Abstract
Arousal and valence are proposed to represent fundamental dimensions of emotion. The neural substrates for processing these aspects of stimuli are studied widely, with recent studies of chemosensory processing suggesting the amygdala processes intensity (a surrogate for arousal) rather than valence. However, these investigations have assumed that a valence effect in the amygdala is linear such that testing valence extremes is sufficient to infer responses across valence space. In this study, we tested an alternative hypothesis, namely that valence responses in the amygdala are nonlinear. Using event-related functional magnetic resonance imaging, we measured amygdala responses to high- and low-concentration variants of pleasant, neutral, and unpleasant odors. Our results demonstrate that the amygdala exhibits an intensity-by-valence interaction in olfactory processing. In other words, the effect of intensity on amygdala activity is not the same at all levels of valence. Specifically, the amygdala responds differentially to high (vs low)-intensity odor for pleasant and unpleasant smells but not for neutral smells. This implies that the amygdala codes neither intensity nor valence per se, but a combination that we suggest reflects the overall emotional value of a stimulus.
Keywords: amygdala, arousal, emotion, odor, olfactory, fMRI, chemosensory
Introduction
A widespread assumption in psychology is that emotional experience is reducible to dimensions of arousal and valence (Russell, 1980; Lang, 1995). The chemosensory system is ideally suited to disentangle representations of elements reflecting these dimensions (Anderson et al., 2003). Because odors and tastes can be independently classified in terms of hedonics [valence (Schiffman, 1974)] or intensity [as a potential index of arousal (Bensafi et al., 2002)], such stimuli have been used to elucidate the stimulus properties that engage the amygdala, a brain area key to processing emotive stimuli (Zald, 2003). As used here, valence operates along a linear continuum of pleasantness, whereby stimuli of low (i.e., more negative) valence represent a less pleasant sensory experience than those of higher (i.e., more positive) valence.
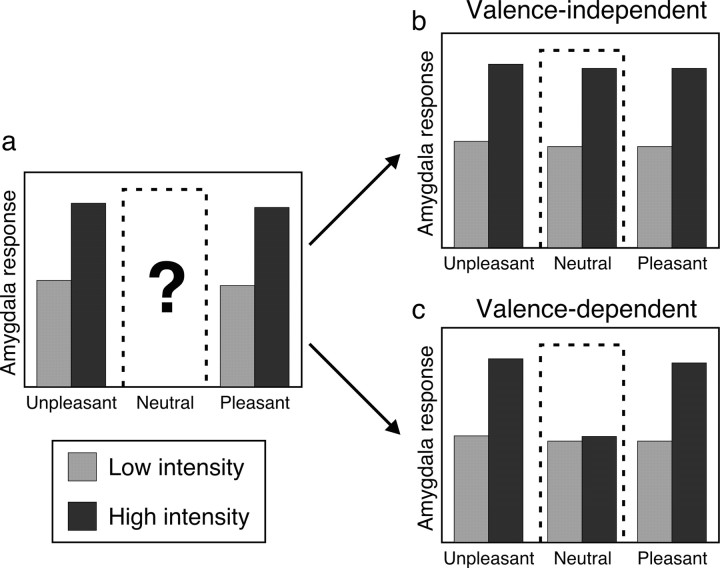
Recent reports that the human amygdala is activated by stimulus intensity but not valence (Anderson et al., 2003; Small et al., 2003) imply that the feature best characterizing amygdala function is intensity/arousal (McGaugh et al., 1996; Anderson and Sobel, 2003; Hamann, 2003). However, because these investigations assumed response invariance along the valence dimension, without sampling the mid-range of “valence space” (corresponding to neutral), it remains unclear whether the amygdala selectively encodes intensity or responds to intensity only with respect to valence extremes (Fig. 1). Given previous data demonstrating that the amygdala is engaged by both positive- and negative-valence stimuli, relative to neutral items (Garavan et al., 2001; Hamann and Mao, 2002; Winston et al., 2003), we reasoned that intensity-evoked responses within the amygdala might depend on the level of valence (Fig. 1c). On theoretical grounds, we also predicted that amygdala activation might show a reliance on valence extremes, given that chemosensory strength or intensity conceivably takes on greater importance when the stimulus is pleasant or unpleasant.
