Abstract
The receptor protein tyrosine phosphatase PTPRO may be involved in axon guidance both as a ligand and as a neuronal receptor. We have begun to characterize signaling by PTPRO as a receptor by screening for proteins interacting with the intracellular domain of PTPRO. In a yeast-two hybrid screen, we identified a novel class of protein, which we named neuronal pentraxin with chromo domain (NPCD), as a PTPRO-interacting protein. We have shown recently that NPCD has multiple cytoplasmic isoforms as a result of alternative splicing and that these proteins are present in many neurons, mainly associated with the inner side of the plasma membrane. Through additional two-hybrid experiments, cotransfection and reciprocal coprecipitation, glutathione S-transferase pulldown, and immunoprecipitation in vivo, we confirm that NPCD isoforms interact with the catalytic phosphatase domain of PTPRO. We also find that at least one NPCD isoform is tyrosine phosphorylated in vivo and can serve as a substrate for PTPRO in vitro. Analysis of PTPRO knock-out mice demonstrates that normal localization of NPCD at the plasma membrane requires PTPRO expression, suggesting a physiological role for the NPCD/PTPRO interaction. NPCD is likely to be relevant to axon growth and/or guidance, because RNA interference mediated knock-down of NPCD expression in pheochromocytoma cells inhibits NGF-induced neuronal process outgrowth without affecting NGF-dependent survival or initial NGF signaling.
Keywords: tyrosine phosphorylation, neurite outgrowth, pentraxin, chromo domain, PC12, neuronal differentiation, nerve growth factor
Introduction
At least two major classes of receptor protein tyrosine phosphatases (RPTPs), type IIa and type III, play important roles in axon patterning during embryogenesis (Bixby, 2000; Johnson and Van Vactor, 2003). Of the five vertebrate type III RPTPs, only PTPRO is selectively expressed in neurons and has been implicated in axon growth and guidance. The recombinant extracellular domain of PTPRO inhibits neurite outgrowth, collapses growth cones, and is a repulsive guidance cue for retinal axons (Stepanek et al., 2001). Knock-down of PTPRO in developing motor neurons causes aberrant motor axon guidance (L. Stepanek, A. Stoker, E. Stoeckli, and J. L. Bixby, unpublished observations). Thus, PTPRO may guide axons both as a ligand and as a receptor.
The signaling mechanisms through which type III RPTPs act are primarily unknown. A promising approach to this issue is the identification of substrates and other intracellular interacting proteins. Substrates for the hematopoietic type III RPTP, density-enhanced phosphatase-1 (DEP-1)/CD148, include the PDGF receptor, the hepatocyte growth factor (HGF) receptor, and p120ctn (Kovalenko et al., 2000; Holsinger et al., 2002; Palka et al., 2003). At present, there are no known substrates for PTPRO (Beltran et al., 2003). To identify PTPRO-related signaling proteins, we have used the intracellular domain of PTPRO as bait in a yeast two-hybrid screen. In this screen, we identified a fragment of the gene previously known as the neuronal pentraxin receptor (NPR), which we have renamed neuronal pentraxin with chromo domain (NPCD). NPCD has a number of different isoforms, formed by alternative splicing, with an unusual motif structure combining a chromatin organizer modifier (chromo) domain and a neuronal pentraxin domain (Chen and Bixby, 2005).
Neuronal pentraxins are selectively expressed in the nervous system and have been suggested to be involved in synaptic functions. NP2/Narp, apparently in a complex with NP1, is involved in the clustering of AMPA-type glutamate receptors at the excitatory CNS synapse (Kirkpatrick et al., 2000; O'Brien et al., 2002). In general, neuronal pentraxins act outside of cells either as secreted proteins or as type II transmembrane proteins (Dodds et al., 1997; O'Brien et al., 2002). Because NPCD has numerous cytoplasmic isoforms, possesses a chromo domain, and appears to interact with the cytoplasmic domain of PTPRO, it is likely to mediate unique activities compared with other pentraxins.
We have now begun to investigate the interaction of NPCD with PTPRO and to examine potential functions of NPCD in neuronal differentiation. Here, we report that (1) NPCD proteins interact with the catalytic domain of PTPRO, (2) PTPRO expression is required for appropriate NPCD localization, and (3) NPCD is tyrosine phosphorylated in vivo and can be dephosphorylated by PTPRO in vitro. Interestingly, NPCD is required for NGF-induced process outgrowth in pheochromocytoma (PC12) cells, suggesting that it may be involved in regulation of axon growth by PTPRO.
Materials and Methods
Antibody sources. Affinity-purified antibodies to the chromo domain (anti-CD) and to the pentraxin domain (anti-Ptx) of NPCD have been described (Chen and Bixby, 2005). MAP2 monoclonal antibody and actin monoclonal antibody were from Sigma (St. Louis, MO), GFAP polyclonal antibody was from DakoCytomation (Carpinteria, CA), L1 polyclonal antibody was a generous gift from Dr. Vance Lemmon (University of Miami, Miami, FL), anti-phosphotyrosine (4G10) was from Upstate Biotechnology (Lake Placid, NY), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody was from Ambion (Austin, TX). Phospho-ERK (extracellular signal-regulated kinase) 1/2, phospho-p90RSK (ribosomal S6 kinase), phospho-Akt, and phospho-S6 ribosomal protein antibodies were from Cell Signaling Technology (Beverly, MA). Antibodies were diluted according to manufacturers' instructions. An antibody to NPR (NPCD pentraxin domain) was a generous gift from Dr. Mark Perin (Lerner Institute, Cleveland, OH).
Yeast two-hybrid screen. A 1.1 kb cDNA encoding the entire intracellular domain of PTPRO was fused to the GAL4 DNA-binding domain in bait vector pGBKT7. The yeast two-hybrid screen (Matchmaker System3; Clontech, Cambridge, UK) was based on the mating of two yeast strains: AH109 transformed with pGBKT7-bait and Y187 pretransformed with an embryonic day 17 (E17) mouse embryonic cDNA library. Library screening and subsequent confirmation of interactions were performed under high-stringency selection, with positive clones identified surviving double nutritional deficiency SD/-His/-Ade. X-gal was added to the plate for the colorimetric detection of the melibiase (MEL1) reporter gene product α-galactosidase.
Site-directed mutagenesis and glutathione S-transferase pull-down assay. A catalytically inactive mutant of PTPRO was created by site-directed mutagenesis of two invariant residues (Xie et al., 2002) of the wild-type intracellular domain of PTPRO (GeneEditor; Promega, Madison, WI). cDNAs encoding the wild-type and the mutant PTPRO were inserted in frame into pGEX6P vector (Amersham Biosciences, Arlington Heights, IL) to make glutathione S-transferase (GST)-PTPRO fusion proteins. GST-PTPRO fusion proteins were purified from Escherichia coli BL21 lysates with glutathione columns. Equal amounts of purified GST and GST-PTPRO fusion proteins were incubated with mouse brain lysates (5 mg/ml) overnight at 4°C and precipitated with glutathione Sepharose beads (Amersham Biosciences). After extensive washing with the lysis buffer, bound proteins were eluted with boiling electrophoresis sample buffer and probed with anti-Ptx antibody on Western blots.
