Abstract
Neurosteroids are produced de novo in neuronal and glial cells, which begin to express steroidogenic enzymes early in development. Studies suggest that neurosteroids may play important roles in neuronal circuit maturation via autocrine and/or paracrine actions. However, the mechanism of action of these agents is not fully understood. We report here that the excitatory neurosteroid pregnenolone sulfate induces a long-lasting strengthening of AMPA receptor-mediated synaptic transmission in rat hippocampal neurons during a restricted developmental period. Using the acute hippocampal slice preparation and patch-clamp electrophysiological techniques, we found that pregnenolone sulfate increases the frequency of AMPA-mediated miniature excitatory postsynaptic currents in CA1 pyramidal neurons. This effect could not be observed in slices from rats older than postnatal day 5. The mechanism of action of pregnenolone sulfate involved a short-term increase in the probability of glutamate release, and this effect is likely mediated by presynaptic NMDA receptors containing the NR2D subunit, which is transiently expressed in the hippocampus. The increase in glutamate release triggered a long-term enhancement of AMPA receptor function that requires activation of postsynaptic NMDA receptors containing NR2B subunits. Importantly, synaptic strengthening could also be triggered by postsynaptic neuron depolarization, and an anti-pregnenolone sulfate antibody scavenger blocked this effect. This finding indicates that a pregnenolone sulfate-like neurosteroid is a previously unrecognized retrograde messenger that is released in an activity-dependent manner during development.
Keywords: steroid, neurotransmitter, release, channel, presynaptic, plasticity
Introduction
Steroid hormones produce many metabolic effects in nonneuronal tissues by interacting with nuclear receptors that regulate gene transcription. In addition, these agents affect the brain, where they have enduring organizational effects that are particularly important during development. For instance, sex steroids permanently program immature neuronal circuits (Hutchison, 1997), and developmental exposure to glucocorticoids induces long-term changes in neurotransmitter systems leading to persistent hyperactivity of the hypothalamic-pituitary-adrenal axis (Welberg and Seckl, 2001). Steroids can also be produced locally in the brain independently of peripheral glands, and these agents are known as the neurosteroids (Baulieu et al., 2001). The first step in the biosynthesis of neurosteroids is the conversion of cholesterol into pregnenolone by the cytochrome P450 side-chain cleavage enzyme. Pregnenolone itself is a neurosteroid along with several of its derivatives, including pregnenolone sulfate (PREGS), dehydroepiandrosterone, dehydroepiandrosterone sulfate (DHEAS), progesterone, and allopregnanolone. Several lines of evidence suggest that neurosteroids play important roles during neurodevelopment. First, the enzymes necessary for neurosteroidogenesis are expressed in the immature brain, where levels of some neurosteroids are higher than in the mature brain (Ukena et al., 1998; Mellon and Vaudry, 2001; Grobin et al., 2003; Ibanez et al., 2003; Caldeira et al., 2004). Second, treatment of cultured neuronal and/or glial cells with neurosteroids has trophic effects (Compagnone and Mellon, 1998; Schumacher et al., 2000). Third, in vivo or in vitro treatment with exogenous progesterone promotes dendritic outgrowth of Purkinje neurons (Sakamoto et al., 2001). Finally, developmental exposure of rat pups to allopregnanolone alters interneuronal distribution in the adult prefrontal cortex (Grobin et al., 2003). The mechanisms by which neurosteroids produce these effects, however, are not fully understood, and it has yet to be determined whether endogenous neurosteroids have any physiological and/or pathophysiological roles in neurodevelopment.
Activity-dependent synaptic plasticity refines immature neuronal circuits by generating, stabilizing, or eliminating synapses (Katz and Shatz, 1996; Hua and Smith, 2004). A number of mechanisms are responsible for synaptic strengthening, including the activity-dependent postsynaptic secretion of factors that retrogradely influence neurotransmitter release from presynaptic terminals. Candidate retrograde messengers that could participate in developmental synaptic plasticity at central synapses have been identified and include nitric oxide, brain-derived neurotrophic factor, arachidonic acid, cannabinoids, and glutamate (Tao and Poo, 2001; Duguid and Smart, 2004; Schmidt, 2004). Production and/or secretion of these messengers is regulated by elevations in intracellular Ca2+ levels in which NMDA receptor activation plays a central role. Given that neurosteroid synthesis can be regulated by NMDA receptor-dependent intracellular Ca2+ elevations (Guarneri et al., 1998; Kimoto et al., 2001), we investigated whether neurosteroids might represent a novel class of retrograde messenger that could be involved in neuronal circuit maturation.
To this end, we recorded from CA1 pyramidal neurons in hippocampal slices from developing rats. We found (1) that exogenous PREGS strengthens synaptic transmission during a restricted developmental period, (2) that this effect involves potentiation of presynaptic and postsynaptic NMDA receptors with different subunit compositions, and (3) that depolarization of postsynaptic neurons releases a PREGS-like neurosteroid that retrogradely modulates synaptic transmission.
Materials and Methods
Unless indicated, all chemicals were from Sigma (St. Louis, MO) or Tocris Cookson (Bristol, UK). Coronal slices (350-400 μm) were prepared from Sprague Dawley rats that were deeply anesthetized with 250 mg/kg ketamine. After a recovery period of ≥80 min, slices were transferred to a chamber perfused at a rate of 2 ml/min with artificial CSF (ACSF) equilibrated with 95% O2/5% CO2 and containing the following (in mm): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, 10 glucose, and 0.02 bicuculline methiodide. Whole-cell patch-clamp electrophysiological recordings from CA1 hippocampal pyramidal cells were performed under infrared-differential interference contrast microscopy at 32°C with an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Patch electrodes (3-5 MΩ) were filled with an internal solution containing the following (in mm): 135 Cs-gluconate, 10 MgCl2, 0.1 CaCl2, 2 Mg-ATP, 1 EGTA, and 10 HEPES, pH 7.25. For the studies with l-AP-5, the internal solution contained the following (in mm): 110 Cs-gluconate, 5 NaCl, 10 TEA-Cl, 4 Mg-ATP, 0.6 EGTA, and 10 HEPES, pH 7.25. Access resistance was between 10 and 30 MΩ; if access resistance changed >20%, the recording was discarded. Miniature EPSCs (mEPSCs) were recorded at a holding potential of -70 mV in the presence of 500 nm tetrodotoxin (Calbiochem, La Jolla, CA). Evoked EPSCs were elicited by stimulation of Schaffer collaterals with a concentric bipolar stimulating electrode placed 100-200 μm from the patched cell in the presence of 4 mm N-(2,6-dimethylphenolcarbamoylmethyl)triethyl-ammonium bromide in the internal solution. A pneumatic picopump (World Precision Instruments, Sarasota, FL) was used to apply puffs of AMPA. The puffing pipette was placed ∼200 μm from the cell, the pressure was 7 psi, and the duration was 500 ms. BAPTA (10 mm) was added to the internal solution for the experiments shown in Figure 4a. (+)-5-Methyl-10,11-dihydro-5H-dibenzo [a,d]cyclohepten-5,10-imine maleate (MK-801) (5 mm) was included in the internal solution for the studies shown in Figure 4b. For the studies shown in Figure 5a, neurons were patched, and a ∼5 min baseline mEPSC recording was obtained. ACSF containing BAPTA-AM (50 μm; Calbiochem) was then perfused for 10 min followed by a 5 min washout before PREGS (Steraloids, Newport, RI) application. Anti-PREGS antiserum was purchased from MP Biomedicals (Orangeburg, NY), and the IgG fraction was purified with a protein G spin chromatography kit (Pierce, Rockford, IL). According to the manufacturer, the immunogen used to raise this antiserum was pregnenolone-3-monohemisuccinate, and the antiserum has the following cross-reactivity profile (in %): 100 pregnenolone, 100 PREGS, 3 progesterone, 0.85 5α-dihydroprogesterone, 0.03 desoxycorticosterone, 0.02 17α-hydroxypregnenolone, dehydroepiandrosterone, and <0.025 other adrenal and gonadal steroids. Protein G-purified rabbit IgG was purchased from Jackson ImmunoResearch (West Grove, PA). Data were acquired and analyzed with pClamp-9 (Axon Instruments). mEPSCs were analyzed with Minis Analysis program (Synaptosoft, Decatur, GA). The Kolmogorov-Smirnov (KS) test was used initially to test for a significant difference (level of significance, p ≤ 0.01) between treated and control cells. Unless indicated, statistical analyses of pooled data were performed by one-way ANOVA followed by Tukey's post hoc test (Prism 4; GraphPad Software, San Diego, CA). Unless indicated, post hoc analyses were performed with respect to initial values (i.e., in time course experiments) or vehicle control values. All values are expressed as mean ± SEM.
