Abstract
Integrative medicine refers to the blending of conventional and evidence-based complementary medicines and therapies with the aim of using the most appropriate of either or both modalities for ultimate patient benefits. One of the major hurdles for the same is the chances of potential herb–drug interactions (HDIs). These HDIs could be beneficial or harmful, or even fatal; therefore, a thorough understanding of the eventualities of HDIs is essential so that a successful integration of the modern and complementary alternative systems of medicine could be achieved. Here, we summarize all the important points related to HDIs, including types, tools/methods for study, and prediction of the HDIs, along with a special focus on interplays between drug metabolizing enzymes and transporters. In addition, this article covers future perspective, with a focus on background endogenous players of interplays and approaches to predict the drug–disease–herb interactions so as to fetch the desired effects of these interactions.
Keywords: Ayurveda, drug metabolizing enzymes–transporter interplays, herb–drug–disease interactions, integrative medicine
Introduction
Modern system of medicine has emerged as the primary choice for the treatment of nearly all types of health-related issues, although, it is mainly based on the nonholistic/bug killing/target-based approach, which ultimately leads to future side effects (notable in case of chronic disorders such as cancer, diabetes, arthritis, etc).1–3 However, patients with such chronic illnesses directly/indirectly undergo combinational/multimodal therapy with or without the knowledge of physicians, leading to potential herb–drug interactions (HDIs).1 The MD Anderson Cancer Centre, in USA, reported that 52% of their cancer patients had used at least one form of complementary and alternative medicine (CAM), and 77% of those were using herbs.4 While, according to the World Health Organization (WHO) and other reviews >80% world's population uses CAM for their health care needs and particularly in western countries CAM has become increasingly popular over the last few decades.5–8 However, concomitant usage of herbs and conventional medicines globally could be much higher as healthcare professionals often do not ask about herbal remedies when prescribing and patients do not volunteer that they are taking them.1,9–11 Indeed, such a scenario of concomitant usage of herbs/CAM and conventional medicines brings with it the potential problem of HDIs and this issue has emerged as a major hurdle/problem in our journey toward integrative medicine (IM).1,12,13 IM refers to the blending of conventional and evidence-based complementary medicines and therapies with the aim of using the most appropriate of either or both modalities for efficient patient care.14 In short, IM uses all appropriate, evidence-based therapies to achieve health.15 For example, Withania somnifera, have been widely accepted as a novel complementary therapy for integrative oncology care.16W somnifera not only helps in controlling the tumor growth but also exerts antioxidant, anti-inflammatory, immunemodulating, and antistress properties that help in combating the cancer and associated complications. It has also been found that, W somnifera enhances the effectiveness of radiation therapy and chemotherapy while potentially mitigating their undesirable side effects.16–18 Similar experiences were also observed by Patil et al and Borse et al for Asparagus racemosus and/or Tinospora cordifolia accepted as novel complementary therapy for integrative oncology care.18–22 IM/care practices are getting increased day by day throughout the world. For instance, IM is being practiced at BSDT's Ayurvedic Hospital & Research Centre, India, The Osher Center for Integrative Medicine, Arizona Center for Integrative Medicine, and many other places in the world.23–26 However, integrative management of the disease is far bigger challenge in spite of high scientific efforts proceeding globally mainly because of the potential risk associated with HDIs.27–33 Hence, the focus needs to be shifted on potential interactions between herbs and pharmaceuticals because of the growing popularity of herbal medicines/CAM. Here, it must be highlighted that the probability of HDIs can be much higher than drug–drug interactions, since most herbal medicines (even single-herb products) contain mixtures of pharmacologically active constituents compared to conventional/modern medicines.1,34 These HDIs could be beneficial or harmful, or even fatal; therefore, a thorough understanding of the eventualities of HDIs is essential so that a successful integration of the modern and complementary alternative systems of medicine could be achieved. Here, in the present review, we summarize all the important points related to HDIs, including types, tools/methods for study, and prediction of the HDI, along with a special focus on interplays between drug metabolizing enzymes (DMEs) and transporters. The interplays between 2 or more things may affect the functioning of each other. Indeed, interplay between DMEs and transporters hold potential to not only alter the pharmacokinetics (PK)–pharmacodynamics (PD) of herb/drug but also their safety profile. In this context, this article also covers future perspective, with a focus on background endogenous players of interplays and approaches to predict the drug–disease–herb interactions so as to fetch the desired effects of these interactions.
Methodology
Both online and offline literature searches were carried out to compile this review. We searched Medline, PubMed, the Cochrane library, ResearchGate, and Google Scholar, for mainly original research articles published between 1970 and 2017 for HDIs studies with focus on role of interplays in it. The main search terms which were used alone or in combination with each other includes but may not be limited to HDI, phytopharmaceuticals–drug–metabolite interactions, drug–herb–disease–metabolite–phytochemical interactions, types, mechanism, tools and techniques, databases, novel approaches, integrative approaches, regulatory guidelines or requirements, mechanistic PK–PD interactions, substrate overlap, enzyme–transporter interplays, cytokines–hormones–neurotransmitter–enzymes–transporter (CHNET) interplays, personalized medicine, and IM, We identified full-text articles without imposing any language restrictions. Reference lists of original studies, narrative reviews, and previous systematic reviews and meta-analyses were also searched carefully. Letters were sent to experts in the field requesting additional information on ongoing or unpublished data. Conference proceedings, dissertation abstracts, and reference lists from included and relevant articles were also searched.
Herb–drug interactions?
It has become clear that both conventional and herbal medicines are often used concomitantly35–37 and this can lead to clinically relevant HDIs.38 The HDI can be seen commonly and these may be beneficial, harmful, or even fatal. Usually the HDI either causes some beneficial or unsuspecting effects. The latter may turn into adverse effects, which may be fatal.39 A systematic approach is required for minimizing the untoward consequences and to reap out the potential benefits of these interactions.
Mechanisms of HDIs
Indeed, a single herb contains multiple phytoconstituents that may be biologically active and capable of modulating physiological actions, similar to therapeutic drugs, through complex synergistic and/or antagonistic effects.39 HDIs are mediated by pharmacodynamic and/or pharmacokinetic mechanisms. Pharmacokinetic interactions are much more difficult to anticipate than pharmacodynamic interactions.40,41 Most commonly reported HDIs are pharmacokinetic interactions, especially those resulting from the functional modulation of DMEs mainly cytochromes (CYPs); drug transporters such as P-gp; and protein binding. While, pharmacodynamic interaction involves antagonism, addition/summation, synergism, and even sometimes modulation of drug targets. However, there could be some other type of interactions, that is, multiple/complex HDIs which may lead to pharmacokinetic as well as pharmacodynamic interactions and may or may not be mediated through interplays involving alteration of CHNET.41–43Figure 1 gives the overview of HDIs, and Figure 2 describes the mechanisms of HDIs.
Figure 1.
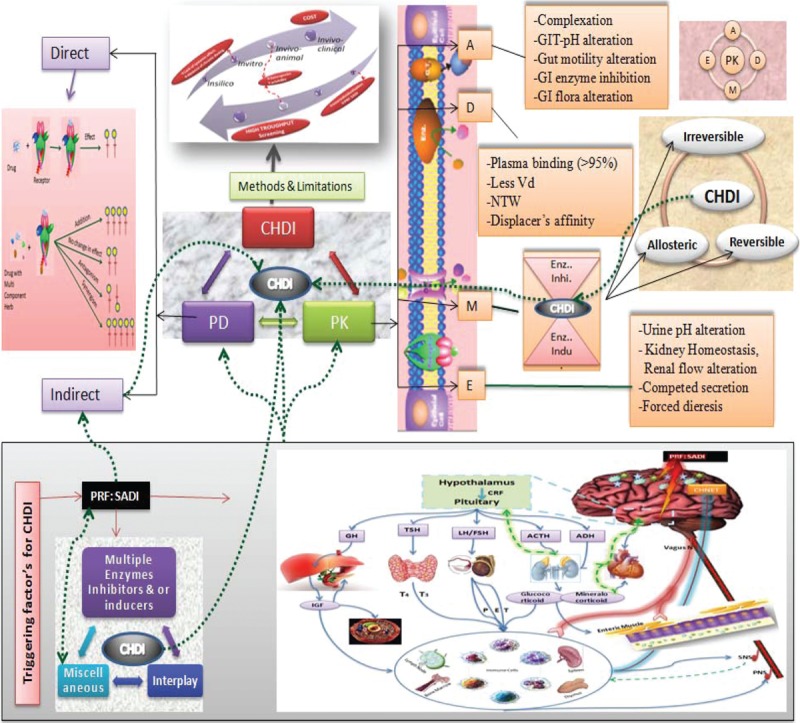
Overview of herb-drug interaction: herb-drug interactions (HDI) have direct and/or indirect effect on pharmacokinetics (PK)-pharmacodynamics (PD) of drug and/or herb which may lead to complex HDI (CHDI). Type and intensity of HDI depends upon the properties of the herb and drug under consideration along with indirect role of PRF:SADI (Patient-related factors: sex, age, disease/disorder, and individualization). Note: Straight line indicates main types and/or main effect, whereas dotted line indicates background interaction/effect. GIT = gastrointestinal tract, NTW = narrow therapeutic window.
Figure 2.
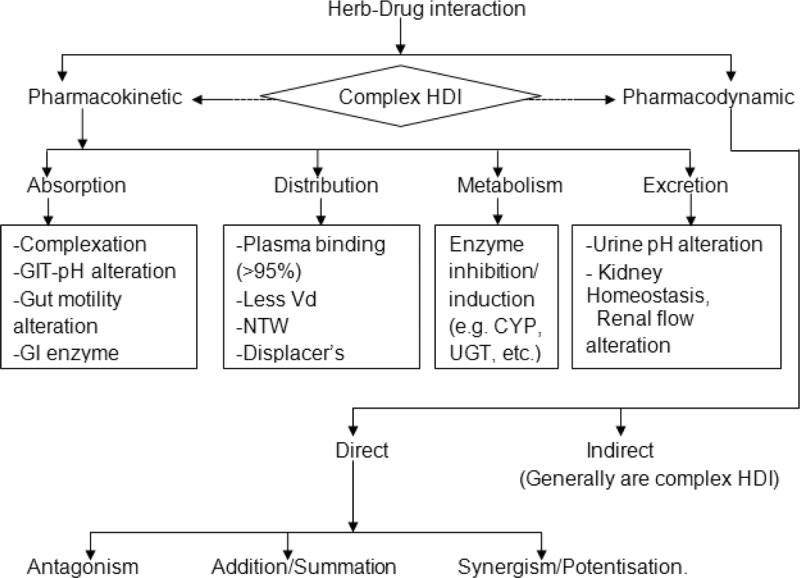
Mechanism of herb-drug interactions. CYP = cytochrome, GIT = gastrointestinal tract, Vd = volume of distribution, UGT = UDP-glucuronosyltransferase.
Pharmacokinetic HDIs
Pharmacokinetic HDIs may occur at any step of absorption, distribution, metabolism, and excretion (ADME), which have been explained section wise. Table 1 covers some representative examples.
Table 1.