Immunoprecipitation and immunoblotting. Two micrograms of antibody were added to 500 μg of 1 mg/ml protein lysates (lysis buffer: 1% Triton X-100, 150 mm NaCl, 20 mm Tris, pH 7.4, 1 mm EDTA, 1 mm EGTA, 1 mm sodium orthovanadate, and various protease inhibitors). Mixed lysates were incubated on ice for 30 min. Twenty microliters of Gammabind Plus Sepharose beads (Amersham Biosciences) were added to each sample and incubated at 4°C overnight with agitation. Beads were washed three times with lysis buffer and boiled in electrophoresis sample buffer. Eluted immunoprecipitates were resolved in 10% SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with appropriate antibodies. HRP-conjugated secondary antibodies were visualized with ECL (Amersham Biosciences).
Immunohistochemistry and imaging. Fresh-frozen tissue sections were fixed in freshly prepared 4% paraformaldehyde for 5 min at room temperature. For double immunolabeling, two primary antibodies from different species were allowed to bind to the 5% goat serum-blocked sections overnight at 4°C. The sections were rinsed with PBS, and 1:200 dilutions of goat anti-primary Alexa 594 and Alexa 488 (Molecular Probes, Eugene, OR) were added. Images were scanned using a Zeiss (Oberkochen, Germany) LSM510 confocal microscope (Miami Project to Cure Paralysis Core Imaging Facility, Miami, FL).
Dephosphorylation assay. The 41 kDa NPCD protein was isolated by immunoprecipitation with the affinity-purified anti-Ptx antibody from mouse brain lysates. Equal amounts of the 41 kDa NPCD protein were mixed with 5 μg of purified GST, GST-PTPRO, or GST-PTPRO* (inactive mutant) and incubated at 37°C for 1 h in a buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% Triton X-100, 5% glycerol, and protease inhibitor tablets (Roche Products, Welwyn Garden City, UK). After incubation, NPCD was precipitated and subjected to antiphosphotyrosine immunoblotting.
RNA interference. NPCD short interfering RNAs (siRNAs) were designed so as not to have significant sequence similarity with other known mRNA sequences. Twenty-one-mer siRNAs with 19 complementary nucleotides and 3′ terminal noncomplementary dimers of uridine were synthesized with the Silencer siRNA Construction kit (Ambion). Synthesized siRNAs were screened for knock-down efficacy on the 41 kDa NPCD protein expressed by a cotransfected 1.1 kb NPCD expression construct in COS cells. These assays were done by transfecting COS cells as described below for PC12 cells but with the inclusion of NPCD cDNA with the siRNAs. Knock-down of NPCD species in transfected cells was assessed by Western blot. One siRNA with the best knock-down efficacy (90%) was used for later analysis in PC12 cells. Synthesized siRNAs were fluorescently labeled with cyanine 3 (Cy3) using the Silencer siRNA Labeling kit (Ambion). Cy3-labeled siRNAs were transfected into PC12 cells using Lipofectamine 2000 (Invitrogen, San Diego, CA). Twelve hours later, transfection medium was replaced with the normal growth medium. Transfected PC12 cells were maintained for 24 h and then replated and treated with 100 ng/ml NGF (Sigma) for 3 d. In a second series of experiments, PC12 cells were “primed” with NGF for 1 week, treated with siRNAs for 3 d, and then replated in the presence of NGF for 12 h. Cells were fixed and incubated with the anti-Ptx antibody overnight using goat anti-chicken Alexa 488 as a secondary antibody. Hoechst 33342 dye (Molecular Probes) was coapplied with the secondary antibody to visualize the cell nuclei. Cells were counted as having neurites if the neurite length exceeded one cell body diameter. Neurite growth was quantified in three independent experiments; between 250 and 500 cells were examined in each experiment.
Signaling scan of ERK and Akt pathway activation. PC12 cells were treated with either control or NPCD siRNAs for 3 d and changed to fresh growth medium (without NGF) for 2 h before being treated with 100 ng/ml NGF. NGF-stimulated cells were lysed with protein sample buffer and boiled. Cell lysates were probed immediately on Western blots with phospho-specific antibodies to detect activation and with anti-Ptx antibody to confirm NPCD knock-down.
Results
A yeast two-hybrid screen identifies the neuronal pentraxin receptor as an interacting protein with the intracellular domain of PTPRO
As a first step in elucidating the intracellular signaling mechanisms initiated from PTPRO, the entire intracellular domain of PTPRO was used as bait in a yeast two-hybrid screen of an E17 mouse embryonic cDNA library. We screened 4 × 106 cDNA clones and identified a total of 53 positive clones. Of these, 27 were strongly interacting clones as assessed by intense blue color (MEL1); all 27 represented a 1.1 kb partial cDNA clone of the neuronal pentraxin receptor (Dodds et al., 1997; Chen and Bixby, 2005).
The PTPRO phosphatase domain interacts with the conserved neuronal pentraxin domain
NPR is a member of a family of neuronal pentraxins consisting of NP1, NP2/Narp, and NPR. As discussed previously, these proteins have multiple suggested functions in neuronal development. NPR mRNA is strongly expressed in neurons in which PTPRO expression is detected, such as the CA1 and CA3 pyramidal neurons of the hippocampus, and multiple layers of the cerebral cortex (Dodds et al., 1997; Beltran et al., 2003), suggesting that the interaction we observed could be physiologically relevant. The postulated membrane topology for NPR is entirely extracellular (Dodds et al., 1997), though, and other neuronal pentraxins are secreted proteins. Although this argues against a relevant interaction between NPR and the intracellular domain of PTPRO, we have discovered recently that NPR is a splice variant of the extended NPCD gene, alternative splicing of which produces multiple isoforms encoding intracellular pentraxins fused to an N-terminal chromatin organization modifier (chromo) domain. These NPCD proteins are localized in the cytoplasm and can be associated with the plasma membrane (Chen and Bixby, 2005). The 1.1 kb cDNA from the two-hybrid screen encodes a fragment of NPCD comprising aa 136-494 of the published sequence. Because the conserved pentraxin domain is included in this sequence, we tested whether the Ptx domain is necessary and sufficient to render a full interaction with PTPRO. In fact, the Ptx domain interacted with PTPRO as well as the original NPCD fragment (supplementary Fig. 1A, available at www.jneurosci.org as supplemental material). Neither the N-terminal half nor the C-terminal half of the Ptx domain was sufficient for this interaction (supplementary Fig. 1A, available at www.jneurosci.org as supplemental material). In reciprocal experiments, we found that the PTPRO catalytic phosphatase domain, and not the alternatively spliced juxtamembrane domain, conferred binding to the Ptx domain of NPCD (supplementary Fig. 1B, available at www.jneurosci.org as supplemental material).
