Abstract
Levothyroxine (LT4) therapy has a long history, a well-defined pharmacological profile and a favourable safety record in the alleviation of hypothyroidism. However, questions remain in defining the threshold for the requirement of treatment in patients with subclinical hypothyroidism, assessing the dose adequacy of the drug, and selecting the best treatment mode (LT4 monotherapy versus liothyronine [LT3]/LT4 combinations) for subpopulations with persisting complaints. Supplied as a prodrug, LT4 is enzymatically converted into the biologically more active thyroid hormone, triiodothyronine (T3). Importantly, tetraiodothyronine (T4) to T3 conversion efficiency may be impaired in patients receiving LT4, resulting in a loss of thyroid-stimulating hormone (TSH)-mediated feed-forward control of T3, alteration of the interlocking equilibria between serum concentrations of TSH, free thyroxine (FT4), and free triiodothyonine (FT3), and a decrease in FT3 to FT4 ratios. This downgrades the value of the TSH reference system derived in thyroid health for guiding the replacement dose in the treatment situation. Individualised conditionally defined setpoints may therefore provide appropriate biochemical targets to be clinically tested, together with a stronger focus on clinical presentation and future endpoint markers of tissue thyroid state. This cautionary note encompasses the use of aggregated statistical data from clinical trials which are not safely applicable to the individual level of patient care under these circumstances.
Keywords: ergodicity, hypothyroidism, LT4 treatment, personalised medicine, setpoint
Introduction
Levothyroxine (LT4) is one of the most widely used drugs worldwide. Its prescription has increased over the last 15 years and is projected to increase further over the next decade according to nationwide data from the National Health Service in the United Kingdom.1 Its primary indication as a prescription drug is in the treatment of hypothyroidism.2–4 The synthetic hormone has almost completely replaced earlier preparations derived from desiccated thyroid extracts (DTE) of bovine or porcine origin.5,6 The treatment goal can be generally defined as substitution of a hormonal deficit, aiming at restoring the previous euthyroid state.7 The drug has a long history of successful use and favourable safety record since its broad introduction to clinical medicine in the 1970s.6,8 Nevertheless, the use of LT4 still raises unresolved issues and substantial controversy.9–11 Important areas of the current debate raise simple questions that are not so easy to answer12. For example, when to initiate substitution, which is equivalent to asking at what point a person is deemed truly hypothyroid, requiring hormonal substitution for optimum health? What is the adequate dose and therapeutic range for LT4, as hormones, unlike other drugs, do not work on a fixed-dose regimen, rather requiring variable dosing adjusted to the specific needs of each patient? Which may be the best mode of treatment, as LT4 replaces only the main hormone physiologically secreted, but not its sister hormone triiodothyronine (T3) co-secreted with tetraiodothyronine (T4) in a lesser amount by the human thyroid13,14? Apart from its well-known pharmacological properties, the practical administration of LT4 as a drug requires consideration of two main aspects: evaluating the presence of hypothyroidism to define indication for treatment and assessing subsequent success in the restoration of euthyroidism.
Methods
A literature search was performed on PubMed in April 2019 with the English search terms ‘hypothyroidism AND levothyroxine treatment’ and a focus on more recent publications within the last 5 years, retrieving 1569 references. There were 225 published prospective and 287 retrospective studies identified over the last decade. Titles, abstracts, and selective full texts were screened by the authors with a focus on obtaining novel insights into pharmacological properties, efficacy, efficiency in practical use, and adverse effects. Thematic review articles were prioritised over citing multiple single studies. Unlike in previous systematic reports and meta-analyses on the topic, prior classification according to evidence-based medicine was not a limitation for selection, because high-ranking evidence may be equally compromised for more fundamental reasons and due to particularities unique to thyroid parameters, discussed later, such as individuality index, lack of ergodicity, and interlocking homeostatic equilibria. When dealing with current controversies and unresolved issues, we employed mathematical principles, methodological rigour, and subjective judgment.
Pharmacological properties of LT4
LT4 is a long-lived drug with a plasma half-life of approximately 7 days, which amongst other influences is modulated by thyroid function itself.15 The drug is commonly administered as a single oral dose taken in the fasting state 30 minutes before breakfast in the morning.16 Bedtime use is less common but may be equally effective according to some studies.17–20 LT4 is readily absorbed via an active, energy-dependent, and saturable mechanism in the small intestine (duodenum, jejunum, and ileum) with an absorption rate >80%, although some issues may occasionally arise in the presence of gastrointestinal diseases, for example, coeliac disease, or due to the interferences of other drugs.7,21 Replacement required in athyreotic patients may be commenced at full dose (approximately 1.6 μg/kg body weight) in most cases, except for patients with severe or unstable cardiac disease, and individually adjusted during follow-up according to clinical symptoms and biochemical parameters.7,22 In the presence of functional residual thyroid tissue, substitution with lower doses may suffice. Of note, biochemical markers should be monitored in equilibrium, which for thyroid-stimulating hormone (TSH) is only achieved with a delay of 4–6 weeks after the commencement of LT4 treatment or a change in dose. The volume of LT4 distribution is approximately 0.2 L/kg and the metabolic clearance is approximately 1.32 L/d, occurring mainly in the liver, kidney, brain and muscle tissue.15,23 In the circulation, LT4 is reversibly bound in excess of 99% to three plasma proteins, namely thyroxine-binding globulin, transthyretin, and albumin.15 Only a minute fraction of the free unbound hormone is biologically active. Being mainly a prodrug, LT4 is activated though metabolisation by enzymatic 5′-deiodination into the biologically more active derivative T3, due to the actions of two types of iodothyronine deiodinases, type 1 and type 2, which are differentially expressed by various organs.24,25 Another deiodinase (type 3) catalyses the inner ring deiodination of T4, inactivating it to reverse triiodothyronine (rT3), and also of T3, degrading it to 3,3′-diiodothyronine (T2).24 T4 and T3 are actively transported across the cell membrane through specific thyroid hormone transporters, and within the cell T3 is subject to further trafficking to the nucleus, where it binds with an approximately 10 times higher affinity than T4 in a complex with thyroid hormone receptors to DNA.26,27 It thereby up- or down-regulates a plethora of genes to exert its genomic actions.28 Although genomic T4 actions termed type 1 signalling according to a recently proposed classification by Flamant and colleagues29 depend on T3 activating pathways, non-genomic effects of the hormone – although still not well understood – also exist, mostly mediated by indirect binding to nuclear receptors via adaptor proteins (type 2 signalling), binding to cytosolic receptors (type 3 signalling) and to membrane-based integrin receptors (type 4 signalling).30 Of note, these signalling types are differently controlled by T4 and T3, and the feedback loop is closed by type 1 signalling only.31
LT4 as a pharmacological compound is synthesised to be identical to the natural hormone,32 and, for that reason, is generally well tolerated when used correctly. Although the drug has been proven efficacious in the treatment of hypothyroidism,2,4 optimum efficiency in eliminating hypothyroidism and fully restoring euthyroidism as a treatment goal is more difficult to ascertain. Lower quality-of-life scores were reported in studies by patients receiving LT4, compared to the healthy population.33–36
Adverse effects arise mainly from either underdosing, not restoring a euthyroid state, or overdose, mimicking signs and symptoms of hyperthyroidism. Symptoms of overtreatment revert after pausing and adjusting daily LT4 dose. Although intolerance to the base formulation may rarely occur, interference with other drugs or comorbidities is more prevalent.21 For treating severe conditions of hypothyroidism and in the case of resorption problems, injectable and liquid formulations are available.37,38 It should be noted that although different brands of LT4 share the same pharmacological substance, they may differ in their formulations and galenics and are therefore not readily interchangeable.39
Disease definition of hypothyroidism
Hypothyroidism can be best defined formally as a state of undersupply of the body with thyroid hormones and/or a resulting lack of response of the organism to hormonal actions. This definition has been frequently used in older text books.40 Depending on the severity of the deficiency, the manifestation of clinical disease may vary from an entirely asymptomatic presentation to the typical yet complex hypothyroid syndrome produced by the many signs and symptoms of thyroid hormone deficiencies, and, more rarely, to the extreme of myxoedemic coma. Clinical scores have been developed but have demonstrated only limited sensitivity and specificity.41,42
Hypothyroidism is a prevalent endocrine disease with a female preponderance of four-fold, compared to men, increasing with older age. Disease prevalence in European countries and the United States is estimated to be 1–3% for overt hypothyroidism and 6–10% for subclinical hypothyroidism.43–48 The disease is commonly classified according to the localisation of the deficiency, primary hypothyroidism resulting from thyroid gland failure to produce sufficient amounts of thyroid hormones. Secondary hypothyroidism arises from regulatory deficiencies and a lack of TSH stimulation to the thyroid gland in pituitary failure – frequently accompanied by other pituitary deficiencies such as hypocortisolism and hypogonadism.49 Tertiary hypothyroidism may occur in the presence of hypothalamic disorders.49 Despite an adequate glandular supply of the body with T4, decreased responsiveness of target organs may be caused by hereditary or acquired syndromes of resistance to thyroid hormone or failure of conversion of T4 into T3.50–54
Ambiguities in the disease definition have left practitioners confused as to the scope of pertinent recommendations. The most prevalent aetiology of primary hypothyroidism in iodine-sufficient areas is a chronic autoimmune process, autoimmune thyroiditis, or Hashimoto’s thyroiditis if accompanied by an enlarged thyroid gland despite a hormonal deficit in thyroid hormone production.45,48 The second frequent cause is ablative or disruptive therapeutic measures such as thyroidectomy, radioiodine treatment, or medical interventions. It is prognostically important both to unequivocally establish the presence of a thyroid disease during the diagnostic workup and to differentiate the aetiology and origin of the disease. Transient forms of hypothyroidism such as deQuervain thyroiditis, silent thyroiditis, TSH-R-Ab-mediated Hashimoto’s thyroiditis or Hashitoxicosis are infrequent, but have to be recognised, as those conditions may not require lifelong thyroid hormone replacement. It is controversial if treatment with thyroid hormones is beneficial in non-thyroidal illness syndrome with low T3 or if it confers detrimental effects, as suggested by some studies.55,56
Contraindications are few, including thyrotoxicosis of any aetiology, the early phase of acute myocardial infarction, and a hypersensitivity to levothyroxine sodium or any component of the formulation. In patients with adrenal insufficiency (Schmidt syndrome or pituitary failure), LT4 administration may cause an acute life-threatening adrenal crisis,57 and the treatment must therefore be deferred until the adrenal insufficiency has been stably corrected.