Figure 1.
Rationale and experimental hypotheses. a, Previous experiments using pleasant and unpleasant stimuli (Anderson et al., 2003; Small et al., 2003) have shown a main effect of intensity in chemosensory processing, suggesting that the amygdala is preferentially tuned to intensity. However, because these studies did not use valence-neutral stimuli, it remains unclear how the amygdala would respond to the neutral mid-range of valence space (dashed box), which has important implications for interpretation of amygdala coding. In the current study, odors of pleasant, neutral, and unpleasant valence were each presented at high and low intensity, permitting a test of two competing hypotheses. b, Hypothesis I: main effect of intensity. Here, the amygdala responses are enhanced to high (vs low)-intensity odors, independent of valence, which would fit with previous findings suggesting the amygdala operates as an intensity detector across the entire valence spectrum. c, Hypothesis II: interaction of intensity and valence. Here, amygdala responses are enhanced only to high-intensity versions of pleasant and unpleasant, but not neutral, odor, implying a greater complexity to amygdala coding involving combined sensitivity to both intensity and valence. Thus, although the diagrams in b and c show equivalent response profiles at the extremes of valence, the inclusion of neutral odor allows the two hypotheses to be distinguished.
Here, we describe the results from a functional magnetic resonance imaging (fMRI) study wherein healthy volunteers received high- and low-concentration variants of pleasant, neutral, and unpleasant odors. Using this experimental design, we could dissociate the dimensions of intensity and valence across a broader hedonic spectrum than achieved previously, enabling a test of two competing models of amygdala function: a valence-independent hypothesis in which the amygdala response to intensity is similar at all levels of valence and a valence-dependent hypothesis in which the amygdala is sensitive to odor intensity only at the outer bounds of valence.
Materials and Methods
Volunteers. Participants were 18 right-handed, healthy volunteers, recruited by local advertisement. All reported a good sense of smell. One participant was excluded after a technical failure, leaving 17 subjects for further data analysis (age range, 20–29 years; mean age, 24 years; 12 women). All gave informed consent to take part in the study, which was approved by the local ethics committee.
Stimuli. The olfactory stimuli (Sigma-Aldrich, Dorset, UK) included one pleasant odor (citral; “lemon-like”), two neutral odors (anisole; “phenolic, gasoline, ethereal;” 2-heptanol, “earthy, oily”), and one unpleasant odor (valeric acid; “putrid, fecal, sweaty, rancid”) [quality descriptions based on Arctander (1994)]. Because the behavioral ratings indicated no significant difference in valence between the two neutral odors nor any interaction with concentration (all p > 0.12), these were collapsed into a single neutral category in subsequent analyses.
Odor delivery and calibration. Stimuli were delivered using a 10-channel computer-controlled air-dilution olfactometer, which permits rapid odor presentation in the absence of tactile, thermal, or auditory cues (Gottfried et al., 2002), with the following differences: (1) odors were absorbed at 100% concentration into 2–3 diethyl phthalate pellets, rather than onto perfumer strips; (2) each odor channel was twinned with an air-dilution channel, permitting delivery of high- and low-concentration stimuli, with constant total airflow (3.0 L/min). High-concentration odors were typically achieved using a combination of 2.0–2.5 L/min (odor channel) and 0.5–1.0 L/min (dilution channel); low-concentration odors, 0.5–1.0 L/min (odor channel) and 2.0–2.5 L/min (dilution channel). Airflow ratios were calibrated for each subject before scanning to ensure that perceived odor intensities were matched across the four different high (or low)-concentration odorants. Odor delivery was controlled using Cogent 2000 software (Wellcome Department of Imaging Neuroscience, London, UK), implemented in Matlab (Math-Works, Natick, MA).
