-RER involving either PLCβ1 or PLCβ4 appears to be a general mechanism by which postsynaptic neuronal activity retrogradely modulates presynaptic function.
Ca-RER can be explained by the property of PLCβ activation. PLCβ activity is dependent not only on the extent of Gq/11-coupled receptor activation (i.e., agonist dose) but also on [Ca2+]i (Rebecchi and Pentyala, 2000; Rhee, 2001). PLCβs have G-protein-binding regions and a conserved catalytic region that requires Ca2+ binding for catalytic activity. Biochemical data suggest that the required Ca2+ level might be within a submicromolar range (Rebecchi and Pentyala, 2000; Rhee, 2001). Recently, we directly examined Ca2+ dependence of PLCβ1 activity in intact hippocampal neurons by using exogenously expressed TRPC6 (canonical transient receptor potential channel 6) channel as a biosensor for the PLC product, DAG (Hashimotodani et al., 2005). PLCβ1 activation by Gq/11-coupled receptors exhibits a similar Ca2+ dependence to that of receptor-driven endocannabinoid release and is augmented by depolarization-induced transient elevation of [Ca2+]i. These results strongly suggest that the Ca2+ dependency of PLCβ1 and presumably that of PLCβ4 are responsible for Ca-RER in the hippocampus and cerebellum, respectively.
2-AG formation by PLCβ activity
In accordance with biochemical data that 2-AG production involves PLC activity (Stella et al., 1997; Sugiura and Waku, 2000; Piomelli, 2003), our present data from analysis of PLCβ4-/- and mGluR1-rescue mice indicate that mGluR1 activation in PCs causes 2-AG production through PLCβ4. In cultured cortical neurons, activation of acetylcholine receptors is reported to enhance 2-AG production when combined with NMDA receptor-mediated Ca2+ influx (Stella and Piomelli, 2001). This is analogous to our experiments that coapplication of a lower dose of DHPG and 20 mm K+ produced 2-AG from PCs. In electrophysiological studies, it is reported that pharmacological blockade of PLC or DAG lipase partially blocks mGluR1-induced suppression of an IPSC in cerebellar PCs (Galante and Diana, 2004) and abolishes endocannabinoid-mediated synaptic plasticity dependent on group I mGluR in hippocampal pyramidal cells (Chevaleyre and Castillo, 2003). We also found that a lipase inhibitor, THL, blocked suppression of PF-EPSPs by a PF train. Thus, it is highly likely that group I mGluR-PLCβ cascade is involved in the production of 2-AG that mediates retrograde signaling. Conversely, the enzymatic inhibitors were reported to have no effect on DSI (Chevaleyre and Castillo, 2003), suggesting that another endocannabinoid might mediate DSI. However, inhibition of cyclooxygenase 2, which can degrade 2-AG, prolongs DSI in CA1 pyramidal cells (Kim and Alger, 2004), and 2-AG can be produced by Ca2+ influx alone (Stella et al., 1997; Stella and Piomelli, 2001). In our preliminary experiments, we found that depolarization of cerebellar slices with 50 mm K+ induced significant 2-AG production even in PLCβ4-/- mice but did not produce AEA. Furthermore, we found that three repetitive voltage steps (100 ms to 0 mV; 1 Hz) did not induce DSE of CF-EPSCs (peak DSE; 4 ± 1%; n = 5) in slices incubated with 10 μm THL, whereas DSE normally occurred in the vehicle-treated slices (peak DSE; 55 ± 8%; n = 4). These results suggest that 2-AG may also contribute to CaER. If 2-AG mediates CaER, it must be produced by Ca2+ elevation alone without activation of Gq/11-coupled receptors. PLCδs can be good candidates, because they can be activated by Ca2+ elevation alone (Rebecchi and Pentyala, 2000; Rhee, 2001).
Possible physiological significance of Ca-RER
We found that a short PF burst could induce endocannabinoid-mediated transient suppression of PF synaptic inputs in mouse PCs. This suppression required mGluR1, Ca2+ elevation, and PLCβ4 but was not affected by depletion of internal Ca2+ stores. We therefore conclude that PF-mediated excitatory synaptic inputs to PCs can activate mGluR1 and simultaneously induce local Ca2+ influx and thereby cause release of endocannabinoid (Ca-RER), presumably 2-AG (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Although contribution of Ca2+ release from internal stores was negligible under our whole-cell recording condition, Ca2+ release might also contribute to endocannabinoid release in a less-invasive recording condition.
We reported previously that repetitive PF stimulation (100 Hz; 25-50 pulses) can induce endocannabinoid-mediated suppression of CF-EPSCs in the presence of intracellular BAPTA (Maejima et al., 2001b), which is presumably through the PF-mediated RER that heterosynaptically suppresses CF inputs. This result indicates that glutamate concentration in the PF synaptic cleft may rise to the level sufficient to trigger RER if a rigorous repetitive stimulus is applied. Such a stronger PF burst also triggered the other mode of endocannabinoid release that required higher-Ca2+ elevation under the blockade of mGluR1 activation (CaER). Nevertheless, because such a repetitive firing of input fibers may not usually occur in vivo, we assume Ca-RER that can occur with relatively weak PF synaptic activity is more physiological than RER or CaER as a mechanism of endocannabinoid release. Besides this endocannabinoid-mediated homosynaptic modulation of PF synapses, it has been reported recently that conjunctive CF and PF stimulation triggers endocannabinoid-mediated transient suppression of PF to PC transmission (Brenowitz and Regehr, 2005). This associative short-term synaptic plasticity requires local Ca2+ elevation in PC dendrites at the site of suppressed PF synapses and is blocked by an mGluR1 antagonist. Therefore, this endocannabinoid-mediated associative plasticity may also result from Ca-RER.