Laboratory-based definition of hypothyroidism
The thyroid gland is physiologically not independently capable of maintaining adequate thyroid hormone supply, requiring thyroid hormone production rates to be raised by pituitary TSH stimulation to both escape hypothyroidism and increase T4 to T3 conversion efficiency.58 Thyroid hormones, in turn, exert a negative feedback on pituitary TSH secretion to prevent their overproduction.59 Modern thyroid test strategies have exploited the relationship between TSH and thyroid hormones for diagnostic purposes.60 Other TSH receptor agonists may mimic the actions of TSH in an uncontrolled manner, such as human chorionic gonadotropin in pregnancy and TSH receptor antibodies in Graves’ disease.61,62 Notably, the contemporary definition of hypothyroidism is exclusively laboratory-based, relying heavily on the measurement of pituitary TSH.7 Formally, a confirmed elevated serum TSH level in the presence of free thyroxine (FT4) concentrations still within the population reference range is termed subclinical hypothyroidism, whereas a combination of FT4 measurement below its range with an elevated TSH concentration defines primary hypothyroidism. Although TSH measurement using modern assay technology is advantageous in providing a sensitive screening tool for primary hypothyroidism, this TSH-centred strategy applies only to the diagnosis of primary hypothyroidism, failing in the cases of secondary hypothyroidism and non-thyroidal illness.63
The TSH-centred strategy has not remained unchallenged, and the deficiencies of this approach in its lack of diagnostic specificity and reliability have been reviewed elsewhere.64 The problem is more fundamentally rooted in the nature of thyroid hormone activity and the principles guiding their regulation.59,65,66 Consequently, recent guidelines have separated therapeutic targets from diagnostic criteria, recommending to withhold LT4 substitution in a substantial portion of patients with subclinical hypothyroidism until TSH concentrations exceed a higher threshold of 10 mIU/L.7 This is higher than the upper limit of the diagnostic reference range defining the disease, which for most assays is approximately 4 mIU/L.7 This elevated decision point setting appears to stem from a desire to minimise the application of therapy in patients who may not require it.7,67,68 Accordingly, as for indicating treatment of hypothyroidism, clinicians are left with a paradox that establishing a diagnosis of subclinical hypothyroidism no longer implies that the disease may warrant treatment. This has created a dilemma for the disease understanding of practitioners and patient communication, reigniting the debate whether subclinical hypothyroidism should be regarded as a laboratory constellation rather than a clinical disease entity.46,69 To appreciate the strengths and deficiencies of the process, the evaluation of a patient with hypothyroidism must be reassessed.
Assessment of a patient with hypothyroidism
Although a disease is commonly characterised by specific signs and distinctive symptoms, this is different for hypothyroidism. A person with the hormone deficiency may typically complain about the full complex, yet characteristic spectrum, of hypothyroid symptoms, including fatigue, tiredness, lack of energy, weakness, cold intolerance, decreased body temperature, weight gain, loss of appetite, hair loss, slow heart rate, muscle aches, constipation, menstrual irregularities, change in sexual desire and function, and depressive mood. Physical examination may uncover hypothyroid signs such as swollen eyelids, puffy face, reduced facial expression, pale dry skin, coarse hair, enlarged tongue, coarse voice, slow speech, slow pulse rate, muscle weakness, and sluggish reflexes. However, many patients present less conspicuously with few and minor signs of the disease, and in about 50% of cases apparent symptoms are absent. Furthermore, hypothyroid symptoms tend to overlap with a multitude of other conditions, their differential diagnosis involving a vast spectrum of other diseases.70–73 Clinicians have therefore embraced the convenience of serum TSH measurement, giving it a pre-eminent position in diagnosing primary hypothyroidism and also defining treatment success as achievement of a TSH target rather than a favourable clinical outcome.7,74
As a laboratory test, third-generation TSH assays perform very well, yielding sensitivities above 95% and specificities between 93 and 97%.60,75,76 However, this excellent test performance does not equivalently translate into diagnostic performance, as diagnostic accuracy in post-test probability also depends on disease prevalence.77 When screening a population with a 1% prevalence of hypothyroidism without any further information with a 97% accurate test, the post-test probability for a person with a positive test result to be truly hypothyroid is only 24%.77 TSH measurements must therefore be critically reviewed in the context of the specific situation considering the patient’s full history and presentation.
Another critical area in the diagnostic process of hypothyroidism is the meaning of the reference range of TSH and thyroid hormones, defined as 95% of healthy subjects drawn from that population. Importantly, thyroid parameters are exceptional in two ways: (1) highly individual personal traits, as their variation within a subject is narrow, compared to the population’s range or between-subject variation78; (2) interlocked, as FT4 is raised by TSH to a healthy level appropriate for each individual.58
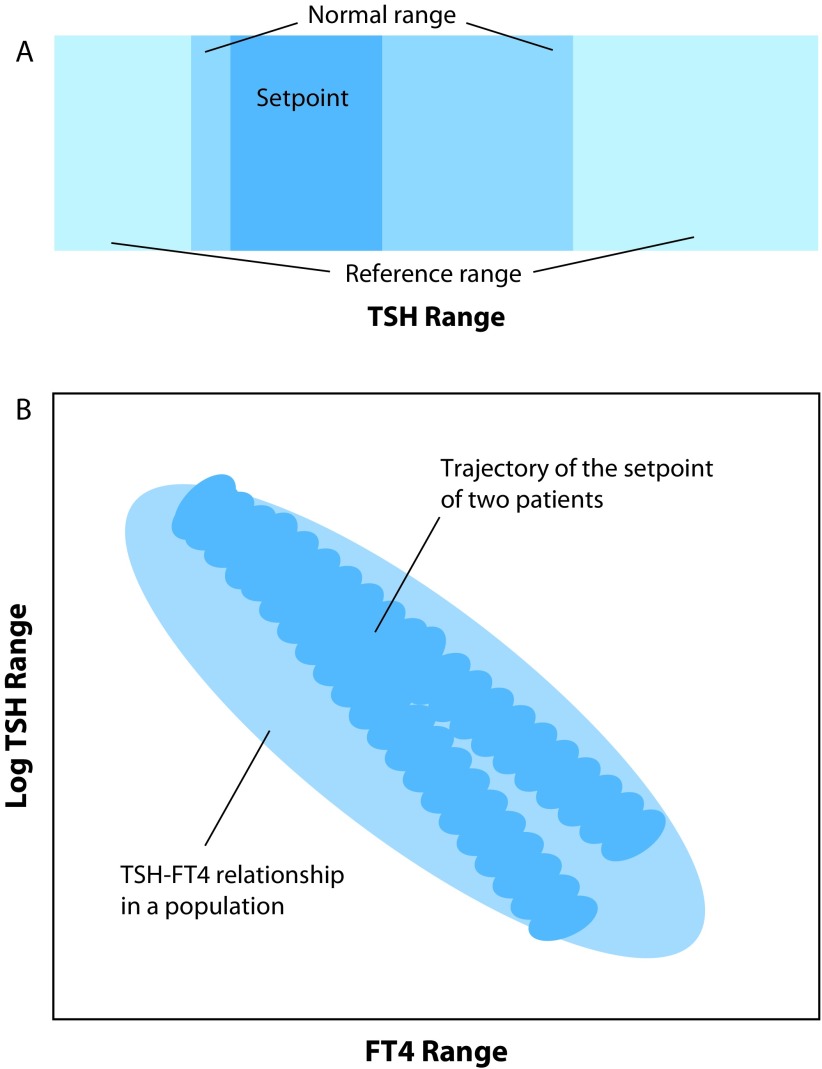
The imbalance between contributors to the biologic variation compromises the reliability of a reference range to detect a pathological test result in a person. According to Andersen and colleagues, the reference range works as intended with a ratio of the intra-individual variation to the inter-individual variation greater than 1.4, but for TSH and thyroid hormones where the ratio is less than 0.6 the population-based reference range is an insensitive measure in most individuals.79 The conditional equilibrium formed between TSH and FT4 is called the setpoint, a narrow individual integrator of the stimulation of thyroid hormone production by TSH and negative feedback control of thyroid hormones upon TSH.62 Figure 1a depicts the relatively wide reference range, the narrower (approximately half as wide) normal range of a single person, and the even narrower setpoint of a person in a defined condition. The interlocking equilibria of TSH and FT4 result in a kite-shaped distribution of the setpoints.66 This is profoundly different from assuming two independent univariate reference ranges for TSH and FT4 whose graphical interaction describes a rectangle. Unlike a fixed TSH threshold for all patients in the population, it renders the discriminatory TSH threshold between the euthyroid and hypothyroid state variable amongst the individuals in a population and, in an individual, conditionally dependent on paired FT4 concentrations or setpoints.66,80
Figure 1.
Range considerations for TSH measurement. Scheme A illustrates three TSH ranges of increasing width and uncertainty, a narrow range defined by the personal setpoint (region of the interlocking homeostatic equilibria including allostatic modifications and hysteresis, all of those being small in healthy individuals), a wider normal range observed when repeating measurements in an individual (within-subject variation including assay imprecision, setpoint imprecision, and biological variation) and a much wider (approximately twice as wide as the former) cross-sectional reference range observed in a healthy population (between-subject variation representing an overlay of genetic traits modifying individual setpoints, variation in peripheral parameters of thyroid hormones and biological random effects). The marked differences in these ranges arise because of the high individuality expressed by TSH. Scheme B indicates the transition from the euthyroid to the hypothyroid state, contrasting the cross-sectional logTSH–FT4 relationship with the path of the setpoint in two individuals. This simplified scheme demonstrates the non-ergodic behaviour of thyroid hormones. A progressive disaggregation of the individual correlations towards the euthyroid range produces stratification collider bias (Simpson’s paradox) in statistically averaged outcome studies. For further explanations refer to the text.
FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Another controlling element and major contributor to variation arises from TSH providing feed-forward stimulation of the enzymes deiodinase 1 and 2, adjusting T4 to T3 conversion rates to genetically determined personal needs and varying conditional requirements.81–84 This pathway is important in providing relational T3 stability in thyroid health, rendering T3 generation to a considerable extent independent of T4 supply.58,85 However, the process fails in LT4-treated athyreotic patients, altering the equilibria between free triiodothyonine (FT3), FT4, and TSH, compared to the healthy state.58,86–88 Consequently, a certain TSH level obtained in a person in thyroid health cannot equally serve as a treatment target for LT4 replacement after the patient has undergone thyroidectomy.87 Similarly, these mechanisms play out in opposite directions in a patient suffering from autoimmune thyroiditis. TSH elevation ensuing with declining thyroid function enhances the proportional production of T3, either synthesised or converted from T4 in the thyroid gland and other organs, thereby protecting the organism against imminent thyroid failure until the destructive disease has progressed to the atrophic end stage.82 Disease progression is generally slow, and many patients with subclinical hypothyroidism may never progress to overt hypothyroidism.89
For these fundamental reasons, the TSH reference range obtained in a healthy population cannot be used as a reliable indicator for an individual person’s true thyroid status.59 Nor can it define requirement of treatment alone and serve as adequate target for LT4 therapy.64
Lack of generality and requirement for individualisation
Population-based reference ranges, although able to describe the distribution of thyroid parameters in populations, are by design unable to reliably define the thyroid status at the individual level. Applying averaged findings from a population requires their applicability to each and every member of it.90–92 As a mathematical prerequisite for a generalisation from the population to the personal level – specified in the framework of ergodicity90 – all subjects are required to share the population moments. This does not hold true for TSH and thyroid hormones where trait-like differences in the genetically determined and conditionally modified setpoints prevail amongst individuals with structural change occurring over time.
The consequences are far reaching. Disease transition must be redefined according to criteria of structural change amongst the system parameters. The latter constitute trait-like characteristic of each subject and produce personally defined boundaries for TSH and thyroid hormones (Figure 1b). The appropriateness of a TSH response is defined by adjusted setpoints, not by statistically invariant relationships.64 Patients follow individual trajectories from the euthyroid to the hypothyroid state or vice versa when treated with LT4.93 This behaviour produces a wide ‘grey’ zone at the population level where the thyroid state is imprecisely defined for an individual (Figure 1). In that grey zone, ranging from approximately 2 to 10 mIU/L TSH, it is virtually impossible to determine the underlying thyroid status from a TSH measurement alone.
The resulting ambiguity and limited value of TSH measurements in defining the euthyroid status has been convincingly documented by large epidemiological studies. For instance, a systematic review concluded that higher circulating FT4 levels, but not TSH levels, were associated with an increased risk of incident atrial fibrillation in euthyroid individuals.94,95 A prospective study in LT4-treated athyreotic patients with thyroid cancer found that patients with mildly suppressed TSH levels were closest to euthyroid, whereas TSH levels within the reference range were still indicative of tissue hypothyroidism in these patients as determined by surrogate markers.96
The current state of affairs has left both practitioners and patients increasingly dissatisfied. A large online survey amongst thyroid patients conducted by the American Thyroid Association revealed widespread dissatisfaction with standard LT4 treatment, and in their subjective view, respondents indicated a preference for satisfying alternatives.97 In another recent survey amongst members of the American Thyroid Association, most thyroid specialists favourably considered therapies alternative to LT4 treatment in hypothyroid patients, but which are not endorsed by official society guidelines.11
It has become increasingly apparent that a historic experiment using an ill-founded ‘one size fits all’ approach and a simplistic TSH-centred method for defining a prevalent disease such as hypothyroidism has failed.98 TSH measurement cannot be dissociated from either the clinical presentation of the patient, the specific underlying condition it applies to, its intricate interlocking pattern with thyroid hormones, or its personal trait-like nonergodicity (Figure 1).
A revisitation of basic science and endocrine principles in dealing with a control parameter such as TSH may offer a way to improving the definition of hypothyroidism in the future as a variably manifesting disease with a wide clinical spectrum of signs and symptoms and non-ergodic hormone profiles. Consideration of individual trajectories when a patient is transitioning from the euthyroid to the hypothyroid state and the reconstruction of personal setpoints seems to be a better alternative to reliance on the population range, awaiting further exploration and confirmation of their clinical utility.
Drug selection in the treatment of hypothyroidism
Although LT4 has been the guideline-endorsed standard treatment for hypothyroidism since its introduction in the 1970s, the universality of this choice has been disputed.99,100 Critics have argued that the transition from the earlier use of thyroid extracts, which still remain a popular choice amongst patients, to LT4 was not supported by strict evidence-based criteria at that time, and a few rigorous comparative studies have only emerged recently.98,101 Comparisons of two synthetic drugs, LT4 and a combination of liothyronine (LT3)/LT4 have been attempted by numerous randomised controlled trials and derived meta-analyses, but could not confirm a clear clinical benefit of one drug regimen over the other, mostly based on quality-of-life measures.13,102–106 However, limitations in the pharmacokinetics of T3 and serious flaws in design and analysis of these trials have also been pointed out.10,16 Experimental treatments in rodents have fundamentally challenged the ability of LT4 as a drug to restore true euthyroidism at the tissue level in various organs, which in these studies was only achieved by combinations of LT4 and LT3.107–109 Such invasive studies cannot readily be repeated in humans, and differences in thyroid physiology between rodents and humans have been emphasised.110,111 In particular, the proportional amount of T3 co-secreted with T4 by the thyroid gland is generally assumed to be much lower in humans, compared to rodents, making the T3 supply to human organs putatively autonomously independent of the glandular T3 secretion and locally controlled by the respective organs.112 Although true in thyroid health where FT3 levels remain stable over wide variations in T4 supply, this appears to be no longer the case in LT4-treated athyreotic patients where T3 levels become unstable and correlated with T4 supply.58,85 In the absence of thyroid remnant tissue, the adjustable intra-thyroidal control of T3 generation by TSH via both de novo synthesis and conversion from T4 becomes deficient and, unlike in the healthy thyroid state, fails to readily achieve the required compensation.86,87,113 A lack of T3-generating capacity, normally contributed by the thyroid gland itself, can apparently not be adequately compensated by other organs in the absence of a functioning thyroid remnant.59,114 This challenges a key assumption and pharmacological principle underlying LT4 monotherapy that the supply of the prodrug LT4 would routinely suffice to guarantee adequate autoregulated derivation of sufficient amounts of T3 by the various organs. Notably, a landmark study by Pilo and colleagues, reporting a relatively low direct thyroidal contribution to the T3 pool in human volunteers, has in its design not adequately considered effects on thyroidal T3 secretion when blocking thyroid hormone secretion overall with the administration of Lugol’s solution to the study participants.115 Apart from its quantitative contribution to the thyroid hormone pool, glandular co-secretion of a minor amount of T3 with T4 may also have an important regulatory role in adjusting the sensitivity of the hypothalamic–pituitary–thyroid feedback control and balancing system equilibria.116 A major homeostatic breakdown in the absence of a functioning thyroid gland may, at least in part, explain the demonstrated inability of LT4 replacement alone to restore euthyroidism in experimental animals, together with the reported failure of patients to make a full recovery in their quality-of-life in prospective studies, and dissatisfaction expressed by patients in various formats.9,33–36,97,107–109,117
Awaiting future improved trials and availability of improved drug formulations such as slow-release preparation for LT3, which would better facilitate the practical use of LT3-based drugs,118,119 the choice of treatment of hypothyroidism is expected to remain controversial in the near future. In view of a lack of clear evidence favouring one drug over the other keeping alternative modes of prescription drugs, such as LT3/LT4 combinations and standardised DTE, available seems currently to be the best option to meet different objective requirements of subpopulations with vastly different physiological conditions, for example, athyreotic patients with impaired conversion ability and patients with autoimmune thyroiditis and compensatory T3 production. This may also address the variable needs and expectations of individual thyroid patients, allowing doctors and patients to agree on the most satisfactory solution for each person by trial and error. The role of patient expectations is poorly studied, although it not only influences the subjective perception of the therapy received but also impacts directly on evidence derived from clinical trials, potentially introducing serious bias to reported trial outcomes, including randomised controlled trials (RCTs).120
Patients displaying apparent conversion deficiencies on LT4 monotherapy, either genetically predetermined or acquired through the administration of the drug itself, are natural candidates for a combined replacement regimen with LT3 and LT4. Although this strategy has been empirically used as a criterion of drug selection by practitioners, it awaits further formal proof in this subpopulation. In particular, polymorphic alleles of the DIO1 and DIO2 genes have been linked with altered concentrations of TSH, free or total T4 and free or total T3.121 Examples associated with decreased conversion efficiency and setpoint elevation of thyroid homeostasis include rs11206244 of the DIO1 gene and rs225014 of the DIO2 gene.122–126 In carriers of the SNP p.Thr92Ala of DIO2 (rs225014), deiodinase activity may be especially decreased postsurgery, and, if the SNP rs17606253 of the MCT10 transporter for thyroid hormones is also present, accompanied with a preference for LT3–LT4 combination therapy.127,128 These and other studies suggest a potential role for genetic testing in tailoring the mode of thyroid replacement.129 From a pharmacological point of view, a currently unavailable slow-release preparation could greatly improve the pharmacological properties of LT3 in a combination drug with LT4 to be clinically tested in these patients.119,130,131
Dose adequacy
Evaluating dose adequacy for LT4 treatment presents another clinical challenge. LT4 dose requirements vary according to various influences including aetiology of disease, body weight, age, time of intake, proximity to food intake, pregnancy, comorbidity, comedication, adherence to medication, and individual responsiveness to the hormone.3,21,132–135 (Table 1). LT4 treatment is generally commenced with a weight-adjusted dose, estimated either empirically (approximately 1.6 μg kg body weight) or using elaborate algorithms, and further adjusted during follow-up.136–141 Higher TSH-suppressive doses have long been used in patients with thyroid cancer but recent recommendations have changed this practice, relaxing dose requirements for patients with favourable long-term prognosis of their cancer.142,143 Increased physiological requirements of thyroid hormones in pregnancy demand an adjustment of exogenous thyroid hormone supply (approximately 30% increase of the LT4 dose) in hypothyroid women with the onset of pregnancy or ideally even before conception.144 For this condition, other particularities in paediatric populations, and patients with rare genetic disorders of thyroid hormone resistance, we refer to dedicated reviews.53,145,146
Table 1.
