Abstract
Radial glia are integral components of the developing neocortex. During corticogenesis, they form an important scaffold for neurons migrating into the cortical plate. Recent attention has focused on neuregulin (NRG1), acting through erbB receptors, in maintaining their morphology. We developed a model of developmental radial glial disruption by delivering an antimitotic [methylazoxy methanol (MAM)] to pregnant ferrets on embryonic day 24 (E24). We previously found that normal ferret cortex contains a soluble factor capable of realigning the disorganized radial glia back toward their normal morphology. Characterization of the reorganizing activity in normal cortex demonstrated that the probable factor mediating these responses was a 30–50 kDa protein. To test whether this endogenous soluble factor was NRG1, we used organotypic cultures of E24 MAM-treated ferret neocortex supplemented with the endogenous factor obtained from normal cortical implants, exogenous NRG1β, antibodies that either blocked or stimulated erbB receptors, or a soluble erbB subtype that binds to available NRG1. We report that exogenous NRG1 or antibodies that stimulate erbB receptors dramatically improve the morphology of disrupted radial glia, whereas blockade of NRG1-erbB signaling prevents the radial glial repair. Our results suggest that NRG1 is an endogenous factor in ferret neocortex capable of repairing damaged radial glia and that it acts via one or more erbB receptors.
Keywords: neocortical development, cerebral cortex, erbB receptors, MAM, organotypic culture, schizophrenia
Introduction
Nearly two decades ago, Hatten (1985) showed that contact with granule neurons induces cerebellar astroglia to adopt the morphology of radial glia, suggesting that neuronal derived signals are important for radial glia development. In a later study, Hunter and Hatten (1995) raised the possibility that this process may be regulated by a soluble “radialization factor.” Through a series of cell culture, morphological, and biochemical studies, they showed that embryonic neocortical cells produce a soluble substance of 50–60 kDa molecular weight (MW) (called RF60), which regulates radial glial and astrocytic morphology. Previous studies in our laboratory also strongly suggested that one or more radializing factors may be present during the period that radial glia are needed as a scaffold for cells migrating into the neocortex. We reported that injection of the antimitotic drug methylazoxy methanol (MAM) into pregnant ferrets early during corticogenesis [embryonic day 24 (E24)] resulted in a cluster of symptoms that included disrupted migration, altered distribution of Cajal Retzius cells, disorganized radial glial cells, and the premature differentiation of radial glia into astrocytes (Hasling et al., 2003). Furthermore, we observed that the morphology of the radial glia could be “rescued” in tissue culture either by coculture with normal cortical slices or by administration of medium conditioned by normal cortical tissues. We subsequently characterized this cortical-derived “factor” as a diffusible protein of nominal molecular weight of ∼66 kDa (Gierdalski and Juliano, 2003).
Looking for potential candidates to be responsible for the cortically derived activity, we identified NRG1, a soluble factor that signals through the erbB receptor tyrosine kinases erbB2, erbB3, and erbB4 (Marmor et al., 2004), which influences the morphology of radial glia in the cerebellum and the cortex. Rio et al. (1997) showed that NRG1 is expressed by migrating cerebellar granule neurons (whereas Bergmann glia express erbB receptors) and demonstrated that NRG1-erbB signaling mediates the conversion of cerebellar astroglia into radial glia in response to neuronal contact. Anton et al. (1997) found that NRG1 is also expressed by migrating neocortical neurons and that in vitro NRG1 promotes the maintenance of radial glia morphology in cortical imprint cultures (Anton et al., 1997).
In the present study, we demonstrate that NRG1 is a substance endogenous to normal ferret cerebral cortex and is capable of repairing disrupted radial glial morphology. We show that medium conditioned by organotypic cultures of normal ferret cortex contains NRG1 and that NRG1, or antibodies activating erbB receptors, repairs the morphology of radial glia after MAM treatment. Furthermore, we found that reagents blocking NRG1-erbB signaling also block the ability of medium conditioned by normal cortical slices to repair the morphology of MAM-treated radial glia. Together, these results show that NRG1 is responsible for at least part of the activity that repairs radial glia disrupted by MAM.
Materials and Methods
MAM treatment and organotypic cultures. Timed pregnant ferrets obtained from Marshall Farms (New Rose, NY) were anesthetized with isoflurane (3%) and nitrous oxide (0.05%). They were injected intraperitoneally with 16 mg/kg MAM on E24 as described by Noctor et al. (1999). Brain slices from postnatal day 0 (P0) ferret kits were prepared as described previously (Gierdalski and Juliano, 2003; Hasling et al., 2003). In brief, kits were anesthetized with pentobarbital Na (50 mg/kg, i.p.), and the brains were removed and sliced at 500 μm in thickness using a tissue chopper. The most rostral and caudal portions of the brain were discarded, and the cultures were prepared predominantly of the presumptive parietal cortex. Each slice was placed in a 70 μm nylon mesh cell strainer (Becton Dickinson-Falcon, Bedford, MA), which was then positioned in a six-well tissue culture plate (Becton Dickinson-Falcon). In some cases, the cortical plate was dissected from corresponding slices of normal cortex at P0, and the explants were placed adjacent to the E24 MAM-treated slice as reported by Hasling et al. (2003). Neurobasal medium (supplemented with B27 and N2; Invitrogen, Carlsbad, CA) with the addition of gentamicin and l-glutamine was placed in each well to just cover the slice as described by Stoppini et al. (1991). The slice cultures were maintained in an incubator (37°C; 95% O2/5% CO2) for the duration of the culture period.