Behavioral data. Ratings for intensity and valence were recorded using modified “Labeled Magnitude Scales” (see Fig. 2) (Green et al., 1993, 1996), bounded at each end by “barely detectable” and “strongest imaginable” (intensity) or by “best imaginable” and “worst imaginable” (valence). Within the MRI scanner, subjects used a keypad to move a cursor along the scale displayed on a computer screen. Calibrated intensity ratings were first collected for each stimulus at each concentration in pseudorandom order, and valence ratings were collected subsequently after presenting the stimuli again. Intensity ratings were also collected at the end of each session to assess habituation.
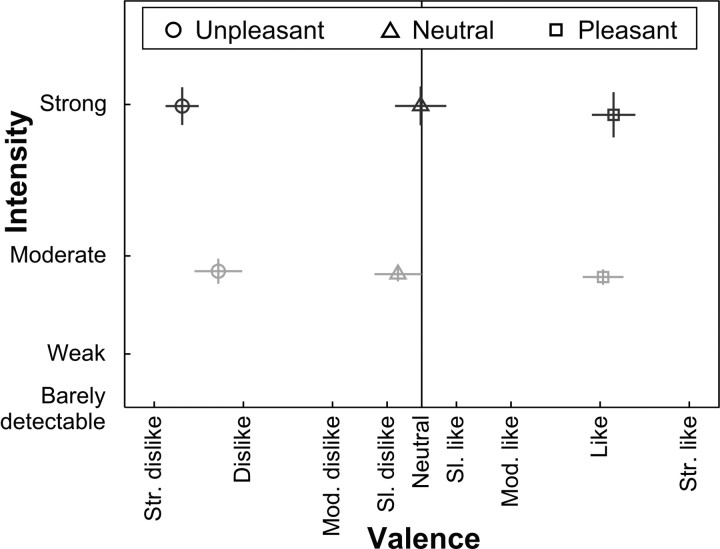
Figure 2.
Experimental design and behavioral data. The experiment corresponded to a three-by-two factorial design. The factors were odor type with three valence levels (pleasant, neutral, or unpleasant) and concentration with two levels (high or low). The plot shows the psychophysical odor space: valence on the abscissa, intensity on the ordinate. Ratings were collected using modified Labeled Magnitude Scales (see Materials and Methods). Both scales are truncated for display. Sl., Slightly; Mod., moderately; Str., strongly. Dark symbols, High-concentration odors; light symbols, low-concentration odors. Error bars show SEM; vertical bars represent error in intensity dimension; horizontal bars represent error in valence ratings.
Respirations were monitored as described previously (Gottfried et al., 2002), and breathing data were analyzed off-line in Matlab. One concern was that subjects might use different patterns of sniffing for the different odors. Therefore, peak height, latency to peak, and sniff volume were measured for each trial and included as nuisance covariates in fMRI data analysis. This rendered imaging data independent of linear effects of between-condition sniffing differences. fMRI scanning procedure. General details of scanning were similar to previous work (Gottfried et al., 2002). Data acquisition was divided into two sessions for subject comfort and to reduce olfactory habituation. In each session, 10 trials of each odor at each of two intensities were presented, along with 16 “no-odor” trials that served as target trials, because subjects' task was to indicate (by button-press) which trials contained no odor. Subjects were cued to sniff after viewing the words “sniff now,” which appeared for 1.5 s. Odor delivery began 450 ms before the sniff instruction and coterminated with it. Minimum stimulus-onset asynchrony was 8.64 s, with an additional random jitter ranging between 0 and 5.4 s.