Pharmacokinetic–pharmacodynamic HDIs

Absorption interactions
Any herb which affects the normal gastrointestinal tract environment will be responsible for the changes in the expected absorption pattern of the drug and will lead to HDI (see Fig. 1 absorption box). For example, any herbal laxative or bulk-forming agent will speed up the intestinal transit, and thus may interfere with the intestinal absorption. The most popular laxative herbs are anthranoid-containing herbs such as senna (Cassia senna and C angustifolia) and cascara sagrada (Rhamnus purshiana).43,85 In addition, in the presence of the drugs belonging to the class of antacids, systemic antiulcer agents, which will increase the pH of stomach, the absorption of weak acidic herbal extracts/formulations may get affected and vice versa.43,85
Distribution interactions
These interactions may occur with drugs having higher plasma protein-binding property (>95%), less Vd (volume of distribution), and narrow therapeutic window (NTW).86 For instance, warfarin a well known anticoagulant remains 98% plasma protein bound with less Vd of 0.11 to 0.18 L/kg and NTW of 1 to 2 which varies with respect to polymorphism of cytochrome P450 (CYP450) enzymes.30,87,88 Some known examples of agents that interact with warfarin include vitamin K, some types of tea and green leafy vegetables. Agrimonia eupatoria has been reported to interfere with the efficacy of anticoagulants.89 These agents interact with warfarin by either increasing or decreasing its effectiveness and thus, leading to prolonged bleeding or increasing the risk of blood clotting, respectively.90–92 Hence, patients on warfarin need to be extremely cautious while taking herbs concomitantly as HDIs pose immense risk which could be even fatal. For instance, PK–PD of warfarin in healthy subjects is insignificantly affected at recommended doses of ginkgo and ginger.91 Echinacea, significantly reduces plasma concentrations of S-warfarin.93 St John's wort decreases the anticoagulant effect of warfarin,90 whereas Allium sativum increases the bleeding risk.90
Metabolism interactions
Metabolism is the biochemical modification of xenobiotics by living organisms, usually through specialized enzymatic systems to eliminate the same.94 The rate of metabolism determines the duration and intensity of a drug's pharmacological action. A large number of phytochemicals that gain access to the systemic circulation tend to be lipophilic, and consequently are difficult to excrete; thus, the body renders them hydrophilic through metabolism to facilitate their excretion.95 This is done in 2 phases, phase I involves CYP450 isoenzyme system, which oxidizes, reduces, or hydrolyzes the drug/xenobiotic, whereas phase II involves conjugation reactions such as glucuronidation, acetylation, and sulfation reactions that increase water solubility of drug with a polar moiety glucuronate, acetate, and sulfate, respectively.96Table 2 covers important metabolizing enzymes with their functional role.97 Many DMEs shows polymorphic nature and intensity of the same varies with respect to patient-related factors: sex, age, disease/disorder, and individualization (PRF:SADI).101 Phytochemicals/xenobiotics can modulate the hepatic and extrahepatic expression of DMEs resulting in marked changes in the metabolism of drugs that leads to HDIs.95,102 Considering these facts Food and Drug Administration (USFDA) asks for the data of drug interactions.103 The significance of the individual CYP enzyme in human drug metabolism varies, with CYP3A, CYP2D, and CYP2C being responsible for the metabolism of 50%, 25%, and 20%, respectively, of most of the pharmaceuticals/xenobiotics.102 Herbal ingredients can alter metabolizing enzymes through induction and/or inhibition.104 Induction of CYPs by herbal products usually requires several days; however, induction of the enzyme(s) may lead to decreased drug plasma levels (through increased drug metabolism), and subsequently to reduced drug effects.38,95,105 Conversely, the inhibition of CYPs is often immediate and may lead to increased drug plasma levels (through decreased drug metabolism), resulting in an enhanced drug effect, that may result in significant adverse reactions or toxicities.95,105,106 In case of prodrugs, opposite may happen, for both induction and inhibition.95,105 Many clinical adverse events have been reported to be associated with CYP-mediated HDIs.107,108 Metabolic pharmacokinetic HDIs occur by various mechanisms (Fig. 3).
Table 2.
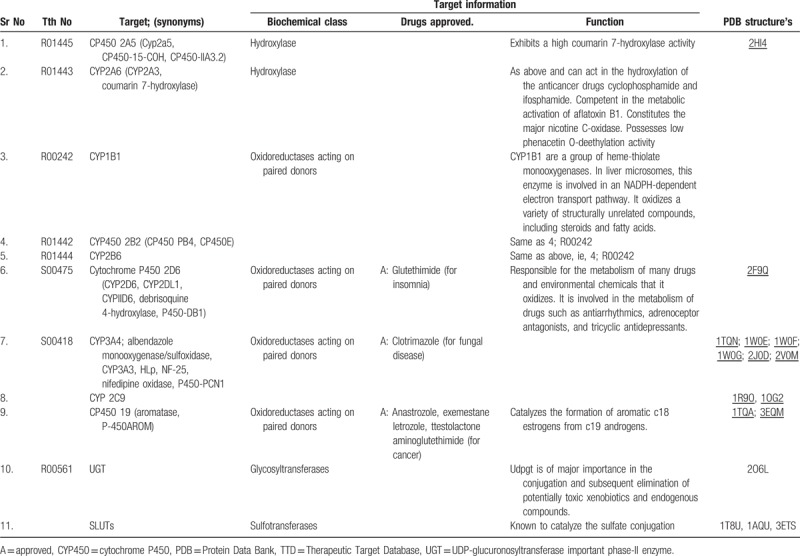
Figure 3.
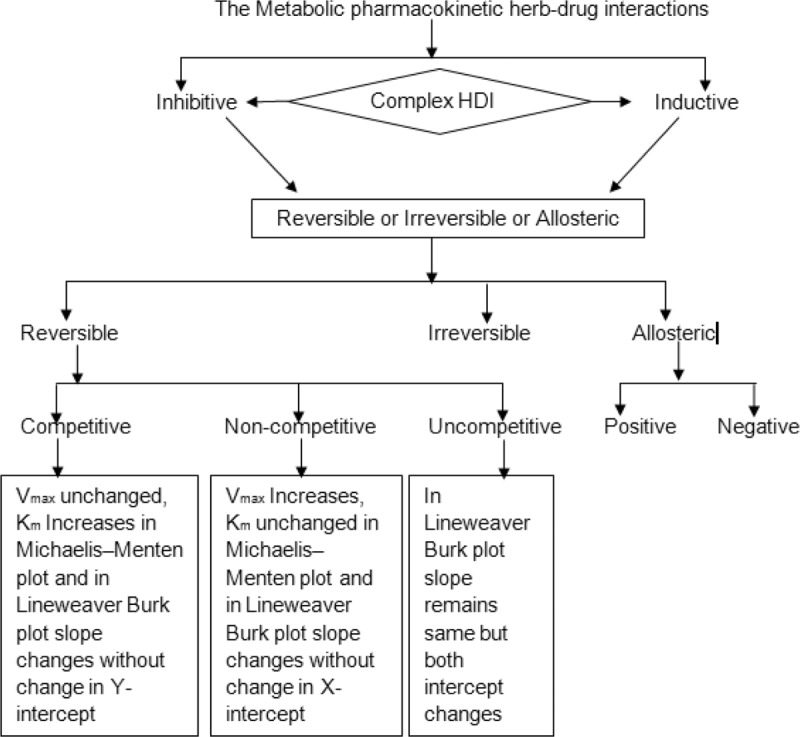
The metabolic pharmacokinetic herb-drug interactions. Vmax = maximum reaction velocity in enzyme kinetics, Km = the substrate concentration required to produce half Vmax in enzyme kinetics.
Mutual competitive inhibition may occur between herbal constituent and a drug, as both are often metabolized by the same CYP isoform. For example, diallyl sulfide from garlic is a competitive inhibitor of CYP2E1.108 Noncompetitive inhibition is caused by the binding of herbal constituents containing electrophilic groups (eg, imidazole or hydrazine group) to the heme portion of CYPs. For example, piperine inhibits CYP1A and CYP2A by noncompetitive mechanism.109 Hyperforin present in St John's wort is also a potent noncompetitive inhibitor of CYP2D6.110 The mechanism-based inhibition of CYP is due to the formation of a complex between herbal metabolite with CYP under consideration. For example, diallyl sulfone derived from diallyl sulfide is a suicide inhibitor of CYP2E1 by forming a complex via an epoxide metabolite,111 leading to autocatalytic destruction of CYP2E1.112 Therefore, the drugs that get metabolized by CYP2E1 are needed to be taken/monitored cautiously while concomitant administration with garlic.112,113
It is evident that, the formation of reactive metabolite of drug/xenobiotic is associated with toxicity.113 Toxicity mediated by herbal metabolites mostly happens via multiple pathways such as cytotoxicity, oncogene activation, and hypersensitivity reactions.113 For instance, CYP1A1/2-mediated bioactivation of aristolochic acid present in Aristolochia spp. produces nitrenium ion that activates H-ras oncogene and finally results in carcinogenesis.114 Similarly, Germander (Teucrium chamaedrys), which is a folk medicine was used as antiseptic and adjuvant to slim diet.114 In 1991 Germander has been found to be hepatotoxic and fatal.115 The furan ring of diterpenoids present in the Germander gets metabolized by CYP3A4 to form reactive epoxide radicals.114 These epoxide radicals react with CYP3A and epoxide hydroxylase which, further causes mitochondrial permeability transition, caspase activation, and apoptosis of hepatocytes.114,115
Elimination interactions
The sources of drug elimination from the body are urine, feces, sweat, tears, semen, menstrual discharge, etc. The main players in drug or xenobiotic elimination are cell transporter protein/enzymes such as P-gp, organic anion transporting polypeptide (OATP), organic anion transporter, OCTP, breast cancer resistance protein, and others in this process. However, these may get affected by concomitant administration of herb and drug resulting in HDIs.43 Furthermore, some herbs are known diuretic, which can affect the excretion of medicinal drugs.116 The nephrotoxic drug induces kidney damage resulting in slow rate of elimination leading to an accumulation of herbs and drugs in the body. Important examples of drugs that damage the kidneys include gentamicin, amphotericin B, methotrexate, and tobramycin. Hence, a close monitoring is required to avoid the unwanted HDIs. Furthermore, in case of elimination interactions the role of transporters needs to be focused as transporters govern the transport of xenobiotics in and out of the cells.
Although less well-recognized than DMEs, membrane transporters can have important effects on PK–PD of the drugs/herbs. However, in contrast to DMEs, which are largely concentrated in the liver and intestine, transporters are present with varying abundance in all tissues in the body and play an important role in drug absorption, distribution, tissue-specific drug targeting, and elimination (Fig. 4).10,101,117
Figure 4.
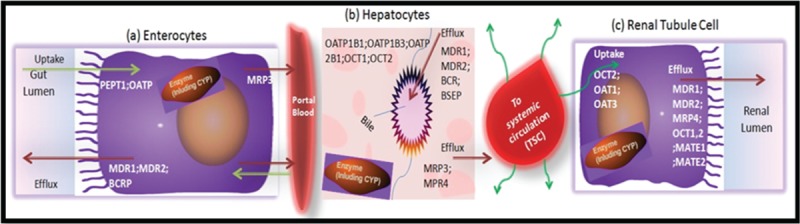
Illustration of examples of efflux and uptake transporters in the gut wall (A), liver (B), and kidneys (C) that may be involved in a drug's absorption, distribution, metabolism, and excretion. BCRP = breast cancer resistance protein, MATE = multidrug and toxic compound extrusion protein, MDR1 = multidrug resistance 1(P-glycoprotein (P-gp)), MRP = multidrug resistance associated protein, OAT = organic anion transporter, OATP = organic anion transporting polypeptide, OCT = organic cation transporter,PEPT1 = peptide transporter 1.