To assess the ability of recombinant or endogenous NRG1 to restore the morphology of radial glia and act through ErbB receptors, we used organotypic cultures of neonatal ferret cortex. Slices of cortex were obtained from normal or E24 MAM-treated kits at P0. They were placed in culture alone, as controls, treated with neuregulin or other substances, or in coculture of MAM-treated slices with isochronic explants from normal cortex. The cultures received additives of human recombinant NRG1β (1 nm; R&D Systems, Minneapolis, MN), anti-erbB-3 blocking antibody, anti-erbB4 activating antibody (20 μg/ml, MS-303-PABX and MS-270-PABX, respectively; Neomarkers, Fremont, CA), or the neuregulin inhibitor IgB4, a chimeric protein composed of the extracellular domain of erbB4 and the Fc portion of human IgG1 (Dong et al., 1995).
Injections of fluorescent dextrans. After being in culture for 2 d, each organotypic culture was removed from the incubator and placed in a chamber used to maintain living slices as described previously (Juliano et al., 1996). Each culture received an iontophoretic injection (4 μA; alternating positive current for 3 min) of fluorescently labeled dextrans (Molecular Probes, Eugene, OR) (we primarily used Fluororuby) into the intermediate zone at a depth of 50–100 μm, as described by Noctor et al. (1999). The injections were made using a glass pipette with a tip diameter of ∼20 μm. After an additional 5–8 h of incubation in the chamber at room temperature, the slices were placed in 4% buffered paraformaldehyde for ≥12 h. Before mounting on subbed slides, the slices were counterstained with bisbenzimide trihydrochloride (Sigma, St. Louis, MO) and subsequently analyzed.
Analysis of radial glial morphology. The label resulting from the dextran injections was quantified by measuring the angular deviation of labeled processes. Each dextran injection was digitally imaged at 25× to allow individual processes to be traced and converted to line drawings. To trace entire glial processes labeled by the dextran injections, several focal planes for each injection were imaged and collapsed. The angles were measured along a chord of each radial glial process, as described previously in detail (Hasling et al., 2003). The average of the absolute differences represented the mean angular deviation of each injection. The mean angular deviation was compared for statistically significant differences using an ANOVA followed by Tukey's test for multiple comparisons between conditions.
Western blots. The fractionation of the conditioned medium was conducted as described by Gierdalski and Juliano (2003). In brief, after 2–3 d of culturing cortical slices from normal P0 ferrets in serum-free medium (Neurobasal plus N2 plus B27 plus gentamicin/l-glutamine; Invitrogen, Carlsbad, CA), the medium was collected and centrifuged while passing through a centrifugal filter device (Amicon Centriprep YM; Millipore, Billerica, MA) with cutoff limits of 50, 30, and 10 kDa MW, consecutively. The fractions were then concentrated and buffer-exchanged three times by centrifugation, washing with replacement TBS buffer (20 mm Tris HCl, 20 mm NaCl, pH 7.2; containing Complete Protease Inhibitor Cocktail tablets; Roche, Indianapolis, IN) using filter devices (Amicon Centricon YM-3; Millipore, Billerica, MA). All centrifugations were done at 4°C. Filtrates were collected and immediately frozen at –80°C.
The samples of concentrated fractions (in equal amounts equivalent to one original cultured slice; i.e., one slice equivalent) were mixed with 4× NuPAGE LDS Sample Buffer (Invitrogen) and 10× NuPAGE Sample Reducing Agent (Invitrogen), boiled for 10 min, loaded onto NuPAGE BisTris 10% minicasette, and run in 3-(N-morpholino)propanesulfonic acid SDS running buffer with the addition of NuPAGE antioxidant. The gel was transferred onto a nitrocellulose membrane (0.45 μm pore; Millipore, Bedford, MA) using XCell Surelock. The membrane was incubated overnight at 4°C in blocking buffer (PBS, 0.5% casein, 0.1% Tween 20) and then incubated with an anti-neuregulin monoclonal primary antibody Ab-1 (MS-272; 1:100 in blocking buffer; Neomarkers) for 2 h at room temperature, washed three times for 15 min in PBS with 0.1% Tween 20, incubated with secondary antibody (goat anti-mouse HRP; 1:3000 in blocking buffer; Pierce, Rockford, IL), and finally washed four times for 15 min in PBS with 0.1% Tween 20. Detection was performed using a SuperSignal West Pico Chemiluminescence kit (Pierce, Rockford, IL) according to the manufacturer. The blots were then exposed to Hyperfilm ECL (Amersham Biosciences, Little Chalfont, UK). Developed films were digitized using LS-1000 Imaging System (Fujifilm, Tokyo, Japan).