Important influences on dose requirements of LT4.
| Factor | Influence |
|---|---|
| Gender | Mainly indirectly influential through lean weight |
| Age | Reduced LT4 requirements in older patients Older patients may be less tolerant to the full dose of a younger person, requiring a more cautious approach |
| Body weight | Higher dose requirements with increasing body weight |
| Time of LT4 intake | Morning versus evening medication equally effective |
| Intake of food | Best absorption rate in the fasting state, recommended LT4 intake 30 minutes before breakfast |
| Thyroid remnant | T3 instability following complete loss of functional thyroid tissue, requiring increased LT4 dose or addition of LT3 |
| Regulatory deficiencies | Altered equilibria between FT3, FT4, and TSH on LT4, compared to thyroid health Secondary hypothyroidism due to hypothalamic or pituitary disease |
| Conversion efficiency | Impaired activity of deiodinases type 1 and 2 in patients receiving LT4, genetic polymorphisms in thyroid health and disease |
| Resistance to thyroid hormones | Hereditary resistance syndromes, acquired resistance due to interference of endocrine disruptors (environmental pollutants) |
| Disease aetiology | Higher dose requirements in athyreotic patients with thyroid cancer, TSH-mediated increase of deiodinase activities and proportional T3 production in patients with autoimmune thyroiditis |
| Pregnancy | Increased LT4 dose requirements due to larger physiological thyroid hormone demand |
| Comorbidity | Decreased gastrointestinal absorption of thyroid hormones in prevalent gastrointestinal diseases, such as coeliac disease |
| Use of other drugs | Interference of many common drugs with the absorption of LT4 |
| Adherence | Approximately 20% of nonadherence rate to regular LT4 intake Less influential single-dose omissions due to the long plasma half-life of the drug |
FT3, free triiodothyonine; FT4, free thyroxine; LT3, liothyronine; LT4, levothyroxine; T3, triiodothyronine; TSH, thyroid-stimulating hormone.
Treating an older population with LT4 presents a particular diagnostic and therapeutic challenge.68 Whilst the prevalence of hypothyroidism increases with age, so does the physiological TSH range in patients over 65 years, although not all studies have confirmed this trend.45,147,148 Hypothyroidism was retrospectively associated with increased all-cause mortality in an older Taiwanese population, and LT4 treatment reduced this risk.149 In contrast, another case–control study reported that LT4 treatment-related mortality is increased in the elderly.150 In centenarians, levels of TSH abnormally high for younger individuals were even linked to longevity.151 A double-blinded, randomised, placebo-controlled trial found no benefit of LT4 treatment for older individuals with subclinical hypothyroidism, but the absence of clinical symptoms, lack of information on antibody status, and only moderately elevated TSH levels for that age group have been of concern.67 The presence of multimorbidity may mask hypothyroid symptoms, and frailty may be aggravated by higher circulating FT4 concentrations.152 Of potential relevance for older patients taking LT4, T4 has also been shown to promote the proliferation of cancer cells in vitro through its interaction with the αvß3 integrin receptor.153 Deiodinase activity estimated by the FT3/FT4 ratio was also documented as an independent marker of both frailty and survival in a geriatric population, even in the presence of normal FT3 values ruling out low-T3 syndrome.154 This exemplifies the complexity of the treatment decision in older patients.
Guidelines have recommended using the population’s reference range of TSH as a treatment target, assuming the pituitary gland of a patient would under all circumstances reflect a correct assessment of the respective thyroid status of the individual.7,155 However, upon closer scrutiny, this strategy and assumption does not hold up. The equilibria between FT3, FT4, and TSH have been shown to be strongly displaced in patients receiving LT4 treatment after thyroidectomy compared to their previous healthy state. Because LT4 treatment fails to re-enact normal euthyroid physiology, based on physiological principles discussed earlier, a disconnect between the FT4–TSH feedback and T3 production arises in these patients receiving LT4, which persists even when sufficient amounts of LT4 are supplied to apparently restore biochemical euthyroidism.88,112,156 Measurement of pituitary TSH can therefore not be regarded as an adequate measure of thyroid hormone-controlled homoeostasis during LT4 treatment.64 If anything, serum FT3 serum concentrations, but not TSH, appear to be the most useful single biochemical indicator for symptom relief in patients treated with LT4, except for patients with concomitant non-thyroidal illness (low-T3 syndrome) where T3 measurements are compromised or in rare syndromes of thyroid hormone resistance.63,95,156
The simplistic interpretation of the role of TSH merely as a passive reflector of peripheral thyroid status has been profoundly misguided. The pituitary is not following but leading the way, providing anticipatory corrective action before the system fails.58,108,157,158 This is also exemplified by situations of thyroid allostasis, where the setpoint of the feedback loop is downregulated in situations representing energy deprivation (type 1 allostatic load) and upregulated in anticipated stress (type 2 allostatic load).63 In these common scenarios, TSH concentration predominantly mirrors the setpoint, and non-normal TSH concentrations rarely indicate a dysfunction of the thyroid.64 Hypothyroidism only ensues if an ongoing corrective action by the system is unsuccessful. A slightly elevated serum TSH level, as observed in subclinical hypothyroidism, is therefore an ambiguous signal, as it can either represent an elevated setpoint, be an expression of successful corrective action by the central system in impending hypothyroidism with slightly reduced (but compensable) thyroid output or a consequence of system failure.63,66 Subclinical hypothyroidism as per the current definition cannot therefore be used as a disease classifier of true hypothyroidism. Only the bivariate placement of the conjoined FT4–TSH setpoint at equilibrium, but not the reference range for TSH alone, is able to deliver a mathematically correct interpretation of the interlocked response (Figure 1).66,80
Reconstruction of personal setpoints and the use of calculated homeostatically derived structural parameters may therefore provide suitable additional tools for wider clinical exploration and testing.159,160 This strategy also allows to compare follow-up measurements with archived markers before the onset of disease whenever available.58,87 Measurement of FT3, unless compromised in patients with non-thyroidal illness, may be additionally useful for estimating global deiodinase activity.58,87,95,113,156,160
Trial and error in finding the optimum treatment and empirical corrections to find the right balance between under- and over-treatment must still be expected, given the uncertainty in defining the euthyroid state for an individual by biochemical means and the often nonspecific nature of patient complaints. Such complaints may be unrelated to thyroid function, rather being associated with other factors including the underlying aetiology of the disease (autoimmune process or malignancy), psychological issues of coping with the disease (cancer fear), or epiphenomena such as fatigue and weight gain.35,161–163 Thyroid autoimmunity per se may be an independent reason for low quality-of-life scores in affected patients, overlapping with a decline in thyroid function.164 The development of reliable endpoint markers of tissue hypothyroidism is needed for future direction.109 This is not to deny that, for example, in the absence of any thyroid failure, LT4 may display mild antidepressant pharmacological properties, which has different implications and should be evaluated against other antidepressants.165
Adverse drug effects
Acting predominantly as a prodrug, LT4 may be expected to have a superior pharmacological safety profile, compared to the biologically more active LT3. Pharmacologically, the long-lived LT4 compound provides stable long-term serum concentrations with a single daily dosing regimen whereas the shorter-lived drug LT3 displays spikes in serum FT3 concentrations, requiring inconvenient administration in divided daily doses.131,166 However, the biochemical responses will be non-instantaneous and metabolically damped, making the impact of such spikes less dangerous. Fixed combinations of LT3 and LT4 in a drug face similar challenges of different half-lives of the components, multiple daily dosing requirements for the T3 component, and optimisation of the ratio of the components to be administered. Pharmacodynamics was one of the main aspects in promoting a shift to the synthetic hormone LT4 from earlier T3-containing alternatives such as DTE, although comparative clinical assessment was widely lacking at that time.5,6 Although the safety profile of LT4 is well-known, few studies have addressed the long-term safety of LT3 administration.167,168 Over 17 years of observation, Leese did not identify increased clinical risks of atrial fibrillation, cardiovascular disease, or fractures for LT3, compared to LT4.167 Only antipsychotic comedication was increased in patients taking LT3.167 Tariq and colleagues compared synthetic and natural T3–T4 combinations with LT4, and found no increase in risk with the prescription of the alternatives.168 Although the cardiovascular and skeletal risks of hyperthyroidism are well documented,2,3,169 it should be emphasised that factitious thyrotoxicosis caused by LT4 overdose is an entity of thyrotoxicosis entirely different from endogenous hyperthyroidism. In clinical studies, the two conditions, displaying different equilibria, must not be aggregated and have to be analysed separately for the results to be informative on LT4 treatment. A failure to discriminate between subclinical and overt thyroid dysfunctions relates any study outcomes to the case mix rather than thyroid function, severely limiting the information contributed by a plethora of studies that have measured TSH only and reported on associated cardiovascular or bone risk.2,170,171 The dissociation between TSH and FT4 in predicting atrial fibrillation in euthyroid subjects has already been discussed earlier.