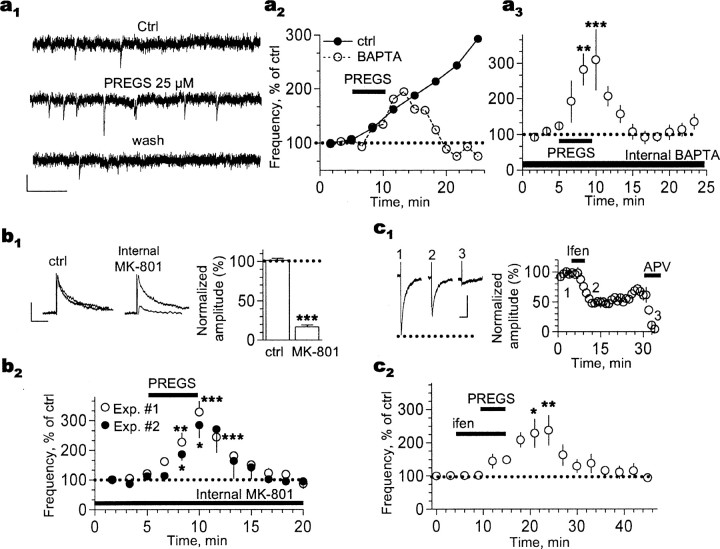
Figure 4.
The PREGS-induced long-term enhancement of postsynaptic AMPA responses in P3-P4 slices is NMDA receptor and Ca2+ dependent. a1, Traces illustrating that the late phase of the 25 μm PREGS-induced increase of AMPA mEPSC frequency cannot be observed when the Ca2+ chelator BAPTA (10 mm) is internally dialyzed via the patch electrode. Calibration: 15 pA, 100 ms. a2, time course of the effect of 25 μm PREGS on a neuron internally dialyzed with BAPTA and a control neuron from the same batch of slices. a3, Summary of the effect of 25 μm PREGS on neurons internally dialyzed with 10 mm BAPTA (**p < 0.01; ***p < 0.001; n = 6). b1, NMDA receptor-mediated EPSCs (Vhold = +40 mm; calibration: 20 pA, 200 ms) are significantly inhibited by intracellular dialysis of MK-801 (5 mm; see Results for more details; ***p < 0.001; n = 11 by t test). To promote entrance of MK-801 to the pore, neurons were depolarized from -70 to -15 mV (5 times for 10 s each). b2, In six of these 11 neurons, after NMDA EPSC inhibition was confirmed, 500 nm tetrodotoxin was applied, and the effect of 25 μm PREGS on mEPSC frequency was assessed [experiment (Exp.) 1]. Under these conditions, application of PREGS did not induce a long-lasting increase in mEPSC frequency. In a separate batch of neurons, tetrodotoxin was present from the start of the experiment, and basal mEPSC frequency was recorded (experiment 2; see Results for more details). Neurons were then depolarized as described above to promote entrance of MK-801 to the pore. mEPSC frequency was recorded again and found to be unchanged by the depolarization procedure (see Results). As shown in the summary graph, 25 μm PREGS did not induce a long-lasting increase in mEPSC frequency under these conditions (n = 4). *p < 0.05; **p < 0.01; ***p < 0.001. c1, Extracellular application of 10 μm ifenprodil (ifen) induces a long-lasting decrease in the amplitude of NMDA EPSCs (recorded at Vhold = -10 mV). Note that these events are blocked by 100 μm dl-AP-5. Calibration: 20 pA, 200 ms. c2, In a separate batch of neurons, mEPSC frequency was recorded in the presence of tetrodotoxin (500 nm). Under these conditions, ifenprodil blocked the late phase of the 25 μm PREGS effect on mEPSC frequency. Ifenprodil alone did not have an effect on mEPSC frequency (see Results). *p < 0.05; **p < 0.01; n = 6. Ctrl, Control. Error bars represent SEM.
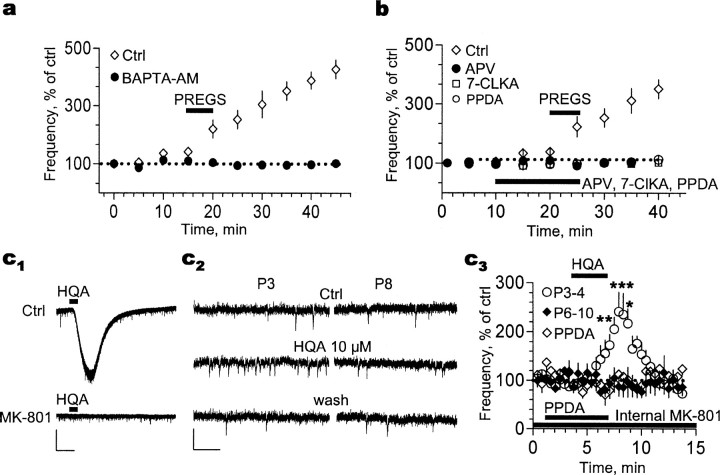
Figure 5.
The PREGS effect in P3-P4 slices requires influx of Ca2+ through presynaptic NMDA receptors likely containing NR2D subunits. a, Preincubation with the membrane-permeable Ca2+ chelator BAPTA-AM (50 μm) blocks the early and late phases of the 25 μm PREGS-induced increase of mEPSC frequency (n = 3 for control and n = 4 for BAPTA-AM). b, A similar effect was observed in slices incubated with the nonselective NMDA receptor antagonists dl-APV (100 μm; n = 3 for control and n = 5 for dl-APV) and 7-chlorokynurenate (7-CLKA; 30 μm; n = 5) and the antagonist of NMDA receptors containing NR2D subunits, PPDA (0.1 μm; n = 5). c1, Bath application of the NMDA receptor agonist homoquinolinic acid (HQA; 10 μm) induces an inward current in a P4 neuron that is blocked by intracellular dialysis of MK-801 (5 mm). Calibration: 100 pA, 300 s. c2, Sample traces illustrating that, in the presence of internal MK-801, homoquinolinic acid mimics the PREGS-induced increase of mEPSC frequency in slices from P3 but not P8 rats. Calibration: 20 pA, 300 ms. c3, Summary graph illustrating the effect of homoquinolinic acid, in the presence of internal MK-801, on P3-P4 (n = 6). Lack of an effect of homoquinolinic acid on P6-P10 slices (n = 5) and blockade of its effect by PPDA (0.1 μm) in P3-P4 slices (n = 5) are also shown. *p < 0.05; **p < 0.01; ***p < 0.001. Ctrl, Control. Error bars represent SEM.
Results
PREGS induces a long-lasting increase in mEPSC frequency during a restricted developmental period
We recorded mEPSCs in the whole-cell patch-clamp configuration from CA1 pyramidal neurons in hippocampal slices from postnatal day 3-4 (P3-P4) rats. Recordings were obtained at a holding membrane potential of -70 mV in the presence of 1 mm Mg2+. Under these conditions, mEPSCs were blocked by 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline (NBQX) (10 μm; n = 4; data not shown), indicating that these events are predominantly mediated by AMPA receptors. Brief (5 min) exposure to 25 μm PREGS increased mEPSC frequency but not amplitude (Fig. 1a) or half-width (102 ± 4 and 109 ± 6% of control during and 15 min after PREGS application, respectively; n = 8). The effect of brief exposure to 25 μm PREGS was long lasting, reaching a plateau by 40 min in some neurons (Fig. 1a) but not all neurons (for instance, see Fig. 5 below). In contrast, the effect of 17 μm PREGS reached a plateau by ∼25 min (Fig. 1a).
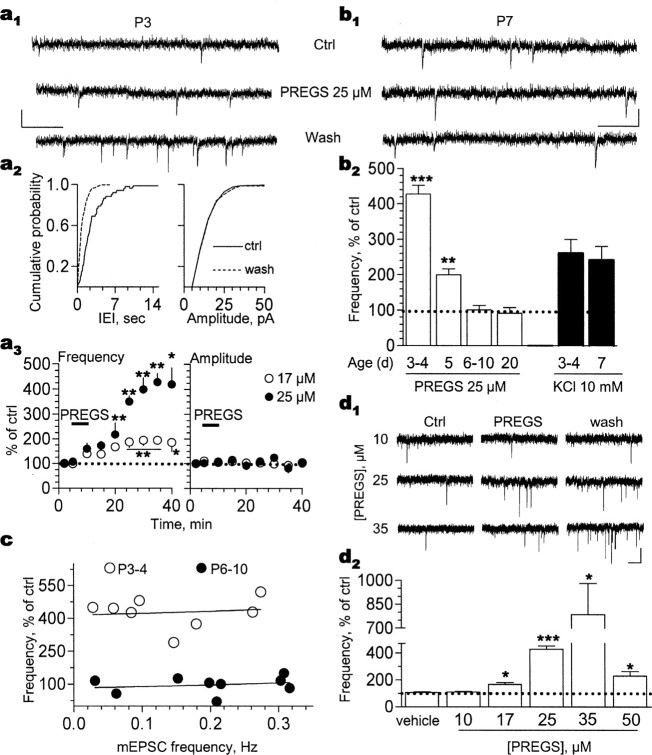
Figure 1.