NPCD interacts with PTPRO in mammalian cells
All NPCD isoforms are predicted to be able to interact with PTPRO, because they contain the neuronal pentraxin domain that is necessary and sufficient for conferring a full-strength interaction with PTPRO. However, the two-hybrid interaction could be an artifact of the yeast system. To test protein interactions in a mammalian cell system, we coexpressed the PTPRO intracellular domain and the 41 kDa NPCD protein (Chen and Bixby, 2005) in COS cells. Immunoprecipitation of PTPRO brought down NPCD, and immunoprecipitation of NPCD brought down PTPRO (supplementary Fig. 1C, available at www.jneurosci.org as supplemental material). Thus, the NPCD/PTPRO interaction is robust even in mammalian cells, because the majority of either protein expressed in COS cells can be found complexed with the other (supplementary Fig. 1C, available at www.jneurosci.org as supplemental material).
PTPRO interacts with multiple NPCD isoforms and is complexed with NPCD in brain
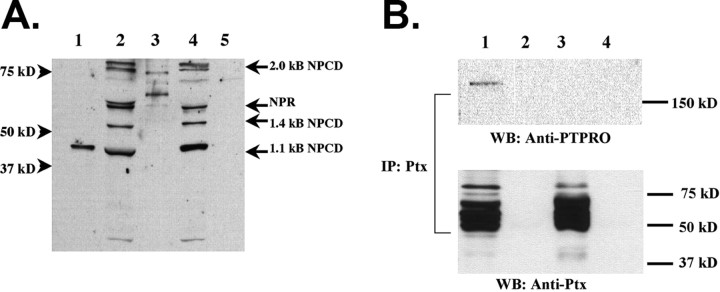
Our initial studies demonstrated that PTPRO and NPCD can interact when overexpressed in heterologous cells. We next used GST pulldown assays to investigate the interaction of native NPCD protein species with PTPRO. The NPCD gene encodes multiple alternatively spliced mRNA isoforms, and protein species corresponding to most of these (41, 52, 55, 65, 76 kDa) can be detected in mouse brain lysates (Chen and Bixby, 2005). A GST fusion protein containing the full-length intracellular domain of PTPRO was used to precipitate proteins from mouse brain lysates, and the precipitated complexes were probed with an NPCD antibody that recognizes a common epitope in the Ptx domain (Dodds et al., 1997). Multiple bands of sizes predicted from cloned NPCD cDNAs (41, 52, 55-60 kDa) were specifically precipitated by GST-PTPRO (but not control GST) from either neonatal or adult brain (Fig. 1A). In addition, two Ptx-immunoreactive bands of ∼76 kDa were seen in the GST pulldowns; these matched the predicted size of proteins encoded by the 2.0 kb NPCD cDNA, which appears as a doublet in brain (Chen and Bixby, 2005). The 41 kDa NPCD protein comigrates with the NPCD protein marker produced in COS cells transfected with the 1.1 kb NPCD cDNA (Fig. 1A, lane 1). These experiments demonstrate that multiple native NPCD isoforms can specifically associate with PTPRO. This result is predicted from our yeast experiments demonstrating that the neuronal pentraxin domain is responsible for the interaction with the PTPRO intracellular domain.
Figure 1.
PTPRO interacts with multiple NPCD isoforms and is present in a complex with NPCD in neonatal brain. A, The intracellular domain of PTPRO was produced as a GST fusion protein. Pulldowns were performed with GST-PTPRO (lanes 2, 4) or GST alone (lanes 3, 5) using either neonatal (lanes 2, 3) or adult (lanes 4, 5) mouse brain lysates. NPCD proteins were detected in the pulldowns by immunoblotting with an antibody recognizing a common epitope present in the NPCD pentraxin domain (a generous gift from Dr. Mark Perin). Multiple bands (41, 52, 55, 76 kDa) of sizes predicted from NPCD cDNAs (labeled arrows) were specifically precipitated by GST-PTPRO from either neonatal or adult brain. The 41 kDa NPCD protein produced in COS cells transfected with the 1.1 kb NPCD cDNA (lane 1) comigrates with one precipitated band. B, Immunoprecipitations were performed using our Ptx antibody (lanes 1, 3) or preimmune serum (lanes 2, 4) from the soluble fraction of either neonatal (lanes 1, 2) or adult (lanes 3, 4) brain lysates. Western blotting (WB) with anti-PTPRO revealed that PTPRO specifically coprecipitates from neonatal brain with NPCD. PTPRO could not be detected in the NPCD precipitates from adult brain (lane 3), although similar amounts of NPCD were precipitated. This result is likely attributable to downregulation of PTPRO expression in the adult brain. Note that the spectrum of NPCD species apparent in the anti-Ptx precipitate is a subset of those pulled down by the GST-PTPRO fusion protein.
As final confirmation of the association of NPCD and PTPRO in native tissue, we performed coimmunoprecipitations from brain lysates. Anti-Ptx antibody was used to immunoprecipitate NPCD proteins from either neonatal or adult brain, and the precipitates were examined on Western blots for the presence of PTPRO. PTPRO (170 kDa) was specifically coprecipitated with NPCD from neonatal but not adult brain (Fig. 1B). The lack of discernible PTPRO in the adult brain immunoprecipitates is consistent with the dramatic downregulation of PTPRO in adult brain (Bodden and Bixby, 1996; Beltran et al., 2003). Thus, PTPRO and NPCD are present in a complex in native tissue. Together with the yeast two-hybrid data and the interaction seen in heterologous cells, these data strongly suggest that PTPRO and NPCD are binding partners in vivo.
NPCD colocalizes with PTPRO in brain and kidney
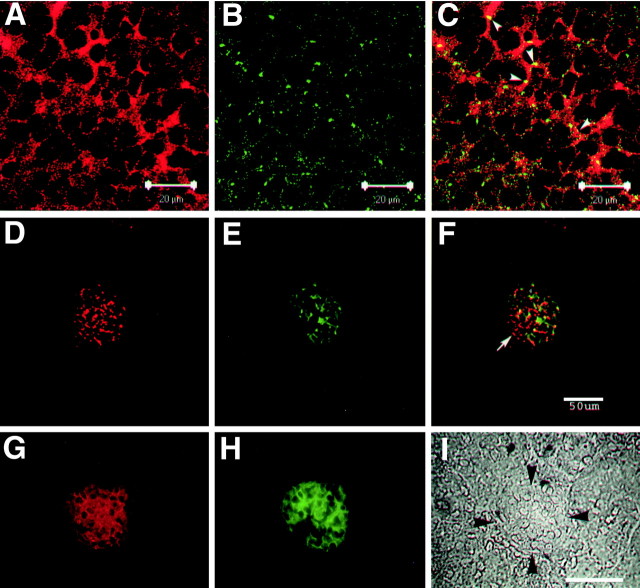
To obtain evidence regarding the physiological function of NPCD/PTPRO interactions, we examined the colocalization of NPCD and PTPRO in vivo. PTPRO expression is strongly developmentally regulated in the brain, peaking around the time of birth (Bodden and Bixby, 1996; Beltran et al., 2003); we therefore examined localization in neonatal brain. Colocalization was assessed by double immunostaining with anti-PTPRO and the anti-Ptx NPCD antibody, followed by confocal microscopy. In the neonatal hippocampus, NPCD (Ptx epitope) immunoreactivity was mostly at or near the plasma membrane (Fig. 2A) (Chen and Bixby, 2005). PTPRO immunoreactivity was highly punctate and as expected, also plasma membrane associated (Fig. 2B). The two proteins were colocalized to some extent, but colocalization was not extensive (Fig. 2C). In particular, there were many regions of NPCD (Ptx) staining not associated with PTPRO. The only nonneuronal tissue known to express PTPRO is the kidney, in which expression is restricted to the glomerular epithelial cells (Thomas et al., 1994; Beltran et al., 2003). Staining for NPCD was also at or near the plasma membrane in glomeruli of the adult kidney (Fig. 2D-I). Although colocalization of NPCD with PTPRO could be seen, there again were extensive regions of NPCD staining not associated with PTPRO (Fig. 2F). These results suggest that there are both PTPRO-dependent and PTPRO-independent functions of NPCD isoforms.