The clinically relevant question arises when overtreatment may have occurred in a patient. Clinical symptoms may often provide an indication. As for biochemical monitoring, studies have documented TSH concentrations that exceed the populations’ reference range in approximately 20% of patients receiving LT4.44,172,173 However, LT4-induced thyrotoxicosis is difficult to verify biochemically based on a TSH measurement alone in a patient on LT4, given the uncertainty surrounding both the upper and lower limits of the TSH reference range.65,66,174,175 FT4 serum concentration is a poor marker of clinical T4 excess, because in many patients receiving LT4, FT4 levels have to be raised above the upper limit of the healthy reference range to facilitate adequate conversion of the precursor into sufficient active T3. Conversion inefficiency of T3 from T4, prevalent in LT4-treated patients, produces unphysiologically high FT4 to FT3 ratios of unknown significance.86,112,176,177 In this situation, elevated serum FT3 levels may provide a more specific, but less sensitive indicator of overdosing, when obtained with a delay of several hours before the intake of the next daily LT4 dose.156 FT3 concentrations tend to be relative or absolutely low in LT4-treated patients, due to the thyroidal deficiency in the TSH-T3 shunt or the suppression of this pathway by T4.86,177 This is a drug-related phenomenon in otherwise healthy subjects, unrelated to non-thyroidal illness and methodological interference.55,63,175
TSH suppression by itself is frequently uninterpretable, being supported by some retrospective and prospective studies as clinically optimally euthyroid, whereas declared a major risk factor by others.2,3,95,156,178,179 A wide disjoint arising between FT3 and TSH on LT4 treatment subdivides patients into three categories of conversion efficiency, as estimated by global deiodinase activity, strong converters achieving appropriate FT3 concentrations without TSH suppression, intermediate converters associated with FT4 levels above the upper reference and as a result suppressed TSH, and poor converters showing a progressive FT3–TSH disjoint whilst failing to adequately raise or even paradoxically lowering their FT3 serum concentrations.113 To complicate things further, although overt hypothyroidism was found to be associated with increased all-cause mortality, several studies have also linked longevity with a slightly hypothyroid biochemical thyroid state.149,180–182 However, it is unknown if this is a genetic advantage and whether it may be prognostically related to LT4 treatment in any way.
In the absence of proper stratification, wide variations amongst individuals in treatment efficiency will produce statistical aggregation bias (Simpson’s paradox) in clinical studies, including RCTs.10 Undue statistical averaging of treatment effects across nonuniform populations with different mathematical moments causes serious bias, not rectified by study design, in non-ergodic parameters such as thyroid hormones.91
Despite their apparent failure to account for collider stratification bias, numerous studies associating TSH concentrations with adverse treatment-related outcomes may still contribute epidemiological data and provide valuable information in relation to a population.2,3,169–171,183 However, conflicting and irreproducible results may be expected, depending on the respective patient mix aggregated in the study.10 More importantly, results obtained from such studies are however not generalisable to the individual level, and become unusable in personalised patient care.58,92,96,184,185 Notably, inherent non-ergodic properties of thyroid hormones and individual traits expressed in setpoints pose serious challenges for the interpretation of clinical studies,91 demanding a sharply-defined distinction between epidemiological strategies and personalised outcome research.
Future directions
Major diagnostic improvements should aim at revising the definition of hypothyroidism as a disease including novel clinical endpoint markers to avoid the current use of different diagnostic and therapeutic cut-offs. Treatment targets should be personalised taking into account the highly individual non-ergodic nature of thyroid parameters. When evaluating different modes of treatment, meta-analyses, systematic reviews and guidelines must be less reliant on statistical averaging, avoiding statistical aggregation bias and considering different needs of subpopulations. Another priority is the development of novel formulas to improve the pharmacokinetic profile of available LT3 drugs.
Conclusions
Recent surveys have highlighted considerable dissatisfaction with the standard LT4 treatment of hypothyroidism and indicated a willingness of most thyroidologists, depending on case presentation, to circumvent treatment recommendations endorsed by current guidelines.11 Expression of strong diverging patient and expert opinions coincides with recent advances in our understanding of thyroid hormone regulation in health and disease.97,186 A long-held tenet has been falsified that replacing only the prodrug LT4 in the treatment of primary hypothyroidism in a dose guided by the TSH reference range in thyroid health, would under all circumstances guarantee derivation of adequate amounts of T3 by various organs for their autonomous utilisation and proper functioning.33,36,85,117,187 The unexpected collapse of the conventional reference system, particularly for TSH,88 in the treatment situation makes current biochemical definitions of hypothyroidism unreliable tools for guiding substitution therapy of the deficiency. This requires a thorough reassessment of basic principles applicable to LT4 use in patient care, extending statistical aggregation of population results obtained from many epidemiological studies that are not readily applicable to the individual level.91 Trait-like personal markers such as setpoints, together with a renewed focus on clinical disease definition and tissue markers of hypothyroidism, may offer a future perspective for making personalised treatment decisions.66,159 Apparent shortcomings in the standard therapy with LT4, discussed in this and other reviews, should stimulate more interest in the development of modern T3-based drugs, such as slow-release T3 preparations.
Acknowledgments
None.
Footnotes
Contributions: RH drafted the manuscript. JEM, RL and JWD contributed additional ideas, text passages, edits, and references. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: JWD is co-owner of the intellectual property rights for the patent ‘System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual’ (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940-5, WIPO number WO/2014/088516). All other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/08/dic.212597-COI.pdf
Funding declaration: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Correct attribution: Copyright © 2019 Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 2 July 2019
References
- 1.Razvi S, Korevaar T, Taylor P. Trends, determinants and associations of treated hypothyroidism in the United Kingdom, 2005–2014. Thyroid. 2019;29:174–182. doi: 10.1089/thy.2018.0251. [DOI] [PubMed] [Google Scholar]
- 2.Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- 3.Jonklaas J. Update on the treatment of hypothyroidism. Curr Opin Oncol. 2016;28:18–25. doi: 10.1097/CCO.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessey JV. Historical and current perspective in the use of thyroid extracts for the treatment of hypothyroidism. Endocr Pract. 2015;21:1161–1170. doi: 10.4158/EP14477.RA. [DOI] [PubMed] [Google Scholar]
- 6.McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164:50–56. doi: 10.7326/M15-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman SC, Gross TP, Kennedy DL. Thyroid hormone use: trends in the United States from 1960 through 1988. Thyroid. 1991;1:285–291. doi: 10.1089/thy.191.1.285. [DOI] [PubMed] [Google Scholar]
- 9.Wiersinga WM. Therapy of endocrine disease: T4 + T3 combination therapy: is there a true effect. Eur J Endocrinol. 2017;177:R287–R296. doi: 10.1530/EJE-17-0645. [DOI] [PubMed] [Google Scholar]
- 10.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Lessons from randomised clinical trials for triiodothyronine treatment of hypothyroidism: have they achieved their objectives. J Thyroid Res. 2018;2018 doi: 10.1155/2018/3239197. 3239197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonklaas J, Tefera E, Shara N. Prescribing therapy for hypothyroidism: influence of physician characteristics. Thyroid. 2019;29:44–52. doi: 10.1089/thy.2018.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladenson PW. Precision medicine comes to thyroidology. J Clin Endocrinol Metab. 2016;101:799–803. doi: 10.1210/jc.2015-3695. [DOI] [PubMed] [Google Scholar]
- 13.Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinol. 2014;10:164–174. doi: 10.1038/nrendo.2013.258. [DOI] [PubMed] [Google Scholar]
- 14.Citterio CE, Targovnik HM, Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol. 2019;15:323–338. doi: 10.1038/s41574-019-0184-8. [DOI] [PubMed] [Google Scholar]
- 15.Colucci P, Yue CS, Ducharme M, Benvenga S. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur Endocrinol. 2013;9:40–47. doi: 10.17925/EE.2013.09.01.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94:3905–3912. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajput R, Chatterjee S, Rajput M. Can levothyroxine be taken as evening dose? Comparative evaluation of morning versus evening dose of levothyroxine in treatment of hypothyroidism. J Thyr Res. 20112011 doi: 10.4061/2011/505239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderpump M. Pharmacotherapy: hypothyroidism—should levothyroxine be taken at bedtime. Nat Rev Endocrinol. 2011;7:195. doi: 10.1038/nrendo.2011.29. [DOI] [PubMed] [Google Scholar]
- 19.Akın O. Morning vs. Bedtime levothyroxine administration: what is the ideal choice for children. J Pediatr Endocrinol Metab. 2018;31:1249–1255. doi: 10.1515/jpem-2018-0168. [DOI] [PubMed] [Google Scholar]
- 20.Jonklaas J. Treatment of hypothyroidism. In: Lauter M, Duntas L, Wartofsky L, editors. The thyroid and its diseases. Basel, Switzerland: Springer International Publishing AG; 2019. pp. 265–281. [Google Scholar]