The order of event types was pseudorandomized for each subject. Two constraints were applied to the event order: specifically, that no odor should appear in successive trials (independent of whether it was high or low concentration) and that every 16 odor trials should contain two each of the eight odor/concentration combinations with no-odor trials randomly interspersed. fMRI data were collected on a Siemens AG (Erlangen, Germany) 1.5 T scanner. Echoplanar imaging (EPI) parameters were optimized for signal recovery in ventral frontal and anterior temporal regions, including amygdala (Deichmann et al., 2003). Each session comprised 720 volumes (18 slices per volume, tilted 30° rostral > caudal), the first eight of which were discarded to permit T1 equilibration. Other parameters were the following: in-plane resolution, 3 × 3 mm; slice thickness, 2 mm (+1 mm gap), repetition time, 1.64 s; echo time, 35 ms; field of view, 192 × 192 mm. A whole-brain echoplanar image was also collected, along with a high-resolution T1-weighted structural scan used for drawing regions of interest (ROIs).
Data analysis. fMRI data were preprocessed in SPM2 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). Realignment of the scans preceded normalization to a standard EPI template in Montreal Neurological Institute space (Friston et al., 1995a). Normalization parameters were estimated from each subject's whole-brain echoplanar image and applied to the fMRI time series after coregistration of the two EPI datasets. These normalization parameters were also applied to subjects' T1-weighted scans.
ROIs for the left and right amygdala were drawn using MRIcro (Rorden and Brett, 2000) using the method described by Anderson et al. (2003) with reference to an atlas (Sakamoto et al., 1999). Left and right amygdala were outlined on each subject's normalized structural scan. These ROIs were divided into three approximately equal-sized subunits (basomedial, basolateral, and lateral). Because individual subnuclei of the amygdala are not identifiable in human MRI scans, these divisions are only loosely based on anatomical criteria and were used in an effort to replicate previous methodology (Anderson et al., 2003). Note that portions of anterior and dorsal amygdala were drawn conservatively to prevent inclusion of adjacent piriform cortex. ROIs were restricted to odor-responsive voxels by masking with the main effect of odor (at p < 0.5) (Anderson et al., 2003). fMRI data were analyzed initially in SPM2 to estimate effect sizes for the different event types (Friston et al., 1995b) using the canonical hemodynamic response function (HRF) as a basis set. Regressors of interest were assembled by convolving each event onset with the HRF. In addition, regressors modeling condition-by-time interactions were included in the design matrix to accommodate temporal changes in response to the odors. Target (no-odor) trials and button presses were also modeled. A final set of regressors of no interest modeled the effects of sniff volume, height and latency, and subject movement (estimated in the realignment). In a subsequent step, parameter estimates for voxels within each of the ROIs were averaged (by taking the arithmetic mean) for each subject and entered into a two (hemisphere)-by-three (amygdala subregion)-by-three (odor type)-by-two (odor concentration) ANOVA.
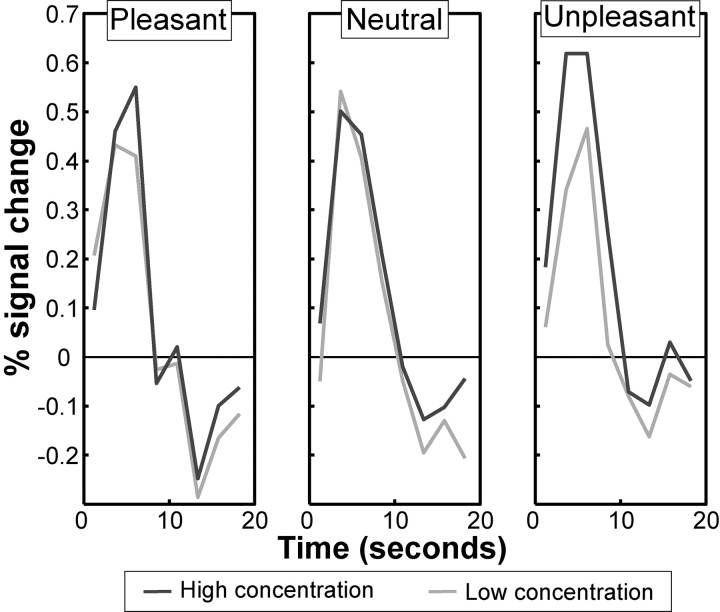
In a supplementary model calculated for illustration of response time course profiles, the first-level model was calculated using a finite impulse response (FIR) basis set with 10 bins of 2.4 s width. This model, which is unconstrained and unfitted with respect to the shape of response, was used to calculate the data presented in Figure 3.
