Abstract
In recent years, circular RNAs (circRNAs) have been revealed to have important roles in carcinogenesis. Metastasis is the leading cause of lung adenocarcinoma (LUAC) death. However, the contributions of circRNA to the metastasis of LUAC remain largely unknown. Based on circBase data and our biobank tissues, we identified circCRIM1 (a circRNA derived from exons 2, 3 and 4 of the CRIM1 gene, hsa_circ_0002346) as having a significantly decreased expression in LUAC samples compared with matched normal control samples. Both in vivo and in vitro experiments revealed that circCRIM1 suppresses the invasion and metastasis of LUAC. In vitro precipitation of circRNAs, luciferase reporter assay, and biotin‐coupled microRNA capture were carried out to investigate the Ago2‐dependent interaction of circCRIM1 and microRNA (miR)‐93/miR‐182. Mechanistically, we found that circCRIM1 could promote the expression of leukemia inhibitory factor receptor, a well‐known tumor suppressor, by sponging miR‐93 and miR‐182. In the clinical and pathological analyses, the downregulation of circCRIM1 in LUAC was significantly correlated with lymphatic metastasis and TNM stage, which served as an independent risk factor for the overall survival of patients with LUAC. Our study showed that circCRIM1 inhibits the invasion and metastasis of lung adenocarcinoma cancer cells, which makes it a potential therapeutic target.
Keywords: circCRIM1, has‐miR‐93 and has‐miR‐182, invasion and metastasis, leukemia inhibitory factor receptor, lung adenocarcinoma
Abbreviations
- Ago2
Argonaute
- ceRNA
competing endogenous RNA
- CI
confidence interval
- circRNAs
circular RNA
- CISH
chromogenic in situ hybridization
- CRIM1
cysteine‐rich motor neuron 1
- HR
hazard ratio
- LIFR
leukemia inhibitory factor receptor
- LUAC
lung adenocarcinoma
- miR
microRNA
- miRNA
microRNA
- qRT‐PCR
quantitative real‐time PCR
- RTCA
real‐time cell analysis
- TMA
tissue microarray
1. INTRODUCTION
Lung cancer has a high incidence and mortality and threatens public health worldwide.1 Adenocarcinoma is the most common histologic type of lung cancer. Although many achievements have been made in diagnosis and therapy, the high rate of recurrence and metastasis in LUAC leads to a very poor prognosis, and the 5‐year overall survival rate of patients with lung cancer is lower than 20%.2, 3 Therefore, the high mortality rate and the variable treatment responses of LUAC urge us to search for novel molecular targets to develop more effective therapeutic strategies for LUAC.
Circular RNAs represent a novel class of widespread transcripts that form a covalently closed continuous loop.4, 5 Circular RNAs arise from exonic, intronic, or intergenic regions; exonic circRNAs, which are mainly located in the cytoplasm, are the end products of splicing and are the most studied type of circRNAs to date.6 Although some recent studies revealed that some circRNAs possess protein translation ability, circRNAs are mostly regarded as miRNA sponges,7, 8 because they contain conserved miRNA target sites, such as the Ago2 protein.9, 10 Studies have shown that the sequences of circRNAs are somewhat conserved, and their expression profiles are cell type‐ or developmental stage‐specific, suggesting that some circRNAs have regulatory functions in biological processes.11, 12 There is growing evidence that circRNAs might play an important role in the progression of some diseases, such as vascular disease risk, neurological disorders, prion diseases, osteoarthritis, diabetes, Parkinson's disease, Alzheimer's disease, and tumor development.9, 13, 14, 15 Although it has been reported that many genes relate to the carcinogenesis of LUAC, the molecular mechanisms of this process remain mostly obscure, and the function of circRNAs in LUAC tumor initiation and progression remains unclear.
In our study, we identified differentially expressed circRNAs in LUAC by analyzing data derived from circBase. We further characterized a circular RNA hsa_circ_0002346 that is produced from the CRIM1 amplicon at chromosome 2p22.2. Therefore, we termed it circCRIM1. We validated that circCRIM1 is almost 6.47‐fold downregulated in 40 pathologically diagnosed LUAC samples compared with their paired peripheral normal lung tissues by using quantitative PCR. In vitro experiments indicated that circCRIM1 repressed the invasion and metastasis of LUAC cell lines by interacting with miR‐182 and miR‐93. These findings contribute to an understanding of the processes involved in migration. Mechanistically, circCRIM1 could promote the expression of LIFR, a well‐known tumor suppressor, by sponging miRNAs. Taken together, we can conclude that circCRIM1 could act as a ceRNA to regulate LIFR expression by interacting with miR‐182 and miR‐93. This study describes a novel regulatory mechanism of circRNAs and provides a potential therapeutic target for lung cancer.
2. MATERIALS AND METHODS
2.1. Patient samples
Tumor tissues and paired peripheral normal lung tissues from LUAC patients who underwent surgery at the Department of Thoracic Surgery, Jiangsu Cancer Hospital (Nanjing, China) were collected subjected to qRT‐PCR, immunohistochemistry, and immunofluorescence analyses. All tumors and paired normal tissues were confirmed by pathologists. None of the patients received neoadjuvant therapy. The clinical and pathological characteristics of each patient were collected after surgery. This study was approved by the Ethics Committee of Jiangsu Cancer Hospital in accordance with the ethical standards. All participants provided written informed consent.
2.2. Cell culture
The human LUAC cell lines A549, H1299, H2228, pc9, and H1975 were purchased from ATCC and maintained in RPMI‐1640 (KeyGen), except for SPCA‐1 which was maintained in DMEM (KeyGen), and both media were supplemented with 10% FBS (Life Technologies) at 37°C in a humidified atmosphere with 5% CO2.
2.3. Quantitative PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen). Quantification of circRNA and mRNA was carried out using PrimeScript RT Master Mix (cat. #RR036A; Takara), and miRNA concentrations were determined using PrimeScript RT Master Mix (cat. #RR037A; Takara). Before calculation using the ΔΔCt method, the levels of GAPDH were used to normalize the relative expression levels of circRNA and mRNA, and the levels of small nuclear U6 were used to normalize the miRNA expression levels. The primers are provided in Table S1.
2.4. Tissue microarray and CISH
Tissue microarray was constructed as described.16 Ninety‐two pairs of LUAC tissues and their paired peripheral normal lung tissues were used to construct the TMA. RNA CISH was undertaken to detect circCRIM1 expression in TMA using digoxigenin labelled probe 3 (5′‐AGTCCAGTTCTCATCTTGTTGG‐3′). Briefly, the samples were fixed in 4% paraformaldehyde and hybridized with the digoxin‐labeled probe overnight at 55°C. The samples were then incubated overnight at 4°C with an anti‐digoxin mAb (Roche Applied Science). The sections were stained with nitro blue tetrazolium/5‐bromo‐4‐chloro‐3‐indolyl phosphate in the dark, mounted and observed.