erbB phosphorylation assay. The activity of the fractions was assessed on L6 rat myoblast cells by analysis of the induction of p185 tyrosine phosphorylation, as described by Corfas et al. (1993). Briefly, cells were grown in DMEM containing 10% fetal bovine serum, Pen/Strep, and l-glutamine. Cells were plated in 24-well plates and treated with NRG1 (1 nm) or conditioned media fractions for 5 min. Cells were washed with cold PBS on ice, lysed in lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerolphosphate, 1 mm Na3VO4, 1 mm Pefabloc), and centrifuged for 10 min at 1000 × g. Lysates were incubated overnight with erbB2 and erbB3 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and immunoprecipitated with protein A/G beads (Pierce) for 1 h. Samples were then washed three times with lysis buffer before the beads were resuspended in SDS-sample buffer and boiled for 2 min. Samples were loaded onto 5% polyacrylamide-SDS gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes and subjected to immunoblotting using the monoclonal phosphotyrosine antibody 4G10 (a gift from O. Gjoerup and T. M. Roberts, Dana-Farber Cancer Institute, Boston, MA). The blots were then incubated with HRP-conjugated anti-mouse secondary antibody and developed using enhanced chemiluminescence. Blots were stripped and subjected to immunoblotting using erbB2 antibody to verify loading.
Results
Medium conditioned by ferret cortical slices contain NRG1
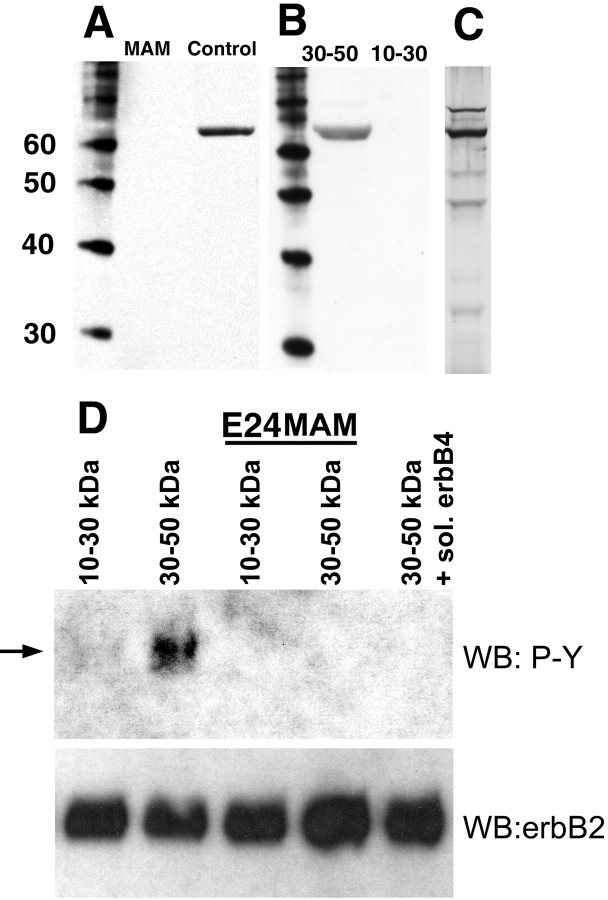
To assess whether NRG1 was present in the neonatal ferret cortex, medium conditioned for 48 h by organotypic cultures of normal cortical slices was fractioned into aliquots of different molecular weight by centrifugation. Then, the fractions containing molecules of 10–30 and 30–50 kDa nominal molecular weight were subjected to Western blot analysis with antibodies against the extracellular domain of NRG1. We previously demonstrated that fractionated conditioned media of nominal molecular weight 30–50 kDa could restore radial glia disrupted by MAM treatment to normal morphology (Gierdalski and Juliano, 2003). The blots shown in Figure 1A show the existence of soluble NRG1 in the conditioned media obtained from normal (control) slices but not in the media obtained from E24 MAM-treated cultures. The reactivity is of slightly higher molecular weight than we expected, ∼66 kDa. The range of molecular weight is shown in Figure 1C, which illustrates a silver stain obtained from a gel of this media fraction.
Figure 1.
The fraction of medium conditioned by ferret cortical slices, which has radializing activity, contains NRG1. A, Conditioned media obtained from normal organotypic cultures of ferret cortex were fractioned into aliquots of different molecular weight by centrifugation and then subjected to Western blot analysis with NRG1 antibodies. NRG1 immunoreactivity was observed in the 30–50 kDa fraction from the conditioned media obtained from normal (control) but not E24 MAM (MAM)-treated organotypic cultures. MW markers are indicated in the left lane. B, In the conditioned media obtained from normal cortical slices, the 30–50 kDa fraction was immunoreactive for NRG1, whereas the 10–30 kDa fraction was not. MW markers are shown in the left lane. C, A silver stain on a gel obtained from the normal 30–50 kDa conditioned media fraction. The molecular weights correspond to the markers shown in A and B. D, L6 muscle cells in culture were treated with conditioned media fractions 30–50 and 10–30 kDa at eight slice equivalents/milliliter for 5 min (see Materials and Methods for details). Cells were lysed and subjected to Western blot analysis with anti-phosphotyrosine antibodies (WB:P-Y). Only the fraction that possesses the radial glia-orienting activity (30–50 kDa fraction obtained from normal tissue) induced the tyrosine phosphorylation of an 185 kDa protein (arrow), similar to that induced by NRG1. Neither the inactive fraction from normal tissue nor either molecular weight fraction from MAM-treated tissue induced phosphorylation. Addition of a soluble form of the erbB4 receptor also blocked activity from the normal active fraction. The blots were stripped and subjected to immunoblotting with erbB2 to demonstrate equivalent loading (WB: erbB2). sol., Soluble.