Figure 3.
Differential effects of odor valence on intensity coding in the amygdala. Response time courses of amygdala activation for each level of odor concentration and odor type are shown. Data are taken from an unconstrained FIR model and highlight the selective effects of intensity on amygdala activity only at the extremes of odor valence.
Supplementary ROI analyses. As a post hoc test to assess whether odor-evoked responses were similar across olfactory regions, we constructed additional ROIs on a group-averaged T1 scan for orbitofrontal cortex (OFC) and piriform cortex. ROIs covered anterior, medial, lateral, and posterior OFC (Anderson et al., 2003) and anterior and posterior piriform. These ROIs were otherwise analyzed in a manner identical to the amygdala data.
Supplementary psychophysical data. To establish whether odor characteristics other than valence or intensity could explain the results, another subject cohort was asked to rate the odorants for familiarity [because this feature has been shown to modulate amygdala activity (Small et al., 1997; Plailly et al., 2005)] and pungency (as an approximate measure of trigeminality). An independent group of 17 volunteers took part in this subsidiary study (13 women; age range, 18–40 years; mean age, 29 years). Data from one woman were excluded because of a reversed pattern of valence ratings.
Odorants were presented in bottles and matched for low and high intensities on an individual basis. Subjects first rated the intensity of low-concentration odors using the Labeled Magnitude Scale, and different concentrations were tested until perceived intensity was equal. Intensity matching for the high-concentration odors proceeded in a similar manner. Ratings of valence (anchors “extremely unpleasant” and “extremely pleasant”), pungency (“not at all” and “extremely pungent”), and familiarity (“extremely unfamiliar” and “extremely familiar”) were then made on computerized visual analog scales.
Results
Importantly, intensity ratings for the high-concentration odors (pleasant, neutral, unpleasant) were all equated, as were the low-concentration odors. Valence ratings for the pleasant, neutral, and unpleasant odors were each equated across high and low concentrations (Fig. 2). There was a main effect of concentration on intensity ratings (F(1,16) = 98.9; p < 0.001), a main effect of odor type on valence ratings (F(2.0,31.4) = 195.0; p < 0.001), and no significant interaction between concentration and odor type on valence ratings (F(1.7,26.6) = 3.3; p = 0.063) or on intensity ratings (F(1.4,22.0) = 0.2; p = 0.744). The trend to an interaction between concentration and odor type on valence ratings cannot be easily explained, because inspection of simple effects revealed a uniformly balanced odor space for dissociating valence and intensity. In particular, there was no significant simple effect of concentration on the valence ratings for unpleasant odor (p = 0.15), neutral odor (p = 0.12), or pleasant odor (p = 0.42). We additionally used sign tests to assess whether there was a systematic difference between high- and low-concentration odors in valence ratings. For none of the odorants was such an effect evident (unpleasant, sign = 6, p = 0.3; neutral, sign = 6, p = 0.3; pleasant, sign = 8, p = 1). In summary, high-concentration unpleasant odors were not systematically rated more unpleasant than the low-concentration unpleasant odors, nor were the high-concentration pleasant odors rated more pleasant than the low-concentration pleasant odors, ruling out the possibility that ratings were systematically splayed at the extremes of valence.