A number of transporter-based interactions have been documented in recent years.118–120 To date, most of the identified transporters belong to 1 of the 2 super families: ATP-binding cassette, that is, P-gp and solute carrier. Transporters and DMEs show substrate specific interplay (due to substrate overlapping) and may affect each other's functional efficacy. P-gp is a plasma membrane–bound drug efflux protein found primarily in drug-eliminating organs and presumably functions as a detoxifying transporter,121 because, P-gp actively extrudes xenobiotics from the body.121,122 In the small intestine, P-gp is localized to the apical membrane of the intestinal epithelial cells, having a role of effluxing the compounds back into the intestinal lumen.122 Pharmacokinetic studies of paclitaxel, and digoxin, in mdr1a knockout mice have revealed the importance of intestinal P-gp in limiting the oral bioavailability of these drugs.123 Phytochemicals are also known to interact with the ATP-dependent transporter proteins such as the intestinal P-gp and other multidrug resistance proteins that facilitate the efflux of the drugs.124–126 It has been shown that various drugs (eg, quinidine, verapamil, and itraconazole) increase plasma levels of digoxin by inhibiting the efflux transporter P-gp at the intestinal level. Plasma levels of many β-Hydroxy β-methylglutaryl-CoA reductase inhibitors including rosuvastatin, pravastatin, and pitavastatin, are increased by co-administration of inhibitors of hepatic uptake transporters (eg, OATP1B1), such as cyclosporine, rifampin, and flavonoids (a commonly found herbal constituents, such as quercetin, curcumin, etc) are competitive inhibitor of OATP1B1/1B3.127 In this purview, Tucker et al42 has discussed various key points to improve the ability to predict HDIs at the transporter level.
Multiple/complex HDIs: importance of interplay(s) in context to HDIs
The HDIs related to ADME and transporters have been discussed separately, but, in some cases drug interactions may occur by combination of these mechanisms called multiple/complex HDI and such scenarios include but are not limited to102:
-
(1)
Concurrent inhibition and induction of 1 enzyme or concurrent inhibition of enzyme and transporter by a drug and/or herb
-
(2)
Increased inhibition of drug elimination by the use of more than 1 inhibitor of the same enzyme that metabolizes the drug and/or herb
-
(3)
Increased inhibition of drug elimination by use of inhibitors of more than 1 enzyme that metabolizes the drug and/or herb
-
(4)
Inhibition by a drug and its metabolite(s), both of which inhibit the enzyme that metabolizes the substrate drug and/or herb
-
(5)
Inhibition of an enzyme other than the genetic polymorphic enzyme in poor metabolizers taking substrate that is metabolized by both enzymes
-
(6)
Use of enzyme/transporter inhibitors in subjects with varying degrees of impairment of xenobiotics eliminating organs (eg, liver or kidney).
However, when multiple mechanisms are involved in HDIs then interplays may happen. In general, as interplays increase, complexity of HDIs is also increased. Figure 5 explains the different ways of multiple/complex HDIs. The size of the quadrant reflects its probable contribution in it.
Figure 5.
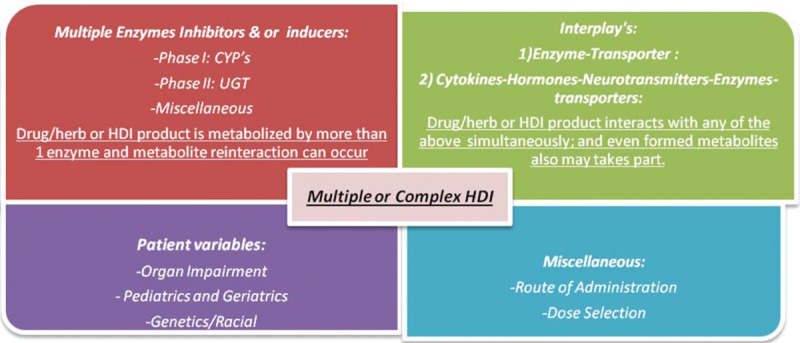
Multiple or complex HDIs: size of the quadrant reflects its probable contribution in it. CYP = cytochrome, HDI = herb-drug interaction, UGT = UDP-glucuronosyltransferase.
Interplay is said to happen when 2 or more things have an effect on each other. As discussed above there are many factors that affect ADME of drug leading/contributing to HDIs. When these confounding factors and/or players of ADME affect each other and/or show substrate overlapping this leads to interplay. The most studied and common interplays are enzyme-transporter interplay(s).
DMEs-transporter interplays
In early 1900 the concept of interplay started to fertilize in the laboratory of University of California, San Francisco resulting from the efforts by Benet and his coworkers.128 Benet and his coworkers first studied the effects of a high-fat meal on cyclosporine pharmacokinetics in healthy subjects,128 which led them to believe that the unusual effects resulting from a high-fat meal, that is, no change in the absorption rate but a significant increase in the extent of absorption128,129 and an increase in the clearance of cyclosporine,130 could be explained by a lipid effect in the liver.131 They were first to note and publish the striking overlap of substrate specificity and the tissue distribution for CYP3A and Pgp. They proposed that CYP3A and P-gp played complementary roles in ADME of the drug by biotransformation and counter transport, particularly in the villi of the small intestine. Shortly following publication of this coordinated protective mechanism,132 Schuetz et al133 demonstrated that modulators and substrates of P-gp and CYP3A coordinately upregulated these proteins in human colon carcinoma cells and that P-gp was a major determinant of rifampicin-inducible expression of CYP3A in mice and humans.134 Similar studies to those described above for cyclosporine were also reported for tacrolimus and sirolimus.135–137 Herbal medicines are often administered orally and they can attain moderate to high concentrations in the gut lumen (the primary site of absorption for most orally administered drugs) and liver, and may exert a significant effect on enterocytes and hepatocytes. Both P-gp and CYP3A4 are abundantly expressed in the villus tip of enterocytes and hepatocytes. The interplay of both intestinal P-gp and CYP3A4 has a strong effect on the bioavailability of most orally administered drugs including cyclosporine, midazolam, talinolol, statins, HIV protease inhibitors, and verapamil.138 Many studies have suggested that both CYP3A4 and P-gp have cosubstrates and the drugs which interact with apical efflux pump P-gp may enhance CYP3A4 mediated disappearance of substrates during intestinal secretory detoxification.139,140 As well as P-gp possibly influences first-pass metabolism in a co-operative manner.141 Thus, the modulation of intestinal and hepatic P-gp and CYP3A4 by herbal medicines represents a potentially important mechanism by which the bioavailability of coadministered drug(s) can be modulated.142 Time-dependant HDIs mainly depend on the primary metabolite formed, which generally interacts with the same parent metabolizing enzyme and leads to its inhibition or induction which is time dependant and/or concentration dependant and ultimately responsible for complex HDIs (Fig. 4).34,143 On the contrary, their evidence in support of interplay between CYP3A4 and P-gp mainly comes from limited in vitro and preclinical studies, which generally gets extrapolated for humans.140 These PK interactions may alter the pharmacodynamic responses. In addition, there could be cases in which, cytokine, hormone, neurotransmitter, enzyme, and transporters all interact and lead to complex interplays, which may result in potential HDIs.
Cytokine–hormone–neurotransmitter–enzyme–transporter interplay(s)
The interplay between endogenous molecules such as cytokines, hormones, neurotransmitter, enzymes, etc is important to maintain the normal homeostasis through feedback loops and healthy condition.144,145 This interplay indirectly affects the functional ability of the DMEs and transporters too.146 In diseased state this interplay gets altered and these defects lead to alteration in the entire CHNET interplay and sometimes affecting PK–PD of the administered drugs. (Figs. 6 and 7).144–157 The CHNET interplay is very difficult to study and use as compared to simply DMEs-transporters interplay (Figs. 6 and 7).
Figure 6.
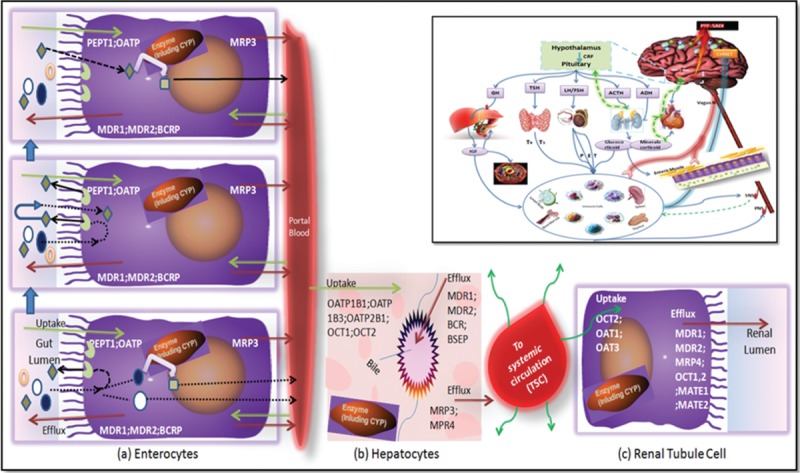
Interplays: (A) CYP3A4 and P-glycoprotein interplay in the enterocytes, (B) hepatocytes, (C) renal tubule cell and the embedded small square contains PRF: SADI (Patient related factors: sex, age, disease/disorder, individualization) which are responsible for alteration in normal physiological balance as a result of imbalanced level of CHNET, which finally responsible for altered pharmacokinetics (PK)-pharmacodynamics (PD) of single drug and even occurrence of Adverse Drug Event/Adverse Drug Reaction (ADE/ADR) and/or suspected unsuspected serious adverse reaction (SUSAR) resulting from DI/herb-drug interaction (HDI). For instance in diabetes the expression of CYP2C11 is decreased, and CYP2E1 increases which might have been triggered or done by altered level of insulin and other hormones as well as altered normal body physiology and hence owing this all the HDI or DI occurs which might be beneficial/harmful/or even fatal. Figure A explains conception of the interaction between CYP3A and P-glycoprotein in the intestine. Three drug molecules are depicted ( ). They are all the same drug and only differentiated by their outcome. Drug is absorbed by passive processes into the enterocytes where it may be metabolized by the enzyme. However, the drug is also subject to active efflux back into the intestine thereby allowing further access to the enzyme upon subsequent passive absorption. The open circle (
). They are all the same drug and only differentiated by their outcome. Drug is absorbed by passive processes into the enterocytes where it may be metabolized by the enzyme. However, the drug is also subject to active efflux back into the intestine thereby allowing further access to the enzyme upon subsequent passive absorption. The open circle ( ) molecule enters the enterocytes, is not metabolized by CYP3A or efflux back into the lumen by P-glycoprotein. It then proceeds in the hepatic portal vein to the liver. The solid circle (
) molecule enters the enterocytes, is not metabolized by CYP3A or efflux back into the lumen by P-glycoprotein. It then proceeds in the hepatic portal vein to the liver. The solid circle ( ) molecule is absorbed into the enterocytes and is metabolized to the open square product upon its first encounter with the enzyme. The open square (
) molecule is absorbed into the enterocytes and is metabolized to the open square product upon its first encounter with the enzyme. The open square ( ) metabolite either passes into the hepatic portal blood or back into the gut lumen. However, the shaded diamond molecule is absorbed (
) metabolite either passes into the hepatic portal blood or back into the gut lumen. However, the shaded diamond molecule is absorbed ( ); it is not metabolized by the enzyme; it is effluxed back into the gut lumen by P-glycoprotein (
); it is not metabolized by the enzyme; it is effluxed back into the gut lumen by P-glycoprotein ( ) and this cycling occurs twice again, where upon the fourth entry into the enterocytes the shaded diamond molecule is metabolized. While the influx transporter helps the drug molecule in absorption by carrier mediated and/or active transporter, and even others like Hsp (
) and this cycling occurs twice again, where upon the fourth entry into the enterocytes the shaded diamond molecule is metabolized. While the influx transporter helps the drug molecule in absorption by carrier mediated and/or active transporter, and even others like Hsp ( ) which helps during attachment of drugs/ligand to receptors, for instance Hsp helps during its binding to aromatic hydrocarbon receptor and they has main role in synthesis, transportation, and folding of proteins especially during the stress. This fig explains that the transporter controlling the access of the drug to the enzyme, giving the enzyme multiple opportunities to prevent the intact xenobiotics from entering the bloodstream. Thus, the enzyme and the transporter and other proteineous and nonproteineous molecules are working in a coordinated manner as a protective process to keep foreign substances out of the body.
