We provide evidence for the effectiveness of acupuncture in an animal model of alcohol dependence.
Abstract
A withdrawal-associated impairment in β-endorphin neurotransmission in the arcuate nucleus (ARC) of the hypothalamus is associated with alcohol dependence characterized by a chronic relapsing disorder. Although acupuncture activates β-endorphin neurons in the ARC projecting to the nucleus accumbens (NAc), a role for ARC β-endorphin neurons in alcohol dependence and acupuncture effects has not been examined. Here, we show that acupuncture at Shenmen (HT7) points attenuates behavioral manifestation of alcohol dependence by activating endorphinergic input to the NAc from the ARC. Acupuncture attenuated ethanol withdrawal tremor, anxiety-like behaviors, and ethanol self-administration in ethanol-dependent rats, which are mimicked by local injection of β-endorphin into the NAc. Acupuncture also reversed the decreased β-endorphin levels in the NAc and a reduction of neuronal activity in the ARC during ethanol withdrawal. These results suggest that acupuncture may provide a novel, potential treatment strategy for alcohol use disorder by direct activation of the brain pathway.
INTRODUCTION
Alcohol use disorder (AUD) is characterized by chronic relapse after periods of abstinence and remains one of the world’s substantial public health problems. The most important method for successful treatment of AUD is to prevent the high rate of craving and relapse. Unfortunately, there is no satisfactory medical intervention to prevent alcohol relapse. Chronic ethanol consumption induces a reduction in β-endorphin neuron activity in the mediobasal hypothalamus, as indicated by the low levels of proopiomelanocortin messenger RNA in ethanol-dependent rats (1). The reduced β-endorphinergic activity in the hypothalamus may be responsible for the dysphoric and depressive states associated with ethanol withdrawal and may lead to the continued ethanol use (2). In addition, it has been proposed that β-endorphin in the nucleus accumbens (NAc) can modulate the behavioral response to stress (3) that can contribute to the development of alcohol dependence (4). Withdrawal from chronic ethanol administration causes the reduction of extracellular dopamine (DA) levels in the NAc, which is considered one of the critical underlying causes of a negative affect state and physical withdrawal signs associated with ethanol withdrawal (5, 6) and provokes relapse to alcohol drinking behavior (7). There is considerable evidence supporting the interaction between the endogenous opioid system and dopaminergic neurotransmission in the NAc (8). Thus, it is expected that β-endorphin may play an important role in attenuating ethanol dependence.
Acupuncture stimulates β-endorphinergic and enkephalinergic fibers in the arcuate nucleus (ARC) of the hypothalamus (9), and endorphinergic neurons originating from the ARC can, in turn, activate opioid receptors expressed on γ-aminobutyric acid (GABA) neurons in the NAc (10)—structures that are involved in ethanol reinforcement. It has been demonstrated that the endogenous opioid system is implicated in the acupuncture’s ability in suppressing the reinforcing effects of abused drugs (11). This is supported by studies showing that systemic administration of the nonselective opioid receptor antagonist naloxone inhibits ethanol intake in ethanol-dependent rats (12).
We have previously observed that acupuncture at Shenmen (HT7) point, located on the ulnar aspect of the wrist, may play an important role in modulating mesolimbic DA release and ethanol self-administration through activation of endorphinergic input to the NAc (13) and in reducing behavioral withdrawal signs by restoring DA levels in the NAc (14). As β-endorphin enhances DA release in the NAc (15), it was hypothesized that acupuncture might stimulate hypothalamic endorphinergic fibers and normalize the decreased β-endorphin levels in the NAc during withdrawal from chronic ethanol exposure, thus preventing physical withdrawal signs and negative emotional state induced by alcohol withdrawal by restoring DA levels in the NAc. To test this hypothesis, we evaluated (i) the effect of acupuncture on physical and psychological signs of ethanol withdrawal in physically ethanol-dependent rats and (ii) the roles of the endogenous opioid system in the NAc in acupuncture and ethanol withdrawal effects.
RESULTS
Induction of physical dependence on ethanol and measurement of tremor
There were no significant differences in body weights between the control group and ethanol groups during the consumption period of total 16 days (fig. S1A). This suggests that their dietary intake of calories was not affected by the liquid ethanol diet. The average of ethanol consumption during the 9-day period was 10.3 ± 0.2 g kg−1 day−1 and 10.3 ± 0.4 g kg−1 day−1 in the Con-ethanol and HT7-ethanol group, respectively (fig. S1B). The mean blood ethanol concentrations (BECs) for the control group, ethanol group, and ethanol + HT7 group were 5.7 ± 1.3 mg/dl, 198.9 ± 9.0 mg/dl, and 180.7 ± 9.1 mg/dl, respectively. BEC was higher in the ethanol groups than in the control group (F2,18 = 122.976, P < 0.001; P < 0.001 versus control group; fig. S1C). These BECs were sufficiently high to produce physical and psychological withdrawal signs of dependence, as described by others (16). Moreover, rats receiving acupuncture at HT7 had BEC that did not differ significantly from the control rats, suggesting that acupuncture did not affect their ethanol metabolism. In the present study, it was shown that small amount of ethanol has been measured in the samples taken from the control rats. The commercial assay we used to measure the ethanol level actually measures the level of hydrogen peroxide, which is the product of ethanol oxidation. The assay includes alcohol oxidase, which oxidizes ethanol into hydrogen peroxide and measures the plasma level of hydrogen peroxide in terms of the ethanol level. Because small amount of hydrogen peroxide is present in every organism as a result of metabolism, the measurement is due to hydrogen peroxide naturally present in the rats and it is not the product of ethanol oxidation. Therefore, because hydrogen peroxide is the one that reacts with the assay probe, samples of the control rats show fluorescence.
Tremor was quantified in a real-time manner using automatic tremor activity monitoring system (ATAMS; fig. S2A). Power spectra for tremor were estimated by testing the effects of harmaline (10 mg/kg), the tremorgenic compound (17), on the distribution of frequency power generated from ATAMS force transducer in normal rats. Harmaline induced a motion power peak in the 10- to 22-Hz band (P < 0.05 versus pre-harmaline; fig. S2, B and C). Similar to harmaline data, the motion power between 10 and 22 Hz was greater in the ethanol diet rats than in the control diet rats during the withdrawal period (treatment F1,126 = 145.037, P < 0.001; time F20,126 = 8.826, P < 0.001; interaction F20,126 = 5.339, P < 0.001; fig. S2, D and E). On the basis of the results from validation of ATAMS using harmaline, ethanol withdrawal tremor was measured in motion power between 10 and 22 Hz for 5-min segment at each time point.
Effects of acupuncture on ethanol withdrawal tremor
Tremor behaviors were monitored through ATAMS up to 24 hours after ethanol withdrawal. The ethanol-withdrawn rats exhibited significant increases in tremor ranging from 172 to 270% above baseline tremor (0 hour) between 2 and 5 hours after ethanol withdrawal (group F1,36 = 6.697, P = 0.061; time F9,36 = 2.489, P = 0.025; interaction F9,36 = 4.459, P < 0.001; P < 0.05 versus control group; Fig. 1, A and B). On the basis of these data, we chose the 2-hour time point after withdrawal to evaluate the effects of acupuncture on ethanol withdrawal tremor. The ethanol diet rats receiving HT7 acupuncture, but not those receiving LI5 acupuncture, showed a significant decrease in tremor compared with their pair-fed controls (F4,30 = 6.418, P < 0.001; P < 0.05, Con-ethanol group versus HT7-ethanol group; Fig. 1D). However, animals in the Con-HT7 group had tremor that did not differ from their control rats (P = 0.997, Con-control group versus HT7-control group).
Fig. 1. Effects of acupuncture on ethanol withdrawal tremor.
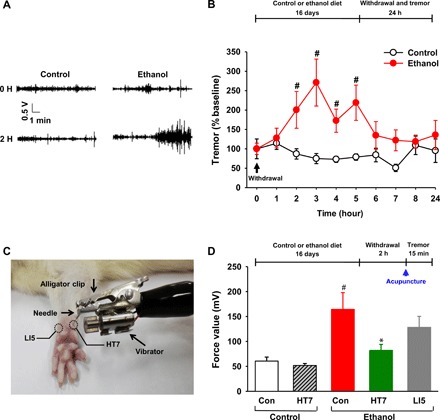
Rats on the chronic ethanol diet (ethanol group) received an ethanol-containing diet, and their pair-fed rats (control group) received an isocaloric diet with dextran substituted for ethanol. Rats received acupuncture at 2 hours after withdrawal from chronic ethanol consumption. Tremor was measured after acupuncture. (A and B) Representative force signals (filter, 10 to 22 Hz) at 0- and 2-hour time points in the control (n = 5) or ethanol (n = 5) group (A) and tremor activity expressed as a percentage of baseline tremor power at different time points (B). (C) Mechanical acupuncture instrument (MAI). (D) Effect of acupuncture on ethanol withdrawal tremor. Tremor was measured in rats given acupuncture at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. Another group of control diet rats (Con-control group) and ethanol diet rats (Con-ethanol group) did not receive any needle insertion. Con-control, n = 8; HT7-control, n = 7; Con-ethanol, n = 7; HT7-ethanol, n = 7; LI5-ethanol, n = 6. Graphs represent mean ± SEM. #P < 0.05 versus Con-control group, *P < 0.05 versus Con-ethanol group. Photo credit: Suchan Chang, College of Korean Medicine, Daegu Haany University.