Having established a balanced behavioral dissociation between odor intensity and valence, we next examined the amygdala response profiles evoked by this stimulus set. The fMRI data were analyzed using an ROI analysis centered on the amygdala. Subject-wise parameter estimates from amygdala ROIs were examined in a four-way factorial design with factors of hemisphere (left/right), amygdala subdivision (basomedial, basolateral, lateral), odor concentration (high/low), and odor type (pleasant/neutral/unpleasant). Repeated-measures ANOVA revealed a significant main effect of concentration (F(1,16) = 11.1; p = 0.004), in agreement with previous findings (Anderson et al., 2003; Small et al., 2003). Critically, however, we also detected an odor concentration-by-type interaction (F(2.0,31.7) = 4.7; p = 0.017), whereby high concentrations of pleasant and unpleasant odors evoked significantly greater amygdala activity than low concentrations in the absence of significant response differences between high and low concentrations of neutral odor (Fig. 3). The mean ± SEM signal change differences between high and low concentration odors were the following: pleasant, 0.14% ± 0.061, t(16) = 2.3, p = 0.017; unpleasant, 0.23% ± 0.058, t(16) = 4.1, p < 0.001; neutral, 0.0001% ± 0.057, t(16) = 0.005, p = 0.50. Thus, the amygdala response to odor intensity is not independent of valence but depends on the outer bounds of valence (positive or negative). We note that there were no main effects or interactions with hemisphere or amygdala subdivision (Anderson et al., 2003), nor was there an overall main effect of odor type (all p > 0.3).
Habituation in intensity perception was assessed by calculating the slope of a regression from the first rating (before scanning started) to the third (after the second scanning session) for each odor type for each subject. Estimated slopes were entered into repeated-measures ANOVA. There was a significant main effect of odor concentration (F(1,16) = 34.5; p < 0.001), resulting from greater habituation to high-concentration odors. There was also a significant main effect of odor type (F(1.9,30.7) = 3.7; p = 0.038), reflecting reduced habituation to neutral odors compared with pleasant and unpleasant odors (although neutral odors still habituated; t(16) = –2.3; p < 0.05). Importantly, there was no concentration-by-odor type interaction (p > 0.4), such that the habituation difference between high- and low-concentration neutral odorants was no different from the differences for the pleasant and unpleasant odorants. Therefore, the habituation patterns described here cannot explain the amygdala concentration-by-odor type interaction.
Although amygdala showed a concentration-by-odor type interaction, other brain regions did not. Separate ANOVAs performed on ROI data from additional olfactory areas revealed other response patterns. In posterior piriform cortex, a main effect of concentration was evident (F(1,16) = 6.1; p = 0.025) with no effect of valence or any interaction (all p > 0.25). Similarly, medial OFC showed a main effect of concentration (F(1,16) = 6.7; p = 0.020) with no other effect observed (all p > 0.5). These findings would be in keeping with the idea that valence-independent intensity coding is actually preserved in other regions of primary and secondary olfactory cortex, as opposed to the valence-dependent effects observed in the amygdala. Conversely, anterior OFC showed a complex pattern of response with a three-way interaction of hemisphere, concentration, and odor type (F(1.9,30.9) = 5.9; p < 0.01), resulting from a significant interaction between concentration and odor type in the right hemisphere only (F(1.7,27.7) = 5.6; p = 0.012). The lack of intensity-independent valence-specific effects in OFC stands in contrast with previous data suggesting valence coding is localized within this region (Gottfried et al., 2002; Anderson et al., 2003; Small et al., 2003).
Supplementary behavioral data gathered from the second cohort of volunteers implied that the fMRI results could not easily be explained by other odor characteristics such as familiarity or pungency (trigeminality). ANOVAs on ratings of intensity and valence from this cohort of subjects replicated our primary results from the scanned cohort. For intensity ratings, there was a main effect of concentration (F(1,15) = 39.2; p < 0.001) but no effect of odor type (F(1.2,18.9) = 2.1; p = 0.16) or interaction (F(1.6,24.0) = 0.4; p = 0.61). For valence ratings, there was a main effect of odor type (F(1.7,25.6) = 112.3; p < 0.001) but no main effect of concentration (F(1,15) = 3.0; p = 0.10) or interaction (F(1.4,20.4) = 1.4; p = 0.27).