- 21.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23:781–792. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Roos A, Linn-Rasker SP, Van Domburg RT, Tijssen JP, Berghout A. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Int Med. 2005;165:1714–1720. doi: 10.1001/archinte.165.15.1714. [DOI] [PubMed] [Google Scholar]
- 23.Younis IR, Ahmed MA, Burman KD, Soldin OP, Jonklaas J. Stable isotope pharmacokinetic studies provide insight into effects of age, sex, and weight on levothyroxine metabolism. Thyroid. 2018;28:41–49. doi: 10.1089/thy.2017.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen PR, Zavacki AM. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J. 2012;1:232–242. doi: 10.1159/000343922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeshige K, Sekido T, Kitahara J-I, et al. Cytosolic T3-binding protein modulates dynamic alteration of T3-mediated gene expression in cells. Endocr J. 2014;6:561–570. doi: 10.1507/endocrj.EJ13-0418. [DOI] [PubMed] [Google Scholar]
- 27.Zevenbergen C, Meima ME, Lima De Souza EC, et al. Transport of iodothyronines by human l-type amino acid transporters. Endocrinology. 2015;156:4345–4355. doi: 10.1210/en.2015-1140. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S-Y, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flamant F, Cheng SY, Hollenberg AN, et al. Thyroid hormone signaling pathways: time for a more precise nomenclature. Endocrinology. 2017;158:2052–2057. doi: 10.1210/en.2017-00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senese R, Cioffi F, De Lange P, Goglia F, Lanni A. Thyroid: biological actions of ‘nonclassical’ thyroid hormones. J Endocrinol. 2014;221:R1–12. doi: 10.1530/JOE-13-0573. [DOI] [PubMed] [Google Scholar]
- 31.Hönes GS, Rakov H, Logan J, et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc Natl Acad Sci USA. 2017;114:E11323–E11332. doi: 10.1073/pnas.1706801115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harington CR, Barger G. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J. 1927;21:169. doi: 10.1042/bj0210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab. 2006;91:3389–3393. doi: 10.1210/jc.2006-0414. [DOI] [PubMed] [Google Scholar]
- 34.Wekking EM, Appelhof BC, Fliers E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. [DOI] [PubMed] [Google Scholar]
- 35.Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid. 2014;24:802–808. doi: 10.1089/thy.2013.0555. [DOI] [PubMed] [Google Scholar]
- 36.Winther KH, Cramon P, Watt T, et al. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One. 2016;11:e0156925. doi: 10.1371/journal.pone.0156925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallahi P, Ferrari SM, Ruffilli I, et al. Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: an update. Exp Op Drug Delivery. 2017;14:647–655. doi: 10.1080/17425247.2016.1227782. [DOI] [PubMed] [Google Scholar]
- 38.Virili C, Giovanella L, Fallahi P, et al. Levothyroxine therapy: changes of TSH levels by switching patients from tablet to liquid formulation. A systematic review and meta-analysis. Front Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krehan A, Kahaly GJ. Randomized, double-blind crossover study of bioavailability of levothyroxine. Med Klin. 2008;97:522–527. doi: 10.1007/s00063-002-1190-4. [DOI] [PubMed] [Google Scholar]
- 40.Braverman LE, Utiger RD. Introduction to thyrotoxicosis. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 6th ed. Philadelphia: J.B. Lippincott Company; 1995. pp. 645–647. [Google Scholar]
- 41.Zulewski H, Müller B, Exer P, Miserez AR, Staub JJ. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab. 1997;82:771–776. doi: 10.1210/jcem.82.3.3810. [DOI] [PubMed] [Google Scholar]
- 42.Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med. 1997;12:544–550. doi: 10.1046/j.1525-1497.1997.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 44.Canaris GJ, Manowitz NR. The Colorado thyroid disease prevalence study. Arch Int Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 45.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National health and nutrition examination survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 46.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 47.Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99:923–931. doi: 10.1210/jc.2013-2409. [DOI] [PubMed] [Google Scholar]
- 48.Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 49.Beck-Peccoz P, Rodari G, Giavoli C, Lania A. Central hypothyroidism – a neglected thyroid disorder. Nat Rev Endocrinol. 2017;13:588–598. doi: 10.1038/nrendo.2017.47. [DOI] [PubMed] [Google Scholar]
- 50.Solter D, Solter M. Benefit of combined triiodothyronine (LT3) and thyroxine (LT4) treatment in athyreotic patients unresponsive to LT4 alone. Exp Clin Endocrinol Diabetes. 2012;120:121–123. doi: 10.1055/s-0031-1297253. [DOI] [PubMed] [Google Scholar]
- 51.Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 2014;10:582–591. doi: 10.1038/nrendo.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schug TT, Johnson AF, Birnbaum LS, et al. Minireview: endocrine disruptors: past lessons and future directions. Mol Endocrinol. 2016;30:833–847. doi: 10.1210/me.2016-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grasberger H, Refetoff S. Resistance to thyrotropin. Best Pract Res Clin Endocrinol Metab. 2017;31:183–194. doi: 10.1016/j.beem.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lauretta R, Sansone A, Sansone M, Romanelli F, Appetecchia M. Endocrine disrupting chemicals: effects on endocrine glands. Front Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3:816–825. doi: 10.1210/jc.2004-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pingitore A, Mastorci F, Piaggi P, et al. Usefulness of triiodothyronine replacement therapy in patients with ST elevation myocardial infarction and borderline/reduced triiodothyronine levels (from the THIRST study) Am J Cardiol. 2019;123:905–912. doi: 10.1016/j.amjcard.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Lakhani OJ, Tripathi S, Indu KC, Desai M. Levothyroxine replacement before glucocorticoid replacement leading to adrenal crisis in a case of autoimmune polyendocrine syndrome type II (Schmidt syndrome) Thyr Res Pract. 2015;12:116–118. doi: 10.4103/0973-0354.157932. [DOI] [Google Scholar]
- 58.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Relational stability in the expression of normality, variation, and control of thyroid function. Front Endocrinol (Lausanne) 2016;7:142. doi: 10.3389/fendo.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Homeostatic control of the thyroid-pituitary axis: perspectives for diagnosis and treatment. Front Endocrinol (Lausanne) 2015;6:177. doi: 10.3389/fendo.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2004;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 61.Hoermann R, Broecker M, Grossmann M, Mann K, Derwahl M. Interaction of human chorionic gonadotropin (hCG) and asialo-hCG with recombinant human thyrotropin receptor. J Clin Endocrinol Metab. 1994;78:933–938. doi: 10.1210/jcem.78.4.8157724. [DOI] [PubMed] [Google Scholar]
- 62.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyroid Res. 2012;2012 doi: 10.1155/2012/351864. 351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatzitomaris A, Hoermann R, Midgley JEM, et al. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol (Lausanne) 2017;8:163. doi: 10.3389/fendo.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol (Lausanne) 2017;8:364. doi: 10.3389/fendo.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross HA, Den Heijer M, Hermus ARMM, Sweep FCGJ. Composite reference interval for thyroid-stimulating hormone and free thyroxine, comparison with common cutoff values, and reconsideration of subclinical thyroid disease. Clin Chem. 2009;55:2019–2025. doi: 10.1373/clinchem.2009.124560. [DOI] [PubMed] [Google Scholar]
- 66.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Advances in applied homeostatic modelling of the relationship between thyrotropin and free thyroxine. PLoS One. 2017;12:e0187232. doi: 10.1371/journal.pone.0187232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534–2544. doi: 10.1056/NEJMoa1603825. [DOI] [PubMed] [Google Scholar]
- 68.Calsolaro V, Niccolai F, Pasqualetti G, et al. Overt and subclinical hypothyroidism in the elderly: when to treat. Front Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med. 2001;345:260–265. doi: 10.1056/NEJM200107263450406. [DOI] [PubMed] [Google Scholar]
- 70.Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: randomised double blind placebo controlled crossover trial. BMJ. 2001;323:891–895. doi: 10.1136/bmj.323.7318.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlé A, Pedersen IB, Knudsen N, et al. Hypothyroid symptoms fail to predict thyroid insufficiency in old people: a population-based case-control study. Am J Med. 2016;129:1082–1092. doi: 10.1016/j.amjmed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Massolt ET, Van Der Windt M, Korevaar TIM, et al. Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2016;85:781–788. doi: 10.1111/cen.13101. [DOI] [PubMed] [Google Scholar]
- 73.Stadje R, Dornieden K, Baum E, et al. The differential diagnosis of tiredness: a systematic review. BMC Fam Pract. 2016;17:147. doi: 10.1186/s12875-016-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldwell G, Kellett HA, Gow SM, et al. A new strategy for thyroid function testing. Lancet. 1985;1:1117–1119. doi: 10.1016/s0140-6736(85)92429-8. [DOI] [PubMed] [Google Scholar]
- 75.Spencer C, Eigen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33:1391–1396. [PubMed] [Google Scholar]
- 76.Carvalho GAD, Perez CLS, Ward LS. The clinical use of thyroid function tests. Arquivos Brasileiros de Endocrinologia & Metabologia. 2013;57:193–204. doi: 10.1590/s0004-27302013000300005. [DOI] [PubMed] [Google Scholar]
- 77.Sox HC, Higgins MC, Owens DK. Chapter 4: understanding new information: Bayes theorem. In: Sox HC, Higgins MC, Owens DK, editors. Medical decision making. Oxford, UK: Wiley-Blackwell; 2013. [Google Scholar]
- 78.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 79.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–1078. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 80.Hoermann R, Larisch R, Dietrich JW, Midgley JEM. Derivation of a multivariate reference range for pituitary thyrotropin and thyroid hormones: diagnostic efficiency compared with conventional single-reference method. Eur J Endocrinol. 2016;174:735–743. doi: 10.1530/EJE-16-0031. [DOI] [PubMed] [Google Scholar]