Figure 2.
NPCD colocalizes with PTPRO in brain and kidney. P0 mouse hippocampal sections (A-C) and adult kidney sections (D-F) were doubly immunostained with anti-Ptx (A,D) and anti-PTPRO (B,E), and confocal analysis (0.5 μm optical sections) was performed. Overlays are shown in C and F. Ptx-containing isoforms of NPCD appear localized mainly to the plasma membrane in CA3 hippocampal pyramidal neurons, where there is clear overlap (arrowheads, yellow; C) but not complete colocalization with PTPRO. Coexpression is present in the kidney, but there is extensive NPCD staining not associated with PTPRO (arrow, F). Conventional fluorescence microscopy of adult kidney sections (G-I) shows that both NPCD staining (red; G) and PTPRO staining (green; H) are confined to glomeruli (bordered by arrowheads in phase-contrast image; I) in adult kidney (Beltran etal., 2003). Scalebars: A-C,20 μm; F, I,50 μm.
PTPRO expression is required for appropriate localization of NPCD
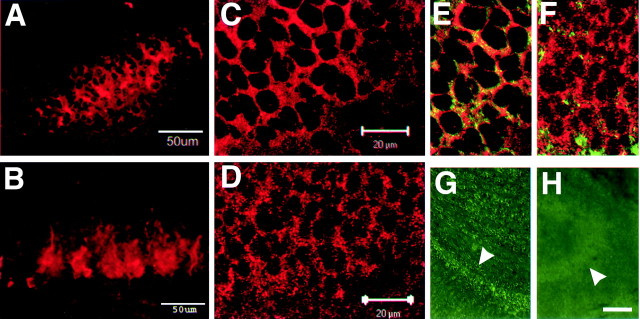
The cellular distribution of NPCD in hippocampal neurons is closely associated with the plasma membrane at the neonatal stage but is more diffusely cytosolic in adulthood (Chen and Bixby, 2005). If interactions with PTPRO served to tether cytosolic NPCD to the inner plasma membrane, the developmental downregulation of PTPRO might contribute to this altered expression pattern. To test the idea that PTPRO expression regulates NPCD localization, we compared the distribution of NPCD immunoreactivity in neonatal hippocampus from wild-type and PTPRO knock-out mice (Wharram et al., 2000). Sections from postnatal day 0 (P0) hippocampus were immunostained with the anti-Ptx antibody, and NPCD localization was examined by confocal microscopy. Consistent with our hypothesis, the highly organized localization of NPCD was disrupted in the PTPRO knock-out hippocampus. Extended projections of 18 μm image stacks showed a more diffuse pattern of NPCD distribution in the PTPRO knock-out hippocampus (Fig. 3B) compared with that in the wild-type hippocampus (Fig. 3A). When 0.5 μm optical sections were examined, Ptx staining in the wild-type hippocampus (Fig. 3C) could be seen to be evenly distributed and primarily restricted to domains at or near the cell membrane, as assessed by the distribution of the neural cell adhesion molecule NCAM (Fig. 3E). In contrast, Ptx staining was more punctate and spread into the cytosol in the PTPRO knock-out neurons (Fig. 3D). This change in Ptx membrane staining was not the result of generally altered neuronal plasma membranes in the PTPRO knock-out, because staining of NCAM was unaltered in the knock-out (data not shown). As a result, the general codistribution of NPCD with NCAM was disrupted in the PTPRO knock-out (Fig. 3F). Comparison of PTPRO knock-out tissue and wild-type tissue stained with anti-PTPRO also demonstrated that the punctate PTPRO staining we observed in the hippocampus was specific (Fig. 3G,H). It should be noted that a proportion of NPCD appeared to be membrane associated even in the PTPRO knock-out. This suggests that at least some NPCD isoforms are localized to neuronal plasma membranes by PTPRO-independent mechanisms.
Figure 3.
PTPRO expression is required for appropriate localization of NPCD. A-F, Hippocampal sections from the CA3 region of P0 wild-type (A, C, E) and PTPRO null (B, D, F) mice were stained with anti-Ptx (A-D) or double stained with anti-Ptx and anti-NCAM (E, F) and examined by confocal fluorescence microscopy. A, B, Extended projections of 18 μm image stacks show that NPCD staining is less organized and appears less tightly associated with cell borders in PTPRO knock-out hippocampus (B) than in the wild type (A). Single optical sections show that NPCD is at or near the neuronal plasma membrane in wild-type hippocampus (C) but becomes more punctate and less tightly localized in the neurons of the PTPRO knock-out mice (D). The membrane association of NPCD in wild-type tissue can be assessed by general overlap with NCAM staining (green) in the same sections (E, F). Staining of wild-type (G) and PTPRO null hippocampus (H) with anti-PTPRO demonstrates the specificity of the punctate PTPRO staining in Figure 3. Arrowheads, Pyramidal layer. Scale bars: (in C, D) C-F,20 μm; H, 100 μm.
The 41 kDa NPCD protein is a PTPRO substrate
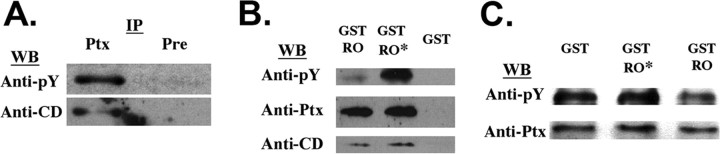
The interaction of NPCD with PTPRO suggests the possibility that NPCD could be a PTPRO substrate. We first examined whether NPCD proteins could be tyrosine phosphorylated in vivo. NPCD proteins were immunoprecipitated from mouse brain lysates with the anti-Ptx antibody, and the precipitates were probed on Western blots with an anti-phosphotyrosine antibody. The 41 kDa NPCD protein precipitated with the Ptx antibody was tyrosine phosphorylated and was also recognized by the CD antibody (Fig. 4A). Thus, at least some fraction of the 41 kDa NPCD protein is tyrosine phosphorylated in brain. Phosphorylation of NPCD in neurons appears to be tightly regulated, because no detectable phosphotyrosine was found on the 41 kDa NPCD protein precipitated from transfected COS cells (data not shown). Recombinant NPCD expressed in COS cells was not detectably tyrosine phosphorylated even when cells were cotransfected with constitutively active v-Src, a procedure that led to a large increase in phosphorylation of a number of other cellular proteins (data not shown). Because this transfected NPCD can interact efficiently with PTPRO (supplementary Fig. 1C, available at www.jneurosci.org as supplemental material), the interaction is evidently not dependent on tyrosine phosphorylation of NPCD.