2.5. Fluorescence in situ hybridization
We used FISH to detect the subcellular location of circCRIM1 in LUAC cells and also to detect the expression of circCRIM1 in LUAC tissues compared with normal tissues. A FISH probe was designed to detect the splicing junction of 2 exons, and the probe sequence was 5′‐FAM‐AGTCC AGTTC TCATC TTGTT GGCAA AGTAC‐FAM‐3′.
2.6. Small interfering RNA and plasmid construction and cell transfection
The circCRIM1 and LIFR siRNAs were provided by Life Technologies. The cDNA of circCRIM1 and LIFR was synthesized by Invitrogen and cloned into the expression vector pcDNA3.1. The miR‐182 and miR‐93 mimics were provided by RiboBio. The nucleotide sequences are listed in Table S1. Transfection was carried out using Lipofectamine 3000 (Invitrogen) according to the manufacturer's instructions.
2.7. In vitro cell migration and invasion assays
We harvested SPCA‐1 cells that had been transfected for 24 hours with si‐circCRIM1 or control siRNA. The transfected cells were plated in the upper chambers of Transwell assay inserts (Millipore) containing serum‐free DMEM with a membrane to test migration. The lower chambers were filled with RPMI DMEM containing 10% FBS. The cells on the filter surface were fixed with methanol, stained with crystal violet, and photographed with a digital microscope after 24 hours. The transfected cells were also plated in the top chamber containing a Matrigel‐coated membrane (BD Biosciences) in serum‐free DMEM to test cell invasion. There was also 10% FBS‐DMEM in the bottom chambers. The invasion ability was determined after 48 hours.
We also harvested H1299 cells that had been transfected for 24 hours with circCRIM1 plasmid or vector pcDNA3.1 and carried out cell migration and invasion assays.
2.8. In vivo metastasis assays
For in vivo metastasis assays, H1299 cells were inoculated into nude mice (4 mice per group) through the tail vein. After 4 weeks, the mice were killed, necropsies were carried out, and the lung metastatic nodules were counted. Staining with H&E confirmed that the nodules were metastatic tumors. The protocol used for these studies was approved by the Institutional Animal Care and Use Committee of the Affiliated Cancer Hospital of Nanjing Medical University.
2.9. Real‐time cell analysis
The RTCA system was applied to monitor cell migration by using cell migration plates. Then RPMI containing 10% FBS was placed in the lower chamber. The upper chamber was mounted, and 30 μL serum‐free medium was added to each well at 37°C and 5% CO2. Cells (40 000) were plated into each well of the upper chamber of the cell migration plates. Readings were recorded at 15‐minute intervals until the end of the experiment (up to 36 hours).
2.10. Separation of cytoplasm and nucleus fraction
The subcellular localization of circCRIM1 was detected using the PARIS Kit according to the manufacturer's protocol (Ambion, Life Technologies).
2.11. Luciferase reporter assay
The LIFR binding sites of miR‐RNA were predicted by TargetScan (http://www.targetscan.org/vert_71/). The different fragment sequences were synthesized and then inserted into the pGL3‐basic vector (Promega). All vectors were verified by sequencing and luciferase activity was assessed using the Dual Luciferase Assay Kit (Promega) according to the manufacturer's instructions.
2.12. RNA immunoprecipitation assay
RNA immunoprecipitation was carried out using anti‐Ago2 or control anti‐IgG Abs with Magna RIP RNA‐Binding Protein Immunoprecipitation Kit (Millipore). Beads were washed and purified RNA was subjected to qRT‐PCR analysis and determined circCRIM1 and miR‐93 and miR‐182 expression levels.
2.13. Biotin‐coupled miRNA capture
The biotin‐coupled miRNA pull‐down assay was carried out as previously described.17, 18 Briefly, the 3′‐end biotinylated miR‐RNA mimic or control biotin‐RNA (RiboBio) was transfected into SPCA1 cells at a final concentration of 20 nmol/L for 1 day. The biotin‐coupled RNA complex was pulled down by incubating the cell lysate with streptavidin‐coated magnetic beads (Ambion, Life Technologies). The abundance of circCRIM1 and LIFR in bound fractions was evaluated by qRT‐PCR analysis.
2.14. RNA sequencing
The Agilent human mRNA array was designed with 8 identical arrays per slide (8 × 60 K format), with each array containing probes interrogating approximately 27 958 Entrez Gene RNAs and 7419 long intergenic noncoding RNA. The array also contains 1280 Agilent control probes. Total RNA containing small RNA was extracted from cells by using TRIzol reagent (Invitrogen) and purified with the mirVana miRNA Isolation Kit (Ambion) according to the manufacturer's protocol. The purity and concentration of RNA were determined from optical density 260/280 readings using a spectrophotometer (NanoDrop ND‐1000). RNA integrity was determined by 1% formaldehyde denaturing gel electrophoresis.
2.15. Western blot analysis
Western blot analyses were carried out according to standard protocols. Anti‐β‐actin and anti‐LIFR were purchased from Abcam.
2.16. Statistical analysis
The numerical data are presented as the mean ± SD of at least 3 determinations. Statistical comparisons between groups of normalized data were undertaken using t tests and the Spearman correlation. Multivariate Cox regression was used to identify factors associated with survival of LUAC. Values of P < .05 were considered statistically significant with a 95% confidence.
3. RESULTS
3.1. CircCRIM1 is downregulated and correlated with poor clinical outcomes in LUAC
In our study, we first characterized circRNA transcripts in LUAC by analyzing data derived from circBase (http://www.circbase.org/) (Figure 1A).
Figure 1.