- 81.Panicker V, Cluett C, Shields B, et al. A common variation in deiodinase 1 gene Dio1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab. 2008;93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Relational stability of thyroid hormones in euthyroid subjects and patients with autoimmune thyroid disease. Eur Thyroid J. 2016;5:171–179. doi: 10.1159/000447967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castagna MG, Dentice M, Cantara S, et al. Dio2 thr92ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102:1623–1630. doi: 10.1210/jc.2016-2587. [DOI] [PubMed] [Google Scholar]
- 84.Berberich J, Dietrich JW, Hoermann R, Müller MA. Mathematical modeling of the pituitary-thyroid feedback loop: role of a TSH-T3-shunt and sensitivity analysis. Front Endocrinol (Lausanne) 2018;9:91. doi: 10.3389/fendo.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm Metab Res. 2015;47:674–680. doi: 10.1055/s-0034-1398616. [DOI] [PubMed] [Google Scholar]
- 86.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito M, Miyauchi A, Morita S, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. 2012;167:373–378. doi: 10.1530/EJE-11-1029. [DOI] [PubMed] [Google Scholar]
- 88.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment. Eur J Endocrinol. 2013;168:271–280. doi: 10.1530/EJE-12-0819. [DOI] [PubMed] [Google Scholar]
- 89.Rosário PWS, Carvalho M, Calsolari MR. Natural history of subclinical hypothyroidism with TSH ≤10 mIU/l: a prospective study. Clin Endocrinol (Oxf) 2016;84:878–881. doi: 10.1111/cen.12939. [DOI] [PubMed] [Google Scholar]
- 90.Molenaar PCM, Campbell CG. The new person-specific paradigm in psychology. Curr Direct Psychol Sci. 2009;18:112–117. https://doi.org/1111/j.1467-8721.2009.01619.x. [Google Scholar]
- 91.Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci USA. 2018;115:E6106–E6115. doi: 10.1073/pnas.1711978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lowie WM, Verspoor MH. Individual differences and the ergodicity problem. Lang Learn. 2019;69:184–206. doi: 10.1111/lang.12324. [DOI] [Google Scholar]
- 93.Leow MKS, Goede SL. The homeostatic setpoint of the hypothalamus-pituitary-thyroid axis--maximum curvature theory for personalized euthyroid targets. Theor Biol Med Model. 2014;11:35. doi: 10.1186/1742-4682-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaker L, Heeringa J, Dehghan A, et al. Normal thyroid function and the risk of atrial fibrillation: the Rotterdam study. J Clin Endocrinol Metab. 2015;100:3718–3724. doi: 10.1210/jc.2015-2480. [DOI] [PubMed] [Google Scholar]
- 95.Baumgartner C, Da Costa BR, Collet TH, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136:2100–2116. doi: 10.1161/CIRCULATIONAHA.117.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito M, Miyauchi A, Hisakado M, et al. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid. 2017;27:484–490. doi: 10.1089/thy.2016.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28:707–721. doi: 10.1089/thy.2017.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Midgley JEM, Toft AD, Larisch R, Dietrich JW, Hoermann R. Time for a reassessment of the treatment of hypothyroidism. BMC Endocr Dis. 2019;19:37. doi: 10.1186/s12902-019-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kraut E, Farahani P. A systematic review of clinical practice guidelines’ recommendations on levothyroxine therapy alone versus combination therapy (LT4 plus LT3) for hypothyroidism. Clin Invest Med. 2015;38:E305–13. doi: 10.1186/s12902-019-0365-4. [DOI] [PubMed] [Google Scholar]
- 101.Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982–1990. doi: 10.1210/jc.2012-4107. [DOI] [PubMed] [Google Scholar]
- 102.Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91:2592–2599. doi: 10.1210/jc.2006-0448. [DOI] [PubMed] [Google Scholar]
- 103.Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics. 2007;48:379–384. doi: 10.1176/appi.psy.48.5.379. [DOI] [PubMed] [Google Scholar]
- 104.Ma C, Xie J, Huang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun. 2009;30:586–593. doi: 10.1097/MNM.0b013e32832c79e0. [DOI] [PubMed] [Google Scholar]
- 105.Michaelsson LF, Medici BB, La Cour JL, et al. Treating hypothyroidism with thyroxine/triiodothyronine combination therapy in Denmark: following guidelines or following trends. Eur Thyroid J. 2015;4:174–180. doi: 10.1159/000437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hennessey JV, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. Int J Clin Pract. 2018;72:e13062–14. doi: 10.1111/ijcp.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Escobar-Morreale HF, Del Rey FE, Obregón MJ, De Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 108.Werneck De Castro JP, Fonseca TL, Ueta CB, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125:769–781. doi: 10.1172/JCI77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103:4533–4542. doi: 10.1210/jc.2018-01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Escobar-Morreale HF, Botella-Carretero JI, Escobar Del Rey F, Morreale De Escobar G. Review: treatment of hypothyroidism with combinations of levothyroxine plus liothyronine. J Clin Endocrinol Metab. 2005;90:4946–4954. doi: 10.1210/jc.2005-0184. [DOI] [PubMed] [Google Scholar]
- 111.Gereben B, McAninch EA, Ribeiro MO, Bianco A. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11:642–652. doi: 10.1038/nrendo.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 113.Midgley JEM, Larisch R, Dietrich JW, Hoermann R. Variation in the biochemical response to L-thyroxine therapy and relationship with peripheral thyroid hormone conversion efficiency. Endocr Connect. 2015;4:196–205. doi: 10.1530/EC-15-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ito M, Miyauchi A, Kang S, et al. Effect of the presence of remnant thyroid tissue on the serum thyroid hormone balance in thyroidectomized patients. Eur J Endocrinol. 2015;173:333–340. doi: 10.1530/EJE-15-0138. [DOI] [PubMed] [Google Scholar]
- 115.Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol. 1990;258:E715–26. doi: 10.1152/ajpendo.1990.258.4.E715. [DOI] [PubMed] [Google Scholar]
- 116.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. The role of functional thyroid capacity in pituitary thyroid feedback regulation. Eur J Clin Invest. 2018;48:e13003. doi: 10.1111/eci.13003. [DOI] [PubMed] [Google Scholar]
- 117.Dietrich JW, Midgley JEM, Larisch R, Hoermann R. Of rats and men: thyroid homeostasis in rodents and human beings. Lancet Diabetes Endocrinol. 2015;3:932–933. doi: 10.1016/S2213-8587(15)00421-0. [DOI] [PubMed] [Google Scholar]
- 118.Jonklaas J, Burman KD, Wang H, Latham KR. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit. 2015;37:110–118. doi: 10.1097/FTD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Da Conceição RR, Fernandes GW, Fonseca TL, Bocco BMLC, Bianco AC. Metal coordinated poly-zinc-liothyronine provides stable circulating triiodothyronine levels in hypothyroid rats. Thyroid. 2018;28:1425–1433. doi: 10.1089/thy.2018.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.George BJ, Li P, Lieberman HR, et al. Randomization to randomization probability: estimating treatment effects under actual conditions of use. Psychol Methods. 2018;23:337–350. doi: 10.1037/met0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dayan CM, Panicker V. Novel insights into thyroid hormones from the study of common genetic variation. Nat Rev Endocrinol. 2009;5:211–218. doi: 10.1038/nrendo.2009.19. [DOI] [PubMed] [Google Scholar]
- 122.Peeters RP, van Toor H, Klootwijk W, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 123.de Jong FJ, Peeters RP, den Heijer T, et al. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab. 2007;92:636–640. doi: 10.1210/jc.2006-1331. [DOI] [PubMed] [Google Scholar]
- 124.Torlontano M, Durante C, Torrente I, et al. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab. 2008;93:910–913. doi: 10.1210/jc.2007-1067. [DOI] [PubMed] [Google Scholar]
- 125.Yalakanti D, Dolia PB. Association of type II 5′ monodeiodinase Thr92Ala single nucleotide gene polymorphism and circulating thyroid hormones among type 2 diabetes mellitus patients. Indian J Clin Biochem. 2016;31:152–161. doi: 10.1007/s12291-015-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arici M, Oztas E, Yanar F, Aksakal N, Ozcinar B, Ozhan G. Association between genetic polymorphism and levothyroxine bioavailability in hypothyroid patients. Endocr J. 2018;65:317–323. doi: 10.1507/endocrj.EJ17-0162. [DOI] [PubMed] [Google Scholar]
- 127.Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102:1623–1630. doi: 10.1210/jc.2016-2587. [DOI] [PubMed] [Google Scholar]
- 128.Carlé A, Faber J, Steffensen R, Laurberg P, Nygaard B. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment – data using a blind, randomized, clinical study. Eur Thyroid J. 2017;6:143–151. doi: 10.1159/000469709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bianco AC, Kim BS. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes. 2018;25:341–346. doi: 10.1097/MED.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Santini F, Giannetti M, Ricco I, et al. Steady-state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3′-triiodothyronine sulfate (T3s) Endocr Pract. 2014;20:680–689. doi: 10.4158/EP13331.OR. [DOI] [PubMed] [Google Scholar]
- 131.Jonklaas J, Burman KD. Daily administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid. 2016;26:770–778. doi: 10.1089/thy.2015.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. 1999;5:233–238. doi: 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 133.Sullivan SD, Downs E, Popoveniuc G, Zeymo A, Jonklaas J, Burman KD. Randomized trial comparing two algorithms for levothyroxine dose adjustment in pregnant women with primary hypothyroidism. J Clin Endocrinol Metab. 2017;102:3499–3507. doi: 10.1210/jc.2017-01086. [DOI] [PubMed] [Google Scholar]
- 134.Velasco I, Taylor P. Identifying and treating subclinical thyroid dysfunction in pregnancy: emerging controversies. Eur J Endocrinol. 2018;178:D1–D12. doi: 10.1530/EJE-17-0598. [DOI] [PubMed] [Google Scholar]
- 135.Cappelli C, Castello R, Marini F, et al. Adherence to levothyroxine treatment among patients with hypothyroidism: a northeastern Italian survey. Front Endocrinol (Lausanne) 2018;9:699. doi: 10.3389/fendo.2018.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90:124–127. doi: 10.1210/jc.2004-1306. [DOI] [PubMed] [Google Scholar]