) which helps during attachment of drugs/ligand to receptors, for instance Hsp helps during its binding to aromatic hydrocarbon receptor and they has main role in synthesis, transportation, and folding of proteins especially during the stress. This fig explains that the transporter controlling the access of the drug to the enzyme, giving the enzyme multiple opportunities to prevent the intact xenobiotics from entering the bloodstream. Thus, the enzyme and the transporter and other proteineous and nonproteineous molecules are working in a coordinated manner as a protective process to keep foreign substances out of the body.
Figure 7.
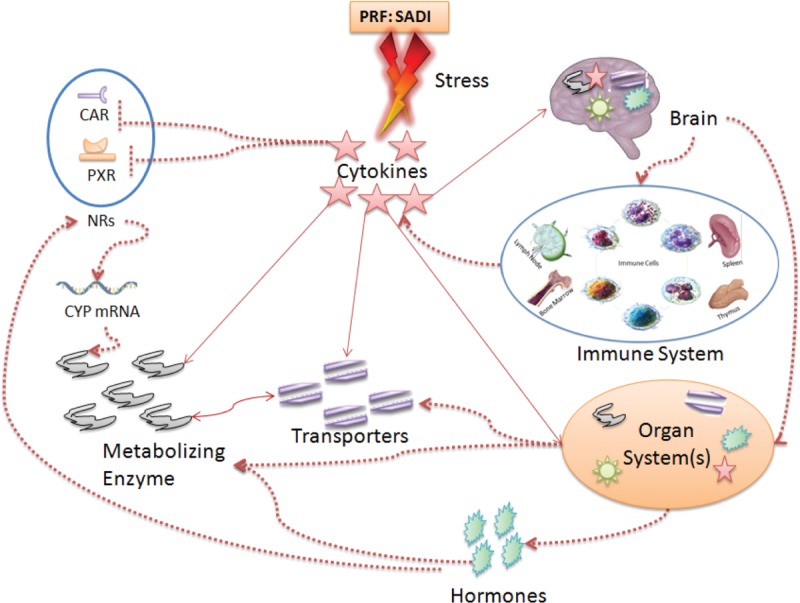
Effect of cytokines–hormones–neurotransmitter–enzymes–transporter (CHNET) interplay(s) on pharmacokinetics: During the disease and/or illness alters the balanced network of CHNET and affects the overall pharmacokinetics (PK)–pharmacodynamics (PD) of the administered drug. The solid lines shows direct impact on functional expression/ability on the corresponding counterpart while dotted lines indirect impact through involvement of other factors such as ROS, NF-kB, gp-130 etc. CAR = constitutive androstane receptor, NF-kB = nuclear factor-kappaB, NRs = other nuclear receptor like: FXR, NR1H4, PRF: SADI = patient-related factors: sex, age, disease, and individualization, PXR = pregnane X receptor, ROS = reactive oxygen species.
Shapiro LE and Shear NH have reviewed that, apart from posological factors, polypharmacy, and organ dysfunction, pharmacogenetic risk factors and/or individualization also affects HDIs.158 In the maintenance of normal body physiological condition, the CHNET has central role and in diseased/unhealthy condition these gets altered which changes not only psychophysiological159–161 and social behavior but also changes receptor pharmacology and may induce newer receptor targets162 in patients. Thus, it can be concluded from the work done by many reviewers and researchers that there can be strong relation between homeostasis and interaction between host–microbiome–virobiota along with external factors which alters normal psychophysiological condition of a patient.163–166 Finally, because of these all, the level and intensity of CHNET gets altered which ultimately creates individualized CHNET cascade affecting normal PK–PD of the drug along with DI/HDI. These observations call for a fresh look on the topic focusing on drug–disease–drug/herb interactions.
Pharmacodynamic HDIs
Pharmacodynamic interactions occur mainly at receptor level and are classified as direct and indirect HDIs (Fig. 2).167,168 The direct HDIs are easy to understand and even predict than indirect HDIs.104,168,169 Medication could be of further risk when used with dietary supplements/herbal medicines that share these pharmacological activities.170 Few examples of direct interactions are mentioned in Table 1. One of the good examples of indirect pharmacodynamic HDIs is cranberry which potentiates the anticoagulant activity of warfarin.73 Doubling of the plasma drug concentration may lead to enhanced drug effects and/or adverse effects depending on the therapeutic and safety window.73,104,168,169 However, less marked changes may still be clinically important for drugs with a steep concentration–response relationship or a narrow therapeutic index.168 The clinical importance of HDIs depends on factors that are related to co-administered drug/herb (dose, dosing regimen, administration route, pharmacokinetic, and therapeutic range) and PRF:SADI.104,169,171 In other words the extent of drug interactions with herbs varies markedly among individuals, depending on individualization in drug metabolism and transporters, comedication with other drugs, age, and many of other factors.104,169
Methods for herb–drug interaction studies: an overview
There are 3 types of methods to study HDIs, namely in silico, in vitro, and in vivo methods. In silico is a term used for experiments done using a high-performance computer, whereas in vitro and in vivo refers to the experiments done outside of living organism and in living organism, respectively. Each method has pros and cons/limitations (Fig. 8) over the other; hence, sometimes to gain overall picture of HDIs, these methods are used in combination.
Figure 8.
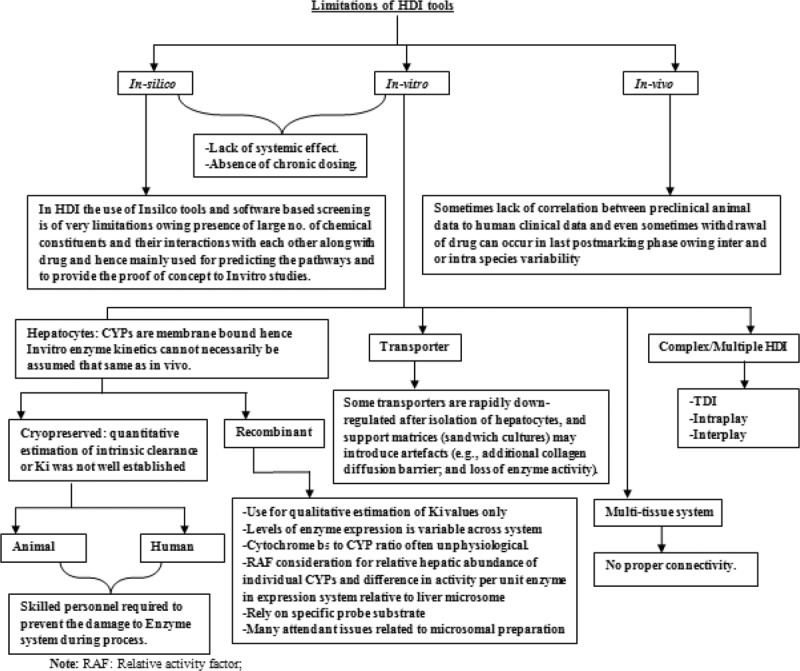
Limitations of HDI tools. HDI = herb–drug interaction.
In silico methods
There is an increasing use of in silico methods to study the CYPs and their interactions with xenobiotics.172 In silico approaches have also been used to study the herb–CYP interactions.173–175 The major in silico methods include simple rule-based modeling, structure–activity relationships, and 3-dimensional quantitative structure–activity relationships.176 All represent useful tools for understanding the reactions catalyzed by CYPs, predicting possible metabolic HDIs, pharmacokinetic parameters such as clearance, and toxicity.177 The resulting data based on in silico approaches may be of clinical relevance.178 A structure–activity relationship analysis was used to investigate the effect of structural modifications of piperine (pentadienyl/piperidine) on the inhibition of the CYP-catalyzed reactions, arylhydrocarbon hydroxylation (CYP1A), and 7-methoxycoumarin-O-demethylation (CYP2) in microsomes prepared from untreated, 3-methylcholanthrene and phenobarbital-treated rat liver.179 This study has indicated that saturation of the side chain resulted in a marked increase in the inhibition of CYPs, whereas modifications in the phenyl and basic moieties in a few analogs led to maximum selectivity in inhibiting either constitutive or inducible CYP activities.178,179 Although it is a virtual screening system, in silico studies could provide some early indications of the possible involvement of CYPs in context to HDIs.
In vitro methods
A number of in vitro systems have been established to investigate the drug interactions. For metabolic interactions, the major models include subcellular fractions (ie, liver microsomes, cytosols, and homogenates), B-lymphoblastoid cells, precision-cut liver slices, isolated and cultured hepatocytes or liver cell lines, and cDNA-expressed enzymes.180 For transporter's studies, Caco-2 and Madin-Darby canine kidney-II cells, oocytes, membrane vesicles, and cDNA-expressed drug transporters are widely used.181 Each of these systems has advantages cum limitations. However, combination of these methods can provide the most accurate information on how herbal medicines affect CYPs and P-gp. For example, cultured human hepatocytes provide cellular integrity with respect to enzyme architecture and allow the study of phase I and II reactions and transporter.182,183 There are several CYP screening kits aimed to offer a simple “mix-and-read” fluorescent assay that is designed for high throughput screening in multiwell plates.184 There are >25 human CYP enzymes having commercial screening kits containing recombinant cDNA-expressed CYP enzymes.184 cDNA-expressed enzyme systems provide high level of catalytic activity (6-fold higher than an average human liver microsomes sample) and are used for screening of diverse compounds related to metabolism in vitro. However, induction effect of test compounds on CYP enzymes could not be investigated by these systems.184–186
Novel approaches such as IdMOC (independent discrete multiple organ co-culture) have been developed to overcome the conventional in vitro systems, in which a critical interaction between organs or cell types gets ignored. Li et al187 have developed the IdMOC system. The IdMOC allows the coculturing of cells from different organs as physically separated cultures that are interconnected by an overlying medium, akin to the blood circulation connecting the multiple organs in the human body.188 This allows, the evaluation of organ-specific effects a drug and its metabolites.189
In vivo methods
Although in silico and in vitro models may provide quick screening methods for the herb–CYP interactions, in vivo interaction studies are usually necessary to provide evidence of adjudging their clinical importance. Probe substrates and selective inhibitors can be used to explore the effects of herbs on the activity of specific CYP enzyme in vivo, for example, erythromycin for CYP3A4; USFDA has given a comprehensive list for the same.190 In clinical trial, there are 2 basic strategies to handle probe drugs, individual administration of a specific probe targeting 1 CYP enzyme and cocktail strategy in which simultaneous administration of multiple probes targeting multiple enzymes at 1 trial session. The cocktail of probe drugs has been used to explore the activities of multiple CYPs190–192 and could provide information on several metabolism pathways in a single session of clinical trial. This minimizes the complicating influence of intraindividual variability over time.193 For instance, a cocktail containing tolbutamide (CYP2C9), caffeine (CYP1A2), dextromethorphan (CYP2D6), oral midazolam (intestinal wall and hepatic CYP3A), and intravenous midazolam (hepatic CYP3A) has been used to investigate the effects of St John's wort on the activities of various CYPs in humans.194 However, the value of the “cocktail approach may be limited due to marked intrasubject variability and the possibility of interaction between the coadministered probes.195 Zhou et al196,197 have discussed various factors that determine the degree of change in the steady-state plasma concentration caused by the HDIs in vivo.