PREGS persistently increases mEPSC frequency in immature synapses in an age-dependent and concentration-dependent manner. a1, Brief (5 min) exposure to 25 μm PREGS persistently increases mEPSC frequency in a P3 CA1 pyramidal neuron. Calibration: 10 pA, 100 ms. a2, Cumulative probability plots for interevent interval (IEI) and amplitude corresponding to the recording shown above. Note the shift to the left in the IEI plot observed after a 20 min washout period after brief exposure to 25 μm PREGS. a3, Time course of the effect of brief (5 min) exposure to 17 μm (n = 4) and 25 μm (n = 8) PREGS on mEPSC frequency (*p < 0.05; **p < 0.01) and amplitude (error bars are smaller than the symbols) in P3-P4 neurons. b1, PREGS does not affect mEPSC frequency in a P7 neuron. Calibration: 10 pA, 100 ms. b2, PREGS increases mEPSC frequency only in P3-P5 neurons (**p < 0.01 and ***p < 0.001 vs P20 neurons; n = 4-12). KCl (10 mm in ACSF) increases mEPSC frequency to a similar extent in P3-P4 and P7 neurons (n = 4). c, The effect of 25 μm PREGS is independent of basal mEPSC frequency. Note that the range of basal mEPSC frequency is similar in slices from P3-P4 and P6-P10 rats. d1, Traces illustrating the effect of increasing PREGS concentrations on mEPSC frequency in P3-P4 neurons. Calibration: 10 pA, 500 ms. d2, PREGS increases mEPSC frequency at concentrations between 17 and 50 μm (*p < 0.05 and ***p < 0.001; n = 4-9). Note the lack of an effect of vehicle (0.025% Me2SO, which was the concentration delivered to the cells in the experiments with 50 μm PREGS). Ctrl, Control. Error bars represent SEM.
The PREGS-induced increase of mEPSC frequency was robust in slices from P3-P4 rats and gradually decreased at P5 to become undetectable by P6 (Fig. 1b). KCl (10 mm in ACSF) increased mEPSC frequency to a similar extent in slices from P3-P4 and P7 rats (Fig. 1b). In slices from P3-P4 rats, PREGS (25 μm) increased the frequency of spontaneous EPSCs, recorded in the absence of tetrodotoxin, to 284 ± 19% of control (15-20 min after PREGS; n = 4; data not shown).
In P3-P4 neurons, there was no correlation between the effect of PREGS and basal mEPSC frequency (Fig. 1c). Although the range of mEPSC frequencies in P6-P10 neurons was similar to that of P3-P4 neurons, PREGS failed to increase mEPSC frequency in P6-P10 neurons (Fig. 1c).
In P3-P4 neurons, PREGS induced a significant effect on mEPSC frequency at concentrations between 17 and 50 μm (Fig. 1d), which have been shown to modulate NMDA receptor function (Gibbs et al., 1999). Analysis of cumulative probability distributions of individual cells by means of the KS test revealed a statistically significant effect of PREGS on mEPSC frequency in zero of nine neurons (10 μm), three of four neurons (17 μm), seven of eight neurons (25 μm), six of six neurons (35 μm), and seven of eight neurons (50 μm). mEPSC frequency in the absence of 10 μm PREGS was 0.24 ± 0.06 versus 0.27 ± 0.07 Hz in its presence (n = 9). mEPSC frequency in the absence of 17 μm PREGS was 0.25 ± 0.08 versus 0.42 ± 0.1 Hz in its presence (n = 4). mEPSC frequency in the absence of 25 μm PREGS was 0.15 ± 0.03 versus 0.63 ± 0.1 Hz in its presence (n = 8). mEPSC frequency in the absence of 35 μm PREGS was 0.18 ± 0.03 versus 1.42 ± 0.5 Hz in its presence (n = 6). mEPSC frequency in the absence of 50 μm PREGS was 0.19 ± 0.03 versus 0.43 ± 0.08 Hz in its presence (n = 8). Potentiation of mEPSC frequency by 50 μm PREGS was maximal ∼25 min after a brief exposure to this agent (data not shown) (compare Fig. 1a). The time course of the effect of 35 μm PREGS was similar to the time course observed with 25 μm PREGS (data not shown) (compare Fig. 1a).
PREGS transiently increases the probability of glutamate release
To determine whether PREGS increases mEPSC frequency by enhancing glutamate release, we used a previously described method that makes use of l-AP-5, a low-affinity NMDA receptor competitive antagonist (Choi et al., 2000). We recorded NMDA receptor-mediated EPSCs in CA1 pyramidal neurons evoked by stimulation of the Schaffer collaterals. Under control conditions, application of a nonsaturating concentration of l-AP-5 (250 μm) reversibly reduced NMDA EPSC amplitude (Fig. 2a). After exposure to PREGS, this concentration of l-AP-5 was unable to antagonize NMDA EPSCs, indicating that PREGS increases synaptic glutamate levels, thereby preventing l-AP-5 binding to the NMDA receptor.
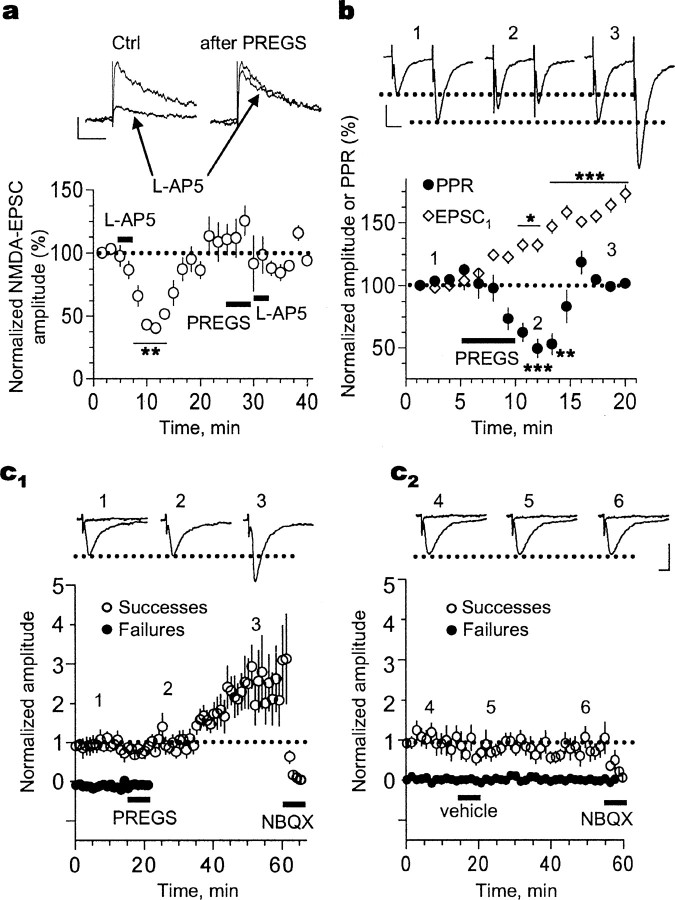
Figure 2.
PREGS transiently increases glutamate release and triggers a long-term enhancement of AMPA receptor-mediated synaptic responses in P3-P4 slices. a, Evoked NMDA EPSCs are not inhibited by the weak antagonist l-AP-5 (250 μm) after exposure to 25 μm PREGS because of increased glutamate release probability. Calibration: 20 pA, 200 ms (**p < 0.01; n = 4). b, Time course of the effect of PREGS on the amplitude of the first EPSC (EPSC1) and paired-pulse ratio (PPR; 50 ms interpulse interval; stimulation delivered every 20 s). Calibration: 20 pA, 25 ms (*p < 0.05, **p < 0.01, and ***p < 0.001; n = 8). c1, Time course of the effect of 25 μm PREGS on AMPA EPSC failures and successes (n = 9). c2, Time course illustrating the lack of an effect of vehicle (0.012% Me2SO) on AMPA EPSC failures and successes (n = 9). NBQX (10 μm) was applied at the end of the recording to confirm that responses were mediated by AMPA receptors. Calibration: 27 pA, 15 ms. The bin size for the data shown in c1 and c2 is 1 min (stimulation was delivered every 20 s), and successes were defined as events with amplitude ≥4 pA. Amplitude of both successes and failures was normalized with respect to the average amplitude of successes obtained during the first 3 min of recording. Ctrl, Control. Error bars represent SEM.