To determine whether the fractions of cortical conditioned medium that can repair the morphology of radial glia after MAM treatment contain active NRG1, we tested whether they induce the activation of erbB receptors. L6 muscle cells, which express erbB2 and erbB3 receptors, were stimulated with the inactive (10–30 kDa) or active (30–50 kDa) conditioned media fractions from normal cortex and then subjected to phosphotyrosine Western blot analysis. The muscle cells were also stimulated using the normal active fraction with a soluble form of erbB4 added and with the 10–30 and 30–50 kDa fractions obtained from MAM-treated animals. The soluble erbB4 would be expected to bind to NRG1 and block its function. Only the active fraction obtained from normal conditioned medium induced tyrosine phosphorylation of a 185 kDa band, corresponding to the molecular weight of the erbB receptors (Fig. 1D). Together, these results show that NRG1 is present in the 30–50 kDa conditioned media fraction obtained from normal ferret cortex but not in MAM-treated cortex. In addition, the ability of the 30–50 kDa fraction to phosphorylate L6 muscle cells was blocked by the addition of soluble erbB4.
Recombinant soluble neuregulin repairs radial glia
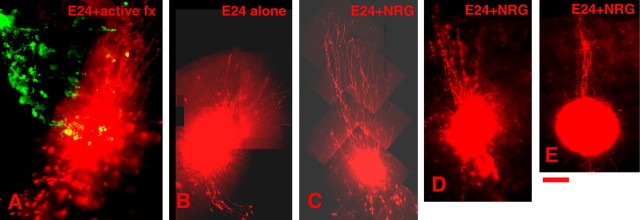
To assess whether NRG1 could be responsible for elongating the disrupted radial glia in E24 MAM-treated cortex, organotypic cultures from P0 E24 MAM-treated cortex were maintained in the presence or absence of 1 nm NRG1 for 2 d. Slices were then injected with FluoroRuby, which selectively labels a small population of radial glial cells and their processes (Noctor et al., 1999), and the angular deviation of radial glia was measured. Addition of NRG1 to the cultures had a strong radializing effect, as indicated by the straightening and elongation of radial glial morphology in E24 MAM-treated cortex compared with the morphology of cultures without added NRG1 (Fig. 2B–E). An example of the elongation effect of using the fraction of normal conditioned media of 30–50 kDa in an E24 MAM-treated slice is shown in Figure 2A. To show this effect, the media fraction was adhered to fluorescent microspheres, which were injected into the organotypic culture.
Figure 2.
NRG1 treatment of E24 MAM-treated organotypic cultures restores radial glia alignment. Examples of radial glial morphology in E24 MAM-treated organotypic cultures are shown. A, An example of an E24 MAM-treated culture with injections of fluorescent microspheres with the active fraction of conditioned medium adhered to the spheres. As the microspheres release the fraction slowly, the radial glia realign toward normal orientations. The microspheres are green. B, An example of an E24 MAM culture with no additional treatment. C–E, Examples of E24 MAM-treated cultures with NRG1 added. After treatment with NRG1, the radial glial processes elongate and orient their processes toward the pia. Scale bar, 200 μm.
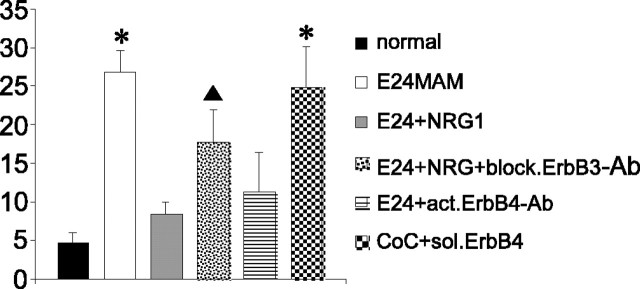
The radializing effect of NRG1 is quantified in Figure 3, which shows that normal radial glia have a low angular deviation, with well aligned fibers (black bar). The angular deviation of E24 MAM-treated slices is high, with the glial fibers arrayed at many angles (Fig. 3, white bar), but when NRG1 is added to MAM-treated tissue, the angular deviation is no longer significantly different from the normal angles (gray bar).
Figure 3.