Effects of naloxone on HT7 inhibition of ethanol withdrawal tremor
Tremor induced by ethanol withdrawal was alleviated by acupuncture at HT7 (P < 0.01, saline-ethanol group versus saline + HT7-ethanol group). In addition, there was a significant difference between naloxone and saline on inhibition of tremor produced by HT7 acupuncture (F4,34 = 15.040, P < 0.001; P < 0.001, saline + HT7-ethanol group versus naloxone + HT7-ethanol group; Fig. 2B). Naloxone slightly, but not significantly, increased tremor in the ethanol diet rats, compared to saline. Saline had no effect on HT7 suppression of tremor.
Fig. 2. Involvement of accumbal β-endorphin in acupuncture inhibition of ethanol withdrawal tremor.
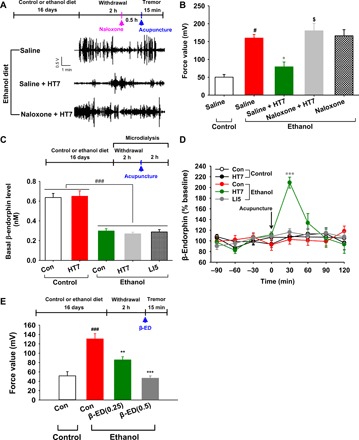
(A and B) Effect of naloxone on HT7 inhibition of ethanol withdrawal tremor. Representative examples of force signals in the ethanol group (filter: 10 to 22 Hz) (A) and tremor activity, expressed as mean values of tremor power for 15 min in the control and ethanol groups (B). Tremor was measured in ethanol-dependent rats given acupuncture at 30 min after intraperitoneal injection of naloxone. #P < 0.05 versus saline-control group (n = 8), *P < 0.05 versus saline-ethanol group (n = 7), $P < 0.05 saline + HT7-ethanol group (n = 7); naloxone + HT7-ethanol group, n = 7; naloxone-ethanol, n = 9. (C and D) Effect of HT7 acupuncture on extracellular β-endorphin levels in the NAc. Basal β-endorphin levels of ethanol groups were significantly lower than those of all other groups (###P < 0.001 versus all other groups, n = 6). Accumbal β-endorphin levels were measured in rats given acupuncture at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. ***P < 0.001 versus all other groups, n = 6 per group. (E) Effect of intra-NAc infusion of β-endorphin on ethanol withdrawal tremor. Tremor was measured in rats given intra-NAc infusions of β-endorphin at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. ###P < 0.001 versus saline-control group, **P < 0.01 and ***P < 0.001 versus saline-ethanol group; saline-control group, n = 6; saline-ethanol group, n = 7; β-ED(0.25)-ethanol group, n = 6; β-ED(0.5)-ethanol group, n = 5. Graphs represent mean ± SEM.
Effects of acupuncture on extracellular β-endorphin levels in the NAc
There was a significant difference in basal β-endorphin levels in the NAc between the control diet rats (0.64 ± 0.01 ng/ml) and the ethanol diet rats (0.30 ± 0.01 ng/ml) (P < 0.001; Fig. 2, C and F). The ethanol diet rats receiving HT7 acupuncture, but not those receiving LI5 acupuncture, showed a significant elevation in extracellular β-endorphin levels compared with their control rats (treatment F4,140 = 2.428, P = 0.082; time F7, 140 = 6.042, P < 0.001; interaction F28,140 = 4.567, P < 0.001; P < 0.001 versus all groups; Fig. 2D). On the other hand, acupuncture at HT7 did not affect extracellular β-endorphin levels in the NAc of the control diet rats.
Effects of intra-NAc infusions of β-endorphin on ethanol withdrawal tremor
We then evaluated the effects of β-endorphin on tremor in the ethanol diet rats using intra-NAc infusions of β-endorphin. Intra-NAc infusions of β-endorphin significantly decreased tremor activity in a dose-dependent fashion. There was a significant difference between β-endorphin and saline on tremor in the ethanol group (F3,20 = 20.414, P < 0.001; Fig. 2E).
Effects of acupuncture on c-Fos expression in ARC neurons
Rats receiving HT7 stimulation, but not rats receiving LI5 stimulation, showed a significant increase in the number of c-Fos–positive cells in the ARC compared to the control rats (F2,17 = 10.001, P = 0.002; P < 0.01 versus Con, P < 0.05 versus LI5; Fig. 3, A and B). c-Fos–positive cells in the retrogradely labeled ARC neurons were demonstrated in HT7-stimulated rats (Fig. 3A), showing activation of ARC neurons projecting to the NAc by acupuncture.
Fig. 3. Effects of acupuncture on hypothalamic ARC neuron activities.
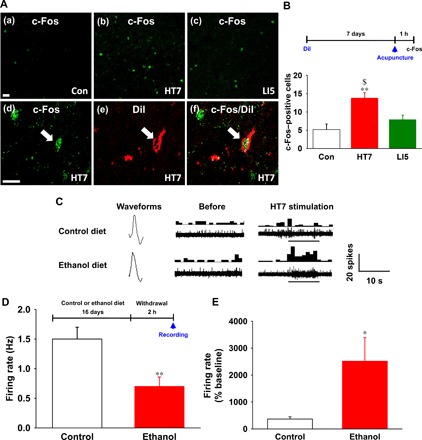
(A and B) Immunohistochemical staining of c-Fos in ARC neurons of control rats (a) and rats given acupuncture at HT7 or LI5 (b and c). A significant increase in the number of c-Fos–positive cells in the ARC was shown in rats subjected to HT7 acupuncture compared to control rats or rats subjected to LI5 acupuncture [#P < 0.05 versus Con, n = 6 per group; (B)]. c-Fos–positive cells (d) in ARC neurons labeled with DiI (e) in HT7 acupuncture–treated rats (f). Scale bars, 50 μm (200×). (C) Typical waveforms of spontaneous and evoked activity of ARC neurons before and during HT7 acupuncture in control (n = 7) or ethanol (n = 7) diet rats. Left, single action potential; middle and right, the continuous single action potential of real-time (waveform) (bottom) and peri-stimulus time histogram (one bin width per second, top). (D) Basal firing rates in the control and ethanol diet rats before acupuncture stimulation. **P < 0.01 versus control group. (E) Effect of acupuncture on firing rate of control and ethanol diet rats. Single-cell activity was analyzed by graphic recording for 20 s at rest and 10 s during acupuncture treatment. *P < 0.05 versus control group. Graphs represent mean ± SEM (n = 7 per group).
Effects of acupuncture on ARC neuron firing rate
A significant reduction in spontaneous activity of the ARC was shown in the ethanol group (0.70 ± 0.16 spikes/s) compared with the control group (1.50 ± 0.2 spikes/s; P = 0.009). Acupuncture at HT7 significantly elevated spontaneous discharge rates of ARC neurons in both the control diet rats and ethanol diet rats compared to those of baseline. The discharge rates of ARC neurons in the ethanol groups (10.92 ± 1.43 spikes/s) were significantly higher than those in the control group after acupuncture treatment (4.92 ± 0.87 spikes/s; P = 0.031, Fig. 3, C to E).
Effects of acupuncture or intra-NAc infusions of β-endorphin on anxiety-like behavior
Rats given yohimbine showed significantly lower percentage of time spent in the open arms than control rats given saline (P < 0.01 versus saline; Fig. 4A). Thus, the elevated plus maze apparatus can be used as an index of anxiety. While animals in the ethanol group had significantly higher percentage of time spent in open arm compared to animals in the control group (P < 0.001 versus Con-control or HT7-control group; Fig. 4B), rats (HT7-ethanol group) exhibited a marked elevation of percentage of time spent in the open arms following acupuncture (diet F1,31 = 21.362, P < 0.001; treatment F1,31 = 4.555, P = 0.041; interaction F1,31 = 3.554, P = 0.069; P < 0.05 versus Con-ethanol group; Fig. 4B). On the other hand, acupuncture at HT7 did not alter the time spent in open arm in the control diet rats, suggesting that acupuncture at HT7 may reduce anxiety-like behaviors without affecting the motor function. Similar to HT7 acupuncture, intra-NAc infusions of β-endorphin markedly increased the percentage of time spent in the open arms in ethanol diet–fed rats (F2,15 = 5.434, P < 0.05; Fig. 4C) or yohimbine-treated rats (fig. S4A). Intra-NAc infusions of β-endorphin itself did not affect anxiety-like behavior in control diet–fed rats (fig. S6). In addition, neither acupuncture (F3,31 = 2.049, P = 0.127; fig.S5, A and B) nor β-endorphin (F2,15 = 1.919, P = 0.181; fig.S5, C and D) affected the total distance of movement in the maze (fig. S5).