Figure 4.
The 41 kDa NPCD protein is a PTPRO substrate. A, Immunoprecipitations were performed using the Ptx antibody (Ptx) or preimmune serum (Pre) from adult brain. The precipitates were probed with anti-phosphotyrosine (anti-pY) and were then stripped and reprobed with the CD antibody. An NPCD band at 41 kDa that is precipitated by the Ptx antibody and recognized by the CD antibody is tyrosine phosphorylated in vivo. B, GST pulldowns were performed from mouse brain lysates using GST-PTPRO (GST-RO), a catalytically inactive mutant of GST-PTPRO (GST-RO*), or GST alone. Precipitates were probed successively with a phosphotyrosine antibody, the Ptx antibody, and the CD antibody. Similar amounts of the 41 kDa NPCD protein were specifically associated with either the wild-type or inactive form of PTPRO. However, phosphotyrosine levels were much lower when the wild-type PTPRO was used, indicating that the 41 kDa NPCD isoform is a PTPRO substrate. C, Native 41 kDa NPCD protein was isolated from brain by immunoprecipitation using the Ptx antibody and was then incubated at 37°C for 1 h with 5 μg of purified GST, catalytically inactive GST-PTPRO, or GST-PTPRO. After incubation, NPCD was immunoprecipitated and probed successively with a phophotyrosine antibody and the Ptx antibody. NPCD is directly dephosphorylated by GST-PTPRO but not by the inactive mutant. WB, Western blot; IP, immunoprecipitation.
When the 41 kDa NPCD protein was precipitated from brain using our fusion protein containing the intracellular domain of PTPRO (GST-PTPRO), it was poorly tyrosine phosphorylated compared with immunoprecipitated NPCD (Fig. 4A,B). This suggests that NPCD can be dephosphorylated by PTPRO. To test this idea, we performed GST pulldowns with a catalytically inactive form of GST-PTPRO (Flint et al., 1997; Xie et al., 2002). A quantitatively similar amount of the 41 kDa NPCD protein associated with the mutant PTPRO as with wild-type PTPRO, as assessed by anti-Ptx Western blotting. However, the NPCD associated with the catalytically inactive protein was strongly tyrosine phosphorylated (Fig. 4B), confirming that NPCD/PTPRO interactions are not regulated by tyrosine phosphorylation of NPCD and suggesting that PTPRO dephosphorylates NPCD. To confirm that NPCD is a direct substrate for PTPRO, we performed in vitro dephosphorylation experiments (detailed in Materials and Methods) using purified PTPRO-GST and purified NPCD. Tyrosine-phosphorylated NPCD protein immunoprecipitated from brain with anti-Ptx antibody was incubated with GST alone, wild-type PTPRO-GST, or catalytically inactive PTPRO-GST. After 1 h at 37°C, wild-type PTPRO-GST, but not the inactive mutant, appreciably dephosphorylated the 41 kDa NPCD protein (Fig. 4C). These results, coupled with our interaction and colocalization data, strongly suggest that NPCD is a physiological substrate for PTPRO.
NPCD is required for NGF-induced neurite extension in PC12 cells
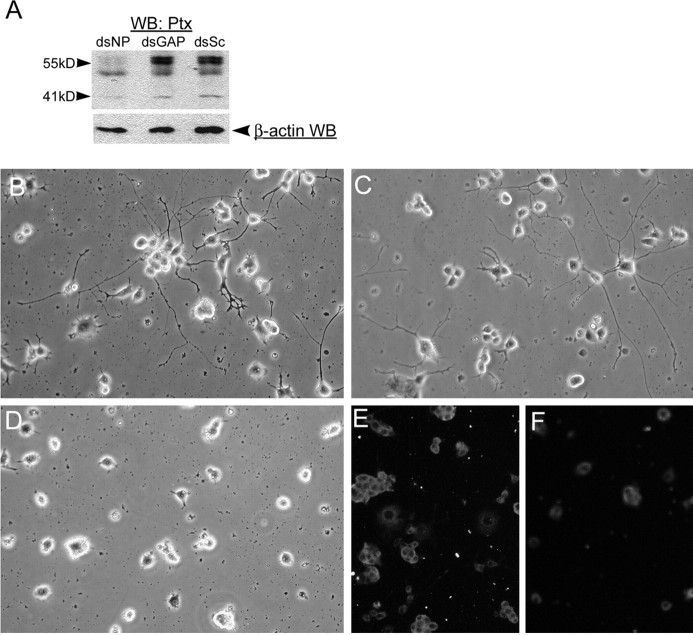
The association of NPCD with PTPRO, combined with the expression of NPCD on neurites and growth cones (Chen and Bixby, 2005), suggests the possible involvement of NPCD in axon growth and/or other aspects of neuronal differentiation. Because both NCPD and PTPRO are expressed in PC12 cells (Chen and Bixby, 2005; P. Beltran, unpublished observations), we used this well-studied model of neuronal differentiation to test the role of NPCD in neuronal differentiation. We used doubled-stranded RNA (dsRNA) interference (Elbashir et al., 2001; Sharp, 2001) to knock down NPCD expression in PC12 cells undergoing NGF-induced neuronal differentiation. In initial experiments, siRNAs corresponding to four different locations within the Ptx domain were produced and tested for efficacy in a COS cell cotransfection assay (see Materials and Methods). In these experiments, one siRNA knocked down expression of the 41 kDa NPCD protein (produced from a transfected cDNA) by 90% (data not shown); this siRNA was chosen for the PC12 experiments. We devised a transfection procedure that reliably transfected >90% of PC12 cells, as assessed by transfection of Cy3-labeled dsRNA (data not shown). When this procedure was used to transfect dsRNAs, NPCD siRNAs, but not control siRNAs, were capable of knocking down expression of NPCD isoforms within 24 h after transfection. The 55 kDa NPCD expression was almost eliminated, and the 41 kDa NPCD expression was knocked down by ∼40% (Fig. 5A). The relative lower efficiency of knock-down of the 41 kDa NPCD isoform at this early time may be attributable to a slower turnover rate. By 72 h after transfection, the 41 kDa NPCD isoform was knocked down by at least 60-75% (Figs. 5, 6 and data not shown).
Figure 5.
NPCD proteins are required for NGF-induced neuronal differentiation in PC12 cells. A, PC12 cells were transfected with siRNAs directed against NPCD (dsNP), GAPDH (dsGAP), or scrambled siRNAs (dsSc), and after 24 h, cell lysates were run on SDS-PAGE and sequentially blotted with anti-Ptx and anti-β-actin as a loading control. NPCD siRNAs severely reduced expression of the 55 kDa NPCD protein doublets (>90%) and caused a 40% reduction in expression of the 41 kDa NPCD protein compared with controls at this early time. B-F, PC12 cells were transfected with siRNAs that were scrambled so as not to match known sequences (B), siRNA capable of knocking down GAPDH (C, E), or siRNA for NPCD (D, F) and were then replated and grown in NGF for 3 d. Cells were examined by phase contrast (B-D) or fixed and stained for NPCD (E, F). Knock-down of NPCD was demonstrable after 4 d(F) and led to a lack of neurite growth (D). Hoechst staining of transfected cells revealed no apoptosis induced by NPCD siRNA (data not shown). These experiments were performed five times with similar results. Note that NPCD staining in PC12 cells requires permeabilization and is therefore cytosolic; the “ring” staining pattern suggests membrane association (Chen and Bixby, 2005). WB, Western blot.