The persistence of the PREGS-induced elevation of mEPSC frequency could be attributable to a long-lasting increase in glutamate release probability. Therefore, we evaluated the time course of the PREGS effect on the paired-pulse ratio of AMPA EPSCs evoked by stimulation of Schaffer collaterals (Fig. 2b). Application of PREGS significantly decreased the paired-pulse ratio by ∼50% (from 1.5 ± 0.2 to 0.73 ± 0.1; n = 9). Together with the results shown in Figure 2a, this finding confirms that PREGS increases the basal probability of glutamate release. However, this effect was transient and the amplitude of the first EPSC continued to increase even after the paired-pulse ratio had returned to baseline.
We next evoked single AMPA EPSCs using a stimulation intensity that yielded a mixture of synaptic transmission successes and failures (Fig. 2c). A few minutes after application of PREGS, but not vehicle (Me2SO), we could only record successes, again consistent with an increase in basal glutamate release probability. At later time points (∼20 min after PREGS application), EPSC amplitude also gradually increased and remained elevated.
PREGS induces a delayed potentiation of postsynaptic AMPA receptors
We bath applied a submaximal concentration of AMPA (1 μm); AMPA EC50 for CA1 hippocampal neurons was previously determined to be 5 μm by Blake et al. (1988). These studies were performed in the presence of cyclothiazide (30 μm), to minimize desensitization, and tetrodotoxin, to block action potential-dependent neurotransmitter release (Fig. 3a). Application of AMPA induced inward currents that were eliminated by the antagonist GYKI-53655 (30 μm). After exposure to vehicle, AMPA-evoked currents were not significantly affected. However, these currents were dramatically increased ∼20 min after brief exposure to PREGS.
Figure 3.
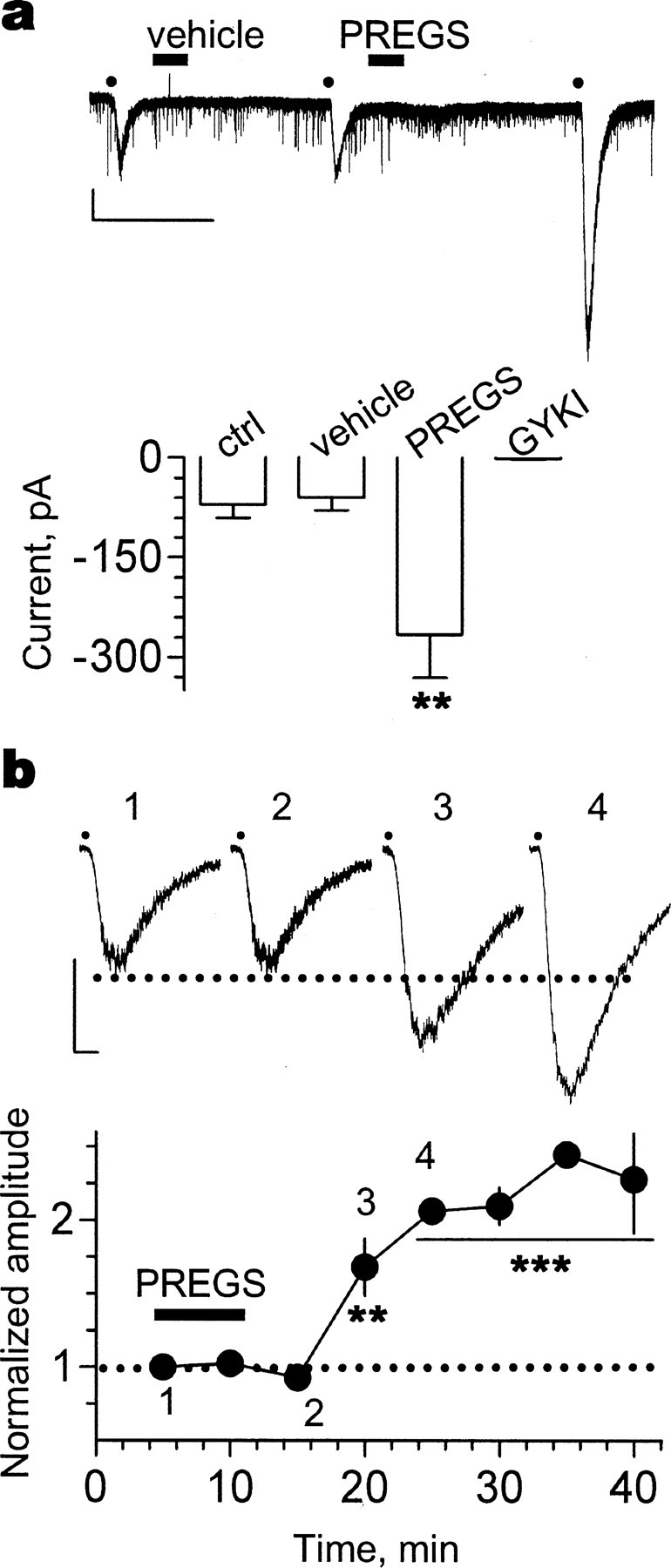
PREGS induces a delayed potentiation of postsynaptic AMPA receptors. a, Effect of vehicle (0.012% Me2SO) and 25 μm PREGS on currents evoked by bath application of AMPA for 30 s (represented by the dots) in the presence of cyclothiazide (30 μm) and tetrodotoxin (500 nm). Currents were blocked by the AMPA receptor antagonist GYKI-53655 (30 μm). Calibration: 200 pA, 800 s (**p < 0.01; n = 4). b, Effect of 25 μm PREGS on currents evoked by pressure application of AMPA (5 μm in a micropipette located ∼200 μm from the soma) in the presence of both tetrodotoxin (500 nm) and bicuculline (20 μm) and in the absence of cyclothiazide. Currents were blocked by the AMPA receptor antagonist NBQX (10 μm; data not shown). AMPA puffs of 500 ms duration were delivered every 40 s, and their onset is represented by the dots above the traces. The summary graph shows average amplitudes in bins of 5 min. Amplitude was normalized with respect to the average amplitude of the responses obtained during the first 3 min of recording. Calibration: 40 pA, 500 ms (**p < 0.01, ***p < 0.001; n = 4). Error bars represent SEM.
To further characterize the time course of the PREGS-induced potentiation of postsynaptic AMPA receptors, a pneumatic picopump was used to apply brief (500 ms) puffs of AMPA in the absence of cyclothiazide and in the presence of both tetrodotoxin (500 nm) and bicuculline (20 μm). Pressure application of AMPA (5 μm in the patch pipette located ∼200 μm away from the patched neuron) evoked inward currents (Fig. 3b) that were blocked by 10 μm NBQX (data not shown). Brief (5 min) bath application of PREGS (25 μm) significantly increased the amplitude of these currents. Importantly, this effect was first observed ∼10 min after brief exposure to PREGS (n = 4) (Fig. 3b).
The PREGS-induced long-term enhancement of postsynaptic AMPA responses is NMDA receptor and Ca2+ dependent
It has been demonstrated that long-term potentiation of AMPA receptor-mediated responses involves changes in phosphorylation and/or trafficking of this receptor that are triggered by Ca2+ influx via postsynaptic NMDA receptors (Soderling and Derkach, 2000; Gomes et al., 2003; Malinow, 2003). Therefore, we tested whether postsynaptic NMDA receptor-mediated Ca2+ influx was required for the late phase of PREGS-induced plasticity. We found that this phase did not occur in neurons intracellularly dialyzed with the Ca2+ chelator BAPTA, although the early phase was unaffected (Fig. 4a). The KS test revealed a significant transient increase in mEPSC frequency in four of six cells. mEPSC frequency was 0.24 ± 0.08 Hz in the absence of PREGS and 0.45 ± 0.14 Hz in its presence (n = 6). This result is consistent with our previous finding that PREGS does not induce a long-term change in the frequency of mEPSCs recorded from cultured hippocampal neurons using an intracellular solution containing 10 mm EGTA (Meyer et al., 2002). Moreover, the reversibility of the PREGS effect observed under conditions of postsynaptic Ca2+ chelation indicates that the long-lasting nature of the PREGS effect cannot be explained by slow washout of the neurosteroid from the slice.