Fig. 4. Effects of acupuncture or intra-NAc infusions of β-endorphin on anxiety-like behavior in the elevated plus maze in ethanol-dependent rats.
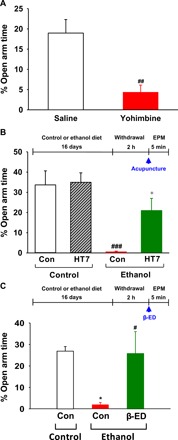
(A) Effect of yohimbine on the time spent in the open arms. The percentage of time spent in the open arms was measured in ethanol-naïve rats 30 min after injection of yohimbine (5 mg/kg, intraperitoneally) (##P < 0.01 versus saline, n = 5 per group). (B) Effect of acupuncture on the time spent in the open arms. The percentage of time spent in the open arms was measured in rats given acupuncture at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. ###P < 0.001 versus Con-control group (n = 9) or HT7-control group (n = 9), *P < 0.05 versus Con-ethanol group (n = 9); HT7-ethanol group (n = 8). (C) Effect of intra-NAc infusions of β-endorphin on the time spent in the open arms. The percentage of time spent in the open arms was measured in rats given intra-NAc infusions of β-endorphin at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. #P < 0.05 Con-control group, *P < 0.05 versus Con-ethanol group, n = 6 per group. Graphs represent mean ± SEM of the percentage of time spent in the open arms.
In another set of animals, to see whether acupuncture effects are associated with hypothalamic-pituitary-adrenal axis, we performed an additional experiment concerning the effect of acupuncture on plasma corticosterone levels in yohimbine (an anxiogenic drug)–treated rats. The rats were randomized into control (saline, n = 8), yohimbine (yohimbine only, n = 7), and acupuncture (yohimbine + acupuncture, n = 8) groups. Acupuncture was given at HT7 acupuncture points 30 min after an intraperitoneal injection of yohimbine. Plasma levels of corticosterone were determined before (baseline) an intraperitoneal injection of yohimbine 10 and 40 min after acupuncture treatment. Yohimbine increased plasma corticosterone levels to about 240 ng/ml 40 min after injection, which was significantly suppressed by acupuncture at HT7 (group factor F2,12 = 48.73, P < 0.001; time factor F2,12 = 49.77, P < 0.001; interaction F4,24 = 25.55, P < 0.001; fig. S4B).
Effects of HT7 acupuncture or intra-NAc infusions of β-endorphin on ethanol self-administration
The numbers of active lever presses were almost two times higher in the ethanol diet rats than in the control diet rats. There was a significant difference in ethanol-reinforced responding between the ethanol diet rats and the control diet rats (Fig. 5B). Acupuncture significantly reduced ethanol-paired lever responding in the ethanol diet rats (diet F1,25 = 2.740, P < 0.110; treatment F1,25 = 14.124, P < 0.001; interaction F1,25 = 5.928, P = 0.022; P < 0.05 versus Con-ethanol group; Fig. 5B). In addition, a decreased number of inactive lever responses were observed following acupuncture at HT7 in the control or ethanol diet rats (diet F1,25 = 1.005, P = 0.326; treatment F1,25 = 11.172, P = 0.003; interaction F1,25 = 0.208, P = 0.653; Fig. 5C). Animals receiving intra-NAc infusions of β-endorphin showed a significant decrease in ethanol self-administration rate compared to animals given saline (F2,18 = 10.316, P < 0.001; P < 0.05 versus Con-ethanol group; Fig. 5D). The inactive lever responding was not altered in animals receiving intra-NAc infusions of β-endorphin (Fig. 5E).
Fig. 5. Effects of acupuncture or intra-NAc infusions of β-endorphin on ethanol self-administration in ethanol-dependent rats.
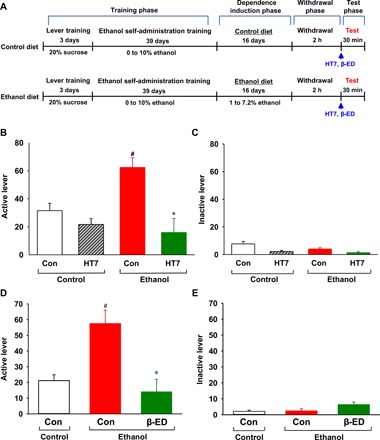
(A) Experimental procedures. (B and C) Effect of acupuncture on ethanol self-administration in dependent rats. Ethanol self-administration was measured in rats given acupuncture at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. #P < 0.05 versus Con-control group; *P < 0.05 versus Con-ethanol group, n = 6 per group. (D and E) Effect of intra-NAc infusions of β-endorphin on ethanol self-administration in ethanol-dependent rats. Ethanol self-administration was measured in rats given intra-NAc infusions of β-endorphin at 2 hours after withdrawal from chronic consumption of an ethanol liquid diet. #P < 0.05 versus Con-control group; *P < 0.05 versus Con-ethanol group; Con-control group, n = 6; Con-ethanol group, n = 8; β-ED-ethanol group, n = 7. Graphs represent mean ± SEM of lever presses.
To see whether acupuncture or β-endorphin itself influences general consummatory behaviors, we have performed further experiments with water self-administration using the same operant ethanol self-administration. Results showed that neither acupuncture nor intra-NAc infusions of β-endorphin affect the amount of water consumption in water-deprived rats. There was no significant difference (P = 0.671) in the baseline number of water infusions between the control group (n = 6) and the HT7 group (n = 6). Acupuncture at HT7 did not alter the number of water infusions compared to the control group (P = 0.501). In addition, there was no difference (P = 0.492) between control (n = 6) and β-endorphin (n = 6) in the baseline number of water infusions. Compared to saline, intra-NAc infusions of β-endorphin slightly, but not significantly, decreased the number of water infusions (P = 0.579) (fig.S7).
Effects of intra-NAc infusions of β-endorphin on levels of tyrosine hydroxylase immunoreactivity and phosphorylation state in the ventral tegmental area
While animals receiving intra-NAc infusions of artificial cerebrospinal fluid in the ethanol group (Con-ethanol group) had significantly decreased tyrosine hydroxylase (TH) expression of the ventral tegmental area (VTA) compared with the control diet rats (Con-control group), the number of TH-positive cells in the VTA in the ethanol diet rats receiving intra-NAc infusions of β-endorphin was significantly increased compared with the control rats (Con-ethanol group) (F2,17 = 33.196, P < 0.001; P < 0.05 versus Con-ethanol group; Fig. 6, A and B). To study the phosphorylation state of TH in the VTA, we analyzed the immunoresponse of THser40 (serine 40–phosphotyrosine hydroxylase), the phosphorylation form of TH, in the VTA. Figure 6 (C and D) shows that the ethanol diet rats receiving intra-NAc infusions of β-endorphin significantly increased the number of THser40-positive cells in the VTA compared to the control rats (Con-ethanol group) (F2,17 = 8.472, P = 0.003; P < 0.05 versus Con-ethanol group; Fig. 6, C and D).
Fig. 6. Effects of intra-NAc infusions of β-endorphin on TH expression and phosphorylation in the VTA.