Prediction of HDI and softwares for clinical use
The prediction of HDI appears to be more challenging than predicting DDI. However, there has been some success in the prediction of phytoconstituent–drug interaction and/or DDI from in vitro metabolic inhibition data, when the following criteria are met190,198:
-
(1)
Drug clearance must be primarily by metabolism.
-
(2)
Drug is not subject to substantial conjugation or other non-CYP metabolism.
-
(3)
The liver is the primary organ of metabolic clearance.
-
(4)
The compound does not possess physicochemical properties that are associated with absorption problems (ie, limited solubility, low gastroenteral permeability).
However, prediction of HDI may be halted by the following factors: (1) Herbal medicines often contain hundreds of constituents with differential quantitative presence of active constituents along with inhibition and/or induction potency for DMEs, transporters, and receptors as a whole formulation; (2) In some cases in which indirect HDI are involved owing to interplays between 1 or more components of CHNET; (3) the inhibition and/or induction of CYPs and P-gp by herbal medicines, which may vary based on related confounding factors; (4) many herbal medicines are used chronically; (5) considerable variability in the active contents of herbal constituents due to quality control problems; (6) presence of extrahepatic metabolism; and active transport in liver; and (7) PRF:SADI. All these factors will contribute to the final outcome of HDIs.
There are several softwares and Web sites available based on traditional Chinese system of medicine and Ayurveda which can help directly and/or indirectly in the development of IM, screening and predicting probable HDIs (Table 3).
Table 3.
Database useful for herb–drug interaction studies100

Future perspective
Drug–disease–herb interactions
Expression and activity of several important DMEs and transporters gets altered in special population and/or conditions such as pediatric, geriatric, pregnancy, renal, and hepatic failure. Now, it has been well accepted that the alteration in the PK–PD can occur in various pathophysiological conditions as well.145,146 To understand drug–disease–herb interaction there is a need of tools/techniques, which can focus on pharmacogenetic–drug interaction data from the disease point of view199; so that drug–disease–herb interaction can be considered to next level of safety and personalization.200,201 But, yet no tools/techniques have been developed or used to focus from this aspect. In upcoming time, a comprehensive database (by integrating novel approaches and all the available databases including but not limited to those are mentioned in Table 3) needs to be developed. Such databases will not only be helpful to reduce the time and efforts to understand/predict HDI but also will be helpful to save the resources and minimize/rationalize the preclinical research related to HDIs.
Novel approach to predict HDIs for integrative medicine (whole system strategy)
Scientific fraternity has created well established guidelines for the industry to study drug interaction, drug–drug interactions, but there are no such well established guidelines for the study of HDIs.202 Hence, there is a need to develop novel approaches, algorithms, databases, and/or integrative tools and techniques to cover all the aspects related to HDIs. The major problem in developing such draft guidance for industry to understand the real clinical scenario of HDIs is that, the presence of n number of phytochemicals in the herb/herbal formulations.
Therefore, the whole herbal formulation needs to be screened for HDI studies. But, for predictive and clinically translational use, one may have to understand and/or develop the connecting link between allopathic and CAM drug(s)/formulation(s). Once that connecting link gets developed using various drug/formulation evaluation criteria's/properties of modern science and CAM system such as Ayurveda, we will be able to study the each other's drug/formulation from each other's point of view.203–205
For instance, Ayurveda is one of the most ancient CAM, and Ayurvedic materia medica has been developed based on its basic principles and evaluation criteria to give/predict fate and detailed medicinal properties of the substance/formulation (as a whole) under evaluation.206,207For instance, Rasa (∼taste), Guna (∼organoleptic and physiochemical properties) used to predict Vipaka (∼rasa after digestion and metabolism) and Virya (∼Potency), which are useful to understand the probable metabolic path and its pharmacological actions along with possible adverse drug interactions and/or side effects.206–211 Few attempts have been made in this direction in past by Nanal et al and recently we have coined the new term Ayurnization for the same205 and predicted phytoconstituents from plants which were unstudied/not well studied based on Ayurveda, ethnopharmacology, and reverse pharmacology.204
Hence, in today's perspective, more of such approaches are needed to be developed so that, one may be directly able to correlate, predict, and integrate the available systems of medicine for better result with optimum and rational use of interactions and minimal use of preclinical and clinical studies. Ultimately, that is what the aim of the scientific research is.
Discussion and conclusion
People with chronic disorders want to do everything they can to combat the disease, manage its symptoms, and cope with the side effects of treatment. Because patients of such chronic diseases take the simultaneous treatment by more than one physician and/or system of medicine with or without prior consent of physician, this may lead to the harmful/beneficial/fatal HDIs. Hence, for the safe use of IM there is an ardent need to understand the importance and consequences of HDIs, then only we can reap out the benefits from all the available systems of medicine viz ayurveda, allopathy, naturopathy, traditional Chinese medicines, etc.16–18 For instance, the importance of HDIs can be highlighted based on in vitro–in vivo studies performed by Patil D et al20 in which they have shown that the concomitant administration of aqueous stems extract of one of the most widely used Ayurvedic rasayana botanical namely T cordifolia with cyclophosphamide significantly reverses the mylosuppression without affecting the pharmacokinetics of cyclophosphamide.18,21,22 They further that such studies could be very helpful to design adjuvant treatment for chronic diseases such as cancer and so on.
Another point worth mentioning here is that there could be differences while studying the effects of crude extract and herbal molecules in context to HDIs and their importance in IM. It has been observed that sometimes, a single component may give action but may not be as desired as by CAM and may be responsible for unknown side effects and/or SUSARs (suspected unsuspected serious adverse reactions), which are even not expected by the CAM.18,20–22,212 For instance, Hudson et al212 have shown that Muscadine grapes skin extracts (MSKEs) contain resveratrol despite of that, when MSKE and resveratrol were separately studied for prostate cancer cell growth inhibition, MSKE and resveratrol targeted distinct pathways to inhibit prostate cancer cell growth. Therefore, one cannot surely predict the effect or pharmacological response of whole extract based on their major phytoconstituents only. The effects of inhibition and/or induction of DMEs and/or transporters on in vivo pharmacokinetics are highly variable and depend on several factors associated with the drug, herbal medicine, and individualization. Therefore, the strategies such as “Whole system strategy” are needed to be developed to focus on real-time clinical scenario. In upcoming time, development of a comprehensive database (by integrating novel approaches and all the available databases including but not limited to those are mentioned in Table 3) for predicting and understanding HDIs will not only be helpful to reduce the time and efforts to understand/predict HDI but also will be helpful to save the resources and minimize/rationalize the preclinical research related to HDI. Apart from this, the pharmacovigilance program also needs to be reenergized. Indeed, in this perspective, WHO has also widen their pharmacovigilance program to include herbals, traditional and complementary medicines, blood products, biological, medical devices, and vaccines.213,214In conclusion, we believe that to extract maximum benefits from IM the apt understanding of the potential threats/benefits and/or consequences of HDIs could go long way in alleviating most of the human sufferings.
Acknowledgments
None.
Conflicts of interest
Authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1].Greener M. The hidden problem of herb-drug interactions. Prescriber. 2016;27:22–27. [Google Scholar]
- [2].Chattopadhyay P, Das KE. Practice of Medicine (Alopathic). Kolkata: Rajendra Library; 2013. [Google Scholar]
- [3].Rakel D. Integrative Medicine E-Book. Albuquerque, New Mexico: Elsevier Health Sciences; 2017. [Google Scholar]
- [4].Zeller T, Muenstedt K, Stoll C, et al. Potential interactions of complementary and alternative medicine with cancer therapy in outpatients with gynecological cancer in a comprehensive cancer center. J Cancer Res Clin Oncol. 2013;139:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Posadzki P, Watson LK, Alotaibi A, et al. Prevalence of use of complementary and alternative medicine (CAM) by patients/consumers in the UK: systematic review of surveys. Clin Med (Lond). 2013;13:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xue CC. Traditional, complementary and alternative medicine: policy and public health perspectives. Bull World Health Organization. 2008;86:77–78. [Google Scholar]
- [7].Reid R, Steel A, Wardle J, et al. Complementary medicine use by the Australian population: a critical mixed studies systematic review of utilisation, perceptions and factors associated with use. BMC Complement Altern Med. 2016;16:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Center WM. Traditional Medicine Fact Sheet No 134, December 2008t. Available at: http://www.who.int/mediacentre/factsheets/fs134/en/. April 5, 2017 [Google Scholar]
- [9].Williamson EM. Drug interactions between herbal and prescription medicines. Drug Saf. 2003;26:1075–1092. [DOI] [PubMed] [Google Scholar]
- [10].Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. [DOI] [PubMed] [Google Scholar]
- [11].Wold RS, Lopez ST, Yau CL, et al. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J Am Diet Assoc. 2005;105:54–63. [DOI] [PubMed] [Google Scholar]
- [12].Singh DP, Borse SP, Nivsarkar M. Co-administration of quercetin with pantoprazole sodium prevents NSAID-induced severe gastroenteropathic damage efficiently: evidence from a preclinical study in rats. Exp Toxicol Pathol. 2017;69:17–26. [DOI] [PubMed] [Google Scholar]
- [13].Singh DP, Borse SP, Nivsarkar M. Overcoming the exacerbating effects of ranitidine on NSAID-induced small intestinal toxicity with quercetin: providing a complete GI solution. Chem Biol Interact. 2017;272:53–64. [DOI] [PubMed] [Google Scholar]