We next examined the effect of intracellular dialysis of the NMDA receptor channel blocker MK-801. We performed two types of experiments with this agent (Fig. 4b). In experiment 1, neurons were patched with internal solution containing MK-801 (5 mm) and allowed to equilibrate with this solution for 5-10 min at -70 mV. NMDA receptor-mediated EPSCs were then recorded at +40 mV to eliminate the Mg2+ block and promote rapid blockade of the channel by MK-801. Indeed, rapid inhibition of the NMDA EPSCs was evident (∼75% decrease after 100 s). To further promote entrance of MK-801 to the pore, the cell was depolarized from -70 to -15 mV (five times for 10 s each), as described previously (Woodhall et al., 2001; Grimwood et al., 2002). After this procedure, the amplitude of NMDA receptor-mediated EPSCs recorded at +40 mV was reduced by ∼80% (Fig. 4b, top), in agreement with previous reports (Berretta and Jones, 1996; Woodhall et al., 2001; Grimwood et al., 2002; Humeau et al., 2003). We performed this procedure on a total of 11 neurons. A subset of these neurons (Fig. 4b, bottom, experiment 1) (n = 6) were subsequently exposed to ACSF containing tetrodotoxin (500 nm). Although baseline mEPSC frequency in these neurons was within the range of frequencies observed in control neurons (0.11 ± 0.06 Hz) (compare with Fig. 1c), the late phase of the PREGS effect was undetectable under these conditions (KS test showed a significant transient increase in mEPSC frequency in four of six cells) (Fig. 4b).
To eliminate potential confounds derived from the depolarization to +40 mV that was performed in experiment 1, we performed an additional experiment. In experiment 2, recording was started in ACSF containing 500 nm tetrodotoxin, and baseline mEPSC frequency was recorded. Neurons were then depolarized from -70 to -15 mV (five times for 10 s each), and mEPSC frequency was again recorded. In four neurons, we found that mEPSC frequency was not significantly different before (0.08 ± 0.01 Hz) and after (0.07 ± 0.02 Hz) depolarization. Subsequently, 25 μm PREGS was bath applied for 5 min, which increased mEPSC frequency to 0.17 ± 0.03 Hz. However, under these conditions, the late phase of the PREGS effect could not be observed (Fig. 4b). To confirm that NMDA receptors were blocked by MK-801, we bath applied the nonselective NMDA receptor agonist homoquinolinic acid (10 μm) at the end of the experiment; this agent produced an inward current shift of 11 ± 4 pA (n = 4). In the absence of internal MK-801, this agent produced an inward current shift of ∼200 pA (Fig. 5c).
Postsynaptic NMDA receptors in developing hippocampal neurons contain the NR2B subunit, and therefore, we examined the effect of ifenprodil, a selective antagonist of receptors containing this subunit (Tovar and Westbrook, 1999; Li et al., 2002). Bath application of ifenprodil reduced NMDA EPSC amplitude for at least 15 min (Fig. 4c, top). In a separate batch of neurons, we determined that ifenprodil alone does not affect mEPSC frequency; raw mEPSC frequencies in the absence and presence of 10 μm ifenprodil were 0.24 ± 0.07 and 0.22 ± 0.08 Hz, respectively (n = 5). We then determined that in the presence of ifenprodil, the late phase of the PREGS effect did not occur (Fig. 4c, bottom). The KS test revealed a significant transient increase in mEPSC frequency in six of seven cells.
The presynaptic mechanism of action of PREGS is also NMDA receptor and Ca2+ dependent
We initially used BAPTA-AM to chelate Ca2+ at both presynaptic and postsynaptic sites. In the presence of this agent, both the early and late phases of PREGS-induced plasticity were abolished (KS test showed that PREGS induced a significant long-lasting increase in mEPSC frequency in three of three control neurons) (Fig. 5a). Notably, basal mEPSC frequency (0.164 ± 0.04 Hz; n = 4) was not affected by BAPTA-AM; mEPSC frequency after a 3-4 min application of this agent was 0.174 ± 0.05 Hz (n = 4).
We next assessed the effects of bath application of NMDA receptor antagonists. As shown in Figure 5b, PREGS produced the expected increase in mEPSC frequency in the absence of these antagonists (KS test indicated that PREGS induced a significant long-lasting increase in mEPSC frequency in three of three control neurons). The competitive antagonist of the glutamate binding site, dl-APV, blocked both phases of the PREGS effect without affecting basal mEPSC frequency (0.23 ± 0.07 Hz in the absence of dl-APV vs 0.23 ± 0.06 Hz in its presence; n = 4). The competitive antagonist of the glycine coagonist binding site, 7-chlorokynurenate, had a similar effect on the PREGS effect and also did not affect basal mEPSC frequency (0.106 ± 0.02 Hz in the absence of 7-chlorokynurenate vs 0.11 ± 0.02 Hz in its presence; n = 5). Finally, we tested the effect of 1-(phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid (PPDA), a compound that preferentially antagonizes NR2C/D subunit-containing receptors (Feng et al., 2004). We elected to test this antagonist in light of studies indicating that the NR2D subunit can be expressed presynaptically and that this subunit, but not NR2C, is transiently expressed during the first week of life in the murine hippocampus (Wenzel et al., 1997a,b; Okabe et al., 1998; Thompson et al., 2002). We found that, like dl-APV and 7-chlorokynurenate, PPDA eliminated both phases of the PREGS effect without affecting basal mEPSC frequency (0.144 ± 0.03 Hz in the absence of PPDA vs 0.141 ± 0.03 Hz in its presence; n = 5). Given that postsynaptic NMDA receptor-dependent Ca2+ influx is required for only the late phase of PREGS-induced plasticity (Fig. 4), the experiments with BAPTA-AM and NMDA antagonists collectively indicate that a presynaptic elevation in [Ca2+]i is required for the early phase of this phenomenon and that it is likely mediated by presynaptic NR2D-containing NMDA receptors. However, an uncertainty must be kept in mind when interpreting our findings with PPDA. Although it is the most selective pharmacological tool currently available to antagonize NR2C/D-containing receptors, PPDA only has a threefold to fivefold selectivity for these receptors, and its pharmacological actions at heterodimeric receptors have yet to be characterized (Feng et al., 2004). Therefore, the involvement of presynaptic NR2D-contaning receptors in the mechanism of action of PREGS will have to be confirmed using more selective agents and/or knock-out animals. We are planning to perform these studies in the future. It should also be emphasized that the effect of PPDA on receptors containing NR3A subunits has not been assessed, and, consequently, we cannot rule out that they are also targeted by this compound. Although the NR3A subunit is expressed in the developing hippocampus, it is unlikely to be involved in the mechanism of the presynaptic action of PREGS because it is not expressed in presynaptic terminals and its expression levels peak after the first week of neonatal life (Wong et al., 2002).
The presence of NMDA receptors in presynaptic terminals was confirmed by bath application of homoquinolinic acid, which has been used previously to activate presynaptic NMDA receptors (Woodhall et al., 2001; Grimwood et al., 2002). As shown in Figure 5c, bath application of homoquinolinic acid induced a large inward current (231 ± 42 pA; n = 4) that was virtually abolished by intracellular dialysis of MK-801 (14 ± 3 pA; n = 6). In the presence of intracellular MK-801, homoquinolinic acid mimicked the PREGS-induced increase of mEPSC frequency in slices from P3-P4 rats, but not P6-P10 rats, and this effect was blocked by PPDA (KS test indicated that, in P3-P4 slices and in the absence of PPDA, homoquinolinic acid induced a significant transient increase in mEPSC frequency in four of six neurons) (Fig. 5c). In P3-P4 neurons, mEPSC frequency was 0.103 ± 0.02 Hz in the absence of homoquinolinic acid versus 0.226 ± 0.05 Hz in its presence (n = 6). In P6-P10 neurons, mEPSC frequency was 0.14 ± 0.03 Hz in the absence of homoquinolinic acid versus 0.135 ± 0.04 Hz in its presence (n = 6). In P3-P4 neurons, mEPSC frequency was 0.15 ± 0.03 Hz in the absence of PPDA versus 0.165 ± 0.04 Hz in its presence (n = 5). In these neurons, mEPSC frequency in the presence of both PPDA and homoquinolinic acid was 0.16 ± 0.03 Hz.