Neither familiarity nor pungency ratings suggested these odor characteristics might have driven the response profile in amygdala. For familiarity ratings, there was a main effect of odor type (F(1.6,23.5) = 27.6; p < 0.001), resulting from increased familiarity of the pleasant odor, but there was no effect of concentration or concentration-by-type interaction (both p > 0.8). For pungency ratings, there were main effects of odor type and concentration (F(1.6,24.0) = 12.5, p < 0.001; F(1,15) = 12.4, p = 0.003) but no significant interaction (F(2.0,29.2) = 1.1; p = 0.34). Greater pungency was reported for high-concentration than low-concentration odors. Additionally, the unpleasant odor was rated more pungent than the neutral odor (t(15) = 3.1; p < 0.01), and the neutral odor was rated more pungent than the pleasant odor (t(15) = 2.6; p < 0.05). Therefore, because ratings for familiarity and pungency did not exhibit a pattern reminiscent of the fMRI data, these odor features cannot explain the amygdala response profile in a parsimonious manner.
Discussion
We demonstrate that when valence is held constant, the amygdala responds robustly to odor intensity for pleasant and unpleasant smells but does not show similar reactivity for neutral smells. In turn, when intensity is held constant (at high concentrations), the amygdala is preferentially activated by positive and negative valence but not by neutral valence. The finding of an odor intensity-by-valence interaction has important implications for interpretation of previous results (Anderson et al., 2003; Small et al., 2003) and argues against the amygdala simply encoding representations of emotional arousal (as indexed by perceived intensity) rather than emotional valence (Anderson and Sobel, 2003; Hamann, 2003).
In keeping with our results, emerging evidence challenges a view of the functional role of the amygdala as a mere intensity detector. For example, as Small et al. (2003) point out, amygdala responses are still evident even when emotive visual stimuli are rendered invisible [i.e., of very low intensity (Morris et al., 1998; Whalen et al., 1998; Williams et al., 2004)]. Additionally, the amygdala shows preference for emotional over neutral-valence stimuli in the domain of face perception (Winston et al., 2003, 2004). Finally, even within the realm of chemosensory processing, certain manipulations alter responsiveness in amygdala independent of changes in stimulus intensity [e.g., in cross-modal flavor processing (Small et al., 1997) or selective satiety (Gottfried et al., 2003)]. Other results also suggest that the amygdala is not insensitive to the dimension of valence (Zald and Pardo, 1997; Royet et al., 2003).
It has been suggested that chemosensory stimuli are ideal to dissociate valence and arousal as components in emotion processing (Anderson et al., 2003), given that perceived intensity is related to arousal (Bensafi et al., 2002). It is important to note that the current study and others have used chemosensory intensity as a surrogate for arousal (which represents an internal state engendered by a stimulus) but have not demonstrated direct measures of arousal. Indeed, Bensafi et al. (2002) demonstrate that chemosensory intensity is correlated only with some measures of arousal (skin conductance), whereas pleasantness is correlated with others (heart-rate variability). However, perceived intensity is strongly correlated with self-rated arousal.
The specific form of the interaction between intensity and valence, with differential responses to high (vs low) intensity in the amygdala only for pleasant and unpleasant odors, reflects a greater complexity in amygdala processing than suggested previously. Previous attempts to dissociate valence and arousal have been limited by neglecting to test neutral valence (Anderson et al., 2003; Small et al., 2003) or by use of stimulus modalities in which valence and arousal are highly correlated. For example, although pictures and words are commonly used to examine emotional processing, items of high arousability are typically limited to the extremes of valence (e.g., images of aggressive animals or erotica), whereas neutral-valence items generally rate low on arousal (e.g., pictures of furniture or tools) and defy attempts to identify highly arousing neutral exemplars (Garavan et al., 2001; Hamann and Mao, 2002; Dolcos et al., 2004). In the present study, by using olfactory stimuli across a range of valences and intensities, we have been able to circumvent the limitations of previous studies and better characterize the response profile of the amygdala.