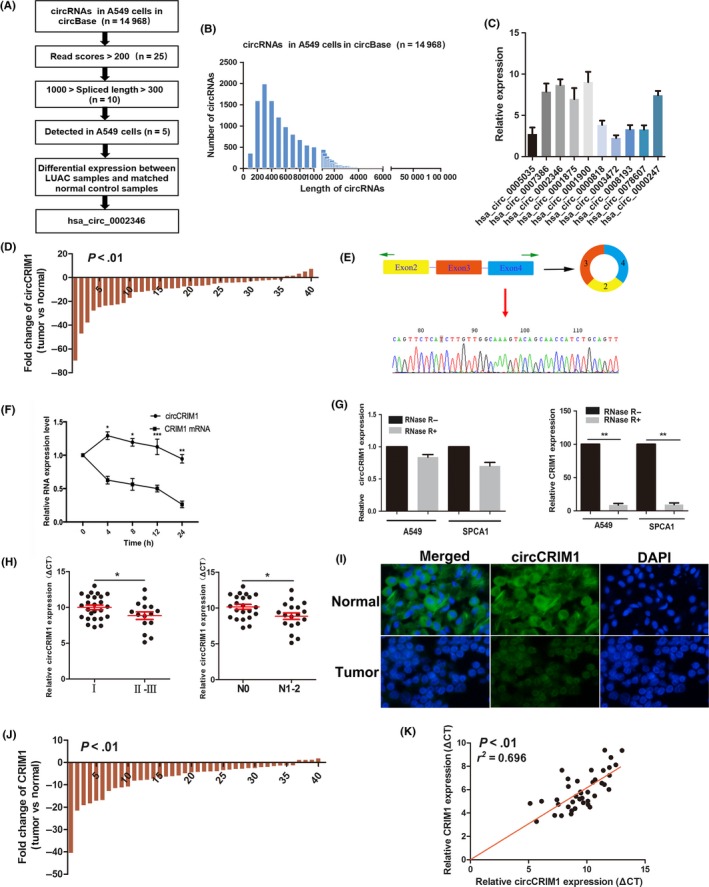
Characterization of circular RNA (circRNA) circCRIM1 downregulated in lung adenocarcinoma (LUAC) cancer tissues. A, Flowchart delineating the identification of circCRIM1. B, Length distribution of circRNAs in A549 cells in circBase in total. C, Five highly expressed circRNAs in A549 cells. D, hsa_circ_0002346 the most downregulated in LUAC samples compared with matched normal control samples. E, Schema illustrating the production of circCRIM1. F, Quantitative real‐time PCR was used to analyze the half‐life of circCRIM1 and CRIM1 mRNAs in H1299 cells. G, circCRIM1 was resistant to RNase‐R digestion but not linear CRIM1. H, Patients with TNM stage I and N0 showed higher expression of circCRIM1. I, circCRIM1 was downregulated in LUAC samples as determined by FISH assays. J, CRIM1 expression in 40 pairs of LUAC tumor tissues. K, CRIM1 expression was positively correlated with circCRIM1 expression
We detected 14 968 distinct circRNAs in A549 cells in circBase in total (Table S2), and the length of most these circRNAs was larger than 300 nt and less than 1000 nt (Figure 1B). After excluding the low abundance (read score <200) ones, we identified 25 circRNAs (Table S3), with 10 whose length is between 300 and 1000 nt. Further validating qRT‐PCR with divergent primers revealed 5 highly expressed circRNAs (hsa_circ_0002346, hsa_circ_0007836, hsa_circ_0001875, hsa_circ_0001900, and hsa_circ_0000247) in LUAC A549 cell (Figure 1C). Among these, hsa_circ_0002346 was the most downregulated in 30 pairs of LUAC samples compared with matched normal control samples (Figure S1). We added 10 more pairs of tumor tissue specimens and validated that circCRIM1 was almost 6.47‐fold downregulated in 40 pathologically diagnosed LUAC samples by using qRT‐PCR (Figure 1D).
The existence of 538‐nt has_circ_0002346 generated from 3 exons (exons 2, 3 and 4) of the CRIM1 gene was further confirmed with Sanger sequencing. Thus, we named hsa_circ_0002346 as “circCRIM1” (Figure 1E). Furthermore, we used actinomycin D to inhibit transcription and then measured the half‐life of circCRIM1 and CRIM1 in H1299 cells. The results showed that circCRIM1 was more stable than CRIM1 mRNA (Figure 1F). We detected that circCRIM1 was resistant to RNase‐R digestion, as measured by real‐time PCR. RNase‐R treatment decreased the linear CRIM1 mRNA but not circCRIM1 (Figure 1G); in general, circRNAs are more stable than their linear counterparts.
Patients with TNM stage I LUAC showed higher circCRIM1 expression than patients with TNM stage II and III LUAC (P = .029; Figure 1H). Patients with lymph node metastasis showed reduced expression of circCRIM1 (P = .013; Figure 1H). We also confirmed that circCRIM1 was downregulated in LUAC samples with FISH assays (Figure 1H). These results revealed that circCRIM1 might play a vital role in the tumor invasion and metastasis of LUAC.
Next, we examined CRIM1 expression in 40 pairs of LUAC tumor tissues. The CRIM1 expression was 4.73‐fold downregulated in tumor tissues compared with paired nontumor tissues and positively correlated with circCRIM1 expression (r 2 = 0.696, P < .01; Figure 1J,K). These results suggested that downregulated expression of circCRIM1 is partially due to CRIM1 gene downregulation.
3.2. CircCRIM1 inhibits LUAC cell migration and invasion
We first examined the expression of circCRIM1 in LUAC cell lines by qRT‐PCR before investigating its function in vitro. We found circCRIM1 was upregulated the most in SPCA‐1 and A549 cells and downregulated the most in H1299, pc9, and H2228 cells (Figure 2A). We designed siRNAs and cDNAs of circCRIM1 and tested their efficiency (Figure 2B).
Figure 2.
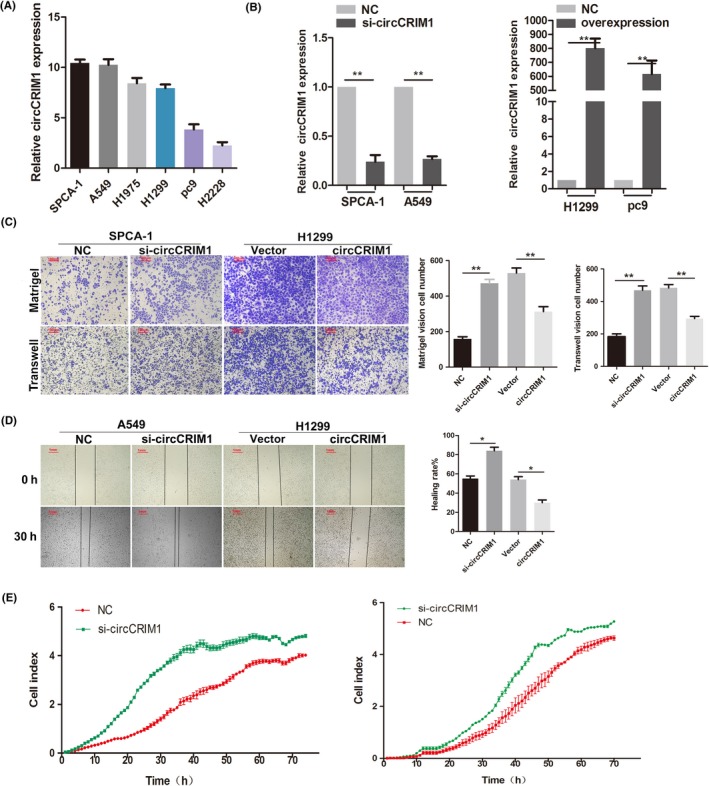
Circular RNA circCRIM1 suppresses the invasion and migration of lung adenocarcinoma cell lines in vitro. A, Expression of circCRIM1 in LUAC cell lines as determined by quantitative real‐time PCR. B, Quantitative real‐time PCR analysis of circCRIM1 RNA expression after treatment with circCRIM1 siRNAs and overexpression plasmids. C‐E, circCRIM1 reduced cell migration and invasion as determined by Transwell and Matrigel (C), wound healing (D), and real‐time cell analysis (E) assays. NC, negative control
Transwell and Matrigel assays were undertaken to further determine the role of circCRIM1 in LUAC progression. Our data showed that circCRIM1 overexpression significantly reduced cell migration and invasion (Figure 2C). The wound healing assay also showed similar results (Figure 2D). The RTCA also identified that circCRIM1 could suppress the migration ability of LUAC cells in silencing and overexpression experiments (Figure 2E). Taken together, these data suggest that circCRIM1 might serve as a tumor suppressor in LUAC progression.