We showed previously that PREGS increases quantal glutamate release in cultured hippocampal neurons from neonatal rats and that this effect is mediated by σ1-like receptors (Meyer et al., 2002). Therefore, we tested the effect of the σ1 receptor antagonist 1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride (BD-1063) on the PREGS-induced increase of mEPSC frequency in P3-P4 slices. In the presence of 2 μm BD-1063, mEPSC frequency after 25 μm PREGS application was 410 ± 19% of control; mEPSC frequency was 0.23 ± 0.13 Hz in the absence of 2 μm BD-1063, 0.21 ± 0.13 Hz in its presence, and 0.87 ± 0.18 Hz in the presence of BD-1063 plus PREGS (n = 5; compare this with the effect of PREGS shown in Fig. 1b). Even in the presence of a higher concentration of BD-1063 (4 μm), PREGS increased mEPSC frequency by 351 ± 25% of control; mEPSC frequency was 0.096 ± 0.017 Hz in the absence of 4 μm BD-1063, 0.088 ± 0.018 Hz in its presence, and 0.309 ± 0.07 Hz in the presence of BD-1063 plus PREGS (n = 4). 1-(4-iodophenyl)-3-(2-adamantyl)guanidine (IPAG), another antagonist of σ1 receptors (Kimes et al., 1992), also failed to prevent the PREGS-induced increase of mEPSC frequency; mEPSC frequency was 0.108 ± 0.02 Hz in the absence of 50 nm IPAG, 0.113 ± 0.03 Hz in its presence, and 0.36 ± 0.08 Hz in the presence of IPAG plus 25 μm PREGS (n = 4). We determined whether σ1 receptor agonists could mimic the effect of PREGS on mEPSC frequency. In P3-P4 slices, we found that mEPSC frequency was not significantly different in the absence (0.153 ± 0.04 Hz) versus the presence (0.16 ± 0.03 Hz) of the σ1 receptor agonist 2-(4-morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride (100 nm; n = 4) (Hayashi et al., 2000). Moreover, application of 10 μm DHEAS, which activates σ1 receptors and enhances mEPSC frequency in cultured hippocampal neurons (Meyer et al., 2002), did not have an effect on mEPSC frequency in P3-P4 slices (mEPSC frequency in the absence of DHEAS was 0.24 ± 0.07 and 0.25 ± 0.08 Hz in its presence; n = 3; data not shown). Together, these results indicate that σ1 receptors are not involved in the mechanism of the PREGS-induced increase of mEPSC frequency in neonatal slices.
Postsynaptic depolarization induces the release of a PREGS-like retrograde messenger
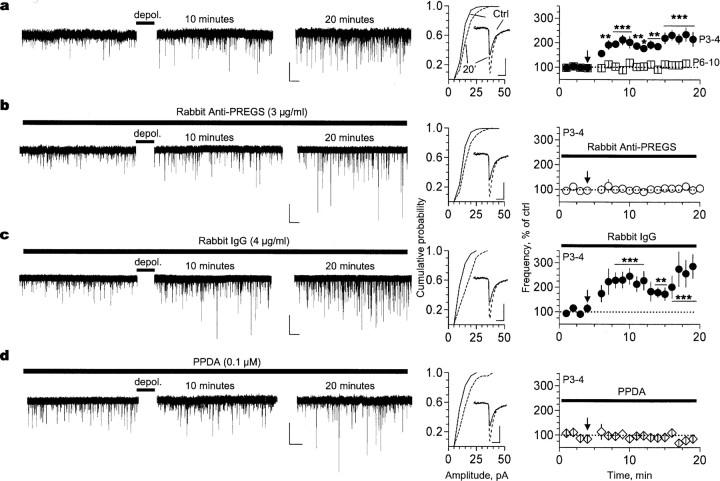
Given that immature neurons express the enzymes required for neurosteroid biosynthesis and an NMDA receptor-dependent elevation of intracellular Ca2+ was shown to increase PREGS levels in rat hippocampus (Kimoto et al., 2001; Mellon and Vaudry, 2001), we investigated whether depolarization could trigger the release of an endogenous PREGS-like neurosteroid. We elected to depolarize for 15 s to mimic early network-driven oscillatory bursts of approximately this duration that are prevalent in developing CA1 pyramidal neurons and produce Ca2+ transients that reach average concentrations of ∼250 nm (Garaschuk et al., 1998). Importantly, these bursts are rarely observed after P5, and, consequently, their developmental time course coincides with the presence of PREGS-sensitive NR2D-containing NMDA receptors in presynaptic terminals (Garaschuk et al., 1998). As observed after exogenous PREGS application, depolarization produced a long-lasting increase in mEPSC frequency in P3-P4 slices (Fig. 6a). KS test revealed that depolarization induced a significant long-lasting increase in mEPSC frequency in 14 of 19 P3-P4 neurons; mEPSC frequencies were 0.27 ± 0.09 and 0.54 ± 0.12 Hz before and after depolarization, respectively (n = 19). This effect was not observed in slices from P6-P10 rats (mEPSC frequencies were 0.21 ± 0.08 and 0.22 ± 0.09 Hz before and after depolarization, respectively; n = 7). The magnitude of the effect of depolarization in P3-P4 slices was similar to that observed with 17 μm PREGS (Fig. 1a,d). Notably, the depolarization-induced increase of mEPSC frequency was blocked by 14 min application of rabbit anti-PREGS IgG, which acts as a neurosteroid scavenger (Fig. 6b). In the presence of anti-PREGS IgG, mEPSC frequencies were 0.19 ± 0.09 and 0.19 ± 0.08 Hz before and after depolarization, respectively (n = 14). This blocking effect was not observed with rabbit IgG (Fig. 6c). KS test revealed that, in the presence of rabbit IgG, depolarization induced a significant long-lasting increase in mEPSC frequency in five of six neurons; mEPSC frequencies were 0.17 ± 0.03 and 0.355 ± 0.02 Hz before and after depolarization, respectively (n = 6). In agreement with the results obtained with exogenous PREGS application, the depolarization-induced increase of mEPSC frequency could not be observed in the presence of PPDA (Fig. 6d). In the presence of PPDA, mEPSC frequencies were 0.28 ± 0.18 and 0.24 ± 0.08 Hz before and after depolarization, respectively (n = 5). These findings indicate that this effect is mediated by potentiation of presynaptic NMDA receptor function via an endogenous PREGS-like compound that acts in a retrograde manner.
Figure 6.
A PREGS-like neurosteroid, which retrogradely modulates NMDA receptors, mediates the depolarization-induced increase of mEPSC frequency. a, Depolarization (depol.)-induced increase of mEPSC frequency can be observed in CA1 pyramidal neurons from P3-P4 (n = 19) but not P6-P10 (n = 7; traces not shown) rats. The cumulative probability plots and average traces (inset) illustrate that mEPSC amplitude also increased 20 min after depolarization in P3-P4 neurons (compare solid vs dashed traces). A similar result was obtained with P6-P10 neurons (n = 7; data not shown). b, Preincubation (14 min) with rabbit anti-PREGS IgG blocks the depolarization-induced increase of mEPSC frequency but not the increase in amplitude in P3-P4 neurons (n = 14). c, Incubation with rabbit IgG neither affects the depolarization-induced increase in frequency nor the increase in amplitude in P3-P4 neurons (n = 6). d, Incubation with PPDA blocks the depolarization-induced increase of mEPSC frequency but not the increase in amplitude in P3-P4 neurons (n = 5). Calibration: a-d, 20 pA, 5 s (left); 8 pA, 12 ms (right). *p < 0.05; **p < 0.01; ***p < 0.001. Ctrl, Control. Error bars represent SEM.
Depolarization was also associated with an increase in mEPSC amplitude that could be observed in P3-P4 slices, even in the presence of anti-PREGS antibodies or PPDA (Fig. 6a,b,d) and in P6-P10 slices (data not shown; percentage increase in amplitude was 32 ± 12%; n = 7). Therefore, the increase in mEPSC amplitude is independent of the actions of the neurosteroid retrograde messenger. This effect could be attributable to Ca2+-dependent phosphorylation of AMPA receptors that is triggered by depolarization-induced removal of Mg2+ blockade of postsynaptic NMDA receptors (Soderling and Derkach, 2000).