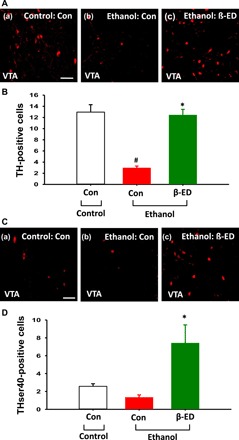
(A and B) Immunohistochemical staining of TH in VTA neurons of the ethanol group given intra-NAc infusions of β-endorphin at 2 hours after ethanol withdrawal (c). Another group of control diet rats (a) (Con-control group, n = 7) and ethanol diet rats (b) (Con-ethanol group, n = 7) received artificial cerebrospinal fluid infusion in place of β-endorphin infusion. A significant increase in the number of TH-positive cells in the VTA was shown in rats (β-ED-ethanol group, n = 6) subjected to intra-NAc infusions of β-endorphin compared to control rats [#P < 0.05, Con-control versus Con-ethanol; *P < 0.05, Con-ethanol versus β-ED-ethanol; (B)]. Scale bar, 50 μm (200×). (C and D) Immunohistochemical staining of THser40, the phosphorylation form of TH, in VTA neurons of ethanol group given intra-NAc infusions of β-endorphin at 2 hours after ethanol withdrawal (c). Another group of control diet rats (a) (Con-control group, n = 7) and ethanol diet rats (b) (Con-ethanol group, n = 7) received artificial cerebrospinal fluid infusion in place of β-endorphin infusion. A significant increase in the number of THser40-positive cells was shown in the VTA of rats (β-ED-ethanol group, n = 6) subjected to intra-NAc infusions of β-endorphin compared to control rats [*P < 0.05, Con-ethanol versus β-ED-ethanol; (E)]. Scale bar, 50 μm (200×).
DISCUSSION
Our present data showed that acupuncture at HT7, but not at LI5, significantly decreased ethanol withdrawal tremor. This agrees with previous reports that acupuncture can attenuate AUD-associated withdrawal syndrome in the ethanol-withdrawn animals and alcoholics (14, 18). As insertion of needles into acupuncture points could cause motor impairment and produce generalized effects on tremor activity, the present study attempted to control for this possibility by comparing HT7 acupuncture with LI5 acupuncture. HT7 acupuncture reduced tremor activity in ethanol-dependent rats, whereas it had no effect on tremor activity in the control diet–fed rats. Similar trends were demonstrated for anxiety-like behavior, ethanol self-administration, and a decrease in extracellular β-endorphin levels in the NAc. This suggests that there is an interaction between HT7 acupuncture and ethanol withdrawal responses. In the present study, systemic administration of naloxone, a nonselective opioid receptor antagonist, blocked HT7 suppression of ethanol withdrawal tremor. Furthermore, HT7 acupuncture prevented a decrease in extracellular β-endorphin levels in the NAc in the ethanol-dependent rats. These findings imply that HT7 stimulation produces accumbal β-endorphin–mediated inhibition of ethanol withdrawal tremor. Our results showed that HT7 stimulation inhibited a reduction of neuron firing rate in the ARC of the hypothalamus in the ethanol-withdrawn rats. Similarly, HT7 acupuncture caused neuronal activation in the ARC projecting to the NAc as demonstrated by a marked increase in c-Fos expression in the ARC. These results provide strong evidence that acupuncture enhances opioid-mediated transmission in the ARC, which projects to the NAc. As β-endorphin–containing fibers highly exist in the ARC (19), innervate the NAc (10), and mediate acupuncture inhibition of morphine withdrawal syndromes (11), we speculated that β-endorphinergic neurons projecting to the NAc from the ARC might contribute to the ability of acupuncture to reduce ethanol dependence. Accordingly, a local injection of β-endorphin into the NAc mimicked acupuncture-mediated suppression of ethanol withdrawal symptoms including tremor, anxiety-like behavior, and ethanol self-administration. These results support the acupuncture’s ability in suppressing ethanol withdrawal symptoms by activating β-endorphin–mediated transmission in the ARC projecting to the NAc. We have previously proposed a possible mechanism that might explain how acupuncture at HT7 might activate β-endorphin neurons in the ARC of the hypothalamus (13). HT7 acupuncture may activate peripheral mechanoreceptors, and the signals are conveyed through large A-fibers within the ulnar nerve, which lies adjacent to HT7 points (20) and the dorsal column somatosensory pathway (21). Sensory stimulation induced by acupuncture triggers β-endorphin neurons in the ARC (9) projecting to the NAc, thereby activating μ-opioid receptors on accumbal GABA neurons (10).
The present study indicated that acupuncture attenuated the decrease in open-arm exploration of the elevated plus maze in ethanol-dependent rats, suggesting an anxiolytic-like function for acupuncture. In addition, acupuncture reduced ethanol self-administration during ethanol withdrawal. Results showed that neither acupuncture nor β-endorphin altered water self-administration and locomotor activity in the elevated plus maze, implying that suppression of ethanol-reinforced responding and anxiety-like behavior by acupuncture and β-endorphin may not be due to reduction in general consummatory behavior and motor impairment. Together with the previous observation that anxiety-like state causes relapse to alcohol drinking in dependent rats (16), the present results suggest that the decreased alcohol drinking behavior induced by acupuncture might result from a diminished anxiety-like state. In addition, although the precise relationship between physical withdrawal syndrome and alcohol drinking behavior remains to be elucidated, ethanol withdrawal tremor has been suggested to be a powerful stressor (22). This supports the idea that tremor may play an important role as a driving factor for ethanol relapse (22). Together, results suggest that acupuncture may attenuate relapse to ethanol seeking in dependent rats by reducing tremor and anxiety-like state. In addition, a local injection of β-endorphin into the NAc decreased anxiety-like behavior and acupuncture reduced plasma corticosterone levels in yohimbine-treated rats, respectively. Yohimbine has been suggested to increase stress through noradrenergic control of the hypothalamo-pituitary adrenal system (23). Thus, this implies the possibility that the reduced stress response produced by acupuncture is mediated via an inhibition of the hypothalamo-pituitary adrenal system in response to β-endorphin in the NAc. Several studies have suggested that β-endorphin in the NAc would play an important role in mediating an adaptive response to stress with DA-independent mechanism. For example, it has been shown that extracellular levels of β-endorphin in the NAc increased in response to the stressful component of extinction of heroin-reinforced behavior and an aversive stimulus (3). A similar result was obtained in another study in which stress facilitates aversion to novelty feeding in mice with lowered β-endorphin (24). The exact neurochemical mechanism mediating a role for β-endorphin is unknown, but some evidence suggests that β-endorphin attenuates stress response by suppressing the secretion of corticotrophin-releasing factor (CRF) (25). Thus, it can be suggested that β-endorphin in the NAc may reduce relapse to ethanol seeking via an inhibition of stress response. Similarly, reduction in β-endorphinergic activity in the brain after chronic exposure to ethanol was suggested to be responsible for the dysphoric symptoms and anxiety state of ethanol withdrawal in alcoholics that might contribute to negative reinforcement (2, 8). In addition, the reduction in hypothalamic β-endorphinergic neuronal activity during ethanol withdrawal might mediate physical withdrawal syndrome and anxiety-like behavior (26). Together, these findings support the hypothesis that β-endorphin neurons in the ARC may mediate the acupuncture’s ability in suppressing ethanol withdrawal symptoms and relapse to alcohol drinking.
Our previous study has shown that HT7 acupuncture prevented a decrease of DA release in the NAc and physical signs of withdrawal during ethanol withdrawal (14). This study raised the possibility that acupuncture may play a functional role in reducing ethanol dependence by normalizing the release of DA in the NAc. Withdrawal from chronic ethanol administration causes a reduction of DA transmission in the mesolimbic pathway, suggesting a likely neurochemical mechanism of the negative affective state and somatic withdrawal signs during ethanol abstinence (5, 6), the intense ethanol craving experienced by addicts (27), and ethanol-seeking behavior in ethanol-dependent rats (7). As the negative affective state could motivate continued ethanol-seeking behavior in ethanol-dependent rats (7), the reduced ethanol self-administration produced by acupuncture or infusions of β-endorphin into the NAc is likely mediated via an inhibition of DA depletion in the NAc, as revealed by the parallel decreases of anxiety-like behavior. Our results showed that intra-NAc infusions of β-endorphin restored deficient TH expression in the VTA and increased TH phosphorylation in the VTA during ethanol withdrawal. This suggests that infusions of β-endorphin into the NAc induce the reversal of the hypodopaminergic state during ethanol withdrawal. Conditions that induce synaptic activation of the cell appear to facilitate the expression and phosphorylation of TH in peripheral sympathetic neurons and central catecholaminergic neurons (28). As accumbal GABA input to VTA DA neurons is modulated by opioid receptors (29), we speculate that β-endorphin may enhance DA transmission in the mesolimbic pathway. Thus, the increased VTA TH expression and phosphorylation in response to β-endorphin is likely due to an enhancement of VTA DA neuron activity. Together with the present results that local injection of β-endorphin into the NAc mimics acupuncture effects on ethanol withdrawal symptoms, it is likely that HT7 acupuncture reduces the negative affective state and alcohol drinking behavior in ethanol-dependent rats via activation of DA neurons in the VTA, which is caused by activation of endorphinergic input into the NAc from the ARC.