Quantitative analysis of angular deviations of radial glial fibers after different treatments. Cortical slices were subjected to the treatments detailed on the x-axis and then injected with FluoroRuby, and the angular deviation of radial glia processes was measured. Low-angular deviations (y-axis) indicate that the processes are oriented in similar directions, such as those in the normal injections (black bar; n = 7). High-angular deviations indicate that the radial glial processes are divergent from one another, as in the E24 MAM-treated injections (whitebar; n=5). Besides the normal slices, all measurements were done on E24MAM-treated cultures. Although angular deviations in E24 MAM-treated tissues are very high, angular deviations in slices treated with NRG1 (gray bar; n = 5) or an erbB4 activating antibody (horizontally striped bar; n = 7) are not significantly different from normal. In contrast, the addition of an antibody that blocks NRG1 binding to erbB3 blocks the NRG1 effects (dotted bar; n = 4). Furthermore, the presence of soluble erbB4 receptors abolishes the ability of normal explants to improve radial glial morphology of E24 MAM slices in coculture (checkered bar; n = 4). Note that E24 MAM-treated slices placed in coculture with normal cortex alone (CoC; vertical stripes; n = 7) have angular deviations similar to normal. The error bars indicate SD. The significance values are those different from the normal values; *p ≤ 0.0001; ▴p ≤ 0.02.
To further assess the effects of NRG1 on the repair of MAM-treated radial glia, we tested whether blockade of NRG1 binding to erbB3 would affect the ability of this factor to repair radial glia morphology. E24 MAM-treated organotypic cultures were treated with NRG1 for 48 h in the presence or absence of the blocking erbB3 antibody. Although radial glial morphology in slices treated with NRG1 had improved morphology, addition of the erbB3 blocking antibody abolished the NRG1 effect (Figs. 3, 4). These results show that NRG1 is sufficient to induce the radial glia repair of MAM-treated slices and that erbB3 may be involved in this process.
Figure 4.
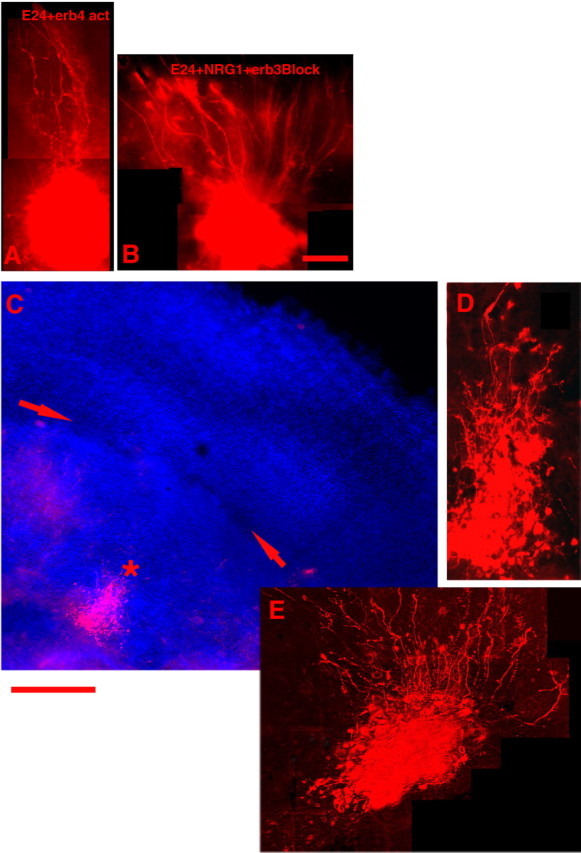
ErbB receptor activation or blockade. A illustrates the effect of adding an activating antibody to the media during organotypic culture of a slice of E24 MAM-treated ferret cortex. The erbB4 activation results in improved morphology of radial glia. B, An example of an E24 MAM-treated culture that received supplemental NRG1 in the medium. The addition of an erbB3 blocking antibody prevents improved morphology usually afforded by the addition of NRG1. C, A coculture with the E24 MAM-treated slice and a normal cortical implant; the red arrows indicate the boundary between the two. Soluble erbB4 receptors were added to the culture media. The normal implant would normally release a factor that improves radial morphology of the radial glia. The soluble erbB4 binds to available NRG1 and prevents radialization by the endogenous factor, suggesting that the diffusible factor in normal cortex is NRG1. D, A higher-power view of the injection shown in C (asterisk). E, An example of a dextran injection taken from a different coculture treated in the same way. Scalebars: (in B) A, B, 200μm; (below C) C, 400 μm; (below C) D, E, 200 μm.
Activation of erbB receptor signaling is sufficient for repair of radial glia morphology
To further test the roles of NRG1-erbB signaling in radial glia repair and to test the roles of erbB4, the other NRG1-binding receptor in this process, we asked whether activation of erbB4 receptor signaling independent of NRG1 could also repair radial glia morphology. E24 MAM-treated slices were treated with an erbB4 antibody that binds and activates the receptor. This treatment was sufficient to cause glial processes to elongate, reducing the angular deviation of radial glial fibers toward normal levels; the angular deviation values after erbB4 activation are not significantly different from the normal values (Figs. 3, 4). These results suggest that erbB4 is also involved in the NRG1 effects on MAM-treated slices.