Discussion
We reported previously that PREGS increases basal glutamate release probability in cultured hippocampal neurons from neonatal rats but not in slices from adult rats (Partridge and Valenzuela, 2001; Meyer et al., 2002). In agreement with these findings, we report here that PREGS increases the probability of glutamate release only in hippocampal slices from rats younger than P6. Importantly, this effect is mediated by presynaptic NMDA receptors likely containing NR2D subunits, which are transiently expressed during the first week of life in the murine hippocampus (Wenzel et al., 1997a,b; Okabe et al., 1998). It is well established that PREGS is a positive allosteric modulator of NMDA receptors and it is expected to act by facilitating the actions of glutamate at presynaptic NMDA receptors (Gibbs et al., 1999). Given that the PREGS effect can be seen in the presence of tetrodotoxin, in which neurotransmitter release occurs in an action potential-independent manner, this neurosteroid must significantly increase the efficacy and/or potency of low glutamate levels generated by quantal release. This effect of PREGS should enhance Ca2+ influx through presynaptic NMDA receptors leading to an increase in the probability of glutamate release. Although tonic activation of presynaptic NMDA receptors in the absence of PREGS was not detectable under our recording conditions, as demonstrated by the lack of an effect of NMDA receptor antagonists on basal mEPSC frequency, our finding that homoquinolinic acid increases quantal glutamate release in the presence of Mg2+ indicates that NMDA receptors are not blocked by this ion in presynaptic terminals. This finding is consistent with the results of a previous study demonstrating activation of presynaptic NMDA receptors in the entorhinal cortex, even in the presence of Mg2+ (Berretta and Jones, 1996). Moreover, it was shown recently that presynaptic NMDA receptors in molecular layer interneurons of the cerebellum can also be activated in the presence of Mg2+ without the need for depolarization to remove blockade by this ion (Duguid and Smart, 2004). Together, these results suggest that axonal terminals are partly depolarized and/or that the NMDA receptors present in these terminals are less sensitive to blockade by Mg2+. The latter scenario is plausible in immature presynaptic terminals expressing NR2D-containing NMDA receptors, which are less sensitive to Mg2+ block than those containing NR2A or NR2B subunits (Monyer et al., 1994).
It is somewhat unexpected that NMDA receptors containing NR2D subunits are potentiated by PREGS given the recent demonstration that Xenopus oocyte-expressed recombinant NR1100/NR2D receptors are inhibited by this neurosteroid (Malayev et al., 2002; Jang et al., 2004). However, NMDA receptors expressed in neonatal rat axonal terminals could contain a different NR1 splice variant than NR1100 or be part of a ternary complex containing other NR2 subunits (Buller and Monaghan, 1997; Dunah et al., 1998; Cheffings and Colquhoun, 2000). Alternatively, these NMDA receptors may undergo posttranslational modifications or be associated with proteins or lipid domains that are specific to immature presynaptic terminals. These characteristics could confer PREGS sensitivity to presynaptic NMDA receptors containing NR2D subunits.
Another unforeseen finding of our study is the lack of an involvement of σ1-like receptors in the presynaptic actions of PREGS in neonatal slices, given our previous demonstration that these receptors are important for its actions in neonatal hippocampal neurons maintained in culture for 8-14 d (Meyer et al., 2002). Moreover, we found that NMDA receptors are not required for the presynaptic actions of PREGS in cultured hippocampal neurons (Meyer et al., 2002). The reasons for the differences in the receptors involved in the mechanism of action of PREGS in acute neonatal slices versus cultured hippocampal neurons are currently under investigation and are likely related to the conditions required for the long-term maintenance of neurons in culture.
The transient presynaptic NMDA receptor-dependent increase of glutamate release induced by PREGS is followed by a long-lasting strengthening of postsynaptic AMPA receptor-mediated transmission that resembles long-term potentiation. This delayed phase of the PREGS effect involves activation of ifenprodil-sensitive postsynaptic NMDA receptors containing NR2B subunits. These receptors should become activated during the PREGS-induced transient increase of quantal glutamate release via AMPA receptor-mediated membrane depolarization in distal dendrites that are not effectively voltage clamped by a somatic patch electrode. Thus, a localized elevation in Ca2+ influx through NMDA receptors could be responsible for the induction of plasticity. Two factors should contribute to this localized Ca2+ elevation: (1) currents mediated by receptors containing the NR2B subunit have slow decay kinetics (Flint et al., 1997), and (2) currents mediated by these receptors should be potentiated during the brief application of PREGS (Gibbs et al., 1999). The Ca2+ elevation could then activate protein kinases that are functional during development, such as cAMP-dependent protein kinase, which phosphorylates AMPA receptors and increases their conductance (Wang et al., 1991; Banke et al., 2000; Yasuda et al., 2003). Alternatively, Ca2+ could induce delivery of AMPA receptors to active synapses already expressing these receptors and to silent synapses lacking AMPA receptors (Isaac, 2003; Malinow, 2003). Although our finding that currents evoked by application of exogenous AMPA dramatically increase after exposure to PREGS is compatible with either mechanism, the fact that PREGS did not affect mEPSC amplitude or half-width is more consistent with an increase in synaptic delivery of AMPA receptors to silent synapses (Fitzjohn et al., 2001; Pickard et al., 2001). These AMPA receptors are likely to contain the GluR4 subunit, which is transiently expressed in neonatal CA1 pyramidal neurons and is incorporated into silent synapses in response to increases in spontaneous synaptic activity in an NMDA-dependent manner (Zhu et al., 2000). It is noteworthy, however, that we cannot rule out that changes in AMPA receptor function also occur at established synapses. Analysis of the rectification properties, single-channel properties, and/or surface expression of the receptors will be required to eliminate this possibility.
Bulk PREGS concentrations in tissue homogenates have been estimated to be in the nanomolar range, yet NMDA receptors are significantly modulated by micromolar concentrations of this neurosteroid (Gibbs et al., 1999). Therefore, it has been hypothesized that PREGS may be focally released, and, using an antibody scavenger, we demonstrated that a PREGS-like neurosteroid is synaptically released in a retrograde manner from depolarized postsynaptic CA1 neurons. Release of this endogenous neurosteroid produced a similar effect to that observed with 17 μm PREGS. Assuming that PREGS efficiently penetrates into the slice, that the endogenous PREGS-like neurosteroid has a similar potency to that of exogenous PREGS, and that PREGS is not degraded by extracellular enzymes present in the slice preparation, these findings suggest that synaptic concentrations of the endogenous equivalent of this neurosteroid are in the micromolar range. Although the antibody scavenger used in these experiments recognizes both pregnenolone and PREGS, it is unlikely that endogenously released pregnenolone mediates the effect of depolarization given that this neurosteroid is devoid of activity at NMDA receptors (Gibbs et al., 1999). However, an uncertainty is that the antibody may recognize another compound chemically related to PREGS. In fact, recent studies suggest that brain levels of PREGS per se are lower than previously thought and that a closely related sulfated neurosteroid may actually be the endogenous counterpart of PREGS (Higashi et al., 2003a,b; Liu et al., 2003; Caldeira et al., 2004).
It is noteworthy that, in addition to modulating NMDA receptors, PREGS regulates the function of other ligand-gated ion channels, including GABAA and glycine receptors (for review, see Rupprecht and Holsboer, 1999). GABAA receptors are particularly important targets of PREGS that are directly inhibited by low micromolar concentrations of this neurosteroid (for review, see Lambert et al., 2003). Moreover, GABA release in cultured hippocampal neurons is reduced by nanomolar concentrations of PREGS (Mtchedlishvili and Kapur, 2003). Although these effects are not expected to be involved in the mechanism of action of PREGS under our recording conditions (i.e., in the presence of bicuculline), they are likely to play a role in the actions of PREGS in vivo. Early in development, GABAA receptors exert dual excitatory and inhibitory influences on neurons (Ben-Ari, 2002), and negative modulation of GABAergic transmission by PREGS is therefore expected to have complex effects. Experiments are currently in progress in our laboratory to address this important issue.
Unlike unconjugated steroids, PREGS cannot freely cross the plasma membrane because of the presence of the negatively charged sulfate group. Moreover, extracellular expression of the enzyme involved in the conversion of pregnenolone into PREGS (3β-hydroxysteroid sulfotransferase) has not been demonstrated. Therefore, our finding that a PREGS-like neurosteroid retrogradely modulates presynaptic NMDA receptors implies that this compound must be released via an active process. It will be of interest to determine whether it involves vesicular release or transporter-mediated extrusion across the plasma membrane. Overall, our findings open a new perspective in developmental neurobiology by placing NMDA receptor-modulating neurosteroids as novel retrograde messengers that may participate in the maturation of neuronal circuits. We recently discovered that prenatal ethanol exposure increases PREGS levels in the developing brain, which could contribute to the abnormalities associated with fetal alcohol syndrome (Costa et al., 2000; Caldeira et al., 2004). It will be important to determine whether alterations in neurosteroid function play a role in other neurodevelopmental disorders.