- 137.Baehr KM, Lyden E, Treude K, Erickson J, Goldner W. Levothyroxine dose following thyroidectomy is affected by more than just body weight. Laryngoscope. 2012;122:834–838. doi: 10.1002/lary.23186. [DOI] [PubMed] [Google Scholar]
- 138.De Lima JG, De Mesquita DJTM, Da Costa Fernandes F, et al. Comparison among the daily levothyroxine doses according to the etiology of hypothyroidism. J Endocrinol Metab. 2013;3:1–6. doi: 10.4021/jem165w. [DOI] [Google Scholar]
- 139.Ojomo KA, Schneider DF, Reiher AE, et al. Using body mass index to predict optimal thyroid dosing after thyroidectomy. J Am Coll Surg. 2013;216:454–460. doi: 10.1016/j.jamcollsurg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin J, Allemang MT, Mchenry CR. Levothyroxine replacement dosage determination after thyroidectomy. Am J Surg. 2013;205:360–363. doi: 10.1016/j.amjsurg.2012.10.015. discussion 363. [DOI] [PubMed] [Google Scholar]
- 141.Di Donna V, Santoro MG, De Waure C, et al. A new strategy to estimate levothyroxine requirement after total thyroidectomy for benign thyroid disease. Thyroid. 2014;24:1759–1764. doi: 10.1089/thy.2014.0111. [DOI] [PubMed] [Google Scholar]
- 142.Cooper DS, Specker B, Ho M, Sperling M. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the national thyroid cancer treatment cooperative registry. J Clin Endocrinol Metab. 1998;8:337–345. doi: 10.1007/s12020-015-0842-0. [DOI] [PubMed] [Google Scholar]
- 143.Carhill AA, Litofsky DR, Ross DS, et al. Long-term outcomes following therapy in differentiated thyroid carcinoma: Ntctcs registry analysis 1987–2012. J Clin Endocrinol Metab. 2015;100:3270–3279. doi: 10.1210/JC.2015-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 145.Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 146.Leung AKC, Leung AAC. Evaluation and management of the child with hypothyroidism. World J Pediatr. 2019;15:124–134. doi: 10.1007/s12519-019-00230-w. [DOI] [PubMed] [Google Scholar]
- 147.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 148.Chaker L, Korevaar TIM, Medici M, et al. Thyroid function characteristics and determinants: the Rotterdam study. Thyroid. 2016;26:1195–1204. doi: 10.1089/thy.2016.0133. [DOI] [PubMed] [Google Scholar]
- 149.Huang HK, Wang JH, Kao SL. Association of hypothyroidism with all-cause mortality: a cohort study in an older adult population. J Clin Endocrinol Metab. 2018 doi: 10.1210/jc.2018-00408. [DOI] [PubMed] [Google Scholar]
- 150.Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65–68. doi: 10.1016/j.ejim.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 151.Mariotti S, Franceschi C, Cossarizza A. The aging thyroid. Endocr Rev. 1995;16:686–715. doi: 10.1210/edrv-16-6-686. [DOI] [PubMed] [Google Scholar]
- 152.Bano A, Chaker L, Schoufour J, et al. High circulating free thyroxine levels may increase the risk of frailty: the Rotterdam study. J Clin Endocrinol Metab. 2018;103:328–335. doi: 10.1210/jc.2017-01854. [DOI] [PubMed] [Google Scholar]
- 153.Davis PJ, Tang HY, Hercbergs A, Lin HY, Keating KA, Mousa SA. Bioactivity of thyroid hormone analogs at cancer cells. Front Endocrinol (Lausanne) 2018;9:739. doi: 10.3389/fendo.2018.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pasqualetti G, Calsolaro V, Bernardini S, et al. Degree of peripheral thyroxin deiodination, frailty, and long-term survival in hospitalized older patients. J Clin Endocrinol Metab. 2018;103:1867–1876. doi: 10.1210/jc.2017-02149. [DOI] [PubMed] [Google Scholar]
- 155.Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association executive committee. Clin Endocrinol (Oxf) 2016;84:799–808. doi: 10.1111/cen.12824. [DOI] [PubMed] [Google Scholar]
- 156.Larisch R, Midgley JEM, Dietrich JW, Hoermann R. Symptomatic relief is related to serum free triiodothyronine concentrations during follow-up in levothyroxine-treated patients with differentiated thyroid cancer. Exp Clin Endocrinol Diabetes. 2018;126:546–552. doi: 10.1055/s-0043-125064. [DOI] [PubMed] [Google Scholar]
- 157.Wan W, Farboud B, Privalsky ML. Pituitary resistance to thyroid hormone syndrome is associated with T3 receptor mutants that selectively impair beta2 isoform function. Mol Endocrinol. 2005;19:1529–1542. doi: 10.1210/me.2005-0014. [DOI] [PubMed] [Google Scholar]
- 158.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf) 2014;81:633–641. doi: 10.1111/cen.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goede SL, Leow MKS, Smit JW, Klein HH, Dietrich JW. Hypothalamus-pituitary-thyroid feedback control: implications of mathematical modeling and consequences for thyrotropin (TSH) and free thyroxine (FT4) reference ranges. Bull Math Biol. 2014;76:1270–1287. doi: 10.1007/s11538-014-9955-5. [DOI] [PubMed] [Google Scholar]
- 160.Dietrich JW, Landgrafe-Mende G, Wiora E, et al. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol (Lausanne) 2016;7:57. doi: 10.3389/fendo.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Watt T, Groenvold M, Rasmussen ÅK, et al. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–510. doi: 10.1530/eje.1.02124. [DOI] [PubMed] [Google Scholar]
- 162.Sawka AM, Naeem A, Jones J, et al. Persistent posttreatment fatigue in thyroid cancer survivors: a scoping review. Endocrinol Metab Clin North Am. 2014;43:475–494. doi: 10.1016/j.ecl.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 163.Boesen VB, Feldt-Rasmussen U, Bjorner JB, et al. How should thyroid-related quality of life be assessed? Recalled patient-reported outcomes compared to here-and-now measures. Thyroid. 2018;28:1561–1570. doi: 10.1089/thy.2018.0210. [DOI] [PubMed] [Google Scholar]
- 164.Watt T, Hegedüs L, Bjorner JB, et al. Is thyroid autoimmunity per se a determinant of quality of life in patients with autoimmune hypothyroidism. Eur Thyroid J. 2012;1:186–192. doi: 10.1159/000342623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012 doi: 10.1155/2012/590648. 590648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goede SL, Latham KR, Leow MKS, Jonklaas J. High resolution free triiodothyronine-thyrotropin (FT3-TSH) responses to a single oral dose of liothyronine in humans: evidence of distinct inter-individual differences unraveled using an electrical network model. J Biol Systems. 2017;25:119–143. doi: 10.1142/S0218339017500073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leese GP, Soto-Pedre E, Donnelly LA. Liothyronine use in a 17 year observational population-based study – the TEARS study. Clin Endocrinol (Oxf) 2016;85:918–925. doi: 10.1111/cen.13052. [DOI] [PubMed] [Google Scholar]
- 168.Tariq A, Wert Y, Cheriyath P, Joshi R. Effects of long-term combination LT4 and LT3 therapy for improving hypothyroidism and overall quality of life. South Med J. 2018;111:363–369. doi: 10.14423/SMJ.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Brix TH, Hegedüs L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res. 2014;29:2040–2050. doi: 10.1002/jbmr.2244. [DOI] [PubMed] [Google Scholar]
- 170.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018;28:566–574. doi: 10.1089/thy.2017.0517. [DOI] [PubMed] [Google Scholar]
- 171.Hung CL, Yeh CC, Sung PS, et al. Is partial or total thyroidectomy associated with risk of long-term osteoporosis: a nationwide population-based study. World J Surg. 2018;42:2864–2871. doi: 10.1007/s00268-018-4573-2. [DOI] [PubMed] [Google Scholar]
- 172.Somwaru LL, Arnold AM, Cappola AR. Predictors of thyroid hormone initiation in older adults: results from the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2011;66:809–814. doi: 10.1093/gerona/glr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174:32–39. doi: 10.1001/jamainternmed.2013.11312. [DOI] [PubMed] [Google Scholar]
- 174.Dickey RA, Wartofsky L, Feld S. Optimal thyrotropin level: Normal ranges and reference intervals are not equivalent. Thyroid. 2005;15:1035–1039. doi: 10.1089/thy.2005.15.1035. [DOI] [PubMed] [Google Scholar]
- 175.Hoermann R, Midgley JEM. TSH measurement and its implications for personalised clinical decision-making. J Thyroid Res. 2012;2012 doi: 10.1155/2012/438037. 438037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoermann R, Midgley JEM, Giacobino A, et al. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014;81:907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 177.Peterson SJ, Mcaninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy. J Clin Endocrinol Metab. 2016;101:4964–4973. doi: 10.1210/jc.2016-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Louwerens M, Appelhof BC, Verloop H, et al. Fatigue and fatigue-related symptoms in patients treated for different causes of hypothyroidism. Eur J Endocrinol. 2012;167:809–815. doi: 10.1530/EJE-12-0501. [DOI] [PubMed] [Google Scholar]
- 179.Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98:3562–3571. doi: 10.1210/jc.2013-1315. [DOI] [PubMed] [Google Scholar]
- 180.Atzmon G, Barzilai N, Surks MI, Gabriely I. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab. 2009;94:4768–4775. doi: 10.1210/jc.2009-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bowers J, Terrien J, Clerget-Froidevaux MS, et al. Thyroid hormone signaling and homeostasis during aging. Endocr Rev. 2013;34:556–589. doi: 10.1210/er.2012-1056. [DOI] [PubMed] [Google Scholar]
- 182.Jansen SW, Roelfsema F, Van Der Spoel E, et al. Familial longevity is associated with higher TSH secretion and strong TSH-FT3 relationship. J Clin Endocrinol Metab. 2015;100:3806–3813. doi: 10.1210/jc.2015-2624. [DOI] [PubMed] [Google Scholar]
- 183.Wang LY, Smith AW, Palmer FL, et al. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid. 2015;25:300–307. doi: 10.1089/thy.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hamaker EL. Why researchers should think “within-person”: a paradigmatic rationale. In: Mehl MR, Conner TS, editors. Handbook of research methods for studying daily life. New York: Guilford Press; 2012. pp. 43–61. [Google Scholar]
- 185.Fitzgerald SP, Bean NG. The relationship between population T4/TSH setpoint data and T4/TSH physiology. J Thyr Res. 2016;2016 doi: 10.1155/2016/6351473. 6351473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ebook: Homeostasis and allostasis of thyroid function. Dietrich JW, Midgley JEM, Hoermann R, editors. Front Endocrinol (Lausanne) 2018;9:287. doi: 10.3389/978-2-88945-570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McAninch EA, Bianco AC. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol. 2015;3:756–758. doi: 10.1016/S2213-8587(15)00325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]