However, ectopic expression of circCRIM1 did not affect cell proliferation (Figure S2).
Epithelial‐mesenchymal transition markers were analyzed in circCRIM1 overexpressed cells using immunofluorescence. The results showed that the E‐cadherin expression level was increased, and vimentin and N‐cadherin expression levels were decreased in circCRIM1‐overexpressing A549 cells (Figure 3A). These results also confirmed that circCRIM1 could suppress the migration and invasion of LUAC cells.
Figure 3.
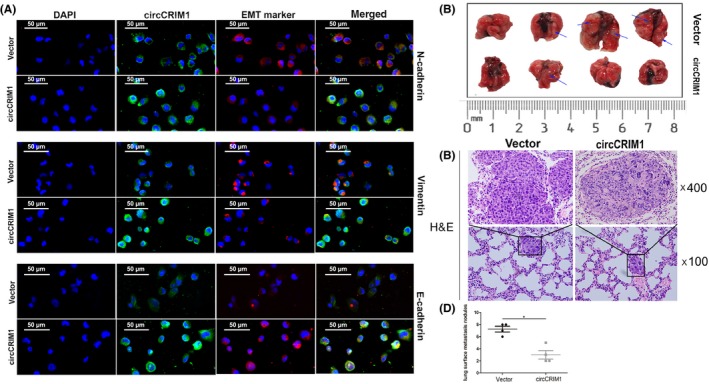
Circular RNA circCRIM1 suppresses lung adenocarcinoma cell metastasis in vitro and in vivo. A, Epithelial‐mesenchymal transition (EMT) markers were analyzed in cells overexpression circCRIM1 using immunofluorescence. B, Overexpression of circCRIM1 inhibited lung metastasis in the mouse i.v. injection model (n = 4 per group). C, H&E staining images. D, Number of lung metastases. *P < .05
Consistent with findings in vitro, using mice i.v. injection metastasis models, we evaluated the effect of circCRIM1 on LUAC metastasis. In the i.v. injection model, all of the mice injected with H1299 cells had metastatic nodules in the lung (Figure 3B,C). The number of lung metastases in mice in the circCRIM1 overexpression group was less than that in the control group (Figure 3D).
3.3. circCRIM1 serves as a sponge for both miR‐93 and miR‐182
To further explore the molecular mechanisms involved in the modulation of circCRIM1, we determined the subcellular location of circCRIM1. Both FISH and separation of cytoplasm and nucleus fraction RNA analyses showed that circCRIM1 predominantly resided in the cytoplasm of LUAC cells (Figure 4A,B).
Figure 4.
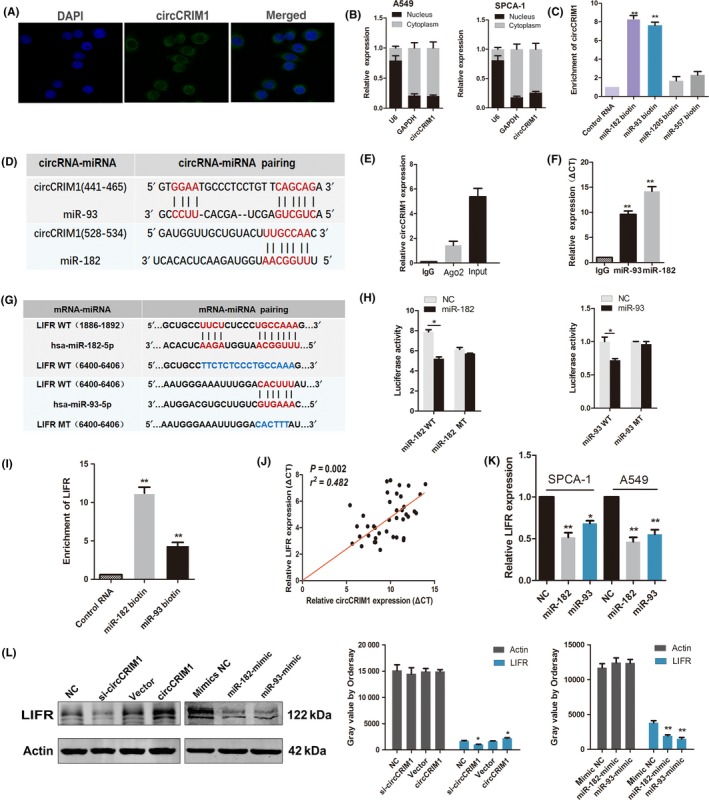
Circular RNA circCRIM1 suppresses cell metastasis through the circCRIM1‐microRNA (miR)‐182/93‐leukemia inhibitory factor receptor (LIFR) axis. A,B, FISH (A) and separation of cytoplasm and nucleus fraction RNA analysis (B) show that circCRIM1 predominantly resides in the cytoplasm of lung adenocarcinoma cells. C, Pull‐down assay using biotin‐coupled miR‐182, miR‐93, miR‐1205, and miR‐557. D, Through bioinformatics analysis, hsa‐miR‐93 and hsa‐miR‐182 were predicted to be the targets of circCRIM1. E,F, RIPA using an Ab against Ago2. Input was used as positive control. G,H, Luciferase activities of WT LIFR 3′‐UTR and mutant LIFR 3′‐UTR after transfection with miR‐182‐5p mimic and miR‐93‐5p mimic. I, Quantitative real‐time PCR analysis of the expression of LIFR in A549 lysates after biotin‐coupled miR‐182 and miR‐93 pull‐down assays. J, circCRIM1 is positively correlated with LIFR in the expression analysis of 40 lung adenocarcinoma patients. K, Quantitative real‐time PCR of the expression levels of LIFR. L, Western blot analysis of the expression levels of LIFR after cotransfection with si‐circCRIM1, circCRIM1 overexpression plasmid, and miR‐182 and miR‐93 mimics. NC, negative control
Recent studies show that circRNAs exert functions in multiple ways, such as binding to proteins or possessing protein‐coding abilities.9 First, we explored the possibility that circCRIM1 might interact with proteins. We searched circRNADB (http://202.195.183.4:8000/circrnadb/circRNADb.php), a comprehensive database of human circRNAs with protein‐coding annotation, and found no results for circCRIM1. It is generally accepted that circRNAs contain binding sites complementary to miRNAs and act as miRNA “sponges”. MicroRNAs usually silence gene expression by combining with the Ago2 protein and form the RNA‐induced silencing complex. The ceRNA mechanism showed a prevalent phenomenon that Ago2 could bind with both circRNAs and miRNAs, based on previous studies.17, 19, 20 Considering the relatively low abundance of circRNAs and the various miRNA binding sites within mRNA and circRNA transcripts, sponging multiple miRNAs by a single circRNA could be a general mechanism in cells.18 Through bioinformatics analysis (miRanda software, the CircInteractome database, and RNAhybrid software),21 we undertook a miRNA pull‐down assay using biotin‐coupled miRNA mimics (miR‐182, miR‐93, miR‐1205, and miR‐557; Figures 4D and S3). Interestingly, circCRIM1 was only efficiently enriched with miR‐182 and miR‐93, but not the other miRs (Figure 4C). hsa‐miR‐93 and hsa‐miR‐182 were predicted to be the targets of circCRIM1 (Figure 4D). To validate the direct binding of circCRIM1 with miR‐93 and miR‐182, we undertook RIPA with anti‐Ago2 or control anti‐IgG Abs. In addition, miRNA and circCRIM1 expression were analyzed using qRT‐PCR. Results showed that circCRIM1, miR‐182, and miR‐93 were significantly enriched in the RNA retrieved with the Ago2 Ab compared with that of the control (Figure 4E,F).