In conclusion, we show that the amygdala is not exclusively engaged by a pure representation of olfactory intensity but instead reflects intensity only for emotionally salient (positive or negative) odors. We speculate that this effect results from the added importance of odor strength in appraising stimulus value for valenced odors. In other words, the more intense an emotionally salient odor, the more likely it is to be relevant to survival, which is not the case for emotionally neutral odors. Indeed, the musky smell of a predator (or prey) that progressively intensifies in the downstream wind is surely a more provocative behavioral cue than a similarly intensifying smell of savannah dirt. Thus, whatever the subjective rating of valence, stronger unpleasant or pleasant odors have added value, and to the amygdala, it is this integrated affective dimension that matters most.
Footnotes
J.A.G. was supported by a Physician–Scientist Postdoctoral fellowship from the Howard Hughes Medical Institute. This work was performed under a Wellcome Trust Programme grant to R.J.D. We thank Nadine Ravel at the Institut des Sciences Cognitives (Lyon, France) for the pellets used in odor delivery, Anthonia Lloyd for collecting pilot data on olfactory stimuli, and Erin Luxenberg for collecting subsidiary behavioral data.
Correspondence should be addressed to Joel S. Winston, Wellcome Department of Imaging Neuroscience, 12 Queen Square, London WC1N 3BG, UK. E-mail: j.winston@fil.ion.ucl.ac.uk.
DOI:10.1523/JNEUROSCI.1569-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258903-05$15.00/0
References
- Anderson AK, Sobel N (2003) Dissociating intensity from valence as sensory inputs to emotion. Neuron 39: 581–583. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N (2003) Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Arctander S (1994) Perfume and flavor chemicals (aroma chemicals). Carol Stream, IL: Allured Publishing.
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A (2002) Autonomic nervous system responses to odours: the role of pleasantness and arousal. Chem Senses 27: 703–709. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R (2003) Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19: 430–441. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R (2004) Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage 23: 64–74. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ (1995a) Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston K, Holmes AP, Worsley K, Poline J-B, Frith C, Frackowiak RSJ (1995b) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC (2001) Amygdala response to both positively and negatively valenced stimuli. Neuro-Report 12: 2779–2783. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ (2002) Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci 22: 10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2003) Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM (1993) Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses 18: 683–702. [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J (1996) Evaluating the `Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses 21: 323–334. [DOI] [PubMed] [Google Scholar]
- Hamann S (2003) Nosing in on the emotional brain. Nat Neurosci 6: 106–108. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H (2002) Positive and negative emotional verbal stimuli elicit activity in the left amygdala. NeuroReport 13: 15–19. [DOI] [PubMed] [Google Scholar]
- Lang PJ (1995) The emotion probe. Studies of motivation and attention. Am Psychol 50: 372–385. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B (1996) Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA 93: 13508–13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1998) Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Plailly J, Bensafi M, Pachot-Clouard M, Delon-Martin C, Kareken DA, Rouby C, Segebarth C, Royet JP (2005) Involvement of right piriform cortex in olfactory familiarity judgments. NeuroImage 24: 1032–1041. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000) Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C (2003) fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. NeuroImage 20: 713–728. [DOI] [PubMed] [Google Scholar]
- Russell JA (1980) A circumplex model of affect. J Pers Soc Psychol 39: 1161–1178. [Google Scholar]
- Sakamoto N, Pearson J, Shinoda K, Alheid GF, de Olmos JS, Heimer L (1999) The human basal forebrain. Part I: an overview. In: The primate nervous system, Part III (Bloom FE, Bjorklund A, Hokfelt T, eds), pp 15–56. Amsterdam: Elsevier.
- Schiffman SS (1974) Physicochemical correlates of olfactory quality. Science 185: 112–117. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC (1997) Flavor processing: more than the sum of its parts. NeuroReport 8: 3913–3917. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T (2003) Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39: 701–711. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB (2004) Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci 24: 2898–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ (2003) Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage 20: 84–97. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Perrett DI, Dolan RJ (2004) Arousal, attractiveness, and the amygdala. Presented at the Cognitive Neuroscience Society Annual Meeting, San Francisco, April.
- Zald DH (2003) The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV (1997) Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 94: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]