- [14].Pirotta M, Kotsirilos V, Brown J, et al. Complementary medicine in general practice: a national survey of GP attitudes and knowledge. Aust Fam Physician. 2010;39:946. [PubMed] [Google Scholar]
- [15].Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420. [DOI] [PubMed] [Google Scholar]
- [16].Winters M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern Med Rev. 2006;11:269–278. [PubMed] [Google Scholar]
- [17].Cassileth BR, Deng G. Complementary and alternative therapies for cancer. Oncologist. 2004;9:80–89. [DOI] [PubMed] [Google Scholar]
- [18].Abrams D, Weil A. Integrative Oncology. Oxford, United Kingdom: Oxford University Press; 2008. [Google Scholar]
- [19].Borse SP, Kamble BB. Effects of Ayurvedic rasayana botanicals on CYP3A4 isoenzyme system. J Integr Med. 2015;13:165–172. [DOI] [PubMed] [Google Scholar]
- [20].Patil D, Gautam M, Gairola S, et al. Effect of botanical immunomodulators on human CYP3A4 inhibition: implications for concurrent use as adjuvants in cancer therapy. Integr Cancer Ther. 2014;13:167–175. [DOI] [PubMed] [Google Scholar]
- [21].Dada Patil SJ, Bhushan, Patwardhan Botanical Drug Interactions: Case Studies From Traditional Medicines. Pune: LAP Lambert Academic Publishing; 2013. [Google Scholar]
- [22].Wesa K, Gubili J, Cassileth B. Integrative oncology: complementary therapies for cancer survivors. Hematol Oncol Clin North Am. 2008;22:343–353. [DOI] [PubMed] [Google Scholar]
- [23].Ubale S. President of India Inaugurates Integrated Cancer Treatment and Research Centre. J Ayurveda Integr Med. 2011;2:97. [Google Scholar]
- [24].Horrigan B, Lewis S, Abrams DI, et al. Integrative medicine in America—how integrative medicine is being practiced in clinical centers across the United States. Glob Adv Health Med. 2012;1:18–52. [Google Scholar]
- [25].Edelman D, Oddone EZ, Liebowitz RS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maizes V, Rakel D, Niemiec C. Integrative medicine and patient-centered care. Explore (NY). 2009;5:277–289. [DOI] [PubMed] [Google Scholar]
- [27].University of Maryland SoM. Cochrane CAM Field. Available at: http://www.compmed.umm.edu/cochrane.asp. April 7, 2017 [Google Scholar]
- [28].Belsey J, Snell T. What is evidence-based medicine? 2nd ed. Hayward Medical Communications Publisher, United Kingdom; 2009. Available: http://www.bandolier.org.uk/painres/download/whatis/ebm.pdf Accessed March 27, 2017. [Google Scholar]
- [29].Baer HA. The work of Andrew Weil and Deepak Chopra—two holistic health/new age gurus: a critique of the holistic health/new age movements. Med Anthropol Q. 2003;17:233–250. [DOI] [PubMed] [Google Scholar]
- [30].Drugs.Com, Warfarin. Available: https://www.drugs.com/pro/warfarin.html Accessed February 18, 2019. [Google Scholar]
- [31].The University of Arizona. The University of Arizona Center for Integrative Medicine. 2019. Available: https://integrativemedicine.arizona.edu/index.html Accessed February 18, 2019. [Google Scholar]
- [32].Harvard Medical School and Brigham and Women's Hospital. Osher Center for Integrative Medicine. Available: https://oshercenter.org Accessed February 18, 2019. [Google Scholar]
- [33].Shankar D. Conceptual framework for new models of integrative medicine. J Ayurveda Integr Med. 2010;1:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thomford NE, Dzobo K, Chopera D, et al. In vitro reversible and time-dependent CYP450 inhibition profiles of medicinal herbal plant extracts Newbouldia laevis and Cassia abbreviata: implications for herb-drug interactions. Molecules. 2016;21:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harrison RA, Holt D, Pattison DJ, et al. Who and how many people are taking herbal supplements? A survey of 21923 adults. Int J Vitam Nutr Res. 2004;74:183–186. [DOI] [PubMed] [Google Scholar]
- [36].Brownie S, Rolfe M. Health characteristics of older Australian dietary supplement users compared to non-supplement users. Asia Pac J Clin Nutr. 2004;13:365–371. [PubMed] [Google Scholar]
- [37].Bruno JJ, Ellis JJ. Herbal use among US elderly: 2002 national health interview survey. Ann Pharmacother. 2005;39:643–648. [DOI] [PubMed] [Google Scholar]
- [38].Hu Z, Yang X, Ho PCL, et al. Herb-drug interactions. Drugs. 2005;65:1239–1282. [DOI] [PubMed] [Google Scholar]
- [39].Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs. Drugs. 2009;69:1777–1798. [DOI] [PubMed] [Google Scholar]
- [40].Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370:185–191. [DOI] [PubMed] [Google Scholar]
- [41].SB. BDaJ. Biopharmaceutics and Pharmacokinetics—A Treatise. New Delhi, Vallabh Prakashan, 2009; 2:140–150. Available at: http://shodhganga.inflibnet.ac.in/bitstream/10603/2314/11/11_part%201.pdf. April 7, 2017 [Google Scholar]
- [42].Tucker GT, Houston JB, Huang SM. Optimizing drug development: Strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin Pharmacol Ther. 2001;70:103–114. [DOI] [PubMed] [Google Scholar]
- [43].Harle U, Gaikwad N. Emerging challenge of herb-drug interaction. Indian J Pharmaceutical Education. 2005;39:71–81. [Google Scholar]
- [44].Dürr D, Stieger B, Kullak-Ublick GA, et al. St John's wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. [DOI] [PubMed] [Google Scholar]
- [45].Henderson L, Yue Q, Bergquist C, et al. St John's wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Haddadian K, Haddadian K, Zahmatkash M. A review of Plantago plant. Indian J Trad Knowledge. 2014;13:681–685. [Google Scholar]
- [47].Fernandez N, Lopez C, Díez R, et al. Drug interactions with the dietary fiber Plantago ovata husk. Expert Opin Drug Metab Toxicol. 2012;8:1377–1386. [DOI] [PubMed] [Google Scholar]
- [48].Yang J-M, Ip S-P, Xian Y, et al. Impact of the herbal medicine Sophora flavescens on the oral pharmacokinetics of indinavir in rats: the involvement of CYP3A and P-glycoprotein. PLoS One. 2012;7:e31312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ge B, Zhang Z, Zuo Z. Updates on the clinical evidenced herb-warfarin interactions. Evid Based Complement Alternat Med. 2014;2014:957362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ingram KD, Dragosavac GB, Benner KG, et al. Risks of drug interactions with St. John's wort. Am J Gastroenterol. 2000;95:3323–3324. [DOI] [PubMed] [Google Scholar]
- [51].Brinker F. Herb contraindications and drug interactions. J Altern Complemnt Med. 2002;8:215–217. [Google Scholar]
- [52].Brinker FJ. Herb contraindications and drug interactions: with appendices addressing specific conditions and medicines. University of Michigan, USA: Eclectic Medical Publications; 1998. [Google Scholar]
- [53].Takanaga H, Ohnishi A, Matsuo H, et al. Inhibition of vinblastine efflux mediated by P-glycoprotein by grapefruit juice components in caco-2 cells. Biol Pharm Bull. 1998;21:1062–1066. [DOI] [PubMed] [Google Scholar]
- [54].Bailey D, Spence J, Munoz C, et al. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–269. [DOI] [PubMed] [Google Scholar]
- [55].Fuhr U. Drug interactions with grapefruit juice. Drug Safety. 1998;18:251–272. [DOI] [PubMed] [Google Scholar]
- [56].Ishihara K, Kushida H, Yuzurihara M, et al. Interaction of drugs and Chinese herbs: pharmacokinetic changes of tolbutamide and diazepam caused by extract of Angelica dahurica. J Pharm Pharmacol. 2000;52:1023–1029. [DOI] [PubMed] [Google Scholar]
- [57].Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–178. [DOI] [PubMed] [Google Scholar]
- [58].Piscitelli SC, Burstein AH, Welden N, et al. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–238. [DOI] [PubMed] [Google Scholar]
- [59].Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2003;55:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dandekar U, Chandra R, Dalvi S, et al. Analysis of a clinically important interaction between phenytoin and Shankhapushpi, an Ayurvedic preparation. J Ethnopharmacol. 1992;35:285–288. [DOI] [PubMed] [Google Scholar]
- [61].Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine. Expanded Commission E monographs. National Library of Australia, Australia: Integrative Medicine Communications; 2000. [Google Scholar]
- [62].Lambrecht JE, Hamilton W, Rabinovich A. Review of herb-drug interactions: documented and theoretical. US Pharmacist (USA). 2000;42, 44-45, 48-50, 53. Available: https://dspace2.creighton.edu/xmlui/handle/10504/67814. Acessed February 18, 2019 [Google Scholar]
- [63].Wooltorton E, Sibbald B. Ephedra/ephedrine: cardiovascular and CNS effects. CMAJ. 2002;166:633. [PMC free article] [PubMed] [Google Scholar]
- [64].Fugh-Berman A, Ernst E. Herb–drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lantz MS, Buchalter E, Giambanco V. St. John's wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol. 1999;12:7–10. [DOI] [PubMed] [Google Scholar]
- [66].Gordon JB. SSRIs and St. John's wort: possible toxicity? Am Fam Physician. 1998;57:950, 953. [PubMed] [Google Scholar]
- [67].Barbenel D, Yusufi B, O'shea D, et al. Mania in a patient receiving testosterone replacement post-orchidectomy taking St John's wort and sertraline. J Psychopharmacol. 2000;14:84–86. [DOI] [PubMed] [Google Scholar]
- [68].Dannawi M. Possible serotonin syndrome after combination of buspirone and St John's Wort. J Psychopharmacol. 2002;16:401. [DOI] [PubMed] [Google Scholar]
- [69].Jiang X, Williams KM, Liauw WS, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Singh IP, Bharate SB, Bhutani K. Interactions of herbs and food products with drugs grapefruit juice as an example. IJNPR. 2005;4:107–112. [Google Scholar]
- [71].Phillipson JD. Chinese Drugs of Plant Origin—Chemistry, Pharmacology and Use in Traditional and Modern Medicine: By W. Tang and G. Eisenbrand, Springer: New York, Heidelberg, 1992. DM248, p. 1056. ISBN 3-540-19309-X. Pergamon; 1993. [Google Scholar]
- [72].Chavez ML, Jordan MA, Chavez PI. Evidence-based drug–herbal interactions. Life Sci. 2006;78:2146–2157. [DOI] [PubMed] [Google Scholar]
- [73].Mohammed Abdul M, Jiang X, Williams K, et al. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br J Pharmacol. 2008;154:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Borrelli F, Capasso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res. 2007;51:1386–1397. [DOI] [PubMed] [Google Scholar]
- [75].Majewski M. Allium sativum: facts and myths regarding human health. Rocz Panstw Zakl Hig. 2014;65:1–8. [PubMed] [Google Scholar]
- [76].Shaw D, Leon C, Kolev S, et al. Traditional remedies and food supplements. Drug Safety. 1997;17:342–356. [DOI] [PubMed] [Google Scholar]
- [77].Galluzzi S, Zanetti O, Binetti G, et al. Coma in a patient with Alzheimer's disease taking low dose trazodone and Ginkgo biloba. J Neurol Neurosurg Psychiatry. 2000;68:679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Matthews MK. Association of Ginkgo biloba with intracerebral hemorrhage. Neurology. 1998;50:1933–1934. [DOI] [PubMed] [Google Scholar]
- [79].Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch Intern Med. 1998;158:2200–2211. [DOI] [PubMed] [Google Scholar]
- [80].Jones B, Runikis A. Interaction of ginseng with phenelzine. J Clin Psychopharmacol. 1987;7:201–202. [DOI] [PubMed] [Google Scholar]