3.4. Both miR‐182 and miR‐93 decrease LIFR expression
To identify the potential target genes of miR‐182 and miR‐93, we used the TargetScan prediction program. We filtered genes that were positively correlated with circCRIM1 expression in our microarray data (Figure S4 and Table S4). We found that LIFR was suggested to be a target gene of both hsa‐miR‐93 and hsa‐miR‐182 (Figure 4G). To confirm this finding, a luciferase reporter assay was carried out. A significant decrease in luciferase activity was observed when cells were cotransfected with the miR‐182 and miR‐93 mimics but not with the mutant luciferase reporter (Figure 4H). Biotin pull‐down assays indicated that the miR‐182‐ and miR‐93‐captured fractions were distinctly enriched with LIFR (Figure 4I). There was also a positive correlation between circCRIM1 expression and LIFR expression in the cohort of 40 LUAC patients (Figure 4J). Furthermore, the transfection of miR‐93 and miR‐182 mimics reduced the expression of circCRIM1 (Figure 4K). These results suggest that circCRIM1 functions as a sponge for LUAC‐related tumor promoter miRNAs. Correspondingly, LIFR protein levels coincided with the change in mRNA levels in miR‐182‐ and miR‐93‐overexpressing cells. The knockdown of circCRIM1 decreased LIFR expression, whereas the overexpression of circCRIM1 produced the opposite results (Figure 4L). We found expression levels of both miR‐93 and miR‐182 are negatively correlated with LIFR in the expression cohort of 20 LUAC patients (Figure S5). These results indicated that LIFR was a direct target of miR‐182 and miR‐93.
3.5. Leukemia inhibitory factor receptor inhibits LUAC cell migration and invasion
Given that miR‐182 and miR‐93 targeted LIFR and suppressed its expression, we investigated the biological function of LIFR in LUAC cells. Leukemia inhibitory factor receptor was almost 3.7‐fold downregulated in pathologically diagnosed LUAC samples compared with their paired peripheral normal lung tissues from The Cancer Genome Atlas database (http://www.targetscan.org/vert_71/) (Figure 5A). These data were also utilized to visualize the association between LIFR expression and the overall survival of LUAC patients. The results showed that patients with high LIFR expression in tumors had a trend towards improved survival compared with patients with low LIFR expression (HR = 0.43, P < .01; Figure 5B). We validated that LIFR was almost 5.42‐fold downregulated in 40 pathologically diagnosed LUAC samples compared with their paired peripheral normal lung tissues by using qRT‐PCR (Figure 5C).
Figure 5.
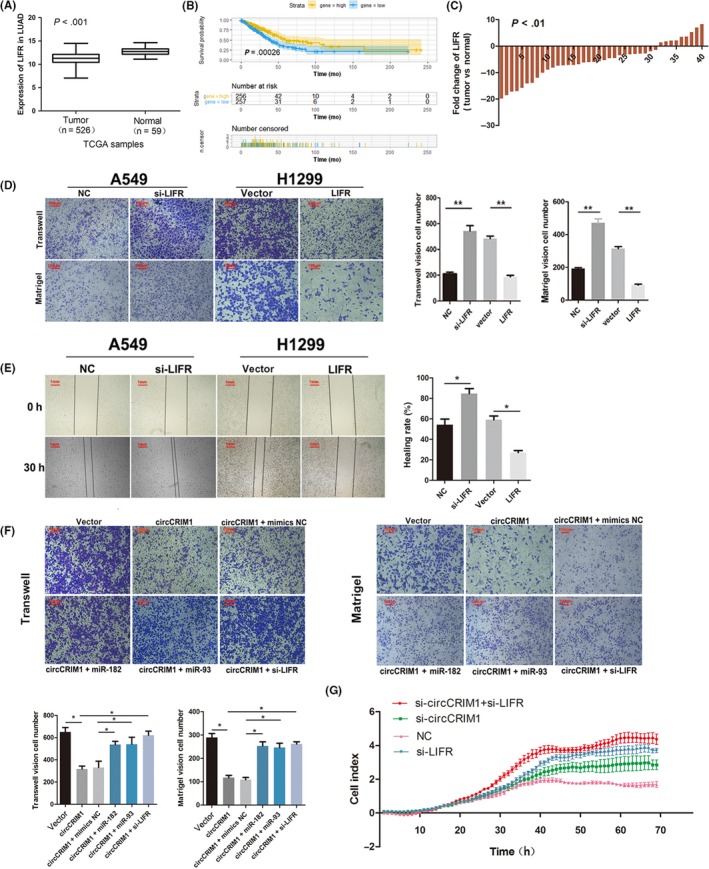
Leukemia inhibitory factor receptor (LIFR) is the direct target of miR‐182 and miR‐93 and suppresses leading cause of lung adenocarcinoma (LUAC) cell metastasis. A, LIFR was downregulated in LUAC samples compared with their paired peripheral normal lung tissues from The Cancer Genome Atlas (TCGA) database. B, Data from TCGA showed that patients with high LIFR expression in tumors had improved survival (P < .01). C, Quantitative real‐time PCR assay indicated the low expression of LIFR in 40 pairs of human LUAC tissues compared with their adjacent normal tissues. D,E, Matrigel and Transwell (D) and wound healing assays (E) showed LIFR markedly inhibited cell migratory abilities. F, Rescue experiments (Transwell and Matrigel assays) showed that circCRIM1 suppresses cell metastasis through the circCRIM1‐microRNA (miR)‐182/93‐LIFR axis. G, Real‐time cell analysis experiment identified the migration ability of LUAC cells. NC, negative control
To evaluate the biological effects of LIFR, we undertook Matrigel, Transwell, and wound healing assays in A549 and H1299 cells. The LIFR‐overexpressing cells showed markedly inhibited cell invasion and migration (Figure 5D,E). Both miR‐182 and miR‐93 act as oncogenic miRNAs to regulate diverse biological processes in lung cancer. Previous studies have confirmed that the overexpression of miR‐182 and miR‐93 could significantly promote cell proliferation, migration, and invasion by directly targeting antioncogenes. Moreover, the high expression of miR‐182 and miR‐93 is significantly correlated with poor prognosis in LUAC and many other carcinomas.22, 23, 24, 25, 26 Subsequent rescue experiments showed that transfecting miR‐182 and miR‐93 mimics into circCRIM1‐overexpressing LUAC cells significantly reduced the circCRIM1‐mediated suppression of cell invasion and migration. And LIFR downregulation significantly increased the circCRIM1 overexpression‐induced suppression of cell invasion and migration (Figure 5F). Thus, RTCA experiments identified the migration ability of LUAC cells comparing the outcome of si‐circCRIM1, si‐LIFR, and si‐circCRIM1 + si‐LIFR. It was clarified that circCRIM1 suppresses invasion and migration through the miR‐182/93‐LIFR axis and, according to the rescue experiments, circCRIM1's function partly depended on LIFR (Figure 5G).