Neuregulin released by normal cortex is necessary for their radialization effects
Finally, to determine whether NRG1 is responsible for the repairing activity released by normal cortical slices, we tested whether addition of soluble erbB4 receptors (IgB4), which binds NRG1 and thus prevents it from binding to and activating the cellular receptors (Dong et al., 1995), could prevent the repair of radial glia morphology in MAM-treated slices by coculture with normal cortical slices. E24 MAM-treated slices were cocultured with normal cortical explants either in the presence of IgB4 or with no additives to the normal medium. Our previous studies demonstrated that after 2 d in culture, the E24 MAM-treated cortex cocultured with normal cortical explants showed the typical elongation and improvement that occurs in the presence of a normal cortical plate (Hasling et al., 2003). The presence of the IgB4 receptor, however, completely abolished these effects (Figs. 3, 4).
Discussion
Is neuregulin present in conditioned medium of a normal ferret?
Our data suggest that neuregulin is endogenous to neonatal ferret cortex and acts via erbB receptors to maintain an elongated morphology in radial glial cells. The substance we previously identified in conditioned medium obtained from neonatal ferret cerebral cortex acts similarly to exogenous NRG1. In the current study, the addition of NRG1 to cultures with disrupted radial glia results in dramatic improvement of their morphology, as does the addition of the identified fraction (Gierdalski and Juliano, 2003). The activity of the conditioned media fraction is concentrated in a nominal MW 30–50 kDa, within the range of NRG1 molecular weight (Gierdalski and Juliano, 2003). Western blots reveal that NRG1 is present only in the media fraction that contains the radial glia-reorganizing activity. In addition, the active fraction is capable of acting in a manner similar to NRG1 by causing erbB receptor tyrosine phosphorylation in muscle cells. We further observed that blockade of endogenous NRG1 by a soluble erbB4 receptor also prevents its action in our model of disrupted radial glia. These observations strongly indicate that NRG1 is present in the developing ferret cortex, is likely to be the factor we previously identified, and acts to elongate radial glia.
NRG1 is a multiform protein and an active integral membrane proprotein. Alternative splicing of a single gene yields three different types (I, II, and III) that differ in the existence of specific domains, glycosylation sites, and molecular topology (cf. Falls, 2003). Furthermore, it has been shown that type I and, possibly, type II of the NRG1 molecule can be cleaved proteolytically by metalloproteinases 17 and 19 containing a disintegrin and metalloprotease domain (ADAM17 and ADAM19) (Shirakabe et al., 2001; Montero et al., 2002) and an extracellular portion released to act as a soluble agent (cf. Buonanno and Fischbach, 2001; cf. Falls, 2003). The size of released, soluble NRG1 forms found in conditioned media varies considerably depending on the experimental system being used, the domain structure, and glycosylation level and may range from 40 to 80 kDa (Holmes et al., 1992; Ethier et al., 1996; Pollock et al., 1999; Villegas et al., 2000; Wang et al., 2001; Malave et al., 2003; Stove et al., 2003; Kuramochi et al., 2004). The size of the NRG1 isoform that we found in normal cortex-conditioned medium falls within this range. The fact that the apparent molecular weight of the NRG1-immunoreactive band (66 kDa) is higher than expected from the filtration device (30–50 kDa) most likely reflects the differences between the tools used: membrane filtration versus gel electrophoresis. For example, the accuracy of membrane filtration depends not only on the size of the molecule but also the shape, which may result in molecules of higher molecular weight included in the sample.
What is the source of NRG1 in our model?
In the rodent CNS, NRG1 is produced predominantly by neurons (Chen et al., 1994; Corfas et al., 1995). Neuregulin is expressed by postmitotic and migrating neurons during corticogenesis and decreases in expression as neurons become mature, whereas radial glia express erbB receptors (Anton et al., 1997; Rio et al., 1997; Longart et al., 2004). Thus, it is likely that in the developing ferret cortex, migrating neurons are the main source of NRG1 and participate in maintaining their elongated morphology (i.e., provide a radialization factor) (Rio et al., 1997). As neurons reach their cortical target and radial glia are no longer needed as a scaffold, they express less neuregulin, and the radial glia differentiate into astrocytes. MAM treatment early during corticogenesis may result in reduced NRG1 expression, which could contribute to the documented disruption of the radial glia.
Neuregulin acts as a radialization factor through ErbB3 and ErbB4 receptors
Members of the erbB receptor family are important in the regulation of cell growth and differentiation (cf. Neve et al., 2001). These receptors interact with each other forming dimers in a seemingly nonrandom manner (cf. Rubin and Yarden, 2001). The content of a dimer has great impact on its signal transduction abilities, with heterodimers being more potent (Wang et al., 1998). In our model, direct application of NRG1 results in radial glial remodeling, whereas blockade of endogenous NRG1 (by soluble erbB4) results in failure of radial glial elongation. The “rescue” of radial glia morphology seems to be mediated via both erbB3 and erbB4, because activation of erbB4 causes an extension of radial glia and blockade of erbB3 prevents radial glial transformation. The potency of these receptors may be increased by dimerization, as described above. Because the kinase domain of the erbB3 receptor is catalytically inactive, this implies that the result seen here is caused by heterodimerization with erbB4 (Citri et al., 2003; Marmor et al., 2004). Our results also suggest that erbB3 and erbB4 together are sufficient for radializing activity. We cannot rule out that erbB2 is also important in this process, because these receptors are also present in the cerebral cortex and often dimerize with other erbB receptors, but they were not tested in our assay. Interestingly, Schmid et al. (2003) demonstrated, using a dominant-negative erbB2 construct, that NRG1-erbB2 signaling can influence the morphology and maintenance of radial glia in the mouse.