- [81].Anoja S, Ji A, Chun-Su Y. Ginseng pharmacology. Biochem Pharmacol. 1999;58:1685–1693. [DOI] [PubMed] [Google Scholar]
- [82].Chan TY. Interaction between warfarin and danshen (Salvia miltiorrhiza). Ann Pharmacother. 2001;35:501–504. [DOI] [PubMed] [Google Scholar]
- [83].Tam L, Chan T, Leung WK, et al. Warfarin interactions with Chinese traditional medicines: danshen and methyl salicylate medicated oil. Aust N Z J Med. 1995;25:258. [DOI] [PubMed] [Google Scholar]
- [84].Aslam M, Stockley I. Interaction between curry ingredient (karela) and drug (chlorpropamide). Lancet. 1979;313:607. [DOI] [PubMed] [Google Scholar]
- [85].Katchamart S, Stresser DM, Dehal SS, et al. Concurrent flavin-containing monooxygenase down-regulation and cytochrome P-450 induction by dietary indoles in rat: implications for drug-drug interaction. Drug Metab Dispos. 2000;28:930–936. [PubMed] [Google Scholar]
- [86].Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].O’Reilly R, Welling P, Wagner J. Pharmacokinetics of warfarin following intravenous administration to man. Thromb Diath Haemorrh. 1970;25:178–186. [PubMed] [Google Scholar]
- [88].Hewick DS, McEwen J. Plasma half-lives, plasma metabolites and anticoagulant efficacies of the enantiomers of warfarin in man. J Pharm Pharmacol. 1973;25:458–465. [DOI] [PubMed] [Google Scholar]
- [89].Mahéo K, Morel F, Langouët S, et al. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- [90].Hodges PJ, Kam P. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57:889–899. [DOI] [PubMed] [Google Scholar]
- [91].Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Patel JA, Gohil KJ. Warfarin-herb interactions: a review and study based on assessment of clinical case reports in literature. B Latinoam Caribe Pl. 2008;7. [Google Scholar]
- [93].Abdul MIM, Jiang X, Williams KM, et al. Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol. 2010;69:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gurley BJ, Gardner SF, Hubbard MA, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. [DOI] [PubMed] [Google Scholar]
- [95].Ioannides C. Xenobiotic metabolism and bioactivation by cytochromes P-450. Biomolecular free radical toxicity: causes and prevention. 2000:103–144. [Google Scholar]
- [96].Rendic S, Carlo FJD. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29:413–580. [DOI] [PubMed] [Google Scholar]
- [97].Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. [DOI] [PubMed] [Google Scholar]
- [98].Bioinformatics and Drug Design Group. Therapeutic Target Database (TTD). Avialable: www.bidd.nus.edu.sg/group/cjttd/ Accessed February 18, 2019. [Google Scholar]
- [99].RCSB PDB Protein Data Bank. Research Collaboratory for Structural Bioinformatics: Rutgers and UCSD/SDSC. Avialable: https://www.rcsb.org Accessed February 18, 2019. [Google Scholar]
- [100].Good Practice in Traditional Chinese Medicine Research Association. GPTCM research Asoociation. Available: www.gp-tcm.org/links/ Accessed February 18, 2019. [Google Scholar]
- [101].Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340:715–722. [PMC free article] [PubMed] [Google Scholar]
- [102].Food and Drug Administration. Guidance for industry: drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations. Center for Drug Evaluation and Research (CDER); 2012. [Google Scholar]
- [103].Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. [DOI] [PubMed] [Google Scholar]
- [104].Zhou S, Gao Y, Jiang W, et al. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003;35:35–98. [DOI] [PubMed] [Google Scholar]
- [105].Martignoni M, Groothuis GM, De Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. [DOI] [PubMed] [Google Scholar]
- [106].Kumar GN, Surapaneni S. Role of drug metabolism in drug discovery and development. Med Res Rev. 2001;21:397–411. [DOI] [PubMed] [Google Scholar]
- [107].Li CG, Yang L, Zhou S-F. Interactions between Chinese herbal medicines and drugs. Aust J Acupunct Chin Med. 2007;2:17. [Google Scholar]
- [108].Teyssier C, Guenot L, Suschetet M, et al. Metabolism of diallyl disulfide by human liver microsomal cytochromes P-450 and flavin-containing monooxygenases. Drug Metab Dispos. 1999;27:835–841. [PubMed] [Google Scholar]
- [109].Dalvi R, Dalvi P. Comparison of the effects of piperine administered intragastrically and intraperitoneally on the liver and liver mixed-function oxidases in rats. Drug Metabol Drug Interact. 1991;9:23–30. [DOI] [PubMed] [Google Scholar]
- [110].Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- [111].Premdas PD, Bowers RJ, Forkert P-G. Inactivation of hepatic CYP2E1 by an epoxide of diallyl sulfone. J Pharmacol Exp Ther. 2000;293:1112–1120. [PubMed] [Google Scholar]
- [112].Jin L, Baillie TA. Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chem Res Toxicol. 1997;10:318–327. [DOI] [PubMed] [Google Scholar]
- [113].Chen X-W, Serag ES, Sneed KB, et al. Herbal bioactivation, molecular targets and the toxicity relevance. Chem Biol Interact. 2011;192:161–176. [DOI] [PubMed] [Google Scholar]
- [114].Zhou S, Koh H-L, Gao Y, et al. Herbal bioactivation: the good, the bad and the ugly. Life Sci. 2004;74:935–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kouzi SA, McMurtry RJ, Nelson SD. Hepatotoxicity of germander (Teucrium chamaedrys L.) and one of its constituent neoclerodane diterpenes teucrin A in the mouse. Chem Res Toxicol. 1994;7:850–856. [DOI] [PubMed] [Google Scholar]
- [116].Newall CA, Anderson LA, Phillipson JD. Herbal medicines. A guide for health-care professionals. London, UK: The Pharmaceutical Press; 1996. [Google Scholar]
- [117].Huang SM. ea. Adverse Drug Reactions and Pharmacokinetic Drug Interactions. Chapter 20. Adverse Drug Reactions and Drug Interactions in Part I (Section 4), Pharmacology and Therapeutics: Principles to Practice, Waldman SA, Terzic A, Eds., Philadelphia, PA: Elsevier, 2009. [Google Scholar]
- [118].Zhang L, Zhang Y, Strong J, et al. A regulatory viewpoint on transporter-based drug interactions. Xenobiotica. 2008;38:709–724. [DOI] [PubMed] [Google Scholar]
- [119].Pang KS, Rodrigues AD, Peter RM. Enzyme- and Transporter-based Drug-Drug Interactions. New York, NY: Springer; 2014. [Google Scholar]
- [120].Dash RP, Jayachandra Babu R, Srinivas NR. Therapeutic potential and utility of elacridar with respect to p-glycoprotein inhibition: an insight from the published in vitro, preclinical and clinical studies. Eur J Drug Metab Pharmacokinet. 2017;42:915–933. [DOI] [PubMed] [Google Scholar]
- [121].Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter 1. Annu Rev Pharmacol Toxicol. 1999;39:361–398. [DOI] [PubMed] [Google Scholar]
- [122].Dash RP, Ellendula B, Agarwal M, et al. Increased intestinal P-glycoprotein expression and activity with progression of diabetes and its modulation by epigallocatechin-3-gallate: evidence from pharmacokinetic studies. Eur J Pharmacol. 2015;767:67–76. [DOI] [PubMed] [Google Scholar]
- [123].Schinkel AH, Wagenaar E, Van Deemter L, et al. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hunter J, Hirst BH. Intestinal secretion of drugs. The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv Drug Deliv Rev. 1997;25:129–157. [Google Scholar]
- [125].Morris ME, Zhang S. Flavonoid–drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. [DOI] [PubMed] [Google Scholar]
- [126].Wong IL, Chan K-F, Tsang KH, et al. Modulation of multidrug resistance protein 1 (MRP1/ABCC1)-mediated multidrug resistance by bivalent apigenin homodimers and their derivatives. J Med Chem. 2009;52:5311–5322. [DOI] [PubMed] [Google Scholar]
- [127].Mandery K, Balk B, Bujok K, et al. Inhibition of hepatic uptake transporters by flavonoids. Eur J Pharm Sci. 2012;46:79–85. [DOI] [PubMed] [Google Scholar]
- [128].Gupta SK, Manfro RC, Tomlanovich SJ, et al. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J Clin Pharmacol. 1990;30:643–653. [DOI] [PubMed] [Google Scholar]
- [129].Gupta SK, Benet LZ. Absorption kinetics of cyclosporine in healthy volunteers. Biopharm Drug Dispos. 1989;10:591–596. [DOI] [PubMed] [Google Scholar]
- [130].Gupta SK, Benet LZ. High-fat meals increase the clearance of cyclosporine. Pharm Res. 1990;7:46–48. [DOI] [PubMed] [Google Scholar]
- [131].Benet LZ. The drug transporter—metabolism alliance: uncovering and defining the interplay. Mol Pharm. 2009;6:1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–134. [DOI] [PubMed] [Google Scholar]
- [133].Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49:311–318. [PubMed] [Google Scholar]
- [134].Schuetz EG, Schinkel AH, Relling MV, et al. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc Natl Acad Sci U S A. 1996;93:4001–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Floren LC, Bekersky I, Benet LZ, et al. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62:41–49. [DOI] [PubMed] [Google Scholar]
- [136].Hebert MF, Fisher RM, Marsh CL, et al. Effects of rifampin on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol. 1999;39:91–96. [DOI] [PubMed] [Google Scholar]
- [137].Floren L, Christians U, Zimmerman J, et al. Sirolimus oral bioavailability increases ten-fold with concomitant ketoconazole. Clin Pharmacol Therapeut. 1999;65:159. [Google Scholar]
- [138].Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. [DOI] [PubMed] [Google Scholar]
- [139].Chan LM, Cooper AE, Dudley AL, et al. P-glycoprotein potentiates CYP3A4-mediated drug disappearance during Caco-2 intestinal secretory detoxification. J Drug Target. 2004;12:405–413. [DOI] [PubMed] [Google Scholar]
- [140].Tandon VR, Kapoor B, Bano G, et al. P-glycoprotein: Pharmacological relevance. Indian J Pharmacol. 2006;38:13. [Google Scholar]
- [141].Kato M, Chiba K, Hisaka A, et al. The intestinal first-pass metabolism of substrates of CYP3A4 and P-glycoprotei—quantitative analysis based on information from the literature. Drug Metab Pharmacokinet. 2003;18:365–372. [DOI] [PubMed] [Google Scholar]
- [142].Zhou S-F, Zhou Z-W, Li C-G, et al. Identification of drugs that interact with herbs in drug development. Drug Discov Today. 2007;12:664–673. [DOI] [PubMed] [Google Scholar]
- [143].Food and Drug Administration. 2012. Draft guidance for industry—drug interaction studies, study design, data analysis, implications for dosing, and labeling recommendations. Food and Drug Administration, Silver Spring, MD. Available at: http://www fda gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362 pdf Accessed on December 16, 2013. [Google Scholar]
- [144].Homeostasis and Regulation. 2015. Avalable at: http://www.ck12.org/book/CK-12-Biology-I-Honors-CA-DTI3/r2/section/19.2/ Accessed August 27, 2015. [Google Scholar]
- [145].Donath MY, Böni-Schnetzler M, Ellingsgaard H, et al. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–267. [DOI] [PubMed] [Google Scholar]
- [146].Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012;51:481–499. [DOI] [PubMed] [Google Scholar]
- [147].Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Curr Drug Metab. 2012;13:1327–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Pickup J, Crook M. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. [DOI] [PubMed] [Google Scholar]