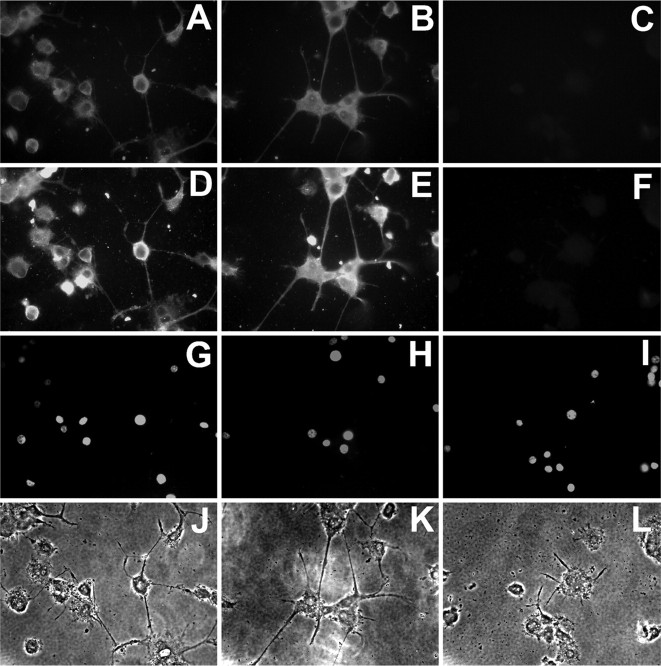
Figure 6.
NPCD proteins are required for neurite outgrowth of differentiated PC12 cells. Primed PC12 cells were treated with siRNAs for 3 d and were then replated onto poly-d-lysine-coated coverslips for an additional 12 h culture in the presence of NGF (100 ng/ml). Cells were treated with scrambled siRNAs (A, D, G, J), siRNAs directed against GAPDH (B, E, H, K), or siRNAs directed against NPCD (C, F, I, L). Staining of permeabilized cells with anti-Ptx (A-C) or anti-CD (D-F) demonstrates that NPCD protein expression is greatly reduced by NPCD siRNAs. Whereas primed PC12 cells treated with controls iRNA extended neurites within 12 h exposure to NGF (J, K), those treated with siRNAs directed against NPCD showed a minimum neurite growth response to NGF (L). Neurites were both shorter and less numerous after NPCD knock-down. There was no sign of apoptosis from siRNA treatment as shown by Hoescht-stained nuclei in the treated cells (G-I).
PC12 cells were transfected with control or NPCD siRNAs and were then replated and treated with 100 ng/ml NGF for 3 d. Cells treated with siRNAs capable of knocking down GAPDH (Ambion) or with “scrambled” siRNAs (not related to known mRNAs) grew long neurites over the 3 d period (Fig. 5B,C). However, neurite growth was essentially abolished in cells treated with NPCD siRNAs (Fig. 5D). Quantitative assessment of the percentage of cells with neurites showed a significant inhibition of neurite growth in cells treated with NPCD siRNA (46 ± 5.3% with neurites, untreated cells; 44 ± 7.6%, scrambled siRNA; 43 ± 5.4%, GAPDH siRNA; 1.5 ± 5.3%, NPCD siRNAs; p < 0.001). Staining of the treated cells demonstrated that NPCD expression was decreased with NPCD siRNA after the 3 d treatment period (Fig. 5F) compared with control RNAs (Fig. 5E). Importantly, PC12 cells treated with NPCD siRNAs were as numerous as control cells and showed no signs of apoptosis as assessed by nuclear staining with Hoechst dye (Fig. 6 and data not shown).
NGF induces a sympathetic-like neuronal differentiation in PC12 cells over a period of 7-10 d. In addition, acute addition of NGF to differentiated (primed) PC12 cells induces rapid neurite growth. Because NPCD knock-down in our initial experiments took place before any addition of NGF, it was not clear whether initial neuronal differentiation or neurite growth per se was primarily affected by the loss of NPCD. We addressed the issue by using primed PC12 cells, which were differentiated before NPCD knock-down. Primed PC12 cells were treated with control or NPCD siRNAs for 3 d before replating in the presence of NGF. Knock-down of NPCD expression in these cells was striking, leading to almost complete loss of NPCD immunoreactivity (Fig. 6 A-F). Whereas primed PC12 cells treated with control siRNAs extended long neurites within 12 h after replating, acute neurite extension was significantly inhibited when NPCD expression was knocked down (Fig. 6 J-L). Quantitative analysis showed a significant inhibition of neurite growth in differentiated PC12 cells treated with NPCD siRNA (11 ± 5.3% with neurites) compared with controls (61 ± 6.3%, untreated cells; 56 ± 7.6%, scrambled-siRNA; 53 ± 4.2%, GAPDH siRNA; p < 0.001). Similarly, no signs of apoptosis were observed as assessed by nuclear Hoechst staining (Fig. 6G-I). Together, the results indicate that intracellular NPCD proteins are required for NGF-induced neurite extension in PC12 cells. It will be important to test the degree to which this requirement is shared by NPCD-expressing primary neurons.
NPCD knock-down does not affect initial NGF signaling
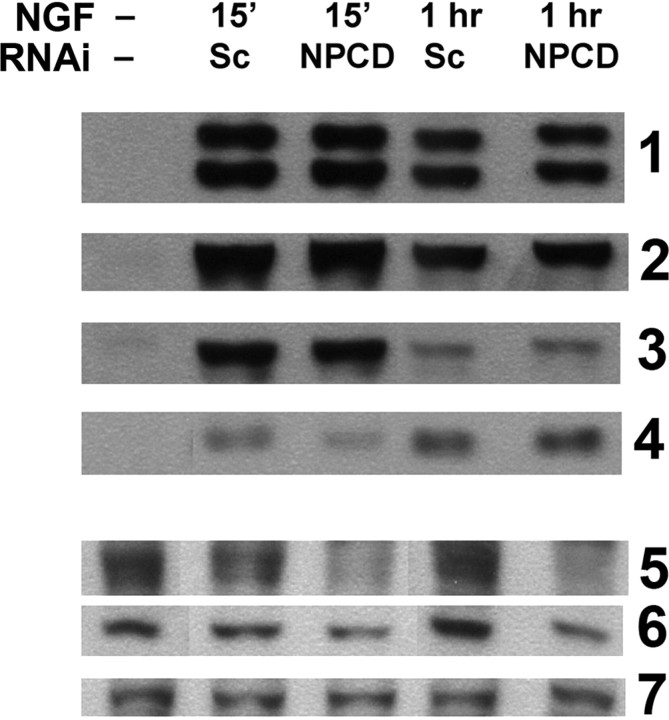
The results with differentiated PC12 cells indicate that NPCD is important in NGF-induced neurite extension independent of any potential effects on initial neuronal differentiation. However, this does not rule out additional roles for NPCD in NGF-induced differentiation. To assess whether NPCD is required for initial signaling responses to NGF, we analyzed the activation of signaling proteins in the ERK and Akt pathways, two pathways known to be important in the NGF response (Ashcroft et al., 1999; Zhang et al., 2000). In control cells (treated with scrambled siRNAs), a strong activation of ERK1/2, p90RSK (downstream target of ERK), Akt, and S6 ribosomal protein (downstream target of Akt) was achieved 15 min after NGF stimulation; this activation was attenuated but still evident 1 h after stimulation (Fig. 7). Treatment with siRNAs that knocked down expression of NPCD (and that were previously shown to inhibit neurite extension at this time) did not affect activation of any of these signaling proteins (Fig. 7). These results suggest that the role of NPCD in NGF-induced neurite extension is downstream of these initial signals or is part of a distinct signaling pathway (Burry, 2001).