The present results demonstrated a significant increase in tremor, anxiety-like behavior, and ethanol self-administration during withdrawal from chronic ethanol. In addition, significant β-endorphin reduction in the NAc was observed in the ethanol-dependent rats. Previous studies have shown that the hypothalamic β-endorphinergic activity was decreased in the ethanol-dependent animals (1, 26). These findings raise the possibility that the diminished β-endorphin release in the NAc may be attributed to suppression of endorphinergic input to the NAc during withdrawal from chronic ethanol. Similarly, it has been shown that NAc β-endorphin levels in the ethanol-intoxicated state were significantly decreased compared with water-intoxicated state (30). As hypothalamic endorphinergic neurons innervate the NAc (10), the decreased β-endorphinergic neuron activity during ethanol withdrawal would result in the excitation of NAc GABA neurons in the NAc. In support of this hypothesis, previous studies using whole-cell recording and in vivo microdialysis revealed that NAc GABA neurons become hyperexcitable during withdrawal from repeated cocaine exposure (31). Although how β-endorphin modulates DA levels in the NAc still remains elusive, it has been shown that morphine inhibits GABA neurons in the NAc and consequently disinhibits DA neurons in the VTA (29). In addition, NAc GABA neurons synapse into DA neurons in the VTA (32, 33). Thus, we can speculate that β-endorphin exerts its inhibitory action on GABA neurons in the NAc projecting to VTA DA neurons via opioid receptors. Accordingly, it can be expected that the lower level of NAc β-endorphin, endogenous opioid peptides, during ethanol withdrawal than normal states may disinhibit GABA neurons in the NAc and subsequently cause decrement of DA neuronal activity in the VTA. A marked deficiency of DA release in the NAc and reduced firing rates of DA neurons in the VTA was shown in the ethanol-dependent rats, and reversals of the hypodopaminergic state in the NAc during ethanol withdrawal attenuated affective and somatic withdrawal signs (5, 6). On the basis of interactions of the endogenous opioid system and DAergic neurotransmission, inhibition of β-endorphin neurons by chronic exposure to ethanol might lead to reduction of DAergic activity in the mesolimbic DA system. Thus, the present results suggest that a significant decrease of accumbal β-endorphinergic activity may represent the underlying mechanism of, at least in part, the dysphoric and neurological symptoms of ethanol abstinence. It has been proposed that the dysphoric and depressive state during ethanol withdrawal may result from the reduced β-endorphinergic activity in the hypothalamus (2). While our and other animal studies revealed consistent results that acupuncture suppresses alcohol-related behaviors such as alcohol craving or consumption (13, 34), human studies have shown mixed results in the effects of acupuncture on reducing clinical symptoms related to alcohol addiction. For examples, controlled studies showed that severe recidivist alcoholics treated with acupuncture at points specific for the treatment of substance abuse expressed less need for alcohol and less drinking episodes, compared to the control group given acupuncture at nonspecific acupoints (35–37). However, Worner et al. (38) reported negative results of acupuncture treatment on alcohol-related symptoms. This discrepancy may be due to many vagaries including acupoint location, treatment duration, and stimulation frequency and intensity, leading to low reproducibility and high individual variations among acupuncturists in human studies. In support of this, our previous study demonstrated that considerable variations in intensity and frequency of acupuncture needle stimulation are observed within each acupuncturist or across acupuncturists (20).
In conclusion, we found that acupuncture inhibited tremor, anxiety-like behavior, and alcohol drinking behavior by restoring decreased β-endorphin levels in the NAc in physically ethanol-dependent rats. However, determination of the specific mechanisms involved in the neuronal links between acupuncture-induced sensory stimulation and hypothalamic β-endorphin neurons will require future study. Last, acupuncture may be an effective therapeutic intervention to treat AUD by direct activation of brain pathways.
MATERIALS AND METHODS
Subjects
Male Wistar rats (Orient Bio, Busan, Korea), weighing 250 to 280 g at the beginning of the experiment, were housed on a 12-hour light and dark cycle and received ad libitum food and water throughout the course of the experiment. All experimental procedures were approved by Daegu Haany University Institutional Animal Care and Use Committee and met National Institutes of Health guidelines for the care and use of laboratory animals.
Drugs
The following drugs were used: β-endorphin [a μ-opioid receptor agonist; 0.25 and 0.5 μg per side, dissolved in sterile saline (site), Bachem, Switzerland]; yohimbine (an anxiogenic compound; 5 mg/kg, dissolved in distilled water, intraperitoneal injection; Sigma-Aldrich, USA); naloxone (a nonselective opioid receptor antagonist; 1.0 mg/kg, dissolved in sterile saline, intraperitoneal injection; Sigma-Aldrich, USA); harmaline (a tremogenic compound; 10 mg/kg, dissolved in sterile saline, subcutaneous injection; Sigma-Aldrich, USA); and a retrograde tracer, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) (2%, dissolved in methanol, Life Technologies, USA).
Induction of physical dependence on ethanol
Rats were housed individually and given ad libitum access to a commercially available liquid diet (Dyets, Bethlehem, PA, USA), known as the Lieber-DeCarli diet. Rats in the chronic ethanol group (ethanol group) were exposed to 1 to 7% ethanol by gradually increasing ethanol concentration over the 7-day period and then maintained at a concentration of 7.2% for 9 days. Rats in the control group (control group) received ethanol-free isocaloric diets (or control diets) in which maltose dextrin replaced ethanol and the same amount of the standard liquid diet as their ethanol-paired rats consumed during the previous day.
Blood ethanol levels were measured 2 hours after removal of the ethanol diet on the last day of the ethanol liquid diet (16 days). To test the possibility that acupuncture might lower the blood ethanol level directly and attenuate alcohol dependence, we investigated whether acupuncture affected serum ethanol concentration in ethanol-dependent rats. Under isoflurane anesthesia, blood samples (0.5 ml) were taken by cardiac puncture 5 min after acupuncture stimulation. Serum ethanol levels were assayed using a commercially available colorimetric/fluorometric assay kit (BioVision, Milpitas, CA, USA).
Ethanol withdrawal tremor
This experiment examined the effects of acupuncture on ethanol withdrawal tremor in ethanol-dependent rats. Acupuncture was given for 20 s after withdrawals from chronic ethanol consumption. Ethanol withdrawal was precipitated by replacement of the ethanol-containing diet with the control liquid diet for 2 hours. To pharmacologically characterize the effects of acupuncture on ethanol withdrawal tremor, we gave rats an intraperitoneal injection of the nonselective opioid receptor antagonist naloxone (1.0 mg/kg) 30 min before acupuncture treatment. Rats also received intra-NAc infusions of β-endorphin (0.25 and 0.5 μg per side) to determine whether β-endorphin in the NAc can mimic acupuncture effect. As GABA neurons to the VTA projecting from the NAc were more abundant and more sensitive to μ-opioid receptor agonist in the shell than core (39), intra-infusions of β-endorphin were administered into the shell. Tremor was quantified in a real-time manner after acupuncture treatment by using a custom-made ATAMS (Fig. 2A), as previously described (40). In brief, the system was designed to monitor tremor activity through a force transducer (Grass Instruments, Braintree, MA, USA) mounted under a clear plastic cylinder (20 cm × 6 cm). The degree of tremor was measured for 15 min after placing each rat in the plastic cylinder. The second 5-min recording was performed for data. The signals from the force transducer were fed into bridged amplifiers (ETH-200, CB Sciences Inc., Dover, NH, USA), filtered between 10 and 22 Hz, and quantified using a LabChart and Scope program (ADInstruments). Power spectra for tremor were estimated by testing a tremogenic compound, harmaline. Harmaline, a derivative of β-carboline, is one of the most frequently used tremorgenic compound, and harmaline-induced tremor is regarded as a model for essential tremor in animals. It has been shown that subcutaneous or intraperitoneal injection of harmaline induces tremors within a few minutes in forelimb, hindlimb, head, or whole body, and the tremors last up to several hours (41). To find out specific oscillation frequencies to differentiate tremor-like activity from other movements, we assessed the motion powers during a 5-min pretreatment baseline and a 5-min posttreatment 10 min after subcutaneous injection of harmaline (10 mg/kg) in ethanol-naïve rats. On the basis of the results from validation of ATAMS using harmaline, ethanol withdrawal tremor was measured between 10 and 22 Hz. Tremor was expressed using the mean of motion power taken from a 5-min-long graphic recording.