- [149].Sickmann HM, Waagepetersen HS, Schousboe A, et al. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab. 2010;30:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Olchovsky D, Bruno JF, Wood TL, et al. Altered pituitary growth hormone (GH) regulation in streptozotocin-diabetic rats: a combined defect of hypothalamic somatostatin and GH-releasing factor. Endocrinology. 1990;126:53–61. [DOI] [PubMed] [Google Scholar]
- [151].Yasui T, Murakami T, Maeda T, et al. Involvement of gonadal steroid hormone disturbance in altered prolactin receptor gene expression in the liver of diabetic mice. J Endocrinol. 1999;161:33–40. [DOI] [PubMed] [Google Scholar]
- [152].Oltmanns KM, Dodt B, Schultes B, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325–331. [DOI] [PubMed] [Google Scholar]
- [153].Bitar M, Koulu M, Rapoport SI, et al. Diabetes-induced alteration in brain monoamine metabolism in rats. J Pharmacol Exp Ther. 1986;236:432–437. [PubMed] [Google Scholar]
- [154].Di Giulio A, Tenconi B, La Croix R, et al. Denervation and hyperinnervation in the nervous system of diabetic animals. II Monoaminergic and peptidergic alterations in the diabetic encephalopathy. J Neurosci Res. 1989;24:362–368. [DOI] [PubMed] [Google Scholar]
- [155].Benko B. Influence of diabetes on cytochrome P450 enzyme mediated drug metabolism–case studies on diclofenac and K-48. PhD Thesis. Semmelweis University. Budapest, 2008. Available: citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.418.8648&rep=rep1&type=pdf Acessed February 18, 2019. [Google Scholar]
- [156].DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Hull RL, Westermark GT, Westermark P, et al. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. [DOI] [PubMed] [Google Scholar]
- [158].Shapiro LE, Shear NH. Drug interactions: proteins, pumps, and P-450s. J Am Acad Dermatol. 2002;47:467–488. [DOI] [PubMed] [Google Scholar]
- [159].Veldhuis JD. Changes in pituitary function with ageing and implications for patient care. Nat Rev Endocrinol. 2013;9:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. [DOI] [PubMed] [Google Scholar]
- [162].Mansi R, Fleischmann A, Mäcke HR, et al. Targeting GRPR in urological cancers—from basic research to clinical application. Nat Rev Urol. 2013;10:235–244. [DOI] [PubMed] [Google Scholar]
- [163].Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- [164].Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. [DOI] [PubMed] [Google Scholar]
- [165].Duerkop BA, Hooper LV. Resident viruses and their interactions with the immune system. Nat Immunol. 2013;14:654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Lecuit M, Eloit M. The human virome: new tools and concepts. Trends Microbiol. 2013;21:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Izzo AA. Herb–drug interactions: an overview of the clinical evidence. Fundam Clin Pharmacol. 2005;19:1–16. [DOI] [PubMed] [Google Scholar]
- [168].Izzo AA, Borrelli F, Capasso R. Herbal medicine: the dangers of drug interaction. Trends Pharmacol Sci. 2002;23:358–359. [DOI] [PubMed] [Google Scholar]
- [169].Zhou S, Lim LY, Chowbay B. Herbal Modulation of P-Glycoprotein. Drug Metab Rev. 2004;36:57–104. [DOI] [PubMed] [Google Scholar]
- [170].Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31:326–343. [DOI] [PubMed] [Google Scholar]
- [171].Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. [DOI] [PubMed] [Google Scholar]
- [172].Hutter M. In silico prediction of drug properties. Curr Med Chem. 2009;16:189–202. [DOI] [PubMed] [Google Scholar]
- [173].Wilson A, White A, Mueller R. Role of predictive metabolism and toxicity modeling in drug discovery--a summary of some recent advancements. Curr Opin Drug Discov Devel. 2003;6:123–128. [PubMed] [Google Scholar]
- [174].De Groot MJ, Kirton SB, Sutcliffe MJ. In silico methods for predicting ligand binding determinants of cytochromes P450. Curr Top Med Chem. 2004;4:1803–1824. [DOI] [PubMed] [Google Scholar]
- [175].De Graaf C, Vermeulen NP, Feenstra KA. Cytochrome P450 in silico: an integrative modeling approach. J Med Chem. 2005;48:2725–2755. [DOI] [PubMed] [Google Scholar]
- [176].Krejsa CM, Horvath D, Rogalski SL, et al. Predicting ADME properties and side effects: the BioPrint approach. Curr Opin Drug Discov Devel. 2003;6:470–480. [PubMed] [Google Scholar]
- [177].Harris D. In silico predictive metabolism: a structural/electronic filter method. Curr Opin Drug Discov Devel. 2004;7:43–48. [PubMed] [Google Scholar]
- [178].Koul S, Koul JL, Taneja SC, et al. Structure–activity relationship of piperine and its synthetic analogues for their inhibitory potentials of rat hepatic microsomal constitutive and inducible cytochrome P450 activities. Bioorg Med Chem. 2000;8:251–268. [DOI] [PubMed] [Google Scholar]
- [179].Anonymous. Chapter 7 In Silico Studies Of Selected Biomarkers and Controls Within CYP3A4 and CYP2D6 Active Site. PhD Thesis. SRM University. Available: shodhganga.inflibnet.ac.in/bitstream/10603/77660/10/chapter%207.pdf Accessed February 18, 2019. [Google Scholar]
- [180].Ekins S, Ring BJ, Grace J, et al. Present and future in vitro approaches for drug metabolism. J Pharmacol Toxicol Methods. 2000;44:313–324. [DOI] [PubMed] [Google Scholar]
- [181].Eddershaw PJ, Dickins M. Advances in in vitro drug metabolism screening. Pharm Sci Technolo Today. 1999;2:13–19. [DOI] [PubMed] [Google Scholar]
- [182].Moore LB, Goodwin B, Jones SA, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Goodwin B, Moore LB, Stoltz CM, et al. Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol. 2001;60:427–431. [PubMed] [Google Scholar]
- [184].LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. [DOI] [PubMed] [Google Scholar]
- [185].Crespi CL, Penman BW. Use of cDNA-expressed human cytochrome P450 enzymes to study potential drug-drug interactions. Adv Pharmacol. 1997;43:171–188. [DOI] [PubMed] [Google Scholar]
- [186].Li AP, Maurel P, Gomez-Lechon MJ, et al. Preclinical evaluation of drug—drug interaction potential: present status of the application of primary human hepatocytes in the evaluation of cytochrome P450 induction. Chem Biol Interact. 1997;107:5–16. [DOI] [PubMed] [Google Scholar]
- [187].Li AP, Bode C, Sakai Y. A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem Biol Interact. 2004;150:129–136. [DOI] [PubMed] [Google Scholar]
- [188].Li AP. In vitro evaluation of human xenobiotic toxicity: scientific concepts and the novel integrated discrete multiple cell co-culture (IdMOC) technology. ALTEX. 2008;25:43–49. [DOI] [PubMed] [Google Scholar]
- [189].Li AP, Uzgare A, Saminathan H, Doshi U. Integrated Discrete Multiple Organ Co-culture (IdMOC) System for the Evaluation of Multiple Organ Drug Distribution, Drug Metabolism, and Organ Specific Drug Toxicity. Drug Metabolism Reviews; 2011: Informa Healthcare Telephone House, 69-77 Paul Street, London EC2A 4LQ, England. pp.141–142. [Google Scholar]
- [190].Obach RS. Metabolism of ezlopitant, a nonpeptidic substance P receptor antagonist, in liver microsomes: enzyme kinetics, cytochrome P450 isoform identity, and in vitro-in vivo correlation. Drug Metab Dispos. 2000;28:1069–1076. [PubMed] [Google Scholar]
- [191].Frye RF, Matzke GR, Adedoyin A, et al. Validation of the five-drug “Pittsburgh cocktail” approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther. 1997;62:365–376. [DOI] [PubMed] [Google Scholar]
- [192].Dierks EA, Stams KR, Lim H-K, et al. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab Dispos. 2001;29:23–29. [PubMed] [Google Scholar]
- [193].Zhu B, Ou-Yang D-S, Chen X-P, et al. Assessment of cytochrome P450 activity by a five-drug cocktail approach. Clin Pharmacol Ther. 2001;70:455–461. [DOI] [PubMed] [Google Scholar]
- [194].Wang Z, Gorski JC, Hamman MA, et al. The effects of St John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Therapeut. 2001;70:317–326. [PubMed] [Google Scholar]
- [195].Palmer J, Scott R, Gibson A, et al. An interaction between the cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A). Br J Clin Pharmacol. 2001;52:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Zhou S, Huang M, Xu A, et al. Prediction of herb–drug metabolic interactions: a simulation study. Phytother Res. 2005;19:464–471. [DOI] [PubMed] [Google Scholar]
- [197].Zhou S, Chan E, Li SC, et al. Predicting pharmacokinetic herb-drug interactions. Drug Metabol Drug Interact. 2004;20:143–158. [DOI] [PubMed] [Google Scholar]
- [198].Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47:1469–1479. [DOI] [PubMed] [Google Scholar]
- [199].Aquilante CL, Kosmiski LA, Bourne DW, et al. Impact of the CYP2C8∗ 3 polymorphism on the drug–drug interaction between gemfibrozil and pioglitazone. Br J Clin Pharmacol. 2013;75:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Melzer D, Raven A, Ling T, et al. Pharmacogenetics: policy needs for personal prescribing. J Health Serv Res Policy. 2005;10:40–44. [DOI] [PubMed] [Google Scholar]
- [201].Pestian J, Spencer M, Matykiewicz P, et al. Personalizing drug selection using advanced clinical decision support. Biomed Inform Insights. 2009;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Food and Drug Administration. Drug Interaction Studies–Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Bethesda, MD: Center for Drug Evaluation and Research, FDA; 2012. [Google Scholar]
- [203].Borse SP, Shripat A, Sharma S, Shrivastava N, Nivsarkar M. Ayurveda based novel integrative approach and/or technique to predict chemical constituents from any substance and/or sample (Indian Patent Application No.: 201621021084). [Google Scholar]
- [204].Borse SP, Shripat A, Sharma S, et al. Ayurnization (Indian Trademark Application No.: 201621021084). [Google Scholar]
- [205].Nanal VM, Nanal RM. Evaluation of custard based on Ayurvedic principles. Anc Sci Life. 1992;12:267. [PMC free article] [PubMed] [Google Scholar]
- [206].Satyavati G. Ayurvedic Concepts of Nutrition and Dietary Guidelines for Promoting/Preserving Health and Longevity. 2008;Nutrition Foundation of India, New Delhi: 210–228. [Google Scholar]
- [207].Rath SK, Panja AK, Nagar L, et al. The scientific basis of rasa (taste) of a substance as a tool to explore its pharmacological behavior. Anc Sci Life. 2014;33:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Rastogi S. Building bridges between Ayurveda and modern science. Int J Ayurveda Res. 2010;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Katiyar C. Ayurpathy: a modern perspective of Ayurveda. Ayu. 2011;32:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Sujatha V. Innovation within and between traditions dilemma of traditional medicine in contemporary India. Sci Technol Society. 2011;16:191–213. [Google Scholar]
- [211].Nanal MVNR. Evolution of custard on Ayurvedic principles. Anc Sci Life. 1992;12:267–270. [PMC free article] [PubMed] [Google Scholar]
- [212].Hudson TS, Hartle DK, Hursting SD, et al. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67:8396–8405. [DOI] [PubMed] [Google Scholar]
- [213].Shetti S, Kumar CD, Sriwastava NK, et al. Pharmacovigilance of herbal medicines: Current state and future directions. Pharmacogn Mag. 2011;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Shaw D, Graeme L, Pierre D, et al. Pharmacovigilance of herbal medicine. J Ethnopharmacol. 2012;140:513–518. [DOI] [PubMed] [Google Scholar]