3.6. circCRIM1 serves as a biomarker of LUAC
Expression of circCRIM1 was also detected in LUAC tissues with CISH of the TMA of 92 pairs of LUAC and adjacent normal tissues. There was also a negative correlation between circCRIM1 expression and lymph node metastasis and TNM stage (Figure 6A, Table 1). Kaplan‐Meier survival curves showed that patients with higher levels of circCRIM1 had a longer overall survival (HR = 0.5896; 95% CI, 0.3647‐0.9532, P = .03; Figure 6B). Multivariate analyses indicated that low circCRIM1 levels are an independent poor prognosis factor for LUAC patients (HR = 2.502; 95% CI, 1.307‐4.792, P = .006; Figure 6C).
Figure 6.
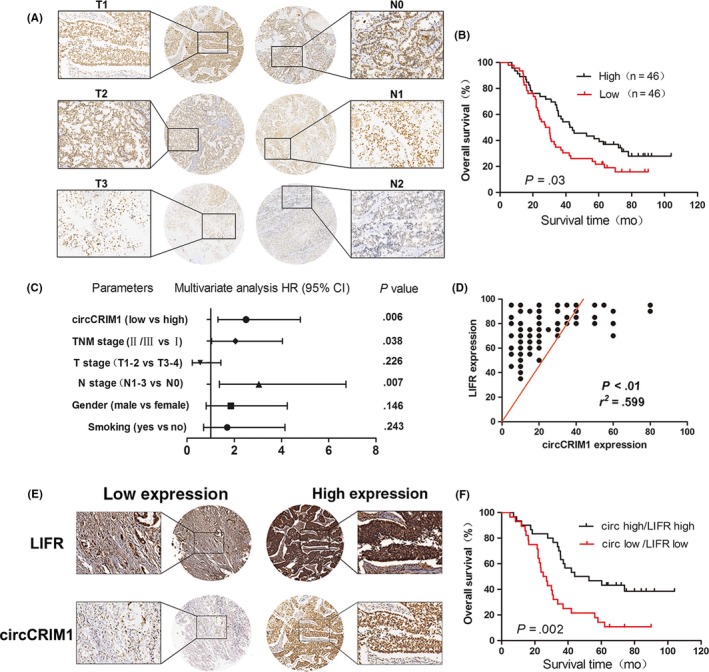
Analysis of circular RNA circCRIM1 expression in lung adenocarcinoma (LUAC) tissues and clinical parameters. A, circCRIM1 expression was detected in LUAC tissues by chromogenic in situ hybridization using tissue microarray (n = 92). B, Kaplan‐Meier survival curves showed that patients with higher levels of circCRIM1 had increased overall survival (OS) (hazard ratio [HR] = 0.5896; 95% confidence interval [CI], 0.3647‐0.9532; P = .03). C, Multivariate analyses of HRs for OS. D,E, Tissue microarray (n = 92) confirmed the interaction between circCRIM1 and leukemia inhibitory factor receptor (LIFR). F, Kaplan‐Meier plots of the OS rates of 92 LUAC patients with high (black) or low (red) expression levels of circCRIM1/LIFR (HR = 0.3663; 95% CI, 0.1942‐0.6909, P = .002)
Table 1.
Correlation between expression of circular RNA circCRIM1 and the clinicopathological features of patients with lung adenocarcinoma
| Variables |
Cases (n) (total n = 92) |
circCRIM1 | P value | |
|---|---|---|---|---|
| Low (n) | High (n) | |||
| Age (y) | ||||
| ≤60 | 42 | 24 | 18 | .209 |
| >60 | 50 | 22 | 28 | |
| Gender | ||||
| Male | 48 | 25 | 23 | .676 |
| Female | 44 | 21 | 23 | |
| Smoking status | ||||
| Ever and current | 40 | 18 | 22 | .400 |
| Never | 52 | 28 | 24 | |
| Tumor size | ||||
| ≤5 cm | 54 | 24 | 30 | .204 |
| >5 cm | 38 | 22 | 16 | |
| Lymph node invasion | ||||
| Present | 43 | 27 | 16 | .022 |
| Absent | 49 | 19 | 30 | |
| TNM stage | ||||
| I | 41 | 15 | 26 | .021 |
| II‐III | 51 | 31 | 20 | |
Furthermore, we detected the expression of both circCRIM1 and LIFR using the same TMA of 92 pairs of LUAC samples. The results confirmed the interaction between circCRIM1 and LIFR (Figure 6D,E). Kaplan‐Meier analysis showed that the high expression of circCRIM1/LIFR was associated with increased overall survival in 92 LUAC patients. The median survival times were 48.28 months and 26 months in the high and low groups, respectively (HR = 0.3663; 95% CI, 0.1942‐0.6909, P = .002; Figure 6F).
4. DISCUSSION
Although surgical techniques and radiochemotherapy regimens have improved in recent decades, the survival rate of lung cancer has not increased as we expected. Therefore, it is urgent to explore the molecular mechanisms of the carcinogenesis and progression of LUAC to develop novel strategies to improve LUAC prognosis.