Figure 7.
Initial NGF signaling through ERK and Akt pathways is unaffected by NPCD knock-down. PC12 cells were transfected with either scrambled (Sc) or NPCD siRNAs for 3 d before stimulation with NGF (100 ng/ml). Activation of signaling proteins was assessed 15 min and 1 h after NGF stimulation by Western blot with phospho-specific antibodies. We tested activation of ERKs 1 and 2 (row 1), the ERK target p90RSK (row 2), Akt (row 3), and the Akt target S6 ribosomal protein (row 4). No consistent effects on activation of any of these proteins at either time point was observed (a slight decrease seen here at 15 min for phosphoS6 was not seen in other experiments). NPCD knock-down of the 55 kDa doublet (row 5) and the 41 kDa band (row 6) were monitored in the same lysates; knock-down by NPCD siRNAs was clearly evident (85% knock-down compared with scrambled, 55 kDa; 60% knock-down, 41 kDa). GAPDH Western blotting was used in these blots as a loading control (row 7). This experiment was repeated twice with similar results. RNAi, RNA interference.
Discussion
This major conclusions from this study are that cytoplasmic isoforms of the novel pentraxin NPCD interact with the catalytic phosphatase domain of PTPRO in brain neurons, that PTPRO expression is required for appropriate localization of NPCD in brain, that NPCD is a phosphoprotein in vivo and a likely PTPRO substrate, and that NPCD is required for NGF-induced process outgrowth in PC12 cells. These findings identify NPCD as the first known substrate of PTPRO in any cell type and simultaneously implicate NPCD in important processes of neuronal differentiation.
RPTPs comprise a major class of proteins regulating axon growth and guidance. In particular, type III RPTPs (PTP99A, PTP10D, PTP52F) have been shown to regulate motor axon guidance as well as guidance of CNS axons in Drosophila (Sun et al., 2000; Schindelholz et al., 2001). Although less is known concerning the involvement of vertebrate type III RPTPs in axon growth, the pattern of expression of PTPRO and the known functions of PTPRO in axon growth and growth cone steering in vitro make it a strong candidate to regulate vertebrate axon guidance (Bodden and Bixby, 1996; Stepanek et al., 2001; Beltran et al., 2003). In support of this idea, we have recently used in ovo electroporation and dsRNA interference to show that knock-down of PTPRO expression during chick embryogenesis interferes with appropriate motor axon guidance (Stepanek, Stoker, Stoeckli, and Bixby, unpublished observations). A mouse knock-out of PTPRO has been made; it has a kidney phenotype (Wharram et al., 2000), but its neural phenotype has not been examined in detail.
RPTPs are likely to interact with a variety of intracellular proteins to exert their functional activities. A large number of potential physiological substrates have now been identified, including growth factor receptors, intracellular tyrosine kinases of the Src and Abl families, adhesion-related proteins such as catenins and p130Cas, and regulators of small GTP proteins such as GIT-1, an Arf-GAP, and Trio, a Rho-guanine nucleotide exchange factor (Bixby, 2001; Beltran and Bixby, 2003). In the case of type III RPTPs, the PDGF receptor, the HGF receptor, and p120ctn have been identified as substrates for the hematopoietic RPTP known as DEP-1/CD148 (Kovalenko et al., 2000; Holsinger et al., 2002; Palka et al., 2003). NPCD is the first substrate identified for PTPRO, although it is possible that tropomyosin-related kinase (Trk) receptors comprise relevant substrates (Beltran et al., 2003; Beltran, unpublished observations). The fact that the catalytic phosphatase domain of PTPRO, which is conserved among RPTPs, is responsible for NPCD interaction leaves open the possibility that other RPTPs may also bind NPCD. Potential binding of NPCD to other membrane receptors is consistent with our finding that NPCD, although mislocalized, can still be found at or near the plasma membrane in PTPRO null mice. Although no pentraxin has previously been shown to reside at the cytoplasmic membrane face, it has been reported recently that another, primarily nuclear, chromo domain protein called Eed can be recruited to the plasma membrane from the nucleus. Like NPCD, Eed is likely to be recruited by multiple transmembrane receptors (Witte et al., 2004).
Most proteins identified as interacting with the intracellular domains of RPTPs are common components of adhesion-related signaling systems and appear to be transiently associated with RPTPs in a phosphotyrosine-dependent manner (Beltran and Bixby, 2003; Johnson and Van Vactor, 2003). Such results have led investigators interested in signaling to search for proteins whose binding to RPTPs depends on phosphorylation. Although NPCD is a phosphoprotein and can serve as a substrate for PTPRO, the association between NPCD and PTPRO does not depend on the phosphorylation state of NPCD. In this last respect, NPCD is similar to members of the liprin-α family, a group of proteins that interact with type IIa RPTPs (Serra-Pages et al., 1998). Genetic experiments in flies and worms, along with recent experiments in mammalian neurons, demonstrate that liprin-α homologs are critical for assembly of both presynaptic and postsynaptic elements of the synapse (Zhen and Jin, 1999; Kaufmann et al., 2002; Wyszynski et al., 2002). Presumably, the liprins act to organize RPTPs together with functionally important signaling proteins. This idea may provide a clue as to the role of NPCD in PTPRO function.
Our results using dsRNA interference demonstrate the functional requirement for NPCD in NGF-induced PC12 cell differentiation, but the isoform(s) involved is not clear. The siRNAs we used to target NPCD knocked down a number of NPCD isoforms in PC12 cells. Because we did not detect cell-surface (transmembrane) NPCD in these cells, it is unlikely that this form (NPR) mediates the activity we have identified. However, it is not possible to implicate any one isoform as being important in NGF-induced neurite outgrowth. Similarly, we do not know the relationship between NPCD function in neurite growth and its interaction with the receptor tyrosine phosphatase PTPRO. PTPRO appears to be involved in axon growth and guidance (Stepanek et al., 2001; Stepanek, Stoker, Stoeckli, and Bixby, unpublished observations). Because the pentraxin domain is the PTPRO binding domain, most NPCD isoforms are predicted to bind PTPRO. Current investigations include analysis of the possible link between PTPRO and NPCD function. In this regard, it will be of interest to determine both the tyrosine kinase(s) responsible for NPCD phosphorylation and the NPCD residues phosphorylated. Because we have been unable to obtain NPCD phosphorylation by Src overexpression in cotransfected COS cells, a specialized neuronal tyrosine kinase may be involved.