In vivo microdialysis and measurement of extracellular β-endorphin levels
Microdialysis was conducted as previously described (14). One week before the start of the ethanol diet, a single guide cannula was stereotaxically implanted into the NAc shell [anteroposterior (AP), 1.7; mediolateral (ML), 0.8; and dorsoventral (DV), 6.0 from bregma] under anesthesia (42). On the day of testing, a microdialysis probe (2 mm length, 20 kDa cutoff value, polyarylethersulfone membrane, CMA 12 Elite, Carnegie Medicine, Stockholm, Sweden) was inserted into the guide cannula in the brain of unanesthetized rats before starting the microdialysis session. Artificial cerebrospinal fluid (150 mM NaCl, 3.0 mM KCl, 1.4 mM CaCl2, and 0.8 mM MgCl2 in 10 mM phosphate buffer at pH 7.4) was perfused at a rate of 2.0 μl/min using a microinfusion pump (CMA 100, Carnegie Medicine, Stockholm, Sweden) 2 hours before ethanol withdrawal. The microdialysis samples were collected at 30-min intervals from 2 hours before and up to 2 hours after the start of ethanol abstinence and stored frozen at −80°C until assayed. Acupuncture was given 2 hours after the start of ethanol withdrawal. Levels of β-endorphin were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Peninsula Laboratories Inc., Belmont, CA, USA) as described by others (3). Data in the microdialysates were reported from β-endorphin levels within the linear segment of the standard curve (0.05 to 5 ng/ml). The intra-assay coefficient was 4%, and the inter-assay coefficient was 13%. Baseline levels of β-endorphin were defined as the average value in four samples collected before acupuncture treatment.
Effects of acupuncture on neuronal activity in ARC neurons
To identify whether ARC neurons projecting to the NAc were activated by HT7 acupuncture, we assessed the expression of c-Fos in ARC neurons. DiI (2%) (a fluorescent retrograde neuronal tracer; Life Technologies, MA, USA) in 0.1 μl per side over 5 min was infused bilaterally into the NAc (AP, 1.7; ML, ±0.75; and DV, 5.7 from bregma) using a 10-μl Hamilton syringe under intraperitoneal sodium pentobarbital anesthesia (50 mg/kg). The tracer was allowed to spread for at least 7 days. After acupuncture treatment, rats were sacrificed and brains were prepared for c-Fos immunohistochemistry. Preparation of the brain tissue began with transcardial perfusion of 4% paraformaldehyde in 0.1 M phosphate buffer under pentobarbital anesthesia (80 mg/kg) 1 hour after acupuncture stimulation. Subsequently, brains were removed, immersed in 4% buffered paraformaldehyde for 2 hours, allowed to stand in 30% sucrose overnight, and cryosectioned at 30 μm (between 3.3 and 3.6 mm posterior to bregma). The brain slices were then mounted on gelatin-coated glass slides. The ARC labeled by DiI was identified with an Olympus AX70 fluorescence microscope (Olympus, Tokyo, Japan). For c-Fos immunohistochemistry study, the brain slices were incubated in blocking solution containing 3% bovine serum albumin for 1 hour at room temperature and incubated with anti–c-Fos rabbit polyclonal antibodies (1:1000, Santa Cruz Biotechnology, Santa Cruz, USA) for 24 hours at 4°C, followed by incubation with a biotinylated donkey anti-rabbit Alexa Fluor 488 (1:500, Invitrogen, Grand Island, NY, USA). Last, the sections were mounted onto gelatin-coated slides. The numbers of c-Fos immunoreactive neurons were counted under 200× with a confocal laser scanning microscope (LSM700, Carl Zeiss, Oberkochen, Germany) and averaged from three to four brain slices per rat.
Effects of acupuncture on ARC neuron firing rates
This experiment was carried out to investigate the effects of acupuncture on neuronal firing rates in the ARC in ethanol-dependent rats. Two hours after withdrawals from the chronic ethanol diet, rats were placed in a stereotaxic apparatus under pentobarbital anesthesia (50 mg/kg, intraperitoneally) for in vivo extracellular recordings of ARC neurons. Anesthesia level was maintained by intravenous infusion of sodium pentobarbital (15 mg kg−1 hour−1) via an indwelling catheter into the jugular vein during the recording. The responsiveness of ARC neurons (AP, −3.3 to 3.6; ML, 2.0 to 2.2; and DV, 10 to 11.1 from bregma) was recorded by in vivo extracellular recording technique as previously described (43). ARC neurons were triggered by acupuncture stimulation. A single microcarbon filament-filled glass electrode (carbon fiber electrode; impedence, 0.4 to 1.2 megohms; Kation Scientific, Minneapolis, MN, USA) was aimed at the ARC (AP, −3.3 to −3.6; ML, 2.0 to 2.2; and DV, 10 to 11.1 from bregma) at an angle of 10° with a one-axis water hydraulic micromanipulator (Narishige, Amityville, NY, USA) following needle insertion into HT7 point. After identification of a single ARC neuron, single-cell activity was amplified (voltage gain, 104), filtered at 0.3 to 10 kHz with an ISO-80 amplifier (World Precision Instruments, Sarasota, FL, USA), fed directly into the data acquisition unit (CED Micro1401; Cambridge Electronic Design, UK), and stored on computer to construct the waveforms or plot peri-stimulus time histograms (spikes per second bin width, Spike2 software). To ensure that a single ARC neuron was held for the duration of recording, we used the Spike2 program to confirm the same action potential shape and amplitude. Single-cell activity was expressed using the average value in three samples obtained from a 10-s-long graphic recording during acupuncture at an interval of 20 s between samples. Baseline single-cell activity was recorded for a 20-s-long graphic recording at rest following stabilization of single-unit firing rate for at least 10 min.
Elevated plus maze
Two hours after withdrawal from the chronic ethanol diet for 16 days, the elevated plus maze test was carried out for 5 min immediately after acupuncture or intra-NAc infusions of β-endorphin using an automated video tracking method as described earlier (16). For validation of open arm entries as an index of anxiety in our elevated plus maze apparatus (44), ethanol-naïve rats were given an intraperitoneal injection of yohimbine (5 mg/kg), an anxiogenic compound, 30 min before the elevated plus maze test. The elevated plus maze test was carried out after 2 hours of withdrawal from the chronic ethanol diet for 16 days (Fig. 5A), according to a previous method (16) with slight modifications. The elevated plus maze was made of dark acrylic consisted of two closed arms (50 cm × 10 cm) with 50-cm-high dark walls and two open arms (50 cm × 10 cm) with 0.5 cm high ledges. The maze was positioned 50 cm above floor under a condition of low-intensity light (1.5 to 2.0 lux) and white noise (70 dB). Before maze test, rats were habituated to the dark anteroom for 2 hours. Each rat was placed into the center of the maze facing a closed arm, and then the time spent in the open arms was measured for 5 min using an automated video-tracking method (EthoVision, Noldus Information Technology BV, Wageningen, The Netherlands). The percentage of time spent in the open arms was used as an index of anxiety (45).
Ethanol self-administration
The experimental procedure for operant ethanol self-administration in ethanol-dependent rats was based on a slight modification of methods used in earlier studies (7). Ethanol self-administration was carried out in daily 30-min sessions for 5 days a week during the dark cycle. After rats met criteria for stable responding with less than 20% variation in three consecutive sessions, rats were given 16 days of daily access to the ethanol liquid diet. Two hours after ethanol withdrawal on day 17, rats were given the opportunity to self-administer ethanol for 30 min after acupuncture treatment or intra-NAc infusions of β-endorphin. After completion of this test session, each rat was exposed again to the ethanol liquid diet for 4 to 5 days for the subsequent sessions identical to the first test. Each experiment was conducted using a within-subjects Latin square design, as described previously (7) (Fig. 5A). Operant test chambers (Med Associates Inc., Georgia, VT, USA) were equipped with two response levers and the house light that was illuminated during each self-administration session. Each press on the active lever delivered 0.1 ml of 10% (v/v) ethanol solution for 5 s to one of two drinking cups in the center panel of the operant chamber. Responses on the inactive lever had no programmed consequence but were recorded. During the lever training with sucrose solution, rats were subjected to a 22-hour water restriction schedule, with freely available food, in their home cage for successful acquisition of lever responding. The rats were trained in daily 30-min session to press a lever for a 20% (w/v) sucrose solution on a fixed ratio (FR1) reinforcement schedule until an acquisition criteria of 100 lever presses for three consecutive sessions was achieved. The rats were then trained to orally self-administer ethanol using a modified sucrose-fading method, as described previously (13). Following lever press training, rats self-administered 20% sucrose solution for the next 3 days. After establishment of stable lever responses, concentration of sucrose was gradually lowered to 0% and that of ethanol was raised to 10%. The detailed procedure was as follows: three to four sessions with 10% sucrose, three sessions with 2% ethanol in 10% sucrose, three sessions with 5% ethanol in 10% sucrose, and four sessions with 10% ethanol in 5% sucrose. Last, 10% ethanol without sucrose was introduced as the reinforcer. The rats met an established criterion for stable active lever responding with less than 20% variation in three consecutive responses. Typically, this required approximately 21 days following initiation of 10% ethanol self-administration. Different groups of animals were also subjected to water self-administration in an attempt to control for the effects of acupuncture or β-endorphin on general consummatory behaviors. Water self-administration took place using the same operant ethanol self-administration. Responses on the active lever produce a 0.1-ml drop of water, whereas responses on the inactive lever were recorded but had no consequence. Rats were trained to self-administer water for 30 min to facilitate lever pressing on an FR1 schedule at least three consecutive days. During the course of the entire sessions, rats were water-deprived for 16 hours. After rats exhibited stable water infusions in three consecutive responses with less than 20% variation, they were exposed to acupuncture or intra-NAc infusions of β-endorphin.