Conclusion
Once neuronal migration is complete, some radial glia transform into astrocytes (Voigt, 1989; Culican et al., 1990; deAzevedo et al., 2003). This transformation has been reported to be bidirectional, in that astrocytes can revert to an elongated radial glia-like state both in vitro (Hatten, 1985; Hunter and Hatten, 1995; Soriano et al., 1997; Leprince and Chanas-Sacre, 2001) and in vivo (Leavitt et al., 1999). NRG1 appears to be important to this reversion, because it can induce cerebellar astrocytes in culture to adopt a radial glia phenotype (Rio et al., 1997). In this study, we demonstrate that NRG1 induces partially differentiated radial glia to revert to an elongated state in organotypic cultures. Thus, NRG1 may be involved in the other instances in which astrocytes transform into radial glia in vivo.
For several years, radial glia have been accepted as neural progenitor cells (Malatesta et al., 2000; Noctor et al., 2002; Tamamaki et al., 2001). NRG1 may play a role in the function of radial glia as such progenitors, because with maturation and conversion into astrocytes, they appear less able to generate neurons. NRG1-erbB signaling is clearly part of a mechanism that maintains the radial glial phenotype as long as needed for proper development, including their function as progenitor cells.
NRG1-erbB signaling has received particular recent attention, because the genes encoding neuregulin and receptors have been implicated in predisposition to schizophrenia (Moises et al., 2002; Stefansson et al., 2002; Corfas et al., 2004). The results from the present study provide one mechanism that could contribute to a deficit seen in schizophrenia: failure of migration in various cortical regions (Akbarian et al., 1996; Rioux et al., 2003). Reduced NRG1 contributes to disruption of radial glia, which in turn influences the ability of neurons to migrate properly into the cerebral cortex. Additional analysis may clarify the brain regions involved and whether NRG1 has a precise role in this process.
Footnotes
This work was supported by National Institute of Mental Health Grant RO1 MH 62721 (S.L.J.) and National Institute of Neurological Disorders and Stroke Grant RO1 NS35884 (G.C.).
Correspondence should be addressed to Dr. Sharon L. Juliano, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814. E-mail: esjuliano@usuhs.mil.
DOI:10.1523/JNEUROSCI.1476-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258498-07$15.00/0
References
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney Jr WE, Jones EG (1996) Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry 53: 425–436. [DOI] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P (1997) Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124: 3501–3510. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD (2001) Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11: 287–296. [DOI] [PubMed] [Google Scholar]
- Chen MS, Bermingham-McDonogh O, Danehy Jr FT, Nolan C, Scherer SS, Lucas J, Gwynne D, Marchionni MA (1994) Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol 349: 389–400. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y (2003) The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 284: 54–65. [DOI] [PubMed] [Google Scholar]
- Corfas G, Falls DL, Fischbach GD (1993) ARIA, a protein that stimulates acetylcholine receptor synthesis, also induces tyrosine phosphorylation of a 185-kDa muscle transmembrane protein. Proc Natl Acad Sci USA 90: 1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD (1995) Differential expression of ARIA isoforms in the rat brain. Neuron 14: 103–115. [DOI] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD (2004) Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 7: 575–580. [DOI] [PubMed] [Google Scholar]
- Culican SM, Baumrind NL, Yamamoto M, Pearlman AL (1990) Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci 10: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R (2003) Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol 55: 288–298. [DOI] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR (1995) Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 15: 585–596. [DOI] [PubMed] [Google Scholar]
- Ethier SP, Langton BC, Dilts CA (1996) Growth factor-independent proliferation of rat mammary carcinoma cells by autocrine secretion of neudifferentiation factor/heregulin and transforming growth factor-alpha. Mol Carcinog 15: 134–143. [DOI] [PubMed] [Google Scholar]
- Falls DL (2003) Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284: 14–30. [DOI] [PubMed] [Google Scholar]
- Gierdalski M, Juliano SL (2003) Factors affecting the morphology of radial glia. Cereb Cortex 13: 572–579. [DOI] [PubMed] [Google Scholar]
- Hasling TA, Gierdalski M, Jablonska B, Juliano SL (2003) A radialization factor in normal cortical plate restores disorganized radial glia and disrupted migration in a model of cortical dysplasia. Eur J Neurosci 17: 467–480. [DOI] [PubMed] [Google Scholar]
- Hatten ME (1985) Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol 100: 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, Shephard HM, Kuang W-J, Wood WI, Goeddel DV, Vandlen RL (1992) Identification of heregulin, a specific activator of p185erbB2. Science 256: 1205–1210. [DOI] [PubMed] [Google Scholar]