One aspect of NPCD function appears to be linked to neurite growth itself. NGF-dependent neurite outgrowth in PC12 cells was severely reduced with knock-down of NPCD, whether this was done in naive cells or in primed, differentiated cells. Thus, NPCD is required in the neurite growth pathway independent of any involvement in initial neuronal differentiation. Whether NPCD is also involved in initial differentiation cannot be determined from our results. Examination of proximal NGF-induced signaling events, however, suggests that NPCD action is downstream of the major early signals through TrkA and is consistent with the idea that initial differentiation is unaffected. In line with this idea, NGF-dependent survival of primed PC12 cells is not dependent on NPCD function.
In summary, our studies on NPCD provide the first example of a predominantly cytoplasmic localization for a pentraxin or a chromo domain-containing protein; the linkage between these two protein interaction domains strongly suggests a novel function. NPCD is also the first protein shown to bind to, and be dephosphorylated by, the catalytic domain of PTPRO and seems likely to be important in PTPRO function. Finally, NPCD is required for NGF-dependent process outgrowth in PC12 cells. In vivo, PTPRO expression strongly overlaps with that of the NGF receptor TrkA and with the NT3 receptor TrkC in various neuronal populations (Beltran et al., 2003). The PC12 results therefore suggest a functional link between PTPRO and the Trk receptors. Our results so far suggest that NPCD does not regulate initial signaling through TrkA; additional studies will be required to determine the level at which NPCD could interact with signaling pathways regulated by Trks.
Footnotes
This work was supported by a grant to J.L.B. from the National Institutes of Health (NS 38920). We thank Dr. Mark Perin, Dr. Paul Worley, and Richard Cho for their generous gifts of neuronal pentraxin antibodies; Beata Frydel and the imaging facility of the Miami Project to Cure Paralysis for help with confocal microscopy; Dr. Pedro Beltran for production of the PTPRO inactive mutant; Drs. Beltran, Antero So, and Konstant in Levay for technical advice; and Drs. Kerry Burnstein and Vance Lemmon for critical comments on this manuscript. Dr. Lemmon also supplied useful experimental advice. We are grateful to Dr. Roger Wiggins for the gift of PTPRO knock-out mice.
Correspondence should be addressed to John L. Bixby, The Miami Project to Cure Paralysis, University of Miami School of Medicine, Lois Pope LIFE Center, Room 4-17, 1095 Northwest 14th Terrace, Miami, FL 33136. E-mail: jbixby@miami.edu.
B. Chen's present address: Department of Genetics, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250880-09$15.00/0
References
- Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR (1999) The selective and inducible activation of endogenous PI 3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite out-growth. Oncogene 18: 4586-4597. [DOI] [PubMed] [Google Scholar]
- Beltran PJ, Bixby JL (2003) Receptor protein tyrosine phosphatases as mediators of cellular adhesion. Front Biosci 8: D87-D99. [DOI] [PubMed] [Google Scholar]
- Beltran PJ, Bixby JL, Masters BA (2003) Expression of PTPRO during mouse development suggests involvement in axonogenesis and differentiation of NT-3 and NGF-dependent neurons. J Comp Neurol 456: 384-395. [DOI] [PubMed] [Google Scholar]
- Bixby JL (2000) Receptor tyrosine phosphatases in axon growth and guidance. NeuroReport 11: R5-R10. [DOI] [PubMed] [Google Scholar]
- Bixby JL (2001) Ligands and signaling through receptor-type tyrosine phosphatases. IUBMB Life 51: 157-163. [DOI] [PubMed] [Google Scholar]
- Bodden K, Bixby JL (1996) CRYP-2: a receptor-type tyrosine phosphatase selectively expressed by developing vertebrate neurons. J Neurobiol 31: 309-324. [DOI] [PubMed] [Google Scholar]
- Burry RW (2001) p21(ras) stimulates pathways in addition to ERK, p38, and Akt to induce elongation of neurites in PC12 cells. J Neurosci Res 63: 45-53. [DOI] [PubMed] [Google Scholar]
- Chen B, Bixby JL (2005) NPCD (neuronal pentraxin with chromo domain) is a novel class of protein expressed in multiple neuronal domains. J Comp Neurol 481: 391-402. [DOI] [PubMed] [Google Scholar]
- Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS (1997) Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J Biol Chem 272: 21488-21494. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494-498. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK (1997) Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA 94: 1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Ward K, Duffield B, Zachwieja J, Jallal B (2002) The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn). Oncogene 21: 7067-7076. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D (2003) Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev 83: 1-24. [DOI] [PubMed] [Google Scholar]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D (2002) Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron 34: 27-38. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS (2000) Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem 275: 17786-17792. [DOI] [PubMed] [Google Scholar]
- Kovalenko M, Denner K, Sandstrom J, Persson C, Gross S, Jandt E, Vilella R, Bohmer F, Ostman A (2000) Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem 275: 16219-16226. [DOI] [PubMed] [Google Scholar]
- O'Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P (2002) Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci 22: 4487-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palka HL, Park M, Tonks NK (2003) Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J Biol Chem 278: 5728-5735. [DOI] [PubMed] [Google Scholar]
- Schindelholz B, Knirr M, Warrior R, Zinn K (2001) Regulation of CNS and motor axon guidance in Drosophila by the receptor tyrosine phosphatase DPTP52F. Development 128: 4371-4382. [DOI] [PubMed] [Google Scholar]
- Serra-Pages C, Medley QG, Tang M, Hart A, Streuli M (1998) Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem 273: 15611-15620. [DOI] [PubMed] [Google Scholar]
- Sharp PA (2001) RNA interference-2001. Genes Dev 15: 485-490. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Sun QL, Wang J, Wang C, Bixby JL (2001) CRYP-2/cPTPRO is a neurite inhibitory repulsive guidance cue for retinal neurons in vitro. J Cell Biol 154: 867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Bahri S, Schmid A, Chia W, Zinn K (2000) Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. Development 127: 801-812. [DOI] [PubMed] [Google Scholar]
- Thomas PE, Wharram BL, Goyal M, Wiggins JE, Holzman LB, Wiggins RC (1994) GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J Biol Chem 269: 19953-19962. [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Gillespie PJ, Wiggins JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC, Wiggins RC (2000) Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest 106: 1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte V, Laffert B, Rosorius O, Lischka P, Blume K, Galler G, Stilper A, Willbold D, D'Aloja P, Sixt M, Kolanus J, Ott M, Kolanus W, Schuler G, Baur AS (2004) HIV-1 Nef mimics an integrin receptor signal that recruits the polycomb group protein Eed to the plasma membrane. Mol Cell 13: 179-190. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M (2002) Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron 34: 39-52. [DOI] [PubMed] [Google Scholar]
- Xie L, Zhang YL, Zhang ZY (2002) Design and characterization of an improved protein tyrosine phosphatase substrate-trapping mutant. Biochemistry 41: 4032-4039. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA (2000) Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci 20: 5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Jin Y (1999) The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans Nature 401: 371-375. [DOI] [PubMed] [Google Scholar]