Intracranial infusions
To identify the effects of intra-NAc infusions of β-endorphin on ethanol tremor, anxiety-like behavior, ethanol self-administration, and levels of TH immunoreactivity in ethanol-dependent rats, bilateral guide cannulae (Plastic One, Roanoke, VA, USA) aimed at the NAc were implanted under anesthesia stereotaxically using the following coordinates: NAc shell region (AP, 1.7; ML, ±0.75; and DV, 5.7 from bregma). The atlas of Paxinos and Watson (42) was used as a reference. After 7 days of recovery, rats were fed by an ethanol-containing diet using the same ethanol diet schedule and then withdrawn from ethanol for 2 hours. Following a 2-hour ethanol withdrawal, β-endorphin (0.25 μg/0.5 μl per side) was injected bilaterally into the NAc more than 1 min with a 33-gauge injection needle extending 1 mm beyond the guide cannulae tip. The injectors were left in place for 2 min to allow diffusion into brain tissues before removal. β-Endorphin was dissolved in 0.9% sterile saline. An equal volume of saline was injected into the NAc for comparative purposes.
Histological confirmation of injection sites and microdialysis probes
After the completion of behavioral testing, rats were perfused transcardially with 4% paraformaldehyde under sodium pentobarbital (50 mg/kg, intraperitoneally) anesthesia. Brains were removed and fixed with 4% paraformaldehyde solution. The brains were cryosectioned at a thickness of 30 μm. Locations of microdialysis probe and infusion site were identified with 1% cresyl violet stain. Only those rats with correct placement were included in the data.
TH immunohistochemistry
Two hours after withdrawal from the chronic ethanol diet for 16 days, rats were given intra-NAc infusions of β-endorphin and sacrificed 5 min later. An equal volume of artificial cerebrospinal fluid was administered into the NAc for comparisons of β-endorphin effects. Brains were then prepared for measuring levels of TH immunoreactivity and phosphorylation state in the VTA. Brains were removed after transcardial perfusion of 4% paraformaldehyde solution at the time of euthanasia and allowed to stand in formalin fixative solution for 1 week. Brains were then cryosectioned into 30-μm-thick sections at the level of the VTA (coordinates: anterior, −5.8 to − 6.3 mm; lateral, ±0.8 mm; deep, −8.2 to − 8.5 mm). Coronal brain sections were incubated overnight (approximately 16 hours) at 4°C with rabbit polyclonal antibodies anti-TH (1:500; Millipore, USA) and anti-THser40 (1:500; Millipore, USA), followed by a 2-hour incubation at room temperature with a biotinylated donkey anti-rabbit Alexa Fluor 594 (red; 1:500; Sigma, USA), and mounted onto gelatin-coated slides. The sections were photographed and quantified using confocal laser scanning microscopy (LSM700, Carl Zeiss, Oberkochen, Germany). The three sections were collected at the level of the VTA in each brain for TH immunoreaction. The cells were counted and averaged from three sections.
Acupuncture treatment
Acupuncture was performed using our recently developed mechanical acupuncture instrument (MAI), as described in our previous publications (20). Acupuncture was given bilaterally at HT7 or LI5 acupuncture points for 20 s, while an assistant lightly held conscious rat without the use of anesthetics or sedatives. The chronic ethanol rats (ethanol-HT7 group) and their control rats (control-HT7 group) were withdrawn from ethanol for 2 hours and then immediately acupunctured. Another group of control diet rats (Con-control group) and ethanol diet rats (Con-ethanol group) did not receive any needle insertion. Stainless steel needles (0.10 mm in diameter and 7 mm in length; Dongbang Medical Co., Korea) were inserted vertically to a depth of 3 mm at acupuncture points and stimulated for 20 s at stimulus of 1.3 m/s2 in intensity and 85 Hz in frequency using MAI. MAI produced the vibration similar to that of manual acupuncture by delivering mechanical stimulation to the needle through an alligator clip attached to a cell phone vibrator (MB-1203 V, Motor Bank, Korea). Needle depths were controlled with a rubber grommet fixed to the needle at a distance of 3 mm from the tip. HT7 point is located on the transverse crease of the wrist of the forepaw, radial to the tendon of the flexor carpi ulnaris muscle. LI5 point, on the opposite side of HT7, was used as a control acupuncture point to control for nonspecific actions due to mechanical stimulation at certain area. HT7 acupuncture point on the heart channel has been used to treat mental and psychiatric disorders in clinic of oriental medicine. LI5 acupuncture point on the large intestine channel is known to be effective in treating disorders of the large intestine and the lung and skin disease. Locations of stimulated acupuncture points were anatomically those corresponding to acupuncture points in man (46). We also controlled for generalized effects of immobilized stress with rats lightly restrained by hands as the same method of each acupuncture treatment without insertion of needles. Before the application of acupuncture treatment, all rats were handled daily for 5 min during five consecutive days to facilitate handling and reduce stress.
Measurement of plasma corticosterone
Rats were slightly anesthetized with isoflurane. Blood samples (0.5 ml) were taken via jugular vein before (baseline) and 40 min and via abdominal vein 70 min after administration of yohimbine (5 mg/kg, intraperitoneally). Acupuncture was given 30 min after an intraperitoneal administration of yohimbine. Blood samples were collected in EDTA coating tube and centrifuged at 3500g for 10 min, and plasma was stored in refrigerator until analyzed. Plasma corticosterone levels were quantified using an ELISA kit (ADI-900-097, Enzo Life Sciences Inc., USA).
Statistical analysis
Statistical analysis was carried out using SPSS 11.0 software (IBM, Somers, NY, USA). All data were presented as mean ± SEM. Statistical significance was assessed using one- or two-way repeated-measures analysis of variance with post hoc Tukey tests or Student t tests, where appropriate. P values below 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank J. B. Kang for his helpful advice and B. Park for her English grammar correction. Funding: This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government MSIT (no. 2018R1A5A2025272). Author contributions: H.Y.K. and C.H.Y. designed the experiments. S.C., D.H.K., E.Y.J., S.S.Y., Y.S.G., Y.J.Y., J.Y.L., S.H.A., J.M.K., B.H.L., Y.-H.R., S.-N.K., H.S.R., M.-Y.L., and S.C.K. carried out the experiments and the data analysis. S.C., H.Y.K., and C.H.Y. drafted the manuscript. H.Y.K. and C.H.Y. were responsible for the overall direction of the project and for edits to the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data need to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax1342/DC1
Fig. S1. Effects of chronic ethanol consumption on growth rate and BEC.
Fig. S2. Measurement of ethanol withdrawal tremor.
Fig. S3. Schematic localization of microdialysis probes and infusion sites.
Fig. S4. Effects of intra-NAc infusions of β-endorphin on anxiety-like behavior in the elevated plus maze and acupuncture at HT7 on plasma corticosterone levels in yohimbine-treated rats.
Fig. S5. Effects of HT7 acupuncture and intra-NAc infusions of β-endorphin on locomotor activity in the elevated plus maze.
Fig. S6. Effects of intra-NAc infusions of β-endorphin on anxiety-like behavior in the elevated plus maze in control diet–fed rats.
Fig. S7. Effects of HT7 acupuncture and intra-NAc infusions of β-endorphin on water self-administration.