Circular RNAs are promising potential biomarkers because of their unique structure, high stability, and specific expression patterns.27 It has been reported that circRNAs are aberrantly expressed in tumors and function as tumor suppressors or oncogenes in the progression of cancer pathogenesis. Several studies suggest that circRNAs regulate gene expression and participate in numerous cellular processes by sponging miRNAs.6, 28 In addition, one circRNA could sponge multiple miRNAs. circHIPK3 has been confirmed to sponge 9 miRNAs (miR‐124, miR‐152, miR‐29a, miR‐29b, miR‐193a, miR‐338, miR‐379, miR‐584, and miR‐654), as evidenced by luciferase activity assay. Furthermore, the overexpression of circHIPK3 has an antagonistic effect on miR‐124 in cell proliferation.29 circCCDC66 sponges multiple miRNAs to promote colorectal cancer growth and metastasis.12
To date, only a few circRNAs have been reported in LUAC. circPRKCI functioned as a sponge for both miR‐545 and miR‐589 and abrogated their suppression of the protumorigenic transcription factor E2F7.18 Similarly, the echinoderm microtubule associated protein‐like 4—anaplastic lymphoma kinase (EML4‐ALK)‐v3b‐derived circRNA, F‐circEA‐2a, promotes cell migration and invasion, supporting the significant diagnostic value of EML4‐ALK‐positive non‐small‐cell lung cancer.30 These studies exemplify circRNAs as oncogenes contributing to LUAC progression. However, the function of downregulated circRNAs in LUAC still comprises unexplored territory. Thus, our study indicates that circCRIM1 is downregulated in LUAC and plays crucial inhibitory roles in LUAC metastasis.
In this study, circBase and CircInteractome were used to predict circRNAs, and the circRNAs were selected with the relatively unbiased approach of computational algorithms. We gradually confirmed the regulatory role of circCRIM1 and its sponging effect on miRNAs in LUAC through functional and molecular experiments. We found that circCRIM1 was downregulated and correlated with poor clinical outcomes in LUAC. Moreover, we found that circCRIM1 was negatively correlated with TNM stage and LUAC lymph node metastasis. Further experiments showed that circCRIM1 could inhibit the invasion and metastasis of LUAC cells in vitro and in vivo. Vimentin, E‐cadherin, and N‐cadherin are mesenchymal markers responsible for maintaining cell shape and stabilizing cytoskeletal interactions. They function as organizers of a number of critical proteins involved in cell attachment and migration.31 These changes of epithelial‐mesenchymal transition markers in our study also confirmed that circCRIM1 could suppress the migration and invasion of LUAC cells.
These results revealed that circCRIM1 plays a tumor‐suppressive role in LUAC progression and could represent a potential novel biomarker and a therapeutic target of LUAC.
Many studies validated that circRNA could bind with Ago2 and sponge miRNAs.19 In the current study, in vitro precipitation, luciferase reporter assay, and biotin‐coupled miRNA capture were undertaken to reveal the Ago2‐dependent interaction of circCRIM1 and miR‐93/miR‐182.
MicroRNA, a key component of the noncoding RNA family, plays multifaceted roles in controlling cellular functions by repressing target genes. Recently, several studies have implicated miR‐182 in numerous pathways and diseases. MicroRNA‐182 can promote cancer metastasis by targeting Forkhead box O3, micropthalmia‐associated transcription factor, metastasis suppressor protein 1, and Reversion‐inducing cysteine‐rich protein with Kazal motifs.32, 33, 34 MicroRNA‐182 is dysregulated and inversely correlated with Pyruvate dehydrogenase kinase 4 (PDK4) in human lung adenocarcinomas. The miR‐182‐PDK4 axis regulates lung cancer cell growth by modulating the activity of pyruvate dehydrogenase and subsequently promotes lung tumorigenesis.22 MicroRNA‐93‐5p is also upregulated in non‐small‐cell lung cancer and plays an oncogenic role by inhibiting PTEN and retinoblastoma 1.26
Mechanistically, we found that circCRIM1 could promote the expression of LIFR, a well‐known tumor suppressor, by sponging miR‐93 and miR‐182. As shown in Figure 7, circCRIM1 binds to both miRNAs and subsequently inhibits their ability to suppress LIFR.
Figure 7.
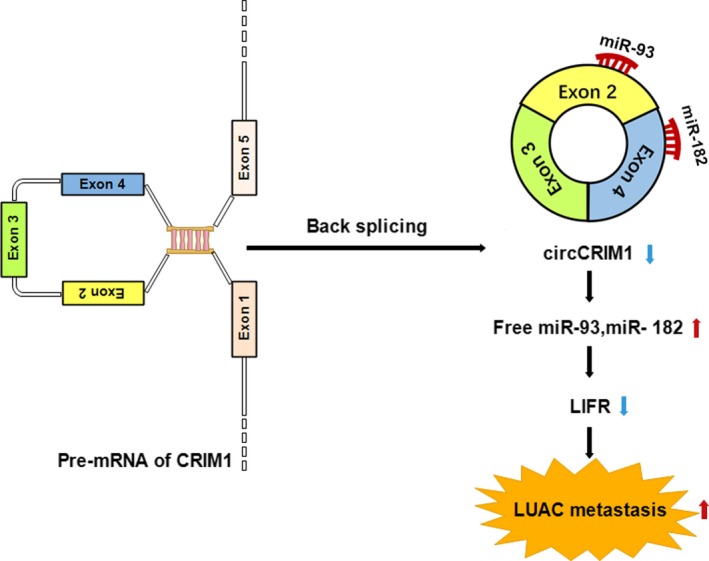
Proposed working model of circular RNA circCRIM1 in lung adenocarcinoma (LUAC). Upregulation of circCRIM1 exerts the invasion‐suppression effect dependent on the circCRIM1‐microRNA (miR)‐182/93‐leukemia inhibitory factor receptor (LIFR) axis
Recently, a few studies have implicated that LIFR functions as a novel metastasis suppressor in cancer metastasis. The metastasis of tumor cells involves a complex series of biological steps that enable cells to overcome multiple barriers erected by normal tissues. Luo et al reported that LIFR knockdown could activate PI3K/AKT signaling through enhancing the phosphorylation of JAK1, which subsequently promoted MMP13 expression and hepatocellular carcinoma metastasis. The combination of LIFR and p‐AKT or MMP13 was a powerful predictor of poor prognosis for hepatocellular carcinoma patients.35 Additionally, LIFR is downregulated in human breast cancer, and it functions as a breast cancer metastasis suppressor. The downregulation of LIFR also significantly correlates with poor clinical outcomes of breast cancer patients.36, 37 We found that LIFR was downregulated and correlated with poor clinical outcomes in LUAC according to the Kaplan‐Meier calculations and The Cancer Genome Atlas database (http://www.targetscan.org/vert_71/). We confirmed this result by quantifying LIFR expression in LUAC tissues using a TMA of 92 pairs of LUAC and adjacent nontumor tissues. Our experiments indicated that LIFR is associated with a suppressed LUAC phenotype in LUAC cells in vitro and that LIFR could be a potential therapeutic target against LUAC metastasis.