- Hunter KE, Hatten ME (1995) Radial glial cell transformation to astrocytes is bidirectional: regulation by a diffusible factor in embryonic forebrain. Proc Natl Acad Sci USA 92: 2061–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SL, Palmer SL, Sonty RV, Noctor S, Hill Jr GF (1996) Development of local connections in ferret somatosensory cortex. J Comp Neurol 374: 259–277. [DOI] [PubMed] [Google Scholar]
- Kuramochi Y, Cote GM, Guo X, LeBrasseur NK, Cui L, Liao R, Sawyer DB (2004) Cardiac endothelial cells regulate ROS-induced cardiomyocyte apoptosis through neuregulin-1beta /erbB4 signaling. J Biol Chem 279: 51141–51147. [DOI] [PubMed] [Google Scholar]
- Leavitt BR, Hernit-Grant CS, Macklis JD (1999) Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons. Exp Neurol 157: 43–57. [DOI] [PubMed] [Google Scholar]
- Leprince P, Chanas-Sacre G (2001) Regulation of radial glia phenotype. Prog Brain Res 132: 13–22. [DOI] [PubMed] [Google Scholar]
- Longart M, Liu Y, Karavanova I, Buonanno A (2004) Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol 472: 156–172. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127: 5253–5263. [DOI] [PubMed] [Google Scholar]
- Malave C, Villegas GM, Hernandez M, Martinez JC, Castillo C, Suarez de Mata Z, Villegas R (2003) Role of glypican-1 in the trophic activity on PC12 cells induced by cultured sciatic nerve conditioned medium: identification of a glypican-1-neuregulin complex. Brain Res 983: 74–83. [DOI] [PubMed] [Google Scholar]
- Marmor MD, Skaria KB, Yarden Y (2004) Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 58: 903–913. [DOI] [PubMed] [Google Scholar]
- Moises HW, Zoega T, Gottesman II (2002) The glial growth factors deficiency and synaptic destabilization hypothesis of schizophrenia. BMC Psychiatry 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A (2002) Mitogen-activated protein kinase-dependent and -independent routes control shedding of transmembrane growth factors through multiple secretases. Biochem J 363: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Lane HA, Hynes NE (2001) The role of overexpressed HER2 in transformation. Ann Oncol 12 [Suppl 1]: S9–S13. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Palmer SL, Hasling T, Juliano SL (1999) Interference with the development of early generated neocortex results in disruption of radial glia and abnormal formation of neocortical layers. Cereb Cortex 9: 121–136. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR (2002) Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 15: 3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock GS, Franceschini IA, Graham G, Marchionni MA, Barnett SC (1999) Neuregulin is a mitogen and survival factor for olfactory bulb ensheathing cells and an isoform is produced by astrocytes. Eur J Neurosci 11: 769–780. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G (1997) Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron 19: 39–50. [DOI] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE (2003) Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippocampal white matter in subjects with schizophrenia. Am J Psychiatry 60: 149–155. [DOI] [PubMed] [Google Scholar]
- Rubin I, Yarden Y (2001) The basic biology of HER2. Ann Oncol 12 [Suppl 1]:S3–S8. [DOI] [PubMed]
- Schmid RS, McGrath B, Berechid BE, Boyles B, Marchionni M, Sestan N, Anton ES (2003) Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci USA 100: 4251–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A (2001) Roles of Meltrin beta /ADAM19 in the processing of neuregulin. J Biol Chem 276: 9352–9358. [DOI] [PubMed] [Google Scholar]
- Soriano E, Alvarado-Mallart RM, Dumesnil N, Del Rio JA, Sotelo C (1997) Cajal-Retzius cells regulate the radial glia phenotype in the adult and developing cerebellum and after granule cell migration. Neuron 18: 563–577. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, et al. (2002) Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37: 173–182. [DOI] [PubMed] [Google Scholar]
- Stove C, Stove V, Derycke L, Van Marck V, Mareel M, Bracke M (2003) The heregulin/human epidermal growth factor receptor as a new growth factor system in melanoma with multiple ways of deregulation. J Invest Dermatol 121: 802–812. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T (2001) Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res 41: 51–60. [DOI] [PubMed] [Google Scholar]
- Villegas R, Villegas GM, Longart M, Hernandez M, Maqueira B, Buonanno A, Garcia R, Castillo C (2000) Neuregulin found in cultured-sciatic nerve conditioned medium causes neuronal differentiation of PC12 cells. Brain Res 852: 305–318. [DOI] [PubMed] [Google Scholar]
- Voigt T (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289: 74–88. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL (2001) The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem 276: 2841–2851. [DOI] [PubMed] [Google Scholar]
- Wang LM, Kuo A, Alimandi M, Veri MC, Lee CC, Kapoor V, Ellmore N, Chen XH, Pierce JH (1998) ErbB2 expression increases the spectrum and potency of ligand-mediated signal transduction through ErbB4. Proc Natl Acad Sci USA 95: 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]