REFERENCES AND NOTES
- 1.Scanlon M. N., Lazar-Wesley E., Grant K. A., Kunos G., Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol. Clin. Exp. Res. 16, 1147–1151 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Gianoulakis C., Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr. Top. Med. Chem. 9, 999–1015 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Zangen A., Shalev U., Nucleus accumbens β-endorphin levels are not elevated by brain stimulation reward but do increase with extinction. Eur. J. Neurosci. 17, 1067–1072 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Stephens M. A. C., Wand G., Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 34, 468–483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diana M., Pistis M., Carboni S., Gessa G. L., Rossetti Z. L., Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: Electrophysiological and biochemical evidence. Proc. Natl. Acad. Sci. U.S.A. 90, 7966–7969 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossetti Z. L., Isola D., De Vry J., Fadda F., Effects of nimodipine on extracellular dopamine levels in the rat nucleus accumbens in ethanol withdrawal. Neuropharmacology 38, 1361–1369 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Weiss F., Parsons L. H., Schulteis G., Hyytiä P., Lorang M. T., Bloom F. E., Koob G. F., Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 16, 3474–3485 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sher L., Alcoholism, anxiety, and opioid-dopaminergic interactions. Psychopharmacology 165, 202–203 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z.-Q., Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85, 355–375 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J., Anatomy of CNS opioid receptors. Trends Neurosci. 11, 308–314 (1988). [DOI] [PubMed] [Google Scholar]
- 11.Cui C.-L., Wu L.-Z., Luo F., Acupuncture for the treatment of drug addiction. Neurochem. Res. 33, 2013–2022 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Overstreet D. H., Cui C.-L., Ma Y.-Y., Guo C.-Y., Han J.-S., Lukas S. E., Lee D. Y.-W., Electroacupuncture reduces voluntary alcohol intake in alcohol-preferring rats via an opiate-sensitive mechanism. Neurochem. Res. 33, 2166–2170 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Yang C. H., Yoon S. S., Hansen D. M., Wilcox J. D., Blumell B. R., Park J. J., Steffensen S. C., Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol. Clin. Exp. Res. 34, 2137–2146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B. H., Zhao R. J., Moon J. Y., Yoon S. S., Kim J.-A., An H., Kwon Y. K., Hwang M., Choi S. H., Shim I., Kim B. H., Yang C. H., Differential involvement of GABA system in mediating behavioral and neurochemical effect of acupuncture in ethanol-withdrawn rats. Neurosci. Lett. 443, 213–217 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Spanagel R., Herz A., Bals-Kubik R., Shippenberg T. S., β-Endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology 104, 51–56 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Valdez G. R., Sabino V., Koob G. F., Increased anxiety-like behavior and ethanol self-administration in dependent rats: Reversal via corticotropin-releasing factor-2 receptor activation. Alcohol. Clin. Exp. Res. 28, 865–872 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Martin F. C., Thu Le A., Handforth A., Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov. Disord. 20, 298–305 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Karst M., Passie T., Friedrich S., Wiese B., Schneider U., Acupuncture in the treatment of alcohol withdrawal symptoms: A randomized, placebo-controlled inpatient study. Addict. Biol. 7, 415–419 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Smyth D. G., 60 YEARS OF POMC: Lipotropin and beta-endorphin: A perspective. J. Mol. Endocrinol. 56, T13–T25 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kim S. A., Lee B. H., Bae J. H., Kim K. J., Steffensen S. C., Ryu Y.-H., Leem J. W., Yang C. H., Kim H. Y., Peripheral afferent mechanisms underlying acupuncture inhibition of cocaine behavioral effects in rats. PLOS ONE 8, e81018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang S., Ryu Y., Gwak Y. S., Kim N. J., Kim J. M., Lee J. Y., Kim S. A., Lee B. H., Steffensen S. C., Jang E. Y., Yang C. H., Kim H. Y., Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Sci. Rep. 7, 5359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Witte P., Pinto E., Ansseau M., Verbanck P., Alcohol and withdrawal: From animal research to clinical issues. Neurosci. Biobehav. Rev. 27, 189–197 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Johnston A. L., Baldwin H. A., File S. E., Measures of anxiety and stress in the rat following chronic treatment with yohimbine. J. Psychopharmacol. 2, 33–38 (1988). [DOI] [PubMed] [Google Scholar]
- 24.Barfield E. T., Moser V. A., Hand A., Grisel J. E., β-Endorphin modulates the effect of stress on novelty-suppressed feeding. Front. Behav. Neurosci. 7, 19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawasai O., Tadano T., Tan-No K., Niijima F., Sakurada S., Endo Y., Kisara K., Changes in β-endorphin and stress-induced analgesia in mice after exposure to forced walking stress. Methods Find. Exp. Clin. Pharmacol. 21, 471–476 (1999). [PubMed] [Google Scholar]
- 26.Rasmussen D. D., Boldt B. M., Wilkinson C. W., Mitton D. R., Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol. Clin. Exp. Res. 26, 535–546 (2002). [PubMed] [Google Scholar]
- 27.Weiss F., Porrino L. J., Behavioral neurobiology of alcohol addiction: Recent advances and challenges. J. Neurosci. 22, 3332–3337 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guitart X., Hayward M., Nisenbaum L. K., Beitner-Johnson D. B., Haycock J. W., Nestler E. J., Identification of MARPP-58, a morphine-and cyclic AMP-regulated phosphoprotein of 58 kDa, as tyrosine hydroxylase: Evidence for regulation of its expression by chronic morphine in the rat locus coeruleus. J. Neurosci. 10, 2649–2659 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui A., Jarvie B. C., Robinson B. G., Hentges S. T., Williams J. T., Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82, 1346–1356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granholm L., Segerström L., Nylander I., Episodic ethanol exposure in adolescent rats causes residual alterations in endogenous opioid peptides. Front. Psych. 9, 425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi Z.-X., Ramamoorthy S., Shen H., Lake R., Samuvel D. J., Kalivas P. W., GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J. Neurosci. 23, 3498–3505 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y., Ostlund S. B., James A. S., Park C. S., Ge W., Roberts K. W., Mittal N., Murphy N. P., Cepeda C., Kieffer B. L., Levine M. S., Jentsch J. D., Walwyn W. M., Sun Y. E., Evans C. J., Maidment N. T., Yang X. W., Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat. Neurosci. 17, 254–261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watabe-Uchida M., Zhu L., Ogawa S. K., Vamanrao A., Uchida N., Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Li J., Zou Y., Ye J.-H., Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res. Bull. 86, 428–434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullock M. L., Umen A. J., Culliton P. D., Olander R. T., Acupuncture treatment of alcoholic recidivism: A pilot study. Alcohol. Clin. Exp. Res. 11, 292–295 (1987). [DOI] [PubMed] [Google Scholar]
- 36.Bullock M. L., Culliton P. D., Olander R. T., Controlled trial of acupuncture for severe recidivist alcoholism. Lancet 1, 1435–1439 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Shin N. Y., Lim Y. J., Yang C. H., Kim C., Acupuncture for alcohol use disorder: A meta-analysis. Evid. Based Complement. Alternat. Med. 2017, 7823278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worner T. M., Zeller B., Schwarz H., Zwas F., Lyon D., Acupuncture fails to improve treatment outcome in alcoholics. Drug Alcohol Depend. 30, 169–173 (1992). [DOI] [PubMed] [Google Scholar]
- 39.Ma Y.-Y., Cepeda C., Chatta P., Franklin L., Evans C. J., Levine M. S., Regional and cell-type-specific effects of DAMGO on striatal D1 and D2 dopamine receptor-expressing medium-sized spiny neurons. ASN Neuro 4, e00077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.In S. L., Gwak Y. S., Kim H. R., Razzaq A., Lee K.-S., Kim H. Y., Chang S., Lee B. H., Grimes C. A., Yang C. H., Hierarchical micro/nano-porous acupuncture needles offering enhanced therapeutic properties. Sci. Rep. 6, 34061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miwa H., Rodent models of tremor. Cerebellum 6, 66–72 (2007). [DOI] [PubMed] [Google Scholar]
- 42.G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition (Elsevier, 2006). [Google Scholar]
- 43.Gwak Y. S., Kim H. K., Kim H. Y., Leem J. W., Bilateral hyperexcitability of thalamic VPL neurons following unilateral spinal injury in rats. J. Physiol. Sci. 60, 59–66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walf A. A., Frye C. A., The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruz A. P. M., Frei F., Graeff F. G., Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 49, 171–176 (1994). [DOI] [PubMed] [Google Scholar]
- 46.B. Pomeranz, G. Stux, B. Berman, Basics of Acupuncture (Springer, 2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax1342/DC1
Fig. S1. Effects of chronic ethanol consumption on growth rate and BEC.
Fig. S2. Measurement of ethanol withdrawal tremor.
Fig. S3. Schematic localization of microdialysis probes and infusion sites.
Fig. S4. Effects of intra-NAc infusions of β-endorphin on anxiety-like behavior in the elevated plus maze and acupuncture at HT7 on plasma corticosterone levels in yohimbine-treated rats.
Fig. S5. Effects of HT7 acupuncture and intra-NAc infusions of β-endorphin on locomotor activity in the elevated plus maze.
Fig. S6. Effects of intra-NAc infusions of β-endorphin on anxiety-like behavior in the elevated plus maze in control diet–fed rats.
Fig. S7. Effects of HT7 acupuncture and intra-NAc infusions of β-endorphin on water self-administration.