The mGluR1-PLCβ4 cascade in PCs is crucial for developmental elimination of redundant CF synapses, long-term depression, and motor learning (Aiba et al., 1994; Kano et al., 1997, 1998; Offermanns et al., 1997; Ichise et al., 2000; Hashimoto et al., 2001; Miyata et al., 2001). It remains to be elucidated whether and how the mGluR1-induced endocannabinoid release is related to these aspects of cerebellar functions. Most neurons in the CNS possess PLCβs (Watanabe et al., 1998), which can be activated by various Gq/11-coupled receptors. CB1 receptors are also strongly expressed at presynaptic fibers in various regions of the CNS (Howlett et al., 2002). Thus, endocannabinoid release by conjoint activation of Gq/11-coupled receptors and Ca2+ elevation can occur widely throughout the CNS and may regulate synaptic transmission. Importantly, because this mechanism depends on the degree of receptor activation (that reflects the amount of transmitter release) and [Ca2+]i level (that reflects postsynaptic activities), it can detect coincidence of presynaptic and postsynaptic activities and therefore may play important roles in the induction of synaptic plasticity.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research and Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Sports, Culture, Science and Technology of Japan. This work was also supported by the Japan Society for the Promotion of Science (JSPS) and the Toyota RIKEN Foundation. T.M. was a recipient of JSPS Research Fellowships for Young Scientists and the Research Aid of Inoue Foundation for Science. We thank S. Arai for 2-AG estimation and Drs. K. Hashimoto and T. Tabata for comments on this work.
Correspondence should be addressed to Masanobu Kano, Department of Cellular Neurophysiology, Graduate School of Medical Science, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-8640, Japan. E-mail: mkano@med.kanazawa-u.ac.jp.
Copyright © 2005 Society for Neuroscience 0270-6474/05/256826-10$15.00/0
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377-388. [PubMed] [Google Scholar]
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68: 247-286. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163: 463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG (2003) Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci 23: 6373-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG (2005) Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron 45: 419-431. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG (2003) Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci 6: 1048-1057. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38: 461-472. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F (1994) Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372: 237-243. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946-1949. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A (2002) Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci 22: 200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686-691. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M (2004) Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci 19: 2682-2692. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA (2004) Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci 24: 4865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM (2003) Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol 140: 781-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM (2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5: 446-451. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440-3450. [PubMed] [Google Scholar]
- Hashimoto K, Kano M (2003) Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron 38: 785-796. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Miyata M, Watanabe M, Kano M (2001) Roles of phospholipase C β4 in synapse elimination and plasticity in developing and mature cerebellum. Mol Neurobiol 23: 69-82. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M (2005) Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45: 257-268. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161-202. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A (2000) mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288: 1832-1835. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon MI, Wu D (1996) Phospholipase C β4 is involved in modulating the visual response in mice. Proc Natl Acad Sci USA 93: 14598-14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S (1997) Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron 18: 71-79. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Watanabe M, Kurihara H, Offermanns S, Jiang H, Wu Y, Jun K, Shin HS, Inoue Y, Simon MI, Wu D (1998) Phospholipase C β4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci USA 95: 15724-15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE (2004) Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci 7: 697-698. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE (2002) Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10182-10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sugiura T, Kodaka T, Kudo N, Waku K, Tokumura A (1998a) Accumulation of various N-acylethanolamines including N-arachidonoylethanolamine (anandamide) in cadmium chloride-administered rat testis. Arch Biochem Biophys 354: 303-310. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kondo H, Nakane S, Kodaka T, Tokumura A, Waku K, Sugiura T (1998b) 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+-dependent and -independent mechanisms. FEBS Lett 429: 152-156. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S (2004) Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279: 10408-10412. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2001a) Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717-727. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2001b) Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21: RC174(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A (1991) Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6: 565-574. [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M (2001a) Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res 40: 205-210. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M (2001b) Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31: 463-475. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530-534. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL (1992) Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9: 259-270. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50: 83-90. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kim HT, Hashimoto K, Lee TK, Cho SY, Jiang H, Wu Y, Jun K, Wu D, Kano M, Shin HS (2001) Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C β4 mutant mice. Eur J Neurosci 13: 1945-1954. [DOI] [PubMed] [Google Scholar]
- Neale SA, Garthwaite J, Batchelor AM (2001) mGlu1 receptors mediate a post-tetanic depression at parallel fibre-Purkinje cell synapses in rat cerebellum. Eur J Neurosci 14: 1313-1319. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson RF, Inoue Y, Kano M, Simon MI (1997) Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq. Proc Natl Acad Sci USA 94: 14089-14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M (2001) Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729-738. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M (2002) Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15: 953-961. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M (2003) Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci 18: 109-116. [DOI] [PubMed] [Google Scholar]
- Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873-884. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80: 1291-1335. [DOI] [PubMed] [Google Scholar]
- Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ (2002) Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA 99: 8384-8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Piomelli D (2001) Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol 425: 189-196. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773-778. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Waku K (2000) 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids 108: 89-106. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215: 89-97. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Yoshinaga N, Kondo S, Waku K, Ishima Y (2000) Generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in picrotoxinin-administered rat brain. Biochem Biophys Res Commun 271: 654-658. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A (1998) A new class of synaptic response involving calcium release in dendritic spines. Nature 396: 757-760. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE (2001) Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zucker RS (2001) Photolysis-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiol (Lond) 533: 757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y (1998) Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci 10: 2016-2025. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588-592. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296: 678-682. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M (2002) The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci 22: 1690-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]