Footnotes
This work was supported by grants from the National Institute of Mental Health and the National Institute of Alcohol Abuse and Alcoholism. We thank Dr. Daniel T. Monaghan (Department of Pharmacology, University of Nebraska Medical Center, Omaha, NE) for generously providing PPDA and Jeff Weiner, Enrico Sanna, John Connor, Mike Wilson, Bill Shuttleworth, and Alex Smith for helpful discussions.
Correspondence should be addressed to Dr. C. Fernando Valenzuela, Department of Neurosciences, MSC08 4740, 1 University of New Mexico, Albuquerque, NM 87131-0001. E-mail: fvalenzuela@salud.unm.edu.
M. Carta's present address: Department of Experimental Biology, University of Cagliari, 09042 Monserrato, Caagliari, Sardinia, Italy.
Copyright © 2005 Society for Neuroscience 0270-6474/05/252285-10$15.00/0
References
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE, Robel P, Schumacher M (2001) Neurosteroids: beginning of the story. Int Rev Neurobiol 46: 1-32. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3: 728-739. [DOI] [PubMed] [Google Scholar]
- Berretta N, Jones RS (1996) Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75: 339-344. [DOI] [PubMed] [Google Scholar]
- Blake JF, Brown MW, Collingridge GL (1988) A quantitative study of the actions of excitatory amino acids and antagonists in rat hippocampal slices. Br J Pharmacol 95: 291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AL, Monaghan DT (1997) Pharmacological heterogeneity of NMDA receptors: characterization of NR1a/NR2D heteromers expressed in Xenopus oocytes. Eur J Pharmacol 320: 87-94. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF (2004) Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem 90: 1530-1539. [DOI] [PubMed] [Google Scholar]
- Cheffings CM, Colquhoun D (2000) Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol (Lond) 526: 481-491. [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW (2000) Postfusional regulation of cleft glutamate concentration during LTP at “silent synapses.” Nat Neurosci 3: 330-336. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH (1998) Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci USA 95: 4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ET, Olivera DS, Meyer DA, Ferreira VM, Soto EE, Frausto S, Savage DD, Browning MD, Valenzuela CF (2000) Fetal alcohol exposure alters neurosteroid modulation of hippocampal N-methyl-d-aspartate receptors. J Biol Chem 275: 38268-38274. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG (2004) Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci 7: 525-533. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB (1998) Subunit composition of N-methyl-d-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol 53: 429-437. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT (2004) Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141: 508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Pickard L, Duckworth JK, Molnar E, Henley JM, Collingridge GL, Noel J (2001) An electrophysiological characterisation of long-term potentiation in cultured dissociated hippocampal neurones. Neuropharmacology 41: 693-699. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H (1997) NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17: 2469-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A (1998) Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol (Lond) 507: 219-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs TT, Yaghoubi N, Weaver Jr CE, Park-Chung M, Russek SJ, Farb DH (1999) Modulation of ionotropic glutamate receptors by neuroactive steroids. In: Neurosteroids: a new regulatory function in the nervous system (Baulieu EE, Robel P, Schumacher M, eds), pp 167-190. Totowa, NJ: Humana.
- Gomes AR, Correia SS, Carvalho AL, Duarte CB (2003) Regulation of AMPA receptor activity, synaptic targeting and recycling: role in synaptic plasticity. Neurochem Res 28: 1459-1473. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Wafford KA, Macaulay A, Hutson PH (2002) N-methyl-d-aspartate receptor subtype-selectivity of homoquinolinate: an electrophysiological and radioligand binding study using both native and recombinant receptors. J Neurochem 82: 794-800. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Heenan EJ, Lieberman JA, Morrow AL (2003) Perinatal neurosteroid levels influence GABAergic interneuron localization in adult rat prefrontal cortex. J Neurosci 23: 1832-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri P, Russo D, Cascio C, De Leo G, Piccoli F, Guarneri R (1998) Induction of neurosteroid synthesis by NMDA receptors in isolated rat retina: a potential early event in excitotoxicity. Eur J Neurosci 10: 1752-1763. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP (2000) Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther 293: 788-798. [PubMed] [Google Scholar]
- Higashi T, Daifu Y, Ikeshima T, Yagi T, Shimada K (2003a) Studies on neurosteroids XV. Development of enzyme-linked immunosorbent assay for examining whether pregnenolone sulfate is a veritable neurosteroid. J Pharm Biomed Anal 30: 1907-1917. [DOI] [PubMed] [Google Scholar]
- Higashi T, Sugitani H, Yagi T, Shimada K (2003b) Studies on Neurosteroids XVI. Levels of pregnenolone sulfate in rat brains determined by enzyme-linked immunosorbent assay not requiring solvolysis. Biol Pharm Bull 26: 709-711. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ (2004) Neural activity and the dynamics of central nervous system development. Nat Neurosci 7: 327-332. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A (2003) Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 426: 841-845. [DOI] [PubMed] [Google Scholar]
- Hutchison JB (1997) Gender-specific steroid metabolism in neural differentiation. Cell Mol Neurobiol 17: 603-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C, Guennoun R, Liere P, Eychenne B, Pianos A, El-Etr M, Baulieu EE, Schumacher M (2003) Developmental expression of genes involved in neurosteroidogenesis: 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase in the rat brain. Endocrinology 144: 2902-2911. [DOI] [PubMed] [Google Scholar]
- Isaac JT (2003) Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology 45: 450-460. [DOI] [PubMed] [Google Scholar]
- Jang MK, Mierke DF, Russek SJ, Farb DH (2004) A steroid modulatory domain on NR2B controls N-methyl-d-aspartate receptor proton sensitivity. Proc Natl Acad Sci USA 101: 8198-8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274: 1133-1138. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Wilson AA, Scheffel U, Campbell BG, London ED (1992) Radiosynthesis, cerebral distribution, and binding of [125I]-1-(p-iodophenyl)-3-(1-adamantyl)guanidine, a ligand for sigma binding sites. J Med Chem 35: 4683-4689. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S (2001) Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-d-aspartate and calcium-dependent synthesis. Endocrinology 142: 3578-3589. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA (2003) Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71: 67-80. [DOI] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA (2002) Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci 5: 833-834. [DOI] [PubMed] [Google Scholar]
- Liu S, Sjovall J, Griffiths WJ (2003) Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem 75: 5835-5846. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH (2002) Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol 135: 901-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R (2003) AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358: 707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H (2001) Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol 46: 33-78. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF (2002) Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. Possible role of metabotropic sigma1-like receptors. J Biol Chem 277: 28725-28732. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529-540. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J (2003) A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol 64: 857-864. [DOI] [PubMed] [Google Scholar]
- Okabe S, Collin C, Auerbach JM, Meiri N, Bengzon J, Kennedy MB, Segal M, McKay RD (1998) Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci 18: 4177-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF (2001) Neurosteroid-induced enhancement of glutamate transmission in rat hippocampal slices. Neurosci Lett 301: 103-106. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnar E (2001) Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology 41: 700-713. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F (1999) Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 22: 410-416. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Tsutsui K (2001) Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci 21: 6221-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JT (2004) Activity-driven sharpening of the retinotectal projection: the search for retrograde synaptic signaling pathways. J Neurobiol 59: 114-133. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE (2000) Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol 29: 307-326. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA (2000) Postsynaptic protein phosphorylation and LTP. Trends Neurosci 23: 75-80. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo M (2001) Retrograde signaling at central synapses. Proc Natl Acad Sci USA 98: 11009-11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Drewery DL, Atkins HD, Stephenson FA, Chazot PL (2002) Immunohistochemical localization of N-methyl-d-aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR2D subunit. Brain Res Mol Brain Res 102: 55-61. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro J Neurosci 19: 4180-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K, Usui M, Kohchi C, Tsutsui K (1998) Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology 139: 137-147. [DOI] [PubMed] [Google Scholar]
- Wang LY, Salter MW, MacDonald JF (1991) Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science 253: 1132-1135. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR (2001) Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13: 113-128. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D (1997a) NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem 68: 469-478. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Benke D, Mohler H, Fritschy JM (1997b) N-methyl-d-aspartate receptors containing the NR2D subunit in the retina are selectively expressed in rod bipolar cells. Neuroscience 78: 1105-1112. [DOI] [PubMed] [Google Scholar]
- Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ (2002) Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol 450: 303-317. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Cunningham MO, Jones RS (2001) NR2B-containing NMDA autoreceptors at synapses on entorhinal cortical neurons. J Neurophysiol 86: 1644-1651. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC (2003) A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6: 15-16. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R (2000) Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci 3: 1098-1106. [DOI] [PubMed] [Google Scholar]