As a member of the gp130 receptor family, LIFR is structurally similar to gp130, which is an interleukin‐6 signal transducer.38 After Leukemia inhibitory factor (LIF) forms multimeric complexes with gp130, LIF binds the complexes and subsequently activates the JAK/STAT, MAPK, and PI3K/AKT signaling pathways.39 Future studies concerning LIFR‐mediated PI3K and AKT phosphorylation are needed to fully elucidate the mechanism underlying LIFR function in LUAC.
In summary, our findings provide new insights into LUAC metastasis and a novel strategy for LUAC diagnosis and treatment.
DISCLOSURE
The authors declare that they have no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81702892, 81672294, 81572261, and 81802902) and the scientific research project of Jiangsu Provincial Commission of Health and Family Planning (Nos. H2018112 and Z201606).
Wang L, Liang Y, Mao Q, et al. Circular RNA circCRIM1 inhibits invasion and metastasis in lung adenocarcinoma through the microRNA (miR)‐182/miR‐93‐leukemia inhibitory factor receptor pathway. Cancer Sci. 2019;110:2960–2972. 10.1111/cas.14131
Lin Wang, Yingkuan Liang, and Qixing Mao contributed equally to this work.
Contributor Information
Lin Xu, Email: xulin_83@hotmail.com.
Feng Jiang, Email: zengnljf@hotmail.com.
Gaochao Dong, Email: ilsyvm@163.com.
REFERENCES
- 1. Dougan M, Li D, Neuberg D, et al. A dual role for the immune response in a mouse model of inflammation‐associated lung cancer. J Clin Invest. 2011;121:2436‐2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Didkowska J, Wojciechowska U, Manczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med. 2016;4:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meng YB, He X, Huang YF, et al. Long noncoding RNA CRNDE promotes multiple myeloma cell growth by suppressing miR‐451. Oncol Res. 2017;25:1207‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Li Y, Zheng Q, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208‐219. [DOI] [PubMed] [Google Scholar]
- 5. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333‐338. [DOI] [PubMed] [Google Scholar]
- 6. Guo JU, Agarwal V, Guo H, et al. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu S, Yang X, Li X, et al. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141‐148. [DOI] [PubMed] [Google Scholar]
- 8. Zhao ZJ, Shen J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017;14:514‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Z, Huang C, Bao C, et al. Exon‐intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256‐264. [DOI] [PubMed] [Google Scholar]
- 10. Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsiao KY, Lin YC, Gupta SK, et al. Non‐coding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang HD, Jiang LH, Sun DW, et al. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2017;25:1‐7. [DOI] [PubMed] [Google Scholar]
- 14. Burd CE, Jeck WR, Liu Y, et al. Expression of linear and novel circular forms of an INK4/ARF‐associated non‐coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;12:10012‐10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosal S, Das S, Sen R, et al. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang X, Zhang Z, Qiu M, et al. Glypican‐5 is a novel 5 metastasis suppressor gene in non‐small cell lung cancer. Cancer Lett. 2013;341:265‐273. [DOI] [PubMed] [Google Scholar]
- 17. Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu M, Xia W, Chen R, et al. The circular RNA circPRKCI promotes tumor growth in lung adenocarcinoma. Cancer Res. 2018;78:2839‐2851. [DOI] [PubMed] [Google Scholar]
- 19. Yu CY, Li TC, Wu YY, et al. The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat Commun. 2017;8:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang K, Gan TY, Li N, et al. Circular RNA mediates cardiomyocyte death via miRNA‐dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24:1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu H, Gong Z, Shen Y, et al. Circular RNA expression in extracellular vesicles isolated from serum of patients with endometrial cancer. Epigenomics. 2018;10:187‐197. [DOI] [PubMed] [Google Scholar]
- 22. Li G, Li M, Hu J, et al. The microRNA‐182‐PDK4 axis regulates lung tumorigenesis by modulating pyruvate dehydrogenase and lipogenesis. Oncogene. 2017;36:989‐998. [DOI] [PubMed] [Google Scholar]
- 23. Wang D, Lu G, Shao Y, et al. MiR‐182 promotes prostate cancer progression through activating Wnt/β‐catenin signal pathway. Biomed Pharmacother. 2018;99:334‐339. [DOI] [PubMed] [Google Scholar]
- 24. Cao MQ, You AB, Zhu XD, et al. Correction to: miR‐182‐5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018;1:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C, Lyu J, Meng QH. MiR‐93 promotes tumorigenesis and metastasis of non‐small cell lung cancer cells by activating the PI3K/Akt pathway via inhibition of LKB1/PTEN/CDKN1A. J Cancer. 2017;8:870‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang W, Bai J, Liu D. MiR‐93‐5p up‐regulation is involved in non‐small cell lung cancer cells proliferation and migration and poor prognosis. Gene. 2018;647:13‐20. [DOI] [PubMed] [Google Scholar]
- 27. Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42‐51. [DOI] [PubMed] [Google Scholar]
- 28. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;7441:333‐338. [DOI] [PubMed] [Google Scholar]
- 29. Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan S, Sun D, Pu W, et al. Circular RNA F‐circEA‐2a derived from EML4‐ALK fusion gene promotes cell migration and invasion in non‐small cell lung cancer. Mol Cancer. 2018;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol. 2014;50:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segura MF, Hanniford D, Menendez S, et al. Aberrant miR‐182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia‐associated transcription factor. Proc Natl Acad Sci USA. 2009;106:1814‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiang CH, Hou MF, Hung WC. Up‐regulation of miR‐182 by beta‐catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta. 2013;1830:3067‐3076. [DOI] [PubMed] [Google Scholar]
- 34. Lei R, Tang J, Zhuang X, et al. Suppression of MIM by microRNA‐182 activates RhoA and promotes breast cancer metastasis. Oncogene. 2014;33:1287‐1296. [DOI] [PubMed] [Google Scholar]
- 35. Luo Q, Wang C, Jin G, et al. LIFR functions as a metastasis suppressor in hepatocellular carcinoma by negatively regulating phosphoinositide 3‐kinase/AKT pathway. Carcinogenesis. 2015;36:1201‐1212. [DOI] [PubMed] [Google Scholar]
- 36. Chen D, Sun Y, Wei Y, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo‐YAP pathway and a prognostic marker. Nat Med. 2012;18:1511‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hergovich A. YAP‐Hippo signalling downstream of leukemia inhibitory factor receptor: implications for breast cancer. Breast Cancer Res. 2012;14:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolf J, Waetzig GH, Chalaris A, et al. Different soluble forms of the Interleukin‐6 family signal transducer gp130 fine‐tune the blockade of Interleukin‐6 trans‐signaling. J Biol Chem. 2016;291:16186‐16196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kosfeld A, Wolf J, Waetzig GH, et al. Mutations in the leukemia inhibitory factor receptor (LIFR) gene and Lifr deficiency cause urinary tract malformations. Hum Mol Genet. 2017;26:1716‐1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


